Abstract
Fluorescence microscopic analysis of newly replicated DNA has revealed discrete granular sites of replication (RS). The average size and number of replication sites from early to mid S-phase suggest that each RS contains numerous replicons clustered together. We are using fluorescence laser scanning confocal microscopy in conjunction with multidimensional image analysis to gain more precise information about RS and their spatial-temporal dynamics. Using a newly improved imaging segmentation program, we report an average of ∼1,100 RS after a 5-min pulse labeling of 3T3 mouse fibroblast cells in early S-phase. Pulse-chase-pulse double labeling experiments reveal that RS take ∼45 min to complete replication. Appropriate calculations suggest that each RS contains an average of 1 mbp of DNA or ∼6 average-sized replicons. Double pulse–double chase experiments demonstrate that the DNA sequences replicated at individual RS are precisely maintained temporally and spatially as the cell progresses through the cell cycle and into subsequent generations. By labeling replicated DNA at the G1/S borders for two consecutive cell generations, we show that the DNA synthesized at early S-phase is replicated at the same time and sites in the next round of replication.
Keywords: replication sites, replication timing, cell nucleus, chromosomes, computer image segmentation
In pioneering autoradiographic studies, it was determined that regions of euchromatin in the mammalian cell nucleus replicated early in S-phase and regions of heterochromatin replicate later in S-phase (Hay and Revel, 1966; Milner, 1969; Williams and Ockey, 1970; Ockey, 1972; Huberman et al., 1973; Comings and Okada, 1973; Fakan and Hancock, 1974; Sparvoli et al., 1976; Smith et al., 1984). Immunofluorescence microscopy of incorporated biotin labeled dUTP (Nakayasu and Berezney, 1989; Berezney, 1991) or 5-bromo-2′-deoxyuridine (BrdU1; Nakamura et al., 1986; Nakayasu and Berezney, 1989; Volgel et al., 1989; Fox et al., 1991; Kill et al., 1991; O'Keefe et al., 1992) further revealed discrete granular sites termed replication sites (RS) that vary in number and location at different times of the S-phase in the mammalian nucleus.
Analysis of the temporal and spatial order of RS has defined three major morphological patterns termed type I, type II, and type III (Nakayasu and Berezney, 1989). These three distinct replication patterns correspond to DNA synthesis in early to mid S-phase, mid to late S-phase, and very late S-phase, respectively. In cells of type I, DNA replication proceeds predominately over the extranucleolar euchromatic regions. Type II RS are concentrated over the perinucleolar and perinuclear heterochromatic regions. Type III RS correspond to DNA replication at satellite DNA heterochromatic regions (Nakayasu and Berezney, 1989; Kill et al., 1991). Other studies have demonstrated further division of the replication sites into five different intranuclear staining patterns (van Dierendonck et al., 1989; Neri et al., 1992; O'Keefe et al., 1992).
It is known that eukaryotic chromosomal DNA is divided into hundreds to thousands of independent subunits of replication termed replicons (Hand, 1978). Moreover DNA sequences are replicated at precise times within the S-phase of eukaryotic cells (Goldmen et al., 1984; Hatton et al., 1988), a phenomenon termed replication timing (RT). Previous studies concluded that a gene's position in the chromosome, rather than its sequence, determines the timing of replication (Calza et al., 1984). Thus, changes in replication timing after gene rearrangement may be mediated by changes in the proximity of the affected gene to sites that control the temporal order of replication during S-phase (Calza et al., 1984; Hatton et al., 1988; Dhar et al., 1989). These studies provided the first indication that replicon cluster synthesis may be temporally and spatially regulated along the chromosomal DNA.
Previous studies from our laboratory and others showed that the arrangement of replicated DNA into discrete RS-like structures persist throughout the cell cycle and subsequent daughter cells (Sparvoli et al., 1994; Berezney et al., 1995a ,b; Jackson and Pombo, 1998). These results suggest that the organization of replicating DNA at distinct RS represents a fundamental feature of the higher order arrangement of chromatin in the cell nucleus. One important factor in this higher order arrangement may be the nuclear matrix. Previous studies demonstrated a remarkable preservation of RS and their S-phase–specific patterns after extraction for nuclear matrix (Nakayasu and Berezney, 1989; Neri et al., 1992; Berezney et al., 1995b ).
In this study, we use fluorescence laser scanning confocal microscopy coupled to multidimensional image analysis to investigate the arrangement of individual replication sites in synchronized mammalian cells. The average number of replication sites in early S-phase and their average lifetime have been determined with great precision. Our results lead us to conclude that each RS corresponds to an in vivo replicon cluster. Further, we study the spatial and temporal dynamic of early and later replicated DNA throughout the cell cycle, and replicating timing of the early S-phase replicated DNA at two consecutive G1/S borders. We report a striking maintenance of the spatial arrangement and timing of replication sites from one cell generation to another.
Materials and Methods
Cell Culture and Synchronization
Mouse 3T3 fibroblasts (American Type Culture Collection [ATCC], Rockville, MD) were grown as monolayers in DME (GIBCO BRL, Gaithersburg, MD) plus 10% FCS (GIBCO BRL). Kangaroo kidney PtK1 cells (ATCC) were grown in MEM (GIBCO BRL) plus 10% FBS (Summits Biotechnology) supplemented with sodium pyruvate acid (GIBCO BRL) and MEM nonessential amino acids (GIBCO BRL). Cells were synchronized by serum starvation as previously reported (Nakayasu and Berezney, 1989). In brief, cells were cultured in 0.5% FCS for 3T3 cells, or 0.5% FBS for PtK1 cells for 72 h, and were released from the nonproliferating G0 phase by the addition of 10% serum into the culture medium. Cells were pulsed for early replication site labeling 10 h after serum addition.
To synchronize cells at the G1/S border, mouse 3T3 fibroblasts were serum deprived as described above. The cells were then cultured with 10% serum in the presence of 2 μg/ml aphidicolin (Cordeiro-Stone and Kaufman 1985; Sorscher and Cordeiro-Stone, 1991; Brylawski et al., 1993) for 20 h. After aphidicolin release and an additional 10 h growth, the cells were blocked at the G1/S border in the second cell cycle by adding 2 μg/ml aphidicolin for 20 h.
Replication Site and Immunolabeling
Single Pulse or Pulse–Chase Experiments.
Mouse 3T3 fibroblasts, either exponentially growing or synchronized in early S-phase by serum deprivation were pulsed with BrdU (10 μM) for 2–30 min. For chromosome spreads, PtK1 cells were synchronized by serum deprivation. 10 h after serum readdition (early S-phase), the cells were labeled with BrdU (10 μM) for either 2 or 15 min, chased for 10 h in fresh medium and processed for chromosomes (see Preparation of Chromosome Spreads). RS labeling was performed according to the instructions of the 5-Bromo-2-Deoxy-Uridine Labeling and Detection Kit I (Boehringer Mannheim Corp., Indianapolis, IN) including reaction with mouse anti-BrdU and FITC conjugated anti– mouse IgG (both 1:10 dilution; Boehringer Mannheim Corp.) for 30 min at 37°C. Chromosomes were counterstained with 0.025 μg/ml propidium iodide (PI).
Pulse-Chase-Pulse Experiments.
In the double labeling (pulse-chase-pulse) experiments, synchronized 3T3 cells in early S-phase were labeled for 2 min with CldU (10 μM), chased for up to 10 h, and pulsed again for 5 min with IdU (10 μM).
Double replication site labeling were performed according to the method developed by Aten et al., (Aten et al., 1992) and modified in our laboratory (Berezney et al., 1995b ). In brief, cells were fixed in 70% ethanol for at least 20 min in −20°C. The halogenated deoxyuridines incorporated DNA was denatured with 4 N HCl for 30 min at room temperature. Antibody reactions were carried out successively with rat anti-BrdU (1:10 dilution; Sera-Lab, Crawley Down, UK) and mouse anti-BrdU monoclonal antibodies (1:50 dilution) for 30 min at 37°C. FITC (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and Texas red (GIBCO BRL) secondary antibodies (both diluted 50-fold with PBS-0.02% Tween 20), respectively, were used for detection. High salt buffer (29.2 g/liter NaCl; 4.44 g/liter Tris-HCl, pH 7.5; and 0.5% Tween 20) was used to remove low affinity cross-reactive anti-BrdU antibodies.
