Abstract
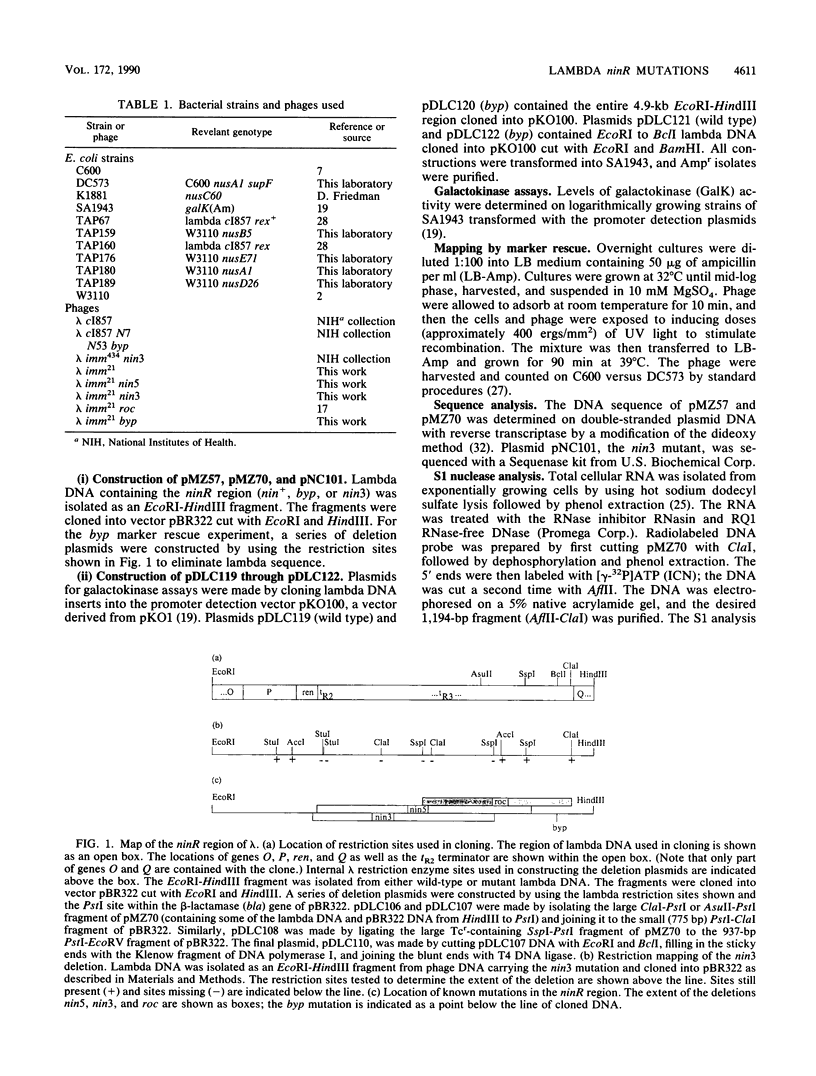
Two mutations in the ninR region of bacteriophage lambda that bypass a requirement for antitermination have been studied. One mutation, byp, has been cloned and mapped by marker rescue to a 417-base-pair segment in the ninR region of the genome. Analysis of the byp mutation by using promoter detection vectors, DNA sequencing, and S1 nuclease analysis showed that the byp mutation created a new promoter that transcribed gene Q. The second mutation analyzed was the deletion nin3. Sequence analysis revealed that 2,485 base pairs of the ninR region were removed, beginning within the ren gene and ending in an open reading frame termed ninG. The tR2 and tR3 terminators, and probably others, were removed by the nin3 deletion, thereby allowing the phage to be N independent and to grow in hosts defective for Nus antitermination factors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbás P., Soberón X., Merino E., Zurita M., Lomeli H., Valle F., Flores N., Bolivar F. Plasmid vector pBR322 and its special-purpose derivatives--a review. Gene. 1986;50(1-3):3–40. doi: 10.1016/0378-1119(86)90307-0. [DOI] [PubMed] [Google Scholar]
- Butler B., Echols H. Regulation of bacteriophage lambda development by gene N: properties of a mutation that bypasses N control of late protein synthesis. Virology. 1970 Feb;40(2):212–222. doi: 10.1016/0042-6822(70)90396-x. [DOI] [PubMed] [Google Scholar]
- Court D., Brady C., Rosenberg M., Wulff D. L., Behr M., Mahoney M., Izumi S. U. Control of transcription termination: a rho-dependent termination site in bacteriophage lambda. J Mol Biol. 1980 Apr;138(2):231–254. doi: 10.1016/0022-2836(80)90285-5. [DOI] [PubMed] [Google Scholar]
- Court D., Sato K. Studies of novel transducing variants of lambda: dispensability of genes N and Q. Virology. 1969 Oct;39(2):348–352. doi: 10.1016/0042-6822(69)90060-9. [DOI] [PubMed] [Google Scholar]
- Dambly C., Delstanche M., Gathoye A. M. qin101: Promoter mutation which allows the constitutive expression of the late genes. J Virol. 1979 Apr;30(1):14–20. doi: 10.1128/jvi.30.1.14-20.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Court D., Green L. On the nature of cis-acting regulatory proteins and genetic organization in bacteriophage: the example of gene Q of bacteriophage lambda. Genetics. 1976 May;83(1):5–10. doi: 10.1093/genetics/83.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollifield W. C., Kaplan E. N., Huang H. V. Efficient RecABC-dependent, homologous recombination between coliphage lambda and plasmids requires a phage ninR region gene. Mol Gen Genet. 1987 Dec;210(2):248–255. doi: 10.1007/BF00325690. [DOI] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. A cII-dependent promoter is located within the Q gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1985 May;82(10):3134–3138. doi: 10.1073/pnas.82.10.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N. Bypassing a positive regulator: isolation of a lambda mutant that does not require N product to grow. Virology. 1970 Feb;40(2):223–229. doi: 10.1016/0042-6822(70)90397-1. [DOI] [PubMed] [Google Scholar]
