Abstract
Glutamylation is the major posttranslational modification of neuronal and axonemal tubulin and is restricted predominantly to centrioles in nonneuronal cells (Bobinnec, Y., M. Moudjou, J.P. Fouquet, E. Desbruyères, B. Eddé, and M. Bornens. 1998. Cell Motil. Cytoskel. 39:223–232). To investigate a possible relationship between the exceptional stability of centriole microtubules and the compartmentalization of glutamylated isoforms, we loaded HeLa cells with the monoclonal antibody GT335, which specifically reacts with polyglutamylated tubulin. The total disappearance of the centriole pair was observed after 12 h, as judged both by immunofluorescence labeling with specific antibodies and electron microscopic observation of cells after complete thick serial sectioning. Strikingly, we also observed a scattering of the pericentriolar material (PCM) within the cytoplasm and a parallel disappearance of the centrosome as a defined organelle. However, centriole disappearance was transient, as centrioles and discrete centrosomes ultimately reappeared in the cell population.
During the acentriolar period, a large proportion of monopolar half-spindles or of bipolar spindles with abnormal distribution of PCM and NuMA were observed. However, as judged by a quasinormal increase in cell number, these cells likely were not blocked in mitosis.
Our results suggest that a posttranslational modification of tubulin is critical for long-term stability of centriolar microtubules. They further demonstrate that in animal cells, centrioles are instrumental in organizing centrosomal components into a structurally stable organelle.
Keywords: centrosome, centriole, tubulin polyglutamylation, mitosis, mitotic spindle
The centrosome was discovered at the start of modern cell biology over a century ago (Boveri, 1901), but it has remained largely resistant to molecular investigation (for recent reviews see Paoletti and Bornens, 1997; Stearns and Winey, 1997). Because of this, the two major properties of the centrosome, i.e., its capacity to reproduce by duplication and its ability to nucleate microtubules, are still poorly understood.
Within the centrosomes of animal cells, the centriole pair remains without a defined role. The only well-established function of the centriole is to act as a template for the growth of the primary or motile cilium axoneme (Rieder and Borisy, 1982). The absence of centrioles in the centrosome from other eukaryotic organisms has led to the dominant view that the centriole pair is not relevant to centrosome activity. This view is also based on the fact that centrioles can be dispensable for spindle assembly, for example during the female meiosis in some species (Szöllösi et al., 1972; Calarco-Gilliam et al., 1983; Theurkauf and Hawley, 1992), or even in an established Drosophila cell line (Debec et al., 1982). However, several results support the alternative view. First, isolated centrosomes from somatic cells reveal that the centrosomal matrix tightly binds to the proximal wall and the proximal end of both centrioles and links them together (Bornens et al., 1987; Paintrand et al., 1992). Recent experiments suggest that NuMA redistribution at the onset of mitosis, which is essential for spindle pole stabilization (Gaglio et al., 1997), depends upon the correct segregation of pericentriolar material (PCM)1 between centriole pairs at the onset of mitosis (Paoletti et al., 1997). Second, the ability of centrioles to replicate by orthogonal budding (Robbins et al., 1968; Kuriyama and Borisy, 1981; Kochanski and Borisy, 1990) has long been postulated to be essential for centrosome continuity. In agreement with this view, the reproductive capacity of centrosomes in sea urchin eggs depends upon the number of centrioles present (Sluder and Rieder, 1985a ,b), and cells artificially deprived of centrioles by microsurgery cannot reform centrioles (Maniotis and Schliwa, 1991). Similar conclusions were drawn from experiments designed to identify the centrosome-associated activity responsible for triggering parthenogenetic development in Xenopus (Klotz et al., 1990).
However, centriole biogenesis other than by parental budding exists, for example, during differentiation of ciliated cells (Sorokin, 1968), at the beginning of development in parthenogenetic species (for a review see Beatty, 1967) or at the blastocyst stage of mouse embryo (Maro et al., 1985; Schatten et al., 1986). There are also numerous examples of so-called de novo assembly of centrioles in unicellular organisms, such as the ameboflagellate Naegleria (Dingle and Fulton, 1966), or multicellular organisms like the fern Marsilea during spermatogenesis (Mizukami and Gall, 1966). The molecular basis of centriole generation in these cases as well as in the classical duplication pathway is still largely unknown.
Microtubules of centrioles are highly stable structures that resist all depolymerizing agents. This particular subset of microtubules shares with basal bodies and axonemes a large number of tubulin modifications, such as acetylation (Piperno and Fuller, 1985), detyrosination (Gundersen and Bulinski, 1986), and glutamylation (Eddé et al., 1990; Bobinnec et al., 1998). This latter modification consists in the formation of a lateral chain of glutamate units linked to a glutamate residue near the COOH terminus of both α- and β-tubulin.
In this paper, we investigate the relationship between glutamylation and centriole stability. Such an investigation was motivated by two sets of data. First, it has been demonstrated that the glutamate lateral chain acts as a regulator for the binding of microtubule-associated proteins (MAPs) and motors to microtubules (Boucher et al., 1994; Larcher et al., 1996). Second, antibodies directed against glutamylated tubulin have been shown to block flagellar motility of reactivated sperm axonemes, probably through the inhibition of microtubule–dynein binding (Gagnon et al., 1996). The polyglutamate side chain is thus likely to be essential for the interaction of these highly stable microtubule subsets with stabilizing factors or with molecular motors.
We have introduced an antiglutamylated tubulin antibody into mammalian cells and checked for a possible effect on centrioles. We observed a complete disappearance of centrioles and the scattering of the associated pericentriolar material. This disappearance was transient, as centrioles reappeared and a centrosome reformed. During the acentrosomal phase, cells displayed no significant abnormalities of their microtubule network in interphase and apparently proceeded through mitosis with disorganized spindles. Our results confirm that centrioles act as organizers for pericentriolar material. Thus, the experimental system developed in this work provides a favorable situation to investigate the continuity of the centrosome as well as the function of centrioles during spindle pole assembly and mitotic progression.
Materials and Methods
Cell Culture and Cell Cycle Analysis
KE37 cell line was grown in RPMI 1640 medium containing 7% fetal calf serum, 2 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. HeLa cells were maintained in DME medium supplemented with 10% fetal calf serum, 2 mM glutamine, penicillin, and streptomycin. Cells were grown in a humidified incubator with a 5% CO2 atmosphere at 37°C.
Microtubule Regrowth In Vivo
Microtubules were depolymerized by incubation of the cells with 5 μM nocodazole for 2 h on ice. Cells were washed thoroughly with fresh medium at 4°C to eliminate nocodazole before incubation at 37°C with prewarmed medium. Microtubules were allowed to grow for 2 or 15 min, and then cells were extracted in PHEM buffer (45 mM Pipes, 45 mM Hepes both adjusted to pH 6.9, 10 mM EGTA, 5 mM MgCl2, and 1 mM PMSF) containing 0.7% Triton X-100 and fixed by cold methanol.
Indirect Immunofluorescence Microscopy
Cells grown on coverslips were washed in PBS and routinely fixed in methanol −20°C for 7 min. For a better visualization of centrioles by immunofluorescence, cells on coverslips were incubated with 5 μM nocodazole for 2 h on ice, extracted in PHEM buffer containing 0.7% Triton X-100 to remove soluble tubulin, and then fixed by cold methanol (−20°C) for 7 min. Visualization of microtubule cytoskeletons was enhanced by extraction of the cells in PHEM–Triton X-100 0.7% before cold methanol fixation.
After fixation, coverslips were washed in PBS containing 0.1% Tween 20. Primary antibodies diluted in PBS containing 3% BSA were added for 30 min at room temperature. Coverslips were then washed in PBS-Tween 0.1%. The same procedures were used for fluorescein- or rhodamine-labeled secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Coverslips were dehydrated in ethanol and mounted in Citifluor (City University, London, UK). Centrosome preparations, obtained as described (Moudjou and Bornens, 1994), were centrifuged on coverslips and fixed in −20°C methanol for immunofluorescence procedure.
Preparations were visualized on a Leica DMRD microscope (Leica, Inc., Heidelberg, Germany) and images recorded with a CCD camera (Hamamatsu, Japan).
Antibodies
GT335 is a monoclonal antibody raised against a synthetic glutamylated peptide and has been shown to react specifically with polyglutamylated α- and β-tubulin (Wolff et al., 1992). Anti–γ-tubulin polyclonal antibody was raised against bacterially expressed human γ-tubulin (Tassin et al., 1997). Anticentrin polyclonal antibody 26-14.2 was generously given by Dr. J. Salisbury (Mayo Clinic, Rochester, MN) (Baron et al., 1992). Centrioles in mitosis were detected with a rabbit serum raised against bacterially expressed HsCen3 protein (Middendorp et al., 1997; Tassin et al., 1998). Anti-NuMA serum is an autoimmune human antibody. Anti–α- and –β-tubulin monoclonal antibodies (clones DM1A and DM1B, respectively) were obtained from Amersham International (Little Chalfont, UK). Monoclonal antibody 1-6.1 specific for acetylated α-tubulin (Thompson et al., 1984) and monoclonal anti-NuMA antibody were purchased from ICN Pharmaceuticals (Costa Mesa, CA). L3 rabbit serum specific for detyrosinated α-tubulin is a kind gift from Dr. L. Paturle-Lafanechère (Centre d'Etudes Nucleaires, Grenoble, France) (Paturle-Lafanechère et al., 1994). Centrosomes were detected by rabbit serum No. 013 (Gosti et al., 1986). Purified nonimmune human IgGs were purchased from Nordic Immunology (Tilburg, The Netherlands). Dilutions used for immunoblotting and immunofluorescence experiments were 1:5,000 for DM1A and DM1B and 1:10,000 for GT335 (ascite fluid at 3 mg/ml IgGs). For immunofluorescence, dilution was 1:100 for serum 013, 1:500 for anti–γ-tubulin, 1:1,000 for 26-14.1, 1:100 for anti-HsCen3p antibody, and 1:500 for anti-NuMA antibody.
