Abstract
A critical role for the small GTPase Rho and one of its targets, p160ROCK (a Rho-associated coiled coil-forming protein kinase), in neurite remodeling was examined in neuroblastoma N1E-115 cells. Using wild-type and a dominant-negative form of p160ROCK and a p160ROCK-specific inhibitor, Y-27632, we show here that p160ROCK activation is necessary and sufficient for the agonist-induced neurite retraction and cell rounding. The neurite retraction was accompanied by elevated phosphorylation of myosin light chain and the disassembly of the intermediate filaments and microtubules. Y-27632 blocked both neurite retraction and the elevation of myosin light chain phosphorylation in a similar concentration-dependent manner. On the other hand, suppression of p160ROCK activity by expression of a dominant-negative form of p160ROCK induced neurites in the presence of serum by inducing the reassembly of the intermediate filaments and microtubules. The neurite outgrowth by the p160ROCK inhibition was blocked by coexpression of dominant-negative forms of Cdc42 and Rac, indicating that p160ROCK constitutively and negatively regulates neurite formation at least in part by inhibiting activation of Cdc42 and Rac. The assembly of microtubules and intermediate filaments to form extended processes by inhibitors of the Rho–ROCK pathway was also observed in Swiss 3T3 cells. These results indicate that Rho/ROCK-dependent tonic inhibition of cell process extension is exerted via activation of the actomysin-based contractility, in conjunction with a suppression of assembly of intermediate filaments and microtubules in many cell types including, but not exclusive to, neuronal cells.
Axonal guidance is regulated by interaction of a growth cone with various environmental cues. Extracellular signals critical in this process include diffusible chemoattractants and chemorepellants, or various kinds of extracellular matrix proteins and cell adhesion molecules. Once the growth cone receives these signals, it moves either toward or away from them (for review see Keynes and Cook, 1995). Such signal-induced motion is, at least in part, mediated by protrusion and retraction of the filopodia and lamellipodia present in the growth cone. The shape and movement of these structures is presumably determined by the reorganization of the actin cytoskeleton (for review see Bently and O'Connor, 1994; Lin et al., 1994; Tanaka and Sabry, 1995).
Rho family proteins including Rho, Rac, and Cdc42 participate in the reorganization of actin cytoskeleton from yeast to mammals (for review see Hall, 1994). In cultured fibroblasts, Rho regulates the formation of focal adhesions and stress fibers, whereas Rac and Cdc42 regulate the growth factor–induced membrane ruffling and filopodia formation, respectively (Ridley and Hall, 1992; Ridley et al., 1992; Kozma et al., 1995; Nobes and Hall, 1995). Recently, several lines of evidence suggested that Rho family proteins play a critical role in axonal and dendritic outgrowth. In Drosophila melanogaster, constitutively active and dominant-negative Rac mutants both induced the disruption of axon outgrowth (Luo et al., 1994). Furthermore, axon terminal formation was significantly reduced in Purkinje cells of mice expressing a constitutively active form of Rac as a transgene (Luo et al., 1996). mig-2 gene product, also a member of the Rho family proteins, was shown to be critical for cell migration and axon outgrowth in Caenorhabditis elegans (Zipkin et al., 1997). In vitro, microinjection of Cdc42 and Rac into cultured N1E-115 neuroblastoma cells, respectively, induced filopodia and lamellipodia in the growth cone (Kozma et al., 1997); these GTPases mediated the action of acetylcholine to induce these membrane structures in this cell line. On the other hand, other types of agonists such as lysophosphatidic acid (LPA),1 thrombin, sphingosine-1-phosphate, or serum itself induced rapid growth cone collapse and neurite retraction in several cultured neuronal cell lines such as N1E-115 cells, NG108-15 neuroblastoma-glioma hybrid cells, and PC12 pheochromocytoma cells (Jalink et al., 1992, 1994; Postma et al., 1996; Tigyi et al., 1996a ). This dramatic morphological change was inhibited by pretreatment of the cells with botulinum C3 exoenzyme, which inactivates Rho by ADP ribosylation, indicating the involvement of Rho in this process. Consistently, overexpression or microinjection of constitutively active Rho into these cells induced growth cone collapse and marked cell rounding (Kozma et al., 1997; Kranenburg et al., 1997). These findings supported the hypothesis that the Rho family GTPases mediated the protrusion and the collapse of the growth cone and perhaps regulated its motility. However, the intracellular signaling mechanisms by which these GTPases exert their actions in the growth cone have remained unknown.