Double Pulse–Double Chase Experiments.
In the double pulse–double chase experiments, synchronized 3T3 cells in early S-phase were pulsed for 2 min with CldU, chased for 2 h and pulsed again for 5 min with IdU. The cells were then chased a second time for 1–48 h before fixation. Double labeling of RS was performed as described above in Pulse-Chase-Pulse Experiments.
For RS and chromosome three color labeling experiments, chromosomes were counterstained with PI (0.025 μg/ml) and displayed in pseudocolor blue. The secondary antibody against the first pulsed CldU was FITC conjugated (green color). The secondary antibody against the second pulsed IdU was Cy-5 conjugated goat anti–mouse IgG (diluted 50-fold with PBS-0.02% Tween 20; Jackson ImmunoResearch Laboratories, Inc.) and displayed in pseudocolor red.
Labeling Early Replication Site at Two Consecutive G1/S Borders.
3T3 cells synchronized at G1/S in the first cell cycle were pulsed for 30 min with CldU (10 μM) immediately after the release from aphidocolin, chased to the second G1/S border and pulsed again for 30 min with IdU (10 μM). Cell fixation, DNA denaturation, and double RS labeling was performed as described above in Pulse-Chase-Pulse Experiments.
Preparation of Chromosome Spreads
After the pulse–chase protocol for RS labeling, kangaroo kidney PtK1 cells were treated with 0.015 μg/ml of colcemid (Sigma Chemical Co., St. Louis, MO) for 2 h at 37°C (Trask, 1991). The cell pellet was then incubated at 37°C for 17 min with prewarmed 75 mM KCl, followed by fixation in three changes of fresh 3:1 methanol/acetic acid (10 min for the first fixation). Cells in suspension were dropped onto clear glass coverslips and incubated in ddH2O at 4°C for at least 12 h in a humid environment to promote spreading of chromosomes. Slides were air dried overnight at room temperature. Chromosome spreads were hardened by incubated for 4 h at 65°C, and then fixed in 70% ethanol in glycine buffer, 50 mM, pH 2.0, for at least 20 min at −20°C.
Confocal Microscopy
All samples were mounted in SlowFade medium (Molecular Probes, Inc., Eugene, OR) and examined by laser scanning confocal microscopy. The confocal imaging was performed on a three channel laser scanning confocal imaging system (MRC-1024 ; Bio-Rad Laboratories, Hercules, CA) equipped with a Nikon Optiphot 2 microscope, a Nikon 60×, 1.4 NA objective and an argon/krypton laser used to excite FITC (488 nm), Texas red or PI (568) and Cy-5 (649 nm). In some cases we used an Olympus GB200 laser scanning confocal microscope system equipped with a plan apo 60×, 1.4 NA objective and an argon laser (λ = 514 nm) to excite green and red fluorescence simultaneously. Optical sections of 512 pixels × 512 pixels × 8 bits/pixel (MRC-1024) or 1536 pixels × 1536 pixels × 24 bits/pixel (Olympus GB200) were collected through the samples at 0.5-μm intervals. 3-D images were reconstructed and pseudo-colored using Sterecon or VMLSM software.
Results
Measuring Replication Sites in the Mammalian Cell Nucleus
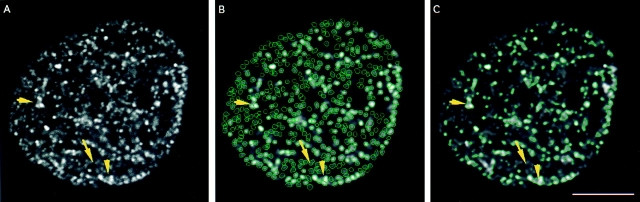
We have combined double labeling techniques, laser scanning confocal microscopy, and a newly developed image segmentation method to investigate the three-dimensional spatial and temporal properties of in vivo DNA RS in the mammalian cell nucleus. In the first series of experiments, we labeled mouse 3T3 fibroblasts with BrdU for 2–30 min. Then we analyzed the confocal images from cells engaged in early S-phase (type I; Nakayasu and Berezney, 1989) DNA replication with a spot based segmentation method (Samarabandu et al.,et al., 1995). This is distinct from the commonly used threshold based methods for segmentation and enabled us to segment virtually all RS visible in the original images despite the wide range of intensities and the close proximity of many of the individual RS (Fig. 1 B). In contrast, application of a commonly used threshold-based algorithm (Samarabandu et al., 1991) resulted in very significant inaccuracies due largely to many small groups of closely positioned RS being segmented as single sites (Fig. 1 C, arrowheads) and the elimination of more weak staining RS during the thresholding (Fig. 1 C, arrow). Indeed, the total number of measured sites averaged less than one-half of that detected with our spot based segmentation in both individual optical sections (e.g., 495 versus 189 contours in Fig. 1, B and C, respectively) and in the overall nuclear volume (1,856 versus 788 sites for the image shown in Fig. 1).
Figure 1.
Computer image analysis of individual replication sites. Replication sites were single labeled for 5 min with BrdU in unsynchronized 3T3 fibroblast cells. (A) Confocal image of replication sites in 3T3 cells in a typical 0.5-μm midplane section. (B) Contours of replication sites in the optical section shown in A after the spot based segmentation method. 495 RS were counted. (C) Contours of replication sites for A using the threshold based segmentation method. 189 RS were counted. Yellow arrow indicates one cluster of weak RS that were imaged well in B but missed in C. Yellow arrowheads indicate closely spaced RS that were imaged as individual RS in B, but grouped into a single contour in C. Bar, 5 μm.
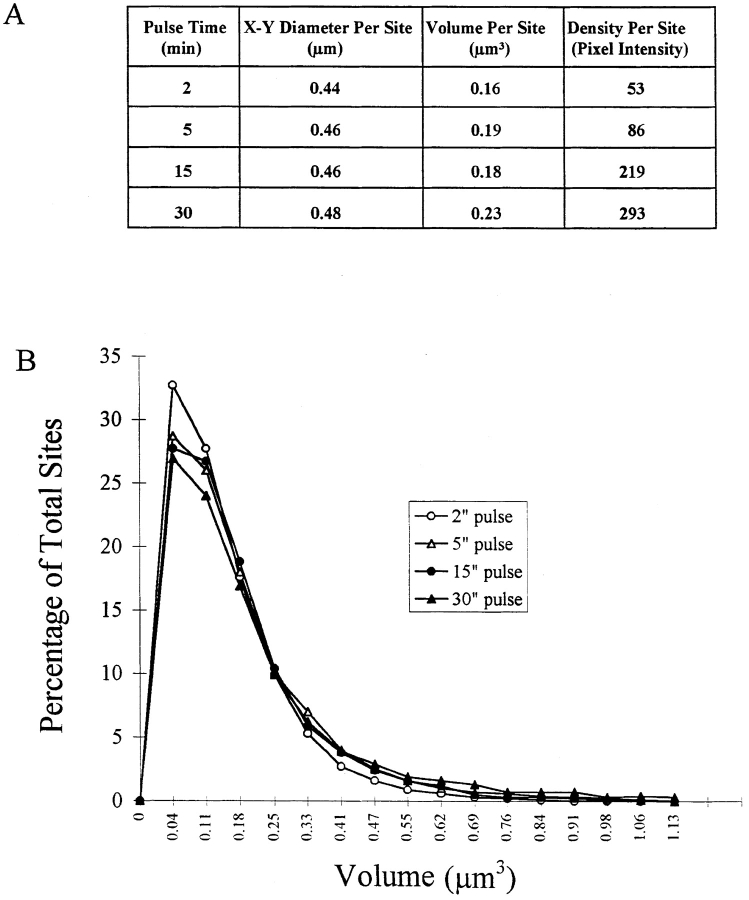
Using these approaches, we have determined that the early S-phase (type I) replication patterns labeled after a 5-min BrdU pulse, contain an average of 1,080 individual sites per nucleus with an average diameter in the X-Y plane of 0.46 μm ± 0.01 (n > 12,000). Moreover, neither the X-Y diameter nor the overall volume of a RS, which represent the average of >12,000 sites for each pulse period, significantly changed between 2–15-min pulses with only a slight increase after a 30-min pulse (Fig. 2 A). There was also a striking similarity in the volume distribution of the total population of RS for each pulse time (Fig. 2 B). In contrast, the relative intensity of the RS populations increase proportionally with pulse time (Fig. 2 A).
