Abstract
Cadherins are involved in a variety of morphogenetic movements during animal development. However, it has been difficult to pinpoint the precise function of cadherins in morphogenetic processes due to the multifunctional nature of cadherin requirement. The data presented here indicate that homophilic adhesion promoted by Drosophila E-cadherin (DE-cadherin) mediates two cell migration events during Drosophila oogenesis. In Drosophila follicles, two groups of follicle cells, the border cells and the centripetal cells migrate on the surface of germline cells. We show that the border cells migrate as an epithelial patch in which two centrally located cells retain epithelial polarity and peripheral cells are partially depolarized. Both follicle cells and germline cells express DE-cadherin, and border cells and centripetal cells strongly upregulate the expression of DE-cadherin shortly before and during their migration. Removing DE-cadherin from either the follicle cells or the germline cells blocks migration of border cells and centripetal cells on the surface of germline cells. The function of DE-cadherin in border cells appears to be specific for migration as the formation of the border cell cluster and the adhesion between border cells are not disrupted in the absence of DE-cadherin. The speed of migration depends on the level of DE-cadherin expression, as border cells migrate more slowly when DE-cadherin activity is reduced. Finally, we show that the upregulation of DE-cadherin expression in border cells depends on the activity of the Drosophila C/EBP transcription factor that is essential for border cell migration.
Keywords: cadherin, intercellular motility, cell migration, Drosophila, oogenesis
Cell motility plays a pivotal role in shaping the animal body. Adhesive interactions between cells and extracellular matrix or between neighboring cells are required to convert the forces produced by the cytoskeleton into actual cell motility. Among the most extensively studied cell movements are integrin mediated migration processes on extracellular substrates (Hynes, 1992; Lauffenburger and Horwitz, 1996). Integrins, a family of extracellular matrix receptors, are connected via cytoplasmic adaptor proteins to F-actin allowing to transmit forces directly from the actin cytoskeleton to an extracellular substrate. In contrast, adhesion mechanisms involved in cell rearrangement movements in epithelia and other solid tissues, as they occur, for example, during gastrulation in many animals, are still very poorly understood. Cadherins are major cell–cell adhesion molecules in epithelial tissues, and, similar to integrins, cadherins are connected to the actin cytoskeleton by cytoplasmic adaptor proteins. These findings have led to the hypothesis that cadherins might play a similar role in intercellular motility as integrins in cell migration on extracellular substrates (Gumbiner, 1992). The notion that cadherins can mediate intercellular motility is supported by the finding that cadherins can promote the migration of neuronal growth cones on cellular substrates (Bixby and Zhang, 1990; Riehl et al., 1996; Iwai et al., 1997).
Cadherins are multifunctional transmembrane proteins that have well-established roles in cell adhesion and in epithelial polarization. In animal morphogenesis, cadherins act as Ca2+-dependent homophilic cell–cell adhesion molecules that mediate tissue-specific adhesion of embryonic and adult cells (Takeichi, 1991, 1995; Gumbiner 1996). In epithelial tissues cadherins are important regulators of epithelial cell structure. This function of cadherins has been well studied for E-cadherin, which is important for the assembly of the lateral cytocortex (McNeil et al., 1990; Eaton and Simmons, 1995; Drubin and Nelson, 1996). E-cadherin belongs to a subfamily of cadherins known as classic cadherins. These molecules interact with cytoplasmic catenins to form the cadherin–catenin complex that is concentrated at cell–cell adherens junctions at which it is connected to microfilaments. The role of the cadherin– catenin complex for epithelial adhesion and polarization explains its importance in transitions of mesenchymal cells to epithelial cells and vice versa (e.g., Hirano et al., 1992; Burdsal et al., 1993), and might account for its function as tumor suppressor gene in human (Birchmeier, 1995; Guilford et al., 1998). Lack of cadherin function in epithelial tissues typically compromises tissue integrity (e.g., Larue et al., 1994; Levine et al., 1994; Riethmacher et al., 1995; Tepass et al., 1996; Uemura et al., 1996). This makes it difficult to determine whether the disruption of cell rearrangements in those tissues is a secondary effect of the structural defects or a direct consequence of the loss of cadherin function. To overcome such difficulties and to study the function and regulation of cadherins in cell movements, it is desirable to identify cell populations in which a particular cadherin has a primary or sole function in cell motility. The data presented in this paper suggest that a group of cells in the Drosophila ovary known as border cells is such a cell population.
The recent isolation of mutations in the gene shotgun (shg)1 that encodes the Drosophila E-cadherin homologue, DE-cadherin, has opened a fruitful avenue to test the role classic cadherins play in animal morphogenesis. Analysis of shg/DE-cadherin revealed that it is the major epithelial cadherin in Drosophila and that its biochemical properties and interactions with catenins, its subcellular localization, and its requirement for epithelial differentiation are similar to vertebrate E-cadherin (Peifer, 1993; Oda et al., 1993, 1994; Tepass et al., 1996; Uemura et al., 1996). Analysis of the shg/DE-cadherin mutant phenotype revealed a striking correlation between the degree of cell rearrangement in a particular tissue and the amounts of DE-cadherin that are required for maintaining the epithelial integrity during cell rearrangements. Experimental suppression of cell rearrangements leads to a reduced requirement for DE-cadherin in maintaining tissue integrity (Tepass et al., 1996). These findings demonstrate that DE-cadherin is important for stabilizing tissues during morphogenetic processes, and suggest the need for regulation of cadherin activity during such events. However, these data do not reveal whether cadherin function plays a permissive role or whether cadherins are directly involved in promoting intercellular motility.
Here, we study the function of shg/DE-cadherin in cell rearrangement and cell migration processes during Drosophila oogenesis. In recent years, the Drosophila ovary has been used to analyze the involvement of the cytoskeleton in pattern formation and morphogenesis (e.g., Cooley and Theurkauf, 1994; Ray and Schüpbach, 1996). Drosophila ovaries are composed of bundles of ovarioles that each consist of an anteriorly located germarium and a series of follicles of increasing developmental age towards posterior. Each follicle contains 16 germline cells, one of which is the oocyte. The oocyte occupies the most posterior position among the germline cells of a follicle. Recently, it has been shown that the posterior localization of the oocyte is controlled by a cell sorting process that is driven by differential DE-cadherin-based adhesion (Godt and Tepass, 1998). The germline cells are surrounded by somatic follicle cells that initially form a uniform cuboidal monolayered epithelium. Follicle cells undergo various morphogenetic movements later in development. During mid-oogenesis most follicle cells move posteriorly, and form a highly columnar epithelium covering the oocyte. The few cells that surround the nurse cells become squamous. Also during midoogenesis, two subpopulations of follicle cells, the border cells and the centripetal cells undergo specific migration movements on the surface of germline cells. The border cells migrate from the anterior tip of a follicle in between nurse cells through the center of a follicle towards the oocyte. The centripetal cells penetrate between the nurse cells and the oocyte and eventually cover the anterior side of the oocyte. Finally, during late oogenesis, two groups of anterior-dorsal follicle cells conduct conversion extension movements and form two long tubes, the so-called dorsal appendages (for review on oogenesis see King, 1970; Spradling, 1993). Thus, Drosophila follicle cells are an excellent model system to study genetic mechanisms of morphogenesis.
The two morphogenetic processes that we analyze in this study are the migration of border cells and centripetal cells. In particular the work of Montell and colleagues has established the border cells as a genetic model system for the analysis of cell migration (Montell, 1994). A number of genes were shown to be involved in border cell migration including the Drosophila CCAAT/enhancer binding protein (DC/EBP) encoded by slow border cells (slbo; Montell et al., 1992), a number of cytoplasmic factors, namely the GTPases Drac1 (Murphy and Montell, 1996), Dras1, and ralA (Lee et al., 1996), Myosin II (Edwards and Kiehart, 1996), and the Drosophila FGF receptor encoded by breathless (btl; Murphy et al., 1995). However, a transmembrane adhesion receptor that provides adhesion and traction during border cell migration has not been identified. Our data indicate that DE-cadherin fulfills this role in border cell and centripetal cell migration. We show that DE-cadherin expression is required in border cells and centripetal cells as well as in germline cells for migration. In case of the border cells we find that the speed of migration depends on the level at which DE-cadherin is expressed. Our analysis also shows that DE-cadherin has no essential role in border cell formation, or in adhesion between border cells during migration. Taken together, our results indicate a specific requirement of DE-cadherin for border cell migration that is mediated by homophilic interactions between cell surfaces of border cells and germline cells. Our observations also suggest that the border cell cluster is not a mesenchymal group of cells, as previously believed, but an epithelial patch in which two central cells retain epithelial polarity and peripheral cells are partially depolarized similar to epithelia that have a free edge.
Materials and Methods
Drosophila Strains
shg alleles used are shgP34-1, shgR69, and shgR6 (Tepass et al., 1996; Godt and Tepass, 1998). slbo alleles used are slbo1, slboe2b, and slboe7b (Montell et al., 1992; Murphy et al., 1995). P[ry+; hs-neo; FRT]42D (FRT42D) and hsFLP1 lines (Xu and Harrison, 1994) were used for mosaic analysis. Oregon R was used as a wild-type strain. Flies were raised at 25°C if not otherwise indicated.
Mosaic Analysis
Site-directed mitotic recombination was catalyzed by the heat shock inducible FLP yeast recombinase at a FRT target element (Golic, 1991; Xu and Harrison, 1994). For the experiment FRT42D homozygous females were crossed to hsFLP1/Y; FRT42D shgR69/+ males. Eggs were collected on apple juice agar plates for 2 h each, and the developing animals were kept at 25°C before and after the 2 h heat shock in a 37°C air incubator. To induce shg mutant germline and follicle stem cells heat shocks were applied at 50 and 68 h after oviposition, which corresponds to the early and late second larval instar, respectively. Larvae were transferred to vials at late third instar. Adult females of the genotype hsFLP1/+; FRT42D shgR69/+FRT42D were easily recognized because they display a rough eye phenotype due to shg mutant patches in the eye. To induce shg mutant clones during oogenesis heat shocks were applied to adult females that were dissected 48–72 h later.
Tissue In Situ Hybridization
In situ hybridizations to adult ovaries of the genotypes wild-type, slbo1/ slbo1, slbo1/slboe7b, and slboe2b/slboe7b were performed using a full-length shg cDNA (Oda et al., 1994). Ovaries from 2–4-d-old well-fed female flies were dissected in PBS, and fixed in PBS containing 10% formaldehyde, 5% dimethylsulfoxide, and 50 mM EGTA for 30 min. The following steps were done according to a standard protocol for in situ hybridization (Tautz and Pfeifle, 1989), with the following modifications. Proteinase K treatment was done for 10 min. Hybridization with a digoxygenin-labeled DNA probe (50 ng DNA/100 μl hybridization solution), prepared as suggested by the manufacturer (Boehringer), was performed at 50°C for 48 h and followed by washes in hybridization solution, 1:1 mixture of hybridization solution and PBT (PBS, 0.1% Tween-20), and PBT at 50°C for 1 h each. The stained ovaries were mounted in 50% glycerol in PBS.
