Abstract
An emerging family of kinases related to the Drosophila Aurora and budding yeast Ipl1 proteins has been implicated in chromosome segregation and mitotic spindle formation in a number of organisms. Unlike other Aurora/Ipl1-related kinases, the Caenorhabditis elegans orthologue, AIR-2, is associated with meiotic and mitotic chromosomes. AIR-2 is initially localized to the chromosomes of the most mature prophase I–arrested oocyte residing next to the spermatheca. This localization is dependent on the presence of sperm in the spermatheca. After fertilization, AIR-2 remains associated with chromosomes during each meiotic division. However, during both meiotic anaphases, AIR-2 is present between the separating chromosomes. AIR-2 also remains associated with both extruded polar bodies. In the embryo, AIR-2 is found on metaphase chromosomes, moves to midbody microtubules at anaphase, and then persists at the cytokinesis remnant. Disruption of AIR-2 expression by RNA- mediated interference produces entire broods of one-cell embryos that have executed multiple cell cycles in the complete absence of cytokinesis. The embryos accumulate large amounts of DNA and microtubule asters. Polar bodies are not extruded, but remain in the embryo where they continue to replicate. The cytokinesis defect appears to be late in the cell cycle because transient cleavage furrows initiate at the proper location, but regress before the division is complete. Additionally, staining with a marker of midbody microtubules revealed that at least some of the components of the midbody are not well localized in the absence of AIR-2 activity. Our results suggest that during each meiotic and mitotic division, AIR-2 may coordinate the congression of metaphase chromosomes with the subsequent events of polar body extrusion and cytokinesis.
Keywords: cytokinesis, Aurora, chromosomes, meiosis, polar bodies, RNAi
Central to the accurate execution of each cell cycle and the faithful segregation of chromosomes to each daughter cell are the precise controls that govern microtubule dynamics (Merdes and Cleveland, 1997; Sorger et al., 1997; Waters and Salmon, 1997). During each cell cycle, the interphase array of microtubules must be broken down, centrosomes must be duplicated, separated, and their microtubule nucleating capacity increased to seed the formation of a microtubule spindle (Verde et al., 1990; Ault and Rieder, 1994; Zhai et al., 1996; Boleti et al., 1996; Gabrielli et al., 1996). Microtubules emanating from these centrosomes connect with chromosomes via their kinetochores. A number of opposing forces generated by motor proteins residing at the kinetochore, on the chromosome arms, on the spindle microtubules, and at the centrosomes induce the formation of a bipolar spindle and the congression of chromosomes to the metaphase plate (Sawin et al., 1992; Walczak et al., 1996; Cottingham and Hoyt, 1997; Gaglio et al., 1997; Heald et al., 1997; Saunders et al., 1997; Stearns, 1997). The congression of chromosomes to a well-defined metaphase plate is required for chromosome separation at anaphase and is also necessary for the subsequent events that result in cytokinesis (Cassimeris et al., 1994; Williams et al., 1995; Zhang and Nicklas, 1995; Glotzer, 1997; Wheatley et al., 1997).
Phosphorylation of a number of proteins contributes to these dynamic events and ensures that they occur in the proper temporal and spatial order (Verde et al., 1990; Nicklas et al., 1993; Ookata et al., 1995; Nigg et al., 1996; Blangy et al., 1997; Drewes et al., 1997; Larsson et al., 1997; Renzi et al., 1997; Gradin et al., 1998). However, many of the proteins involved in the phosphorylation cascades controlling these processes have not yet been identified (Nasmyth, 1996).
Recently, an emerging family of protein kinases that appears to regulate microtubule-based mitotic events has been described (Chan and Botstein, 1993; Glover et al., 1995; Niwa et al., 1996; Gopalan et al., 1997; Kimura et al., 1997; Sen et al., 1997; Yanai et al., 1997; Roghi et al., 1998; Terada et al., 1998). The founding members of this kinase family, Saccharomyces cerevisiae Ipl1 and Drosophila Aurora, were both identified in mutant screens for mitotic defects (Chan and Botstein, 1993; Glover et al., 1995). Mutations in ipl1 and aurora result in the generation of severely aneuploid cells, and in the case of aurora, monopolar spindles arising from a failure in centrosome separation (Chan and Botstein, 1993; Glover et al., 1995).
Another family member, Xenopus Eg2, has recently been shown to be associated with the centrosomes and microtubules of the mitotic spindle in cultured cells (Roghi et al., 1998). Addition of a kinase-inactive form of Eg2 to Xenopus egg extracts prevented the formation of mitotic spindles (Roghi et al., 1998). Two highly related mammalian members of the Ipl1/Aurora family, mouse IAK-1 and human AIK-1, have also been found to be associated with the centrosomes and microtubules of the mitotic spindle in mouse and human cell lines respectively (Gopalan et al., 1997; Kimura et al., 1997). The kinase activities of IAK-1 and AIK-1 were also shown to be cell cycle–regulated, peaking at midmitosis. Neither of these proteins could complement ipl1 mutant yeast cells, suggesting a functional diversification in this protein kinase family (Gopalan et al., 1997; Kimura et al., 1997).
Multiple members of this kinase family have been found in mammals and C. elegans. Recently, a second mammalian orthologue, rat AIM-1 (highly related to mouse STK-1 [Niwa et al., 1996]), was found to be associated not with centrosomes, but rather to be specifically localized to midbody microtubules during anaphase (Terada et al., 1998). Expression of a kinase-inactive form of AIM-1 in rat tissue culture cells resulted in defects in cytokinesis. In addition, a third murine family member, IAK-3, has been found to be exclusively expressed in the testis and ovary, and in situ analysis has revealed high levels of IAK-3 mRNA in meiotic spermatocytes (Gopalan et al., 1999).
We have identified two members of the Ipl1/Aurora kinase family in C. elegans, AIR-1 and AIR-2.1 AIR-1, like its relatives Eg2, IAK-1, and AIK-1, was also found to be associated with mitotic centrosomes (Schumacher et al., 1998). Disruption of AIR-1 expression resulted in embryonic lethality, apparently due to defects in nucleating functional mitotic spindles. Here we report the localization and the consequence of disrupting the expression of the AIR-2 protein during C. elegans gametogenesis and embryogenesis. Our results suggest that AIR-2, via its association with meiotic and mitotic chromosomes, may couple chromosome alignment during each meiotic and mitotic metaphase with the subsequent events of polar body extrusion and cytokinesis.
Materials and Methods
C. elegans Strains
N2 (wild-type) and tra-2(g122gf) nematode strains were obtained from the C. elegans Genetics Center (St. Paul, MN). Standard culture conditions were used for all strains (Brenner, 1974).
air-2 cDNA Synthesis
Total RNA was prepared from N2 gravid hermaphrodites by standard methods. First-strand cDNA was prepared with a GeneAmp reverse transcription (RT)-PCR kit (Perkin-Elmer, Branchburg, NJ) following the manufacturer's instructions. air-2–specific cDNAs were PCR amplified as described in the text. PCR products were gel purified using Wizard columns (Promega, Madison, WI) and cloned into a pBluescript vector (Stratagene, La Jolla, CA). Automated DNA sequencing of cDNAs was performed using standard methods. Except as described in the text, cDNA sequences confirmed the predictions of Genefinder, a program used by the C. elegans Genome Consortium to predict open reading frames. Amino acid sequence alignments were performed using the NCBI BLAST Program, and the Clustal W1.7 Multiple Sequence Alignment Program (Higgins et al., 1996), accessed through the Baylor College of Medicine Search Launcher (Houston, TX). The air-2 cDNA sequence and predicted protein product have been submitted under GenBank/EMBL/ DDBJ accession number AF071207.
AIR-2 Antibody Production
A peptide corresponding to the COOH-terminal 12 amino acids of the predicted AIR-2 protein was coupled to keyhole limpet hemocyanin (KLH) by standard methods and injected into rabbits. Rabbits were boosted for a period of 6 mo and final bleeds were collected. For affinity purification, the AIR-2 antigenic peptide was coupled to BSA and immobilized using an AminoLink Kit (Pierce, Rockford, IL) as described by the manufacturer. Using this immobilized AIR-2 peptide, AIR-2–specific antibodies were purified as described in the protocol of Walczak and Mitchison (1996).
Western Blot Analysis
Protein lysates of large populations of C. elegans embryos were prepared as follows: 10 100-mm Petri plates were seeded with OP-50 and N2 adult hermaphrodites which were allowed to reproduce at 20°C until the plates were confluent with gravid adult hermaphrodites. Adults were washed off the plates with distilled water, lightly pelleted, and then washed two times in distilled water. The animals were then resuspended and gently rocked for 3 min in a solution of 1 N NaOH and 10% bleach, gently pelleted, and then subjected to bleaching for another 3 min. Released embryos were gently pelleted and washed three times in distilled water. Washed embryos were resuspended in cold PBS + 1% NP-40 and briefly sonicated on ice (two sonications of 15 s each). Lysates were transferred to eppendorf tubes and spun in a microfuge at high speed for 10 min at 4°C to pellet insoluble material. Supernatants were collected and assayed for protein concentration using Bradford reagent (Bio-Rad Laboratories, Hercules, CA). Lysates were quick-frozen in a dry ice/ethanol bath and stored at −20°C.
