Abstract
Lymphocytes accumulate within the extracellular matrix (ECM) of tumor, wound, or inflammatory tissues. These tissues are largely comprised of polymerized adhesion proteins such as fibrin and fibronectin or their fragments. Nonactivated lymphoid cells attach preferentially to polymerized ECM proteins yet are unable to attach to monomeric forms or fragments of these proteins without previous activation. This adhesion event depends on the appropriate spacing of integrin adhesion sites. Adhesion of nonactivated lymphoid cells to polymeric ECM components results in activation of the antigen receptor-associated Syk kinase that accumulates in adhesion-promoting podosomes. In fact, activation of Syk by antigen or agonists, as well as expression of an activated Syk mutant in lymphoid cells, facilitates their adhesion to monomeric ECM proteins or their fragments. These results reveal a cooperative interaction between signals emanating from integrins and antigen receptors that can serve to regulate stable lymphoid cell adhesion and retention within a remodeling ECM.
Keywords: integrin, lymphocyte, extracellular matrix, protein tyrosine kinase, cell adhesion
Lymphocytes traverse between the tissue and circulatory compartments in a regulated manner requiring the expression of cell adhesion receptors and their ligands (Springer, 1994; Butcher and Picker, 1996). Chronic inflammatory diseases are marked by both the deposition of provisional extracellular matrix (ECM),1 such as fibrin, and the accumulation of lymphocytes within affected tissues (Postigo, 1993). Lymphoid adhesion and migration on purified ECM components is largely dependent on integrins, heterodimeric (α/β) proteins that integrate extracellular interactions of cells with internal signal-transducing elements, including the cytoskeleton (Aplin et al., 1998). Lymphoid integrins, like those on other hematopoietic cells, are regulated at both the level of expression and function (Springer, 1994), suggesting that lymphoid cell interaction with, and adhesion to, ECM proteins is itself dependent upon initiating signaling events.
The induction of lymphoid adhesion in vitro has been associated with the activation of a variety of signaling molecules, yet in most cases these stimuli result in transient adhesion. However, it remains unclear how sustained lymphoid interaction with the ECM is maintained in vivo. Although conformational changes in integrin structure may occur in response to some activational protocols, these are often associated with ligand binding itself (Bazzoni and Hemler, 1998), and the precise relationship between affinity modulation and adhesion is still open to interpretation (Stewart and Hogg, 1996).
During wound repair, cancer, and inflammation, the ECM is comprised of provisional matrix proteins, including fibrinogen, fibronectin, and vitronectin. Each is capable of self-assembly and polymerization after vascular leakage (Dvorak et al., 1995). The rapid, organized deposition of these molecules results in binding sites for adhesion molecules, including integrins, being separated by only nanometers (Mosesson et al., 1995), which provides a mechanism for local avidity modulation within affected tissues. During tissue remodeling, proteolytic activity provides a complementary mechanism to digest the polymeric ECM. Provisional ECM, when intact, is immunopotentiating (Postigo et al., 1991; Halvorson et al., 1996), whereas proteolytic fragments are immunosuppressive (Robson et al., 1993; Edgington et al., 1985), suggesting an important role for ECM deposition and remodeling in lymphocyte responses (Ratner et al., 1992).
To investigate whether the induction of a polymeric status in ECM components could facilitate lymphoid adhesion and attachment, we examined the capacity of lymphocytes and lymphoid lines to interact with polymerized or unpolymerized ECM proteins or with structurally defined integrin-specific ligands. Lymphoid cells required activation to adhere to unpolymerized matrix components, but readily attached to multimeric matrix components. Lymphoid adhesion did not require affinity modulation, but rather was dependent upon the appropriate spacing of integrin adhesive sites. Engagement of integrin by polymeric adhesive sites initiated signaling through the hematopoietic kinase Syk. Activation of this kinase by mutagenesis or through antigenic stimulation resulted in cellular adherence to monomeric ligand. These results provide a mechanism for cooperation between antigen receptors and integrins during the activation of lymphocyte adhesion to the ECM.
Materials and Methods
Cells and Cell Lines
DT40 cells deficient in Syk and those reconstituted with Syk have been previously established (Keshvara et al., 1996). Human M21 melanoma cells and established lymphoblastoid cell line (LCL) (JY) and lymphoid tumor cells (RPMI 8866, RPMI 8226; Ramos), with adhesion and integrin expression as published (Felding-Haberman et al., 1992; Stupack et al., 1992) were obtained from J. Wilkins (University of Manitoba, Manitoba, Canada). Clone E6 of the Jurkat cell line was obtained from American Type Culture Collection. All lines were cultured in RPMI 1640, supplemented with glutamine/geneticin (Sigma) and 10% fetal bovine serum (GIBCO-BRL). The DT40 cell lines were further supplemented with 1% chicken serum (GIBCO-BRL). LCL were established by outgrowth of freshly isolated, purified B cells infected with EBV-B95 (Huang et al., 1997). Murine γδ T cells were established from lymphocytic choriomeningitis virus (LCMV) preimmunized animals cocultured with LCMV-infected, irradiated helper cell populations as described (Hahn et al., 1994). In brief, splenic lymphocytes were harvested from infected mice, and cytotoxic γδ T cells (CTLs) established by outgrowth in the presence of 10 U/ml murine interleukin (IL)-2 and irradiated LCMV-infected autologous helper cells. Cells were used in the assay after 5 d in culture. Sorted human lymphocytes expressing integrin αvβ3 were isolated from PBMC after ficoll-hypaque differential gradient purification followed by flow cytometry (FACStar™; Becton Dickinson) using murine monoclonal P4C10 (5 μg/ ml) purified from the hybridoma and rabbit antisera to integrin αvβ3 (1: 1,200), detected with secondary goat anti–mouse (PE-conjugated) or goat anti–rabbit (FITC-conjugated) preadsorbed antisera, respectively (Southern Biotechnology). Populations expressing high levels of both integrins were principally monocytes and were identified and excluded by forward scatter, side scatter, and fluorescence (phycoerythrin [PE]) gating. Analysis of these populations with direct-labeled (FITC-conjugated) reagents 24 h later confirmed these cells were a mixed lymphocyte population (∼65% CD19+, ∼20% CD3+) without monocytes (CD14−) or progenitor cells (CD38−). Antibodies were purchased from PharMingen.
Cell Adhesion
Cell adhesion to 48-well plates precoated with substrate ligand was performed as previously described (Filardo et al., 1995). In brief, cells were suspended at 106/ml in RPMI 1640, 1.0% BSA. 200-μl aliquots of cells were added to wells which had been precoated overnight with the specified ligands in PBS, pH 8.0, at 4°C and then blocked for 1 h at room temperature with RPMI, 3% BSA (Sigma). Fibronectin (GIBCO-BRL) and pronectin (Stratagene) were purchased; superfibronectin and monomeric vitronectin were gifts of E. Ruoslahti (The Burnham Institute, La Jolla, CA) and D. Sieffert (The Scripps Research Institute, La Jolla, CA). Recombinant, pentameric penton base (PB) was expressed in insect cells as described previously (Wickham et al., 1993). Monomeric PB integrin-binding domain (Mathias et al., 1994) was the gift of T. Muir (The Rockefeller University, New York, NY). Adhesion was allowed to proceed for 30 min at 37°C followed by washing to remove nonadherent cells and then quantitation of adherence by staining adherent fractions with crystal violet (0.1% in 0.15 M NaCl, 20% ethanol). Cell-bound stain was resolubilized in methanol and quantitated at 600 nm. Soluble fibrin was formed by the addition of 0.2 U thrombin in DME (50 U/ml stock) (Calbiochem-Novabiochem) to the specified fibrinogen (American Diagnostica) concentrations during coating. The addition of thrombin to BSA- or fibrinogen-coated wells served as a control and did not differ from thrombin-free coated surfaces. Molar concentrations of pentameric PB were calculated based upon the five individual molecules within each pentamer (therefore, five integrin-binding sites). To express PB concentration as an aggregate unit (pentamer), concentration may be reduced fivefold. Purified lymphocyte adhesion to substrate-coated chamber slides was quantitated by direct counting of six fields at 100× after sorting on a FACStar™ (Becton Dickinson). Three independent sorts were performed. Adhesion to cryosections from three different sources was performed with Cell Tracker Green-labeled LCL (5 μM for 15 min; Molecular Probes) in binding buffer (DME, 3% BSA, 100 U/ml heparin) as previously described (Stamper and Woodruff, 1976) and was quantitated similarly.
Radioimmunoassay
Adhesion plates (48 well) were coated exactly as described for the adhesion assay and blocked with 1.0% BSA in DME (assay buffer) for 1 h at 37°C. Wells were probed by the addition of with 0.5 μg of [125I] radiolabeled mAb DAV-1, which recognizes an epitope (IRGDTFAT) within the integrin-binding domain (Stewart et al., 1997). DAV-1 was radiolabeled with [125I] (ICN) using Iodobeads (Pierce) according to the manufacturer's instructions. The specific binding of DAV-1 (< 1%) was assessed after 1 h. Specific binding was resolubilized by the addition of 1% SDS, 50 mM Tris, pH 6.8, 10% betamercaptoethanol (100°C) (Sigma) after extensive washing (five times) in assay buffer.
DAV-1 binding to BSA alone was not significantly different from background, and was subtracted from all values to get specific binding. Radioactivity bound per unit area was used to calculate sites present after normalizing specific activity per mol of DAV-1 in each experiment.