Figure 2.
Quantitative image analysis of replication sites. (A) Volumes and labeling intensities of RS after two 30-min pulses. (B) Distribution of RS volumes after two 30-min pulses. Over 12,000 individual sites were measured for each pulse time.
Kinetic Analysis of Replication Site Lifetimes
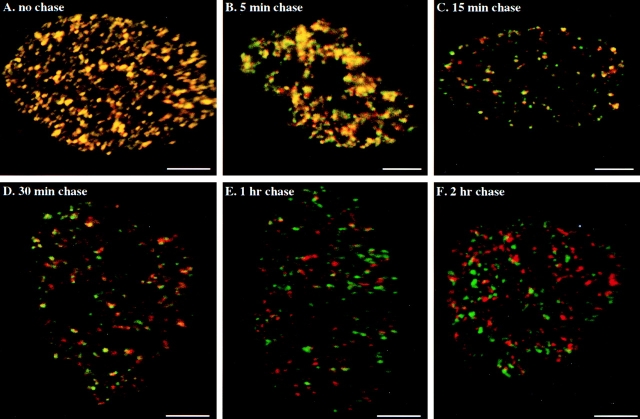
A series of pulse-chase-pulse experiments were performed to directly determine the average time to complete replication at individual RS. As described in Materials and Methods, synchronized 3T3 cells were pulsed in early S-phase with CldU (2 min) followed by a variable chase time (5–120 min) and a second pulse with IdU for 5 min. The strategy of this experiment is to measure the progressive decrease in overlap of early replication sites (first pulse, green color) with later replication sites (second pulse, red color). Thus the zero chase control experiment (simultaneous labeling with CldU and IdU) shows that all the labeled replication sites overlap (Fig. 3, 100% yellow sites). However, with increasing chase time between pulses there should be a progressive decrease in the number of labeled yellow sites (early and later replication at the same site) and a corresponding increase in the number of separately labeled green sites (sites labeled only with the first pulse) and red sites (sites labeled only with the second pulse). The kinetics of this progressive decay in the distribution of double labeled (Fig. 3, yellow) sites compared with singly labeled sites is a characteristic property of the average time it takes to complete replication at each site. As shown in Fig. 3, most of the replication sites are labeled as mixed yellow sites after a 5-min chase but this decreases to a small percentage after a 30-min pulse. By 1 h, all the sites are separated into red and green.
Figure 3.
Double labeling experiments in early S-phase. Replication sites in synchronized 3T3 fibroblasts were first labeled for 2 min with CldU (FITC secondary antibody, green sites), chased for 0–60 min and, pulsed again for 5 min with IdU (Texas red secondary antibody, red sites). (A) Simultaneously pulsed; chased for (B) 5 min; (C) 15 min; (D) 30 min; (E) 1 h; (F) 2 h. Bars, 5 μm.
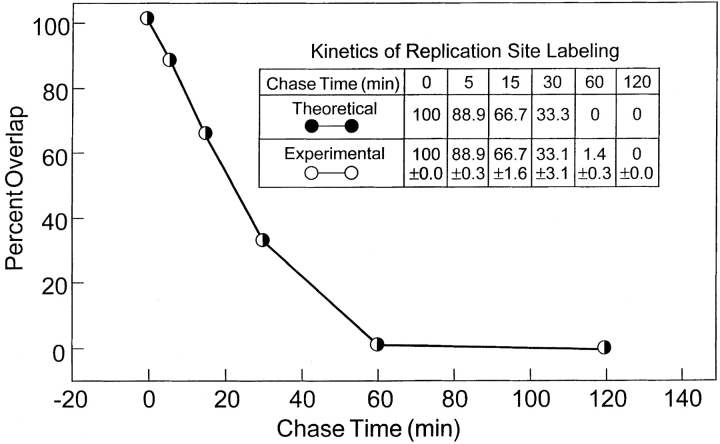
Next, we quantitated the progressive decay in mixed yellow labeled sites by direct counting of >10,000 RS for each chase time and calculating the percentage of yellow sites in the total population of yellow (early and late replication) plus green sites (early replication only). This was performed manually by direct comparison of individual sites of the separate channel and merged images. This enabled a more rigorous determination of overlap at individual sites than the simpler method of counting yellow sites in the merged images. The later method depends on a good balance of signals between the two channels whereas our method directly determines overlap without the need to visualize a yellow color. The results shown in Fig. 4, perfectly match the theoretical values obtained by assuming a replication lifetime of 45 min per site.
Figure 4.
Kinetics of replication sites. The yellow RS and green RS were counted after chases from 0–120 min (>10,000 RS were counted for each chase time). The x axis indicates the chase time between the early and later RS. The y axis indicates the percentage overlap and is defined as the percentage of yellow RS (overlap of early and later RS labeling) compared with the yellow plus green (early S-phase labeling) sites. A theoretical decay chase curve based on a 45-min labeling time for a RS completely coincides with the experimental curve. Individual values ± SEM are shown in the insert.
Estimating the Amount of DNA and the Number of Replicons Per Replication Site
The data generated in this study can then be used to estimate the average amount of DNA encompassed by each RS. Assuming that an average of 1,000 new replication sites are activated every 45 min during a typical 8-h S-phase, we estimate ∼10,000 RS in the 3T3 cell. Since the 3T3 cell nucleus has ∼10,000 mbp of DNA, each RS contains an average of 1 mbp. With a bidirectional replicative fork rate of ∼3.4 kb (Jackson and Pombo, 1998), only ∼150 kb of DNA can be replicated in 45 min and it would take ∼300 min to replicate 1 mbp of DNA. Therefore, the average 1 mbp RS must be composed of at least six replicons that replicate in a relatively synchronous manner.
Replication Sites Persist throughout the Cell Cycle
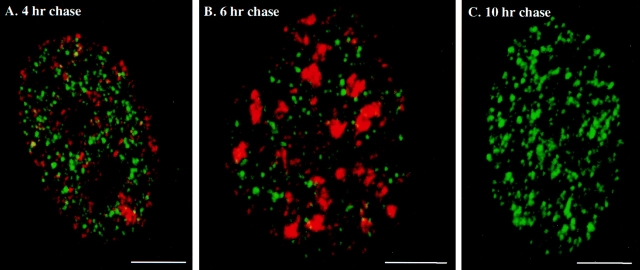
Increasing the chase time up to 6 h in the pulse-chase-pulse experiments enabled simultaneous visualization of replication sites labeled in early S- versus mid to late S-phase. We observed type II RS around the nuclear and nucleolar periphery (Fig. 5 A, red RS) and type III RS at typical mouse centromeric heterochromatin regions (Fig. 5 B, red RS). Longer chase times (e.g., 10 h) enabled us to visualize early S-phase replication sites in the G2 phase where later S-phase replication sites are absent.
Figure 5.
Double labeling experiments in early S-phase versus later S-phase and the G2-phase. Replication sites in synchronized 3T3 fibroblasts were first labeled for 2 min with CldU (FITC secondary antibody, green sites), chased for 4–10 h and pulsed again for 5 min with IdU (Texas red secondary antibody, red sites). (A) 4-h chase (mid S-phase, type II for red RS); (B) 6-h chase (late S-phase, type III for red RS); (C) 10-h chase (G2-phase, there are no red RS). Bars, 5 μm.