Staining Procedures
For immunostainings the following primary antibodies were used: rat monoclonal antibody anti-DE-cadherin (DCAD2, 1:50; Oda et al., 1994), mouse monoclonal antibodies anti-Crumbs (Cq4, 1:25; Tepass and Knust, 1993), anti–Fasciclin III (7G10, 1:50, Patel et al., 1987), and anti-Armadillo (N2-7A1, 1:100; Peifer, et al., 1994), and the rabbit polyclonal antibody anti-DC/EBP (C143, 1:100; Montell et al., 1992). Ovaries from 2–4-d-old well-fed female flies were dissected in PBS and fixed in 5% formaldehyde in phosphate buffer (PB), pH 7.4 for 10 min. For anti-Crumbs stainings ovaries were treated with methanol for 5 min after fixation. Tissues were washed in PB-T (PB, 0.3% Triton X-100) for 2× 15 min, followed by an 1 h incubation in PB-TB (PB-T, 0.2% BSA, 5% goat serum). Incubation with primary antibody, diluted in PB-TB was done at 4°C overnight. Ovaries were washed in PB-T for 4× 15 min and blocked in PB-TB for 1 h. Secondary antibodies conjugated with Cy3 or FITC (Jackson Laboratories) were used at a dilution of 1:400 in PB-TB at 4°C overnight. Ovaries were washed in PB-T for 4× 15 minutes and mounted in Antifade (70% glycerol + 2.5% DABCO [Sigma] in PBS).
To monitor lacZ expression of the P-lacZ insertion mutations shgP34-1 and shgR69 ovaries were fixed in 1% glutaraldehyde in PBS for 5 min, washed in PBT (PBS, 0.1% Triton X-100), and incubated in prewarmed X-gal staining solution (Bellen et al., 1989) containing 0.2% X-gal (US-Biological) at 37°C for 4–5 h. Tissues were washed in PBT and mounted in 50% glycerol in PBS.
F-actin filaments were detected with phalloidin. After antibody staining ovaries were washed with PBS, incubated in Oregon Green 488-phalloidin (Molecular Probes) at a dilution of 1:20 in PBS at 4°C overnight, washed in PBS, and mounted in Antifade.
Cell nuclei were visualized with Picogreen. After antibody staining ovaries were treated with 0.4 mg RNase A/ml PB-T for 1 h, rinsed with PB-T, incubated with Picogreen (Molecular Probes) at a dilution of 1:1,000 in PB-T at 4°C overnight, washed in PB-T and mounted in Antifade.
Light microscopic images were taken with a Zeiss Axiophot 2 microscope equipped with differential interference contrast optics using a Plan-Neofluar 20×/0.5 or a Plan-Neofluar 40×/1.30 oil objective and a condenser with a numerical aperture of 0.9. Confocal images were obtained with a Zeiss LSM420 laser confocal scanning microscope equipped with an Argon/Krypton laser (488/568 nm), 480 nm or 568 nm excitation filters, and 515- or 590-nm emission filters, respectively. Objectives used were Plan-Neofluar 40×/1.30 oil and Plan-Apochromat 100×/1.40 oil.
Results
DE-Cadherin Expression during Follicular Morphogenesis
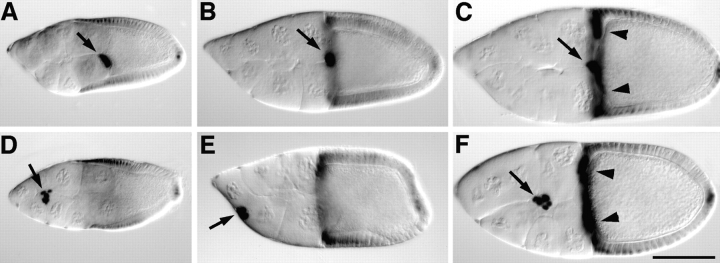
DE-cadherin shows a dynamic expression pattern during follicular morphogenesis. DE-cadherin is expressed in the germline throughout oogenesis, with the possible exception of the germline stem cells and early cystoblasts in the germarium. Furthermore, DE-cadherin is expressed in all somatic cells in the germarium and the follicles, except for the terminal filaments in which DE-cadherin was not detected (Fig. 1, A–D). High levels of DE-cadherin are found in a honeycomb pattern in the follicular epithelium that corresponds to the zonulae adherentes (Fig. 1 E). Lower levels of DE-cadherin are seen along the lateral surfaces of follicle cells and on the surfaces of the germ-line cells (Fig. 1, C and D). Expression of DE-cadherin is upregulated in various cell populations during oogenesis. In the germarium increased concentrations of DE-cadherin are seen in the oocyte and the anterior and posterior follicle cells. This differential expression of DE-cadherin promotes a cell sorting process that is responsible for posterior oocyte localization (Godt and Tepass, 1998). From stage 4/5 of oogenesis onwards increased levels of DE-cadherin are seen in a pair of follicle cells at the anterior and posterior pole, respectively, called the polar cells (Fig. 1 C). The anterior polar cells become part of the border cell cluster that forms during stage 8. The border cells and the centripetal cells express high levels of DE-cadherin during their migration as described in more detail below (Fig. 1 D). The distribution of the shg transcript as assayed by tissue in situ hybridization and a lacZ reporter (shgP34-1; Tepass et al., 1996; Godt and Tepass, 1998) is similar to the protein distribution. An exception are the dorsal appendages in which only the level of the mRNA but not the level of the protein is increased (Fig. 1 F; data not shown). Taken together, the shg/DE-cadherin expression profile during oogenesis suggests a possible role for DE-cadherin in maintaining the integrity of the follicular epithelium and, in particular, in the dynamic movements of border cells and centripetal cells.
Figure 1.
Expression of DE-cadherin during wild-type oogenesis. (A and B) A germarium, double-stained for DE-cadherin (A) and F-actin (B). DE-cadherin expression is seen throughout the germarium (g), in germline cells and somatic cells except for the terminal filament. A previously unidentified group of 6–7 somatic cells close to the base of the terminal filament is strongly stained (arrow). Low levels of DE-cadherin are detected in the anterior region of the germarium where germline stem cells and early cystoblasts are located. Posterior to this region strong DE-cadherin expression is seen in the germline cells and all somatic cells. The DE-cadherin expression in the germarium was described in more detail elsewhere (Godt and Tepass, 1998). (C) Throughout oogenesis DE-cadherin is expressed in the follicular epithelium and the germline cells of follicles. Anterior and posterior polar cells (arrows) show a higher level of expression than the remaining follicle cells during stages 4–7. (D) The highest amounts of DE-cadherin are seen in two migrating follicle cell populations, the border cells (arrow) and the centripetal cells (arrowheads). (E) shows a top view of the DE-cadherin expression pattern in the follicular epithelium at stage 14. (F) shows DE-cadherin distribution in the dorsal appendages (arrow) and in the part of the follicular epithelium that covers the anterior side of the oocyte including the micropyle (arrowhead). This part of the follicular epithelium is composed of the border cells and the centripetal cells. Anterior is to the left in all panels. s, stage; TF, terminal filament; IS, interfollicular stalk. Bars: (A and B) 50 μm; (C, D, and F) 50 μm; (E) 15 μm.
Border Cells Migrate as an Epithelial Patch
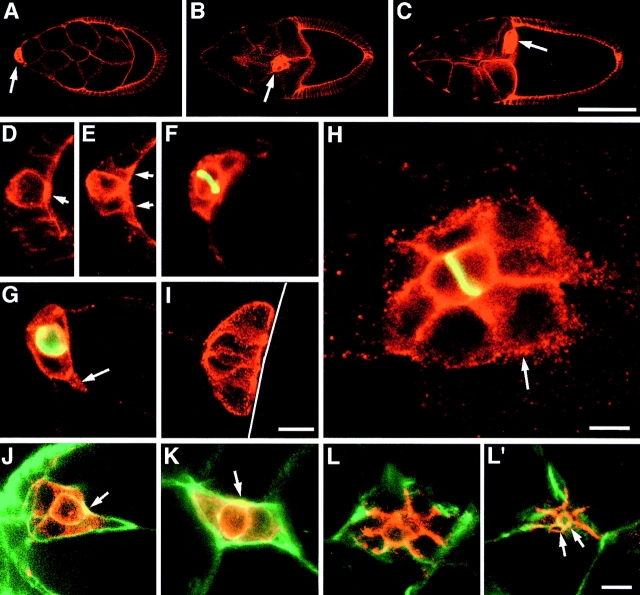
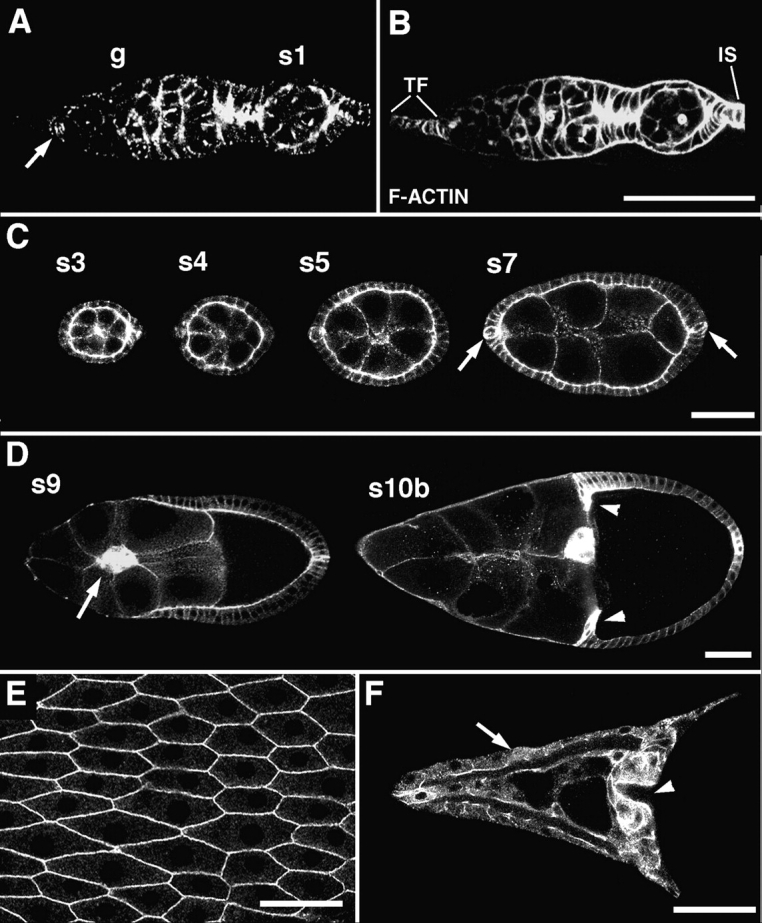
The border cells consist of the two anterior polar cells and an average of 6 additional follicle cells. DE-cadherin expression increases in these cells shortly before they segregate from the follicular epithelium (Fig. 2). Closer examination of migrating border cells revealed that the polar cells behave very differently from the other cells in the cluster. The polar cells upregulate DE-cadherin expression, constrict their apical surfaces, and assume a round shape at stage 4/5, long before the border cell cluster forms (Fig. 2 D). At stage 8, follicle cells next to the polar cells upregulate DE-cadherin expression (Fig. 2 E). At late stage 8, the border cells segregate from the follicular epithelium and DE-cadherin becomes distributed rather uniformly over the surface of the border cells (Fig. 2 F). At early stage 9, border cell migration is initiated by a single border cell that extends a process between nurse cells. This border cell is never a polar cell (Fig. 2, G and J). In fact, here and during the entire migration process the polar cells occupy a central position in the border cell cluster (see also Edwards et al., 1997), and maintain their constricted apical surface and round shapes (Fig. 2, H and J–L′). The polar cells can be specifically addressed with anti–Fasciclin III antibodies (Brower et al., 1980; Patel et al., 1987) that label most prominently the contact surface of the two polar cells (Fig. 2, F and H). The other border cells in the cluster form a single layered rosette that surrounds the polar cells (we will refer to these cells as rosette cells; Fig. 2, H, K, and L). In the migrating border cell cluster the highest concentration of DE-cadherin is found at the contact sites between rosette cells and polar cells and between adjacent rosette cells (Fig. 2 H). At the interface between rosette cells and nurse cells substantially lower amounts of DE-cadherin are seen. Here, DE-cadherin is distributed in a punctate pattern that might represent surface clusters or intracellular vesicles (Fig. 2 H), and might be a consequence of a high turn-over rate of DE-cadherin at the interface between rosette cells and nurse cells. In contrast to DE-cadherin, the highest concentration of F-actin in rosette cells is found in the region of the cytocortex that contacts the nurse cells whereas lower levels of F-actin are seen at contact sites between border cells (Fig. 2, J–L). The high concentration of F-actin in the periphery of the border cell cluster is consistent with a role of actin polymerization in border cell migration (see also Murphy and Montell, 1996; Oda et al., 1997). Our results show that the border cell cluster has a two-dimensional organization and suggest that the polar cells maintain aspects of epithelial polarity during migration.
