Abstract
Neural cell adhesion molecules composed of immunoglobulin and fibronectin type III-like domains have been implicated in cell adhesion, neurite outgrowth, and fasciculation. Axonin-1 and Ng cell adhesion molecule (NgCAM), two molecules with predominantly axonal expression exhibit homophilic interactions across the extracellular space (axonin- 1/axonin-1 and NgCAM/NgCAM) and a heterophilic interaction (axonin-1–NgCAM) that occurs exclusively in the plane of the same membrane (cis-interaction). Using domain deletion mutants we localized the NgCAM homophilic binding in the Ig domains 1-4 whereas heterophilic binding to axonin-1 was localized in the Ig domains 2-4 and the third FnIII domain. The NgCAM–NgCAM interaction could be established simultaneously with the axonin-1–NgCAM interaction. In contrast, the axonin-1–NgCAM interaction excluded axonin-1/axonin-1 binding. These results and the examination of the coclustering of axonin-1 and NgCAM at cell contacts, suggest that intercellular contact is mediated by a symmetric axonin-12/NgCAM2 tetramer, in which homophilic NgCAM binding across the extracellular space occurs simultaneously with a cis-heterophilic interaction of axonin-1 and NgCAM. The enhanced neurite fasciculation after overexpression of NgCAM by adenoviral vectors indicates that NgCAM is the limiting component for the formation of the axonin-12/NgCAM2 complexes and, thus, neurite fasciculation in DRG neurons.
Keywords: axonal fasciculation, axon guidance molecules, cell adhesion molecules, cell contact, cell recognition
The precise wiring of the neuronal network is essential for the proper information-processing function of the nervous system. During the formation of the basic network neurons send out processes that reach their target regions in a highly stereotyped and precise manner and very few errors of navigation occur (Dodd and Jessell, 1988; Jessell, 1988; Hynes and Lander, 1992; Luo et al., 1993; Goodman and Shatz, 1993). The correct trajectory of growing fibers is determined by the growth cone, a specialized sensory motor organelle at the tip of the growing axon. Growth cones are capable of axon elongation in response to diffusible or surface-bound growth-promoting molecules and to make the correct decisions at pathway intersections and crossings (Tessier-Lavigne and Goodman, 1996; Cook et al., 1998). Axons growing out after the so-called pioneers tend to make use of the scaffold formed by earlier axons, by virtue of the growth-promoting molecules displayed along their axonal shaft, giving rise to axon fascicles. Selective axonal fasciculation represents a simplifying strategy in the assembly of complex neuroanatomical structures (Van Vactor, 1998).
Two large families of neural cell adhesion molecules (CAMs)1, the cell adhesion molecules of the Ig superfamily (IgSF) and the cadherins, represent local cues for axon guidance and specific fasciculation (Rathjen and Jessel, 1991). A large subfamily of IgSF CAMs, the immunoglobulin/fibronectin type-III (IgFnIII) class of molecules, is composed of Ig domains in conjunction with FnIII-like domains (Williams and Barclay, 1988; Chothia and Jones, 1997). One group of IgFnIII CAMs is attached to the cell membrane by a GPI-anchor and includes axonin-1/TAG-1 (Ruegg et al., 1989a ; Stoeckli et al., 1989; Furley et al., 1990; Zuellig et al., 1992), F11 (contactin)/F3 (Bruemmendorf et al., 1989; Gennarini et al., 1989), and PANG/BIG1 (Connelly et al., 1994; Yoshihara et al., 1994, 1995). The molecules of this group are composed of six Ig domains in conjunction with four FnIII-like domains in their extracellular part. A second group includes transmembrane glycoproteins composed of six Ig domains and five FnIII-like domains, including NgCAM/L1 (Moos et al., 1988; Burgoon et al., 1991), neurofascin (Volkmer et al., 1992), and NrCAM (Grumet et al., 1991; Kayyem et al., 1992). The molecules of these subgroups are expressed predominantly on axons and are involved in neurite extension (Lemmon et al., 1989; Rathjen, 1991; Stoeckli et al., 1991; Lemmon et al., 1992), axonal pathfinding (Stoeckli and Landmesser, 1995; Stoeckli et al., 1997; Stoeckli and Landmesser, 1998), and neurite fasciculation (Rathjen et al., 1987; Ruegg et al., 1989b ; Rathjen and Jessell, 1991; Stoeckli and Landmesser, 1995). They exhibit a complex pattern of interactions: e.g., axonin-1 interacts with NgCAM (Kuhn el al., 1991), NrCAM (Suter et al., 1996), neurocan, and phosphacan/protein-tyrosine phosphatase β (Milev et al., 1996; Holland et al., 1998), whereas NgCAM binds to axonin-1, F11 (contactin; Bruemmendorf et al., 1993), DM-GRASP (DeBernardo and Chang, 1997), laminin (Grumet et al., 1993a ) and the proteoglycans 3F8, neurocan, and phosphacan (Grumet et al., 1993b ; Friedlander et al., 1994; Milev et al., 1994). Axonin-1 and NgCAM, in addition, exhibit homophilic interactions (Lemmon et al., 1989; Rader et al., 1993).
The role of axonin-1 and NgCAM in specific neurite fasciculation has been demonstrated by in vitro and in vivo studies (Ruegg et al., 1989a ; Stoeckli et al., 1991; Stoeckli and Landmesser, 1995) and is further supported by the specific coclustering of these molecules at sites of contact between adjacent neurites in vitro (Honig and Kueter, 1995). A crucial role of NgCAM-mediated cell adhesion in the organization of the axonal cytoskeleton has been suggested by the fact that all vertebrate and invertebrate members of the NgCAM/L1 family contain two highly conserved amino acid sequences in their cytoplasmic domains, which were found to bind ankyrin, a protein that acts as an adaptor between membrane proteins and the actin spectrin-based cytoskeleton (Davis et al., 1993; Davis and Bennett, 1994) The intracellular association of ankyrin with neuroglian, the Drosophila homologue of NgCAM/L1, depends on the extracellular homophilic interaction (Dubreuil et al., 1996). Thus, members of the NgCAM/L1 family may be able to recruit cytoskeletal components to cell contact sites, and, thus, shape the connections between the cell surface and the cytoskeleton, depending on their pattern of extracellular interactions. We have recently observed that the progressing fasciculation in cultured sensory neurons is accompanied by the formation of heterodimeric and heterotetrameric complexes of NgCAM and axonin-1 (Kunz et al., 1996). Based on these results, we have speculated that heterooligomeric complexes composed of IgFnIII CAMs, such as NgCAM and axonin-1, may represent the functional units of cell recognition. It is, therefore, conceivable that the different functional roles of these molecules like those in axon growth-promotion, axon guidance, and axonal fasciculation, are determined by the composition of the CAM complexes at the sites of contact and the nature of the intracellular signals elicited by the specific complexes. The investigation of the structural and functional features of such complexes is therefore of pivotal importance for an understanding of CAM function at the molecular level.
In this study, we identified the NgCAM domains involved in the homophilic NgCAM/NgCAM interaction and the heterophilic axonin-1–NgCAM interaction. We investigated the binding pattern of axonin-1 and NgCAM in the areas of cell–cell contacts and found that homophilic NgCAM/NgCAM interaction can occur simultaneously with the heterophilic axonin-1–NgCAM binding, whereas the heterophilic axonin-1–NgCAM interaction and the homophilic axonin-1/axonin-1 binding are mutually exclusive. Based on these results, we suggest a model for a symmetric tetrameric axonin-12/NgCAM2 complex that may represent the functional unit of cell adhesion and signal transduction of neurite fasciculation by axonin-1 and NgCAM.
Materials and Methods
Proteins and Antibodies
Axonin-1 was purified from chicken ocular vitreous fluid as described previously (Ruegg et al., 1989a ). NgCAM was immunoaffinity purified from embryonic day 14 chicken brain membranes using the monoclonal antibody 12-I-14-E 311, provided by Fritz Rathjen (Rathjen et al., 1987). Laminin (mouse, isolated from Engelbreth-Holm-Swarm sarcoma) was purchased from GIBCO-BRL laboratories (Gaithersburg, MD). Polyclonal serum G67 against axonin-1 (Ruegg et al., 1989a ) and G4 against NgCAM was raised in goat (Kuhn et al., 1991). IgG and Fab were obtained as previously specified (Stoeckli et al., 1991). Cy3-conjugated anti– mouse IgG and Texas red–conjugated anti–rabbit IgG was from Zymed Laboratories Inc. (South San Francisco, CA), FITC-conjugated anti–goat IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA), and anti–sheep/goat IgG secondary antibodies for enhanced chemiluminescence (ECL) detection were from Boehringer Mannheim (Mannheim, Germany).
Production of Soluble Axonin-1 Variants in Myeloma Cells
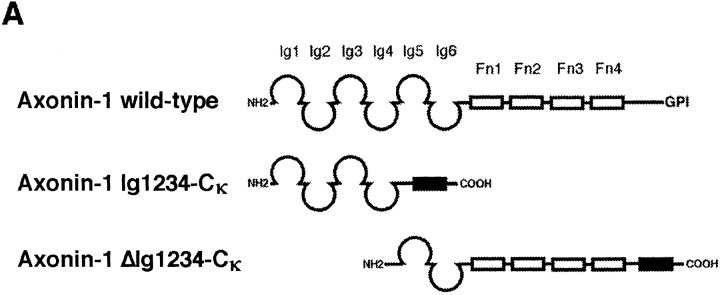
The production of the myeloma cell lines that constitutively express and secrete the fusions of axonin-1 (or fragments thereof) and the constant domain of the mouse Ig κ-light chain, axonin-1 Ig1234-Cκ and ΔIg1234-Cκ is described by Rader et al. (1996). For the isolation of the axonin-1 variants, transfected myeloma cells were adapted to 2% FCS in DME and cultivated in roller bottles (Costar, Cambridge, MA). Supernatants were concentrated by ultrafiltration (Skanette; Skan AG, Basel, Switzerland) and loaded on an immunoaffinity column with rat anti–mouse Cκ mAb 187.1 (American Type Culture Collection, Rockville, MD) coupled to Sepharose 4B. The column was washed with 40 column volumes of PBS and eluted with 50 mM diethylamine, 100 mM NaCl, pH 11.0. The eluate was immediately neutralized by the addition of 1 M Tris-HCl, pH 7.0, and dialysis against PBS. The integrity of the fusion proteins was tested by SDS-PAGE and Western blot analysis using anti-axonin-1 and anti-Cκ antibodies for detection.
Construction of NgCAM Deletion Mutants
The domain deletion mutants of NgCAM were inserted downstream of the cytomegaloviral promotor into the eucaryotic expression vector pSCT (Buchstaller et al., 1996). Domain borders were defined according to the homology of the NgCAM domains to the Ig domains or the type-III domains of fibronectin (Burgoon et al., 1991). To construct the domain deletion mutants XhoI restriction sites were introduced at the domain borders by PCR cloning. In brief, the cDNA fragments 1Fc-Ig01B, Ig12F-10B, 1Fc-Ig12B, Ig23F-10B, 1Fc-Ig23B, Ig34F-10B, 1Fc-Ig34B, Ig45F-4B, 1Fc-Ig45F, Ig56F-4B, 13F-Ig56B, Ig6Fn1F-4B, 13F-Ig6Fn1B, Fn12F-Fn45B, Ig6Fn1F-Fn12B, Fn23F-Fn45B, Ig6Fn1F-Fn23B, Fn34F-2B, Ig6Fn1F-Fn34B, Fn45F-2B, Ig6Fn1F-Fn45B, FN5TMF-2B were amplified using the polymerase chain reaction on the full-length NgCAM cDNA cloned into the pSCT vector (Buchstaller et al., 1996) as a template. PCR amplification was performed as described (Rader et al., 1993).
The following primer sequences were used: 1Fc: 5′CGTGAAAGCTTCCGCCATGGCTCTGCCCCATGGGCTCG3′; Ig01B: 5′CGGGGGGCTCGAGGAAATCGTGTGC3′; Ig12F: 5′CCAACGTCATCCTCGAGAACACTCCG3′; Ig12B: 5′GGAGTGTTCTCGAGGATGA-CGTTGGC3′; 13F: 5′CGAAGCTTCCGCAGTGGCCGGAGAAGA-AGG3′; Ig23F: 5′GAAGGAGCCCCTCGAGCTCCGCG3′; Ig23B: 5′GCCACGCGGAGCTCGAGGGGCTCC3′; Ig34F: 5′CCACAGCGT-CACCCTCGAGGCCGCCC3′; Ig34B: 5′GGGGCGGCCTCGAGGG-TGACGC3′; Ig45F: 5′CCTGCACGTCCTCGAGCTGCCGGTCC3′; Ig45B: 5′GGACCGGCAGCTCGAGGACGTGCAGG3′; 10B: 5′GAA-GGATCGATCGTCCTGCAGAGC3′; Ig56F: 5′CGGCACTCCTCGA-GGTCAGAGCC3′; Ig56B: 5′GGCCCTGACCTCGAGGAGCGCCG3′; Ig6Fn1F: 5′CCGGCCCCCTCGAGGACCTCC3′; Ig6Fn1B: 5′CTT-GGAGGTCCTCGAGGGGGCCG3′; Fn12F: 5′CCCCGCGGCTCTCG-AGCGCAACC3′; Fn12B: 5′CCGGGTTGCGCTCGAGAGCCGCG3′; Fn23F: 5′CGTTGGTTTACCTCGAGAATGTGGG3′; Fn23B: 5′CCCC-ACATTCTCGAGGTAAACCAACGG3′; 4B: 5′GGAAGCTTACTTA-CCTATTGTCGACGACTGCGCTCCCCC3′; Fn34F: 5′GGCCCCCCC-CTCGAGCCCATCGCC3′; Fn34B: 5′GAAGGCGATGGGCTCGA-GGGGGGG3′; Fn45F: 5′CGGCCCCGCCCTCGAGACGGTGGG3′; Fn45B: 5′GCTCCCCACCGTCTCGAGGGCG3′; Fn5TMF: 5′CCGTG-GTGCGCAGCCTCGAGGG3′.
The construction of ΔIg1 was performed as follows: 1Fc-Ig01B was digested with XbaI/XhoI and Ig12F-10B with XhoI/RsrII. The two fragments were then cloned into the expression vector pSCTNgCAM (Buchstaller et al., 1996), previously digested with XbaI/RsrII. Using the same strategy, all the other NgCAM domain deletion mutants were generated: ΔIg2: 1Fc-Ig12B digested with XbaI/XhoI, Ig23F-10B with XhoI/RsrII, cloned into pSCTNgCAM XbaI/RsrII; ΔIg3: 1Fc-Ig23B digested with MluI/XhoI, Ig34F-10B with XhoI/SfiI, cloned into pSCTNgCAM MluI/ SfiI; ΔIg4: 1Fc-Ig34B digested with XbaI/XhoI, Ig45F-4B with XhoI/ RsrII, cloned into pSCTNgCAM XbaI/RsrII; ΔIg5: 1Fc-Ig45F digested with MluI/XhoI, Ig56F-4B with XhoI/HpaI, cloned into pSCNgCAM MluI/HpaI; ΔIg6: 13F-Ig56B digested with RsrII/XhoI, Ig6Fn1F-4B with XhoI/HpaI, cloned into pSCTNgCAM RsrII/HpaI; ΔFn1: 13F-Ig6Fn1B digested with RsrII/XhoI, Fn12F-Fn45B with XhoI/SacI, cloned into pSCTNgCAM RsrII/SacI; ΔFn2: Ig6Fn1F-Fn12B digested with HpaI/ XhoI, Fn23F-Fn45B with XhoI/SacI, cloned into pSCTNgCAM HpaI/ SacI; ΔFn3: Ig6Fn1F-Fn23B digested with HpaI/XhoI, Fn34F-2B with XhoI/NarI, cloned into pSCTNgCAM HpaI/NarI; ΔFn4: Ig6Fn1F-Fn34B digested with HpaI/XhoI, Fn45F-2B with XhoI/NarI, cloned into pSCTNgCAM HpaI/NarI; ΔFn5: Ig6Fn1F-Fn45B digested with HpaI/XhoI, FN5TMF-2B with XhoI/NarI, cloned into pSCTNgCAM HpaI/NarI.
The double domain deletion mutants were constructed as follows: ΔIg12: 1Fc-Ig01B digested with XbaI/XhoI, Ig23F-10B with XhoI/RsrII, cloned into pSCTNgCAM XbaI/RsrII; ΔIg23: 1Fc-Ig12B digested with XbaI/XhoI, Ig34F-10B with XhoI/SfiI, cloned into pSCTNgCAM XbaI/ SfiI; ΔIg34: 1Fc-Ig23B digested with MluI/XhoI, Ig45F-4B with XhoI/ RsrII, cloned into pSCTNgCAM MluI/RsrII; ΔIg45: 1Fc-Ig34B digested with XbaI/XhoI, Ig56F-4B with XhoI/HpaI, cloned into pSCTNgCAM XbaI/HpaI. ΔIg56: 1Fc-Ig45F digested with MluI/XhoI, Ig6Fn1F-4B with XhoI/HpaI, cloned into pSCTNgCAM MluI/HpaI; ΔIg6Fn1: 13F-Ig56B digested with RsrII/XhoI, Fn12F-Fn45B with XhoI/SacI, cloned into pSCTNgCAM RsrII/SacI; ΔFn12: 13F-Ig6Fn1B digested with RsrII/XhoI, Fn23F-Fn45B with XhoI/SacI, cloned into pSCTNgCAM RsrII/SacI; ΔFn23: Ig6Fn1F-Fn12B digested with HpaI/XhoI, Fn34F-2B with XhoI/ NarI, cloned into pSCTNgCAM HpaI/NarI; ΔFn34: Ig6Fn1F-Fn23B digested with HpaI/XhoI, Fn45F-2B with XhoI/NarI, cloned into pSCTNgCAM HpaI/NarI; ΔFn45: Ig6Fn1F-Fn34B digested with HpaI/XhoI, FN5TMF-2B with XhoI/NarI, cloned into pSCTNgCAM HpaI/NarI.