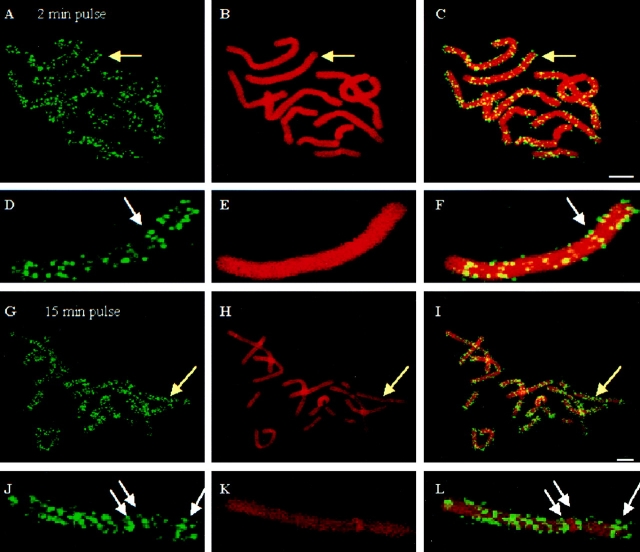
To study the fate of early S-phase RS in mitosis and the next cell generation, we labeled PTK1 cells with BrdU in early S-phase and chased the labeled cells into mitosis and the next cell generation. As previously reported by our group and others (Sparvari et al., 1994; Berezney et al., 1995b ; Jackson and Pombo, 1998; Zink et al., 1998), the RS appear to be maintained during mitosis and into future cell generations (data not shown). Because it is difficult to see specific RS in the highly condensed mitotic spindle structure, we further analyzed chromosome spreads after these labeling protocols. As shown in Fig. 6, individual RS are easily resolved with this technique and are arranged along the chromosomes in a pattern reminiscent of chromosome bands. Remarkably this band-like pattern of individual RS along the chromosomes was even apparent at the earliest pulse period investigated (Fig. 6, A, C, D, and F, 2 min pulse). Previous studies have also reported band-like arrangement of BrdU labeled replication sites on mitotic chromosomes (Sparvoli et al., 1994; Ferreira et al., 1997). The doublets or sister chromatid replication pairs characteristic of RS labeling on mitotic chromosomes (Ferreira et al., 1997; Jackson and Pombo, 1998) were also observed (Fig. 6, D and J, arrows). However, the limited degree of swelling in these chromosome spreads compromised the efficiency of identifying sister chromatid replication pairs.
Figure 6.
Analysis of early S-phase RS in mitotic chromosomes. Replication sites in synchronized kangaroo kidney PtK1 were labeled with BrdU (FITC secondary antibody, green sites) for 2 min (A–F) or 15 min (G–L), and chased for 12 h (M-phase). (A, D, G, and J) Replication sites (green probe); (B, E, H, and K) chromosomes were counterstained with PI (red); (C, F, I, and L) merged images of replication sites (green) and chromosomes (red). (D–F) are displayed at 8× higher magnification as compared with the metaphase spreads (A–C). (J–L) are displayed at 10× higher magnification as compared with the metaphase spreads (G–I). White arrows indicate replication sites forming a chromosome band structure. Bars, 5 μm.
DNA from Replication Sites Labeled in One Cell Generation Does Not Significantly Mix with Later Replication Sites in Future Cell Generations
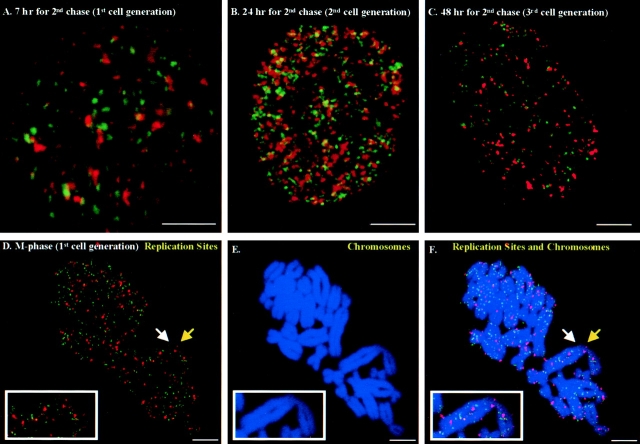
It was previously demonstrated (Figs. 3 and 4) that early replicated DNA is completely distinguishable from later replicated DNA by occupying separate RS after a 1- or 2-h chase. Therefore, we designed double pulse–double chase experiments (see Materials and Methods) to determine whether the DNA sequences replicated at individual RS maintain their separation or mix together as the cells proceed through subsequent cell generations. After the first 2-h chase between early and later labeling of RS, the cells are secondarily chased into late S- or G2-phase (Fig. 7 A), M-phase (Fig. 7, D–F) and into subsequent cell generations (Fig. 7, B and C). The results demonstrate that temporally distinct early S-phase RS (Fig. 7, green) and later replicated sites (red) are totally maintained in spatially distinct domains throughout the S-phase and in future cell generations. As a control, the replication sites are pulsed with CldU and IdU simultaneously in early S-phase and chased through the same time span. Yellow RS are maintained completely throughout this extensive chase period (data not shown). Separation of the early and later labeled RS is especially evidence in chromosome spreads that reveal repeating band-like clusters of green versus red sites (Fig. 7, D and F, arrows) and confirm the previous observations by Ferreira et al. (1997) who used longer pulse labeling. We also observe a significant decrease in the number of replication sites in the third generation cells (Fig. 7 C) compared with first and second generation cells (Fig. 7, A and B), which is consistent with semiconservative replication (Sparvoli et al., 1994; Ferreira et al., 1997; Jackson and Pombo, 1998).
Figure 7.
Double pulse–double chase experiments. Replication sites in synchronized mouse 3T3 fibroblasts were first labeled for 2 min with CldU (FITC secondary antibody, green sites), chased for 2 h, and then pulsed again for 5 min with IdU (Texas red secondary antibody, red sites). The cells were then chased a second time for (A) 7 h (late S- or G2-phase); (B) 24 h (2nd cell generation); (C) 48 h (3rd cell generation); or (D–F) 12 h (M-phase). (D) Replication sites (green for early replication sites and pseudocolor red for later replication sites); (E) Chromosomes were counterstained with PI, and pseudocolored to blue. The secondary antibody for the IdU labeling was Cy-5 conjugated and pseudocolored to red. (F) Merged image of replication sites and chromosomes. One chromosome is enlarged 4× and indicated by the white boxed window. White and yellow arrows indicate replication sites forming chromosome band structures. Bars, 5 μm.
DNA Replicated in Early S-Phase Is Replicated at the Same Time and Site in the Next Round of Replication
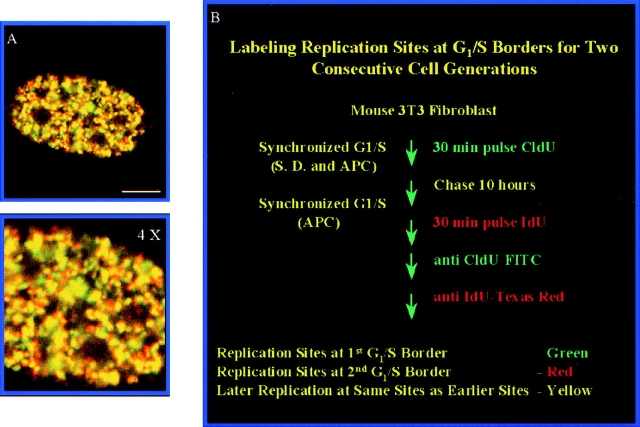
The previous results demonstrate that the DNA replicated at individual RS in early S-phase is spatially maintained at distinct sites in mitotic chromosomes and in subsequent cell generations (Fig. 7). We next addressed whether this spatially distinct group of RS replicate at the same time in the next cell generation. For these experiments, mouse 3T3 cells were synchronized at the G1/S border with aphidicolin for two successive cell generations and pulsed with CldU and IdU, respectively, immediately after release from the G1/S border (see Materials and Methods and Fig. 8 B for details). Under these conditions, we achieved a high degree of synchrony in early S-phase with >99% of the S-phase cells (507 counted) having type I early S-phase replication patterns immediately after release and >90% of the cells entering S-phase within 2 h of releasing the aphidicolin block (data not shown). The results shown in Fig. 8 A demonstrate complete overlap of the RS labeled at two successive early S-phases. We conclude that DNA synthesized in early S-phase is replicated at the same time and at the same site in the next round of replication.
