Abstract
We have investigated the role of the small GTP-binding protein Rho in cytokinesis by microinjecting an inhibitor, C3 ribosyltransferase, into cultured cells. Microinjection of C3 into prometaphase or metaphase normal rat kidney epithelial cells induced immediate and global cortical movement of actin toward the metaphase plate, without an apparent effect on the mitotic spindle. During anaphase, concentrated cortical actin filaments migrated with separating chromosomes, leaving no apparent concentration of actin filaments along the equator. Myosin II in injected epithelial cells showed a diffuse distribution throughout cell division. All treated, well-adherent cells underwent cleavage-like activities and most of them divided successfully. However, cytokinesis became abnormal, generating irregular ingressions and ectopic cleavage sites even when mitosis was blocked with nocodazole. The effects of C3 appeared to be dependent on cell adhesion; less adherent 3T3 fibroblasts exhibited irregular cortical ingression only when cells started to increase attachment during respreading, but managed to complete cytokinesis. Poorly adherent HeLa cells showed neither ectopic cleavage nor completion of cytokinesis. Our results indicate that Rho does not simply activate actin–myosin II interactions during cytokinesis, but regulates the spatial pattern of cortical activities and completion of cytokinesis possibly through modulating the mechanical strength of the cortex.
Keywords: GTP-binding protein, cytoskeleton, cell division, actin, cytokinesis
Cytokinesis is believed to involve localized cortical contraction through interactions of actin and myosin II filaments. After chromosomal separation at anaphase onset, cortical actin and myosin II begin to move toward the equatorial region, where they form a contractile cleavage furrow (for reviews see Mabuchi, 1986; Salmon, 1989; Satterwhite and Pollard, 1992; Fishkind and Wang, 1995; Glotzer, 1997). However, the cleavage process may involve more than actin–myosin II interactions along the equator. For example, detailed three-dimensional studies of the organization of actin and myosin do not support a simple “purse string” model in cultured mammalian cells and Dictyostelium (Fishkind and Wang, 1993; Neujahr et al., 1997b). In addition, myosin II–null Dictyostelium are capable of initiating and completing a furrow when attached to a substrate (Neujahr et al., 1997a), and the budding yeast Saccharomyces cerevisiae can undergo cytokinesis in the absence of myosin II or an intact F-actin cytoskeleton (Bi et al., 1998).
Equally important are the signaling events that coordinate cortical dynamics with chromosomal separation. Previous studies have indicated that Rho, a ubiquitous protein of the Ras family (for reviews see Narumiya, 1996; Ridley, 1996; Hall, 1998), plays an important role in regulating the actin cytoskeleton. In interphase Swiss 3T3 fibroblasts, Rho regulates the actin cytoskeleton by promoting the assembly of stress fibers and focal adhesions (Ridley and Hall, 1992; Horiguchi et al., 1995), possibly through elevating the level of phosphorylation of the myosin II regulatory light chain (Kimura et al., 1996). However, there are additional, poorly characterized effectors of Rho (Kinoshita et al., 1997; Narumiya et al., 1997; Madaule et al., 1998), which might account for the variability of observations among different cell types (Nishiki et al., 1990; Allen et al., 1997; Kozma et al., 1997).
Several studies with embryos indicate that Rho is required for the reorganization of actin cytoskeleton and the maintenance of cleavage activities during cell division (Kishi et al., 1993; Mabuchi et al., 1993; Drechsel et al., 1996). Inhibition of Rho prevented cytoplasmic division from taking place and caused existing furrows to regress. Inhibition of one of the downstream effectors of Rho, a novel protein kinase that localizes to the cleavage furrow, also caused inhibition of cytokinesis (Madaule et al., 1998). These observations, together with the disassembly of stress fibers and focal contacts during cell division, lead to the hypothesis that Rho may be deactivated as cells enter mitosis. Its reactivation during cytokinesis may then be responsible for the generation and maintenance of cell ingression.
We have investigated the role of Rho in cultured cells during division by microinjecting C3 ribosyltransferase, a Clostridium botulinum toxin that specifically inactivates Rho by ADP ribosylating its Asp41 residue (Sekine et al., 1989), into mitotic normal rat kidney (NRK)1 epithelial cells, Swiss 3T3 fibroblasts, and HeLa cells. We found that C3 inhibited the cleavage of only poorly adherent HeLa cells. The majority of firmly attached NRK cells cleaved successfully, but showed irregular ingressions without an apparent concentration of F-actin or myosin II along the equator. Our results suggest that Rho may be involved in defining the spatial and temporal pattern of cleavage. In addition, the intriguing adhesion-dependent responses of dividing cells to C3 provide new insights into the mechanism of cytokinesis.
Materials and Methods
Cell Culture
A well spread subclone (NRK-52E) of NRK epithelial cells, Swiss 3T3 fibroblasts, and HeLa cells (American Type Culture Collection) were cultured on glass coverslips as described previously (McKenna and Wang, 1989). NRK cells were grown in Kaighn's modified F12 medium (Sigma Chemical Co.) containing 10% FCS (JRH Biosciences), 1 mM l-glutamine, 50 μg/ml streptomyosin, and 50 U/ml penicillin. 3T3 fibroblasts and HeLa cells were cultured in Dulbecco's modified Eagle's medium (Sigma Chemical Co.) supplemented with 10% donor calf serum (JRH Biosciences), l-glutamine, and antibiotics as above.