For the construction of the NgCAM mutant ΔIg56Fn1 the fragment XbaI(565)-XhoI(1815), obtained from the XbaI/XhoI digest of ΔIg5, and the fragment XhoI(2716)-SacI(3422), from a XhoI/SacI digest of ΔFn1, was cloned into pSCTNgCAM XbaI/SacI. For the construct ΔIg6Fn12, XbaI(565)-XhoI(2122), from a XbaI/XhoI digest of ΔIg6, and XhoI- (3040)-SacI(3422), from a XhoI/SacI digest of ΔFn2, were cloned into pSCTNgCAM XbaI/SacI. For the cloning of ΔIg56Fn12, XbaI(565)- XhoI(1815), obtained from XbaI/XhoI digest of ΔIg5, and XhoI(3040)- SacI(3422), from a XhoI/SacI digest of ΔFn2, were cloned into pSCTNgCAM XbaI/SacI.
All Deletion Mutants Were Verified by Double Strand DNA Sequencing
Based on the numbering of the amino acid sequence of NgCAM given by Buchstaller et al. (1996), the following segments were deleted in the mutants: ΔIg1: Q35-A129; ΔIg2: E130-L222; ΔIg3: D223-V325; ΔIg4: E326-V418; ΔIg5: E419-L508; ΔIg6: E509-R610; ΔFn1: R610-P707; ΔFn2: E708-P816; ΔFn3: E817-S926; ΔFn4: E927-L1024; ΔFn5: Q1025-L1137; ΔIg12: Q35-L222; ΔIg23: E130-V325; ΔIg34: D223-V418; ΔIg45: E326-L508; ΔIg56: E419-R619; ΔIg6+Fn1: E509-P707; ΔFn12: R610-P816; ΔFn23: E708-S926; ΔFn34: E817-L1024; ΔFn45: E927-L1134; ΔIg56Fn1: E419-P707; ΔIg6Fn12: E509-P816; ΔIg56Fn12: E419-P816.
The insertion of an XhoI site resulted in most of the cases in silent or conservative mutations of amino acids flanking the deletions. The following replacements were made: ΔIg2: A129-L129, D223-E223; ΔIg4: V325-L325; ΔIg5: V418-L418; ΔIg6: R610-E610; ΔFn1: I609-L609; ΔFn2: P707-L707; ΔFn3: P816-L816; ΔFn4: S926-L926, Q1025-E1025; ΔFn5: V1137-E1137; ΔIg12: D223-E223; ΔIg23: A129-L129; ΔIg45: V325-L325; ΔIg56: V418-L418, R610-E610; ΔFn12: I609-L609; ΔFn23: P707-L707; ΔFn34: P816-L816, Q1025-E1025; ΔFn45: S926-L926, V1137-E1137; ΔIg56Fn1 and ΔIg56Fn12: V418-L418.
Construction of the Point Mutants NgCAM PM1-PM3 and the Deletion Mutant NgCAMΔP871-V888
For the construction of NgCAM variants deficient for proteolytic processing, the following point mutations were introduced to the cleavage site: NgCAM PM1: R865A; NgCAM PM2: R865A and R864S; and NgCAM PM3: R865A, R864S, and R862A. R865, R864, and R862 correspond to the conserved Arg residues at the positions −1, −2, and −4 of the putative furin cleavage site described by Grumet and Sakurai (1996). The point mutations were introduced by PCR using the primers PM1, PM2, and PM3 that contained the necessary base exchanges.
The following primer sequences were used: PM1, 5′GTGGGGGAG-CGCAGTCGTGCACAAGCCCCCCCC3′; PM2, 5′GTGGGGGAGC-GCAGTAGTGCACAAGCCCCCCCC3′; PM3, 5′GTGGGGGAGGC-CAGTAGTGCACAAGCCCCCCCC3′; PMd, 5′GGGGAGCTCAGT-CGTGCACAAGCCCCCCCC3′.
The introduction of R865A resulted in a new ApaLI restriction site in position 3186 of pSCTNgCAM. The PCR fragments Fn12F-PM1, Fn12F-PM2, and Fn12F-PM3 were digested with KpnI/ApaLI. The PCR fragment PMd-Fn45B was digested with ApaLI/SacI. Fn12F-PM1, Fn12F-PM2, and Fn12F-PM3 KpnI/ApaLI and PMd-Fn45B ApaLI/SacI were cloned into pSCTNgCAM KpnI/SacI.
To construct the deletion mutant ΔP871-V888, a SacI restriction site was introduced at positions 870 and 889 of the amino acid sequence by PCR cloning, resulting in the exchanges D870E and A889L. The following primer sequences were used: LF, 5′GATTTGGGGGAGCTCGGGG-GGGGCTTG3′ and LB, 5′CCCCCCGAGCTCCTGACAGTGGGGG-GGGAC3′.
In brief, the PCR fragments Fn12F-LB, digested with KpnI/SacI, and the fragment LF-Fn45B, digested by SacI/SacI were cloned into pSCTNgCAM KpnI/SacI. The NgCAM mutants were verified by double strand DNA sequencing.
Transient Transfection of COS7 Cells
Transient transfectants were obtained by electroporation according to Chu et al. (1987) and Rols et al. (1994). In brief, 106 COS7 cells were trypsinized and resuspended in 700 μl of PBS and mixed with 100 μl of PBS containing 10 μg of vector DNA purified using the EndoFree plasmid purification kit (QuiaGen, Hilden, Germany). After a 10-min incubation on ice, the electroporation was carried out in a 1-cm cuvette by applying 960 μF and 230 V (Bio-Rad Gene Pulser). After 10 min of incubation at 37°C, the cells were plated on 28 cm2 cell culture dishes in DME supplemented with 10% FCS at 37°C in 5% CO2. After 8 h the cells were washed twice with PBS and incubated for 16 h with 10 ml DME 10% FCS.
Immunoblotting
Transfected COS7 cells were washed twice with PBS and detached by treatment with 2 mM EDTA in PBS 2 d after transfection. For solubilization of membrane proteins the detached cells were lysed in 1% (wt/vol) Triton X-100, 1% (wt/vol) CHAPS, 0.1% (wt/vol) SDS, 5 mM EDTA, 1 mM PMSF, 1 mM iodacetamide, 100 mM NaCl, 50 mM Tris-HCl, pH 7.5. Proteins were precipitated (Wessel and Fluegge, 1984). After addition of sample buffer and boiling for 5 min, the samples were examined by gel electrophoresis and transferred to nitrocellulose. The nitrocellulose was blocked overnight with 1% (wt/vol) blocking reagent for chemiluminescence detection (Boehringer Mannheim) in TBS, then incubated for 2 h in 1% (wt/vol) blocking reagent containing the primary antibody (polyclonal serum G4 anti-NgCAM in a dilution of 1:5,000), and, subsequently, incubated for 1 h at room temperature in 0.5 μg/ml anti–sheep/goat IgG Fab coupled to peroxidase in 0.5% (wt/vol) blocking reagent in TBS. After several washings in TBS and followed by one washing with 0.1% (wt/vol) Tween-20 in TBS, the chemiluminescence system from Boehringer Mannheim was used for detection. Exposure times for the autoradiographs was 10 s.
To assess the extent of proteolytic cleavage of the NgCAM mutants PM1, PM2, PM3, ΔP871-V888, and ΔFn3 as well as wild-type NgCAM, quantitative Western blot analysis was performed, using the monoclonal anti-NgCAM antibody 12-I-14-E 311, that recognizes the 135-kD fragment of NgCAM, and enhanced chemiluminescence for detection. For the relative quantification of the NgCAM fragments of 135 and 200 kD, densitometric analysis with the Image QanNT software (version 4.1; Molecular Dynamics, Sunnyvale, CA) was performed. The extent of proteolytic cleavage was calculated as the ratio of cleaved NgCAM (represented by the 135-kD band) relative to total NgCAM (represented by the sum of the 135- and the 200-kD form): I(135 kD)/(I(200 kD) + I(135 kD)), where I(200 kD) and I(135 kD) represent the intensity of the bands of 135 and 200 kD, measured in densitometric units. The following percentages of cleavage were found: 61% (± 7.1) for wild-type NgCAM, 9.1 (± 2.8) for PM1, 8.7 (± 3.5) for PM2, and 2.6 (±1.6) for PM3. No detectable cleavage was found for ΔP871-V888 and ΔFn3.
Binding of Protein-conjugated Covaspheres to Transfected COS7 Cells and Immunofluorescence Staining
24 h after transfection, the cells were washed twice with PBS, detached by treatment with 2 mM EDTA in PBS, and resuspended in DME, 10% FCS. The cells were transferred to Lab-Tek glass slides coated with 250 μg/ml poly-d-lysine (Nunc Inc., Naperville, IL), incubated at 37°C in 5% CO2 for 24 h and then washed twice with DME, 5 mg/ml BSA. TRITC-Covaspheres were coupled to axonin-1, NgCAM, and the recombinant axonin-1-Cκ fusion proteins Ig1234-Cκ and ΔIg1234-Cκ as described elsewhere (Kuhn et al., 1991). The Covaspheres were sonicated for 2 min, diluted 1:1,000 in DME, 5 mg/ml BSA, and again sonicated for 3 min immediately before use. The cells were incubated with the prepared Covaspheres for 1 h at 37°C, washed twice with DME, 5 mg/ml BSA, and fixed with 2% formaldehyde for 1 h at 37°C. After washing twice with PBS, 1% FCS, the NgCAM expressing cells were visualized in direct immunofluorescence staining using a polyclonal anti-NgCAM serum (1:500) and FITC-labeled rabbit anti–goat IgG (1:100). The preparation was examined with a fluorescence microscope (Leitz DMR; Leica AG, Heerbrugg, Switzerland) using a green/red filter (Leica AG) for the simultaneous detection of FITC and TRITC fluorescence. For quantification, green fluorescing cells binding at least four Covaspheres were scored as binding cells (nonexpressing cells were found to bind 0-1 Covaspheres). In antibody blocking experiments the cells were preincubated with goat polyclonal anti-axonin-1 or anti-NgCAM Fab (250 μg/ml) in DME, 10% FCS for 1 h. The cells were washed three times with DMEM, 5 mg/ml BSA. Incubation with protein-conjugated Covaspheres and fixation were carried out as described above.
Confocal Laser Scanning Microscopy of Stably Transfected CV-1 Cells
CV-1 cells stably transfected with the full-length cDNA of axonin-1, NgCAM, or both (Buchstaller et al., 1996) were cultured in Lab-Tek glass slides coated with 250 μg/ml poly-d-lysine in DME, supplemented with 2.5% FCS and 2.5% NBCS, to a confluency of 20%. The cells were fixed in the culture medium by adding fixation solution to a final concentration of 2% formaldehyde and 0.1% glutaraldehyde for 1 h at 37°C. This fixation protocol was described to result in a complete immobilization of membrane proteins without permeabilization of the cells (Dubreuil et al., 1996). Axonin-1 and NgCAM were stained with goat anti-axonin-1 (1:500) and rabbit anti-NgCAM (1:1,000) serum in PBS, 10% FCS for 1 h at room temperature and rabbit anti-goat FITC and donkey anti–rabbit Texas red antibodies for detection. Confocal laser scanning microscopy was performed using a Multi Probe 2001 CLSM (Molecular Dynamics, Sunnyvale, CA) equipped with an argon/krypton laser and the single scanning mode. For the detection of FITC, the band-pass excitation filter 488DF10 was used in combination with a 510DLRP primary beam splitter and a long-pass barrier filter 510EPLP. For the detection of Texas red, the band-pass excitation filter 568DF10 was used in combination with a 488/ 568 dual dichronic filter as a primary beam splitter and a 590EFLP long-pass barrier filter. To obtain cell layers with comparable geometry, identical numbers of confocal sections were integrated for image generation and quantitative analysis of the distribution of axonin-1 and NgCAM. For quantification distribution coefficients were calculated. In cultures consisting of only one type of cells the distribution coefficient was calculated as: q = 2 × Icell-contact/Icell1 + Icell2 and, in cases of contacts formed by different cell types (e.g., in a mixture of axonin-1 and NgCAM expressing cells), q* = Icell-contact/Iexpressing cell, where the terms Icell-contact represents the fluorescence intensity per unit area at the cell contact, and the terms Icell1, Icell2, and Iexpressing cell represent the fluorescence intensity per unit area at the surface of the cells forming the contacts.
Cultivation of DRG Explants and Infection with Adenoviral Vectors for Gene Transfer
Dorsal root ganglia (DRG) were dissected from neonatal mice (ICR) and 2–3 ganglia were cultivated per well in Lab-Tek glass slides coated with 20 μg/ml poly-d-lysine and 20 μg/ml laminin in DME supplemented with 10% FCS, 1% N3 (Ruegg et al., 1989a ; Stoeckli et al., 1991), and 40 ng/ml NGF. For infection the cultures were inoculated with 107 pfu/well AdCMVax-1, AdCMVNgCAM, or AdCMVlacZ. For description of the adenoviral vectors see Giger et al. (1996) and Vogt et al. (1996). After 12 h the ganglia were washed three times with in DME supplemented with 10% FCS, 1% N3, and 40 ng/ml NGF and incubated for another 36 h. The explant cultures were fixed in the culture medium by adding fixation solution to a final concentration of 2% formaldehyde and 0.1% glutaraldehyde. For immunofluorescence staining the axonin-1–specific monoclonal antibody X7C11 or the NgCAM-specific monoclonal antibody 12-I-4E-311 (10 μg/ml in PBS, 1% FCS for 1 h) and a Cy3-labeled donkey anti-mouse antibody (1:200, 30 min) were used. For β-galactosidase staining, the explants were fixed in 2% (wt/vol) formaldehyde for 10 minutes and incubated with staining solution: 5 mM K3FeIII(CN)6, 5 mM K4FeII(CN)6, 10 mM MgCl2, and 5 mg/ml X-Gal in PBS at 37°C.
In antibody blocking experiments the explants were incubated with anti-axonin-1 or anti-NgCAM monoclonal antibodies (100 μg/ml IgG) during the whole duration of the experiment. After 48 h the explants were fixed and stained with Cy3-labeled donkey anti–mouse antibody.
For quantification of neurite fasciculation individual immunostained neurites were tracked from the periphery of the ganglion to a point at one-third of the total neurite length. Neurites that run into bundles consisting of >4 neurites formed one category and neurites found in bundles of <4 neurites the other (in each sample, 50 neurites were scored per ganglion and 6 ganglia were analyzed).
