Abstract
LY-A strain is a Chinese hamster ovary cell mutant resistant to sphingomyelin (SM)-directed cytolysin and has a defect in de novo SM synthesis. Metabolic labeling experiments with radioactive serine, sphingosine, and choline showed that LY-A cells were defective in synthesis of SM from these precursors, but not syntheses of ceramide (Cer), glycosphingolipids, or phosphatidylcholine, indicating a specific defect in the conversion of Cer to SM in LY-A cells. In vitro experiments showed that the specific defect of SM formation in LY-A cells was not due to alterations in enzymatic activities responsible for SM synthesis or degradation. When cells were treated with brefeldin A, which causes fusion of the Golgi apparatus with the endoplasmic reticulum (ER), de novo SM synthesis in LY-A cells was restored to the wild-type level. Pulse–chase experiments with a fluorescent Cer analogue, N-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-d-erythro-sphingosine (C5-DMB-Cer), revealed that in wild-type cells C5-DMB-Cer was redistributed from intracellular membranes to the Golgi apparatus in an intracellular ATP-dependent manner, and that LY-A cells were defective in the energy-dependent redistribution of C5-DMB-Cer. Under ATP-depleted conditions, conversion of C5-DMB-Cer to C5-DMB-SM and of [3H]sphingosine to [3H]SM in wild-type cells decreased to the levels in LY-A cells, which were not affected by ATP depletion. ER-to-Golgi apparatus trafficking of glycosylphosphatidylinositol-anchored or membrane-spanning proteins in LY-A cells appeared to be normal. These results indicate that the predominant pathway of ER-to-Golgi apparatus trafficking of Cer for de novo SM synthesis is ATP dependent and that this pathway is almost completely impaired in LY-A cells. In addition, the specific defect of SM synthesis in LY-A cells suggests different pathways of Cer transport for glycosphingolipids versus SM synthesis.
Keywords: sphingomyelin, ceramide, trafficking, mutant, Chinese hamster ovary cells
Lipids serve not only as essential constituents of the fluid matrix of biological membranes, but also as modulators regulating various cellular events. Membrane lipids of mammalian cells consist mainly of three different classes of lipids (glycerolipids, sterols, and sphingolipids), which are categorized according to the structure of their hydrophobic backbones. In living cells, the membranes of different intracellular organelles have different lipid compositions, and various biomembranes show an asymmetric distribution of lipid types across the membrane bilayer (Alberts et al., 1994). Furthermore, various steps in lipid biosynthesis can occur in different intracellular compartments; for example, ceramide (Cer)1 produced in the ER is sorted to the Golgi apparatus for conversion to sphingomyelin (SM) or glucosylceramide (GlcCer) (reviewed in Hoekstra and Kok, 1992; Rosenwald and Pagano, 1993; van Meer, 1993). Phosphatidylserine produced in the ER is sorted to the mitochondria where phosphatidylserine is converted to phosphatidylethanolamine, which is translocated to the plasma membrane (reviewed in Trotter and Voelker, 1994). Thus, the synthesis, translocation, and sorting of lipids represent an important set of problems relating to membrane biogenesis and homeostasis. However, little is known about the mechanisms underlying lipid transport and sorting, although various pathways for intracellular transport of newly synthesized lipids have been suggested (reviewed in Moreau and Cassagne, 1994; Trotter and Voelker, 1994).
Sphingolipid biosynthesis is initiated by condensation of l-serine with palmitoyl CoA, a reaction catalyzed by serine palmitoyltransferase to generate 3-ketodihydrosphingosine, which is then converted to dihydrosphingosine (see Merrill and Jones, 1990 for review of sphingolipid biosynthesis). Dihydrosphingosine is N-acylated to produce dihydroceramide (DCer), followed by desaturation to form Cer. These early synthetic steps occur at the cytosolic surface of the ER (Mandon et al., 1992; Hirschberg et al., 1993; Michel and van Echten-Deckert, 1997). Cer, a common intermediate for both SM and glycosphingolipid syntheses, is translocated from the ER to the Golgi apparatus by yet-undefined mechanisms, and then converted to SM by the enzyme phosphatidylcholine:ceramide cholinephosphotransferase (SM synthase) in the lumenal side of the Golgi apparatus or to GlcCer on the cytosolic surface of the Golgi apparatus (Coste et al., 1986; Futerman et al., 1990; Futerman and Pagano, 1991; Trinchera et al., 1991; Jeckel et al., 1992). After translocation into the Golgi lumen, GlcCer is further converted to lactosylceramide and more complex glycosphingolipids. SM and glycosphingolipids produced in the Golgi lumen are predominantly delivered to the plasma membrane, most likely via the bulk flow of membranes from the Golgi apparatus to the plasma membrane (reviewed in Hoekstra and Kok, 1992; Rosenwald and Pagano, 1993; van Meer, 1993), while a portion of newly synthesized GlcCer reaches the plasma membrane by a non-Golgi pathway (Warnock et al., 1994).
A powerful approach towards understanding the metabolic mechanisms and biological functions of membrane phospholipids is the biochemical and cell-biological characterization of cell mutants with specific defects in phospholipid metabolism (Nishijima et al., 1997). Lysenin, derived from the coelomic fluid of the earthworm Eisenia foetida, is a cytolysin that binds to SM with a high affinity (Yamaji et al., 1998). Recently, we isolated several types of lysenin-resistant CHO cell mutants, two of which (designated LY-A and LY-B strains) are defective in SM production, and the LY-B strain has been found to lack the LCB1 subunit of serine palmitoyltransferase (Hanada et al., 1998). In this study, we further characterize the LY-A strain and find that this mutant is defective in an ATP-dependent trafficking pathway of Cer from the ER to the Golgi apparatus for de novo SM synthesis. As far as we are aware, this study is the first report providing genetic evidence for the existence of specific machinery for intracellular trafficking of Cer. We also discuss models for ER-to-Golgi apparatus trafficking of de novo synthesized Cer toward SM or glycosphingolipids.
Materials and Methods
Materials
N-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)- d-erythro-sphingosine (C5-DMB-Cer) and 6-[N-(7-nitrobenzo-2-oxa-1,3-diazol-4-yl)amino]caproyl-d-erythro-sphingosine (C6-NBD-Cer) were purchased from Molecular Probes. Brefeldin A, fatty acid–free BSA, and endoglycosidase H (Endo H) were from Sigma Chemical Co. l-[U- 14C]serine (5.85 GBq/mmol) was from Amersham Pharmacia Biotech. [Methyl-14C]choline chloride (2.04 GBq/mmol) and [3-3H]d-erythro-sphingosine (0.74 GBq/mmol) were from American Radiolabeled Chemicals Inc.
Cells and Cell Cultures
The CHO-K1 cell line was obtained from the American Type Culture Collection (ATCC CCL 61), and LY-A strain, a CHO-K1–derived mutant cell line, has been established previously by us (Hanada et al., 1998). Cells were routinely maintained in Ham's F-12 medium supplemented with 10% NBS, penicillin G (100 U/ml), streptomycin sulfate (100 μg/ml), and sodium hydrogen carbonate (1.18 g/liter) at 33°C (Hanada et al., 1990). Nutridoma medium (Ham's F-12 medium containing 1% Nutridoma-SP [Boehringer Mannheim] and gentamicin [25 μg/ml]) was used as serum-free medium. Cells constitutively expressing a glycosylphosphatidylinositol (GPI)-anchored human placental alkaline phosphatase (PLAP) (Berger et al., 1987) or a membrane-spanning PLAP (PLAP-HA), which is a chimera of PLAP with influenza hemagglutinin (Arreaza and Brown, 1995), were established as described (Hanada et al., 1995).
Metabolic Labeling of Lipids with [14C]Serine and [14C]Choline in Cells
Subconfluent cell monolayers in 6-cm dishes were incubated in 2 ml of Nutridoma medium containing 37 kBq of [14C]serine or [14C]choline at 33°C for various times. After washing twice with 2 ml of cold PBS, cells were lysed in 1 ml of 0.1% SDS at 4°C, and 0.1 and 0.8 ml of the cell lysate were used for protein determination and lipid extraction, respectively. Lipids extracted from the cells (Bligh and Dyer, 1959) were separated on TLC plates with solvent of methyl acetate/n-propanol/chloroform/methanol/0.25% potassium chloride (25:25:25:10:9, vol/vol) in the [14C]serine-labeling experiments, or chloroform/methanol/acetic acid/water (25:15:4:2, vol/vol) in the [14C]choline-labeling experiments. After collecting gels from the plates by scraping, radioactivity in each lipid was determined by liquid scintillation counting. The values were normalized to cell protein.
Since DCer and Cer were not separated by the TLC systems used, composition of metabolically labeled ceramides was determined after acid hydrolysis of them. Cells were labeled with [14C]serine for 2 h at 33°C and labeled lipids were separated by the TLC as described above. Lipids containing both [14C]DCer and [14C]Cer were reextracted from the TLC fractions, hydrolyzed in 0.5 ml of 10 N hydrochloric acid/water/methanol (8.6:9.4:100, vol/vol) for 18 h at 70°C (Gaver and Sweeley, 1965), and then the liberated sphingoid bases were separated on TLC plates with a solvent of chloroform/methanol/2N ammonia solution (80:20:1, vol/vol) (Williams et al., 1984). Radioactivity in separated dihydrosphingosine or sphingosine was determined by liquid scintillation counting.
For determination of distribution of SM to the external surface of cells, cells were labeled with [14C]serine for 48 h at 33°C, fixed with 0.125% glutaraldehyde in PBS for 30 min at room temperature, and treated with or without 250 mU/ml of recombinant Bacillus cereus sphingomyelinase (Higeta Shoyu) for 30 min at 37°C. Radioactive SM extracted from the cells was analyzed as described above.
Metabolic Labeling of Lipids with [3H]Sphingosine in Cells
For preparation of a complex of 100 μM [3H]sphingosine (1.85 MBq/ml) with 200 μM fatty acid–free BSA in PBS, a stock solution of [3H]sphingosine (370 KBq, 20 nmol) in ethanol was dried under a stream of nitrogen gas. The dried lipid was dispersed in 200 μl of PBS containing 200 μM fatty acid–free BSA by vortex mixing and sonication. Subconfluent cell monolayers were incubated in Nutridoma medium containing 1 μM [3H]sphingosine complexed with BSA for various times. Cellular lipids were extracted under mild alkaline conditions for efficient recovery of the sphingoid bases (Williams et al., 1984) or mild acidic conditions for efficient recovery of N-acetylneuramyl lactosylceramide (GM3), and were separated on TLC plates with a solvent of chloroform/methanol/water (65:25:4, vol/vol). Radioactivity in each lipid was determined as described above.
