Abstract
The optic disk–directed growth of retinal ganglion cell axons is markedly disturbed in the presence of polyclonal antineurolin antibodies, which mildly affect fasciculation (Ott, H., M. Bastmeyer, and C.A.O. Stuermer, 1998. J. Neurosci. 18:3363–3372).
New monoclonal antibodies (mAbs) against goldfish neurolin, an immunoglobulin (Ig) superfamily cell adhesion/recognition molecule with five Ig domains, were generated to assign function (guidance versus fasciculation) to specific Ig domains. By their ability or failure to recognize Chinese hamster ovary cells expressing recombinant neurolin with deletions of defined Ig domains, mAbs were identified as being directed against Ig domains 1, 2, or 3, respectively. Repeated intraocular injections of a mAb against Ig domain 2 disturb the disk-directed growth: axons grow in aberrant routes and fail to reach the optic disk, but remain fasciculated. mAbs against Ig domains 1 and 3 disturb the formation of tight fascicles.
mAb against Ig domain 2 significantly increases the incidence of growth cone departure from the disk-oriented fascicle track, while mAbs against Ig domains 1 and 3 do not. This was demonstrated by time-lapse videorecording of labeled growth cones.
Thus, Ig domain 2 of neurolin is apparently essential for growth cone guidance towards the disk, presumably by being part of a receptor (or complex) for an axon guidance component.
Keywords: axon guidance, receptor domain, neurolin, immunoglobulin superfamily, retinal axons
During their growth to distant targets, elongating axons receive multiple molecular signals which allow growth cones to navigate in predestined directions. Recognition/receptor components on the surface of the growth cone and the axon guarantee that the signals are perceived and relayed to intracellular signalling cascades.
Cell adhesion molecules (CAMs)1 of the immunoglobulin superfamily (IgSF) are surface recognition molecules whose characteristic structural features include one to several Ig-like domains. IgSF CAMs promote growth cone elongation and the selective fasciculation between developing axons (for review see Brümmendorf and Rathjen, 1994) through homo- and heterophilic interaction with members of this superfamily. However, specific members of the IgSF have been identified as receptors for guidance molecules of unrelated families such as the IgSF CAM DCC (deleted in colon rectal cancer). DCC is part of a receptor complex for the “netrins” (Chan et al., 1996; Keino-Masu et al., 1996; Kolodziej et al., 1996; Deiner et al., 1997), axon guidance molecules which are expressed in specific regions of the developing central nervous system, such as the floor plate (Kennedy et al., 1994; Serafini et al., 1994).
Several mechanisms have been proposed to account for the directed growth of a developing axon from its origin (the retinal ganglion cell [RGC]) towards the optic disk (Ramon y Cajal, 1972; Halfter, 1996; Deiner et al., 1997; Ott et al., 1998) and long-range attractive guidance cues have been postulated but have not yet been identified. Theoretically, most RGC axons could reach the disk by selectively fasciculating with their forerunners and indeed, the IgSF CAMs L1, NCAM, and a fish L1 homologue, the E587 antigen, subserve such functions (Bastmeyer et al., 1995; Brittis and Silver, 1995; Brittis et al., 1995). However, the tracking of forerunners may not suffice for guidance to the optic disk. At least the first differentiating RGCs whose growth cone pioneers the pathways to the disk seem to require directional guidance information to find their first intermediate target.
More recently, the IgSF CAM neurolin (Paschke et al., 1992; Laessing et al., 1994), the teleost homologue of DM-GRASP/SC-1/BEN/ALCAM of birds and mammals (Burns et al., 1991; Tanaka et al., 1991; Pourquié et al., 1992; Bowen et al., 1997), has been discovered to contribute to the disk-directed growth of RGC axons in the goldfish retina (Ott et al., 1998). Neurolin, like its avian and mammalian homologues, consists of five extracellular Ig domains, a transmembrane segment, and a short cytoplasmic domain (see Fig. 1 A). The outcome of in vitro assays, probing for DM-GRASP/SC-1/BEN functions, shows that IgSF CAM promotes axon fasciculation by homo- and/ or heterophilic binding mechanisms (Burns et al., 1991; Tanaka et al., 1991; DeBernado and Chang, 1996). In the chick retina, DM-GRASP participates in the growth of new growth cones along preexisting axons (Pollerberg and Mack, 1994) but a contribution to the oriented growth of axons by this CAM has not been recognized in these earlier studies. That neurolin contributes to the disk-directed growth of RGC axons was evidenced by intraocular injections of Fab fragments of a polyclonal antineurolin antiserum (neurolin Fabs) into the goldfish eye (Ott et al., 1998).
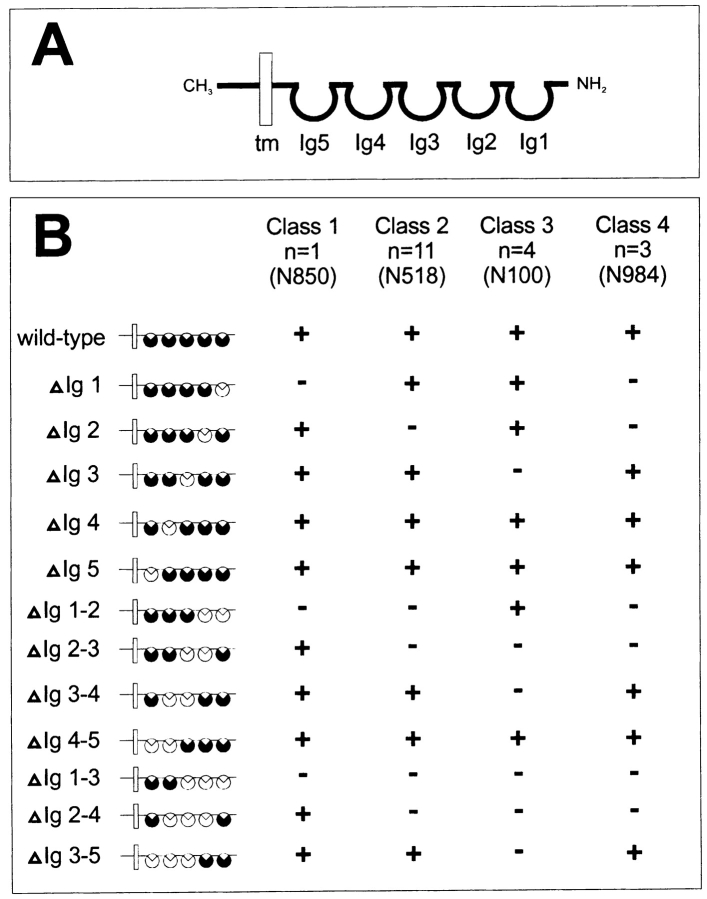
Figure 1.
Schematic representation of neurolin deletion clones and the binding properties of neurolin mAbs. (A) Wild-type neurolin consists of five Ig domains (Ig1–Ig5, black circles), a transmembrane domain (tm), and a short intracellular domain (carboxy terminus, CH3). (B) mAbs against neurolin were screened for their ability (+) or failure (−) to bind to CHO cells expressing forms of neurolin (ΔIg1, ΔIg2, etc.) from which either one, two, or three consecutive Ig domains are deleted (white circles). mAbs fall into four classes: mAbs of class 1 require the presence of Ig domain 1; mAbs of class 2, Ig domain 2; mAbs of class 3, Ig domain 3; and mAbs of class 4, Ig domains 1 and 2 (n, number of mAbs in this class). mAbs N850, N518, N100, and N984 of classes 1, 2, 3, and 4, respectively, were used in functional assays.
The goldfish visual system is well suited for in vivo functional analyses, because it continues to grow throughout life. New RGCs are added at the retinal peripheral margin (Johns, 1977) and young emerging axons grow in strict peripherocentral orientation towards the optic disk by fasciculating with other young axons and axons of the preceeding generation (Easter et al., 1984; Stuermer et al., 1992; Bastmeyer et al., 1995). The spatiotemporally regulated expression of several CAMs (being synthesized at high levels by young RGCs and being downregulated by RGCs of older generations) facilitates the selective association of new with young axons (Stuermer et al., 1992). This selective fasciculation is evidently mediated by several IgSF CAMs and results in the establishment of the age-related order of RGC axons in the fish retinotectal pathway (Easter et al., 1984; Stuermer and Easter, 1984).
The rate of neuronal proliferation in the retina is dependent on the overall growth of the fish (Fernald, 1990); rapidly growing goldfish add a substantially larger number of RGCs and RGC axons than slowly growing individuals. In 3–4-cm rapidly growing fish, roughly 50,000 new RGCs are generated over a period of 10 wk (Bastmeyer et al., 1995).
In a recent study, we injected Fab fragments of a polyclonal antiserum against E587 antigen (E587 Fabs) or neurolin Fabs repeatedly (twice per week) into the eyes of these fast growing fish. RGC axons in neurolin Fab- injected fish exhibit severe pathway mistakes (Ott et al., 1998). Many axons fail to reach the optic disk after leaving their fascicle of origin, turning in the opposite direction or growing in loops and circles (Ott et al., 1998). In addition to causing aberrant routes, neurolin Fabs interfere with the tight fasciculation of axons, but less severely than Fabs against the L1-like CAM E587 antigen. E587 Fabs disrupt the fascicle order (Bastmeyer et al., 1995; Ott et al., 1998) but do not prevent RGC axons from reaching the disk.