Figure 8.
Labeling replication sites at early S-phase for two consecutive cell generations. Replication sites in synchronized mouse 3T3 fibroblasts were first labeled for 30 min with CldU (FITC secondary antibody, green sites) at the 1st G1/S border, chased, and blocked at the 2nd G1/S border, and then pulsed again for 30 min with IdU (Texas red secondary antibody, red sites). (A) Original image (yellow replication sites mean DNA replicated at the second G1/S border occurs at same site as at the first G1/S border); (B) 4× enlargement of original replication sites; (C) Protocol for labeling replication sites at the G1/S borders for two consecutive cell generations. APC stands for aphidicolin. Bar, 5 μm.
Discussion
Fluorescence microscopic analysis of newly replicated DNA has revealed discrete granular sites of replication (Nakamura et al., 1986; Nakayasu and Berezney, 1989; Mills et al., 1989; Berezney, 1991; Kill et al., 1991; Fox et al., 1991; O'Keefe et al., 1992). Moreover, the pioneering studies of Nakamura et al. (1986) showed that increasing times of BrdU in vivo labeling of DNA RS leads to progressively more elongated structures and to increased difficulty in resolving individual sites. We are using fluorescence laser scanning confocal microscopy in conjunction with a spot-based segmentation algorithm (Samarabandu et al., 1995) to address these issues and to gain more precise spatial information about individual RS and their potential organization into higher order domains.
We report an average of ∼1,100 replication sites after a 5-min pulse of 3T3 mouse fibroblasts synchronized in early S-phase. Similar results were obtained in exponentially dividing cells where selection for measurement was based solely on visible type I patterns (data not shown). Our results, therefore, suggest that ∼1,000 RS are active at any time in early S-phase. This number is several fold higher than previously reported values (100–300) using both epifluorescence and laser scanning confocal microscopy (Nakamura et al., 1986; Mills et al., 1989; Nakayasu and Berezney, 1989; Fox et al., 1991; Kill et al., 1991; Hozak et al., 1993; Hassan and Cook, 1993) as well as when the very same samples were analyzed by a widely adopted threshold-based segmentation algorithm (Samarabandu, 1991; see Fig. 1). Direct visual comparison demonstrates that our newly developed spot-based segmentation method (Samarabandu et al., 1995) is extremely accurate and sensitive in distinguishing individual sites over a broad range of intensities and in close spatial apposition (Fig. 1). In contrast, threshold-based methods result in nondetection of more weakly stained sites and the merging of closely positioned individual sites into one contour (see Fig. 1). Recently, Jackson and Pombo (1998) have applied a direct spread method for manually counting RS in early S-phase. This resulted in higher numbers than many previous reports (750 sites), albeit significantly lower than reported here.
Our segmentation analysis also revealed that individual sites of RS do not significantly grow in size with increasing pulse time as initially reported by Nakamura et al. (1986). Indeed, we report a remarkable similarity in the volume distributions and average X-Y diameters of RS over pulse times ranging from 2 to 30 min (Fig. 2). This suggests that each RS site corresponds to a discrete domain of DNA whose replication occurs uniformly over the individual RS. Consistent with this interpretation, the overall intensity of the individual RS increase progressively with increasing pulse times up to 30 min (Fig. 2). Moreover, as previously reported, double labeling experiments revealed that new sites of replication are preferentially in close proximity with previously replicated sites (Berezney et al., 1995a ,b; Manders et al., 1996). Thus dual labeled sites in close apposition often give the appearance of elongated single sites when singly labeled (data not shown).
We have determined the average time of replication at a RS using the double halogen labeling method developed by Aeten et al. (1992). The results of these pulse-chase-pulse experiments demonstrate an overall average time of 45 min (Fig. 4) and are consistent with the previous but less precise measurements of Manders et al. (1992, 1996) and the recent estimation by Jackson and Pombo (1998) in HeLa cells using DNA fiber analysis. From this we estimate ∼10,000 RS in S-phase or an average of 1 mbp per RS in the 3T3 cell nucleus. Whereas replicons are very heterogeneous, ranging in size from <20 to >300 kb (Edenberg et al., 1976; Hand, 1978), the overall population average is ∼100–150 kb in mammalian cells. Thus each mbp RS is composed of at least six average-sized replicons. Jackson and Pombo (1998) have come to similar conclusions based on DNA fiber analysis of replicon clusters size: the RS observed in the cell nucleus directly correspond to replicon cluster observed with DNA fiber analysis.
Previous studies have shown that postreplicated DNA maintains a discrete RS pattern throughout the cell cycle and into subsequent cell generations (Sparvoli et al., 1994; Berezney et al., 1995a ,b; Jackson and Pombo, 1998; Zink et al., 1998). This has lead to the view that DNA arranged in domains of approximately mbp size may be a fundamental feature of the higher order arrangement of chromatin in the cell nucleus (Berezney et al., 1995a ,b). An important question is whether the DNA that composes the individual RS is also maintained in these sites as the cell progresses through the cell cycle and from one cell generation to another. In other words, whereas individual RS may be reflecting a fundamental feature of higher order chromatin organization, the actual DNA sequences that compose individual sites may fluctuate. To address this we labeled the replicating DNA in early S-phase in two colors with a 2-h chase between the brief pulses. We then chased the spatially separate red and green sites through S-phase, mitosis and the next cell generation (Fig. 7). If the DNA in individual RS is promiscuous, then we should observe mixing of the separate green and red RS with increasing chase time. However, no significant mixing (Fig. 7, yellow) was observed as the cells progressed through S-phase, G2-phase, mitosis and into the next two cell generations (Fig. 7). We conclude that the DNA at individual RS is maintained with a high degree of fidelity through at least several cell generations.
There is growing evidence that DNA replication in the eucaryotic cell proceeds in a precisely choreographed manner whereby specific groups of replicons are active at different times in S-phase. Studies of specific gene sequences have shown that most active genes replicate in early S-phase, whereas inactive ones tend to replicate in late S-phase (Goldman et al., 1984; Hatton et al., 1988). The higher order arrangement of chromatin into ∼1-mbp domains of replication (RS) may represent the spatial basis for this replication timing. In pioneering studies, Schildkraut and his associates demonstrated that the position of a gene on a chromosome determines its replication timing (Calza et al., 1984; Dhar et al., 1988, 1989; Hatton et al., 1988). Specific timing was measured for up to several hundred kilobases in several multi-gene families (Brown et al., 1987; Dhar et al., 1988, 1989; Hatton et al., 1988). More recently, Selig et al. (1992) identified replication time (control) zones exceeding 500 kb within and flanking the cystic fibrosis gene on human chromosome 7 and suggested that these replication zones correspond to basic units of chromosome structure for both DNA replication and transcriptional regulation. Consistent with this view, replication control zones that range from several hundred kilobases to >1 mbp have been identified in the delayed replication of the FMR1 gene associated with fragile X syndrome (Hansen et al., 1993) and in the imprinted human Igf2 gene domain (Kitsberg et al., 1993).
Based on these findings and relationships, we suggest that replication time (control) zones correspond to individual RS, which are, in turn, composed of a cluster of replicons that are subject to replication timing control. To test this concept experimentally, we labeled RS at two consecutive S-phases after release from the G1/S borders. The results strikingly demonstrate complete colocalization of the RS (Fig 8). In other words, the large subset of RS at this discrete time in very early S-phase (∼1,000 sites) behave as a collection of replication time zones and lead to the replication of the same subset of RS from one cell generation to another. However, due to the limited resolution of our approach, we can not rule out at this time a degree of fluctuation among the specific DNA sequences at individual sites. Future studies designed to map DNA sequences at individual replication sites will provide a more rigorous test. Moreover, Jackson and Pombo (1998) have come to similar conclusions using DNA fiber analysis after double labeling. This enables direct visualization of overlap at the level of individual replicons.
