Abstract
While the Vpr protein of HIV-1 has been implicated in import of the viral preintegration complex across the nuclear pore complex (NPC) of nondividing cellular hosts, the mechanism by which Vpr enters the nucleus remains unknown. We now demonstrate that Vpr contains two discrete nuclear targeting signals that use two different import pathways, both of which are distinct from the classical nuclear localization signal (NLS)- and the M9-dependent pathways. Vpr import does not appear to require Ran-mediated GTP hydrolysis and persists under conditions of low energy. Competition experiments further suggest that Vpr directly engages the NPC at two discrete sites. These sites appear to form distal components of a common import pathway used by NLS- and M9-containing proteins. Together, our data suggest that Vpr bypasses many of the soluble receptors involved in import of cellular cargoes. Rather, this viral protein appears to directly access the NPC, a property that may help to ensure the capacity of HIV to replicate in nondividing cellular hosts.
Keywords: Vpr, nuclear import, HIV, nuclear pore complex, preintegration complex
Acrucial event in the early stages of the human immunodeficiency virus (HIV)1 life cycle in nondividing cells is the active transport of the viral preintegration complex (PIC) across the nuclear envelope, thereby permitting integration of HIV DNA into the host chromosome. The ability of HIV to infect such nondividing cells as terminally differentiated macrophages distinguishes this and the other primate lentiviruses from the oncoretroviruses that only infect proliferating cells (Humphries and Temin, 1974). For example, nuclear entry and integration of murine leukemia virus DNA requires passage of the host cell through mitosis, at which time the nuclear membrane breaks down and the virus gains entry into the nucleus (Roe et al., 1993; Lewis and Emerman, 1994). In the case of HIV, the matrix (Bukrinsky et al., 1993; von Schwedler et al., 1994; Gallay et al., 1995), integrase (Gallay et al., 1997), and Vpr (Heinzinger et al., 1994; Popov et al., 1998; Vodicka et al., 1998) proteins have all been identified as possible mediators of viral PIC nuclear localization. The matrix and integrase proteins contain classical SV-40–like nuclear localization signal (NLS) sequences and appear to use importin α/importin β (see below) for transport across the nuclear pore complex (NPC) (Bukrinsky et al., 1993; Gallay et al., 1996, 1997; Popov et al., 1996). However, the precise contribution of matrix and integrase to PIC import remains unclear (Freed et al., 1995; Fouchier et al., 1997). While Vpr is believed to contribute to nuclear targeting of the viral PIC, nuclear import of Vpr is a poorly understood process. The signal within Vpr responsible for nuclear localization is not known, and the import pathway used by Vpr is also not defined. Vpr significantly enhances viral replication in terminally differentiated macrophages (Connor et al., 1995), a function believed to be related to its karyophilic properties (Heinzinger et al., 1994).
Vpr is a small protein that is composed of only 96 amino acids and is efficiently packaged into virions (Cohen et al., 1990; Yuan et al., 1990) because of its association with the p6 region of the p55gag precursor (Paxton et al., 1993; Lu et al., 1995). In addition to its enhancement of replication in nondividing macrophages, Vpr also causes proliferating human cells to undergo an arrest or delay in the G2 phase of the cell cycle (He et al., 1995; Jowett et al., 1995; Re et al., 1995; Emerman, 1996). This property of Vpr may serve to amplify viral gene expression since the HIV-1 long terminal repeat is more active during G2 (Goh et al., 1998).
Secondary structure analysis of Vpr predicts the presence of two α-helical domains within the NH2-terminal 70 amino acids (Mahalingam et al., 1995). Vpr binding to p6gag and virion incorporation appear to require the first of these two α-helical domains. In contrast, many of the determinants of cell cycle arrest reside in the arginine-rich COOH-terminal portion of the protein (Di Marzio et al., 1995; Zhou et al., 1998). This COOH-terminal region of Vpr most closely resembles a canonical NLS; however, this region can be deleted with no impairment of nuclear localization (Di Marzio et al., 1995). Nevertheless, a recent study indicates that substitution of five arginines located within the COOH-terminal region with glutamine residues leads to a cytoplasmic pattern of Vpr localization, suggesting that the COOH-terminal region participates in nuclear targeting (Zhou et al., 1998). The NH2-terminal portion of Vpr does not bear any resemblance to a canonical NLS; amino acid mutations scattered throughout this region disrupt the nuclear targeting function of Vpr (Mahalingam et al., 1997). Furthermore, mutational analysis suggests the importance of the predicted α-helical secondary structure for nuclear localization of Vpr (Di Marzio et al., 1995; Mahalingam et al., 1995).
The previously characterized pathways for signal-mediated import of proteins into the nucleus share several features. First, the signal-bearing karyophilic protein is recognized by a specific transport receptor, and secondly, the protein complex is actively translocated through the NPC. Finally, once in the nucleus, the transported cargo dissociates from the receptor, and the receptor is recycled to the cytoplasm (Nigg, 1997; Ohno et al., 1998). The best-characterized nuclear import pathway is for proteins containing a basic residue–rich NLS, the prototype being the NLS present in the large T antigen of SV-40. Proteins containing such a classical, positively charged NLS bind to a heterodimeric receptor complex composed of importin α (karyopherin α; for alternate names see Nigg, 1997) (Görlich et al., 1994; Weis et al., 1995) and importin β (karyopherin β, p97) (Chi et al., 1995; Görlich et al., 1995a ; Radu et al., 1995a ). Importin α is responsible for binding to the NLS-bearing protein, while importin β mediates binding of the transport complex to the NPC (Görlich et al., 1995b ). The interaction of importin α with importin β occurs through the NH2 terminus of importin α termed the importin β binding domain (IBB). When fused to heterologous proteins, the IBB domain alone is sufficient to produce nuclear entry via importin β (Görlich et al., 1996a ; Weis et al., 1996). The importin α/importin β system also requires the participation of the small Ras-related GTPase, Ran/TC4 (Moore and Blobel, 1993; Melchior et al., 1993), and p10 (NTF2) (Moore and Blobel, 1994; Paschal and Gerace, 1995). However, the mechanism of cargo translocation and the exact contribution of Ran-mediated GTP hydrolysis to the import reaction are not well understood. The asymmetric distribution of the GTPase activating protein, RanGAP (cytoplasm), and the nucleotide exchange factor, RCC1 (nucleus), is predicted to generate a steep gradient of RanGTP between these two cellular compartments, and this gradient may confer vectoriality to the various transport pathways (Görlich et al., 1996b ; Izaurralde et al., 1997a ). In this regard, once the importin α/importin β/cargo complex reaches the nucleus, RanGTP binding to importin β dissociates the ternary complex, allowing cargo delivery and recycling of importin α and importin β to the cytoplasm (Rexach and Blobel, 1995; Görlich et al., 1996b ; Koepp and Silver, 1996).
A distinct nuclear import pathway has been identified for the RNA binding protein hnRNP A1. This protein contains a signal sequence termed M9 that bears no sequence homology to the classical NLS and mediates both nuclear import and export of hnRNP A1 (Siomi and Dreyfuss, 1995; Michael et al., 1995). Transportin, a novel receptor distantly related to importin β, binds to the M9 domain and mediates the import of hnRNP A1 in vitro (Aitchison et al., 1996; Pollard et al., 1996; Fridell et al., 1997). As in the classical NLS pathway, import of hnRNP A1 is dependent on the presence of Ran/TC4 (Nakielny et al., 1996). Binding of RanGTP to transportin in the nucleus also leads to the dissociation of hnRNP A1 and recycling of transportin to the cytoplasm (Izaurralde et al., 1997b ; Siomi et al., 1997).
Importin β and transportin are members of a large superfamily of proteins that contain RanGTP binding domains and are believed to function as nuclear transport receptors for unknown cellular cargoes (Fornerod et al., 1997a ; Görlich et al., 1997). More recently, members of this superfamily have been implicated in the import of ribosomal proteins (Rout et al., 1997; Schlenstedt et al., 1997), the import of proteins involved in RNA processing (Pemberton et al., 1998; Rosenblum et al., 1998; Senger et al., 1998), as well as the export of importin α (Kutay et al., 1997a ), the export of tRNA (Arts et al., 1998; Kutay et al., 1998), and cytoplasmic delivery of proteins containing a leucine-rich nuclear export signal like that found in HIV Rev (Fornerod et al., 1997b; Fukuda et al., 1997; Ossareh-Nazari et al., 1997; Stade et al., 1997).