Results
The Ig Domains 2-4 and the Third FnIII Domain of NgCAM Are Essential for Axonin-1 Binding
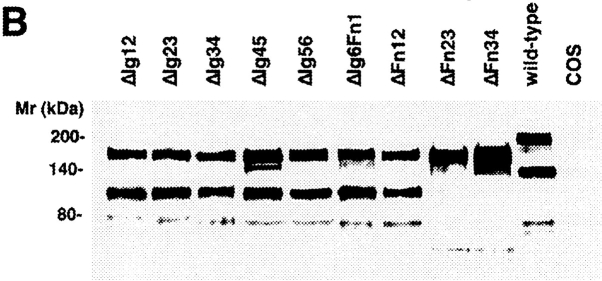
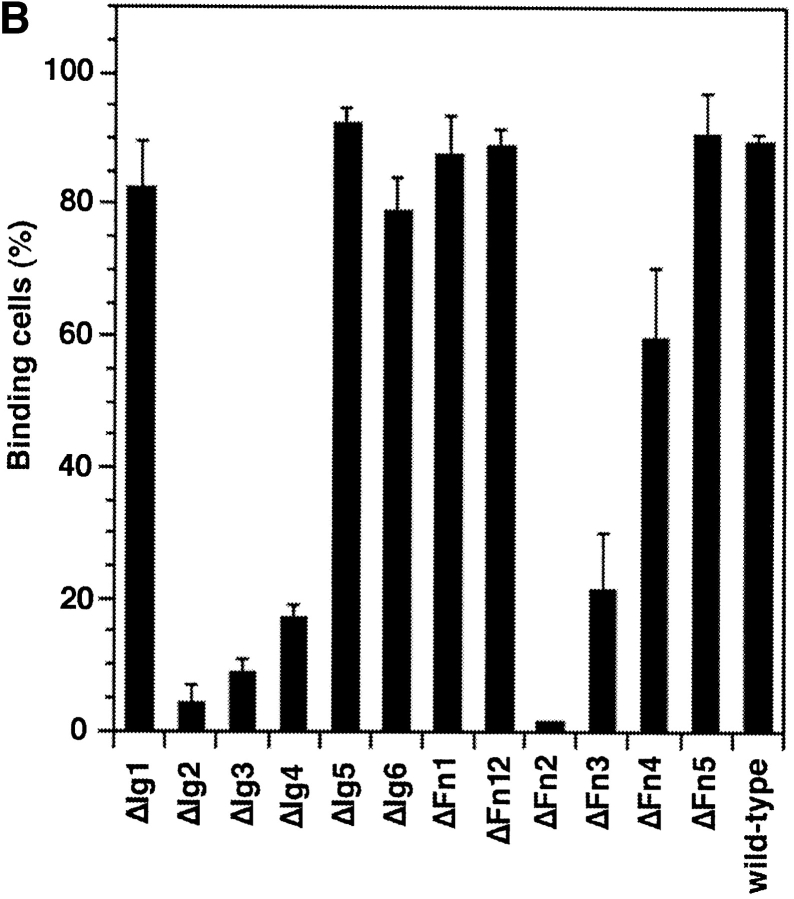
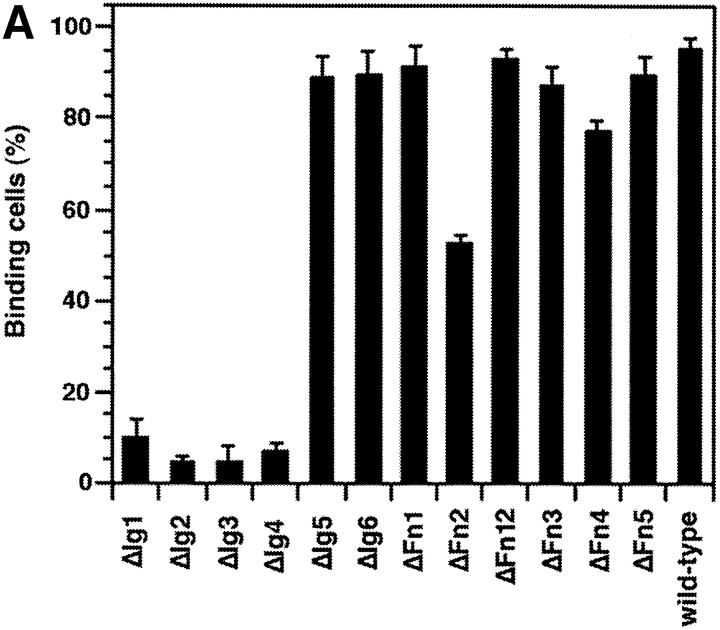
Entire domains of NgCAM, as defined by their homology to the Ig domains or the type-III domains of fibronectin (Grumet et al., 1991; Buchstaller et al., 1996), were deleted. Two different sets of deletion mutants were generated, a complete set of single domain deletions and a complete set of double deletions of adjacent domains. This strategy was based on the observation that in many IgFnIII class molecules some of the folding units comprise two adjacent domains (Huber et al., 1994; Vaughn and Bjorkman, 1995). The NgCAM mutants were cloned into the NgCAM expression vector pSCTNgCAM. Transfection of COS7 cells by electroporation resulted in 30–40% of the cells expressing heterologous protein. The cells were solubilized 48 h after transfection and analyzed by immunoblotting using a polyclonal anti-NgCAM antibody. The molecular masses of the NgCAM domain deletion mutants were in agreement with the expected values (Fig. 1). In accordance with the localization of the natural proteolytic cleavage site of NgCAM in the third FnIII domain (Burgoon et al., 1991), proteolytic cleavage was absent in all mutants lacking the third FnIII domain. All mutants, except ΔFn45, were expressed in similar quantities at the cell surface, as revealed by immunofluorescence analysis. 2 d after electroporation the cells were incubated with axonin-1–conjugated polystyrene microspheres (Covaspheres). After washing and fixation the NgCAM-expressing cells were identified by immunostaining. The deletion of each of the Ig domains Ig2-4 resulted in an almost complete loss of axonin-1 binding. Deletion of Fn2 and Fn3 resulted in strong reduction of axonin-1 binding, deletion of Fn4 in a much weaker, but still significant, reduction (Fig. 2 B). The axonin-1 binding capacities of the mutants with deleted Ig2, Ig3, Ig4, and Fn3, were in accordance with the data from the double deletion mutants lacking Ig1+2, Ig2+3, Ig3+4, Ig4+5, and the membrane proximal domain tandems Fn2+3 and Fn3+4 (data not shown). In contrast, the lack of axonin-1 binding by ΔFn2 contradicts the observation that axonin-1 binding is unaffected by the deletion of Fn1 and Fn2 in ΔFn12 (Fig. 2 B). In consideration of the latter finding, a direct involvement of Fn2 in the binding to axonin-1 must be excluded. Interestingly, x-ray crystallographic analysis of the domain-tandem Fn1+2 of neuroglian, the homologue of NgCAM in Drosophila melanogaster, revealed an extended hydrophobic contact area between the two domains, which is conserved in the vertebrate isologues (Huber et al., 1994). The deletion of Fn2 places Fn1 adjacent to the putative binding domain Fn3. It is conceivable that, in the absence of the cognate area on Fn2, the hydrophobic contact area of Fn1 prevents a correct folding of Fn3. Thus, we conclude that the Ig domains 2-4 and the third FnIII domain of NgCAM are essential for axonin-1 binding. The deletion of each of these domains resulted in a loss of binding. This indicates that Ig2-4 and Fn3 do not represent two alternative binding sites, but must engage cooperatively in a direct or indirect manner in order to establish a binding of axonin-1.
Figure 1.
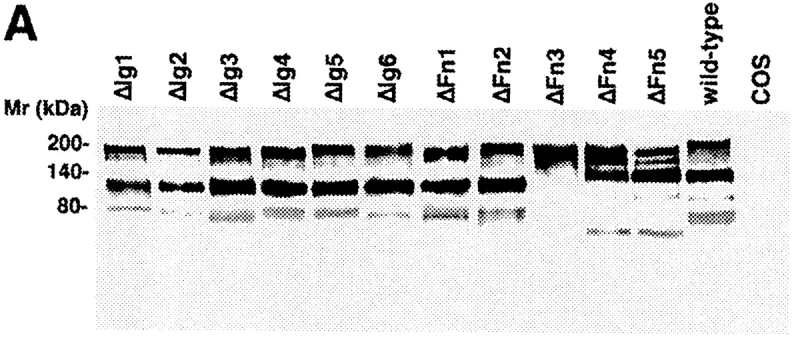
Analysis of the molecular masses of wild-type NgCAM and NgCAM domain deletion mutants transiently expressed in COS7 cells. Total protein of nontransfected COS cells (COS) and COS cells transfected with wild-type NgCAM (wild-type) and the and NgCAM single (A) and double (B) domain deletion mutants was separated by SDS-PAGE, transferred to nitrocellulose and immunostained using a polyclonal anti-NgCAM antibody followed by enhanced chemiluminescence detection. Molecular masses are indicated.
Figure 2.
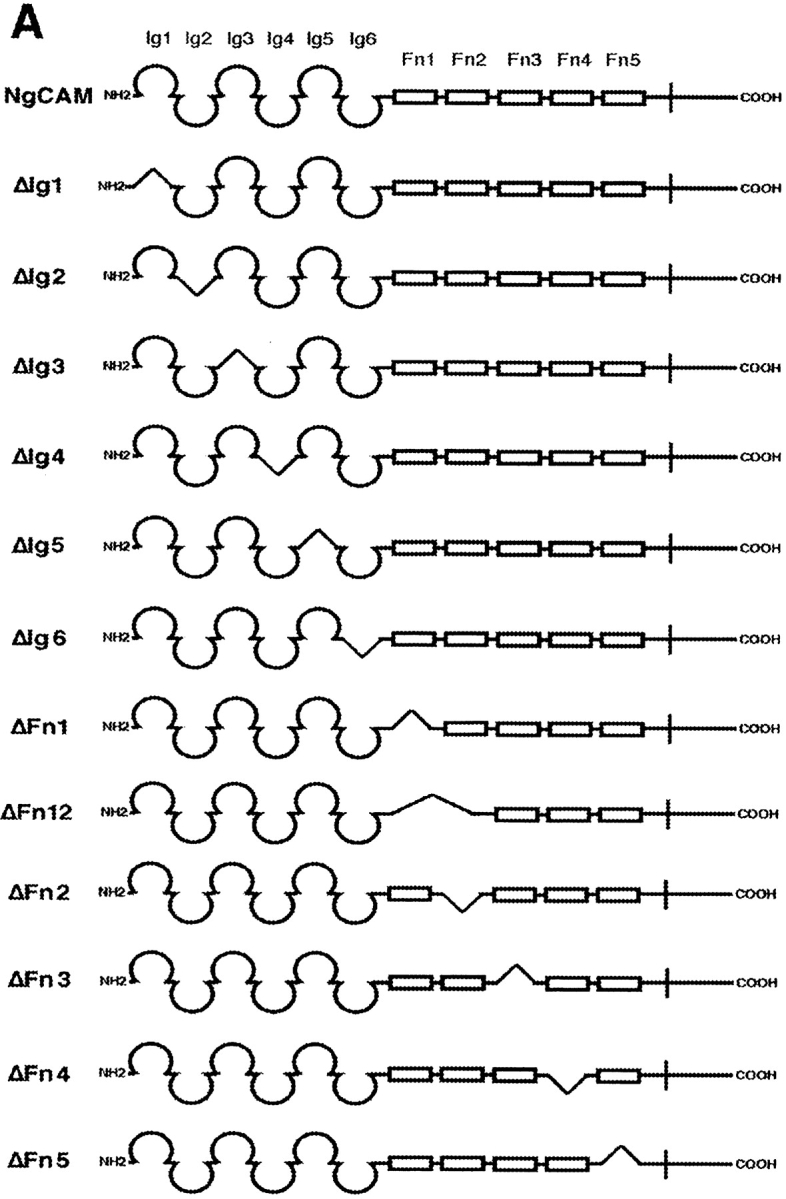
Axonin-1 binding to wild-type and domain deletion mutants of NgCAM. (A) Schematic representation of the NgCAM domain deletion mutants. NH2-termini are on the left (NH2) and COOH termini on the right (COOH). Ig domains are represented by half-circles and FnIII-like domains by rectangular boxes. The lines indicate the domain deletions. (B) Binding of axonin-conjugated TRITC Covaspheres to COS cells expressing wild-type NgCAM and the domain deletion mutants. NgCAM expressing cells were identified by indirect immunofluorescence staining using a polyclonal anti-NgCAM antibody and a FITC-labeled secondary antibody. For each mutant 100 green fluorescing cells were analyzed. Green fluorescing cells that bound four or more red fluorescing Covaspheres were scored as positive cells. Cells that did not express NgCAM were used as internal negative controls and were found to bind on the average 0.2 axonin-conjugated Covaspheres per cell. Each column corresponds to four independent experiments (± SD).
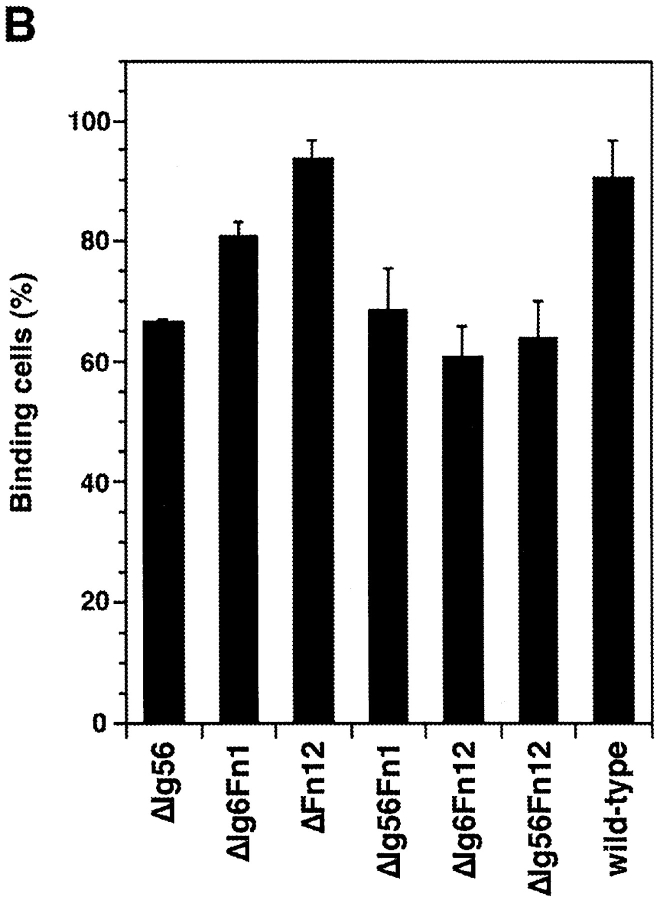
The axonin-1 binding properties of ΔIg5, ΔIg6, and ΔFn12 show clearly that the domains Ig5, Ig6, Fn1, and Fn2, which link the NgCAM domains Ig2-4 and Fn3, are not essential for axonin-1 binding. However, a marked reduction of axonin-1 binding was observed with the mutant ΔIg56 (Fig. 3 B). Because the deletions of Ig5 and Ig6 individually do not reduce axonin-1 binding, the possibility of an involvement of Ig5 or Ig6 in the axonin-1–NgCAM interaction must be excluded (Fig. 2 B). The effect of the deletion of the domain tandem Ig5+Ig6 cannot be ascribed to a reduced spacing between Ig2-4 and Fn3, because no comparable effect was observed with the deletion mutants ΔIg6Fn1 and ΔFn12. The mutants ΔIg56Fn1, ΔIg6Fn12, which lack three domains, and ΔIg56Fn12, which lacks all four domains between Ig2-4 and Fn3 exhibit axonin-1 binding comparable to ΔIg56 (Fig. 3 B). This indicates that the domains Ig5-Fn2 do not participate directly in axonin-1 binding but rather provide the conformational flexibility required for an optimal arrangement of Ig2-4 and Fn3 with respect to the corresponding binding areas on axonin-1.
Figure 3.
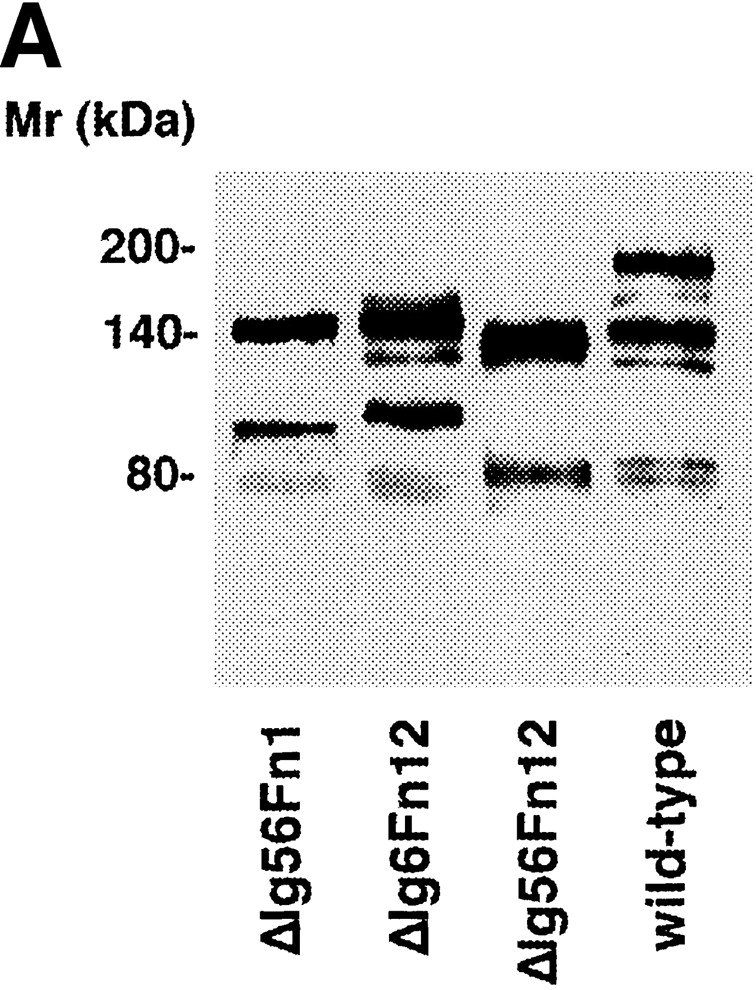
Axonin-1 binding to wild-type NgCAM and the domain deletion mutants ΔIg56, ΔIg6Fn1, ΔFn12, ΔIg56 Fn1, ΔIg6Fn12, and ΔIg56Fn12. (A) Analysis of the molecular masses of the NgCAM domain deletion mutants ΔIg56Fn1, ΔIg6Fn12, and ΔIg56Fn12 transiently expressed in COS7 cells. Western blot analysis was performed as described in Fig. 1, using a polyclonal anti-NgCAM antibody followed by enhanced chemiluminescence for detection. Molecular masses are indicated. (B) Binding of axonin-conjugated TRITC Covaspheres to COS cells expressing wild-type NgCAM and the domain deletion mutants ΔIg56, ΔIg6Fn1, ΔFn12, ΔIg56Fn1, ΔIg6Fn12, and ΔIg56Fn12. The detection of NgCAM expressing cells and the quantification was carried out as described in Fig. 2. Each column corresponds to three independent experiments (± SD).
Proteolytic Cleavage of NgCAM Is Not Required for Axonin-1 Binding
In neural tissue, NgCAM is proteolytically processed, resulting in a NH2-terminal fragment of 135 kD and a transmembrane fragment of 80 kD (Grumet et al., 1984). The cleavage site is localized within the third FnIII-type domain, between the residues Q866 and A867 (Burgoon et al., 1991). NgCAM from COS7 cells exhibits a similar, although less pronounced, proteolytic processing (Buchstaller et al., 1996). The function of the proteolytic cleavage of NgCAM is currently unclear. As the deletion of Fn3 resulted in an uncleaved form of NgCAM that exhibited a strongly reduced binding of axonin-1, a possible effect of the proteolytic processing of NgCAM on the interaction with axonin-1 was investigated.