Whereas these studies are an important advance in understanding the relationship of chromatin organization and function in the cell nucleus, they do not directly address the nature of this higher order arrangement of chromatin. Indeed, our knowledge of how chromatin is arranged in the nucleus beyond the arrangement of nucleosomes into a 30-nm solenoid or zigzag structure (McGhee et al., 1980; McGhee et al., 1983; Butler, 1984; Pienta and Coffey, 1984; Thomas, 1984; Sen et al., 1986; Horowitz et al., 1994) is still in its infancy. However, direct ultrastructural studies of chromatin inside the cell nucleus have suggested a higher level of organization that is consistent with the ∼1-mbp sites that we can identify with replication labeling (Lau and Arrighi, 1981; Mullinger and Johnson, 1983; Belmont et al., 1989; Manuelidis, 1990).
The relationship of functional domains of replicon clusters to chromosomes bands has attracted particular attention. Indeed, the discrete patterns of R-bands versus G/Q bands observed in early versus later S-phase has lead to the proposal that individual bands correspond to replicon clusters (Klevecz and Keniston, 1975; Latt, 1975; Stubblefield, 1975; Lau and Arrighi, 1981; Holmquist et al., 1982). Our results, however, suggest that chromosome bands may be composed of several RS (see Figs. 6 and 7) and are consistent with previous findings (Sparvari et al., 1994; Ferreira et al., 1997). Thus the equivalent of a chromosome band in the interphase nucleus may be a small cluster of several RS. Recently we have used quantitative image analysis to demonstrate that a large percentage of individual RS are indeed arranged in clusters of higher order domains or zones in the nucleus (Wei et al., 1998). Moreover, these higher order replication zones are spatially segregated from analogous zones containing clusters of transcription sites (Wei et al., 1998). Distinct clusters of RS have also been observed after BrdU labeling of RS followed by chase times of up to 10 cell generations and are believed to represent chromosome territories or territorial sub-regions (Jackson and Pombo, 1998; Zink et al., 1998). The relationship of the replication and transcription zones elucidated by Wei et al. (1998) to chromosome territories remains to be elucidated.
Numerous findings point to the nuclear matrix as a key player in organizing the higher order domain structure of chromatin (Berezney and Jeon, 1995). Chromatin domains are believed to be attached to the nuclear matrix and chromosome scaffold in repeating 50–200-kb loops (Vogelstein et al., 1980; Goldberg et al., 1983; Nelson and Coffey, 1987; Chai and Sandberg, 1988; Laemmli et al., 1992; Roberge et al., 1992; Hiraoka et al., 1993). These loop domains may provide the structural basis for both domains of transcription and individual replicons (Vogelstein et al., 1980; Jackson and Cook, 1995). Models have been proposed that describe the replication of DNA on the nuclear matrix as a cluster of replicons in loop form (Dijkwel et al., 1979; Pardoll et al., 1980; Berezney and Buchholtz, 1981; Berezney, 1984; Cook, 1991). Consistent with these biochemical findings and models, the RS and the various patterns of RS characteristic of different stages in S-phase are strikingly maintained after extraction of cells for nuclear matrix (Berezney and Nakayasu, 1989; Neri et al., 1992).
These results suggest that the matrix may be involved in chromatin organization at levels even higher than the 100-kb loop domains such as the 1 mbp RS elucidated in this study. However, it is important to recognize that the precise role(s) of the nuclear matrix in chromatin organization remain(s) to be defined. For example, it can be argued that nuclear matrix organization may be regulated by chromosome territory organization rather than vice versa (Cremer et al., 1995). In this regard, we recently found that the integrity of chromosome territories is dependent on the corresponding integrity of the nuclear matrix (Ma, H., and R. Berezney, manuscript submitted for publication).
Abbreviations used in this paper
- BrdU
5-bromo-2′-deoxyuridine
- CldU
5-chloro-2′-deoxyuridine
- IdU
5-iodo-2′-deoxyuridine
- PI
propidium iodide
- RS
replication sites
- RT
replication timing
Footnotes
We are extremely grateful to Dr. R. Summers for his assistance in confocal microscopy and to A. Siegel for use of the Microscopic Imaging Facility in our department. Special thanks to Dr. G. Mayers and Dr. R. Bankert for the gift of mouse anti-BrdU IgG antibodies; S. Somanathan and V. Sarangan for help with computer imaging; and J. Stamos for illustrations and photography.
The experiments were funded by National Institutes of Health Grant GM 23922 to R. Berezney, and a grant from the Mark Diamond Fund of the State University of New York at Buffalo Graduate School to H. Ma (27-S-98).
Dr. Ma's present address is Vollum Institute, L-474, Oregon Health Sciences University, 3181 SW Sam Jackson Park Rd., Portland, OR 97201-3098.
Dr. Samarabandu's present address is Life Imaging Systems Inc., 195 Dufferin Ave., Suite 300, London, ON N6A 1K7, Canada.
References
- Aten JA, Bakker PJM, Stap J, Boschman GA, Veenhof CHN. DNA double labeling with IdUrd and CldUrd for spatial and temporal analysis of cell proliferation and DNA replication. Histochem J. 1992;24:251–259. doi: 10.1007/BF01046839. [DOI] [PubMed] [Google Scholar]
- Belmont AS, Braunfeld MB, Sedat JW, Agard DA. Large-scale chromatin structural domains within mitotic and interphase chromosomes in vivo and in vitro. Chromosoma. 1989;98:129–143. doi: 10.1007/BF00291049. [DOI] [PubMed] [Google Scholar]
- Berezney, R. 1984. Organization and functions of the nuclear matrix. In chromosomal nonhistone proteins. L.S. Hnilica, editor. CRC Press Inc., Boca Raton, FL. 4:119–180.
- Berezney R. Visualizing DNA replication sites in the cell nucleus. Semin Cell Biol. 1991;2:103–115. [PubMed] [Google Scholar]
- Berezney R, Buchholtz LA. Dynamic association of replicating DNA fragments with the nuclear matrix of regenerating liver. Exp Cell Res. 1981;132:1–13. doi: 10.1016/0014-4827(81)90076-8. [DOI] [PubMed] [Google Scholar]
- Berezney, R., and K.W. Jeon. 1995. Structural and functional organization of the nuclear matrix. Int. Rev. Cytol. 162A:1–595. [DOI] [PubMed]
- Berezney R, Ma H, Meng C, Samarabandu J, Cheng PC. Connecting genomic architecture and DNA replication in three dimensions. Zool Stud. 1995a;34:29–32. [Google Scholar]
- Berezney, R., M.J. Mortillaro, H. Ma, X. Wei, and J. Samarabandu. 1995b The nuclear matrix: a structural milieu for genomic function. Int. Rev. Cytol. 162A:1–65. [DOI] [PubMed]
- Brown EH, Iqbal MA, Stuart S, Hatton KS, Valinsky J, Schildkraut CL. Rate of replication of the murine immunoglobulin heavy-chain locus: evidence that the region is part of a single replicon. Mol Cell Biol. 1987;7:450–457. doi: 10.1128/mcb.7.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brylawski BP, Tsongalis GJ, Cordeiro-Stone M, May WT, Comeau LD, Kaufman DG. Association of putative origins of replication with the nuclear matrix in normal human fibroblasts. Cancer Res. 1993;53:3865–3868. [PubMed] [Google Scholar]
- Butler PJ. A defined structure of the 30 nm chromatin fibre which accommodates different nucleosomal repeat lengths. EMBO (Eur Mol Biol Organ) J. 1984;3:2599–2604. doi: 10.1002/j.1460-2075.1984.tb02180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza RE, Eckhardt LA, DelGiudice T, Schidkraut CL. Changes in gene position are accompanied by a change in time of replication. Cell. 1984;36:689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Chai LS, Sandberg AA. Chromosomes and their relationship to nuclear components during the cell cycle in Chinese hamster cells. Cell Tissue Res. 1988;251:197–204. doi: 10.1007/BF00215465. [DOI] [PubMed] [Google Scholar]
- Comings DE, Okada TA. DNA replication and the nuclear membrane. J Mol Biol. 1973;75:609–618. doi: 10.1016/0022-2836(73)90295-7. [DOI] [PubMed] [Google Scholar]
- Cook PR. The nucleoskeleton and the topology of replication. Cell. 1991;66:627–635. doi: 10.1016/0092-8674(91)90109-c. [DOI] [PubMed] [Google Scholar]
- Cordeiro-Stone M, Kaufman DG. Kinetics of DNA replication in C3H 10T1/2 cells synchronized by aphidicolin. Biochemistry. 1985;24:4815–4822. doi: 10.1021/bi00339a015. [DOI] [PubMed] [Google Scholar]
- Cremer, T., S. Dietzel, R. Eils, P. Licher, and C. Cremer. 1995. Chromosome territories, nuclear matrix filaments and inter-chromatin channels: a topological view on nuclear architecture and function. P.E. Brandham, and M.D. Bennett, editors. Kew Chromosome Conference IV. 63–81.
