Abstract
Two splice variants of the α6 integrin subunit, α6A and α6B, with different cytoplasmic domains, have previously been described. While α6B is expressed throughout the development of the mouse, the expression of α6A begins at 8.5 days post coitum and is initially restricted to the myocardium. Later in ontogeny, α6A is found in various epithelia and in certain cells of the immune system. In this study, we have investigated the function of α6A in vivo by generating knockout mice deficient for this splice variant. The Cre- loxP system of the bacteriophage P1 was used to specifically remove the exon encoding the cytoplasmic domain of α6A in embryonic stem cells, and the deletion resulted in the expression of α6B in all tissues that normally express α6A. We show that α6A−/− mice develop normally and are fertile. The substitution of α6A by α6B does not impair the development and function of the heart, hemidesmosome formation in the epidermis, or keratinocyte migration. Furthermore, T cells differentiated normally in α6A−/− mice. However, the substitution of α6A by α6B leads to a decrease in the migration of lymphocytes through laminin-coated Transwell filters and to a reduction of the number of T cells isolated from the peripheral and mesenteric lymph nodes. Lymphocyte homing to the lymph nodes, which involves various types of integrin–ligand interactions, was not affected in the α6A knockout mice, indicating that the reduced number of lymph node cells could not be directly attributed to defects in lymphocyte trafficking. Nevertheless, the expression of α6A might be necessary for optimal lymphocyte migration on laminin in certain pathological conditions.
Keywords: integrin, laminin receptor, knockout, migration, lymphocyte
The adhesion receptors of the integrin family play a major role in various physiological and developmental processes by regulating cell adhesion, migration, differentiation, and proliferation (Hynes, 1992). Integrins are transmembrane heterodimers formed by noncovalently linked α and β subunits. While the extracellular domain of integrins mediates cell–cell or extracellular matrix–cell interactions, the cytoplasmic domain provides a link with proteins of the cytoskeleton and is involved in the transmission of intracellular signals (Clark and Brugge, 1995).
16 α and 8 β subunits have been identified so far, constituting a family of over 20 distinct receptors. A further degree of diversity arises from alternative RNA splicing, which produces extracellular and cytoplasmic variants of some of these subunits. Splice variants of the extracellular domain of α6 (Delwel et al., 1995), α7 (Ziober et al., 1993), and αIIβ (Bray et al., 1990) and of the cytoplasmic domain of α3 (Takada et al., 1991), α6 (Cooper et al., 1991; Hogervorst et al., 1991), α7 (Song et al., 1993; Ziober et al., 1993), β1 (Altruda et al., 1990; Languino and Ruoslahti, 1992; van der Flier et al., 1995; Zhidkova et al., 1995), β3 (van Kuppevelt et al., 1989; Kumar et al., 1997), and β4 (Hogervorst et al., 1990; Suzuki and Naitoh, 1990; Tamura et al., 1990; Clarke et al., 1994; van Leusden et al., 1997) subunits have been described.
The α6 subunit dimerizes with either the β1 or the β4 subunit to form receptors for various laminin isoforms (Delwel and Sonnenberg, 1996). Two splice variants of the cytoplasmic domain of α6, α6A and α6B, have been identified (Cooper et al., 1991; Hogervorst et al., 1991), the cytoplasmic domains of which are encoded by separate exons and which present entirely different sequences with the exception of the GFFKR motif, present in all α subunits. The α6A and α6B mRNA variants are generated by pre-mRNA splicing in such a way that exon A sequences are either retained in or removed from the primary transcript. In the α6A transcripts, a stop codon at the 3′ end of exon A prevents translation to continue further into exon B. The cytoplasmic sequences of α6A and α6B are conserved in mammalian species, suggesting that the existence of the two forms may be functionally advantageous. This hypothesis is further supported by the homologies existing between the α6 splice variants and the variants of two other laminin-binding subunits, α3 and α7.
Little is known about the functions of α6A and α6B. Transfection experiments have shown that whether α6β1 or α6β4 contain α6A or α6B makes no difference in the regulation of their binding activity or ligand specificity (Delwel et al., 1993; Shaw et al., 1993 a) or the transduction of inside-out signals, although only the variant A is phosphorylated upon phorbolester treatment (Hogervorst et al., 1993a ; Shaw and Mercurio, 1993 b). The subcellular localization of the two variants and their interaction with the cytoskeleton appear to be cell type specific: while both variants distribute to the focal contacts in many cell lines, staining of α6B revealed a punctate pattern distinct from focal adhesions in embryonic fibroblasts (Cattelino et al., 1995). Differential interactions with cytoskeleton proteins were further suggested by the finding that α6A induced the formation of pseudopodia in a macrophage cell line and promoted cell migration on laminin-1 to a greater extent than α6B (Shaw et Mercurio, 1994). This might be related to the quantitative differences in tyrosine phosphorylation of certain proteins, including paxillin, upon ligation of integrins containing either of the two variants in these cells (Shaw et al., 1995). This property of α6A to facilitate cell migration was also observed in embryonic stem (ES)1 cells (Domanico et al., 1997) and could be crucial during development and tissue remodeling.
The hypothesis that α6A and α6B differentially regulate cell behavior is further supported by their specific distribution patterns in both embryonic and adult tissues. While omnipotent mouse ES cells only express the α6B variant in vitro, their differentiation was found to be correlated with the expression of α6A (Cooper et al., 1991; Hierck et al., 1993). Similarly, α6B is found in the earliest stages of embryonic development, whereas expression of α6A does not start until 8.5 days post coitum (dpc) and is initially restricted to the myocardium (Collo et al., 1995; Thorsteinsdóttir et al., 1995). At this stage, α6A is present in the heart in a gradient from strong expression in the atrium to a weaker expression in the ventricle. By 12.5 dpc, this gradient has disappeared, and α6A is found in other tissues, including the epidermis, the gonads, and the epithelium of the digestive tract (Thorsteinsdóttir et al., 1995). In the adult mouse, α6A is expressed in the epidermis, in the epithelia of the mammary gland and the digestive tract, in the mature gonads, and in Schwann cells (Hogervorst et al., 1993b ; Salanova et al., 1995). However, it is no longer present in the adult myocardium. From early development to the adult stage, α6B is expressed in the kidney, in endothelia, in certain epithelia, and in the nervous system (Hogervorst et al., 1993b ).
Interestingly, the expression of α6 splice variants is regulated during the development of thymic endothelial and stromal cells and cells of the thymocyte/T cell lineage. Thus, while α6B is the first variant detected in the thymus at 10 dpc, α6A becomes expressed later during ontogeny, in association with both β1 and β4 subunits (Ruiz et al., 1995). The expression of α6 on endothelial thymic cells appears to be important for proper maturation of the immune system since anti-α6 antibodies block homing of T cell progenitors to the thymus (Ruiz et al., 1995), where differentiation into CD4+ or CD8+ cells occurs. In the T cell lineage, α6A and α6B are present on immature thymocytes, but mature cells no longer express α6 (Ruiz et al., 1995). However, both splice variants are found on human peripheral blood T lymphocytes (Chang et al., 1995).
Although the spatial and temporal regulation of α6A and α6B expression strongly suggests that the splice variants have specific functions during embryogenesis as well as in various organs at the adult age, this has never been studied in vivo. To answer this question and to test the tentative conclusions from results obtained in vitro, we have generated exon-specific knockout mice in which the exon encoding the cytoplasmic domain of α6A is deleted. As a consequence, only exon B is inserted in the α6 mRNA, which results in the replacement of α6A by α6B in all tissues that normally express only α6A. Classical ES cell technology allows the generation of null mutations by simple ablation and replacement of the gene of interest by a selection marker cassette through homologous recombination. This approach could not be used in the case of an exon-specific knockout since the selection marker remaining in the gene after homologous recombination might affect the splicing of the remaining exons. Therefore, we made use of the Cre-loxP system of the bacteriophage P1 (Sauer and Henderson, 1988) to subsequently excise the selection marker cassette in the ES cells after replacement of exon A.
The analysis of the phenotype of the α6 exon A–specific knockout mice confirmed the involvement of α6A in some aspects of cell migration but also revealed a number of surprising and unexpected results.
Materials and Methods
Construction of the Targeting Vector
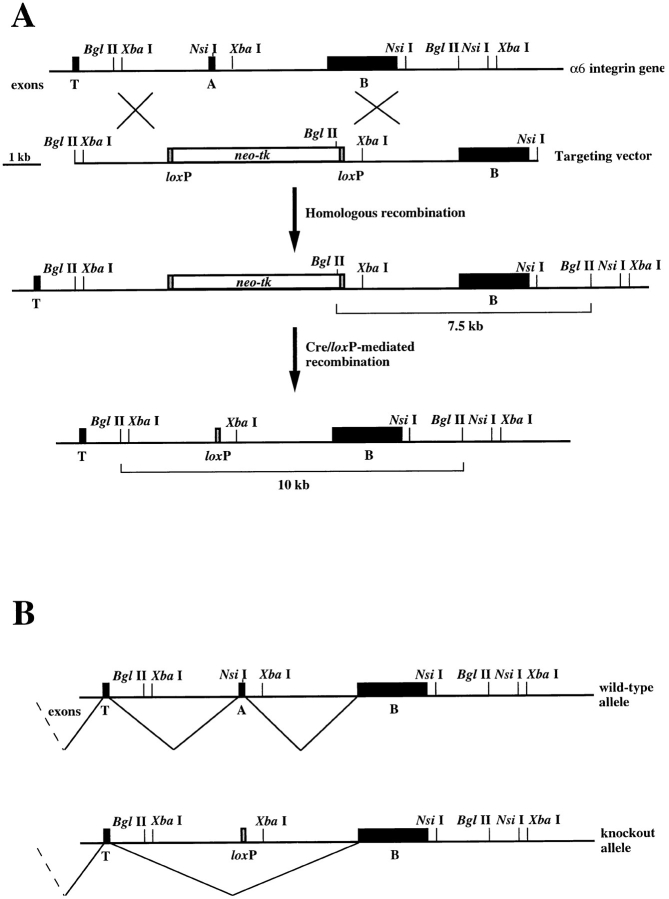
A 14-kb clone containing the exon A encoding the cytoplasmic domain of α6A and the exon B encoding the cytoplasmic domain of α6B was isolated from the λFIX-II 129/Sv mouse genomic library (Stratagene, La Jolla, CA) and used to construct the targeting vector. The exon A and the exon– intron boundaries were replaced by a BamHI site and a XhoI site using PCR in the 3-kb XbaI-XbaI DNA fragment (Fig. 1 A). Next, a gene cassette containing the neo r gene and the HSV-tk gene flanked by two loxP sites (a gift of Dr. R. Fässler, Lund University Hospital, Lund, Sweden) was inserted into the introduced BamHI and XhoI sites. Each selectable marker gene is under the control of a PGK promoter and contains PGK poly-adenylation sequences. The final targeting construct contained 2.8 kb of flanking genomic sequences further upstream from the exon A to the BglII site, and a 5.5-kb fragment including the exon B downstream from the exon A to the NsiI site.
Figure 1.