In Vitro Enzyme Assays
Cell lysates were used as the enzyme source for assays. Subconfluent cells were washed twice with PBS, harvested by scraping, and precipitated by centrifugation (400 g, 5 min). The cells were washed with buffer A (10 mM Hepes-Na, pH 7.5, containing 0.25 M sucrose and 5 mM EDTA), suspended in buffer A, and lysed with a probe-type sonicator. After removal of unbroken cells by centrifugation (1,000 g, 5 min), cell lysates were stored at −80°C until use.
An assay for SM synthase activity using C5-DMB-Cer was performed as described (Hanada et al., 1991), except that C5-DMB-Cer was used instead of C6-NBD-Cer. GlcCer synthase activity was determined as described (Hanada et al., 1991), except that 500 μM UDP-glucose was included in the assay mixture. Dihydrosphingosine N-acyltransferase (DCer synthase) activity was determined as described (Morell and Radin, 1970) except for using [3H]sphingosine instead of [14C]acyl-CoA. Acid and neutral sphingomyelinase activities were determined as described (Schütze and Krönke, 1995).
Metabolism of Fluorescent Cer Analogues in Cells
Metabolism of C5-DMB-Cer and C6-NBD-Cer in cells was examined under the pulse–chase conditions as described (Pagano and Martin, 1988; Pagano et al., 1991) with some modifications. In brief, subconfluent cell monolayers were incubated in Ham's F-12 containing 1.25 μM C5-DMB-Cer complexed with 1.25 μM BSA, or 5 μM C6-NBD-Cer complexed with 5 μM BSA at 4°C for 30 min. Monolayers were washed three times with Ham's F-12 and subsequently chased in Nutridoma medium containing 0.34 mg/ml fatty acid–free BSA at 33°C for various times. Lipids extracted from the cells were separated on TLC plates with a solvent of chloroform/ methanol/15 mM calcium chloride (60:35:8, vol/vol). After collecting the fluorescent spots by scraping, lipids were extracted with 0.5 ml of chloroform/methanol/0.1 M potassium chloride (1:2:0.8, vol/vol). NBD (excitation at 470 nm; emission at 530 nm) or DMB (excitation at 480 nm; emission at 515 nm) fluorescence of each lipid was measured with a spectrofluorometer. Values are presented as percentages of total fluorescence recovered (the sum of C5-DMB-Cer and C5-DMB-SM, or the sum of C6-NBD-Cer, C6-NBD-SM, and C6-NBD-GlcCer).
Fluorescence Microscopy
Cells grown on glass coverslips (22-mm diameter) were prelabeled with fluorescent lipids at 4°C and chased at 33°C under essentially the same conditions for the labeling of cells grown on culture dishes. After washing with PBS, the cells were fixed with 0.125% glutaraldehyde solution in PBS for 5 min at 4°C. The fixed specimens were observed and photographed under a fluorescence microscope. Note that the specimens were photographed soon after fixation, because redistribution of fluorescent lipid analogues might occur even in glutaraldehyde-fixed cells (Pagano et al., 1989).
Radiolabeling, Immunoprecipitation, and Endo H Treatment
CHO transfectants expressing PLAP or PLAP-HA were pulsed with 9.25 MBq/ml of EXPRESS 35S-protein labeling mix (DuPont-New England Nuclear) for 15 min at 33°C and chased for various times in Nutridoma medium containing 10 mM methionine. Radiolabeled cells were washed and lysed in 0.9 ml of lysis buffer, 50 mM Tris-HCl (pH 8.0) containing 150 mM NaCl, 0.1% SDS, and 1% Triton X-100. After addition of 100 μl of 10% BSA, the cell lysates were boiled for 5 min and clarified by centrifugation twice at 10,000 g for 10 min. The clarified extracts were incubated with 2 μl of rabbit antisera against human PLAP (Biomeda Corp.) at 4°C for 2 h. Resultant immunocomplexes were incubated with 50 μl of a 50% slurry of protein A–Sepharose CL-4B (Amersham Pharmacia Biotech) at 4°C for 2 h, pelleted, and washed once with lysis buffer containing 1% BSA and three times with lysis buffer at room temperature. Immunoprecipitates were resuspended in 50 mM sodium citrate (pH 5.5) containing 0.5% SDS, 1% 2-mercaptoethanol, and 1 mM 4-amidinophenylmethanesulfonyl fluoride, and boiled for 5 min. Immunoprecipitated proteins released from resin were incubated in the presence or absence of 6 mU of Endo H at 37°C for 16 h and separated by SDS-PAGE.
Protein Determination
Protein was determined according to Lowry et al. (1951) or with a BCA protein assay reagent kit (Pierce Chemical Co.), using BSA as standard.
Results
Metabolic Labeling of Lipids with [14C]Serine in LY-A Cells
We examined the effects of cell culture temperature on the activity of de novo SM synthesis in LY-A and wild-type cells by metabolic labeling of lipids with [14C]serine for 2 h, and found that the relative levels of SM synthesis in LY-A cells to that in wild-type cells at 33, 37, and 40°C were 9.5, 10, and 16%, respectively. Thus, further characterizations of LY-A cells were performed with cells cultured at 33°C, unless otherwise described.
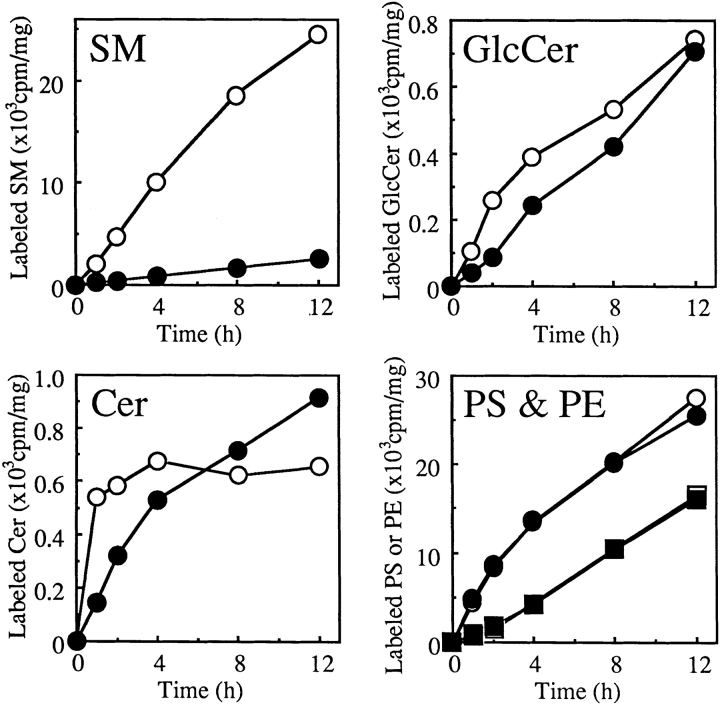
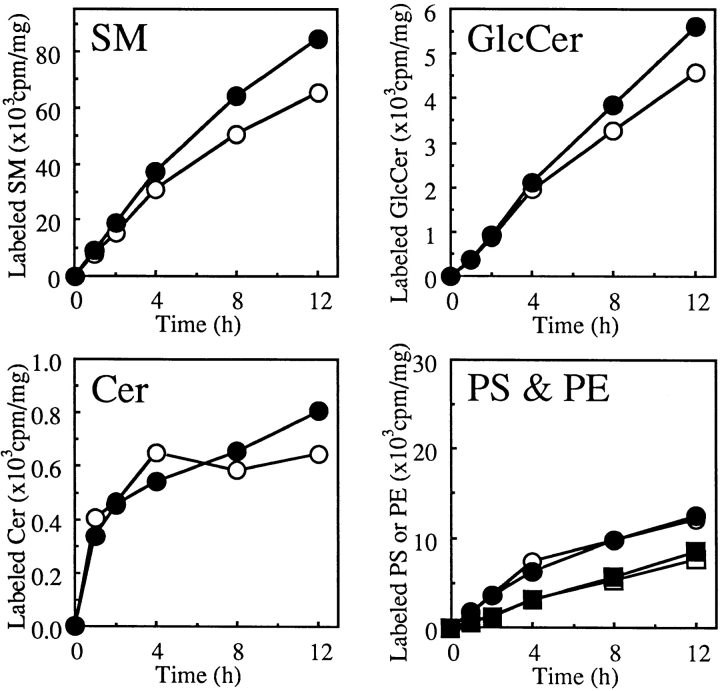
Time courses of metabolic labeling of lipids with [14C] serine up to 12 h were compared between LY-A and wild-type cells. As shown in Fig. 1, the SM labeling in LY-A cells was <10% of the wild-type level throughout the incubation. In contrast, time courses for Cer and GlcCer labeling in LY-A cells were comparable to those in wild-type cells, although the early labeling rates of these lipids in LY-A cells were slower than wild-type cells. LY-A and wild-type cells were virtually identical in the labeling of phosphatidylserine or phosphatidylethanolamine, both of which incorporate serine via metabolic pathways distinct from the pathway for sphingolipids. These results confirmed that LY-A cells are defective in SM synthesis, but not in glycosphingolipid synthesis.
Figure 1.
Metabolic labeling of lipids with [14C]serine in wild-type and LY-A cells. Cells were incubated with [14C]serine for the indicated times at 33°C. Radioactivity of each labeled lipid was determined as described in Materials and Methods. Open symbols, wild-type CHO cells; closed symbols, mutant LY-A cells. Circles, phosphatidylserine; squares, phosphatidylethanolamine, in the panel named PS & PE.
Although DCer comigrated with Cer in the TLC system used, analysis of sphingoid base composition of metabolically labeled lipids in the TLC fraction containing both DCer and Cer showed that >80% of sphingoid bases derived from the fraction was sphingosine in both LY-A and wild-type cells, indicating that conversion of DCer to Cer is normal in LY-A cells.
For determination of the distribution of SM at the cell surface, cells were labeled with [14C]serine for 48 h, fixed with glutaraldehyde, and treated with or without B. cereus sphingomyelinase. Analysis of radioactive lipids extracted from the cells showed that the pool size of sphingomyelinase-sensitive SM among the total pool of cellular SM was 56% in wild-type cells and 50% in LY-A cells. Thus, SM appeared to be preferentially distributed in the outer leaflet of the plasma membrane bilayer in LY-A cells, as in wild-type cells.