Using an established in vitro nuclear import assay, we now demonstrate that HIV Vpr contains two distinct nuclear targeting signals that use different receptors for entry into the nucleus. Furthermore, we show that neither of these Vpr signals is dependent on importin α/importin β or transportin for nuclear entry and that Ran/TC4-mediated GTP hydrolysis does not appear to be required. We further find that both signals in Vpr operate efficiently under conditions of minimal energy. Finally, competition experiments suggest that Vpr directly engages the NPC at two discrete sites in a pathway of nuclear import also used by NLS- and M9-containing proteins. Our data support an entry mechanism in which Vpr bypasses the soluble receptors involved in import of cellular cargoes and, instead, directly targets factors located within the NPC itself.
Materials and Methods
Plasmid Construction and Expression and Purification of Recombinant Proteins
The plasmid encoding the βgalactosidase (βgal) fusion protein was constructed by inserting a PCR fragment corresponding to the lacZ sequence of Escherichia coli into the XbaI/XmaI restriction sites in the pCMV4 eukaryotic expression vector. The 5′ PCR primer incorporated a thrombin cleavage site (Leu-Val-Pro-Arg-Gly-Ser) located between the XbaI site and the first codon of lacZ. Full-length Vpr and the Vpr deletion mutants were constructed by inserting the appropriate PCR fragments into the HindIII/XbaI sites of pCMV-3′ βgal. All Vpr sequences were derived from the NL4-3 allele of HIV-1. Expression of recombinant fusion proteins in E. coli was performed using the pET28 vector (Novagen, Madison, WI). All Vpr constructs were confirmed by DNA sequencing, and protein expression was confirmed by immunoblotting with an anti-βgal monoclonal antibody (Boehringer Mannheim Corp., Indianapolis, IN). The plasmid encoding glutathione-S-transferase (GST)-M9 was prepared by inserting the M9 sequence (amino acids 268–305 of hnRNP A1) amplified from EST clone 81773 into the EcoRI/XhoI sites in pGEX-4T1 (Pharmacia Biotech, Piscataway, NJ). The prokaryotic expression vector for importin β (71–876) was a gift from Dr. S. Adam (Northwestern University, Chicago, IL).
Recombinant fusion proteins were expressed in E. coli strain BL21(DE3). Cultures were grown to an OD600 nm of 0.9 and induced with 1 mM IPTG for 2–5 h at 30°C. After washing with PBS, bacterial pellets were resuspended in 20 mM Hepes, pH 7.3, 100 mM NaCl, 1 mM βME, and 10% glycerol (buffer A) and sonicated. Fusion proteins containing Vpr or Vpr fragments consistently partitioned into the insoluble fraction when prepared by this method. Inclusion bodies were solubilized by sonication in 5 mM imidazole, 0.5 M NaCl, 20 mM Tris-HCl, pH 7.9, and 6 M urea (buffer B) and incubated overnight at 0°C. After ultracentrifugation of lysates, soluble fusion proteins were isolated using Ni-NTA chromatography (Invitrogen Corp., Carlsbad, CA). Proteins were eluted from resin using buffer B containing 4 M imidazole. The denatured proteins were refolded by slow dialysis into buffer A. After completion of the experiments included in this manuscript, Vpr(73–96)–βgal was subsequently produced in a soluble form. Partial characterization in vitro of soluble FITC-labeled Vpr(73–96)–βgal showed no apparent differences from the denatured/ renatured form of the protein. IBB–βgal, importin β (71–876), RanQ69L, and GST-M9 were produced as previously described (Bischoff et al., 1994; Pollard et al., 1996; Weis et al., 1996; Chi et al., 1997). Peptides encoding Vpr(73–96) or the same sequence in reverse order as a control were obtained from Research Genetics (Huntsville, AL). Purity of peptides was confirmed by mass spectrometry and HPLC analysis. Fluorescent labeling of proteins was performed as detailed in the instructions included with the N-hydroxylsuccinimide esters of either rhodamine isothiocyanate or fluorescein (Pierce Chemical Co., Rockford, IL). Excess label was removed by dialysis into buffer A.
Indirect Immunofluorescence
Before transfection, HeLa cells (American Type Culture Collection, Rockville, MD) were plated onto glass coverslips. Transfections were performed using calcium phosphate precipitates. After 48 h, cells were stained as described previously (Weis et al., 1996). The primary antibody was an anti-βgal monoclonal antibody used at a dilution of 1:500 in 10% BSA in PBST (0.05% Tween-20 in PBS). FITC- or LRSC-conjugated secondary anti–mouse antibodies (Jackson ImmunoResearch, West Grove, PA) were used at a dilution of 1:100 in 10% BSA in PBST. Coverslips were mounted using Gel Mount (Biomeda Corp., Foster City, CA), and samples were imaged using a scanning confocal microscope (model MRC-600; Bio-Rad Labs, Hercules, CA).
In Vitro Nuclear Transport Assay
HeLa cells were plated onto glass coverslips 24 h before treatment for 5 min at room temperature with 50 μg/ml digitonin (Fluka AG, Buchs, Switzerland) in transport buffer (20 mM Hepes, KOH, pH 7.3, 110 mM potassium acetate, 5 mM sodium acetate, 2 mM magnesium acetate, 1 mM EGTA, and 2 mM DTT) (Adam et al., 1990). Import reactions were mixed in a volume of 10 μl, spun at 10,000 rpm for 15 min at 4°C, and then overlaid on top of the coverslips containing the plated HeLa cells. Rabbit reticulocyte lysate containing an energy-regenerating system (Promega Corp., Madison, WI) was used as a source of cytosolic factors. Transport reactions were always performed in parallel with IBB–βgal as a control. Vpr fusion proteins and GST-M9 were used at a concentration of 20 ng/μl, while IBB–βgal was used at 40 ng/μl. Protein concentrations were doubled for competition reactions using the Vpr peptides. Transport reactions were set up on ice and then allowed to proceed at room temperature for 1 h. Coverslips were washed with PBST, fixed, mounted, and then imaged as described above.
Results
Nuclear Localization of Vpr Is a Signal-mediated Process
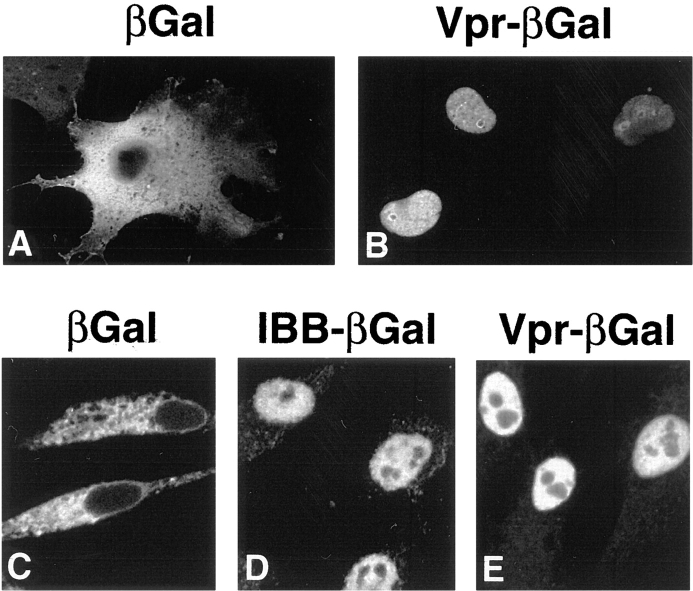
Vpr has been previously shown to localize to the nucleus, both in transiently transfected cells and in cells infected with HIV (Lu et al., 1993; Zhao et al., 1994; Yao et al., 1995). Since the molecular mass of Vpr (14 kD) is substantially smaller than the 40–60-kD size limit for passive diffusion of proteins through the NPC, the observed nuclear location of Vpr could result from at least two possible mechanisms: (a) Vpr is imported via a nuclear targeting signal, or (b) Vpr enters the nucleus by passive diffusion and is retained in that cellular compartment by binding to nuclear proteins. To distinguish between these two possibilities, we constructed a chimeric protein consisting of Vpr fused at its COOH terminus to βgal, and analyzed its subcellular localization both in vivo after transfection in HeLa cells and in vitro using digitonin-permeabilized HeLa cells and fluorescently labeled recombinant Vpr–βgal fusion proteins (Fig. 1). The size of the resulting fusion protein (∼130 kD for the monomer and ∼520 kD for the tetramer) is sufficiently large to preclude passive diffusion into the nucleus.
