Abstract
The clustering of acetylcholine receptors (AChR) on skeletal muscle fibers is an early event in the formation of neuromuscular junctions. Recent studies show that laminin as well as agrin can induce AChR clustering. Since the α7β1 integrin is a major laminin receptor in skeletal muscle, we determined if this integrin participates in laminin and/or agrin-induced AChR clustering. The alternative cytoplasmic domain variants, α7A and α7B, and the extracellular spliced forms, α7X1 and α7X2, were studied for their ability to engage in AChR clustering. Immunofluorescence microscopy of C2C12 myofibers shows that the α7β1 integrin colocalizes with laminin-induced AChR clusters and to a much lesser extent with agrin-induced AChR clusters. However, together laminin and agrin promote a synergistic response and all AChR colocalize with the integrin. Laminin also induces the physical association of the integrin and AChR. High concentrations of anti-α7 antibodies inhibit colocalization of the integrin with AChR clusters as well as the enhanced response promoted by both laminin and agrin. Engaging the integrin with low concentrations of anti-α7 antibody initiates cluster formation in the absence of agrin or laminin. Whereas both the α7A and α7B cytoplasmic domain variants cluster with AChR, only those isoforms containing the α7X2 extracellular domain were active. These results demonstrate that the α7β1 integrin has a physiologic role in laminin-induced AChR clustering, that alternative splicing is integral to this function of the α7 chain, and that laminin, agrin, and the α7β1 integrin interact in a common or convergent pathway in the formation of neuromuscular junctions.
Keywords: integrin, laminin, acetylcholine receptor, agrin, α7β1
The postsynaptic membrane of the neuromuscular junction is a specialized structure characterized by a high density of acetylcholine receptors (AChRs)1 on the surface of myofibers (Fertuck and Salpeter, 1976; Anderson and Cohen, 1997; Frank and Fischbach, 1979). These clusters mediate the effects of acetylcholine released from the nerve on muscle fibers. Clustering of AChRs takes place during synaptogenesis and involves the migration of pre-existing dispersed AChRs in the sarcolemma to the postsynaptic membrane and an increase at the synapse in receptor synthesis (Hall and Sanes, 1993; Bowe and Fallon, 1995).
Migration of AChRs to the nerve terminal is mediated by agrin, a component of the synaptic basal lamina. Agrin isoforms are generated by alternative RNA splicing and each appears to have different capacities for clustering. Although agrin is synthesized by both muscle cells and motor neurons, the isoforms that most actively cluster AChRs are synthesized and released by motor neurons (McMahan, 1990; Ruegg and Bixby, 1998). Neural agrin plays a central role in synaptogenesis by binding to a receptor on the muscle cell surface. The αvβ1 integrin has been shown to bind agrin and participate in agrin-induced AChR clustering (Martin and Sanes, 1997), and MuSK, a muscle-specific tyrosine kinase, is required for the formation of neuromuscular junctions in vivo (Ganju et al., 1995; Valenzuela et al., 1995; DeChiara et al., 1996). Neural agrin is unable to cluster AChRs in myofibers that develop in vitro from MuSK-deficient myoblasts (Glass et al., 1996; Sugiyama et al., 1997).
Recent studies show that laminin can induce AChR clusters both in wild-type and in MuSK-deficient myotubes (Sugiyama et al., 1997; Montanaro et al., 1998). This induction is specific to laminin-1 and the effect of laminin on clustering is additive to those of agrin. These observations led to the suggestion that two alternative pathways participate in the formation of AChR clusters, one mediated by neural agrin and one by laminin.
The α7β1 integrin is a major laminin receptor in skeletal muscle (von der Mark et al., 1991; Song et al., 1992; George-Weinstein et al., 1993; Martin et al., 1996). Different isoforms of the α7 chain (α7AX1, α7AX2, α7BX1, and α7BX2) are produced by developmentally regulated alternative RNA splicing (Collo et al., 1993; Song et al., 1993; Ziober et al., 1993; Hodges and Kaufman, 1996). The alternative cytoplasmic (A, B, and C) and extracellular (X1 and X2) domains are predicted to affect either signal transduction and/or ligand binding. Evidence to date suggests that the α7 integrins play multiple roles in skeletal muscle development and in adult fibers. During development, the α7Bβ1 isoform is believed to mediate myoblast migration (George-Weinstein et al., 1993; Echtemeyer et al., 1996; Yao et al., 1996; Crawley et al., 1997) and the maintenance of myoblast proliferation on laminin (Foster et al., 1987). As a consequence, this population of precursor cells expands and becomes localized at sites of myofiber formation. After birth, specific isoforms of the α7β1 integrin are present at myotendinous and neuromuscular junctions, and serve as a transmembrane link between laminin in the basal lamina and the myofiber cytoskeleton (Hodges and Kaufman, 1996; Martin et al., 1996). Structural variants of the β1 integrin chain in skeletal muscle also contribute to the functional repertoire of this receptor (Van der Flier et al., 1995; Zhidkova et al., 1995).
To further understand the mechanism of neuromuscular junction formation, we have investigated whether the α7β1 integrin participates in the formation of AChR clusters. Our results demonstrate that the α7β1 integrin does participate in laminin-induced AChR clustering, that specific alternative splice isoforms of the integrin are integral to this process, and that laminin, agrin, and the α7β1 integrin interact through a common pathway in the formation of neuromuscular junctions.
Materials and Methods
MCK Promoter α7 Constructs
The 298-bp DNA sequence encoding the mouse α7 integrin 5′ untranslated region (UTR) and signal peptide was amplified by reverse transcriptase (RT)-PCR using the proof reading enzyme Pfu polymerase (Stratagene, La Jolla, CA). The forward primer (5′-ggtcaccttagctggtcctggggcagc-3′) was engineered to contain a BstEII restriction site and this product was cloned into pCRScript (Stratagene). The reverse primer was 5′-ggcaccccatgacgtccagattgaagg-3′. The following amplification conditions were used: 95°C for 4 min, followed by 35 cycles of 95°C for 1 min, 65°C for 30 s, and 72°C for 1 min. The 3.3-kb mouse muscle creatine kinase (MCK) promoter (Jaynes et al., 1986) was subcloned 5′ to the α7 insert using PstI and BstEII restriction sites. The MCK–5′UTRα7 was subcloned into pBKRSV containing the neoR gene. cDNAs encoding the rat α7AX1, AX2, BX1, and BX2 isoforms were subcloned into MCK–5′UTRα7 using AatII and KpnI sites to produce the complete mouse MCK–rat α7 constructs. DNA sequence analysis of each construct verified these clones.
Cell Lines and Transfection
C2C12 mouse myoblasts were cultured in Dulbecco's medium (low glucose) containing 20% FCS, 0.5% chick embryo extract (GIBCO BRL, Gaithersburg, MD), 2 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10 μg/ml kanamycin (P/S/K). These cells were transfected with 5 μg of linearized MCK–α7 plasmid using Superfect Reagent (QIAGEN Inc., Valencia, CA), as per company directions for the production of stable cell lines. Cells were selected in growth medium containing 500 μg/ml G418 (GIBCO BRL), and analyzed by Western blots and immunofluorescence (Song et al., 1992, 1993). RMo rat myoblasts (Merrill, 1989) were grown in Dulbecco's medium (high glucose) containing 15% horse serum, 2 mM glutamine, 0.5% chick embryo extract, and P/S/K.