Thus, we propose that neurolin has more than one function. It seems to be part of a receptor complex for a molecule that guides axons to the optic disk, and it promotes axon fasciculation by binding neighboring axons together, presumably through a homo- or heterophilic adhesion mechanism.
Defects in the normal axon order caused by neurolin Fabs were prominent in the dorsal retina. Ventral axons were unaffected despite the fact that antibodies have access to both ventral and dorsal axons. This suggests that additional factors participate in intraretinal growth cone guidance (Ott et al., 1998).
We have generated new mAbs against immunoaffinity purified neurolin. The new mAbs were used to examine whether neurolin's function in axon guidance and fasciculation can be assigned to distinct domains. This would support the notion that neurolin is a multifunctional IgSF CAM. Moreover, the identification of a specific domain involved in the long-distance guidance function facilitates the future search for the relevant guidance cues which are expected to be present in various species, not just in fish (Deiner et al., 1997). We find that the disk-directed growth of RGC axons is severely affected by a mAb against Ig domain 2. Disruption of tight fasciculation is observed with mAbs against Ig domains 1 and 3 which also reduce the preference of growth cones to fasciculate with neighboring axons in vitro.
Materials and Methods
Animals
Common goldfish (Carrassius auratus, 5–7 cm body length) obtained from a local supplier were used for protein isolation and neuronal tissue cultures. In vivo antibody pertubation experiments were performed with juvenile goldfish from our breeding colony at the University of Konstanz. For these in vivo tests, groups of 10 individuals were kept in 100-liter tanks at 22°C and fed twice a day to accelerate their growth. For intraocular injections of antibodies and for optic nerve transection (for in vitro assays), fish were anesthetized in MS 222 (3-aminobenzoic acid ethyl ester; Sigma Chemical Co.) in compliance with animal welfare legislation.
Antibodies
mAb E21 (Paschke et al., 1992) was used for immunoaffinity purification of neurolin as described. Purified neurolin was used to immunize a Balb-c mouse according to established protocols (Vielmetter et al., 1991). Supernatants of the resulting hybridoma clones from this immunization were tested on cryosections through the goldfish optic tectum and those producing an immunostaining pattern identical with that of mAb E21 (Paschke et al., 1992) were selected. The specificity of the new mAbs against neurolin was confirmed in Western blots with a fraction enriched in cell surface proteins from goldfish brain (Vielmetter et al., 1991). Hybridoma clones that produced neurolin-specific antibodies of the IgG subclass were further subcloned and tested on CHO cells transfected with neurolin (see below). From 20 new mAbs, four were selected: N518, N100, N850, requiring the presence of Ig domains 1, 2, and 3, respectively, and N984 that requires the presence of Ig domains 1 and 2 (see below). A purified IgG fraction in citrate buffer was prepared for functional assays. Neurolin Fabs were produced and used as previously described (Ott et al., 1998).
Neurolin Deletion Mutants
The full-length neurolin cDNA clone P19 (Laessing et al., 1994) including the signal and the Kozak sequences was amplified in a standard PCR. The PCR product was directly ligated into the eukaryotic expression vector pCR3 (Invitrogen Corp.) and the integrity of the construct was confirmed by double stranded sequencing (T7 sequencing kit; Amersham Pharmacia Biotech).
Neurolin deletion mutants were generated (ExSite PCR-based site directed mutagenesis kit; Stratagene) following standard protocols provided by the manufacturer. A selected fragment of the full-length neurolin cDNA was deleted in a PCR with opposing oligonucleotide primer pairs flanking the corresponding segment. The PCR amplifications were performed under conditions that limit incorrect incorporations. A typical reaction mixture contained 1 mM dATP, dGTP, dCTP, and dTTP, 1.2 μg neurolin-cDNA in pCR3, 0.2 μg of each primer, 2.5 U Taq DNA polymerase, and 2.5 U Taq extender (Stratagene). The amplification protocol consisted of two cycles of denaturation at 94°C for 4 min, annealing at 50°C for 2 min, and elongation at 72°C for 2 min plus 15 cycles with an elevated annealing temperature (94°C for 1 min, 56°C for 2 min, 72°C for 1 min) and a final elongation step at 72°C for 10 min. The template was then removed by digestion with 10 U DpnI endonuclease for 30 min at 37°C. The PCR product was polished with 1.25 U Pfu DNA polymerase for 30 min at 72°C, religated with 4 U T4 DNA ligase for 60 min at 37°C, and transformed into competent Escherichia coli cells (Epicurian Coli XL1-Blue; Stratagene). The resulting neurolin deletion clones were confirmed by double stranded sequencing.
Compared to wild-type neurolin (Laessing et al., 1994), neurolin mutants have deletions in amino acids as follows: ΔIg1 = Δ(Y8 − F93), ΔIg2 = Δ(E131 − G202), ΔIg3 = Δ(V237 − L287), ΔIg4 = Δ(L314 − S366), ΔIg5 = Δ(H399 − V450), ΔIg1 − 2 = Δ(Y8 − G202), ΔIg1 − 3 = Δ(Y8 − L287), ΔIg1 − 4 = Δ(Y8 − S366), ΔIg2 − 3 = Δ(E131 − L287), ΔIg2 − 4 = Δ(E131 − S366), ΔIg3 − 4 = Δ(V237 − S366), ΔIg3 − 5 = Δ(V237 − V450), ΔIg4 − 5 = Δ(L314 − V450) (see also Fig. 1).
Transfection of CHO Cells
The neurolin full-length expression clone, the neurolin deletion constructs, and the pCR3 vector without an insert (mock control) were transfected into CHO cells using the calcium-phosphate precipitation method (Ausubel et al., 1994). Stable transfectants were selected by their resistance to 500 μg/ml geneticin (Gibco BRL). Expression of full-length neurolin and the deletion mutants by the transfected CHO cells was confirmed by immunostaining and immunoblot experiments using the polyclonal antineurolin antiserum. The new neurolin mAbs were then screened for their ability to immunostain transfected CHO cells. CHO cells were seeded into 48-well plates (Costar Corp.). After 24 h, cells were washed once in HBSS (Gibco BRL), fixed in methanol (1 min, −20°C) and rinsed in PBS (3 × 5 min) and then incubated with neurolin mAbs for 90 min at room temperature. Cells were washed again (3 × 10 min) with PBS and incubated with HRP-coupled goat anti–mouse antibodies (1:20,000 in 1% BSA/PBS) (Dianova). After 1 h cells were rinsed in PBS (3 × 10 min), incubated for 5–10 min with the HRP substrate “True Blue” (Kirkegaard & Perry Laboratories, Inc.) and analyzed with an inverted microscope.
Goldfish Retinal Explants
In vitro functional assays were performed with regenerating retinal axons which extend from goldfish retinal explants when the optic nerve is transected 14–17 d before preparation. Goldfish retinal explants were prepared as previously described (Vielmetter and Stuermer, 1989). In brief, the retina was isolated and attached to a Hybond nylon filter (Amersham Pharmacia Biotech). Retina and filter were cut into strips 300 μm wide and explanted, ganglion cell layer down, onto coated coverslips. Small metal blocks were placed on the ends of the strips to keep the retina in contact with the substrate. Retinal explants were kept in culture medium (Ham's F12; Gibco BRL) supplemented with 10% FCS (Sigma Chemical Co.) and 0.4% methyl cellulose at 22°C.
To test whether neurolin has axon growth-promoting properties, immunopurified neurolin was applied to coverslips either directly or after precoating with polylysine according to procedures successfully applied with E587 antigen. Moreover, as described for E587 antigen (Bastmeyer et al., 1995), neurolin was applied in stripes with the aid of a silicone matrix to examine whether growing axons have a preference for neurolin stripes over polylysine. The negative outcome of these experiments lead to a third assay to examine which of the three mAbs affects axon fasciculation.
Neurolin mAbs (N100, N518, or N850, each 100 μg/ml diluted in culture medium) were added to the cultures when retinal explants were placed on polylysine-coated coverslips. Growing axons extending from retinal strips in the presence of mAbs and controls (with no mAbs added) were monitored with time-lapse videomicroscopy. Living axons were viewed with a 40× phase-contrast lens in an inverted microscope (Zeiss Axiovert) to which a camera (Newicon; Hamamatsu Phototonics) was attached. The camera was connected to an image processor (Hamamatsu Phototonics) and an S-VHS time-lapse recorder (Panasonic). To avoid continuous illumination, a shutter which opened every 5 s for 200 ms was inserted into the light path. Four images were taken, averaged, and recorded. Axon growth was recorded in randomly selected fields for 3–6 h to determine if growth cones elongating on polylysine fasciculate with another axon when they make contact. Growth cones that changed their direction and elongated along the other axons for at least 1 h were counted as fasciculating as opposed to growth cones that continued to elongate on the polylysine substrate for at least 1 h after contact with another axon (Ott et al., 1998).