Strategy for exon A targeting. (A) Restriction maps of the 3′ end of the mouse α6 integrin gene, the targeting vector, the homologous recombinant allele, and the exon A–deleted mutant are shown. The targeting vector was designed to replace exon A by a neo-tk cassette flanked by two loxP sites. After homologous recombination, ES cells were transiently transfected with a plasmid encoding the Cre enzyme, which resulted in the removal of the neo-tk cassette, leaving a single loxP site behind. Homologously recombined clones were identified by Southern blot analysis as those containing a new 7.5-kb BglII fragment on hybridization with a cDNA probe corresponding to exon B. (B) In wild-type cells expressing α6A, exon A is spliced into the mature α6 mRNA together with exon B, which contains the polyadenylation sequence. In knockout cells, the exon encoding the transmembrane region (exon T) and exon B are connected in the mature α6 mRNA, leading to the exclusive expression of α6B.
Generation of α6A-deficient Mice
E14 ES cells were transfected with 80 μg of the targeting construct by electroporation. The transfected cells were grown on gelatin and selected in the presence of G418 (200 μg/ml). Homologous recombinant clones were identified by Southern blot hybridization with a cDNA probe corresponding to exon B. To excise the neo-tk cassette, several homologous recombinant clones were transfected with 10 μg of Cre-encoding plasmid by electroporation. Transfected cells were grown on irradiated mouse embryonic fibroblasts for 48 h. Cells were then trypsinized and seeded on a fibroblast monolayer at a density of 3 × 103 ES cells/cm2. Selection with 1.5 mM gancyclovir started 3 d later. Resistant clones were screened by PCR for the Cre-mediated recombination events using oligonucleotides in the intronic sequences adjacent to the exon A, 83 bp upstream of the exon A (5′-ACGGCACAGTGACTGCTCGCT-3′), and 36 bp downstream of the exon A (5′-GCCACCACAACCACAGCAGGT-3′). Two independent clones were isolated and expanded, and their karyotype was checked before injection into C57BL6 blastocysts. Male chimeric mice were bred with 129/OLA and FVB females to obtain heterozygous α6A+/− mice, identified by PCR. Intercrossing of these mice produced offspring homozygous for the mutation.
Histological Analysis
Whole embryos of 10.5 or 12.5 dpc and tissues from adult mice were collected and fixed in 20% ethanol/5% acetic acid/5% formalin for 48 h, embedded in paraffin, cut into 7-μm sections, and stained with haematoxylin/ eosin.
Immunofluorescence
Whole embryos of 12.5 dpc and skin samples from adult mice were collected and fixed in Tissue-Tek OCT compound (Miles Inc., Elkhart, IN) and frozen in liquid nitrogen. Cryosections (5 μm) were prepared and air dried. After blocking with PBS, 2% bovine serum albumin for 1 h, unfixed sections were incubated with primary antibodies for 1 h at 37°C. The following monoclonal antibodies were used: rat anti–integrin α6 (GoH3; Sonnenberg et al., 1987), mouse anti–α6A (1A10; Hogervorst et al., 1993a ,b) and mouse anti–α6B (PB36; de Melker et al., 1997), mouse anti-α3A (29A3; de Melker et al., 1997), and rat anti–mouse α7 (CA5; Yao et al., 1996). After washing in PBS, sections were incubated with FITC-conjugated and TRITC-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 45 min at 37°C, washed again in PBS, and mounted in Vectashield (Vector Laboratories, Inc., Burlingame CA).
Ultrastructural Analysis
Skin samples from adult mice were collected and fixed in 2.5% glutaraldehyde/0.1 M cacodylate buffer, pH 7.2, post-fixed in 1% OsO4/0.1 M cacodylate, stained en bloc with UO2Ac2, and embedded in a mixture of LX112 and Araldite. Thin sections were examined with an electron microscope (model CM10; Philips Electron Optics, Mahwah, NJ).
Wound Healing Experiments
Wounding by tail amputation was conducted as previously described (Guo et al., 1996). A small section of the tail end was amputated from age- and sex-matched wild-type and α6A−/− mice. 2 or 4 d after wounding, an additional segment of the tail was amputated, fixed in 20% ethanol/5% acetic acid/5% formalin for 48 h, embedded in paraffin, cut into 7-μm sections, and stained with haematoxylin/eosin.
Primary Keratinocyte Culture and Immunoprecipitations
Keratinocytes were isolated and cultured as previously described (Hennings, 1994) with some modifications. In brief, newborn mice were killed by decapitation, and the tail and limbs were removed. Skin was removed from the base of the tail to the head and rinsed in PBS containing penicillin/streptomycin, and fat tissue underlying the dermis was discarded. Skins were floated, dermis side down, on a 0.25% trypsin solution overnight at 4°C. Dermis was then removed with forceps and discarded. Epidermis was minced, and keratinocytes were mechanically isolated by stirring at 4°C for 30 min. The cell suspension was then filtered through a 70-μm nylon filter, and keratinocytes were centrifuged and seeded on Matrigel (Collaborative Biomedical Products, Becton Dickinson Labware, Mountain View, CA) in SFM keratinocyte medium (GIBCO BRL, Paisley, UK). From the first passage, cells were grown on tissue culture–treated plastic dishes.
Immunoprecipitation from lysates of 125I surface-labeled cells was performed as previously described (Hogervorst et al., 1993b ) using the following antibodies: anti-α6 (GoH3), anti-α6A (1A10), anti-α6B (PB36), anti-α3A (29A3), anti–mouse β4 (346-11A; Kennel et al., 1986), and rat anti–mouse β1 (MB1.2; von Ballestrem et al., 1996).
Keratinocyte Adhesion and Migration
For adhesion assays, subconfluent keratinocytes were trypsinized, washed twice in SFM medium, seeded in 96-well plates (105 cells/well) previously coated with laminin-1 (Collaborative Biomedical Products), laminin-5 (a gift from Dr. P. Rousselle, Institut de Biologie et Chimie des Protéines, Lyon, France), or fibronectin (Sigma Chemical Co., St. Louis, MO), and blocked with 1% BSA. After 50 min incubation at 37°C, cells were washed with PBS, fixed with 1% glutaraldehyde, and stained with crystal violet for 30 min. Cells were then thoroughly washed and solubilized in 0.2% Triton X-100. Adhesion was quantified as a measure for the OD at 540 nm.
For migration assays, 105 keratinocytes were seeded in the upper compartment of 8-μm-pore Transwell filters (Costar, Cambridge, MA) previously coated with 10 μg/ml of laminin-1, laminin-5, or fibronectin on the lower face of the filter only. After 6 or 18 h of migration, cells in the upper chamber of the filter were removed, and keratinocytes on the lower side of the filter were fixed in methanol and stained with crystal violet. The number of cells under the microscope was assessed by counting a minimum of five fields/filter.
Flow Cytometry
Total cell suspensions from the various lymphoid organs were isolated, and red blood cells were lysed by routine techniques. Cells were washed twice in PBS, incubated with purified anti–mouse CD32/CD16 (2.4G2; PharMingen, San Diego, CA) to block Fcγ receptors for 30 min at 4°C, and stained for flow cytometry with the following antibodies from PharMingen for 30 min at 4°C: PE-conjugated anti–mouse CD3ε (145-2C11), FITC-conjugated anti–mouse CD4 (L3T4, RM4-5), PE-conjugated anti– mouse CD8b.2 (Ly-3.2), FITC-conjugated anti–mouse IgM (R6-60.2), and biotin-conjugated anti–mouse B220/CD45R. The samples were washed three times in PBS and analyzed in a FACScan® using CellQuest software (Becton Dickinson Labware).
T Cell Purification and Proliferation
Axillary, brachial, inguinal, and mesenteric lymph nodes from 2-mo-old wild-type and α6A−/− mice were collected, and cells were extracted in Iscove's medium (GIBCO BRL) supplemented with 5% heat inactivated FCS, penicillin, streptomycin, and 30 μM β-mercaptoethanol. After lysis of red cells, cell suspensions were first depleted from large adhering cells by passing them through a nylonwool column (Polysciences, Inc., Warrington, PA) for 45 min at 37°C. Unbound cells (containing T lymphocytes) were subjected to further purification on anti-MHCII–coupled beads (anti–mouse I-Ad/I-Ed antibody 2G9 from PharMingen; beads from PerSeptive Biosystems, Inc., Framingham, MA). Purity was checked by FACS® analysis using a PE-conjugated anti–mouse CD3ε antibody, and cell preparations containing less than 97% of CD3-positive cells were discarded.
For proliferation assays, 96-well plates were first coated overnight at 4°C with anti-CD3ε followed by coating with mouse laminin-1 for 4 h at 37°C. T lymphocytes (105 cells/well) were cultured in 5% FCS Iscove's medium for 48, 72, or 96 h, pulsed with [3H]thymidine (0.5 μCi/well) for the last 12 h, and harvested onto glass fiber filter paper. Radioactivity was determined by liquid scintillation counting. In some cases, anti-CD28 antibody (1 μg/ml) was added to the wells.
In Vitro T Cell Migration
5-μm-pore Transwells (Costar) were coated overnight at 4°C with laminin-1 or fibronectin at the indicated concentrations on both sides of the filter and subsequently washed in PBS. SDF-1 or MCP-1 was added to 0.5% BSA Iscove's medium (GIBCO BRL) in the lower compartment of the Transwell only. 105 purified lymph node T lymphocytes (isolated from the axillary, brachial, inguinal, and mesenteric lymph nodes) were seeded in the upper compartment of the Transwell in 0.5% BSA Iscove's medium and allowed to migrate for 2 h. Migration was terminated by removal of the filter and counting of the cells collected on the bottom of the well.
In Vivo Homing Assay
Single cell suspensions were prepared from peripheral (axillary, brachial, and inguinal) and mesenteric lymph nodes from age- and sex-matched wild-type and α6A−/− mice and used as lymph node cells. Cells were washed twice in 0.5% Iscove's medium (GIBCO BRL) and labeled with either PKH2 or PKH26 fluorescent cell linkers (Sigma Chemical Co., St. Louis, MO) according to the manufacturer's instructions. In brief, cells were resuspended in diluent C, after which they were immediately added to an equal volume of a 4 μM PKH2 or PKH26 solution in diluent C. The final cell concentration was 107 cells/ml. The cells were then incubated at room temperature for 2 min, after which the staining reaction was stopped by the addition of an equal volume of FCS. After 1 min, an equal volume of Iscove's medium supplemented with 10% FCS was added to the cells. Cells were then washed four times in the same medium. Labeled α6A+/+ and α6A−/− cells were mixed in equal number and immediately injected into the tail vein of wild-type recipient animals (3 × 107 cells for each cell type). The percentage of PKH2- and PKH26-labeled cells in the cell mixture was checked by FACS® analysis. After 2 or 20 h, mice were killed, and the spleen, peripheral, and mesenteric lymph nodes were isolated. Cell suspensions were extracted from each organ, and the percentages of labeled wild-type (α6A+/+) and α6A−/− cells were determined by FACS® analysis.