Metabolic Labeling of Lipids with [14C]Sphingosine and [14C]Choline in LY-A Cells
A defect in SM formation might result in some repression of de novo sphingoid base formation. Thus, to prevent such possible feedback repression from affecting Cer-to-SM conversion by metabolic labeling, we used [3H]sphingosine as an alternative precursor for monitoring SM synthesis, since it has been shown that CHO cells are able to utilize exogenously supplied sphingosine for synthesis of Cer and complex sphingolipids (Hanada et al., 1992). When cells were labeled with 1 μM [3H]sphingosine at 33°C for various times up to 2 h, wild-type and LY-A cells similarly accumulated free [3H]sphingosine, with a plateau level at 15–30 min after incubation, and showed almost equal levels of the total radioactivity of cell-associated lipids (Fig. 2), indicating that the two cell types had an equal efficiency of sphingosine uptake. Under these conditions, formation of radioactive SM in LY-A cells was <30% of the wild-type level throughout the incubation, whereas formations of Cer, GlcCer, and GM3 were slightly higher in LY-A cells than in wild-type cells (Fig. 2). When [3H]dihydrosphingosine was used as another precursor, we obtained results similar to those obtained with [3H]sphingosine (data not shown).
Figure 2.
Metabolic labeling of sphingolipids with [3H]sphingosine in wild-type and LY-A cells. Cells were incubated with 1 μM [3H]sphingosine for the indicated times at 33°C. Radioactivity of each labeled lipid was determined as described in Materials and Methods. Open symbols, wild-type CHO cells; closed symbols, mutant LY-A cells; So, sphingosine.
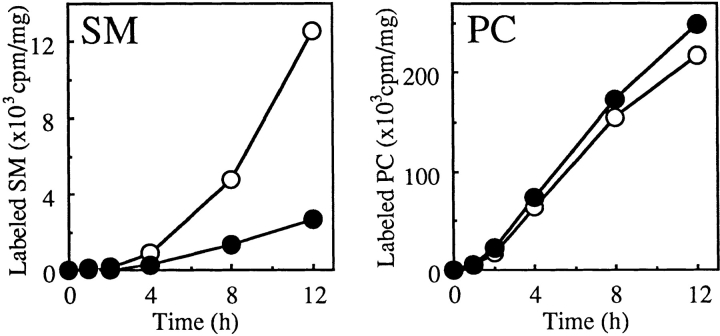
Synthesis of SM was also examined by metabolic labeling with [14C]choline. Incorporation of [14C]choline into SM in LY-A cells was ∼20% or less of the wild-type level, while incorporation of [14C]choline into phosphatidylcholine (the phosphorylcholine donor for conversion of Cer to SM) was slightly higher in LY-A cells than in wild-type cells (Fig. 3), eliminating the possibility that LY-A cells had a defect in phosphatidylcholine synthesis.
Figure 3.
Metabolic labeling of lipids with [14C]choline in wild-type and LY-A cells. Cells were incubated with [14C]choline for the indicated times at 33°C. Radioactivity of each labeled lipid was determined as described in Materials and Methods. Open symbols, wild-type CHO cells; closed symbols, mutant LY-A cells; PC, phosphatidylcholine.
Cell-free Assays for Activities of Enzymes Involved in SM Metabolism
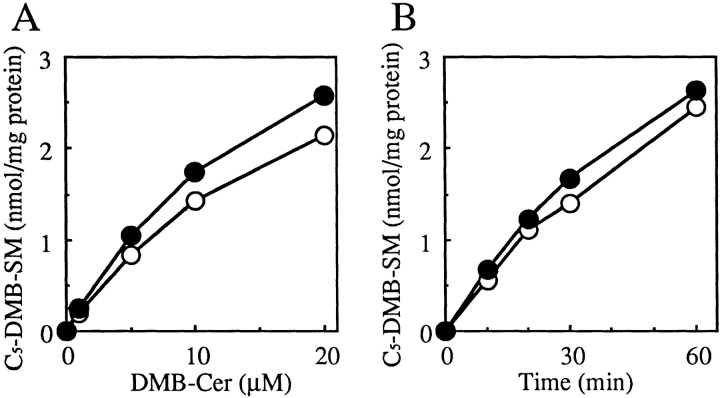
To identify the mechanism underlying the defect in conversion of Cer to SM in LY-A cells, we examined SM synthase activity in cell lysates by using C5-DMB-Cer, a fluorescent Cer analogue, as the substrate. LY-A cells showed essentially the same level of SM synthase activity as wild-type cells, as there was no difference in the dependence of C5-DMB-SM formation on C5-DMB-Cer concentration or in the time course of C5-DMB-SM formation between wild-type and LY-A cells (Fig. 4, A and B). Similarly, when C6-NBD-Cer or N-[14C]palmitoyl-sphingosine was used as the substrate, no difference in activity of SM synthase between the two cell types was observed (data not shown).
Figure 4.
SM synthase activity in wild-type and LY-A cell lysates. (A) The dependence of C5-DMB-SM formation on a dose of C5-DMB-Cer. Cell lysate (100 μg protein) was incubated in an SM synthase assay mixture containing the indicated amounts of C5-DMB-Cer for 30 min. (B) Time course of C5-DMB-SM formation. Cell lysate (100 μg protein) was incubated in an SM synthase assay mixture containing 10 μM C5-DMB-Cer for the indicated times. C5-DMB-SM produced was determined as described in Materials and Methods. Open symbols, wild-type CHO lysate; closed symbols, mutant LY-A lysate.
We also measured in vitro activities of various other enzymes related to SM metabolism including serine palmitoyltransferase, dihydroceramide synthase, GlcCer synthase, and acid and neutral sphingomyelinases. However, significant differences in these activities between wild-type and LY-A cells were not detected (data not shown; see also Hanada et al., 1998 for analysis of serine palmitoyltransferase activity). Collectively, the results of these in vivo and in vitro experiments suggested that the specific defect of SM formation in LY-A cells was not due to alterations in enzymatic activities responsible for SM synthesis or degradation.
Effect of Brefeldin A on De Novo SM Synthesis in LY-A Cells
We next explored the possibility that LY-A cells had a defect in translocation of Cer from the ER to the Golgi apparatus where SM synthase exists. Treatment of CHO cells with brefeldin A, leading to fusion of the Golgi membrane with the ER (Klausner et al., 1992), enhances the rate of conversion of Cer to SM, suggesting that in brefeldin A–treated cells Cer is accessible to the normally Golgi apparatus–localized SM synthase without the ER-to-Golgi apparatus trafficking process (Warnock et al., 1994; van Helvoort et al., 1997). Thus, if LY-A cells have a defect in ER-to-Golgi apparatus trafficking of Cer, brefeldin A treatment should restore SM synthesis in LY-A cells to the wild-type level.
After pretreatment with 1 μg/ml brefeldin A for 30 min at 33°C, cells were labeled with [14C]serine in the presence of brefeldin A, and the labeling rates of lipids were compared between wild-type and LY-A cells. Under conditions without brefeldin A, the rate of SM synthesis in LY-A cells was only 10% of the wild-type level, while the labeling rates of other lipids in LY-A cells were comparable to the wild-type levels (Fig. 1). In contrast, under brefeldin A–treated conditions, the rate of SM synthesis in LY-A cells was quite similar to the wild-type level, which was about threefold higher than the rate in brefeldin A–untreated wild-type cells, while labeling rates of Cer, GlcCer, phosphatidylserine, and phosphatidylethanolamine were also similar between wild-type and LY-A cells (Fig. 5). In addition, we used [3H]sphingosine and [3H]dihydrosphingosine for monitoring the rate of Cer-to-SM conversion and observed restoration of SM synthesis in LY-A cells to the wild-type levels under brefeldin A–treated conditions (data not shown). These results demonstrated that LY-A cells had the Golgi apparatus–localized SM synthase normally and suggested that LY-A cells were defective in translocation of Cer from the ER to the Golgi apparatus.
Figure 5.
Effect of brefeldin A on metabolic labeling of lipids with [14C]serine in wild-type and LY-A cells. Cells were preincubated in Nutridoma medium containing 1 μg/ml brefeldin A for 30 min at 33°C and then labeled with [14C]serine for the indicated times at 33°C. Radioactivity of each labeled lipid was determined as described in Materials and Methods. Open symbols, wild-type CHO cells; closed symbols, mutant LY-A cells. Circles, phosphatidylserine; squares, phosphatidylethanolamine, in the panel named PS & PE.
Metabolism of a Fluorescent Cer Analogue, C5-DMB-Cer, in LY-A Cells
To examine further whether LY-A cells were defective in ER-to-Golgi apparatus trafficking of Cer, we used C5-DMB-Cer, a fluorescent Cer analogue, as a probe. Since the critical micellar concentration of C5-DMB-Cer is higher than that of natural Cer (Pagano et al., 1991), externally supplied C5-DMB-Cer can be readily transferred to the plasma membrane and, after movement across the plasma membrane, this probe fluorescently labels various intracellular membranes including the ER membrane in living cells at 4°C. In addition, when cells prelabeled with C5-DMB-Cer are warmed up to physiological temperatures, C5-DMB-Cer is efficiently accumulated into the Golgi region and metabolized to C5-DMB-SM (Pagano et al., 1991; Ktistakis et al., 1995), indicating the possibility that the ER-to-Golgi apparatus trafficking behavior of C5-DMB-Cer mimics that of natural Cer produced at the ER.
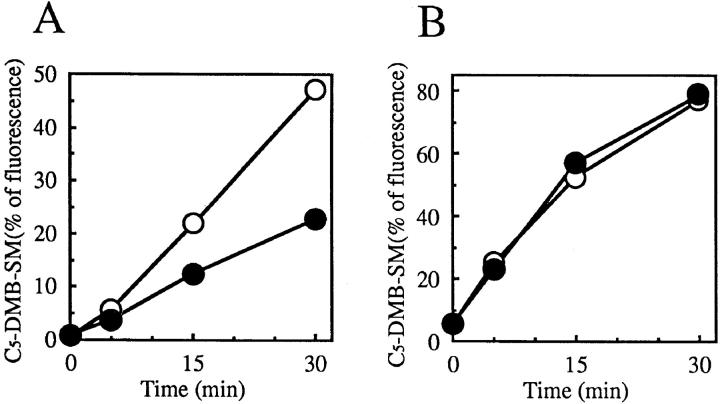
For testing whether the behavior of C5-DMB-Cer in cells reflected the defect of SM synthesis in LY-A cells, the metabolic rate of C5-DMB-Cer to C5-DMB-SM was compared between wild-type and LY-A cells. Cells were preincubated with C5-DMB-Cer for 30 min at 4°C for transferring the probe to intracellular membranes and, after washing the extracellular C5-DMB-Cer, cells were incubated at 33°C for various times to start conversion of intracellular C5-DMB-Cer to C5-DMB-SM. During the chase, C5-DMB-Cer was metabolized to C5-DMB-SM in a time-dependent manner, resulting in conversion of 50% of the total lipidic fluorescence to C5-DMB-SM in wild-type cells 30 min after the chase, while the metabolic rate of C5-DMB-Cer to C5-DMB-SM in LY-A cells was about half of the wild-type level (Fig. 6 A). In addition, the metabolic rate of C5-DMB-Cer to C5-DMB-SM in LY-A cells was restored to the wild-type level by brefeldin A treatment (Fig. 6 B). Note that the level of total lipidic DMB fluorescence associated with cells was identical in the two cell types during the pulse and chase (data not shown). These results indicated that the behavior of C5-DMB-Cer at least partly reflects that of natural Cer in cells.