Immunoprecipitation and Immunoblot Analysis
Approximately 2 × 105 C2C12 myoblasts transfected with the MCK–rat α7BX2 construct were seeded on fibronectin-coated (20 μg/ml), 100-mm tissue culture dishes. At confluence, differentiation media was added to induce myotube formation. AChR clustering was induced with 100 nM laminin-1, 0.5 nM agrin, or 100 nM laminin-1 and 0.5 nM agrin for 18 h. Cells were washed once in cold PBS (without Ca+2 or Mg+2) and harvested in PBS containing 2 mM PMSF. Cells were extracted at 4°C in 200 mM octyl-β-d-glucopyranoside, 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 2 mM PMSF, 20 μg/ml aprotinin, and 12.5 μg/ml leupeptin. Extracts were incubated at 4°C with anti-AChR antibody K-20 (Santa Cruz Biotechnology, Santa Cruz, CA) at 10 μg antibody/mg of total protein for 12 h. Protein G–agarose (Sigma, St. Louis, MO) was added at 1 mg protein G/100-mm plate of cells for 4 h at 4°C. The beads were then centrifuged at 4°C, at 12,000 rpm and washed three times in extraction buffer. Beads were mixed with 1× SDS sample loading buffer and centrifuged. The supernatants were collected and samples were separated on an 8% SDS acrylamide gel at 40 mA for 50 min. The protein was transferred to nitrocellulose filters. Blocked filters were probed with a 1:500 dilution of polyclonal anti-α7CDB (347) antibody that recognizes the α7B cytoplasmic domain (Song et al., 1993). The filters were washed and incubated with alkaline phosphatase–conjugated goat anti–rabbit immunoglobulin. Specificity of the antibody was determined using the immunizing peptide to block the antibody. Immunoreactive protein was visualized with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate.
AChR Cluster Assay
LAB-TEK eight-well chamber slides (No. 177445; Nalge Nunc International, Rochester, NY) were coated with purified fibronectin (20 μg/ml) for 24 h, washed with PBS, seeded with 1.5 × 104 myoblasts in growth medium per well, and then incubated 3 d at 37°C, in 5% CO2. Differentiation medium containing 2% horse serum and 2 mM glutamine was then added to induce myotube formation (Sugiyama et al., 1997). 3 d later, mature C2C12 myotubes were treated with 60 nM laminin-1 (GIBCO BRL) and/or 0.5 nM recombinant COOH-terminal (N2) agrin, for 18 or 5 h. RMo-8 myotubes were treated with 120 nM laminin and/or 1.0 nM agrin. To determine the effect of anti-α7 antibody on colocalization and cluster formation, 50–150 μg H36 mouse anti–rat α7 antibody or 026 mouse anti–rat α7 antibody (Kaufman et al., 1985; Song et al., 1992) was added at the time of cluster induction. To determine if anti-α7 antibody induced cluster formation, 5–250 μg/ml H36-α7 antibody was added in the absence of exogenous agrin or laminin. AChR clusters and α7β1 integrin localization were then determined by immunofluorescence. Agrin was prepared from transfected COS cells as described (Hoch et al., 1994).
Immunofluorescence and Quantitation
To detect the exogenous rat α7β1 integrin, transfected C2C12 myotubes were incubated with 10 μg/ml purified O26 anti-rat α7 mAb (Song et al., 1992) and a 1:1,000 dilution of rhodamine–α-bungarotoxin (Molecular Probes, Inc., Eugene, OR) for 1 h at 37°C, washed three times, and incubated with a 1:100 dilution of FITC-donkey anti–mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 45 min, and then fixed and mounted as described (Song et al., 1992). RMo myotubes were processed as above to quantitate the effect of anti-α7 antibody on endogenous rat integrin-mediated AChR clustering. Alternatively, to identify the endogenous mouse α7β1 integrin, C2C12 myotubes were incubated with labeled α-bungarotoxin, the cells were then fixed in 70% methanol and reacted with either 10 μg/ml purified rabbit anti-α7B cytoplasmic domain antibody (Song et al., 1993), or with a 1:2,000 dilution of rabbit anti-β1 integrin chain antisera (Chemicon International, Inc., Temecula, CA), followed by a 1:100 dilution of FITC-goat anti–rabbit F(ab′)2 (Jackson ImmunoResearch Laboratories), or with 10 μg/ml rat anti–mouse β1 integrin mAb (MAB-1997; Chemicon International, Inc.), followed by a 1:50 dilution of FITC-donkey anti–rat IgG (Jackson ImmunoResearch Laboratories). Quantitation of α7β1 and AChR colocalization was performed in triplicate cultures, using a Zeiss Photomicroscope III (Carl Zeiss, Inc., Thornwood, NY) and 63× Planapo objective. Between 266 and 437 clusters were scored per experimental group. Images of immunofluorescence localization were acquired using a Sony DXC9000 color video CCD camera.
Results
The α7β1 Integrin Localizes at Laminin-induced AChR Aggregates
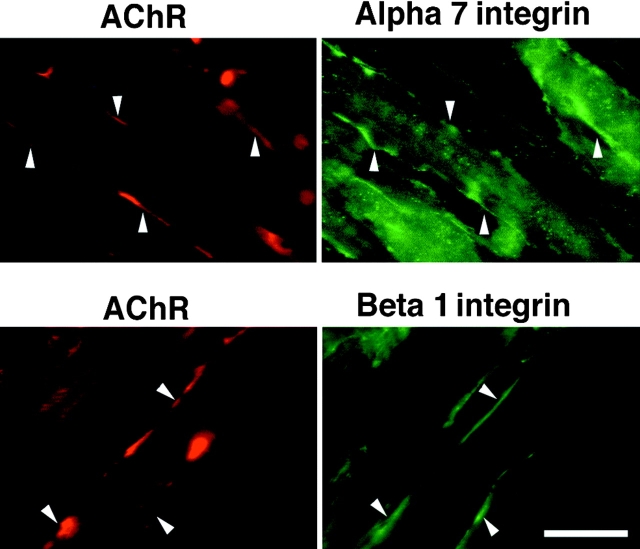
To further understand the process of neuromuscular junction formation, we have investigated whether the α7β1 integrin participates in the formation of AChR clusters. AChR clustering was induced in C2C12 myotubes with laminin-1. 18 h after the initiation of clustering, the myotubes were stained using rhodamine-labeled α-bungarotoxin to detect AChR clusters and the polyclonal antibodies specific for the α7B cytoplasmic domain and the β1 integrin chain. Both the α7 and β1 integrin chains were detected at laminin-induced AChR clusters (Fig. 1).
Figure 1.
Immunofluorescence localization of endogenous α7 and β1 integrin chains (green) at sites of laminin-induced clusters of AChRs (red) in mouse C2C12 myofibers. Rhodamine–bungarotoxin was used to identify AChR clusters. Bar, 15 μm.