Recently, several putative target molecules of Rho were isolated (for review see Narumiya et al., 1997). Among them, a family of Rho-associated serine/threonine kinase isozymes named p160ROCK (Ishizaki et al., 1996) and ROKα/Rho-kinase/ROCK-II (Leung et al., 1995; Matsui et al., 1996; Nakagawa et al., 1996) has been identified as a new class of Rho effectors, which induced focal adhesions and stress fibers in cultured fibroblasts and epithelial cells (Leung et al., 1996; Ishizaki et al., 1997; Amano et al., 1997). Kimura et al. (1996) showed that Rho-kinase phosphorylated and inactivated myosin phosphatase in vitro and suggested that it thereby regulated myosin light chain (MLC) phosphorylation. We recently identified a new pyridine derivative named Y-27632 as a specific inhibitor of the ROCK/ROK family of protein kinases (Uehata et al., 1997). This compound inhibited smooth muscle contraction both in vitro and in vivo, as well as the formation of stress fibers and focal adhesions induced by p160ROCK in cultured cells. These findings suggested that ROCK mediated the Rho effect on the formation of focal adhesions and stress fibers, at least partly through the regulation of actomyosin system.
In this study, we used Y-27632 and wild-type and a dominant-negative form of p160ROCK, and examined the role of p160ROCK in the neurite remodeling of N1E-115 cells. Our findings indicate that p160ROCK not only mediates the agonist-induced neurite retraction but also tonically suppresses neurite outgrowth in the presence of serum. The former action appears to be exerted through both the activation of the actomyosin system and the disassembly of the intermediate filaments and microtubules. The latter suppression appears partly due to inactivation of Cdc42 and Rac, because the inhibition of the p160ROCK leads to the activation of Cdc42 and Rac and to the neurite induction. Our findings further indicate that the opposing actions of p160ROCK on the actin cytoskeleton and the other two types of cytoskeletons are not limited to the cells of neuronal origin.
Materials and Methods
Materials
LPA was purchased from Sigma Chemical Co (St. Louis, MO). Rabbit polyclonal anti-peripherin antibody, mouse monoclonal anti-vimentin antibody (LN-6), and mouse monoclonal anti–β-tubulin antibody (N-357) were purchased from Chemicon (Temecula, California), Sigma Chemical Co., and Amersham (Little Chalfont, UK), respectively. Mouse monoclonal anti-FLAG M2 antibody was obtained from Eastman Kodak Co. (New Haven, CT). pEXV-myc-V14RhoA and Swiss 3T3 cells were kindly provided by A. Hall (University College London, London, UK). pCMV-myc-N17Cdc42 and pCMV5-FLAG-N17Rac1 were provided by M. Symons (Onyx Pharmaceuticals, Richmond, CA) and Y. Kaziro (Tokyo Institute of Technology, Tokyo, Japan). Y-27632 was supplied by Yoshitomi Pharmaceutical Industries (Saitama, Japan). Botulinum C3 exoenzyme was purified as described (Jalink et al., 1994). Rabbit polyclonal anti-ROCK antibody 20490 and anti-myc antibody 9E10 were prepared as described (Fujita et al., 1997; Ishizaki et al., 1997).