Integrin-binding Assay
Adhesion plates (96 well) were coated exactly as described for the adhesion assay (0.5 μg/ml of fibrinogen/fibrin) and blocked with 1.0% BSA in DME (assay buffer) for 1 h at 37°C. Wells were probed by the addition of soluble, biotinylated integrin generously provided by S.L. Goodman (Merck KGaA; Mehta et al., 1998) for 2 h at 37°C, washed with PBS five times, and then bound integrin was detected by the addition of secondary horseradish peroxidase-conjugated anti-biotin secondary antibody (Sigma). After four further washes, the chromogenic substrate 3,3′, 5,5′-tetramethyl benzidine (Bio-Rad) was added and absorbance quantitated at 450 nm after a 10-min incubation.
Immunoprecipitation and Kinase Activity
LCL were washed twice in PBS, then aliquots (5 × 106 in 4,000 μl) were plated for 15 min in DME on surfaces coated with different substrate as indicated. Excess media was then aspirated, leaving the bulk of the cells (adherent and nonadherent) in the final 800 μl. Cells were lysed by the addition of 700 μl 1.0% Nonidet P-40, 50 mM Tris-buffered lysates, pH 7.4, containing 2× protease inhibitor cocktail (Boehringer Mannheim) 2 mM PMSF, 2 mM EGTA, and 2 mM sodium vanadate (Sigma). Specific kinases were immunoprecipitated after preclearing lysates with protein A–Sepharose (Pierce) by the addition of monoclonal antibody 4D10 (Syk) (Santa Cruz Biotechnology), or polyclonal antisera against phosphoinositide-3-kinase (PI[3]K)–p85 (Grb-1) (Upstate Biotechnology) or Syk (Santa Cruz Biotechnology) (2–4 μg/ml) and the addition of protein A– or protein G–Sepharose, as appropriate. Alternatively, immunoprecipitations were performed with agarose-coupled polyclonal antisera to Syk or Lyn (Santa Cruz Biotechnology). Immunoprecipitated proteins were resolved by 10–12% SDS-PAGE and transferred to polyvinylene difluoride membranes to facilitate phosphotyrosine analysis via immunodetection with monoclonal antibodies 4G10 (Upstate Biotechnology) and PY72 (prepared from the hybridoma). Similarly, Syk or Lyn autophosphorylation activity was determined from the immunoprecipitates as described by the addition of exogenous γ32P-ATP (5 μCi; ICN) in kinase buffer (20 mM Hepes, pH 7.4, 10 mM MnCl2, 150 mM NaCl) as described (Burg et al., 1994). All activity was normalized by reprobing to account for small variations in protein loading of individual lanes. The p85-associated PI(3)K activity was determined after immunoprecipitation by the addition of exogenous γ32P-ATP and (4,5) PIP2 substrate (Calbiochem-Novabiochem) followed by silica gel thin layer chromatography (1:1:1, CHCl3, CH3OH, HCl [1 M]) as previously described (Li et al., 1998) and subsequent visualization via autoradiography or ImageQuant phosphorimager analysis (Molecular Dynamics).
Results
Nonadherent Lymphoid Cells Spontaneously Attach to Tissue-immobilized ECM
Lymphoid cells can be detected in association with the ECM of tumor and inflammatory tissues. Fibrin, a polymeric constituent of tumor ECM (Dvorak et al., 1995), is recognized by integrin αvβ3 expressed on a subpopulation of lymphoid cells (Halvorson et al., 1996). To examine lymphoid interaction with a tumor-associated ECM, nonactivated lymphoblastoid B cells (LCL) were assessed for their attachment to fibrin-rich cryosections of human breast tumors (Fig. 1, A and B). LCL attachment localized to regions of fibrin deposition (Fig. 1 C), and the observed adhesion was significantly blocked (Fig. 1 D) with monoclonal (mAb) LM609 directed to integrin αvβ3 (∼65%, Fig. 1 E), a known receptor for fibrinogen/fibrin (Cheresh, 1987) (and other provisional ECM components) expressed on LCL. However, these tissues also contain other adhesive ligands for integrin αvβ3. Thus, fibrin does not account for all the adhesive interactions observed.
Figure 1.
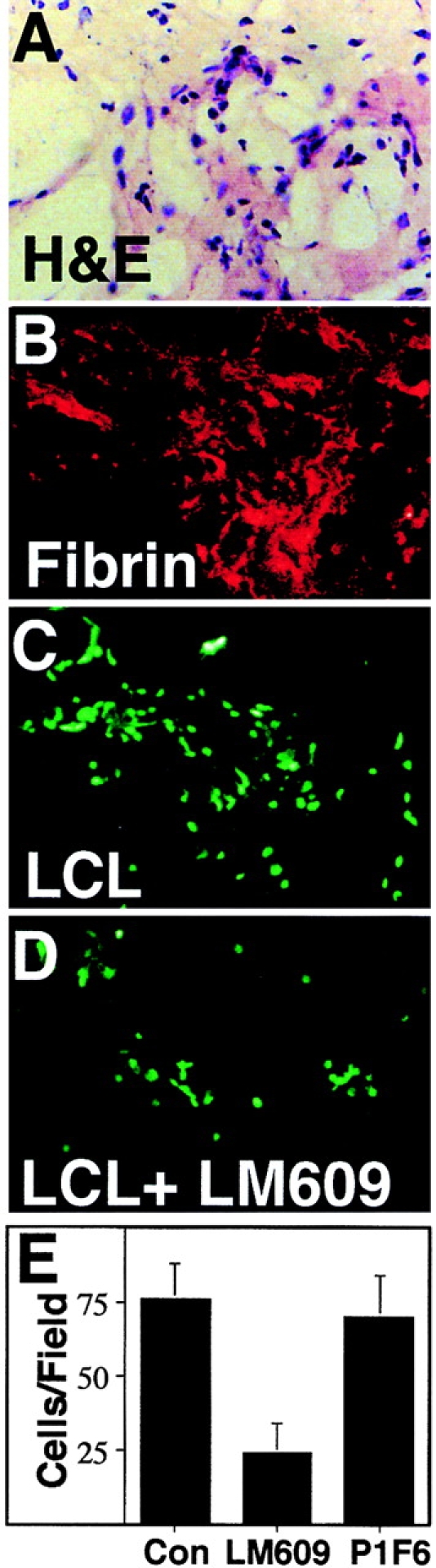
Colocalization of LCL attachment with fibrin deposition in tissue sections. (A) Serial cryosections of a human breast tumor were stained with hematoxylin and eosin or (B) stained with mAb 17B4, a monoclonal antibody specific for fibrin (10 μg/ml) as detected with secondary rhodamine-conjugated goat anti–mouse polyclonal antibody (red). Fluorescent-labeled LCL (105/ slide) were allowed to attach to immediate serial sections in DME, 3% BSA, 100 U/ml heparin and either 50 μg/ml nonspecific murine IgG (C) or monoclonal antibody LM609 (anti-αvβ3) (D). Attached cells per field in the presence of control, LM609, or P1F6 (anti-αvβ5) antibodies were quantified by direct counting of fluorescent cells (E). Results are expressed as the mean ± SE of six fields counted. A representative experiment of three is shown.
To characterize this interaction more carefully, LCL were allowed to attach to microtiter wells coated with either fibrin or fibrinogen. Although LCL attached to immobilized fibrin in a concentration-dependent manner, they failed to attach to fibrinogen at all concentrations tested (Fig. 2 A). The adhesion to fibrin, in vitro, was primarily dependent upon both αvβ3 and β2 integrins, as determined using function-blocking mAbs LM609 and TS1/ 18, respectively (Fig. 2 B). In contrast, adherent M21 melanoma cells readily attached to fibrinogen or its polymeric form, fibrin, in a dose-dependent manner (Fig. 2 A) and this was blocked by mAb LM609 (data not shown), as previously reported (Felding-Habermann et al., 1992).
Figure 2.