Specific α7 Integrin Isoforms Colocalize with AChR Clusters
To study the colocalization of individual α7 integrin isoforms with the laminin-induced AChR clusters, C2C12 mouse myoblasts were transfected with cDNAs encoding rat α7AX1, AX2, BX1, and BX2 under control of the mouse MCK promoter. This promoter is active in differentiated myotubes but not in myoblasts (Jaynes et al., 1986). The rat-specific mouse anti-α7 mAb O26 was used to detect the rat α7 isoforms expressed in mouse myotubes. This antibody also enables more definitive localization and quantitation of the integrin than afforded by conventional antisera. Western blot analysis (not shown) and immunofluorescence (Fig. 2) showed that the transfected cell populations produced equivalent amounts of the appropriate isoforms of rat α7 protein with the exception that α7BX1 was ∼1.5–2-fold higher. The rat integrin was not detected by immunoblot or immunofluorescence in untransfected C2C12 cells or in control cells transfected with the parent plasmid pBKRSV. All the cell populations differentiated at approximately the same time and were morphologically indistinct from the C2C12 control cells. Likewise, all cell populations also formed approximately equivalent numbers of AChR clusters.
Figure 2.
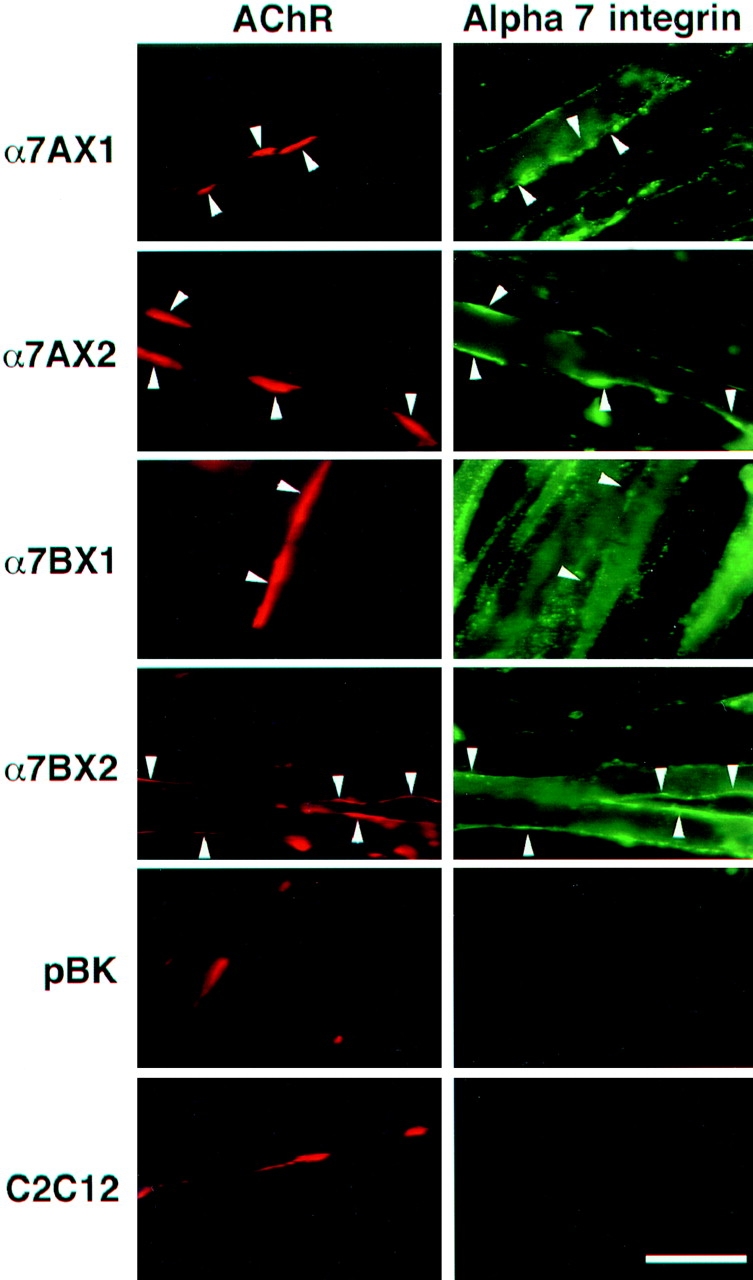
Immunofluorescence localization of rat α7 integrin isoforms (green) in mouse C2C12 myofibers at sites of laminin-induced clusters of acetylcholine receptors (red) using rat-specific anti-α7 antibody. The rat α7AX2 and α7BX2 isoforms, but not the α7AX1 or BX1 isoforms, colocalized with rhodamine–bungarotoxin at AChR clusters. Endogenous α7 integrin in cells transfected with the pBK vector and control C2C12 cells does not react with the 026 antibody. Bar, 15 μm.
The transfected and control cells were analyzed for laminin- and agrin-induced AChR clustering to determine which α7 integrin isoforms colocalized with AChR clusters. Fig. 2 shows the results of laminin-induced AChR clustering in C2C12 and α7 integrin–transfected myotubes. Both the α7A and α7B alternative cytoplasmic domain isoforms that contain the X2 extracellular domain colocalize with AChR clusters. In striking contrast, α7A and α7B chains bearing the alternative X1 extracellular domain showed little or no colocalization. Thus, there is a marked functional difference in the capacity of the X1 and X2 extracellular domains of the α7 integrin to participate in laminin-induced AChR clustering. This is consistent with the predominance of endogenous X2 isoforms in myotubes (Ziober et al., 1993; Hodges and Kaufman, 1996) that become localized at these sites (Martin et al., 1996).
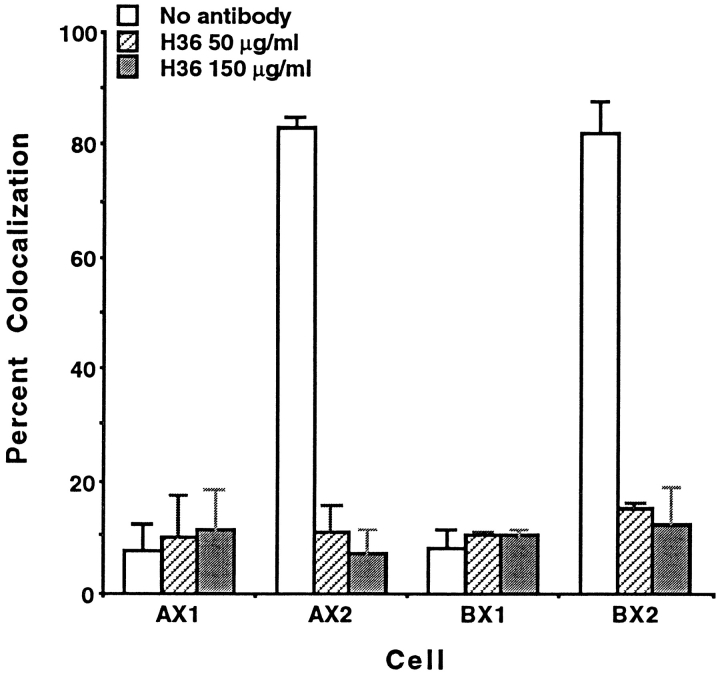
Quantitation of colocalization confirms our visual observations. The extent of colocalization of α7 isoforms and AChR clusters promoted by laminin was analyzed in three separate experiments by scoring AChR clusters and determining the number of clusters with α7 integrin. Clusters with <50% coincident α7 or β1 integrin and AChR signals were not scored as colocalized. By this stringent criterion, >80% of the AChR clusters were colocalized with α7AX2 and α7BX2 integrins upon induction with 60 nM laminin (Fig. 3). This increase was not observed in α7AX1 or α7BX1 transfected cell lines, again indicating an essential role for the α7X2 extracellular domain in laminin-induced AChR clustering. When antibody specific for the rat α7 integrin was added to cultures at the same time cluster formation was initiated with laminin, the anti-α7 antibody inhibited colocalization of the rat integrin with AChRs. Furthermore, this inhibition was restricted to colocalization of the α7AX2 and α7BX2 isoforms.