Assay for Neurite Retraction
Mouse neuroblastoma N1E-115 cells were maintained in DME supplemented with 10% FBS. For neurite induction, N1E-115 cells were cultured in serum-free DME for 24 h. More than 90% of the cells became flattened and extended neurites under these conditions. Neurite retraction was evoked by incubating cells with 1 μM LPA for 10 min, with or without preincubation with indicated concentrations of Y-27632 for 30 min. Phase-contrast photomicrographs were taken before the Y-27632 pretreatment and after the LPA stimulation. More than 200 cells were identified in randomly chosen fields of view, and the percentage of cells that retracted neurites after LPA application was calculated.
p160ROCK Expression Constructs and Cell Transfection
Myc-tagged wild-type and dominant–negative mutant of p160ROCK (KD-IA) were constructed in the pCAG mammalian expression vector as previously described (Ishizaki et al., 1997) (see Fig. 1). N1E-115 cells were plated at a density of 2 × 104 cells per 3.5-cm dish and of 105 cells per 3.5-cm dish for immunofluorescence and immunoblotting studies, respectively. After culture for 1 d, cells were transfected with the plasmid DNA (1 μg) by the application of lipofectamine-DNA coprecipitates in Opti-MEM (Gibco Laboratories, Grand Island, NY) (Ishizaki et al., 1996). For cotransfection of KD-IA with either N17Rac1 or N17Cdc42, 1 μg of each plasmid DNA was used. For transfection with V14Rho, wild-type p160ROCK, the medium was changed to serum-free DME at 4 h and then the cells were cultured for another 12 h. For transfection with the KD-IA mutant, the medium was replaced by DME containing 10% FBS at 4 h and the cells were cultured for another 16 h in the presence of serum.
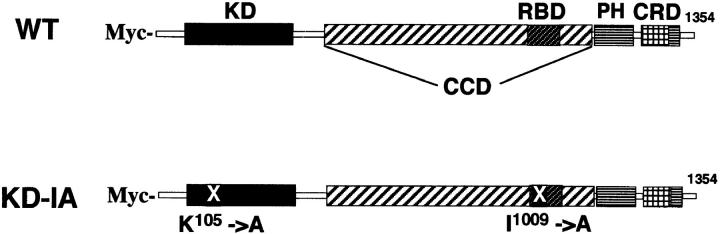
Figure 1.
Schematic representation of the wild-type and the KD-IA mutant of p160ROCK. Domains of p160ROCK and the point mutations in the KD-IA mutant are illustrated. KD, serine/threonine protein kinase domain; CCD, coiled-coil forming amphiphatic α-helical domain; RBD, Rho-binding domain; PH, pleckstrin homology domain; CRD, cysteine-rich zinc-finger domain; X, positions of mutations with amino acid numbers.
Immunofluorescent Staining of N1E-115 Cells and Swiss3T3 Cells
N1E-115 cells were cultured as described above. Swiss 3T3 cells were plated on glass coverslips at a density of 3 × 104 cells per 6-cm dish in DME containing 10% FBS. Incubation of Swiss 3T3 cells with 30 μg/ml of C3 exoenzyme for 72 h or with 10 μM Y-27632 for 2 h was carried out in the presence of serum. Fixation, permeabilization, and blocking steps for actin staining and immunofluorescence with anti-myc, anti-FLAG, anti-ROCK, and anti-peripherin antibodies were performed as described (Stokoe et al. 1994). For immunostaining with anti-vimentin and anti- tubulin antibodies, cells were fixed with 3% paraformaldehyde in PBS (−) at room temperature for 20 min, permeabilized with 0.2% Triton X-100 in PBS (−), and then blocked with 1% BSA in PBS (−). N1E-115 cells were first incubated with primary antibodies in a Blotto solution (5% skim milk, 0.1% Triton X-100, 0.1% Tween-20 in PBS[−]); anti-peripherin polyclonal antibody (1:200 in Blotto), anti-myc (9E10) monoclonal antibody (1:300 in Blotto), anti-FLAG antibody (1:100 in Blotto), anti- tubulin monoclonal antibody (1:100 in PBS containing 1% BSA) and anti-p160ROCK polyclonal antibody (1:300 in Blotto). Anti-myc (9E10) and anti-FLAG antibodies were detected with FITC-conjugated anti–mouse IgG (Vector Laboratories, Burlingame, CA). Rhodamine-conjugated anti–rabbit IgG (Vector Laboratories) was used to detect the signal of anti-peripherin antibody. For F-actin staining, rhodamine-conjugated phalloidin (Molecular Probes, Inc., Eugene, OR) was added after incubation with the second antibody. Anti-tubulin antibody and anti-p160ROCK antibody were detected by rhodamine-conjugated anti–mouse IgM (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) and FITC-conjugated anti–rabbit IgG (Vector Laboratories), respectively. Swiss 3T3 cells were first incubated with either anti-vimentin antibody (1:100 in Blotto) or anti-tubulin antibody (1:100 in Blotto). Anti-vimentin antibody were detected with rhodamine-conjugated anti–mouse IgM. For F-actin staining, OregonGreen-conjugated phalloidin (Molecular Probes, Inc.) was added after incubation with the secondary antibody. Cells were analyzed at 0.36-μm optical sections on a laser scanning confocal microscope imaging system (model MRC-1024; Bio-Rad Laboratories, Hercules, CA) and built-up images were constructed.
