Abstract
Laminin 5 (α3β3γ2) distribution in the human thymus was investigated by immunofluorescence on frozen sections with anti-α3, -β3, and -γ2 mAbs. In addition to a linear staining of subcapsular basal laminae, the three mAbs give a disperse staining in the parenchyma restricted to the medullary area on a subset of stellate epithelial cells and vessel structures. We also found that laminin 5 may influence mature human thymocyte expansion; while bulk laminin and laminin 2, when cross-linked, are comitogenic with a TCR signal, cross-linked laminin 5 has no effect. By contrast, soluble laminin 5 inhibits thymocyte proliferation induced by a TCR signal. This is accompanied by a particular pattern of inhibition of early tyrosine kinases, including Zap 70 and p59fyn inhibition, but not overall inhibition of p56lck. Using a mAb specific for α6β4 integrins, we observed that while α3β1 are known to be uniformly present on all thymocytes, α6β4 expression parallels thymocyte maturation; thus a correspondence exists between laminin 5 in the thymic medulla and α6β4 on mature thymocytes. Moreover, the soluble Ab against α6β4 inhibits thymocyte proliferation and reproduces the same pattern of tyrosine kinase phosphorylation suggesting that α6β4 is involved in laminin 5–induced modulation of T cell activation.
Keywords: laminin 5, thymus, T cell, integrins, cellular activation
Laminins are the major constituents of basal laminae; together with other extracellular matrix components (ECM)1 they contribute, by binding cell surface receptors such as integrins, to maintain epithelial tissue integrity (3, 38, 76). In addition to their mechanical role, they are important signaling molecules with the ability to strongly influence cellular programs, promoting differentiation and migration, proliferation and activation (13, 39, 40). Thus, they are influential molecules in the development (1, 59, 62) and repair (29, 30, 53) of many tissues.
Laminins are heterotrimers where the α chain is critical for cell/matrix interactions (37). Among laminin isoforms (4, 67), laminin 5 (epiligrin, kalinin, nicein) is somewhat unique and is preferentially found in basement membranes underlying squamous and transitional epithelia (4). From a structural viewpoint, the molecule displays a particular chain composition (α3β3γ2) and has been characterized as a 105-nm rod-like molecule from the conditioned medium of normal human keratinocytes (49). Studies performed with extracts of human amnion revealed that, in addition to monomeric molecules, much of the laminin 5 isolated is covalently associated with laminin 6 (α3β1γ1) and 7 (α3β2γ1) (8). Whether these complexes are located in other basement membranes remains to be shown. Laminin 5 has the ability to bind the NH2-terminal domain of type VII collagen and is believed to strongly connect cells to anchoring fibrils in skin (51). Laminin 5 has been identified as a ligand for the integrins α3β1 (7, 48) and α6β4 (44, 60). From a functional viewpoint, laminin 5 plays a major mechanical role in maintaining the basal layer of epithelia (1, 3, 6, 7, 14, 15, 23, 49, 51, 52), and it is also increased in the early stages of wound repair (30, 53). Furthermore, accumulated evidence suggests that laminin 5 is a signaling molecule that is particularly involved in controlling cell migration and tumor cell expansion (16, 26, 27, 39, 46).
The thymus can be regarded as a mesh of epithelial cells surrounding maturing T cells; thus, ECM components and integrins displayed by thymocytes appear to strongly influence T cell differentiation/maturation (5, 10, 12, 20, 31, 43, 54–56, 69, 73, 74). Mice deficient in merosin, for example, suffer from abnormal T cell development (31). Since the presence of laminin 5 has been found within the human thymus (25, 42, 72), this work was undertaken to document expression and functional influence of laminin 5 and its cellular receptors during thymocyte maturation. We show here that laminin 5 displays a peculiar tissue distribution in the human thymus. In addition, we show that laminin 5, in soluble form, provides mature thymocytes with an inhibitory signal upon stimulation via the CD3–TCR complex which can be reproduced by an anti-α6 mAb specific for α6β4. We have already shown that ligands to β1 integrins, when used in soluble form, inhibit T cell activation triggered via the CD3–TCR complex (19, 34, 65). We now report that α6β4 integrins can also trigger a similar effect after ligation with soluble laminin 5.
Materials and Methods
Materials
Human laminin 5 was obtained by immunopurification of the conditioned medium of SCC25 cells (squamous cell carcinoma) using the 6F12 mAb coupled to CNBr-activated Sepharose 4B (Pharmacia Fine Chemicals). Before this step, medium was passed sequentially over 25 ml of gelatin– Sepharose (Pharmacia Fine Chemicals) in order to remove fibronectin. Molecular mass of the eluted laminin 5 was analyzed by SDS-PAGE and revealed the presence of the trimer α3β3γ2 only. Purity of the molecule preparation was checked by immunoblotting using a panel of several monoclonal and polyclonal antibodies as previously described (48, 50). Laminin 2 was obtained from Chemicon. Human fibronectin, protein A–Sepharose CL 4B, PMSF, leupeptin, aprotinin, NP-40, and BSA were all purchased from Sigma Chemical Co.; human IL-2 was from R&D Systems Inc. Chemicals for PAGE were from Bio-Rad Laboratories. Antifading mounting medium Citifluor was obtained from Citifluor Chemical Laboratory. [3H]thymidine and 125I were obtained from Commisariat á l'Energie Atomique. ECL reagents were purchased from Amersham.
Antibodies
The BM165 mAb (mouse IgG1), specific for α3 chain of human laminin 5 was produced as previously described (49). The 6F12 mAb, specific for β3 chain of human laminin 5, was produced as previously described (33). The GB3 mAb (70) specific for γ2 chain of human laminin 5, was from Accurate and Specific Corporation. The human CD49f (α6 integrin chain) mAb S3-41 (mouse, IgG1) was produced as previously described (24). The human CD49f mAb GOH3 (rat IgG1; 61) was obtained from Immunotech. The human CD49f mAbs 135 13C (rat IgG2a), J8H (mouse IgG1), BQ16 (mouse IgG1), and 450 30A1 (mouse IgG1) were obtained from the 5th International Workshop of Leukocyte Typing. The CD49c mAb (α3 integrin chain) P1B5 was obtained from Becton Dickinson. Human CD104 (β4 integrin chain) mAb 3E1F6 was a gift from Dr. Kennel (Oak Ridge National Laboratory, Oak Ridge, Tennessee). Anti-p67 high affinity non-integrin laminin receptor mAb MPLR2 was provided by Dr. Colnaghi (Istituto Nazionale Tumori, Milan, Italy) as ascites fluid. CD28 mAb 9.3 (mouse IgG2a) was kindly provided by Dr. Ledbetter (Pharmaceutic Research Institute, Bristol Myers Squibb, Seattle, WA). Human CD29 (β1 integrin chain) mAb K20 (mouse IgG2a), CD3 mAb X3 (mouse IgG2a), and CD2 mAbs GT2 (mouse IgG1) and D66 (mouse IgM) were generated in our laboratory. The anti–Zap 70 rabbit antibody was directed against amino acids 485 to 499 of the human ZAP 70 sequence as previously described (68). Rabbit anti-human p56lck and anti-p59fyn Abs and peroxidase (HRP) conjugated anti-phosphotyrosine (4G10) were obtained from Upstate Biotechnology Inc. The goat anti–mouse, anti–rat, or anti– rabbit antibodies, alone or coupled with fluorochromes or peroxidase, as well as irrelevant mouse IgG1, were purchased from Dakopatts.
Cell Culture and Tissue
Normal thymi were obtained from children (<4 yr) undergoing cardiac surgery. Thymocytes were prepared by physical disruption of the tissue and washes in RPMI/10% FCS. Jurkat (T lymphoma), HT29 (human colon carcinoma), and K562 (myelogenous leukemia) cell lines were obtained from the American Type Culture Collection.
Thymocytes, human cell lines Jurkat and K562 were cultured in RPMI 1640 (GIBCO BRL) supplemented with 10% FCS. Human colon carcinoma cell line HT29 was cultured in DME (GIBCO BRL) 5% FCS. All media were supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM l-glutamine, and 1 mM pyruvate (Merck).
Immunohistochemistry
For immunohistochemistry, specimens of human thymi were fragmented, immediately frozen in liquid nitrogen, and stored at −70°C.
Frozen sections (4 μm thick) were processed for indirect immunofluorescence, using a single label fluorescein technique. Air-dried cryostat sections were incubated for 30 min with the primary antibody diluted in PBS, at room temperature. Antibody binding was then detected by incubating the sections with FITC-conjugated rat anti–mouse secondary antibody (F(ab)′2) fragments (Dakopatts), diluted 1:50 in PBS, for 30 min at room temperature. Cell nuclei were labeled with 0.5% propidium iodide (Sigma Chemical Co.). After final washing in PBS, the sections were mounted in Citifluor (Plano) and examined with a laser scanning confocal microscope (Ultima Meridian; DGL Bioscience). Indirect double staining of thymic epithelial cells was performed with the anti-laminin 5 mAb BM165 (mouse IgG1) and the anti-keratin mAb CK19 (mouse IgM) using isotype-specific secondary antibodies, i.e., FITC-conjugated goat anti–mouse IgM, obtained from Chemicon, and rabbit anti–mouse IgG1 (Dakopatts) revealed with phycoerythrin (PE)-conjugated goat anti–rabbit Ig (Dakopatts). After final washing in PBS, sections were mounted in Citifluor (Plano) and examined with a laser scanning confocal microscope (Ultima Meridian; DGL Bioscience).
Proliferation Assays
Cell proliferation assays were performed in triplicate in 96-well culture plates (Nunc). Thymocytes at 105 cells/well were cultured in 0.2 ml of RPMI 1640 with 10% FCS. Coating of the CD3 mAb was carried out by overnight incubation at 4°C with CD3 mAb (×3, 10 μg/ml) diluted in PBS. Each well was then washed three times with PBS. To saturate the plastic before using soluble reagents, RPMI with 10% FCS was added for 2 h, then the wells were washed three more times with PBS. Cells were added in culture medium containing soluble laminins or Abs before the addition of rIL-2 (5 ng/ml).
