Abstract
The α6β4 integrin promotes carcinoma in-vasion by its activation of a phosphoinositide 3-OH (PI3-K) signaling pathway (Shaw, L.M., I. Rabinovitz, H.H.-F. Wang, A. Toker, and A.M. Mercurio. Cell. 91: 949–960). We demonstrate here using MDA-MB-435 breast carcinoma cells that α6β4 stimulates chemotactic migration, a key component of invasion, but that it has no influence on haptotaxis. Stimulation of chemotaxis by α6β4 expression was observed in response to either lysophosphatidic acid (LPA) or fibroblast conditioned medium. Moreover, the LPA-dependent formation of lamellae in these cells is dependent upon α6β4 expression. Both lamellae formation and chemotactic migration are inhibited or “gated” by cAMP and our results reveal that a critical function of α6β4 is to suppress the intracellular cAMP concentration by increasing the activity of a rolipram-sensitive, cAMP-specific phosphodiesterase (PDE). This PDE activity is essential for lamellae formation, chemotactic migration and invasion based on data obtained with PDE inhibitors. Although PI3-K and cAMP-specific PDE activities are both required to promote lamellae formation and chemotactic migration, our data indicate that they are components of distinct signaling pathways. The essence of our findings is that α6β4 stimulates the chemotactic migration of carcinoma cells through its ability to influence key signaling events that underlie this critical component of carcinoma invasion.
Keywords: integrin, migration, cyclic AMP, phosphodiesterase, cytoskeleton
Carcinoma invasion is a complex process that involves directed migration and localized proteolysis (24). Although the mechanistic basis of invasion had been elusive, recent advances in molecular cell biology have facilitated a much more rigorous analysis of this important and critical component of cancer progression. In particular, insight into the function and regulation of cell adhesion receptors, as well as proteases, has fueled significant progress in our understanding of the invasive process. Studies aimed at defining specific signal transduction pathways that determine the behavior of invasive carcinoma cells are also contributing to an uncovering of the molecular basis of invasion.
Recent work by our group and others has implicated a key role for the α6β4 integrin in carcinoma invasion (3, 10, 32, 35, 40, 47). This integrin, which is a receptor for the laminins, is essential for the organization and maintenance of epithelial structure. In many epithelia, α6β4 mediates the formation of stable adhesive structures termed hemidesmosomes that link the intermediate filament cytoskeleton with the extracellular matrix (2, 12). The importance of this integrin in epithelial structure has been reinforced by the generation of β4-nullizygous mice that exhibit gross alterations in epithelial morphology and anchorage to the basement membrane (9, 46). In contrast to its function in normal epithelia, α6β4 can stimulate carcinoma migration and invasion through its ability to interact with the actin cytoskeleton and mediate the formation and stabilization of lamellae (32). This dynamic function of α6β4 in enhancing the migration of invasive carcinoma cells is quite distinct from its role in maintaining stable adhesive contacts in normal epithelia by associating with intermediate filaments. In fact, we have established that the ability of α6β4 to stimulate carcinoma migration and invasion depends upon its preferential activation of a phosphoinositide 3-OH kinase (PI3-K)1/Rac signaling pathway that we (40) and others (18) have shown is necessary for invasion. In essence, our studies have defined an integrin-mediated mechanism of carcinoma invasion that involves the stimulation of carcinoma migration by the dynamic association of α6β4 with F-actin and the activation of a specific signaling pathway by this integrin.
Although we have established the involvement of α6β4 in the migration of invasive carcinoma cells, the nature of this migration has not been well defined. Moreover, signaling pathways distinct from PI3-K/Rac that are also regulated by α6β4 are likely to contribute to carcinoma migration. For these reasons, we sought to examine the migration mediated by α6β4 in more detail and to identify other signaling pathways regulated by this integrin that contribute to migration. The results obtained indicate that α6β4 stimulates the chemotactic migration of invasive carcinoma cells but that it has no influence on their haptotactic migration. Importantly, we demonstrate that the ability of α6β4 to suppress the intracellular cAMP concentration ([cAMP]i) by activating a cAMP-specific phosphodiesterase (PDE) is essential for its enhancement of lamellae formation and chemotactic migration. Although PI3-K and cAMP-specific PDE activities are required for lamellae formation and chemotactic migration, we conclude that they are components of distinct signaling pathways.
Materials and Methods
Cell Culture and Antibodies
We used stable subclones of MDA-MB-435 human breast carcinoma cells that had been transfected with either the expression vector alone (mock transfectants), a full-length β4 cDNA (MDA/β4 transfectants), or a mutated β4 cDNA that lacked the entire cytoplasmic domain with the exception of four amino acids distal to the transmembrane sequence (MDA/β4-ΔCYT). The characterization of these transfectants has been described previously (38, 40). Both the β4 transfectants and the β4-ΔCYT transfectants expressed the α6β4 heterodimer on the cell surface as assessed by FACS® analysis and immunoprecipitation of surface-labeled extracts (40). The surface expression of α6β4 in these transfectants was comparable to the expression seen in other breast carcinoma cell lines that express this integrin endogenously such as MDA-MB-231 cells (Shaw, L.M., unpublished observation). All MDA-MB-435 cells were cultured in Dulbecco's modified Eagle's medium (DME) with 10% fetal calf serum plus 1% l-glutamine, 1% penicillin, and 1% streptomycin (GIBCO BRL, Gaithersburg, MD). Clone A cells, originally isolated from a human, poorly differentiated colon adenocarcinoma (7) and were cultured in RPMI 1640 medium containing 10% fetal calf serum plus 1% l-glutamine, 1% penicillin, and 1% streptomycin.
NIH-3T3 cells were cultured in DME containing 10% newborn calf serum plus 1% l-glutamine, 1% penicillin, and 1% streptomycin. NIH-3T3 conditioned medium was prepared from normal culture medium incubated with cells for 2 d before harvest with cellular debris removed by centrifugation.
The following function blocking, integrin-specific monoclonal antibodies (mAb) were used: mAb 13 (mouse anti-β1; S. Akiyama, National Institutes of Health, Research Triangle Park, NC), G0H3 (rat anti-α6; Immunotech, Westbrook, ME) and 2B7 (mouse anti-α6, prepared by our laboratory [39]). Non-specific mouse IgG was purchased from Sigma Chemical Co. (St. Louis, MO).
Migration and Invasion Assays
Cells were harvested using trypsin, rinsed three times with serum-free DME containing 250 μg/ml heat-inactivated BSA (DME/BSA), and then resuspended in DME/BSA. For migration assays, the lower surface of the membrane in each Transwell chamber (6.5-mm-diam, 8 μm pore size; Costar, Cambridge, MA) was coated for 30 min with either 15 μg/ml laminin-1 purified from Englebreth-Holm-Swarm tumor (19), 15 μg/ml collagen I (Vitrogen®; Collagen Biomaterials, Palo Alto, CA), or NIH-3T3 conditioned medium. For chemotaxis assays, either NIH-3T3 conditioned medium or lysophosphatidic acid (LPA) was added to the lower chamber. For haptotaxis assays, DME/BSA was added to the lower chamber. Cells (5 × 104) suspended in DME/BSA were added to the upper chamber. After incubating for 4 h at 37°C, nonmigrating cells were removed from the upper chamber with a cotton swab and cells that had migrated to the lower surface of the membrane were fixed with 100% methanol and stained with 0.2% (wt/vol) crystal violet in 2% ethanol. Migration was quantified by counting cells per square millimeter using bright-field optics. For antibody inhibition experiments, cells were incubated with 20 μg/ml of antibody for 30 min and then added to the Transwell chambers. The effects of pertussis toxin, IBMX (Calbiochem-Novabiochem, La Jolla, CA), forskolin, and rolipram (Sigma Chemical Co.) on migration were assessed by preincubating the cells with these reagents for 30 min before assay and including them in the assay medium at the concentrations noted in the figure legends.
Invasion assays were performed as described previously (40). In brief, 10 μg of Matrigel (Collaborative Research, Bedford, MA) was diluted with cold water and dried onto each Transwell filter. The Matrigel was reconstituted with DME for 1 h before its use in the assays. Cells were prepared as above and then added to the upper chamber of each well. NIH-3T3 conditioned medium was added to the lower chamber. Cells were allowed to invade for 4 h and then cells that had invaded were stained and quantified as described above.
cAMP Assays
Culture dishes (35-mm) were coated overnight with 20 μg/ml of collagen I in PBS and then blocked with serum-free RPMI containing 250 μg/ml BSA (RPMI/BSA). Cells (1.5 × 106) were then plated for 2 h and harvested by quickly removing the medium and extracting them directly with 80% (vol/vol) ethanol. Cell extracts were collected, cleared by centrifugation in a microcentrifuge for 10 min, dried in a SpeedVac (Savant Instruments, Farmingdale, NY) for 1.5 h, and then resuspended in 50 mM phosphate buffer, pH 6.2. The intracellular cAMP concentration was quantified using a cAMP enzyme-linked immunoabsorption assay (cAMP EIA; Cayman Biochemicals, Ann Arbor, MI) following the manufacturer's recommendation using nonacetylated cAMP as a standard and acetylcholine esterase-linked cAMP as a competitor. Values were corrected for cell number as determined from replicate plates. In some experiments, either 50 μM forskolin alone or forskolin plus 1 mM IBMX was added to cells 15 min before harvesting. To determine the cAMP content of cells under normal culture conditions, cells were plated in 35-mm dishes in DME plus 10% FCS, incubated for 18 h, and then processed as described above.