For microinjection and electropermeabilization, pure 1-6.1 immunoglobulins were prepared by the caprylic acid method, and GT335 IgGs were affinity-purified on brain tubulin bound to a Hi-Trap column (Pharmacia Biotech, Piscataway, NJ). Purified GT335 IgGs, 1-6.1, and human immunoglobulins were used at 5 mg/ml for microinjection experiments. Immunoglobulins from the L3 serum were microinjected at 13 mg/ml. For electropermeabilization, GT335 mAb and nonimmune human immunoglobulins were concentrated at 10 mg/ml and dialyzed against electropermeabilization buffer containing 50 mM NaCl to avoid antibody precipitation. Final IgG concentration was brought to 6 mg/ml by addition of the cell suspension.
Protein Analysis
One-dimensional SDS-PAGE was performed according to Laemmli (1970) using 8% polyacrylamide gels. Transfer of proteins onto nitrocellulose membranes was performed as described (Towbin et al., 1979). After electrotransfer, nitrocellulose sheets were stained with Ponceau red to register the location of proteins and saturated for 1 h in PBS-Tween 0.1%.
Protein content was determined according to Bradford (1976), using bovine serum albumin as a standard.
Microinjection Experiments
Cells were seeded onto glass coverslips for at least 2 d until 50% confluent. Coverslips were transferred into fresh medium before injection of immunoglobulins into the cytoplasm of interphase cells. Injection was carried out using an Eppendorf (Madison, WI) semiautomatic microinjection device coupled to the Zeiss (Thornwood, NY) Automated Injection System. Cell fixation (methanol) and immunofluorescence were routinely performed 3 and 24 h after injection. Injected immunoglobulins were detected with the corresponding secondary antibody. In parallel, detection of centrosomes was performed using a rabbit serum directed against pericentriolar material (serum No. 013; Gosti et al., 1986).
Cell Electropermeabilization
Cell electropermeabilization was performed using a PS-15 electropulsator (Jouan, Saint-Herblay, France). Electrodes are composed of two stainless steel plates placed 2 mm apart. Cell populations were pulsed in volumes as reduced as 50 μl, which allows the use of small amounts of antibodies even at large external antibody concentration. HeLa cells were harvested, transferred into low salt electropermeabilization buffer (10 mM Tris-HCl, pH 7.4, 250 mM sucrose, and 1 mM MgCl2), centrifuged, and resuspended in the buffer containing the antibody at a final concentration of 5 × 107 cells/ml. Final NaCl concentration should not exceed 30 mM. After a 5-min incubation at 4°C, 50 μl of the suspension was transferred between the electrodes. 10 square-wave electric pulses of 5 ms each were then immediately delivered at a voltage of 600 V/cm and a frequency of 1 Hz. Voltage can be adjusted to favor cell survival. Cells were then left for 5–10 min in a Petri dish at room temperature, pelleted to remove excess immunoglobulins, resuspended in fresh culture medium, and seeded in Petri dishes with or without glass coverslips. Under these conditions, cell viability is ∼30%. Surviving cells spread out within the first 3 h, allowing us to eliminate floating dead cells by washing the Petri dishes. Centrosomes were detected with the 013 serum, and the percentage of cells displaying a centrosomal labeling was determined on at least 200 cells from randomly selected fields.
Electron Microscopy
Cells were fixed at different times with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.3, for 30 min. The coverslip culture was then washed twice in cacodylate buffer and postfixed in 2% aqueous OsO4 for 60 min at 4°C. After three washes in buffer, the cells were treated with 0.15% tannic acid (in buffer) for 1 min, washed once in buffer, and then washed twice in distilled water. Next, they were stained in 1% uranyl acetate (4°C; 60 min), washed in distilled water, dehydrated in a graded series of ethanols, and flat-embedded in Epon. Cells were then serially thick-sectioned (0.25 μm). Ribbons of sections were mounted in the center of formvar-coated slot grids, on which 25-nm colloidal gold had been lightly deposited to facilitate subsequent micrograph alignment. After staining in uranyl acetate and lead citrate, the sections were viewed and photographed on a Zeiss 910 electron microscope. Selected thick sections were reconstructed by intermediate voltage electron microscope (IVEM) tomography as detailed by McEwen et al. (1993) using an IVEM (model JEM 4000 FX; JEOL USA, Inc., Peabody, MA) equipped with a computer-controlled tilt/rotation specimen holder.
Results
Centriole Microtubules Are Heavily Glutamylated
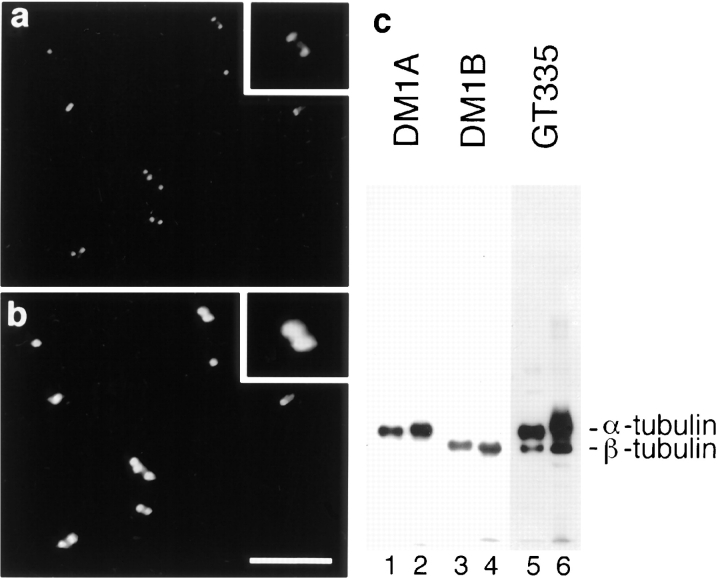
A previous investigation of the presence of glutamylated tubulin in nonneuronal cell lines demonstrated that this modification is mainly detected on centriole α- and β-tubulin, and to a much lesser extent on interphasic and spindle β-tubulin (Bobinnec et al., 1998). When decorated with GT335, an mAb specific to glutamylated tubulin, isolated centrosomes from human KE37 cells were detected as small pairs of dots corresponding to centrioles (Fig. 1 a), whereas a specific serum, which has been shown to stain pericentriolar material (Gosti et al., 1986), decorated bigger dots (Fig. 1 b).
Figure 1.
Centriole microtubules are heavily glutamylated. Double-immunofluorescence on isolated centrosomes with the antiglutamylated tubulin antibody GT335 (a) and 013 anticentrosome antibody (b). Glutamylated centrioles were decorated as pairs of dots embedded in pericentriolar material (twofold magnification of a selected pair in inset). Glutamylated tubulin isoforms were detected in similar amounts in tubulin from brain (c, lanes 1, 3, and 5) and isolated centrosomes (lanes 2, 4, and 6). Detection of α-tubulin (lanes 1 and 2: DM1A), β-tubulin (lanes 3 and 4: DM1B), or glutamylated tubulin (lanes 5 and 6: GT335). Similar amounts of tubulin were loaded in all lanes. Bar, 10 μm.
In brain, as in centriole preparations, GT335 mAb detected both α- and β-tubulin (Fig. 1 c). α-Tubulin appeared more labeled than the β-subunit and was detected as a smear, suggesting that centrioles contained glutamyl chains longer than those of brain microtubules. As glutamylated α-tubulin isoforms are likely to represent >50% of the total tubulin in brain (Eddé et al., 1990; Audebert et al., 1994), we conclude that glutamylation is a major modification of centriole α-tubulin.