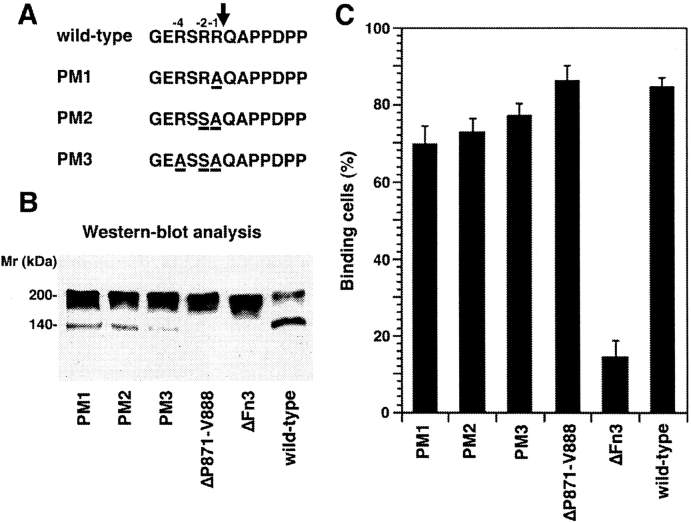
The cleavage site that is localized within the third FnIII domain is preceded by conserved Arg residues at positions −1 (R865), −2 (R864), and −4 (R862). This pattern of conserved residues resembles the recognition sequences for furin-type proteases (Burgoon et al., 1991; Hosaka et al., 1991; Grumet and Sakurai, 1996). To generate NgCAM variants deficient in proteolytic cleavage we introduced the following point mutations: NgCAM PM1, R865; NgCAM PM2, R865A and R864S; and NgCAM PM3, R865A, R864S, and R862A (Fig. 4 A). In another construct, the segment P871-V888 that is localized COOH terminally to the cleavage site was deleted. Sequence alignments revealed that this Pro-rich sequence occurs only in NgCAM but not in its putative mammalian homologue L1, which is found primarily in the uncleaved 200-kD form (Grumet et al., 1991). Based on comparisons of the sequences of NgCAM and L1 (Grumet et al., 1991) and considering the recently published hypothetical outline structure of Fn3 of mammalian L1 (Bateman et al., 1996), we expected the deletion of the sequence P871-V888 to have an effect on proteolytic cleavage without extensive changes in the tertiary structure of Fn3.
Figure 4.
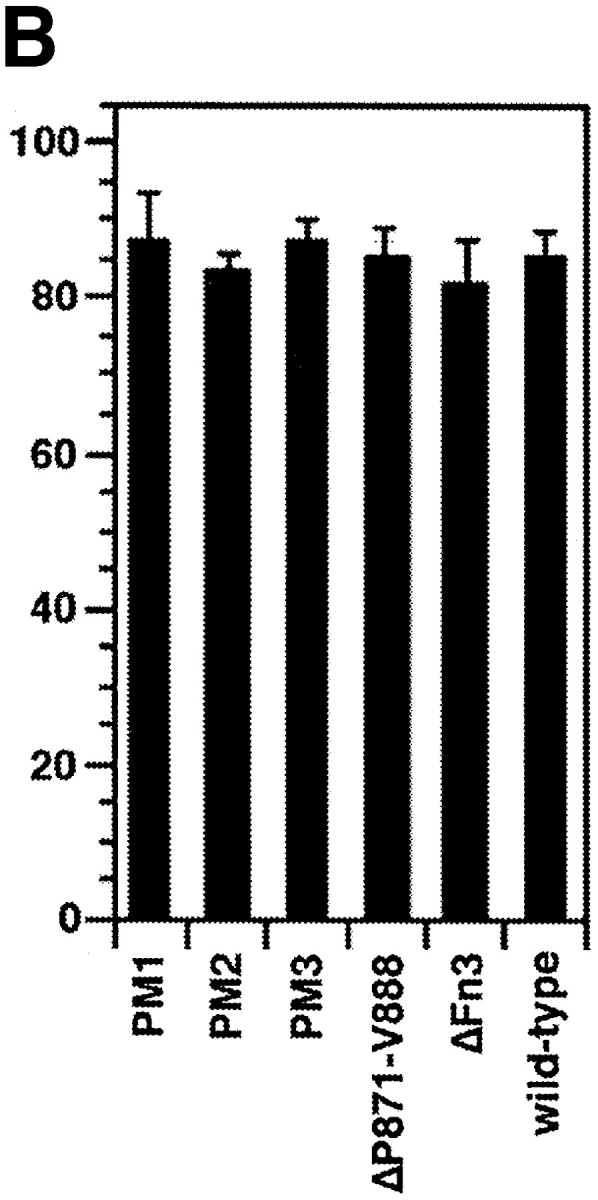
Binding of axonin-1 to the NgCAM mutants NgCAM PM1-3, NgCAM ΔP871-V888, and wild-type NgCAM. (A) Schematic representation of the point mutations introduced into positions R865, R864, and R862 of NgCAM PM1, NgCAM PM2, and NgCAM PM3. Amino acid exchanges are underlined and the site of cleavage marked by an arrow. The relative positions of the residues with respect to the cleavage site are indicated. (B) Western blot analysis. The NgCAM mutants were transiently expressed in COS7 cells, total protein separated by SDS-PAGE, transferred to nitrocellulose and immunostained using a monoclonal anti-NgCAM antibody against the 135-kD fragment followed by enhanced chemiluminescence for detection. Molecular masses are indicated. (C) Binding of axonin-conjugated TRITC Covaspheres to COS cells expressing wild-type NgCAM and the NgCAM mutants NgCAM PM1-3, NgCAM ΔP871-V888. The identification of NgCAM expressing cells and the quantification of the binding of axonin-1 Covaspheres was carried out as described in Fig. 2. Each column corresponds to three independent experiments (± SD).
The NgCAM mutants were expressed in COS7 cells as described above. Proteolytic cleavage of these NgCAM variants was assessed by quantitative Western blot analysis using a monoclonal anti-NgCAM antibody that recognizes only the 135-kD fragment for detection. The extent of proteolytic processing is reflected by the relative intensities of the 135-kD band compared with the sum of the 135- and the 200-kD band, that represents the uncleaved form of NgCAM (Fig. 4 B). Compared with wild-type NgCAM that was cleaved to ∼60%, densitometric analysis revealed a sixfold reduction of proteolytic cleavage for PM1, carrying the point mutation R865A in position −1. No further reduction was observed with the additional point mutation R864S in position −2. In contrast, a strong additional reduction in cleavage, to ∼25-fold compared with wild-type, was observed after the additional exchange R862A in position −4. Cleavage was entirely absent from NgCAMΔP871-V888 (for details see Materials and Methods). To investigate the possible effects of the reduced cleavage of these NgCAM variants on their interaction with axonin-1, binding studies using axonin-1–conjugated Covaspheres were performed. Surprisingly, none of the NgCAM mutants exhibited a significant reduction in axonin-1 binding when compared with the wild-type (Fig. 4 C). These data clearly demonstrate that proteolytic cleavage of NgCAM is not required for the binding to axonin-1. Thus, the strongly reduced binding of ΔFn3 cannot be ascribed to the lack of proteolytic processing of this mutant but must be caused by the actual deletion of the domain.
Ig2-4 and Fn3 of NgCAM Interact with the Four NH2-terminal Domains of Axonin-1
Because the four NH2-terminal Ig domains of axonin-1 were previously found to form a domain conglomerate that is necessary and sufficient for NgCAM binding (Rader et al., 1996), we also tested the binding of the domain deletion mutants of NgCAM with two truncated forms of axonin-1, axonin-1 Ig1234-Cκ, and axonin-1 ΔIg1234-Cκ. The soluble axonin-1 fragments were produced as fusion proteins with the mouse Cκ domain, as described by Rader et al. (1996), coupled to fluorescent microspheres, and analyzed for their NgCAM binding as described for wild-type axonin-1 (Fig. 5). The binding pattern of axonin-1 Ig1234-Cκ to the NgCAM mutants (Fig. 5 C) was very similar to that of wild type axonin-1 (Fig. 2). In contrast, the complementary axonin-1 fragment axonin-1 ΔIg1234-Cκ did not show any binding to the NgCAM mutants (Fig. 5 C). This result provides experimental evidence that the binding of axonin-1 to both, Ig2-4 and Fn3 of NgCAM is mediated by the four NH2-terminal Ig domains of axonin-1. The loss of binding of axonin-1 Ig1234-Cκ to NgCAM by deletion of one of the domains Ig2-4 and Fn3 indicates that the domain conglomerate formed by the four NH2-terminal Ig domains of axonin-1 must interact simultaneously with Ig2-4 and Fn3 of NgCAM.
Figure 5.
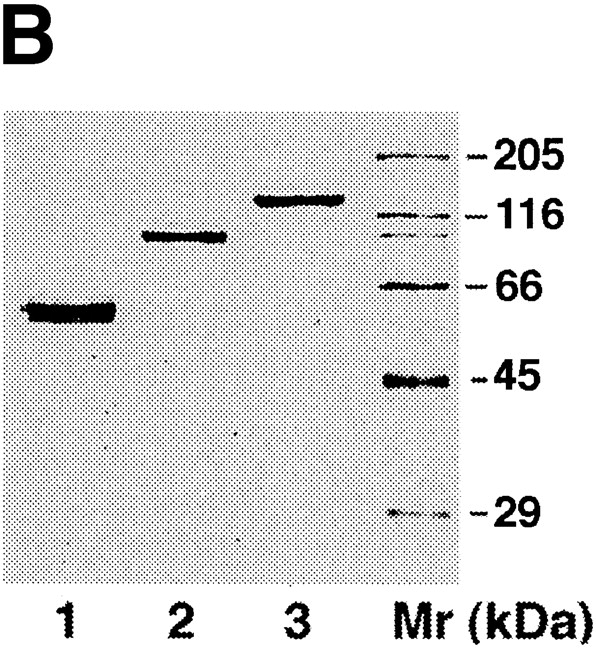
Binding of axonin-1 Ig1234-Cκ and axonin-1 ΔIg1234-Cκ to wild-type NgCAM and the two series of domain deletion mutants. (A) Schematic representation of the soluble axonin-1-fragments fused to mouse Ig Cκ domain (filled bars). As in Fig. 2, Ig domains are represented by half-circles and FnIII-like domains by empty bars. The molecules were expressed in stably transfected myeloma cells and purified from the supernatants by immunoaffinity-chromatography using an anti-mouse Ig Cκ monoclonal antibody. (B) Purified proteins were subjected to SDS-PAGE and visualized by silver staining. Lane 1, axonin-1 Ig1234-Cκ; lane 2, axonin-1 ΔIg1234-Cκ; lane 3, native axonin-1 purified from the vitreous fluid of E14 chicken embryos; lane Mr represents the molecular mass standard. (C) The soluble fusion proteins axonin-1 Ig1234-Cκ and axonin-1 ΔIg1234-Cκ were coupled to TRITC-conjugated Covaspheres and tested for binding to the NgCAM mutants expressed on COS cells. The experiment was carried out as described in Fig. 2. Again, green fluorescing cells that bound four or more red fluorescing Covaspheres were scored as positive cells. Binding of Ig1234-Cκ is represented by the empty bars, ΔIg1234-Cκ by the filled bars. Cells that did not express NgCAM were used as internal negative controls and were found to bind on the average 0.1 Covaspheres per cell for Ig1234-Cκ and 0.5 Covaspheres per cell in case of ΔIg1234-Cκ. Each column corresponds to four independent experiments (± SD).
The Four NH2-terminal Ig Domains of NgCAM Are Involved in NgCAM Homophilic Binding
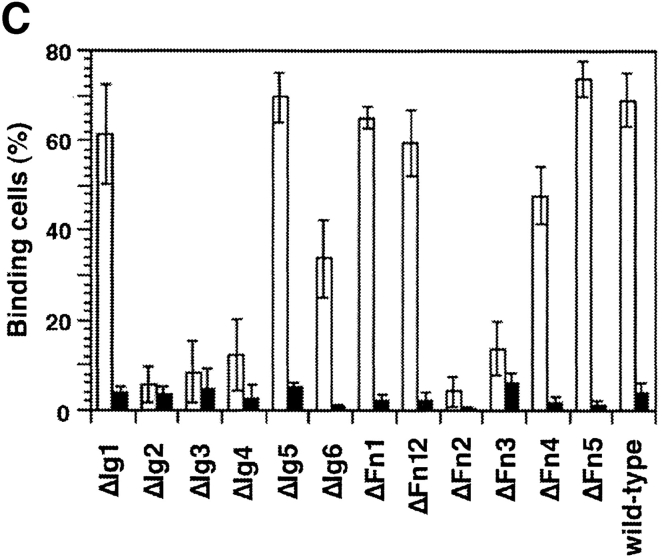
For the identification of the NgCAM domains involved in homophilic binding we investigated the binding of NgCAM-conjugated microspheres to both series of domain deletion mutants of NgCAM (Fig. 6). The data derived from both sets of NgCAM deletion mutants show clearly that deletions of the four NH2-terminal Ig domains of NgCAM resulted in a strong reduction of the homophilic binding. In addition, a significant reduction of the homophilic binding was observed with ΔFn2. In contrast, no reduction of binding was found with the mutant lacking the domain tandem Fn1+Fn2, ΔFn12. This result resembles the situation regarding the binding of axonin-1 described above. The reduced homophilic binding of ΔFn2 could reflect an impaired folding of the NgCAM mutant due to the deletion of Fn2 rather than an involvement of Fn2 in the homophilic interaction.
Figure 6.
Binding of NgCAM-microspheres to wild-type NgCAM, the domain deletion mutants, and the NgCAM variants deficient in proteolytic processing. Binding of NgCAM-conjugated TRITC Covaspheres to COS cells expressing wild-type NgCAM and the domain deletion mutants (A) and the mutants NgCAM PM1-3 and NgCAMΔP871-V888 (B). NgCAM expressing cells were identified as described in Fig. 2. For each mutant 100 green fluorescing cells were analyzed. Green fluorescing cells that bound four or more red fluorescing Covaspheres were scored as positive cells. Cells that did not express NgCAM were used as internal negative controls and were found to bind on the average 0.1 NgCAM-conjugated Covaspheres per cell. Each column corresponds to three independent experiments (± SD).
To address the question whether proteolytic cleavage of NgCAM in Fn3 has an influence on the homophilic interaction we tested the binding of NgCAM-conjugated Covaspheres to the NgCAM mutants NgCAM PM1-3 and NgCAMΔP871-V888. All NgCAM mutants, including ΔFn3 exhibited homophilic binding comparable to the wild-type (Fig. 6 B). As the axonin-1–NgCAM interaction, NgCAM homophilic binding was not affected in mutants that were not processed.
Coexpression of Axonin-1 and NgCAM in the Same Membrane Does Not Affect the Homophilic Binding Capacity of NgCAM, but Reduces Homophilic Binding of Axonin-1
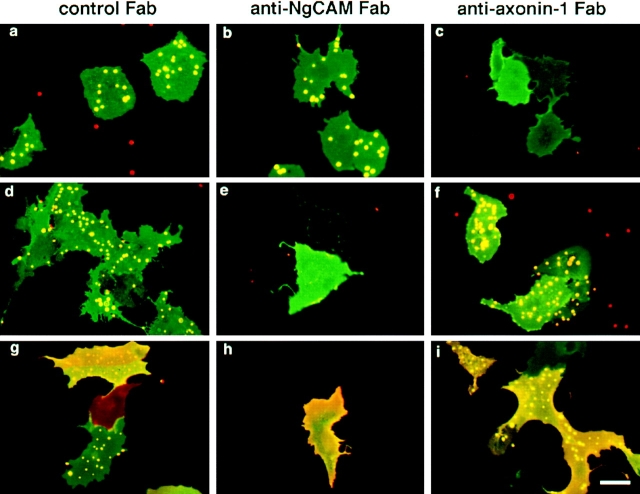
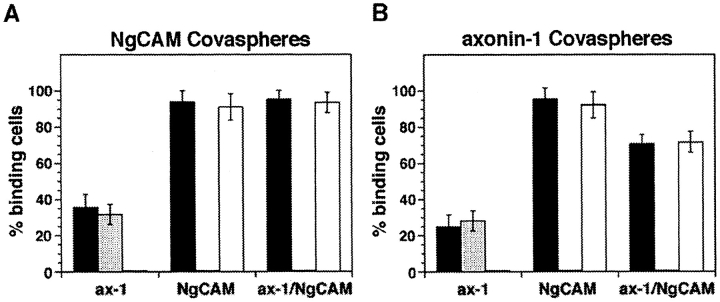
The results of the domain mapping studies described above indicate an overlap of the domains of NgCAM involved in axonin-1 binding, Ig2-4 and Fn3, and the domains involved in the homophilic NgCAM interaction, Ig1-4. Likewise, localization of the binding sites on axonin-1 had revealed that the axonin-1 domains Ig1-4, which mediate NgCAM binding, overlap with those identified recently to be involved in axonin-1 homophilic binding, Ig1 and Fn4 (Kunz, B., R. Lierheimer, C. Rader, U. Ziegler, L. Vogt, M. Spirig, and P. Sonderegger, manuscript submitted for publication). The participation of some domains of both axonin-1 and NgCAM in homophilic and heterophilic interaction rises the possibility of a competition between the axonin-1–NgCAM binding and the homophilic interactions of each of the two molecules. To address this question, we investigated the effect of a coexpression of axonin-1 with NgCAM on the homophilic interactions of axonin-1 and NgCAM. For this purpose, the expression constructs pSCTaxonin-1 (Rader et al., 1993) and pSCTNgCAM were introduced individually or together into COS7 cells by electroporation, resulting in the expression of either axonin-1 or NgCAM alone or the coexpression of axonin-1 and NgCAM. 48 h after transfection the cells were preincubated with either control Fab (prepared from preimmune serum), anti-axonin-1, or anti-NgCAM Fab, washed extensively, and subsequently incubated with microspheres conjugated to NgCAM or axonin-1. The expression of axonin-1 and NgCAM was detected by immunofluorescence staining using goat anti-axonin-1 and rabbit anti-NgCAM antibodies and the corresponding secondary antibodies conjugated to FITC or Texas red, respectively. The coexpressing cells were clearly identified by their yellow staining using a narrow band-pass double filter for analysis. For quantification, we selected cells with comparable intensity of the individual immunofluorescence staining of axonin-1 and NgCAM. The results of this study are partially presented in Fig. 7. A summary of all results is represented by Table I and a quantitative analysis is shown in Fig. 8.