Figure 6.
Metabolism of C5-DMB-Cer in wild-type and LY-A cells in the presence or absence of brefeldin A. Wild-type CHO (open circles) and mutant LY-A (closed circles) cells were preincubated in Nutridoma medium without (A) or with (B) 1 μg/ml brefeldin A for 30 min at 33°C, then incubated with 1.25 μM C5-DMB-Cer for 30 min at 4°C. After washing, cell monolayers were further incubated in Nutridoma medium containing 0.34 mg/ml BSA without or with 1 μg/ml brefeldin A for the indicated times at 33°C. Cellular lipids were extracted and analyzed by TLC. The formation of the DMB derivative of GlcCer was too low (<5% of the level of C5-DMB-SM) to be determined accurately.
Intracellular Behavior of Pulse-labeled C5-DMB-Cer in LY-A Cells
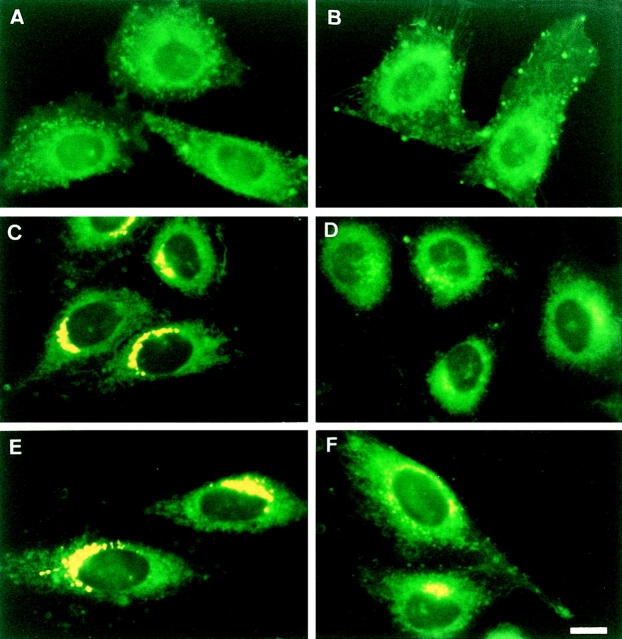
Distribution patterns of intracellular DMB fluorescence were compared between LY-A and wild-type cells under the pulse–chase conditions. After prelabeling of cells with C5-DMB-Cer at 4°C, intracellular fluorescence was distributed throughout intracellular membranes including the ER in both wild-type and LY-A cells (Fig. 7, A and B). After the chase at 33°C for 15–30 min, most of the intracellular fluorescence in wild-type cells accumulated in the perinuclear region corresponding to the Golgi complex (Fig. 7, C and E), in agreement with previous studies (Pagano et al., 1991; Ktistakis et al., 1995). Interestingly, accumulation of intracellular fluorescence in the Golgi region was lower in LY-A cells than wild-type cells (Fig. 7, D and F vs. C and E). The lower level of the Golgi fluorescence in LY-A cells was not due to an enhanced secretion of intracellular DMB fluorescence to the medium, because there was no significant difference in the total amount of DMB lipids associated with cells between the two cell types during the pulse and chase periods (data not shown). These observations also provided evidence that LY-A cells are defective in ER-to-Golgi apparatus trafficking of Cer.
Figure 7.
Intracellular distribution of DMB fluorescence in wild-type and LY-A cells over time at 33°C after labeling with C5-DMB-Cer. Wild-type CHO (A, C, and E) and mutant LY-A (B, D, and F) cells were incubated with 1.25 μM C5-DMB-Cer for 30 min at 4°C, washed, further incubated in Nutridoma medium containing 0.34 mg/ml BSA for 0 (A and B), 15 (C and D), or 30 (E and F) min at 33°C, and photographed. All photomicrographs were exposed and printed identically. Bar, 10 μm.
Energy Dependence of Redistribution of Intracellular C5-DMB-Cer to the Golgi Apparatus
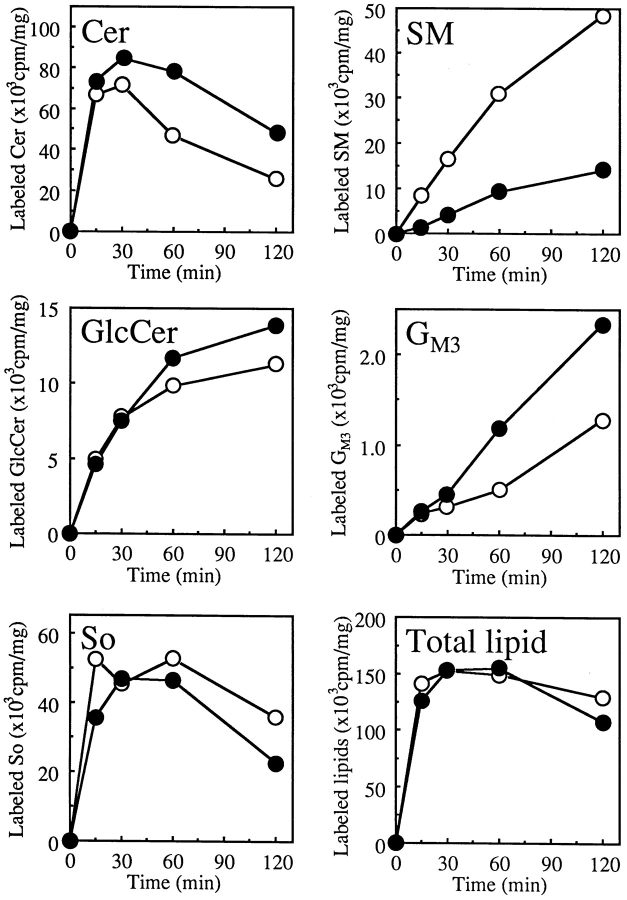
For examination of the energy dependence of intracellular redistribution of C5-DMB-Cer, cells were pretreated with or without energy inhibitors (50 mM 2-deoxy-d-glucose and 5 mM NaN3) for 15 min at 33°C, incubated with C5-DMB-Cer for 30 min at 4°C, washed, and chased for 15 min at 33°C in the presence or absence of the energy inhibitors. Measurements of intracellular ATP showed that wild-type and LY-A cells had the same ATP levels under the inhibitor-minus control conditions, and ATP levels in both cell types were reduced by >95% in the presence of the inhibitors. ATP depletion did not affect the level and distribution of C5-DMB-Cer during the prelabeling of cells at 4°C; DMB fluorescence was distributed to various intracellular membranes (data not shown), as seen under the normal conditions (Fig. 7, A and B). However, after the chase at 33°C, the level of DMB fluorescence accumulated into the Golgi region in wild-type cells was strikingly lower under the ATP-depleted conditions than under the control conditions, while in LY-A cells ATP depletion did not appreciably affect the Golgi level of DMB fluorescence (Fig. 8, A–D). Consequently, ATP depletion reduced the DMB fluorescence level associated with the Golgi apparatus in wild-type cells to the level of LY-A cells.
Figure 8.
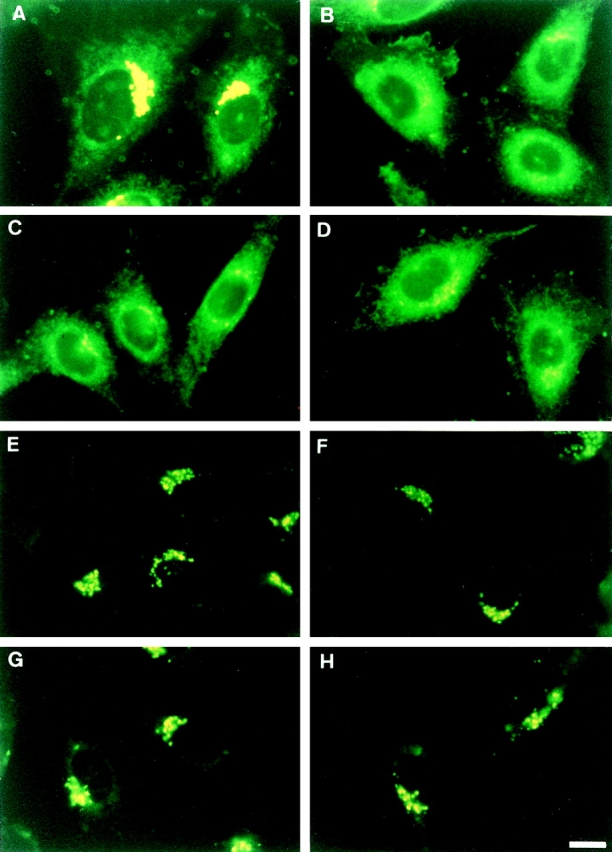
Effect of ATP depletion on redistribution of intracellular fluorescence in wild-type and LY-A cells labeled with C5-DMB-Cer or C6-NBD-Cer. Cells were preincubated in Ham's F-12 with or without energy inhibitors (50 mM 2-deoxy-d-glucose and 5 mM NaN3) for 15 min at 33°C, then incubated with 1.25 μM C5-DMB-Cer (A–D) or 5 μM C6-NBD-Cer (E–H) for 30 min at 4°C. After washing, cell monolayers were further incubated in Nutridoma medium containing 0.34 mg/ ml BSA with or without the inhibitors for 15 min at 33°C, and photographed. (A and E) Wild-type cells without inhibitors; (B and F) mutant LY-A cells without inhibitors; (C and G) wild-type cells with inhibitors; (D and H) mutant LY-A cells with inhibitors. For each fluorescent probe, all photomicrographs were exposed and printed identically. Bar, 10 μm.