100 μg of protein were boiled in SDS-PAGE loading buffer and loaded into each lane of a 10% NuPAGE polyacrylamide gel (Novex, San Diego, CA). The gel was run and blotted onto nitrocellulose according to the manufacturer's instructions. Blots were blocked in TBS (137 mM NaCl, 20 mM Tris, pH 8.0) + 2% BSA for 60 min at room temperature. They were then washed two times for 10 min in TBS + 5% Tween-20 (TBST). Affinity-purified primary antibody was diluted 1:200 in TBST. For peptide competition, the antigenic peptide was added to a final concentration of 100 μg/ml. Blots were incubated in primary antibody with and without competing peptide at 4°C overnight. Blots were washed three times for 15 min each in TBST at room temperature. A horseradish peroxidase-conjugated anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) was diluted 1:20,000 in TBST and incubated with the blots for 1 h at room temperature. Blots were washed three times for 15 min each in TBST at room temperature. Proteins were visualized using a chemiluminescence reagent kit (Amersham, Arlington Heights, IL). Blots were stripped in 2.2 M glycine, pH 4.0, 0.5 M NaCl for 60 min at room temperature with intermittent changes of the stripping buffer. Blots were then blocked and reprobed with an α-tubulin antibody (Sigma, St. Louis, MO), as a protein loading control.
Immunocytochemistry
Immunocytochemistry experiments and gonad dissections were performed on gravid N2 hermaphrodites, air-2 double-stranded RNA-injected hermaphrodites, and virgin or mated tra-2(g122gf) females. Single adult animals were placed in 15 μl of PBS on a subbed glass slide. For gonad dissections, animals were cut with two fine-gauge needles to release the gonad and embryos. A coverslip was then gently placed over the dissected gonad and the released embryos. For isolation of embryos alone, a coverslip was placed over an intact animal and gently tapped to release embryos from the uterus. For both gonad and embryo isolations, slides were placed on frozen metal blocks on dry ice for 25 min. Coverslips were removed and slides were immediately placed in −20°C methanol for 5 min. Slides were then transferred to 10% formaldehyde fix for 30 min and washed as described by Seydoux and Dunn (1997). α-Tubulin antibodies were purchased from Sigma, actin antibodies from ICN (Aurora, OH), and the nuclear pore antibody from Berkeley Antibody (Berkeley, CA). The NMY-2 and C. elegans MKLP-1 antibodies were obtained from K. Kemphues (Cornell University, Ithaca, NY) and S. Strome (University of Indiana, Bloomington, IN), respectively. Immunofluoresence microscopy was performed with a Nikon Microphot FXA (Tokyo, Japan) microscope. Images were processed using Adobe Photoshop (Adobe Systems, Mountain View, CA).
Video Microscopy
For video microscopy, single adult wild-type or air-2 double-stranded RNA-injected animals were placed in 15 μl of PBS on a painted glass slide. Animals were cut with two fine-gauge needles to release the gonad and embryos. The area surrounding the released embryos was then swabbed with vaseline to prevent the embryos being crushed by the coverslip that was gently placed over the dissected gonad and the released embryos. The development of wild-type and air-2(RNAi) embryos was recorded on videotape using Nomarski optics under low-light conditions.
Double-stranded RNA Injections
A pBluescript vector containing the entire air-2 cDNA was linearized by cutting with restriction endonucleases that were specific for sites within the multiple cloning site at either end of the cDNA. The DNA templates were phenol/chloroform extracted and ethanol precipitated. Sense and antisense RNAs corresponding to the entire coding region of the air-2 cDNA were synthesized from the appropriate DNA template using T7 and T3 in vitro transcription kits (Ambion, Austin, TX) according to the manufacturer's instructions. Equal volumes of each single-stranded RNA were mixed, placed at 65°C to denature the RNAs, and slow cooled at room temperature to anneal the complementary strands. L4 and adult N2 hermaphrodites were injected by standard methods (Mello et al., 1991) and were allowed to recover for 12–24 h before dissection for vidoerecording or fixation for immunocytochemistry.
Results
Cloning of an Aurora/Ipl1-related Kinase from C. elegans
We have identified two new members of the Aurora/Ipl1 protein kinase family, AIR-1 and AIR-2, in the C. elegans genome database. Further analysis of AIR-1 has been presented elsewhere (Schumacher et al., 1998). The air-2 genomic sequence was found on cosmid B0207 from chromosome I (B0207; GenBank/EMBL/DDBJ accession number U97196). B0207 maps to the right of dpy-5 and to the left of bli-4. An oligonucleotide primer corresponding to the SL1 trans-spliced leader RNA (found on the majority of C. elegans mRNAs) (Blumenthal, 1995), and a primer specific for the region surrounding the predicted translation stop codon of the air-2 sequence were used to RT-PCR amplify an air-2 cDNA from adult C. elegans hermaphrodite total RNA. Sequencing of the air-2 cDNA revealed that it lacked the first exon predicted by the C. elegans Genome Consortium, but otherwise reflected the predicted exon structure. An alignment of the predicted protein sequences for C. elegans AIR-2 and AIR-1, mammalian AIM-1 and IAK-1, Drosophila Aurora, and yeast Ipl1, showed that all six coding regions share a high degree of homology throughout the predicted kinase domain and only diverge significantly at their amino termini (Fig. 1).
Figure 1.

AIR-2 is member of a highly conserved family of protein kinases. An alignment of the predicted protein product sequences for C. elegans AIR-2 and AIR-1, mammalian AIM-1 and IAK-1, Drosophila Aurora, and budding yeast Ipl1 is shown. Black shading, identical residues; gray shading, similar residues; underline, predicted kinase domain. The AIR-2 kinase domain has the following amino acid identities and similarities with the kinase domains of (a) AIM-1: 63% identical, 76% similar; (b) IAK-1: 62% identical, 75% similar; (c) Aurora: 55% identical, 71% similar; (d) AIR-1: 51% identical, 69% similar; and (e) Ipl1: 50% identical, 70% similar. Nucleotide and predicted protein sequences for the air-2 cDNA have been submitted to GenBank/EMBL/DDBJ under accession number AF071207.
AIR-2 Is Associated with Meiotic Chromosomes
To assess the location of the AIR-2 protein during C. elegans gametogenesis and early embryogenesis, a rabbit polyclonal antiserum was raised against a synthetic peptide corresponding to the COOH-terminal 12 amino acids of AIR-2. Western blot analysis of total protein lysates from C. elegans embryos probed with affinity-purified AIR-2 antisera revealed a single protein of the predicted size of 34.7 kD (Fig. 2). Antibody recognition of this protein was entirely competed by addition of the antigenic peptide to the primary antibody incubation (Fig. 2).
Figure 2.
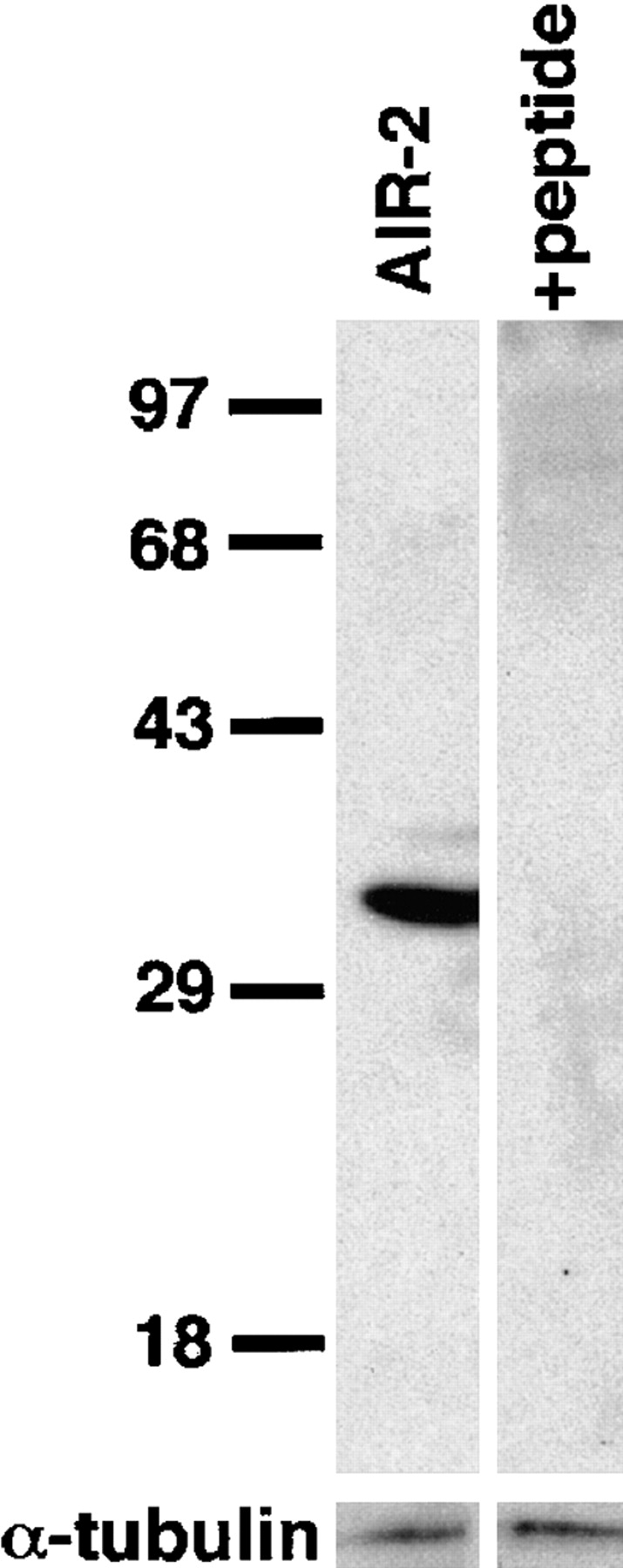
Specificity of the AIR-2 antibody. Western blots of protein lysates from C. elegans embryos were prepared as described in Materials and Methods. Affinity-purified AIR-2 antiserum specifically recognizes a protein of the predicted size of 34.7 kD. Antibody recognition of the 34.7-kD band is completely eliminated by addition of the antigenic peptide to the primary antibody incubation. α-Tubulin was used as a protein loading control.