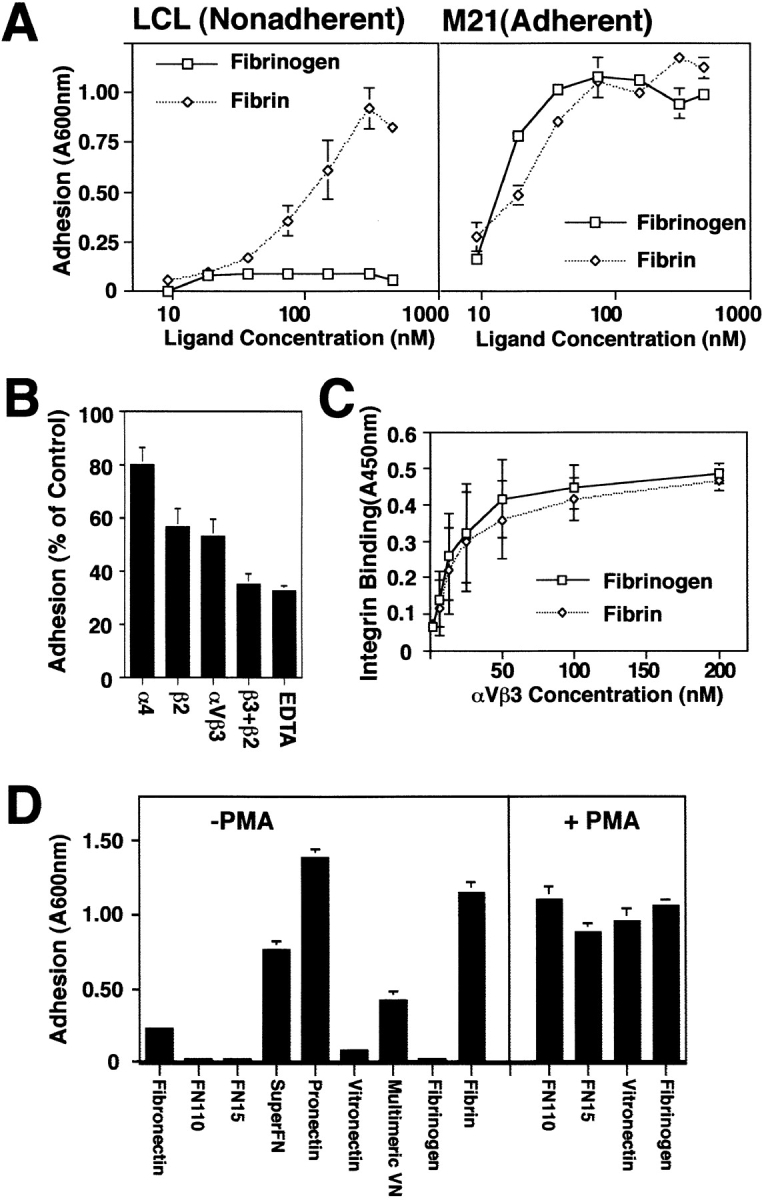
Preferential attachment of unactivated LCL to multimeric ECM components. (A) Nonadherent LCL or adherent M21 cells were allowed to attach to wells coated overnight with various concentrations of either fibrinogen or fibrin. Cells remaining attached after washing were stained with crystal violet and quantitated by reconstituting cell-bound dye with methanol and reading absorbance at 600 nm. The mean ± SE of triplicate determinations from one of three similar experiments is shown. (B) Attachment to polymerized fibrin was assessed in the presence of monoclonal antibodies specific for integrin α4 (P4C2), β2 (TS1/ 18), or αvβ3 (LM609) (25 μg/ml) or EDTA (10 mM) added to cell suspensions immediately before adhesion assay. (C) Relative affinity of the ligands for receptor was determined by binding of soluble, biotinylated integrin to fibrin or fibrinogen-coated wells that was detected by the addition of horseradish peroxidase–conjugated secondary anti-biotin monoclonal antibody as described in Materials and Methods. (D) Multivalent ECM ligands, including fibrin, superfibronectin (SuperFN), pronectin, and multimeric vitronectin (Multimeric VN) or unpolymerized/proteolyzed forms of these molecules forms including fibronectin fragments of 110 (FN110) and 15 kD (FN15), fibrinogen, and monomeric vitronectin (Vitronectin) were coated on adhesion assay wells overnight (0.5–5 μg/ml) and assessed for their capacity to support LCL adhesion (left). To determine if activation could rescue LCL adhesion to unpolymerized substrates, LCL were treated with PMA (20 ng/ml) immediately before adhesion assay on plates coated as described above (right). Representative results (mean ± SE of triplicate determinations) are shown; each substrate was tested three or more times.
M21 cells did not appear to prefer either form of fibrinogen/fibrin, suggesting that both ligand forms offered similar affinity sites for adhesion. To confirm this, the binding of soluble biotinylated integrin (Mehta et al., 1998) to fibrin or fibrinogen-coated surfaces was tested. No difference in ligand capacity to support integrin binding was detected (Fig. 2 C) as both ligand forms supported half maximal integrin binding at receptor concentrations of ∼15–20 nM. These results suggested that polymerization of fibrinogen to fibrin did not result in exposure of adhesive sites with increased affinity for integrin. Importantly, preactivation of LCL by phorbol ester (Shimizu, 1996), which has been shown to promote integrin clustering in lymphoid cells (Haverstack et al., 1992; van Kooyk et al., 1994), activated lymphoid attachment to fibrinogen, demonstrating that this ligand supported lymphoid adhesion (Fig. 2 D). In contrast, attachment to fibrinogen (or BSA) was not rescued by pretreating either the plates or the LCL with thrombin, suggesting that thrombin was not itself an activator of LCL attachment (data not shown). LCL also gained the ability to bind unpolymerized fibronectin or vitronectin after exposure to PMA (Fig. 2 D). However, in all cases examined, multivalent and polymerized forms of fibronectin and vitronectin, like fibrin, supported LCL adhesion without previous stimulation (Fig. 2 D) suggesting that LCL adhesion was inducible by polymeric integrin ligands, and that this may be a general property of lymphoid cell integrins.
Multivalent Integrin Ligands Mimic Polymeric ECM and Facilitate Lymphoid Adhesion
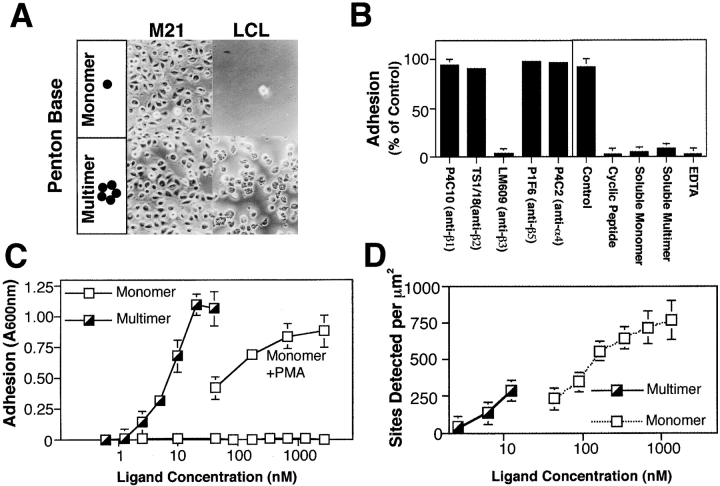
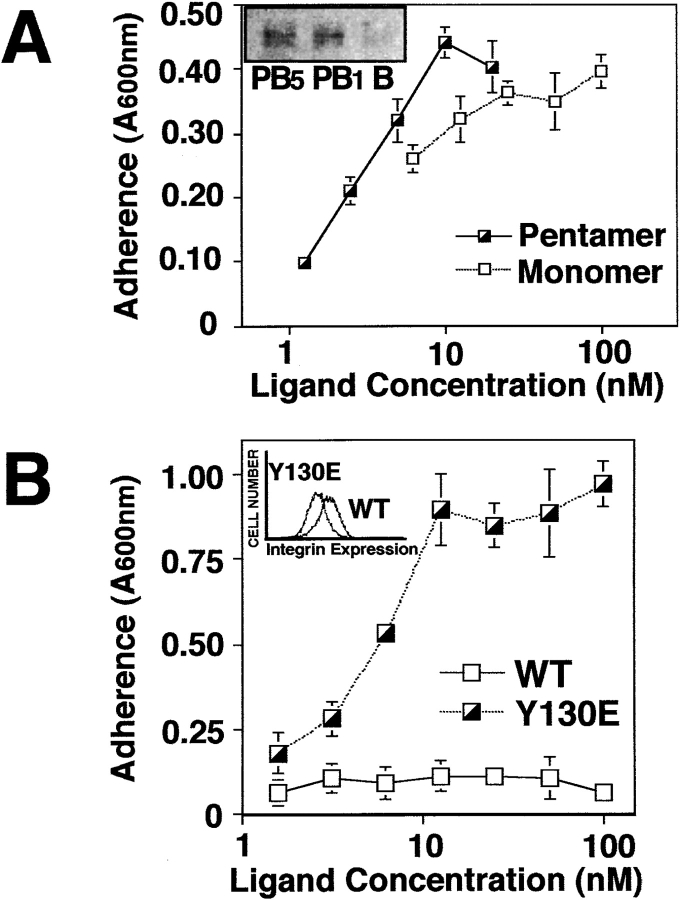
To further establish the structural basis of lymphoid-mediated integrin function, LCL were allowed to attach to one of two structurally-defined αvβ3 ligands. In this case, microtiter wells were coated with a substrate comprised of either a monovalent or pentavalent form of the adenovirus PB protein (Mathias et al., 1994). The pentavalent form of PB contains five available αvβ3-binding sites equally separated by ∼60 Å (Stewart et al., 1997), whereas the monomeric construct contains only a single αvβ3-binding site (Mathias et al., 1994). As shown in Fig. 3 A, the adherent M21 cell line attached to either form of PB, whereas LCL selectively attached to the pentamer. Adhesion to multimeric PB was entirely dependent on integrin αvβ3, since either LM609 or a peptide antagonist of this integrin completely blocked adhesion while antibodies to other integrins showed no effect (Fig. 3 B). The addition of soluble monomeric or multimeric PB also disrupted adhesion (Fig. 3 B), indicating that either form of PB was an effective soluble inhibitor of integrin αvβ3.
Figure 3.
