Abstract
Cytokinesis is the part of the cell cycle in which the cell is cleaved to form two daughter cells. The unicellular yeast, Schizosaccharomyces pombe is an excellent model organism in which to study cell division, since it shows the general features of eukaryotic cell division and is amenable to genetic analysis. In this manuscript we describe the isolation and characterization of a new protein, imp2, which is required for normal septation in fission yeast. imp2, which colocalizes with the medial ring during septation, is structurally similar to a group of proteins including the S. pombe cdc15 and the mouse PSTPIP that are localized to, and thought to be involved in actin ring organization. Cells in which the imp2 gene is deleted or overexpressed have septation and cell separation defects. An analysis of the actin cytoskeleton shows the lack of a medial ring in septating cells that overexpress imp2, and the appearance of abnormal medial ring structures in septated cells that lack imp2. These observations suggest that imp2 destabilizes the medial ring during septation. imp2 also shows genetic interactions with several, previously characterized septation genes, strengthening the conclusion that it plays a role in normal fission yeast septation.
Keywords: fission yeast, septation, actin, cell cycle, cytokinesis
Cytokinesis is the cellular process by which eukaryotic cells divide after mitosis to form two daughter cells (Fishkind and Wang, 1995). This process requires the assembly of a contractile actin ring at the plasma membrane, where cell division is going to take place. To achieve correct separation of the genetic material, the cell has to monitor the position of the medial ring as well as the timing of its formation and contraction. Identification of components of the medial ring containing structural components such as actin and myosin, as well as regulators of its assembly, disassembly, and contraction are important for understanding cell division in molecular detail. Fission yeast is a unicellular organism that exhibits the features of cytokinesis typical of other eukaryotic cells and, as it is amenable to genetic manipulation, is extensively used as a model organism to study this process.
Schizosaccharomyces pombe cells have a cylindrical shape, grow by elongation at their tips and divide by medial septation. Both of these processes, cell growth and septation, are dependent on the proper organization of the actin cytoskeleton. There are three types of filamentous actin (F-actin)1 structures in fission yeast (Balasubramanian et al., 1997). One is the actin contractile ring or primary ring, that is formed in the middle of the cell during M phase at the site where the septum will be positioned. The ring constricts as the septum is formed, and then it disappears and cannot be detected until the next division. A second type of F-actin structure is the actin patch. In interphase these patches localize to the growing tips of the cell and during septum formation they relocalize in the middle of the cell (Marks et al., 1986). Interphase cells also contain a third type of F-actin, actin cables, but these structures are difficult to observe using standard methods and their function is not clear (Balasubramanian et al., 1997).
Genetic analysis identified several groups of genes required for the sequential steps of septation in fission yeast: medial ring formation, initiation and deposition of the septum, and cell separation (Gould and Simanis, 1997). A large group includes genes whose products are required for the formation of the medial ring. Among these, cdc3 (Balasubramanian et al., 1994) and cdc8 (Balasubramanian et al., 1992), which encode profilin and tropomyosin, respectively, have a more general role in organizing actin filaments; they colocalize with actin in all phases of the cell cycle. The products of cdc4 (a myosin light chain) (McCollum et al., 1995), cdc12 (a formin) (Chang et al., 1997), myo2 (Kitayama et al., 1997) and myp2 (two myosin heavy chains) (Bezanilla et al., 1997), and cdc15 (an SH3-containing protein) (Fankhauser et al., 1995) have a more specialized role in the formation of the medial ring and colocalize only with this structure. Once the medial ring is in place and mitosis is complete, the ring contracts and septum deposition is initiated. This process is thought to involve the products of cdc7, a protein kinase (Fankhauser and Simanis, 1994), cdc11, not yet cloned (Nurse et al., 1976), cdc14, a novel protein (Fankhauser and Simanis, 1993) and spg1, a small GTPase (Schmidt et al., 1997). Once septum formation is complete, two genes are required to turn off synthesis of the septum, cdc16 and byr4, both of which encode novel proteins. Although mutations in genes involved in other aspects of septation can lead to septa that are not cleaved during cell separation, the products of sep1, a forkhead-like transcription factor (Sipiczki et al., 1993; Ribar et al., 1997), and spn1, one of six septins identified in fission yeast (Longtine et al., 1996) might be more closely involved with cell separation. Several pathways involving kinases and phosphatases also affect cell separation: spm1, a mitogen-activated protein (MAP) kinase (Zaitsevskaya-Carter and Cooper, 1997); ppb1, a calcineurin-like phosphatase (Yoshida et al., 1994); and pkc1, a protein kinase C homologue (Mazzei et al., 1993), suggesting regulation of this step.
In this article we describe the isolation of a new component of the fission yeast septation machinery. imp2 encodes a new fission yeast protein that shows homology to a family of proteins of similar domain organization including the fission yeast cdc15 (Fankhauser et al., 1995) and the mouse protein PSTPIP (Spencer et al., 1997). The imp2 protein colocalizes with the medial ring as it contracts during septum formation. The gene is not essential for growth but its deletion leads to multiple septation and cell separation defects. Defects in medial ring structures in cells overproducing or lacking imp2 suggest that it is involved in disassembly of the medial ring during septation.
Materials and Methods
Strains, Media, and Genetic Methods
The strains used in this study are listed in Table I. Standard cell culture, media, and genetic techniques were used (Moreno et al., 1991). Double mutants were created by either tetrad dissection or random spore analysis. In the latter case, the presumed double mutants were verified by backcrossing to a wild-type strain, germinating the resulting spores, and then recovering colonies showing the phenotypes of both of the original single mutants. For ectopic expression of proteins we used the regulatable nmt1 promoter (Forsburg, 1993; Maundrell, 1993). Expression was repressed by the addition of 10 μg/ml of thiamine to Edinburgh minimal media (EMM) and induced by washing, and then incubating the cells in EMM lacking thiamine.
Table I.
List of Strains Used in This Study
| JD86 | ade6-M210/ade6-M216 ura4-D18/ura4/D18 leu1-32/leu1-32 imp2::ura4/imp2 h − /h + | |
| JD123 | cdc8-110 imp2::ura4 leu1-32 ade6-M210 h − | |
| JD140 | imp2::ura4 leu1-32 ade6-M216 h + | |
| JD141 | imp2::ura4 leu1-32 ade6-M216 h − | |
| JD124 | cdc4-8 leu1-32 ura4-D18 ade6-M210 h + | |
| JD143 | cdc15-140 leu1-32 ura4-D18 ade6-M120 h − | |
| SS67 | cdc11-119 leu1-32 ura4-D18 h − | |
| SS168 | cdc25-22 leu1-32 ura4-D18 h − | |
| SS364 | nda3-311 leu1-32 ura4-D18 ade6-M216 h − | |
| SS134 | ade6-M210/ade6-M216 ura4-D18/ura4-D18 leu1-32/leu1-32 h − /h + | |
| SS137 | leu1-32 ura4-D18 h − | |
| SS226 | leu1-32 ura4-D18 ade6-M216 h + | |
| SS227 | leu1-32 ura4-D18 ade6-M216 h − | |
| SS377 | cdc8-110 ura4-D18 leu1-32 h + |
To construct pREP41X-imp2 and pREP81X-imp2 to express the gene at different levels, the XhoI–BamHI fragment of the imp2 cDNA was subcloned from pREP3X-imp2, in which expression is driven by the high strength nmt1 promoter, into the same sites in pREP41X and pREP81X, in which expression is directed by mutant versions of the nmt1 promoter providing medium and low level expression (Basi et al., 1993; Forsburg, 1993). The pGFP42-imp2 construct was created by first subcloning the BspLUIII–BamHI fragment from pREP3X-imp2, containing the complete open reading frame (ORF), into the pAS1 vector (Durfee et al., 1993) digested with NcoI and BamHI. The cDNA was removed from this construct by digestion with NdeI–BamHI and subcloned into pGFP42, which has a ura4 +-selectable marker (gift of T. Carr, University of Sussex, Sussex, UK) digested with the same enzymes. It was subsequently subcloned by inserting the PstI–SacI fragment into pREP3X, to create pGFP41-imp2 with LEU2-selectable marker. The pREP42-GFP-cdc4 and pREP81-GFP-cdc4 fusion constructs (Balasubramanian et al., 1997) were gifts of K. Gould (Howard Hughes Medical Institute, Vanderbilt University, Nashville, TN). We obtained a hemagglutinin (HA)-tagged form of cdc15 (Fankhauser et al., 1995) expressed from pREP41, and the pREP3Δ-PSTPIP-FLAG construct (Spencer et al., 1997) from V. Simanis (Swiss Institute for Experimental Cancer Research (ISREC), Epalinges, Switzerland). The pSGP573-myp2 construct (Bezanilla et al., 1997) expressing green fluorescent protein (GFP)-myp2 was obtained from M. Bezanilla and T.D. Pollard (The Salk Institute for Biological Studies, La Jolla, CA).