Figure 2.
Expression of DE-cadherin in border cells of wild-type ovaries. (A–C) Arrows point to border cells. (A) At early stage 9 border cells have accumulated high levels of DE-cadherin before migration is initiated. (B) Border cells expressing elevated levels of DE-cadherin migrate on a straight path through the center of the follicle towards the oocyte during stage 9. (C) After border cells have reached the oocyte that fills the posterior half of a follicle at stage 10a, they maintain high levels of DE-cadherin expression for some time and move slightly dorsally. (D–I) show high resolution images of DE-cadherin (red) expression in border cells. (F–H) show follicles that are counterstained for Fasciclin III (green) that serves as a marker for the two polar cells. Fasciclin III accumulates at the contact surface between polar cells. (D) Stage 6. Anterior polar cells contain more DE-cadherin than neighboring follicle cells. Polar cells are constricted apically (arrow) and have a rounded shape. DE-cadherin is concentrated at the zonula adherens. (E) Stage 8. Follicle cells adjacent to polar cells have upregulated DE-cadherin expression (arrows). (F) Early stage 9. Same follicle as in A. DE-cadherin distribution in follicle cells adjacent to polar cells (the rosette cells) has depolarized. (G) Early stage 9. Migration is initiated by a rosette cell penetrating between nurse cells (arrow). (H) Late stage 9. Same follicle as in B. During migration polar cells have a central position and are surrounded by rosette cells. Highest concentration of DE-cadherin is seen at the contact surfaces between rosette cells and polar cells and between rosette cells. Lower amounts of DE-cadherin are seen at the interface of rosette cells and nurse cells in a punctate pattern (arrow). (I) Stage 10a. Border cells have established contact to the surface of the oocyte (white line). The polar cells are centrally located and their constricted apical surface contacts the oocyte (see also Peifer et al., 1993). (J–L′) show distribution of DE-cadherin (red) and F-actin (green) in border cells at stage 9. F-actin is concentrated in the periphery of the border cell cluster. (J) Border cells at the onset of migration and (K) during mid migration. Note the round centrally located polar cells with constricted apical cell surfaces that are rich in DE-cadherin and F-actin. (L) and (L′) show two confocal sections of the same cluster. The arrows in L′ point to the constricted apical surfaces of the polar cells. Anterior is to the left in all panels. Bars: (A–C) 50 μm; (D–G, J–L′) 10 μm; (H and I) 10 μm.
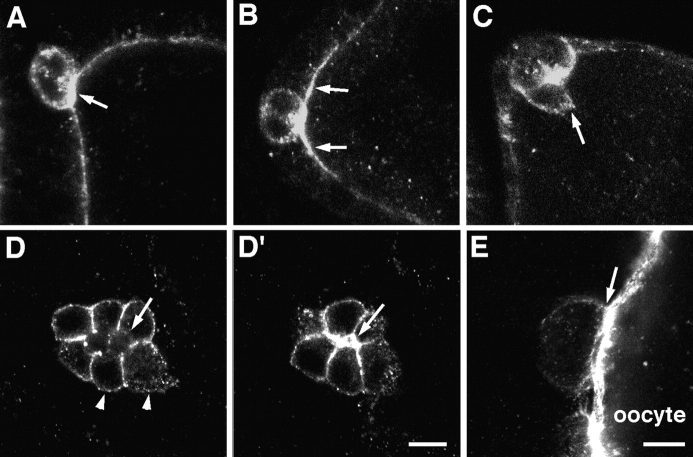
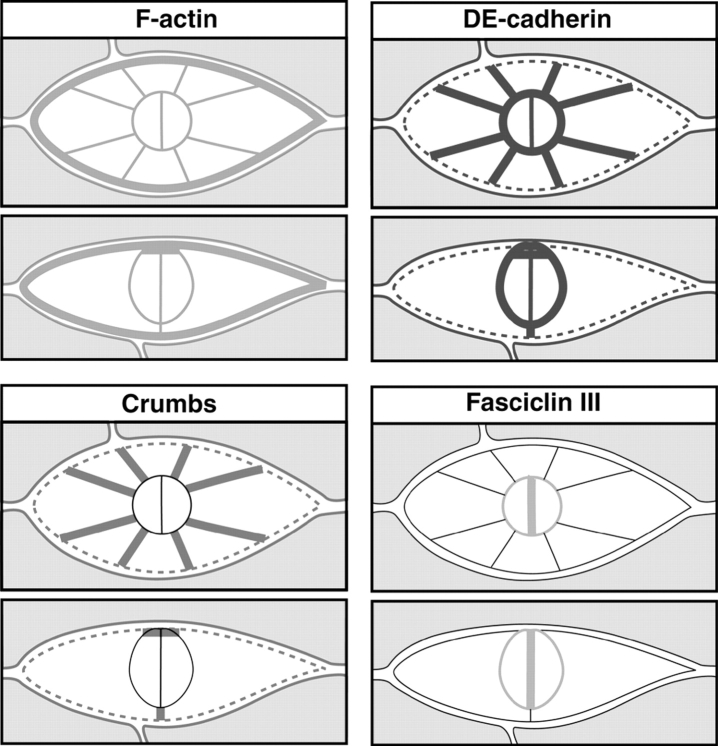
To further analyze border cell structure we examined border cells with antibodies recognizing the Crumbs protein, a marker for the apical membrane of epithelial cells including the follicular epithelium (Tepass and Knust, 1990; Tepass et al., 1990). Crumbs is expressed in the border cells during migration (Fig. 3). Similar to DE-cadherin, increased levels of Crumbs are seen in the polar cells and in surrounding follicle cells before border cells segregate from the epithelium (Fig. 3, A and B). Upon segregation of the border cells Crumbs distribution depolarizes in rosette cells (Fig. 3, C and D). Crumbs is highly expressed at the interface of adjacent rosette cells, and at lower levels at the interface of rosette cells and nurse cells, a pattern similar to DE-cadherin. Crumbs remains restricted to the apical surface of polar cells during migration. Crumbs is not found at the lateral surface of polar cells or at the cell surface of the rosette cells that are in contact with the polar cells (Fig. 3, D and D′). At the end of migration when the border cell cluster contacts the oocyte, the apical surface of the polar cells faces the oocyte. The distribution of Crumbs in the rosette cells becomes restricted again to the apical cell surface before these cells become confluent with the ingrowing centripetal cells (Fig. 3 E). These results indicate that polar cells and, to a lesser degree, rosette cells retain epithelial polarization during migration. Fig. 4 summarizes the expression and distribution of molecular markers that together with the morphology of the border cells suggest that these cells form a migrating epithelial patch rather than a mesenchymal cluster. Our findings suggest that only the rosette cells are actively migrating and that the polar cells are carried by the rosette cells during the migration process.
Figure 3.
Expression of Crumbs in border cells of wild-type ovaries. (A) Crumbs is found at the apical cell surface in all follicle cells. At early stage 8 anterior polar cells have upregulated Crumbs expression (arrow). Crumbs protein is also seen in the cytoplasm of the polar cells. (B) Follicle cells next to the polar cells upregulate Crumbs during stage 8. (C) At early stage 9 when a rosette cell initiates migration (arrow), Crumbs has a nonpolarized distribution in rosette cells. D and D′ show two confocal sections of the same cluster during mid migration. In polar cells Crumbs is concentrated at the apical cell surface (arrow in D′) and not found at the lateral cell surface that contacts the rosette cells (arrow in D). In rosette cells Crumbs accumulates at the contact sides between neighboring rosette cells and is found in a punctate pattern at the interface of rosette cells and nurse cells (arrowheads). (E) At stage 10 when the border cells are in contact with the oocyte Crumbs distribution in rosette cells is again restricted to the apical surface. Bars: (A–D′) 10 μm; (E) 10 μm.
Figure 4.
Schematic summary of marker distribution in migrating border cells. For each marker a top view (upper panels) and a side view (lower panels) is shown (see text for further explanations).