Figure 7.
Binding of NgCAM-microspheres to cells expressing axonin-1, NgCAM or coexpressing axonin-1 and NgCAM. COS cells were transfected with axonin-1 (a– c), NgCAM (d–f) and cotransfected with axonin-1 and NgCAM (g–i). The cells were preincubated with anti-axonin-1, anti-NgCAM, and control Fab as indicated. Binding of TRITC-conjugated NgCAM Covaspheres was assessed. Axonin-1 expressing cells in a–c were identified by indirect immunofluorescence staining using a polyclonal anti-axonin-1 antibody and a FITC-labeled secondary antibody. An identical staining protocol was applied for the detection of NgCAM in d–f, using a polyclonal anti-NgCAM antibody. Axonin-1–NgCAM coexpressing cells were detected in g and h by the application of goat polyclonal anti-NgCAM antibody (green fluorescence due to the FITC labeled secondary antibody) combined with rabbit anti-axonin-1 antibody (red fluorescence due to the Texas red–labeled secondary antibody). In i, axonin-1 was detected by a goat polyclonal antibody, combined with a the FITC-labeled secondary antibody and NgCAM by a rabbit anti-NgCAM antibody using a the Texas red–labeled secondary antibody. Axonin-1–NgCAM coexpressing cells appear in yellow as a Texas red/FITC double filter device was used. TRITC-conjugated NgCAM Covaspheres can be easily recognized on coexpressing cells. Bar, 20 μm.
Table I.
Binding of Axonin-1 and NgCAM to Cells That Express Axonin-1, NgCAM, or Coexpress Axonin-1 or NgCAM
| Expression on COS cells | Preincubation with Fab | Binding of microspheres | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Anti-NgCAM | Anti-axonin-1 | NgCAM | Axonin-1 | ||||||
| Axonin-1 | X | + | + | |||||||
| Axonin-1 | X | + | + | |||||||
| Axonin-1 | X | − | − | |||||||
| NgCAM | X | + | + | |||||||
| NgCAM | X | − | − | |||||||
| NgCAM | X | + | + | |||||||
| Axonin1/NgCAM | X | + | +* | |||||||
| Axonin1/NgCAM | X | − | − | |||||||
| Axonin1/NgCAM | X | + | +* | |||||||
COS cells were transfected with axonin-1 (Axonin-1), NgCAM (NgCAM), and cotransfected with axonin-1 and NgCAM (Axonin-1/NgCAM). The cells were preincubated with control, anti-axonin-1, and anti-NgCAM Fab as indicated (X). Binding of TRITC-conjugated Covaspheres conjugated to axonin-1 and NgCAM was assessed as described in Fig. 2. Binding comparable to controls is represented by +, no detectable binding by −, and partially reduced binding by +
, according to the quantitative analysis represented in Fig. 8.
Figure 8.
Binding of NgCAM and axonin-1-microspheres to cells that express axonin-1, NgCAM or coexpressed axonin-1 and NgCAM: Quantitative analysis of the binding of NgCAM (A) and axonin-1 (B) Covaspheres to COS cells expressing axonin-1 (ax-1), NgCAM (NgCAM) and coexpressing axonin-1 and NgCAM (ax-1/NgCAM). Cells were preincubated with control- (filled bars), anti-NgCAM- (shaded bars), and anti-axonin-1- (empty bars) Fab. In case of the single axonin-1 or NgCAM transfections, green fluorescing cells that bound four or more red fluorescing Covaspheres were scored as positive cells. In axonin-1–NgCAM cotransfection cells of comparable fluorescence for axonin-1 and NgCAM staining were selected by examination with the corresponding single-pass filters. Again, cells that bound four or more red fluorescing Covaspheres were scored as positive. Cells that did not express NgCAM or axonin-1 were used as internal negative controls and were found to bind on the average 0.1 axonin-1 or NgCAM Covaspheres. Each column corresponds to four independent experiments (± SD). The capability of NgCAM to bind axonin-1 microspheres is only partially reduced in axonin-1–NgCAM coexpressing cells. This may be due to a stoichiometric excess of NgCAM versus axonin-1 in the coexpressing cells we selected for quantification. Combined analysis using immunofluorescence microscopy and quantitative immunoblotting, performed on axonin-1–NgCAM coexpressing cells indeed revealed a comparably weaker immunostaining of NgCAM on cells with similar amounts of axonin-1 and NgCAM protein (data not shown).
The binding of NgCAM microspheres to cells coexpressing axonin-1 and NgCAM was similar to the binding observed with cells expressing NgCAM alone (Fig. 7). This indicates that the capability of NgCAM to undergo homophilic binding is not altered by the coexpression of axonin-1 in the same cell. However, a change in the accessibility of axonin-1 in the axonin-1–NgCAM coexpressing cells was observed by antibody pertubation experiments. Binding of axonin-1- and NgCAM-microspheres to cells coexpressing NgCAM and axonin-1 was completely abolished after masking of cellular NgCAM by preincubation with anti-NgCAM Fab (Fig. 7 h, data not shown). No effect was observed with anti-axonin-1 Fab (Fig. 7 i, data not shown). This indicates that axonin-1- and NgCAM-microspheres bind to coexpressing cells exclusively via cellular NgCAM. In contrast, cellular axonin-1 seems to be inaccessible for an interaction with axonin-1 or NgCAM when coexpressed with NgCAM. These observations suggest that the heterophilic axonin-1–NgCAM interaction, which is thought to take place in the plane of the membrane (Stoeckli et al., 1996; Buchstaller et al., 1996), excludes homophilic axonin-1 binding but does not interfere with the homophilic NgCAM interaction.
Coclustering of Axonin-1 and NgCAM at Cell Contacts Is Mediated by NgCAM Homophilic Binding Across the Extracellular Space and an Interaction of Axonin-1 with NgCAM in the Plane of the Same Membrane
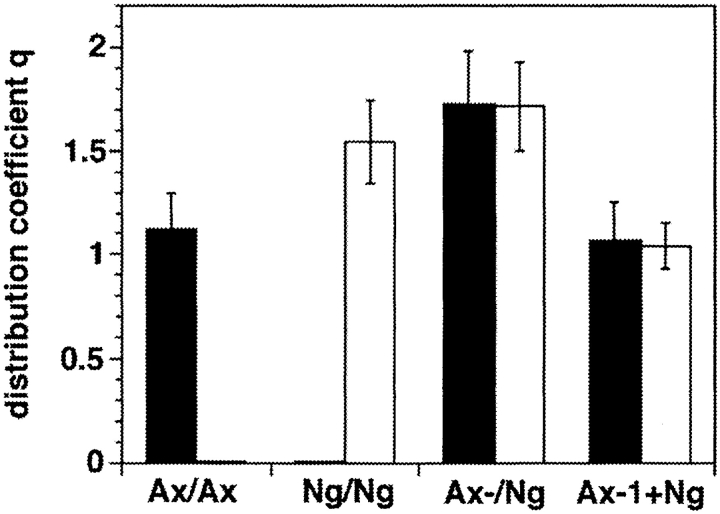
To investigate the molecular interactions underlying the formation of complexes of axonin-1 and NgCAM at cell contacts we used CV-1 cells stably transfected with full-length cDNAs of axonin-1 or NgCAM alone or stably cotransfected with both cDNAs. Cultures of single transfectants, double transfectants and mixtures of single transfectants were kept for 24 h to allow the formation of extended cell contacts. Subsequently, the cells were fixed using 2% (wt/vol) formaldehyde and 0.1% (wt/vol) glutaraldehyde to obtain complete immobilization of membrane proteins without permeabilization of the cells (Dubreuil et al., 1996). Axonin-1 and NgCAM were localized by immunofluorescence staining using goat anti-axonin-1 and rabbit anti-NgCAM antibodies and the corresponding secondary antibodies conjugated to FITC or Texas red, respectively. The distribution of axonin-1 and NgCAM was quantitatively analyzed by confocal laser scanning microscopy. To obtain cell layers of comparable thickness we integrated identical numbers of confocal sections for image generation and quantitative analysis (Figs. 9 and 10). The accumulation of axonin-1 and NgCAM in the different situations is reflected by the distribution coefficient that represents the ratio of protein detected at the cell contact versus protein at the surface of the adjacent cells (Fig. 10). These distribution coefficients represent underestimations of the actual amount of protein accumulated at the cell contact region due to a limited accessibility of the molecules localized in closely apposed membranes (for details see Materials and Methods).
Figure 9.
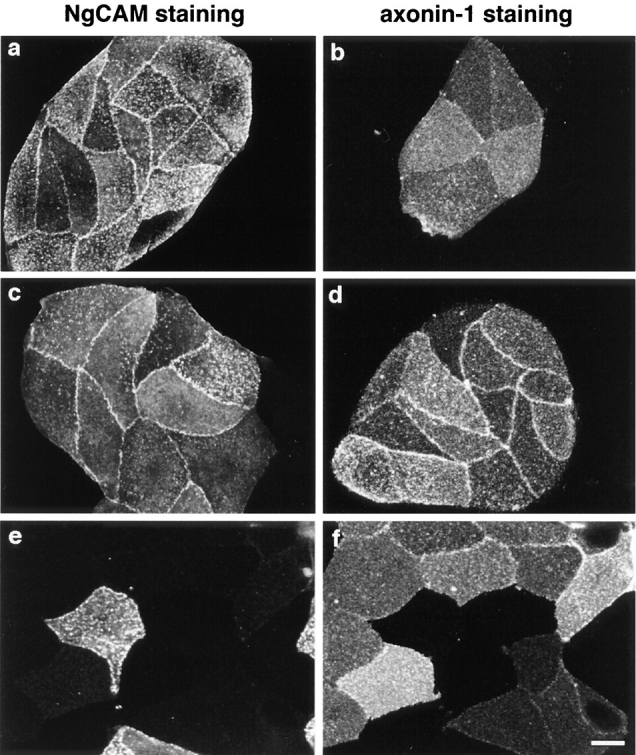
Localization of axonin-1 and NgCAM at cell contacts of stably transfected CV-1 cells: CV-1 cells were stably transfected with NgCAM (a) and axonin-1 (b) or stably cotransfected with axonin-1 and NgCAM (c and d). e and f represent mixtures of single axonin-1 and NgCAM transfectants. Axonin-1 and NgCAM were stained using a rabbit polyclonal anti-axonin-1 antibody (axonin-1 staining) combined with a Texas red–labeled secondary antibody and a goat anti-NgCAM antibody (NgCAM staining) combined with a FITC-labeled secondary antibody. Images were generated by confocal laser-scanning microscopy. To examine cell layers of equal thickness, equal numbers of confocal sections were integrated for image generation. Bar, 20 μm.
Figure 10.
Quantitative analysis of the distribution of axonin-1 and NgCAM in stably transfected CV-1 cells: The distributions of axonin-1 (filled bars) and NgCAM (empty bars) were quantified by analysis of the fluorescence intensity of the axonin-1 and NgCAM immunofluorescence staining in axonin-1 single transfectants (Ax), NgCAM single transfectants (Ng), axonin-1–NgCAM cotransfectants (Ax-/Ng) and mixtures of axonin-1 and NgCAM single transfectants (Ax-1+Ng). The combinations of antibodies used correspond to those in Fig. 9. To obtain cell layers with comparable geometry, identical numbers of confocal sections were integrated for quantitative analysis of the distribution of axonin-1 and NgCAM. For quantification distribution coefficients were calculated. In case of cultures consisting of only one type of cell the distribution coefficient was calculated as: q = 2 × Icell-contact/ Icell1 + Icell2 and in cases of contacts formed by different cell types (e.g., axonin-1 and NgCAM expressing cells) q* = Icell-contact/ Iexpressing cell. The term Icell-contact represents the fluorescence intensity per area at the cell contact and the terms Icell1, Icell2, and Iexpressing cell represent the fluorescence intensity per area at the surface of the cells forming the contacts. Bars represent means ± SD, n = 30 for NgCAM and n = 24 for axonin-1 single transfectants, n = 24 for axonin-1–NgCAM double transfectants, and n = 24 and for the mixtures of axonin-1 and NgCAM single transfectants.
Accumulation of NgCAM was observed at contacts formed by cells expressing NgCAM alone (Fig. 9 a) or NgCAM in combination with axonin-1 (Fig. 9 c). Accumulation of axonin-1 at cell contacts was found to be critically dependent on the presence of NgCAM in the same cell. Strong axonin-1 accumulation was detected at cell contacts formed by cells coexpressing axonin-1 and NgCAM (Fig. 9 d), but only a slight accumulation was found at contacts formed by axonin-1 single transfected cells (Fig. 9 b). No accumulation of axonin-1 and NgCAM was found at mixed contacts formed by axonin-1 and NgCAM single transfectants (Fig. 9, e and f). This indicates the absence of a heterophilic interaction between axonin-1 and NgCAM, when the two molecules are present in different cells (trans-interaction). Based on these findings and the results of the preceding section, we propose that the coclustering of NgCAM and axonin-1 at the cell contact area of axonin-1–NgCAM coexpressing cells is driven by a trans-homophilic NgCAM binding. The accumulation of axonin-1 at the same sites results from an interaction of axonin-1 with NgCAM in the plane of the membrane that occurs simultaneously with the trans-homophilic NgCAM binding.
Overexpression of NgCAM Enhances Neurite Fasciculation in DRG Explants
The data presented above indicate that in the fasciculation of neurites bearing axonin-1 and NgCAM it is a homophilic NgCAM interaction that mediates the contacts between adjacent neurite membranes. The cis-axonin-1–NgCAM interactions that occur simultaneously in both membranes would result in the formation of a symmetric tetrameric axonin-1–NgCAM complex. Complexes of the expected size and composition were indeed detected after chemical cross-linking on strongly fasciculated but not on unfasciculated neuronal cultures (Kunz et al., 1996). This distinctive interactive pattern of axonin-1 and NgCAM in fascicles formed by neurons coexpressing the two molecules introduced the possibility that changes in the stoichiometric ratio of the two molecules might modulate neurite fasciculation. To test this hypothesis we changed the stoichiometric ratios of axonin-1 and NgCAM in DRG explants using adenoviral gene transfer vectors of axonin-1 (AdCMVax-1) and NgCAM (AdCMVNgCAM). Mouse DRG explants were used because they develop long neurites that extend away from the ganglion in a radial orientation and are highly susceptible to adenoviral infection. Cross-species studies demonstrated that the mouse homologues of axonin-1 and NgCAM, TAG-1, and L1, can undergo functional interactions with their chicken counterparts (Lemmon et al., 1989; Felsenfeld et al., 1994). Therefore, heterologous expression of axonin-1 and NgCAM in mouse DRG neurons corresponds functionally to an overexpression of TAG-1/axonin-1 and L1/NgCAM.
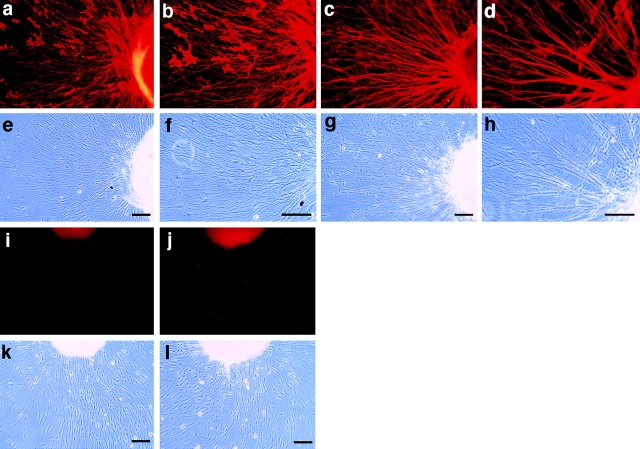
Adenoviral gene transfer was initiated after 12 h in culture by infection of the DRG explants with AdCMVax-1, AdCMVNgCAM, and AdCMVlacZ. At the time of infection, the ganglia had already initiated neurite outgrowth. 48 h after infection the DRG explants were fixed by 2% formaldehyde and 0.1% glutaraldehyde. Axonin-1 and NgCAM that appeared on the extracellular face of the axonal membrane were visualized by immunofluorescence staining using specific monoclonal antibodies that did not cross-react with TAG-1 and L1. Strong heterologous expression of axonin-1 and NgCAM was detected on ∼80% of the neurites. β-galactosidase staining of ganglia infected with AdCMVlacZ revealed a similar rate of infection. The morphological integrity of the explants infected with the various viral constructs in comparison to uninfected explants was examined microscopically using Nomarski optics. No significant morphological alterations due to viral cytotoxicity were detected. After 60 h in culture most neurites stained homogeneously for axonin-1 and NgCAM from the growth cone at the leading tip to the neurite shaft proximal to the ganglion. In contrast, at an earlier time point, after only 20 h in culture, a predominant staining of the growth cone tip and the neurite shaft distally from the ganglion was detected. This is in good accordance with previous studies indicating that newly synthesized axonin-1 and NgCAM are inserted into the axonal membrane of the growth cone (Vogt et al., 1996).