Characterization of local valency in promoting LCL adhesion via integrins. (A) LCL or M21 cells were assessed for attachment to wells coated with either recombinant adenovirus penton base (PB) protein (10 nM), a pentamer, or a monomeric PB construct (100 nM) containing the integrin-binding domain, and phase– contrast images captured at 200× magnification. (B) LCL adhesion to multimeric PB was assessed in the presence of function-blocking integrin-specific monoclonal antibodies specific to β1 (P4C10), β2 (TS1/18), αvβ3 (LM609), αvβ5 (P1F6), α4β1/β7 (P4C2) (25 μg/ml), or soluble antagonists of integrin adhesion including a cyclic peptide antagonist (cyclo Arg-Gly-Asp-dPhe-Val) (5 μM), control antagonist (cyclo Arg-βAla-Asp-dPhe-Val) (5 μM), EDTA (10 μM), and both monomeric and multimeric forms of PB in solution (10 μM). (C) The adhesion of LCL to pentameric PB was determined by attachment assay as described above, as a function of increasing coating concentration of pentavalent or monovalent PB. To rescue attachment to monomeric PB, LCL were treated with PMA (20 ng/ml) immediately before assay. (D) The density of native integrin-binding sites on BSA-blocked, substrate-coated adhesion assay plates was assessed on monomer and pentamer coated wells by the specific binding of radio-iodinated mAb DAV-1 (0.5 μg), which recognizes the Ile-Arg-Gly-Asp-Thr-Phe-Ala-Thr sequence found in the integrin-binding domain of PB. Data is expressed as the mean ± SE of triplicate determinations from one of three separate experiments.
Examination of the role of ligand density in adhesion to the pentamer revealed that LCL adhesion was dose-dependent with a half-maximal coating concentration of 10 nM (Fig. 3 C). In contrast, LCL adhesion to the monomer could not be achieved at up to a 500-fold increased coating concentration unless the cells were first activated (Fig. 3 C). The available density of adhesive sites were determined by radioimmunoassay with a monoclonal antibody specific for the IRGDTFAT adhesive sequence within the integrin-binding domain of PB (Stewart et al., 1997). Significant adhesion to the pentamer was observed at concentrations where only 125 adhesive sites/mm2 were available, yet no adhesion occurred on monomer-coated wells where more than 800 sites/mm2 were detected (Fig. 3 D). The failure of LCL to attach to the monomer was therefore not due to limiting availability or absolute density of adhesive sites. These findings suggest that it is the relative spatial distribution of the integrin adhesive sites that facilitates LCL adhesion to substrate.
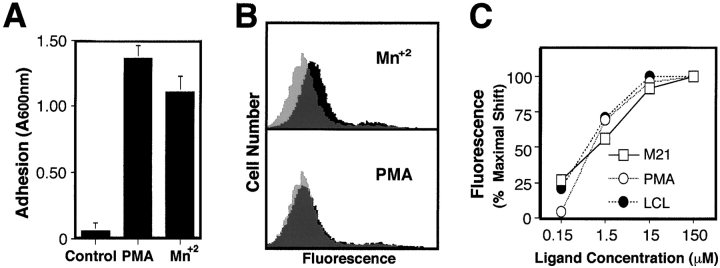
Integrin organization on the cell surface may be an important factor contributing to adhesion, since the activation of LCL attachment to monomeric PB (Fig. 3 B) did not influence αvβ3 expression (our unpublished data). Lymphoid integrins can be activated by conformational (affinity) changes by a variety of reagents, including manganese (Bazzoni and Hemler, 1998). Manganese activated the attachment of LCL to monomeric PB similarly to PMA (Fig. 4 A), and caused conformational changes in integrin αvβ3 which were detected with monoclonal antibody ligand-induced binding site (LIBS)-1 (Fig. 4 B) (Frelinger et al., 1990). Although LIBS-1 detects the ligand-occupied form of αvβ3, the presence of manganese elicited conformational changes which exposed this epitope on LCL even in the absence of ligand (Fig. 4 B, top). In contrast, LCL activated with PMA did not differ from basal LIBS-1 expression (Fig. 4 B, bottom). Further, PMA did not stabilize integrin binding of soluble ligand (a relative measurement of affinity) (Frelinger et al., 1990; Filardo et al., 1995). Nonactivated or PMA-activated LCL were allowed to bind PB monomer, and LIBS-1 was used to detect ligand binding as a function of ligand concentration. No difference in half-maximal ligand occupancy was evident between PMA-activated and untreated LCL for soluble monomeric PB (∼1.5 μM) (Fig. 4 C). We observed that LCL displayed the same relative affinity for ligand as determined for M21 cells (Fig. 4 C) and other adherent cells (Filardo et al., 1995). Thus, activation of LCL by PMA leading to stable adhesion on monomer does not appear to result from preexisting affinity changes.
Figure 4.
Rescue of integrin-mediated attachment to monomeric PB. (A) The attachment of LCL to monomeric PB substrate (200 nM) was assessed in untreated cells or in the presence of PMA (20 ng/ml) or manganese chloride (5 mM). (B) Manganese-treated LCL with rescued adhesion were tested for relative expression of the LIBS-1 neoepitope, an indicator of conformational changes in β3 integrin associated with ligand binding. Increased FITC–LIBS-1 binding (indicating integrin conformational change) was detected by FACS™ in manganese-treated LCL (black histogram, upper panel) relative to untreated controls (light gray histogram, top) in the absence of ligand. PMA-activated LCL (black histogram, bottom) were also compared with unactivated LCL (light gray histogram, bottom). Dark gray indicates regions where activated or unactivated histograms overlap. (C) The influence of PMA on LCL integrin affinity was examined using soluble ligand binding as a relative indicator. PMA-treated LCL, untreated LCL, or adherent M21 melanoma cells were assessed for their capacity to bind soluble PB monomer as a function of ligand concentration using FACS™ analysis of FITC–LIBS-1 binding. The relative shift in mean fluorescence intensity is plotted for each ligand concentration as a percentage of the maximum shift observed. A representative experiment from three experiments is shown.
Preferential Attachment to Multimeric Integrin Substrate Is Conserved in Lymphoid Cells
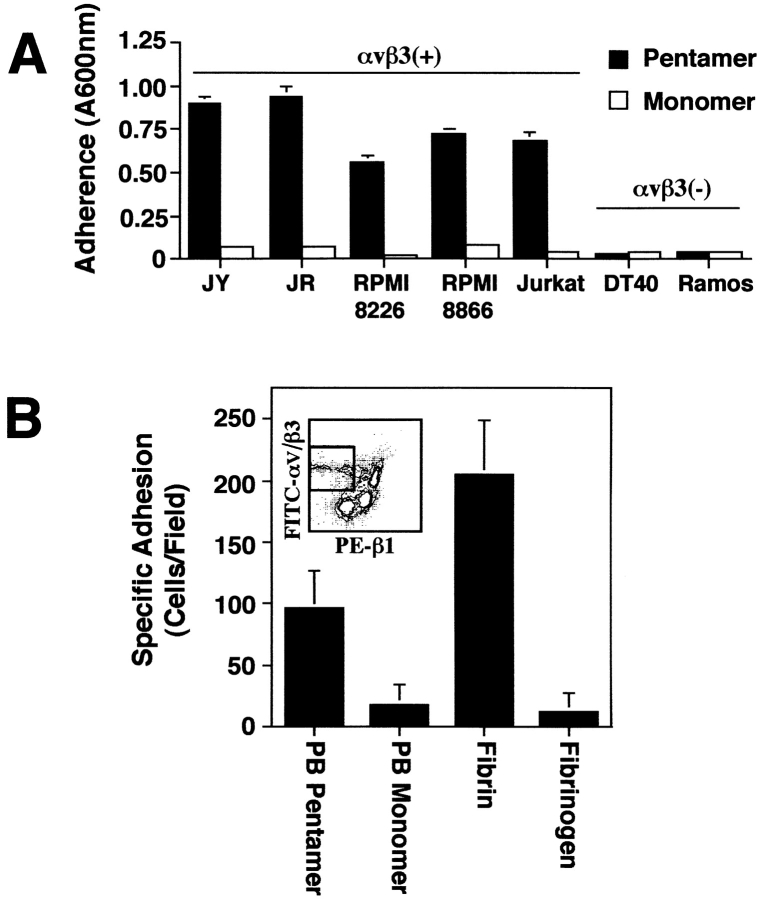
Importantly, other LCL and nonadherent B and T lymphoid cells, such as RPMI 8866, RPMI 8226, and Jurkat, also attached selectively to multimeric αvβ3 ligands (Fig. 5 A). In each case, attachment could be inhibited with monoclonal antibody LM609 (data not shown). In contrast, B cell tumor lines which do not express αvβ3 such as DT40 and Ramos, failed to attach to either form of PB (Fig. 5 A). Although integrin αvβ3 has been described in tissue-associated lymphocyte populations (Halvorson et al., 1996), it is only minimally expressed on circulating lymphocytes (Hemler, 1990). However, when the subpopulation of lymphocytes expressing αvβ3 were isolated from peripheral blood (< 2%) (Fig. 5 B, inset), these cells also demonstrated selective adhesion to both multimeric PB and fibrin relative to monomeric PB and fibrinogen, respectively (Fig. 5 B). Thus, lymphocytes which express appropriate receptors can attach to multivalent ligands without a requirement for exogenous activation.
Figure 5.
Integrin αvβ3 mediates selective attachment to pentameric PB. (A) The lymphoblastoid cell lines (LCL) JY and JR, as well as the lymphoid tumor cell lines RPMI 8226, RPMI 8866, and Jurkat (all αvβ3+), and the cell lines DT40 and Ramos (αvβ3−) were assessed for attachment to pentameric or monomeric PB by adhesion assay as previously described. (B) Human peripheral blood mononuclear cells were sorted for positive expression of αvβ3 (FITC), and low expression of β1 (PE) (gated box, inset) allowing for depletion of monocyte contamination during purification of αvβ3-expressing lymphocyte populations. Sorted lymphocytes were assessed for attachment to penton base coated (10–20 nM) or fibrin/fibrinogen coated (100 nM) chamber slides (105 cells/slide) and attached cells per field were quantified by direct counting. Data are expressed as the mean specific attachment ± SE from six fields counted. A representative experiment from three experiments is shown.