Microinjection and Video Microscopy
Swiss 3T3 cells were plated on a glass coverslip at 3 × 104 cells per 3.5-cm dish and then cultured in DME containing 10% FBS for 24 h. Medium was changed to Hepes-buffered DME containing 10% FBS and the cells were incubated for 1 h. A coverslip was then transferred onto a heated stage maintained at 37°C in the Hepes-buffered medium. C3 exoenzyme at 150 μg/μl was injected into Swiss cells by the use of Eppendorf Microinjector 5242 (Eppendorf Scientific, Inc., Hamburg, Germany) as described previously (Watanabe et al., 1997). Time-lapse analysis was carried out by phase contrast on an Axiovert microscope (model 100; Carl Zeiss, Inc., Thornwood, NY) with a Hamamatsu charge-coupled device camera (model C2400; Hamamatsu Phototonics, Hamamatsu City, Japan) and recorded by a SONY laser video disc recorder (model LVR-3000AN; Sony Corp., Park Ridge, NJ) Images were printed by a SONY color video printer (model UP-1850).
Western Blotting Analysis of p160ROCK in N1E-115 Cells
N1E-115 cells were lysed in Laemmli's SDS-PAGE sample buffer. The cell lysates were subjected to SDS-PAGE on a 8% polyacrylamide gel, and then separated proteins were transferred onto a nitrocellulose membrane (BA85; Schleicher & Schuell, Inc., Keene, NH). The membrane was blocked with 5% BSA in Tris-buffered saline. The membrane was probed with anti-p160ROCK rabbit antibody 20490 (Fujita et al., 1997) and then the signal was detected by alkaline phosphatase-conjugated anti–rabbit IgG antibody (Kirkegaard & Perry Laboratories, Inc.).
Detection of MLC Phosphorylation
N1E-115 cells were allowed to extend neurites in serum-free DME and were preincubated with or without various concentrations of Y-27632 for 30 min. The cells were then stimulated for indicated times with 1 μM LPA and then lysed in Laemmli's SDS-PAGE sample buffer. The cell lysates were subjected to immunoblotting with anti-phosphorylated MLC (p-MLC) rabbit antibody (Matsumura et al., 1998) using alkaline phosphatase staining system as described above.