Protein Preparation and Microinjection
C3 transferase was either purchased from Calbiochem or received as a gift from Dr. S. Narumiya (Kyoto University, Kyoto, Japan). The cells responded the same to C3 from both sources. It was stored as 1.0 mg/ml aliquots in 5 or 10 mM Hepes at −80°C and was microinjected at concentrations ranging from 0.2 to 1.0 mg/ml with fluorescein dextran as a marker (Molecular Probes). Injection of fluorescein dextran alone had no effect on mitosis or cytokinesis. Rhodamine tubulin was prepared as described by Wheatley and Wang (1996), and cells were microinjected as described previously (Wang, 1992).
pGEX RhoA, RhoAV14, and RhoAL63 constructs were expressed in the Escherichia coli strain XL-1 blue and proteins were purified essentially as described in Self and Hall (1995). Proteins were stored at −70°C in aliquots of 50 μl and activities were verified based on their ability to induce cell rounding as described by Allen et al. (1997). Wild-type RhoA was microinjected at a concentration of 2.0 mg/ml, and the dominant positive mutant proteins were microinjected at 0.1–0.5 mg/ml.
Labeling of Surface Receptors and Drug Treatments
Carboxylated polystyrene beads were prepared as described previously (Wang et al., 1994). Briefly, 0.1-μm yellow-green fluorescent beads (Molecular Probes) were washed three times by centrifugation in an Airfuge (Beckman Instruments) and resuspended by sonication in PBS with 1% BSA (Sigma Chemical Co.). Surface receptors were labeled by incubating cells with beads at 10–100× dilution for 2 min, followed by at least five rinses with medium.
Cells were treated with cytochalasin D (Sigma Chemical Co.) by replacing the medium with 2 or 5 μM cytochalasin D in culture medium. Depolymerization of microtubules was induced by treating cells with 2.5 μM nocodazole (Sigma Chemical Co.). Both cytochalasin D and nocodazole were diluted into the medium immediately before use, from frozen stock solutions of 2 and 10 mM, respectively, in DMSO. In most experiments cells were microinjected with C3 at prometaphase or metaphase, then treated with the drugs at specified stages of division.
Fixation and Staining
Fixation solutions for actin, tubulin, and myosin were prepared with cytoskeleton buffer (Small, 1981, 1982). Actin filaments were preserved by fixing cells in warm 0.5% glutaraldehyde and 0.2% Triton X-100, followed by postfixation in 1% glutaraldehyde. Cells were treated with 0.5 mg/ml NaBH4 to reduce autofluorescence and stained with 200 nM FITC-phalloidin or TRITC-phalloidin (Molecular Probes) for 30 min. Microtubules were preserved similarly, except Triton X-100 was at a concentration of 0.1%. Tubulin was immunostained using anti–β-tubulin mAb (Amersham Corp.) at a dilution of 1:10 and FITC or TRITC-conjugated secondary antibodies (Sigma Chemical Co.) at a dilution of 1:100. Myosin II was preserved by fixing cells for 1 min in warm 1% formaldehyde, 0.1% glutaraldehyde, and 0.3% Triton X-100, followed by postfixation for 15–20 min in 0.5% glutaraldehyde. Myosin II was immunostained with an anti–platelet myosin II antiserum (a gift from Dr. K. Fujiwara, National Cardiovascular Center, Osaka, Japan) at a dilution of 1:100.
Spindle poles were located by methanol fixation and subsequent staining with anti–γ tubulin (Sigma Chemical Co.) at a dilution of 1:1,000. Fixation and staining of Telophase Disc 60 protein (TD60) were performed as described by Wheatley and Wang (1996).
Microscopy and Data Collection
Imaging of cells was done using either an Axiovert 10 or an IM35 inverted microscope (Carl Zeiss), with a ×40, N.A. 0.75 plan-achroplan phase-contrast lens or a ×100, N.A. 1.3 neofluar lens. Images were acquired and processed using a cooled CCD camera (model TE/CCD-576EM; Princeton Instruments) and custom designed hardware and software. Optical sectioning and three-dimensional reconstruction were performed with custom software and a computer-controlled stepping motor (Fishkind and Wang, 1993; Wang, 1998).
Results
C3 Transferase Induces Poorly Regulated Cleavage Activities
The effects of Rho on cell cortical activities were investigated by microinjecting 0.2 mg/ml C3 transferase, a specific inhibitor of Rho proteins. Identical results were observed using 1.0 mg/ml C3, indicating that the injection exerted saturating effects on cells. Upon exposure to the toxin, interphase 3T3 fibroblasts and isolated NRK cells retracted and produced long, dendritic processes, similar to what was documented in previous studies (Rubin et al., 1988; Chardin et al., 1989; Ridley and Hall, 1992).
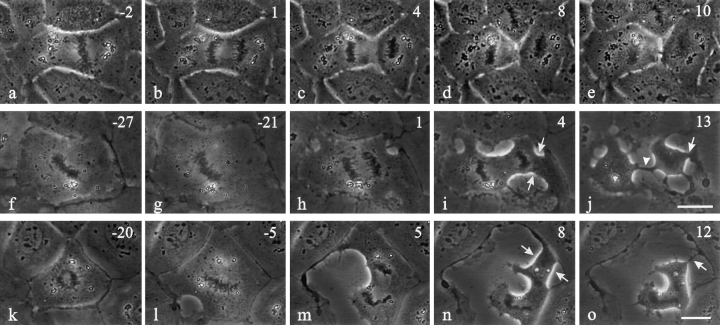
Both interphase and mitotic NRK cells within a monolayer showed a transient increase in surface area upon microinjection with C3 (Fig. 1, f and g, k and l). Mitotic cells then developed an elongated cleavage furrow between separating chromosomes (95%, n = 20 Fig. 1, h–j and l–o) as if the cleavage activity were spread over a wide area. In addition, while most injected NRK cells completed cytokinesis (55%, n = 20 Fig. 1 j), cleavage became poorly coordinated with mitosis. Ingressions in many cells started at random sites well before the onset of anaphase (25%, n = 16 Fig. 1 l). Moreover, 57% of C3-injected cells developed randomly placed ectopic furrows, which lead to the formation of anuclear fragments (Fig. 1, i and j and n and o, arrows). Cytokinesis failed in 45% of injected cells, not due to the lack of ingression but apparently to the disorganization of cortical activities. As for cleavage in normal cells, ingression in the presence of C3 was inhibited by cytochalasin D.