Phosphodiesterase Assays
cAMP PDE assays were performed according to the protocol of Sette et al. (37). In brief, cells were plated onto collagen I coated dishes as described for cAMP assays. Cells were then scraped from the dishes in a hypotonic lysis buffer (PDE lysis buffer: 20 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.2 mM EGTA, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, 4 μg/ml aprotinin, and 2 mM PMSF) and then sonicated. Cellular debris was removed by centrifugation and the supernatants were assayed immediately for PDE activity. PDE activity of cell extracts (2–4 μg protein) was assayed in cAMP PDE assay buffer (20 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 1.25 mM β-mercaptoethanol, 0.14 mg/ml BSA, 1 μM cAMP, and 0.2 μCi [3H] cAMP) for 10 min at 34°C. The reaction was stopped by adding 40 mM Tris, pH 7.5, containing 10 mM EDTA and heating for 2 min at 100°C. The reaction products were then digested with 50 μg Crotalus atrox snake venom (Sigma Chemical Co.) for 30 min at 34°C, separated from substrate using SpinZyme acidic alumina devices (Pierce Chemical Co., Rockford, IL), and quantified using a scintillation counter (Wallace, Gaithersburg, MD). Values were corrected for protein content and are reported as pmol cAMP hydrolyzed/min per milligram of protein. Protein content of cell extracts was determined using the Bio-Rad protein reagent (Hercules, CA) with BSA as a protein standard. Where noted, cells were incubated either for 30 min with 50 μM forskolin or 100 nM wortmannin or for 5 min with 100 nM LPA before harvest. For rolipram inhibition of PDE activity, 100 nM rolipram was added to cell extracts for 5 min before assaying for PDE activity.
Assessment of Lamellae Formation
Glass coverslips were coated overnight at 4°C with 20 μg/ml collagen I or laminin-1 and then blocked with BSA (0.25% in RPMI). The MDA-MB-435 transfectants were trypsinized and rinsed as described above, and then plated onto the coverslips for 2 h. As noted, cells were then treated with either 1 mM IBMX or 0.2% DMSO for 30 min. Subsequently, the cells were either treated with 100 nM LPA for 5 min or left untreated and then fixed for 10 min with 4% paraformaldehyde containing 10 mM Pipes, pH 6.8, 2 mM EGTA, 2 mM MgCl2, 7% sucrose, and 100mM KCl. The coverslips were rinsed three times with PBS and mounted in glycerol. For the analysis of clone A cells, the cells were treated with IBMX or DMSO for 30 min, plated on laminin-1–coated coverslips, incubated for 45 min at 37°C, and then fixed. Clone A cells were then rinsed three times with PBS and incubated with blocking solution containing 1% BSA/5% normal donkey serum for 30 min. Cells were incubated with 20 μg/ml TRITC-labeled phalloidin in blocking solution for 30 min. Cells were rinsed four times with PBS over 30 min and then mounted in glycerol containing 1× PBS, pH 8.5, and 0.1% propylgallate. All cells were imaged with a Nikon Diaphot 300 inverted microscope (Tokyo, Japan) using either Nomarski differential–interference contrast (DIC) or phase–contrast optics. Images were captured with a charge-coupled device camera (Dage-MTI, Michigan City, IN), a frame grabber (Scion, Frederick, MD) and a 7600 Power Macintosh computer (Apple Computer, Cupertino, CA). Images were analyzed and lamellar area quantified using IPLab Spectrum image analysis software (Signal Analytics, Vienna, VA) using the criteria for defining lamellae used previously by our group (32). Lamellae were defined as broad, flat cellular protrusions rich in F-actin and devoid of membrane-bound vesicles. The lamellar area of each cell was determined using both phase contrast optics and FITC-phalloidin staining.
Analysis of PDE Expression
To determine the relative expression of PDE in the cells used in this study, cell extracts (40 μg of protein) were resolved by SDS-PAGE (8%), transferred to nitrocellulose, and then immunoblotted with PDE4-specific antibodies provided by M. Conti (Stanford University, Stanford, CA) (15). Immune complexes were detected with horseradish peroxidase-conjugated secondary antibodies and visualized using SuperSignal chemiluminescent substrate (Pierce Chemical Co.).
Analysis of PI3-K Activation
The activation of PI3-K by the integrin α6β4 was assessed as described previously (40). In brief, cells were trypsinized and rinsed as above, resuspended in RPMI/BSA at a concentration of 2 × 106 cells/ml and incubated for 30 min with integrin-specific antibodies or in buffer alone. Either IBMX (1 mM), forskolin (50 μM) or DMSO (0.2%) was added for 10 min before plating the cells onto tissue culture dishes coated with goat anti–rat IgG Ab. After incubation for 30 min at 37°C in the presence of IBMX, forskolin, or DMSO, the cells were washed twice with cold PBS and solubilized at 4°C for 10 min with 20 mM Tris, pH 7.4, 137 mM NaCl, 1% NP-40, 10% glycerol, 1 mM sodium orthovanadate, 2 mM PMSF, and 5 μg/ml of aprotinin, pepstatin, and leupeptin. Equivalent amounts of protein from each extract were incubated for 3 h at 4°C with the antiphosphotyrosine mAb, 4G10 (Upstate Biotechnology, Lake Placid, NY) and protein A–Sepharose (Pharmacia Biotech, Piscataway, NJ). The Sepharose beads were washed twice with lysis buffer then twice with 10 mM Hepes, pH 7.0, and 0.1 mM EGTA. Beads were then resuspended with kinase buffer plus 100 μM ATP, 25 μM MgCl2, 10 μCi [γ-32P]ATP, and 10 μl sonicated brain lipids and incubated for 10 min at room temperature. The reaction was stopped using 60 μl 2N HCl and 160 μl chloroform/methanol (1:1). Lipids were resolved using potassium oxalate-coated thin layer chromatography plates.
Results
Expression of the α6β4 Integrin in MDA-MB-435 Cells Enhances Their Chemotactic Migration
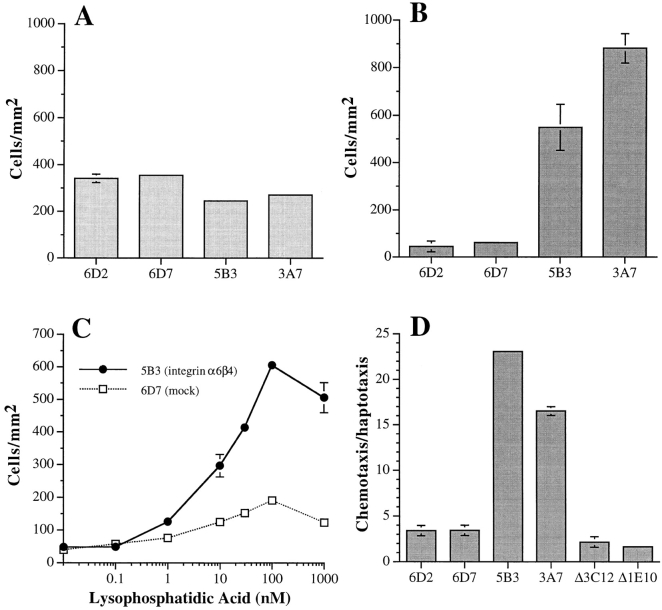
The possibility that expression of the α6β4 integrin influenced the rate of either haptotactic or chemotactic migration was assessed. For this purpose, stable transfectants of MDA-MB-435 cells were used that expressed either the α6β4 integrin (MDA/β4) or a deletion mutant of α6β4 (MDA/β4-ΔCYT) that retains only four amino acids of the β4 cytoplasmic domain, immediately proximal to the transmembrane domain (40). As shown in Fig. 1 A, subclones of the MDA/β4 transfectants (5B3 and 3A7) exhibited a rate of haptotactic migration toward laminin-1 that was slightly lower than the rate observed for subclones of the mock transfectants (6D7 and 6D2). In marked contrast, expression of α6β4 induced a substantial increase in the rate of chemotaxis of these cells towards conditioned medium from NIH-3T3 cells (Fig. 1 B). The rate of chemotaxis of the MDA/β4 transfectants (5B3 and 3A7) was 15– 20-fold greater than that of the mock transfectants (6D7 and 6D2) over a 4-h time period. These data indicate that expression of α6β4 potentiates chemotactic migration of MDA-MB-435 cells without substantially altering their rate of haptotaxis.
Figure 1.
Expression of the α6β4 integrin in MDA-MB-435 carcinoma cells stimulates chemotaxis but not haptotaxis. The migration of the MDA/β4 (5B3 and 3A7) MDA/β4-ΔCYT (Δ3C12, Δ1E10), and MDA/mock (6D2 and 6D7) transfectants toward laminin-1 (haptotaxis; A), 3T3 conditioned medium (chemotaxis; B), or LPA (chemotaxis; C and D) was assessed using a modified Boyden chamber. The lower surfaces of Transwell membranes were coated with either laminin-1 (A), conditioned medium (B), or collagen I (C and D), and then either BSA (A) 3T3 conditioned medium (B) or LPA (C and D) was added to the lower chambers. Cells (105 [A and B] or 5 × 104 [C and D]) were placed in the upper chambers. After 4 h at 37°C, cells that did not migrate were removed from the upper chamber with a cotton swab and cells on the opposite side of the membrane were fixed, stained, and quantified manually as described in the Materials and Methods. (A) Haptotaxis toward laminin-1; (B) chemotaxis toward NIH-3T3 conditioned medium; (C) dose response of MDA-MB-435 subclones 5B3 (β4 transfected; solid circles) and 6D7 (mock transfected; open squares) chemotaxis toward LPA; (D) Chemotaxis toward 100 nM LPA. Data are reported as fold increases over haptotactic migration on collagen I in the absence of LPA. Data (all panels) are shown as mean ± standard deviation from triplicate determinations.