For costimulation assays, CD3 mAb was coimmobilized with another mAb or with an ECM. A first overnight incubation at 4°C was carried out with an ECM component (laminin 5, laminin 2, or fibronectin) or mAbs diluted in PBS followed, after washing, by immobilization of CD3 mAb.
Cells were incubated at 37°C in a 5% CO2 humidified atmosphere for 4 d, and 1 μCi of [3H]thymidine (2 Ci/mmol; CEA) was added for the final 18 h. Cells were harvested onto filter paper using a semiautomatic cell harvester (Skatron) and thymidine incorporation was measured in a liquid scintillation counter (Beckman Instruments Inc.).
Apoptosis Assays
Apoptosis was determined by incubating cells previously cultivated as described above with Hoechst 33342 (Interchim), 30 min at 37°C. Results were analyzed on a FACStar® cytometer (Becton Dickinson).
Immunofluorescence Analysis of Isolated Cells
Cells were washed three times with PBS and incubated at 4°C for 30 min in the dark in 100 μl PBS, 0.1% NaN3, and 0.1% BSA with saturating concentration of mAbs, washed three times and resuspended at a density of 3 × 106 cells/ml. Indirect labeling was performed by adding goat anti– mouse PE-conjugated F(ab)′2 fragments (Dako). This reagent also binds to rat IgG. Double staining was performed using indirect labeling with a primary mAb revealed with anti–mouse (PE) conjugated F(ab)′2 fragments (Dako) followed, after washes, by staining with (FITC) conjugated CD3. Controls included cells incubated with mouse IgG of the relevant subclasses. Analysis was performed on a FACScan® (Becton Dickinson).
Radioiodination of Cells, Immunoprecipitation, and Immunoblot Analysis
Surface labeling of cells with 125I (1 mCi; Amersham) was performed by the lactoperoxidase method, as previously described (11). For immunoprecipitation, cells were washed three times, and lysed in ice-cold lysis buffer (stop buffer with 1% NP-40, 10 μg/ml leupeptin, 1 mM PMSF, and 1,000 U/ml aprotinin) for 30 min on ice. Immunoprecipitation with indicated mAbs (10 μg) adsorbed on protein A–Sepharose 3 h at 4°C under agitation was then performed on precleared lysates. Sepharose-bound immune complexes were washed three times in TNN buffer (50 mM Tris [pH 7.8], 250 mM NaCl, 0.1% NP-40), then eluted into reduced Laemmli buffer. Immunoprecipitated proteins were analyzed by SDS-PAGE on a 7.5% gel and revelation was performed by autoradiography. Unlabeled immunoprecipitated proteins were analyzed by SDS-PAGE on a 7.5% gel and then electroblotted onto Immobilon P membrane (Millipore). The blotted membrane was saturated overnight at 4°C in a buffer containing 5% BSA, 100 mM Tris, 1.4 M NaCl, pH 7.4, then incubated overnight with the anti-α6 mAb S3-41. Immunolabeling was revealed by chemiluminescence (ECL) after reaction with goat anti–mouse immunoglobulins in conjunction with HRP.
Anti-Phosphotyrosine Immunoblot Analysis
Cells at 20 × 106/ml in RPMI 5% FCS were incubated with Abs at 37°C for various time intervals. Activation was stopped by addition of 1 ml of ice-cold stop buffer (20 mM Hepes [pH 7.4], 150 mM NaCl, 100 mM NaF, 10 mM EDTA, 10 mM Na4P2O7, and 2 mM Na3VO4). Cells were spun down and the pellets were resuspended in lysis buffer (stop buffer with 1% NP-40, 10 μg/ml leupeptin, 1 mM PMSF, and 1,000 U/ml aprotinin) and incubated on ice for 30 min. After centrifugation (15,000 g for 15 min) supernatants were incubated with Abs for 4 h at 4°C with shaking, followed by 1 h with RAM adsorbed on protein A–Sepharose. Pellets were washed four times with lysis buffer containing 1% NP-40, then once in stop buffer without detergent before mixing with Laemmli buffer containing 3% (final volume) SDS. For reduction, the loading buffer contained 0.75 M 2 ME. Proteins were analyzed by SDS-PAGE, then electroblotted onto Immobilon P membrane (Millipore). The blots were saturated overnight at 4°C in a buffer containing 5% BSA, 100 mM Tris, 1.4 M NaCl, pH 7.4, then incubated overnight with the appropriate antibodies (HRP conjugated anti-phosphotyrosine mAb [4G10], pAb anti-lck, pAb anti-fyn, or pAb anti–Zap 70). For anti-lck, anti-fyn, and anti–Zap 70 immunoblots, membranes were washed and incubated 1 h with a HRP-conjugated goat anti–rabbit antibody. After washing, the immunolabeling was revealed by ECL (enhanced chemiluminescence) analysis system. HRP-conjugated anti-phosphotyrosine Ab was also used followed by ECL. For reprobing, the membranes were submerged in stripping buffer (100 mM 2 NE, 2% SDS, 62.5 mM Tris-HCl, pH 6.7) and incubated at 50°C for 30 min. After washing, the membrane was blocked and immunodetection was performed as described.
Results
Immunohistolocalization of Laminin 5 on Post-natal Human Thymus Sections
We investigated laminin 5 (α3β3γ2) expression in the human thymus by immunofluorescence, using mAbs specific for the α3 chain (BM165 [49]; Fig. 1, a and b), for the β3 chain (6F12 [33]; Fig. 1 c), or for the γ2 chain (GB3, [35]; Fig. 1 d). Staining of laminin 5 was performed together with a nuclear counter-staining with propidium iodide (Fig. 1, a–d; Fig. 2, a and b) in order to recognize the cortical (C) and the medullary (M) regions. All three mAbs stained strongly the medullary area of parenchyma and the basal laminae from subcapsular cortex, consistent with the presence of laminin 5 in these areas. More precisely, laminin 5 was localized in stellate keratin positive epithelial cells (Fig. 2, c and d) and in the basal laminae of vessels, including small vessels and capillary structures, together with endothelial cells of the larger vessels (Fig. 1 b, arrows; Fig. 2, a and b). Of note, a more intense staining at the corticomedullary junction was only visible with the anti-α3 mAb BM165, suggesting that laminin 6 (α3β1γ1) and/or 7 (α3β2γ1) could be associated with laminin 5 in this area. As to the cortex, mAbs stained very scattered structures that corresponded for the most part to vascular structures.
Figure 1.
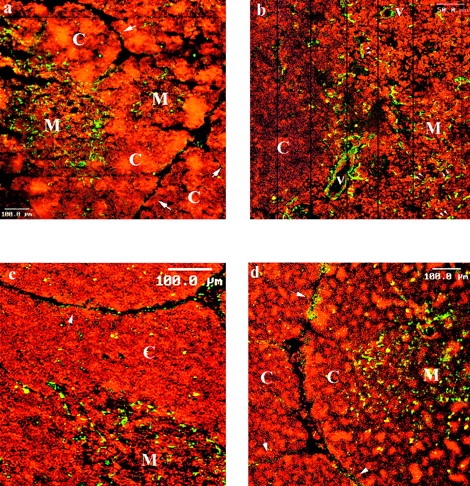
Immunofluorescence on frozen human thymus section was performed with anti-laminin 5 chain mAbs: the anti-α3 chain BM165 (a and b), the anti-β3 chain 6F12 (c); the anti-γ2 chain GB3 (d). mAb staining was revealed by FITC conjugated goat anti–mouse F(ab)′2 fragments and analysis was performed by laser scanning confocal microscopy. Variations of cellular density between cortex (C) and medulla (M), were revealed by cell nuclear staining with propidium iodide. Single arrows designate the subcapsular basal laminae. Double arrows show examples of laminin 5 positive epithelial cells. b is a view constructed by the juxtaposition of successive areas scanned at a larger magnification.
Figure 2.
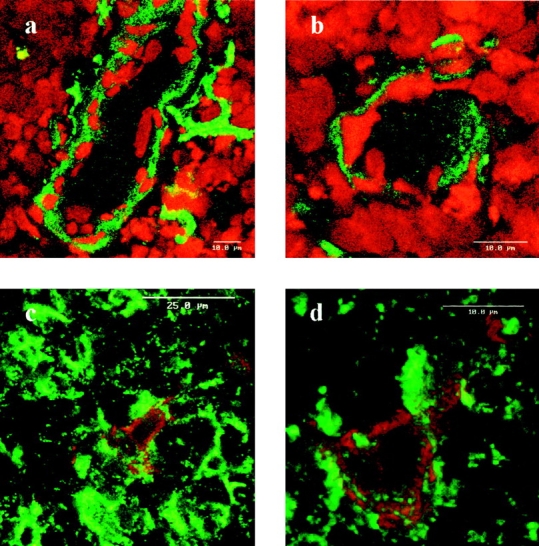
Frozen human thymus section stained with anti-laminin 5 α3 chain mAb BM165. (a and b) BM165 staining on vessels; staining was revealed by FITC conjugated goat anti–mouse F(ab)′2 fragments and analysis was performed by laser scanning confocal microscopy. Cell nuclei were stained with propidium iodide. (c and d) BM165 staining on stellate thymic epithelial cells; double staining obtained with mAb BM165 revealed by rabbit anti– mouse IgG1 F(ab)′2 fragments followed by PE conjugated goat anti–rabbit F(ab)′2 fragments, and the anti-keratin CK19 revealed by FITC conjugated goat anti–mouse IgM Ab. Controls made with secondary Abs gave no staining (not shown).