Microinjection of GT335 mAb Leads to the Loss of Centrosome Labeling
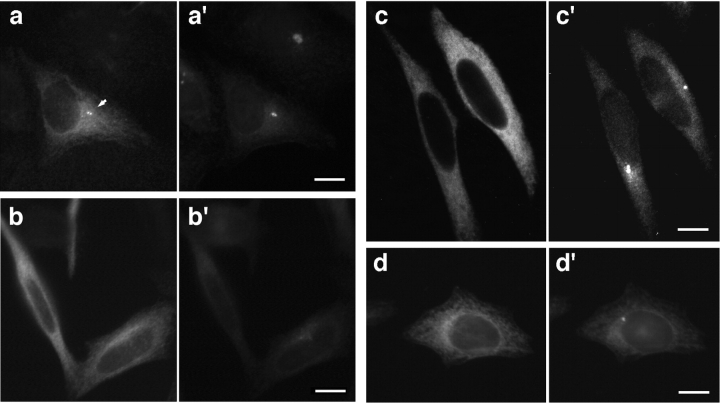
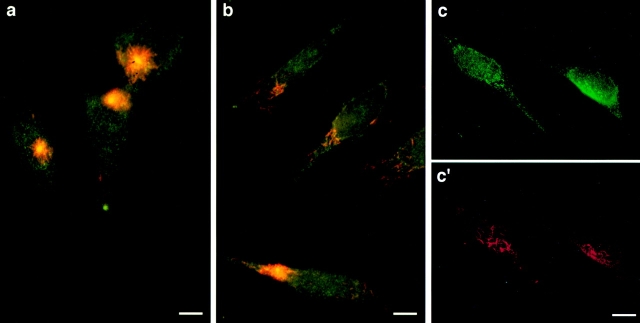
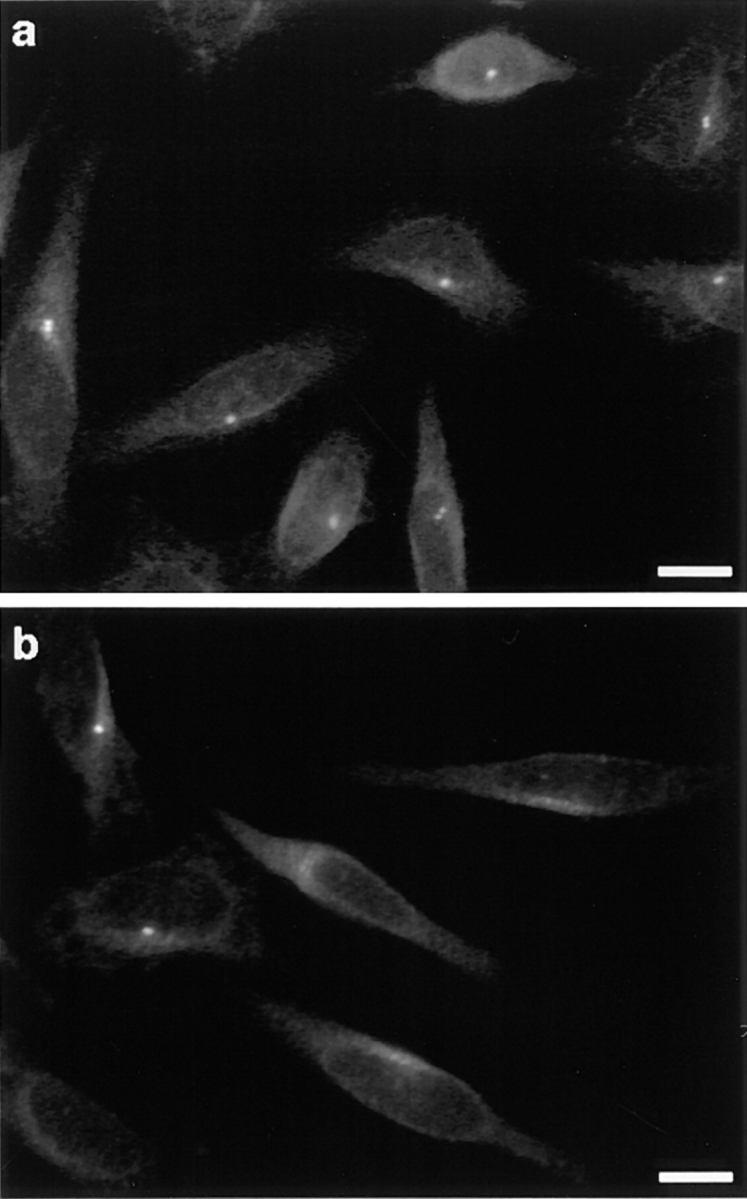
We conducted a series of experiments in which we studied the effect of GT335 mAb microinjection on the structural and functional properties of glutamylated microtubules. In all microinjected cells, GT335 immunoglobulins (IgGs) are detected on cytoplasmic microtubules 3 h after microinjection and decorate the centrioles (Fig. 2 a). Immunostaining of the pericentriolar material with the 013 antibody confirmed a localization of the injected IgGs to the centrioles (Fig. 2 a′). By contrast, after 24 h most microinjected cells (67%, n = 452), identified by the staining of the cytoplasm, did not show a concentration of injected GT335 IgGs at the centrioles (Fig. 2 b). Double immunofluorescence labeling with the anticentrosome serum 013 (Gosti et al., 1986) showed that the cells lacking a labeling of the centrioles by GT335 IgGs did not display any centrosomal staining either (Fig. 2 b′). All control cells, injected with purified IgGs from a nonimmune human serum (Fig. 2, c and c′), exhibited a labeling of the centrosome identical to that of the non-injected cells (Table I). This was also the case with IgGs directed against different posttranslational modifications of tubulin present on HeLa centriole microtubules: L3, a rabbit serum directed against detyrosinated α-tubulin (Paturle-Lafanechère et al., 1994), and 1-6.1, a monoclonal antibody against acetylated α-tubulin (Thompson et al., 1984) (Fig. 2, d and d′). These results suggest a specific effect of GT335 mAb that leads to a loss of centrosomal labeling.
Figure 2.
Microinjection of GT335 immunoglobulins in HeLa cells. Cells were processed for double immunofluorescence with a secondary antibody to localize injected immunoglobulins (a–d) and with 013 anticentrosome antibody (a′–d′). 3 h after injection, GT335 IgGs were detected at the centrioles (arrow) and on cytoplasmic microtubules (a); centrosomes were also detected in the same cells (a′). No centriole or centrosome staining were visible 24 h after microinjection (b and b′). 24 h after injection of mock IgGs, control cells showed a background staining of the cytoplasm and displayed a centrosome labeling (c and c′). 24 h after injection, cells injected with antiacetylated tubulin antibody showed an intense staining of the cytoplasmic microtubules; centrioles were not visible due to this staining (d). Centrosomes were clearly detected (d′). Bars, 10 μm.
Table I.
Effect of Antibody Injection on Centrosome
| Antibody injected | Hours after injection | |||
|---|---|---|---|---|
| + 3 h | + 24 h | |||
| Cells with centrosome (%) | ||||
| GT335 | 194/194 (100) | 150/452 (33) | ||
| Human IgGs | 458/458 (100) | 337/337 (100) | ||
| 1-6.1 | 109/111 (98) | 177/178 (99) | ||
| L3 | 171/171 (100) | 210/210 (100) | ||
Cells were microinjected with GT335 IgGs or control IgGs and fixed after 3 or 24 h. Cells were decorated by immunofluorescence with secondary antibody to detect the injected IgGs, and with anticentrosome antibody to determine the percentage of cells that presented a centrosomal labeling. These results were obtained from several independent experiments (percentages are shown in parentheses).
To further characterize the effect of GT335 IgGs on centrosomes, we set up a protocol that allowed us to load a large population of HeLa cells with an antibody.
GT335 mAb Promote a Massive Disappearance of Centrioles and Centrosomes
We combined previous electropermeabilization protocols (Teissié and Rols, 1988; Casabianca-Pignède et al., 1991) and improved the internalization of antibodies in HeLa cells. The resulting protocol (see Materials and Methods) is more efficient and uses less antibody than classical electroporation methods. As measured by introducing mock IgGs (which were detected with a fluorescent secondary antibody), electropermeabilization allowed us to load 80– 90% of the cells. IgGs could be detected up to 1 wk after electropermeabilization (data not shown), with a progressive decrease in the intensity of the labeling. This suggests that the internalized IgGs are not rapidly degraded, but are rather diluted by successive division of the cells.
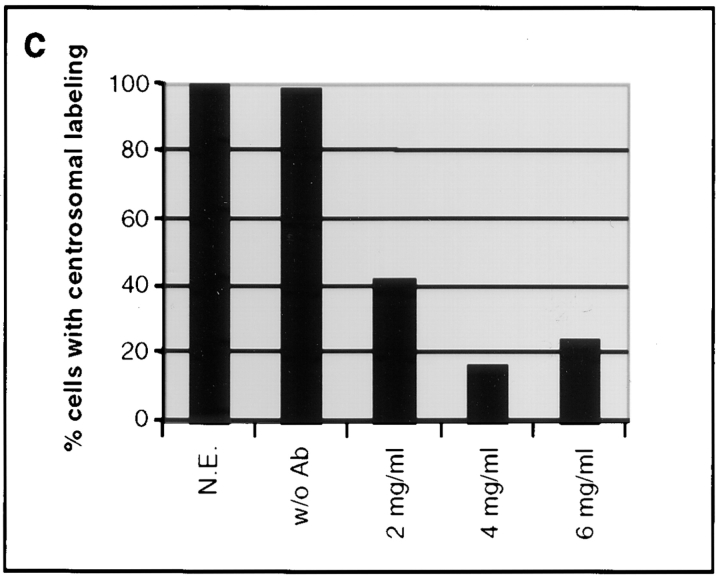
As a first step, we determined the optimal concentration of GT335 IgGs required to induce a loss of centrosome labeling. The proportion of cells that displayed a centrosome labeling was determined 24 h after loading by use of the specific anticentrosome antibody 013 (Fig. 3). As a control, cells were electropermeabilized in presence of human purified IgGs. In this case, the centrosomal labeling could be detected in all cells (Fig. 3 a). When electropermeabilized with GT335 antibody, immunofluorescence analysis (Fig. 3 b) showed that most of the cells had lost the centrosomal labeling. A maximum effect of GT335 IgGs (up to 80%) was reached at a concentration of 4 mg/ml (Fig. 3 c). We thus chose to routinely use IgG concentrations of 6 mg/ml to ensure a better reproducibility of the experiments. Similar percentages of acentrosomal cells were scored using different anticentrosome antibodies from the laboratory, including an anti–γ-tubulin antibody.
Figure 3.
Percentage of cells with a centrosome 24 h after loading was determined by immunofluorescence with the 013 antibody. All cells loaded with mock IgGs presented a centrosomal staining (a), whereas the vast majority of GT335-loaded cells did not (b). All detected centrosomes were in the same focus on these selected fields. Percent of cells displaying a centrosomal labeling was determined under various conditions 24 h after electropermeabilization (c). N.E., nonelectropermeabilized cells; w/o Ab, cells electropermeabilized without antibody; 2–6 mg/ml, final antibody concentration for cell electropermeabilization. A maximal effect was reached at an antibody concentration of 4 mg/ml. Bars, 10 μm.
Since centrioles were no longer decorated by GT335 IgGs in these cells, we investigated if these structures could still be detected with other anticentriole antibodies. To distinguish centrioles from the cytoplasmic microtubule network, cells were incubated with nocodazole at 4°C for 2 h and further extracted with Triton X-100 to remove all soluble tubulin. Under these conditions, centrioles are the only microtubule structures resistant to nocodazole. We then carried out double immunofluorescence experiments with an antibody directed against centrin, an intracentriolar protein (Paoletti et al., 1996), and antitubulin antibody. Immunostaining of control cells (loaded with human IgGs) with anti–α-tubulin antibody showed two or four bright spots corresponding to centrioles, which colocalized with the anticentrin labeling. By contrast, double immunostaining with anti–α-tubulin and anticentrin antibodies failed to reveal such a typical centriole staining in 72% of the cells loaded with GT335 IgGs (not shown). In the same experiment, the use of an anti-PCM antibody showed an absence of centrosome labeling in 76% of the cells.
These results suggest that GT335 IgGs induce the complete disappearance of the centrioles and of the surrounding pericentriolar material. These conclusions were supported by electron microscopy analysis of complete serial section of GT335-loaded cells (see below).