Fluorescent polystyrene microspheres (diameter of 0.5 μm; Duke Scientific Corp.) were conjugated with immunopurified neurolin, E587 antigen (Bastmeyer et al., 1995) or BSA (Sigma Chemical Co.) according to Kuhn et al. (1991), and tested for the ability to bind to one another. Neurolin microspheres were also tested for their ability to bind to RGC axons in retinal whole mounts, to RGC axons in vitro, and to neurolin expressing CHO cells.
In Vivo Functional Assays
For in vivo functional assays, 6-mo-old goldfish of approximately equal size (length: ∼3 cm, eye diameter: ∼3.5 mm) were selected from our breeding colony. Antibodies in citrate buffer (50 mM citric acid, 150 mM NaCl, pH 7.5) were injected into the vitreous chamber of the left eye (Ott et al., 1998). The sclera and iris were penetrated with a syringe and antibody solution was pressure-injected through the preformed hole using a glass micropipette connected to a picospritzer (Transjector; Eppendorf) as previously described (Bastmeyer et al., 1995; Ott et al., 1998). Fish received injections twice a week for 10 wk. Four groups of fish were injected through the temporal aspect of the left eye as follows: (group 1) 9 fish, a mixture of mAbs N984 (7.7 mg/ml), N518 (6 mg/ml), and N100 (20 mg/ml); (group 2) 16 fish, mAb N100 (7 or 20 mg/ml); (group 3) 16 fish, mAb N518 (6 mg/ml); (group 4) 10 fish, mAb N850 (12 mg/ml). The volume injected ranged from 0.1 to 0.4 μl (depending on the concentration of each antibody) and was adjusted so that the concentration of each mAb was 70 μg/ml in the vitreous chamber after each injection. During these 10 wk, the goldfish grew by roughly 40% in length to a mean body length of 5 cm. The right control eye received no injections in these experiments. In earlier studies with the same injection protocol we provided evidence that buffer injections had no visible effect on the order of RGC axons (Bastmeyer et al., 1995; Ott et al., 1998). As in earlier experiments with similar procedures (Bastmeyer et al., 1995; Ott et al., 1998), the injections had no negative effect on growth of the eye or retina. Both the injected (left) and the control (right) eye had the same size and both retinae had the same diameters at the end of the experiment.
Immunohistochemistry on Retinal Whole Mounts
Eyes were isolated, cornea and pigment epithelium removed, and the retinae (photoreceptor layer down) attached to a Hybond nylon filter (Amersham Pharmacia Biotech) by suction. The vitreous layer was carefully removed, but the inner-limiting membrane and the retinal blood vessels were left intact to exclude the possibility that abnormal axonal growth patterns happened because of rupturing of axons (which sometimes occurs when vessels and basal lamina are removed). The retinae were fixed in methanol (−20°C for 10 min) and treated with a polyclonal neurolin antiserum (Ott et al., 1998) overnight at 4°C. After three washes in PBS (20 min each) retinae were incubated in a mixture of dichlorotriazinyl aminofluorescein (DTAF)-coupled goat anti–rabbit antibodies and TRITC-coupled goat anti–mouse antibodies (Dianova) at 4°C overnight. After three washes in PBS (20 min each), retinae were coverslipped in Mowiol (Hoechst AG), and viewed in a fluorescence microscope (Zeiss Axiophot) using the appropriate filter sets.
Ex Vivo Functional Assays
To observe living RGC growth cones in situ, retinae were carefully removed, kept in F12 medium and flattened by attaching them to a nylon filter as described above. At 6 and 2 d before retina excision, the eyes received injections of either mAb N100, mAb N518, mAb N850, neurolin Fabs, or an equal volume of buffer, as described above. To label growth cones of young RGC axons, several small crystals of DiO (N,N-dioctadecyloxacarbocyanine 4-toluenesulfonate; Serva) were placed all around the retinal peripheral margin under the inner limiting membrane and close to the cell bodies of young RGCs. Retinae were maintained in culture medium for 12 h at 25°C to allow incorporation of the dye into RGCs and their axons. Labeled growth cones were observed with a 40× water immersion lens (AchroplanW; Zeiss Axioplan) in a microscope equipped with epifluorescence (Zeiss Axioplan). Images were collected with a silicon intensified target (SIT) camera (Hamamatsu Phototonics). Neutral density filters were placed in the light path to minimize photodamage to the living tissue. For time-lapse recording, images of selected growth cones were taken every 5 min, and stored in a computer using Metamorph software. To quantify the effects caused by the injected neurolin antibodies, images of all labeled growth cones found in one retina were taken. 1–6 h later the same growth cones were examined again and their growth direction determined. For growth cones that were observed for 2–6 h the growth velocity (micrometers per hour) was determined. Growth cones that had elongated directly towards the optic disk were categorized as directed growth. Growth cones deviating by >10° from the direction towards the optic disk were categorized as misrouted growth. Individual growth cones were monitored up to 24 h.
All figures in this study were produced either from directly digitized video images or from original negatives that had been digitized with a Microtec ScanMaker (Polaroid). Images were processed with Adobe Photoshop software and printed (Pictography 3000; Fuji).
Results
Characterization of mAbs Against Neurolin
Neurolin appears to function in both the disk-directed growth of RGC axons (presumably as a receptor for a guidance molecule) and in axon fasciculation. To determine whether neurolin functions can be assigned to specific Ig domains of the protein, mAbs requiring the presence of specific Ig domains for their binding to neurolin were selected for antibody pertubation experiments.
20 mAbs of the IgG subclass were obtained that fulfilled the following criteria: (a) they bind to the same structures on cryosections of adult goldfish brain as the original mAb E21, which had been used earlier to isolate and characterize neurolin (Paschke et al., 1992); (b) they recognize a protein of the appropriate molecular mass (86 kD) in immunoblots with cell surface proteins obtained from adult goldfish brains; and (c) they bind to (i.e., immunostain) transfected CHO cells that express full-length goldfish neurolin (Laessing et al., 1994).
These mAbs were screened on transfected CHO cells expressing various truncated forms of neurolin which lack either one, two consecutive, or three consecutive Ig domains (Fig. 1). In their ability or failure to bind to CHO cells expressing mutant forms of neurolin, mAbs fall into four classes (Fig. 1 B). One antibody (mAb N850) binds to CHO cells expressing neurolin deletion mutants only when Ig domain 1 is present (class 1). Thus, mAb N850 requires Ig domain 1 to recognize neurolin. Class 2 antibodies (n = 11), including mAb N518, require the presence of Ig domain 2. Class 3 antibodies (n = 4), including mAb N100, require Ig domain 3 for binding. Class 4 antibodies (n = 3) include the original mAb E21 and mAb 984, and require both Ig domains 1 and 2 for binding. Thus, they may recognize epitopes to which these two Ig domains contribute. The binding specificity of these mAbs with regard to Ig domains 1–3 of neurolin was verified in immunoblots with proteins from the various CHO cell clones.
mAbs specific for Ig domain 4, Ig domain 5, or the intracellular domain were not found. However, Ig domains 4 and 5 are recognized by the polyclonal antineurolin antiserum. The antiserum labeled CHO cells expressing neurolin with deletion of Ig domains 1–3, and recognized the recombinant protein of these cells in Western blots. Conversely, all mAbs failed to immunolabel these cells, and did not recognize this truncated form of neurolin in Western blots. mAbs N850, N518, and N100 requiring the presence of (tentatively called “mAbs against”) Ig domains 1, 2, and 3, respectively (see Discussion), were selected for in vivo and in vitro assays.
In Vitro Assays for Tests of Axon Fasciculation in Dependence of mAbs Against Ig Domains 1, 2, or 3
To determine which of the selected mAbs decrease the tendency of growth cones to fasciculate with other axons (Ott et al., 1998), we monitored the growth of goldfish RGC axons in the presence of mAbs of classes 1, 2, and 3 with time-lapse videomicroscopy. Growth cones elongating on polylysine that changed their direction upon contact with other axons and continued to elongate along this axon were counted as fasciculating. Control cultures received no mAbs since we have shown in an earlier study that control mAbs have no effect on axonal fasciculation (Bastmeyer et al., 1995).