Laminin 5 and Laminin 2 Have Distinct Effects on Thymocyte Proliferation: The Soluble Form of Laminin 5 Inhibits Proliferation of Human Thymocytes Stimulated via the CD3–TCR Complex
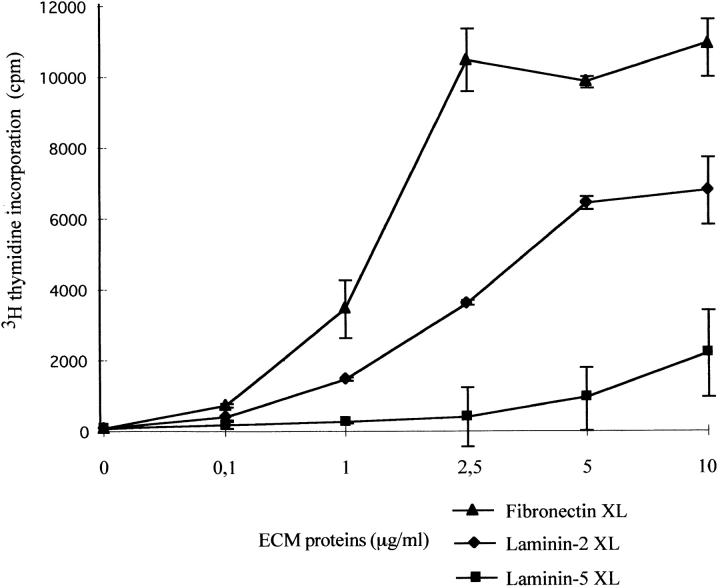
Since laminins were shown to influence the functional program of numerous cell types, and in particular bulk preparations of laminins were shown to deliver coactivation signals to T cells (10, 58, 66), we investigated the effects of purified laminin 5 on thymocytes. When we coimmobilized laminin 5 on plastic, this induced a weak costimulation with a CD3–TCR signal, as compared with coated fibronectin or laminin 2 (α2β1γ1), another laminin isoform found in the thymus (10; Fig. 3). We then investigated the effect of laminin 5 and laminin 2 presented in soluble form; soluble laminin 5 clearly inhibited the proliferation of thymocytes stimulated via the CD3–TCR (Fig. 4 a). The inhibition was dose-dependent reaching a maximum of 50%, in terms of [3H]thymidine incorporation, at 5 μg/ml. Over six independent experiments we saw a quite significant inhibition down to 1 μg/ml (∼30%). We checked the specificity of laminin 5–induced inhibition by adding the anti-α3 chain mAb BM165, which recognizes an epitope involved in the interaction of laminin 5 with cells. BM165 abolished the inhibiting effect observed with soluble laminin 5 alone (Fig. 4 a). Conversely, no inhibition was seen when we added, instead of laminin 5, increasing amounts of soluble human laminin 2 (Fig. 4 a).
Figure 3.
Comparison of costimulating effects of fibronectin, laminin 2 and laminin 5 when presented in cross-linked form to thymocytes. Indicated amounts of purified ECM components were first added to culture wells. After overnight incubation at 4°C, ECM solutions were discarded and wells were washed. CD3 mAb was next incubated in the culture wells for an additional 12 h at 4°C. After further washes, cells were added (105/well) to the culture wells and incubated for 4 d with addition of [3H]thymidine for the final 18 h.
Figure 4.
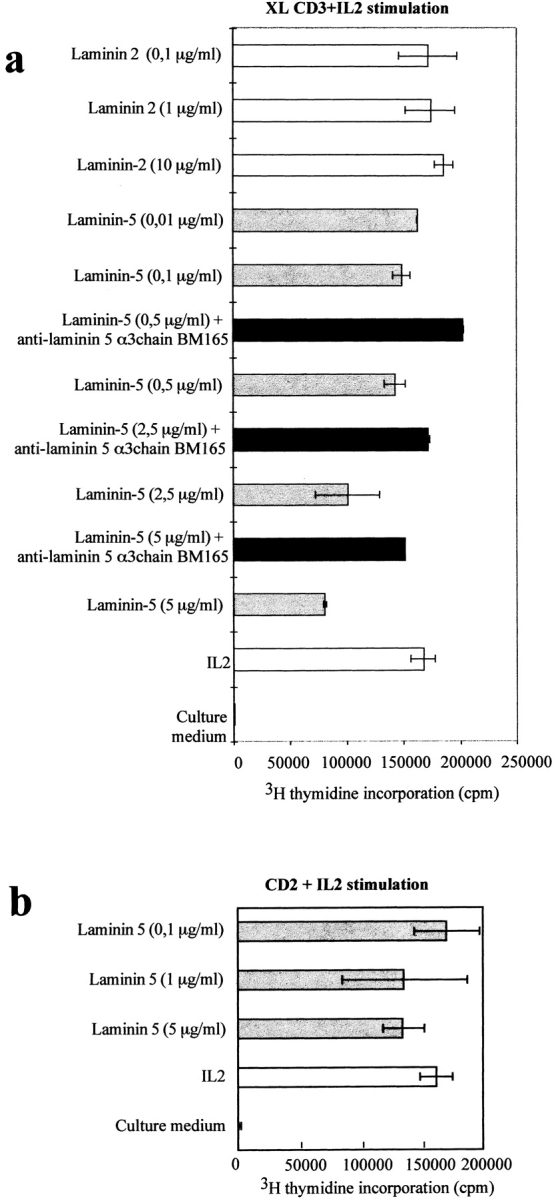
Laminin 5 but not laminin 2, when added in soluble form, inhibits human thymocyte proliferation induced by CD3 + IL-2. (a) CD3 antibodies were first incubated overnight in culture wells. Next, 10% FCS in RPMI was added and incubated in order to minimize further protein adsorption on plastic. After washing, cells (105/well) were added in culture medium containing IL-2 (5 ng/ml) and laminin 5 or laminin 2. Cultures were also performed in the presence of 10 μg/ml anti-laminin 5 mAb BM165. (b) Effect of soluble laminin 5 on thymocyte proliferation induced by a mitogenic CD2 mAb pair (GT2 + D66, 10 μg/ml) plus IL-2.
Inhibition was seen only when T cells were stimulated via the CD3–TCR; we observed no inhibition when thymocytes were induced to proliferate via a mitogenic pair of CD2 mAbs (Fig. 4 b). We verified that inhibition of proliferation was not related to apoptosis by incubating cells with Hoechst 342 30 min at 37°C. Results analyzed on a FACStar® cytometer (Becton Dickinson) did not reveal any sign of apoptosis (not shown).
Inhibition by Soluble Laminin 5 of Early Kinase Phosphorylations Activated via the CD3–TCR Complex
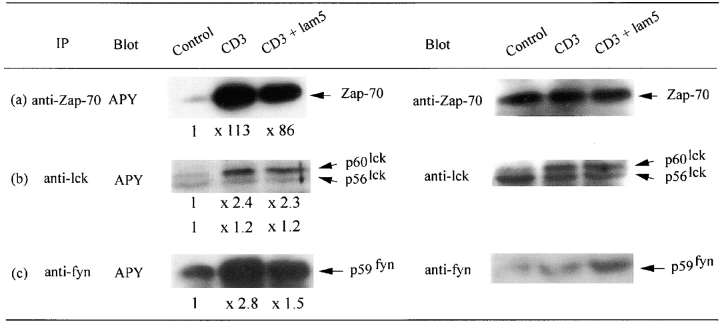
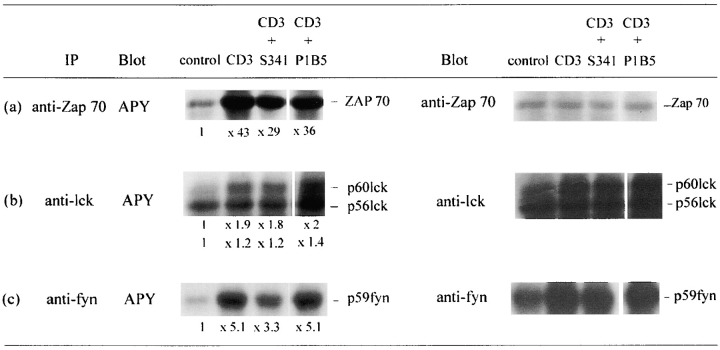
We tested soluble laminin 5 effects on early kinase phosphorylation events. Since Jurkat cells express a low density of α6β4 we selected by cytofluorometry cells expressing α6β4 in similar density as CD3high thymocytes (not shown). These cells were activated with a CD3 mAb (×3, 10 μg/ml) during 2.5 min at 37°C, with or without soluble laminin 5. Immunoprecipitation of Zap 70, p56lck, or p59fyn was carried out on activated cell lysates, after which precipitated proteins were transferred on Immobilon. Membranes were then incubated with HRP conjugated anti-phosphotyrosine antibody. Results were revealed by ECL system. We observed that the addition of 1 μg/ml of soluble laminin 5 was sufficient to induce an inhibition of Zap 70 (Fig. 5 a) and p59fyn tyrosine phosphorylation (Fig. 5 c). By contrast, the addition of soluble laminin 5, up to 5 μg/ml, a concentration fully efficient in proliferation assays, did not induce a significant inhibition of p56lck phosphorylation (Fig. 5 b). Membranes were stripped, blocked, and reprobed with the precipitating antibody, i.e., respectively anti–Zap 70 (Fig. 5 a), anti-lck (Fig. 5 b), and anti-fyn (Fig. 5 c), respectively, in order to verify amounts of precipitated material in each lane.
Figure 5.