GT335 mAb-loaded Cells Have No Microtubules Nucleating Center
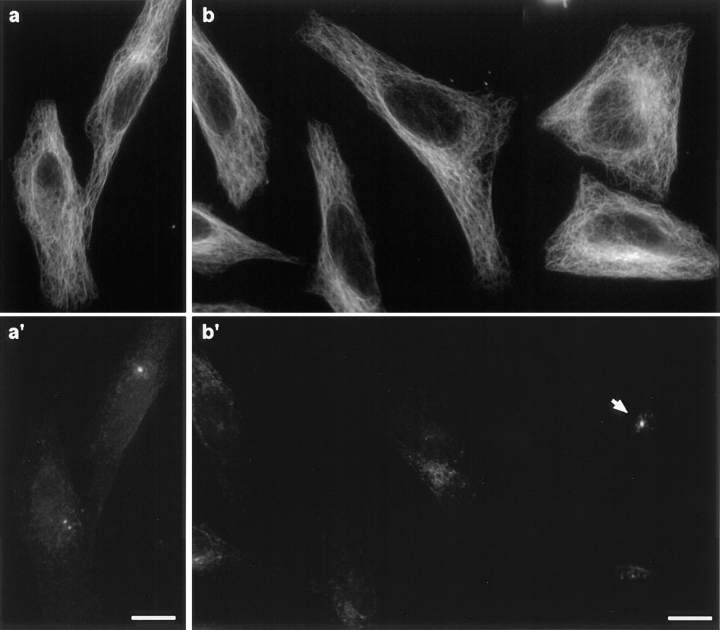
Centrosomes are the main microtubule organizing centers of interphase cells. To investigate a possible disorganization of the microtubule cytoskeleton in acentrosomal cells, cells were detergent-extracted and processed for immunofluorescence 24 h after electropermeabilization. Decoration of microtubules and centrosomes revealed no obvious differences in the organization of the interphasic microtubule network of centrosomal and acentrosomal cells, although a radial aster focused on the centrosome could be seen in some control cells and never in acentrosomal cells (Fig. 4). Interestingly, numerous detergent-extracted acentrosomal cells presented small foci of pericentriolar material scattered in the juxtanuclear region (Fig. 4 b′).
Figure 4.
GT335-loaded cells present no visible microtubule disorganization. Cells collected 24 h after antibody loading were detergent-extracted and processed for immunofluorescence with antitubulin (a and b) and 013 anticentrosome (a′ and b′) antibodies. Comparison of microtubule networks from mock IgG–loaded cells (a) and GT335-loaded cells (b) showed no obvious differences. Centrosome staining was detected as discrete spots in control cells (a′) and as scattered foci in most GT335-loaded cells (b′). One cell in (b′) still possessed a centrosome (arrow). Bar, 10 μm.
To reveal nucleating capabilities of acentrosomal cells, we conducted microtubule regrowth experiments in cells after microtubule depolymerization. 24 h after electropermeabilization, cells were incubated for 2 h with nocodazole, and microtubule regrowth from endogenous tubulin was allowed for 2 or 15 min. After 2 min, the formation of a small aster centered on the centrosome was observed in all control cells (Fig. 5 a). By contrast, no aster was found in the majority of the cells loaded with GT335 IgGs. Instead, very short microtubules were found disseminated within the cytoplasm (Fig. 5, b and c′). As expected, these cells presented no focused centrosomal labeling. Scattered pericentriolar material was detected in the juxtanuclear region, in the vicinity of growing microtubules (Fig. 5, c and c′), and the microtubule network appeared to be reconstituted when GT335 mAb-loaded cells were allowed to recover from nocodazole for 15 min (data not shown), suggesting that the discrete cytoplasmic nucleation sites observed in the cells, or spontaneous assembly, were sufficient for the reassembly of the microtubule network.
Figure 5.
Loaded cells have no functional microtubules nucleating centers. Control and GT335-loaded cells were treated with nocodazole and extensively washed to allow microtubule regrowth. Cells were further processed for double immunofluorescence with antitubulin (red) and anticentrosome (green) antibodies. Microtubules regrew as an aster focused on the centrosome in the control (a), whereas GT335 mAb–loaded cells displayed short individual microtubules disseminated in the cytoplasm (b). Pericentriolar material was detected by rabbit serum 013 close to the nucleus (c), where single microtubules were nucleated (c′). Bars, 10 μm.
Together, the above-mentioned results imply that GT335 IgGs induce the disassembly of the centrioles, which in turn leads to the scattering of the pericentriolar material. Thus, the absence of centrioles does not seem to abolish the capacity of pericentriolar material to organize and nucleate microtubules.
Centrosome and Centriole Disappearance Is a Transient Phenomenon
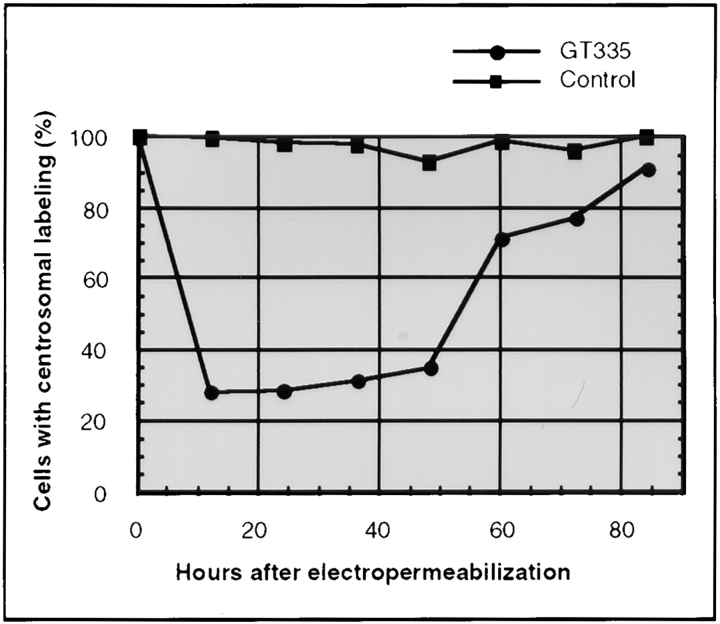
Next we determined the time course of the GT335 mAb effect on centrosome labeling. Cells were fixed every 12 h after loading with GT335 IgGs and analyzed by immunofluorescence with the 013 rabbit serum to decorate centrosomes. A representative experiment is shown in Fig. 6. A maximum effect of GT335 IgGs was reached 12 h after electropermeabilization and maintained for 36 h. Strikingly, this effect was reversible, as centrosome labeling was progressively recovered in the whole cell population. 84 h after cell loading, almost all cells displayed normal centrosome labeling. As a control, a cell population was processed in parallel with mock IgGs. No loss of centrosomal labeling was observed at any time.
Figure 6.
Centrosome disappearance is transient. Cells loaded with GT335 antibody were collected every 12 h and processed for immunofluorescence with an anticentrosome antibody. Percent of cells with a centrosomal labeling was determined over time (n = 200). Data presented here are means from two independent experiments. 97–100% of the control cells loaded with mock immunoglobulins displayed a centrosomal labeling (squares). By contrast, cells loaded with GT335 IgGs lost centrosomal labeling (circles) in the first 12 h. At this time, only 28% of the cells displayed a centrosomal labeling. Centrosomes were progressively detected anew within the cell population. At 84 h, almost all cells presented a centrosomal labeling.
Thus, the disappearance of the centrioles and the disorganization of the centrosome is a transient event. We postulate that this is related to the decrease of GT335 mAb content in the cytoplasm.
Mitotic Spindles in Acentriolar Cells
We investigated the organization of mitotic spindles in GT335 mAb-loaded cells by immunofluorescence using several antibodies, including a rabbit serum raised against human centrin 3, a protein constitutive of the centrioles, and GT335 mAb itself, as it represents a convenient way to visualize both centrioles and spindle microtubules.
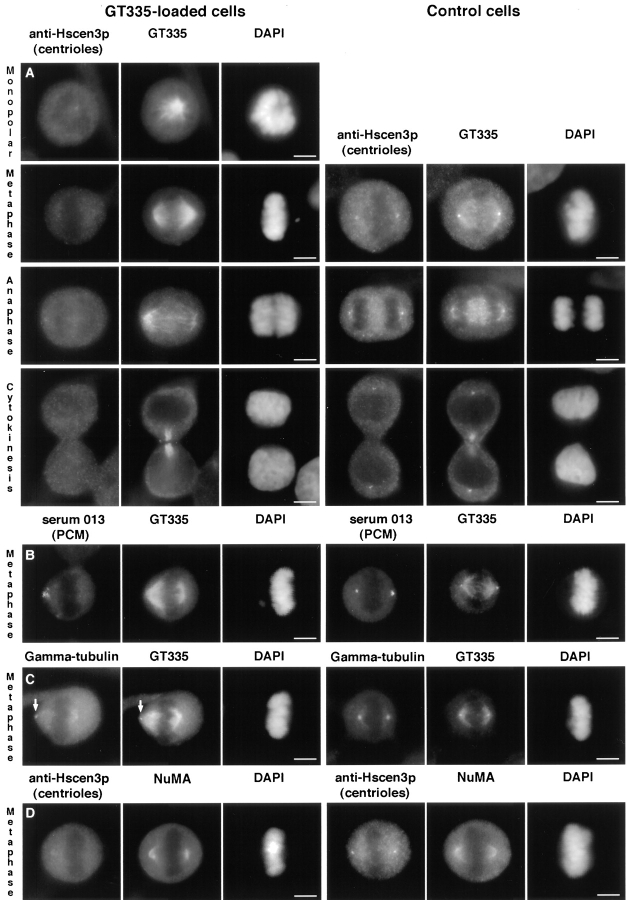
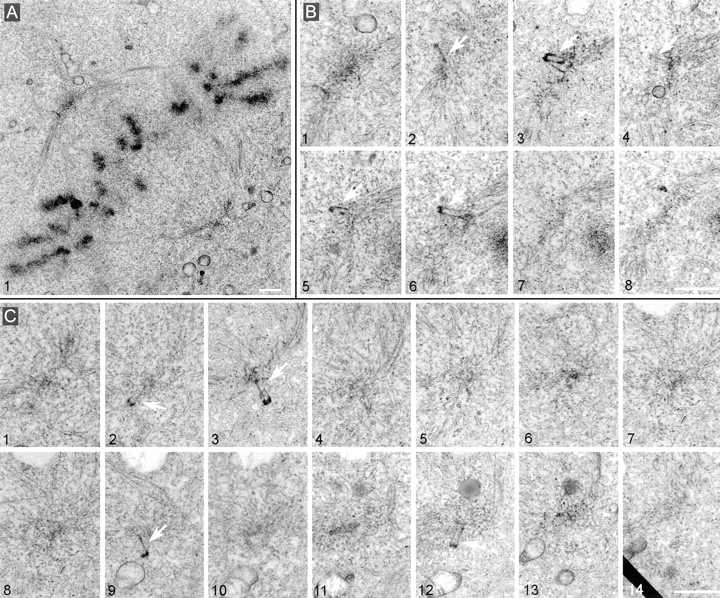
Immunofluorescent analysis of GT335 mAb-loaded cells showed that 58% (n = 200) of the mitotic cells displayed a monopolar spindle, compared with 2% for control cells, whereas the other 42% displayed bipolar metaphase spindles (Fig. 7 A). Some bipolar spindles presented a centriole at one pole. Acentriolar spindles were often characterized by an asymmetry between the two half-spindles. Most poles appeared loosely organized, sometimes barrel-shaped at one pole (compare for example metaphase of Fig. 7, A and C, the latest still possessing one centriole at the leftmost pole). The pericentriolar material showed an unequal distribution at the acentriolar poles (Fig. 7 B). γ-Tubulin could be faintly detected at the acentriolar poles but appeared segregated in variable amount to each pole, just like the pericentriolar material (Fig. 7 C). We further checked for the presence of NuMA, a protein involved in spindle pole stabilization (Gaglio et al., 1995). Double immunofluorescence with antipericentriolar material antibody showed that NuMA was indeed a component of the acentriolar spindle poles, but it did not show its usually sharp focused distribution (Fig. 7 D).