Results
Y-27632 Inhibits Agonist-induced Neurite Retraction
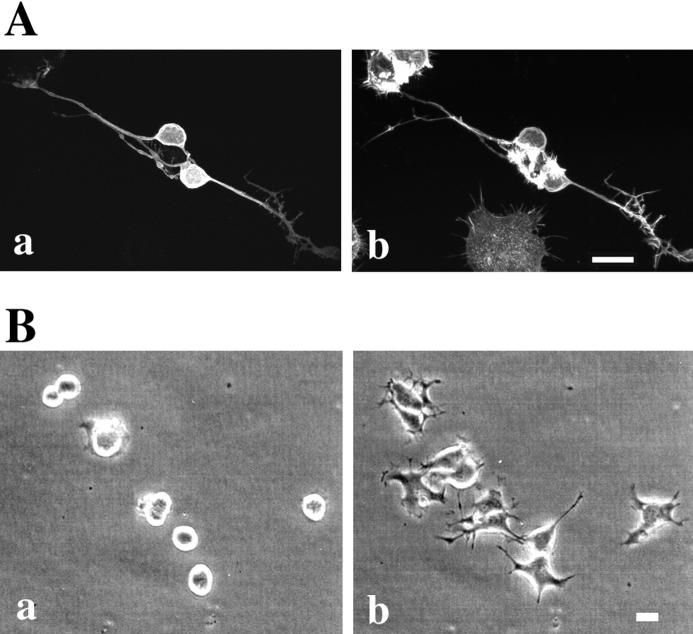
Under serum starvation, most N1E-115 cells became flattened and extended neurites with prominent growth cones (Fig. 2, B and D). As reported previously (Suidan et al., 1992; Jalink et al., 1993), the addition of LPA to these cells caused rapid collapse of growth cones and neurite retraction. This morphological change was usually complete within 5 min, and in 10 min most cells became round and few, if any, neurites remained extended (Fig. 2 C). We previously demonstrated that this neurite retraction was blocked completely by a previous treatment of the cells with C3 exoenzyme, indicating the involvement of Rho in this process (Jalink et al., 1994).
Figure 2.
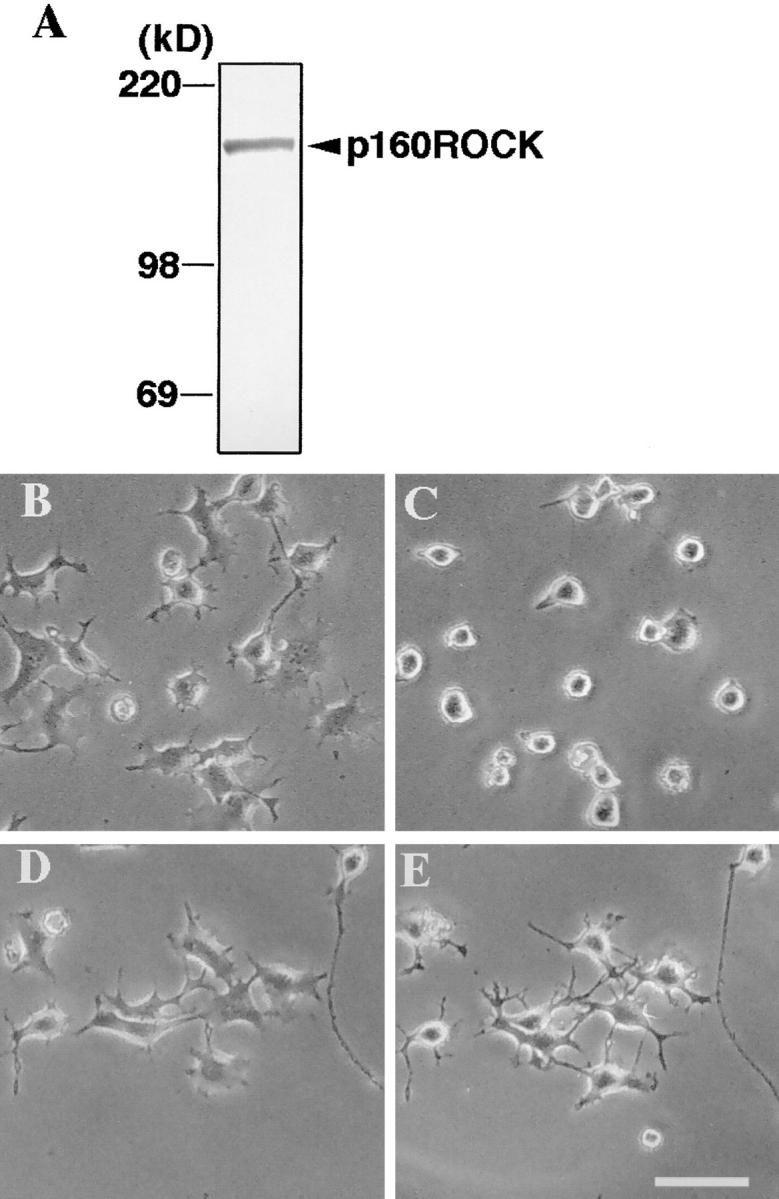
Western blotting of p160ROCK in N1E-115 cells (A) and inhibition of LPA-induced neurite retraction and cell rounding by Y-27632 (B–E). (A) N1E-115 cells were lysed in Laemmli– SDS-PAGE sample buffer and then subjected to immunoblotting using anti-p160ROCK antibody. (B–E), N1E-115 cells were maintained in serum-free DME for 24 h and allowed to extend neurites. The cells were pretreated without (B and C) or with 10 μM Y-27632 (D and E) for 30 min, and exposed to 1 μM LPA for 10 min. The same fields were photographed before the drug addition (Band D) and after LPA stimulation (C and E). Bar, 100 μm.