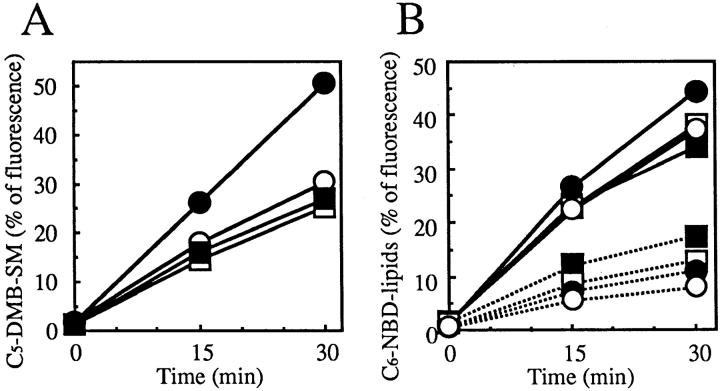
Biochemical analysis showed that the ATP-depleted conditions caused an ∼50% reduction of the conversion rate of C5-DMB-Cer to C5-DMB-SM in wild-type cells (Fig. 9), indicating that the ATP-dependent redistribution of C5-DMB-Cer is responsible for synthesis of C5-DMB-SM in wild-type cells. The reduced rate in wild-type cells was almost identical to the rate in LY-A cells, which was not affected by ATP depletion (Fig. 9), being consistent with the similar level of DMB fluorescence in Golgi apparatus between ATP-depleted wild-type and normally cultured LY-A cells (Fig. 8, B and C). There was no difference in the total amount of lipidic DMB fluorescence associated with cells between the normal and ATP-depleted cells even after the 33°C chase (data not shown). Collectively, these results indicated that, in wild-type cells, redistribution of intracellular C5-DMB-Cer to the Golgi apparatus for C5-DMB-SM synthesis consists of an ATP-dependent pathway(s) and an ATP-independent (or less ATP-dependent) pathway(s), and that the ATP-dependent trafficking pathway of C5-DMB-Cer is almost completely impaired in LY-A cells.
Figure 9.
Metabolism of C5-DMB-Cer and C6-NBD-Cer in normal and ATP-depleted cells over time. Cells were preincubated in Ham's F-12 with or without energy inhibitors (50 mM 2-deoxy- d-glucose and 5 mM NaN3) for 15 min at 33°C, then incubated with 1.25 μM C5-DMB-Cer (A) or 5 μM C6-NBD-Cer (B) for 30 min at 4°C. After washing, cell monolayers were further incubated in Nutridoma medium containing 0.34 mg/ml BSA with or without the inhibitors for the indicated time at 33°C. Lipids extracted from the cells were separated by TLC, and the amounts of C5-DMB-SM (solid lines in A), C6-NBD-SM (solid lines in B), and C6-NBD-GlcCer (dotted lines in B) were determined. Formation of C5-DMB-GlcCer was too low to be quantified. Closed circles, wild-type cells without inhibitors; open circles, wild-type cells with inhibitors; closed squares, mutant LY-A cells without inhibitors; open squares, mutant LY-A cells with inhibitors.
Metabolism and Intracellular Behavior of C6-NBD-Cer in Cells
While C5-DMB-Cer is a poor substrate for GlcCer synthase (Pagano et al., 1991; Paul et al., 1996), C6-NBD-Cer, another fluorescent analogue of Cer, serves as an efficient substrate for both GlcCer and SM syntheses (Futerman and Pagano, 1991). C6-NBD-Cer has an ∼10-fold faster rate at spontaneous transfer between artificial membranes than C5-DMB-Cer (Rosenwald and Pagano, 1993; Bai and Pagano, 1997), and C6-NBD-Cer rapidly targets to the Golgi membrane even in glutaraldehyde-fixed cells, suggesting that no active process is required for ER-to-Golgi apparatus transfer of C6-NBD-Cer (Pagano et al., 1989).
Under normal culture or ATP-depleted conditions, wild- type and LY-A cells were preincubated with C6-NBD-Cer at 4°C to label intracellular membranes and, after washing extracellular C6-NBD-Cer, cells were incubated at 33°C for the chase. During the chase, C6-NBD-Cer was converted to C6-NBD-SM or C6-NBD-GlcCer in a time-dependent manner with redistribution of NBD fluorescence to the Golgi apparatus, in agreement with a previous study (Lipsky and Pagano, 1983). In contrast to the cases with C5-DMB-Cer (Figs. 8, A–D, and 9 A), redistribution of C6-NBD-Cer to the Golgi region or conversion of C6-NBD-Cer to C6-NBD-SM was not affected by ATP depletion even in wild-type cells, and C6-NBD-Cer behaved almost identically between LY-A and wild-type cells (Figs. 8, E–H, and 9 B). The conversion rate of C6-NBD-Cer to C6-NBD-GlcCer under the ATP-depletion conditions was ∼70–75% of the normal level, probably due to a decrease in the intracellular UDP-glucose level by ATP depletion. These results indicated that C6-NBD-Cer is efficiently redistributed to the Golgi apparatus not via the ATP-dependent pathway, which is involved in the redistribution of C5-DMB-Cer to the Golgi apparatus. However, this nature of C6-NBD-Cer allowed us to demonstrate that the ATP-depleted conditions used did not cause an inhibition of SM synthase activity itself or extensive destruction of the Golgi apparatus.
Effects of ATP Depletion on SM and GlcCer Formation from [3H]Sphingosine
We next examined the effect of ATP depletion on conversion of natural Cer to SM by metabolic labeling with [3H]sphingosine. ATP depletion of wild-type cells resulted in the reduction of the [3H]SM formation to the level of LY-A cells, which was not affected by ATP depletion (Fig. 10), being consistent with the results of the pulse–chase experiments using C5-DMB-Cer (Fig. 9 A). Although the [3H]SM formation in wild-type cells under the ATP-depleted conditions was only 30% of the normal level, the [3H]GlcCer formation under the ATP-depleted conditions was ∼65% of the normal level both in wild-type and LY-A cells (Fig. 10). Considering that GlcCer formation appeared to be partially inhibited by a reduction of the UDP-glucose level under the ATP-depletion conditions, effects of ATP depletion on accessibility of [3H]Cer to SM and GlcCer synthases could be estimated by the corrected values of [3H]SM/C6-NBD-SM and [3H]GlcCer/C6-NBD-GlcCer, respectively, although we did not eliminate the possibility that access of C6-NBD-Cer to SM and GlcCer synthases was not completely ATP independent. The corrected values suggested that the influence of ATP depletion on [3H]Cer trafficking caused ∼70% and ∼20% inhibition of [3H]SM and [3H]GlcCer formation, respectively (Fig. 10, bottom). ATP depletion did not reduce the level of [3H]sphingosine associated with cells and did cause an increase in the level of [3H]Cer along with the striking decrease in [3H]SM, ruling out the possibility that the decreased formation of [3H]SM under the ATP-depleted conditions was due to a decrease in cellular uptake of [3H]sphingosine or formation of [3H]Cer from [3H]sphingosine. These results demonstrated evidence that trafficking of natural Cer from the ER to the site for SM synthesis is mainly ATP dependent and that this ATP-dependent trafficking of Cer is defective in LY-A cells.
Figure 10.
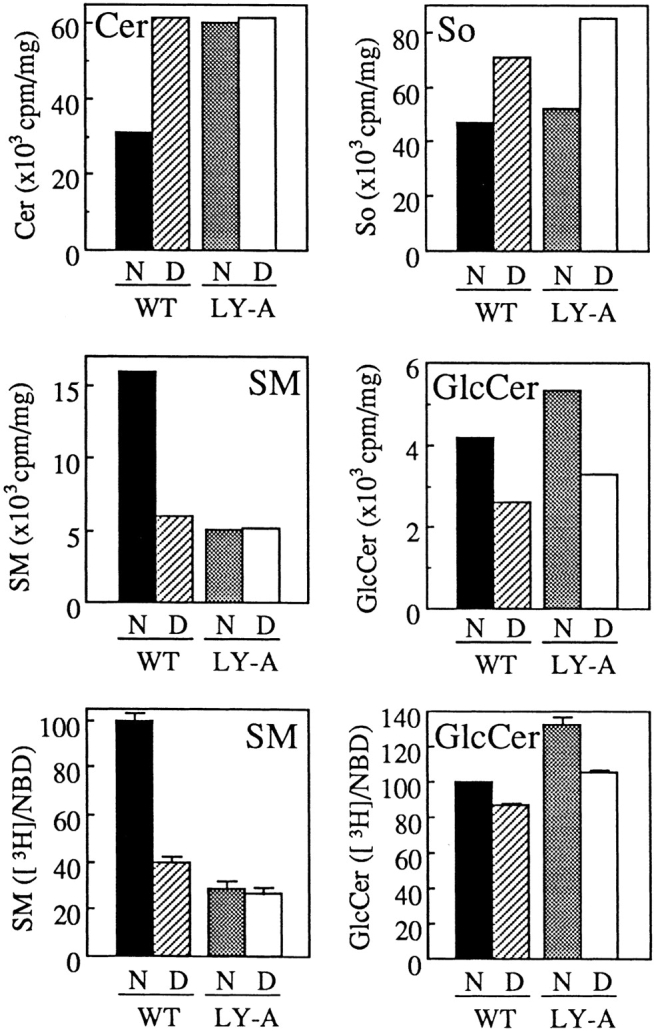
Effect of ATP depletion on metabolism of sphingolipids labeled by [3H]sphingosine in wild-type and LY-A cells. (Top and middle) After ATP depletion of cells as described in the legend to Fig. 9, wild-type CHO (WT) and mutant LY-A cells were incubated with 1 μM [3H]sphingosine for 30 min at 33°C. Radioactivity of each labeled lipid was determined as described in Materials and Methods. The values of [3H]lactosylceramide and [3H]GM3 were both <10% of those of [3H]GlcCer. (Bottom) For the estimation of specific effects of ATP depletion on accessibility of [3H]Cer to SM and GlcCer synthases, the corrected values of [3H]SM/C6-NBD-SM and [3H]GlcCer/C6-NBD-GlcCer are shown as percentages of the value of normal cultured wild-type cells. The values of NBD-SM and -GlcCer formation are from the data shown in Fig. 9 B. N, normal culture conditions; D, ATP-depleted conditions; So, sphingosine.