Figure 1.
Time lapse images of control NRK cells and cells microinjected with C3 transferase during prometaphase or metaphase. Approximate time in minutes relative to anaphase onset is located in the upper right corner. Control cells (a–e) developed a narrow cleavage furrow along the equatorial plane (c–e). Cells microinjected with C3 underwent immediate spreading in all directions (f and g, k and l; f and k were recorded immediately after microinjection). Ingressions were poorly regulated and initiated at several points around the cell after anaphase onset. An elongated cleavage furrow (j, arrowhead) formed as a result of the broad ingression that developed between separating chromosomes. Another cell initiated an ingression before anaphase onset (l), but did not complete cleavage between the sets of chromosomes. Ectopic furrows were common in C3-injected cells (i and j and n and o, arrows). Bars, 20 μm.
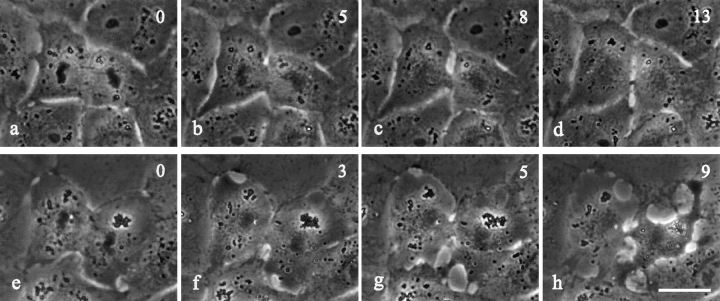
The effects of C3 were dependent on the timing of microinjection. When C3 was microinjected into NRK cells already undergoing cytokinesis (Fig. 2, n = 15), the cleavage continued to completion 100% of the time (Fig. 2, d and h). In 53% of these cells cytokinesis showed no detectable abnormalities (compare Fig. 2, a–d with Fig. 1, c–e). The remaining cells showed some abnormal cortical activities outside the equator (Fig. 2, g and h), without impairing the morphology or progression of the cleavage furrow.
Figure 2.
Time lapse images of NRK cells microinjected with C3 transferase after the initiation of cytokinesis. Time in minutes after microinjection is shown in the upper right corner. Roughly half of the cells injected after the initiation of furrowing proceeded through cytokinesis without any apparent effects (compare a–d with Fig. 1, c–e). The remaining cells showed increased cortical activities outside the equatorial region (f–h). All the injected cells completed cytokinesis with a normal-looking furrow and midbody (d and h). Bar, 20 μm.
Both transient spreading and irregular ingression induced by C3 appeared to depend on cell–cell adhesions and associated mechanical interactions. Isolated NRK cells and cells located at the edge of a colony showed neither transient increase in surface area nor extensive ectopic cortical activities along the free boundary, although the cleavage furrows were still elongated (not shown).
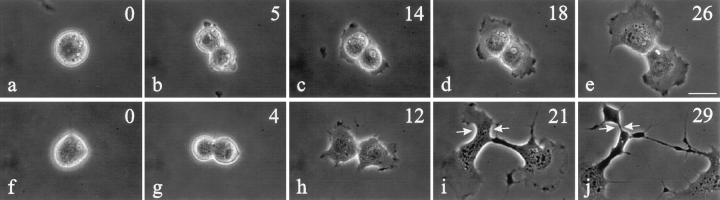
To further investigate the role of cell adhesion, C3 was injected into 3T3 fibroblasts that round up during mitosis (Fig. 3). Unlike NRK cells, no spreading was observed immediately after the microinjection of C3. The initiation of cytokinesis in C3-injected cells appeared indistinguishable from that of control 3T3 cells (Fig. 3 g). However, the effects of C3 became clear as the cell started to spread out. The furrow elongated as in NRK cells, while other regions of the cell developed additional sites of ingression or ectopic furrows (Fig. 3, i and j). Control cells never developed such broad or ectopic furrows. All C3-injected 3T3 cells completed cytokinesis (n = 5; Fig. 3 j).
Figure 3.
Time lapse images of control 3T3 fibroblasts and cells microinjected with C3 at metaphase. Approximate time in minutes relative to anaphase onset is located in the upper right corner. Control cells (a–e) maintained a very short cleavage furrow. C3-injected cells (f–j) developed abnormalities very similar to those in NRK cells when they started to respread (compare h–j with Fig. 1, h–j), forming both elongated furrows and ectopic ingressions. The latter resulted in the formation of anuclear fragments (i and j, arrows). Bar, 20 μm.
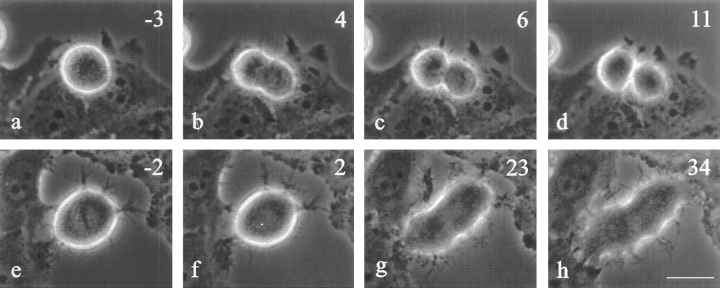
C3 was also microinjected into HeLa cells that are very weakly adherent to the substrate and their neighbors (Fig. 4). A large fraction of injected HeLa cells either detached from the dish during microinjection or failed to initiate anaphase. Of the limited number of cells injected with C3 that proceeded into anaphase, which we took as an indication of proper injection, all failed cytokinesis (n = 5; Fig. 4, g and h). Some of them showed clear signs of the initiation of cleavage, but never made further progress (n = 2; Fig. 4, g and h). HeLa cells that were microinjected with buffer alone were able to cleave successfully (Fig. 4 d).
Figure 4.