DE-Cadherin Expression in Border Cells Is Required for Migration
To study the role of shg/DE-cadherin in border cells we generated homozygous shg mutant follicle cell clones using the FLP/FRT system and the embryonic lethal null allele shgR69 (see Materials and Methods). Cells mutant for shgR69 do not express detectable levels of protein allowing the identification of shg mutant cell clones by assaying for DE-cadherin expression (Godt and Tepass, 1998). In a first set of experiments clones were induced in the second larval instar to generate shg mutant follicle stem cell precursors. As each germarium in adult females contains only two follicle stem cells, cell clones derived from a single stem cell populate large portions of the follicular epithelium or in many cases the entire follicular epithelium of a single follicle (Margolis and Spradling, 1995). Follicles that do not express DE-cadherin in follicle cells show a variety of defects, and such follicles eventually degenerate. One prominent defect is a mispositioning of the oocyte that normally is the most posterior germline cell in a follicle (Godt and Tepass, 1998; González-Reyes and St. Johnston, 1998b). Surprisingly, many follicles do not show defects in the integrity of the follicular epithelium until late oogenesis. A reduced but significant amount of Armadillo/β-catenin is found at the level of the zonula adherens in shg mutant follicle cells suggesting that Armadillo interacts with a different cadherin than DE-cadherin which might account for the maintenance of epithelial cell structure (Godt and Tepass, 1998).
Border cell clusters in which all cells lack DE-cadherin do not migrate between nurse cells towards the oocyte. We examined a total of 62 shg mutant follicle cell clones. shg mutant border cell clusters formed in all clones and segregated from the follicular epithelium as revealed, for example, by the expression of the border cell specific marker DC/EBP (Fig. 5, A–C). shg mutant clusters contain a normal number of DC/EBP positive border cells (8.3; n = 15) as compared with wild-type clusters (8.0; n = 40), and show an overall normal cell arrangement with a pair of Fasciclin III positive central polar cells (Fig. 5, D–F). In all follicles examined, the border cell cluster was located between follicular epithelium and nurse cells indicating that shg mutant border cell clusters cannot penetrate between nurse cells. The clusters were located either near the anterior tip of the follicle or at the boundary between the first and second nurse cell. These findings indicate that DE-cadherin expression in border cells is required for border cell migration.
Figure 5.
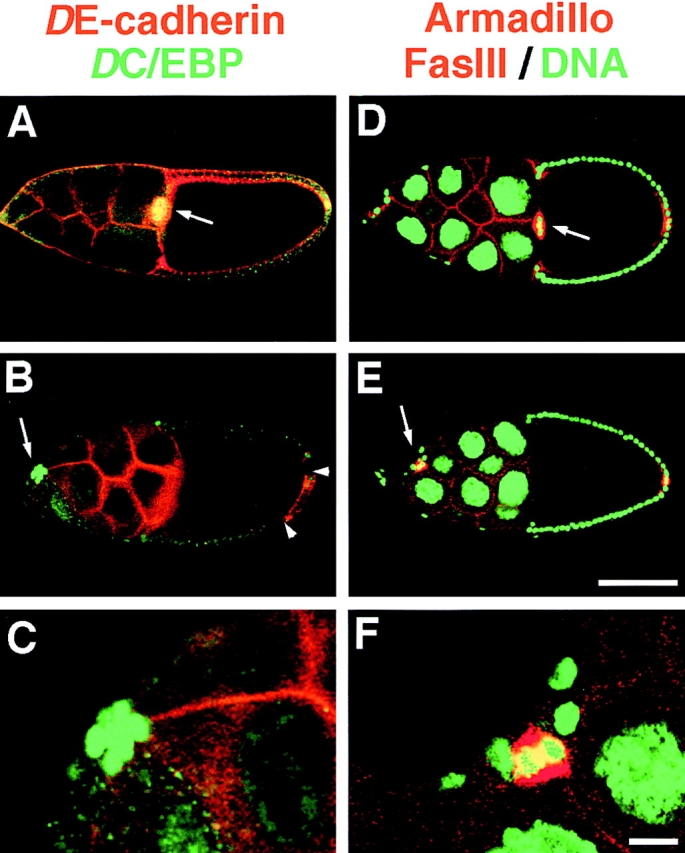
DE-cadherin expression in border cells is required for their migration between the nurse cells. (A–C) Double staining of stage 10 follicles with DE-cadherin (red) and the nuclear border cell marker DC/EBP (green). Arrows point to border cell clusters. (A) In a wild-type follicle the border cell cluster that expresses DE-cadherin and DC/EBP has reached the oocyte. (B) shows a shg mutant follicle cell clone that encompasses most of the follicle cells, except for a small patch of DE-cadherin positive cells at the posterior pole (arrowheads). The germline cells express DE-cadherin. A shg mutant border cell cluster expressing DC/EBP has formed that has not moved between the nurse cells towards the oocyte. The cluster is located between nurse cells and the follicular epithelium close to the anterior tip of the follicle. (C) Close-up of the shg mutant border cell cluster shown in B. (D–F) Triple staining of stage 10 follicles for Armadillo (red) that is expressed in the same pattern as DE-cadherin, for the polar cell marker Fasciclin-III (FasIII; also red), and the nuclear marker Picogreen (green). Arrows point to border cell clusters. (D) In the wild-type follicle the border cell cluster has reached the oocyte. (E) shows a shg mutant follicle cell clone derived from shg mutant follicle stem cells that comprises all follicle cells, including the border cells, as indicated by the absence of Armadillo. Red staining in anterior and posterior polar cells is due to expression of Fasciclin III. The anterior polar cells are part of the border cell cluster that has not migrated to the oocyte but remained attached to follicle cells close to the anterior pole of the follicle. (F) Closeup of the shg mutant border cell cluster shown in (E). Bars: (A, B, D, and E) 100 μm; (C and F) 10 μm.
A few border cell clusters (n = 11) were found that contained DE-cadherin positive and negative cells (Fig. 6). These clusters emerged in mosaic follicles in which the clone boundary separating DE-cadherin positive and negative cells runs along the anterior tip of the follicle from where the border cells derive. These clusters, which contained 3–5 DE-cadherin positive and 2–4 DE-cadherin negative cells, migrated between the nurse cells towards the oocyte. The DE-cadherin expressing cells were always found at the leading edge of the cluster while the cells that lack DE-cadherin were trailing behind (Fig. 6, D–F). This cell behavior suggests that the DE-cadherin positive cells are actively migrating whereas the DE-cadherin negative cells are pulled along. It also indicates that DE-cadherin is not essential for maintaining adhesion between border cells during migration despite its prominent accumulation between these cells. These results indicate that in border cells DE-cadherin might have a specific role in cell migration.
Figure 6.
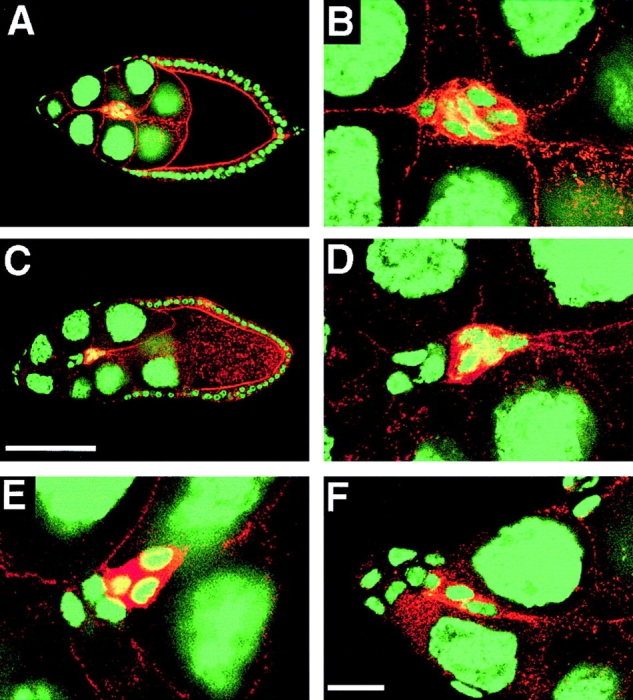
Migration of shg mutant mosaic border cell clusters. Stage 9 follicles were stained with anti-DE-cadherin (red) and the nuclear marker Picogreen (green). (A) A wild-type follicle showing a migrating border cell cluster. (B) Closeup of the border cell cluster shown in A. All border cells express DE-cadherin. (C) shg mutant follicle cell clone covering part of the follicular epithelium. (E) Closeup of the mosaic border cell cluster shown in C. The migrating border cell cluster contains DE-cadherin positive cells at the front, and DE-cadherin negative cells trailing behind. D shows another example of a mosaic border cell cluster with the DE-cadherin expressing border cells moving ahead. F shows a shg mutant mosaic border cell cluster in which the DE-cadherin positive cells have started migrating between the nurse cells and the shg mutant cells are still located at the anterior end of the follicle. Anterior is to the left in all panels. Bars: (A and C) 100 μm; (B, D, E, and F) 20 μm.
Differences in the Expression Level of DE-Cadherin Might Play a Role in Border Cell Recruitment
In a second set of experiments, clones were induced in adult females in order to generate a higher clone frequency and hence a larger number of border cell clusters that are composed of shg mutant and wild-type cells (see Materials and Methods). To determine the clone frequency in border cells we took advantage of the fact that the shgR69 allele contains a lacZ reporter gene in the shg locus that is expressed in follicle cells including all border cells. We find that 75% of border cell clusters (n = 79) contain lacZ negative wild-type cells. Surprisingly, no migrating border cell clusters were found that contained shg mutant cells as assayed by anti-DE-cadherin staining, although there were numerous DE-cadherin negative clones in the follicular epithelium that ranged in size from 2–3 to more than 25 cells. In this experiment the number of cells in border cell clusters (7.8 cells per cluster, n = 166) was not significantly reduced in comparison to wild-type (8.0 cells per cluster, n = 40). This finding suggests that during recruitment of follicle cells into a border cell cluster the DE-cadherin negative follicle cells are discriminated against the DE-cadherin positive follicle cells.
One possible explanation for the differences in cell behavior of shg mutant cells derived from follicle stem cell clones as opposed to clones induced during oogenesis are differences in the availability of DE-cadherin positive cells at the anterior tip of a follicle. If the clone boundary of a large clone, derived from a mutant stem cell passes through the anterior tip of a follicle the number of DE-cadherin positive cells competent to form border cells might be below 8. Since DE-cadherin is not essential for the formation of a border cell cluster as shown above, a cluster forms and migrates that contains both DE-cadherin positive and negative cells (Fig. 6). On the other hand, if only a small shg mutant patch is present at the anterior tip of the follicle enough DE-cadherin positive cells might still exist at this position to form a normal cluster. Our results indicate that DE-cadherin positive cells are preferentially recruited into the border cell cluster and suggests a possible role of a sorting process in border cell recruitment that depends on the level of DE-cadherin expression.