Results
Generation of α6A Integrin–deficient Mice
The two cytoplasmic variants α6A and α6B result from alternative splicing of the α6 pre-mRNA (Cooper et al., 1991; Hogervorst et al., 1991). In the case of the variant A, exon A is inserted in the α6 mRNA, and the presence of a stop codon at the 3′ end of exon A prevents translation to continue further into exon B. In the case of α6B, an α6 RNA transcript is produced that lacks exon A sequences.
To define the function of the two α6 variants in the development of the mouse and to determine whether α6B could functionally replace α6A, we specifically deleted the exon A. To this end, we have used a gene targeting approach combining classical methods of gene inactivation by homologous recombination and the use of the Cre-loxP system (Fig. 1 A). This technique has been successfully used to generate knockout mice in which the exon coding for a splice variant of the β1 integrin subunit was selectively deleted (Baudoin et al., 1998). The Cre recombinase of the bacteriophage P1 excises DNA residing between repeats of 34 bp termed loxP sites, leaving one loxP site in the gene locus (Sauer and Henderson, 1988). We made use of this property to delete the selection marker cassette after inactivation of exon A. We predicted that the removal of the 4.8-kb cassette would allow normal splicing between the exon coding for the transmembrane domain of the integrin subunit and exon B (Fig. 1 B), and thus only α6B was expected to be expressed in all tissues that normally express α6A.
In the first step, a λFIX-II 129/Sv mouse genomic library was screened with a cDNA probe corresponding to the exon coding for the cytoplasmic domain of α6A. One of the resulting clones, mα6Aλ1, was characterized further, and its restriction map is shown in Fig. 1 A. The position of exon A and exon B was determined by PCR using oligonucleotides specific for these exons. The vector was designed to replace exon A and the exon–intron boundaries by a selection marker cassette containing the neo r gene for positive selection and the HSV-thymidine kinase gene for negative selection.
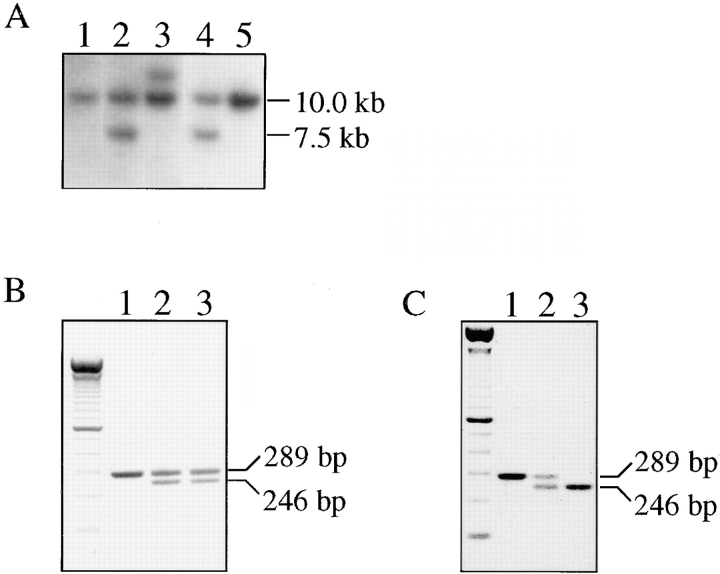
The linearized targeting vector was transfected in E14 ES cells by electroporation, and cells were subjected to positive selection (G418). Cell clones containing a recombined α6 allele were identified by Southern blot analysis after digestion of genomic DNA with BglII and hybridization with a cDNA probe corresponding to exon B. Targeted clones yielded a 7.5-kb band in addition to the 10-kb band corresponding to the wild-type allele (Fig. 2 A). 30% of the resistant clones were positive for homologous recombination.
Figure 2.
(A) Identification of homologous recombination in ES cells by Southern blot analysis. Lanes 1 and 5, a BglII digest of ES cell clones in which recombination did not occur; lane 3, a clone in which random recombination had occurred; lanes 2 and 4, two ES cell clones (6A4 and 9H4) in which homologous recombination had occurred. (B) Identification of Cre-loxP–mediated recombination in ES cells by PCR. The 289-bp band in lane 1 represents the wild-type allele, whereas the 246-bp band in lanes 2 and 3 represents the Cre-loxP–targeted allele. (C) PCR analysis of the genotypes. Lane 1, the genotype of a wild-type mouse; lane 2, a mouse heterozygous; lane 3, one homozygous for the mutation.
Clones that had undergone homologous recombination were transfected with a Cre-encoding plasmid and subjected to negative selection (gancyclovir). As the size of the loxP site remaining in the gene is smaller than that of exon A, PCR was used to identify the resistant colonies in which Cre-loxP–mediated recombination had occurred (Fig. 2 B), and the results were confirmed by Southern blot analysis (not shown). 90% of the resistant clones scored positive for Cre-loxP–mediated recombination. Two independent α6A+/− clones were used to generate chimeric males, which transmitted the mutated allele to their progeny. Mice heterozygous for the mutation in the α6 integrin gene were identified by PCR analysis on tail DNA (Fig. 2 C).
Deletion of Exon A Causes Replacement of α6A by α6B in Tissues That Normally Express α6A
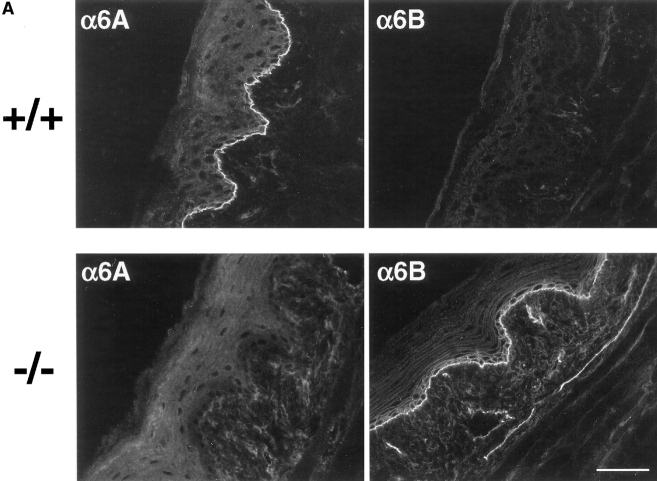
Intercrossing heterozygous animals produced offspring homozygous for the deletion, which occurred in the expected mendelian frequency. Such animals developed apparently normally and were fertile. These surprising results indicate that α6B can fully functionally replace α6A in mouse development. We first verified that the deletion of exon A caused α6A to be replaced by α6B in the embryonic myocardium by using antibodies directed against the cytoplasmic domain of α6A or α6B on tissue sections of wild-type (α6A+/+) and α6A−/− embryos collected at 12.5 dpc (Fig. 3). As previously described (Collo et al., 1995; Thorsteinsdóttir et al., 1995), the α6A variant is expressed in the myocardium of the atria and the ventricles of 12.5 dpc α6A+/+ embryos, and although a reaction was seen throughout the ventricle wall, the protruding cells at the inner face of the ventricular myocardium reacted more intensely than the peripheral cells. No staining for α6B could be detected in the myocardium. A weak staining of the blood vessels feeding the heart was observed with anti-α6B antibodies and probably corresponded to the expression of this variant in endothelial cells (Thorsteinsdóttir et al., 1995). This was in agreement with finding α6B mRNA by reverse transcription PCR in heart preparations (Thorsteinsdóttir et al., 1995). In contrast, the somites, the kidneys, and the head of the embryo strongly reacted with the anti-α6B antibody (not shown).
Figure 3.
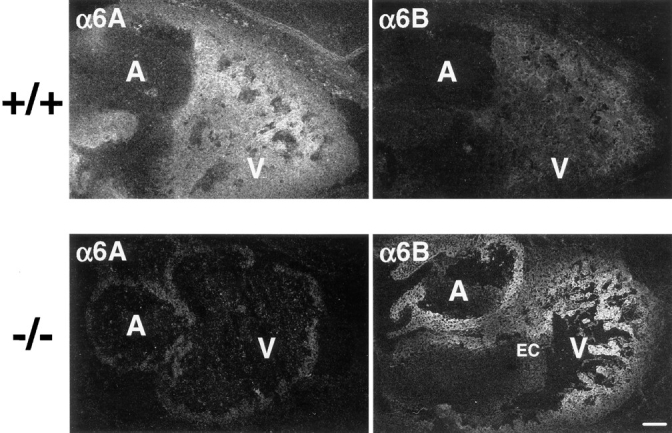
Deletion of exon A leads to replacement of α6A by α6B in the embryonic heart. Embryonic heart sections from control (+/+) and knockout (−/−) mice were prepared and subjected to immunohistochemistry with anti-α6A (1A10) or anti-α6B (PB36) antibodies, followed by incubation with an FITC-conjugated secondary anti– mouse antibody. Note that the atria (A) and ventricles (V) but not the endocardiac cushion (EC) express α6. Bar, 100 μm.
As predicted, no α6A was detected in the myocardium of α6A−/− embryos of 12.5 dpc. In contrast, α6B was found to have replaced α6A and was now present in the atria and ventricles in a gradient, the expression being strongest at the inner face of the ventricular myocardium and weakening towards the peripheral cells, similar to the gradient of expression of α6A in control mice.
These data demonstrate that the specific removal of exon A using homologous recombination and the Cre- loxP system resulted in the replacement of α6A by α6B in a tissue that normally expresses α6A.
α6B Takes Over the Function of α6A in Heart Development
The viability of the α6A−/− mice and the normal morphology of atria and ventricles of 12.5-dpc α6A−/− embryos suggested that cardiogenesis was not affected by the replacement of α6A by α6B. Careful histological analysis of the heart was conducted on 10.5- and 12.5-dpc embryos to investigate whether there were any subtle morphological defects due to the mutation, but no aberrations in the morphology of the heart and cardiomyocyte organization were observed at these stages (not shown). We tested the possibility that the normal development of the heart in the α6A−/− mice could be the result of compensatory mechanisms involving other laminin-binding integrins such as α3β1 and α7β1. These integrins were found to be absent in the myocardium of both control and knockout 12.5-dpc embryos (not shown), suggesting that α6B alone can take over the function of α6A in the formation of the heart.
Replacement of α6A by α6B Does Not Impair the Differentiation of the Epidermis, Hemidesmosome Formation, or Wound Closure
Although α6B is present in the epidermis of the embryo (Thorsteinsdóttir et al., 1995), only α6A is expressed there after birth and in the adult, in association with the β4 integrin subunit. However, no obvious abnormalities were detected in histological sections of the epidermis of α6A−/− mice (not shown). The substitution of α6A by α6B in the epidermis of the knockout mice was first confirmed with antibodies directed against α6A or α6B (Fig. 4 A). In control animals, the staining for α6A was restricted to the basal keratinocyte layer, and a weak staining of the blood vessels in the dermis corresponding to that of α6B in endothelial cells was observed. As expected, α6A was not expressed in the epidermis of the knockout mice and was replaced by α6B. The absence of α6A in skin was confirmed by Northern blot analysis (not shown).