Time lapse images of control (a–d) and C3- injected HeLa cells (e–h). Approximate time in minutes relative to anaphase onset is located in the upper right corner. Cells injected with C3 were not able to complete cytokinesis (h), although some did attempt a weak furrow that could not progress (g and h). Control cells injected with 10 mM Hepes, pH 7.5, had no difficulty completing division (c and d). Bar, 20 μm.
The opposite manipulation, artificial activation of Rho, can be achieved by microinjecting either excess wild-type or GTP-independent, dominant positive mutant forms. In the present study this approach was limited to one isoform, RhoA. Neither excess wild-type nor dominant positive mutants of RhoA caused visible effects on dividing NRK cells (not shown), even though the same proteins were found to induce rounding of interphase macrophages as reported by Allen et al. (1997). In addition, no effect was observed when cells were treated with lysophosphatidic acid, which activates Rho proteins in interphase cells (Ridley and Hall, 1992).
Microinjection of C3 Disrupts the Organization of Cortical Actin and Myosin
The spread morphology of dividing NRK cells facilitated a more detailed investigation of cortical organization and dynamics. Fig. 5 shows the distribution of actin and myosin II in control cells (a and a′, c and c′) and C3-injected cells (b and b′, d and d′). In control cells a band of concentrated actin filaments is visible in the cleavage furrow (Fig. 5 a′). In C3-injected cells, despite the formation of cortical ingressions and elongated furrows, phalloidin staining showed no apparent concentration of actin filaments at the sites of furrowing or ingression. Actin filaments in the equatorial region are oriented predominantly along the spindle axis (Fig. 5 b′). In addition, all injected cells developed a curious local concentration of cortical actin filaments in the vicinity of one or both sets of separated chromosomes and associated spindle poles (Fig. 5 b′, arrows). In most cells these concentrated actin filaments appeared as a cluster of aggregates.
Figure 5.
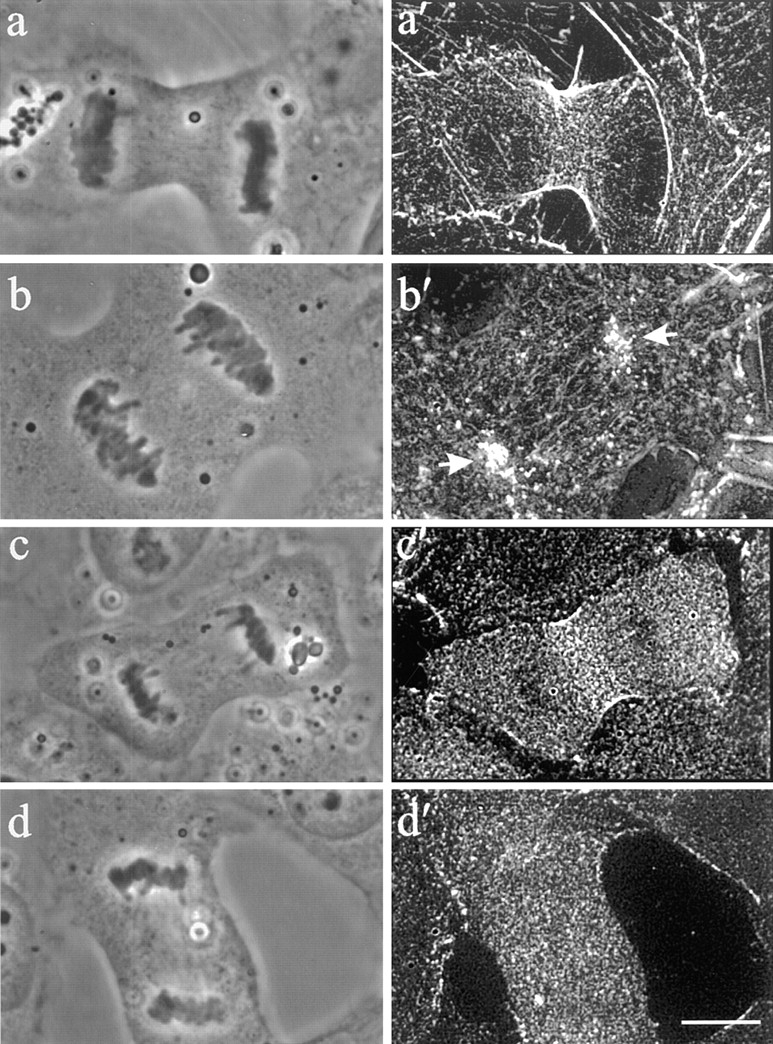
The organization of F-actin and myosin II in control (a′ and c′) and Rho inhibited (b′ and d′) cells. Stacks of optical sections were deconvolved and used for reconstruction of the 90° view. Phalloidin staining of ingressions in C3-injected cells revealed clusters of cortical actin localized above separated chromosomes (b and b′, arrows). No equatorial accumulation of actin as found in control cells was visible (a and a′). Immunofluorescence of myosin II similarly demonstrated a lack of localization to the equatorial plane (d and d′) compared to control cells (c and c′). Myosin II remained diffuse throughout the cell and did not colocalize with cortical actin over the chromosomes. Bar, 10 μm.
Immunofluorescence staining of myosin II indicated that all control cells developed a concentrated band of myosin II along the equator (Fig. 5 c′). However, no C3-injected cells showed a detectable concentration of myosin II in the furrow or at the cluster of cortical actin filaments (Fig. 5 d′).
Inhibition of Rho Causes Premature Cortical Flow in Dividing Cells
The reorganization of cortical actin during normal cell division is known to involve directional movements toward the equator (Cao and Wang, 1990), which can be easily followed by tracking associated surface receptors using charged fluorescent latex beads (Wang et al., 1994). In control NRK cells, such movement occurs only after anaphase onset, and is limited in a region roughly defined by the spindle interzone (Wang et al., 1994).