- Kröger M., Hobom G. A chain of interlinked genes in the ninR region of bacteriophage lambda. Gene. 1982 Nov;20(1):25–38. doi: 10.1016/0378-1119(82)90084-1. [DOI] [PubMed] [Google Scholar]
- Leason K. R., Friedman D. I. Analysis of transcription termination signals in the nin region of bacteriophage lambda: the roc deletion. J Bacteriol. 1988 Nov;170(11):5051–5058. doi: 10.1128/jb.170.11.5051-5058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Naito A., Naito S., Ikeda H. Homology is not required for recombination mediated by DNA gyrase of Escherichia coli. Mol Gen Genet. 1984;193(2):238–243. doi: 10.1007/BF00330674. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. Replication of bacteriophage DNA. I. Replication of DNA of lambda phage defective in early functions. J Mol Biol. 1968 Dec 14;38(2):217–225. doi: 10.1016/0022-2836(68)90407-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D., Shimatake H., Brady C., Wulff D. L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978 Mar 30;272(5652):414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- Sarmientos P., Sylvester J. E., Contente S., Cashel M. Differential stringent control of the tandem E. coli ribosomal RNA promoters from the rrnA operon expressed in vivo in multicopy plasmids. Cell. 1983 Apr;32(4):1337–1346. doi: 10.1016/0092-8674(83)90314-8. [DOI] [PubMed] [Google Scholar]
- Schmidt M. C., Chamberlin M. J. nusA protein of Escherichia coli is an efficient transcription termination factor for certain terminator sites. J Mol Biol. 1987 Jun 20;195(4):809–818. doi: 10.1016/0022-2836(87)90486-4. [DOI] [PubMed] [Google Scholar]
- Silva L. H., Jacob F. Etude genétique d'une mutation modifiant la sensibilité à l'immunité chez le bacteriophage lambda. Ann Inst Pasteur (Paris) 1968 Aug;115(2):145–158. [PubMed] [Google Scholar]
- Snyder L., McWilliams K. The rex genes of bacteriophage lambda can inhibit cell function without phage superinfection. Gene. 1989 Sep 1;81(1):17–24. doi: 10.1016/0378-1119(89)90332-6. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Enquist L. Analysis of coliphage lambda mutations that affect Q gene activity: puq, byp, and nin5. J Virol. 1979 Apr;30(1):1–13. doi: 10.1128/jvi.30.1.1-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toothman P., Herskowitz I. Rex-dependent exclusion of lambdoid phages. II. Determinants of sensitivity to exclusion. Virology. 1980 Apr 15;102(1):147–160. doi: 10.1016/0042-6822(80)90077-x. [DOI] [PubMed] [Google Scholar]