Plasmid transformations into S. pombe were done using either electroporation (Prentice, 1992) or LiOAc transformation procedures (Elble, 1992). pREP3X-imp2 integrants were obtained after LiOAc transformation and screening for stable transformants, and were confirmed by Southern blot analysis. One strain contained multiple copies of the integrated imp2 gene and was used for the time lapse experiment shown in Fig. 3. Synthetic lethal interactions involving the expression of imp2 cDNA from the pREP3X or pREP41X plasmids were done by growing transformants in liquid cultures to mid-log phase at their respective permissive temperatures in EMM supplemented with thiamine to repress transcription from the nmt1 promoter. The cells were washed three times with thiamine-free media, and then grown for 24 h in the absence of thiamine to allow transcription. Cells were counted, brought to a concentration of 1.5 × 106/ml, and a fivefold dilution series was prepared. Equal aliquots of these samples were applied to EMM plates without thiamine, and incubated at temperatures ranging from 25°C to 36°C to test for synthetic interactions.
Figure 3.

imp2 overexpression results in the disappearance of the medial ring during its contraction. Septation was observed in an imp2-overexpressing integrant strain before (a) and after (b) full induction of imp2 by time-lapse microscopy by following the localization of GFP-cdc4 to visualize the medial ring and by CCF staining to visualize the septum. Before full induction of imp2, cells showed normal medial ring contraction ahead of the developing septum (a), but after full induction (b) the initially normal medial ring (1 and 5 min, arrowheads) became unstable and abnormal ring structures were seen during its contraction (9 and 10 min, arrows), before it completely disappeared from the middle of the cell (12 min, arrowheads). Frequently, new rings appeared adjacent to the partially formed septum (12 min, arrows). t = 0 min is the beginning of observation of the field. Arrowheads indicate the site of the originally formed, normal medial ring, while arrows point to abnormal structures. Bar, 5 μm.
The 5′ and 3′ ends of imp2 were sequenced using Sequenase (United States Biochemical Corp., Cleveland, OH) and found to be identical to the ends of a S. pombe ORF in the S. pombe Genome Database: SPAC13F4.08c (http://www.sanger.ac.uk/Projects/S_pombe/).
Microscopy
For actin staining, cells were fixed in 3.3% formaldehyde in PBS for 30 min (Balasubramanian et al., 1997) and to 50 μl of fixed cell suspension 1 μl of 100 μg/ml TRITC-phalloidin (rhodamine-labeled phalloidin) (Sigma Chemical Co., St. Louis, MO) was added. After 30 min at room temperature the excess phalloidin was washed away with PBS, cells were dried on coverslips treated with poly-l-lysine (Sigma Chemical Co.) and counterstained with 4,6-diamidino-2-phenylindole (DAPI) dissolved in PBS to visualize the DNA. For anti-Arp3 immunofluorescence localization cells were fixed in methanol (McCollum et al., 1996) and incubated overnight with a 1:200 dilution of anti-Arp3p antibody, a generous gift of K. Gould, and then with Texas red–labeled goat anti–rabbit secondary antibody (Jackson Labs, Bar Harbor, ME) diluted 1:200 for 2 h. Calcofluor white (CCF) (Sigma Chemical Co.) staining of the septum was performed either by adding an equal volume of 100 μg/ml dye dissolved in 50% glycerol to the live cell suspension, and then directly observing them on microscope slides, or mounting fixed cells dried on coverslips in 100 μg/ml CCF solution. All microscopic and photographic work was done using a Zeiss Axioskop fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) except for the localization of GFP-imp2 (see Fig. 7) and the time-lapse experiments (see Figs. 3 and 6), which were carried out using a Deltavision deconvolution microscope system (Applied Precision Inc., Issaquah, WA).
Figure 7.

GFP-imp2 localizes to a medial ring during septation that constricts with the progression of septation. (a and β) GFP-imp2 expression was induced in a wild-type strain (SS137) and live cells were stained with Hoechst dye that stains the chromatin and septa. Images of serial sections were collected on a Deltavision deconvolution microscope and the result of a three-dimensional reconstruction of GFP-imp2 (green) and Hoechst (blue; small arrow, developing septum; arrowheads, nuclei) images is shown (a). The image on the right (b) displays only GFP-imp2 to show its localization relative to the contour of the cell. (c) The ring formed by GFP-imp2 constricts as septation proceeds. The upper panel shows the localization of GFP-imp2 to a medial ring in a cell as it progresses through septation, while the lower panel shows Calcofluor staining of the same cell. (d–f) In a separate experiment GFP-imp2–expressing cells (d–f) were stained with rhodamine–phalloidin, to visualize F-actin (d, red). Besides forming a ring during septation, GFP-imp2 localizes to cytoplasmic spots (d–f, green) that are different from actin patches (c, red); the Figure also shows DAPI staining of the chromatin (c, blue). The localization of GFP-imp2 is shown in interphase (d), septating (e) and septated (f) cells. Bars, 5 μm.
Figure 6.

Septum formation in germinating Δimp2 spores expressing GFP-cdc4 was followed by time-lapse microscopy. The series shows a representative example of the medial ring organization defects we observed during both the first and later septations after germination. The original spore is the compartment, whose cell wall is brightly stained by CCF. Although abnormal actin filaments were observed during ring contraction (14 and 21 min, arrows), ring contraction and septum formation did go to completion (26 min, CCF). At this stage the medial ring did not disappear (26 min, dotted arrows), and additional transient filaments (29 and 36 min, arrows) or rings formed (29 and 36 min, arrowheads). t = 0 min is the beginning of observation of the field. Bar, 5 μm.
Mapping of the imp2 Gene
Data deposited in the S. pombe Genome Database indicated that imp2 was identical to an ORF (SPAC13F4.08c) found on cosmid ICRFc60F0413 from chromosome I (Hoheisel et al., 1993). However, by Southern blot analysis, the internal BstXI fragment of the imp2 cDNA failed to hybridize to this cosmid, provided by Resource Center/Primary Database of the German Human Genome Project (RZPD) (http://www.rzpd.de/). Subsequently, the same internal fragment of the imp2 cDNA was hybridized to an S. pombe cosmid library filter (RZPD) (Hoheisel et al., 1993). The RZPD mapped the positive clones to the right arm of chromosome II. 6 of the 12 cosmids mapping to this location (obtained from RZPD) hybridized to the internal BstXI fragment of the imp2 cDNA. The PstI–EcoRV fragment containing the imp2 gene in four of these six cosmids (ICRFc60C0515, ICRFc60F0731, ICRFc60F0313, and ICRFc60D0616) was 3.5 kb, the size expected based on the sequence of the regions flanking the imp2 gene reported for cosmid ICRFc60F0413 that was mis-assigned to chromosome I.