When cells were injected with C3 transferase at pro-metaphase or metaphase, surface-bound beads immediately started directional movement toward a central region of the cell, near the congregated chromosomes (Fig. 6, a and b). In addition, the movements were observed over most of the, if not the entire, top surface. During anaphase, the cluster of beads either followed the movement of one set of chromosomes (Fig. 6 c), or split into two and moved with each set (see Fig. 5 b′), as if they were tethered to the chromosomes. This cortical movement clearly accounts for the lack of concentration of actin filaments along the equator and the concentration over the segregated chromosomes/ spindle poles as described above.
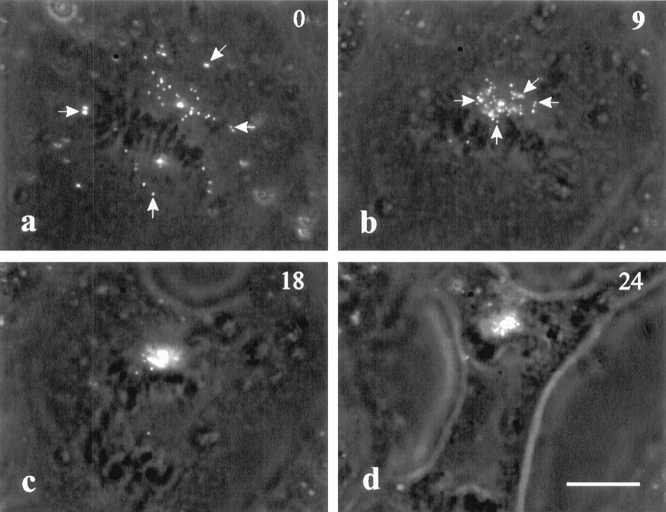
Figure 6.
Dynamics of surface receptors on a C3-injected cell. Surface receptors were labeled with fluorescent latex beads. Movements toward chromosomes initiated immediately upon the injection of C3 (a and b, arrows). During anaphase (c), the cluster of beads followed the top set of chromosomes and remained in the vicinity of the chromosomes (d). Bar, 10 μm.
C3-induced Cortical Ingression Persists upon the Depolymerization of Microtubules
The above observations suggest that C3 may disrupt the coordination between mitosis and cytokinesis. In control NRK cells, depolymerization of microtubules before anaphase onset causes arrest of mitosis and inhibition of cytokinesis. Depolymerization after the initiation of the cleavage furrow causes it to regress or to follow an irregular path (Wheatley and Wang, 1996; Wheatley et al., 1998). However, all C3-injected NRK cells proceeded with cleavage when treated with the microtubule depolymerizing drug nocodazole after anaphase onset (Fig. 7, c–e). Even when nocodazole was added at metaphase, broad ingressions developed despite the inhibition of mitosis (Fig. 7, f–j). Unlike NRK cells, 3T3 cells showed no C3-induced cleavage activity in the presence of nocodazole.
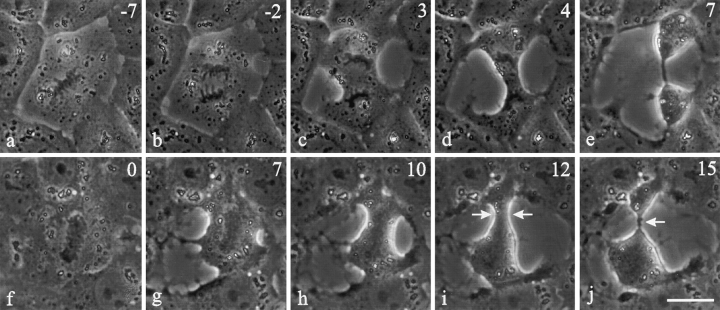
Figure 7.
Effects of microtubule depolymerization on C3-induced cleavage. NRK cells were microinjected with C3 during metaphase followed by treatment with 2.5 μM nocodazole. Time in minutes after the addition (t = 0) of nocodazole is located in the upper right corner. When cells were treated with nocodazole after anaphase onset or at the initiation of cytokinesis (a–e) the furrow continued to ingress. Those locked in metaphase with nocodazole treatment (f–j) underwent cortical ingression without entering anaphase (g and h). This cell eventually divided into a daughter cell containing all of the chromosomes and an anuclear fragment (i and j, arrows). Bar, 20 μm.
C3 Transferase Causes No Direct Effect on Mitosis
Contrary to the dramatic effects on the cortex and actin organization, no apparent effect was observed in C3-injected cells with regard to chromosomal separation or nuclear envelope reformation, confirming that the effects on cortex were not caused by the disruption of mitosis or cell cy-cle. Moreover, imaging of microinjected fluorescent tubulin showed no change in microtubule organization during metaphase or anaphase (Fig. 8, f and g), nor was there apparent effect on the elongation of astral microtubules. During telophase, wavy interzonal microtubules formed along the equator, most likely due to irregular cortical ingressions (Fig. 8, h and i). In addition, the elongated furrows were probably responsible for the formation of an extended bundle of microtubules flanking the midbody (Fig. 8 j). However, no prominent microtubule bundle was detected near ectopic furrows.
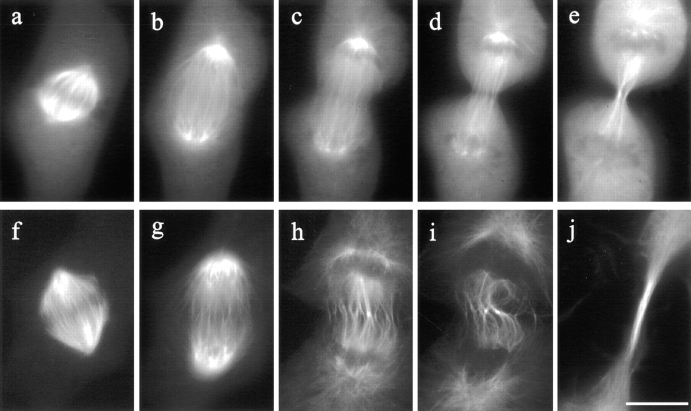
Figure 8.