Figure 1.
Nuclear import of Vpr is a signal-mediated process. Indirect immunofluorescence of HeLa cells transfected with an expression plasmid encoding either βgal (A) or Vpr–βgal fusion protein (B). Import of FITC-labeled βgal (C), IBB–βgal (D), and Vpr–βgal (E) in digitonin-permeabilized cells. Reactions shown here and in all of the following figures contain rabbit reticulocyte lysate with an energy-regenerating system unless otherwise indicated.
As expected, in transiently transfected HeLa cells, βgal alone localized to the cytoplasm (Fig. 1 A). In contrast, the Vpr–βgal fusion protein was almost exclusively localized to the nucleus (Fig. 1 B) of the transfected cells. Using indirect immunofluorescence, we observed nucleoplasmic but nonnucleolar staining of more than 85% of the transfected cells. These subcellular localization results were additionally confirmed in biochemical fractionation studies where Vpr–βgal was principally detected in the nuclear fraction while βgal alone was cytoplasmic (data not shown). From these experiments, we conclude that Vpr contains a nuclear targeting signal that mediates nuclear uptake.
We next evaluated whether Vpr-mediated nuclear import could be reconstituted in vitro using digitonin-permeabilized HeLa cells. As a positive control, we used a chimeric protein consisting of βgal fused to the IBB domain of importin α. The IBB–βgal protein, as previously reported (Görlich et al., 1996a ; Weis et al., 1996), was efficiently localized to the nucleus (Fig. 1 D), while βgal alone was cytoplasmic (Fig. 1 C). Consistent with the in vivo observations, fusion of the βgal reporter protein to Vpr effectively redirected this normally cytoplasmic protein to the nucleus (Fig. 1 E). While localized to the nucleoplasm, the Vpr–βgal protein was excluded from the nucleoli.
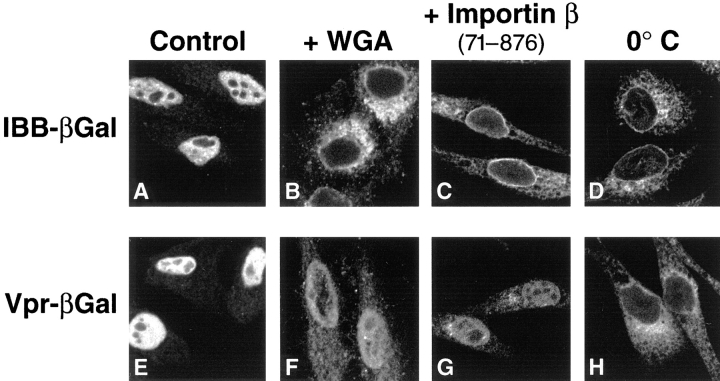
To investigate whether Vpr–βgal nuclear uptake reflected active transport across the nuclear pore, the effects of wheat germ agglutinin (WGA) were examined. This lectin binds to N-acetyl-d-glucosamine residues present on many of the nucleoporins and blocks NLS-mediated import without restricting passive diffusion of small molecules (Finlay et al., 1987). Addition of WGA markedly inhibited nuclear uptake of both IBB–βgal (Fig. 2 B) and Vpr–βgal (Fig. 2 F). Vpr-mediated nuclear import was also examined in the presence of a dominant-negative importin β deletion mutant, importin β (71–876). This mutant lacks a RanGTP binding domain and has been shown to inhibit multiple pathways of nuclear import and export across the NPC (Izaurralde et al., 1997a ; Kutay et al., 1997b ). Addition of importin β (71–876) effectively blocked nuclear localization of both IBB–βgal (Fig. 2 C) and Vpr–βgal (Fig. 2 G). Finally, we examined the temperature dependence of the Vpr-mediated import reaction. When the import reaction was performed on ice (0°C) instead of at room temperature (25°C), nuclear localization of both IBB–βgal (Fig. 2 D) and Vpr–βgal was inhibited (Fig. 2 H). Taken together, these results indicate that nuclear import of Vpr is a temperature-dependent, signal-mediated process that proceeds through the NPC.
Figure 2.
Comparison of NLS-mediated (A–D) and Vpr-mediated (E–H) nuclear import in vitro. Import of IBB–βgal as a probe of the NLS pathway or Vpr–βgal was performed in the presence of lysate alone (A and E), lysate supplemented with WGA (50 μg/ml) (B and F), or lysate containing 8 μM importin β (71–876) (C and G). Import reactions containing IBB–βgal (D) or Vpr–βgal (H) fusion proteins were also performed at 0°C instead of 25°C.
Vpr Contains Two Independent Nuclear Targeting Signals with Receptor Requirements Distinct from NLS- and M9-mediated Import
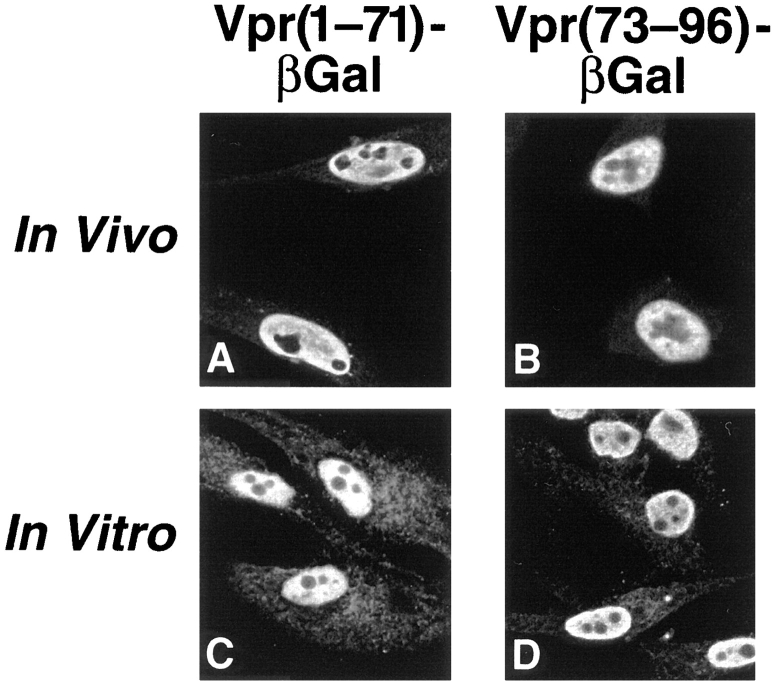
We next investigated what sequences within Vpr are both necessary and sufficient for the observed nuclear uptake of Vpr–βgal. As a first step, two Vpr fragments containing amino acids 1–71 and 73–96 were individually fused to βgal and tested. Unexpectedly, both of these Vpr fragments effectively targeted the βgal protein to the nucleus in vivo (Fig. 3, A and B) and in vitro (Fig. 3, C and D). These findings suggest that Vpr contains at least two functional nuclear targeting signals.
Figure 3.
Vpr contains two independent nuclear targeting signals. (A and B) Subcellular localization was determined for Vpr (1–71)–βgal and Vpr(73–96)–βgal in transiently transfected HeLa cells. Fusion proteins were detected by indirect immunofluorescence using antibodies specific for βgal. (C and D) Import of Vpr(1–71)–βgal and Vpr(73–96)–βgal fusion proteins was also analyzed in vitro in the digitonin-permeabilized HeLa cell system.
A prior study had indicated that saturation of the classical NLS pathway using a peptide corresponding to the SV-40 large T antigen sequence had no inhibitory effect on Vpr import (Gallay et al., 1996). This result suggests that Vpr does not bind to importin α at the NLS cargo site but does not exclude direct binding to importin β. In this regard, importin β–dependent and importin α–independent nuclear entry has been reported for translocation of U snRNPs (Palacios et al., 1997). To further confirm the lack of importin α involvement and to probe the use of importin β by Vpr, we performed nuclear import assays in the presence of excess IBB as an unlabeled competitor. Inclusion of the IBB domain potently inhibited NLS-mediated import in vitro (Fig. 4, A vs. B) by saturating the importin α binding site on importin β (Görlich et al., 1996; Weis et al., 1996). In contrast, the addition of the IBB competitor did not impair nuclear uptake of Vpr–βgal (Fig. 4, C vs. D). However, since one but not both of the Vpr signals might involve the NLS pathway, each signal was individually tested. The addition of the IBB competitor did not inhibit either Vpr signal (Vpr[1–71], Fig. 4, E vs. F, or Vpr[73–96], Fig. 4, G vs. H). Together, these findings indicate that Vpr does not use the classical NLS pathway for nuclear entry via either of its nuclear targeting signals.