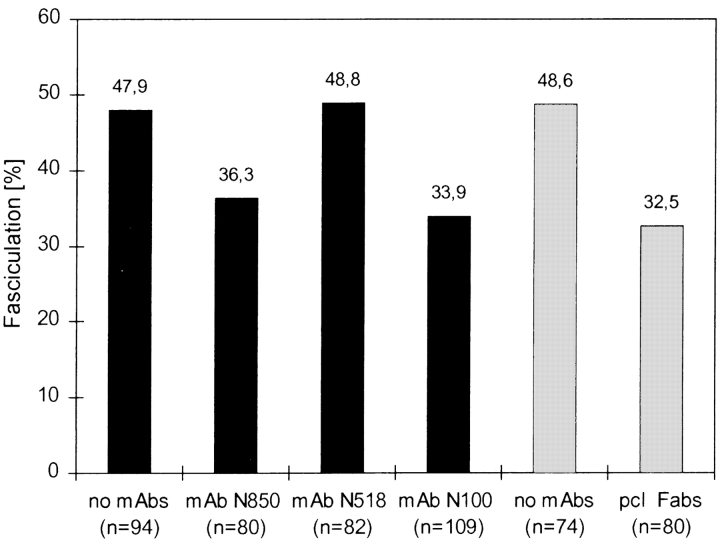
In the presence of mAb N850 (requiring Ig domain 1) 36.3% of the growth cones (n = 29) fasciculated with another axon, whereas 63.7% (n = 51) continued to elongate on polylysine (Fig. 2). Similar ratios were obtained with mAb N100 (requiring Ig domain 3) where 33.9% of the growth cones (n = 37) fasciculated, and 66.1% of the growth cones (n = 72) elongated on the polylysine substrate (Fig. 2). In the presence of mAb N518 (requiring Ig domain 2), however, 48.8% of the growth cones (n = 40) fasciculated, and 51.2% (n = 42) continued to grow on polylysine (Fig. 2). These ratios were similar in control culture where no mAbs were added: 47.9% of the growth cones (n = 45) fasciculated, and 52.1% (n = 49) elongated on polylysine. These findings show that mAbs against neurolin Ig domains 1 and 3 affect axonal fasciculation to the same extent as neurolin Fabs (Ott et al., 1998), whereas mAb N518 against Ig domain 2 does not notably interfere with the choice of growth cones for growth along another axon versus growth on polylysine. The average growth velocity of axons was not affected by neurolin Fabs (Ott et al., 1998) or by our mAbs.
Figure 2.
Ig domains 1 and 3 of neurolin contribute to axon fasciculation in vitro. When growth cones elongating on polylysine meet another axon, 47.9% of the growth cones fasciculate with the other axon in control cultures. In the presence of mAb N850 (against Ig domain 1) 36.3% of the growth cones fasciculate with another axon, and 33.9% do so in the presence of mAb N100 (against Ig domain 3). Similar values (32.5%) were obtained in an earlier study (Ott et al., 1998) with neurolin Fabs (shaded bars). mAb N518 (against Ig domain 2) does not affect the level of fasciculation (48.8%).
The present results provide hints as to the involvement of Ig domains 1 and 3 (but not Ig domain 2) in axon fasciculation but the contribution of neurolin to axon fasciculation is weak, especially compared to that of E587 antigen (Bastmeyer et al., 1995; Ott et al., 1998).
As axon fasciculation often rests on homo- or heterophilic adhesion of IgSF CAMs on the axonal surfaces, attempts were made to assess the putative adhesive property of neurolin with polystyrene microspheres conjugated with the protein and to assay their binding to one another and to the surface of axons and CHO cells. Parallel experiments were performed with E587 antigen–conjugated microspheres which did form aggregates. However, the same microspheres conjugated with neurolin failed to aggregate, nor did they preferentially adhere to retinal axons in vivo or in vitro or to neurolin-expressing CHO cells. Together with the weak contribution of neurolin (Ig domains 1 and 3) to axon fasciculation, the failure of neurolin-conjugated microspheres to adhere may mean that adhesive interactions of neurolin are too weak to become evident in this assay.
Perturbation of the RGC Axon Order by mAbs Against Ig Domains 1, 2, or 3 In Vivo
Defects in RGC Axon Order Caused by Coinjections of Three mAbs.
To determine whether mAbs interfere with the tight fasciculation of young RGC axons in vivo and contribute to their aberrant routes, initial experiments were performed by simultaneous injections of three mAbs: N984, N518, and N100. mAb N984 was used because at the beginning of these tests, we had not yet realized that mAbs requiring only Ig domain 1 (instead of Ig domains 1 and 2) existed. mAbs were injected intraocularly twice a week for 10 wk. When retinal wholemounts were exposed to secondary antibodies, 4–6 d after a single intraocular injection, the young axons were labeled throughout their intraretinal path, and in all sectors of the retina, indicating that the injected mAbs had access to and are bound to young axons for at least 6 d.
All eight retinae that were successfully prepared as wholemounts from group 1 fish had defects in their fascicle order. Axons in aberrant routes and loss of tight fasciculation were predominantly found in the dorsal, nasal, and temporal areas of the retina. Thus, injections of the three mAbs combined cause essentially the same defects in axon order as neurolin Fabs (Ott et al., 1998). These findings indicate that the first three Ig domains of neurolin are involved in axonal fasciculation and in the disk-directed growth of young RGC axons.
mAbs Against Ig Domains 1 and 3 Disturb the Formation of Tight Fascicles.
After injections of either mAb N850 or mAb N100 (requiring the presence of Ig domains 1 and 3, respectively), the pattern of peripherocentrally oriented fascicles was preserved (Fig. 3, A and B) but the tight adherence of young axons within their fascicles was disturbed. The association of axons in loose bundles is illustrated in Fig. 3, C and D, and compared to the tighter fascicles in normal control (Fig. 3 F) and mAb N518 (Fig. 3 E) injected retinae. The defasciculation effect was most pronounced in the temporal retina and occurred in 8 out of 10 retinae after mAb N850 injections. Three retinae showed occasional (6–10) misoriented axon fascicles in the dorsal half of the retina, and two retinae appeared entirely normal.
Figure 3.
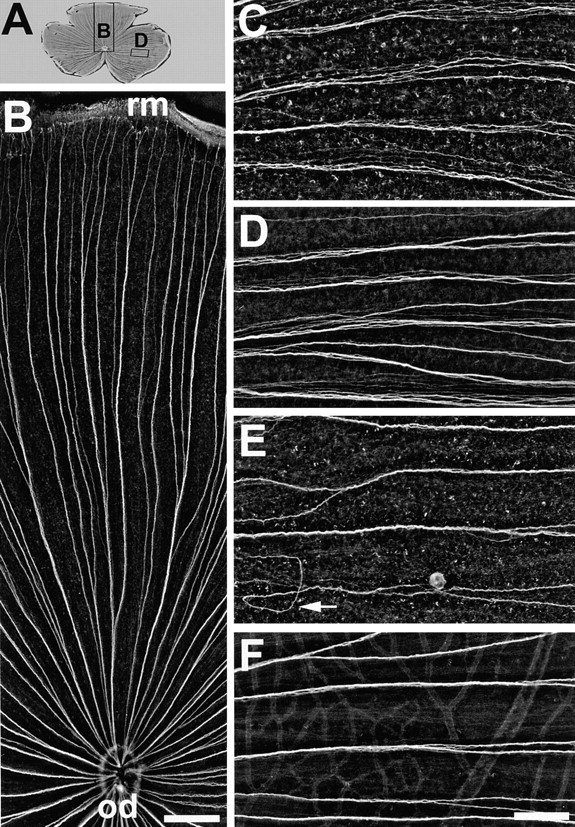
Ig domains 1 and 3 of neurolin contribute to axonal fasciculation in vivo. (A) Wholemount of a retina injected with mAb N100 against Ig domain 3. The positions of B and D are indicated by rectangles. (B) Dorsal segment of the retina in A. The directed growth of young RGC axons along fascicles from the retinal margin (rm) to the optic disk (od) is maintained and is as orderly as in control retinae. In retinae injected with mAb N850 against Ig domain 1 (C) or mAb N100 against Ig domain 3 (D) young RGC axons in fascicles fail to adhere tightly to each other. The distance between neighboring axons is increased compared to controls (F). (E) mAb N518 against domain 2 does not interfere with tight fasciculation, but causes pathfinding errors of young RGC axons (arrow). C–F are from the temporal retina, oriented with the retinal margin to the right, and the optic disk to the left. Bars, B, 300 μm; C–F, 100 μm.
After mAb N100 injections, 14 out of 16 retinae contained many axon fascicles in which axons failed to adhere tightly to each other. Seven of these retinae contained occasional (1–10) misoriented axon fascicles in the dorsal retinal half. Thus, mAb N850 against Ig domain 1 and mAb N100 against Ig domain 3 interfere with axonal fasciculation in vivo, and cause occasional defects in the oriented growth of young axons.
mAb Against Ig Domain 2 Causes Severe Pathfinding Errors.
After injections of mAb N518, misrouted axons were abundant in all 16 retinae. Axons in aberrant routes occurred from nasal over dorsal to temporal retinal aspects, and were most abundant in the inner retinal aspect, i.e., when axons had covered roughly 50% of their path from the periphery to the optic disk (Fig. 4). Axons that were previously associated with other axons in distinct fascicles no longer preserved this order. They formed new subfascicles with more distant axons and traveled in loops and other irregular pathways (Figs. 4 and 5). Quite frequently, axons that left their fascicle of origin grew away from the disk, turned again, associated with bundles of other misoriented or disk-oriented fascicles, or ended somewhere between other axon bundles (Fig. 5). Despite their errant path, a fraction of axons reached the optic disk (Figs. 4 and 5 A), yet others appeared to get lost (Fig. 5, A, B, and D). Most axons were able to form tight fascicles over the first 50% of the path from the margin to the disk, but even here subfascicles separated from the major fascicle (Fig. 4). These subfascicles then often followed curvy routes and crossed other fascicles until they merged again with one of the prominent bundles. Yet, as they approached the inner 50% of the retina, most axons apparently lost their ability to travel along disk-directed fascicles (Fig. 5). This suggests that many young growing axons in mAb N518-treated retinae fail to respond to guidance cues that they normally would perceive.