Construction of the Δimp2 Strain
Cosmid ICRFc60F0313 (Hoheisel et al., 1993) was used to create a null mutation of the imp2 gene (Fig. 1 d). The PstI–PshAI fragment from the cosmid was subcloned into the PstI–EcoRV sites of a modified Bluescript vector from which the HindIII site had been eliminated. The internal HindIII fragment of the imp2 gene, containing 1,988 bp of the 2,100-bp coding region, was replaced by a 1.8-kb HindIII fragment containing the S. pombe ura4 + gene (Grimm et al., 1988). The 3.4-kb BamI–PstI fragment, carrying the imp2::ura4 construct, was gel purified and used to transform by electroporation a diploid S. pombe strain containing a homozygous deletion of the ura4 gene (SS134). To enrich for stable integrants, the ura+ transformants were replica plated three times in the absence of selection onto yeast extract (YE) plates and then onto ura− EMM plates to identify the transformants that were still ura+. These colonies were further tested by streaking on YE and then replica plating again to ura− EMM plates. Transformants that gave rise to only ura+ colonies were candidates for stable integrants. Genomic DNA was prepared from four of these strains, digested with ScaI, and then probed with the ura4 gene on a Southern blot. Two of the transformants gave the 1.6- and 4.3-kb fragments, expected for a homologous integrant (Fig. 1 d), identifying these as potential deletion strains. Subsequently, colony PCR was used to verify that these strains were heterozygous imp2 deletion strains using two reactions: one reaction used oligonucleotides 1 and 2, the other oligonucleotides 1 and 3 (Fig. 1 c). Oligonucleotide 1 hybridizes between the PstI and ScaI site upstream of the coding region, outside the region used for the construction of the null mutant (GGG CGT TTT GTA TGT ACC), oligonucleotide 2 hybridizes immediately upstream of the imp2 gene (CCC AAG CTT GTA AAC GGA AAA AAA CAC G), and oligonucleotide 3 hybridizes inside the ura4 gene, close to its 3′ end (CAT TGG TGT TGG AAC AG). In the first reaction with oligonucleotides 1 and 2, both of the two candidate strains as well as the original wild-type diploid gave the expected 850-bp PCR fragment. In the other reaction with oligonucleotides 1 and 3, the two potential integrants gave a 910-bp band indicative of the null allele, while the wild-type diploid gave no band. One of these strains (JD86) was used for all subsequent experiments. This diploid was sporulated on a malt extract (ME) plate and tetrads were analyzed on YE media at 32°C. Of eight complete tetrads that were analyzed, all four spores gave rise to colonies, two of which were ura−, and two of which were ura+, indicating that the imp2 gene is not essential for viability.
Figure 1.

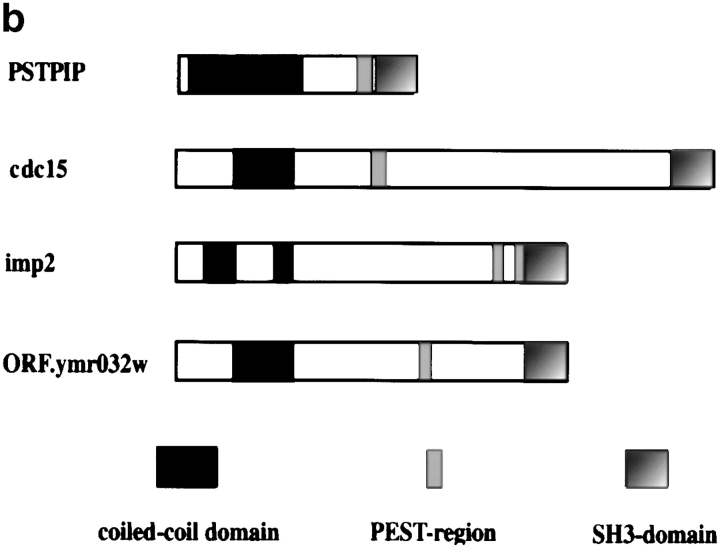
Sequence characteristics of imp2. (a) Amino acid sequence comparison between the predicted products of S. pombe imp2, S. cerevisiae ORF ymr032w, mouse PSTPIP, and S. pombe cdc15. The DNA sequence data imp2 are available from GenBank/EMBL/DDBJ under accession No. Z69379. (b) Schematic representation of domain structure of the proteins encoded by ymr032w, cdc15, imp2, and PSTPIP. Regions showing the highest similarity are the NH2-terminal domains with propensity to form coiled-coil structure and the COOH-terminal SH3 domains. All four proteins have high scoring PEST sequences, but their localization is not conserved. (c) Map of the genomic imp2 locus and the imp2 deletion construct. The internal HindIII fragment of the imp2 gene was replaced by the ura4 + gene carried on a HindIII fragment. Small arrows, oligonucleotides 1–3 with their direction, that were used to verify the null mutation.
For germination experiments, asci were digested with glusulase (DuPont-NEN, Boston, MA), and the resulting debris removed by centrifugation of the spores through a 40% glycerol cushion. Spores were inoculated into EMM media lacking uracyl at 36°C and cells were fixed 13 and 17 h later. To obtain spores lacking any possible residual imp2 protein, the haploid deletion strains JD140 and JD141 were crossed and the resulting spores were germinated as described above.
To remove the cell wall, JD141 cells were digested with a mixture of Novozyme and Zymolyase in buffer containing 1.2 M sorbitol. After the cell wall was removed, protoplasts were washed and transferred to growth media containing 0.8 M sorbitol, and then were plated at 36°C on YE plates at 36°C to assay for the presence of cell wall, or YE plates containing 0.8 M sorbitol to allow recovery of protoplasts. The recovery of cylindrical-shaped cells was tested by microscopically examining the growing colonies on the YE–sorbitol plates.
Results
Isolation and Identification of imp2
We performed a screen to identify S. pombe cDNAs that are weak overexpression suppressors of pim1-d1ts. pim1 encodes the guanine nucleotide exchange factor for the small GTPase, spi1, which is a structural and functional homologue of the mammalian GTPase Ran. Cells with temperature-sensitive mutations in pim1 arrest with nuclear defects, including hypercondensed chromosomes and nuclear envelope fragmentation (Sazer and Nurse, 1994; Demeter et al., 1995), and cytoplasmic phenotypes, including an abnormal, wide septum and medial ring structures that are not disassembled after the septum is formed (Demeter, J., and S. Sazer, unpublished results). Here we describe the characterization of one of these genes that we named imp2 (for increased maximal permissive temperature for pim1).
The imp2 cDNA insert was sequenced and found to be identical to the ORF of an S. pombe gene, identified by the S. pombe Genome Project as SPAC13F4.08c (Hoheisel et al., 1993), which is located on chromosome II (see Materials and Methods). The 2,100-bp imp2 gene is predicted to contain three short introns and a 2,010-bp ORF, which encodes a 670–amino acid protein product. Analysis of the protein sequence revealed that imp2 contains two NH2-terminal regions predicted to form coiled-coil structures, a COOH-terminal SH3 domain, and two high scoring PEST regions, the second of which partially overlaps the SH3 domain. A database search revealed a high degree of similarity between imp2 and a previously characterized S. pombe protein, cdc15, the predicted protein product of an uncharacterized S. cerevisiae ORF deposited in the Saccharomyces cerevisiae Genome Database (http://genome-www.stanford.edu/Saccharomyces/) under the name of ymr032w, and a mouse protein, PSTPIP (Spencer et al., 1997). An alignment of these proteins shows that the most conserved regions are the NH2-terminal coiled coil and the COOH-terminal SH3 domains (Fig. 1 a). The predicted protein products of these three genes show similar structural organization: they all contain coiled-coil NH2-terminal domains, a COOH-terminal SH3 domain, and internal PEST regions (Fig. 1 b). The overall sequence identity is 26% between imp2 and Cdc15, 19% between imp2 and PSTPIP, and 15% for cdc15 and PSTPIP.
Ectopic Expression of imp2 Promotes Medial Ring Disassembly in Septating Cells
The amino acid similarity between imp2 and cdc15, and the previously characterized involvement of cdc15 in septation, suggested the possibility that imp2 also has a role in this process. To test this possibility, we expressed the imp2 cDNA in wild-type cells from the high expression level thiamine-regulatable nmt1 promoter in pREP3X. Cells were fixed after 24 h of transcriptional de-repression at 32°C and were stained with Calcofluor to visualize the septum and DAPI to visualize the DNA. Under these conditions, 50% of wild-type cells expressing imp2 were septated and these cells exhibited the following defects in septation and cell separation: 10% showed filamentation, in which three septa separated four nucleus-containing compartments (Fig. 2 a); 28% showed defective septum formation, resulting in abnormal, wide septa, some of which appeared not fully closed (Fig. 2 b); and 35% exhibited partial septa (Fig. 2 c). In wild-type cells these abnormal phenotypes were never observed.