Figure 4.
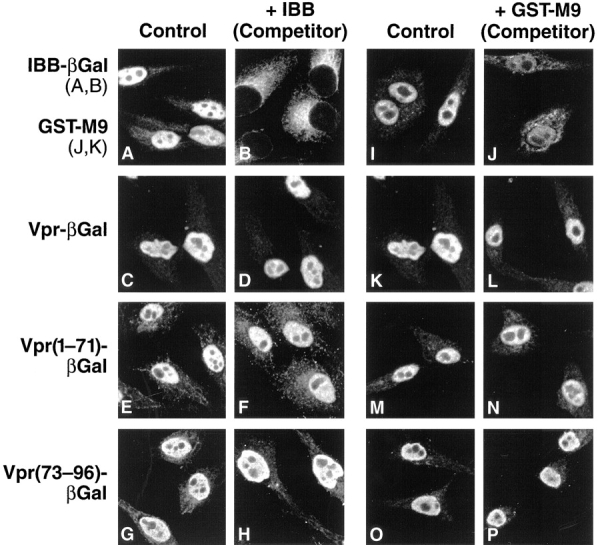
Vpr does not use the classical NLS or the M9 pathway for entry into the nucleus. (A–H) Import of IBB– βgal, Vpr–βgal, Vpr(1–71)– βgal, and Vpr(73–96)–βgal with lysate (control) (A, C, E, and G) or with lysate containing 30 μM unlabeled IBB as a competitor (B, D, F, and H). (I–P) Import of GST-M9 (FITC-labeled), Vpr–βgal, Vpr(1–71)–βgal, and Vpr(73– 96)–βgal with lysate (control) (I, K, M, and O) or with lysate containing 15 μM unlabeled GST-M9 (J, L, N, and P). Inhibition of the nuclear import reaction may be manifested by a cytoplasmic pattern of staining (B) or a general decrease in the fluorescence intensity reflecting exit from the permeabilized cell (J).
After the classical NLS pathway, the best-characterized import process is the M9 pathway used by the hnRNP A1 protein and its receptor transportin. To probe Vpr's use of the M9 pathway, competition experiments using the M9 nuclear targeting sequence were performed. As expected, saturation of the M9 pathway using an excess of the M9 signal sequence inhibited nuclear import of fluorescently labeled GST-M9 (Fig. 4, I vs. J). However, addition of excess GST-M9 had no effect on protein import mediated by full-length Vpr (Fig. 4, K vs. L), Vpr(1–71) (Fig. 4, M vs. N), or Vpr(73–96) (Fig. 4, O vs. P). Consistent with previously reported data (Izaurralde et al., 1997b ), addition of the M9 competitor also had no inhibitory effect on nuclear import of IBB–βgal (data not shown). Thus, Vpr nuclear entry does not appear to involve the transportin-dependent M9 pathway.
Vpr Nuclear Import Does Not Require GTP Hydrolysis by Ran/TC4
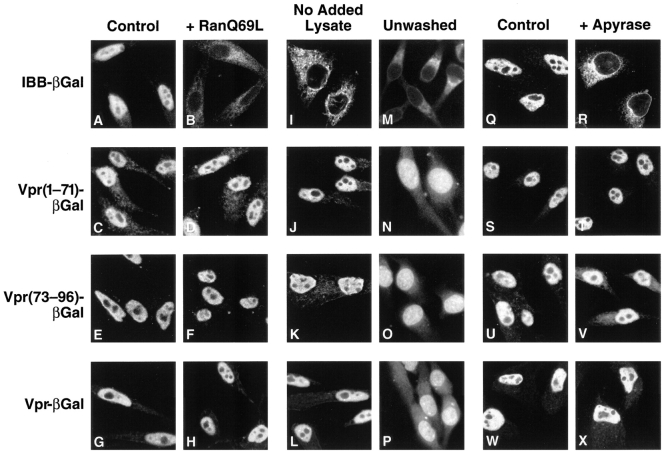
To further characterize the transport pathway(s) used by the two signals of Vpr, the requirement for GTP hydrolysis by Ran/TC4 was studied using a dominant-negative mutant of Ran, RanQ69L. This mutant binds GTP but is unable to hydrolyze this nucleotide triphosphate and consequently remains in the GTP-bound state. Addition of RanQ69L has been shown to block nuclear import of NLS- (Palacios et al., 1996) and M9-containing proteins (Nakielny et al., 1996) as well as nuclear import of U snRNPs (Palacios et al., 1996). Consistent with these results, inclusion of the RanQ69L mutant blocked IBB–βgal import (Fig. 5, A vs. B). In contrast, the RanQ69L mutant displayed no inhibitory effects on nuclear import mediated by either Vpr(1–71)–βgal (Fig. 5, C vs. D), Vpr(73–96)– βgal (Fig. 5, E vs. F), or full-length Vpr–βgal (Fig. 5, G vs. H). These results suggest that nuclear import mediated by either Vpr signal proceeds independently of Ran-mediated GTP hydrolysis.
Figure 5.
The two nuclear targeting signals of Vpr exhibit similar biological properties. (A–H) Nuclear import of IBB–βgal, Vpr(1–71)– βgal, Vpr(73–96)–βgal, and full-length Vpr–βgal in the presence of lysate (control) (A, C, E, and G) or with lysate containing 6 μM of the RanQ69L mutant, which is unable to hydrolyze bound GTP (B, D, F, and H). (I–L) Nuclear import of IBB–βgal (I), Vpr(1–71)–βgal (J), Vpr(73–96)–βgal (K), and Vpr–βgal (L) in the absence of added lysate. (M–P) Nuclear uptake of fluorescently labeled proteins IBB–βgal (M), Vpr(1–71)–βgal (N), Vpr(73–96)–βgal (O), and Vpr–βgal (P) from a buffered solution 30 min into the import reaction. In this study, cells were visualized without prior washing to evaluate the extent of nuclear accumulation. Images were photographed on a Leitz diaplan fluorescence microscope illuminated by a mercury arc lamp. (Q–X) Import of IBB–βgal, Vpr(1–71)–βgal, Vpr(73–96)– βgal, and Vpr–βgal in the presence of lysate (control) (Q, S, U, and W) or lysate containing apyrase (2.5 U/ml) (R, T, V, and X) to deplete high-energy phosphates in the lysates. Lysates were preincubated with apyrase for 20 min at room temperature before their use in the nuclear import assays.
The Two Nuclear Targeting Signals of Vpr Exhibit Similar Properties
To further compare the two targeting signals within Vpr, we examined nuclear import of the Vpr(1–71)–βgal and Vpr(73–96)–βgal proteins in vitro under different conditions. First, we investigated the dependence of the import reactions on protein factors provided by the rabbit reticulocyte lysate. Consistent with prior studies, nuclear import of IBB–βgal was entirely dependent on the addition of the reticulocyte lysate (Fig. 5 I) (Weis et al., 1996; Görlich et al., 1996a ). In contrast, both Vpr(1–71)–βgal and Vpr(73–96)– βgal readily entered the nucleus in the presence of buffer alone (Fig. 5, J and K). Similarly, the full-length Vpr–βgal protein entered the nucleus in a lysate-independent manner (Fig. 5 L). These results indicate that no rate-limiting factors required for import of either Vpr targeting signal are lost during the digitonin permeabilization procedure.