Tyrosine Phosphorylation of p85 and Syk Follows Integrin Contact with Ligand
It has been previously reported that agonists can facilitate both lymphoid and platelet “outside-in” signaling through the activation of signaling intermediates including Syk, FAK, and PI(3)K (Shattil et al., 1994; Ma et al., 1997; Rabinowich et al., 1997). Although nonactivated LCL are capable of recognizing monomeric integrin ligands in solution (Fig. 4 C), the specific presentation of immobilized multimeric adhesive sites was required to facilitate stable adhesion. This suggests that integrin-dependent signaling may be sufficient to initiate postreceptor-binding events that lead to stable lymphoid cell adhesion. Kinetic analysis showed that LCL adhesion occurred principally (∼75%) during the initial 5–15 min (data not shown). Therefore, to investigate possible mechanisms leading to adhesion, lysates from LCL plated on monomeric or multimeric ligands for 15 min were subjected to immunoprecipitation and phosphotyrosine analysis.
Although LCL failed to express detectable levels of FAK (data not shown), they expressed both Syk and PI(3)K. Attachment of LCL to pentavalent PB resulted in enhancement of Syk phosphorylation compared with Syk isolated from cells plated on the monomeric substrate or on BSA (4.5 ± 1.7 fold) (Fig. 6 A). Importantly, there was a corresponding two- to threefold increase in Syk kinase activity in cells adherent to the pentamer compared with cells plated on the monomer or the nonadherent substrate protein BSA (Fig. 6 B). In contrast, an increase in phosphorylation of the p85 subunit of PI(3)K (Fig. 6 C) and PI(3)K activity (Fig. 6 D) was observed in cells interacting with either pentamer or monomer relative to cells plated on BSA. These data suggest that lymphoid cell PI(3)K becomes activated with a low threshold of integrin ligation in general, whereas integrin ligation leading to (stable) adhesion is specifically associated with activation of Syk.
Figure 6.
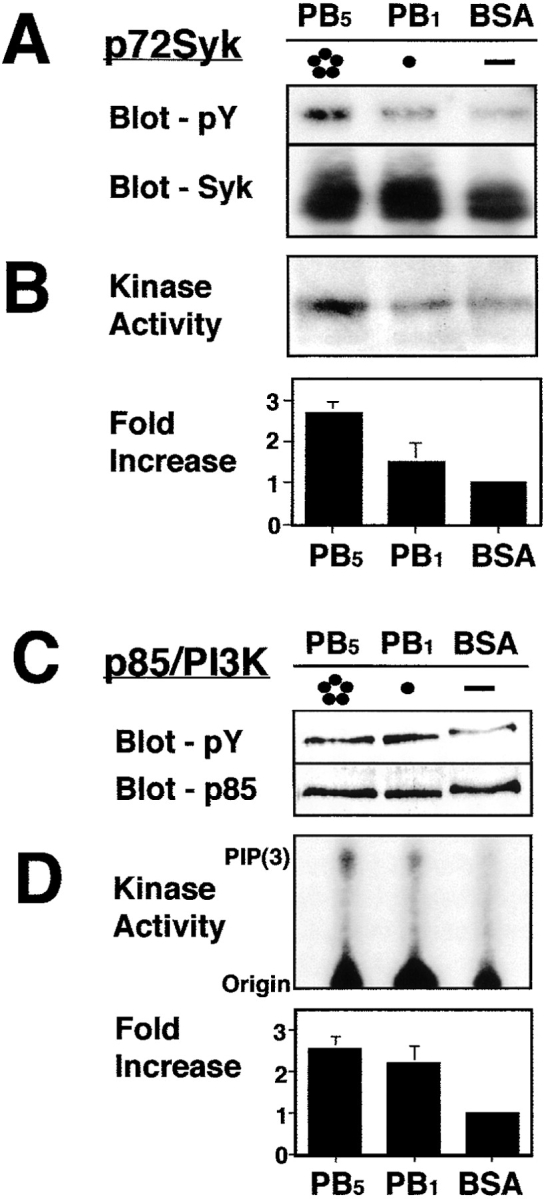
Activation of Syk and PI(3)K after integrin ligation. (A) NP-40 lysates of LCL plated on wells coated with either BSA (B), monomeric penton base (PB1) or pentameric penton base (PB5) generated 15 min after plating were subjected to immunoprecipitation for Syk, followed by immunoblot analysis for phosphotyrosine or Syk with monoclonal antibodies 4G10 or 4D10, respectively. (B) Autokinase activity of immunoprecipitated Syk was assessed by in vitro kinase assay after immunoprecipitation. The mean relative increase and SEM from three separate experiments is plotted. (C) NP-40 lysates of LCL plated on wells coated with either BSA or monomeric or pentameric PB were similarly subjected to immunoprecipitation for the p85 subunit of PI(3)K, followed by immunoblotting analysis for phosphotyrosine with monoclonal antibody 4G10. (D) The p85-associated PI(3)K activity was assessed after immunoprecipitation of p85 and the addition of exogenous 4,5 PIP2 and 5 μCi 32P-ATP. Lipids were resolved on TLC-silica plates (1:1:1, CHCl3, CH3OH, 1 M HCl). The mean relative increase and SEM from three separate experiments is plotted.
Syk Activity Regulates Integrin-mediated Attachment by LCL
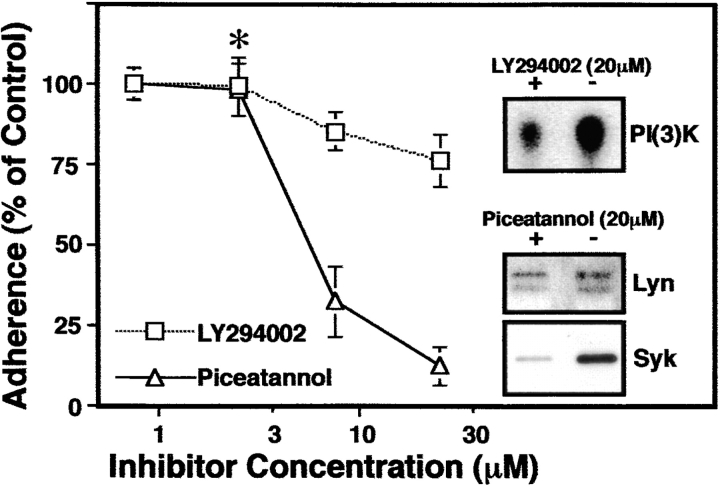
As an initial approach to testing whether Syk was involved in regulating adhesion to the ECM, LCL were allowed to bind to PB in the presence of either pharmacological inhibitors known to inhibit Syk (piceatannol) or PI(3)K (LY294002 and wortmannin). Adhesion of the LCL to PB was blocked by piceatannol (IC50 ∼5 mM), but was only slightly impacted by the PI(3)K inhibitor LY294002 (Fig. 7). Although LY294002 treated cells attached, they exhibited reduced PI(3)K activity (Fig. 7, inset), failed to spread (data not shown), and were more susceptible to shear force during washing, resulting in an apparent slight inhibition of adhesion. Similar results were obtained with an alternative inhibitor of PI(3)K, wortmannin (increasing effect from 70–80 nM, data not shown). Although selective for the kinase Syk, piceatannol has pleiotropic effects on cells, including the blockade of src family kinases at increased concentrations. However, no significant inhibition of the related src family kinase Lyn (the major src kinase in LCL) was found in these studies at the concentrations of piceatannol used (Fig. 7, inset).
Figure 7.
Inhibition of LCL binding by piceatannol. Increasing concentration of LY294002 or piceatannol were incubated with LCL for 15 min. Cells were then assessed for adhesion as described above. LY294002 caused visible decreases in LCL spreading at concentrations as low as 2.5 μM (asterisk), and inhibited PI(3)K activity, (inset), but did not block attachment at concentrations below 100 μM. The derived IC50 for piceatannol inhibition of adhesion was ∼5 μM. Inhibition of Syk (> 90%), but not the related src family kinase Lyn (18 ± 11%) was assessed by autokinase activity (inset).
Surprisingly, if LCL were allowed to attach and spread, no reversal of adhesion was observed after the addition of piceatannol (data not shown), suggesting that activation of Syk was important in the initial phase of adhesion of lymphoid cells to the multimeric ligands. Therefore, Syk localization was examined by immunofluorescent confocal microscopy during attachment. In nonadherent LCL cells Syk is present diffusely in the cytosol and does not significantly colocalize with actin in the microspikes present ubiquitously across the cell surface (Fig. 8 A, arrow). Similarly, there is only modest colocalization of Syk and actin in adherent cells above the plane of interaction with substrate (Fig. 8 A, +10-μm Z section). However, we consistently observed colocalization of Syk and actin in the podosome near the cell–substrate interface during early phases of cell attachment (Fig. 8 B), although this colocalization disappeared upon full cell spreading (Fig. 8 C). Together, these results suggest that the activation of Syk plays a role in the initial polarization of cells leading to productive attachment on multimeric ligand.
Figure 8.