Figure 4.

Ig domain 2 of neurolin participates in axon guidance to the optic disk. Wholemount of a retina injected with mAb N518 against Ig domain 2. The dorsal inner area where pathway mistakes are most frequent is indicated by the dashed semi-circle. Occasional aberrant fascicles in the peripheral retina are marked by arrows. Dorsal retina is up and temporal to the right. Bar, 1 mm.
Figure 5.
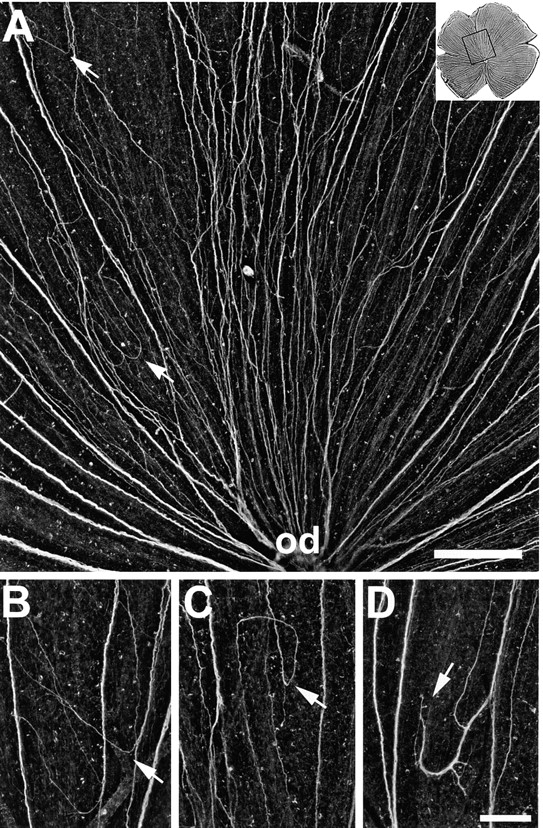
Aberrant routes in retinae injected with mAb N518 against Ig domain 2. (A) Higher magnification of the dorsal retina shown in Fig. 4, as indicated by the box in the upper right corner. Instead of growing directly towards the optic disk (od), young RGC axons form new subfascicles, change direction (arrows), and follow irregular pathways. (B–D) Examples of pathfinding errors. Young RGC axons depart from disk-oriented fascicles (arrow in B), grow back towards the retinal margin, establish circular routes (arrow in C), and form subfascicles that end in between fascicles (arrow in D). The retinal margin is up and the optic disk down. Bars, A, 200 μm; B–D, 100 μm.
Growth Cone Behavior Analyzed by Time-Lapse Videorecordings in Ex Vivo Retina Wholemounts: Effects of mAbs Against Ig Domains 1–3 and Neurolin Fabs
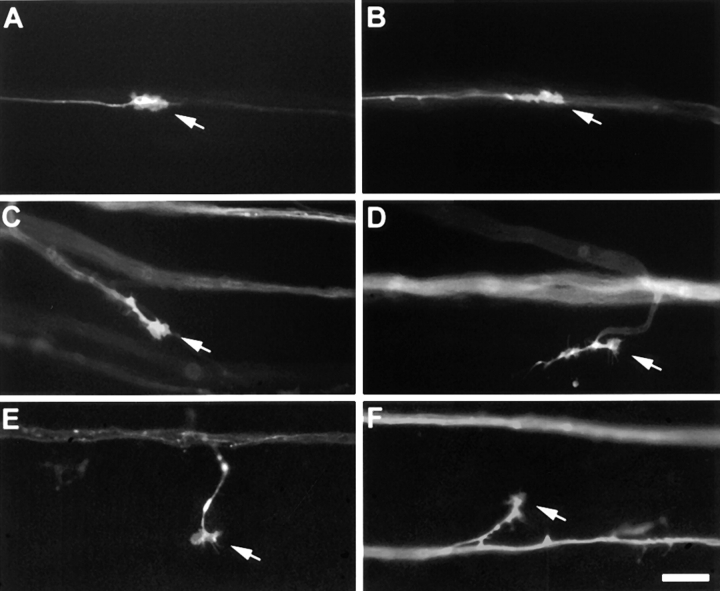
To quantitatively assess axonal pathfinding errors caused by each of the three mAbs and neurolin Fabs, we isolated retinae after two intraocular injections of either mAb N850, N518, N100, or neurolin Fabs, applied DiO to the retinal margin, and observed the DiO-labeled growth cones by videomicroscopic recordings for 2–6 h. We determined how many growth cones deviate for >10° from the fascicle pathway for each antibody injected and in buffer-injected and in noninjected retinae. Growth cones traveling along their fascicle and examples of errant growth cones in mAb N518-treated retinae are presented in Fig. 6. Growth cones depart from their fascicle of origin obliquely (Fig. 6, C and F), or at right angles (Fig. 6 E), cross other fascicles (Fig. 6 D), and show signs of growth in the wrong direction, i.e., away from the optic disk (Fig. 6 D).
Figure 6.
Representative frames from time-lapse sequences showing growing RGC axons in a noninjected control retina, and after injections of mAb N518. The optic disk is to the right. (A and B) DiO-labeled growth cones (arrows) elongate towards the optic disk in association with other labeled axons of the same fascicle (control retina). (C–F) DiO-labeled growth cones (arrows) in mAb N518-injected retinae depart from their fascicle of origin (bright band) either obliquely (C and F), or at right angles (E), and turn away from the optic disk (D). Bar, 20 μm.
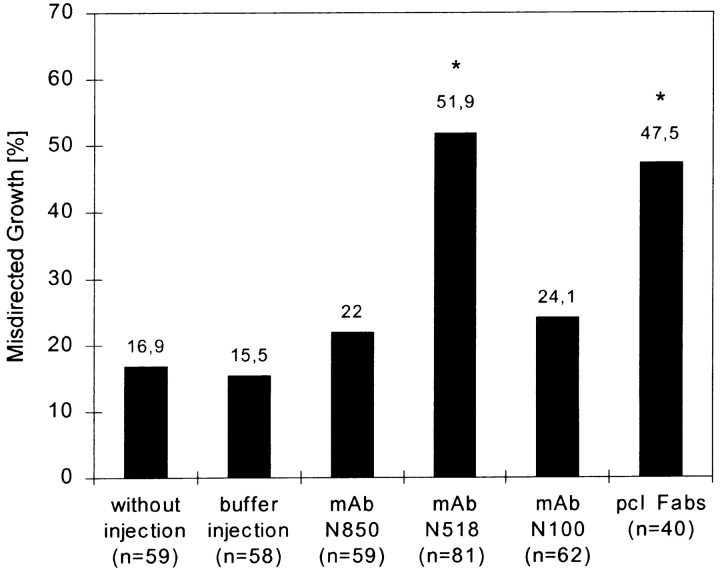
In buffer-injected and noninjected control retinae, the majority of growth cones (>80%) grew towards the optic disk in association with other labeled (and unlabeled) axons of the same fascicle (Fig. 7). Between 15.5 and 16.9% of the growth cones deviated from the disk-directed route in both types of control retinae (Fig. 7). This relatively high occurrence of growth outside the fascicle may result from the flattening of the retina during preparation, from suboptimal culture conditions, or from labeling and illuminating the growth cones, but is probably not caused by the earlier intraocular injections of fluid, since the amount of off-track growth was similar in retinae of injected and uninjected eyes. In retinae isolated from mAb N850- and mAb N100-injected eyes, the fraction of growth cones deviating from the fascicle track amounted to 22 and 24.1%, respectively, and thus, was similar to that in controls. After neurolin Fab and mAb N518 injections, the fraction of deviant growth cones increased to 47.5 and 51.9%, respectively. The difference between mAb N518, and mAb N850 and N100 effects is statistically significant (P < 0.001, χ2 test). Thus, mAb 518 and neurolin Fabs cause roughly twice as many growth cones to depart from their fascicle as in controls, and in mAb N850- and mAb N100-injected fish. Moreover, as in vivo, the departure of dorsal RGC growth cones from their fascicle was significantly more frequent (n = 21) than that of ventral axons (n = 3). This was determined from 24 deviant growth cones with known positions.
Figure 7.
Quantification of growth cones departing from their fascicle in the presence of different antineurolin antibodies. To quantify the effects caused by the injected neurolin antibodies, images of all DiO-labeled growth cones found in one retina were taken. Growth cones elongating directly towards the optic disk were categorized as directed growth. Growth cones deviating by >10° from the disk-directed path were categorized as misdirected growth. The percentage of misdirected growth was determined in retinae isolated from eyes injected with either mAb N850, mAb N518, mAb N100, or neurolin Fabs (from a polyclonal antiserum, pcl Fabs) and compared to control eyes that received either buffer injections or no injections. mAb N518 (*) or pcl Fabs (*) caused significantly more misdirected growth than mAb N850 or mAb N100 (P < 0.001, mAb N518; P < 0.05, pcl Fabs; χ2 test), in which off-track growth cones were numerically similar to controls.