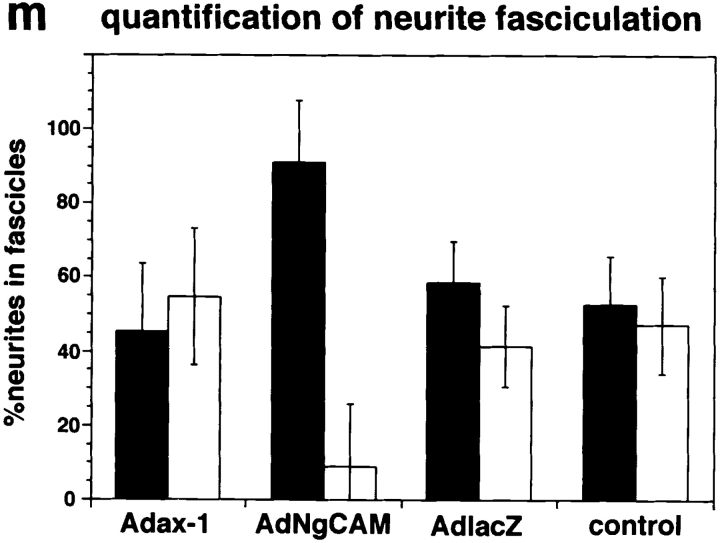
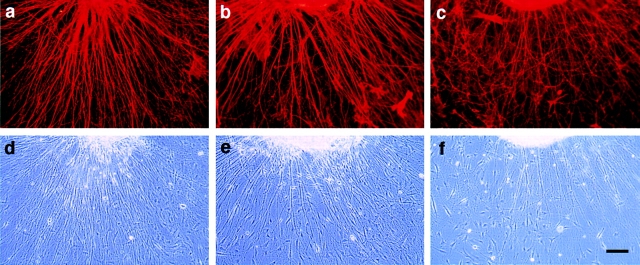
Overexpression of axonin-1 resulted in a slightly reduced neurite fasciculation (Fig. 11, a, b, e, and f) compared with control explants treated with AdCMVlacZ (not shown) or without virus (Fig. 11, i–m). In contrast, overexpression of NgCAM resulted in strongly enhanced fasciculation (Fig. 11, c, d, g, and h). Quantitative analysis of the effects of axonin-1 and NgCAM overexpression on neurite fasciculation are summarized in Fig. 11 m (for details see Materials and methods). The effect of NgCAM overexpression on neurite fasciculation was blocked specifically by the addition of monoclonal anti-NgCAM IgG (Fig. 12, c and f) but not by control IgG (Fig. 12, b and e). The enhanced fasciculation observed with neurites overexpressing NgCAM can therefore be ascribed specifically to the enhanced NgCAM expression in the neuronal membrane. The observed effects of axonin-1 and NgCAM overexpression on neurite fasciculation are in accordance with the observation that NgCAM but not axonin-1 can efficiently mediate contact across the extracellular space in the context of heterooligomeric axonin-1–NgCAM complexes.
Figure 11.
Expression of chicken axonin-1 and NgCAM in mouse DRG explants: Cultured mouse DRG explants were infected with the adenoviral vectors AdCMVax-1 (a, b, e, and f) and AdCMVNgCAM (c, d, g, and h), i–l represent uninfected controls. 60 h after infection axonin-1 and NgCAM were detected on the cell surface by indirect immunofluorescence. For immunofluorescence staining the axonin-1–specific monoclonal antibody X7C11 and the NgCAM-specific monoclonal antibody 12-I-4E-311, and a Cy3-labeled donkey anti–mouse secondary antibody were used. Axonin-1 staining: a, b, and i (fluorescence optics; e, f, and k phase optics); NgCAM staining: c, d, and j (fluorescence optics; g, h, and l phase optics). Quantitative analysis of neurite fasciculation is represented by m. In brief, for quantification individual neurites that stained positively for the heterologously expressed protein were tracked from the periphery of the ganglion to a point at one-third of the total neurite length. Neurites that coalesced into bundles consisting of more than 4 neurites were scored as the stronger fasciculated category (filled bars) and neurites found in bundles of less than 4 neurites belonged to the other category (empty bars). 50 neurites were scored per ganglion, n = 6 (6 ganglia were analyzed per sample). Bars represent means ± SD. Bars, 100 μm.
Figure 12.
Pertubation of neurite fasciculation with species-specific anti-NgCAM antibodies. Cultured mouse DRG explants were infected with the adenoviral vector AdCMVNgCAM. The explants were incubated in the presence of control mouse IgG (a and d), monoclonal anti-axonin-1 IgG X7C11 (b and e) and the monoclonal mouse anti-NgCAM IgG 12-I-4E-311 (c and f). NgCAM was detected on the cell surface by indirect immunofluorescence as described in Fig. 11; a–c represent fluorescence optics and d–f the corresponding phase optics. Bar, 100 μm.
Discussion
The Ig Domains 2-4 and the Third FnIII Domain of NgCAM Are Essential for the Binding to Axonin-1 in the Plane of the Membrane
A direct binding between axonin-1 and NgCAM was originally demonstrated using the purified proteins coupled to the surface of microspheres (Kuhn et al., 1991). Further studies, including this paper, revealed that axonin-1 and NgCAM cannot interact when present on the surface of different cells (Buchstaller et al., 1996). In contrast, antibody-induced cocapping and chemical cross-linking indicated a specific and direct binding of the two molecules in the plane of the same membrane (cis-interaction; Buchstaller et al., 1996). In the Covaspheres-binding assays performed in this study, membrane-bound NgCAM can engage in a heterophilic binding with axonin-1 that is presented in the Covaspheres-conjugated form. This indicates that at least one fraction of the axonin-1 molecules on Covaspheres is bound in an orientation appropriate for binding cell surface exposed NgCAM, whereas membrane-anchored axonin-1 is presented only on one orientation, that allows cis- but not trans-interaction. We suggest that the fraction of axonin-1 on Covaspheres that binds to cellular NgCAM is oriented in a way that it approaches NgCAM in the same relative orientation as axonin-1 present in the same membrane. The binding of axonin-1–conjugated Covaspheres corresponds therefore most probably to a cis-axonin-1–NgCAM interaction and does not represent an actual trans-binding.
Binding studies revealed that the Ig domains 2-4 (Ig2-4) and the third FnIII-type domain (Fn3) are necessary for axonin-1 binding. Both Ig2-4 and Fn3 were required for axonin-1 binding as demonstrated by the loss of axonin-1 binding after deletion of any one of the domains. These results indicate that Ig2-4 and Fn3 may contribute cooperatively to axonin-1 binding and do not represent alternative axonin-1 binding sites. The question arises whether Ig2-4 and Fn3 undergo a direct interaction with axonin-1 in a cooperative manner or whether the deletion of Fn3 simply results in a changed conformation of Ig2-4 that is incompatible with the binding of axonin-1. An extensive conformational change in the NH2-terminal Ig domains of NgCAM due to the deletion of Fn3 appears rather unlikely in the light of the absence of any effect on the homophilic NgCAM binding that is mediated by the Ig domains 1-4. However, since the third FnIII domain of NgCAM is subject to proteolytic cleavage (Burgoon et al., 1991) we addressed the possibility that the absence of proteolytic cleavage in ΔFn3 rather than the actual lack of the domain may be responsible for the loss of axonin-1 binding.
Proteolytic Processing of NgCAM Is Not Required for Binding to Axonin-1 and NgCAM Homophilic Binding
The strong and specific reduction in cleavage after amino acid exchanges at positions −1 (R865A) and −4 (R862A) from the cleavage site is in good accordance with previous studies (Hosaka et al., 1991; Jiang et al., 1993) and makes furin family proteases likely candidates for proteolytic processing of NgCAM in COS7 cells. The absence of proteolytic processing after deletion of the sequence P871-V888 that is localized COOH terminally from the cleavage site, was also not unexpected. According to sequence alignments, this Pro-rich sequence occurs specifically in NgCAM and is absent from mammalian L1, which occurs mainly in the uncleaved 200-kD form (Grumet et al., 1991). Considering the recently published outline structure of Fn3 from mammalian L1 (Bateman et al., 1996) this sequence may represent an NgCAM-specific extension of the polypeptide chain that makes the cleavage site more accessible for proteolytic enzymes. Despite their pronounced effect on proteolytic cleavage, none of these mutations had a significant effect on the heterophilic binding to axonin-1 or the homophilic interaction. These results clearly demonstrate that proteolytic processing of NgCAM is not required for binding to axonin-1 or NgCAM. It is conceivable, that after cleavage, the third FnIII domain may retain enough tertiary structure to maintain its overall conformation and the association between the two fragments of 135 and 80 kD. However, a possible functional relevance of the proteolytic processing of NgCAM cannot be excluded. Proteolytic cleavage may affect other functionally important interactions of NgCAM. Alternatively, it may exert more subtle influence on the conformational dynamics of the molecule that could be relevant for example in signal transduction.
In the context of our investigation, the reduced binding of axonin-1 to the NgCAM mutant ΔFn3 cannot be ascribed to he lack of proteolytic processing but may rather reflect the loss of an actual binding domain. Thus, a direct interaction of axonin-1 with both, Ig2-4 and Fn3 of NgCAM appears likely.
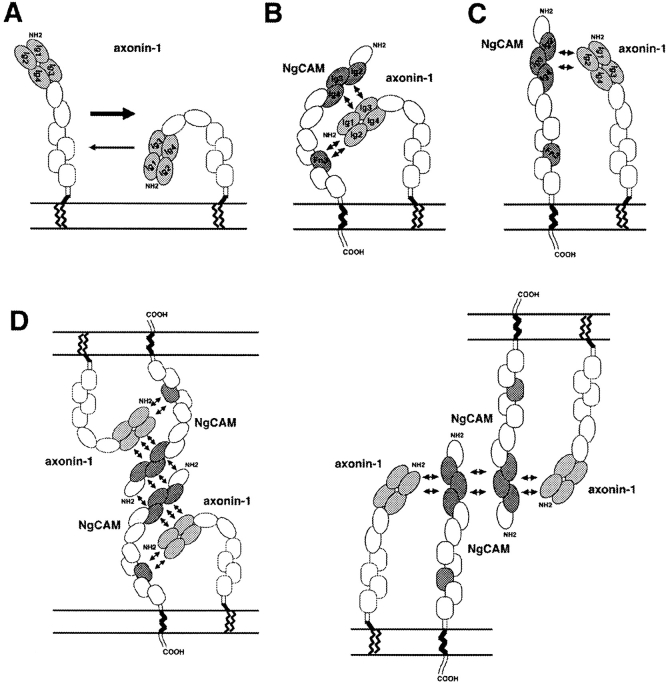
Implications for the Conformations of Axonin-1 and NgCAM within the Postulated Cis-Axonin-1–NgCAM Complex
Domain deletion studies had revealed that the first four NH2-terminal Ig domains of axonin-1 are necessary and sufficient for NgCAM binding (Rader et al., 1996). In this study, evidence was provided that the four NH2-terminal Ig domains of axonin-1 form a conglomerate that maintains its native structure only when all domains are present. Our results indicate that the binding sites for Ig2-4 and Fn3 of NgCAM reside also on this domain conglomerate. A possible simultaneous interaction of the NH2-terminal domain conglomerate of axonin-1 with Ig2-4 and Fn3 of NgCAM may bend the NgCAM molecule around the Ig1-4 conglomerate of axonin-1. This conformational change of NgCAM due to the binding of the Ig1-4 conglomerate of axonin-1 within a cis-axonin-1–NgCAM complex may require a flexible hinge between Ig2-4 and Fn3. Some evidence for this is derived from the reduced axonin-1 binding of the mutant ΔIg56 that cannot be due to a simple space effect, because no reduction was observed after deletion of the domain tandems Ig6+Fn1 and Fn1+Fn2 (Fig. 2 B). The binding properties of ΔIg56 suggests a requirement for conformational flexibility in order to fit Ig2-4 and Fn3 to the domain conglomerate of axonin-1. The comparably strong axonin-1 binding of the NgCAM mutants ΔIg56Fn1, ΔIg6Fn12, and ΔIg56Fn12 may therefore reflect a close proximity of Ig2-4 and Fn3 in the axonin-1–NgCAM complex.
Based on these results we suggest the following molecular model: to establish a concomitant binding of Ig2-4 and Fn3 of NgCAM with the NH2-terminal Ig1-4 domain conglomerate of axonin-1, with both molecules being anchored to the same membrane by their COOH-terminal end, a backfolded structure of axonin-1 would be required. Interestingly, negative staining electron microscopy had revealed that monomeric axonin-1 has a backfolded, horseshoe-shaped domain-arrangement (Fig. 13 A; Rader et al., 1996). Thus, monomeric axonin-1 can readily fit its NH2-terminal domain conglomerate into a hypothetical binding pocket of NgCAM when both molecules are bound to the same membrane (Fig. 13 B). Although a direct interaction between Fn3 of NgCAM and the Ig1-4 domain conglomerate appears likely, we cannot exclude the possibility that axonin-1 interacts with NgCAM exclusively via a binding site localized in Ig2-4 of NgCAM. In the latter case a partial upfolding of axonin-1 from its horseshoe like conformation in the monomeric state would be required (Fig. 13 C). However, compared with the situation in a cis-axonin-1–NgCAM complex, a considerable conformational change of the axonin-1 molecule would be required to establish a contact with NgCAM residing in the membrane of an opposed cell.
Figure 13.
Molecular models of complexes formed by axonin-1 and NgCAM. (A) Previous studies revealed that a major proportion of monomeric axonin-1 has a backfolded, horseshoe-like conformation in which the six Ig domains fold back towards the membrane. The four NH2-terminal Ig domains (light gray) are shown in close physical association. This conglomerate bears the NgCAM binding site (after Rader et al., 1996). (B) In one model for a cis-axonin-1–NgCAM complex the domain conglomerate formed by the four NH2-terminal Ig domains of axonin-1 interacts directly with the Ig domains 2-4 and the third FnIII-like domain of NgCAM, Fn3 (dark gray). In this model, a partially backfolded conformation of axonin-1 would be required. NgCAM is thought to bend around the Ig1-4 conglomerate of axonin-1 to allow a simultaneous interaction of Ig2-4 and Fn3 of NgCAM with Ig1-4 of axonin-1. (C) An alternative model for the cis-axonin-1–NgCAM complex in which the axonin-1 domains Ig1-4 interact only with Ig2-4 of NgCAM. The absence of a direct interaction between Fn3 of NgCAM and axonin-1 Ig1-4 suggests a partially upfolded conformation for axonin-1 in this model of the complex. (D) Models for the presumptive axonin-1–NgCAM tetramer that is formed at cell contacts. Intercellular binding is mediated by NgCAM homophilic interaction that can occur simultaneously with the cis-binding between axonin-1 and NgCAM. The cis interaction between axonin-1 and NgCAM may involve a direct interaction of the axonin-1 domains Ig1-4 with Ig2-4 and Fn3 of NgCAM (B, left model) or only a binding between axonin-1 and Ig2-4 of NgCAM (C, right model).
Homophilic Interaction of NgCAM but Not Axonin-1 Can Occur Simultaneously with the Heterophilic Axonin-1–NgCAM Binding
The domain deletion studies also revealed that the four NH2-terminal Ig domains of NgCAM are essential for its homophilic interaction. Thus, in the segment of the Ig domains 2-4, binding sites for both the homophilic binding as well as the NgCAM/axonin-1 binding are located. Domain localization studies of the homophilic axonin-1 interaction revealed an involvement of Ig1 and Fn4 of axonin-1 and, interestingly, a heterotypic binding modus (Ig1 of one axonin-1 molecule binds to Fn4 of the other molecule and vice versa) and prompted the postulation of an extended conformation of axonin-1 in the homodimeric complex (Kunz, B., R. Lierheimer, C. Rader, U. Ziegler, L. Vogt, M. Spirig, and P. Sonderegger, manuscript submitted for publication). In analogy to NgCAM, axonin-1 exhibited domains that contributed to both the homophilic binding as well as the heterophilic axonin-1–NgCAM binding. The locations of the binding sites and the different conformational requirements for axonin-1 in the heterophilic versus homophilic complex indicate the possibility of competition between the heterophilic axonin-1–NgCAM binding and the homophilic interactions of axonin-1 and NgCAM, respectively.
In this study we found that coexpression of axonin-1 and NgCAM in the same cell, and, thus, the formation of heterodimeric axonin-1–NgCAM complexes, reduced the accessibility of axonin-1 for interactions with axonin-1 and NgCAM presented on the surface of microspheres. The inacessibility of axonin-1 for trans-homophilic interaction in this situation indicates that the axonin-1–NgCAM cis-interaction excludes the axonin-1 homophilic binding. Considering the domain arrangement of axonin-1 and NgCAM within the cis-axonin-1–NgCAM complex as proposed in the model of Fig. 13 B, it is conceivable that the heterodimeric complex stabilizes the bent conformation of axonin-1 and prevents its transition into the extended conformation required for homophilic binding. In contrast, the capability of NgCAM to undergo homophilic binding was not altered by the complexation with axonin-1, suggesting that the cis-heterophilic axonin-1–NgCAM interaction does not require a major conformational change of NgCAM. A cis-interaction of axonin-1 with NgCAM that is further involved in a trans-homophilic binding may have important physiological functions. Two independent studies demonstrated a requirement for axonin-1 for neurite outgrowth on NgCAM (Stoeckli et al., 1996; Buchstaller et al., 1996). In this situation growth cone-substratum contact is thought to be mediated by homophilic NgCAM interaction (Lemmon et al., 1989). Axonin-1 interacting in cis with NgCAM of the growth cone may act as an essential costimulatory factor for neurite growth. A ternary axonin-1–NgCAM2 complex could represent a minimal functional unit in neurite outgrowth on NgCAM substratum.