Figure 7.
GT335 mAb–loaded cells display mitotic figures with acentriolar poles. Cells were processed for immunofluorescence 24 h after antibody loading. The left columns represent GT335-loaded cells, and the right columns are control cells. (A–C) All cells were costained with GT335 antibody to detect microtubules and DAPI for nuclei staining. (A) Detection of the centrioles with an antibody against human centrin3 at different stages of mitosis. (B) Detection of the pericentriolar material by the 013 serum. (C) Detection of γ-tubulin. (D) Cells were costained with HsCen3p and with NuMA antibodies. Nuclei were stained by DAPI. Bipolar mitotic spindles with no centrioles were observed, showing some disorganization of the poles. However, these spindles seemed functional, as anaphase and cytokinesis figures were observed (A). Pericentriolar material was segregated in asymmetrical amounts and presented a nonfocussed organization. The selected cell presented no centrioles at both poles, as judged by the GT335 staining (B). γ-Tubulin was barely detectable at the poles, and it stained centrioles when present (arrow in C). NuMA was present at the acentriolar poles but showed some disorganization (D). Bars, 5 μm.
Taken together, these results indicate that the acentriolar spindle poles are organized by the same components as the centriole-containing poles, but these proteins appeared less focused. In spite of this, spindles could be functional, as anaphase and cytokinesis figures (Fig. 7 A) in which both daughter cells were devoid of centriole staining were observed.
Thus, it seems likely that in acentriolar cells distribution of pericentriolar material was modified at mitosis, being partially focused at minus ends of spindle microtubules, together with the recruitment of other spindle pole components.
Electron Microscopy Analysis Confirms a Complete Loss of the Centrioles
We further investigated by electron microscopy if any structure reminiscent of the centriole or centrosome could be seen. Complete cell reconstructions were obtained by serial thick sectioning (0.25 μm). Cells were prepared for electron microscopy 24 and 60 h after electropermeabilization.
Interphase Cells.
Of a total of 12 interphase cells analyzed at 24 h, 9 had no detectable centrioles or centriole-like structures. In the three others, two possessed a pair of normal centrioles, and one had a single centriole to which a procentriole was attached. In the latter case, the single centriolar cylinder appeared to be thinner and slightly kinked at the distal end. 60 h after treatment, we found centrioles in 8 out of 12 interphase cells. However, three cells contained one single centriole: one of these was normal, one was twice shorter than normal, and one showed a single microtubule triplet in its vicinity. Although obtained on a small number of cells, these data are in agreement with the percentage of acentriolar cells determined by immunofluorescence analysis of the same experiment (Table II).
Table II.
Percent Acentriolar Interphase Cells
| Time after treatment | IF | EM | ||
|---|---|---|---|---|
| 24 h | 148/200 (71.5%) | 9/12 | ||
| 60 h | 57/200 (28.5%) | 4/12 |
Comparison of the percentage of acentriolar interphase cells estimated by immunofluorescence (IF) and electron microscopy (EM) 24 and 60 h after loading the cells with GT335 IgGs.
Mitotic Cells.
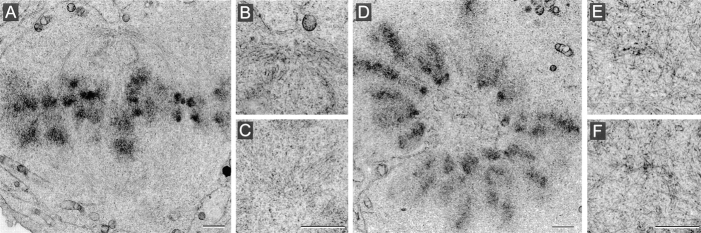
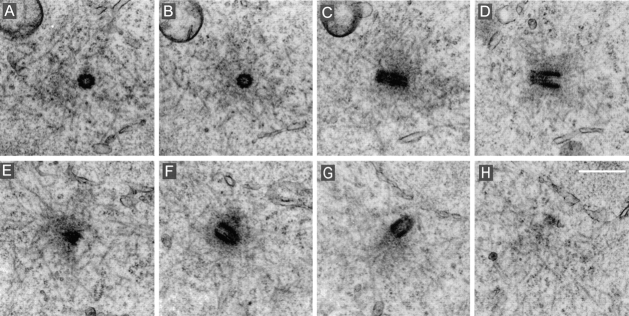
We carried out the complete reconstruction of three mitotic cells fixed 24 h after antibody loading. One of these cells presented a bipolar spindle (Fig. 8, A–C). The poles were easily identified and contained pericentriolar material but no centrioles. The second cell showed a monopolar spindle with chromosomes arranged around the acentriolar pole (Fig. 8, D–F). In the third case, two sister cells still paired through a midbody were found, exhibiting no centriole in both cells (not shown). This latter observation supports our immunofluorescence data (see Fig. 7 A).
Figure 8.
Cells were collected 24 h after electropermeabilization and were processed for electron microscopy. (A) Overview of a bipolar spindle. (B and C) Magnification of the poles from the same section as in A. Centrioles were not visible at both poles. (D) Monopolar spindle with chromosomes displaying a rosette configuration. The center of this mitotic figure contained no centrioles, as shown after magnification of two adjacent sections, presented in E and F. Bars, 1 μm.
We further analyzed by electron microscopy mitotic cells 60 h after loading. For comparison, centriole pairs at both poles of a mitotic cell loaded with mock IgGs are presented in Fig. 9. On the six mitotic cells analyzed from a population loaded with GT335 mAb, one cell displayed a bipolar spindle with normal centriole pairs at the poles. Two cells showed well-organized bipolar spindles, but a careful examination revealed unusual organization of the spindle poles. Centriole-like structures, which resembled highly distorted incomplete cylinders, were present in both poles (one cell, see Fig. 10) or in one only of two mitotic poles (one cell, not shown). These centriole-like structures were not completely surrounded by the pericentriolar material of the pole, but rather were excluded from it.
Figure 9.
Mitotic cell from a control population analyzed 60 h after loading with human nonimmune IgGs. This late prometaphase cell presented centriole pairs at both poles. (A–D) Adjacent sections of one pole. (E–H) Adjacent sections of the other pole. Bar, 500 nm.
Figure 10.
Metaphase spindles assemble in cells collected 60 h after loading with GT335 mAb but present partial centrioles at their poles. (A) Overview of the spindle. (B) Eight adjacent sections of the upper spindle pole. (C) 14 adjacent sections of the lower pole. Note in both cases the presence of several abnormal centrioles (arrows). They seemed excluded from the pericentriolar cloud and presented a weak staining of the walls. Pericentriolar material, however, was visible at both poles. Bars, 1 μm.
Three other cells possessed a monopolar spindle. These monopolar spindles contained one or two distorted centriole-like structures embedded in pericentriolar material in their single poles (Fig. 11, A and B).
Figure 11.
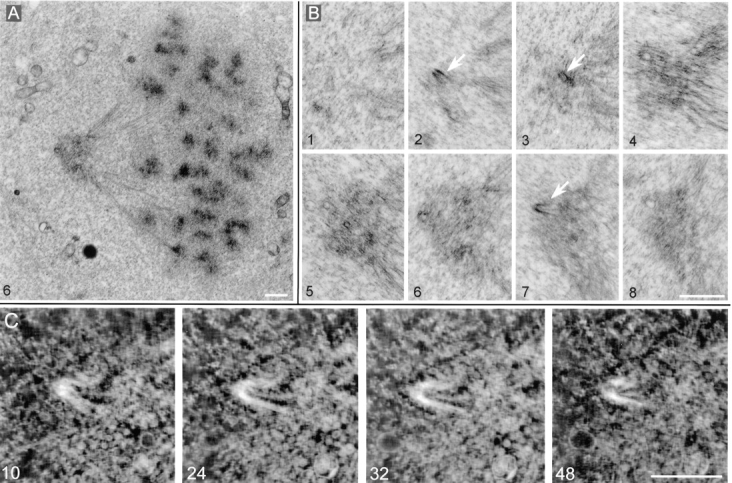
Monopolar spindle observed 60 hours after loading with GT335 mAb. (A) Overview of the spindle. (B) Eight successive sections (thickness 0.25 μm each) through the pole of the spindle. Arrows point to centriole remnants. (C) Tomographic reconstruction of the centriolar remnant seen in B, section 7. Four axial sections of 3–4 nm are presented. Bars: (B) 1 μm; (C) 0.5 μm.