As IgSF CAMs such as E587 antigen increase the rate of growth cone elongation (Bastmeyer et al., 1995) axon growth velocities were determined for growth cones navigating under the influence of the mAbs against neurolin. The average growth velocity of growth cones within the fascicle was 21.5 (± 9 SD) μm/h in mAb N518-treated retinae (n = 16), and 22.1 (± 9.5 SD) μm/h, and 21.6 (± 8.3 SD) μm/h in mAb N100 (n = 17) and N850 (n = 13) treated retinae, respectively. These values are similar to controls (24.1 ± 10.8 SD μm/h, n = 31), indicating that binding of these mAbs to elongating growth cones does not reduce their speed. In retinae exposed to mAb N518, however, many more growth cones grow outside of the fascicles and here their average growth velocity was reduced to 6.2 (± 4 SD μm/h, n = 12). A similar tendency was noted for growth cones outside of the fascicles in the control group, and in mAb N100- and N850-treated retinae, where growth cones progressed with 5.6 (± 3 SD, n = 4), 9.1 (± 4.5 SD, n = 6), and 4.4 (± 2.3 SD, n = 5) μm/h, respectively, after departure from their fascicle.
Therefore, growth cones elongate outside a fascicle roughly three times more slowly than within the fascicle. The few examples of growth cones outside the fascicle in controls and mAb N100- and N850-treated retinae suggest that reduced growth velocities are the rule for growth cones off the fascicle track rather than binding a specific effect of mAb N518.
Some growth cones were videorecorded for up to 24 h to directly visualize the development of the aberrant routes. The growth cone (Fig. 8) left its fascicle of origin, turned away from the direction of the optic disk, coursed towards the periphery, turned again, and continued to grow outside of the fascicles. Its further growth could not be followed because continuous observation of labeled growth cones causes photodamage and eventually leads to the cessation of growth and disintegration of the growth cone and axon. This growth cone is a representative example of how the many abnormal routes develop after neurolin Fab and mAb N518 injections.
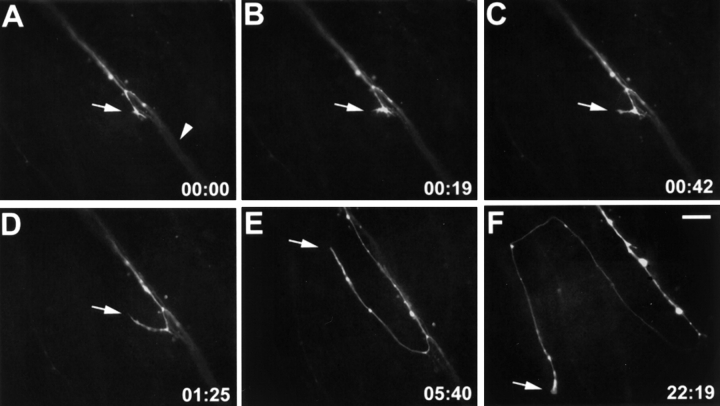
Figure 8.
Time-lapse sequence of an axon after mAb N518 injections. A DiO-labeled growth cone (arrow in A) elongates towards the optic disk (to the lower right) in association with other labeled axons of the same fascicle (arrowhead in A). This growth cone left its fascicle (arrow in B), turned away from the optic disk (C–E), turned again, and continued to grow outside the fascicle (arrow in F). Elapsed time is given in hours and minutes in the lower right corner. Bar, 20 μm.
Discussion
Young RGC axons in the goldfish retina travel in abnormal paths, form loops and circles, and often fail to reach the optic disk when grown in the presence of mAb N518 against Ig domain 2 of the neurolin. This aberrant growth suggests that the binding of mAb N518 to axonally expressed neurolin interferes with the ability of RGC axons to respond to guidance information that normally directs them to the optic disk.
That blockage of domain 2 by mAb N518 affects growth cone navigation is evident in fixed retinal wholemounts and in time-lapse video observations of growing axons in ex vivo retina preparations, where the number of growth cones departing from their fascicle track increases in the presence of this mAb. In contrast, mAbs N850 and N100, which block Ig domains 1 and 3, do not increase the number of deviant growth cones. Likewise, after weekly injections of mAb N850 and N100 into the eye, fixed retinal wholemounts show comparatively mild defects in the order of the axons: the tight fasciculation of RGC axons is disturbed, but they rarely fail to reach the optic disk. This weak but specific effect of mAbs against Ig domains 1 and 3 is also apparent in growth cone behavior in vitro, but is not observed with mAb N518 against Ig domain 2. The present data are consistent with the concept that neurolin Ig domain 2 is responsible for the guided growth of RGC axons, whereas Ig domains 1 and 3 contribute to the formation of tight fascicles.
The function of specific Ig domains was determined through a combination of domain-deletion mutants and mAbs. To ensure that domains adjacent to the deletion remained intact with regard to their primary structure, we excised the domains precisely at their borders by oligonucleotide-directed mutagenesis. The primers were chosen near the sequence which encodes the cysteine residues that determine the Ig fold so that most of the sequence between the Ig domains was preserved.
The ability or failure of the mAbs to recognize mutant forms of neurolin was determined by immunostaining of the CHO cell clones and by immunoblots with proteins of the cells. Both methods gave congruent results.
mAb N518 is considered to be directed against neurolin Ig domain 2 because it fails to recognize neurolin from which domain 2 has been deleted. Clearly, Ig domain 2 is necessary for mAb N518 binding: the deletion of Ig domain 2 is sufficient to prevent binding of mAb N518, and deletion of any other domain or combination of domains is not. This strongly suggests that the epitope of this antibody lies in domain 2.
The same arguments apply for mAbs N850 and N100 against Ig domains 1 and 3, respectively. Each mAb selectively lost its ability to recognize neurolin depleted of the respective domain. Binding to neurolin was unaffected after removal of the neighboring domains as long as domain 1 or 3 was present. This was not the case with class 4 mAbs, which require the presence of both Ig domains 1 and 2. This supports the view that the recognition sites of the other three mAbs are confined to one Ig domain. On the basis of these criteria, we use the term “mAb against” Ig domains 1, 2, or 3.
In the case of the axonally expressed IgSF CAM axonin-1, antibody mapping experiments demonstrated that Ig domains 1–4 together contribute to NgCAM recognition (Rader et al., 1996). On the other hand, functional epitopes are confined to distinct Ig domains or to individual fibronectin type III domains present in several IgSF CAMs (Diamond et al., 1991; Brümmendorf and Rathjen, 1994; Bowen et al., 1997).
In light of the present results, it seems reasonable to suggest that the role of Ig domain 2 in the proposed function of neurolin is that of a receptor or part of a receptor complex for a guiding component. Determination of its participation in axon guidance facilitates the search for the guidance component itself, which has long been postulated to exist (Ramon y Cajal, 1972; Deiner et al., 1997). Although the nature and location of the guidance component is not known, the area where errant RGC growth occurs with the greatest frequency and with the most dramatic phenotypes is toward the center of the retina rather than in the periphery. This suggests that the guidance information which axons recognize via neurolin Ig domain 2 may be located within this inner retinal territory or may be more concentrated there than in the retinal periphery. During the early period of their long-distance growth, axons may rely more on other cues such as repellent components (Brittis et al., 1992).
Since entire mAbs were used (molecules of 150 kD), the possibility remains that bound mAbs sterically hinder interactions of neurolin, be it with the presumed guidance component, with neurolin on the surface of neighboring axons, or with other axonally expressed IgSF CAMs for homo- or heterophilic binding mechanisms. Moreover, bivalent antibodies are known to cross-link IgSF CAMs, and to mimic ligand binding in specific instances (for review see Schuch et al., 1989; Brown, 1993). For these reasons, we would have preferred to use Fabs instead of the entire IgGs, but all three mAbs lost most of their activity when subjected to enzymatic digestion by papain. However, since defects in axon order and growth cone behavior were similar with mAbs and polyclonal Fabs (Ott et al., 1998), it is unlikely that the mAbs had effects other than the blocking of neurolin functions.
For all of the in vivo or ex vivo experiments, an internal control for unspecific effects is present: only the axons of the dorsal retina are affected, although the antibodies bind to young axons in both the dorsal and ventral halves. Reasons for the resistance of ventral RGC axons to Fab or mAb-induced pertubations are presently unknown. One explanation may be that neurolin interacts with molecules that are differentially expressed in the dorsal and ventral retinal halves, such as specific ephrins and ephrin receptors (Nakamoto et al., 1996; Brennan et al., 1997; for review see Drescher et al., 1997; Sefton and Nieto, 1997). Alternatively, other axonal receptors and/or guidance cues may be predominant in the ventral retinal half.
mAbs against Ig domains 4 and 5 were not available for functional analyses but domains 4 and 5 are recognized by neurolin Fabs that, as stated above, produce similar quantitative and qualitative defects in axon order (Ott et al., 1998). This suggests that binding of antibodies to all domains does not produce an effect beyond that seen with mAbs against only the first three domains. Furthermore, the weak defasciculation effect of mAbs N850 and N100 was similar to the effect of neurolin Fabs. The formation of aberrant routes, however, was more pronounced in mAb N518-treated retinae than in retinae injected with neurolin Fabs (Ott et al., 1998). Therefore, it is unlikely (but not entirely impossible) that Ig domains 4 and 5 contribute to aspects of axon guidance or fasciculation.