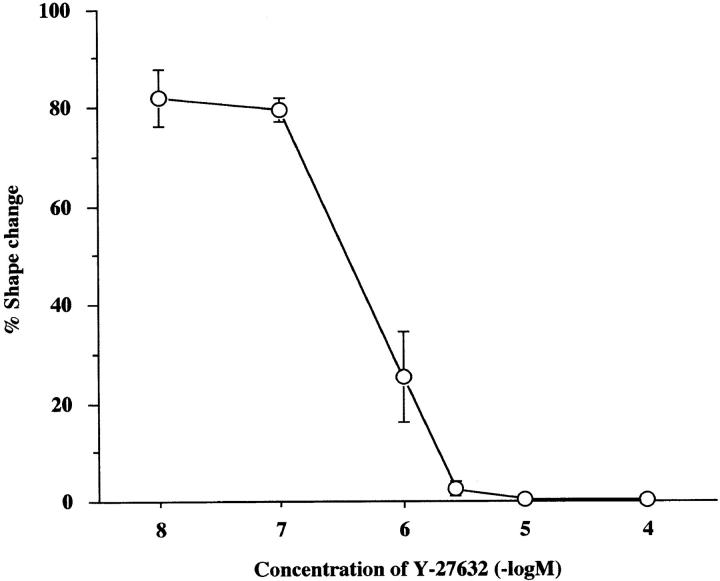
To examine a role of p160ROCK in this Rho-mediated neurite retraction, we first examined the presence of this kinase in N1E-115 cells by Western blotting analysis. As shown in Fig. 2 A, a single, positively stained band was found at 160 kD, suggesting the presence of p160ROCK in this cell line. We then applied a specific p160ROCK inhibitor, Y-27632, onto serum-starved, neurite-extended N1E-115 cells before the LPA addition, and examined its effects on the LPA-induced morphological change. As shown in Fig. 2, D and E, LPA-induced neurite retraction, as well as cell rounding of N1E-115 cells, was completely abolished by the treatment with 10 μM Y-27632. This inhibition occurred in a concentration-dependent manner, with a 50% inhibitory concentration (IC50) value of 0.56 μM (Fig. 3). Y-27632 also inhibited neurite retraction induced by other agonists such as serum and thrombin with similar IC50 values (data not shown). These results strongly indicated that p160ROCK was necessary as a downstream effector of Rho for the agonist-induced neurite retraction in N1E-115 cells.
Figure 3.
Concentration-dependent inhibition by Y-27632 of LPA-induced neurite retraction in N1E-115 cells. Serum-starved N1E-115 cells were pretreated with the indicated concentrations of Y-27632 for 30 min and then exposed to 1 μM LPA for 10 min. The percentages of cells with complete neurite retraction are shown. Results of three experiments are shown as mean ± SEM.