Figure 2.

Ectopic expression of imp2 in wild-type cells causes septation defects and actin mislocalization. imp2 expression (pREP3X- imp2) was induced for 24 h at 32°C in wild-type cells (SS137), and fixed cells were stained with DAPI (a, c) and CCF (a–c) to visualize the DNA and septal material. Calcofluor staining showed the formation of abnormal, wide septa that were not always fully closed (b, arrowhead) and cell separation defects (a–c). Vector-containing (d) or imp2-expressing cells (e, f) were stained with rhodamine-labeled phalloidin to visualize actin. Actin localized normally in the control cells: at the growing tips in interphase cells (d, arrowhead), in a medial ring in septating cells (d, small arrow), and at the septum in septated cells (d, large arrow). Actin localization was disorganized in septating and septated imp2-expressing cells: it was randomly localized at the cell cortex (e) or in abnormal structures, that seemed to be extra rings (f, arrowhead). The colocalization of the medial ring and the septum was visualized with GFP-cdc4 (g, i) and Calcofluor (h, j). GFP-cdc4 did not localize to a medial ring (g) in septating (h) imp2-expressing cells. In wild-type septating cells, GFP-cdc4 always localized to the medial ring (i) in septating cells (j). imp2 expression was induced (k) or repressed (l) in cdc25ts-arrested cells, and phalloidin staining showed the lack of actin ring formation. Bar: (a–j) 5 μm; (k–l) 10 μm.
In S. pombe the localization of F-actin correlates with the sites of localized cell wall deposition during growth and septation. To test whether imp2 expression affects the organization of the actin cytoskeleton, we used rhodamine-labeled phalloidin, that binds to F-actin, to visualize actin in both the ring and the patches. As has been previously reported (Marks et al., 1986), in wild-type cells F-actin, visualized by phalloidin staining, localized to the growing tips in interphase cells (Fig. 2 d, arrowhead), formed a medial ring in septating cells (Fig. 2 d, small arrow) and remained in the center of cells that had fully formed primary septa (Fig. 2 d, large arrow). We found that the localization of actin in cells expressing pREP3X- imp2 was altered in septating or septated cells compared with wild-type cells: actin spots were found randomly distributed at the cell cortex in 40% of cells undergoing septation (Fig. 2 e). In ∼5% of septated cells, we observed an abnormal actin structure that seemed to be an extra ring localized close to the septum (Fig. 2 f, arrowhead). Since phalloidin staining can not distinguish whether a particular actin structure is derived from the patches or the medial ring, we used two other methods to visualize actin containing structures: the gene encoding a GFP-cdc4 fusion protein (Balasubramanian et al., 1997) that, like wild-type cdc4, localizes to the medial ring, was co-expressed with imp2 in cells to specifically visualize the medial ring during cell division; and actin patches were monitored by immunolocalization of arp3, an S. pombe actin-like protein that colocalizes specifically with actin patches, but not the medial ring, in all phases of the cell cycle (McCollum et al., 1996). In imp2-overexpressing cells GFP-cdc4 localization showed medial ring formation as in wild-type cells, but in 62% of the cells that initiated but did not fully finish septum formation, GFP-cdc4 could not be detected at the site of septum formation (Fig. 2, g and h), unlike in septating wild-type control cells in which cdc4 and the developing septum colocalize (Fig. 2, i and j). To exclude the possibility of a specific displacement of GFP-cdc4 from the medial ring in these cells, we confirmed the lack of an actin ring in these cells by phalloidin-staining (data not shown). Immunolocalization of arp3 showed that the scattered actin spots, detected by phalloidin staining in septated or septating imp2-expressing cells, were actin patches (data not shown). The lack of a medial ring in septating cells ectopically expressing imp2 suggests that imp2 may normally destabilize the medial ring in septating cells.
To further show that imp2 expression affects medial ring stability during contraction, we followed the localization of GFP-cdc4 by time-lapse microscopy in cells overexpressing imp2 and undergoing septum formation. In the plasmid transformants used in the previous experiments the plasmid was present in multiple copies but in fission yeast the plasmid copy number is known to vary greatly between cells. To ensure uniformity among the cells, we integrated the pREP3X-imp2 construct in wild-type cells and examined a strain in which the construct integrated in multiple copies according to Southern blot analysis (data not shown). Under repressing conditions this strain grew at a normal rate, but induction of imp2 expression led to growth arrest. The effect of imp2 expression on the medial ring was analyzed in this strain transformed with the GFP- cdc4 plasmid and septum formation was visualized by Calcofluor staining (Fig. 3). Formation and initial contraction of the medial ring occurred normally and symmetrically, as in wild-type cells (Fig. 3 a), but it became unstable at later time points during its contraction and we observed the formation of abnormal medial ring structures (Fig. 3 b, 9 and 10 min, arrows). Eventually, the medial ring disappeared from the leading edge of the incompletely formed septum (Fig. 3 b, 11 and 12 min, arrowheads) and septum formation stopped. We frequently observed the aberrant formation of a second ring after the original one disappeared, at a site close to the original ring (Fig. 3 b, 12 min, arrows). The second ring recapitulated the same process as the original one: it started to contract, was able to direct the deposition of septal material at the site marked by this ring and like the first ring, it also prematurely disassembled before the completion of the septum, leading to the formation of two partially formed septa in one cell (data not shown). After the initial contraction, the medial ring seemed to go through a period of instability, eventually leading to disappearance of the original medial ring and frequently to the appearance of a second ring structure (Fig. 3 b). We do not know the origin of these unstable structures and the second ring, because they are transient. Photobleaching of the GFP-imp2 probe did not allow us to carry out a more extensive analysis. The disappearance of the appropriately formed medial ring from its original site of formation confirms our hypothesis that imp2 overexpression leads to destabilization of the medial ring.
The high proportion of septated cells seen when imp2 is overexpressed could be due to either inappropriate induction of septation or an inability to complete septation. To exclude the first possibility, we asked whether imp2 expression could induce inappropriate septation from different phases of the cell cycle. We found that the expression of imp2 cDNA in a cdc25-22ts–containing strain that at its restrictive temperature arrests the cell cycle at G2 phase (Hiraoka et al., 1984; Russell and Nurse, 1986), did not induce inappropriate septation when arrested: 31 h of imp2 expression increased the septation index from 5% to 15% at the permissive temperature, while after 27 h at the permissive temperature and then 4 h at the restrictive temperature the septation index only increased from 2% to 4%. In cdc25-arrested cells imp2 overexpression did not induce actin ring formation (Fig. 2 k) compared with uninduced cells (Fig. 2 l). We also tested imp2 expression in the nda3-311cs mutant, which at the restrictive temperature arrests the cell cycle in M phase (Hiraoka et al., 1984) and did not observe an increased septation index.
According to our observations, imp2 expression seemed to destabilize the medial ring in septating cells. Next, we wanted to test whether imp2 expression affected the formation of the medial ring. We visualized F-actin with rhodamine-labeled phalloidin in nda3cs cells expressing imp2, since the nda3cs mutant has been shown to arrest with a medial actin ring (Chang et al., 1996). Expression was induced for 10 h at 32°C and cells were shifted to the restrictive temperature of 20°C for 6 h. At this time point 15% of imp2-expressing cells and 12% of the plasmid only control cells localized actin to the middle of the cell. This indicates that ectopic expression of imp2 neither promotes nor interferes with the formation of the medial ring. It destabilizes the ring only during septation.
imp2 Is Not Essential for Viability
To test whether the function of the imp2 protein was essential, we created a null mutant. Cosmid ICRFc60F0313, containing the imp2 gene, was used to create the deletion construct (see Materials and Methods) in which an internal HindIII fragment was replaced by the S. pombe ura4 + gene (Fig. 1 c). This replacement removed 95% of the coding region of imp2 leaving only 7 amino acids at the NH2 terminus and 30 amino acids at the COOH terminus intact.