These studies did not exclude the possibility that the nuclear localization observed with Vpr(1–71)–βgal, Vpr(73– 96)–βgal, and full-length Vpr–βgal in Fig. 5, J–L, could reflect a process of “facilitated diffusion” rather than actual nuclear accumulation of Vpr as a result of vectorial import. Entry based on purely facilitated diffusion would be expected to yield a localization pattern consistent with equilibration of the protein between the nuclear and cytoplasmic compartments. The distinction between a facilitated diffusion mechanism and actual nuclear accumulation cannot be made from the previous experiment performed in buffer; nuclear fluorescence would be observed in both cases because extensive washing of the cells removes the background fluorescence. To distinguish between these two possibilities, we measured the nuclear uptake of FITC-labeled proteins in buffer 30 min into the import reaction in the absence of washing or fixation. Under these conditions, IBB–βgal is excluded from the nucleus (Fig. 5 M). In contrast, Vpr(1–71), Vpr(73–96), and the full-length Vpr protein fused to βgal undergo substantial nuclear accumulation against a concentration gradient even in the absence of an energy-regenerating system (Fig. 5, N–P). Thus, nuclear entry of Vpr does not appear to result from facilitated diffusion but rather reflects a vectorial transport of the protein into the nucleus.
To examine the energy requirement for nuclear import mediated by the two Vpr signals, each of the transport reactions was incubated with apyrase to deplete the nucleotide triphosphate pool. As expected, apyrase treatment of the lysate effectively eliminated IBB–βgal import (Fig. 5, Q vs. R). In contrast, import of Vpr(1–71)–βgal (Fig. 5, S vs. T), Vpr(73–96)–βgal (Fig. 5, U vs. V), or full-length Vpr–βgal (Fig. 5, W vs. X) was not significantly affected by apyrase-induced energy depletion. Comparable results were obtained when hexokinase/glucose instead of apyrase was used to deplete the energy present in the lysates (data not shown). Together, these findings suggest that Vpr(1–71), Vpr(73–96), and full-length Vpr are capable of mediating nuclear import in the presence of minimal energy.
Vpr (1–71) and Vpr (73–96) Target Vpr to Two Distinct Import Pathways
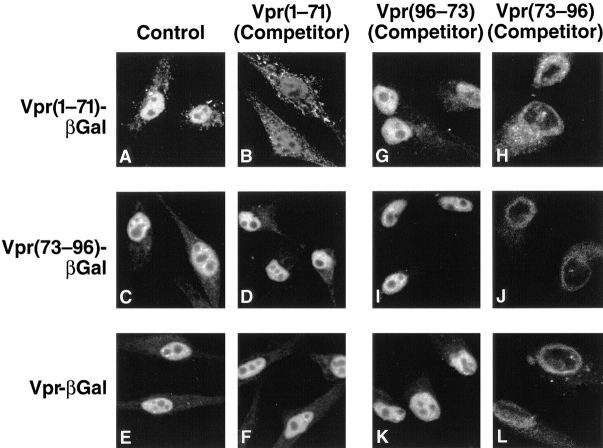
To further examine receptor specificity in the two Vpr pathways, cross-competition experiments using the Vpr fragments were performed. Addition of a 300-fold molar excess of an unlabeled Vpr(1–71) protein fragment inhibited Vpr(1–71)–βgal nuclear import (Fig. 6, A vs. B). However, addition of an equimolar amount of Vpr(1–71) did not prevent nuclear entry mediated by Vpr(73–96) (Fig. 6, C vs. D) or full-length Vpr (Fig. 6, E vs. F). These findings demonstrate that nuclear import occurring via the Vpr (1–71) fragment is a saturable process mediated through a receptor distinct from that involved in Vpr(73–96) import. Additionally, the ability of Vpr–βgal to enter the nucleus in the presence of an excess of the Vpr(1–71) protein fragment indicates that the COOH-terminal signal is functional in the context of the full-length protein.
Figure 6.
The two nuclear targeting signals of Vpr target the protein to distinct import pathways. (A–F) Import of Vpr(1–71)–βgal, Vpr(73–96)–βgal, and full-length Vpr–βgal with lysate (control) (A, C, and E) or with lysate containing 50 μM unlabeled Vpr(1–71) as a competitor (B, D, and F). (G–L) Import of Vpr(1–71)– βgal, Vpr(73–96)–βgal, and Vpr–βgal with lysate containing unlabeled Vpr(96–73) reverse peptide as a control (G, I, and K) or Vpr(73–96) forward peptide (H, J, and L). Peptides were used at a final concentration of 500 μM.
These competition experiments were next extended to the COOH-terminal signal of Vpr using synthetic peptides corresponding to amino acids 73–96 synthesized in the forward or reverse orientation. The Vpr(73–96) peptide effectively inhibited nuclear localization of Vpr(73–96)–βgal (Fig. 6 J). In contrast, nuclear import of Vpr(73–96)–βgal was not inhibited by the control reverse peptide (Fig. 6 I). These findings demonstrate that transport of the Vpr COOH-terminal signal also involves a saturable receptor. Addition of the Vpr(73–96) peptide also blocked nuclear import of both Vpr(1–71)–βgal (Fig. 6, G vs. H) and full-length Vpr–βgal (Fig. 6, K vs. L). These results suggest that the two signals bind to two factors that may participate sequentially in a common nuclear import pathway with Vpr(73–96) interacting with a receptor positioned downstream of the receptor recognized by Vpr(1–71).
Vpr (1–71) and Vpr (73–96) Use Distal Components of the NLS and M9 Nuclear Import Pathways
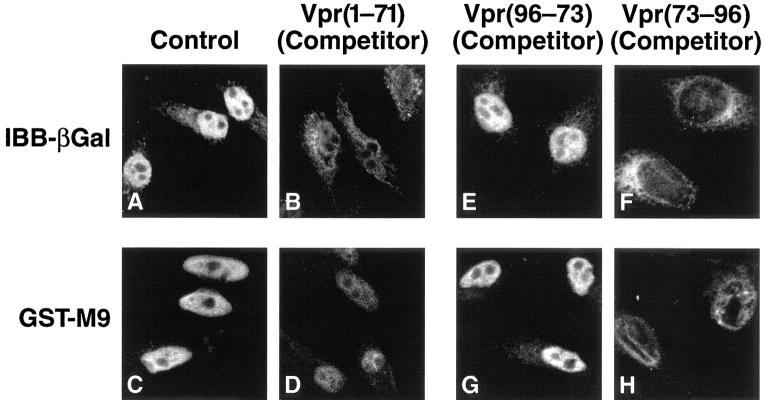
We next analyzed the effects of the Vpr fragments on nuclear import mediated through the NLS and M9 pathways. Although saturation of either the NLS or the M9 pathway failed to block import mediated by Vpr(1–71) or Vpr(73– 96) (Fig. 4), addition of an excess of either Vpr(1–71) or Vpr(73–96) fragments markedly inhibited import mediated by either the classical NLS (Fig. 7, A vs. B) or the M9 (Fig. 7, C vs. D) signal. Together, these experiments suggest that Vpr(1–71) associates with the NPC either at the site where the NLS and the M9 pathways converge or downstream of this junction. Vpr(73–96) also enters this pathway but even further downstream of the site of Vpr(1–71) engagement, thus accounting for the ability of Vpr(73–96) to block import mediated by the Vpr(1–71), NLS, and M9 signals.
Figure 7.
Vpr(1–71) and Vpr(73–96) block nuclear import mediated through both the classical NLS and M9 pathways. (A–D) Import of IBB–βgal and GST-M9 in the presence of lysate (control) (A and C) or with lysate containing 50 μM Vpr(1–71) (B and D). (E–H) Import of IBB–βgal and GST-M9 with lysate containing Vpr(96–73) reverse peptide (control) (E and G) or containing Vpr(73–96) forward peptide (F and H). Peptides were used at a final concentration of 500 μM.