It has been ruled out previously that injection of fluid into the vitreous chamber of the fish eye or repeated injections have negative side effects (Bastmeyer et al., 1995; Ott et al., 1998). Repeated injections of antibodies (Fabs or mAbs) or buffer did not affect the growth of the eye or retina, nor did injections (and the transient increase of intraocular pressure) have any noticeable influence on the axon order. Moreover, defects in axon order after injection of antibodies were always antibody/antigen specific. Furthermore, defects emerging in vivo (from injections into the eye) were consistent with defects observed in vitro. For example, anti-E587 antibodies cause axon defasciculation in vivo and in vitro to an extent that was never obtained with antineurolin antibodies. Fab fragments of a polyclonal E587 antiserum, however, did not prevent RGC axons from navigating towards the optic disk nor did these Fab fragments cause growth in loops and circles or abrupt endings of axons within the retina (Bastmeyer et al., 1995). mAb E587 reduced the velocities of growth cones in culture (Bastmeyer et al., 1995), but this did not occur with antineurolin antibodies, either with neurolin Fabs or mAbs.
The ex vivo functional growth assay allows direct observation of growth cones in a quasinatural environment and in the presence of antibodies (Halfter and Deiss, 1984; Brittis and Silver, 1995; Brittis et al., 1995). The rate of growth cone advance along a fascicle or outside of the fascicle track was also unaffected by neurolin Fabs and mAbs in this assay. Since many more growth cones course outside the fascicles in mAb 518- and neurolin Fab-treated retinae than in mAb N850- and N100-treated and control retinae, representative data on growth velocities outside a fascicle were obtained only for the former, and a tendency was demonstrable for the latter. This shows, however, that growth cones outside a fascicle are at a disadvantage compared to those within the fascicle because their average growth velocities are markedly reduced. From the average velocities determined from growth cones in the ex vivo assays, one can calculate that a growth cone requires 3–4 d to cover the distance from the retinal margin to the optic disk. Outside the fascicle path, growth cone progress is slow.
Growth cones depart from their fascicles twice as frequently with mAb against Ig domain 2 (and with neurolin Fabs) as with mAbs against Ig domains 1 and 3 (or in controls). Moreover, events of off-track growth occurred more often in the dorsal than in the ventral retina. This is consistent with the abundant pathway mistakes of dorsal RGC axons observed in vivo after injection of this mAb (and neurolin Fabs). Both types of experiments provide strong evidence for the role of Ig domain 2 in dorsal RGC axon guidance.
It may be due to the presence of several IgSF CAMs that a substantial number of the affected axons nevertheless reaches the disk by tracking the fascicles. Also, it is for these reasons and/or due to additional cues (see above) that ventral RGC axons are prevented from taking aberrant routes.
However, tracking forerunners does apparently not suffice to guide axons reliably to their first intermediate target (Stuermer et al., 1992). They seem to require additional cues to maintain their disk-oriented growth. This may be compared to a driver on a highway who, in addition to following the paved surface of the road, must also read traffic signs to get to his destination. When he becomes unable to read signs, he may erroneously turn off the highway and lose orientation. mAb N518 against Ig domain 2 seems to block the growth cone's ability to perceive or read the cues. The probability of progressing in aberrant routes is high under these circumstances. Once outside of its fascicle, the growth cone has fewer guides to follow. The chance of finding the disk is low and the danger of getting lost is high. It lacks the highly growth-supportive surface provided by other young axons which are known to express several growth-promoting IgSF CAMs (Bastmeyer et al., 1990; Vielmetter et al., 1991; Paschke et al., 1992).
It has been suggested that the directed growth of RGC axons is the result of their response to repelling components in the periphery of the differentiating RGC (Brittis et al., 1992), and that the extracellular matrix and basal lamina contain directional cues (Krayanek and Goldberg, 1981; Halfter et al., 1996). It has been predicted that attractive cues at or in the vicinity of the optic disk (or optic stalk in the embryonic eye) help axons grow toward these structures (Ramon y Cajal, 1972; Deiner et al., 1997). As discussed earlier, netrins could be the attractive cues. Netrins are known to attract axons at the floor plate and in related structures along the ventral midline of the developing central nervous system. They are synthesized by cells (perhaps glial cells) in the optic stalk (Deiner et al., 1997; Macdonald et al., 1997; Strähle et al., 1997), which later become the optic disk, and retinal axons can respond to them (De la Torre et al., 1997). But retinal axons in netrin knockout mice still grow towards the disk, although they fail to exit the eye (Deiner et al., 1997). Interestingly, however, the receptor or part of the netrin receptor complex is the IgSF CAM DCC (Chan et al., 1996; Keino-Masu et al., 1996; Kolodziej et al., 1996). The netrin-oriented growth of many axons including that of retinal ganglion cells is blocked by anti-DCC antibodies and fails to occur in DCC knockout mice (Deiner et al., 1997; Fazeli et al., 1997).
DCC is probably the first IgSF CAM for which a receptor function for guidance molecules such as the netrins has been shown (Chan et al., 1996; Keino-Masu et al., 1996). Neurolin may be another example. DCC participates in axon fasciculation (Pierceall et al., 1994), the more classic function of IgSF CAMs. Neurolin lacks the growth-accelerating property of E587 antigen (Bastmeyer et al., 1995), and other members of the Ig superfamily (Brümmendorf and Rathjen, 1994). Also, the contribution of neurolin to axon fasciculation is weak in comparison to that of the L1-like E587 antigen (Bastmeyer et al., 1995; Ott et al., 1998), which may also explain why neurolin-coated microspheres as opposed to E587 antigen–conjugated microspheres fail to form aggregates. An alternative explanation could be that neurolin loses conformational properties needed for adhesive functions when removed from the membrane. Moreover, neurolin does not promote outgrowth (a property identified for many IgSF CAMs including the E587 antigen) when offered as the sole substrate to retinal axons. The avian homologue DM-GRASP/SC-1/BEN seems to possess most or all of the functions typical for IgSF CAMs with respect to axons outside of the visual system. Thus, the prime function of neurolin in the retina may be that of a receptor. That neurolin homologues can have ligands other than IgSF CAMs (DeBernado and Chang, 1996) was demonstrated for ALCAM. In the immune system, ALCAM has been shown to interact with CD6, a scavenger receptor, and this function appears to lie in Ig domain 1 (Bowen et al., 1997). The possibility arises that neurolin and its homologues in warm-blooded vertebrates have multiple functions in various contexts. The function of DCC as a netrin receptor requires the presence of unc-5 homologues, suggesting that both cooperate in the netrin response (for review see Kolodziej, 1997). Likewise, there may be coreceptors for neurolin, and these may contribute to the position dependency of the mAb-induced defects.
The identification of Ig domain 2 as a receptor (or part of one) for a guidance component allows a search for this component by applying, for instance, a tagged neurolin Ig domain 2 to the retina, an approach which could provide information on the cellular source and distribution of the guidance component, and ultimately lead to its identification. The proposed guidance molecule is predicted to be relevant not only in the teleosts but also in warm-blooded vertebrates. The high degree of cross-species conservation of identified guidance molecules (Tessier-Lavigne and Goodman, 1996) such as the netrins and ephrins supports this prediction.
Acknowledgments
We thank G. Zimmermann and M. Wiechers for their excellent technical assistance. C. Leppert performed the in vivo experiments and H. Diekmann produced the CHO cell clones.
This work was supported by grants of the Deutsche Forschungsgemeinschaft to C.A.O. Stuermer and the Fonds der Chemischer Industrie.
Abbreviations used in this paper
- CAM
cell adhesion molecule
- IgSF
immunoglobulin superfamily
- RGC
retinal ganglion cell
Footnotes
Address correspondence to Claudia A.O. Stuermer, Department of Biology, University of Konstanz, D-78457 Konstanz, Germany. Tel.: (49) 7531-88-22-36. Fax: (49) 7531-88-38-94. E-mail: claudia.stuermer@uni-konstanz.de
References
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidmann, J.A. Smith, and K. Struhl. 1994. Current Protocols in Molecular Biology. Greene Publishing Associates/Wiley-Interscience, New York.