A heterozygous Δimp2 diploid was isolated (see Materials and Methods) and tetrad analysis of spores derived from this strain gave a 2:2 segregation of ura+ to ura− colonies. Eight complete tetrads gave two ura− wild-type colonies and two ura+ Δimp2 colonies indicating that the imp2 gene was not essential for viability. Random spore analysis showed that both ura+ and ura− colonies formed and all of the ura− colonies were morphologically wild-type while all of the ura+ colonies showed the same abnormal phenotype. However, colony formation by ura+ Δimp2 spores was temperature dependent. On YE plates at 25°C the ratio of ura+:ura− colonies was 1:1, whereas at 36°C it decreased to <1:20. On EMM media, ura+ Δimp2 colonies did not form above 32°C. However, when streaked to a fresh plate, cells from a ura+ colony growing on any media or at any temperature were able to grow at temperatures between 25°C and 36°C on both YE and EMM.
Deletion of imp2 Causes Septation Defects
The phenotype of the haploid strain containing Δimp2 was complex. At 36°C cells formed long branching filaments, that were multiseptated and contained abnormal septa (Fig. 4, a and b). More than 80% of the cells were either septated or were undergoing cell separation. Although more severe, this phenotype is similar to that of cells in which imp2 was ectopically expressed (see Fig. 2 a) and indicated a defect in both the septation and cell separation processes. In <10% of the cells we observed defects in the placement of septa including multiple septa between nuclei (Fig. 4, a and b, arrowheads) or several nuclei in one compartment without septa separating them (Fig. 4 b, small arrow).
Figure 4.
Deletion of imp2 causes the formation of cell filaments with defective septa and the appearance of aberrant medial ring structures. Δimp2 cells (JD141) were grown at 29°C and then shifted to 36°C for 4 h. Cells were stained with Calcofluor to visualize the septum (a), Calcofluor and DAPI to visualize the septum and the DNA (b), or rhodamine-labeled phalloidin to visualize actin (c). GFP-cdc4 was expressed in Δimp2 cells to localize medial ring structures in live cells (d, e). Δimp2 cells showed various septation defects: multinucleate compartments (b, small arrow), multiple septa between nuclei (a and b, arrowheads) and cell separation defects. Branching could also be observed (a). Abnormal actin (arrowheads in c) and contractile ring (arrowheads in d and e) structures were detected. Bar, 5 μm.
Misplaced and abnormal actin structures were observed when cells containing Δimp2 grown at 36°C were stained with rhodamine-labeled phalloidin (Fig. 4 c). The localization of GFP-cdc4 also showed the formation of aberrant filaments and misplaced rings (Fig. 4, d and e).
These observations suggested a defect in actin ring organization, but the actin organization seemed generally disorganized and the cells also formed branches (Fig. 4, a and b). To gain a clearer understanding of the development of the abnormal phenotype caused by the lack of imp2, we followed the germination of Δimp2 spores to determine the first defect in actin organization. To eliminate residual imp2 protein we examined spores from a cross between h + and h− Δimp2 strains. Localization of F-actin during germination was visualized with phalloidin (Fig. 5, a and b). Δimp2-containing and wild-type spores germinated with the same timing. Initially, actin localization in the Δimp2-containing strain was normal: it localized to the growing tips of the germinating spores and formed an actin ring before the first division (data not shown). The first defect we could observe was that after the first septation the two new cells remained attached to each other (Fig. 5 a), whereas wild-type daughter cells separated from one another normally and that after the first division aberrant actin structures appeared in ∼10% of cell compartments (Fig. 5 b). In a separate experiment, spores expressing the GFP-cdc4 fusion protein were germinated to monitor the localization of the medial ring. The observation that GFP-cdc4 localized into abnormal filamentous structures (Fig. 5 c) not seen in wild-type cells (data not shown) confirmed the previous observation obtained by phalloidin staining and suggested that these structures represent abnormal medial ring structures. Branching occurred infrequently during the time course of these experiments. To directly observe the formation of these abnormal medial ring structures, we followed by time lapse microscopy the germination of Δimp2 spores transformed with the GFP- cdc4–expressing plasmid (Fig. 6). The medial ring formed normally and, although anomalous ring structures were observed during its contraction (Fig. 6, 14 and 21 min, arrows), it contracted completely and lead to the formation of a normal septum as shown by the corresponding Calcofluor images. Unlike in wild-type septation, however, the medial ring did not fully disassemble after septum formation was completed (Fig. 6, 26 min, dotted arrows) and subsequently secondary structures, filaments (Fig. 6, 29 and 36 min, arrows), or more frequently rings (Fig. 6, 29 and 36 min, arrowheads) formed near the septum. Since these structures incorporated GFP-cdc4 and seemed to originate from the remnants of the original medial ring (Fig. 6, 29 min, arrowhead), we suppose that they are abnormal medial ring structures. Later, the abnormal rings detached from the septum and became highly mobile: both their distance from and their angle relative to the septum changed with time. These observations demonstrated that imp2 is required for correct disassembly of the medial ring after septation and that when this process is interfered with abnormal ring structures are formed.
Figure 5.
Germination of Δimp2 spores. Spores from a homozygous diploid were germinated at 36°C and cells were fixed after 13 h. Rhodamine-labeled phalloidin staining (a, b) showed that actin localization in the majority of cells was normal (a), but after the first division the cells did not separate from each other and in these unseparated cells we observed extra, misplaced medial rings (b). The appearance of the extra ring structures was further confirmed by the expression of GFP-cdc4 in these germinating spores (c). Bar, 5 μm.
Because the haploid Δimp2 strain showed a generally disorganized actin organization and the cells eventually also formed branches (Fig. 4, a and b), we wanted to test the possibility that imp2 also plays a role during interphase. But the demonstration that imp2 function was not required for the re-establishment of the polarized rod shape after cell wall removal (data not shown), which is a process dependent on interphase actin organization (Kobori et al., 1989), or after spore germination (Fig. 6), suggested that imp2 does not directly affect polarized growth.
imp2 Protein Localizes to the Medial Ring in Septating Cells
To determine the cellular localization of imp2 protein, the imp2 cDNA was subcloned into a GFP fusion vector (pGFP41) in which transcription is regulated by the medium level nmt1 promoter. This construct was transformed into the heterozygous diploid imp2 deletion strain to test for the functionality of the fusion protein. The resulting Δimp2-containing spores were able to form colonies under conditions that induce transcription at 32°C, a temperature at which the deletion strain without the plasmid was unable to do so, indicating that the GFP-imp2 fusion protein was functional. To monitor the localization of imp2, the GFP-imp2 fusion protein was expressed in wild-type cells, where its localization was identical to its localization in Δimp2-containing cells (data not shown). Since S. pombe vectors are mitotically unstable, plasmid copy number is variable within the population, leading to heterogeneous levels of overexpression. In transformants expressing GFP-imp2 from the medium strength nmt1 promoter, we observed a faint cytoplasmic signal in all cells expressing a detectable level of the fusion protein. Additionally in cells with a low signal level we observed a medial ring in dividing cells. Three-dimensional reconstruction of serial sections imaged using a Deltavision deconvolution microscope system demonstrated that the GFP-imp2 protein formed a ring in septated cells (Figs. 7, a and b). We observed the formation of GFP-imp2 rings before septum deposition started (data not shown) and the GFP-imp2 ring constricted progressively as septum formation advanced (Fig. 7 c). The GFP-imp2 ring colocalized with the actin ring that was visualized with rhodamine–phalloidin (data not shown). In addition to the medial ring signal we observed the localization of GFP-imp2 to cytoplasmic spots. Because these spots tended to localize near the ends of the cell in interphase (Fig. 7 d) and near the medial region during septation (Fig. 7, e and f), we tested whether they colocalized with actin patches as visualized by phalloidin staining. We did not see colocalization of these two signals (Fig. 7 d), indicating that imp2 does not associate with actin patches. Since the two structures can be detected in the same cell (Fig. 7 e), the imp2 spots are not likely to be precursors of the ring structure.