Inhibition by soluble laminin 5 (lam5) of early kinases phosphorylation events activated via the CD3–TCR complex. Jurkat cells at 20 × 106/ml in RPMI 5% FCS were incubated with Abs at 37°C for 2.5 min, solubilized, and lysates were immunoprecipitated with (a) a pAb anti–Zap 70, (b) a pAb anti-lck, (c) a pAb anti-fyn. After SDS- PAGE separation and electrotransfer onto Immobilon P membrane, proteins were incubated with HRP conjugated anti-phosphotyrosine Ab (APY) followed by ECL. For densitometric analysis of APY immunoblotting, control values were reduced to 1 in order to compare signals after activation. Values are mentioned under each lane. For reprobing, the membranes were submerged in stripping buffer, blocked and immuno-detected with (a) pAb anti–Zap 70, (b) pAb anti-lck, and (c) pAb anti-fyn was performed. Reactions were revealed by incubating membranes with GAR HRP followed by ECL. Laminin 5 (lam5) was added in soluble form at 1 μg/ml for Zap 70 and fyn analysis and 5 μg/ml for lck analysis.
Identification of an Anti-α6 mAb Specific for α6β4: Evidence for Distinct Forms of α6 Chains Paired with β1 and β4 Chains
Next, we investigated whether the effect of laminin 5 would be mediated by α6β4, α3β1, or both. We tested a battery of anti-α6 mAbs for their reactivity in flow cytometry with dispersed human thymocytes (135 13C [rat IgG2a]; J8H, BQ16, 450 30A1, S3-41 [mouse IgG1]; reference 24) and the widely used anti-α6 GOH3 [rat IgG1]; reference 61). We noticed that the mouse mAb S3-41 displayed a peculiar reactivity as compared with other anti-α6 mAbs, strikingly similar to the reactivity of the anti-β4 mAb 3E1F6 (Fig. 6). S3-41, as 3E1F6, did not react with the most immature CD3low thymocytes. Moreover, staining by S3-41 and 3E1F6 increased with the density of CD3, which indicates the level of maturation of thymocytes (Fig. 6). This pattern of reactivity was in marked contrast to the pattern given by the other anti-α6 mAbs tested including GOH3, which stained immature CD3low thymocytes. Of note, peripheral blood T lymphocytes were equally stained with GOH3 and S3-41 (not shown).
Figure 6.
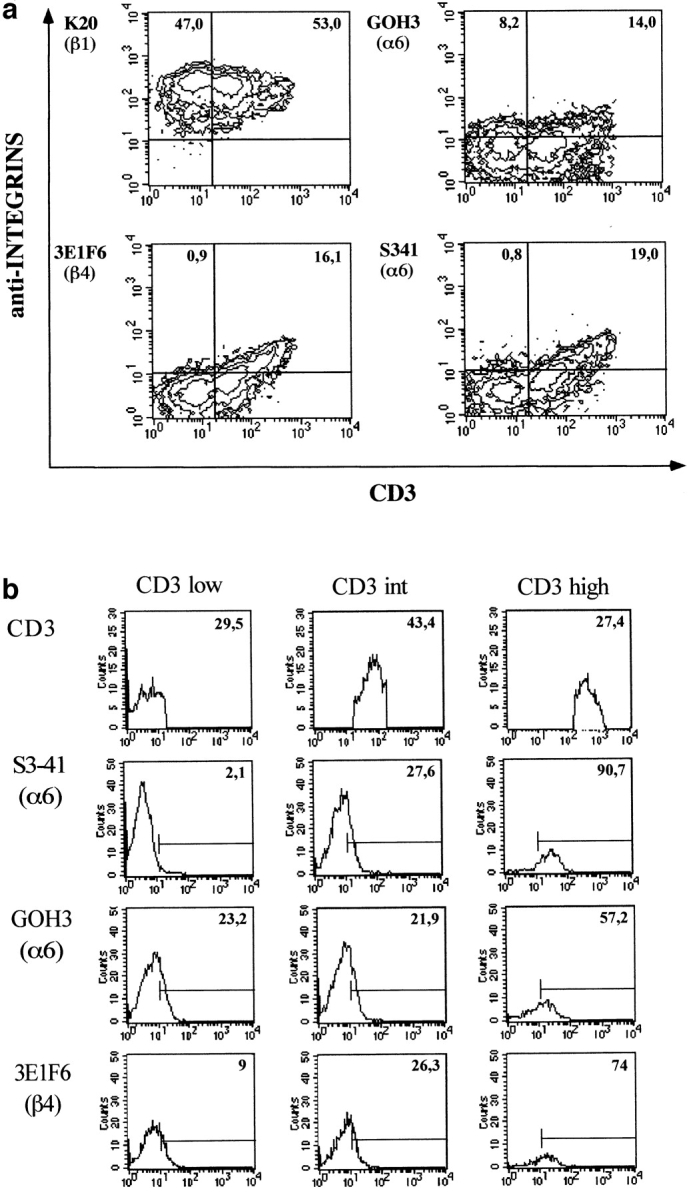
(a) The anti-α6 integrin chain mAb S3-41 has a peculiar pattern of reactivity with human thymocytes, resembling the reactivity of the anti-β4 chain mAb. Two color immunofluorescence was performed on human thymocytes with the CD3 mAb X3 directly conjugated with FITC and with the indicated amounts of anti-integrin mAb that was indirectly labeled with goat anti–mouse PE conjugated F(ab)′2 fragments. The percentage of positive cells for the integrin chain analyzed is noted in the upper corner of the corresponding area. α6 expression was analyzed with the mAbs GOH3 and S3-41; β1 expression was analyzed with the mAb K20 and β4 expression was analyzed with the mAb 3E1F6. (b) Reactivities of the anti-α6 mAb S3-41 and GOH3 and of the anti-β4 mAb 3E1F6 with thymocyte subpopulations according to their surface density in CD3. The percentage in the upper right corner indicates the number of positively stained cells. All antibodies were used at saturating doses, i.e., 10 μg/ml for S3-41, 3E1F6, K20 and 5 μg/ml for GOH3 and X3.
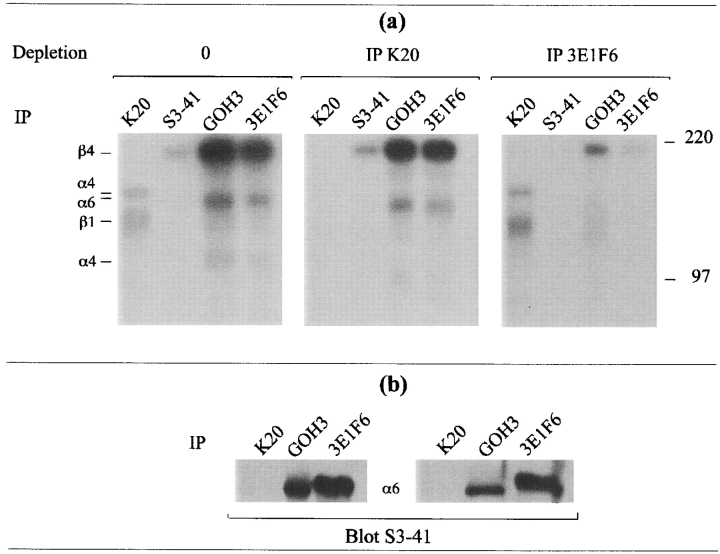
To confirm that S3-41 would react only with the α6 chains linked to β4, we performed sequential immunoprecipitations using the colon carcinoma cell line HT29, which expresses high densities of both α6β1 and α6β4. Cells were lysed with 1% NP-40 and lysates were depleted of β1 or β4 integrins by two successive immunoprecipitations either with the anti-β1 mAb K20 or the anti-β4 mAb 3E1F6. Depleted lysates were then immunoprecipitated with either the anti-α6 mAbs S3-41 or GOH3 (Fig. 7 a). It can be seen that GOH3 was still able to immunoprecipitate residual material when lysates were depleted of either β1 or β4 integrins (Fig. 7 a, middle, lane 3; right, lane 3). In contrast, S3-41 did not precipitate any residual material when lysates were depleted of β4 integrins (Fig. 7 a, right, lane 2) whereas it remained fully reactive when lysates were depleted of β1 integrins (Fig. 7 a, middle, lane 2). Thus, if GOH3 immunoprecipitates α6 chains associated with either β1 or β4 chains, S3-41 recognizes only α6 chains paired with β4. These results were confirmed by immunoblotting experiments with S3-41. Cell lysates were first immunoprecipitated either with the anti-β1 mAb K20 or the anti-β4 3E1F6. Fig. 7 b shows that S3-41 recognized only the α6 chains that coimmunoprecipitated with β4. Note that these experiments were performed on two distinct cell lines that expressed different relative amounts of α6β1 and α6β4 (Fig. 7 b, left and right). Taken together, these experiments demonstrate that the anti-α6 mAb S3-41 reacts with an epitope present only when α6 chains are linked to β4 chains.
Figure 7.
(a) Sequential immunoprecipitations of α6, β1, or β4 chains from HT29 colon carcinoma cell line after anti-β1 or anti-β4 immunoprecipitations (IP). (Left) IP with anti-β1 mAb K20, anti-α6 mAb S3-41, anti-α6 mAb GOH3, and anti-β4 mAb 3E1F6 were performed on total cell lysates. (Middle) To deplete lysates of β1 integrins, a first IP with the anti-β1 mAb K20 was performed on cell lysates and a second IP was then carried out on the supernatants using the same mAb. Finally, a third immunoprecipitation was carried out on the supernatants with the indicated mAbs. (Right) To deplete lysates of α6β4, a first IP with the anti-β4 mAb 3E1F6 was performed on cell lysates and a second IP was then performed on the supernatants using the same mAb. Finally, a third immunoprecipitation was performed on the supernatants with the indicated mAbs. Controls showed that the material recovered was efficiently depleted in β1 (middle panel, lane 1) or β4 chains (right panel, lane 4), respectively. Immunoprecipitated proteins were separated on SDS-PAGE 7.5%, under reducing conditions. Results were revealed by autoradiography. (b) Immunoblots with the anti-α6 mAb S3-41 were performed on immunoprecipitates obtained with mAbs K20 (anti-β1), 3E1F6 (anti-β4) and GOH3 (anti-α6) on lysates from (left) HT29 cells that express α6β4 at higher density than α6β1 or (right) K562 cells that express α6β1 at higher density than α6β4. Immunoprecipitated proteins were separated on SDS-PAGE 7.5%, under reducing conditions before blotting. Immunolabeling was revealed by chemiluminescence (ECL) after reaction with goat anti–mouse immunoglobulins conjugated with HRP.