Expression of DE-Cadherin in the Germline Is Required for Border Cell Migration
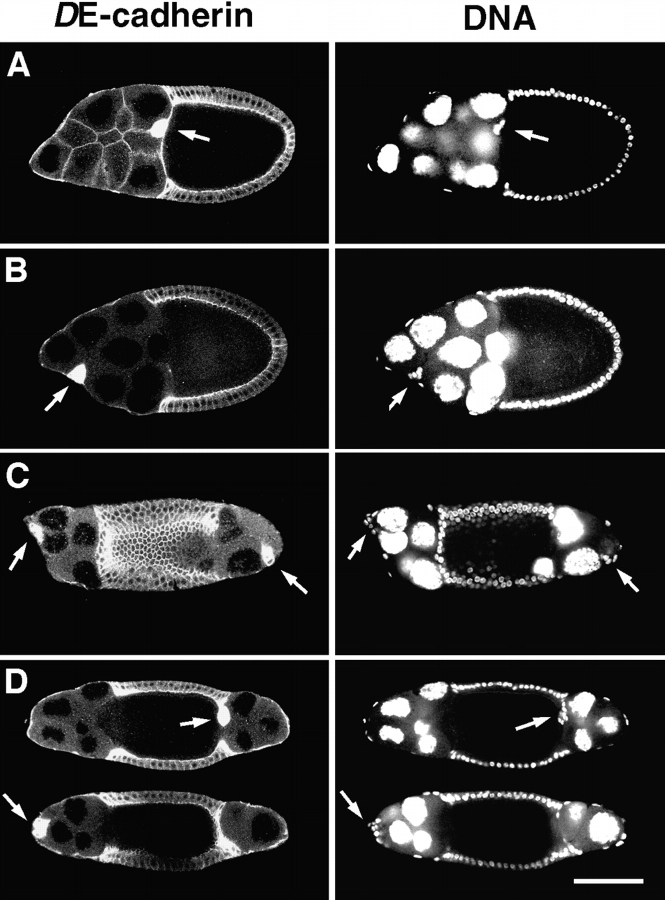
DE-cadherin like other classic cadherins is a homophilic adhesion molecule (Oda et al., 1994). The lack of DE-cadherin in border cells prevents penetration and migration of border cells between nurse cells. This suggests that DE-cadherin on the surface of border cells might directly interact with DE-cadherin expressed by nurse cells to promote migration. To examine this possibility we generated follicles that contain a shgR69 mutant germline (Fig. 7; see Materials and Methods). Follicles with a shg mutant germ-line show a variety of defects including a mislocalization of the oocyte similar to follicle cell clones (Oda et al., 1997; White et al., 1998; Godt and Tepass, 1998; González-Reyes and St. Johnston, 1998b). All follicles eventually degenerate. Among the examined 99 shg germline clones with normally localized oocyte there was no follicle in which the border cell cluster had penetrated between nurse cells. Instead, the border cell clusters always remain attached to the follicular epithelium. Typically, they are found at the boundary between the first and second nurse cell as shown in Fig. 7 B. Sometimes, they move even further posteriorly, and a single case was observed where the border cells had reached the oocyte.
Figure 7.
Disruption of border cell migration in shg mutant germ- line clones. Stage 10 follicles are double stained with DE-cadherin (left panels) and the nuclear marker Picogreen (right panels). Arrows point to border cell clusters. (A) Wild-type follicle with the border cell cluster attached to the oocyte. (B) shg mutant germline clone with a normally located oocyte. The border cell cluster has not invaded between the nurse cells but remained directly beneath the follicular epithelium close to the anterior pole. (C) shg mutant germline clone with a centrally located oocyte. Two border cell clusters have formed, one at each pole of the follicle, which have not migrated towards the oocyte. (D) Two optical sections of the same shg mutant germline clone that has a centrally located oocyte. From the two border cell clusters one has moved and reached the oocyte (upper section). Note that the border cell cluster is in contact with the follicular epithelium. The other one has not moved away from its original polar position (lower section). Anterior is to the left in all panels. Bar, 100 μm.
In follicles that contain a shg mutant germline with centrally localized oocyte a border cell cluster forms at both poles of the follicle. This was also shown for other mutants that cause mislocalization of the oocyte (Gonzáles-Reyes and St. Johnston, 1994). In shg mutant germline clones with central oocyte position (n = 75) border cells are never observed between nurse cells (Fig. 7 C). However, the fraction of clusters that have reached the oocyte is higher (35%, n = 124) than in shg germline clones with posterior oocyte (1%, n = 99). The shorter migration distance in follicles with central oocyte is presumably responsible for the higher fraction of border cell clusters that have reached the oocyte. Clusters that reach the oocyte are still in contact to the follicular epithelium (Fig. 7 D). This observation, together with the finding that border cells in shg mutant germline clones do not penetrate between nurse cells, suggests that the clusters which have reached the oocyte migrated along the follicular epithelium. Taken together, our analysis of shg germline clones is consistent with the results of a previous study on shg mutant germline clones (Oda et al., 1997) and demonstrates that DE-cadherin on the surface of nurse cells is required for border cells to penetrate between nurse cells and to migrate along their normal route.
Reduced Expression of DE-Cadherin Slows Down Border Cell Migration
The level of DE-cadherin expression in border cells is extremely high compared with most other cells in ovaries or elsewhere. To address the question whether the high levels of DE-cadherin expression are required to sustain normal migration we reduced DE-cadherin activity in follicles. We examined border cells in animals that carry the heteroallelic combination shgP34-1/shgR6. shgP34-1 is an allele of moderate strength that causes complete embryonic lethality with an intermediate cuticle phenotype (Tepass et al., 1996). shgR6 is a homozygous viable shg allele that displays an adult wing phenotype. shgP34-1/shgR6 animals are semiviable and ovaries show a substantial reduction in levels of DE-cadherin expression (Godt and Tepass, 1998; data not shown). In shgP34-1/shgR6 follicles border cell migration is in many cases substantially delayed (Fig. 8; Table I). In contrast to shgR69 mutant follicles, ∼65% of border cell clusters that had not reached the oocyte at stage 10 in shgP34-1/shgR6 mutant follicles were located between nurse cells and only 35% of the clusters did not penetrate between nurse cells. Thus, in shgP34-1/shgR6 mutant follicles most clusters migrate along their normal route but many clusters show a substantial decrease in the speed of migration suggesting that the level of DE-cadherin expression determines the speed of border cell migration.
Figure 8.
Reduced motility of border cells in a weak shg mutant. X-gal staining of follicles at late stage 9 (A and D), at stage 10a (B and E) and stage 10b (C and F) reveals lacZ expression of the shgP34-1 P-element insertion in border cells (arrows) and centripetal cells (arrowheads). (A–C) shgP34-1/CyO follicles show normal border cell migration. The border cell cluster is attached to the oocyte in all three follicles. (D–F) shgP34-1/shgR6 mutant follicles. D and F show border cell clusters that have migrated between nurse cells but have not reached the oocyte. E shows a border cell cluster that has not penetrated between nurse cells but remained in contact with the follicular epithelium. Anterior is to the left in all panels. Bar, 100 μm.
Table I.
Reduced Motility of Border Cells (BC) in a Weak shg Mutant
| Genotypes | shgP34-1/CyO | shgP34-1/shgR6 | ||
|---|---|---|---|---|
| Position of the BC cluster in relation to the | ||||
| migrating outer follicle cells during mid/late | ||||
| stage 9* | ||||
| % BC clusters that are ahead or parallel | 93.7 | 52.9 | ||
| % BC clusters that are behind | 6.3 | 47.1 | ||
| (n = 191) | (n = 223) | |||
| Position of the BC cluster at stage 10a | ||||
| % clusters that reached the oocyte | 99.3 | 69.8 | ||
| % clusters that did not reach the oocyte | 0.7 | 30.2 | ||
| (n = 145) | (n = 129) | |||
| Position of BC at stages 10b/11 | ||||
| % clusters that reached the oocyte | 98.5 | 89.4 | ||
| % clusters that did not reach the oocyte | 1.5 | 10.6 | ||
| (n = 136) | (n = 142) |
Also, during stage 9 the cells of the follicular epithelium (outer follicle cells) move towards the oocyte. BC normally migrate parallel or slightly ahead to the posteriorly moving boundary that separates columnar and squamous follicle cells during mid/late stage 9. Migration was considered slower if border cells stayed behind this boundary.
DE-Cadherin Expression in Border Cells Is Controlled by DC/EBP
A number of genes have been implicated in border cell migration most notably the gene slbo that encodes a C/EBP transcription factor (Montell et al., 1992). In follicles mutant for strong slbo alleles the border cell cluster forms but does not migrate. Among genes or genetic markers that are expressed in border cells some depend on slbo, as for example btl that encodes a Drosophila FGF-receptor homologue, while others do not (Murphy et al., 1995). To study interactions between shg and slbo, we analyzed the shg/DE-cadherin expression in slbo mutant follicles (Fig. 9). In anterior polar cells both shg transcript and protein are upregulated in slbo mutants similar to wild-type. This result is not surprising as shg/DE-cadherin expression in polar cells increases before slbo is expressed at stage 8. In rosette cells, on the other hand, transcript and protein concentrations remain at the same level as in the follicular epithelium (Fig. 9, A–D) whereas in wild-type a dramatic increase in shg expression is seen as described above. In contrast, Crumbs expression in slbo mutant follicles is elevated in all border cells as in wild-type (data not shown). This finding indicates that the upregulation of shg/DE-cadherin expression in rosette cells at the transcriptional level depends on DC/EBP.
Figure 9.
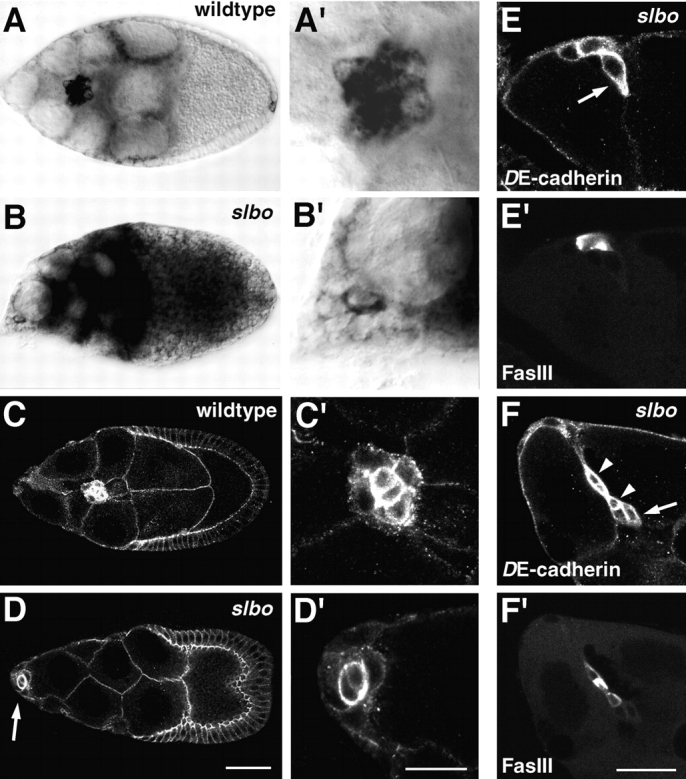
Upregulation of shg expression in border cells depends on slbo. A′–D′ are close-ups of the border cell clusters shown in A–D. (A and A′) shg RNA expression in a wild-type follicle, and (B and B′) a homozygous slbo1 mutant follicle at stage 9 was detected by in situ hybridization using a digoxygenin-labeled shg cDNA probe. The migrating border cell cluster in the wild-type follicle shows high concentration of shg transcript in all border cells. In the slbo mutant follicle the border cell cluster remained at the anterior tip. shg expression in the border cells has not been upregulated, and is much lower than in wild-type. The two polar cells show a higher level of shg expression than the rosette cells (B′). (C and D′) Protein expression as revealed by anti-DE-cadherin staining in a wild-type follicle (C and C′) and a homozygous slbo1 mutant follicle (D and D′) at stage 9. The slbo mutant border cell cluster expresses a much lower level of DE-cadherin than the wild-type cluster. The two polar cells express higher levels of DE-cadherin than the surrounding rosette cells in both genotypes. (E–F′) Double staining of slbo1 mutant follicles with anti-DE-cadherin (E and F) and the polar cell specific marker Fasciclin III (E′ and F′). Migratory activity of border cells (arrows) in stage 10b slbo1 mutant follicles is accompanied by upregulation of DE-cadherin expression. Arrowheads in F point to polar cells. Anterior is to the left in all panels. Bars: (A–D) 50 μm; (A′–D′) 20 μm; (E–F′) 20 μm.