Organization of microtubules in control and C3-injected mitotic NRK cells. In a control cell microinjected with rhodamine-labeled tubulin (a–e), kinetochore microtubules shortened, while midzone microtubules elongated. The spindle poles separated as the cell progressed from metaphase through anaphase (a–c). Prominent microtubule bundles were associated with the midbody during telophase (e). Microtubules in a C3- injected cell (f–j) underwent similar changes, except that midzone microtubules became distorted (compare c and d, with h and i). A long bundle of microtubules formed in the extended cleavage furrow (j). Bar, 10 μm.
TD60 antigen, a chromosomal passenger protein that relocates to the equatorial cortex during telophase, has been implicated in signaling cytokinesis (Andreassen et al., 1991; Margolis and Andreassen, 1993; Wheatley and Wang, 1996). The pattern of TD60 redistribution was unaffected by the microinjection of C3 (Fig. 9, a and b), and no concentration of TD60 was found near ectopic furrows. Similarly, there appeared to be no disruption to spindle poles, as visualized by staining with anti–γ-tubulin antibodies (Fig. 9, c and d).
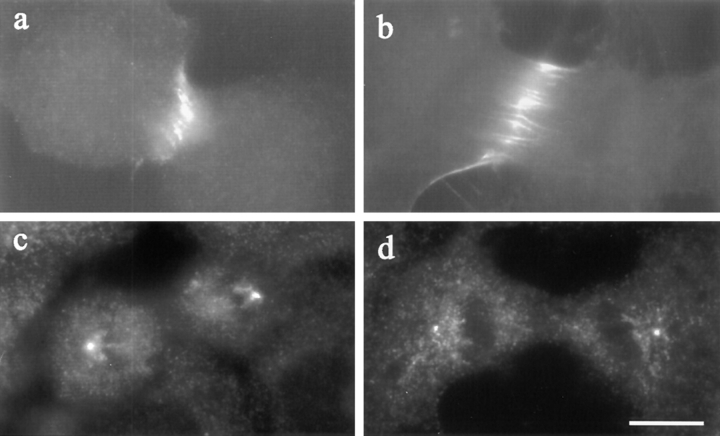
Figure 9.
The distribution of TD60 (a and b) and γ-tubulin (c and d) in Rho-inhibited NRK cells. Both control cells (a) and C3- injected cells (b) showed a localization of TD60 along the equator. Staining of γ-tubulin, used as a marker for centrosomes, showed similar localizations at the spindle poles in a C3-injected cell (d) and a control cell (c). Bar, 10 μm.
Discussion
All observations to date indicate that the mitotic apparatus directs the formation, initiation, and progression of the cleavage furrow in tissue culture cells. There is evidence that at least part of the signal originates from the chromosomal region at anaphase onset (Cao and Wang, 1996; Wheatley and Wang, 1996). However, except for several novel proteins such as TD60 and INCENP (Cook et al., 1987; Andreassen et al., 1991; Earnshaw and Bernat, 1991; Margolis and Andreassen, 1993), few molecules have been identified that may mediate the coordination between mitosis and cytokinesis.
The Rho family of small GTP-binding proteins represents attractive candidates for regulating cortical activities during cell division (Narumiya, 1996; Ridley, 1996; Gerald et al., 1998; Hall, 1998). In smooth muscle cells, Rho is believed to promote contraction through the phosphorylation of myosin II regulatory light chains (Kimura et al., 1996). In dividing echinoderm (Mabuchi et al., 1993) and frog embryos (Kishi et al., 1993; Drechsel et al., 1996), inhibition of Rho prevented cytokinesis from taking place and caused preformed furrows to regress. In one simple model, Rho is deactivated as cells enter mitosis, causing the disassembly of focal adhesions and stress fibers. During anaphase and telophase, localized reactivation of Rho along the equator triggers actin–myosin II interactions and activates cytokinesis.
However, our observations suggest a considerably more complicated picture. We found that adherent NRK cells respond immediately to C3 injection during prometaphase and metaphase, suggesting that some Rho activities are not only maintained during mitosis but are required for a normal cell morphology. In addition, unlike embryos, most cultured cells cleaved successfully upon the injection of C3. Thus, either some actin–myosin II interactions can take place in the presence of high concentrations of C3, or a Rho-independent motor is involved in the cleavage of these adherent cells.
Inhibition of Rho also induces several striking effects on dividing NRK cells. First, cortical actin moved immediately toward the central region during metaphase, accompanied by a transient spreading of cells that adhere to their neighbors. Second, all injected cells showed cleavage or cortical ingressions, without an apparent concentration of actin or myosin filaments along the furrow. Third, the cleavage furrow became elongated and ectopic furrows appeared in many cells, creating multiple anuclear fragments. Fourth, cleavage persisted even when mitosis was blocked with nocodazole. Since these effects were never observed in control cells, they cannot be easily explained as incomplete inhibition of an existing activity by suboptimal concentrations of C3. Moreover, identical results were obtained at increasing concentrations of C3 up to 1.0 mg/ml, which readily causes cell retraction and stress fiber disassembly in interphase cells.
The effects of C3 appeared to be highly dependent on cell adhesion. Ectopic cortical ingressions were observed before anaphase in well adherent NRK cells, but were not apparent in poorly adherent HeLa cells. Such effects took place in 3T3 cells only when the cells started to respread. In addition, while both NRK cells and respreading 3T3 cells developed elongated furrows, cleavage never even passed beyond an early stage in HeLa cells. Our results suggest that Rho is required for cytokinesis in the absence of traction forces, as in embryos and HeLa cells. However, Rho is involved in the spatial and temporal regulation of cortical ingression in the presence of adhesion forces, as in adherent epithelial cells.