Figure 3.
The α7AX2 and α7BX2 integrins exhibit a high level of colocalization with laminin-induced AChR clusters in transfected C2C12 myofibers. H36 anti-α7 antibody added at the time of induction inhibits this colocalization. A low level of colocalization is seen with AChR clusters and the α7AX1 and α7AX2 isoforms. The mean values scored in triplicate samples, ±SE, are given.
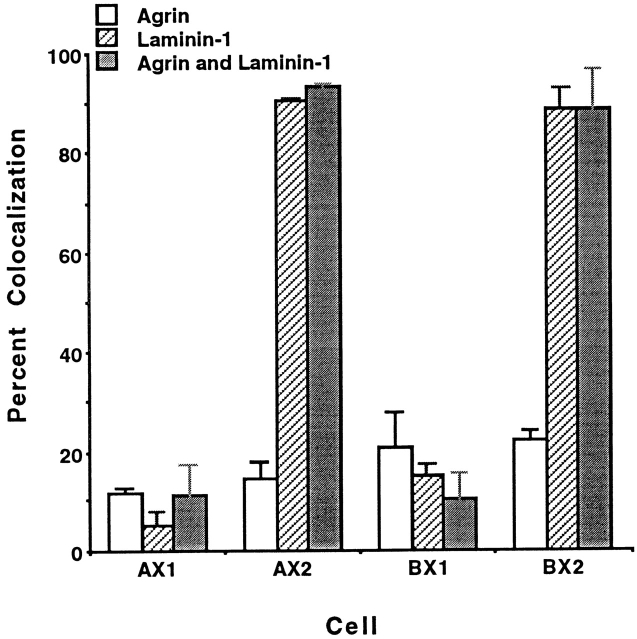
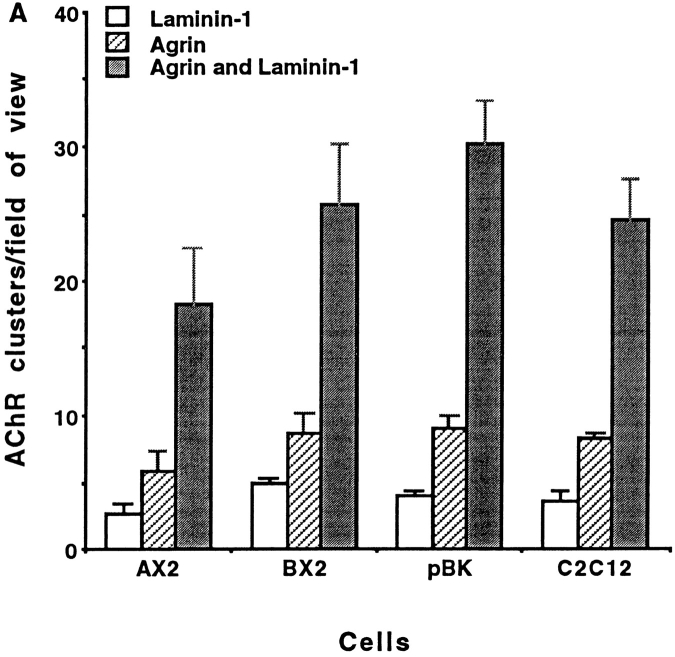
To examine whether the integrin is also present at agrin-induced AChR clusters, colocalization of α7 isoforms and AChR clusters promoted by agrin, laminin, and by a combination of laminin and agrin, was analyzed. Induction with 0.5 nM agrin resulted in relatively low levels of receptor colocalization (8–19%) in myotubes expressing any of the α7 chain isoforms (Fig. 4). In contrast, more than 90% of the AChR clusters were colocalized with α7AX2 and α7BX2 integrins upon induction with 60 nM laminin. The numbers of clusters that formed upon incubation with both 0.5 nM agrin and 60 nM laminin were 30–74% greater than those induced separately by each protein. Anti-α7 antibody will also inhibit this enhancement (see below). Moreover, upon induction with both laminin and agrin virtually all clusters colocalize with the α7AX2 and α7BX2 integrin isoforms. Taken together, these results suggest that laminin, agrin, and the α7β1 integrin interact in a common or convergent pathway. In this pathway, either inducer can independently promote AChR clustering, whereas together, they promote a more vigorous response, with the α7β1 integrin at all clusters.
Figure 4.
Colocalization of the rat α7 integrin is largely restricted to laminin-induced AChR clusters. The exogenously derived integrin is localized at virtually all laminin-induced clusters, but at only 10–20% agrin-induced clusters. In contrast, laminin and agrin together induce ∼30% more clusters than do either separately, and the α7 integrin is associated with essentially all clusters. As in Figs. 1–3, localization of the integrin and AChRs was determined 18 h after induction. The mean values scored in triplicate samples, ±SE, are given.
Because the anti-α7 mAbs react with rat but not mouse integrin, it was necessary to use rat myofibers to determine if the enhancement of AChR clustering fostered by induction with both laminin and agrin could be inhibited with anti-α7 antibody. Myofibers that developed from the RMo line of rat myoblasts were induced with agrin, laminin, or agrin and laminin. O26 anti-α7 antibody was added at the time of induction. In two separate experiments the antibody reduced the numbers of clusters to ∼50% that of control cultures induced with laminin. The number of AChR aggregates promoted by agrin was not diminished by antibody, whereas the enhancement of clustering promoted upon induction with both agrin and laminin was reduced to that promoted by agrin alone (Fig. 5).
Figure 5.
Antibody against endogenous α7β1 integrin inhibits laminin-induced AChR clustering. AChR clustering was initiated in RMo rat myotubes with agrin, laminin, or agrin and laminin. Anti-α7 O26 antibody, reactive with the endogenous rat α7 integrin chain, had no effect on agrin-induced clustering (n = 55). The antibody inhibited ∼50% of laminin-induce AChR clustering (n = 103) as well as the enhanced clustering promoted by laminin and agrin (n = 154). n = number of clusters scored in control wells (no antibody). The mean values scored in six samples per condition, ±SE, are given.