Immunocytochemistry using affinity-purified AIR-2 antisera on fixed gonads dissected from gravid adult wild-type hermaphrodites revealed that the AIR-2 protein was specifically localized to meiotic chromosomes (Fig. 3). The C. elegans gonad can be divided into three separate regions: (a) the distal syncytial gonad, which is populated by mitotic germ cells, (b) the syncytial meiotic region, which is characterized by the presence of hundreds of germ nuclei arrested in pachytene of meiosis I, and (c) the proximal gonad (next to the spermatheca and uterus) where oocytes begin to cellularize around nuclei containing condensed meiotic chromosomes in diakinesis of prophase I (Schedl, 1997).
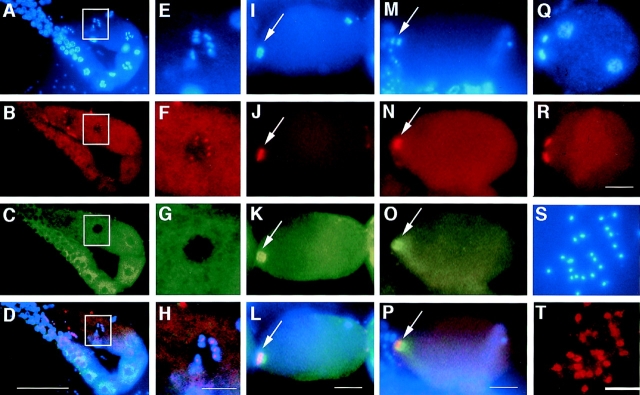
Figure 3.
AIR-2 is localized to meiotic chromosomes. Gonads were dissected from wild-type adult hermaphrodites, fixed, and then stained with DAPI (A, E, I, M, and Q), AIR-2 antisera (B, F, J, N, R, and T), and α-tubulin antisera (C, G, K, and O). Merged images are shown in D, H, L, and P. (A–D) AIR-2 is diffuse throughout the distal and proximal gonad, but is specifically localized to chromosomes in the oocyte most proximal to the spermatheca (white box). The spermatheca is to the left of the white box in A–D, but is out of the focal plane. (E–H) High magnification of the most proximal oocyte (boxed region in A–D). AIR-2 is specifically localized to meiotic chromosomes in the proximal oocyte. (I–L) Meiosis resumes after the oocyte is ovulated into the spermatheca and is fertilized. AIR-2 is associated with the chromosomes undergoing meiotic divisions (arrows). The sperm chromatin is visible at the posterior in I. Left, anterior. (M–P) AIR-2 is localized between the chromosomes during meiotic anaphase (arrows). Left, anterior. (Q and R) Polar bodies are extruded after each meiotic division at the anterior of the embryo. AIR-2 staining remains on the polar bodies throughout their presence during embryogenesis. AIR-2 staining is not associated with maternal or paternal pronuclear chromatin (the two nuclei within the embryo, Q). Left, anterior. (S and T) AIR-2 stains the mature sperm found in hermaphrodites but appears to be distinct from the chromatin. AIR-2 was also found in mature sperm from males (data not shown). Bars: (A–D) 50 μm; (E–H, I–L, M–P, Q, and R) 10 μm.
Diffuse AIR-2 staining was found in the syncytial distal gonad and the cellularized oocytes of the proximal gonad (Fig. 3, A–D). However, in the oocyte most proximal to the spermatheca, a striking localization of AIR-2 to meiotic chromosomes was found (Fig. 3, E–H). Maturation events that occur in this oocyte include the dissolution of the nucleolus, the asymmetric localization of the nucleus to the future anterior of the embryo (opposite from the spermatheca), and nuclear envelope breakdown, which occurs just before fertilization (McCarter et al., 1998). Both meiotic divisions occur at the anterior of the oocyte after it passes through the spermatheca and is fertilized at the posterior. The association of AIR-2 with meiotic chromosomes occurred before nuclear envelope breakdown, fertilization, and formation of the meiotic spindle (assessed by counterstaining with an antibody specific for the nuclear pore complex, 4′,6-diamidino-3-phenylindole dihydrochloride [DAPI], and α-tubulin antibodies) (Fig. 3, A–H and data not shown). Occasionally, we have also noted faint AIR-2 staining of chromosomes in the neighboring oocyte as well, suggesting that the localization of AIR-2 to meiotic chromosomes begins well before nuclear envelope breakdown and fertilization.
Anti–AIR-2 staining of meiotic chromosomes persisted throughout both meiotic divisions and remained on the extruded polar body chromatin throughout embryogenesis (Fig. 3, I–L, Q, and R). The staining of meiotic chromatin appeared to be localized to the interior face of the chromosomes (Fig. 3, I–L). Indeed, during both meiotic anaphases, AIR-2 staining was localized between the separating chromosomes (Fig. 3, M–P). AIR-2 immunostaining of fixed gonads dissected from C. elegans males revealed that AIR-2 was also present on meiotic chromosomes in spermatocytes (data not shown). AIR-2 staining was also found in mature sperm present in both males and hermaphrodites (Fig. 3, S and T). However, in this case staining did not appear to be chromatin associated. Instead, AIR-2 appeared to be present in the cytoplasm and/or on the sperm membrane. All of the staining patterns described above were entirely competed by preincubation of the primary antibody with the antigenic peptide (data not shown).
The Localization of AIR-2 to Meiotic Chromosomes Requires the Presence of Sperm in the Spermatheca
Given the striking localization of AIR-2 to chromosomes in the most mature oocyte residing next to the spermatheca, we determined whether this localization was dependent on the presence of sperm in the spermatheca. Gain-of-function (gf) mutations in tra-2 [tra-2(gf)] result in feminization of the hermaphrodite gonad (Doniach, 1986; Schedl and Kimble, 1988). tra-2(gf) females do not produce sperm, but their oocytes can still be fertilized with sperm introduced upon mating with males. In the absence of sperm, these oocytes will remain in diakinesis and are rarely ovulated. Dissected gonads from virgin and mated tra-2(gf) females were fixed and stained with the AIR-2 antibody. Although diffuse AIR-2 staining was seen in the prophase I–arrested oocytes of virgin tra-2(gf) females, no chromosomal staining was found in any of the oocytes, including the oocyte proximal to the empty spermatheca (>20 gonads were examined) (Fig. 4, A and B). We also noted a few tra-2(gf) oocytes that had been ovulated in the absence of sperm. AIR-2 was not present on the chromosomes of these ovulated oocytes (data not shown). In mated tra-2(gf) females, chromosomal staining of the proximal oocyte was indistinguishable from wild-type staining patterns (Fig. 4, C and D). AIR-2 staining in fertilized oocytes and embryos produced from these matings was also indistinguishable from wild-type controls (see below). Thus, like nuclear envelope breakdown and other hallmarks of oocyte maturation (McCarter et al., 1998), the localization of AIR-2 to meiotic chromosomes is dependent on the presence of sperm in the spermatheca.