Homophilic NgCAM Binding Across the Extracellular Space and Axonin-1–NgCAM Binding in the Plane of the Membrane May Result in the Formation of Axonin-12/NgCAM2 Tetramers at Cell Contacts
Complexes of axonin-1 and NgCAM were implicated in neuronal cell recognition processes serving distinct developmental functions such as axonal pathfinding in vivo (Stoeckli and Landmesser, 1995) and in vitro (Stoeckli et al., 1997), substratum recognition by growth cones (Stoeckli et al., 1996), and neurite fasciculation (Ruegg et al., 1989b ; Stoeckli et al., 1991; Honig and Kueter, 1995; Kunz et al., 1996). To investigate the detailed interactions of axonin-1 and NgCAM at cell contacts we used CV-1 cells transfected with axonin-1 or NgCAM alone as well as cotransfectants expressing both molecules. The main result of these studies was a strong discrepancy in the accumulation of NgCAM and axonin-1 at homophilic cell–cell contact sites. Whereas contacts between cells expressing NgCAM exhibited a pronounced accumulation of NgCAM, only a minor accumulation of axonin-1 was observed when axonin-1–expressing cells were in contact. The accumulation of NgCAM at contacts between NgCAM transfectants reflects the well described trans-homophilic binding capacity of NgCAM (Lemmon et al., 1989; Kuhn et al., 1991; Buchstaller et al., 1996). The weak accumulation of axonin-1 at contacts between axonin-1 single transfected cells was not unexpected despite the clear demonstration of the axonin-1/TAG-1 homophilic interaction in various systems (Rader et al., 1993; Felsenfeld et al., 1994; Kunz, B., R. Lierheimer, C. Rader, U. Ziegler, L. Vogt, M. Spirig, and P. Sonderegger, manuscript submitted for publication). When expressed alone, the favored state of axonin-1 may be the backfolded structure (Rader et al., 1996). Evidence from domain deletion studies suggests that the horseshoe form of axonin-1 is stabilized by an intramolecular interaction of the Ig1-4 domain conglomerate with Fn4, the most membrane proximal domain. In axonin-1 single transfected cells, a major proportion of the axonin-1 molecules may therefore adopt this conformation and the likelihood of the extended state, which would favor the formation of a homophilic interaction and the accumulation of axonin-1 at cell contact regions, may be relatively low. Strong accumulation of axonin-1 at cell contacts was only observed when NgCAM was expressed in the same cell. Because the cis-axonin-1–NgCAM binding makes axonin-1 inaccessible for trans-interactions with axonin-1, the accumulation of axonin-1 at the cell contact regions is unlikely to be driven by a homophilic axonin-1 interaction. We suggest that the coclustering of axonin-1 and NgCAM at the cell contacts is driven by a NgCAM trans-homophilic binding, whereas the accumulation of axonin-1 is due to its cis-heterophilic binding with NgCAM that occurs simultaneously and results in the formation of a symmetric tetrameric axonin-1–NgCAM complex with a composition of axonin-12/NgCAM2 (Fig. 13 D). Chemical cross-linking studies of axonin-1, performed previously on cultured DRG neurons, revealed the aggregation of axonin-1 and NgCAM into a complex with a molecular mass of ∼500 kD during the formation of cell-cell contacts (Kunz et al., 1996; Fig. 9). Based on its size and composition this complex most probably represents the symmetric tetrameric axonin-12/ NgCAM2 complex suggested by the results of this study (Fig. 13 D).
Overexpression of NgCAM but Not Axonin-1 Results in Enhanced Neurite Fasciculation
Several independent pieces of evidence implicate axonin-1 and NgCAM as key players in neurite fasciculation. Immunocytochemical analysis revealed coclustering of axonin-1 and NgCAM at sites of close contact between neurites (Honig and Kueter, 1995). Antibody pertubation experiments as well as chemical cross-liking studies indicated that the formation of neurite fascicles in vitro involves axonin-1 and NgCAM (Rathjen et al., 1987; Ruegg et al., 1989b ; Stoeckli et al., 1991; Kunz et al., 1996). Further, an interaction between axonin-1 and NgCAM was demonstrated to be of crucial importance for the fasciculation of commissural axons in vivo (Stoeckli and Landmesser, 1995). We have previously found a progressive accumulation of heterotetrameric complexes of axonin-1 and NgCAM during the formation of neurite fascicles in cultures of DRG neurons (Kunz et al., 1996). It is an intriguing possibility that heterooligomeric complexes of axonin-1 and NgCAM, such as the tetrameric axonin-12/NgCAM2 complex, represent functional units in neurite fasciculation mediated by axonin-1 and NgCAM. Size and composition of such axonin-1–NgCAM complexes formed at cell contacts would be determined by the stoichiometric ratios of the two molecules in the cells involved. This would open the possibility to regulate axonal fasciculation by changing the relative expression levels of axonin-1 and NgCAM. To test this hypothesis we overexpressed axonin-1 and NgCAM in DRG explants using adenoviral vectors for gene transfer and found that overexpression of axonin-1 resulted in a slight but statistically not significant defasciculation whereas NgCAM overexpression caused strongly enhanced fasciculation. The effects of axonin-1 and NgCAM overexpression on neurite fasciculation correlate very well with the observations in the CV-1 cell model system. NgCAM mediates the contact between adjacent neurite membranes across the extracellular space by homophilic interaction. In contrast, axonin-1 is a cis-interacting component of the heterotetrameric cell contact complex that does not directly engage in an interaction across the extracellular space.
According to these findings one would expect that the extent of neurite fasciculation strongly depends on the level of NgCAM expression. Indeed, strong defasciculation was observed upon down-regulation of axonal L1 in mouse DRG neurons dependent on the pattern of electrical activity (Itoh et al., 1995, 1997). These findings are in line with our results of enhanced neurite fasciculation due to NgCAM overexpression and indicate that NgCAM is the limiting component for neurite fasciculation in DRG neurons.
The role of axonin-1 in the heterotetrameric cell contact complex is currently not clear. Yet, results of the studies of the intracellular signaling associated with axonin-1, NgCAM, and complexes thereof suggest a role of axonin-1 in the modulation of intracellular phosphorylation events. Studies on the signal transduction of axonin-1 and NgCAM revealed that the formation of higher molecular axonin-1–NgCAM complexes during neurite fasciculation was accompanied by a reduction of axonin-1-associated fyn tyrosine kinase activity and an increased NgCAM phosphorylation by a casein kinase II related kinase (Kunz et al., 1996; Wong et al., 1996). The effects of the two distinct protein kinases associated with axonin-1 and NgCAM on the cytoskeleton may contribute to the observed effects of axonin-1 or NgCAM overexpression on neurite fasciculation. The high expression of fyn in developing fiber tracts during the phase of axon outgrowth and a comparably low abundance in consolidated fascicles (Bare et al., 1993) suggests that fyn activity may contribute to a dynamic state of the cytoskeleton in transient cell contacts. In contrast, phosphorylation of specific cytoskeletal proteins by casein kinase II could result in a stabilization of the axonal cytoskeleton. Phosphorylation of microtubule-associated proteins such as MAP1B by casein kinase II was reported to be essential for the consolidation of stable neurite structures (Matus, 1991; Brugg et al., 1993; Ulloa et al., 1993). Therefore, an excess of free axonin-1 could destabilize contacts between adjacent neurite membranes due to the predominance of fyn over casein kinase II. Overexpression of NgCAM would drive more axonin-1 into axonin-1–NgCAM complexes resulting in reduced activity of fyn and predominance of casein kinase II. This could result in an enhanced stability of the neuronal cytoskeleton at the cell contact sites and lead to a stronger association of NgCAM with cytoskeletal components.
Similar mechanisms may underlie the strong defasciculation that is observed after application of soluble axonin-1 to neurite bundles in vitro and in vivo (Stoeckli et al., 1991; Stoeckli and Landmesser, 1995). Soluble axonin-1 may interfere with the complexation of cellular axonin-1 by NgCAM in a competitive manner. This would result in an enhanced concentration of monomeric axonin-1 in the neuronal cell membrane. The enhanced fyn activity that may accompany the excess of monomeric axonin-1 (Kunz et al., 1996) may alter the phosphorylation pattern of cytoskeletal proteins and result in a destabilization of cell contacts. Thus, differential fasciculation that represents a crucial prerequisite for functional target innervation during development and regeneration could be regulated not only by changes in the relative expression of CAMs like axonin-1 and NgCAM but also by variations in the local concentrations of soluble axonin-1 during development.
Axonin-1 and NgCAM Are Implicated in Axonal Fasciculation In Vivo
The findings on the interactions of axonin-1 and NgCAM, suggest an important role of the two molecules in axonal growth and fasciculation. During neural development axonin-1 and NgCAM are coexpressed predominantly in outgrowing fiber tracts (Thiery et al., 1985; Daniloff et al., 1986; Shiga et al., 1990, 1991; Shiga and Oppenheim, 1991; Halfter et al., 1994; Wolfer et al., 1994; Moscoso and Sanes, 1995). A strong coexpression of axonin-1 and NgCAM is found on DRG axons during their entry into the dorsal horn of the spinal cord (Shiga et al., 1997). Antibody pertubation experiments suggest that these CAMs mediate fasciculation among the DRG axons in the prospective dorsal funiculus that is thought to be crucial for the correct pathfinding of these axons in vivo, probably by preventing their entry into the mantle layer of the spinal cord (Shiga et al., 1997). Specific fasciculation mediated by axonin-1 and NgCAM was also demonstrated for the commissural axons of the spinal cord. Several IgFnIII molecules are expressed in the commissural system of the chicken embryo when commissural growth cones are making pathfinding decisions. NgCAM and axonin-1 are both expressed on commissural axons (Shiga et al., 1990; Shiga and Oppenheim, 1991; Stoeckli and Landmesser, 1995) and NrCAM/Bravo is expressed in the floor plate cells (Krushel et al., 1993; Denburg et al., 1995). The pertubation of the in vivo guidance of chick commissural axons with antibodies demonstrated that the heterophilic interaction between axonin-1 and NgCAM and the homophilic binding of NgCAM are involved in the bundling or fasciculation of these axons whereas heterophilic interactions between axonin-1 and NrCAM appear to be involved in pathfinding (Stoeckli and Landmesser, 1995). An important role for axonin-1 and NgCAM in the organization of neuronal projections is further suggested by the analysis of their expression during the development of the retinotectal system. Both are present in the area where retinal ganglion cells are generated and the expression of axonin-1 and NgCAM along the optic nerve, chiasm, optic tract, and in the superficial layers of the optic tectum follows the chronotropic pattern of axon formation. The temporal correlation of the coexpression of these proteins with the onset and the duration of fiber growth makes it highly probable, that these proteins play an important role in the formation of the fiber pathway (Rager et al., 1996; Morino et al., 1996).
The in vivo coexpression suggests that heterooligomeric complexes of axonin-1 and NgCAM that are formed at cell contacts may provide signals for axonal growth and fasciculation during the development of fiber tracts. In early stages of fiber tract development, axons may grow along each other in a way that the membranes of earlier axons may provide the substratum for the growth cones of later ones. This situation may be mimicked in vitro by neurite outgrowth on axonin-1 and NgCAM substratum. Several lines of evidence suggest that complexes formed by axonin-1 and NgCAM mediate the signals for neurite elongation elicited by axonin-1 or NgCAM substratum (Kuhn et al., 1991; Stoeckli et al., 1996; Buchstaller et al., 1996). As mentioned above, neurite outgrowth of DRG neurons on NgCAM substratum for example is thought to involve a heterooligomeric complex, formed by the interaction of a cis-axonin-1–NgCAM heterodimer of the growth cone membrane with substratum NgCAM (Stoeckli et al., 1996; Buchstaller et al., 1996). Similar complexes formed at growth cone/neurite contacts may provide the signals for the specific axonal elongation on preexisting fibers in vivo. The composition of such complexes may be determined by the relative expression of axonin-1 and NgCAM in the axons that form the contact. Contacts between axons that show coexpression of axonin-1 and NgCAM, like the DRG projections of the dorsal horn, the commissural axons of the spinal cord, and the axons of the retinotectal projection mentioned above may result in the formation of a symmetric axonin-1–NgCAM heterooligomer. This assumption is supported by the detection of a presumptive axonin-1/NgCAM tetramer in strongly fasciculated DRG neurons in vitro (Kunz et al., 1996) and the interactive pattern of axonin-1 and NgCAM at cell contacts described in this study. In later stages of fiber tract development, distinct complexes of axonin-1 and NgCAM, that elicit different intracellular signals, may be involved in the consolidation of the fascicle structure.
We expect that the axonin-1–NgCAM complexes involved in axonal growth along preexisting fibers in early stages of fiber tract development are functionally different from those that are involved in the consolidation of the fascicle structure in later phases. Axonin-1–NgCAM complexes of distinct compositions, that elicit different intracellular signals, may be generated by developmental changes in the relative expression of axonin-1 and NgCAM. This assumption is supported by in vivo studies that reveal marked changes in the expression of axonin-1 and NgCAM during fiber tract development (Dodd and Jessell, 1988; Dodd et al., 1988; Stoeckli and Landmesser, 1995). The aggregation of axonin-1 and NgCAM could be further influenced by the expression of other binding partners for the two molecules, that may change their signaling properties or alter the equilibrium of complex formation.
Acknowledgments
We thank Dr. Fritz G. Rathjen for the monoclonal NgCAM antibody and Dr. Esther T. Stoeckli, Dora Fitzli, and Dr. Lukas Leder for helpful discussions.
This work was supported by the Swiss National Science Foundation and the Biotechnology Program of the European Commission.