- Bastmeyer M, Schlosshauer B, Stuermer CAO. The spatiotemporal distribution of N-CAM in the retinotectal pathway of adult goldfish detected by the monoclonal antibody D3. Development (Camb) 1990;108:299–311. doi: 10.1242/dev.108.2.299. [DOI] [PubMed] [Google Scholar]
- Bastmeyer M, Ott H, Leppert CA, Stuermer CAO. Fish E587 glycoprotein, a member of the L1 family of cell adhesion molecules, participates in axonal fasciculation and the age-related order of ganglion cell axons in the goldfish retina. J Cell Biol. 1995;130:969–976. doi: 10.1083/jcb.130.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MA, Bajorath J, D'Egidio M, Whitney GS, Palmer D, Kobarg J, Starling GC, Siadak AW, Aruffo A. Characterization of mouse ALCAM (CD166): the CD6-binding domain is conserved in different homologs and mediates cross-species binding. Eur J Immunol. 1997;27:1469–1478. doi: 10.1002/eji.1830270625. [DOI] [PubMed] [Google Scholar]
- Brennan C, Monschau B, Lindberg R, Guthrie B, Drescher U, Bonhoeffer F, Holder N. Two Eph receptor tyrosine kinase ligands control axon growth and may be involved in the creation of the retinotectal map in the zebrafish. Development (Camb) 1997;124:655–664. doi: 10.1242/dev.124.3.655. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Silver J. Multiple factors govern intraretinal axon guidance: a time-lapse study. Mol Cell Neurosci. 1995;6:413–432. doi: 10.1006/mcne.1995.1031. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;255:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Lemmon V, Rutishauser U, Silver J. Unique changes of ganglion cell growth cone behavior following cell adhesion molecule perturbations: a time-lapse study of the living retina. Mol Cell Neurosci. 1995;6:433–449. doi: 10.1006/mcne.1995.1032. [DOI] [PubMed] [Google Scholar]
- Brown D. The tyrosine kinase connection: how GPI-anchored proteins activate T-cells. Curr Opin Immunol. 1993;5:349–354. doi: 10.1016/0952-7915(93)90052-t. [DOI] [PubMed] [Google Scholar]
- Brümmendorf, T., and F. Rathjen. 1994. Cell Adhesion Molecules 1: Immunoglobulin Superfamily. Academic Press, London. 951–1058. [PubMed]
- Burns FR, Von Kannen S, Guy L, Raper JA, Kamholz J, Chang S. DM-GRASP, a novel immunoglobulin superfamily axonal surface protein that supports neurite extension. Neuron. 1991;7:209–220. doi: 10.1016/0896-6273(91)90259-3. [DOI] [PubMed] [Google Scholar]
- Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. eleganshomolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- De la Torre JR, Höpker VH, Ming GL, Poo MM, Tessier-Lavigne M, Hemmati-Brivanlou A, Holt CE. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–1224. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- DeBernardo AP, Chang S. Heterophilic interactions of DM-GRASP: GRASP-NgCAM interactions involved in neurite extension. J Cell Biol. 1996;133:657–666. doi: 10.1083/jcb.133.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disk: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Drescher U, Bonhoeffer F, Müller BK. The Eph family in retinal axon guidance. Curr Opin Neurobiol. 1997;7:75–80. doi: 10.1016/s0959-4388(97)80123-7. [DOI] [PubMed] [Google Scholar]
- Easter SSJ, Bratton B, Scherer SS. Growth-related order of the retinal fiber layer in goldfish. J Neurosci. 1984;4:2173–2190. doi: 10.1523/JNEUROSCI.04-08-02173.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc)gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Teleost vision: seeing while growing. J Exp Zool Suppl. 1990;5:167–180. doi: 10.1002/jez.1402560521. [DOI] [PubMed] [Google Scholar]
- Halfter W. Intraretinal grafting reveals growth requirements and guidance cues for optic axons in the developing avian retina. Dev Biol. 1996;177:160–177. doi: 10.1006/dbio.1996.0153. [DOI] [PubMed] [Google Scholar]
- Halfter W, Deiss S. Axon growth in embryonic chick and quail retinal whole mounts in vitro. Dev Biol. 1984;102:344–355. doi: 10.1016/0012-1606(84)90199-4. [DOI] [PubMed] [Google Scholar]
- Johns PA. Growth of the adult goldfish eye. III. Source of the new retinal cells. J Comp Neurol. 1977;176:343–357. doi: 10.1002/cne.901760304. [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SSY, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC)encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, De la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA. DCC's function takes shape in the nervous system. Curr Opin Genet Dev. 1997;7:87–92. doi: 10.1016/s0959-437x(97)80114-1. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. frazzled encodes a Drosophilamember of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- Krayanek S, Goldberg S. Oriented extracellular channels and axonal guidance in the embryonic chick retina. Dev Biol. 1981;84:41–50. doi: 10.1016/0012-1606(81)90368-7. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Stoeckli ET, Condrau MA, Rathjen FG, Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4) J Cell Biol. 1991;115:1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laessing U, Giordano S, Stecher B, Lottspeich F, Stuermer CAO. Molecular characterization of fish neurolin: a growth associated cell surface protein and member of the immunoglobulin superfamily in the fish retinotectal system with similarities to chick protein DM-GRASP/SC-1/ BEN. Differentiation. 1994;56:21–29. doi: 10.1046/j.1432-0436.1994.56120021.x. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Scholes J, Strähle U, Brennan C, Holder N, Brand M, Wilson SW. The Pax protein Noi is required for commissural axon pathway formation in the rostral forebrain. Development (Camb) 1997;124:2397–2408. doi: 10.1242/dev.124.12.2397. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Cheng HJ, Friedman GC, McLaughlin T, Hansen MJ, Yoon CH, O'Leary DDM, Flanagan JG. Topographically specific effects of ELF-1 on retinal axon guidance in vitro and retinal axon mapping in vivo. Cell. 1996;86:755–766. doi: 10.1016/s0092-8674(00)80150-6. [DOI] [PubMed] [Google Scholar]
- Ott H, Bastmeyer M, Stuermer CAO. Neurolin, the goldfish homolog of DM-GRASP, is involved in retinal axon pathfinding to the optic disk. J Neurosci. 1998;18:3363–3372. doi: 10.1523/JNEUROSCI.18-09-03363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschke KA, Lottspeich F, Stuermer CAO. Neurolin, a cell surface glycoprotein on growing retinal axons in the goldfish visual system, is reexpressed during retinal axonal regeneration. J Cell Biol. 1992;117:863–875. doi: 10.1083/jcb.117.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierceall WE, Cho KR, Getzenberg RH, Reale MA, Hedrick L, Vogelstein B, Fearon ER. NIH3T3 cells expressing the deleted in colorectal cancer tumor suppressor gene product stimulate neurite outgrowth in rat PC12 pheochromocytoma cells. J Cell Biol. 1994;124:1017–1027. doi: 10.1083/jcb.124.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollerberg GE, Mack TGA. Cell adhesion molecule SC1/ DMGRASP is expressed on growing axons of retina ganglion cells and is involved in mediating their extension on axons. Dev Biol. 1994;165:670–687. doi: 10.1006/dbio.1994.1284. [DOI] [PubMed] [Google Scholar]
- Pourquié O, Corbel C, Le Caer J-P, Rossier J, Le Douarin NM. BEN, a surface glycoprotein of the immunoglobulin superfamily, is expressed in a variety of developing systems. Proc Natl Acad Sci USA. 1992;89:5261–5265. doi: 10.1073/pnas.89.12.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader C, Kunz B, Lierheimer R, Giger RJ, Berger P, Tittmann P, Gross H, Sonderegger P. Implications for the domain arrangement of axonin-1 derived from the mapping of its NgCAM binding site. EMBO (Eur Mol Biol Organ) J. 1996;15:2056–2068. [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal, S. 1972. The Structure of the Retina (English edition). Charles C. Thomas, Springfield, IL. 148–151.
- Schuch U, Lohse MJ, Schachner M. Neural cell adhesion molecules influence second messenger systems. Neuron. 1989;3:13–20. doi: 10.1016/0896-6273(89)90111-6. [DOI] [PubMed] [Google Scholar]
- Sefton M, Nieto MA. Multiple roles of Eph-like kinases and their ligands during development. Cell Tissue Res. 1997;290:243–250. doi: 10.1007/s004410050928. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegansUNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Strähle U, Fischer N, Blader P. Expression and regulation of a netrinhomologue in the zebrafish embryo. Mech Dev. 1997;62:147–160. doi: 10.1016/s0925-4773(97)00657-6. [DOI] [PubMed] [Google Scholar]
- Stuermer CAO, Easter SSJ. Rules of order in the retinotectal fascicles of goldfish. J Neurosci. 1984;4:1045–1051. doi: 10.1523/JNEUROSCI.04-04-01045.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuermer CAO, Bastmeyer M, Bähr M, Strobel G, Paschke K. Trying to understand axonal regeneration in the CNS of fish. J Neurobiol. 1992;23:537–550. doi: 10.1002/neu.480230508. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Matsui T, Agata A, Tomura M, Kubota I, McFarland KC, Kohr B, Lee A, Phillips HS, Shelton DL. Molecular cloning and expression of a novel adhesion molecule, SC1. Neuron. 1991;7:535–545. doi: 10.1016/0896-6273(91)90366-8. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Vielmetter J, Stuermer CAO. Goldfish retinal axons respond to position-specific properties of tectal cell membranes in vitro. Neuron. 1989;2:1331–1339. doi: 10.1016/0896-6273(89)90071-8. [DOI] [PubMed] [Google Scholar]
- Vielmetter J, Lottspeich F, Stuermer CAO. The monoclonal antibody E587 recognizes growing (new and regenerating) retinal axons in the goldfish retinotectal pathway. J Neurosci. 1991;11:3581–3593. doi: 10.1523/JNEUROSCI.11-11-03581.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]