Discussion
Nuclear Entry of Vpr Is Signal-mediated, Occurs via the NPC, and Is Not the Result of Facilitated Diffusion
In this study, we show that nuclear localization of Vpr is a signal-mediated process. Specifically, Vpr efficiently targets an appended protein to the nucleus, even when the fusion partner is a normally cytoplasmic protein larger than the 40–60-kD size limit for passive diffusion. This observed property is consistent with the proposed role of Vpr in mediating nuclear uptake of the large viral PIC. Vpr-mediated transport into the nucleus appears to occur through the NPC since this process is inhibited by the addition of both WGA and a dominant-negative mutant of importin β, importin β (71–876), which blocks multiple independent import and export pathways (Kutay et al., 1997b ). In contrast to a recent report suggesting that Vpr targets proteins to the nuclear envelope (Vodicka et al., 1998), we observe Vpr-mediated targeting to the nucleoplasm. Of note, transient transfection of cells with an untagged Vpr expression vector similarly leads to a nucleoplasm pattern of localization detected by immunofluorescence staining with anti-Vpr antibody (data not shown). We suspect that the use of different alleles of Vpr and/or slight conformational variations between the experimental Vpr fusion proteins may contribute to these different results. In a separate study (Fouchier et al., 1998), a protein chimera composed of Vpr fused to maltose binding protein was reported to localize to the nucleus while a Vpr–βgal chimera was detected both in the nucleus and at the nuclear envelope. For the Vpr–βgal protein chimera used in this study, we find nucleoplasmic localization both for the expressed protein in transiently transfected HeLa cells and for the recombinant protein in digitonin-permeabilized HeLa cells. These results indicate that Vpr is capable of targeting proteins to the nucleus.
Our studies further demonstrate that Vpr contains two functional nuclear targeting signals that reside within the 1–71 and 73–96 fragments and can efficiently direct this HIV protein to the nucleoplasm. Of note, these two regions of Vpr correlate well with regions observed after limited proteolysis, suggesting that they may correspond to structural domains within the protein (Zhao et al., 1994). In contrast to previous studies suggesting a prominent role for the NH2 terminus of Vpr in nuclear targeting (Di Marzio et al., 1995; Mahalingam et al., 1995; Yao et al., 1995), we find that the COOH-terminal signal is also capable of mediating nuclear localization, both alone and in the context of the full-length protein. The ability of Vpr to access the nucleus using two different signals should be taken into consideration when attempting to identify individual amino acid residues critical for such targeting.
A feature of Vpr-mediated nuclear import that sharply contrasts with import mediated through the classical NLS- or M9-dependent pathway is the ability of Vpr to enter the nucleus efficiently under conditions of minimal energy. This property of Vpr is conferred by both of its nuclear targeting signals. Specifically, we show that Vpr-mediated nuclear entry occurs in the absence of exogenously added energy and even persists in energy-depleted lysates. Despite such conditions of limited energy, nuclear localization of Vpr results from net accumulation, rather than simple equilibration through facilitated diffusion.
How is Vpr able to mediate nuclear enrichment in the presence of minimal energy levels? The decreased energy requirement for entry may be a function of the discrete sites along the NPC where Vpr(1–71) and Vpr(73–96) bind. Based on several lines of evidence, both Vpr targeting signals appear to engage the NPC at positions located downstream of the sites of association of the NLS- and M9-transport complexes (see below). Alternatively, the absolute energy requirement for Vpr-mediated nuclear import may simply be much lower than that observed for import via the classical NLS pathway. Finally, digitonin permeabilization may not completely release energy stores associated with the NPC, explaining the ability of Vpr to enter the nucleus in the absence of exogenously added nucleotide triphosphates. The fact that Vpr-mediated translocation does not occur at 0°C suggests that some energy is required. However, we cannot exclude a temperature-dependent conformational change in the NPC that inhibits signal-mediated transport. Future experiments will attempt to distinguish between these various possibilities. Further study of Vpr import also promises to yield new insights into the mechanism by which proteins translocate from one face of the NPC to the other. The ability of Vpr to localize to the nucleus in the absence of exogenously added protein factors and energy provides a simplified model system that should facilitate the elucidation of the precise mechanics of the transport reaction. Finally, the presence of nuclear targeting signals that operate effectively under conditions of limited energy may enhance viral replication and spread in nondividing cells by facilitating nuclear uptake of the viral PIC through increasing its import kinetics.
Vpr Uses an Import Pathway Distinct from NLS or M9 and Does Not Require GTP Hydrolysis by Ran/TC4
Unlike nuclear import occurring through the classical NLS and M9 signals, nuclear entry of Vpr–βgal does not appear to require GTP hydrolysis mediated by Ran/TC4. Specifically, both the Vpr(1–71)–βgal and Vpr(73–96)–βgal fusion proteins are entirely resistant to the inhibitory effects of the RanQ69L mutant, which fails to undergo GTP hydrolysis.
Consistent with the Ran independence of transport, blockade of either the Ran-dependent NLS or the M9 pathways using either an excess of unlabeled IBB- or M9-containing protein competitors produced no inhibitory effects on nuclear import mediated by full-length Vpr or either of the Vpr fragments. These findings confirm that Vpr does not use importin α/importin β– or transportin-mediated pathways for nuclear entry. Binding of Vpr to both yeast and human importin α has been reported (Popov et al., 1998; Vodicka et al., 1998). However, the ability of Vpr to efficiently enter the nucleus in the presence of an excess of the IBB competitor clearly demonstrates that importin α function is not required for import of Vpr itself.
In terms of other receptors possibly used by Vpr, we have not formally eliminated the human homologue of Kap123p, a recently described yeast transporter of ribosomal proteins (Rout et al., 1997). However, Vpr utilization of this pathway seems unlikely since Kap123p, like importin β and transportin, contains a RanGTP binding domain and has been shown to bind RanGTP in vitro (Schlenstedt et al., 1997), suggesting a role for Ran in transport of Kap123p. As noted above, Vpr import is unaffected by the presence of a dominant-negative mutant of Ran, RanQ69L, suggesting that Vpr does not use Kap123p or, by analogy, other members of this superfamily of RanGTP binding proteins.
The Two Nuclear Targeting Signals in Vpr Are Unique and Bind to Receptors Shared by the Classical NLS and M9 Import Pathways
An unusual feature of Vpr is that this small, 96–amino acid protein of HIV contains two distinct nuclear targeting signals that use different receptors. Characterization of the two Vpr nuclear targeting signals reveals that import mediated by either signal in vitro occurs independently of added lysate proteins and, as noted above, under conditions of limited energy. Competition experiments designed to probe the receptors involved demonstrate that the addition of an excess of the Vpr(1–71) protein fragment inhibits import mediated by Vpr(1–71) but does not alter import mediated by Vpr(73–96) or the full-length Vpr protein. In contrast, competition with excess unlabeled Vpr(73–96) peptide, but not the reverse peptide as a control, inhibits import of Vpr(1–71)–βgal and Vpr(73–96)– βgal, as well as full-length Vpr–βgal. These findings suggest a model for nuclear entry of Vpr(73–96) involving a receptor positioned distally in a convergent import pathway used by both Vpr signals.
Further support for such a convergent pathway of nuclear import was also obtained when the Vpr fragments were tested for effects on the NLS and M9 import pathways. Although the NLS- and M9-specific inhibitors exert no inhibitory effects on either Vpr nuclear targeting signal, both the Vpr(1–71) and Vpr(73–96) fragments, when added as competitors, effectively block the NLS and M9 pathways. These findings argue that the two Vpr fragments act at later points in a nuclear import pathway also used by NLS- and M9-bearing proteins and their respective receptors. In this regard, Popov and colleagues (1998) have also reported that Vpr blocks nuclear import of NLS-containing substrates. The eventual convergence of distinct nuclear import pathways at the NPC is also supported by data from Michael and colleagues (1997), who have described inhibition of the NLS and M9 pathways by saturation of the import pathway used by the hnRNP K nuclear targeting signal, and by data from Kutay and coworkers (1997b), showing inhibition of multiple import and export pathways by importin β (71–876).
What is the nature of these Vpr receptors? As noted, both the NH2- and the COOH-terminal signals of Vpr mediate nuclear entry in the absence of added soluble factors, indicating either that the import receptors are not released from the NPC upon digitonin permeabilization or that both Vpr(1–71) and Vpr(73–96) directly associate with components of the NPC. In this regard, binding of full-length Vpr to the yeast nucleoporin Nsp1p (Vodicka et al., 1998) and to the vertebrate nucleoporin POM 121 (Fouchier et al., 1998) has recently been described. In addition, although Vpr-mediated nuclear import is unaffected by the IBB domain of importin α, inclusion of full-length importin β inhibits nuclear localization of Vpr–βgal (data not shown), suggesting that Vpr and importin β may use common intermediates to enter the nucleus. The Vpr(1–71) and Vpr(73–96) fragments will certainly serve as valuable reagents to probe the identity of these two different receptors. It is also possible that the two Vpr nuclear targeting signals interact with different sites on the same protein. Such a model could also explain the observed nonreciprocal pattern of inhibition observed for Vpr(73–96) and Vpr(1–71) binding.