Overexpression of p160ROCK Induces Marked Rounding of N1E-115 Cells
To confirm the involvement of p160ROCK in the neurite retraction and to test whether p160ROCK activation was sufficient for the above change of the N1E cell morphology, we transiently overexpressed either V14Rho or the wild-type p160ROCK in N1E-115 cells and examined the morphology of the cells expressing the respective proteins. After transfection, the cells were incubated in serum-free DME for 12 h. Transfected cells were identified by staining the Myc epitope and F-actin organization was examined by phalloidin staining. In the serum-free medium, nontransfected cells had flattened polygonal cell bodies and extended neurites as above (Fig. 4). On the other hand, cells expressing either V14Rho or the wild-type p160ROCK showed a marked cell rounding. No extended neurites were found in these cells, although very thin spikes were observed on their periphery. In both transfectants, strong actin staining was found under the plasma membrane of the round cell bodies. These experiments clearly showed that activation of the Rho and ROCK pathway is sufficient to block and to completely reverse cell flattening and neurite extension seen in the serum-starved state.
Figure 4.
Morphology of N1E-115 cells expressing active Rho and p160ROCK. N1E-115 cells were transfected with V14Rho (A and B) or wild-type p160ROCK (C and D), and were cultured in serum-free DME for 12 h. Overexpressing cells were identified by the positive Myc-staining (A and C) and their F-actin structures were examined (B and D). Note that the transfected cells do not extend neurites even under serum-starved conditions. In addition, marked bleb formation was also noted in some of p160ROCK-expressing cells. Bar, 20 μm.
Expression of a Dominant-negative Mutant of p160ROCK and Treatment with Y-27632 Induces Neurites in N1E-115 Cells
The round cell morphology induced by p160ROCK is not only similar to that evoked by the agonist-induced neurite retraction in these cells as noted above, but also to that elicited in the continued presence of serum. Because serum can induce the activation of endogenous Rho in a variety of cells (Hall, 1994), these findings suggest that the round cell morphology in the presence of serum is mediated by the Rho–ROCK pathway. However, it does not necessarily indicate that inactivation of the Rho–ROCK pathway is sufficient to induce neurite extension in these cells. We therefore transfected N1E-115 cells with the KD-IA mutant of p160ROCK, and cultured them in the presence of serum. This mutant has point mutations both in the kinase domain and the Rho-binding domain, and is defective in both kinase activity and Rho binding. Our previous study in HeLa cells had established that this mutant inhibits endogenous p160ROCK activity by a dominant-negative mechanism (Ishizaki et al., 1997). As shown in Fig. 5 A, cells expressing KD-IA mutant extended long neurites in a culture medium containing 10% FBS. We next incubated N1E-115 cells with Y-27632 in the presence of serum and followed their morphology. As shown in Fig. 5 B, the cells became polygonal and extended neurites in a time-dependent manner. Thus, the presence of serum makes p160ROCK constitutively active to prevent neurite formation and inactivation of this activity is sufficient for neurite extension. Notably, the cell bodies of the transfected or treated cells were not fully flattened, indicating that the phenotype induced by ROCK inhibition in the presence of serum may not be exactly identical to that induced by serum starvation. This phenotype of N1E-115 cells is reminiscent of that induced by treatment of C3 exoenzyme in the same cells as well as other neuronal cells such as PC-12 cells (Nishiki et al., 1990; Jalink et al., 1994). These results indicate that pathways other than the Rho– ROCK pathway are also activated by serum and may be responsible for the cell rounding found in these cells in the presence of serum.
Figure 5.
Morphology of N1E-115 cells expressing the KD-IA mutant and of N1E-115 cells treated with Y-27632. (A) N1E-115 cells were transfected with the KD-IA mutant, a dominant-negative form of p160ROCK, and were cultured for 16 h in the presence of 10% FBS. The cells were double-stained for the Myc-tag (a) and F-actin (b). Note that the transfected cells extend long neurites in the presence of serum. (B) N1E-115 cells were cultured in the presence of 10% FBS, and treated with 10 μM Y-27632, a specific ROCK inhibitor. Phase-contrast photomicrographs were taken at 0 (a) and 60 min (b) after the addition of the compound. Bar, 20 μm.