Occasionally, border cell clusters penetrate between nurse cells in slbo mutant follicles at stage 10b or 11. Typically, only a few border cells slide between nurse cells while other cells of the same cluster remain at the periphery of the follicle. The cells that initiate migration show intense staining for DE-cadherin (Fig. 9, E and F). This correlation between DE-cadherin expression level and degree of motility of border cells further supports the hypothesis that DE-cadherin plays a key role in border cell migration.
DE-Cadherin Is Required for Centripetal Cell Migration
The centripetal cells are a second migratory cell population in Drosophila follicles that expresses high levels of DE-cadherin during migration (Fig. 10, A and B). During stage 10b of oogenesis the anterior most columnar follicle cells, the centripetal cells undergo a strong apical-basal cell elongation while penetrating between nurse cells and oocyte. Their leading apical edges that penetrate between the oocyte and the nurse cells are rich in DE-cadherin and F-actin (Fig. 10, A–B′). The centripetal cells migrate along the surface of the oocyte towards the border cells. They make lateral contact with the border cells later during oogenesis to form a confluent epithelium that covers the anterior side of the oocyte.
Figure 10.
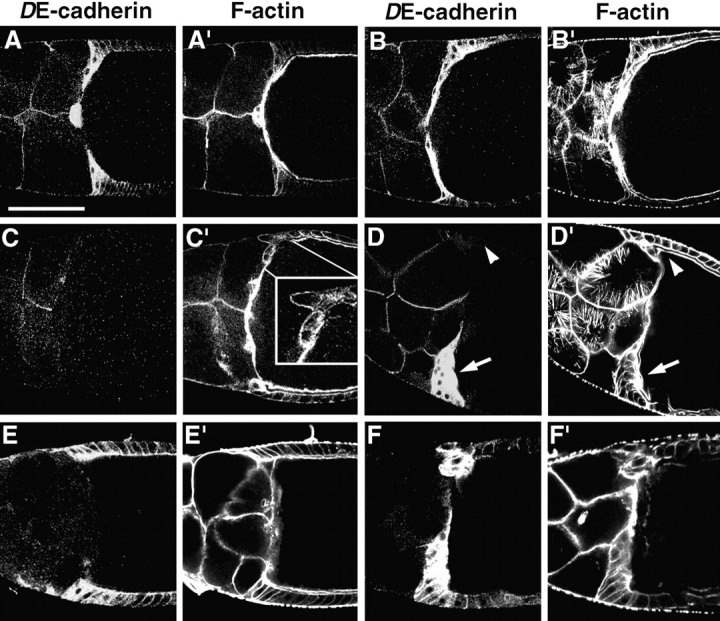
DE-cadherin is required for centripetal cell movement. Follicles are double stained for DE-cadherin (A–F) and F-actin (A′–F′). (A and A′) In an early stage 10b wild-type follicle centripetal cells strongly elongate apical-basally, develop protrusive ends that penetrate between oocyte and nurse cells, and migrate along the surface of the oocyte towards the border cells. Strongest concentration of DE-cadherin is seen in the leading edges of the centripetal cells. (B and B′) At late stage 10b, when cytoplasmic actin fibers have formed in the nurse cells, the invaded centripetal cells form a thin layer covering the anterior surface of the oocyte in a wild-type follicle. (C and C′) Mid-stage 10b follicle with a shgR69 mutant follicle cell clone that comprises all follicle cells. Some shg mutant centripetal cells have segregated from the follicular epithelium but remain in the periphery of the follicle. These cells have a rounded appearance and lack protrusive ends in contrast to wild-type centripetal cells (see inset). (D and D′) The late stage 10b follicle has a shg mutant mosaic follicular epithelium. DE-cadherin negative centripetal cells (arrowhead) show no invasive behavior in contrast to the DE-cadherin positive centripetal cells (arrow) that are elongated and migrated between oocyte and nurse cells. However, the migrating DE-cadherin positive centripetal cells form a thicker layer than in wild-type follicles. (E and E′) Stage 10b follicle with a shg mutant germline. Centripetal cells that show an elevated level of DE-cadherin expression did not move between germline cells. (F and F′) Stage 10b follicle with a shg mutant germline. Centripetal cells moved between oocyte and nurse cells forming clumps of cells with abnormal morphology. Anterior is to the left in all panels. Bar, 100 μm.
To analyze the role of DE-cadherin in the migration of centripetal cells we examined shgR69 mutant follicle cell and germline clones. Centripetal cells showed no or only rudimentary movement in stage 10b follicles that expressed DE-cadherin in the germline but not in the follicle cells (17 follicles examined). A few shg mutant centripetal cells segregate from the follicular epithelium but remain in the periphery of the follicle. These cells neither elongate nor form protrusive ends, and instead take on a rounded shape (Fig. 10, C and C′). Four mosaic follicles were analyzed that contained shg mutant and wild-type centripetal cells. Again, the shg mutant centripetal cells fail to migrate and display a rounded shape, in contrast to the DE-cadherin expressing cells that migrate between nurse cells and oocyte (Fig. 10, D and D′). However, the migrating DE-cadherin positive centripetal cells do not behave completely normal. They display a shorter and bulkier morphology compared with wild-type follicles in which centripetal cells are thin and very elongated (Fig. 10, B and D). This suggests that the migration of centripetal cells is not fully cell-autonomous but depends on a coordinated movement of the centripetal cells. Lack of DE-cadherin in the germline also causes a failure in centripetal cell migration in most follicles (Fig. 10, E–F′). In follicles in which centripetal cells penetrated between germline cells (2 out of 10 germline clones) the morphology of the invading cells was highly abnormal and they formed irregular clusters (Fig. 10, F–F′). In contrast to rosette cells, expression of DE-cadherin in centripetal cells does not depend on slbo (data not shown), consistent with the fact that slbo mutations do not interfere with centripetal cell migration (Montell et al., 1992). Taken together, these results indicate that DE-cadherin mediated contact between centripetal and germline cells is required for centripetal cell migration.
Discussion
The multifunctionality of cadherins complicates the analysis of the role cadherins play in specific morphogenetic processes in vivo. Breakdown of cell or tissue structure might result from a failure of cadherin function in adhesion, linkage of the cytoskeleton to the cell surface, or cell polarization. Lack of cadherin activity resulting in such structural defects is likely to mask cadherin activity in morphogenetic movements that normally take place in these tissues. In this study we have investigated the role of DE-cadherin in two cell migration processes in Drosophila follicles. The border cells and the centripetal cells migrate on the surface of germline cells towards specific targets. The removal of DE-cadherin from the germline cells or from both groups of follicle cells blocks migration demonstrating that DE-cadherin controls intercellular motility in this system.
Formation and Organization of the Border Cell Cluster
The mechanisms that lead to the formation of the border cell cluster are still largely unknown. The anterior and posterior polar cells are apparently specified very early during oogenesis (Margolis and Spradling, 1995) and are morphologically distinct at stage 4. These cells might emit a signal that contributes to the patterning of the terminal follicle cells (Gonzáles-Reyes and St. Johnston, 1998a), and in case of the anterior polar cells to the recruitment of adjacent follicle cells (the future rosette cells) into the border cell cluster. The finding that the level of expression of DE-cadherin and Crumbs (this work) as well as Armadillo (Peifer et al., 1993) is upregulated in follicle cells adjacent to the polar cells is consistent with this model. Thus, the shg and crumbs promoters are presumably targets of a signaling pathway that responds to a putative signal emitted by the polar cells. In fact, there must be two different transcriptional mechanisms that respond to the putative polar cell signal as only DE-cadherin expression but not Crumbs expression requires DC/EBP. DC/EBP is also needed for the expression of the btl FGF receptor, another factor involved in border cell migration, but it is not required for the expression of a variety of other molecular markers expressed in border cells (Murphy et al., 1995) suggesting that DC/EBP might specifically target genes that are required for migration.
Border cell clusters can form in the absence of DE-cadherin suggesting that DE-cadherin is not essential for this process. However, if small DE-cadherin negative follicle cell clones are induced in potential rosette cells, such cells are not recruited into the cluster. This finding suggests that cells with higher levels of DE-cadherin expression are preferentially recruited. We recently found that different DE-cadherin concentrations can promote cell sorting during early oogenesis (Godt and Tepass, 1998). Such a mechanism might ensure that cells with the higher level of DE-cadherin are integrated into the border cell cluster. Ensuring that only those cells that show the highest level of DE-cadherin expression become rosette cells might be important for reaching optimal migration speed as we have shown that a reduction in DE-cadherin expression reduces border cell velocity.
The morphological differentiation and the distribution of markers suggest that the border cell cluster migrates as an epithelial patch that has a two-dimensional organization and that retains its apical basal axis throughout migration. Before migration is initiated, the apical-basal axis of the border cell cluster is oriented parallel to the anterior-posterior axis of the follicle. When the cluster penetrates between nurse cells, it turns by 90° and migrates with its apical-basal axis perpendicular to the anterior-posterior axis of the follicle. As the border cells approach the oocyte a second 90° turn occurs, and the cluster attaches to the oocyte with its apical side. Why epithelial polarity is maintained during migration is not understood. Maintenance of polarity during migration would have the advantage that epithelial polarity does not need to be established de novo when the border cells reach the oocyte. The polar cells are located centrally in the cluster and retain epithelial polarity. Rosette cells surround the polar cells and retain polarity at the contact surface to polar cells but have depolarized their remaining surface area as indicated by the overlapping distribution of an apical marker (Crumbs) and a lateral marker (DE-cadherin). Thus, rosette cells show a mixed cellular morphology that is typical for epithelial cells located at the free edge of an epithelium (e.g., Odland and Ross, 1968; Radice, 1980). The polar cells apparently do not actively migrate but are carried by the rosette cells during the migration process.