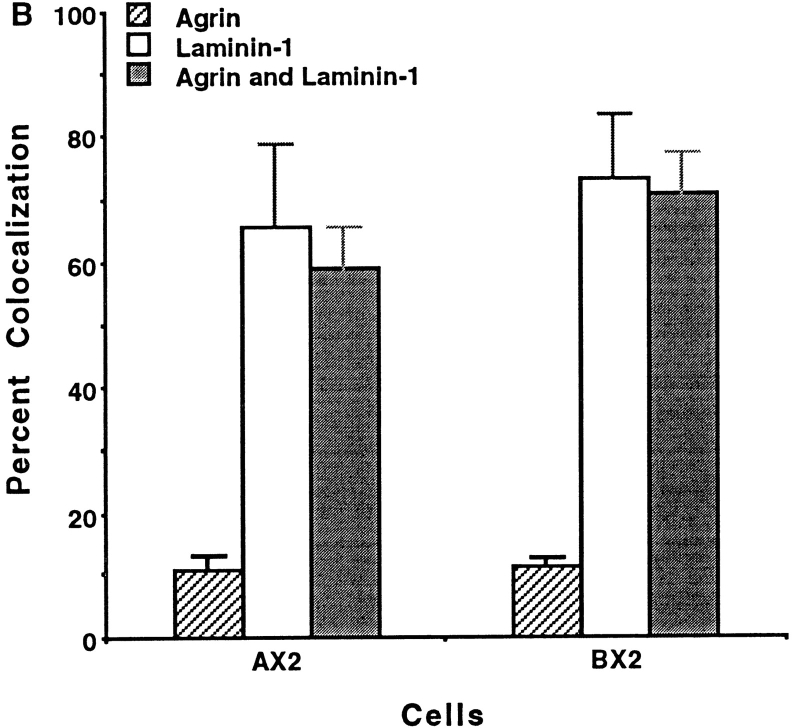
It was recently suggested that laminin and agrin initiate clustering by alternative pathways that can be distinguished both temporally and biochemically: agrin induces clustering more rapidly than laminin and requires MuSK (Sugiyama et al., 1997). We therefore examined early on in the induction process whether we could distinguish the association of the α7β1 integrin with agrin- and laminin-induced AChR clusters. Myofibers were treated with agrin, laminin, or with both agrin and laminin, and cluster formation was determined 5 h later. Initiation of cluster formation with both laminin and agrin results early on in a quantitatively synergistic response compared with induction solely with laminin or agrin (Fig. 6 A). Furthermore, the rat α7β1 integrin was localized at >60% of the AChR clusters induced by laminin and agrin (Fig. 6 B). As the total numbers of clusters formed in response to agrin and laminin were so enhanced, this extent of coclustering is much greater than expected if agrin induced AChR aggregation independently of the integrin. By 18 h, the α7β1 integrin is detected at essentially all AChR clusters that form in the presence of laminin and agrin (Fig. 4). These results suggest that agrin, laminin, and the α7β1 integrin can participate jointly in the formation of AChR clusters and neuromuscular junctions.
Figure 6.
Cluster formation and colocalization of the α7 integrin 5 h after induction of transfected C2C12 myofibers with laminin, agrin, or laminin and agrin. (A) The number of clusters that form in response to agrin and laminin exceeds that produced by either alone. (B) Exogenous α7 does not localize well with agrin-induced clusters. In contrast, the synergistic response evoked by agrin and laminin is characterized by localization of the α7β1 integrin with AChRs, even early in cluster formation. These results suggest that laminin, agrin and the α7β1 integrin interact in a common or convergent pathway to form AChR clusters and neuromuscular junctions. The mean values scored in triplicate samples, ±SE, are given.
β1 Integrin Is Localized at Both Agrin- and Laminin-induced AChR Clusters
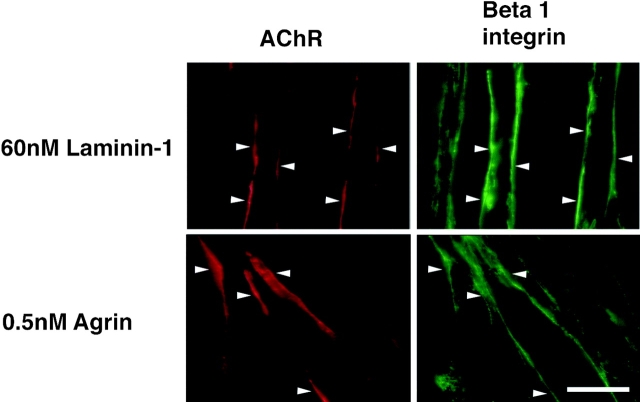
To confirm the localization of β1 integrins at AChR clusters, an additional anti-β1 integrin mAb was used. As seen in Fig. 7, the integrin β1 chain was localized to both agrin and laminin-induced AChR clusters. The β1 integrin colocalized with ∼82% of agrin-induced clusters and 92% of laminin-induced clusters. This result is consistent with the inhibition of agrin-induced clustering by anti-β1 antibody and the role of the αvβ1 integrin in this process (Martin and Sanes, 1997). The β1 integrin has also been localized at agrin-induced AChR clusters in chick myofibers (Bozyczko et al., 1989).
Figure 7.
Immunofluorescence localization of the β1 (green) integrin at AChR clusters (red). Anti-β1 mAb is colocalized at 82% (n = 91) of agrin- induced and 92% (n = 145) of laminin-induced AChR clusters. Bar, 15 μm.
Immunoprecipitation of AChR and the α7β1 Integrin
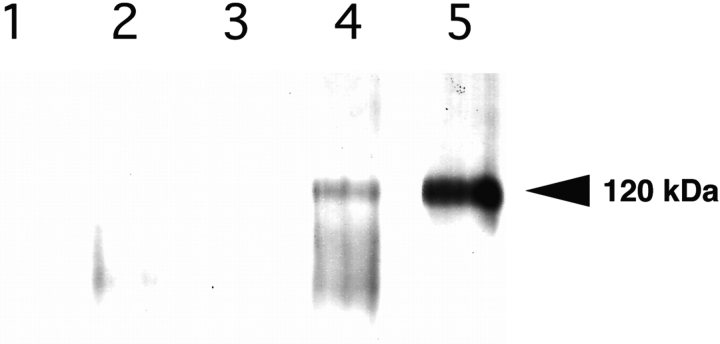
To further demonstrate a physiologic role for the α7β1 integrin in neuromuscular junction formation, immunoprecipitation was carried out to determine whether the AChR and integrin were physically associated. C2C12α7BX2 myotubes were induced with agrin, laminin, or agrin and laminin. Anti-AChR mAb and protein G–agarose were used to immunoprecipitate the AChR and its associated molecules. Western blot analysis reveals that the α7β1 integrin is coprecipitated with the AChR and that their association in this complex is dependent on induction with laminin. Whereas the integrin is not detected in uninduced fibers or upon induction with agrin, it is associated with AChR when laminin or laminin and agrin were used to promote clustering (Fig. 8). In the presence of both agrin and laminin, the α7 chain may undergo increased proteolytic processing (Song et al., 1992). This laminin-dependent association of the α7β1 integrin with AChRs further supports the conclusion that the integrin has a direct physiologic role in the clustering of AChR.
Figure 8.
Coprecipitation of the AChR and α7β1 integrin. Anti-AChR antibody and protein G–agarose were used to immunoprecipitate extracts prepared from C2C12α7BX2 myotubes. Western blot analysis using anti-α7 cytoplasmic domain B antibody of (1) uninduced cells, no anti-AChR antibody, (2) uninduced cells, (3) agrin-induced cells, (4) laminin and agrin-induced cells, and (5) laminin-induced cells, reveals laminin-dependent coprecipitation of the AChR and integrin. The α7 chain may undergo increased proteolytic processing (Song et al., 1992) in the presence of both agrin and laminin.