To ask whether other septation proteins were required for imp2 ring formation, we expressed GFP-imp2 in several different temperature sensitive mutants that are defective in septation. GFP-imp2 formed a medial ring only in cdc11-119 and cdc15-140, which are capable of actin ring formation, but not in cdc4-8 and cdc8-110, which do not form actin ring structures (data not shown). This suggests that imp2 localization to a ring structure is dependent on the formation of the actin ring. Conversely, the actin ring can form in cells in which imp2 has been deleted (Fig. 5 a).
To ask whether the septation defect in imp2 null cells could be explained by the lack of proper localization of some other component of the medial ring, we tested the localization of other known components of the medial ring in the Δimp2 strain. Plasmid constructs encoding the fission yeast cdc15-HA (Fankhauser et al., 1995) and GFP- myp2 (Bezanilla et al., 1997) were transformed into the Δimp2 strain and the proteins were localized by immunofluorescent detection of the HA tag or by fluorescence of the GFP tag, respectively. We found that both cdc15 and myp2 still localized normally to the medial ring in the deletion strain, indicating that their localization is not dependent upon the presence of imp2 (data not shown).
imp2 Interacts Genetically with cdc15 and Other Septation Genes
To test for genetic interactions between imp2 and genes defective in various aspects of actin organization or septation (Table II), we first created double mutants containing Δimp2 and cdc8-110, cdc11-119, cdc4-8, and cdc15-140 (Nurse et al., 1976). We found that Δimp2 had the strongest interaction with cdc15-140 and cdc4-8. By tetrad analysis we found that these double mutants showed synthetic lethality at 25°C. In the case of cdc15ts, out of 30 tetrads analyzed, 7 had the non-parental ditype in which 2 were wild-type colonies and 2 were double mutants. 13 of 14 double-mutant spores germinated, but underwent only a few divisions. We did not recover double-mutant colonies from four nonparental ditype tetrads from a cdc4ts × Δimp2 cross at 25°C indicating that the double mutant is not viable at this temperature. cdc11ts also showed strong genetic interaction with Δimp2. One double mutant isolated by tetrad dissection did not form a colony at 25°C and the five others that did were not able to grow at or above 29°C. This indicates a strong interaction between imp2 and cdc11. cdc8ts showed a weaker interaction with Δimp2. The presence of the imp2 null mutation decreased the restrictive temperature caused by cdc8ts by 3°C (from 32°C to 29°C).
Table II.
Growth Characteristics of Δimp2 Double Mutants and imp2-Overexpressing Strains
| Strains | Growth temperature | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25°C | 29°C | 32°C | 34°C | 36°C | ||||||||
| Δimp2 double mutants: | ||||||||||||
| cdc8-110 Δimp2 (JD123) | + | − | − | |||||||||
| cdc8-110 | + | + | − | |||||||||
| cdc11-119 Δimp2 | ± | − | ||||||||||
| cdc11-119 | + | + | ||||||||||
| cdc15-140 Δimp2 | − | |||||||||||
| cdc15-140 | + | |||||||||||
| cdc4-8 Δimp2 | − | |||||||||||
| cdc4-8 | + | |||||||||||
| imp2 overexpression from pREP3X-imp2: | ||||||||||||
| cdc8-110 | + imp2 | + | − | − | − | |||||||
| + vector | + | + | + | − | ||||||||
| cdc11-119 | + imp2 | + | + | − | − | − | ||||||
| + vector | + | + | + | + | − | |||||||
| cdc15-140 | + imp2 | + | − | − | − | − | ||||||
| + vector | + | + | + | + | − | |||||||
| cdc4-8 | + imp2 | + | − | − | ||||||||
| + vector | + | ± | − | |||||||||
| wild type | + imp2 | + | + | + | + | ± | ||||||
| + vector | + | + | + | + | + | |||||||
PSTPIP is a mouse homologue of imp2 and its expression in fission yeast leads to a phenotype similar to that of the imp2 deletion (Spencer et al., 1997). To test whether it interferes with the imp2 pathway, we expressed it in the Δimp2 strain. Expression of this exogeneous protein led to a noticeable decrease in the growth rate of the deletion strain at all temperatures tested (from 29°C to 36°C) suggesting that it indeed may negatively affect the imp2 pathway.
Additionally, we tested for genetic interactions between ectopic imp2 expression and known septation mutants (Table II). imp2 was expressed in cdc8-110–, cdc11-119–, and cdc15-140–containing mutants, and a wild-type strain from the strong nmt1 promoter in pREP3X. Wild-type cells expressing imp2 grew at close to normal rate at all temperatures, except at 36°C, where they showed a noticeable growth defect. Expression of imp2 showed the strongest interaction with the cdc15-140 mutation. This synthetic lethal interaction decreased the restrictive temperature of the mutant containing cdc15ts from 36°C to 29°C. A less severe decrease in the restrictive temperature of the other two mutants tested was also observed: in the case of the strain with cdc11ts, it was lowered from 36°C to 32°C, and for cdc8ts, it was lowered from 34°C to 32°C. Overexpression of imp2 from the medium strength nmt1 promoter in pREP41X had a much weaker effect: only the cdc15-140–containing strain showed sensitivity, reflected in a decrease in the restrictive temperature from 36°C to 34°C.
Discussion
In this article we describe the identification of a new S. pombe gene, imp2, that is required for septation in this organism. The imp2 protein is colocalized with the medial ring and its overexpression or deletion leads to medial ring abnormalities indicating that it is required for normal medial ring function. Genetic interactions with known septation genes also suggest that imp2 plays a role in septation.
imp2 Affects Medial Ring Stability in Septating Cells
Our phenotypic and genetic analyses suggest that the imp2 protein affects the stability of the medial ring after the initiation of septum formation. This is supported by the phenotypes of both the imp2-overexpressing and the imp2 null cells. Overexpression of imp2 in wild-type cells leads to an accumulation of septated cells with heterogeneous phenotypes that include cells with incompletely closed or wide septa, in which the medial ring is missing, indicating that the inappropriate expression of imp2, in terms of timing or level, results in destabilization of the medial ring. Time-lapse microscopy showed that the correctly formed medial ring disappeared during its contraction, before septation was completed. Lack of imp2 function has an opposite effect: medial ring structures could be detected in ∼10% of the septated cells indicating a defect in ring disassembly. Localization of imp2 to a ring that colocalizes with, and depends on the prior assembly of the medial ring is consistent with the model that the primary target of imp2 function is the medial ring.
imp2 was isolated as a weak overexpression suppressor of the lethality of pim1-d1ts mutation. At the restrictive temperature this mutant arrests with actin ring structures at fully formed septa (Demeter, J., and S. Sazer, unpublished data), suggesting a defect in medial ring disassembly in the absence of pim1 function. Overexpression of imp2 in a pim1-d1ts–containing strain leads to a decrease in the number of cells showing this phenotype (Demeter, J., and S. Sazer, unpublished data). This observation further supports a model in which imp2 plays a role in medial ring disassembly.
In addition to its effect on the medial ring, imp2 overexpression also affects the localization of actin patches and the septa, which appear wide because of septal material deposited around the actual septation site. Since it is believed (Balasubramanian et al., 1997) that the deposition of the septum is a function related to the actin patches, it is possible that ectopic imp2 expression affects the distribution of actin patches around the septation site. However, we believe that this is a secondary consequence of the effect of imp2 expression on the contractile ring. How the coordination between the functions of the contractile ring and the actin patches is achieved during septation is not clear, but it is likely that a normal medial ring is required for the proper localization of actin patches. For example, deletion of cdc4, whose function is required only for the formation of the contractile ring, blocks medial ring formation and leads to a uniform distribution of actin patches around the circumference of these cells (McCollum et al., 1995). By destabilizing the medial ring, ectopic imp2 expression could indirectly lead to an abnormal distribution of actin patches.
Overexpression of imp2, like the deletion of imp2, leads to cell separation defects, as well. Mutations in other proteins whose function is expected to affect the medial ring have also been reported to lead to defects in cell separation (McCollum et al., 1995; Kitayama et al., 1997).