To identify specific factors that could cooperate with α6β4 to promote chemotaxis of MDA-MB-435 cells, we tested several growth factors known to have chemotactic potential including epidermal growth factor, basic fibroblast growth factor, hepatocyte growth factor/scatter factor, insulin-like growth factor type I, transforming growth factor α and β, platelet-derived growth factor (AA and BB), somatostatin, thrombin, and LPA. Of these factors, only LPA was able to mimic the chemotactic effects of NIH-3T3 cell conditioned medium on the MDA-MB-435 transfectants (Fig. 1 C and data not shown). LPA stimulated the chemotaxis of MDA-MB-435 cells in a dose dependent manner with maximal stimulation observed at 100 nM. Of note, LPA stimulation of chemotaxis was five- to sevenfold greater in the MDA/β4 transfectants than in the mock transfectants. Subclones of the MDA/β4-ΔCYT transfectants (Δ3C12 and Δ1E10) exhibited a rate of chemotaxis that was similar to the mock transfectants (Fig. 1 D), indicating that the β4 cytoplasmic domain is critical for mediating the increased chemotaxis seen in the MDA/β4 transfectants.
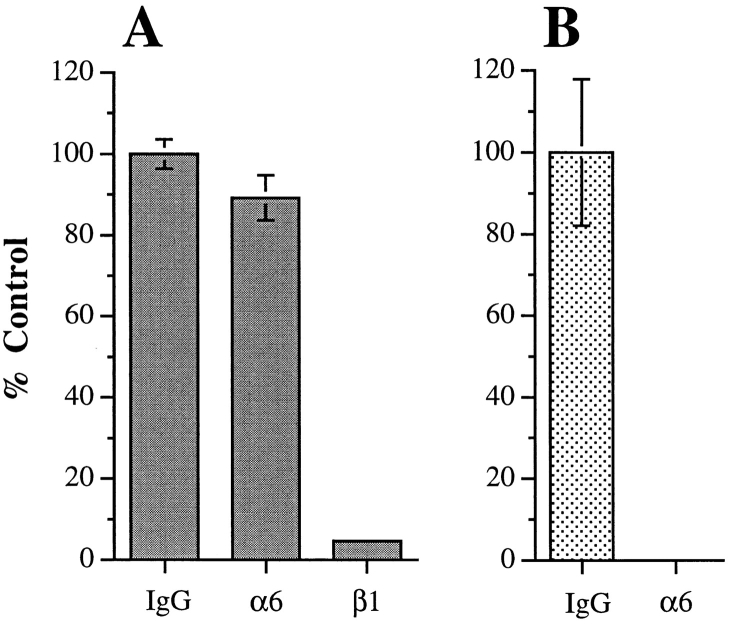
The increased chemotaxis observed for the MDA/β4 transfectants in response to LPA was evident on both collagen I (Fig. 1, C and D) and laminin-1 (data not shown), indicating that α6β4-enhanced migration is independent of the matrix protein used for traction. This possibility was examined further by preincubating the MDA/β4 transfectants with function-blocking mAbs before their use in the chemotaxis assays. As shown in Fig. 2 A, inhibition of α6 integrin function with the mAb 2B7 did not block the chemotaxis of the MDA/β4 transfectants on collagen I towards LPA. However, this mAb inhibited the haptotaxis of MDA-MB-435 cells toward a laminin-1 gradient (Fig. 2 B), a process that is dependent on the α6β1 integrin (38). Chemotaxis toward LPA was inhibited completely, however, by preincubating the cells with the β1 integrin-specific mAb 13 (Fig. 2 A). Collectively, these data indicate that the stimulation of chemotaxis by expression of α6β4 can be independent of the adhesive functions of α6β4, and that the adhesive interactions required for α6β4-enhanced chemotaxis on collagen I are mediated through β1 integrins.
Figure 2.
Inhibition of α6β4-stimulated migration by integrin-specific antibodies. MDA/β4 (5B3; A, gray bars) or mock transfectants (6D7; B, stippled bars) were incubated with the indicated function blocking mAbs for 30 min before their use in a chemotaxis assay using 100 nM LPA on collagen I (A) or a haptotaxis assay on laminin-1–coated wells (B) as described in Fig. 1. Nonspecific mouse IgG was used as a negative control. Data are reported as the percentage of migration observed for the IgG control ± standard deviation from triplicate determinations.
Expression of the α6β4 Integrin Is Required for the Formation of Lamellae in Response to LPA
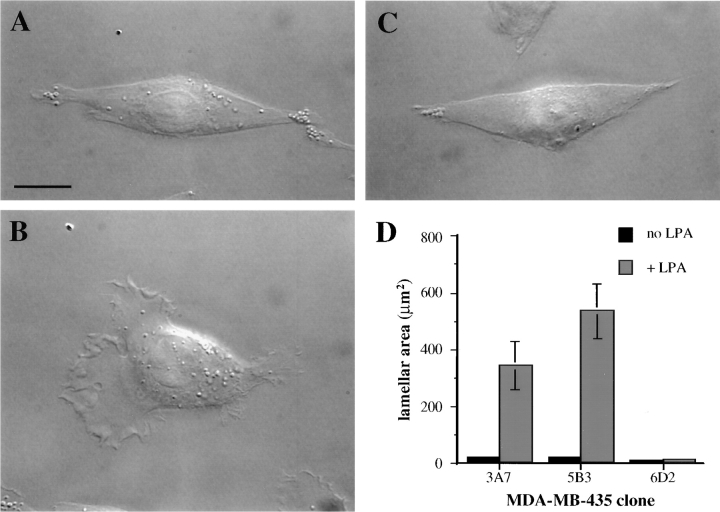
Chemotactic migration frequently involves the formation of broad sheets of polymerized actin at the leading edge of the cell termed lamellae (27). To determine if expression of the α6β4 integrin influenced the formation of such motile structures, we analyzed the morphology of the MDA-MB-435 transfectants plated on collagen I (Fig. 3). Prominent lamellae were not evident in the mock transfectants and treatment with 100 nM LPA did not stimulate a significant increase in lamellar area (Fig. 3, C and D). The MDA/β4 transfectants exhibited a similar morphology to that of the mock transfectants when plated on collagen I (Fig. 3, compare A with C) or laminin-1 (data not shown). Within minutes after LPA treatment, however, the MDA/ β4 transfectants formed large, ruffling lamellae (Fig. 3 C). Quantification of these cells by digital image analysis indicated that LPA stimulated a dramatic increase in the lamellar area of the two subclones of the MDA/β4 transfectants (Fig. 3 D). In contrast, no increase in the lamellar area of the mock transfectants in response to LPA was detected by this analysis (Fig. 3 D).
Figure 3.
The α6β4 integrin is required for the LPA-dependent formation of lamellae in MDA-MB-435 cells. MDA/β4 (A and B) and mock transfectants (C) were plated onto coverslips that had been coated with 20 μg/ ml collagen I. Cells were allowed to adhere for 2 h at 37°C and then treated with LPA for 5 min. (B and C) or left untreated (A). The cells were visualized using Nomarski DIC optics. Note the large lamellae that are formed in response to LPA stimulation of the MDA/β4 transfectants. (D) The effect of LPA on lamellar area was quantified using IPLab Spectrum imaging software. Data are shown as mean lamellar area ± standard error in which n > 20. Bar, 10 μm.
Pharmacological Evidence for the Involvement of cAMP in Chemotaxis
LPA is a bioactive phospholipid that can mediate its effects on cells through a receptor linked to heterotrimeric G proteins, including inhibitory type G (Gi) proteins (29). To assess the possible involvement of a Gi protein in α6β4-enhanced chemotaxis, we used pertussis toxin, which inactivates heterotrimeric Gi-proteins by ADP ribosylation (31). The LPA-stimulated chemotaxis of both the MDA/β4 and mock transfectants was inhibited by pertussis toxin with maximal inhibition observed at 100 ng/ml (data not shown). These data suggested that the α6β4 integrin enhances chemotaxis that is mediated through pertussis toxin-sensitive, Gi-linked receptors. Gi proteins are known to inhibit certain classes of adenyl cyclases and thus limit cAMP production (45). For this reason, we analyzed the impact of stimulating cAMP production on chemotaxis using forskolin. Although forskolin inhibited LPA-stimulated chemotaxis, the MDA/β4 and mock transfectants differed significantly in their response to this activator of adenyl cyclases. LPA-stimulated chemotaxis of the mock transfectants was inhibited to basal levels by 50 μM forskolin (Fig. 4 A). At this concentration of forskolin, the inhibition of chemotaxis of the MDA/β4 transfectants was only 50% and higher concentrations of forskolin (100 μM) did not abrogate chemotaxis of these cells (Fig. 4 A). Interestingly, treatment of the MDA/β4 or mock transfectants with forskolin did not inhibit haptotactic migration on laminin-1 (Fig. 4 B). These data indicate that a cAMP-sensitive pathway plays a key role in LPA-stimulated chemotaxis of MDA-MB-435 cells and they suggest that the α6β4 integrin may regulate this pathway.
Figure 4.