Anti-α7 Integrin Antibody Promotes AChR Clustering
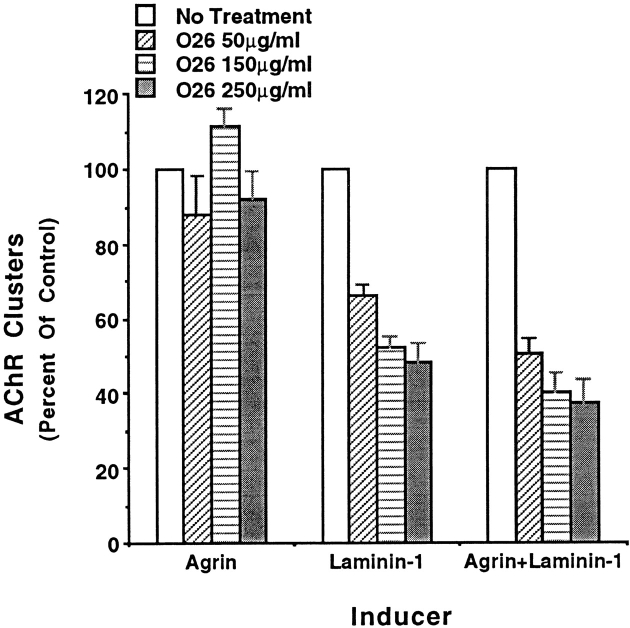
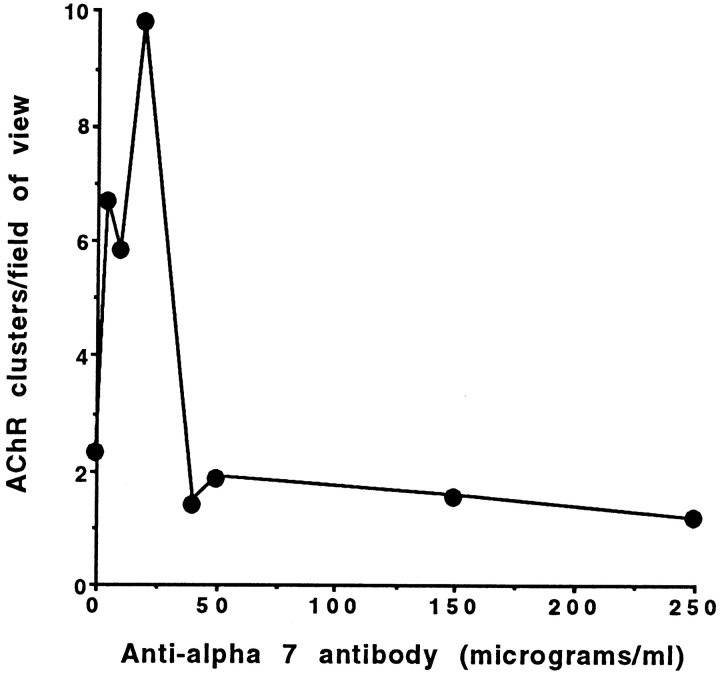
Lastly, engaging the α7β1 integrin with laminin or cross-linking the exogenous integrin with anti-α7 antibody promotes a detergent-stable association of the integrin with the cell cytoskeleton (Lowrey and Kaufman, 1989; Song et al., 1993) and an increase in free intracellular calcium (Kwon, M.S., C.S. Park, K.R. Choi, S.J. Kaufman, and W.S. Song, manuscript in preparation). In the absence of added laminin or agrin, relatively low concentrations of anti-α7 antibody also promotes AChR clustering on α7BX2 transfected C2 myofibers (Fig. 9). At higher antibody concentrations, where excess antibody fails to cross-link the integrin, cluster formation is minimal. Thus, engaging the α7β1 integrin with either its ligand or with antibody is sufficient to promote AChR clustering.
Figure 9.
Anti-α7 antibody induces AChR clustering in the absence of exogenous agrin or laminin. Purified H36-α7 antibody was added to cultures of α7BX2-transfected C2C12 myofibers. Rhodamine–bungarotoxin was used to identify AChR clusters 18 h later. Whereas high concentrations of anti-α7 antibody inhibit laminin-induced AChR clustering (Fig. 5) and colocalization (Fig. 3), low concentrations of antibody cross-link the integrin, promote its association with the cytoskeleton (Lowrey and Kaufman, 1989; Song et al., 1993), and induce AChR clustering.
Discussion
It has become clear that at least two molecules in the skeletal muscle basal lamina can initiate clustering of AChRs, the heparan sulfate proteoglycan agrin and laminin. In this report we have shown that specific isoforms of the α7β1 integrin participate in laminin-induced AChR clustering in C2C12 myotubes as well as clustering induced by both laminin and agrin. Laminin was shown to induce colocalization of the integrin and AChR. In the presence of both laminin and agrin, cluster formation and colocalization with the integrin are markedly enhanced. High concentrations of two different anti-α7 mAbs inhibit colocalization of the integrin with AChR clusters as well as the enhanced response promoted by both laminin and agrin. In addition to the laminin-dependent colocalization of the α7β1 integrin with AChRs, laminin also promotes the physical association of these two receptors, further suggesting that the integrin has a direct physiologic role in AChR aggregation. Engaging the integrin with concentrations of anti-α7 antibody that cross-link the integrin also induces AChR clusters. This is likely a consequence of association of the integrin and cell cytoskeleton (Lowrey and Kaufman, 1989; Song et al., 1993), and an α7β1-mediated increase in intracellular calcium (Kwon, M.S., C.S. Park, K.R. Choi, S.J. Kaufman, and W.S. Song, manuscript in preparation) that is essential to AChR clustering (Megeath et al., 1998). Anti-laminin antiserum will also enhance receptor clustering induced by agrin (Montanaro et al., 1998), presumably by this same mechanism. Although laminin- and agrin- induced clustering of AChR can be studied independently, we suggest that a unified mechanism may underlie receptor aggregation and differentiation of the postsynaptic membrane. The following evidence supports this: (a) Laminin and agrin together produce more vigorous clustering than either protein induces alone and virtually all clusters are associated with the integrin by 18 h. The α7β1 integrin is also localized in vivo at neuromuscular junctions (Martin et al., 1996). As only 60% of clusters formed 5 h after induction are colocalized with the exogenous integrin, agrin and laminin may initiate clustering independently; a common aggregation mechanism predominates thereafter. (b) The enhanced aggregation of AChR clustering induced by agrin and laminin is completely inhibited by anti-α7 integrin antibody. (c) AChR and the α7β1 integrin physically associate upon induction of cluster formation with laminin or with laminin and agrin. (d) AChR aggregates do form in agrin null mice, although they are reduced in number, size and density (Gautam et al., 1996). Thus, agrin appears to be essential, but not sufficient for the normal development of postsynaptic sites in vivo. Our data suggest that laminin and the α7β1 integrin are additional components required for normal synaptic organization. Laminin, agrin, and the α7β1 integrin are also all expressed during formation of the postsynaptic membrane in vivo (Bayne et al., 1984; McMahan, 1990; Hall and Sanes, 1993; Bowe and Fallon, 1995; Martin et al., 1996; Ruegg and Bixby, 1998). Although α7 null mice do form neuromuscular junctions, no detailed structural study of these sites, nor the expression of other integrins at postsynaptic sites, nor physiologic studies of these mice have been reported (Mayer et al., 1997). (e) MuSK, a receptor protein tyrosine kinase (Valenzuela et al., 1995; Ganju et al., 1996), is required for AChR aggregation in vivo: MuSK null mice do not form these clusters (DeChiara et al., 1996). Although laminin can induce limited AChR clustering in MuSK null myotubes in vitro (Sugiyama et al., 1997), MuSK appears to be essential to the rigorous combined laminin and agrin-induced AChR aggregation in vitro and in vivo. (f) Since myotubes synthesize laminin, it is questionable whether any in vitro experiments on AChR clustering have been conducted in the complete absence of laminin. Therefore, it is not surprising that “spontaneous” clusters that form in the absence of exogenous laminin or agrin do have localized α7 integrin, and that 10– 20% of AChR clusters formed in response to agrin do have exogenous α7β1 integrin at those sites. (g) Anti-β1 integrin antibody completely inhibits agrin-induced cluster formation, presumably by inhibiting αvβ1 integrin (Martin and Sanes, 1997) as well as the α7β1 integrin mobilized by endogenous laminin. This is consistent with our localization of the β1 integrin chain at agrin-induced AChR clusters and similar findings in the chick (Bozyzcko et al., 1989). Differences in antibody specificity, determinant accessibility, and expression of endogenous laminin may account for the lower level of β1 integrin detected by Montanaro et al. (1998) at agrin-induced AChR clusters. (h) Laminin