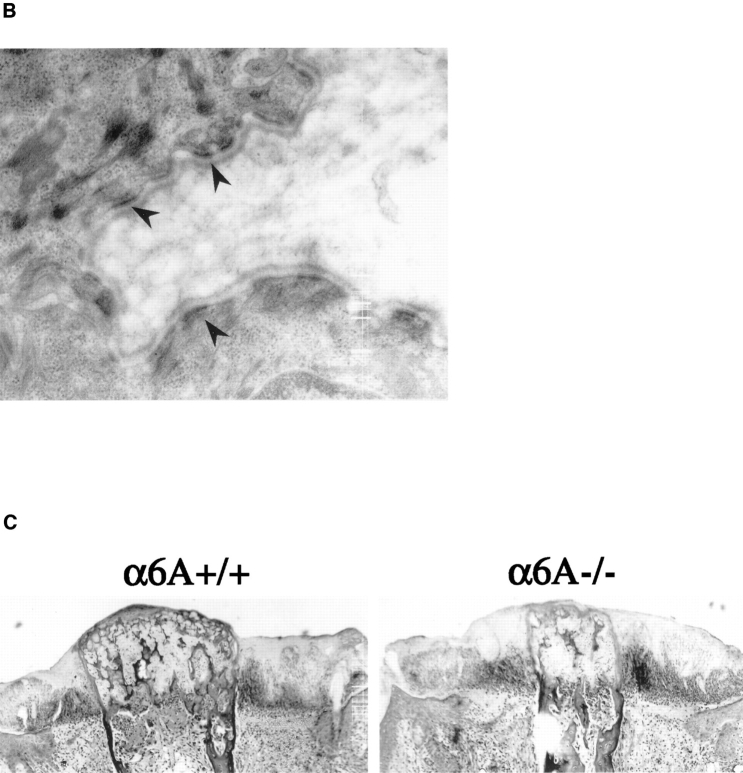
Figure 4.
Differentiation of the epidermis, hemidesmosome formation, and wound closure are not impaired in α6A−/− mice. (A) Skin sections from control (+/+) and knockout mice (−/−) were incubated with anti-α6A or anti-α6B antibodies. Note the presence of an apparently normal pluristratified epithelium in the knockout mice. (B) Electronic microscopy analysis revealed the presence of normal hemidesmosomes in the skin of α6A−/− mice. (C) Wounds were made by amputation of the tail, and reepithelialization was analyzed using hematoxylin/ eosin–stained sections. After 2 d, the cells had migrated half the distance between the wound edge and the protruding bone (shown). After 5 d, cells had completely covered the wounds (not shown). Bar, 50 μm.
Ultrastructural analysis revealed that hemidesmosomes were normal in number and morphology in the skin of knockout animals (Fig. 4 B), indicating that α6B in association with β4 supports hemidesmosome formation. Since the α6β4 integrin is redistributed from the hemidesmosomes to a more even distribution over the membrane during keratinocyte migration (Kurpakus et al., 1991), we investigated whether the replacement of α6A by α6B would lead to the disassembly of hemidesmosomes and, as a consequence, would affect keratinocyte migration during wound healing. Therefore, a wound closure experiment by tail amputation was conducted on α6A+/+ and α6A−/− mice. Keratinocyte migration was assessed at day 2 and 4 after amputation of the tail by hematoxylin/eosin staining. No apparent irregularities were detected in the epidermis of the reepithelializing skin, and wounds healed as quickly in the α6A−/− mice as in the control mice (Fig. 4 C).
Together, these results show that the differentiation of the epidermis, hemidesmosome formation, and reepithelialization after wound healing in mice expressing only the variant α6B are normal.
Keratinocyte Adhesion and Motility Are Not Altered in α6A−/− Mice
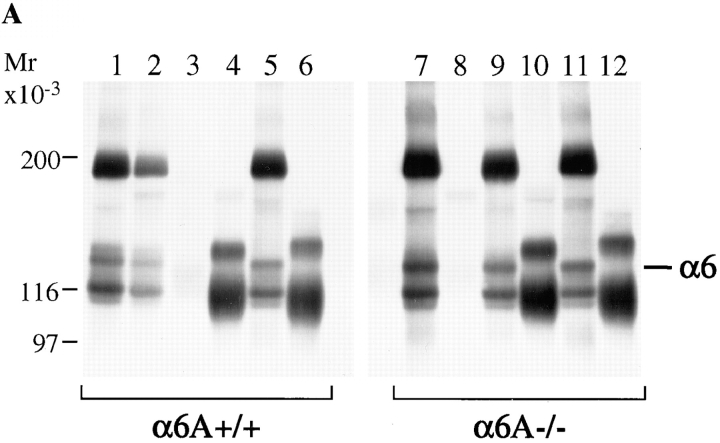
To further confirm the replacement of α6A by α6B in the epidermis and to study the adhesion of the α6A−/− keratinocytes and their migration properties, we isolated keratinocytes from newborn mice and cultured them in vitro. At none of the passages was the morphology of α6A−/− cells different from that of control keratinocytes (not shown). The pattern of expression of laminin-binding integrins on both cell types was analyzed by immunoprecipitation from 125I-labeled cells (Fig. 5 A). The α6A variant but not α6B was precipitated from α6A+/+ keratinocytes. On the contrary, α6A was not expressed at the surface of α6A−/− keratinocytes. It had been replaced by α6B. As expected, the expression of the α3A, β4, and β1 chains was not altered by the deletion. The absence of a band corresponding to the α6 polypeptide in the β1 immunoprecipitates indicated that mouse keratinocytes, like those of humans, do not express α6β1.
Figure 5.
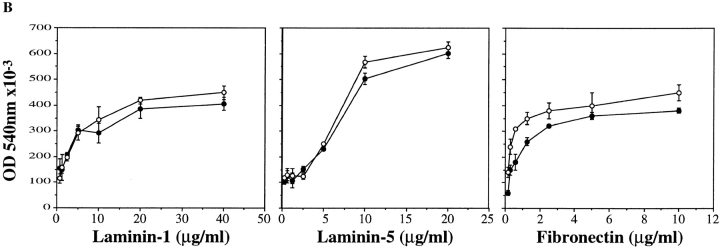
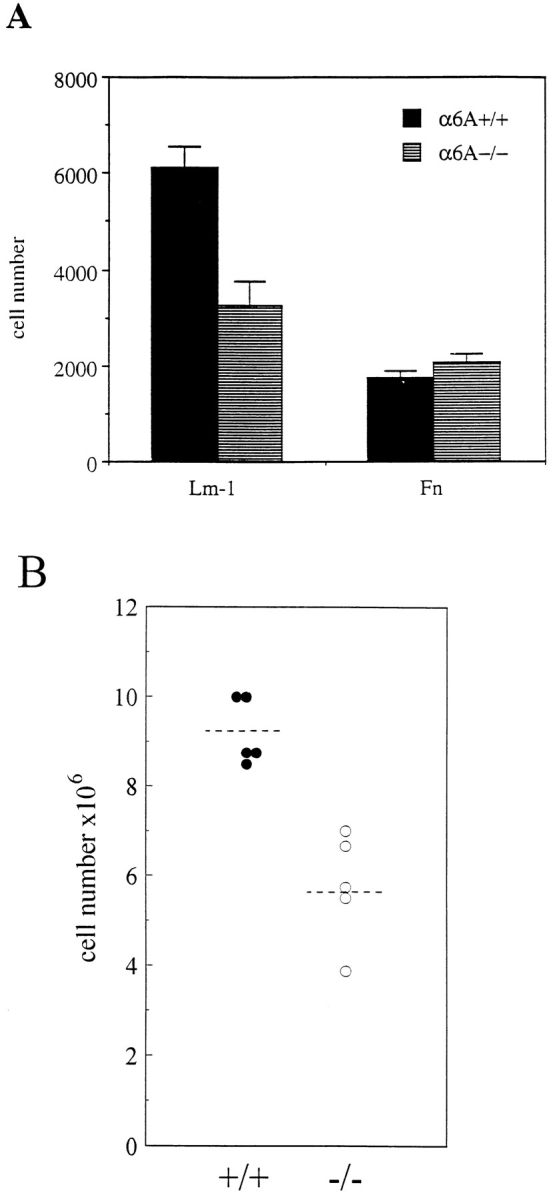
Analysis of integrin expression and adhesion properties of α6A+/+ and α6A−/− keratinocytes. (A) Lysates of 125I-labeled control and knockout keratinocytes were immunoprecipitated with antibodies against the following integrin subunits: α6 (lanes 1 and 7), α6A (lanes 2 and 8), α6B (lanes 3 and 9), α3A (lanes 4 and 10), β4 (lanes 5 and 11), and β1 (lanes 6 and 12). α6A+/+ and α6A−/− keratinocytes express the α6A and the α6B subunits, respectively, in association with the β4 subunit (200 kD). The band around 116 kD in the α6 precipitates is a degradation product of β4. Both types of cells express α3 (150 kD) in association with β1 (120 kD). Samples were analyzed on SDS 5% acrylamide under nonreducing conditions. (B) α6A+/+ (closed circles) and α6A−/− (open circles) primary keratinocytes adhered equally well to laminin-1, laminin-5, or fibronectin.
Data obtained by Tennenbaum et al. (1995) revealed that the introduction of the α6B variant in a papilloma cell line induced an increase of the adhesion of these cells to laminin-1, which is a ligand for α6β4 (Lee et al., 1992; Niessen et al., 1994). We have assessed the adhesion of keratinocytes to laminin-1, which interacts with α6β4, to laminin-5, involving both α3β1 and α6β4, and to fibronectin, mediated by α5β1 and αv integrins. As shown in Fig. 5 B, the adhesion properties of α6A+/+ and α6A−/− keratinocytes on all three substrates are similar.
Finally, because in vivo wound healing involves both cell migration and proliferation, we investigated the migratory properties of α6A−/− keratinocytes in vitro using Transwell filters coated with laminin-1, laminin-5, or fibronectin on the lower side. We could not observe any significant difference in the motility of the two cell types after 6 or 18 h of migration (not shown). These results indicate that substitution of α6A by α6B does not modify keratinocyte adhesion and motility.
Replacement of α6A by α6B Does Not Affect Lymphocyte Differentiation and Proliferation but Reduces the Motility of These Cells
In the thymus during development, the expression of α6A and α6B is regulated in both the thymocytes and the endothelial cells (Chang et al., 1995; Ruiz et al., 1995). In addition, it has been previously shown that the homing of T cell precursors into the thymus is blocked by anti-α6 antibodies (Ruiz et al., 1995). Therefore, it was expected that T lymphocyte differentiation might be impaired in our α6A−/− mice. Furthermore, laminin has been shown to promote the proliferation of thymocytes and T cells plated on anti-CD3 antibody (Shimizu et al., 1990a ; Chang et al., 1995). Finally, lymphocytes encounter different laminin isoforms as they recirculate and transmigrate through the basement membranes underlying the endothelial cells in secondary lymphoid organs and at sites of inflammation. The receptor for laminin on T cells is α6β1 (Shimizu et al., 1990b ), and because the cytoplasmic domain of α6A might give a specific signal to lymphocytes upon interaction with laminin, we investigated the ability of α6B to sustain T cell differentiation, proliferation, and migration.