When Used in Soluble Form the α6β4-specific mAb S3-41 Inhibits Proliferation of Thymocytes Stimulated via the CD3–TCR Complex
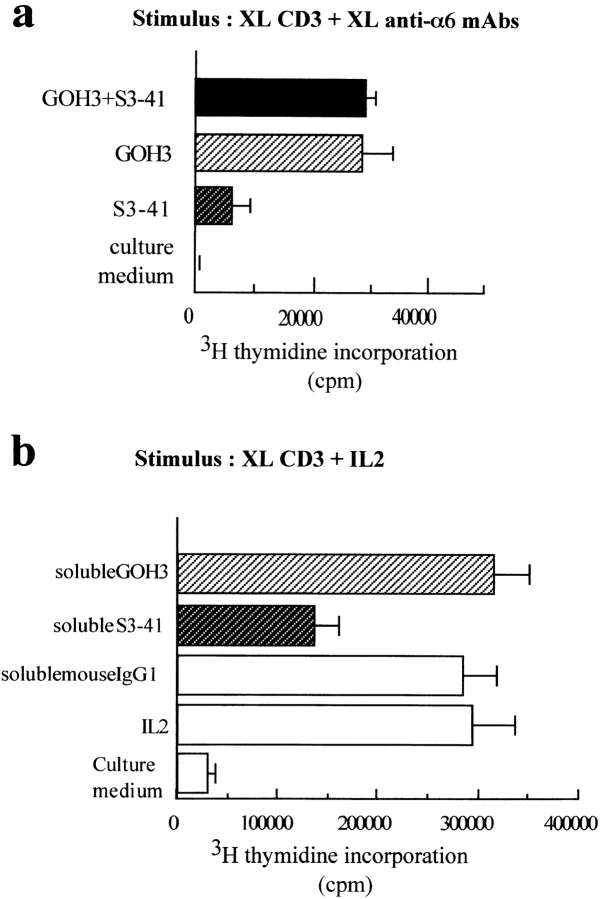
Anti-α6 antibodies were used in proliferation assays performed on human thymocytes. To determine which laminin 5 receptor is involved in the modulation of thymocyte proliferation, we used GOH3 mAb which recognizes all α6 chains and S3-41 mAb which is α6β4 specific. As previously described (58, 66), the anti-α6 mAb GOH3 immobilized on culture wells delivered a strong costimulus for thymocyte proliferation in the presence of cross-linked CD3 mAb (Fig. 8 a). Under the same conditions, S3-41 delivered a weak proliferative costimulus to thymocytes when compared with GOH3. Moreover, there was no additive or synergistic effect when the two mAbs GOH3 and S3-41 were immobilized on plates with a CD3 mAb. When anti-α6 mAbs were used in soluble form, we observed that only S3-41 inhibited proliferation of thymocytes stimulated via the CD3–TCR complex (Fig. 8 b). Inhibition was very efficient with S3-41 (50%). We tested the same soluble mAbs on thymocytes stimulated with a mitogenic pair of CD2 mAbs (GT2 + D66) (Fig. 9 a), the CD28 mAb and immobilized CD3 mAb (Fig. 9 b), or the CD28 mAb and rIL-2 (Fig. 9 c). Again, an inhibitory effect was seen only when thymocytes were stimulated via the CD3–TCR pathway.
Figure 8.
Effects of anti-α6 chain mAb S3-41, added, either in cross-linked or soluble form, on thymocyte proliferation. (a) S3-41 or GOH3 and CD3 were cross-linked on plastic from culture wells as indicated in Fig. 2 before adding the thymocyte suspension. (b) CD3 was first cross-linked on plastic from culture wells. FCS in culture medium was incubated for 2 h at 37°C to saturate the plastic, before adding IL-2, S3-41, or GOH3 in solution and the thymocyte suspension. The indicated mAbs were added at 10 μg/ml.
Figure 9.
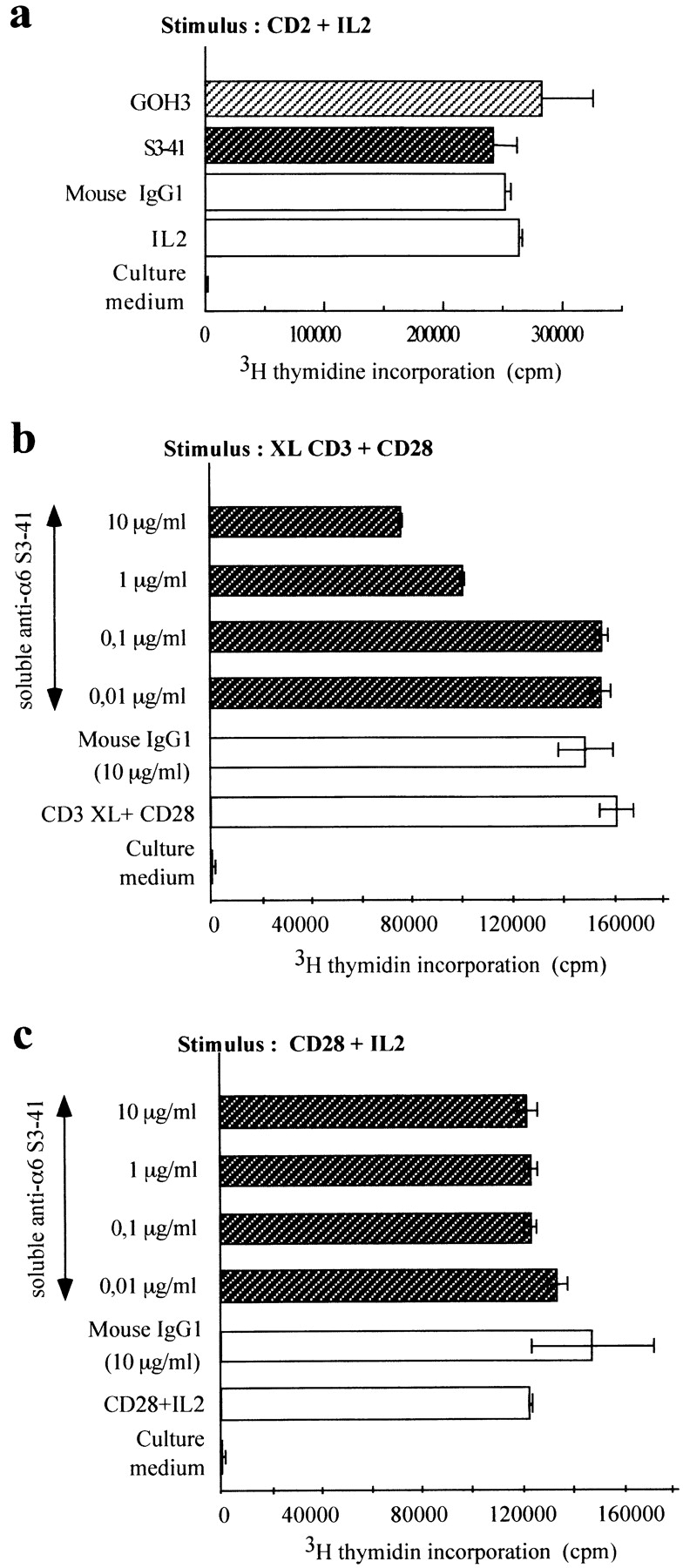
Effects of anti-α6 chain mAb S3-41, added in soluble form, on thymocyte proliferation induced by several pathways. (a) Cells were stimulated with a mitogenic pair of CD2 mAbs (GT2 and D66, 10 μg/ml) plus IL-2. The indicated mAbs were added in soluble form at saturating dose (10 μg/ml). (b) Cells were stimulated with plate-bound CD3 and soluble CD28 mAb (9.3, 1/500). (c) Cells were stimulated with soluble CD28 mAb 9.3 and rIL-2. Various amounts of S3-41 were added in soluble form before addition of cell suspension.
We also used the anti-α3 mAb P1B5, which recognizes the α3β1 epitope involved in laminin 5 binding (7, 48). When added in soluble form in amounts ranging from 0.1 to 10 μg/ml, P1B5 did not inhibit proliferation of thymocytes stimulated via the CD3–TCR complex (not shown).
As it has been described that the non-integrin receptor p67 can associate with α6β4 (2), it was of interest to test the effects of an anti-p67 mAb, namely MPLR2, on T cell proliferation. We did not observe a significant inhibition of soluble MPLR2 on thymocyte proliferation induced via the CD3–TCR complex (not shown).
Inhibition by Soluble S3-41 mAb of Early Kinase Phosphorylations Activated via the CD3–TCR Complex
We tested the effects of S3-41 and P1B5 mAbs, used in soluble form, on early kinase phosphorylation events (Fig. 10). Jurkat cells expressing α6β4 in similar density as CD3high thymocytes were activated with a CD3 mAb (×3, 10 μg/ml) during 2.5 min at 37°C, with or without soluble mAbs. Immunoprecipitation of Zap 70, p56lck, or p59fyn was carried out on activated cell lysates. Precipitated proteins were transferred onto Immobilon, then membranes were incubated with an anti-phosphotyrosine antibody coupled to peroxidase. Results were revealed by ECL. We observed that the addition of S3-41 (10 μg/ml) induced the same pattern of inhibition as soluble laminin 5 with inhibition of Zap 70 and p59fyn tyrosine phosphorylation (Fig. 10, a and c, respectively) but no inhibition of overall p56lck (Fig. 10 b). In contrast, addition of soluble P1B5 (10 μg/ml) did not induce this particular pattern of reactivity, supporting the hypothesis that α6β4 is the laminin 5 receptor involved.