Our results suggest that imp2 does not affect the correct positioning or initial formation of the medial ring, and affects the stability of the ring only at later steps during its contraction. Similarly, microinjection of mutant forms of cdc42, a Rho-like GTPase, in Xenopus embryos (Drechsel et al., 1997) or mutations in an FH protein, cyk-1, in Caenorhabditis elegans (Swan et al., 1998) lead to defective cytokinesis at a late stage, during the contraction of the medial ring. Since several Rho-type GTPases are known to bind FH proteins, both of these observations might indicate the involvement of Rho proteins in a late stage in cytokinesis. Unlike imp2-overexpressing cells, in these examples the medial ring is still present in arrested cells indicating a defect distinct from the one caused by imp2 overexpression.
imp2 Is Similar to the Septation Protein cdc15
imp2 shows substantial homology to cdc15 and all of the recognizable functional motifs of cdc15 are preserved in imp2. Both cdc15 and imp2 proteins localize to the medial ring. The two genes also show genetic interactions: the ectopic expression of imp2 lowers the restrictive temperature of cells containing cdc15ts and the cdc15ts Δimp2 double mutant shows synthetic lethality. Both cdc15ts and Δimp2 are synthetically lethal with cdc4ts. These genetic interactions suggest that cdc15 and imp2 may both act on the same cellular structure, the medial ring. They clearly act at different stages of the septation process: cdc15 acts in early steps of septation before, whereas imp2 acts after septum formation has started. cdc15 has a role in the formation of the medial ring, although its exact role is not clear. It may be required either for the formation of the medial ring (Fankhauser et al., 1995) or for the mobilization of actin patches to the septation site (Balasubramanian et al., 1997). imp2 may have a complementary role in disassembling the medial ring and reorganizing the actin patches after septation.
imp2 is not required for viability, suggesting either that the function it performs is not essential or that there are additional genes capable of substituting for imp2. A candidate is a recently discovered potential ORF (SPAC7D4.02c) sequenced by the S. pombe Genome Project that has homology to imp2 in both the NH2-terminal and COOH-terminal domains. The sequence similarity between imp2 and the predicted translation product of this ORF is substantially lower than that between imp2 and cdc15, but is still significant. The existence of this ORF suggests that there is a family of at least three proteins in S. pombe with similar domain organization that include a coiled-coil, an SH3 domain, and perhaps PEST motifs, as well. Together these proteins may regulate the actin cytoskeleton during septation and may perform partially overlapping functions.
Proteins structurally similar to cdc15 and imp2 exist in other organisms, suggesting that their functions are evolutionarily conserved. Indeed, two structurally similar proteins that have been studied colocalize with actin cytoskeletal structures: PSTPIP, a protein that interacts with the tyrosine phosphatase PTP HSCF (Cheng et al., 1996) in mouse, is associated with the cleavage furrow (Spencer et al., 1997); and FAP52, which shows weaker homology but similar domain structure, is associated with focal adhesions in chicken cells (Merilainen et al., 1997). Although in budding yeast there seems to be only one ORF with homology to cdc15 or imp2, in mouse there are now two potential proteins with this domain structure, PSTPIP and h74, a gene not yet characterized (these sequence data are available from GenBank/EMBL/DDBJ under accession No. X85124) (Fankhauser et al., 1995), suggesting that multiple proteins with this domain structure may regulate actin organization in the same organism.
Both cdc15 and PSTPIP are phosphoproteins, and the latter is known to be modified on tyrosine residues. A critical tryptophane residue (W232) in PSTPIP mediating its interaction with PTP HSCF (Dowbenko et al., 1998), is also conserved among cdc15, PSTPIP, and imp2, suggesting that this residue may be important for imp2 function as well. Overexpression of PSTPIP in S. pombe results in a phenotype similar to the imp2 deletion phenotype (Spencer et al., 1997), and decreases the growth rate of the Δimp2 strain suggesting that PSTPIP might interfere with imp2 function, though further experiments will be needed to verify these predictions.
The observation that PSTPIP interacts with a phosphatase in mouse cells may have a parallel in fission yeast, since several kinases and phosphatases were recently shown to affect septation and lead to the production of short filaments in fission yeast (Zaitsevskaya-Carter and Cooper, 1997; Sugiura et al., 1998). Deletion of ppb1 or pmp1 that encode phosphatases lead to a cytokinesis defect and hypersensitivity to a high concentration of chloride ion. We observed that unlike wild-type cells, the Δimp2 strain is unable to grow in 1 M KCl at any temperature (data not shown), suggesting the possibility that these two pathways interact.
Recently, it was shown that PSTPIP binds to the Wiskott-Aldrich Syndrome Protein (WASP) via its SH3-domain (Wu et al., 1998). WASP was implicated in actin polymerization (Symons et al., 1996; Li, 1997) and deletion of its budding yeast homologue BEE1 leads to both budding and cytokinesis defects. Because the human homologue binds WASP, this raises the possibility that imp2 might affect a similar pathway during septation in fission yeast. It is important to note here that WASP is a potential effector of cdc42 and Rac1, Rho-type small GTPases (Aspenstrom et al., 1996; Symons et al., 1996) suggesting a potential link between imp2 and Rho-type GTPases in fission yeast and the possible involvement of these GTPases in a late aspect of cytokinesis in fission yeast as well (Eng et al., 1998). Manipulations of the fission yeast Rho-like GTPase cdc42 did not seem to result in defects in septation (Miller and Johnson, 1994) indicating that perhaps a different Rho-type GTPase is involved in this process in fission yeast.
Acknowledgments
We thank Dr. K. Gould for the GFP-cdc4 fusion constructs and the α-Arp3 antibody; Dr. T. Carr for the pGFP42 plasmid; Drs. B. Edgar and C. Norbury for the cDNA library; Dr. V. Simanis for the cdc15 and PSTPIP-expressing plasmids; M. Bezanilla and Dr. T. Pollard for the GFP-myp2 construct; RZPD for cosmid mapping; and Dr. K. Gould, U. Mueller, and S. Salus for comments on the manuscript.
This work was supported by National Institutes of Health grant GM49119 to S. Sazer.