Implications for Nuclear Targeting of the HIV Preintegration Complex
It has been proposed that Vpr acts as a specialized importin β for the delivery of the HIV preintegration complex to the nuclear envelope (Vodicka et al., 1998). Vpr has been shown to bind to importin α and the FG repeat–containing nucleoporins Nsp1p and POM 121. Similarly, importin β has also been shown to bind directly to FG repeat–containing nucleoporins in vitro (Radu et al., 1995b ; Rexach and Blobel, 1995). Overexpression of Vpr in yeast resulted in an mRNA export defect, but surprisingly, no import defect was observed (Vodicka et al., 1998).
Our data suggest that Vpr contains minimal elements required for transport, allowing for passage through the NPC. As with importin β, transportin, and exportin-t (Kose et al., 1997; Nakielny and Dreyfuss, 1997; Kutay et al., 1998), nuclear import of Vpr does not require soluble factors. As observed with importin β (71–876), addition of either the NH2- or COOH-terminal nuclear targeting signal of Vpr blocks import of both NLS- and M9-bearing import substrates. Based on experiments performed in vitro, Bukrinsky and coworkers have proposed that Vpr functions by enhancing the association of the classical NLS found in the matrix protein with importin α, thereby making a weak karyophile into a stronger karyophile (Popov et al., 1998). Our results clearly show that Vpr entry into the nucleus occurs independently of importin α function and also demonstrate that Vpr itself is highly karyophilic and is able to mediate nuclear localization on its own through two pathways distinct from the classical NLS pathway.
Although we have identified two import pathways different from the classical NLS pathway that are potentially accessed by the viral PIC, how this assembly localizes to the nucleus is still unknown. Matrix and integrase are believed to target this viral complex to the nucleus via the classical NLS pathway. Our data suggest that the PIC also has access, because of Vpr, to two nonredundant import pathways distinct from that used by classical NLS-containing proteins. The presence of Vpr may serve to facilitate nuclear uptake of the PIC, perhaps by influencing the import kinetics due to the accessibility of an NLS that operates effectively under conditions of minimal energy and/or by enabling the viral PIC to directly engage the NPC. Despite these uncertainties, these findings highlight the evolution of HIV to encode a viral factor that bypasses many of the critical proximal components that govern other nuclear import pathways. Rather, Vpr appears to bind to receptors located within the NPC, a property that may help to ensure the capacity of HIV to replicate in nondividing cellular hosts.
Acknowledgments
We thank Dr. Stephen Adam for the importin β (71–876) expression vector, Dr. Michael Malim (University of Pennsylvania, Philadelphia, PA) for communicating results before publication, and Mr. Robin Givens for administrative assistance in the preparation of this manuscript.
Abbreviations used in this paper
- βgal
βgalactosidase
- GST
glutathione-S-transferase
- HIV
human immunodeficiency virus
- IBB
importin β binding domain
- NLS
nuclear localization signal
- NPC
nuclear pore complex
- PIC
preintegration complex
- WGA
wheat germ agglutinin
Footnotes
Y. Jenkins is supported by a National Institutes of Health postdoctoral fellowship. K. Weis acknowledges support from the Deutsche Forschungsgemeinschaft. We also acknowledge core support from the University of California San Francisco Center for AIDS Research (P30A127763).
Address all correspondence to Warner C. Greene, Gladstone Institute of Virology and Immunology, University of California, San Francisco, CA 94141-9100. Tel.: (415) 695-3800. Fax: (415) 826-1514. E-mail: wgreene@gladstone.ucsf.edu
References
- Adam SA, Marr RS, Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitchison JD, Blobel G, Rout MP. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. doi: 10.1126/science.274.5287.624. [DOI] [PubMed] [Google Scholar]
- Arts G-J, Fornerod M, Mattaj IW. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. doi: 10.1016/s0960-9822(98)70130-7. [DOI] [PubMed] [Google Scholar]
- Bischoff FR, Klebe C, Kretschmer J, Wittinghofer A, Ponstingl H. Ran GAP1 induces GTPase activity of nuclear Ras related Ran. Proc Natl Acad Sci USA. 1994;91:2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Haggerty S, Dempsey MP, Sharova N, Adzhubei A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Adam EJ, Adam SA. Sequence and characterization of cytoplasmic nuclear protein import factor p97. J Cell Biol. 1995;130:265–274. doi: 10.1083/jcb.130.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Adam JH, Adam SA. Different binding domains for RanGTP and RanGDP/Ran BP1 on nuclear import factor p97. J Biol Chem. 1997;272:6818–6822. doi: 10.1074/jbc.272.10.6818. [DOI] [PubMed] [Google Scholar]
- Cohen EA, Dehni G, Sodroski JG, Haseltine WA. Human immunodeficiency virus vprproduct is a virion-associated regulatory protein. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau NR. Mutational analysis of cell cycle arrest, nuclear localization, and virion packaging of human immunodeficiency virus type 1 Vpr. J Virol. 1995;69:7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M. HIV-1, Vpr and the cell cycle. Curr Biol. 1996;6:1096–1103. doi: 10.1016/s0960-9822(02)00676-0. [DOI] [PubMed] [Google Scholar]
- Finlay DR, Newmeyer DD, Price TM, Forbes DJ. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987;104:189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti KG, Fransen J, Grosveld G. The human homolog of yeast Crm1 is in a dynamic subcomplex with Can/Nup214 and a novel nuclear pore component Nup88. EMBO (Eur Mol Biol Organ) J. 1997a;16:807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich export signals. Cell. 1997b;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fouchier RA, Meyer BE, Simon JH, Fischer U, Malim MH. HIV-1 infection of non-dividing cells: evidence that the N-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO (Eur Mol Biol Organ) J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier RA, Meyer BE, Simon JH, Fischer U, Albright AV, González-Scarano F, Malim MH. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virol. 1998;72:6004–6013. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed EO, Englund G, Martin M. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J Virol. 1995;69:3949–3954. doi: 10.1128/jvi.69.6.3949-3954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell RA, Truant R, Thorne L, Benson RE, Cullen BR. Nuclear import of hnRNP A1 is mediated by a novel cellular cofactor related to karyopherin-β. J Cell Sci. 1997;110:1325–1331. doi: 10.1242/jcs.110.11.1325. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. . Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- Görlich D, Prehn S, Laskey RA, Hartmann E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994;79:767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Görlich D, Kostka S, Kraft R, Dingwall C, Laskey RA, Hartmann E, Prehn S. Two different subunits of importin cooperate to recognize nuclear localization signals and bind them to the nuclear envelope. Curr Biol. 1995a;5:383–392. doi: 10.1016/s0960-9822(95)00079-0. [DOI] [PubMed] [Google Scholar]
- Görlich D, Vogel F, Mills AD, Hartmann E, Laskey RA. Distinct functions for the two importin subunits in nuclear protein import. Nature. 1995b;377:246–248. doi: 10.1038/377246a0. [DOI] [PubMed] [Google Scholar]
- Görlich D, Henklein P, Laskey RA, Hartmann E. A 41 amino acid motif in importin-α confers binding to importin-β and hence transit into the nucleus. EMBO (Eur Mol Biol Organ) J. 1996a;15:1810–1817. [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Pante N, Kutay U, Aebi U, Bischoff FR. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO (Eur Mol Biol Organ) J. 1996b;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Dabrowski M, Bischoff FR, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. J Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzinger NK, Bukrinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee M-A, Gendelman HE, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries ER, Temin HM. Requirement for cell division for initiation of transcription of Rous sarcoma virus RNA. J Virol. 1974;14:531–546. doi: 10.1128/jvi.14.3.531-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E, Kutay U, von Kobbe C, Mattaj IW, Görlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO (Eur Mol Biol Organ) J. 1997a;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E, Jarmolowski A, Beisel C, Mattaj IW, Dreyfuss G, Fischer U. A role for the M9 transport signal of hnRNP A1 in mRNA nuclear export. J Cell Biol. 1997b;137:27–35. doi: 10.1083/jcb.137.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowett JBM, Planelles V, Poon B, Shah NP, Chen M-L, Chen ISY. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp DM, Silver PA. A GTPase controlling nuclear trafficking: running the right way or walking RANdomly? . Cell. 1996;87:1–4. doi: 10.1016/s0092-8674(00)81315-x. [DOI] [PubMed] [Google Scholar]