Figure 10.
Inhibition by soluble anti-α6 mAb S3-41 of early kinases phosphorylation events activated via the CD3–TCR complex. Jurkat cells at 20 × 106/ml in RPMI 5% FCS were incubated with Abs at 37°C for 2.5 min, solubilized, and lysates were immunoprecipitated with (a) a pAb anti–Zap 70, (b) a pAb anti-lck, (c) a pAb anti-fyn. After SDS-PAGE separation and electrotransfer onto Immobilon P membrane, proteins were incubated with HRP conjugated anti-phosphotyrosine Ab (APY) followed by ECL. For reprobing, the membranes were submerged in stripping buffer, blocked, and immunodetected with (a) pAb anti–Zap 70, (b) pAb anti-lck, (c) pAb anti-fyn was performed. Reactions were revealed by incubating membranes with GAR HRP followed by ECL. For densitometric analysis of APY immunoblotting, control values were reduced to 1 in order to compare signals after activation. Values are mentioned under each lane. Anti-integrin antibodies were added in soluble form at 10 μg/ml.
Discussion
Using mAbs specific for the three chains of laminin 5 (anti-α3 mAb BM165, anti-β3 mAb 6F12, anti-γ2 mAb GB3), we have investigated the presence and cellular distribution of laminin 5 on frozen human thymus sections. It has been mentioned in earlier publications that laminin 5 is expressed in the human thymus (25, 42, 72). Our data focus on laminin 5 expression in thymic parenchyma and link up with functional effects of this molecule on thymocyte proliferation. Since we have found an identical reactivity of the three mAbs, it can be concluded that a complete form of laminin 5 is displayed by the thymus. Indeed, it is known that β3γ2 modules can be expressed without any detectable α3 chains, such as in choroid plexus (1). We have observed that laminin 5 has quite a particular expression in the thymus; it can be detected in the medulla where a subpopulation of epithelial cells displays laminin 5 as revealed by double staining with keratin. For double staining we used the anti-keratin Ab CK19 that recognizes all epithelial cells (71). Whether the laminin 5 epithelial cells form a specialized set of cells or whether any epithelial cells in these areas could become laminin 5 positive under certain circumstances of stimulus remains to be established. However, this feature is additional to the similarities previously described between thymic epithelial cells and epidermal keratinocytes (45). Thymic vessels are also strongly stained with anti-laminin 5 antibody, particularly at the level of basal laminae, including capillary structures. It is more difficult to ascertain that endothelial cells are also stained; this staining is irregular and is restricted to larger vessels. Interestingly, preliminary results indicate that, in humans, small vessels and capillary structures from other organs appear devoid of laminin 5. In the thymus, vessels are abundant in the medulla, particularly at the corticomedullary junction and it is likely that emigration of thymocytes having reached maturation occurs in this area. Indeed, laminin 5 has been involved in various cell migration phenomena in wound repair (63) or tumor invasion (26, 27, 41) involving cleavage by metalloproteases (17). Therefore, the present data raise the possibility of a role for laminin 5 in T cell migration outside the thymus. The intensity of BM165 staining at the corticomedullary junction suggests that laminin 6 and/or 7 may be also present in these areas. Laminin 5 is also present in subcortical flat epithelial basement membrane that is also known to include hemidesmosome components like collagen type VII and α6β4 integrins (50, 72). It is thus likely that laminin 5 in this region has a different function to the laminin 5 present in the thymus parenchyma, which would be similar to the structural role it exerts in the basement membrane of skin and other epithelia.
Whereas laminin 5 has been identified as a ligand for both α3β1 and α6β4 integrins in vitro, (7, 44, 48, 60), we have observed that α3β1 and α6β4 have a quite distinct distribution on human thymocytes in vivo. Whereas it was found that α3β1 is evenly distributed on all thymocyte subsets (43), we have shown here that α6β4 density on the surface of thymocytes parallels their maturation. We have used the mAb S3-41 (24) because we found that it reacts only with the α6 chains linked to the β4 chain, and not with the α6 chains linked to the β1 chain. Although this mAb was classified as CD49f by the International Workshops on Leucocyte Antigens on the basis of its capacity to recognize human α6 transfected in RBL rat cells (22), a particular reactivity was noticed on skin sections, where α6 is associated with the β4 chain, but not on kidney sections, where α6 is associated with the β1 chain (28, 77). The sequential immunoprecipitations and immunoprecipitations followed by Western blots that were performed in the present study confirmed that S3-41 mAb reacts exclusively with the α6 chain bound to β4. We have noticed that, on total lysates, S3-41 immunoprecipitated much less material than anti-α6 mAb GOH3. This could be explained either by a difference of affinity between the two mAbs and/or by the staining of only a subpopulation of α6 chains with S3-41. Indeed, S3-41 mAb did not precipitate any material when lysates were depleted of β4 integrins but it was fully reactive when lysates were depleted of β1 integrins, and only α6 chains coimmunoprecipitated with β4 carried the S3-41 epitope. Moreover, as S3-41 reacted with α6 chains separated from β4 chains, this suggests that it recognizes an intrinsic isomorphism of the α6 chains and not a conformational epitope. Thus, strikingly, α6β4 upregulation on mature thymocytes coincides with laminin 5 expression in the medullary area, the thymic region where mature thymocytes are located. In mice, it has been described that, on the contrary, downregulation of β4 is simultaneous with the upregulation of CD4, CD8, and CD3 (73), but differences in integrin expression with T cell maturation between human and mouse have already been observed for α4β1 (55).
Since it was described that bulk preparations of laminin coated on plastic are strongly mitogenic for T cells (58), it was of interest to investigate the effect of laminin 5 on human T cell proliferation. We show here that laminin 5 presented in a cross-linked form has only a slight coactivation effect on thymocytes. However, when presented in soluble form, a clear inhibition of proliferation triggered via the CD3–TCR complex was observed. It should be noted that this effect was reproduced by the anti-α6 mAb S3-41; however, it was not reproduced by the typical anti-α3 integrin mAb P1B5 in agreement with the view that the inhibitory influence of soluble laminin 5 on T cell proliferation is mediated via α6β4. In addition, we did not observe an inhibition on TCR triggered proliferation with soluble mAb MPLR2, an antibody reacting with the high affinity non-integrin p67 laminin receptor, reported to be associated with α6β4 (2). Soluble laminin can be detected in the serum of normal individuals, and its level was found to be strongly increased in inflammatory conditions and certain autoimmune disorders (57, 75). Laminin is also present in the extracellular spaces of secreting cells, and it was found that a significant proportion (∼15%) of Engelbreth Holm Swarm matrix proteins is present as a soluble form released into the supernatant (47). It is likely that an equilibrium takes place between the soluble laminins, either released by secreting cells or liberated from the ECM on the one hand, and the laminin deposited as an insoluble fraction in the ECM or on cell surface receptors on the other hand (47). The data presented here disclose a cross-talk event occurring between the laminin 5 receptor α6β4 and the T cell receptor. Of note, inhibition induced by laminin 5 is restricted to signaling via the CD3–TCR complex, and we observed no inhibition when T cells were stimulated via other pathways such as the CD2 or the CD28 pathways. Inhibition by laminin 5 therefore shares similarities with the inhibition generated by β1 integrin ligands we have described, either VCAM 1 or mAbs (19, 65, 34). It has been previously shown by other groups that integrins can negatively regulate T cell activation (21, 36, 64). Our data show that α6β4 can also modulate T cell activation. Consistent with this view, it has been previously suggested that this integrin could, depending on the cell type, either increase or block normal cell proliferation (16). Besides, as an activation pathway was previously described when α6β4 was ligated with coated antibodies or laminin 5 (32), our data obtained with soluble ligand and mAb suggest also that exposing cells to immobilized or soluble ligands can trigger different signaling pathways. Such influences, depending on the form of the ligand, fits well with the known feature of integrins signaling, whose pattern is critically dependent on sole ligand occupancy, sole integrin aggregation or both ligand occupancy and aggregation (40).
Moreover, we have observed here that laminin 5 inhibits the phosphorylation of Zap 70 and p59fyn but has no effect on overall phosphorylation and activation of p56lck, a pattern of kinase inhibition that was also observed in our laboratory by binding β1 integrins with soluble ligand (34). Thus α6β4, under the influence of laminin 5 appears to use, at least in part, the same intracellular signaling pathway as β1 integrins under the influence of VCAM 1. Whether these signaling pathways are common or whether they diverge deserves further investigation.
There is increasing evidence that integrins and their ligands, including ECM components, play a critical role in T cell maturation (5, 10, 12, 20, 31, 43, 54–56, 69, 73, 74). The pattern of expression of the various integrins present in the thymus and the pattern of expression of their ligand are both subtle events that account for their influence at given stages of T cell differentiation/maturation (12, 54). Since, it is clear that thymocyte differentiation involves cycles of proliferation and arrest (18), the coordination of adhesion and proliferation events mediated by laminin sub-types is likely to be significant for T cell maturation. The results reported here suggest that laminin 5 might be an important signaling molecule in the control of expansion of mature thymocytes and possibly their migration outside the thymus, given its presence in vascular structures.
Acknowledgments
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Association pour la Recherche centre le Cancer, the Ligue contre le Cancer, the Programme Hospitalier de Recherche Clinique and the FEGEFLUC.
Abbreviations used in this paper
- APY
anti-phosphotyrosine
- Ab
antibody
- ECM
extracellular matrix
- GAR
goat anti–rabbit immunoglobulins
- IP
immunoprecipitation
- pAb
polyclonal antibody
- PE
phycoerythrin
Footnotes
The authors express gratitude to Dr. S. Kennel for the gift of 3E1F6 mAb, to Hélène Senesi for technical assistance and to Ghislaine Bernard for helpful discussion.