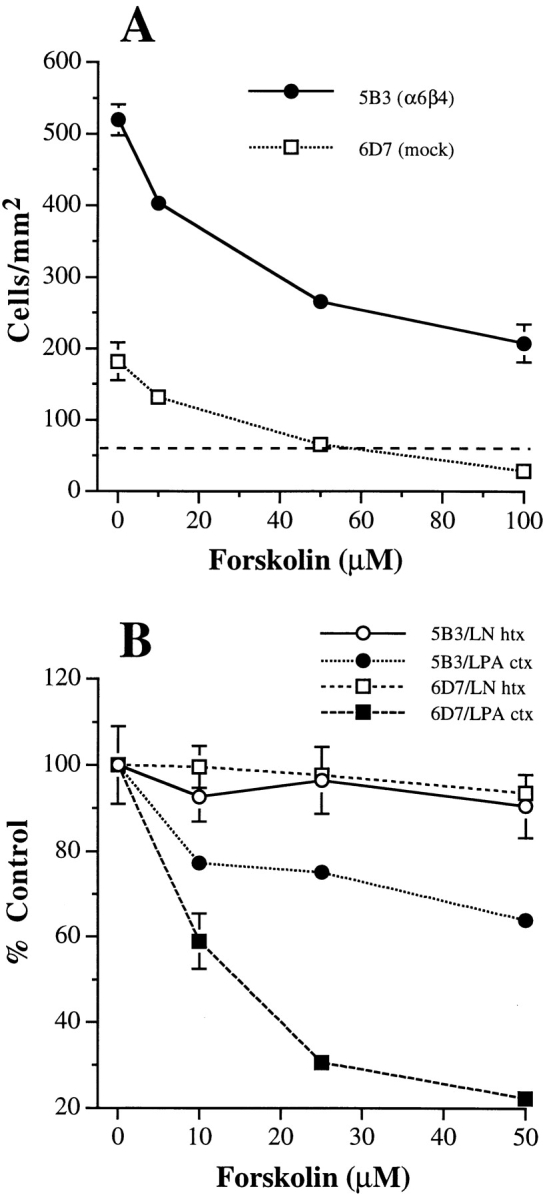
Forskolin stimulation of adenyl cyclase inhibits LPA-mediated chemotaxis differentially in the MDA/β4 and mock transfectants. (A) MDA/β4 transfectants (5B3; solid circles) or mock transfectants (6D7; open squares) were treated with the indicated concentration of forskolin for 30 min before their addition to the upper wells of the Transwell chambers. Cells were assayed for LPA-mediated chemotaxis on collagen I as described in Fig. 1. The dashed line depicts the basal level of migration of both subclones in the absence of LPA. (B) In a separate experiment, the same cells were treated with forskolin for 30 min before assaying for LPA chemotaxis (solid symbols) or laminin haptotaxis (open symbols). Data are reported as the percent migration of cells not treated with forskolin ± standard deviation of triplicate determinations.
Expression of the α6β4 Integrin in MDA-MB-435 Cells Influences cAMP Metabolism
To determine if α6β4 expression influences the [cAMP]i, the [cAMP]i was determined in extracts obtained from subconfluent cultures of MDA/mock, β4, and β4-ΔCYT transfectants using a cAMP enzyme-linked immunoabsorption assay. As shown in Fig. 5 A, the MDA/β4 transfectants had a 30% lower [cAMP]i (2.7 pmol cAMP per 106 cells) than either the mock (3.7 pmol cAMP per 106 cells) or β4-ΔCYT transfectants (3.8 pmol cAMP per 106 cells). This difference was statistically significant (P < 0.001). Of note, neither clustering of α6β4 using the 2B7 mAb and an appropriate secondary Ab nor LPA treatment reduced cAMP levels further (data not shown).
Figure 5.
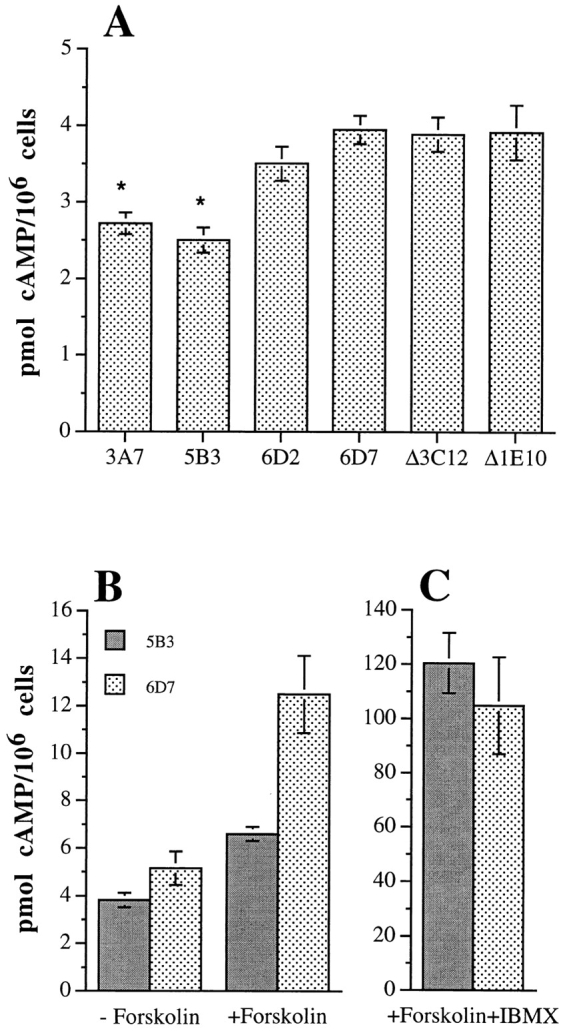
Intracellular cAMP content of the MDA-MB-435 transfectants. The MDA/β4 (3A7, 5B3), MDA/β4-ΔCYT (Δ3C12, Δ1E10) and MDA/mock transfectants (6D2, 6D7) were plated in DME containing 10% FCS. After 18 h, cells were harvested and cAMP content was measured using a cAMP EIA protocol as described in Materials and Methods. Data shown represent the mean of 10 sample determinations ± standard error. The difference in the [cAMP]i between the MDA/β4 and the mock transfectants is significant (P < 0.001; asterisk), but the difference between the mock and the β4-ΔCYT transfectants is not significant (P = 0.2). (B and C) Differential effects of forskolin stimulation on the [cAMP]i in the MDA/β4 and mock transfectants. The [cAMP]i was assayed in the 5B3 (solid bars) and 6D7 (stippled bars) clones plated on collagen I and treated for 15 min with either 50 μM forskolin (B) or forskolin and 1 mM IBMX (C). Note that the MDA/β4 transfectants (5B3) are more resistant to a forskolin-stimulated increase in [cAMP]i than the mock transfectants (6D7). The inhibition of PDE activity with IBMX shown in C reveals that α6β4 expression results in an increase in PDE activity and not a decrease in cAMP synthesis. Data shown are the mean values ± standard error obtained from multiple experiments.
The observation that the MDA/β4 transfectants were more resistant to forskolin inhibition of chemotaxis than the mock transfectants (Fig. 4) suggested that these two populations of cells differ in their ability to metabolize the cAMP generated in response to forskolin stimulation. This possibility was examined by determining the [cAMP]i in forskolin-treated cells. As shown in Fig. 5 B, the MDA/β4 transfectants exhibited a 30% lower [cAMP]i than the mock transfectants when plated on collagen I. With forskolin stimulation, a 2.5-fold greater accumulation of cAMP was observed in the mock transfectants (6.6 pmol per 106 cells) compared with the β4 transfectants (2.6 pmol per 106 cells). When the forskolin-treated cells were also treated with the PDE inhibitor, IBMX, to prevent breakdown of cAMP, the MDA/β4 transfectants exhibited a [cAMP]i comparable to the mock transfectants (120 ± 11 versus 104 ± 18 pmol per 106 cells, respectively; Fig. 5 C). Together, these data suggest that expression of α6β4 integrin suppresses the [cAMP]i by increasing PDE activity.
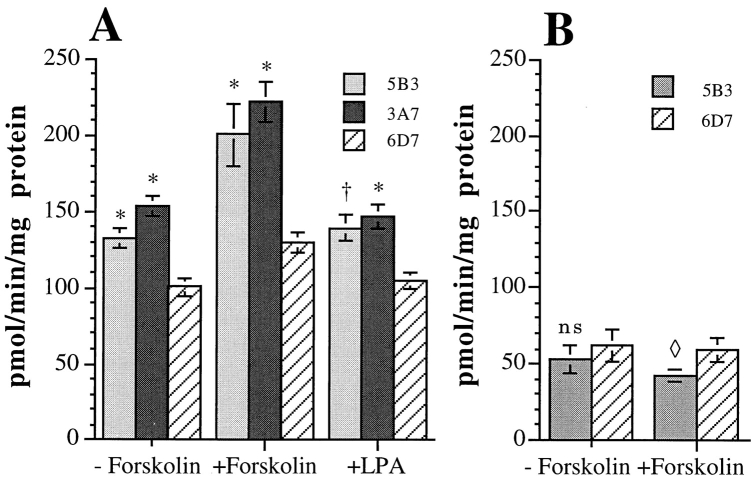
To establish more directly that expression of the α6β4 integrin can regulate cAMP-dependent PDE activity, the activity of this enzyme was assayed in cell extracts obtained from the MDA/mock and β4 transfectants. As shown in Fig. 6 A, the MDA/β4 transfectants exhibited a significantly higher rate of PDE activity than the mock transfected cells. Moreover, the PDE activity of the MDA/ β4 transfectants was markedly increased (51% for 5B3 and 45% for 3A7) in response to forskolin stimulation compared with the mock transfectants (29% for 6D7; Fig. 6 A). The difference in PDE activity between the MDA/β4 and mock transfectants was eliminated by rolipram, a type IV PDE-specific (PDE 4) inhibitor (Fig. 6 B). These data indicate that a cAMP-dependent PDE 4 activity is influenced by α6β4 expression in MDA-MB-435 cells. Also, this activity is likely responsible for the observed decrease in [cAMP]i and the resistance to forskolin-mediated inhibition of LPA chemotaxis observed in the MDA/β4 transfectants.