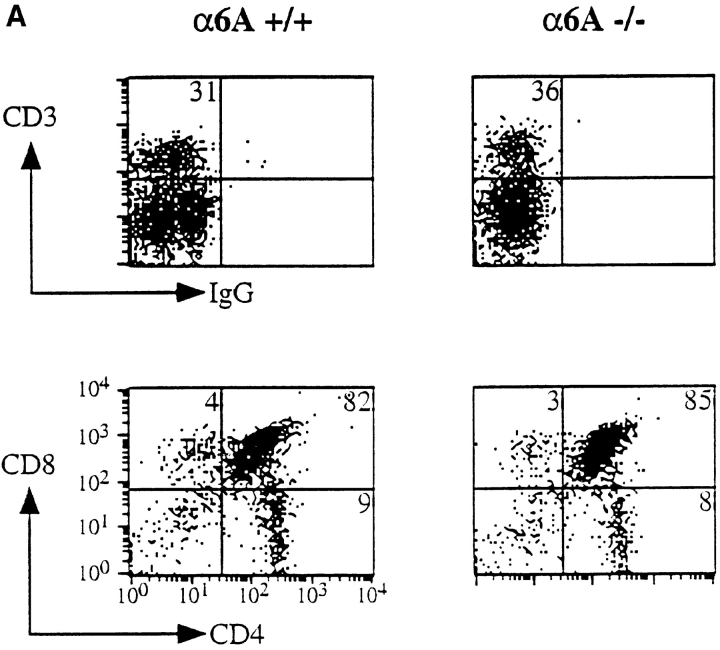
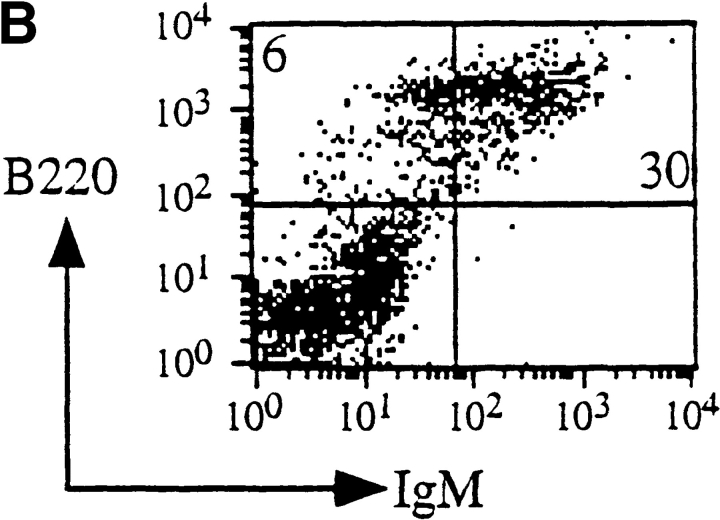
α6A was only present in the T cells of lymph nodes from control mice and not in those from knockout mice, while α6B was expressed in α6A−/− T cells as determined by reverse transcription PCR (not shown). The levels of expression of the α6 subunit were the same on both cell types as determined by FACS® analysis using the GoH3 antibody (not shown). Differentiation of the T cell-lineage was assessed by reactivity of total cell suspensions isolated from the thymus of age- and sex-matched control and knockout mice with anti-CD3 (Fig. 6 A). A normal percentage of strongly CD3-positive T cells was found in the thymus of α6A−/− mice. Moreover, CD4/CD8 ratios were similar in knockout and control mice, indicating that the development of various T cell subsets proceeds normally in the presence of α6B alone. As the presence of α6 integrin on B cells has been reported in one study (Ohguro and Tsubota, 1996), we also studied B cell maturation using antibodies directed against B220/CD45R and IgM on spleen cells. Fig. 6 B shows that their differentiation is not affected in α6A−/− mice.
Figure 6.
T and B lymphocytes lacking α6A develop normally. (A) Thymic cells from control and α6A−/− mice were incubated with either PE-conjugated anti-CD3ε (top) or with a combination of FITC-conjugated anti-CD4 and PE-conjugated anti-CD8 antibodies, and analyzed by flow cytometry. (B) Splenic lymphocytes from control and knockout mice were incubated with biotin-conjugated anti-B220/CD45R and FITC-conjugated anti-IgM antibodies and analyzed by flow cytometry.
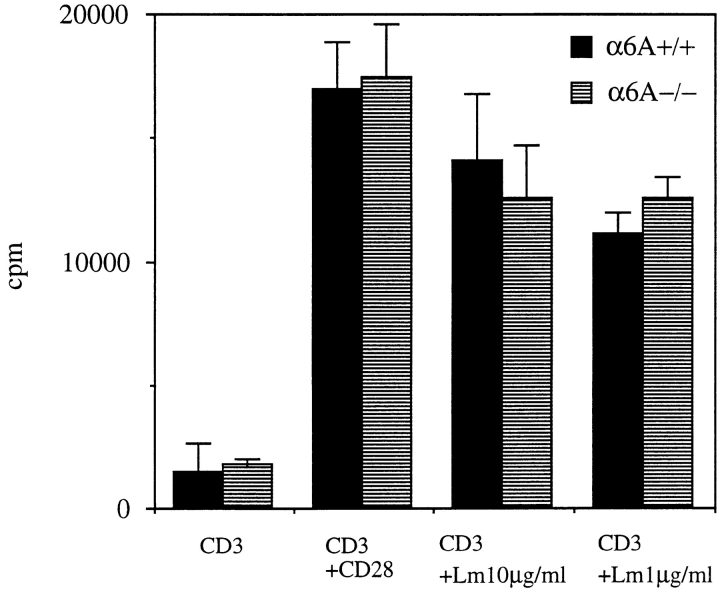
To determine whether the substitution of α6A by α6B affected the proliferative response triggered by laminin-1, we conducted costimulation experiments by incubating purified lymph node T cells on plate-bound laminin-1 and anti-CD3 antibody for 48 h (not shown), 72 h (Fig. 7), or 96 h (not shown). While the incorporation of [3H]thymidine was low in cells stimulated by anti-CD3 antibody alone, costimulation by soluble anti-CD28 antibody (Gross et al., 1992) showed that α6A+/+ and α6A−/− T cells displayed the same ability to proliferate. More importantly, incorporation of [3H]thymidine was found to be increased by binding to laminin-1 to the same extent in T cells isolated from knockout mice and control animals, indicating that the proliferative response to laminin-1, although α6β1 mediated (Shimizu et al., 1990a ), is not dependent on the nature of the cytoplasmic domain of α6.
Figure 7.
Costimulation of CD3-mediated T cell proliferation by laminin-1. Wells of microtiter plates were first coated with anti-CD3 antibodies and, when indicated, with 1 or 10 μg/ml laminin-1. Costimulation of proliferation in the presence of soluble anti-CD28 antibody was taken as a control. The experiment shown is one representative experiment out of five.
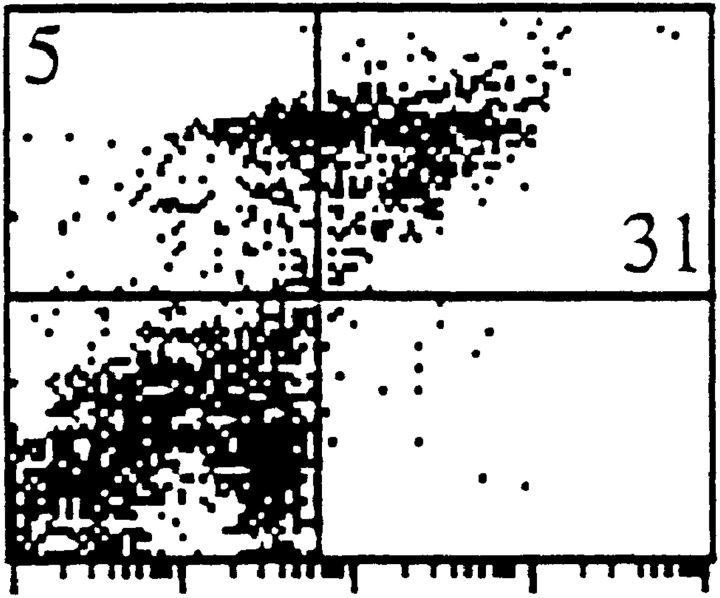
Finally, the ability of α6B to support migration of cells of the immune system was studied. Although α6A has previously been reported to increase motility of cells of a macrophage cell line on laminin-1 (Shaw and Mercurio, 1994), we could not investigate the effects of the absence of α6A in cells of this type because laminin-1 failed to support attachment and migration of peritoneal macrophages isolated from either our control or knockout mice (not shown). Migratory properties of T lymphocytes were first studied in vitro using Transwell filters coated with either laminin-1 or fibronectin, and in the presence of the chemoattractant SDF-1, in the lower compartment of the chamber. Interestingly, we have found that the motility of α6A−/− T cells was reduced by 46% compared with that of control cells on laminin-1–coated filters, whereas migration through fibronectin-coated filters was not affected (Fig. 8 A). Similar results were obtained with another chemoattractant, MCP-1 (not shown). Importantly, the decrease in cell migration was not due to differences in the activation state of T cells from both normal and knockout mice, as verified by the expression of the activation marker CD69 (not shown). Finally, a decrease in cell motility was observed in cells originating from two independent ES cell clones. This indicates that optimal migration of T lymphocytes is dependent on the expression of the cytoplasmic domain of α6A.
Figure 8.
The motility of α6A−/− T lymphocytes is reduced on laminin-1. (A) T lymphocytes from lymph nodes were allowed to migrate through fibronectin- or laminin-1–coated Transwell filters for 2 h in the presence of 10 ng/ml SDF-1 in the lower compartment of the wells. Note that the migration of α6A−/− T cells is reduced on laminin-1 only and not on fibronectin. The experiment shown is one representative experiment out of four. (B) Lymph nodes from α6A−/− mice contain fewer cells than their normal counterparts. T cells from the peripheral and mesenteric lymph nodes of five control and five α6A−/− mice were purified and counted. Mean values are 9.2 × 106 ± 0.7 (α6A+/+) and 5.75 × 106 ± 1.2 (α6A−/−).
After T cell purification, we consistently observed a strong decrease (30–40%) in the absolute number of cells isolated from the peripheral and mesenteric lymph nodes of α6A−/− mice (Fig. 8 B). To test the hypothesis that this could be due to alterations in the migration of T cells to the lymph nodes, we have conducted in vivo lymphocyte homing experiments using PKH2 and PKH26 fluorescent cell linkers. Lymph node cells from α6A+/+ and α6A−/− cells were labeled with one of the fluorescent cell linkers, mixed in equal number, and injected into the tail vein of wild-type animals. The percentage of each cell type in the cell mixture was checked by FACS® analysis and was close to 50% (not shown). Fluorescent-labeled cells homing into spleen, peripheral, and mesenteric lymph nodes was determined by FACS® analysis 2 or 20 h after the injection. Two series of experiments were performed with the injection of either PKH2-labeled α6A+/+ and PKH26-labeled α6A−/− cells or PKH2-labeled α6A−/− and PKH26-labeled α6A+/+ cells. Results obtained with both combinations were similar and are presented in Table I. The frequency of α6A−/− cells in spleen, peripheral, and mesenteric lymph nodes was comparable to that of the α6A+/+ lymphocytes 2 h after injection. A higher percentage of cells were found in the lymph nodes after 20 h of homing, but no significant difference could be observed between the two cell types.