Abbreviations used in this paper
- CAM
cell adhesion molecule
- DRG
dorsal root ganglia
- ECL
enhanced chemiluminescence
- ECM
extracellular matrix
- Fab
fragment with an antigen binding site
- FnIII
fibronectin type-III
- IgSF
immunoglobulin superfamily
- IgFnIII
immunoglobulin/fibronectin type-III class of molecules
- NBCS
newborn calf serum
- pfu
plaque-forming unit
Footnotes
Address all correspondence to Dr. Peter Sonderegger, Institute of Biochemistry, University of Zurich, Winterthurerstr. 190, CH-8057 Zurich, Switzerland. Tel.: 41 1 635 55 41. Fax: 41 1 635 68 31. E-mail: pson@bioc.uniz.ch
References
- Bare DJ, Lauder JM, Wilkie MB, Maness PF. p59fyn in rat brain is localized in developing axonal tracts and subpopulations of adult neurons and glia. Oncogene. 1993;8:1429–1436. [PubMed] [Google Scholar]
- Bateman A, Jouet M, MacFarlane J, Du J-S, Kenwrick S, Chothia C. Outline structure of the human L1 cell adhesion molecule and the sites where mutations cause neurological diseases. EMBO (Eur Mol Biol Organ) J. 1996;15:6050–6059. [PMC free article] [PubMed] [Google Scholar]
- Bruemmendorf T, Wolff JM, Frank R, Rathjen FG. Neural cell recognition molecule F11: homology with fibronectin type III domains and immunoglobulin type C domains. Neuron. 1989;2:1351–1361. doi: 10.1016/0896-6273(89)90073-1. [DOI] [PubMed] [Google Scholar]
- Bruemmendorf T, Hubert M, Treubert U, Leuschner R, Tarnok A, Rathjen FG. The axonal recognition molecule F11 is a multifunctional protein: specific domains mediate interactions with Ng-CAM and restrictin. Neuron. 1993;10:711–727. doi: 10.1016/0896-6273(93)90172-n. [DOI] [PubMed] [Google Scholar]
- Brugg B, Reddy D, Matus A. Attenuation of microtubule-associated protein 1B expression by antisense oligodeoxynucleotides inhibits initiation of neurite outgrowth. Neurosci. 1993;52:489–496. doi: 10.1016/0306-4522(93)90401-z. [DOI] [PubMed] [Google Scholar]
- Buchstaller A, Kunz S, Berger P, Rader C, Ziegler U, Sonderegger P. The cell adhesion molecules NgCAM and axonin-1 form heterodimers in the neuronal membrane and cooperate in neurite outgrowth promotion. J Cell Biol. 1996;135:1593–1607. doi: 10.1083/jcb.135.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoon MP, Grumet M, Mauro V, Edelmann GM, Cunningham BA. Structure of the chicken neuron-glia cell adhesion molecule, NgCAM: origin of the polypeptides and relation to the Ig superfamily. J Cell Biol. 1991;130:733–744. doi: 10.1083/jcb.112.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Rathjen FG, Raper JA. Extension of neurites on axons is impaired by antibodies against specific neural cell surface glycoproteins. J Cell Biol. 1987;104:355–362. doi: 10.1083/jcb.104.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C, Jones YE. The molecular structure of cell adhesion molecules. Annu Rev Biochem. 1997;66:823–862. doi: 10.1146/annurev.biochem.66.1.823. [DOI] [PubMed] [Google Scholar]
- Chu G, Hayakawa H, Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987;15:1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly MA, Grady RC, Mushinski JF, Marcu KB. PANG, a gene encoding a neuronal glycoprotein is ectopically activated by intracisternal A-type particle long terminal repeats in murine plasmacytomas. Proc Natl Acad Sci USA. 1994;91:1337–1341. doi: 10.1073/pnas.91.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G, Tannahill D, Keynes R. Axon guidance to and from choice points. Curr Opin Neurobiol. 1998;8:64–72. doi: 10.1016/s0959-4388(98)80009-3. [DOI] [PubMed] [Google Scholar]
- Daniloff JK, Chuong C-M, Levi G, Edelman GM. Differential distribution of cell adhesion molecules during histogenesis of the chick nervous system. J Neurosci. 1986;6:739–758. doi: 10.1523/JNEUROSCI.06-03-00739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JQ, Bennet V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem. 1994;269:27163–27166. [PubMed] [Google Scholar]
- Davis JQ, McLaughlin T, Bennet V. Ankyrin-binding proteins related to nervous system cell adhesion molecules: candidates to provide transmembrane and intercellular connections in adult brain. J Cell Biol. 1993;121:121–133. doi: 10.1083/jcb.121.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bernardo AP, Chang S. Heterophilic interactions of DM-GRASP: GRASP-NgCAM interactions involved in neurite extension. J Cell Biol. 1996;133:657–666. doi: 10.1083/jcb.133.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg JL, Caldwell RT, Marner JM. Developmental changes in epitope accessibility as an indicator of multiple states of an immunoglobulin-like cell adhesion molecule. J Comp Neurol. 1995;354:533–550. doi: 10.1002/cne.903540405. [DOI] [PubMed] [Google Scholar]
- Dodd J, Jessell TM. Axon guidance and the patterning of neuronal projections in vertebrates. Science. 1988;242:692–699. doi: 10.1126/science.3055291. [DOI] [PubMed] [Google Scholar]
- Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Dubreuil RR, MacViar G, Dissanayake S, Liu C, Homer D, Hortsch M. Neuroglian-mediated cell adhesion induces assembly of the membrane skeleton at cell contact sites. J Cell Biol. 1996;133:647–655. doi: 10.1083/jcb.133.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld DP, Hynes MA, Skoler KM, Furley AJ, Jessell TM. TAG-1 can mediate homophilic binding, but neurite outgrowth on TAG-1 requires a L1-like molecule and β1 integrins. Neuron. 1994;12:675–690. doi: 10.1016/0896-6273(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Friedlander D, Milev RP, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules NgCAM/L1/NILE and NCAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furley AJ, Mortun SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth promoting activity. Cell. 1990;61:157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Gennarini G, Cibelli G, Rougon G, Mattei M, Goridis C. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J Cell Biol. 1989;109:775–788. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Ziegler U, Hermens WTJMC, Kunz B, Kunz S, Sonderegger P. Adenovirus-mediated gene transfer in neurons: construction and characterization of a vector for heterologous expression of the axonal cell adhesion molecule axonin-1. J Neurosci Methods. 1997;71:99–111. doi: 10.1016/s0165-0270(96)00130-6. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Schatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;10:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Grumet M. Structure, expression, and function of Ng-CAM, a member of the immunoglobulin superfamily involved in neuron-neuron and neuron-glia adhesion. J Neurosci Res. 1992;31:1–13. doi: 10.1002/jnr.490310102. [DOI] [PubMed] [Google Scholar]
- Grumet M, Sakurai T. Heterophilic interactions of the neural cell adhesion molecules Ng-CAM and Nr-CAM with neural receptors and extracellular matrix proteins. Sem Neurosci. 1996;8:379–389. [Google Scholar]
- Grumet M, Hoffman S, Chuong C-M, Edelman GM. Polypeptide components and binding functions of neuron-glia cell adhesion molecules. Proc Natl Acad Sci USA. 1984;81:7989–7993. doi: 10.1073/pnas.81.24.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Mauro V, Burgoon MP, Edelman GM, Cunningham BA. Structure of a new nervous system glycoprotein, Nr-CAM, and its relationship to subgroups of neural cell adhesion molecules. J Cell Biol. 1991;113:1399–1412. doi: 10.1083/jcb.113.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Friedlander DR, Edelmann GM. Evidence for the binding of NgCAM to laminin. Cell Adhes Commun. 1993a;7:177–190. doi: 10.3109/15419069309095693. [DOI] [PubMed] [Google Scholar]
- Grumet M, Flaccus A, Margolis RU. Functional characterization of chondroitin sulfate proteoglycans of brain: Interactions with neurons and neurla cell adhesion molecules. J Cell Biol. 1993b;120:815–824. doi: 10.1083/jcb.120.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Yip YPL, Yip JW. Axonin-1 is expressed primarily in subclasses of avian sensory neurons during outgrowth. Dev Brain Res. 1994;78:87–101. doi: 10.1016/0165-3806(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Peles E, Pawson T, Schlessinger J. Cell-contact-dependent signalling in axon growth and guidance: Eph receptor tyrosine kinases and receptor protein tyrosine phosphatase β. Curr Opin Neurobiol. 1998;8:117–127. doi: 10.1016/s0959-4388(98)80015-9. [DOI] [PubMed] [Google Scholar]
- Honig GM, Kueter J. The expression of cell adhesion molecules on the growth cones of chick cutaneous and muscle sensory neurons. Dev Biol. 1995;167:563–583. doi: 10.1006/dbio.1995.1049. [DOI] [PubMed] [Google Scholar]
- Hosaka M, Nagahama M, Kim WS, Watanabe T, Hatsuzawa K, Ikemizu J, Murakami K, Nakayama K. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalysed by furin within the constitutive secretory pathway. J Biol Chem. 1991;266:12127–12130. [PubMed] [Google Scholar]
- Huber AH, Wang Y, Bieber AJ, Bjorkman PJ. Crystal structure of tandem type III fibronectin domains from Drosophilaneuroglian at 2.1 A. Neuron. 1994;12:717–731. doi: 10.1016/0896-6273(94)90326-3. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations and targeting of cells and axons. Cell. 1992;68:303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- Itoh K, Stevens B, Schachner M, Fields RD. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–1372. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- Itoh K, Ozaki M, Stevens B, Fields RD. Activity-dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J Neurobiol. 1997;33:735–748. doi: 10.1002/(sici)1097-4695(19971120)33:6<735::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Adhesion molecules and the hierarchy of neural development. Neuron. 1988;1:3–13. doi: 10.1016/0896-6273(88)90204-8. [DOI] [PubMed] [Google Scholar]
- Jiang YP, Wang H, D'Eustachio P, Musacchio JM, Schlessinger J, Sap J. Cloning and characterization of R-PTP-κ, a new member of the receptor protein tyrosine phosphatase family with a proteolytically cleaved cellular adhesion molecule-like extracellular region. Mol Cell Biol. 1993;13:2942–2951. doi: 10.1128/mcb.13.5.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayyem JF, Roman JM, de la Rosa EJ, Schwarz U, Dreyer WJ. Bravo/NrCAM is closely related to the cell adhesion molecule L1 and NgCAM and has a similar heterodimer structure. J Cell Biol. 1992;118:1259–1270. doi: 10.1083/jcb.118.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krushel LA, Prieto AL, Cunningham BA, Edelman GM. Expression patterns of the cell adhesion molecule NrCAM during histogenesis of the chick nervous system. Neurosci. 1993;53:797–812. doi: 10.1016/0306-4522(93)90625-p. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Stoeckli ET, Condrau MA, Rathjen FG, Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4) J Cell Biol. 1991;115:1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S, Ziegler U, Kunz B, Sonderegger P. Intracellular signaling is changed after clustering of the neural cell adhesion molecules axonin-1 and NgCAM during neurite fasciculation. J Cell Biol. 1996;135:253–267. doi: 10.1083/jcb.135.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon V, Farr KL, Langenaur C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron. 1989;2:1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Matus A. Microtubule-associated proteins and neuronal morphogenesis. J Cell Sci. 1991;15:61–67. doi: 10.1242/jcs.1991.supplement_15.9. [DOI] [PubMed] [Google Scholar]
- Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Maurel P, Haring M, Margolis RK, Margolis RU. TAG-1/axonin-1 is a high-affinity ligand of neurocan, phosphacan/protein-tyrosine phosphatase μ/β and NCAM. J Biol Chem. 1996;271:15716–15723. doi: 10.1074/jbc.271.26.15716. [DOI] [PubMed] [Google Scholar]
- Moos M, Tacke R, Scherer H, Teplow D, Früh K, Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;334:701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Morino P, Buchstaller A, Giger R, Sonderegger P, Rager G. Differential expression of the mRNAs of the axonal glycoproteins axonin-1 and NgCAM in the developing chick retina. Dev Brain Res. 1996;91:252–259. doi: 10.1016/0165-3806(95)00184-0. [DOI] [PubMed] [Google Scholar]
- Moscoso LM, Sanes JR. Expression of four immunoglobulin superfamily adhesion molecules (L1, NrCAM/Bravo, Neurofascin/ABGP, and NCAM) in the developing mouse spinal cord. J Comp Neurol. 1995;352:321–344. doi: 10.1002/cne.903520302. [DOI] [PubMed] [Google Scholar]
- Rader C, Stoeckli ET, Ziegler U, Osterwalder T, Kunz B, Sonderegger P. Cell-cell adhesion by homophilicinteraction of the neural cell recognition molecule axonin-1. Eur J Biochem. 1993;215:133–141. doi: 10.1111/j.1432-1033.1993.tb18015.x. [DOI] [PubMed] [Google Scholar]
- Rader C, Kunz B, Lierheimer R, Giger RJ, Berger P, Tittmann P, Gross H, Sonderegger P. Implications for the domain arrangement of axonin-1 derived from the mapping of its NgCAM binding site. EMBO (Eur Mol Biol Organ) J. 1996;15:2056–2068. [PMC free article] [PubMed] [Google Scholar]
- Rager G, Morino P, Schnitzer J, Sonderegger P. Expression of the axonal cell adhesion molecules axonin-1 and NgCAM during the development of the chick retinotectal system. J Comp Neurol. 1996;365:594–609. doi: 10.1002/(SICI)1096-9861(19960219)365:4<594::AID-CNE7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Rathjen FG. Neural cell contact and axonal growth. Curr Opin Cell Biol. 1991;3:992–1000. doi: 10.1016/0955-0674(91)90119-j. [DOI] [PubMed] [Google Scholar]
- Rathjen FG, Jessell TM. Glycoproteins that regulate the growth and guidance of vertebrate axons: domains and dynamics of the immunoglobulin/fibronectin type III subfamily. Sem Neurosci. 1991;3:297–307. [Google Scholar]
- Rathjen FG, Wolff JM, Frank R, Bonhoeffer F, Rutishauser U. Membrane glycoproteins involved in neurite fasciculation. J Cell Biol. 1987;104:343–353. doi: 10.1083/jcb.104.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg MA, Stoeckli ET, Kuhn TB, Heller M, Zuellig R, Sonderegger P. Purification of axonin-1, a protein that is secreted from axons during neuritogenesis. EMBO (Eur Mol Biol Organ) J. 1989a;8:55–63. doi: 10.1002/j.1460-2075.1989.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg MA, Stoeckli ET, Lanz RB, Streit P, Sonderegger P. A homologue of the axonally secreted protein axonin-1 is an integral membrane protein of nerve fiber tracts involved in neurite fasciculation. J Cell Biol. 1989b;109:2363–2378. doi: 10.1083/jcb.109.5.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga T, Oppenheim PW. Immunolocalization studies of putative guidance molecules used by axons and growth cones of intersegmental interneurons in the chick embryo spinal cord. J Comp Neurol. 1991;310:234–252. doi: 10.1002/cne.903100208. [DOI] [PubMed] [Google Scholar]
- Shiga T, Oppenheim RW, Grumet M, Edelman GM. Neuron-glia cell adhesion molecule (Ng-CAM) expression in the chick embryo spinal cord: Observations on the earliest development intersegmental interneurons. Dev Brain Res. 1990;55:209–217. doi: 10.1016/0165-3806(90)90202-a. [DOI] [PubMed] [Google Scholar]
- Shiga T, Kuenzi R, Oppenheim RW. Axonal projections and synaptogenesis by supraspinal descending neurons in the spinal cord of the chick embryo. J Comp Neurol. 1991;305:83–95. doi: 10.1002/cne.903050109. [DOI] [PubMed] [Google Scholar]
- Shiga T, Lustig M, Grumet M, Shirai T. Cell adhesion molecules regulate guidance of dorsal root ganglion axons in the marginal zone and their invasion into the mantle layer of embryonic spinal cord. Dev Biol. 1997;192:136–148. doi: 10.1006/dbio.1997.8742. [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT. Axonin-1, NrCAM and NgCAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995;14:1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT. Axon guidance at choice points. Curr Opin Neurobiol. 1998;8:73–79. doi: 10.1016/s0959-4388(98)80010-x. [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Lemkin PF, Kuhn TB, Ruegg MA, Heller M, Sonderegger P. Identification of proteins secreted from axons of embryonic dorsal root ganglia neurons. Eur J Biochem. 1989;180:249–258. doi: 10.1111/j.1432-1033.1989.tb14640.x. [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Kuhn TB, Duc CO, Ruegg MA, Sonderegger P. The axonally secreted protein axonin-1 is a potent substratum for neurite outgrowth. J Cell Biol. 1991;112:449–455. doi: 10.1083/jcb.112.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli ET, Ziegler U, Bleiker A, Groscurth P, Sonderegger P. Clustering and functional cooperation of NgCAM and axonin-1 in the substratum-contact area of growth cones. Dev Biol. 1996;177:15–29. doi: 10.1006/dbio.1996.0141. [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Sonderegger P, Pollerberg EG, Landmesser LT. Interference with axonin-1 and NrCAM interactions unmasks a floor-plate activity inhibitory for commissural axons. Neuron. 1997;18:209–221. doi: 10.1016/s0896-6273(00)80262-7. [DOI] [PubMed] [Google Scholar]
- Suter, D.M., G.E. Pollerberg, A. Buchstaller, R.J. Giger, W.J. Dreyer and P. Sonderegger. Binding between the neural cell adhesion molecules axonin-1 and NrCAM/Bravo is involved in neuron-glia interaction. J. Cell Biol. 131: 1067–1081. [DOI] [PMC free article] [PubMed]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Thiery J-P, Delouvee A, Grumet M, Edelman GM. Initial appearance and regional distribution of the neuron-glia cell adhesion molecule in the chick embryo. J Cell Biol. 1985;100:442–456. doi: 10.1083/jcb.100.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L, Diaz-Nido J, Avila J. Depletion of casein kinase II by antisense oligonucleotide prevents neuritogenesis in neuroblastoma cells. EMBO (Eur Mol Biol Organ) J. 1993;12:1633–1640. doi: 10.1002/j.1460-2075.1993.tb05808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vactor D. Adhesion and signaling in axon fasciculation. Curr Opin Neurobiol. 1998;8:80–86. doi: 10.1016/s0959-4388(98)80011-1. [DOI] [PubMed] [Google Scholar]
- Vaughn DE, Bjorkman PJ. The (greek) key to structures of neural cell adhesion molecules. Neuron. 1996;16:261–273. doi: 10.1016/s0896-6273(00)80045-8. [DOI] [PubMed] [Google Scholar]
- Vogt L, Giger RJ, Ziegler U, Kunz B, Buchstaller A, Hermens WTJMC, Kaplitt MG, Rosenfeld MR, Pfaff DW, Verhaagen J, Sonderegger P. Continuous renewal of the axonal pathway sensor apparatus by insertion of new sensor molecules into the growth cone membrane. Curr Biol. 1996;6:1153–1158. doi: 10.1016/s0960-9822(02)70682-9. [DOI] [PubMed] [Google Scholar]
- Volkmer H, Hassel B, Wolff JM, Frank R, Rathjen FG. Structure of the axonal surface recognition molecule neurofascin and its relationship to a neural subgroup of the immunoglobulin superfamily. J Cell Biol. 1992;118:149–161. doi: 10.1083/jcb.118.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel P, Fluegge UI. Method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Willams AF, Barclay AN. The immunoglobulin superfamily-domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Henehan-Beatty A, Stoeckli E, Sonderegger P, Lipp H-P. Distribution of TAG-1/axonin-1 in fibre tracts and migratory streams of the developing mouse nervous system. J Comp Neurol. 1994;345:1–32. doi: 10.1002/cne.903450102. [DOI] [PubMed] [Google Scholar]
- Wong EV, Schaefer AW, Landreth G, Lemmon V. Casein kinase II phosphorylates the neural cell adhesion molecule L1. J Neurochem. 1996a;66:779–786. doi: 10.1046/j.1471-4159.1996.66020779.x. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Kawasaki M, Tani A, Tamada A, Nagata S, Kagamyiama H, Mori K. BIG-1: a new TAG-1/F3-related member of the immunoglobulin superfamily with neurite outgrowth-promoting activity. Neuron. 1994;13:415–426. doi: 10.1016/0896-6273(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Kawasaki M, Tamada A, Nagata S, Kagamyiama H, Mori K. Overlapping and differential expression of BIG-2, BIG-1, TAG-1 and F3: Four members of an axon-associated cell adhesion molecule subgroup of the immunoglobulin family. J Neurobiol. 1995;28:51–69. doi: 10.1002/neu.480280106. [DOI] [PubMed] [Google Scholar]
- Zuellig RA, Rader C, Schroeder A, Kalousek MB, von Bohlen F und Halbach, T. Osterwalder, C. Inan, E.T. Stoeckli, H.U. Affolter, A. Fritz, et al. The axonally secreted cell adhesion molecule, axonin-1: primary structure, immunoglobulin- and fibronectin type-III-like domains, and glycosyl-phosphatidylinositol anchorage. Eur J Biochem. 1992;204:453–463. doi: 10.1111/j.1432-1033.1992.tb16655.x. [DOI] [PubMed] [Google Scholar]