ER-to-Golgi Apparatus Trafficking of GPI-anchored and Membrane-spanning Glycoproteins in LY-A Cells
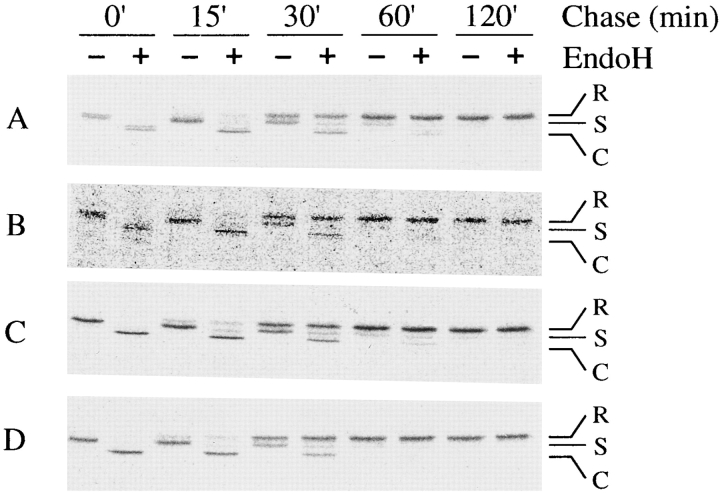
To address the question of whether ER-to-Golgi apparatus trafficking of proteins was affected in LY-A cells, we determined the acquisition rate of Endo H resistance, a well-accepted determinant for arrival in the medial Golgi apparatus (Kornfeld and Kornfeld, 1985). For this, we used wild-type and LY-A cells constitutively expressing GPI-anchored alkaline phosphatase (PLAP) or chimeric alkaline phosphatase (PLAP-HA) having a membrane-spanning region in place of the GPI anchor. PLAP and PLAP-HA in these cells were pulse-labeled with [35S]methionine/cysteine for 15 min and chased for various time at 33°C. Then, extracts from the cells were immunoprecipitated with anti-PLAP antibody, treated with Endo H, and separated by SDS-PAGE for detection of the Endo H–sensitive and –resistant forms. From the data shown in Fig. 11, the acquisition rates of Endo H resistance of not only PLAP but also PLAP-HA were quite similar (t 1/2 = ∼30 min) between wild-type and LY-A cells. Thus, it was likely that the machinery for ER-to-Golgi apparatus trafficking of glycoproteins operated normally in LY-A cells.
Figure 11.
ER-to-Golgi apparatus trafficking of GPI-anchored or membrane-spanning glycoprotein in wild-type and LY-A cells. Cells were pulsed for 15 min with [35S]methionine/cysteine and chased for the indicated time. Immunoprecipitates were treated with Endo H for 16 h at 37°C and separated by SDS-PAGE as described in Materials and Methods. (A and C) Wild-type CHO cell derivatives expressing PLAP and PLAP-HA, respectively; (B and D) mutant LY-A cell derivatives expressing PLAP and PLAP-HA, respectively. R, Endo H–resistant form; S, Endo H–sensitive form; C, cleaved form by Endo H.
Discussion
LY-A Cells Have a Specific Defect in an ATP-dependent Trafficking Pathway of Cer from the ER to the Golgi Compartment for De Novo SM Synthesis
Previously, we isolated the LY-A strain, a CHO cell mutant resistant to an SM-directed cytolysin, and showed that LY-A cells have only a 30% level of SM content, but nearly a 100% level of glycosphingolipid content compared with wild-type cells (Hanada et al., 1998). In this study, we attempted to identify a defective step causing the phenotype of LY-A cells.
Metabolic labeling experiments with [14C]serine, [3H] sphingosine, and [14C]choline indicated that conversion of Cer to SM was impaired in LY-A cells, but that the formations of Cer and phosphatidylcholine, both of which are immediate precursors for SM formation, are normal (Figs. 1–3). However, enzyme assays in cell lysates showed no difference in the activities of SM synthase, neutral sphingomyelinase, or acid sphingomyelinase between LY-A and wild-type cells. Although reduced SM synthesis along with Cer accumulation was observed during the mitotic phase of the cell cycle (Yokoyama et al., 1997), LY-A and wild-type cells grown in Nutridoma medium displayed virtually identical patterns of the phase distribution of the cell cycle; 30–32% of the total population of each cell type was in the G1 phase, 50–54% in the S phase, and 15–17% in the G2 plus M phases (data not shown). These in vivo and in vitro experiments suggested that the defect of SM formation in LY-A cells was not due to alterations in enzymatic activities responsible for SM metabolism nor to a secondary effect of cell-cycle phase on the lipid metabolism, leading us to the possibility that LY-A cells had a defect in translocation of Cer from the ER to the Golgi compartment where SM synthase exists.
Several lines of evidence support this conclusion. First, when cells were treated with brefeldin A, SM synthesis in LY-A cells was restored to the wild-type level (Fig. 5), in agreement with the expectation that in brefeldin A–treated cells Cer is accessible to the normally Golgi apparatus–localized SM synthase without the ER-to-Golgi apparatus trafficking process. Second, pulse–chase experiments with C5-DMB-Cer in intact cells demonstrated that redistribution of intracellular DMB fluorescence from intracellular membranes to the Golgi apparatus is strikingly slower in LY-A cells than in wild-type cells (Fig. 7), along with the defect of conversion of C5-DMB-Cer to C5-DMB-SM in intact LY-A cells (Fig. 6 A). Furthermore, ATP depletion in wild-type cells inhibited the conversion of C5-DMB-Cer to C5-DMB-SM by 50% and also inhibited redistribution of C5-DMB-Cer to the Golgi apparatus, whereas ATP depletion did not affect the behavior of C5-DMB-Cer in LY-A cells (Figs. 8, A–D, and 9 A). Although conversion of C6-NBD-Cer to the SM metabolite may be necessary for trapping NBD fluorescence in the Golgi apparatus (Pütz and Schwarzmann, 1995), at least a portion of the Golgi apparatus–accumulated DMB fluorescence in wild-type cells appears to be attributable to unconverted C5-DMB-Cer, because the amount of C5-DMB-SM formed in LY-A cells after a 30-min chase was equivalent to that of C5-DMB-SM formed in wild-type cells after a 15-min chase (Fig. 6 A), whereas DMB fluorescence in the Golgi region was much denser in the latter cells than in the former (Fig. 7, C and F). By contrast, intracellular labeling with C6-NBD-Cer, which rapidly transfers between membranes, displayed no difference in the Golgi redistribution between LY-A and wild-type cells, or between normally cultured and ATP-depleted cells (Fig. 8, E–H). Thus, the impairment of the Golgi accumulation of C5-DMB-Cer in LY-A cells reflects a defect in the energy-dependent transport of this probe from intracellular membranes to the Golgi apparatus. In addition, the findings that intracellular DMB fluorescence is initially distributed throughout intracellular membranes after prelabeling with C5-DMB-Cer at 4°C (Fig. 7, A and B; see also Pagano et al., 1991) and that >50% of the surface area of intracellular membranes is attributed to the ER membrane (Griffiths et al., 1989) indicate that the energy-dependent redistribution of DMB fluorescence most likely represents an ER-to-Golgi apparatus transport of C5-DMB-Cer. Importantly, this energy-dependent trafficking of C5-DMB-Cer is relevant to physiological trafficking of natural Cer for SM synthesis, because ATP depletion in wild-type cells inhibited [3H]SM formation from metabolically labeled [3H]Cer, whereas [3H]SM formation in LY-A cells was not affected by ATP depletion (Fig. 10). Collectively, we conclude that LY-A cells are defective in ATP-dependent trafficking of Cer from the ER to the Golgi compartment where SM synthase exists.
The defect in LY-A cells appears to be specific to Cer transport. When cellular lipids were metabolically labeled with [14C]serine, the formation rates of [14C]phosphatidylserine and [14C]phosphatidylethanolamine were identical between LY-A and wild-type cells (Fig. 1), indicating that transport of phosphatidylserine from the ER to the mitochondria proceeds normally in LY-A cells. Cholesterol metabolism in LY-A cells is also suggested to be normal, since the amount of free cholesterol in LY-A cells is the wild-type level (Hanada et al., 1998). There was no appreciable difference in the acquisition rate of Endo H resistance of a membrane-spanning or a GPI-anchored protein between LY-A and wild-type cells (Fig. 11), indicating that ER-to-Golgi apparatus trafficking of proteins is not defective in LY-A cells. Both wild-type and LY-A cells display a similar preferential distribution of SM at the plasma membrane (data not shown), suggesting that, once SM is produced in the Golgi apparatus, SM is subsequently sorted to the plasma membrane in LY-A cells similar to wild-type cells.
Our findings that in wild-type CHO cells ATP depletion inhibits conversion of C5-DMB-Cer to C5-DMB-SM by 50% concomitantly with inhibition of redistribution of intracellular DMB fluorescence to the Golgi apparatus (Figs. 8 and 9 A) suggest that a ∼50% portion of ER-to-Golgi apparatus transfer of C5-DMB-Cer is mediated via the ATP-dependent pathway and that another 50% of the C5-DMB-Cer transfer is mediated by ATP-independent (or less ATP-dependent) mechanisms. The ATP-independent redistribution of C5-DMB-Cer to the Golgi apparatus may be at least partly attributable to a nonphysiological spontaneous transfer of C5-DMB-Cer between membranes due to less hydrophobicity of C5-DMB-Cer than natural Cer. For natural Cer, ATP-dependent and -independent (or less ATP-dependent) trafficking mechanisms were estimated to be responsible for 70–80% and 20–30%, respectively, of newly synthesized SM, since ATP depletion caused a 70–80% inhibition of formation of [3H]sphingosine-derived SM without reduction of [3H]Cer formation (Fig. 10). In contrast, redistribution of intracellular C6-NBD-Cer to the Golgi apparatus or conversion of C6-NBD-Cer to C6-NBD-SM was not, or only slightly, affected by ATP depletion. The different trafficking behavior between C5-DMB-Cer and C6-NBD-Cer most likely reflects the fact that C6-NBD-Cer has a much faster rate of spontaneous transfer between artificial membranes than C5-DMB-Cer (Rosenwald and Pagano, 1993; Bai and Pagano, 1997), implying that C5-DMB-Cer is embedded in membranes more firmly and thus mimics natural Cer more accurately, compared with C6-NBD-Cer.