Table I.
Homing of Fluorescence-labeled α6A+/+ and α6A−/− Lymphocytes to Spleen, Peripheral Lymph Nodes, and Mesenteric Lymph Nodes
| 2 h | 20 h | |||||||
|---|---|---|---|---|---|---|---|---|
| Cells | Spleen | Peripheral lymph nodes | Mesenteric lymph nodes | Peripheral and mesenteric lymph nodes | ||||
| α6A+/+ | 3.66 ± 0.69 | 1.18 ± 0.12 | 0.97 ± 0.29 | 2.26 | ||||
| α6A−/− | 3.85 ± 0.76 | 1.21 ± 0.45 | 0.74 ± 0.17 | 2.48 | ||||
Cells were isolated from the peripheral and mesenteric lymph nodes of α6A+/+ and α6A−/− mice and labeled with either PKH2 or PKH26 as described in Materials and Methods. 3 × 107 α6A+/+ and α6A−/− cells were injected into wild-type recipient mice. After 2 or 20 h, cell suspensions were isolated from the spleen, peripheral lymph nodes, and mesenteric lymph nodes of the recipient mice and subjected to FACS® analysis to determine the percentage of α6A−/− versus α6A+/+ cells in each suspension. Values after 2 h homing are the means ± SEM obtained from a total of six recipient mice in two independent experiments. Mean for two mice are indicated after 20 h homing.
Together, these data suggest that, although the expression of α6A is necessary for the optimal migration of T cells on laminin-1 in vitro, it is dispensable for in vivo lymphocyte homing to the secondary lymphoid organs.
Discussion
Development Is Not Impaired in Mice Expressing Only the α6B Variant
Classical ES cell technology combined with the Cre-loxP system of the bacteriophage P1 was used to create exon-specific knockout mice that did not express the A variant of the α6 integrin subunit. The deletion resulted in the replacement of α6A by α6B in all tissues that normally express α6A, such as the developing heart, in which α6A is associated with the β1 subunit, and the adult epidermis, in which α6Aβ4 is expressed in the basal keratinocyte layer. Previous studies have indicated that α6 is apparently not essential for proper prenatal development (Georges-Labouesse et al., 1996). However, the importance of α6A for the function of the epidermis, mature gonads, Schwann cells, and the immune system could not be addressed since α6−/− pups die shortly after birth because of detachment of the epidermis. Moreover, it was not possible to investigate the consequences of the lack of α6A during cardiogenesis on the function of the adult heart. We show here that α6B can sustain normal mouse development until maturity and that α6A−/− animals are fertile. We did not detect any abnormalities in either the morphology or the histology of various organs and tissues, and we argue that the presence of exon A in α6 mRNA is not essential for the proper control of cell migration and proliferation during development. These findings were surprising in the light of the recently published work by Domanico et al. (1997), who have shown that the ectopic expression of α6A in undifferentiated ES cells conferred a highly migratory phenotype to these cells as compared with their normal counterparts, which express only α6B. Importantly, these stimulatory effects on cell migration were found to be independent from adhesion to laminin-1, suggesting that the cytoplasmic domain of α6A was sufficient for promoting motility. However, our own results unequivocally show that the cytoplasmic tail of α6B can support cell migration during development.
Heart Development and Function Are Normal in α6A−/− Mice
Most surprising was the absence of an apparently abnormal phenotype during heart ontogeny in α6A−/− mice. While α6B is expressed throughout development, the expression of α6A begins at 8.5 dpc and is initially restricted to the developing myocardium (Collo et al., 1995; Thorsteinsdóttir et al., 1995). The onset of α6A expression immediately precedes the initiation of the looping process, which converts anterior–posterior patterning of the heart tube into left–right asymmetry (Fishman and Olson, 1997), strongly suggesting a critical role of this integrin subunit in cardiogenesis. Although α6−/− mice did not present any abnormalities of the heart (Georges-Labouesse et al., 1996), we assumed that the replacement of α6A by α6B, while not preventing adhesion to laminin substrata, could result in the transmission of inappropriate molecular signals into the cardiomyocytes and thus could have more profound effects on heart formation than the complete absence of α6. Surprisingly, histological analysis and immunofluorescence data revealed no overt abnormal phenotype of the heart, and the pattern of expression of α6B was similar to that of α6A in control mice, the inner cells of the myocardium being more intensely stained than the peripheral cells. Because an abnormal phenotype is more likely to be detected in a simplified, in vitro differentiation model (Bagutti et al., 1996), double knockout ES cells were generated and aggregated into embryoid bodies. We found that α6A−/− ES cells displayed the same ability to differentiate into cardiac contracting cells as their wild-type counterparts (Gimond, C., unpublished results), further supporting the finding that the substitution of α6A by α6B is compatible with normal cardiogenesis. Finally, this substitution does not seem to impair the function of the adult heart either, since the level of the atrial natriuretic peptide, a marker of heart dysfunction (Edwards et al., 1988), was normal in the ventricles of our α6A knockout mice (our unpublished results).
Although the simplest explanation for the lack of an overt abnormal phenotype in the heart is that α6B, in spite of its different cytoplasmic domain, can substitute for α6A during cardiomyocyte differentiation and reorganization, more complex compensatory mechanisms, involving other laminin-binding receptors, might have occurred during development. However, if compensation occurred, our results show that this is not due to the induction of the expression of two other laminin-binding integrins, α3Aβ1 and α7β1.
Keratinocytes Expressing α6B Assemble Hemidesmosomes and Display Normal Adhesive and Migratory Properties
In contrast to the embryonic heart, in which α6A is complexed with the β1 subunit, in developing epidermis both α6A and α6B are expressed in association with the β4 subunit (Thorsteinsdóttir et al., 1995). Only α6A remains after birth, and although the reasons for the loss of expression of α6B in this tissue is not clear, the expression patterns of the two variants might correspond to key stages in skin development or to specific physiological conditions. However, the epidermis apparently differentiated normally in α6A−/− mice, and more importantly, it contains hemidesmosomes in normal number and of normal morphology. This is interesting because phosphorylation of α6A was thought to be required for the nucleation of hemidesmosome assembly (Baker et al., 1997). Thus, although α6B was never found to become phosphorylated, it is capable, in association with β4, of supporting hemidesmosome formation. This suggests that, for this function of α6, only binding to laminin, which does not depend on the cytoplasmic domain of the subunit, is required. Alternatively, the cytoplasmic domain of α6B may render conformation of α6β4 permissive for hemidesmosome formation. Nevertheless, the substitution of α6A by α6B might have subtle effects on the regulation of the assembly or the disruption of hemidesmosomes, in which β4 is involved (Dowling et al., 1996; van der Neut et al., 1996), and although the migration of keratinocyte per se was found to be independent of α6β4 (Kurpakus et al., 1991), the cytoplasmic domain of this integrin might play a role in hemidesmosome disassembly and the onset of cell movement. However, given our results both in vivo and in vitro, it appears that the expression of only α6B does not perturb keratinocyte migration and wound closure.
Previous work by Tennenbaum et al. (1995) has shown that overexpression of α6B in a papilloma cell line resulted in increased binding to laminin-1, whereas overexpression of α6A had no effect on adhesion. In contrast to those results, we show that when using an in vivo approach in which the expression level of α6 is not modified, adhesion properties of keratinocytes expressing either the one or the other splice variant are not different.
Lymphocyte Motility Is Reduced in α6A−/− Mice
During maturation of the immune system, recirculation, and inflammation, lymphocytes encounter different laminin isoforms as they cross the basement membrane underlining various blood vessels (Springer, 1994). In addition, laminin-1, -3, and -5, together with other extracellular matrix proteins, are present in the stroma of various lymphoid organs such as the thymus and lymph nodes and in the bone marrow (Chang et al., 1993; Jaspars et al., 1996), in which lymphocytes proliferate and differentiate. The production of laminins by these tissues is mirrored by the stronger expression of α6 integrins on immature than on mature T cells (Ruiz et al., 1995), suggesting a critical role of laminin–integrin interactions in the differentiation process. Moreover, α6 expressed by thymic endothelial cells was shown to participate in the homing of T cell progenitors to this organ (Ruiz et al., 1995). A differential role for the cytoplasmic domains of the α5 and α6 subunits in mediating signals for proliferation and differentiation has recently been described in myoblasts (Sastry et al., 1996), and because of their entirely different cytoplasmic domain, we expected the ability of α6A and α6B to regulate these cellular responses to be different. It was therefore surprising to find that T and B cells differentiated normally in α6A−/− mice and that the α6-dependent costimulatory effect of laminin-1 on T cell proliferation (Shimizu et al., 1990a ; Chang et al., 1995) can be transmitted as efficiently by α6B as by α6A. This finding might be explained by the critical function of the cytoplasmic tail of the β subunit in cell growth (Merredith and Schwartz, 1997). Alternatively, the adhesion-dependent activation of integrin-associated protein could be involved in the induction of proliferative signals in T cells regardless of which integrin is ligated (Reinhold et al., 1997). Finally, adhesion of lymphocytes to extracellular matrix proteins in the bone marrow or in the thymus might be sufficient to allow their sustained interaction with stromal cells and stimulation by cytokines, which are the conditions for proper selection and differentiation (Anderson et al., 1996). In that case, substitution of α6A by α6B is not expected to affect maturation, since the ligand-binding activities of the two splice variants are identical.
Interestingly, the motility of α6A−/− T cells through laminin-1-coated filters was markedly decreased. A promoting effect of α6A on cell migration has already been reported (Shaw and Mercurio, 1994; Domanico et al., 1997), but in previous work, the effects of ectopic and overexpression of either one or the variants in two different cell lines was analyzed. In the present paper, we show, for the first time, that the endogenous expression of α6A more efficiently supports the migration of primary T lymphocytes in vitro. When overexpressed in ES cells, α6A was reported to enhance migration not only on laminin-1 but also on other extracellular matrix proteins that do not use α6β1 as a receptor (Domanico et al., 1997). Yet, we show here that the effects of the absence of α6A on T cell migration only occur on laminin-1. We have also observed that the number of T cells isolated from the lymph nodes was consistently lower in the α6A−/− than in wild-type mice. Since lymphocytes must cross basement membranes containing laminins when recirculating through the organism, one hypothesis for this difference is that impaired migration of α6A−/− cells could result in a less efficient homing to the peripheral and mesenteric lymph nodes, causing the number of cells in these organs to be lower. In this regard, it is interesting to note that antilaminin antibodies were found to inhibit lymphocyte trafficking and homing to the peripheral lymph nodes (Kupiec-Weglinski and De Sousa, 1991). However, we could not demonstrate any defects in lymphocyte homing in our α6A−/− mice, which indicates that the reduced cell number in lymph nodes has other causes, e.g., defects in cell proliferation. Although we have shown that the substitution of α6A by α6B does not affect T cell proliferation in vitro in conditions where both CD3 and α6β1 are ligated, the lack of α6A in lymphocytes or in lymph node stromal cells might lead to a decrease in lymphocyte proliferation in vivo.