Role of DE-Cadherin in Border Cell Migration
We examined a large number of follicles that either had shg mutant follicle cells or a shg mutant germline. In none of these follicles did border cells penetrate or migrate between the nurse cells towards the oocyte. This finding not only strongly suggests that DE-cadherin is the key adhesion molecule that mediates adhesion and traction during border cell migration, but also indicates that no other adhesion system is present that can support border cell migration on the surface of germline cells. In contrast, many other morphogenetic processes are promoted by multiple, at least partially redundant adhesion systems (e.g., Hynes, 1996). Defects in border cell migration have also been observed in arm germline clones (Peifer et al., 1993; Oda et al., 1997) suggesting that border cell migration is mediated by the DE-cadherin–catenin complex. In the absence of DE-cadherin from follicle cells, a border cell cluster of normal size forms that contains two polar cells. Moreover, although DE-cadherin (this work) and Armadillo (Peifer et al., 1993) strongly accumulate at the contact sites between border cells, DE-cadherin appears not to be required for maintaining contact between border cells during migration as shg mosaic border cell clusters migrate as coherent clusters. Taken together, these results suggest that the function of DE-cadherin in border cells might be specific for migration. The clear cut phenotype observed in shg mutant follicles, that in the absence of DE-cadherin border cells cannot use the germline cells as a substratum for migration, makes border cell migration a unique system to study cadherin-based intercellular motility.
The fact that DE-cadherin concentration influences migration speed supports the model that DE-cadherin is the key adhesion receptor that mediates border cell migration. A similar connection has been demonstrated for the role of integrins in cell migration on an extracellular substratum (see Palecek et al., 1997; Huttenlocher et al., 1995 and references therein). A final observation that indicates a direct role for DE-cadherin in border cell migration is that in a few slbo mutant follicles some border cells penetrate between nurse cells. These border cells show high levels of DE-cadherin expression whereas border cells of the same cluster that remained outside of the nurse cells express low levels of DE-cadherin.
Border cells seem to retain a low degree of motility if DE-cadherin is absent from either the germline or the border cells. In particular in follicles containing a shg mutant germline some border cells clusters moved a considerable distance towards the oocyte along the follicular epithelium as we and others (Oda et al., 1997) have observed. This suggests that in the absence of DE-cadherin some aspects of the motility apparatus are still functioning in contrast to slbo mutant follicles in which border cells do not move away from the anterior tip of the follicle (Montell et al., 1992). The higher motility observed in germline clones as compared with follicle cell clones might result from DE-cadherin mediated interactions between border cells and follicular epithelium. That border cells in shg mutant follicles show a low level of motility but move along a different pathway also emphasizes that by allowing the border cells to penetrate between nurse cells, DE-cadherin contributes to the choice of the route of migration taken by the border cells (Oda et al., 1997).
DC/EBP causes a transcriptional upregulation of shg/ DE-cadherin. Whether DC/EBP interacts directly with the shg promoter remains to be determined. In addition to DC/EBP, a number of other factors that might modulate cadherin mediated adhesion were shown to effect border cell migration. For example, activity of the small GTPase Drac1 is required for border cell migration (Murphy and Montell, 1996). This finding is not surprising as the activation of vertebrate rac causes the formation of lamellipodia in fibroblasts. In this process rac regulates actin polymerization and the formation of integrin-based adhesive contacts (Van Aelst and D'Souza-Schorey, 1997; Hall, 1998). rac is also needed for the formation of cadherin based adherens junctions in epithelial cell culture (Braga et al., 1997; Takaishi et al., 1997). Thus, Drac1 might exert its effect on border cell migration at least in part by promoting DE-cadherin mediated adhesion.
A function for the btl FGF receptor in border cell migration was revealed through genetic interactions with slbo. The phenotype of weak slbo mutations is enhanced by btl loss of function mutations and suppressed by overexpression of btl. The function of btl in border cell migration might be redundant, however, as loss of btl in a wild-type background does not interfere with migration (Murphy et al., 1995). In addition, the overexpression of other tyrosine kinase receptors can rescue delays in border cell migration caused by slbo mutations suggesting that the effect of the FGF receptor on border cell migration is mediated by a common downstream target of tyrosine kinase receptors (Murphy et al., 1995). This is supported by the finding that the activity of Dras1 is required for border cell migration. However, Dras1 appears not to act through activation of the MAP kinase pathway to promote migration (Lee et al., 1996). As ras was shown to activate rac (Ridley et al., 1992; Nobes et al., 1995), this raises the intriguing possibility that the btl FGF receptor might modulate DE-cadherin activity during migration via a pathway that involves Dras1 and Drac1. Whether this is a valid model needs to be addressed in future experiments.
Mechanism of Centripetal Cell Migration
The ingrowing edges of centripetal cells are rich in F-actin and Myosin II, and Myosin II mutants are known to block centripetal cell migration (Edwards and Kiehard, 1996). Based on these observations, it was suggested that the circumferential inwards movement of the centripetal cells is driven by a purse string mechanism (Edwards and Kiehard, 1996) similar to epithelial wound healing (Bement et al., 1993) or dorsal closure of the embryonic ectoderm in Drosophila (Young et al., 1993). High levels of DE-cadherin expression in centripetal cells might contribute to a purse string-type movement by providing strong adhesion between centripetal cells. However, our findings are inconsistent with a model in which a purse string mechanism is the only driving force of centripetal cell movement. First, we find that in follicles with a shg mosaic follicular epithelium the DE-cadherin positive centripetal cells migrate although the DE-cadherin negative centripetal cells stay behind. Such a cell-autonomous migratory behavior argues against a purse string mechanism as the latter requires the coordinated movement of all centripetal cells. Second, we find that removal of DE-cadherin from the germline interferes with centripetal cell movement. Adhesion between the germline and the centripetal cells would be expected to counteract but not to promote centripetal cell movement if it is driven by a purse string. On the other hand, in follicles in which a fraction of the centripetal cells does not express DE-cadherin, the migrating DE-cadherin positive centripetal cells are broader and shorter than in wild-type follicles suggesting that their penetration between germline cells might be less efficient. This finding suggests that a coordinated movement of the centripetal cells is required for an orderly migration process.
Taken together, our results suggest that centripetal cell migration is mechanistically similar to border cell migration. In both cases do follicle cells move on the surface of germline cells, express high levels of DE-cadherin, and require DE-cadherin in germ line and soma for migration. Whereas we consider adhesion and traction provided by DE-cadherin as the main mechanism for centripetal cell migration, a purse string-type mechanism might coordinate this migration process. Similar to shg, also Myosin II mutations block both border cell and centripetal cell migration (Edwards and Kiehard, 1996) suggesting that Myosin II cooperates with DE-cadherin in follicle cell motility. The mechanism of this interaction remains to be determined.
Cadherins and Intercellular Motility
Recent genetic studies have emphasized roles of cadherins in specific aspects of animal morphogenesis. The characterization of a cadherin–catenin complex from Caenorhabditis elegans suggests that this complex is required for morphogenetic events that occur in the hypodermis during embryogenesis (Costa et al., 1998). Hypodermal cells in C. elegans migrate ventrally to enclose the body and, later on, undergo cell shape changes that cause an elongation of the body. Cadherin or catenin mutants do not prevent the ventral migration of hypodermal cells but prevent stable adhesion at the ventral midline that causes ventral openings in the hypodermis. Moreover, in cadherin and catenin mutants force generating contractile bundles of microfilaments detach from the cell surface causing cell shape changes to fail during body elongation. A similar role to C. elegans cadherin during body closure was found for DE-cadherin in the fusion of tracheal branches in the Drosophila embryo (Tanaka-Matakatsu et al., 1996). Here, individual cells located at the tip of each branch (fusion cells) migrate, approach each other, and form a continuous lumen after establishing a stable contact. Reduction of DE-cadherin activity does not interfere with the motility of fusion cells. However, fusion cells do not establish stable contacts, and no continuous lumen is formed. Further analysis of DE-cadherin mutants (Tepass et al., 1996; Uemura et al., 1996) and dominant negative and knock out experiments for vertebrate cadherins (e.g., Kintner, 1992; Larue et al., 1994; Levine et al., 1994; Riethmacher et al., 1995; Radice et al., 1997) have uncovered a function for cadherins in the formation and maintenance of various embryonic epithelia. Taken together, these and other genetic studies have revealed important functions for cadherins in a number of morphogenetic processes. However, the analysis of cadherin function in these studies did not reveal a direct role of cadherins in promoting intercellular motility because the observed morphogenetic defects can be explained by a lack of stable adhesion or by defects in cytoarchitecture.
The hypothesis that cadherins mediate intercellular motility, where cells move on the surface of neighboring cells, was supported by two observations (Gumbiner, 1992, 1996). First, the actin cytoskeleton is the main force generating system that promotes cell migration and cell rearrangement movements. Second, cadherin based cellular junctions are major anchor points for actin filaments at cell–cell contact sites. This is similar to the role integrins have in providing anchor points for the actin cytoskeleton at contact sites between cell surface and extracellular matrix. Therefore, cadherins are attractive candidates for mediating cell migration or rearrangement by direct cell to cell contact. Experimental evidence for a potentially more direct role for cadherins in cell motility was revealed in cell culture studies. L-cell fibroblasts transfected with E-cadherin show increased intercellular motility (Nagafushi et al., 1994), and growth cone extension of chick brain neurons is promoted on substrates containing N-cadherin (Bixby and Zhang, 1990). A role for both vertebrate and Drosophila N-cadherin in growth cone motility has recently been supported by in vivo studies (Riehl et al., 1996; Iwai et al., 1997). Moreover, the analysis of Xenopus C-cadherin suggests a role for this cadherin in promoting motility in gastrulating embryos (Brieher and Gumbiner, 1994; Lee and Gumbiner, 1995). Reduction in C-cadherin activity blocks gastrulation movements presumably as a consequence of disrupting the conversion extension movement of the dorsal marginal zone that is driven by motile intercalating cells (Shih and Keller, 1992). Our investigation of the function of DE-cadherin in border cell migration strongly supports the hypothesis that classic cadherins can directly participate in intercellular motility in vivo. Border cell migration is an excellent system for the further analysis of cadherin-based intercellular motility as DE-cadherin appears to be the sole adhesion receptor that promotes border cell migration on the surface of germline cells. Since the border cells are intensely studied as a genetic model for cell migration this system is likely to provide insights into function and regulation of cadherin based motility in the near future.
Acknowledgments
We thank Tadashi Uemura, Denise Montell, Mark Peifer, and Pernille Rørth for providing reagents, and the Developmental Studies Hybridoma Bank for the 7G10 antibody. We thank Isabella Stüttem for critical reading of the manuscript.