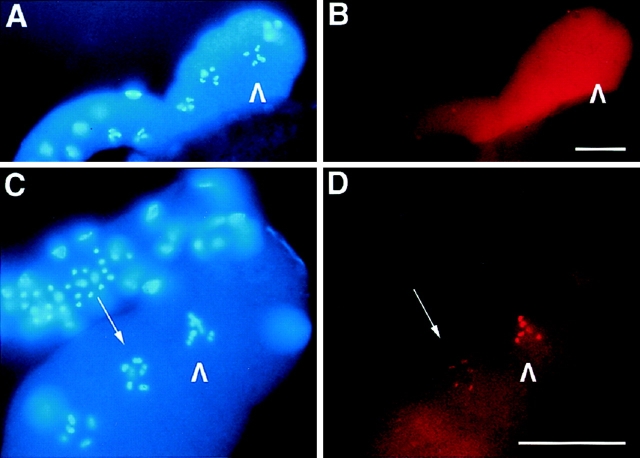
Figure 4.
The localization of AIR-2 to meiotic chromosomes requires the presence of sperm in the spermatheca. (A and B) Gonads dissected from virgin tra-2(gf) females were fixed and stained with DAPI (A) and AIR-2–specific antisera (B). AIR-2 staining is diffuse throughout the cellularized oocytes and does not localize to chromosomes in the proximal oocyte (arrowheads). (C and D) Gonads dissected from mated tra-2(gf) females were fixed and stained with DAPI (C) and AIR-2–specific antisera (D). AIR-2 strongly stains the chromosomes of the proximal oocyte (arrowheads) and fainter staining of chromosomes is also seen in a neighboring oocyte (arrow). Bars, 20 μm.
AIR-2 Is Associated with Metaphase Chromosomes and Midbody Microtubules in Embryos
Double immunostaining of fixed C. elegans embryos of various ages with AIR-2– and α-tubulin–specific antisera revealed that AIR-2 was associated with mitotic chromosomes. Anti–AIR-2 staining of chromatin was apparent just before and during metaphase (Fig. 5, A–D and data not shown). During anaphase and telophase, AIR-2 was no longer associated with chromatin, but became localized to the midbody microtubules (Fig. 5, E–H). After the completion of cytokinesis, AIR-2 persisted at the cytokinesis remnant (Fig. 5, I–L, arrowhead J and L). As above, all of the described staining patterns were entirely competed by preincubation of the primary antibody with the antigenic peptide (data not shown).
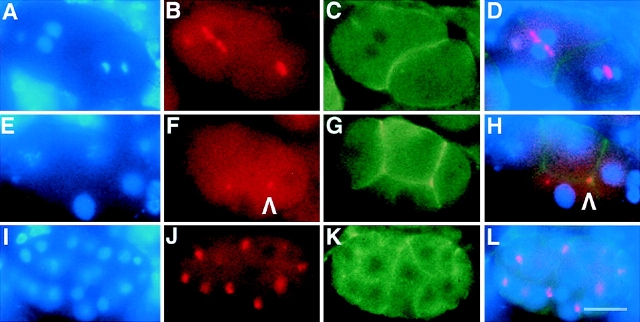
Figure 5.
AIR-2 is localized to metaphase chromosomes and midbody microtubules in C. elegans embryos. (A–L) Embryos were dissected from wild-type adult hermaphrodites, fixed, and then stained with DAPI (A, E, and I), AIR-2 (B, F, and J), and α-tubulin antibodies (C, G, and K). Merged images are shown in (D, H, and L). (A–D) AIR-2 is concentrated at the metaphase plate, and (E–H) moves to the midbody microtubules as anaphase progresses. (I–L) AIR-2 staining (arrowhead) persists at the cytokinesis remnant as seen in this four-cell embryo. The other remnant is out of the focal plane. Bar, 10 μm.
To confirm the various subcellular locations of AIR-2 during the cell cycle, fixed embryos of varying ages were coimmunostained with AIR-2– and actin-specific antibodies. Again, AIR-2 was present between the chromosomes in anaphase and telophase (Fig. 6, A–D), and was found as a small dot of staining on the cell membrane following cytokinesis (Fig. 6, E–H). All of the cell cycle–specific AIR-2 staining patterns described were found throughout the embryo and were not cell lineage-specific (Fig. 6, I–L, and data not shown). Given that the AIR-2 protein was present during gametogenesis and throughout embryogenesis, AIR-2 is likely to be both a maternally supplied and a zygotically expressed gene product.
Figure 6.
AIR-2 is retained at the cytokinesis remnant. (A– L) Embryos were dissected from wild-type adult hermaphrodites, fixed, and then stained with DAPI (A, E, and I), AIR-2 (B, F, and J), and actin antibodies (C, G, and K). Merged images are shown in (D, H, and L). (A–D) AIR-2 is concentrated between separating chromatin at anaphase. The AB cell in anterior (left) is in telophase, the P1 cell in posterior (right) is in anaphase. (E– H) AIR-2 staining persists on the cell membrane once cytokinesis is complete (arrowhead). The dot of staining that is not associated with the membrane is a polar body that is out of the focal plane. (I–L) AIR-2 appears to behave similarly throughout embryogenesis in all cell lineages. Bar, 10 μm.
Disruption of AIR-2 Expression by RNA-mediated Interference
We disrupted the function of AIR-2 during embryogenesis by the injection of double-stranded RNA (corresponding to the entire coding sequence of the air-2 cDNA) into the syncytial gonads of adult C. elegans hermaphrodites. Injection of antisense, sense, or double-stranded RNA into the gonads of wild-type hermaphrodites has been shown to result in gene-specific loss-of-function phenotypes in the embryos of injected mothers (Guo and Kemphues, 1996; Powell-Coffman et al., 1996; Fire et al., 1998). This method of disrupting protein expression is commonly referred to as RNA-mediated interference (RNAi) (Rocheleau et al., 1997). RNAi may act by depleting maternally-provided mRNA and may also prevent the expression of zygotic transcripts (Fire et al., 1998). Injection of C. elegans hermaphrodites with air-2 double-stranded RNA resulted in 100% embryonic lethality in the resultant brood (assessed 12 or more h after injection; n > 200 injected adults).
To further characterize air-2(RNAi) lethality, embryos dissected from injected mothers were fixed and stained with DAPI as well as antibodies specific for AIR-2 and α-tubulin. Immunofluorescent analysis revealed that each embryo consisted of an extremely polyploid single cell (Fig. 7 A). AIR-2 staining was absent (Fig. 7 B), and α-tubulin staining revealed either an interphase-like array of microtubules or numerous microtubule asters in each cell (Fig. 7 C). These results suggest that each air-2(RNAi) embryo has undergone multiple cycles of DNA replication and centrosome duplication without completing a single round of cytokinesis.
Figure 7.
air-2(RNAi) embryos execute multiple cell cycles in the absence of cytokinesis. (A–C) Embryos were dissected from hermaphrodites that had been injected with double-stranded RNA corresponding to the entire air-2 cDNA. air-2 (RNAi) embryos were fixed and stained with DAPI (A), AIR-2 (B), and α-tubulin antibodies (C). (A) air-2(RNAi) embryos accumulate DNA, (B) do not express a detectable level of AIR-2, and (C) contain multiple microtubule asters. The embryos appear to execute multiple cell cycles without completing cytokinesis. As revealed by tubulin staining (C), some of the embryos appear to be in interphase whereas others are in mitosis. Bar, 20 μm.
To analyze the progression of the air-2(RNAi) phenotype, embryos of varying ages were fixed and stained with DAPI and an antibody specific for α-tubulin (n > 200 embryos). In newly fertilized embryos, the meiotic spindle appeared to be normal and no obvious defects were observed (Fig. 8, A and B). However, external polar bodies were never found in the hundreds of air-2(RNAi) embryos we examined. Indeed, in pronuclear stage air-2(RNAi) embryos, we often observed maternal pronuclei that were clearly polyploid with respect to the paternal pronucleus (data not shown). In other cases, what appeared to be internal polar bodies remained separate from the zygotic chromatin and continued to replicate. Thus, although meiotic spindles were found in newly fertilized air-2(RNAi) embryos, polar bodies were not extruded but remained in the embryo where they either fused with the maternal pronucleus or remained separate from the zygotic chromatin.
Figure 8.