The altered cell motility on laminin-1 that we observed in vitro does not seem to have consequences for lymphocyte homing. This might be due to the ability of lymphocytes to use several other integrins from their repertoire to migrate on the various extracellular matrix proteins present in basement membranes, and the lack of α6A is likely to be compensated by other types of integrin–matrix interactions. Such compensatory mechanisms cannot be used by lymphocytes migrating on a laminin-1 matrix, and therefore, defects resulting of the absence of α6A are more likely to be detected in vitro. Redundancy and compensatory mechanisms could also explain the absence of effects of the lack of α6A on the development of the mouse. Nevertheless, we cannot rule out that the promoting role of α6A on cell motility might be important for the fast recruitment of lymphocytes to sites of inflammation or in other pathological conditions.
The molecular basis for the promoting effect of α6A on cell migration has not yet been elucidated, but it might involve the different ability of the two splice variants to trigger the phosphorylation of certain cytoskeletal proteins (such as paxillin; Shaw et al., 1995) that are involved in cell migration (Aznavoorian et al., 1996; Tourkin et al., 1996). In agreement with this notion is the induction of filopodia in cells of both ES and macrophage cell lines by α6A (Shaw and Mercurio, 1994; Domanico et al., 1997), which reflects cytoskeleton rearrangements. Since the only structural differences between α6A and α6B reside in their cytoplasmic domains, it is clear that these unique sequences are responsible for the transmission of distinct signals into the cell. This is not restricted to the α6 subunit since Chan et al. (1992), using chimeric integrin molecules, have previously shown that the unique intracellular domains of the α2 and α4 integrin subunits triggered either collagen gel contraction or cell migration, respectively. Whether the cytoplasmic domain of the α chain itself is able to transduce signals has not yet been elucidated. Alternatively, it could regulate interactions of the cytoplasmic tail of β1 with intracellular proteins involved in transduction. Regulation of the phosphorylation state of α6A, for example by chemoattractants, might also play a role in cell motility. Such a posttranslational regulation has never been reported for α6B and may account partly for their distinct functional properties. Finally, internalization of α6β1, which is an important aspect of cell migration, could be regulated by the cytoplasmic domains of α6A and α6B in a different manner, although previous reports argue against this suggestion (Gaietta et al., 1994).
In conclusion, the specific removal of exon A from the α6 gene allowed us to study in detail the role of endogenously expressed α6A in vivo and to determine whether α6B could functionally replace it. Our results revealed that, in contrast to previous assumptions and despite its remarkable up-regulation at the stage of the heart-looping process, α6A is not essential for proper cardiogenesis and cell migration during development. However, we have demonstrated that the two splice variants α6A and α6B are not equivalent in supporting lymphocyte migration on laminin-1, and thus, although its absence does not impair lymphocyte homing into the lymph nodes, α6A might contribute to some aspects of host defense. The α6A−/− mice will provide a useful tool for future studies aiming at fully understanding the molecular mechanisms activated by the two cytoplasmic variants of α6.
Acknowledgments
We thank A.J. Schrauwers for mice husbandry; M. van der Valk, D. Hoogervorst, J. Bulthuis, and K. de Goeij for their help with histological analysis of the mice; L. Oomen for confocal laser scanning microscopy; H. Janssen for electronic microscopy; N. Ong for artwork; and R. Soede for his helpful comments on lymphocyte migration. A special thank you goes to D. Amsen for his valuable advice on experiments on lymphocyte differentiation and critical reading of the manuscript, and to P. Rousselle for providing us with laminin-5 and for her helpful advice on keratinocyte culture. We also thank B.M.C. Chang for the rat monoclonal antibody to murine β1; S.J. Kennel for the antibody to murine β4; R. Kramer for the rat antibody to murine α7; and R. Fässler for providing us with the loxP-neo-tk-loxP cassette and for useful suggestions.
Abbreviations used in this paper
- dpc
days post coitum
- ES
embryonic stem
Footnotes
All of the ES cell work was carried out in the division of Molecular Genetics (Head Prof. Dr. A. Berns) at the Netherlands Cancer Institute. This work was supported by a fellowship from the European Commission (ERB4050PL930847) to C. Gimond, a Marie Curie Research training grant from the European Commission (ERBFMBICT961823) to C. Baudoin, and grants from the Netherlands Heart Foundation (NHS 96.006) and the Netherlands Organization for Scientific Research (NWO 900-511-043).
Address all correspondence to Arnoud Sonnenberg, Division of Cell Biology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands. Tel.: (31) 20 512 1942. Fax: (31) 20 512 1944. E-mail: asonn@nki.nl
Ronald van der Neut's present address is INSERM U434, 27 rue Juliette Dodu, 75010 Paris, France.
References
- Altruda F, Cervella P, Tarone G, Botta C, Balzac F, Stefanuto G, Silengo L. A human integrin β1 subunit with a unique cytoplasmic domain generated by alternative mRNA processing. Gene. 1990;5:261–266. doi: 10.1016/0378-1119(90)90369-3. [DOI] [PubMed] [Google Scholar]
- Anderson G, Moore NC, Owen JJT, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- Aznavoorian S, Stracke ML, Parsons J, McClanahan J, Liotta LA. Integrin αvβ3 mediates chemotactic and haptotactic motility in human melanoma cells through different signaling pathways. J Biol Chem. 1996;271:3247–3254. doi: 10.1074/jbc.271.6.3247. [DOI] [PubMed] [Google Scholar]
- Bagutti C, Wobus AM, Fässler R, Watt FM. Differentiation of embryonal stem cells into keratinocytes: comparison of wild-type and β1 integrin-deficient cells. Dev Biol. 1996;179:184–196. doi: 10.1006/dbio.1996.0250. [DOI] [PubMed] [Google Scholar]
- Baker SE, Skalli O, Goldman RD, Jones JCR. Laminin-5 and modulation of keratin cytoskeleton arrangement in FG pancreatic carcinoma cells: involvement of IFAP300 and evidence that laminin-5/cell interactions correlate with a dephosphorylation of α6A integrin. Cell Motil Cytoskel. 1997;37:271–286. doi: 10.1002/(SICI)1097-0169(1997)37:3<271::AID-CM9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Baudoin C, Goumans MJ, Mummery C, Sonnenberg A. Knockout and knockin of the β1 exon D define distinct roles for integrin splice variants in heart function and embryonic development. Genes Dev. 1998;12:1202–1216. doi: 10.1101/gad.12.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray PF, Leung CS, Shuman MA. Human platelets and megakaryocytes contain alternately spliced glycoprotein IIb mRNAs. J Biol Chem. 1990;265:9587–9590. [PubMed] [Google Scholar]
- Cattelino A, Longhi R, de Curtis I. Differential distribution of two cytoplasmic variants of the α6β1 integrin laminin receptor in the ventral plasma membrane of embryonic fibroblasts. J Cell Sci. 1995;108:3067–3078. doi: 10.1242/jcs.108.9.3067. [DOI] [PubMed] [Google Scholar]
- Chan BM, Kassner PD, Schiro JA, Byers HR, Kupper TS, Hemler ME. Distinct cellular functions mediated by different VLA integrin α subunit cytoplasmic domains. Cell. 1992;68:1051–1060. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Chang AC, Wadsworth S, Coligan JE. Expression of merosin in the thymus and its interaction with thymocytes. J Immunol. 1993;151:1789–1801. [PubMed] [Google Scholar]
- Chang AC, Salomon DR, Wadsworth S, Hong MJP, Mojcik CF, Otto S, Shevach EM, Coligan JE. α3β1 and α6β1 integrins mediate laminin/merosin binding and function as costimulatory molecules for human thymocyte proliferation. J Immunol. 1995;154:500–510. [PubMed] [Google Scholar]
- Chichester CO, Fernandez M, Minguell JJ. Extracellular matrix gene expression by human bone marrow fibroblasts. Cell Adhes Commun. 1993;1:93–99. doi: 10.3109/15419069309095685. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Lotz MM, Mercurio AM. A novel structural variant of the human β4 integrin cDNA. Cell Adhes Commun. 1994;2:1–6. doi: 10.3109/15419069409014197. [DOI] [PubMed] [Google Scholar]
- Collo G, Domanico SZ, Klier G, Quaranta V. Gradient of integrin α6A distribution in the myocardium during early heart development. Cell Adhes Commun. 1995;3:101–113. doi: 10.3109/15419069509081280. [DOI] [PubMed] [Google Scholar]
- Cooper HM, Tamura RN, Quaranta V. The major laminin receptor of mouse embryonic stem cells is a novel isoform of the α6β1 integrin. J Cell Biol. 1991;115:843–850. doi: 10.1083/jcb.115.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel, G.O., and A. Sonnenberg. 1996. Laminin isoforms and their integrin receptors. In Adhesion Receptors as Therapeutic Targets. M.A. Horton, editor. CRC Press, Boca Raton, FL. 9–36.
- Delwel GO, Hogervorst F, Kuikman I, Paulsson M, Timpl R, Sonnenberg A. Expression and function of the cytoplasmic variants of the integrin α6 subunit in transfected K562 cells. Activation-dependent adhesion and interaction with isoforms of laminin. J Biol Chem. 1993;268:25865–25875. [PubMed] [Google Scholar]
- Delwel GO, Kuikman I, Sonnenberg A. An alternative spliced exon in the extracellular domain of the human α6 integrin subunit. Functional analysis of the α6 integrin variants. Cell Adhes Commun. 1995;3:143–161. doi: 10.3109/15419069509081283. [DOI] [PubMed] [Google Scholar]
- de Melker AA, Sterk LMT, Delwel GO, Fles DLA, Daams H, Weening JJ, Sonnenberg A. The A and B variants of the α3 integrin subunit: tissue distribution and functional characterization. Lab Invest. 1997;76:547–563. [PubMed] [Google Scholar]
- Domanico SZ, Pelletier AJ, Havran WL, Quaranta V. Integrin α6Aβ1 induces CD81-dependent cell motility without engaging the extracellular matrix migration substrate. Mol Biol Cell. 1997;8:2253–2265. doi: 10.1091/mbc.8.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E. β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BS, Ackermann DM, Lee ME, Reeder GS, Wold LE, Burnett JC. Identification of atrial natriuretic factor within ventricular tissue in hamsters and humans with congestive heart failure. J Clin Invest. 1988;81:82–86. doi: 10.1172/JCI113314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman MC, Olson EN. Parsing the heart: genetic modules for organ assembly. Cell. 1997;91:153–156. doi: 10.1016/s0092-8674(00)80397-9. [DOI] [PubMed] [Google Scholar]
- Gaietta G, Redelmeier TEK, Jackson MR, Tamura RN, Quaranta V. Quantitative measurement of α6β1 and α6β4 integrin internalization under cross-linking conditions: a possible role for α6 cytoplasmic domains. J Cell Sci. 1994;107:3339–3349. doi: 10.1242/jcs.107.12.3339. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messadeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- Guo L, Degenstein L, Fuchs E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996;10:165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- Hennings, H. 1994. Primary culture of keratinocytes from newborn mouse epidermis in medium with lowered levels of Ca2+ In Keratinocyte Methods. I. Leigh and F. Watt, editors. Cambridge University Press, Cambridge, UK. 21–23.