Abbreviations used in this paper
- CCF
Calcofluor white
- DAPI
4,6-diaminido-2-phenylindole
- F-actin
filamentous actin
- GFP
green fluorescent protein
- HA
hemagglutinin
- ORF
open reading frame
- PSTPIP
proline, serine, threonine, phosphatase interacting protein
- WASP
Wiskott-Aldrich syndrom protein
- YE
yeast extract
References
- Aspenstrom P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol. 1996;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M, Helfman D, Hemmingsen S. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. . Nature. 1992;360:84–87. doi: 10.1038/360084a0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M, Hirani B, Burke J, Gould K. The Schizosaccharomyces pombe cdc3+ gene encodes a profilin essential for cytokinesis. J Cell Biol. 1994;125:1289–1301. doi: 10.1083/jcb.125.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M, McCollum D, Gould K. Cytokinesis in fission yeast Schizosaccharomyces pombe. . Methods Enzymol. 1997;283:494–506. doi: 10.1016/s0076-6879(97)83039-x. [DOI] [PubMed] [Google Scholar]
- Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombenmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Bezanilla M, Forsburg SL, Pollard TD. Identification of a second myosin-II in Schizosaccharomyces pombe. . Mol Biol Cell. 1997;8:2693–2705. doi: 10.1091/mbc.8.12.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Woollard A, Nurse P. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J Cell Sci. 1996;109:131–142. doi: 10.1242/jcs.109.1.131. [DOI] [PubMed] [Google Scholar]
- Chang F, Drubin D, Nurse P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J Cell Biol. 1997;137:169–182. doi: 10.1083/jcb.137.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Daimaru L, Fennie C, Lasky LA. A novel protein tyrosine phosphatase expressed in lin(lo)CD34(hi)Sca(hi) hematopoietic progenitor cells. Blood. 1996;88:1156–1167. [PubMed] [Google Scholar]
- Demeter J, Morphew M, Sazer S. A mutation in the RCC1-related protein pim1 results in nuclear envelope fragmentation in fission yeast. Proc Natl Acad Sci USA. 1995;92:1436–1440. doi: 10.1073/pnas.92.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowbenko D, Spencer S, Quan C, Lasky LA. Identification of a novel polyproline recognition site in the cytoskeletal associated protein, proline serine threonine phosphatase interacting protein. J Biol Chem. 1998;273:989–996. doi: 10.1074/jbc.273.2.989. [DOI] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopusembryos. Curr Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- Durfee T, Becherer K, Chen P, Yeh S, Yang Y, Kilburn A, Lee W, Elledge S. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Elble R. A simple and efficient procedure for transformation of yeasts. Biotechniques. 1992;13:18–20. [PubMed] [Google Scholar]
- Eng K, Naqvi NI, Wong KC, Balasubramanian MK. Rng2p, a protein required for cytokinesis in fission yeast, is a component of the actomyosin ring and the spindle pole body. Curr Biol. 1998;8:611–621. doi: 10.1016/s0960-9822(98)70248-9. [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Simanis V. The Schizosaccharomyces pombecdc14 gene is required for septum formation and can also inhibit nuclear division. Mol Biol Cell. 1993;4:531–539. doi: 10.1091/mbc.4.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Simanis V. The cdc7 protein kinase is a dosage dependent regulator of septum formation in fission yeast. EMBO (Eur Mol Biol Organ) J. 1994;13:3011–3019. doi: 10.1002/j.1460-2075.1994.tb06600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Reymond A, Cerutti L, Utzig S, Hofmann K, Simanis V. The S. pombecdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell. 1995;82:435–444. doi: 10.1016/0092-8674(95)90432-8. [DOI] [PubMed] [Google Scholar]
- Fishkind DJ, Wang YL. New horizons for cytokinesis. Curr Opin Cell Biol. 1995;7:23–31. doi: 10.1016/0955-0674(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Forsburg S. Comparison of Schizosaccharomyces pombeexpression systems. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K, Simanis V. The control of septum formation in fission yeast. Genes Dev. 1997;11:2939–2951. doi: 10.1101/gad.11.22.2939. [DOI] [PubMed] [Google Scholar]
- Grimm C, Kohli J, Murray J, Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4gene as a selectable marker. Mol Gen Genet. 1988;215:81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, Toda T, Yanagida M. The NDA3 gene of fission yeast encodes β-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984;39:349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Hoheisel J, Maier E, Mott R, McCarthy L, Grigoriev A, Schalkwyk L, Nizetic D, Francis F, Lehrach H. High resolution cosmid and P1 maps spanning the 14 Mb genome of the fission yeast S. pombe. . Cell. 1993;73:109–120. doi: 10.1016/0092-8674(93)90164-l. [DOI] [PubMed] [Google Scholar]
- Kitayama C, Sugimoto A, Yamamoto M. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. . J Cell Biol. 1997;137:1309–1319. doi: 10.1083/jcb.137.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Yamada N, Taki A, Osumi M. Actin is associated with the formation of the cell wall in reverting protoplasts of the fission yeast Schizosaccharomyces pombe. . J Cell Sci. 1989;94:635–646. doi: 10.1242/jcs.94.4.635. [DOI] [PubMed] [Google Scholar]
- Li R. Bee1, a yeast protein with homology to Wiskott-Aldrich syndrome protein, is critical for the assembly of cortical actin cytoskeleton. J Cell Biol. 1997;136:649–658. doi: 10.1083/jcb.136.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, DeMarini DJ, Valencik ML, Al-Awar OS, Fares H, De Virgilio C, Pringle JR. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/s0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- Marks J, Hagan I, Hyams J. Growth polarity and cytokinesis in fission yeast: the role of the cytoskeleton. J Cell Sci Suppl. 1986;5:229–241. doi: 10.1242/jcs.1986.supplement_5.15. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Mazzei GJ, Schmid EM, Knowles JK, Payton MA, Maundrell KG. A Ca(2+)-independent protein kinase C from fission yeast. J Biol Chem. 1993;268:7401–7406. [PubMed] [Google Scholar]
- McCollum D, Balasubramanian M, Pelcher L, Hemmingsen S, Gould K. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J Cell Biol. 1995;130:651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D, Feoktistova A, Morphew M, Balasubramanian M, Gould K. The Schizosaccharomyces pombeactin-related protein, Arp3, is a component of the cortical actin cytoskeleton and interacts with profilin. EMBO (Eur Mol Biol Organ) J. 1996;15:6438–6446. [PMC free article] [PubMed] [Google Scholar]
- Merilainen J, Lehto V, Wasenius V. FAP52, a novel, SH3 domain-containing focal adhesion protein. J Biol Chem. 1997;272:23278–23284. doi: 10.1074/jbc.272.37.23278. [DOI] [PubMed] [Google Scholar]
- Miller PJ, Johnson DI. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. . Mol Cell Biol. 1994;14:1075–1083. doi: 10.1128/mcb.14.2.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. . Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. . Mol Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Prentice H. High efficiency transformation of Schizosaccharomyces pombeby electroporation. Nucleic Acids Res. 1992;20:621. doi: 10.1093/nar/20.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribar B, Banrevi A, Sipiczki M. sep1+ encodes a transcription-factor homologue of the HNF-3/forkhead DNA-binding-domain family in Schizosaccharomyces pombe. . Gene. 1997;202:1–5. doi: 10.1016/s0378-1119(97)00390-9. [DOI] [PubMed] [Google Scholar]
- Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Sazer S, Nurse P. A fission yeast RCC1-related protein is required for the mitosis to interphase transition. EMBO (Eur Mol Biol Organ) J. 1994;13:606–615. doi: 10.1002/j.1460-2075.1994.tb06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Sohrmann M, Hofmann K, Woollard A, Simanis V. The Spg1p GTPase is an essential, dosage-dependent inducer of septum formation in Schizosaccharomyces pombe. . Genes Dev. 1997;11:1519–1534. doi: 10.1101/gad.11.12.1519. [DOI] [PubMed] [Google Scholar]
- Sipiczki M, Grallert B, Miklos I. Mycelial and syncytial growth in Schizosaccharomyces pombeinduced by novel septation mutations. J Cell Sci. 1993;104:485–493. doi: 10.1242/jcs.104.2.485. [DOI] [PubMed] [Google Scholar]
- Spencer S, Dowbenko D, Cheng J, Li W, Brush J, Utzig S, Simanis V, Lasky L. PSTPIP: A tyrosine phosphorylated cleavage furrow-associated protein that is a substrate for a PEST tyrosine phosphatase. J Cell Biol. 1997;138:845–860. doi: 10.1083/jcb.138.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura R, Toda T, Shuntoh H, Yanagida M, Kuno T. pmp1(+), a suppressor of calcineurin deficiency, encodes a novel MAP kinase phosphatase in fission yeast. EMBO (Eur Mol Biol Organ) J. 1998;17:140–148. doi: 10.1093/emboj/17.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan KA, Severson AF, Carter JC, Martin PR, Schnabel H, Schnabel R, Bowerman B. cyk-1: a C. elegansFH gene required for a late step in embryonic cytokinesis. J Cell Sci. 1998;111:2017–2027. doi: 10.1242/jcs.111.14.2017. [DOI] [PubMed] [Google Scholar]
- Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, McCormick F, Francke U, Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- Wu Y, Spencer SD, Lasky LA. Tyrosine phosphorylation regulates the SH3-mediated binding of the Wiskott-Aldrich syndrome protein to PSTPIP, a cytoskeletal-associated protein. J Biol Chem. 1998;273:5765–5770. doi: 10.1074/jbc.273.10.5765. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Toda T, Yanagida M. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J Cell Sci. 1994;107:1725–1735. doi: 10.1242/jcs.107.7.1725. [DOI] [PubMed] [Google Scholar]
- Zaitsevskaya-Carter T, Cooper JA. Spm1, a stress-activated MAP kinase that regulates morphogenesis in S. pombe. . EMBO (Eur Mol Biol Organ) J. 1997;16:1318–1331. doi: 10.1093/emboj/16.6.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]