- Dhar V, Mager D, Iqbal A, Schildkraut CL. The coordinate replication of the human globin β gene domain reflects its transcriptional activity and nuclease hypersensitivity. Mol Cell Biol. 1988;8:4958–4965. doi: 10.1128/mcb.8.11.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar VA, Skoultchi I, Schidkraut CL. Activation and repression of a β-globin gene in cell hybrids is accompanied by a shift in its temporal replication. Mol Cell Biol. 1989;9:3524–3532. doi: 10.1128/mcb.9.8.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel PA, Mullenders LH, Wanka F. Analysis of the attachment of replicating DNA to a nuclear matrix in mammalian interphase nuclei. Nucleic Acids Res. 1979;6:219–230. doi: 10.1093/nar/6.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Waqar MA, Huberman JA. Subnuclear systems for synthesis of simian virus 40 DNA in vitro. Proc Natl Acad Sci USA. 1976;73:4392–4396. doi: 10.1073/pnas.73.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S, Hancock R. Localization of newly-synthesized DNA in a mammalian cell as visualized by high resolution autoradiography. Exp Cell Res. 1974;83:95–102. doi: 10.1016/0014-4827(74)90692-2. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Paolella G, Ramos C, Lamond AI. Spatial organization of large-scale chromatin domains in the nucleus: a magnified view of single chromosome territories. J Cell Biol. 1997;139:1597–1610. doi: 10.1083/jcb.139.7.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MH, Arndt-Jovin DJ, Jovin TM, Baumann PH, Robert-Nicoud M. Spatial and temporal distribution of DNA replication sites localized by immunofluorescence and confocal microscopy in mouse fibroblasts. J Cell Sci. 1991;99:247–253. doi: 10.1242/jcs.99.2.247. [DOI] [PubMed] [Google Scholar]
- Goldberg GI, Collier I, Cassel A. Specific DNA sequences associated with the nuclear matrix in synchronized mouse 3T3 cells. Proc Natl Acad Sci USA. 1983;80:6887–6891. doi: 10.1073/pnas.80.22.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MA, Holmquist GP, Gray MC, Caston L, Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984;224:686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978;15:315–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Hansen RS, Canfield TK, Lamb MM, Gartler SM, Laird CD. Association of fragile X syndrome with delayed replication of the FMR1 gene. Cell. 1993;73:1403–1409. doi: 10.1016/0092-8674(93)90365-w. [DOI] [PubMed] [Google Scholar]
- Hassan AB, Cook PR. Visualization of replication sites in unfixed human cells [published erratum appears 107:1102] J Cell Sci. 1993;105:541–550. doi: 10.1242/jcs.105.2.541. [DOI] [PubMed] [Google Scholar]
- Hatton KS, Dhar V, Gahn TA, Brown EH, Mager D, Schildkraut CL. Temporal order of replication of multigene families reflects chromosomal location and transcriptional activity. Cancer Cells. 1988;6:335–340. [Google Scholar]
- Hay ED, Revel JP. The fine structure of the DNP component of nucleus. An electron microscopic study using autoradiography to localize DNA synthesis. J Cell Biol. 1966;16:29–51. doi: 10.1083/jcb.16.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, Dernburg AF, Parmelee SJ, Rykowski MC, Agard DA, Sedat JW. The onset of homologous chromosome pairing during Drosophila melanogasterembryogenesis. J Cell Biol. 1993;120:591–600. doi: 10.1083/jcb.120.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist G, Gray M, Porter T, Jordan J. Characterization of Giemsa dark- and light-band DNA. Cell. 1982;31:121–129. doi: 10.1016/0092-8674(82)90411-1. [DOI] [PubMed] [Google Scholar]
- Horowitz RA, Agard DA, Sedat JW, Woodcock CL. The three-dimensional architecture of chromatin in situ: electron tomography reveals fibers composed of a continuously variable zig-zag nucleosomal ribbon. J Cell Biol. 1994;125:1–10. doi: 10.1083/jcb.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozak P, Hassan AB, Jackson DA, Cook PR. Visualization of replication factories attached to nucleoskeleton. Cell. 1993;73:361–373. doi: 10.1016/0092-8674(93)90235-i. [DOI] [PubMed] [Google Scholar]
- Huberman JA, Tsai A, Deich RA. DNA replication sites within nuclei of mammalian cells. Nature. 1973;241:32–36. doi: 10.1038/241032a0. [DOI] [PubMed] [Google Scholar]
- Jackson, D.A., and P.R. Cook. 1995. The structural basis of nuclear function. Int. Rev. Cytol. 162A:125–149. [DOI] [PubMed]
- Jackson DA, Pombo A. Replicon clusters are stable units of chromosome structure: evidence that nuclear organization contributes to the efficient activation and propagation of S phase in human cells. J Cell Biol. 1998;140:1285–1295. doi: 10.1083/jcb.140.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kill IR, Bridger JM, Campbell KH, Maldonado-Codina G, Hutchison CJ. The timing of the formation and usage of replicase clusters in S-phase nuclei of human diploid fibroblasts. J Cell Sci. 1991;100:869–876. doi: 10.1242/jcs.100.4.869. [DOI] [PubMed] [Google Scholar]
- Kitsberg D, Selig S, Brandeis M, Simon I, Keshet I, Driscoll DJ, Nicholls RD, Cedar H. Allele-specific replication timing of imprinted gene regions. Nature. 1993;364:459–463. doi: 10.1038/364459a0. [DOI] [PubMed] [Google Scholar]
- Klevecz RR, Keniston BA. The temporal structure of S phase. Cell. 1975;5:195–203. doi: 10.1016/0092-8674(75)90027-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK, Kas E, Poljak L, Adachi Y. Scaffold-associated regions: cis-acting determinants of chromatin structural loops and functional domains. Curr Opin Genet Dev. 1992;2:275–285. doi: 10.1016/s0959-437x(05)80285-0. [DOI] [PubMed] [Google Scholar]
- Latt SA. Fluorescence analysis of late DNA replication in human metaphase chromosomes. Somatic Cell Genet. 1975;1:293–321. doi: 10.1007/BF01538452. [DOI] [PubMed] [Google Scholar]
- Lau YF, Arrighi FE. Studies of mammalian chromosome replication. II. Evidence for the existence of defined chromosome replicating units. Chromosoma. 1981;83:721–741. doi: 10.1007/BF00328530. [DOI] [PubMed] [Google Scholar]
- Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labeling of DNA and confocal microscopy. J Cell Sci. 1992;103:857–862. doi: 10.1242/jcs.103.3.857. [DOI] [PubMed] [Google Scholar]
- Manders EM, Stap J, Strackee J, van Driel R, Aten JA. Dynamic behavior of DNA replication domains. Exp Cell Res. 1996;226:328–335. doi: 10.1006/excr.1996.0233. [DOI] [PubMed] [Google Scholar]
- Manuelidis L. A view of interphase chromosomes. Science. 1990;250:1533–1540. doi: 10.1126/science.2274784. [DOI] [PubMed] [Google Scholar]