Different Pathways of Cer Transport for SM versus GlcCer Synthesis
It has been postulated that a yet-undefined pathway(s) mediates Cer trafficking from the ER to the Golgi apparatus and that, after arrival at the cytoplasmic surface of the Golgi apparatus, Cer molecules are used for GlcCer synthesis and the residual Cer molecules are subsequently used for SM synthesis after translocation to the lumenal side of the Golgi apparatus (reviewed in Hoekstra and Kok, 1992; Rosenwald and Pagano, 1993; van Echten and Sandhoff, 1993; van Meer, 1993). Collins and Warren (1992) have shown that newly synthesized Cer can be delivered to the sites of SM and GlcCer synthases by a different pathway from the major vesicular-mediated trafficking pathway for newly synthesized proteins during the mitotic phase in HeLa cells. Slomiany et al. (1992) have demonstrated that Cer-containing vesicles derived from the ER fuse with the Golgi membrane in an N-ethylmaleimide– sensitive manner under cell-free conditions. On the other hand, studies with another cell-free system and with a semi-intact cell system to assay for the transfer of Cer between the ER and the Golgi membranes have suggested that Cer could be transferred from the ER to the Golgi apparatus through a nonvesicular pathway in ATP-independent and N-ethylmaleimide–insensitive manners (Moreau et al., 1993; Kok et al., 1998).
If ER-to-Golgi apparatus trafficking of Cer is mediated by only one pathway, it would be a paradox that LY-A cells synthesize GlcCer to the wild-type level or more, in spite of the defect of the ATP-dependent ER-to-Golgi apparatus trafficking of Cer. A possible explanation for the paradoxical phenotype of LY-A cells is that the putative sole pathway for ER-to-Golgi apparatus Cer trafficking might be narrowed in LY-A cells, but that the narrowed pathway might still supply enough Cer to produce a normal level of GlcCer in the Golgi apparatus, but not of SM. However, this explanation is not consistent with the observation that formations of [3H]GlcCer and [3H]GM3 from [3H]sphingosine were somewhat higher in LY-A cells than in wild-type cells (Fig. 2). It is unlikely that the reduced synthesis of SM results from an overconsumption of newly synthesized Cer at the GlcCer synthesis step, since the level of [3H]sphingosine-derived Cer was higher in LY-A cells than in wild-type cells (Fig. 2) and there was no difference in GlcCer synthase activity between the two cell types (data not shown). Another possibility is that a transbilayer transport of Cer across the Golgi membranes is impaired in LY-A cells. However, the observation that SM synthesis in LY-A cells was restored to the wild-type level after brefeldin A treatment (Fig. 5) favors the interpretation that the phenotype of LY-A cells is primarily due to a defect in the interorganelle transport of Cer, although we cannot completely exclude the possibility that brefeldin A treatment facilitates a transbilayer transport of Cer across the membrane where SM synthase exists, thereby restoring SM synthesis to LY-A cells. Considering that SM synthase as well as ganglioside synthases are suggested to exist more distally in the Golgi complex than GlcCer synthase (Futerman and Pagano, 1991; Jeckel et al., 1992; van Echten and Sandhoff, 1993), an intra–Golgi apparatus transport of Cer might be specifically blocked in LY-A cells. If so, one could expect accumulation of Cer in a pre- or early Golgi region. However, there was no sign of such specific redistribution of C5-DMB-Cer in LY-A cells (Fig. 7), although this observation does not conflict with the alternative expectation that a block of the intra–Golgi apparatus transport of Cer causes recycling of C5-DMB-Cer to the ER instead of its accumulation in the early Golgi apparatus. Note that LY-A cells normally produce GM3 (Fig. 2), eliminating the possibility that intra–Golgi apparatus transport pathways of various sphingolipid types are nonspecifically blocked in LY-A cells.
Therefore, we considered another possible mechanism that Cer, produced at the ER, is accessible to GlcCer synthase by different pathways from the ATP-dependent trafficking pathway which is impaired in LY-A cells. This mechanism appears to be consistent with the characteristics of LY-A cells; for example, the overflow of Cer to glycosphingolipids in LY-A cells could be mediated by the putative pathway responsible for GlcCer synthesis. The observation that an ATP depletion of wild-type cells caused ∼70% inhibition of [3H]sphingosine-derived [3H]SM formation, but only ∼20% inhibition of [3H]GlcCer formation (Fig. 10), may also be accounted for by the notion that Cer is efficiently accessible to GlcCer synthase by ATP-independent (or less ATP-dependent) trafficking, although there could be the alternative explanation that a minor population of GlcCer synthase is distributed to the ER in CHO cells, so that Cer produced at the ER is accessible to the ER-distributed GlcCer synthase without an interorganelle transport. Collectively, we propose that there are at least two pathways for Cer trafficking from the ER to the site of de novo SM synthesis: the ATP-dependent major pathway, which is impaired in LY-A cells, and the ATP-independent (or less ATP-dependent) minor pathway(s). This scenario appears to occur in various types of mammalian cells since depletion of intracellular ATP in HeLa cells and human skin fibroblasts affected redistribution of C5-DMB-Cer to the Golgi apparatus and conversion of C5-DMB-Cer to C5-DMB-SM (data not shown), as observed in wild-type CHO cells (Figs. 8 and 9 A).
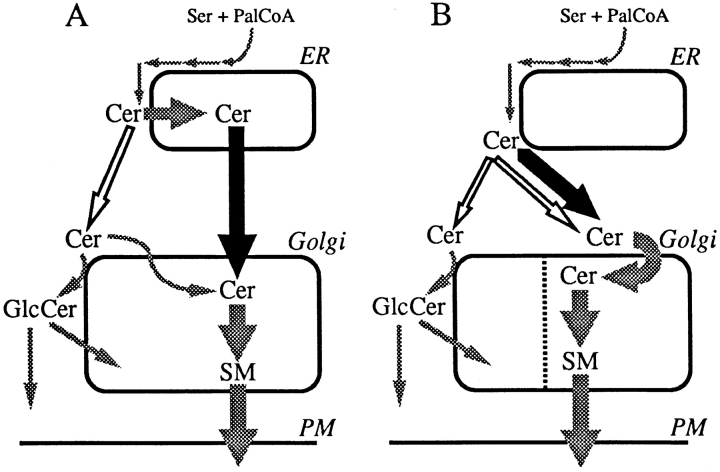
Two hypothetical models may be conceivable to explain the preferential delivery of Cer to SM synthase by the ATP-dependent mechanism. In one model (Fig. 12 A), a portion of newly synthesized Cer molecules is rapidly internalized to the lumenal side of the ER, and the lumenal Cer is delivered to the Golgi apparatus via an ATP-dependent and vesicle-mediated pathway for SM synthesis, while Cer remaining at the cytosolic side of the ER is delivered to the cytosolic side of the Golgi apparatus via an ATP-independent (or less ATP-dependent) pathway for GlcCer synthesis. In another model (Fig. 12 B), regardless of the Cer topology in the ER membrane, a portion of the Cer molecules enters an ATP-dependent pathway, which is specifically directed to the Golgi subcompartment where SM synthase is localized, while Cer molecules associated with an ATP-independent (or less ATP-dependent) pathway have a broader specificity for their destination and are eventually accessible to both GlcCer and SM synthases. If LY-A cells have a deficiency in an intra–Golgi apparatus transport of Cer, it may be conceivable that the ATP-dependent step for specific delivery to the SM synthase– localizing compartment is located at the intra–Golgi apparatus transport of Cer.
Figure 12.
Possible models for ER-to-Golgi apparatus trafficking of Cer responsible for SM and GlcCer biosynthesis. For ER-to-Golgi apparatus trafficking pathways of Cer, at least two distinct pathways are proposed to exist; one is ATP-dependent (black arrows) and another is ATP-independent (or less ATP-dependent) (white arrows). Mutant LY-A cells have a defect in the ATP-dependent pathway. (A) A portion of Cer produced on the cytosolic surface at the ER is rapidly internalized to the lumenal side, and the lumenal Cer is delivered to the Golgi apparatus via an ATP-dependent pathway for SM synthesis, while Cer remaining at the cytosolic side of the ER is delivered to the cytosolic side of the Golgi apparatus via an ATP-independent pathway for GlcCer and SM synthesis. (B) Regardless of the Cer topology in the ER membrane, a portion of newly synthesized Cer is delivered to the Golgi apparatus via an ATP-dependent pathway, which is specifically directed to the Golgi subcompartment where SM synthase is localized, while another portion of Cer is delivered to more various subcompartments of the Golgi apparatus via an ATP-independent pathway and can be converted to both SM and GlcCer. Ser, serine; PalCoA, palmitoyl CoA; PM, plasma membrane.
Membrane proteins are delivered from the ER to the Golgi apparatus by vesicular transport mechanisms (Rothman and Orci, 1990), and the same mechanisms for protein trafficking may be involved in the ATP-dependent trafficking of Cer. However, since LY-A cells exhibit no appreciable defect in ER-to-Golgi apparatus trafficking of proteins, we currently speculate that the machine defective in LY-A cells could be a specific device for Cer transport. There might be multiple, independent vesicular transport pathways from the ER to the Golgi apparatus, one of which is exclusive for Cer. For further elucidation of the molecular mechanism underlying the intracellular trafficking of Cer, additional studies, including identification of the genes related to this function, will be necessary, and the LY-A strain will be a useful tool for this purpose as well.
Acknowledgments
We thank Dr. Osamu Kuge of our laboratory for his helpful discussions and Dr. Richard E. Pagano (Mayo Clinic and Foundation, Rochester, MN) for his critical reading of the manuscript and helpful comments.
This work was supported in part by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan, CREST of Japan Science and Technology Corporation, and the Naito Foundation, and a Special Coordination Fund for Promoting Science and Technology from the Science and Technology Agency of Japan.
Abbreviations used in this paper
- Cer
ceramide
- DCer
dihydroceramide
- Endo H
endoglycosidase H
- GlcCer
glucosylceramide
- GPI
glycosylphosphatidylinositol
- PLAP
human placental alkaline phosphatase
- PLAP-HA
a chimera of PLAP with influenza hemagglutinin
- SM
sphingomyelin
- SM synthase
phosphatidylcholine:ceramide cholinephosphotransferase
References
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J.D. Watson, editors. 1994. Molecular Biology of the Cell. Garland Publishing, New York. 477–485.