and agrin have common structural motifs and thus have the potential to interact with common elements. Furthermore, although the agrin used in many in vitro studies lacks the domain that supports its binding to laminin, native neural and muscle agrin can directly interact with laminin (Hall and Sanes, 1993; Bowe and Fallon, 1995; Sugiyama et al., 1997; Denzer et al., 1998; Ruegg and Bixby, 1998). Thus the two inducers may act in concert both physically and mechanistically. The interaction of laminin and agrin may enhance their association with the MuSK receptor complex (Glass et al., 1996). Our results suggest that the α7β1 integrin may also be part of this complex. (i) The α7β1 integrins provide the potential for signal transduction and for the association with the cytoskeleton that may be significant to cluster formation and the integrity of neuromuscular junctions (Song et al., 1993; Hodges and Kaufman, 1996). (j) Engaging the integrin with laminin or anti-α7 antibody induces AChR aggregation and an increase in intracellular calcium that is required for agrin-induced AChR clustering (Kwon, M.S., C.S. Park, K.R. Choi, S.J. Kaufman, and W.S. Song, manuscript in preparation; Megeath et al., 1998).
We believe these results can best be explained by a unified mechanism of laminin and agrin-induced clustering. Additional molecules also appear important to the aggregation of AChRs. The αvβ1 integrin binds agrin and also appears to be required for clustering (Martin and Sanes, 1997). α-Dystroglycan can bind laminin and agrin and it also may function in laminin and agrin-induced AChR clustering (Bowe and Fallon, 1995; Montanaro et al., 1998; Ruegg and Bixby, 1998). Although Montanaro et al. (1998) suggest that integrins may not be involved in laminin- induced AChR aggregation, our results demonstrate otherwise. It will be interesting to further define the molecular orchestration of this concerted mechanism of receptor aggregation.
With respect to the integrin, both the α7A and α7B alternative cytoplasmic domains engage in AChR clustering, and both are localized at neuromuscular junctions in vivo (Martin et al., 1996). In contrast, the alternative X1 and X2 extracellular domains of the α7 chain have distinct functions. Although both can bind laminin, only those isoforms containing the 39–amino acid X2 sequence engage in AChR clustering. In addition to a role in synaptogenesis, specific isoforms of the α7β1 integrin also appear essential to the maintenance of skeletal muscle and synaptic function (Martin et al., 1996; Hodges et al., 1997). Lastly, based on the involvement of the α7β1 integrin in the formation and integrity of neuromuscular junctions, mutations in the α7 integrin gene might be expected to underlie muscle diseases that exhibit a neurologic component. In this regard, we have recently identified three patients with mutations in the α7 gene that do not express the α7 integrin protein and who show congenital myopathies with delayed motor milestones (Hayashi et al., 1998).
Acknowledgments
We thank Dr. S. Hauschka for providing the MCK promoter used in these experiments and Dr. Jean Buskin for her helpful advice. We also thank Mr. Gregory Wallace for his skillful technical assistance.
This work was supported by the Muscular Dystrophy Association of America and National Institutes of Health.
Abbreviations used in this paper
- AChR
acetylcholine receptor
- MCK
muscle creatine kinase
- MuSK
muscle-specific tyrosine kinase
References
- Anderson MJ, Cohen MW. Nerve-induced and spontaneous redistribution of acetylcholine receptor clusters on cultured muscle cells. J Physiol. 1977;286:757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne EK, Anderson MJ, Fambrough DM. Extracellular matrix organization in developing muscle: correlation with acetylcholine receptor aggregates. J Cell Biol. 1984;99:1486–1501. doi: 10.1083/jcb.99.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe MA, Fallon JR. The role of agrin in synapse formation. Annu Rev Neurosci. 1995;18:443–462. doi: 10.1146/annurev.ne.18.030195.002303. [DOI] [PubMed] [Google Scholar]
- Bozyczko D, Decker C, Muschler J, Horwitz AF. Integrin on developing and adult skeletal muscle. Exp Cell Res. 1989;183:72–91. doi: 10.1016/0014-4827(89)90419-9. [DOI] [PubMed] [Google Scholar]
- Collo G, Starr L, Quaranta V. A new isoform of the laminin receptor integrin α7β1 is developmentally regulated in skeletal muscle. J Biol Chem. 1993;268:19019–19024. [PubMed] [Google Scholar]
- Crawley S, Farrell EM, Wang W, Gu M, Huang H-Y, Huynh V, Hodges BL, Cooper DNW, Kaufman SJ. The α7β1 integrin mediates adhesion and migration of skeletal myoblasts on laminin. Exp Cell Res. 1997;235:274–286. doi: 10.1006/excr.1997.3671. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, et al. The receptor tyrosine kinase MUSK is required for neuromuscular junction formation in vivo. . Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Denzer AJ, Schulthess T, Fauser C, Schumacher B, Kammerer RA, Engel J, Ruegg MA. Electron microscopic structure of agrin and mapping of its binding site in laminin-1. EMBO (Eur Mol Biol Organ) J. 1998;17:335–343. doi: 10.1093/emboj/17.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtemeyer F, Schober S, Poschl E, von der Mark H, von der Mark K. Specific induction of cell mobility on laminin by α7 integrin. J Biol Chem. 1996;271:2071–2075. doi: 10.1074/jbc.271.4.2071. [DOI] [PubMed] [Google Scholar]
- Fertuck HC, Salpeter MM. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-α-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976;69:144–148. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RF, Thompson JM, Kaufman SJ. A laminin substrate promotes myogenesis in rat skeletal muscle cultures; analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Dev Biol. 1987;122:11–20. doi: 10.1016/0012-1606(87)90327-7. [DOI] [PubMed] [Google Scholar]