Polar bodies are not extruded in air-2(RNAi) embryos. (A–L) air-2(RNAi) embryos at various stages of development were dissected from injected mothers, fixed, and then stained with DAPI (left) and an antibody to α-tubulin (right). (A and B) An air-2(RNAi) embryo completing meiosis. (A) The maternal meiotic chromatin is apparent at the anterior (arrowhead), whereas the sperm nucleus can be seen at the posterior of the embryo. (B) By tubulin staining, a morphologically normal meiotic spindle is apparent surrounding the meiotic chromatin at the anterior of the embryo (arrowhead). (C and D) A one-cell air-2 (RNAi) embryo undergoing anaphase. (C) Note the chromatin bridges and hypercondensation of the chromatin. The second chromatin body appears to be a replicating unextruded polar body (arrowhead). (D) The spindle is morphologically normal. (E and F) An air-2(RNAi) embryo that has gone through multiple rounds of DNA replication and centrosome duplication without completing cytokinesis is shown. (E) The chromatin is condensed and (F) multiple microtubule asters are apparent. (G and H) air-2 (RNAi) embryos cycle through interphase. (G) Chromosomes are decondensed and (H) an “interphase array” of microtubules is observed. (I–L) Polar bodies in some air-2(RNAi) embryos proceed through the cell cycle asynchronously from the rest of the chromatin in the embryo (arrowheads). (I) This polar body appears to be in anaphase. (K) The polar body (arrowhead) has microtubule asters surrounding it whereas the nuclear chromatin appears to be interphase (L). Bar, 10 μm.
air-2(RNAi) embryos undergoing the first mitotic division had morphologically normal spindles, but were often found to have overly compact chromatin and sometimes displayed anaphase chromatin bridges (Fig. 8, C and D). Although temporally older embryos still remained as single cells, they had increased amounts of DNA and a number of microtubule asters that apparently reflected the age of each embryo and the number of cell cycles that had been initiated (Fig. 8, E–L). Unlike wild-type cells, the distribution of microtubule asters did not necessarily correspond to chromatin distribution. The chromatin was often found clumped in the middle, or at one end, of the embryos whereas the asters were found distributed throughout each embryo (Fig. 8, E–L). Microtubules appeared to cycle between interphase and mitosis in that asters were not always present and interphase-like arrays of microtubules were found in embryos with decondensed chromatin (Fig. 8, G and H).
Although karyokinesis was apparent in some of these embryos (Fig. 8, G and K), multiple cells, which would have arisen from cellular cytokinesis, were never found in any of the air-2(RNAi) embryos examined. To confirm that nuclear division occurred, we immunostained air-2 (RNAi) embryos with an antibody to the nuclear pore complex (Davis and Blobel, 1986). This staining revealed that nuclear membranes surround the decondensed chromatin found in interphase-like embryos, but is not found around the highly condensed chromatin present in cells with multiple asters (data not shown). Thus, nuclear divisions occur in the absence of AIR-2, and the nuclear envelopes present in these cells breakdown and reform in concert with the cell cycle–regulated behavior of the microtubules.
Except for the presumed polar bodies, each embryo was internally synchronous, with the chromatin and microtubules in each embryo proceeding through the cell cycle together (Fig. 8, G–L). However, the internal polar bodies were often asynchronous with respect to the rest of the chromatin and microtubules in each embryo (Fig. 8, I and K, arrowheads). Despite the independent cycling of the polar bodies, no subcellular compartment surrounding the polar bodies was apparent by actin or tubulin staining (data not shown and Fig. 8). Like the zygotic chromatin, nuclear envelopes were present around decondensed polar body chromatin but were not present around highly condensed polar body chromatin (as assessed by staining with the nuclear pore complex-specific antibody; data not shown).
air-2(RNAi) Embryos Are Defective in the Completion of Cytokinesis
To specifically address the cytokinesis defects seen in air-2 (RNAi) embryos, we stained wild-type and air-2(RNAi) embryos with antibodies specific for the cleavage furrow. Both actin and the C. elegans nonmuscle myosin protein NMY-2 localize to the cleavage furrow in wild-type cells (Fig. 9, A–H) (Guo and Kemphues, 1996). In air-2(RNAi) embryos, we have occasionally found staining indicative of cleavage furrow formation with both the NMY-2 and actin antibodies (Fig. 9, I–P). However, we have never seen multicellular air-2(RNAi) embryos. This suggests that although cleavage furrows form, they regress before the cell membrane is established. To confirm this, we videorecorded several wild-type and air-2(RNAi) embryos undergoing the first few cleavages. Whereas wild-type cleavage furrows persisted, those in air-2(RNAi) embryos formed transiently and then regressed, leaving a multinucleate or polyploid one cell embryo (data not shown). After the first cell cycle, cytokinesis furrows began to form and regress in very dynamic patterns indicative of the tri- and tetrapolar spindles found in air-2(RNAi) embryos by α-tubulin staining (data not shown and Fig. 10 G). Thus, the cytokinesis defects seen in air-2(RNAi) embryos do not arise from a failure in initiating a cleavage furrow, but instead appear to be due to a failure in the completion of or stabilization of the newly formed cell membrane.
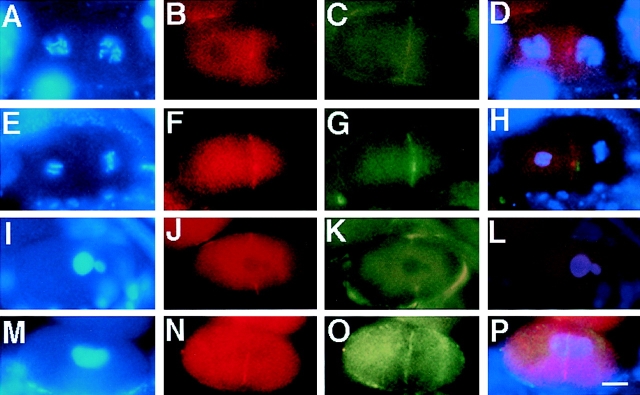
Figure 9.
The cleavage furrow is unstable in air-2 (RNAi) embryos. Wild-type and air-2(RNAi) embryos were dissected from adult hermaphrodites, fixed, and then stained with DAPI (A, E, I, and M), NMY-2 (B, F, J, and N), actin (C, G, and K), and α-tubulin antibodies (O). Merged images are shown in D, H, L, and P. (A–D) A wild-type two-cell embryo in prophase. (B) NMY-2 is faintly seen on the cell membrane and at the cytokinesis remnant. (C) Actin is apparent at the cell membrane. (D) Both proteins overlap at the cell membrane. (E–H) A wild-type two-cell embryo where the AB cell (left) is in anaphase and the P1 (right) is in metaphase. (F) NMY-2 and (G) actin colocalize at the cell membrane (H). (I–L) An air-2(RNAi) embryo undergoing the first cell cycle. (J) NMY-2 and (K) actin colocalize to an incomplete cleavage furrow (L). (M–P) An air-2(RNAi) embryo undergoing the first cell cycle. A transient cleavage furrow bisecting the cell is apparent by (N) NMY-2 and (O) α-tubulin staining that overlaps (P). Bar, 10 μm.
Figure 10.
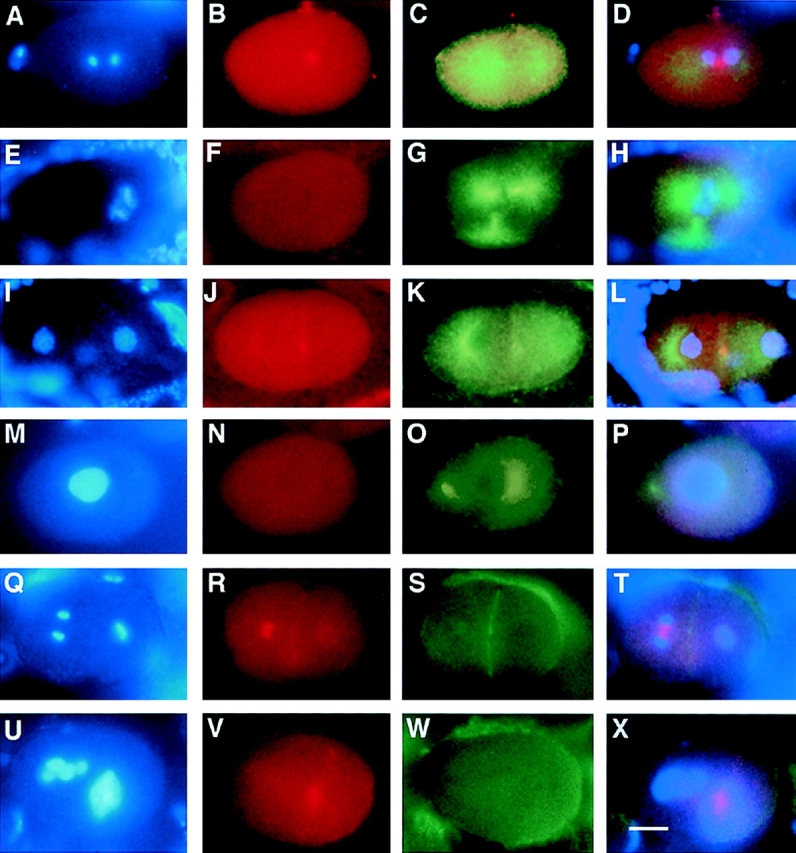
C. elegans MKLP-1/ZEN-4 protein does not localize properly in the absence of AIR-2. Wild-type and air-2(RNAi) embryos were dissected from adult hermaphrodites, fixed, and then stained with DAPI (A, E, I, M, Q, and U), MKLP-1 (B, F, J, N, R, and V), α-tubulin (C, G, K, and O) and actin antibodies (S and W). Merged images are shown in D, H, L, P, T, and X. (A–D) Wild-type one-cell embryo undergoing anaphase. (B) MKLP-1 localizes to the midbody microtubules. (C) Tubulin staining and (D) merged DAPI, MKLP-1, and tubulin. (E–H) air-2 (RNAi) embryo in metaphase/anaphase. (F) No MKLP-1 staining is apparent on the (G) tripolar spindle. (H) merged DAPI, MKLP-1, and tubulin. (I–L) Wild-type two-cell embryo in prophase. (J) MKLP-1 staining is localized to the midbody remnant on the cell membrane. (K) Tubulin staining, (L) merged DAPI, MKLP-1, and tubulin. (M–P) air-2(RNAi) embryo in prophase. (N) No MKLP-1 staining is found in this embryo. (O) Tubulin staining, (P) merged DAPI, MKLP-1, and tubulin staining. (Q–T) Wild-type two-cell embryo where the AB cell (left) is in anaphase, and the P1 cell (right) is in metaphase. (R) MKLP-1 staining is found between the separating chromosomes in the anaphase cell and is found at low levels on the cell membrane and surrounding the metaphase plate in the P1 cell. (S) Actin staining of the cell membrane, (T) merged DAPI, MKLP-1, and actin staining. (U– X) A multinucleate air-2(RNAi) embryo. (V) MKLP-1 staining is found in a “cloud” around the chromatin. (W) Actin staining reveals no cleavage furrows in this embryo, (X) merged DAPI, MKLP-1, and actin staining. Bar, 10 μm.