- Hierck BP, Thorsteindóttir S, Niessen CM, Freund E, Iperen LP, Feyen A, Hogervorst F, Poelmann RE, Mummery CL, Sonnenberg A. Variants of the α6β1 laminin receptor in early murine development: distribution, molecular cloning and chromosomal localization of the mouse α6 integrin subunit. Cell Adhes Commun. 1993;1:33–53. doi: 10.3109/15419069309095680. [DOI] [PubMed] [Google Scholar]
- Hogervorst F, Kuikman I, von dem Borne AEGK, Sonnenberg A. Cloning and sequence analysis of β4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO (Eur Mol Biol Organ) J. 1990;9:765–770. doi: 10.1002/j.1460-2075.1990.tb08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst F, Kuikman I, van Kessel AG, Sonnenberg A. Molecular cloning of the human α6 integrin subunit. Alternative splicing of α6 mRNA and chromosomal localization of the α6 and β4 genes. Eur J Biochem. 1991;199:425–433. doi: 10.1111/j.1432-1033.1991.tb16140.x. [DOI] [PubMed] [Google Scholar]
- Hogervorst F, Kuikman I, Noteboom E, Sonnenberg A. The role of phosphorylation in activation of the α6Aβ1 laminin receptor. J Biol Chem. 1993a;268:18427–18430. [PubMed] [Google Scholar]
- Hogervorst F, Admiraal LG, Niessen CM, Kuikman I, Janssen H, Daams H, Sonnenberg A. Biochemical characterization and tissue distribution of the A and B variants of the integrin α6 subunit. J Cell Biol. 1993b;121:179–191. doi: 10.1083/jcb.121.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jaspars LH, De Melker AA, Bonnet P, Sonnenberg A, Meijer CJLM. Distribution of laminin variants and their integrin receptors in human lymphoid tissue. Cell Adhes Commun. 1996;4:4–5. doi: 10.3109/15419069609010771. [DOI] [PubMed] [Google Scholar]
- Kennel SJ, Foote LJ, Flynn KM. Tumor antigen on benign adenomas and on murine lung carcinomas quantitated by a two-site monoclonal antibody assay. Cancer Res. 1986;46:707–712. [PubMed] [Google Scholar]
- Kumar CS, James IE, Wong A, Mwangi V, Feild JA, Nuthulaganti P, Connor JR, Eichman C, Ali F, Hwang SM, et al. Cloning and characterization of a novel integrin β3 subunit. J Biol Chem. 1997;272:16390–16397. doi: 10.1074/jbc.272.26.16390. [DOI] [PubMed] [Google Scholar]
- Kupiec-Weglinski JW, De Sousa M. Lymphocyte traffic is modified in vivo by anti-laminin antibody. Immunology. 1991;72:312–313. [PMC free article] [PubMed] [Google Scholar]
- Kurpakus MA, Quaranta V, Jones JCR. Surface relocation of α6β4 integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol. 1991;115:1737–1750. doi: 10.1083/jcb.115.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino LR, Ruoslahti E. An alternative form of the integrin β1 subunit with a variant cytoplasmic domain. J Biol Chem. 1992;267:7116–7120. [PubMed] [Google Scholar]
- Lee EC, Lotz MM, Steele GD, Jr, Mercurio AM. The α6β4 integrin is a laminin receptor. J Cell Biol. 1992;117:671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merredith JE, Jr, Schwartz MA. Integrins, adhesion and apoptosis. Trends Cell Biol. 1997;7:146–150. doi: 10.1016/S0962-8924(97)01002-7. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Hogervorst F, Jaspars LH, de Melker AA, Delwel GO, Hulsman EHM, Kuckman I, Sonnenberg A. The integrin α6β4 is a receptor for both laminin and kalinin. Exp Cell Res. 1994;211:360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- Ohguro S, Tsubota H. Expressions of very late antigen-6 and vitronectin receptor, and their interactions to laminin and vitronectin during tonsillar B-cell activation. Auris Nasus Larynx. 1996;23:111–120. doi: 10.1016/s0385-8146(96)80017-4. [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Lindberg FP, Kersh GJ, Allen PM, Brown EJ. Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway. J Exp Med. 1997;185:1–11. doi: 10.1084/jem.185.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz P, Wiles MV, Imhof BA. α6 integrins participate in pro-T cell homing to the thymus. Eur J Immunol. 1995;25:2034–2041. doi: 10.1002/eji.1830250735. [DOI] [PubMed] [Google Scholar]
- Salanova M, Stefanini M, de Curtis I, Palombi F. Integrin receptor α6β1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol Reprod. 1995;52:79–87. doi: 10.1095/biolreprod52.1.79. [DOI] [PubMed] [Google Scholar]
- Sastry S, Lakonishok M, Thomas DA, Muschler J, Horwitz AF. Integrin α subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Mercurio AM. Regulation of α6β1 integrin laminin receptor function by the cytoplasmic domain of the α6 subunit. J Cell Biol. 1993;123:1017–1025. doi: 10.1083/jcb.123.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Mercurio AM. Regulation of cellular interactions with laminin by integrin cytoplasmic domains: the A and B structural variants of the α6β1 integrin differentially modulate the adhesive strength, morphology and migration of macrophages. Mol Biol Cell. 1994;5:679–690. doi: 10.1091/mbc.5.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Lotz MM, Mercurio AM. Inside-out integrin signaling in macrophages. Analysis of the role of the α6Aβ1 and α6Bβ1 integrin variants in laminin adhesion by cDNA expression in an α6 integrin-deficient macrophage cell line. J Biol Chem. 1993;268:11401–11408. [PubMed] [Google Scholar]
- Shaw LM, Turner CE, Mercurio AM. The α6Aβ1 and α6Bβ1 integrin variants signal differences in the tyrosine phosphorylation of paxillin and other proteins. J Biol Chem. 1995;270:23648–23652. doi: 10.1074/jbc.270.40.23648. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, van Seventer GA, Horgan KJ, Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol. 1990a;145:59–67. [PubMed] [Google Scholar]
- Shimizu Y, van Seventer GA, Horgan KJ, Shaw S. Regulated expression and binding of three VLA (β1) integrin receptors on T cells. Nature. 1990b;345:250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Song WK, Wang W, Sato H, Bielser DA, Kaufman SJ. Expression of α7 integrin cytoplasmic domains during skeletal muscle development: alternate forms, conformational change, and homologies with serine/ threonine kinases and tyrosine phosphatases. J Cell Sci. 1993;106:1139–1152. doi: 10.1242/jcs.106.4.1139. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Janssen H, Hogervorst F, Calafat J, Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987;262:10376–10383. [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Naitoh Y. Amino acid sequence of a novel integrin β4 subunit and primary expression of the mRNA in epithelial cells. EMBO (Eur Mol Biol Organ) J. 1990;9:757–763. doi: 10.1002/j.1460-2075.1990.tb08170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Murphy E, Pil P, Chen C, Ginsberg MH, Hemler ME. Molecular cloning and expression of the cDNA for α3 subunit of human α3β1 (VLA-3), an integrin receptor for fibronectin, laminin, and collagen. J Cell Biol. 1991;115:257–266. doi: 10.1083/jcb.115.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura RN, Rozzo C, Starr L, Chambers J, Reichardt LF, Cooper HM, Quaranta V. Epithelial integrin α6β4: complete primary structure of α6 and variant forms of β4. J Cell Biol. 1990;111:1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennenbaum T, Belanger AJ, Glick AB, Tamura R, Quaranta V, Yuspa S. A splice variant of α6 integrin is associated with malignant conversion in mouse skin tumorigenesis. Proc Natl Acad Sci USA. 1995;92:7041–7045. doi: 10.1073/pnas.92.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsdóttir S, Roelen BAJ, Freund E, Gaspar AC, Sonnenberg A, Mummery CL. Expression patterns of laminin receptor splice variants α6Aβ1 and α6Bβ1 suggest different roles in mouse development. Dev Dyn. 1995;204:240–258. doi: 10.1002/aja.1002040304. [DOI] [PubMed] [Google Scholar]
- Tourkin A, Bonner M, Mantrova E, LeRoy EC, Hoffman S. Dot-like focal contacts in adherent eosinophils, their redistribution into peripheral belts, and correlated effects on cell migration and protected zone formation. J Cell Sci. 1996;109:2169–2177. doi: 10.1242/jcs.109.8.2169. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Kuikman I, Baudoin C, van der Neut R, Sonnenberg A. A novel β1 integrin isoform produced by alternative splicing: unique expression in cardiac and skeletal muscles. FEBS Lett. 1995;369:340–344. doi: 10.1016/0014-5793(95)00814-p. [DOI] [PubMed] [Google Scholar]
- van der Neut R, Krimperfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- van Kuppevelt THMSM, Languino LR, Gailit JO, Suzuki S, Ruoslahti E. An alternative cytoplasmic domain of the integrin β3 subunit. Proc Natl Acad Sci USA. 1989;86:5415–5418. doi: 10.1073/pnas.86.14.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leusden MR, Kuikman I, Sonnenberg A. The unique cytoplasmic domain of the human integrin variant β4E is produced by partial retention of intronic sequences. Biochem Biophys Res Commun. 1997;235:826–830. doi: 10.1006/bbrc.1997.6892. [DOI] [PubMed] [Google Scholar]
- von Ballestrem CG, Uniyal S, McCormick JI, Chau T, Singh B, Chan BMC. VLA-β1 integrin subunit-specific monoclonal antibodies MB1.1 and MB1.2: binding to epitopes is not dependent on thymocyte development or regulated by phorbolester and divalent cations. Hybridoma. 1996;15:125–132. doi: 10.1089/hyb.1996.15.125. [DOI] [PubMed] [Google Scholar]
- Yao CC, Liober BL, Sutherland AE, Mendrick DL, Kramer RH. Laminins promote the locomotion of skeletal myoblasts via the α7 integrin receptor. J Cell Sci. 1996;109:3139–3150. doi: 10.1242/jcs.109.13.3139. [DOI] [PubMed] [Google Scholar]
- Zhidkova NI, Belkin AM, Mayne R. Novel isoform of β1 integrin expressed in skeletal and cardiac muscles. Biochem Biophys Res Commun. 1995;214:279–285. doi: 10.1006/bbrc.1995.2285. [DOI] [PubMed] [Google Scholar]
- Ziober BL, Vu MP, Waleb N, Crawford J, Lin CS, Kramer RH. Alternative extracellular and cytoplasmic domains of the integrin α7 subunit are differentially expressed during development. J Biol Chem. 1993;268:26773–26783. [PubMed] [Google Scholar]