- Arreaza G, Brown DA. Sorting and intracellular trafficking of a glycosylphosphatidylinositol-anchored protein and two hybrid transmembrane proteins with the same ectodomain in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1995;270:23641–23647. doi: 10.1074/jbc.270.40.23641. [DOI] [PubMed] [Google Scholar]
- Bai J, Pagano RE. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry. 1997;36:8840–8848. doi: 10.1021/bi970145r. [DOI] [PubMed] [Google Scholar]
- Berger J, Haward AD, Gerber L, Cullen BR, Udenfriend S. Expression of active, membrane-bound human placental alkaline phosphatase by transfected simian cells. Proc Natl Acad Sci USA. 1987;84:4885–4889. doi: 10.1073/pnas.84.14.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Collins RN, Warren G. Sphingolipid transport in mitotic HeLa cells. J Biol Chem. 1992;267:24906–24911. [PubMed] [Google Scholar]
- Coste H, Martel MB, Got R. Topology of glucosylceramide synthesis in Golgi membranes from porcine submaxillary glands. Biochim Biophys Acta. 1986;858:6–12. doi: 10.1016/0005-2736(86)90285-3. [DOI] [PubMed] [Google Scholar]
- Futerman AH, Pagano RE. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem J. 1991;280:295–302. doi: 10.1042/bj2800295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, Stieger B, Hubbard AL, Pagano RE. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990;265:8650–8657. [PubMed] [Google Scholar]
- Gaver RC, Sweeley CC. Methods for methanolysis of sphingolipids and direct determination of long-chain bases by gas chromatography. J Am Oil Chem Soc. 1965;42:294–298. doi: 10.1007/BF02540132. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Fuller SD, Back R, Hollinshead M, Pfeiffer S, Simons K. The dynamic nature of the Golgi complex. J Cell Biol. 1989;108:277–297. doi: 10.1083/jcb.108.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Nishijima M, Akamatsu Y. A temperature-sensitive mammalian cell mutant with thermolabile serine palmitoyltransferase for the sphingolipid biosynthesis. J Biol Chem. 1990;265:22137–22142. [PubMed] [Google Scholar]
- Hanada K, Horii M, Akamatsu Y. Functional reconstitution of sphingomyelin synthase in Chinese hamster ovary cell membranes. Biochim Biophys Acta. 1991;1086:151–156. doi: 10.1016/0005-2760(91)90002-y. [DOI] [PubMed] [Google Scholar]
- Hanada K, Nishijima M, Kiso M, Hasegawa A, Fujita S, Ogawa T, Akamatsu Y. Sphingolipids are essential for the growth of Chinese hamster ovary cells: restoration of the growth of a mutant defective in sphingoid base biosynthesis by exogenous sphingolipids. J Biol Chem. 1992;267:23527–23533. [PubMed] [Google Scholar]
- Hanada K, Nishijima M, Akamatsu Y, Pagano RE. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein, in mammalian membranes. J Biol Chem. 1995;270:6254–6260. doi: 10.1074/jbc.270.11.6254. [DOI] [PubMed] [Google Scholar]
- Hanada K, Hara T, Fukasawa M, Yamaji A, Umeda M, Nishijima M. Mammalian cell mutants resistant to a sphingomyelin-directed cytolysin: genetic and biochemical evidence for complex formation of the LCB1 protein with the LCB2 protein for serine palmitoyltransferase. J Biol Chem. 1998;273:33787–33794. doi: 10.1074/jbc.273.50.33787. [DOI] [PubMed] [Google Scholar]
- Hirschberg K, Rodger J, Futerman AH. The long-chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem J. 1993;290:751–757. doi: 10.1042/bj2900751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D, Kok JW. Trafficking of glycosphingolipids in eucaryotic cells; sorting and recycling of lipids. Biochim Biophys Acta. 1992;1113:277–294. doi: 10.1016/0304-4157(92)90002-r. [DOI] [PubMed] [Google Scholar]
- Jeckel D, Karrenbauer A, Burger KNJ, van Meer G, Wieland F. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J Cell Biol. 1992;117:259–267. doi: 10.1083/jcb.117.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok JW, Babia T, Klappe K, Egea G, Hoekstra D. Ceramide transport from endoplasmic reticulum to Golgi apparatus is not vesicle-mediated. Biochem J. 1998;333:779–786. doi: 10.1042/bj3330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Ktistakis NT, Kao C-Y, Qang R-H, Roth MG. A fluorescent lipid analogue can be used to monitor secretory activity and for isolation of mammalian secretion mutants. Mol Biol Cell. 1995;6:135–150. doi: 10.1091/mbc.6.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky NG, Pagano RE. Sphingolipid metabolism in cultured fibroblasts: microscopic and biochemical studies employing a fluorescent ceramide analogue. Proc Natl Acad Sci USA. 1983;80:2608–2612. doi: 10.1073/pnas.80.9.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mandon EC, Ehses I, Rother J, van Echten G, Sandhoff K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver. J Biol Chem. 1992;267:11144–11148. [PubMed] [Google Scholar]
- Merrill AH, Jr, Jones DD. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta. 1990;1044:1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- Michel C, van Echten-Deckert G. Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum. FEBS Lett. 1997;416:153–155. doi: 10.1016/s0014-5793(97)01187-3. [DOI] [PubMed] [Google Scholar]
- Moreau P, Cassagne C. Phospholipid trafficking and membrane biogenesis. Biochim Biophys Acta. 1994;1197:257–290. doi: 10.1016/0304-4157(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Moreau P, Cassagne C, Keenan TW, Morré DJ. Ceramide excluded from cell-free vesicular lipid transfer from endoplasmic reticulum to Golgi apparatus. Evidence for lipid sorting. Biochim Biophys Acta. 1993;1146:9–16. doi: 10.1016/0005-2736(93)90332-t. [DOI] [PubMed] [Google Scholar]
- Morell P, Radin NS. Specificity in ceramide biosynthesis from long chain bases and various fatty acyl coenzyme A's by brain microsomes. J Biol Chem. 1970;245:342–350. [PubMed] [Google Scholar]
- Nishijima M, Kuge O, Hanada K. Mammalian cell mutants of membrane phospholipid biogenesis. Trends Cell Biol. 1997;7:324–329. doi: 10.1016/S0962-8924(97)01084-2. [DOI] [PubMed] [Google Scholar]
- Pagano RE, Martin OC. A series of fluorescent N-acylsphingosines: synthesis, physical properties, and studies in cultured cells. Biochemistry. 1988;27:4439–4445. doi: 10.1021/bi00412a034. [DOI] [PubMed] [Google Scholar]
- Pagano RE, Sepanski MA, Martin OC. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989;109:2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano RE, Martin OC, Kang HC, Haugland RP. A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J Cell Biol. 1991;113:1267–1279. doi: 10.1083/jcb.113.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P, Kamisaka Y, Marks DL, Pagano RE. Purification and characterization of UDP-glucose:ceramide glucosyltransferase from rat liver Golgi membranes. J Biol Chem. 1996;271:2287–2293. doi: 10.1074/jbc.271.4.2287. [DOI] [PubMed] [Google Scholar]
- Pütz U, Schwarzmann G. Golgi staining by two fluorescent ceramide analogues in cultured fibroblasts requires metabolism. Eur J Cell Biol. 1995;68:113–121. [PubMed] [Google Scholar]
- Rosenwald AG, Pagano RE. Intracellular transport of ceramide and its metabolites at the Golgi complex: insight from short-chain analogs. Adv Lipid Res. 1993;26:101–118. [PubMed] [Google Scholar]
- Rothman JE, Orci L. Movement of proteins through the Golgi stack: a molecular dissection of vesicular transport. FASEB (Fed Am Soc Exp Biol) J. 1990;4:1460–1468. doi: 10.1096/fasebj.4.5.2407590. [DOI] [PubMed] [Google Scholar]
- Schütze, S., and M. Krönke. 1995. Measurement of cytokine induction of PC-specific phospholipase C and sphingomyelinases. In Cytokines. 2nd edition. F.R. Balkwill, editor. IPL Press, Oxford. 93–110.
- Slomiany A, Grzelinska E, Grabska M, Yamaki K, Tamura S, Kasinathan C, Slomiany BL. Intracellular processes associated with glycoprotein transport and processing. Arch Biochem Biophys. 1992;298:167–175. doi: 10.1016/0003-9861(92)90108-9. [DOI] [PubMed] [Google Scholar]
- Trotter PJ, Voelker DR. Lipid transport processes in eukaryotic cells. Biochim Biophys Acta. 1994;1213:241–262. doi: 10.1016/0005-2760(94)00073-5. [DOI] [PubMed] [Google Scholar]
- Trinchera M, Fabbri M, Ghidoni R. Topography of glycosyltransferases involved in the initial glycosylations of gangliosides. J Biol Chem. 1991;266:20907–20912. [PubMed] [Google Scholar]
- van Echten G, Sandhoff K. Ganglioside metabolism. J Biol Chem. 1993;268:5341–5344. [PubMed] [Google Scholar]
- van Helvoort A, Giudici ML, Thielemans M, van Meer G. Transport of sphingomyelin to the cell surface is inhibited by brefeldin A and in mitosis, where C6-NBD-sphingomyelin is translocated across the plasma membrane by a multidrug transporter activity. J Cell Sci. 1997;110:75–83. doi: 10.1242/jcs.110.1.75. [DOI] [PubMed] [Google Scholar]
- van Meer G. Transport and sorting of membrane lipids. Curr Opin Cell Biol. 1993;5:661–673. doi: 10.1016/0955-0674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Warnock DE, Lutz MS, Blackburn WA, Young WW, Jr, Baenziger JU. Transport of newly synthesized glucosylceramide to the plasma membrane by a non-Golgi pathway. Proc Natl Acad Sci USA. 1994;91:2708–2712. doi: 10.1073/pnas.91.7.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RD, Wang E, Merrill AH., Jr Enzymology of long-chain base synthesis by liver: characterization of serine palmitoyltransferase in rat liver microsomes. Arch Biochem Biophys. 1984;228:282–291. doi: 10.1016/0003-9861(84)90069-9. [DOI] [PubMed] [Google Scholar]
- Yamaji A, Sekizawa Y, Emoto K, Sakuraba H, Inoue K, Kobayashi H, Umeda M. Lysenin, a novel sphingomyelin-specific binding protein. J Biol Chem. 1998;273:5300–5306. doi: 10.1074/jbc.273.9.5300. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Suzuki M, Kawashima I, Karasawa K, Nojima S, Enomoto T, Tai T, Suzuki A, Setaka M. Changes in composition of newly synthesized sphingolipids of HeLa cells during the cell cycle. Eur J Biochem. 1997;249:450–455. doi: 10.1111/j.1432-1033.1997.00450.x. [DOI] [PubMed] [Google Scholar]