To further characterize these centriole-like structures, we performed three-dimensional IVEM tomography on two of them. Tomographic analysis revealed that indeed the walls of these “incomplete centrioles” were organized by microtubules (Fig. 11 C). However, these microtubules did not form triplets that are characteristic of normal centrioles. Individual microtubules were not parallel to each other and did not form a complete cylinder. Overall, the structure resembled a “partially disrupted” centriole.
Thus, we concluded that GT335 mAb leads to the disintegration of the centrioles and dispersion of the pericentriolar material.
Acentriolar Cells and Mitotic Progression
By both immunofluorescence and electron microscopy, we obtained evidence that the cells are able to proceed through mitosis in the absence of centrioles. The unique specificity of this system, in contrast to an ablation approach (by laser irradiation, see Koonce et al., 1984, or by microsurgery, see Maniotis and Schliwa, 1991), is that the centrosome disappears only transiently. This raises the possibility that all centrosomal components are still present as scattered functional subcomplexes within the cell.
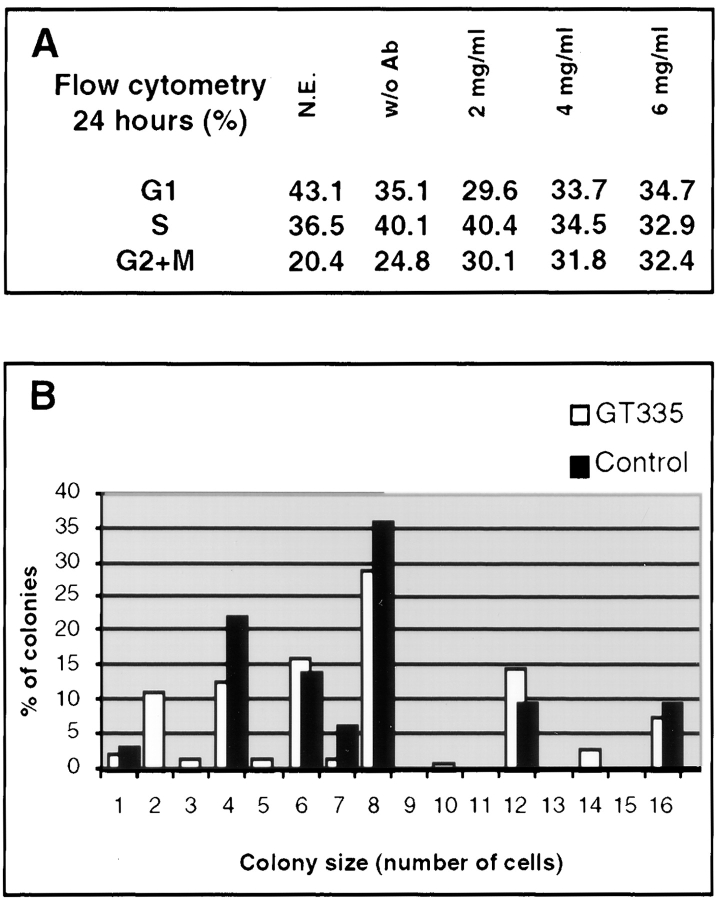
To characterize the behavior of acentriolar cells, the cell cycle distribution of GT335 mAb–loaded cells was analyzed by flow cytometry 24 h after electropermeabilization. The data show a limited increase in G2/M cells for the highest concentrations in IgGs (Fig. 12 A). To extend these results, cells loaded with GT335 mAb were seeded at low density in Petri dishes, and the ability to form colonies was measured 4 d later. In this experiment, no significant differences were visible in terms of size and number of colonies between control and GT335 mAb–treated samples (Fig. 12 B).
Figure 12.
Growth of GT335 mAb–loaded cells. (A) Flow cytometry analysis of cell cycles show an increase in G2/M phases for GT335 mAb–loaded cells after 24 h. N.E., nonelectropermeabilized cells; w/o Ab, cells electropermeabilized without antibody; 2–6 mg/ml, final antibody concentration for cell electropermeabilization. (B) Cells were plated at low density after loading with GT335 mAb, and the number of cells per colony was determined 5 d later. Bars represent the percent of colonies in the corresponding size. For each colony size, the first bar (white) is for GT335 IgGs, and the second bar (black) is for control IgGs. The distribution of the colonies according to their size did not appear significantly different.
Since we scored the acentriolar cells as ∼70% in the whole population for both experiments, these results suggest that most cells proceed through mitosis in the absence of a centrosome and lead to the formation of colonies, whereas a small proportion of the cells presented a mitotic delay.
Discussion
Centrosome Stability Depends upon Polyglutamylation of Centriole Tubulin
Glutamylation is the major posttranslational modification of neuronal and axonemal microtubules (Eddé et al., 1990; Fouquet et al., 1994). In other cell types, we previously found that this modification concerns primarily the centriole microtubules (Bobinnec et al., 1998). In this paper, we investigated the functional significance of this property.
Our data demonstrate that this modification, or at least the tubulin domain immediately proximal, is involved in centriole stability, as the introduction of a monoclonal antibody directed against glutamylated tubulin (GT335) leads to the disappearance of the whole centrosome structure. Other antibodies specific for other posttranslational modifications of tubulin have no effect, in particular L3 antibodies, which recognize a major centriolar epitope corresponding to the detyrosinated form of α-tubulin that localized very near the glutamylation site. However, whether the effect of GT335 mAb is manifested through its binding to the glutamylated site of α-tubulin, β-tubulin, or both is not known. Previous studies reported that the polyglutamate side chain mediates the binding of MAPs (Boucher et al., 1994) and motors (Larcher et al., 1996) to microtubules. Moreover, GT335 monoclonal antibody had been shown to inhibit the motility of reactivated sperm axonemes (Gagnon et al., 1996). Based on these data, we propose that GT335 antibody inhibits the binding of specific centriolar organizing proteins, either directly or by impairing tubulin polyglutamylation of centriole microtubules. Both effects might contribute.
Centriole disintegration could either be induced by loss of interactions between microtubule triplets or by the destabilization of the triplets themselves. Since we did not find, within the first 24 h, intermediate figures that could correspond to abnormal centriole structures (such as disconnected triplets), we believe that GT335 mAb leads to progressive destabilization of the doublet and triplet microtubules. This would fit with the observation that centriole destabilization by GT335 mAb is a rather slow process. No effect was observed 3 h after injection, and 12 h were necessary to reach a maximal effect, a time which is in the range of tubulin turnover in centrioles (Kochanski and Borisy, 1990). In this view, the exceptional stability of the centriole microtubules could be achieved by the regulated binding of stabilizing proteins through the polyglutamate side chain. The inhibition of these interactions by GT335 mAb would modify the dynamics of centriole microtubules and lead to their progressive disassembly and to the dispersal of the whole centrosome structure.
Are Centriole Organizers Segregated to Acentriolar Daughter Cells?
In mammalian cell lines, the generation of a new centriole is believed to arise from a structure associated with the preexisting centriole. As suggested earlier (Mazia et al., 1960; Sluder and Rieder, 1985a ; Sluder et al., 1989), a nontubulin structure could be responsible for the assembly of a new centriole. This conservative mechanism implies that the constitutive loss of the centriolar structure (Debec et al., 1982) or the microsurgical removal of the whole centrosome (Maniotis and Schliwa, 1991) would lead to the inability of the cell to generate a new centriole. In the present work, we observed the regeneration of centrioles after their complete disappearance, suggesting either that centriole organizers were preserved in transiently acentriolar cells, or that de novo centriole formation occurs in this system. We were not able to identify a structure that could correspond to such centriole organizers, but the fact that GT335 antibody acts through the destabilization of microtubules leads us to favor the hypothesis of the maintenance of such a structure. Previous studies reported that a procentriole appears on the parental centriole as a short cylinder that progressively elongates to reach its final size (Dippell, 1968; Gould, 1975; Kuriyama and Borisy, 1981). These observations suggest that centriole microtubules are gradually stabilized and linked to each other concomitantly to their elongation. In our system, we visualized abnormal centrioles 60 h after introduction of GT335 antibody. However, no intermediate structures were observed at earlier stages. We believe that these abnormal centrioles correspond to partially reassembled centrioles. Since we show that the GT335 antibody act in a dose-dependent manner, the progressive dilution of the antibody within the cell at each division might allow the progressive reassembly of the centrioles. 84 h after introduction of GT335 antibody, almost all cells presented a normal centrosome staining. This observation suggests that centriole organizers were segregated in all acentriolar daughter cells.
Do Acentriolar Cells Proceed through Mitosis?
The occurrence of acentriolar cells after loading with the GT335 antibody allowed us to address the question of the role of the centrioles in spindle formation. Our data suggest that acentriolar cells can proceed through mitosis, in agreement with other reports (Debec et al., 1982), even though many abnormal mitotic spindles were observed. We cannot exclude a contribution of GT335 antibody to disorganization of the spindle poles through an effect on spindle microtubules. However, the level of glutamylated tubulin in cytoplasmic microtubules is very low, whereas centriole tubulins are heavily modified (Bobinnec et al., 1998). When cells were fixed 3 h after injection of the GT335 antibody, some cells presented labeled metaphase spindles looking identical to noninjected cells. Abnormal spindles were indeed visualized 24 h after injection, a time where the effect of the antibody on centrioles was maximal. Moreover, spindle defects, such as loosely organized or barrel-shaped poles, were already reported in studies of acentriolar mitosis (Debec et al., 1982; Heald et al., 1997). We thus believe that spindle defects were not caused by the binding of GT335 to spindle microtubules but were rather the consequence of the absence of centrioles. Mitotic markers such as NuMA (Compton and Cleveland, 1994) were always detected at the poles, although in asymmetrical amounts. Pericentriolar material was found in various amounts at the spindle poles and was barely detectable in some cases. These observations suggest that the pathway of spindle assembly with or without centrioles involves indeed the same components (Merdes and Cleveland, 1997). In agreement with previous results that suggested that NuMA redistribution at the onset of mitosis depends upon the correct redistribution of PCM between centriole pairs (Paoletti et al., 1997), the present work indicates that in animal cells, when present, centrioles allow the correct segregation of the pericentriolar material and lead to more defined spindle poles.