Since AIR-2 is a midbody-associated protein that is required for the completion of cytokinesis, we examined the localization of another C. elegans midbody-associated protein in air-2(RNAi) embryos. Like AIR-2, the C. elegans kinesin-like protein MKLP-1 (also known as ZEN-4) is localized to midbody microtubules and the cytokinesis remnant in wild-type cells (Fig. 10, A–D, I–L, Q–T) (Powers et al., 1998; Raich et al., 1998). Like AIR-2, it is also required for cytokinesis in C. elegans embryos (Powers et al., 1998; Raich et al., 1998). Staining of air-2(RNAi) embryos revealed either no staining of the mitotic spindle with the MKLP-1 antibody (Fig. 10, E–H and M–P) or very diffuse staining associated with chromatin (Fig. 10, U–X). Tightly localized MKLP-1 staining indicative of well-organized midbody microtubule bundles were never seen in air-2 (RNAi) embryos (>30 embryos). Thus, as reported for MKLP-1/ZEN-4, the cytokinesis defects seen in air-2 (RNAi) embryos is likely to be due to a failure in the proper organization or function of midbody microtubules (Powers et al., 1998; Raich et al., 1998).
Discussion
AIR-2 Is Required for the Completion of Cytokinesis
Here we have described AIR-2, a new member of the Aurora/Ipl1 family of protein kinases that is required for polar body extrusion and cytokinesis in C. elegans embryos. AIR-2 is initially localized to meiotic and mitotic chromosomes, but moves to the midbody microtubules during each division. Analysis of air-2(RNAi) embryos revealed that although AIR-2 is not required for the initiation of the cleavage furrow, it is required for its stabilization and the completion of cytokinesis. The presence of AIR-2 at the midbody appears to be necessary for the proper localization of at least one other midbody component, the kinesin-like protein MKLP-1/ZEN-4.
Two recent reports have shown that mklp-1(RNAi) and zen-4 genetic mutants phenocopy the polar body and cytokinesis defects we have found in air-2(RNAi) embryos (Powers et al., 1998; Raich et al., 1998). These reports suggest that C. elegans MKLP-1/ZEN-4 function is required at the midbody either for the proper bundling of midbody microtubules (Raich et al., 1998) or for the localization of midbody components that are necessary for the stabilization of the advancing cleavage furrow (Powers et al., 1998). Interestingly, AIR-2 is still well localized to midbody microtubules in mklp-1 (RNAi) embryos, however, the microtubule bundles do not appear to compact to a tight disc (Powers et al., 1998). Taken together with our localization data, it appears that AIR-2 kinase activity may be acting upstream of mklp-1 in a linear pathway to organize the microtubules at the midzone such that they can interact with and stabilize the advancing cleavage furrow.
AIR-2 Is a Chromosomal Passenger Protein
The dynamic cell cycle–regulated character of AIR-2 movement from chromosomes (kinetochores) to midbody microtubules is identical to the movement of a number of chromosomal passenger proteins (Earnshaw and Bernat, 1991; Earnshaw and Mackay, 1994; Mackay et al., 1998). Although the chromosomal passengers centromere-associated protein-E (CENP-E) (a kinesin-like protein) and inner centromere protein (INCENP) have both been shown to be essential for chromosome congression (Duesbery et al., 1997; Schaar et al., 1997; Mackay et al., 1998), recent studies have also implicated INCENP in cytokinesis (Eckley et al., 1997; Mackay et al., 1998). Cytokinesis defects are observed in tissue culture cells expressing a dominant-negative variant of INCENP that remains bound to chromosomes and does not move to microtubules (Mackay et al., 1998). Additional studies in cultured cells have shown that cleavage furrow activity is correlated with midzone microtubules and the distribution of the chromosomal passenger TD60 rather than with the position of the spindle poles (Wheatley and Wang, 1996). Consequently, the correct positioning of chromosomal passengers with respect to the midzone microtubules appears to be critical for the accurate execution of cytokinesis. Thus, our results support previous findings from other systems that suggest that congressed metaphase chromosomes, midbody microtubules, and the machinery required for cleavage furrow formation all communicate to ensure the proper temporal and spatial execution of cytokinesis during each cell division.
Cytokinesis and Phosphorylation
The molecular mechanisms underlying the movements and activities of chromosomal passenger proteins and other midbody components remain unknown. However, phosphorylation has a key role in regulating cytokinesis (as it does in other stages of the cell cycle). Phosphorylation of the regulatory light chain of myosin II by cyclin B-p34cdc2 negatively regulates cleavage furrow formation and entry into cytokinesis (Satterwhite et al., 1992). Additionally, prolonged activity of cyclin B-p34cdc2 by expression of a nondestructible form of cyclin B in tissue culture cells also results in the lack of midbody formation and a subsequent failure in cytokinesis (Wheatley et al., 1997). Treatment of sea urchin eggs with kinase and phosphatase inhibitors have opposite effects on cleavage furrow formation; kinase inhibitors block cleavage furrow formation whereas phosphatase inhibitors result in cytokinesis in the absence of nuclear division (Mabuchi et al., 1993). In C. elegans, the AIR-2 kinase may regulate the activity or localization of fellow chromosomal passenger proteins or other midbody components, like MKLP-1/ZEN-4, that are necessary for cytokinesis.
Meiotic AIR-2 Protein Localization Responds to a Sperm-dependent Signal
In meiotic cells, AIR-2 is localized to chromosomes just before nuclear envelope breakdown. These events precede ovulation and the formation of the meiotic spindle. We have demonstrated that the localization of AIR-2 to meiotic chromosomes requires the presence of sperm in the spermatheca. These data suggest that the AIR-2 protein is responding to a sperm-dependent signal originating from the spermatheca. This signal may be required to prepare the oocyte for fertilization and the completion of the meiotic divisions. Although the nature of this particular signal is unknown, communication between the oocytes of the proximal gonad and the spermatheca is necessary for ovulation (Clandinin et al., 1998). Whether AIR-2 localization requires these same signals is currently under investigation. Additional studies aimed at determining the mode of AIR-2 nuclear translocation in the proximal oocyte are also planned.
The association of AIR-2 with meiotic chromosomes appears to be an early event in meiotic maturation. However, in the absence of AIR-2, meiotic maturation proceeds normally and the meiotic spindle is formed. Although the meiotic divisions proceed, polar bodies are not extruded and remain in the embryo. Similar findings have been found in other cytokinesis-defective mutants in C. elegans, including cyk-1 and zen-4 (Swan et al., 1998; Raich et al., 1998). These results suggest that polar body extrusion and cytokinesis are mechanistically similar events that require the same components.
AIR-2 clearly associates with meiotic chromosomes in the proximal gonad and with meiotic and mitotic chromosomes in the fertilized embryo. However, AIR-2 is not associated with mitotic chromosomes in the distal gonad. The distal gonad contains hundreds of mitotic nuclei dividing in a syncytium that does not require cytokinesis. AIR-2 does not appear to be required for nuclear division as nuclear division still occurs in air-2(RNAi) embryos. In addition, AIR-2 is not localized to meiotic chromosomes in most of the cellularized oocytes of the proximal gonad and does not appear to be required for oocyte cellularization. Taken together, these observations suggest that the association of AIR-2 with chromosomes appears to precede, and only be necessary for, nuclear divisions that are followed by polar body extrusion or cytokinesis.