Figure 6.
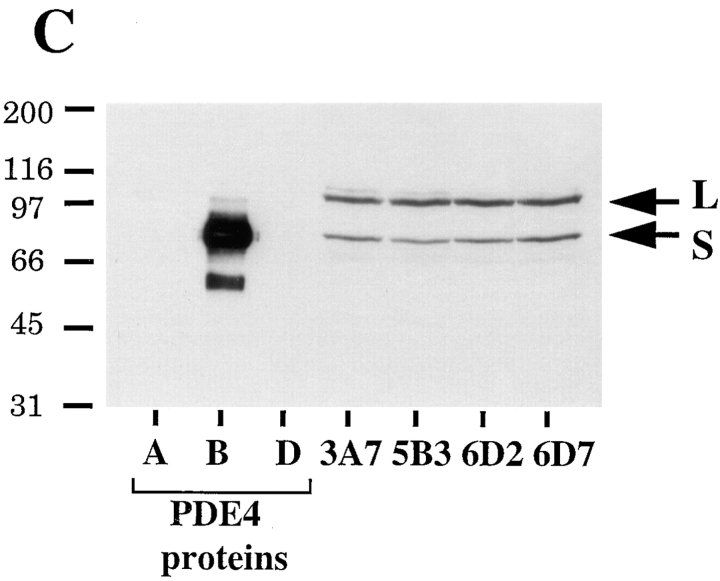
Assay of cAMP-specific PDE activity. (A) MDA/β4 (3A7 and 5B3) or mock transfectants (6D7) plated on collagen I were treated with 50 μM forskolin or 100 nM LPA as noted. Cells were harvested and the cytosolic fraction was assayed for PDE activity as described in Materials and Methods. The PDE activity of the MDA/β4 transfectants was compared with the MDA/mock transfectants for statistical significance: *, P < 0.002; †, P < 0.01. (B) Extracts from cells treated as in A were incubated with 100 μM rolipram before assaying for PDE activity to determine how much of the activity in A constitutes cAMP-specific PDE (PDE 4). Data shown are mean ± standard error of four separate determinations (A and B). ns, not significant; ⋄, P = 0.02. (C) Relative expression of PDE 4B in the MDA-MB-435 transfectants. Extracts (40 μg protein) obtained from the MDA/β4 (3A7 and 5B3) and mock (6D2 and 6D7) transfectants, as well as purified PDE 4 proteins (short form of variants A, B, and D; 10 ng each; provided by M. Conti) were resolved by SDS-PAGE and immunoblotted with a PDE 4B-specific Ab. Arrows, long and short forms of PDE 4B.
To examine the possibility that the MDA/β4 and mock transfectants differed in their level of PDE expression, we assessed PDE 4 expression in these cells using antibodies specific for the various PDE 4 variants (15). The predominant PDE 4 variant expressed in MDA-MB-435 cells is PDE 4B based on results obtained with antibodies specific for PDE 4A, 4B and 4D (data not shown). Importantly, the expression of PDE 4B did not differ significantly between the MDA/β4 and mock transfectants (Fig. 6 C). These data indicate that the increased PDE activity observed in the MDA/β4 transfectants is not the result of increased PDE expression.
PDE Activity Is Necessary for Chemotaxis, Invasion, and Lamellae Formation
The importance of PDE for chemotactic migration was examined by treating the MDA/mock and β4 transfectants with IBMX before their use in the chemotaxis assay. As shown in Fig. 7 A, IBMX inhibited LPA-stimulated chemotaxis with maximal inhibition observed at 1 mM. Similar results were obtained with the cAMP-specific PDE inhibitor, rolipram (data not shown). We also examined the involvement of PDE in carcinoma invasion by treating cells with IBMX before their use in a standard Matrigel invasion assay. A substantial inhibition of invasion was observed in the presence of IBMX in comparison to the solvent control (Fig. 7 B).
Figure 7.
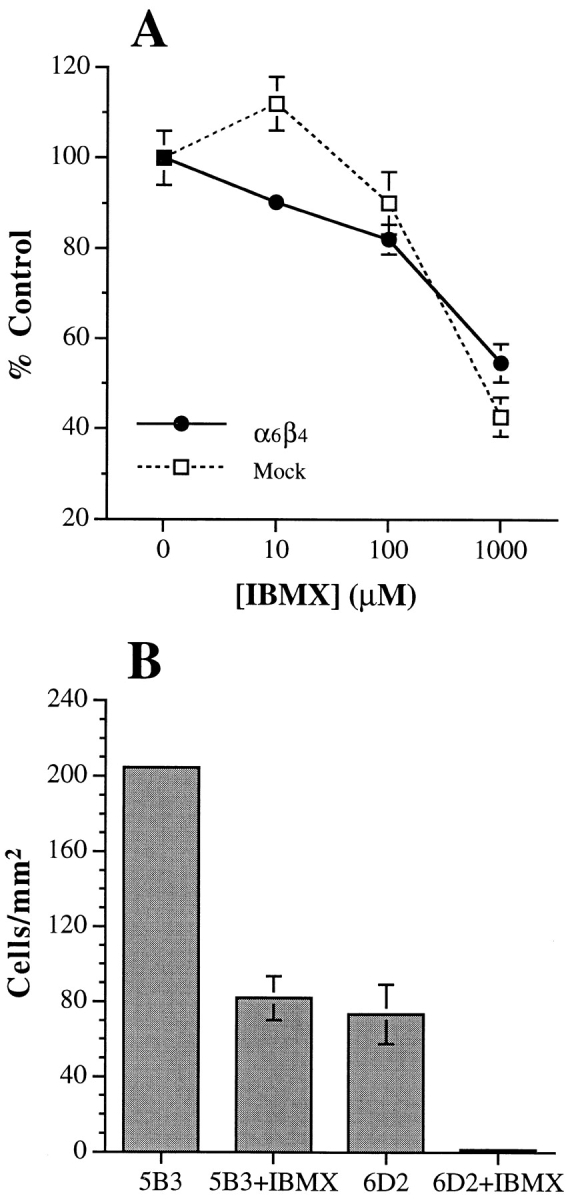
cAMP specific-PDE activity is required for the chemotactic migration and invasion of MDA-MB-435 cells. The MDA/ β4 (5B3; squares) or mock transfectants (6D7; circles) were treated with varying concentrations (A) or 1 mM (B) IBMX for 30 min before their use in either an LPA chemotaxis assay (A) or a Matrigel chemoinvasion assay (B). Data shown represent mean values ± standard deviation of triplicate determinations.
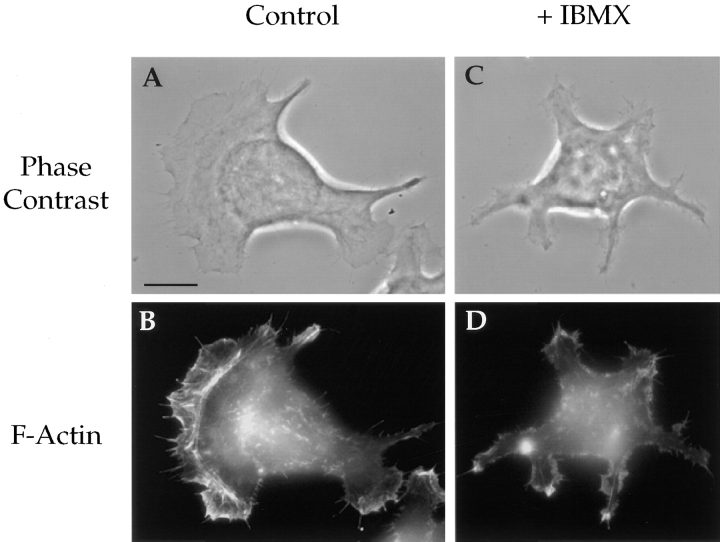
The necessity of cAMP-specific PDE activity in the formation of lamellae was also assessed. IBMX had no effect on the morphology of the MDA/β4 transfectants in the absence of LPA (Fig. 8, compare A with B). However, IBMX-treated cells were unable to form the large, ruffling lamellae in response to LPA stimulation in comparison to untreated cells (Fig. 8, compare C with D). Quantitative analysis of these cell populations revealed that inhibition of PDE activity resulted in an approximate fourfold reduction in the lamellar area of LPA-stimulated MDA/β4 transfectants (Fig. 8 E).
Figure 8.