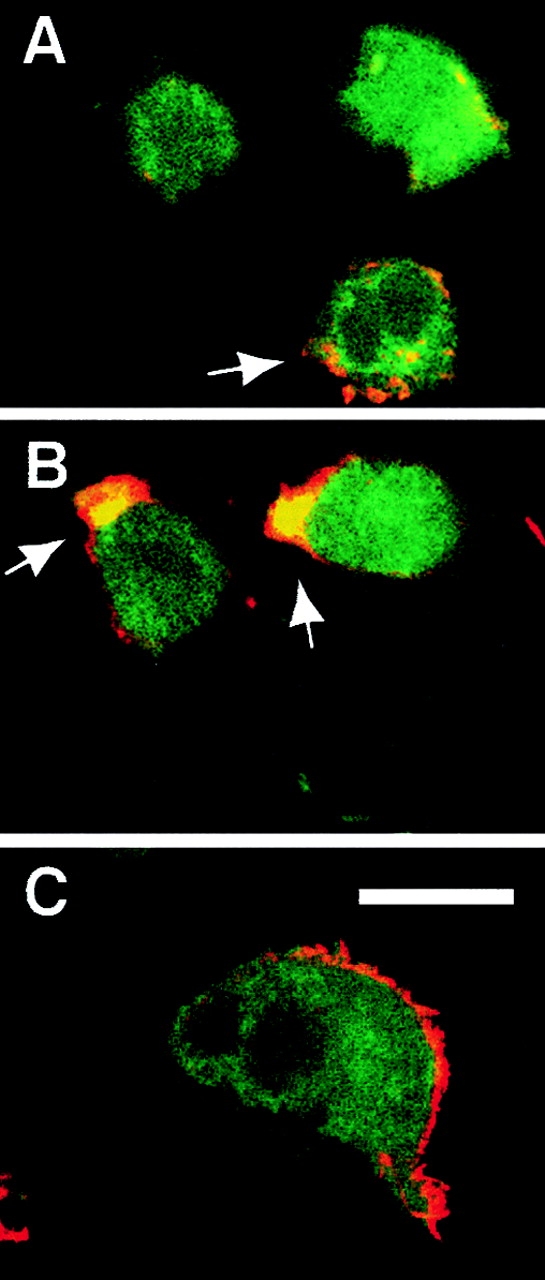
Confocal immunofluorescent microscopic examination of Syk localization during LCL attachment. LCL were allowed to attach for 10 (A and B) or 30 (C) min and subsequently fixed and stained for the presence of endogenous Syk with monoclonal 4D10 followed by secondary detection by FITC-conjugated goat anti– mouse (green), and costained with rhodamine-conjugated phalloidin to label F-actin (red). Significant colocalization of the signals (yellow) did not occur in nonadherent LCL (A, arrow) and was modest in confocal Z sections of adherent LCL viewed above the adhesive substrate (A) (Z plane = 11 μm above the substrate). Colocalization of signal was observed consistently in the podosomes (B, arrows) of actively attaching LCL at the cell–substratum interface (Z plane = 1 μm above the substrate), but not in fully spread cells at 30 min (C) (Z plane = 1 μm above the substrate). All Z sections are 1 μm. Bar, 10 μm.
These results suggest that preactivation of Syk might be able to facilitate attachment to monomeric ligands. To determine whether nonintegrin mediated activation of Syk could regulate lymphoid adhesion, we examined an antigen-specific response in Syk-expressing, tissue-resident T cells. CTL cells specific for LCMV were established by coculture with LCMV-infected, irradiated helper cells, resulting in a proliferative response (Hahn et al., 1994) and the activation of Syk (Fig. 7 A, inset). In these CTL, Syk activation by antigen was sufficient to facilitate attachment to either monomeric or multimeric ligands (Fig. 9 A). To confirm that Syk activation was maintained in these cells, lysates from the CTL were generated and Syk immunoprecipitation and phosphotyrosine analysis performed at the conclusion of the adhesion assay. Interestingly, Syk phosphorylation was maintained in CTL populations plated on monomeric or multimeric substrate, but this was not the case when cells were plated on the nonadhesive substrate BSA (Fig. 9 A, inset). These results indicate that Syk phosphorylation may be maintained by ongoing adhesion, and that Syk activation correlates with stable adhesion to monomeric ligand.
Figure 9.
Activation of Syk is associated with cellular adhesion. (A) Antigen-activated murine γδ cytotoxic T cells were generated by coculture of splenic T cells from LCMV-challenged mice with LCMV-infected helper cells. The γδ cells were tested for their capacity to attach to monomeric or multimeric PB by adhesion assay. To confirm Syk activation, lysates derived from γδ CTL plated on BSA (B), pentamer (PB5), or monomer (PB1) for 1 h were subjected to immunoprecipitation and Syk phosphorylation was assessed (inset). (B) Syk-deficient DT40 cells which were reconstituted with cDNA constructs encoding either Syk (WT) or a constitutively active form of Syk (Y130E), were determined to express similar levels of β1 integrin by flow cytometry using the monoclonal antibody CSAT, which detects chick β1 integrin (inset). Cells were subsequently tested for their capacity to attach to wells coated with varying concentrations of a monomeric fragment of fibronectin containing the Arg-Gly-Asp cell-binding domain.
To determine if Syk activity, independent of antigen activation, was sufficient to promote attachment to ECM, nonadherent B lymphoma cells (DT40) deficient in Syk (Kurosaki et al., 1995) were reconstituted with either wild-type Syk or activated (Y130E) Syk (Keshvara et al., 1996) and then examined for their capacity to adhere to a monomeric substrate. Stable expression of Syk (SykWT) in Syk-deficient DT40 cells failed to promote attachment to a monomeric fragment of fibronectin (Fig. 9 B), however, DT40 cells expressing the constitutively active form of Syk (Y130E) gained the capacity to bind to the monomeric cell-binding domain of fibronectin. Parental DT40 cells and their derivatives lack αvβ3 and do not attach to PB (Fig. 5 A), but do express β1 integrins (Fig. 9 B, inset) which bind this region of fibronectin. Consistent with this, adhesion was blocked by a monoclonal antibody to β1 integrin (data not shown). These data show that β1 integrin-dependent adhesion to fibronectin, like that mediated by αvβ3, can also be upregulated by Syk activation, and that Syk requires activation to promote lymphoid cell adhesion to integrin ligands.
Discussion
Inflammatory processes are characterized by the coordinated deposition of provisional ECM and accumulation of leukocytes within affected tissues. Lymphocyte interaction with components of the ECM plays a crucial role in the chronicity (Postigo et al., 1991; Roman, 1996) and resolution (Edgington et al., 1985; Issekutz, 1993; Hershkoviz et al., 1994) of inflammatory processes. Lymphoid adhesion resulting from integrin-mediated recognition of multimeric ligands such as fibrin, polymerized fibronectin, and vitronectin, as demonstrated, provides a mechanism whereby nonactivated lymphoid cells can become partially activated independent of exposure to antigen or other agonists. The capacity to control adhesion by the multimerization of integrin ligands provides a means to specifically promote the accumulation of lymphoid cells within inflammatory or diseased tissues. Within the tissues, lymphocytes may then undergo further activation by antigen or other agonists leading to the formation of local immune responses or alternatively egress to the draining lymph nodes. Lymphoid activation after tissue invasion exploits the known comitogenic activity of the ECM (Shimizu and Shaw, 1991).
Evidence is presented that a crucial aspect to initiating lymphoid adhesion is the functional multimerization of binding sites for integrins. The current study used the PB protein of adenovirus, a model ligand bound exclusively by integrins (Wickham et al., 1994) (Fig. 3 B), which provided a structurally defined ligand to examine lymphoid interactions with different substrate forms. In fact, the intact PB pentamer is known to have five available integrin-binding sites arranged in a pentagonal array with a separation of 60 Å (Stewart et al., 1997) allowing it to accommodate the binding of five integrins simultaneously. We contend that, like polymerized ECM proteins, PB acts as a clustered ligand and exploits local avidity as a means to initiate signaling events. It is clear that the overall (general) density of interaction is not as efficient in this respect, since much higher levels of monomeric penton base did not support adhesion. In fact, monomer coated surfaces containing 800 integrin sites/mm2 were unable to support lymphoid adhesion, whereas multimer-coated surfaces with less than 125 sites/mm2 available supported LCL adhesion. To demonstrate that the monomer could support adhesion, PMA, an agonist which promotes lymphoid integrin clustering via a PKC-dependent pathway (Haverstack et al., 1992; van Kooyk et al., 1994), was used to rescue attachment to monomer coated surfaces. Alternatively, it is possible that adhesion was observed on the multimeric proteins because the pentameric form of PB, and of ECM proteins in general, present higher affinity sites for integrin interactions upon multimerization. However, at least in the case of αvβ3/fibrinogen interaction, this does not appear to be the case (Fig. 2 B). Competitive blocking studies indicate that soluble PB monomer and the pentamer have a similarly high affinity for integrin (IC50 ∼18 and 5 nM, respectively) although pentameric PB is significantly more potent at inducing integrin-mediated signaling events (Li et al., 1998) (Li, E., and G. Nemerow, unpublished data). Therefore, these findings suggest that appropriately spaced clustering of integrin ligand provides a specific mechanism through which these postreceptor events promote stable adhesion. The fact that adherent M21 cells attached to either substrate suggests that lymphoid cells regulate postreceptor ligation events differently than adherent cell types.