- McGhee JD, Nickol JM, Felsenfeld G, Rau DC. Higher order structure of chromatin: orientation of nucleosomes within the 30 nm chromatin solenoid is independent of species and spacer length. Cell. 1983;33:831–841. doi: 10.1016/0092-8674(83)90025-9. [DOI] [PubMed] [Google Scholar]
- McGhee JD, Rau DC, Charney E, Felsenfeld G. Orientation of the nucleosome within the higher order structure of chromatin. Cell. 1980;22:87–96. doi: 10.1016/0092-8674(80)90157-9. [DOI] [PubMed] [Google Scholar]
- Mills AD, Blow JJ, White JG, Amos WB, Wilcock D, Laskey RA. Replication occurs at discrete foci spaced throughout nuclei replicating in vitro. J Cell Sci. 1989;94:471–477. doi: 10.1242/jcs.94.3.471. [DOI] [PubMed] [Google Scholar]
- Milner GR. Nuclear morphology and the ultrastructural localization of deoxyribonucleic acid synthesis during interphase. J Cell Sci. 1969;4:569–582. doi: 10.1242/jcs.4.3.569. [DOI] [PubMed] [Google Scholar]
- Mullinger AM, Johnson RT. Units of chromosome replication and packing. J Cell Sci. 1983;64:179–193. doi: 10.1242/jcs.64.1.179. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Morita T, Sato C. Structural organizations of replicon domains during DNA synthetic phase in the mammalian nucleus. Exp Cell Res. 1986;165:291–297. doi: 10.1016/0014-4827(86)90583-5. [DOI] [PubMed] [Google Scholar]
- Nakayasu H, Berezney R. Mapping replicational sites in the eucaryotic cell nucleus. J Cell Biol. 1989;108:111. doi: 10.1083/jcb.108.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WG, Coffey DS. Structural aspects of mammalian DNA replication: topoisomerase II. NCI Monographs. 1987;4:23–29. [PubMed] [Google Scholar]
- Neri LM, Mazzotti G, Capitani S, Maraldi NM, Cinti C, Baldini N, Rana R, Martelli AM. Nuclear matrix-bound replicational sites detected in situ by 5-bromodeoxyuridine. Histochemistry. 1992;98:19–32. doi: 10.1007/BF00716934. [DOI] [PubMed] [Google Scholar]
- Ockey CH. Distribution of DNA replicator sites in mammalian nuclei. II. effect of prolonged inhibition of DNA synthesis. Exp Cell Res. 1972;70:203–213. doi: 10.1016/0014-4827(72)90198-x. [DOI] [PubMed] [Google Scholar]
- O'Keefe RT, Henderson SC, Spector DL. Dynamic organization of DNA replication in mammalian cell nucleus: spatially and temporally defined replication of chromosome-specific α-satellite DNA sequence. J Cell Biol. 1992;116:1095–1110. doi: 10.1083/jcb.116.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM, Vogelstein B, Coffey DS. A fixed site of DNA replication in eucaryotic cells. Cell. 1980;19:527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Pienta KJ, Coffey DS. A structural analysis of the role of the nuclear matrix and DNA loops in the organization of the nucleus and chromosome. J Cell Sci Suppl. 1984;1:123–135. doi: 10.1242/jcs.1984.supplement_1.9. [DOI] [PubMed] [Google Scholar]
- Roberge M, Gasser SM. DNA loops: structural and functional properties of scaffold-attached regions. Mol Microbiol. 1992;6:419–423. doi: 10.1111/j.1365-2958.1992.tb01485.x. [DOI] [PubMed] [Google Scholar]
- Samarabandu JK, Acharya R, Cheng PC, Meng C, Berezney R, Summers RG. Three-dimensional structural analysis from biological confocal images. SPIE. 1991;1566:154–164. [Google Scholar]
- Samarabandu J, Ma H, Acharya R, Cheng PC, Berezney R. Image analysis techniques for visualizing the spatial organization of DNA replication sites in the mammalian cell nucleus using multi-channel confocal microscopy. SPIE. 1995;2434:370–375. [Google Scholar]
- Selig S, Okumura K, Ward DC, Cedar H. Delineation of DNA replication time zones by fluorescence in situ hybridization. EMBO (Eur Mol Biol Organ) J. 1992;11:1217–1225. doi: 10.1002/j.1460-2075.1992.tb05162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D, Mitra S, Crothers DM. Higher order structure of chromatin: evidence from photochemically detected linear dichroism. Biochemistry. 1986;25:3441–3447. doi: 10.1021/bi00359a052. [DOI] [PubMed] [Google Scholar]
- Smith HC, Puvion E, Buchholtz LA, Berezney R. Spatial distribution of DNA loop attachment and replicational sites in the nuclear matrix. J Cell Biol. 1984;99:1794–1802. doi: 10.1083/jcb.99.5.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorscher DH, Cordeiro-Stone M. Gene replication in the presence of aphidicolin. Biochemistry. 1991;30:1086–1090. doi: 10.1021/bi00218a030. [DOI] [PubMed] [Google Scholar]
- Sparvoli E, Galli MG, Mosca A, Paris G. Localization of DNA replicator sites near the nuclear membrane in plant cells. Exp Cell Res. 1976;97:74–82. doi: 10.1016/0014-4827(76)90656-x. [DOI] [PubMed] [Google Scholar]
- Sparvoli E, Levi M, Rossi E. Replicon clusters may form structurally stable complexes of chromatin and chromosomes. J Cell Sci. 1994;107:3097–3103. doi: 10.1242/jcs.107.11.3097. [DOI] [PubMed] [Google Scholar]
- Stubblefield E. Analysis of the replication pattern of Chinese hamster chromosomes using 5-bromodeoxyuridine suppression of 33258 Hoechst fluorescence. Chromosoma. 1975;53:209–221. doi: 10.1007/BF00329172. [DOI] [PubMed] [Google Scholar]
- Thomas JO. The higher order structure of chromatin and histone H1. J Cell Sci Suppl. 1984;1:1–20. doi: 10.1242/jcs.1984.supplement_1.1. [DOI] [PubMed] [Google Scholar]
- Trask, B.J. 1991. Functional Organization of the Nucleus: A Laboratory Guide. B.A. Hamkalo, and S.C.R. Elgin, editors. Academic Press, Inc., New York. 3–35.
- van Dierendonck JH, Keyzer R, van de Velde CJH, Cornelisse CJ. Subdivision of S-phase by analysis of nuclear 5-bromodeoxyuridine staining patterns. Cytometry. 1989;10:143–150. doi: 10.1002/cyto.990100205. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Pardoll DM, Coffey DS. Supercoiled loops and eucaryotic DNA replication. Cell. 1980;22:79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Volgel W, Autenrieth M, Mehnert K. Analysis of chromosome replication by a BrdU antibody technique. Chromosoma. 1989;98:335–341. doi: 10.1007/BF00292386. [DOI] [PubMed] [Google Scholar]
- Wei X, Samarabandu J, Rakendu SD, Siegel AJ, Acharya R, Berezney R. Segregation of transcription and replication sites into higher order domains. Science. 1998;281:1502–1505. doi: 10.1126/science.281.5382.1502. [DOI] [PubMed] [Google Scholar]
- Williams CA, Ockey CH. Distribution of DNA replication sites in mammalian nuclei after different methods of cell synchronization. Exp Cell Res. 1970;63:365–372. doi: 10.1016/0014-4827(70)90224-7. [DOI] [PubMed] [Google Scholar]
- Zink D, Cremer T, Saffrich R, Fischer R, Trendelenburg MF, Ansorge W, Stelzer EH. Structure and dynamics of human interphase chromosome territories in vivo. Human Genetics. 1998;102:241–251. doi: 10.1007/s004390050686. [DOI] [PubMed] [Google Scholar]