Different Aurora/Ipl1 Family Members Function at Distinct Stages of Mitosis
Although the exact mechanism and mode of action of the AIR-2 kinase during polar body extrusion and cytokinesis is not understood, our results support an essential role for AIR-2 in late mitotic events in C. elegans. Only one other Aurora/Ipl1-related kinase has been shown to have a role in late mitosis. The rat AIM-1 protein is specifically localized to midbody microtubules and overexpression of a dominant-negative AIM-1 protein in cultured rat cells results in cytokinesis defects (Terada et al., 1998). These results sharply contrast with the localization and function of the other Aurora/Ipl1 family members characterized to date. The mammalian proteins IAK and AIK, Xenopus Eg2, and C. elegans AIR-1 are all associated with mitotic centrosomes (Gopalan et al., 1997; Kimura et al., 1997; Roghi et al., 1998; Schumacher et al., 1998). Wheras mutants in Drosophila aurora appear to have defects in centrosome separation (Glover et al., 1995), loss-of-function of Xenopus Eg2 or C. elegans AIR-1 results in the formation of abnormal bipolar mitotic spindles (Roghi et al., 1998; Schumacher et al., 1998). These abnormal spindles appear to be due to a decrease in the ability of mitotic centrosomes to nucleate and/or stabilize spindle microtubules (Roghi et al., 1998; Schumacher et al., 1998). Mutations in yeast ipl1 also result in improper formation and function of the mitotic spindle (Francisco et al., 1994). Thus, with the exception of AIM-1 and AIR-2, the Aurora/Ipl1-related kinases that have been functionally characterized to date appear to function in the early events of mitosis, including centrosome separation and mitotic spindle formation.
Mammalian AIM-1 and C. elegans AIR-2 define a demarcation within the Aurora/Ipl1 kinase family that separates these mitotically active kinases into two groups, those required early in mitosis in the establishment of the mitotic spindle, and those required for late mitotic events and cytokinesis. However, this family is still very much unified in that all of the members appear to affect microtubule dynamics. Those that act early in mitosis appear to be involved in the transition from interphase microtubule arrays to the short dynamic microtubules characteristic of mitosis. They apparently do so by affecting the function of the centrosomes to separate and/or nucleate microtubules. Those acting late in mitosis appear to act in a pathway that stabilizes the midbody microtubules and their associated proteins, allowing a functional interaction between the midzone and the progressing cleavage furrow. Dissecting the function of these kinases in a genetically-tractable system such as C. elegans will continue to help elucidate the role of different Aurora/Ipl1 family members in the regulation of distinct aspects of karyokinesis and cytokinesis in multicellular organisms.
Acknowledgments
We thank the C. elegans Genome Sequencing Consortium for sequence information, T. Copeland (ABL-Basic Research Program, Frederick, MD) for synthesizing peptides and generating AIR-2 antisera, members of the extended Golden laboratory and D. Garfinkel (all from ABL-Basic Research Program) for critical reading of the manuscript, the Baltimore area C. elegans research community, P. Morgan (Case Western Reserve University, Cleveland, OH), B. Bowerman (University of Oregon, Eugene, OR), T. Schedl (Washington University, St. Louis, MO), and S. Strome (Indiana University, Bloomington, IN) for helpful discussions, and K. Kemphues (Cornell University, Ithaca, NY) and S. Strome for antibodies. We also thank the C. elegans Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources (NCRR), for the strains used in this study.
This work was supported by the National Cancer Institute, Department of Health and Human Services, under contract with ABL-Basic Research Program, NCI-FCRDC. J.M. Schumacher is a fellow of the Leukemia Society of America.
Abbreviations used in this paper
- AIR
Aurora/Ipl1 related protein
- DAPI
4′,6-diamidino-3-phenylindole dihydrochloride
- gf
gain-of-function
- KLH
keyhole limpet hemacyanin
- RNAi
RNA-mediated interference
References
- Ault JG, Rieder CL. Centrosome and kinetochore movement during mitosis. Curr Opin Cell Biol. 1994;6:41–49. doi: 10.1016/0955-0674(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Blangy A, Arnaud L, Nigg EA. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor HsEg5 to the dynactin subunit p150. J Biol Chem. 1997;272:19418–19424. doi: 10.1074/jbc.272.31.19418. [DOI] [PubMed] [Google Scholar]
- Blumenthal T. Trans-splicing and polycistronic transcription in Caenorhabditis elegans. . Trends Genet. 1995;11:132–136. doi: 10.1016/s0168-9525(00)89026-5. [DOI] [PubMed] [Google Scholar]
- Boleti H, Karsenti E, Vernos I. Xklp2, a novel Xenopuscentrosomal kinesin-like protein required for centrosome separation during mitosis. Cell. 1996;84:49–59. doi: 10.1016/s0092-8674(00)80992-7. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. . Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L, Rieder CL, Salmon ED. Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J Cell Sci. 1994;107:285–297. doi: 10.1242/jcs.107.1.285. [DOI] [PubMed] [Google Scholar]
- Chan CS, Botstein D. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics. 1993;135:677–691. doi: 10.1093/genetics/135.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, DeModena JA, Sternberg PW. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. . Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- Cottingham FR, Hoyt MA. Mitotic spindle positioning in Saccharomyces cerevisiaeis accomplished by antagonistically acting microtubule motor proteins. J Cell Biol. 1997;138:1041–1053. doi: 10.1083/jcb.138.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LI, Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986;45:699–709. doi: 10.1016/0092-8674(86)90784-1. [DOI] [PubMed] [Google Scholar]
- Doniach T. Activity of the sex-determining gene tra-2 is modulated to allow spermatogenesis in the C. eleganshermaphrodite. Genetics. 1986;114:53–76. doi: 10.1093/genetics/114.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Duesbery NS, Choi T, Brown KD, Wood KW, Resau J, Fukasawa K, Cleveland DW, Vande GF, Woude CENP-E is an essential kinetochore motor in maturing oocytes and is masked during mos-dependent, cell cycle arrest at metaphase II. Proc Natl Acad Sci USA. 1997;94:9165–9170. doi: 10.1073/pnas.94.17.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC, Bernat RL. Chromosomal passengers: toward an integrated view of mitosis. Chromosoma. 1991;100:139–146. doi: 10.1007/BF00337241. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Mackay AM. Role of nonhistone proteins in the chromosomal events of mitosis. FASEB (Fed Am Soc Exp Biol) J. 1994;8:947–956. doi: 10.1096/fasebj.8.12.8088460. [DOI] [PubMed] [Google Scholar]
- Eckley DM, Ainsztein AM, Mackay AM, Goldberg IG, Earnshaw WC. Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells. J Cell Biol. 1997;136:1169–1183. doi: 10.1083/jcb.136.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. . Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Francisco L, Wang W, Chan CS. Type 1 protein phosphatase acts in opposition to Ipl-1 protein kinase in regulating yeast chromosome segregation. Mol Cell Biol. 1994;14:4731–4740. doi: 10.1128/mcb.14.7.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli BG, De Souza CP, Tonks ID, Clark JM, Hayward NK, Ellem KA. Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J Cell Sci. 1996;109:1081–1093. doi: 10.1242/jcs.109.5.1081. [DOI] [PubMed] [Google Scholar]
- Gaglio T, Dionne MA, Compton DA. Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J Cell Biol. 1997;138:1055–1066. doi: 10.1083/jcb.138.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti MG, Bonaccorsi S, Williams B, Williams EV, Santolamazza C, Goldberg ML, Gatti M. Cooperative interactions between the central spindle and the contractile ring during Drosophilacytokinesis. Genes Dev. 1998;12:396–410. doi: 10.1101/gad.12.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. The mechanism and control of cytokinesis. Curr Opin Cell Biol. 1997;9:815–823. doi: 10.1016/s0955-0674(97)80082-8. [DOI] [PubMed] [Google Scholar]
- Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- Gopalan G, Chan CS, Donovan PJ. A novel mammalian, mitotic spindle-associated kinase is related to yeast and fly chromosome segregation regulators. J Cell Biol. 1997;138:643–656. doi: 10.1083/jcb.138.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalan G, Centanni J, Gilbert DJ, Copeland NG, Jenkins NA, Donovan PJ. Novel mammalian kinase related to yeast and fly chromosome segregation regulators is exclusively expressed in the germline. Mol Reprod Dev. 1999;52:18–28. [Google Scholar]