By itself, affinity modulation of integrin function did not appear to explain the observed agonist-induced adhesion, since soluble integrins maintained the same affinity for either multimeric or monomeric ligand (Fig. 2 C). It remains possible that avidity changes in integrin (clustering) can result in higher affinity through local packing and lateral stabilization of ligated integrin. Such changes might not be detected by conformational/affinity measurements such as LIBS binding due to steric issues of accessibility. Further study will be necessary to differentiate pure affinity changes from local packing effects. However, in addition to activation of PKC and Rho-dependent integrin clustering, PMA activates a variety of cellular signaling pathways, and induction of integrin-mediated adhesion by this agonist has previously been suggested to occur as a result of postreceptor events (Danilov and Juliano, 1989). The current investigation similarly provides evidence for postreceptor signaling events inducing attachment, and suggests basic differences in the response of lymphoid cells and adherent cells to different ligand forms. These findings suggest that ligand polymerization may provide all cues necessary to facilitate stable lymphocyte attachment.
Based upon these results, we propose that integrin ligation-dependent signaling impacts the ability of lymphoid cells to attach to the ECM. Several morphological (e.g., cell spreading) and signaling events were observed to occur during adhesion, including the phosphorylation of both Syk and p85. Although p85-associated PI(3)K activity increased after interaction with PB pentamer, it was similarly observed after contact with monomeric PB where no stable adhesion was detected. Inhibitors of PI(3)K did not block attachment of LCL to pentameric PB, however, they did disrupt spreading and the extension of processes (Fig. 3 A and our unpublished observations). However, some low level of PI(3)K activity may be required for adhesion, and our data supports the possibility that activation of PI(3)K plays a crucial role in attachment under conditions of flow/high shear. Pretreatment with piceatannol blocked adhesion to pentamer at very low concentrations (5 μM), suggesting that activation of Syk was required to induce subsequent attachment (Fig. 7), but was not necessarily involved in spreading or maintenance of LCL adhesion. These results are supported by the colocalization of Syk with actin during initial cell polarization/podosome formation (Fig. 8). The activation of Syk in lymphoid cells by antigenic stimulation or by reconstitution with constitutively active mutants rescued lymphoid cell attachment to monomer-coated surfaces (Fig. 9). Together, these results indicate that integrin postreceptor signaling events are crucial in governing LCL adhesion, and that local clustering of ligand provides a means to elicit integrin-mediated signaling resulting in stable attachment. Parallel studies confirmed that attachment to PB occurred as a result of postreceptor signaling in other cell types. For example, we were unable to observe attachment to PB with melanoma cells bearing a mutant β3 integrin tail (N744A), which is deficient in signaling but not ligand binding (Filardo et al., 1995) (our unpublished observations).
The activation of the hematopoietic kinase Syk occurs via the antigen receptor, soluble agonists, and integrin interactions with immobilized substrate (Burg et al., 1994; Lin et al., 1995; Gotoh et al., 1997; Okazaki et al., 1997). It is clear that qualitative differences exist in the outcome after interaction with different ligands. Activation of Syk through the antigen receptor leads to activation of MAP kinases and proliferation (Wan et al., 1996; Jacinto et al., 1998), whereas our results suggest a role for Syk in regulating adhesion. Adhesion alone was not sufficient to fully activate freshly isolated lymphocytes, since attachment to multimeric integrin ligands did not lead to significant proliferation of nonactivated, sorted peripheral blood lymphocytes (our unpublished observations). However, after initial antigenic stimulation, γδ CTL adhesion to monomer and pentameric ligands was observed, and Syk phosphorylation was maintained throughout the course of the adhesion. Supporting this result, coexpression of integrin αvβ3 and the ζ subunit of the T cell antigen–receptor complex are known to be sufficient for continued γδ CTL clone proliferation and cytokine secretion (Halvorson et al., 1996). The ζ subunit consists of a transmembrane domain and an immunoreceptor tyrosine-based activation motif (ITAM)-containing docking motif which is recognized specifically by the dual SH2 domains of Syk and its homologue ζ-associated protein (ZAP)-70 (Chan and Shaw, 1996), providing a mechanism by which integrin-mediated Syk activation pathways and antigen-stimulated pathways may couple.
Similarities do exist in the initial signaling pathways triggered by integrins or antigen receptors, since both integrins and antigen receptors activate specific members of the src kinase family identified as activators of Syk (Burg et al., 1994; Shattil et al., 1994; El Hillal et al., 1997; Hunter and Shimizu, 1997). Moreover, cross-linking either antigen receptor or integrins results in Syk activation (Rabinowich et al., 1996), suggesting that clustering of integrins by multimeric ligand leads to Syk activation. Syk, in turn, is known to phosphorylate cytosolic target proteins including Pyk-2 (Okazaki et al., 1997), α-tubulin (Peters et al., 1996), and the guanine nucleotide exchange factor Vav (Deckert et al., 1996; Gotoh et al., 1997; Teramoto et al., 1997), supporting a mechanism for rapid cytoskeletal reorganization in lymphoid cells during both antigen presentation and adhesion to the ECM (Kupfer and Singer, 1989; Hahn et al., 1994). However, Syk is not always required for the activation of downstream targets by G protein– coupled receptors or other agonists (Wan et al., 1996). Therefore, modulation of integrin function by chemokines (Carr et al., 1996; Laudanna et al., 1996), multimeric ECM, and/or antigenic stimulation may potentiate adhesion via somewhat distinct signaling pathways.
In general, recognition of ECM by lymphocytes is critical to a variety of processes, including inflammation, lymphoid maturation, tissue retention, trafficking, and surveillance (Butcher and Picker, 1996). The implication of Syk as a crucial effector of lymphocyte–ECM interactions is interesting, since Syk-deficient lymphocytes are defective in tissue entry and distribution, as well as in subsequent maturation steps in vivo (Turner et al., 1994). Thymic maturation of T cells requires both integrins (Crisa et al., 1995) and Syk kinases (Cheng et al., 1995). Taken together, these observations reveal a mechanism whereby lymphoid responses are modulated by the local composition of the ECM, and specifically by the multimeric presentation of integrin binding sites. Integrin-mediated adhesion to polymeric ligands plays a key role in modulating lymphoid responses through the central kinase Syk, which in turn exerts pleiotropic effects upon lymphoid cells. This interplay may be important in the understanding of lymphoid cell behavior in vivo, and suggests a prominent role for the local ECM in governing lymphoid behavior in malignancy and inflammatory processes.
Acknowledgments
The authors wish to thank E. Ruoslahti, R. Pasqualini, D. Sieffert, T. Muir, J. Wilkins, and S. Goodman for the generous gift of reagents, and D. Salomon and S. Shattil (TSRI) for helpful discussion. We thank R. Summers (TSRI) for assistance with the confocal microscopy.
This work was funded by grants from National Institutes of Health to D.A. Cheresh (CA 45726 and CA 50286), G.R. Nemerow (HL 54352 and EY 11431), K. Hahn (AI 39643), R.L. Geahlen (CA 37372) and the Joseph Drown Foundation to D.G. Stupack. D.G. Stupack was a recipient of a fellowship from the Canadian Arthritis Society. This is manuscript number 12080-IMM from The Scripps Research Institute.