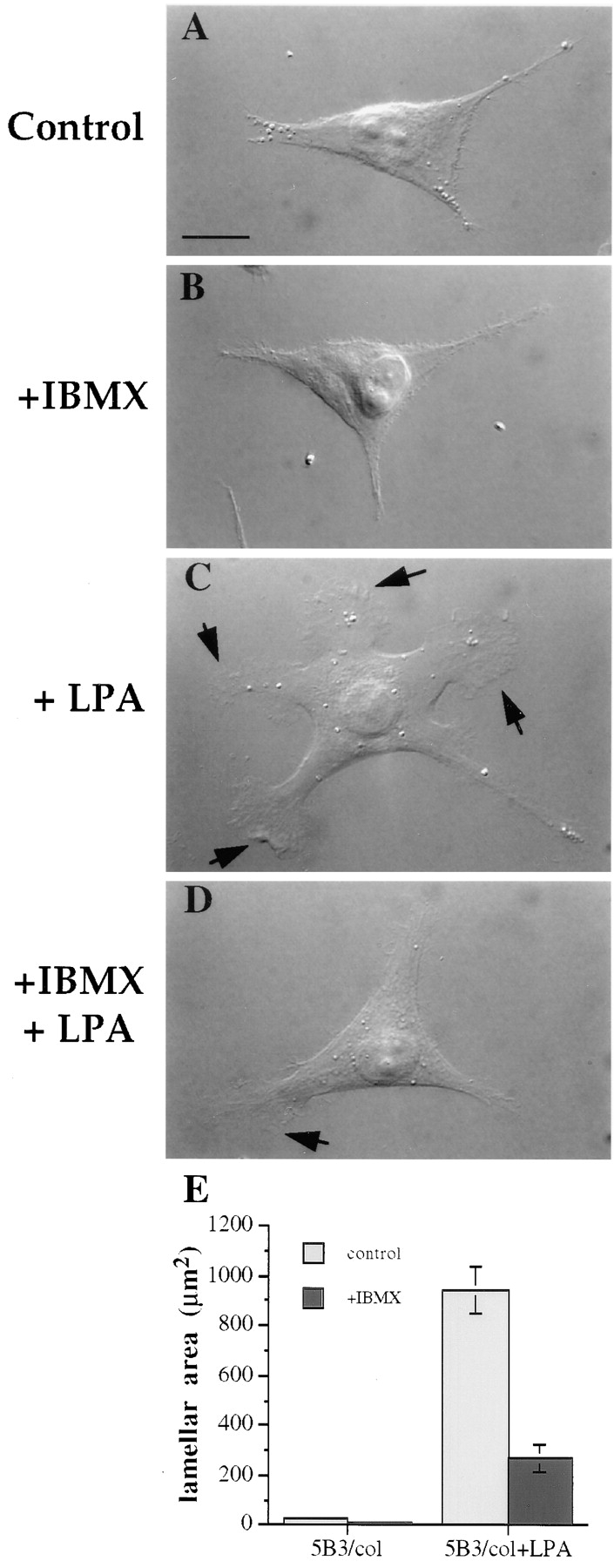
cAMP specific-PDE activity is required for LPA-dependent formation of lamellae in the MDA/β4 transfectants. The MDA/β4 transfectants (5B3) were plated on collagen I–coated coverslips. After 2 h, the cells were either left untreated (A and C) or treated with 1mM IBMX (B and D) for 30 min. Subsequently, the cells were either left untreated (A and B) or treated with 100 nM LPA for 5 min (C and D). The cells were then fixed and visualized using Nomarski DIC optics. (E) The effect of LPA and IBMX on lamellar area was quantified using IPLab Spectrum imaging software. Bars represent mean lamellar area ± standard error in which n > 20. Of note, IBMX inhibited the LPA-dependent formation of lamellae by 70%.
Recently, we reported that α6β4 is necessary for the formation and stabilization of lamellae in clone A colon carcinoma cells plated on laminin-1 (32). If PDE activity is needed for lamellae formation as indicated by the above results, IBMX should inhibit the formation of lamellae in clone A cells. To test this possibility, clone A cells were treated with IBMX or a solvent control and then plated onto laminin-1 for 45 min. The control cells formed large fan-shaped lamellae enriched in F-actin when plated on laminin-1 (Fig. 9, A and B) as we reported previously (32). In contrast, IBMX-treated cells formed small, immature lamellae with a marked reduction in F-actin content (Fig. 9, C and D). Quantitative analysis of these images revealed that IBMX reduced the total lamellar area of clone A cells on laminin-1 by ∼75% (629 ± 74 μm2 for control versus 164 ± 24μm2 with IBMX). Interestingly, inhibition of PDE activity had no effect on the attachment or spreading of clone A cells on laminin-1 (Fig. 9).
Figure 9.
Lamellae formation in clone A colon carcinoma cells requires PDE activity. Clone A colon carcinoma cells were either treated with solvent alone (A and B) or 1 mM IBMX in solvent (C and D) and then plated on laminin-1–coated coverslips. After 45 min the cells were fixed and stained for F-actin using TRITC-phalloidin. (A and C) Phase–contrast images; (B and D) fluorescence images. Bar, 10 μm.
cAMP Metabolism and PI3-K Signaling Are Not Directly Linked in MDA-MB-435 Cells
A possible relationship between cAMP metabolism and PI3-K signaling is of interest given our recent finding that α6β4 stimulates the preferential activation of PI3-K and that this activity is required for invasion and the formation of lamellae (40). To determine if PI3-K activity is required for the cAMP-specific PDE activity we observed in the MDA/β4 transfectants, these cells were incubated in the presence of wortmannin, a specific inhibitor of PI3-K, before extraction and assay of PDE activity. As shown in Fig. 10 A, wortmannin had no effect on PDE activity in these cells and it did not inhibit the marked induction of PDE activity that we had observed in response to forskolin stimulation. The possibility also existed that cAMP influences the α6β4-mediated activation of PI3-K. To address this issue, we used the α6-specific mAb G0H3 to cluster α6 integrins on the MDA/β4 transfectants in the presence of the PDE inhibitor IBMX and forskolin. As shown in Fig. 10 B, mAb-mediated clustering of α6β4 in MDA/β4 transfectants activated PI3-K markedly compared with cells maintained in suspension, in agreement with our previous results (40). However, treatment of MDA/β4 transfectants with either IBMX or forskolin did not inhibit α6β4-mediated activation of PI3-K (Fig. 10 B). In fact, no inhibition of PI3-K was observed when both of these inhibitors were used in combination, a treatment that increases the [cAMP]i from 4 to 120 pmoles per 106 cells (Fig. 5).
Figure 10.
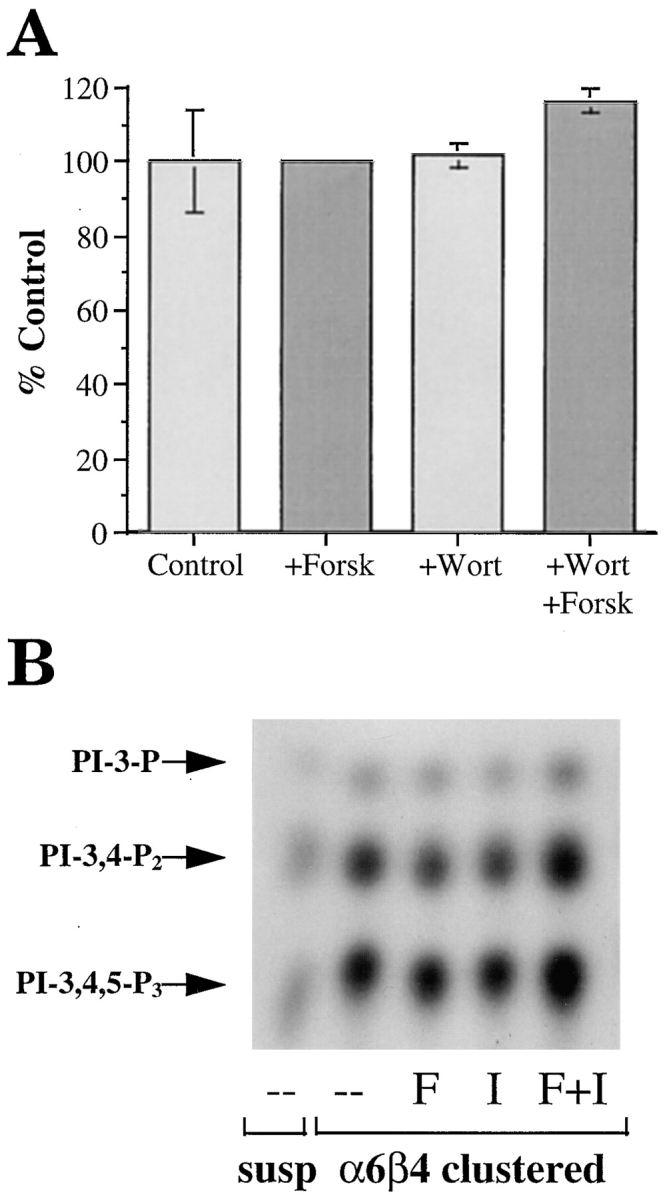
(A) Evaluation of PI3-K involvement in PDE activity. The MDA/β4 transfectants were incubated for 30 min in the presence of either forskolin or wortmannin, or both in combination, before assay of PDE activity as described in Materials and Methods. (B) Evaluation of the cAMP regulation of PI3-K activity. The MDA/β4 and mock transfectants were incubated in suspension with either forskolin or IBMX or both for 10 min. Subsequently, these cells were either maintained in suspension or incubated with a β4 integrin-specific antibody and allowed to adhere to anti-mouse IgG-coated plates or laminin-1–coated plates for 30 min. Aliquots of cell extracts that contained equivalent amounts of protein were incubated with the anti-phosphotyrosine mAb 4G10 and protein A–Sepharose for 3 h. After washing, the beads were resuspended in kinase buffer and incubated for 10 min at room temperature. The phosphorylated lipids were resolved by thin layer chromatography. Arrows, position of the D3-phosphoinositides.