Monopolar spindles observed presented partial centriole-like structures associated with pericentriolar material. This finding suggests that these structures were still able to interact with pericentriolar material, leading to the formation of a pseudocentrosome. The inability of such structures to duplicate could explain the occurrence of these monopolar spindles. However, most of these monopolar spindles displayed chromosomes that did not surround the single pole, but rather were organized as a metaphase plate. These images are quite different from monopolar spindles of cells blocked in metaphase. Therefore, there is a possibility that these monopolar figures, as suggested earlier in Drosophila (Wilson et al., 1997), arose from bipolar spindles from which one pole collapsed. However, the reciprocal movements of centrosomes and chromosomes are highly dynamic at the onset of mitosis, and other possibilities exist to explain the formation of such monopolar spindles (see for example Cassimeris et al., 1994). We were not able to analyze these transitions. In vivo analysis of acentriolar mitosis in cells with fluorescently tagged microtubules will be necessary to explain this point and to ascertain whether cells with such half-spindles are able or not to exit from mitosis.
A Model for Centrosome Assembly
Two features of the GT335 mAb-induced disappearance of centriole structure, and of the whole centrosome organelle as a consequence, are striking: First, it is a slow process. We believe that the observed time course for centrosome disappearance results from interference of the antibody with both the turnover of individual centrosomal components and the duplication cycle of the whole organelle. Centrioles could be more sensitive to destabilization by GT335 mAb during a limited period of the cell cycle. Indeed, important changes in centrosome organization and redistribution of the pericentriolar material are known to take place at the onset of mitosis (Rieder and Borisy, 1982; Vorobjev and Chentsov, 1982). We previously showed that concomitantly, centriole glutamylation is increased during mitosis (Bobinnec et al., 1998). Molecular (Lange and Gull, 1995) and structural differences (Paintrand et al., 1992; Doxsey et al., 1994) between mother and daughter centrioles could account for, or reflect, differential stability within the centriole pair. This could explain our observation of cells with a single centriole at 24 h as well as 60 h after loading.
Second, centrosome disappearance is accompanied by a scattering of the microtubules nucleating material in the cytoplasm and around the nucleus. Our data suggests that the focalization of the centrosomal material depends on centrioles. Also supporting such a role for centrioles, we noted that even abnormal centrioles, observed 60 h after introduction of GT335 mAb, were apparently able to concentrate material around them at the spindle poles (see Figs. 8 and 10). Such a role was also suggested in more physiological conditions. After the loss of centrioles during differentiation of myoblasts into myotubes, the pericentriolar material redistributes around the nucleus, where microtubules are nucleated (Tassin et al., 1985). All these data, as well as ultrastructural features of centrosome organization, suggest that some centrosomal components interact directly with the microtubule triplets of the centrioles.
A minimal model for centrosome assembly could propose that centrioles nucleate the assembly of the centrosomal matrix through two subsets of proteins, one able to bind to the wall, and another able to bind to the proximal end. For the first subset, progressive glutamylation of tubulin during centriole assembly would bring long-term stability to this edifice and trigger the lateral association of specific MAPs (Boucher et al., 1994; Larcher et al., 1996).
In the mean time, the nine triplets of stable and short centriole microtubules would provide at their proximal end a set of highly stable microtubule minus ends able to anchor a second subset of proteins, i.e., minus end–binding proteins, including γ-tubulin–containing complexes (Moritz et al., 1995; Zheng et al., 1995; Martin et al., 1998; Murphy et al., 1998; Tassin et al., 1998). These complexes could in turn trigger the progressive accumulation of centrosomal matrix components. The observation that microtubule-stabilizing drugs induce the redistribution of the centrosomal matrix from centrioles to the minus ends of free cytoplasmic microtubules supports this proposal (for a discussion of this aspect see Bornens, 1992; Paoletti et al., 1997). This model is admittedly highly hypothetical, since our knowledge on centriole composition is still scarce. The recent isolation of the gene UNI3 (which appears necessary for the assembly of basal bodies in Chlamydomonas reinhardtii) as a new tubulin gene, distinct from α-, β-, and γ-tubulin, might represent a new and important piece of information for understanding centriole assembly (Dutcher and Trabuco, 1998).
Whatever the molecular mechanisms controlling the assembly of centrioles, we can conclude from the present work that in animal cells, the centriole pair is a conspicuous part of the centrosome and plays a critical role for centrosome stability and reproduction. One of the important goals in the near future will be to understand how these roles are exerted.
Acknowledgments
We would like to thank Dr. L. Paturle-Lachanefère and Dr. D. Job for L3 antidetyrosinated tubulin serum and Dr. J. Salisbury for 26-14.2 anticentrin antibody. We thank Dany Rouillard for FACS® analysis. We also thank Dr. V. Doye for helpful discussions and critical reading during the preparation of the manuscript, E. Desbruyères for technical assistance, and D. Meur for artwork.
Abbreviations used in this paper
- IVEM
intermediate voltage electron microscopy
- MAP
microtubule-associated protein
- PCM
pericentriolar material
Footnotes
Y. Bobinnec is the recipient of a fellowship from the Association pour la Recherche sur le Cancer. This work was supported in part by National Institutes of Health (NIH) GMS 40198 to C.L. Rieder, by NIH/NCRR P41-RR 01219 for the Wadsworth Center Biological Microscopy and Image Reconstruction Facility as a National Biotechnological Resource, and by Institut Curie, CNRS, and European Community grant CHRX-CT940642 to M. Bornens.
References
- Audebert S, Koulakoff A, Berwald-Netter Y, Gros F, Denoulet P, Eddé B. Developmental regulation of polyglutamylated α- and β-tubulin in mouse brain neurons. J Cell Sci. 1994;107:2313–2322. doi: 10.1242/jcs.107.8.2313. [DOI] [PubMed] [Google Scholar]
- Baron AT, Greenwood TM, Bazinet CW, Salisbury JL. Centrin is a component of the pericentriolar lattice. Biol Cell. 1992;76:383–388. doi: 10.1016/0248-4900(92)90442-4. [DOI] [PubMed] [Google Scholar]
- Beatty, R.A. 1967. Parthenogenesis in vertebrates. In Fertilization. C.B. Metz and A. Monroy, editors. Academic Press, New York. 413–440.
- Bobinnec Y, Moudjou M, Fouquet JP, Desbruyères E, Eddé B, Bornens M. Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil Cytoskel. 1998;39:223–232. doi: 10.1002/(SICI)1097-0169(1998)39:3<223::AID-CM5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bornens, M. 1992. Structure and function of isolated centrosomes. In The Centrosome. V.I. Kalnins, editor. Academic Press, San Diego. 1–43.