Abbreviations used in this paper
- CTL
cytotoxic lymphocyte
- ECM
extracellular matrix
- LCL
lymphoblastoid cell line
- LCMV
lymphocytic choriomenginitis virus
- LIBS
ligand-induced binding site
- MAP kinase
mitogen-activated protein kinase
- PB
penton base
- PE
phycoerythrin
- PI(3)K
phosphoinositide-3-kinase
References
- Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules and selectins. Pharmacol Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- Bazzoni G, Hemler ME. Are changes in integrin affinity and conformation overemphasized? . Trends Biochem Sci. 1998;23:30–34. doi: 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- Burg DL, Furlong MT, Harrison ML, Geahlen RL. Interactions of Lyn with the antigen receptor during B cell activation. J Biol Chem. 1994;269:28136–28142. [PubMed] [Google Scholar]
- Butcher EC, Picker LJ, Lymphocyte homing and homeostasis Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Carr MW, Alon R, Springer TA. The C-C chemokine MCP-1 differentially modulates the avidity of β1 and β2 integrins on T lymphocytes. Immunity. 1996;4:179–187. doi: 10.1016/s1074-7613(00)80682-2. [DOI] [PubMed] [Google Scholar]
- Chan AC, Shaw AS. Regulation of antigen receptor signal transduction by protein tyrosine kinases. Curr Opin Immunol. 1996;8:394–401. doi: 10.1016/s0952-7915(96)80130-0. [DOI] [PubMed] [Google Scholar]
- Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- Cheresh DA. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Nat Acad Sci USA. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisa L, Cirulli V, Ellisman MH, Ishii JK, Elices MJ, Salomon DR. Cell adhesion and migration are regulated at distinct stages of thymic T cell development: the roles of fibronectin, VLA-4, and VLA-5. J Exp Med. 1996;184:215–228. doi: 10.1084/jem.184.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov YN, Juliano RL. Phorbol ester modulation of integrin-mediated cell adhesion: a postreceptor event. J Cell Biol. 1989;108:1925–1933. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Edgington TS, Curtiss LK, Plow EF. A linkage between the hematostatic and immune systems embodies in the fibrinolytic release of lymphocyte suppressive peptides. J Immunol. 1985;134:471–477. [PubMed] [Google Scholar]
- El-Hillal O, Kurosaki T, Yamamura H, Kinet JP, Scharenberg AM. Syk kinase activation by a src kinase-initiated activation loop phosphorylation chain reaction. Proc Natl Acad Sci USA. 1997;94:1919–1924. doi: 10.1073/pnas.94.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B, Ruggeri ZM, Cheresh DA. Distinct biological consequences of integrin αvβ3-mediated melanoma cell adhesion to fibrinogen and its plasmic fragments. J Biol Chem. 1992;267:5070–5077. [PubMed] [Google Scholar]
- Filardo EJ, Brooks PC, Deming SL, Damsky C, Cheresh DA. Requirement of the NPXY motif in the integrin β3 subunit cytoplasmic tail for melanoma cell migration in vitro and in vivo. J Cell Biol. 1995;130:441–450. doi: 10.1083/jcb.130.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger AL, III, Cohen I, Plow EF, Smith MA, Roberts J, Lam SC, Ginsberg MH. Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J Biol Chem. 1990;265:6346–6352. [PubMed] [Google Scholar]
- Gotoh A, Takahira H, Geahlen RL, Broxmeyer HE. Cross-linking of integrins induces tyrosine phosphorylation of the proto-oncogene product Vav and the protein tyrosine kinase Syk in human factor-dependent myeloid cells. Cell Growth Differ. 1997;8:721–729. [PubMed] [Google Scholar]
- Hahn K, DeBiasio R, Tishon A, Lewicki H, Gairin JE, LaRocca G, Taylor DL, Oldstone M. Antigen presentation and cytotoxic T lymphocyte killing studied in individual living cells. Virology. 1994;201:330–340. doi: 10.1006/viro.1994.1298. [DOI] [PubMed] [Google Scholar]
- Halvorson MJ, Coligan JE, Sturmhofel K. The vitronectin receptor (αvβ3) as an example for the role of integrins in T lymphocyte stimulation. Immunol Res. 1996;15:16–29. doi: 10.1007/BF02918281. [DOI] [PubMed] [Google Scholar]
- Haverstack DM, Sakai H, Gray LS. Lymphocyte adhesion can be regulated by cytoskeleton-associated, PMA-induced capping of surface receptors. Am J Physiol. 1992;262:C916–C926. doi: 10.1152/ajpcell.1992.262.4.C916. [DOI] [PubMed] [Google Scholar]
- Hemler ME. VLA proteins in the integrin family: structures, functions and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hershkoviz R, Greenspoon N, Mekori YA, Hadari R, Alon R, Kapustina G, Lider O. Inhibition of CD41 T lymphocyte binding to fibronectin and immune-cell accumulation in inflammatory sites by non-peptidic mimetics of Arg-Gly-Asp. Clin Exp Immunol. 1994;95:270–276. doi: 10.1111/j.1365-2249.1994.tb06522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Stupack D, Mathias P, Wang Y, Nemerow G. Growth arrest of Epstein-Barr virus immortalized B lymphocytes by adenovirus-delivered ribozymes. Proc Nat Acad Sci USA. 1997;94:8156–8161. doi: 10.1073/pnas.94.15.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AJ, Shimizu Y. α4β1 integrin-mediated tyrosine phosphorylation in human T cells: characterization of Crk- and Fyn-associated substrates (pp105, pp115, and human enhancer of filamentation-1) and integrin-dependent activation of p59fyn1. J Immunol. 1997;159:4806–4814. [PubMed] [Google Scholar]
- Issekutz TB. Dual inhibition of VLA-4 and LFA-1 maximally inhibits cutaneous delayed-type hypersensitivity-induced inflammation. Am J Pathol. 1993;143:1286–1293. [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Werlen G, Karin M. Cooperation between Syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity. 1998;8:31–41. doi: 10.1016/s1074-7613(00)80456-2. [DOI] [PubMed] [Google Scholar]
- Keshvara LM, Isaacson C, Harrison ML, Geahlen RL. Syk activation and dissociation from the B-cell antigen receptor is mediated by phosphorylation of tyrosine 130. J Biol Chem. 1997;272:10377–10381. doi: 10.1074/jbc.272.16.10377. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Singer SJ. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu Rev Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- Kurosaki T, Johnson SA, Pao L, Sada K, Yamamura H, Cambier JC. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J Exp Med. 1995;182:1815–1823. doi: 10.1084/jem.182.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna C, Campbell JJ, Butcher EC. Role of rho in chemoattractant-activated leukocyte through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Rosales C, Mondal K, Bolen JB, Haskill S, Juliano RL. Integrin-mediated tyrosine phosphorylation and cytokine message induction in monocytic cells. A possible signaling role for the Syk tyrosine kinase. J Biol Chem. 1995;270:16189–16197. doi: 10.1074/jbc.270.27.16189. [DOI] [PubMed] [Google Scholar]
- Ma EA, Lou O, Berg NN, Ostergaard HL. Cytotoxic T lymphocytes express a β3 integrin which can induce the phosphorylation of focal adhesion kinase and the related PYK-2. Eur J Immunol. 1997;27:329–335. doi: 10.1002/eji.1830270147. [DOI] [PubMed] [Google Scholar]
- Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RJ, Diefenbach B, Brown A, Cullen E, Jonczyk A, Gussow D, Lukenbach GA, Goodman SL. Transmembrane-truncated αvβ3 retains high affinity for ligand binding: evidence for an “inside-out” suppressor? . Biochem J. 1998;330:861–869. doi: 10.1042/bj3300861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson MW, Siebenlist KR, Hainfeld JF, Wall JS. The covalent structure of factor XIIIa crosslinked fibrinogen fibrils. J Struct Biol. 1995;115:88–101. doi: 10.1006/jsbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- Okazaki H, Zhang J, Hamawy MM, Siraganian RP. Activation of protein-tyrosine kinase Pyk2 is downstream of Syk in FceRI signaling. J Biol Chem. 1997;272:32443–32447. doi: 10.1074/jbc.272.51.32443. [DOI] [PubMed] [Google Scholar]
- Peters JD, Furlong MT, Asai DJ, Harrison ML, Geahlen RL. Syk, activated by cross-linking the B-cell antigen receptor, localizes to the cytosol where it interacts with and phosphorylates alpha-tubulin on tyrosine. J Biol Chem. 1996;271:4755–4762. doi: 10.1074/jbc.271.9.4755. [DOI] [PubMed] [Google Scholar]
- Postigo AA, Corbi AL, Sanchez-Madrid F, de Landazuri MO. Regulated expression and function of CDllc/CD18 integrin on human B lymphocytes. Relationship between attachment to fibrinogen and triggering of proliferation through CDllc/CD18. J Exp Med. 1991;174:1313–1322. doi: 10.1084/jem.174.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postigo AA, Garcia-Vicuna R, Laffon A, Sanchez-Madrid F. The role of adheison molecules in the pathogenesis of rheumatoid arthritis. Autoimmunity. 1993;16:69–76. doi: 10.3109/08916939309010649. [DOI] [PubMed] [Google Scholar]
- Rabinowich, H., M. Manciulea, R.B. Herberman, and T.L. Whiteside. β1 integrin-mediated activation of focal adhesion kinase and its association with Fyn and Zap-70 in human NK cells. J. Immunol. 157:3860–3868. [PubMed]
- Ratner S. Lymphocyte migration through extracellular matrix. Invasion Metastasis. 1992;12:82–100. [PubMed] [Google Scholar]
- Robson SC, Saunders R, Purves LR, de Jager C, Corrigall A, Kirsch RE. Fibrin and fibrinogen degradation products with an inteact D domain C-terminal γ chain inhibit an early step in accessory cell-dependent lymphocyte mitogenesis. Blood. 1993;81:3006–3014. [PubMed] [Google Scholar]
- Roman J. Extracellular matrix and lung inflammation. Immunol Res. 1996;15:163–178. doi: 10.1007/BF02918505. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Ginsberg MH, Brugge JS. Adhesive signaling in platelets. Curr Opin Cell Biol. 1994;6:695–704. doi: 10.1016/0955-0674(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Shaw S. Lymphocyte interactions with extracellular matrix. FASEB (Fed Am Soc Exp Biol) J. 1991;135:105–117. doi: 10.1096/fasebj.5.9.1860621. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stamper HB, Jr, Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976;144:828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M, Hogg N. Regulation of leukocyte integrin function: affinity vs. avidity. J Cell Biochem. 1996;61:554–561. doi: 10.1002/(sici)1097-4644(19960616)61:4<554::aid-jcb8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Stewart PL, Chiu CY, Huang S, Muir T, Zhao Y, Chait B, Mathias P, Nemerow GR. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO (Eur Mol Biol Organ) J. 1997;16:1189–1198. doi: 10.1093/emboj/16.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG, Shen C, Wilkins JA. Inducible B cell adherence to extracellular matrix mediated by a β3 integrin. Exp Cell Res. 1992;203:443–448. doi: 10.1016/0014-4827(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Teramoto H, Salem P, Robbins KC, Bustelo XR, Gutkind JS. Tyrosine phosphorylation of the vav proto-oncogene product links FceRI to the Rac1-JNK pathway. J Biol Chem. 1997;272:10751–10755. doi: 10.1074/jbc.272.16.10751. [DOI] [PubMed] [Google Scholar]
- Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1994;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- Van Kooyk Y, Weder P, Heje K, Figdor CG. Extracellular Ca2+modulates leukocyte function-associated antigen-1 cell surface distribution of T lymphocytes and consequently affects cell adhesion. J Cell Biol. 1994;124:1061–1070. doi: 10.1083/jcb.124.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Kurosaki T, Huang XY. Tytosine kinases in activation of the MAP kinase cascade by G-protein-coupled receptors. Nature. 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]