References
- 1.Aberdam D, Aguzzi A, Baudouin C, Galliano MF, Ortonne JP, Meneguzzi G. Developmental expression of nicein adhesion protein (laminin-5) subunits suggests multiple morphogenic roles. Cell Adhes Commun. 1994;2:115–129. doi: 10.3109/15419069409004431. [DOI] [PubMed] [Google Scholar]
- 2.Ardini E, Tagliabue E, Buto S, Castronovo V, Colnaghi MI, Menard S. Co-regulation and physical association of the 67 Kd monomeric laminin receptor and the α6β4integrin. J Biol Chem. 1997;272:2342–2345. doi: 10.1074/jbc.272.4.2342. [DOI] [PubMed] [Google Scholar]
- 3.Baker SE, DiPasquale AP, Stock EL, Quaranta V, Fitchum M, Jones JC. Morphogenetic effects of soluble laminin-5 on cultured epithelial cells and tissue explants. Exp Cell Res. 1996;228:262–270. doi: 10.1006/excr.1996.0325. [DOI] [PubMed] [Google Scholar]
- 4.Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinmann H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, Timpl R, Trygvason K, Yamada Y, Yurchenco PD. A new nomenclature for the laminins. Marix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 5.Cardarelli PM, Crispe IN, Pierschbacher MD. Preferential expression of fibronectin receptors on immature thymocytes. J Cell Biol. 1988;106:2183–2190. doi: 10.1083/jcb.106.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins α3β1 in focal adhesions and α6β4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin α3β1in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- 8.Champliaud MF, Lunstrum GP, Rousselle P, Nishiyama T, Keene DR, Burgeson RE. Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment. J Cell Biol. 1996;132:1189–1198. doi: 10.1083/jcb.132.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang AC, Wadsworth S, Coligan JE. Expression of merosin in the thymus and its interaction with thymocytes. J Immunol. 1993;151:1789–1801. [PubMed] [Google Scholar]
- 10.Chang AC, Salomon DR, Wadsworth S, Hong M-J, Mojcik CF, Otto S, Shevach EM, Coligan JE. α3β and α6β integrins mediate laminin/merosin binding and function as costimulatory molecules for human thymocyte proliferation. J Immunol. 1995;154:500–510. [PubMed] [Google Scholar]
- 11.Cloix JF, Bader CA, Monet JD, Funck-Brentano JL. Radiolabeling (125I) with high specific activity of the 1-34 N-terminal derivative of bovine parathormone without loss of biological activity. Pathol Biol. 1975;23:827–831. [PubMed] [Google Scholar]
- 12.Crisa L, Cirulli V, Ellisman MH, Ishii JK, Elices MJ, Salomon DR. Cell adhesion and migration are regulated at distinct stages of thymic T cell development: the roles of fibronectin, VLA4, and VLA5. J Exp Med. 1996;184:215–218. doi: 10.1084/jem.184.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dipersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. α3β1integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnoux-Palacios L, Vailly J, Durand-Clement M, Wagner E, Ortonne JP, Meneguzzi G. Functional re-expression of laminin-5 in laminin gamma-2-deficient human keratinocytes modifies cell morphology, motility, and adhesion. J Biol Chem. 1996;271:18437–18444. doi: 10.1074/jbc.271.31.18437. [DOI] [PubMed] [Google Scholar]
- 15.Galliano MF, Aberdam D, Aguzzi A, Ortonne JP, Meneguzzi G. Cloning and complete primary structure of the mouse laminin alpha-3 chain. Distinct pattern of the laminin alpha-3A chain and alpha-3B chain isoforms. J Biol Chem. 1995;270:21820–21826. doi: 10.1074/jbc.270.37.21820. [DOI] [PubMed] [Google Scholar]
- 16.Giancotti FG. Signal transduction by the α6β4integrin: charting the path between laminin binding and nuclear events. J Cell Sci. 1996;109:1165–1171. doi: 10.1242/jcs.109.6.1165. [DOI] [PubMed] [Google Scholar]
- 17.Gianelli G, Falk-Mareillier J, Schiraldi O, Stetler-Stevenson G, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 18.Groettrup M, Ungewiss K, Azogui O, Palacios R, Owen MJ, Hayday AC, von Boehmer H. A novel disulphide-linked heterodimer on pre-T cells consists of the TCR β chain and a 33 kD glycoprotein. Cell. 1993;75:283–294. doi: 10.1016/0092-8674(93)80070-u. [DOI] [PubMed] [Google Scholar]
- 19.Groux H, Huet S, Valentin H, Pham D, Bernard A. Suppressor effects and cyclic AMP accumulation by the CD29 molecule of CD4+lymphocytes. Nature. 1989;339:152–154. doi: 10.1038/339152a0. [DOI] [PubMed] [Google Scholar]
- 20.Halvorson MJ, Coligan JE. Enhancement of VLA integrin receptor function on thymocytes by cAMP is dependent on the maturation stage of the thymocytes. J Immunol. 1995;155:4567–4574. [PubMed] [Google Scholar]
- 21.Hemesath TJ, Marton L, Stefansson K. Inhibition of T cell activation by the extracellular matrix protein tenascin. J Immunol. 1994;152:5199–5207. [PubMed] [Google Scholar]
- 22.Hemler, M.E., V. Quaranta, L. Starr, and J. Bodorova. 1995. CD49f cluster report. In Leucocyte Typing. V.S.F. Schlossman, L. Boumsell, W. Gilks, J.M. Harlan, T. Kishimoto, C. Morimoto, J. Ritz, S. Shaw, R. Silverstein, T. Springer, T.F. Tedder, R.F. Todd, editors. Oxford University Press, New York. 2:1619–1620.
- 23.Jones JC, Kurpakus MA, Cooper HM, Quaranta V. A function for the integrin alpha 6 beta 4 in the hemidesmosome. Cell Regul. 1991;2:427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajiji S, Tamura RN, Quaranta V. A novel integrin (αEβ4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO (Eur Mol Biol Organ) J. 1989;8:673–680. doi: 10.1002/j.1460-2075.1989.tb03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallunki P, Sainio K, Eddy R, Byers M, Kallunki T, Sariola H, Beck K, Hirvonen H, Shows TB, Trygvason K. A truncated laminin chain homologous to the B2 chain: structure, spatial expression, and chromosomal assignments. J Cell Biol. 1992;119:679–693. doi: 10.1083/jcb.119.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikkawa Y, Umeda M, Miyazaki K. Marked stimulation of cell adhesion and motility by ladsin, a laminin-like scatter factor. J Biochem. 1994;116:862–869. doi: 10.1093/oxfordjournals.jbchem.a124608. [DOI] [PubMed] [Google Scholar]
- 27.Kikkawa Y, Akaogi K, Mizushima H, Yamanaka N, Umeda M, Miyazaki K. Stimulation of endothelial cell migration in culture by ladsin, a laminin-5-like cell adhesion protein. In Vitro Cell Dev Biol Anim. 1996;32:46–52. doi: 10.1007/BF02722993. [DOI] [PubMed] [Google Scholar]
- 28.Korhonen M, Sariola H, Gould VE, Kangas L, Virtanen I. Integrins and laminins in human renal carcinoma cells and tumors grown in nude mice. Cancer Res. 1994;16:4532–4538. [PubMed] [Google Scholar]
- 29.Kurpakus MA, Quaranta V, Jones JC. Surface relocation of α6β4integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol. 1991;115:1737–1750. doi: 10.1083/jcb.115.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993;92:1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magner W, Chang AC, Owens J, Hong M-JP, Brooks A, Coligan JE. Aberrant differentiation of thymocytes in mice lacking laminin-2. Mol Biol Cell. 1995;6:280a. doi: 10.1155/2000/90943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J, Giancotti FG. Signal transduction by the α6β4integrin: distinct β4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO (Eur Mol Biol Organ) J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marinkovich MP, Lunstrum GP, Keene DR, Burgeson RE. The dermal-epidermal junction of human skin contains a novel laminin variant. J Cell Biol. 1992;119:695–703. doi: 10.1083/jcb.119.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mary, F., M. Ticchioni, J.C. Bendini, T. Venaille, D. Mary, and A. Bernard. 1997. CD29 workshop: a distinctive pattern of tyrosine kinases inhibition accompanies the blocking of T cell activation by β1 integrins. In Leucocyte Typing VI. Garland Publishing, Inc., New York. 361–362.