Discussion
Recently, we established that the α6β4 integrin promotes carcinoma invasion (3, 32, 40). In the current study, we extend this observation by demonstrating that a major function of α6β4 is to stimulate the chemotactic migration of carcinoma cells, a function that is essential for invasion. This function is consistent with our previous finding that α6β4 is involved in the formation and stabilization of lamellae and filopodia (32). Importantly, the data presented here also provide evidence that α6β4 stimulates chemotaxis and lamellae formation by regulating the [cAMP]i by a mechanism that involves activation of a rolipram-sensitive, cAMP-specific PDE. Our finding that elevated [cAMP]i inhibits the formation of lamellae, chemotactic migration, and invasion is in agreement with recent studies indicating that cAMP can function to inhibit or “gate” specific signaling pathways (14, 16, 23). Furthermore, we show that the cAMP-mediated gate does not influence haptotaxis thus providing additional evidence that the signaling events governing chemotaxis and haptotaxis differ (1, 20). Collectively, our results strengthen the hypothesis that α6β4 promotes carcinoma invasion through its ability to regulate signaling pathways required for migration. They also indicate that cAMP metabolism is likely to be an important factor in the regulation of carcinoma invasion and progression.
Although integrins can regulate a number of signaling pathways (4), their ability to influence cAMP metabolism has not been studied extensively. An earlier study, however, did provide evidence that the simultaneous engagement of β2 integrins and tumor necrosis factor (TNF) receptors decreases the [cAMP]i in neutrophils (30). Interestingly, the reduction in [cAMP]i observed in response to β2 integrin and TNF receptor engagement in neutrophils is similar to the level of [cAMP]i suppression that we observed in response to α6β4 expression (∼30%). This level of suppression of the total [cAMP]i is quite impressive given that localized gradients of [cAMP]i are probably required to facilitate chemotactic migration, as well as for other cell functions that are gated by cAMP. For example, localized gradients of cAMP have been implicated in regulating the direction of growth cone turning (41). It is also important to note that we observed an inverse correlation between the [cAMP]i and the rate of chemotaxis (compare values in Fig. 4 A with Fig. 5 B). This observation reinforces the functional significance of α6β4 suppression of the [cAMP]i.
A novel aspect of our study is the finding that integrins, and α6β4 in particular, can regulate the activity of a rolipram-sensitive, cAMP-specific PDE. This family of PDEs is defined as type IV PDE (PDE 4) and consists of a number of structural variants (6). Because all of these variants hydrolyze cAMP with a Km comparable to the [cAMP]i, it is thought that tissue-specific expression and the state of activity of these variants are the major determinants of their responsiveness to extracellular stimuli (6). Indeed, the major focus of work in this area has been hormonal regulation of PDE activity. Regulation of PDE activity can occur rapidly in response to hormone stimulation through a mechanism that involves PKA-dependent phosphorylation of the enzyme (36, 37). In addition, long-term, hormonal stimulation can actually increase de novo synthesis of the cAMP-specific PDEs (6, 43). The data we obtained suggest that expression of α6β4 does not increase the expression of PDE 4B, a predominant PDE variant expressed by MDA-MB-435 cells. For this reason, regulation of PDE 4 activity by α6β4 expression may occur through a mechanism that involves PDE phosphorylation. Another possibility that has been proposed recently is that the subcellular localization of the cAMP-specific PDEs influences their function and activation (17). The possibility that α6β4 increases the association of PDE 4 with either the plasma membrane or cytoskeleton is certainly attractive and could account, at least in part, for its ability to influence cAMP metabolism. Interestingly, LPA stimulation by itself had no effect on either PDE activity or the [cAMP]i in MDA-MB-435 cells. This observation reinforces our hypothesis that a major function of α6β4 is to release cAMP gating of LPA-stimulated chemotaxis.
In previous studies, we established that an important function of α6β4 in invasive carcinoma cells is its ability to stimulate the formation of lamellae (32). This function of α6β4 is highlighted by the observation in the present study that LPA was able to induce significant lamellae formation only in MDA-MB-435 cells that expressed α6β4 (Fig. 3). Importantly, our finding that PDE activity is necessary for lamellae formation promoted by α6β4 expression implies that a localized suppression of the [cAMP]i plays an important role in controlling the signaling and cytoskeletal events that are required for lamellae formation. This hypothesis agrees with studies that have shown an inhibitory effect of cAMP on the organization of the actin cytoskeleton (11, 13, 21, 22). Moreover, the formation of lamellae is a dynamic process that is linked to the mechanism of cell migration. Therefore, it is likely that temporal fluxes in the [cAMP]i regulated by α6β4 contribute to the chemotactic migration of carcinoma cells.
Of particular relevance to our work, Butcher and colleagues (23) reported that cAMP is a negative regulator of leukocyte migration signaled through the classical chemoattractants. In this model, cAMP impedes or gates RhoA-mediated leukocyte integrin activation and adhesion. Our results support their conclusion that cAMP inhibits chemotactic migration. Importantly, we also provide evidence for an integrin-mediated mechanism of regulating the [cAMP]i to facilitate migration. Our findings support the work of the Butcher group because LPA is a potent activator of Rho (29) and, in fact, we have observed that the expression of a dominant-negative Rho can inhibit both LPA-stimulated chemotactic migration and invasion of MDA-MB-435 cells (O'Connor, K.L., and A.M. Mercurio, unpublished observation). Thus, a possibility worth investigating is that LPA-mediated activation of Rho is gated by cAMP and that α6β4 releases cAMP gating by increasing cAMP-specific PDE activity and thereby enhances Rho activation. A likely target of Rho activation is the actin cytoskeleton (44), which is consistent with the reported effects of cAMP on the cytoskeleton (11, 13, 21, 22), as well as our demonstration that cAMP inhibits lamellae formation. Although Rho has been linked to stress fiber formation and not lamellae formation in fibroblasts (25), much less is known about Rho function in epithelial-derived cells. In fact, our preliminary data suggest that LPA-induction of lamellae formation in the MDA/β4 transfectants is inhibited by expression of a dominant-negative Rho. Other integrins, especially the β1 integrins that mediate the adhesive interactions required for chemotactic migration, are another potential target of Rho (25, 34). It is worth noting in this context that expression of α6β4 has been shown to alter the function of collagen I–binding integrins in breast carcinoma cells (42).
The ability of α6β4 to promote lamellae formation and carcinoma invasion is dependent upon its preferential activation of a PI3-K and Rac signaling pathway (40). Our current finding that the release of cAMP gating by α6β4 is also required for these events raised the issue of a possible link between cAMP and the PI3-K/Rac pathway. Such a link was suggested, for example, by the finding that the interleukin-2 dependent activation of PI3-K is inhibited by cAMP (28). In our experiments, however, pharmacological stimulation of cAMP levels had no effect on the ability of α6β4 to activate PI3-K even under conditions in which the [cAMP]i increased 30-fold over basal levels. Our data also indicate that PI3-K probably does not function upstream of cAMP-specific PDE because wortmannin did not inhibit the activity of this enzyme. We conclude from these findings that PI3-K and cAMP-specific phosphodiesterase function in tandem to promote lamellae formation and chemotactic migration but they are components of distinct signaling pathways.
An interesting finding in the present study is that the ability of α6β4 to stimulate chemotactic migration and suppress the [cAMP]i can be independent of the adhesive function of this integrin. Although the laminins are the only known matrix ligands for α6β4 (26), expression of this integrin also stimulated chemotactic migration on a collagen I matrix and this migration was not inhibited by an α6-function blocking mAb. The possibility that the effects of α6β4 expression on migration result from a decrease of α6β1 expression is discounted by the fact that expression of the α6β4 ΔCYT integrin had no effect on either chemotaxis, cAMP levels or PDE activity even though expression of this mutant integrin eliminates α6β1 expression in these cells (38). The observation that the ability of α6β4 to promote chemotactic migration can be independent of its adhesive function is in agreement with several recent studies by our group and others that have revealed ‘ligand-independent' functions for the α6 integrins in carcinoma cells (3, 5, 8). Insight into the possible mechanism of this phenomenon was provided by a recent study that demonstrated self-association of the β4 cytoplasmic domains, a process that could initiate intracellular signaling events independently of ligand binding (33). One important implication of these findings is that the ability of α6β4 to influence cAMP metabolism and stimulate the chemotactic migration of carcinoma cells need not be limited to sites of contact with laminin-containing matrices. This possibility is supported by the numerous studies that have implicated α6β4 as a major determinant of carcinoma invasion and progression (10, 35, 47).
In summary, we have demonstrated that the α6β4 integrin can stimulate lamellae formation and chemotactic migration of invasive carcinoma cells by increasing the activity of a rolipram-sensitive cAMP specific-PDE and lowering the [cAMP]i. This cAMP specific-PDE functions in tandem with a PI3-K/Rac pathway, that is also regulated by α6β4, and is required for carcinoma invasion and lamellae formation. The essence of our findings is that the α6β4 integrin stimulates the chemotactic migration of carcinoma cells through its ability to influence key signaling events that underlie this critical component of carcinoma invasion.
Acknowledgments
This work was supported by grants from the United States Army Medical Research and Material Command to K. O'Connor (DAMD17-98-1-8033), L.M. Shaw (DAMD17-97-1-7313), and A.M. Mercurio (DAMD17- 96-1-6199) and a grant from the National Institutes of Health to A.M. Mercurio (CA-44704).