Abbreviations used in this paper: arm
armadillo
- btl
breathless
- DE-cadherin
Drosophila E-cadherin
- DC/EBP
Drosophila CCAAT/enhancer binding protein
- FGF
fibroblast growth factor
- shg
shotgun
- slbo
slow border cells
Footnotes
This research was supported by a grant to U. Tepass by the National Cancer Institute of Canada with funds from the Terry Fox run.
The first two authors contributed equally to this work.
References
- Bellen HJ, O'Kane CJ, Wilson C, Grossniklaus U, Pearson RK, Gehring WJ. P-element-mediated enhancer detection: a versatile method to study development in Drosophila. . Genes Dev. 1989;3:1288–1300. doi: 10.1101/gad.3.9.1288. [DOI] [PubMed] [Google Scholar]
- Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol. 1993;121:565–578. doi: 10.1083/jcb.121.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier W. E-cadherin as a tumor (invasion) suppressor gene. BioEssays. 1995;17:97–99. doi: 10.1002/bies.950170203. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher WM, Gumbiner BM. Regulation of C-cadherin function during activin induced morphogenesis of Xenopusanimal caps. J Cell Biol. 1994;126:519–527. doi: 10.1083/jcb.126.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower DL, Smith RL, Wilcox M. A monoclonal antibody specific for diploid epithelial cells in Drosophila. . Nature. 1980;285:403–405. doi: 10.1038/285403a0. [DOI] [PubMed] [Google Scholar]
- Burdsal CA, Damsky CH, Petersen RA. The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development. 1993;118:829–844. doi: 10.1242/dev.118.3.829. [DOI] [PubMed] [Google Scholar]
- Cooley L, Theurkauf WE. Cytoskeletal functions during Drosophilaoogenesis. Science. 1994;266:590–595. doi: 10.1126/science.7939713. [DOI] [PubMed] [Google Scholar]
- Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegansembryo. J Cell Biol. 1998;141:297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Eaton S, Simons K. Apical, basal, and lateral cues for epithelial polarization. Cell. 1995;82:5–8. doi: 10.1016/0092-8674(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Edwards KA, Kiehart DP. Drosophilanonmuscle myosin II has multiple essential roles in imaginal disc and egg chamber morphogenesis. Development. 1996;122:1499–1511. doi: 10.1242/dev.122.5.1499. [DOI] [PubMed] [Google Scholar]
- Edwards KA, Demsky M, Montague RA, Weymouth N, Kiehart DP. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. . Dev Biol. 1997;191:103–117. doi: 10.1006/dbio.1997.8707. [DOI] [PubMed] [Google Scholar]
- Godt D, Tepass U. Drosophilaoocyte localization is mediated by differential cadherin-based adhesion. Nature. 1998;395:387–391. doi: 10.1038/26493. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. . Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- González-Reyes A, St. Johnston D. Role of oocyte position in establishment of anterior-posterior polarity in Drosophila. . Science. 1994;266:639–642. doi: 10.1126/science.7939717. [DOI] [PubMed] [Google Scholar]
- González-Reyes A, St. Johnston D. Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophilaoogenesis. Development. 1998a;125:2837–2846. doi: 10.1242/dev.125.15.2837. [DOI] [PubMed] [Google Scholar]
- González-Reyes A, St. Johnston D. The DrosophilaAP axis is polarized by the cadherin-mediated positioning of the oocyte. Development. 1998b;125:3635–3644. doi: 10.1242/dev.125.18.3635. [DOI] [PubMed] [Google Scholar]
- Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familiar gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Epithelial morphogenesis. Cell. 1992;69:385–387. doi: 10.1016/0092-8674(92)90440-n. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992;70:293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Targeted mutations in cell adhesion genes: what have we learned from them? . Dev Biol. 1996;180:402–412. doi: 10.1006/dbio.1996.0314. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophilaembryonic CNS. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- King, R.C. 1970. Ovarian Development in Drosophila melanogaster. Academic Press, New York.
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectodermal epithelium. Proc Natl Acad Sci USA. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lee C-H, Gumbiner BM. Disruption of gastrulation movements in Xenopusby a dominant-negative mutant for C-cadherin. Dev Biol. 1995;171:363–373. doi: 10.1006/dbio.1995.1288. [DOI] [PubMed] [Google Scholar]
- Lee T, Feig L, Montell DJ. Two distinct roles for Ras in a developmentally regulated cell migration. Development. 1996;122:409–418. doi: 10.1242/dev.122.2.409. [DOI] [PubMed] [Google Scholar]
- Levine E, Lee CH, Kintner C, Gumbiner BM. Selective disruption of E-cadherin function in early Xenopusembryos by a dominant negative mutant. Development. 1994;120:901–909. doi: 10.1242/dev.120.4.901. [DOI] [PubMed] [Google Scholar]
- Margolis J, Spradling A. Identification and behavior of epithelial stem cells in the Drosophilaovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- McNeill H, Ozawa M, Kemler R, Nelson WJ. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990;62:309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- Montell DJ, Rørth P, Spradling AC. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes DrosophilaC/EBP. Cell. 1992;71:51–62. doi: 10.1016/0092-8674(92)90265-e. [DOI] [PubMed] [Google Scholar]
- Montell DJ. Moving right along: regulation of cell migration during Drosophiladevelopment. Trends Genet. 1994;10:59–62. doi: 10.1016/0168-9525(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Murphy AM, Montell DJ. Cell type-specific roles for cdc42, Rac, and RhoL in Drosophilaoogenesis. J Cell Biol. 1996;133:617–630. doi: 10.1083/jcb.133.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AM, Lee T, Andrews CM, Shilo B-Z, Montell DJ. The breathless FGF receptor homolog, a downstream target of DrosophilaC/EBP in the developmental control of cell migration. Development. 1995;121:2255–2263. doi: 10.1242/dev.121.8.2255. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin–α-catenin fusion molecules. J Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Oda H, Uemura T, Takeichi M. Phenotypic analysis of null mutants of DE-cadherin and Armadillo in Drosophilaovaries reveals distinct aspects of their functions in cell adhesion and cytoskeletal organization. Genes Cells. 1997;2:29–40. doi: 10.1046/j.1365-2443.1997.d01-284.x. [DOI] [PubMed] [Google Scholar]
- Oda H, Uemura T, Shiomi T, Nagafuchi A, Tsukita S, Takeichi M. Identification of a Drosophilahomologue of α-catenin and its association with the armadillo protein. J Cell Biol. 1993;121:1133–1140. doi: 10.1083/jcb.121.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophilahomolog of cadherin associated with Armadillo and essential for embryonic cell–cell adhesion. Dev Biol. 1994;165:716–726. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- Odland G, Ross R. Human wound repair. I. Epidermal regeneration. J Cell Biol. 1968;39:135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Patel NH, Snow PM, Goodman CS. Characterization and cloning of Fasciclin III: A glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. . Cell. 1987;48:975–988. doi: 10.1016/0092-8674(87)90706-9. [DOI] [PubMed] [Google Scholar]
- Peifer M, Sweeton D, Casey M, Wieschaus E. Winglesssignal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development. 1994;120:369–380. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- Peifer M. The product of the Drosophila segment polarity gene armadillois part of a protein complex resembling the vertebrate adherens junction. J Cell Sci. 1993;105:993–1000. doi: 10.1242/jcs.105.4.993. [DOI] [PubMed] [Google Scholar]
- Peifer M, Orsulic S, Sweeton D, Wieschaus E. A role for the Drosophila segment polarity gene armadilloin cell adhesion and cytoskeletal integrity during oogenesis. Development. 1993;118:1191–1207. doi: 10.1242/dev.118.4.1191. [DOI] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knunden KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Radice GP. The spreading of epithelial cells during wound closure in Xenopuslarvae. Dev Biol. 1980;76:26–46. doi: 10.1016/0012-1606(80)90360-7. [DOI] [PubMed] [Google Scholar]
- Ray R, Schüpbach T. Intercellular signaling and the polarization of body axes during Drosophilaoogenesis. Genes Dev. 1996;10:1711–1723. doi: 10.1101/gad.10.14.1711. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Riehl R, Johnston K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Brinkmann V, Birchmeier C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci USA. 1995;92:855–859. doi: 10.1073/pnas.92.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J, Keller R. Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis. . Development. 1992;116:915–930. doi: 10.1242/dev.116.4.915. [DOI] [PubMed] [Google Scholar]
- Spradling, A.C. 1993. Developmental genetics of oogenesis. In The Development of Drosophila melanogaster. M. Bate and A. Martinez-Arias, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 1–70.
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell–cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Tanaka-Matakatsu M, Uemura T, Oda H, Takeichi M, Hayashi S. Cadherin-mediated cell adhesion and cell motility in Drosophilatrachea regulated by the transcription factor Escargot. Development. 1996;122:3697–3705. doi: 10.1242/dev.122.12.3697. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals a translational control of the segmentation gene hunchback. . Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophilaepithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- Tepass U, Knust E. Phenotypic and developmental analysis of mutations at the crumbs locus, a gene required for the development of epithelia in Drosophila melanogaster. . Roux's Arch Dev Biol. 1990;199:189–206. doi: 10.1007/BF01682078. [DOI] [PubMed] [Google Scholar]
- Tepass U, Knust E. crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. . Dev Biol. 1993;159:311–326. doi: 10.1006/dbio.1993.1243. [DOI] [PubMed] [Google Scholar]
- Tepass U, Gruszynski-de Feo E, Haag TA, Omatyar L, Török T, Hartenstein V. shotgun encodes DrosophilaE-cadherin and is preferentially required during cell rearrangement in the neuroectoderm and other morphogenetically active epithelia. Genes Dev. 1996;10:672–685. doi: 10.1101/gad.10.6.672. [DOI] [PubMed] [Google Scholar]
- Uemura T, Oda H, Kraut R, Hayashi S, Takeichi M. Processes of dynamic epithelial cell rearrangement are the major targets of cadE/shotgun mutations in the Drosophilaembryo. Genes Dev. 1996;10:659–671. doi: 10.1101/gad.10.6.659. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- White P, Aberle H, Vincent J-P. Signaling and adhesion activities of mammalian β-catenin and plakoglobin in Drosophila. . J Cell Biol. 1998;140:183–195. doi: 10.1083/jcb.140.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, T., and S.D. Harrison. 1994. Mosaic analysis using FLP recombinase. In Drosophila melanogaster: Practical Uses in Cell and Molecular Biology. L.S.B. Goldstein and E.A. Fyrberg, editors. Academic Press, San Diego. 655–682. [DOI] [PubMed]
- Young PE, Richman AM, Ketchum AS, Kiehart DP. Morphogenesis in Drosophilarequires myosin heavy chain function. Genes Dev. 1993;7:29–41. doi: 10.1101/gad.7.1.29. [DOI] [PubMed] [Google Scholar]