- 35.Matsui C, Nelson CF, Hernandez GT, Herron GS, Bauer EA, Hoeffler WK. Gamma 2 chain of laminin 5 is recognized by monoclonal antibody GB3. J Invest Dermatol. 1995;105:648–652. doi: 10.1111/1523-1747.ep12324108. [DOI] [PubMed] [Google Scholar]
- 36.McIntyre BW, Woodside DG, Caruso DA, Wooten DK, Simon SI, Neelamegham S, Revelle JK, Vanderslice P. Regulation of human T lymphocyte coactivation with an α4 integrin antagonist peptide. J Immunol. 1997;158:4180–4186. [PubMed] [Google Scholar]
- 37.Mercurio AM. Laminin receptors. Trends Cell Biol. 1995;5:419–423. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- 38.Miner JH, Sanes JR. The functions of laminins: lessons from in vivo studies. 3. Laminin β2. Matrix Biol. 1996;15:369–381. doi: 10.1016/s0945-053x(96)90157-2. [DOI] [PubMed] [Google Scholar]
- 39.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin α chains: expression, developmental transitions and chromosomal locations of a1,5, identification of heterotrimeric laminins 8-11, and cloning of a novel isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyamoto S, Teramoto H, Coso O, Gutkind S, Burbelo P, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyazaki K, Kikkawa Y, Nakamura A, Yasumitsu H, Umeda M. A large cell-adhesive scatter factor secreted by human gastric carcinoma cells. Proc Natl Acad Sci USA. 1993;90:11767–11771. doi: 10.1073/pnas.90.24.11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizushima H, Miyagi Y, Kikkawa Y, Yamanaka N, Yasumitsu H, Misugi K, Miyazaki K. Differential expression of laminin-5/ ladsin subunits in human tissues and cancer cell lines and their induction by tumor promoter and growth factors. J Biochem. 1996;120:1196–1202. doi: 10.1093/oxfordjournals.jbchem.a021541. [DOI] [PubMed] [Google Scholar]
- 43.Mojcik CK, Salomon DR, Chang AC, Shevach EM. Differential expression of integrins on human thymocyte subpopulations. Blood. 1995;86:4206–4217. [PubMed] [Google Scholar]
- 44.Niessen CM, Hogervorst F, Jaspars LH, de Melker AA, Delwel GO, Hulsman EH, Kuikman I, Sonnenberg A. The α6β4integrin is a receptor for both laminin and kalinin. Exp Cell Res. 1994;211:360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- 45.Patel DD, Whichard LP, Radcliff G, Denning SM, Haynes BF. Characterization of human thymic epithelial cell surface antigens: phenotypic similarity of thymic epithelial cells to epidermal keratinocytes. J Clin Immunol. 1995;15:80–92. doi: 10.1007/BF01541736. [DOI] [PubMed] [Google Scholar]
- 46.Pyke C, Salo S, Ralfkiaer E, Romer J, Dano K, Trygvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139. [PubMed] [Google Scholar]
- 47.Riedle B, Kerjaschi D. Reactive oxygen species cause direct damage of Engelbreth-Holm-Swarm matrix. Am J Pathol. 1997;151:215–231. [PMC free article] [PubMed] [Google Scholar]
- 48.Rousselle P, Aumailley M. Kalinin is more efficient than laminin in promoting adhesion of primary keratinocytes and some other epithelial cells and has a different requirement for integrin receptors. J Cell Biol. 1994;125:205–214. doi: 10.1083/jcb.125.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rousselle P, Lunstrum G, Keene DR, Burgeson RE. Kalinin: an epithelium specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rousselle P, Golbik R, van der Rest M, Aumailley M. Structural requirements for cell adhesion to kalinin (laminin-5) J Biol Chem. 1995;270:13766–13770. doi: 10.1074/jbc.270.23.13766. [DOI] [PubMed] [Google Scholar]
- 51.Rousselle P, Keene DR, Ruggiero F, Champliaud MC, van der Rest M, Burgeson RE. Laminin 5 binds the NC-1 domain of type VII collagen. J Cell Biol. 1997;138:719–728. doi: 10.1083/jcb.138.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan MC, Christiano A. The functions of laminins: lessons from in vivo studies. 1. The laminin α3 chain. Matrix Biol. 1996;15:369–381. doi: 10.1016/s0945-053x(96)90157-2. [DOI] [PubMed] [Google Scholar]
- 53.Ryan MC, Tizard R, Van Devanter DR, Carter WG. Cloning of the LamA3 gene encoding the α3 chain of adhesive ligand epiligrin. J Biol Chem. 1994;269:22779–22787. [PubMed] [Google Scholar]
- 54.Salomon DR, Crisa L, Mojcik CF, Ishii JK, Klier G, Shevach EM. Vascular cell adhesion molecule-1 is expressed by cortical thymic epithelial cells and mediates thymocyte adhesion. Blood. 1997;89:2641–2671. [PubMed] [Google Scholar]
- 55.Salomon DR, Mojcik CK, Chang AC, Wadsworth S, Adams DH, Coligan JE, Shevach EM. Constitutive activation of integrin α4β1 defines a unique stage of human thymocytes development. J Exp Med. 1994;179:1573–1584. doi: 10.1084/jem.179.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savagner P, Imhof B, Yamada KM, Thiery JP. Homing of hemopoietic precursor cells to the embryonic thymus: characterization of an invasive mechanism induced by chemotactic peptides. J Cell Biol. 1986;103:2715–2727. doi: 10.1083/jcb.103.6.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoenfeld N, Schmolke B, Fritzsche A, Loddenkemper R. Laminin fragment P1 in the sera of patients with pulmonary sarcoidosis. Sarcoidosis Vasc. . Diffuse Lung Dis. 1996;13:135–138. doi: 10.1007/BF00389837. [DOI] [PubMed] [Google Scholar]
- 58.Shimizu Y, Van SG, Horgan KJ, Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol. 1990;145:59–67. [PubMed] [Google Scholar]
- 59.Smith BE, Bradshaw AD, Choi ES, Rousselle P, Wayner EA, Clegg DO. Human SY5Y neuroblastoma cell interactions with laminin isoforms: neurite outgrowth on laminin-5 is mediated by integrin α3β1 . Cell Adhes Commun. 1996;3:451–462. doi: 10.3109/15419069609081022. [DOI] [PubMed] [Google Scholar]
- 60.Sonnenberg Biochemical characterization and tissue distribution of the A and B variants of the integrin alpha 6 subunit. J Cell Biol. 1993;121:179–191. doi: 10.1083/jcb.121.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonnenberg A, Daams H, van der Valk MA. Development of mouse mammary gland: identification of stages in differentiation of luminal and myoepithelial cell using monoclonal antibodies and polyvalent antiserum against keratin. J Histochem Cytochem. 1986;34:1037–1046. doi: 10.1177/34.8.2426332. [DOI] [PubMed] [Google Scholar]
- 62.Stahl S, Weitzman S, Jones JCR. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J Cell Sci. 1997;110:55–63. doi: 10.1242/jcs.110.1.55. [DOI] [PubMed] [Google Scholar]
- 63.Symington BE, Carter WG. Modulation of epidermal differentiation by epiligrin and integrin α3β1 . J Cell Sci. 1995;108:831–838. doi: 10.1242/jcs.108.2.831. [DOI] [PubMed] [Google Scholar]
- 64.Teague TK, McIntyre BW. mAb 18D3 triggering of integrin β1 will prevent but not terminate proliferation of human T cells. Cell Adhes Commun. 1994;2:169–184. doi: 10.3109/15419069409004435. [DOI] [PubMed] [Google Scholar]
- 65.Ticchioni M, Aussel C, Breittmayer JP, Manié S, Pelassy C, Bernard A. Suppressive effect of T cell proliferation via the CD29 molecule. The CD29 mAb “K20” decreases diacylglycerol and phosphatidic acid levels in activated T cells. J Immunol. 1993;151:119–127. [PubMed] [Google Scholar]
- 66.Ticchioni M, Deckert M, Bernard G, Calandra D, Breittmeyer JP, Imbert V, Peyron JF, Bernard A. Comitogenic effects of very late activation antigens on CD3-stimulated human thymocytes. J Immunol. 1995;154:1207–1215. [PubMed] [Google Scholar]
- 67.Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 68.Tosello AC, Mary F, Amiot M, Bernard A, Mary D. Activation of T cells via CD55: recruitment of early components of the CD3-TCR pathway is required for early IL2 secretion. J Inflamm. 1998;48:13–27. [PubMed] [Google Scholar]
- 69.Utsumi K, Sawada M, Narumiya S, Nagamine J, Sakata T, Iwagami S, Kita Y, Teraoka H, Hirano H, Ogata M, et al. Adhesion of immature thymocytes to thymic stromal cells through fibronectin molecule and its significance for the induction of thymocyte differentiation. Proc Natl Acad Sci USA. 1991;88:5685–5689. doi: 10.1073/pnas.88.13.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verrando P, Hsi BL, Yeh CJ, Pisani A, Serieys N, Ortonne JP. Monoclonal antibody GB3, a new probe for the study of human basement membranes and hemidesmosomes. Exp Cell Res. 1987;170:116–128. doi: 10.1016/0014-4827(87)90121-2. [DOI] [PubMed] [Google Scholar]
- 71.Vieira JL, Meireles de Souza LR, Savino W. Conceptual aspects of the thymic microenvironment. Immunol Today. 1994;15:192–193. [PubMed] [Google Scholar]
- 72.Virtanen I, Lohi J, Tani T, Sariola H, Burgeson RE, Lehto VP. Laminin chains in the basement membranes of human thymus. Histochem J. 1996;28:643–650. doi: 10.1007/BF02331385. [DOI] [PubMed] [Google Scholar]
- 73.Wadsworth S, Halvorson MJ, Coligan JE. Developmentally regulated expression of the beta 4 integrin on immature mouse thymocytes. J Immunol. 1992;149:421–428. [PubMed] [Google Scholar]
- 74.Wadsworth S, Halvorson MJ, Chang AC, Coligan JE. Multiple changes in VLA protein glycosylation, expression, and function occur during mouse T cell ontogeny. J Immunol. 1993;150:847–857. [PubMed] [Google Scholar]
- 75.Wenisch C, Bankl HC, Schonthal E, Myskiw D, Presterl E, Rumpold H, Graninger W. Serum levels of the carboxy-terminal cross-linked telopeptide of type I collagen and laminin are elevated in Graves' disease but not in toxico nodular goiter. Clin Immunol Immunopathol. 1995;75:225–230. doi: 10.1006/clin.1995.1075. [DOI] [PubMed] [Google Scholar]
- 76.Yurchenco PD, O'Rear JJ. Basal lamina assembly. Curr Opin Cell Biol. 1994;6:674–681. doi: 10.1016/0955-0674(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 77.Zambruno, G., A. Baraldi, C. Vaschieri, A. Marconi, E. Lusvarghi, and A. Giannetti. 1994. Immunohistochemical and biochemical characterization of β1 integrins on normal skin and kidney. In Leucocyte Typing. V.S.F. Schlossman, L. Boumsell, W. Gilks, J.M. Harlan, T. Kishimoto, C. Morimoto, J. Ritz, S. Shaw, R. Silverstein, T. Springer, T.F. Tedder, and R.F. Todd, editors. Oxford University Press, New York. 2:1623–1625.