Abbreviations used in this paper
- [cAMP]i
intracellular cyclic AMP concentration
- DIC
differential–interference contrast
- Gi
inhibitory type G protein
- IBMX
isobutylmethylxanthine
- LPA
lysophosphatidic acid
- PDE
phosphodiesterase
- PI3-K
phosphoinositide 3-OH kinase
Footnotes
We would also like to thank M. Conti and S. Iona (Stanford University, Stanford, CA) for their generous contribution of the PDE 4 antibodies, as well as S. Akiyama for the β1 integrin antibody. We also thank R. Falcioni, (Regina Elena Cancer Institute, Rome, Italy), R. Bachelder, I. Rabinovitz, and D. Senger (all three from Beth Israel Deaconess Medical Center, Boston, MA) for their comments and discussions regarding the manuscript.
References
- 1.Aznavoorian S, Stracke ML, Parsons J, McClanahan J, Liotta LA. Integrin αvβ3 mediates chemotactic and haptotactic motility in human melanoma cells through different signaling pathways. J Biol Chem. 1996;271:3247–3254. doi: 10.1074/jbc.271.6.3247. [DOI] [PubMed] [Google Scholar]
- 2.Borradori L, Sonnenberg A. Hemidesmosomes—roles in adhesion, signaling and human disease. Curr Opin Cell Biol. 1996;8:647–656. doi: 10.1016/s0955-0674(96)80106-2. [DOI] [PubMed] [Google Scholar]
- 3.Chao C, Lotz MM, Clarke AC, Mercurio AM. A function for the integrin α6β4 in the invasive properties of colorectal carcinoma cells. Cancer Res. 1996;56:4811–4819. [PubMed] [Google Scholar]
- 4.Clark EA, Brugge JS. Integrins and signal transduction pathways: The road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 5.Clarke AS, Lotz MM, Chao C, Mercurio AM. Activation of the p21 pathway of growth arrest and apoptosis by the β4integrin cytoplasmic domain. J Biol Chem. 1995;270:22672–22676. doi: 10.1074/jbc.270.39.22673. [DOI] [PubMed] [Google Scholar]
- 6.Conti M, Nemoz G, Sette C, Vicini E. Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocrine Rev. 1995;16:370–389. doi: 10.1210/edrv-16-3-370. [DOI] [PubMed] [Google Scholar]
- 7.Dexter DL, Barbosa JA, Calabresi P. N,N-dimethylformamide-induced alterations of cell culture characteristics and loss of tumorigenicity in cultured human colon carcinoma cells. Cancer Res. 1979;39:1020–1025. [PubMed] [Google Scholar]
- 8.Domanico SZ, Pelletier AJ, Havran WL, Quaranta V. Integrin α6Aβ1 induces CD81-dependent cell motility without engaging the extracellular matrix migation substrate. Mol Biol Cell. 1997;8:2253–2265. doi: 10.1091/mbc.8.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowling J, Yu QC, Fuchs E. β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falcioni R, Turchi V, Vittulo P, Navarra G, Ficari F, Cavaliere F, Sacchi A, Mariani-Costantini R. Integrin β4 expression in colorectal cancer. Int J Oncology. 1994;5:573–578. doi: 10.3892/ijo.5.3.573. [DOI] [PubMed] [Google Scholar]
- 11.Glass II, W.F., and J.I. Kreisberg. Regulation of integrin-mediated adhesion at focal contacts by cyclic AMP. J Cell Physiol. 1993;157:296–306. doi: 10.1002/jcp.1041570212. [DOI] [PubMed] [Google Scholar]
- 12.Green KJ, Jones JCR. Desmosomes and hemidesmosomes— structure and function of molecular components. FASEB (Fed Am Soc Exp Biol) J. 1996;10:871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- 13.Han J-D, Rubin CS. Regulation of cytoskeletal organization and paxillin dephosphorylation by cAMP. J Biol Chem. 1996;271:29211–29215. doi: 10.1074/jbc.271.46.29211. [DOI] [PubMed] [Google Scholar]
- 14.Harvath L, Robbins JD, Russell AA, Seamon KB. cAMP and human neutrophil chemotaxis. Elevation of cAMP differentially affects chemotactic responsiveness. J Immunol. 1991;146:244–332. [PubMed] [Google Scholar]
- 15.Iona S, Cuomo M, Bushnik T, Naro F, Sette C, Hess M, Shelton ER, Conti M. Characterization of the rolipram-sensitive, cyclic AMP-specific phosphodiesterases: identification and differential expression of immunologically distinct forms in the rat brain. Mol Pharm. 1998;53:23–32. doi: 10.1124/mol.53.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Iyengar R. Gating by cyclic AMP: expanded role for an old signaling pathway. Science. 1996;271:461–463. doi: 10.1126/science.271.5248.461. [DOI] [PubMed] [Google Scholar]
- 17.Jin S-JC, Bushnik T, Lan L, Conti M. Subcellular localization of rolipram-sensitive, cAMP-specific phospodiesterases. J Biol Chem. 1998;273:19672–19678. doi: 10.1074/jbc.273.31.19672. [DOI] [PubMed] [Google Scholar]
- 18.Keely JP, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 19.Kleinman HK, McGarvey ML, Hassell JT, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane protein complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 20.Klemke RL, Cai S, Giannini AL, Gallageher PJ, deLanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lampugnani MG, Giorgi M, Gaboli M, Dejana E, Marchisio PC. Endothelial cell motility, integrin receptor clustering, and microfilament organization are inhibited by agents that increase intracellular cAMP. Lab Invest. 1990;63:521–531. [PubMed] [Google Scholar]
- 22.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO (Eur Mol Biol Organ) J. 1996;15:510–519. [PMC free article] [PubMed] [Google Scholar]
- 23.Laudanna C, Campbell JM, Butcher EC. Elevation of intracellular cAMP inhibits RhoA activation and integrin-dependent leukocyte adhesion induced by chemoattractants. J Biol Chem. 1997;272:24141–24144. doi: 10.1074/jbc.272.39.24141. [DOI] [PubMed] [Google Scholar]
- 24.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51:5054s–5059s. [PubMed] [Google Scholar]
- 25.Mackay DJG, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- 26.Mercurio AM. Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 1995;5:419–423. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- 27.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 28.Monfar M, Lemon KP, Grammer TC, Cheatham L, Chung J, Vlahos CJ, Blenis J. Activation of pp70/85 S6 kinases in interleukin-2-responsive lymphoid cells is mediated by phosphatidylinositol 3-kinase and inhibited by cyclic AMP. Mol Cell Biol. 1995;15:326–337. doi: 10.1128/mcb.15.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moolenaar WH, Kranenburg O, Postma FR, Zondag GCM. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr Opin Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- 30.Nathan C, Sanchez E. Tumor necrosis factor and CD11/CD18 (β2) integrins act synergistically to lower cAMP in human neutrophils. J Cell Biol. 1990;111:2171–2181. doi: 10.1083/jcb.111.5.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 32.Rabinovitz I, Mercurio AM. The integrin α6β4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containin motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rezniczek GA, dePereda JM, Reipert S, Wiche G. Linking integrin α6β4-based cell adhesion to the intermediate filment cytoskeleton: direct interaction between the β4 subunit and plectin at multiple molecular sites. J Cell Biol. 1998;141:209–225. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 35.Serini G, Trusolino L, Saggiorato E, Cremona O, Derossi M, Angeli A, Orlandi F, Marchisio PC. Changes in integrin and E-cadherin expression in neoplasatic verus normal thyroid tissue. J Natl Cancer Inst. 1996;88:442–449. doi: 10.1093/jnci/88.7.442. [DOI] [PubMed] [Google Scholar]
- 36.Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. J Biol Chem. 1996;271:16526–16534. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- 37.Sette C, Iona S, Conti M. The short-term activation of a Rolipram-sensitive, cAMP-specific phophodiesterase by thyroid-stimulating hormone in thyroid FRTL-5 cells is mediated by a cAMP-dependent phosphorylation. J Biol Chem. 1994;268:9245–9252. [PubMed] [Google Scholar]
- 38.Shaw LM, Chao C, Wewer UM, Mercurio AM. Function of the integrin α6β1 in metastatic breast carcinoma cells assessed by expression of a dominant-negative receptor. Cancer Res. 1996;56:959–963. [PubMed] [Google Scholar]
- 39.Shaw LM, Lotz MM, Mercurio AM. Inside-out integrin signaling in macrophages. J Biol Chem. 1993;268:11401–11408. [PubMed] [Google Scholar]
- 40.Shaw LM, Rabinovitz I, Wang HH-F, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 41.Song H-J, Ming G-L, Poo M-M. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 42.Sun H, Santoro SA, Zutter MM. Downstream events in mammary gland morphogenesis mediated by repression of the α2β1 integrin: the role of the α6 and β4 integrin subunits. Cancer Res. 1998;58:2224–2233. [PubMed] [Google Scholar]
- 43.Swinnen JV, Tsikalas KE, Conti M. Properties and hormonal regulation of two structurally related cAMP phosphodiesterases from the rat Sertoli cell. J Biol Chem. 1991;266:18370–18377. [PubMed] [Google Scholar]
- 44.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1998;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 45.Taussig R, Gilman AG. Mammalian membrane-bound adenylyl cyclases. J Biol Chem. 1995;270:1–4. doi: 10.1074/jbc.270.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Vanderneut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 47.VanWaes C, Kozarsky KF, Warren AB, Kidd L, Paugh D, Liebert M, Carey TE. The A9 antigen associated with aggressive human squamous carcinoma is structurally and functionally similar to the newly defined integrin α6β4. Cancer Res. 1991;51:2395–2402. [PubMed] [Google Scholar]