- Bornens M, Paintrand M, Berges J, Marty MC, Karsenti E. Structural and chemical characterization of isolated centrosomes. Cell Motil Cytoskel. 1987;8:238–249. doi: 10.1002/cm.970080305. [DOI] [PubMed] [Google Scholar]
- Boucher D, Larcher JC, Gros F, Denoulet P. Polyglutamylation of tubulin as a progressive regulator of in vitrointeractions between the microtubule-associated protein Tau and tubulin. Biochemistry. 1994;33:12471–12475. doi: 10.1021/bi00207a014. [DOI] [PubMed] [Google Scholar]
- Boveri T. Uber die Natur der Centrosomen. Jena Z Med Naturw. 1901;28:1–220. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Calarco-Gilliam PD, Siebert MC, Hubble R, Mitchison T, Kirschner M. Centrosome development in early mouse embryos as defined by an auto-antibody against pericentriolar material. Cell. 1983;35:621–629. doi: 10.1016/0092-8674(83)90094-6. [DOI] [PubMed] [Google Scholar]
- Casabianca-Pignède M-R, Mir LM, Le Pecq JB, Jacquemin-Sablon A. Stability of antiricin antibodies introduced into DC-3F Chinese hamster cells by electropermeabilization. J Cell Pharmacol. 1991;2:54–60. [Google Scholar]
- Cassimeris L, Rieder CL, Salmon ED. Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J Cell Sci. 1994;107:285–297. doi: 10.1242/jcs.107.1.285. [DOI] [PubMed] [Google Scholar]
- Compton DA, Cleveland DW. NuMA, a nuclear protein involved in mitosis and nuclear reformation. Curr Opin Cell Biol. 1994;6:343–346. doi: 10.1016/0955-0674(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Debec A, Szöllösi A, Szöllösi D. A Drosophila melanogastercell line lacking centrioles. Biol Cell. 1982;44:133–138. [Google Scholar]
- Dingle AD, Fulton C. Development of the flagellar apparatus of Naegleria. . J Cell Biol. 1966;31:43–54. doi: 10.1083/jcb.31.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippell RV. The development of basal bodies in Paramecium. . Proc Natl Acad Sci USA. 1968;51:461–468. doi: 10.1073/pnas.61.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Dutcher SK, Trabuco EC. The UNI3 gene is required for assembly of basal bodies of Chlamydomonasand encodes δ-tubulin, a new member of the tubulin superfamily. Mol Biol Cell. 1998;9:1293–1308. doi: 10.1091/mbc.9.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddé B, Rossier J, Le Caer JP, Desbruyeres E, Gros F, Denoulet P. Posttranslational glutamylation of α-tubulin. Science. 1990;247:83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Fouquet JP, Eddé B, Kann ML, Wolff A, Desbruyères E, Denoulet P. Differential distribution of glutamylated tubulin during spermatogenesis in mammalian testis. Cell Motil Cytoskel. 1994;27:49–58. doi: 10.1002/cm.970270106. [DOI] [PubMed] [Google Scholar]
- Gaglio T, Saredi A, Compton DA. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T, Dionne MA, Compton DA. Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J Cell Biol. 1997;138:1055–1066. doi: 10.1083/jcb.138.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon C, White D, Cosson J, Huitorel P, Eddé B, Desbruyères E, Paturle-Lafanechère L, Multigner L, Job D, Cibert C. The polyglutamylated lateral chain of α-tubulin plays a key role in flagellar motility. J Cell Sci. 1996;109:1545–1553. doi: 10.1242/jcs.109.6.1545. [DOI] [PubMed] [Google Scholar]
- Gosti F, Marty MC, Berges J, Maunoury R, Bornens M. Identification of centrosomal proteins in a human lymphoblastic cell line. EMBO (Eur Mol Biol Organ) J. 1986;5:2545–2550. doi: 10.1002/j.1460-2075.1986.tb04533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RR. The basal bodies of Chlamydomonas reinhardtii: formation from probasal bodies, isolation, and partial characterization. J Cell Biol. 1975;65:65–74. doi: 10.1083/jcb.65.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Bulinski JC. Distribution of tyrosinated and nontyrosinated α-tubulin during mitosis. J Cell Biol. 1986;102:1118–1126. doi: 10.1083/jcb.102.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Habermann A, Karsenti E, Hyman A. Spindle assembly in Xenopusegg extracts: respective roles of centrosomes and microtubule self-organization. J Cell Biol. 1997;138:615–628. doi: 10.1083/jcb.138.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz C, Dabauvalle MC, Paintrand M, Weber T, Bornens M, Karsenti E. Parthenogenesis in Xenopuseggs requires centrosomal integrity. J Cell Biol. 1990;110:405–415. doi: 10.1083/jcb.110.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanski RS, Borisy GG. Mode of centriole duplication and distribution. J Cell Biol. 1990;110:1599–1605. doi: 10.1083/jcb.110.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce MP, Cloney RA, Berns MW. Laser irradiation of centrosomes in newt eosinophils: evidence of centriole role in motility. J Cell Biol. 1984;98:1999–2010. doi: 10.1083/jcb.98.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Borisy GG. Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J Cell Biol. 1981;91:814–821. doi: 10.1083/jcb.91.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange BM, Gull K. Molecular marker for centriole maturation in the mammalian cell cycle. J Cell Biol. 1995;130:919–927. doi: 10.1083/jcb.130.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher JC, Boucher D, Lazereg S, Gros F, Denoulet P. Interaction of kinesin motor domains with α- and β-tubulin subunits at a Tau-independent manner. J Biol Chem. 1996;271:22117–22124. doi: 10.1074/jbc.271.36.22117. [DOI] [PubMed] [Google Scholar]
- Maniotis A, Schliwa M. Microsurgical removal of centrosomes blocks cell reproduction and centriole generation in BSC-1 cells. Cell. 1991;67:495–504. doi: 10.1016/0092-8674(91)90524-3. [DOI] [PubMed] [Google Scholar]
- Maro B, Howlett SK, Webb M. Non–spindle microtubule organizing centers in metaphase II–arrested mouse oocytes. J Cell Biol. 1985;101:1665–1672. doi: 10.1083/jcb.101.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin OC, Ruwanthi N, Gunawardane AI, Zheng Y. Xgrip109: a tubulin-associated protein with an essential role in tubulin ring complex (TuRC) assembly and centrosome function. J Cell Biol. 1998;141:675–687. doi: 10.1083/jcb.141.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D, Harris PJ, Bibring T. The multiplicity of mitotic centers and the time-course of their duplication and separation. J Biophys Biochem Cytol. 1960;7:1–10. doi: 10.1083/jcb.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BF, Arena J, Liu YU, Frank J, Rieder CL. Three-dimensional ultrastructure of the colcemid-treated PtK1 kinetochore outer plate as determined by high voltage electron microscopic tomography. J Cell Biol. 1993;120:301–312. doi: 10.1083/jcb.120.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, Cleveland DW. Pathways of spindle pole formation: different mechanisms; conserved components. J Cell Biol. 1997;138:953–956. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middendorp S, Paoletti A, Schiebel E, Bornens M. Identification of a new mammalian centrin gene, more closely related to Saccharomyces cerevisiaeCDC31 gene. Proc Natl Acad Sci USA. 1997;1994:9141–9146. doi: 10.1073/pnas.94.17.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami I, Gall J. Centriole replication. II. Sperm formation in the fern, Marsilea and the cycad, Zamia. . J Cell Biol. 1966;56:13–26. doi: 10.1083/jcb.29.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature. 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Moudjou, M., and M. Bornens. 1994. Method of centrosome isolation from cultured animal cells. In Cell Biology: A Laboratory Handbook. J.E. Célis, editor. Academic Press, San Diego. 595–604.
- Murphy SM, Urbani L, Stearns T. The mammalian γ-tubulin complex contains homologues of the yeast spindle pole body components Spc97p and Spc98p. J Cell Biol. 1998;141:663–674. doi: 10.1083/jcb.141.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintrand M, Moudjou M, Delacroix H, Bornens M. Centrosome organization and centriole architecture: their sensitivity to divalent cations. J Struct Biol. 1992;108:107–128. doi: 10.1016/1047-8477(92)90011-x. [DOI] [PubMed] [Google Scholar]
- Paoletti A, Moudjou M, Paintrand M, Salisbury JL, Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J Cell Sci. 1996;109:3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- Paoletti A, Bornens M. Organisation and functional regulation of the centrosome in animal cells. Prog Cell Cycle Res. 1997;3:285–299. doi: 10.1007/978-1-4615-5371-7_23. [DOI] [PubMed] [Google Scholar]
- Paoletti A, Giocanti N, Favaudon V, Bornens M. Pulse treatment of interphasic HeLa cells with nanomolar doses of docetaxel affects centrosome organization and leads to catastrophic exit of mitosis. J Cell Sci. 1997;110:2403–2415. doi: 10.1242/jcs.110.19.2403. [DOI] [PubMed] [Google Scholar]
- Paturle-Lafanechère L, Manier M, Trigault N, Pirollet F, Mazarguil H, Job D. Accumulation of Δ2-tubulin, a major tubulin variant which cannot be tyrosinated, in neuronal tissues and in stable microtubule assembly. J Cell Sci. 1994;107:1529–1543. doi: 10.1242/jcs.107.6.1529. [DOI] [PubMed] [Google Scholar]
- Piperno G, Fuller MT. Monoclonal antibodies specific for an acetylated form of α-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985;101:2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Borisy GG. The centrosome cycle in PtK2 cells: asymmetric distribution and structural change in the pericentriolar material. Biol Cell. 1982;44:117–132. [Google Scholar]
- Robbins E, Jentzsch G, Micali A. The centriole cycle in synchronized HeLa cells. J Cell Biol. 1968;36:329–339. doi: 10.1083/jcb.36.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten H, Schatten G, Mazia D, Balczon R, Simerly C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sci USA. 1986;83:105–109. doi: 10.1073/pnas.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Rieder CL. Centriole number and the reproductive capacity of spindle poles. J Cell Biol. 1985a;100:887–896. doi: 10.1083/jcb.100.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Rieder CL. Experimental separation of pronuclei in fertilized sea urchin eggs: chromosomes do not organize a spindle in the absence of centrosomes. J Cell Biol. 1985b;100:897–903. doi: 10.1083/jcb.100.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Rieder CL. Reproductive capacity of sea urchin centrosomes without centrioles. Cell Motil Cytoskel. 1989;13:264–273. doi: 10.1002/cm.970130405. [DOI] [PubMed] [Google Scholar]
- Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- Stearns T, Winey M. The cell center at 100. Cell. 1997;91:303–309. doi: 10.1016/s0092-8674(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Szöllözi D, Callarco PG, Donahue RP. Absence of centrioles in the first and second meiotic spindles mouse oocytes. J Cell Sci. 1972;11:521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- Tassin AM, Maro B, Bornens M. Fate of microtubule-organizing centers during myogenesis in vitro. J Cell Biol. 1985;100:35–46. doi: 10.1083/jcb.100.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin AM, Celati C, Paintrand M, Bornens M. Identification of an Spc110p-related protein in vertebrates. J Cell Sci. 1997;110:2533–2545. doi: 10.1242/jcs.110.20.2533. [DOI] [PubMed] [Google Scholar]
- Tassin AM, Celati C, Moudjou M, Bornens M. Characterization of the human homologue of the yeast Spc98p and its association with γ-tubulin. J Cell Biol. 1998;141:689–701. doi: 10.1083/jcb.141.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissié, J., and M.P. Rols. 1988. Electropermeabilization and electrofusion of cells. In Dynamics of Membrane Proteins and Cellular Energetics. N. Latruffe, Y. Gaudemer, P. Vignais, and A. Azzi, editors. Springer Verlag, Berlin. 249–268.
- Theurkauf WE, Hawley RS. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nodKinesin-like protein. J Cell Biol. 1992;116:1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WC, Asai DJ, Carney DH. Heterogeneity among microtubules of the cytoplasmic microtubule complex detected by a monoclonal antibody to α-tubulin. J Cell Biol. 1984;98:1017–1025. doi: 10.1083/jcb.98.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoresis transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev IA, Chentsov YS. Centrioles in the cell cycle. I. Epithelial cells. J Cell Biol. 1982;98:938–949. doi: 10.1083/jcb.93.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PG, Fuller MT, Borisy GG. Monastral bipolar spindles: implications for dynamic centrosome organization. J Cell Sci. 1997;110:451–464. doi: 10.1242/jcs.110.4.451. [DOI] [PubMed] [Google Scholar]
- Wolff A, de Néchaud B, Chillet D, Mazarguil H, Desbruyères E, Audebert S, Eddé B, Gros F, Denoulet P. Distribution of glutamylated α- and β-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur J Cell Biol. 1992;59:425–432. [PubMed] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchison M. Nucleation of microtubule assembly by a γ-tubulin–containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]