Taken together, our observations cannot be easily explained with a model of Rho-activated cortical contraction, which would predict an inhibition of cytokinesis in all cell types by C3 as when cells were treated with cytochalasins. They indicate that Rho plays a more delicate role in coordinating the spatial and temporal pattern of cleavage with mitosis. One plausible explanation is that Rho regulates the mechanical integrity and strength of the cortex. Weakening of the cortex by C3 during metaphase causes the collapse of cortical actin toward the cell center, as well as an apparent spreading of the cell due to outward mechanical forces exerted by the neighbors. Subsequent interplay of cleavage forces and cell–cell, cell–substrate adhesive forces then leads to irregular ingressions and ectopic furrowing throughout a weakened cortex. This model suggests that cytokinesis of adherent cells is normally driven not only by localized contractions along the equator, but by localized weakening or “solation” of the cortex coupled to traction or adhesion forces.
The involvement of cortical disintegration in cytokinesis was first suggested based on electron microscopic observation of the thickness of the cortex (Schroeder, 1972). The cortex maintained a constant thickness despite the decrease in the diameter of the furrow, suggesting that materials are being removed continuously during the contraction. There is now evidence that cleavage can take place when such cortical disassembly is coupled to traction forces. In Dictyostelium, myosin II–null mutants cannot divide in suspension, but can divide when cells are adhered to the substrate, through a “traction-mediated cytofission” or “attachment-assisted mitotic cleavage” (Spudich, 1989; Neujahr et al., 1997a). As in the present case, cleavage took place without an apparent concentration of actin or myosin II along the equator (Neujahr et al., 1997a,b).
It is important to determine the spatial and temporal distribution of Rho activities during cell division. We cannot rule out the possibility that high activities of Rho are present along the equator during the early stage of cytokinesis, as suggested by the localization of RhoA (Takaishi et al., 1995; Madaule et al., 1998). However, the lack of effects with excess wild-type RhoA, constitutively active mutant forms of RhoA, or lysophosphatidic acid in the present experiments suggests that RhoA activity may normally be maintained at a high level in cultured cells, and that any localization of activities involves redistribution rather than net activation. Equally important is whether and how Rho activities decrease during subsequent stages of cytokinesis. Our observation that late injections of C3 resulted in the largely normal cleavage is consistent with the idea that Rho activities may decrease normally after furrow initiation, through either a GDP- or proteolysis-mediated pathway. This may lead to the solation of the cortex and allow the cleavage to proceed to completion.
Finally, it is important to determine the relationship between Rho proteins and signals that emanate from the mitotic spindle. Our observations indicate that the effects of C3 did not involve spindle microtubules, since both the mitotic spindle and an associated chromosomal passenger protein, TD60, appear to be unperturbed by the injection of C3 toxin. In addition, while normal cytokinesis requires the continuous presence of interzone microtubules (Fishkind et al., 1996; Wheatley and Wang, 1996), cortical responses to C3 transferase can occur without the proximity of these microtubules and can proceed after microtubule depolymerization. Therefore, Rho-mediated signals are likely to lie downstream from spindle signals. By manipulating directly the activities of Rho, we have likely caused a “short circuit” of the regulatory mechanism and induced cleavage independent of signals from the mitotic spindle.
Acknowledgments
We would like to thank Shuh Narumiya for recombinant C3, Keigi Fujiwara for anti–platelet myosin II antibody, and D. Palmer (University of Washington, Seattle, WA) for antibody against TD60. We are also grateful to D. Fishkind (University of Notre Dame, South Bend, IN) for his myosin II fixation protocol.
Abbreviations used in this paper
- NRK
normal rat kidney
- TD60
Telophase Disc 60 protein
Footnotes
Sally P. Wheatley's current address is Institute of Cell and Molecular Biology, Swann Building, King's Buildings, Mayfield Rd., Edinburgh EH9 3JR, United Kingdom.
This research was supported by a grant from the National Institutes of Health (GM-32476).
References
- Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- Andreassen PR, Palmer DK, Wener MH, Margolis RL. Telophase disc: a new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J Cell Sci. 1991;99:523–534. doi: 10.1242/jcs.99.3.523. [DOI] [PubMed] [Google Scholar]
- Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiaecytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L-G, Wang Y-L. Mechanism of the formation of contractile ring in dividing cultured animal cells. II. Cortical movement of microinjected actin filaments. J Cell Biol. 1990;111:1905–1911. doi: 10.1083/jcb.111.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L-G, Wang Y-L. Signals from the spindle midzone are required for the stimulation of cytokinesis in cultured epithelial cells. Mol Biol Cell. 1996;7:225–232. doi: 10.1091/mbc.7.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P, Boquet P, Madaule P, Popoff MR, Rubin EJ, Gill DM. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinumexoenzyme C3 and affects actin microfilaments in Vero cells. EMBO (Eur Mol Biol Organ) J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke CA, Heck MS, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus. . Curr Biol. 1996;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Bernat RL. Chromosomal passengers: towards an integrated view of mitosis. Chromosoma (Berl) 1991;100:139–146. doi: 10.1007/BF00337241. [DOI] [PubMed] [Google Scholar]