- Kose S, Imamoto N, Tachibana T, Shimamoto T, Yoneda Y. Ran-unassisted nuclear migration of a 97-kD component of nuclear pore-targeting complex. J Cell Biol. 1997;139:841–849. doi: 10.1083/jcb.139.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Bischoff RF, Kostka S, Kraft R, Görlich D. Export of importin α from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997a;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- Kutay U, Izaurralde E, Bischoff FR, Mattaj IW, Görlich D. Dominant-negative mutants of importin-β block multiple pathways of import and export through the nuclear pore complex. EMBO (Eur Mol Biol Organ) J. 1997b;16:1153–1163. doi: 10.1093/emboj/16.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Lipowsky G, Izaurralde E, Bischoff FR, Schwarzmaier P, Hartmann E, Görlich D. Identification of a tRNA-specific nuclear export receptor. Mol Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-L, Spearman P, Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993;67:6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-L, Bennett RP, Wills JW, Gorelick R, Ratner L. A leucine triplet repeat sequence (LXX)4 in p6gagis important for Vpr incorporation into human immunodeficiency virus type 1 particles. J Virol. 1995;69:6873–6879. doi: 10.1128/jvi.69.11.6873-6879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S, Collman RG, Patel M, Monken CE, Srinivasan A. Functional analysis of HIV-1 Vpr: identification of determinants essential for subcellular localization. Virology. 1995;212:331–339. doi: 10.1006/viro.1995.1490. [DOI] [PubMed] [Google Scholar]
- Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Weiner DB. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr. J Virol. 1997;71:6339–6347. doi: 10.1128/jvi.71.9.6339-6347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F, Paschal B, Evans J, Gerace L. Inhibition of nuclear import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993;123:1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael WM, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- Michael WM, Eder PS, Dreyfuss G. The K nuclear shuttling domain: a novel signal for nuclear import and nuclear export in the hnRNP K protein. EMBO (Eur Mol Biol Organ) J. 1997;16:3587–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MS, Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Moore MS, Blobel G. Purification of a Ran-interacting protein that is required for protein import into the nucleus. Proc Natl Acad Sci USA. 1994;91:10212–10216. doi: 10.1073/pnas.91.21.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S, Dreyfuss G. Import and export of the nuclear protein import receptor transportin by a mechanism independent of GTP hydrolysis. Curr Biol. 1997;8:89–95. doi: 10.1016/s0960-9822(98)70039-9. [DOI] [PubMed] [Google Scholar]
- Nakielny S, Siomi MC, Siomi H, Michael WM, Pollard V, Dreyfuss G. Transportin: nuclear transport receptor of a novel protein import pathway. Exp Cell Res. 1996;229:261–266. doi: 10.1006/excr.1996.0369. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Nucleocytoplasmic transport: signals, mechanisms, and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- Ohno M, Fornerod M, Mattaj IW. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- Palacios I, Weis K, Klebe C, Mattaj IW, Dingwall C. Ran/TC4 mutants identify a common requirement for snRNP and protein import into the nucleus. J Cell Biol. 1996;133:484–494. doi: 10.1083/jcb.133.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios I, Hetzer M, Adam SA, Mattaj IW. Nuclear import of U snRNPs requires importin β. EMBO (Eur Mol Biol Organ) J. 1997;16:6783–6792. doi: 10.1093/emboj/16.22.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal BM, Gerace L. Identification of NTF2, a cytosolic factor for nuclear import that interacts with nuclear pore complex protein p62. J Cell Biol. 1995;129:925–937. doi: 10.1083/jcb.129.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton W, Connor RI, Landau NR. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of gagand mutational analysis. J Virol. 1993;67:7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton LF, Rosenblum JS, Blobel G. A distinct and parallel pathway for the nuclear import of an mRNA-binding protein. J Cell Biol. 1998;139:1645–1653. doi: 10.1083/jcb.139.7.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard VW, Michael WM, Nakielny S, Siomi MC, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Popov S, Dubrovsky L, Lee M-A, Pennathur S, Haffar O, Al-Abed Y, Tonge P, Ulrich P, Rexach M, Blobel G, Cerami A, Bukrinsky M. Critical role of reverse transcriptase in the inhibitory mechanism of CNI-HO294 on HIV-1 nuclear translocation. Proc Natl Acad Sci USA. 1996;93:11859–11864. doi: 10.1073/pnas.93.21.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov S, Rexach M, Zybarth G, Reiling N, Lee M-A, Ratner L, Lane CM, Moore MS, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO (Eur Mol Biol Organ) J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A, Blobel G, Moore MS. Identification of a protein complex that is required for nuclear protein import and mediates docking of import substrate to distinct nucleoporins. Proc Natl Acad Sci USA. 1995a;92:1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A, Moore MS, Blobel G. The peptide repeat domain of Nup98 functions as a docking site in transport across the nuclear pore complex. Cell. 1995b;81:215–222. doi: 10.1016/0092-8674(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Re F, Braaten D, Franke EK, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- Roe T, Reynolds TC, Yu G, Brown PO. Integration of murine leukemia virus DNA depends on mitosis. EMBO (Eur Mol Biol Organ) J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum JS, Pemberton LF, Blobel G. A nuclear import pathway for a protein involved in tRNA maturation. J Cell Biol. 1998;139:1655–1661. doi: 10.1083/jcb.139.7.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout MP, Blobel G, Aitchison JD. A distinct nuclear import pathway used by ribosomal proteins. Cell. 1997;89:715–725. doi: 10.1016/s0092-8674(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Schlenstedt G, Smirnova E, Deane R, Solsbacher J, Kutay U, Görlich D, Ponstingl H, Bischoff FR. Yrb4p, a yeast Ran-GTP-binding protein involved in import of ribosomal protein L25 into the nucleus. EMBO (Eur Mol Biol Organ) J. 1997;16:6237–6249. doi: 10.1093/emboj/16.20.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger B, Simos G, Bischoff FR, Podfelejnikov A, Mann M, Hurt E. Mtr10p functions as a nuclear import receptor for the mRNA-binding protein Npl3p. EMBO (Eur Mol Biol Organ) J. 1998;17:2196–2207. doi: 10.1093/emboj/17.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H, Dreyfuss G. A nuclear localization domain in the hnRNP A1 protein. J Cell Biol. 1995;129:551–560. doi: 10.1083/jcb.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi MC, Eder PS, Kataoka N, Wan L, Liu Q, Dreyfuss G. Transportin-mediated nuclear import of heterogeneous nuclear RNP proteins. J Cell Biol. 1997;138:1181–1192. doi: 10.1083/jcb.138.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Vodicka MA, Koepp DM, Silver PA, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwedler U, Kornbluth RS, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis K, Mattaj IW, Lamond AI. Identification of hSRP1α as a functional receptor for nuclear localization sequences. Science. 1995;268:1049–1053. doi: 10.1126/science.7754385. [DOI] [PubMed] [Google Scholar]
- Weis K, Ryder U, Lamond AI. The conserved amino-terminal domain of hSRP1α is essential for nuclear protein import. EMBO (Eur Mol Biol Organ) J. 1996;15:1818–1825. [PMC free article] [PubMed] [Google Scholar]
- Yao X-J, Subbramanian RA, Rougeau N, Boisvert F, Bergeron D, Cohen EA. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal α-helical structure in Vpr nuclear localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X-F, Matsuda Z, Matsuda M, Essex M, Lee T-H. Human immunodeficiency virus vprgene encodes a virion-associated protein. J Virol. 1990;64:5688–5693. doi: 10.1089/aid.1990.6.1265. [DOI] [PubMed] [Google Scholar]
- Zhao L-J, Wang L, Mukherjee S, Narayan O. Biochemical mechanism of HIV-1 Vpr function. J Biol Chem. 1994;269:32131–32137. [PubMed] [Google Scholar]
- Zhou Y, Lu Y, Ratner L. Arginine residues in the C-terminus of HIV-1 Vpr are important for nuclear localization and cell cycle arrest. Virology. 1998;242:414–424. doi: 10.1006/viro.1998.9028. [DOI] [PubMed] [Google Scholar]