- Gradin HM, Larsson N, Marklund U, Gullberg M. Regulation of microtubule dynamics by extracellular signals: cAMP-dependent protein kinase switches off the activity of oncoprotein 18 in intact cells. J Cell Biol. 1998;140:131–141. doi: 10.1083/jcb.140.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Kemphues KJ. Molecular genetics of asymmetric cleavage in the early Caenorhabditis elegansembryo. Curr Opin Genet Dev. 1996;6:408–415. doi: 10.1016/s0959-437x(96)80061-x. [DOI] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Habermann A, Karsenti E, Hyman A. Spindle assembly in Xenopusegg extracts: respective roles of centrosomes and microtubule self-organization. J Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DG, Gibson TJ, Thompson JD. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 1996;266:383–402. doi: 10.1016/s0076-6879(96)66024-8. [DOI] [PubMed] [Google Scholar]
- Kimura M, Kotani S, Hattori T, Sumi N, Yoshioka T, Todokoro K, Okano Y. Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, AIK, related to Aurora of Drosophilaand yeast Ipl1. J Biol Chem. 1997;272:13766–13771. doi: 10.1074/jbc.272.21.13766. [DOI] [PubMed] [Google Scholar]
- Larsson N, Marklund U, Gradin HM, Brattsand G, Gullberg M. Control of microtubule dynamics by oncoprotein 18: dissection of the regulatory role of multisite phosphorylation during mitosis. Mol Cell Biol. 1997;17:5530–5539. doi: 10.1128/mcb.17.9.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi I. Regulation of cytokinesis in animal cells: possible involvement of protein phosphorylation. Biomed Res. 1993;14:155–159. [Google Scholar]
- Mackay AM, Ainsztein AM, Eckley DM, Earnshaw WC. A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J Cell Biol. 1998;140:991–1002. doi: 10.1083/jcb.140.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter, J., B. Bartlett, T. Dang, and T. Schedl. 1998. On the control of oocyte meiotic maturation and ovulation in C. elegans. Dev. Biol. In press. [DOI] [PubMed]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO (Eur Mol Biol Organ) J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, Cleveland DW. Pathways of spindle pole formation: different mechanisms, conserved components. J Cell Biol. 1997;138:953–963. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. At the heart of the budding yeast cell cycle. Trends Genet. 1996;12:405–412. doi: 10.1016/0168-9525(96)10041-x. [DOI] [PubMed] [Google Scholar]
- Nicklas RB, Krawitz LE, Ward SC. Odd chromosome movement and inaccurate chromosome distribution in mitosis and meiosis after treatment with protein kinase inhibitors. J Cell Sci. 1993;104:961–973. doi: 10.1242/jcs.104.4.961. [DOI] [PubMed] [Google Scholar]
- Nigg EA, Blangy A, Lane HA. Dynamic changes in nuclear architecture during mitosis: on the role of protein phosphorylation in spindle assembly and chromosome segregation. Exp Cell Res. 1996;229:174–180. doi: 10.1006/excr.1996.0356. [DOI] [PubMed] [Google Scholar]
- Niwa H, Abe K, Kunisada T, Yamamura K. Cell-cycle-dependent expression of the STK-1 gene encoding a novel murine putative protein kinase. Gene. 1996;169:197–201. doi: 10.1016/0378-1119(95)00809-8. [DOI] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Bulinski JC, Murofushi H, Aizawa H, Itoh TJ, Hotani H, Okumura E, Tachibana K, Kishimoto T. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J Cell Biol. 1995;128:849–862. doi: 10.1083/jcb.128.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Coffman JA, Knight J, Wood WB. Onset of C. elegansgastrulation is blocked by inhibition of embryonic transcription with an RNA polymerase antisense RNA. Dev Biol. 1996;178:472–483. doi: 10.1006/dbio.1996.0232. [DOI] [PubMed] [Google Scholar]
- Powers J, Bossinger O, Rose D, Strome S, Saxton W. A nematode kinesin required for cleavage furrow advancement. Curr Biol. 1998;8:1133–1136. doi: 10.1016/s0960-9822(98)70470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich WB, Moran AN, Rothman JH, Hardin J. Cytokinesis and midzone microtubule organization in Caenorhabditis elegansrequire the kinesin-like protein ZEN-4. Mol Biol Cell. 1998;9:2037–2049. doi: 10.1091/mbc.9.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzi L, Gersch MS, Campbell MS, Wu L, Osmani SA, Gorbsky GJ. MPM-2 antibody-reactive phosphorylations can be created in detergent-extracted cells by kinetochore-bound and soluble kinases. J Cell Sci. 1997;110:2013–2025. doi: 10.1242/jcs.110.17.2013. [DOI] [PubMed] [Google Scholar]
- Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegansembryos. Cell. 1997;90:707–716. doi: 10.1016/s0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- Roghi C, Giet R, Uzbekov R, Morin N, Chartrain I, Le Guellec R, Couturier A, Doree M, Philippe M, Prigent C. The Xenopusprotein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J Cell Sci. 1998;111:557–572. doi: 10.1242/jcs.111.5.557. [DOI] [PubMed] [Google Scholar]
- Satterwhite LL, Lohka MJ, Wilson KL, Scherson TY, Cisek LJ, Corden JL, Pollard TD. Phosphorylation of myosin-II regulatory light chain by cyclin-p34cdc2: a mechanism for the timing of cytokinesis. J Cell Biol. 1992;118:595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RD, Avides MC, Howard T, Gonzalez C, Glover DM. The Drosophilagene abnormal spindle encodes a novel microtubule-associated protein that associates with the polar regions of the mitotic spindle. J Cell Biol. 1997;137:881–890. doi: 10.1083/jcb.137.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- Schaar BT, Chan GK, Maddox P, Salmon ED, Yen TJ. CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl, T. 1997. Developmental genetics of the germ line. In C. elegans II. D. Riddle, T. Blumenthal, B. Meyer, and J.R. Priess, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 241–269. [PubMed]
- Schedl T, Kimble J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. . Genetics. 1988;119:43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher JM, Ashcroft N, Donovan PJ, Golden A. A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in C. elegansembryos. Development (Camb) 1998;125:4391–4402. doi: 10.1242/dev.125.22.4391. [DOI] [PubMed] [Google Scholar]
- Sen S, Zhou H, White RA. A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines. Oncogene. 1997;14:2195–2200. doi: 10.1038/sj.onc.1201065. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Dunn MA. Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. . Development (Camb) 1997;124:2191–2201. doi: 10.1242/dev.124.11.2191. [DOI] [PubMed] [Google Scholar]
- Sorger PK, Dobles M, Tournebize R, Hyman AA. Coupling of cell division and cell death to microtubule dynamics. Curr Opin Cell Biol. 1997;9:807–814. doi: 10.1016/s0955-0674(97)80081-6. [DOI] [PubMed] [Google Scholar]
- Stearns T. Motoring to the finish: kinesin and dynein work together to orient the yeast mitotic spindle. J Cell Biol. 1997;138:957–960. doi: 10.1083/jcb.138.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan KA, Severson AF, Carter JC, Martin PR, Schnabel H, Schnabel R, Bowerman B. cyk-1: a C. elegansFH gene required for a late step in embryonic cytokinesis. J Cell Sci. 1998;111:2017–2027. doi: 10.1242/jcs.111.14.2017. [DOI] [PubMed] [Google Scholar]
- Terada Y, Tatsuka M, Suzuki F, Yasuda Y, Fujita S, Otsu M. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO (Eur Mol Biol Organ) J. 1998;17:667–676. doi: 10.1093/emboj/17.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F, Labbe JC, Doree M, Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopuseggs. Nature. 1990;343:233–238. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ. Kinesin-related proteins at mitotic spindle poles: function and regulation. Cell. 1996;85:943–946. doi: 10.1016/s0092-8674(00)81295-7. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopuskinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- Waters JC, Salmon E. Pathways of spindle assembly. Curr Opin Cell Biol. 1997;9:37–43. doi: 10.1016/s0955-0674(97)80149-4. [DOI] [PubMed] [Google Scholar]
- Wheatley SP, Hinchcliffe EH, Glotzer M, Hyman AA, Sluder G, Wang Y. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J Cell Biol. 1997;138:385–393. doi: 10.1083/jcb.138.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley SP, Wang Y. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BC, Riedy MF, Williams EV, Gatti M, Goldberg ML. The Drosophilakinesin-like protein KLP3A is a midbody component required for central spindle assembly and initiation of cytokinesis. J Cell Biol. 1995;129:709–723. doi: 10.1083/jcb.129.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai A, Arama E, Kilfin G, Motro B. ayk1, a novel mammalian gene related to Drosophilaaurora centrosome separation kinase, is specifically expressed during meiosis. Oncogene. 1997;14:2943–2950. doi: 10.1038/sj.onc.1201144. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Kronebusch PJ, Simon PM, Borisy GG. Microtubule dynamics at the G2/M transition: abrupt breakdown of cytoplasmic microtubules at nuclear envelope breakdown and implications for spindle morphogenesis. J Cell Biol. 1996;135:201–214. doi: 10.1083/jcb.135.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Nicklas RB. The impact of chromosomes and centrosomes on spindle assembly as observed in living cells. J Cell Biol. 1995;129:1287–1300. doi: 10.1083/jcb.129.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]