- Fishkind DJ, Wang Y-L. Orientation and three-dimensional organization of actin filaments in dividing cultured cells. J Cell Biol. 1993;123:837–848. doi: 10.1083/jcb.123.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishkind DJ, Wang Y-L. New horizons for cytokinesis. Curr Opin Cell Biol. 1995;7:23–31. doi: 10.1016/0955-0674(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Fishkind DJ, Silverman JD, Wang Y-L. Function of spindle microtubules in directing cortical movement and actin filament organization in dividing cultured cells. J Cell Sci. 1996;109:2041–2051. doi: 10.1242/jcs.109.8.2041. [DOI] [PubMed] [Google Scholar]
- Gerald NP, Dai J, Ting-Beall HP, DeLozanne A. A role for DictyosteliumRacE in cortical tension and clearage furrow progression. J Cell Biol. 1998;141:483–492. doi: 10.1083/jcb.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. The mechanism and control of cytokinesis. Curr Opin Cell Biol. 1997;9:815–823. doi: 10.1016/s0955-0674(97)80082-8. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Horiguchi Y, Senda T, Sugimoto N, Katahira J, Matsuda M. Bordetella bronchisepticadermonecrotizing toxin stimulates assembly of actin stress fibers and focal adhesions by modifying the small GTP-binding protein rho. J Cell Sci. 1995;108:3243–3251. doi: 10.1242/jcs.108.10.3243. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin-phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, Hiraoka Y, Noda M. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopusembryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi I. Biochemical aspects of cytokinesis. Int Rev Cytol. 1986;101:175–213. doi: 10.1016/s0074-7696(08)60249-1. [DOI] [PubMed] [Google Scholar]
- Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. A rho-like protein is involved in the organization of the contractile ring in dividing sand dollar eggs. Zygote. 1993;1:325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394:491–494. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- Margolis RL, Andreassen PR. The telophase disc: its possible role in mammalian cell cleavage. Bioessays. 1993;15:201–207. doi: 10.1002/bies.950150310. [DOI] [PubMed] [Google Scholar]
- McKenna NM, Wang Y-L. Culturing cells on the microscope stage. Methods Cell Biol. 1989;29:195–205. doi: 10.1016/s0091-679x(08)60195-8. [DOI] [PubMed] [Google Scholar]
- Narumiya S. The small GTPase Rho: cellular functions and signal transduction. J Biochem (Tokyo) 1996;120:215–228. doi: 10.1093/oxfordjournals.jbchem.a021401. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ishizaki T, Watanabe N. Rho effectors and reorganization of actin cytoskeleton. FEBS (Fed Eur Biochem Soc) Lett. 1997;410:68–72. doi: 10.1016/s0014-5793(97)00317-7. [DOI] [PubMed] [Google Scholar]
- Neujahr R, Heizer C, Gerisch G. Myosin II-independent processes in mitotic cells of Dictyostelium discoideum: redistribution of the nuclei, re-arrangement of the actin system and formation of the cleavage furrow. J Cell Sci. 1997a;110:123–137. doi: 10.1242/jcs.110.2.123. [DOI] [PubMed] [Google Scholar]
- Neujahr R, Heizer C, Albrecht R, Ecke M, Schwartz J-M, Weber I, Gerish G. Three-dimensional patterns and redistribution of myosin II and actin in mitotic Dictyosteliumcells. J Cell Biol. 1997b;139:1793–1804. doi: 10.1083/jcb.139.7.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiki TS, Narumiya S, Morii N, Yamamoto M, Fujiwara M, Kamata Y, Sakaguchi G, Kozaki S. ADP-ribosylation of the rho/rac proteins induces growth inhibition, neurite outgrowth and acetylcholine esterase in cultured PC12 cells. Biochem Biophys Res Commun. 1990;167:265–272. doi: 10.1016/0006-291x(90)91760-p. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho: theme and variations. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rubin EJ, Gill DM, Boquet P, Popoff MR. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. . Mol Cell Biol. 1988;8:418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon ED. Cytokinesis in animal cells. Curr Opin Cell Biol. 1989;1:541–547. doi: 10.1016/0955-0674(89)90018-5. [DOI] [PubMed] [Google Scholar]
- Satterwhite LL, Pollard TD. Cytokinesis. Curr Opin Cell Biol. 1992;4:43–52. doi: 10.1016/0955-0674(92)90057-j. [DOI] [PubMed] [Google Scholar]
- Schroeder TE. The contractile ring. II. Determining its brief existence, volumetric changes, and vital role in cleaving Arbaciaeggs. J Cell Biol. 1972;53:419–434. doi: 10.1083/jcb.53.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine A, Fujiwara M, Narumiya S. Asparagine residue in the rhogene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989;264:8602–8605. [PubMed] [Google Scholar]
- Self AJ, Hall A. Purification of recombinant Rho/Rac/G25K from Escherichia coli. . Methods Enzymol. 1995;256:3–10. doi: 10.1016/0076-6879(95)56003-3. [DOI] [PubMed] [Google Scholar]
- Small JV. Organization of actin in the leading edge of cultured cells: influence of osmium tetraoxide and dehydration on the ultrastructure of actin meshworks. J Cell Biol. 1981;91:695–705. doi: 10.1083/jcb.91.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Rinnerthaler G, Hinssen H. Organization of actin meshworks in cultured cells: the leading edge. Cold Spring Harbor Symp Quant Biol. 1982;46:599–611. doi: 10.1101/sqb.1982.046.01.056. [DOI] [PubMed] [Google Scholar]
- Spudich JA. In pursuit of myosin function. Cell Regul. 1989;1:1–11. doi: 10.1091/mbc.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kameyama T, Tsukita S, Tsukita S, Takai Y. Translocation of activated Rhofrom the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- Wang, Y.-L. 1992. Fluorescence microscopic analysis of cytoskeletal organization and dynamics. In The Cytoskeleton: A Practical Approach. K.L. Carraway and C.A.C. Carraway, editors. IRL Press at Oxford University Press, Oxford, UK. 1–22.
- Wang Y-L. Digital deconvolution of fluorescence images for biologists. Methods Cell Biol. 1998;56:305–315. doi: 10.1016/s0091-679x(08)60432-x. [DOI] [PubMed] [Google Scholar]
- Wang Y-L, Silverman JD, Cao L-G. Single particle tracking of surface receptor movement during cell division. J Cell Biol. 1994;127:963–971. doi: 10.1083/jcb.127.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley SP, Wang Y-L. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley SP, O'Connell CB, Wang Y-L. Inhibition of chromosomal separation provides insights into cleavage furrow stimulation in cultured epithelial cells. Mol Biol Cell. 1998;9:2173–2184. doi: 10.1091/mbc.9.8.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]