- Frank E, Fischbach GD. Early events in neuromuscular junction formation in vitro: Induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979;83:143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganju P, Walls E, Brennan J, Reith AD. Cloning and expression of Nsk, a novel receptor tyrosine kinase implicated in skeletal myogenesis. Oncogene. 1995;11:281–290. [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–536. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M, Foster RF, Gerhart JV, Kaufman SJ. In vitro and in vivoexpression of α7 integrin and desmin define the primary and secondary myogenic lineages. Dev Biol. 1993;156:209–229. doi: 10.1006/dbio.1993.1071. [DOI] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattson K, Burden SJ, et al. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–524. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Hall, Z.W., and J.R. Sanes. 1993. Synaptic structure and development: the neuromuscular junction. Neuron. 10(Suppl.):99–121. [DOI] [PubMed]
- Hayashi YK, Chou F-L, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ, et al. Mutations in the integrin α7 gene cause congenital myopathy. Nat Genet. 1998;19:94–97. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- Hoch W, Campanelli JT, Harrison S, Scheller RH. Structural domains of agrin required for clustering of nicotinic acetylcholine receptors. EMBO (Eur Mol Biol Organ) J. 1994;13:479–490. doi: 10.1002/j.1460-2075.1994.tb06575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges BL, Kaufman SJ. Developmental regulation and functional significance of alternative splicing of NCAM and α7β1 integrin in skeletal muscle. Basic Appl Myol. 1996;6:437–447. [Google Scholar]
- Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the α7β1 integrin in human and murine muscular dystrophies. J Cell Sci. 1997;110:2873–2881. doi: 10.1242/jcs.110.22.2873. [DOI] [PubMed] [Google Scholar]
- Jaynes JB, Chamberlin JS, Buskin JE, Johnson JE, Hauschka SD. Transcriptional regulation of the muscle creatine kinase gene and related expression in transfected mouse myoblasts. Mol Cell Biol. 1986;6:2855–2864. doi: 10.1128/mcb.6.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman SJ, Foster RF, Haye KR, Faiman LE. Expression of a developmentally regulated antigen on the surface of skeletal and cardiac muscle cells. J Cell Biol. 1985;100:1977–1987. doi: 10.1083/jcb.100.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey AA, Kaufman SJ. Membrane-cytoskeleton associations during myogenesis deviate from traditional definitions. Exp Cell Res. 1989;183:1–23. doi: 10.1016/0014-4827(89)90414-x. [DOI] [PubMed] [Google Scholar]
- Martin PT, Sanes JR. Integrins mediate adhesion to agrin and modulate agrin signaling. Development (Camb) 1997;124:3909–3917. doi: 10.1242/dev.124.19.3909. [DOI] [PubMed] [Google Scholar]
- Martin PT, Kaufman SJ, Kramer RH, Sanes JR. Synaptic integrins in developing, adult, and mutant muscle: selective association of α1, α7A and αB integrins with the neuromuscular junction. Dev Biol. 1996;174:125–139. doi: 10.1006/dbio.1996.0057. [DOI] [PubMed] [Google Scholar]
- Mayer U, Saher G, Faessler R, Boornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Absence of integrin α7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- McMahan UJ. The agrin hypothesis. Cold Spring Harbor Symp Quant Biol. 1990;50:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- Megeath LJ, Fallon JR. Intracellular calcium regulates agrin-induced acetylcholine receptor clustering. J Neurosci. 1998;18:672–678. doi: 10.1523/JNEUROSCI.18-02-00672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. Clonal derivation of a rat muscle strain that forms contractile-competent myotubes. In Vitro Cell Dev Biol. 1989;25:471–476. doi: 10.1007/BF02624635. [DOI] [PubMed] [Google Scholar]
- Montanaro F, Gee SH, Jacobson C, Lindenbaum MH, Froehner SC, Carbonetto S. Laminin- and α-dystroglycan mediated acetylcholine receptor aggregation via a MuSK-independent pathway. J Neurosci. 1998;18:1250–1260. doi: 10.1523/JNEUROSCI.18-04-01250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg MA, Bixby JL. Agrin orchestrates synaptic differentiation at the vertebrate neuromuscular junction. Trends Neurosci. 1998;21:22–27. doi: 10.1016/s0166-2236(97)01154-5. [DOI] [PubMed] [Google Scholar]
- Song WK, Wang W, Foster RF, Bielser DA, Kaufman SJ. H36-α7 is a novel integrin α chain that is developmentally regulated during skeletal myogenesis. J Cell Biol. 1992;117:643–657. doi: 10.1083/jcb.117.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WK, Wang W, Sato H, Bielser DA, Kaufman SJ. Expression of α7 integrin cytoplasmic domains during skeletal muscle development: alternative forms, conformational change, and homologies with serine/threonine kinases and tyrosine phosphatases. J Cell Sci. 1993;106:1139–1152. doi: 10.1242/jcs.106.4.1139. [DOI] [PubMed] [Google Scholar]
- Sugiyama JE, Glass DJ, Yancopoulos GD, Hall ZW. Laminin-induced acetylcholine receptor clustering: An alternative pathway. J Cell Biol. 1997;139:181–191. doi: 10.1083/jcb.139.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela DM, Stitt TN, DiStefano PS, Rojas E, Mattsson K, Compton DL, Nunez L, Park S, Stark JL, Gies DR, et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron. 1995;15:573–584. doi: 10.1016/0896-6273(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Van der Flier A, Kuikman I, Baudoin C, van der Neut R, Sonnenberg A. A novel β1 integrin isoform produced by alternative splicing: unique expression in cardiac and skeletal muscle. FEBS (Fed Eur Biochem Soc) Lett. 1995;369:340–344. doi: 10.1016/0014-5793(95)00814-p. [DOI] [PubMed] [Google Scholar]
- von der Mark H, Durr J, Sonnenberg A, von der Mark K, Deutzmann R, Goodman SL. Skeletal myoblasts utilize a novel beta-1 series integrin and not α60 β1 for binding to the E8 and T8 fragments of laminin. J Biol Chem. 1991;266:23593–23601. [PubMed] [Google Scholar]
- Yao C-C, Ziober BL, Sutherland AE, Mendrick DL, Kramer RH. Laminins promote the locomotion of skeletal myoblasts via the α7 integrin receptor. J Cell Sci. 1996;109:3139–3150. doi: 10.1242/jcs.109.13.3139. [DOI] [PubMed] [Google Scholar]
- Zhidkova NI, Belkin AM, Mayne R. Novel isoform of β1 integrin expressed in skeletal and cardiac muscle. Biochem Biophys Res Commun. 1995;214:279–285. doi: 10.1006/bbrc.1995.2285. [DOI] [PubMed] [Google Scholar]
- Ziober BL, Vu MP, Waleh N, Crawford J, Lin C-S, Kramer RH. Alternative extracellular and cytoplasmic domains of the integrin α7 subunit are differentially expressed during development. J Biol Chem. 1993;268:26773–26783. [PubMed] [Google Scholar]