Recently, Kozma et al. (1997) reported that the C3 exoenzyme-induced neurite outgrowth is inhibited by microinjection of dominant-negative forms of Cdc42 and Rac, and suggested that inhibition of the Rho pathway leads to activation of either one or both of the Cdc42 and Rac pathways, which in turn by themselves could induce neurite outgrowth. They further showed that neurite extension induced by serum starvation was also blocked by expression of dominant-negative Cdc42 and Rac. We, therefore, examined if activation of Cdc42 and Rac was also involved in the expression of the KD-IA phenotype. As shown in Fig. 6, expression of N17Cdc42 and N17Rac inhibited the neurite generation induced by expression of the KD-IA mutant in N1E-115 cells.
Figure 6.
Inhibition by N17Cdc42 and N17Rac of the neurite generation induced by the KD-IA mutant of p160ROCK. N1E-115 cells were transfected with KD-IA p160ROCK alone (A and B), with N17Cdc42 and KD-IA p160ROCK (C and D) or with N17Rac and KD-IA p160ROCK (E and F). The transfected cells were cultured in DME containing 10% FBS for 16 h. Overexpressing cells were identified with p160ROCK staining (A, C, and E) and F-actin was stained with phalloidin (B, D, and F). Bar, 20 μm. (G) Quantification of cells bearing no neurites, short neurites, and long neurites. N1E cells expressing KD-IA alone, KD-IA and N17Cdc42, or KD-IA and N17Rac were identified as above, and the numbers of cells bearing no neurites, or neurites shorter or longer than 100 μm were determined. *, P < 0.001 compared with KD-IA.
p160ROCK Induces MLC Phosphorylation during Agonist-induced Neurite Retraction
Having established the causal relationship between activation of the Rho–ROCK pathway and the agonist-induced neurite retraction and cell rounding of N1E-115 cells, we next examined whether p160ROCK provided a mechanistic link leading to an increase in the actomyosin-driven contractility, as suggested in our previous study (Jalink et al., 1994). First, using an anti-phospho-MLC antibody which specifically recognized MLC phosphorylated at Ser19 (Matsumura et al., 1998), we examined whether MLC phosphorylation was elevated in N1E-115 cells during neurite retraction. As shown in Fig. 7 A, the immunoblotting analysis using the above antibody revealed a transient increase in phospho-MLC content in response to LPA applied to serum-starved N1E-115 cells. The MLC phosphorylation peaked at 1–2 min and gradually declined thereafter, but the phosphorylation level still remained high at 30 min. This time course was consistent with the rapidity and the stability of the morphological changes induced by LPA. We then asked if this stimulus-evoked MLC phosphorylation was mediated by p160ROCK. The serum-starved cells were pretreated with various concentrations of Y-27632 for 30 min and were then stimulated by 1 μM LPA. Effects on MLC phosphorylation was examined at 2 min after the LPA addition. Y-27632 inhibited the increase of MLC phosphorylation in a concentration-dependent manner; the inhibition was evident at 1 μM and complete at 10 μM (Fig. 7 B). On the other hand, the total amount of MLC did not change in these samples (data not shown). This concentration–effect relationship was quite similar to that seen for inhibition of the LPA-induced neurite retraction and cell rounding (refer to Fig. 3). These results provide compelling evidence for a role of p160ROCK in the regulation of the growth cone contractility via MLC phosphorylation. Whether this is elicited through a Ca2+-sensitization mechanism similar to that found in smooth muscle contraction (Uehata et al., 1997) remains to be established.
Figure 7.
LPA-induced MLC phosphorylation and its inhibition by Y-27632 in N1E-115 cells. (A) Time course of LPA-induced MLC phosphorylation. Serum straved N1E-115 cells were incubated with 1 μM LPA for the indicated times. Cells were collected in Laemmli-SDS-PAGE sample buffer and then subjected to immunoblotting using anti-phosphoMLC antibody. (B) Concentration-dependent inhibition of MLC phosphorylation by Y-27632. Serum-starved N1E-115 cells were treated with the indicated concentrations of Y-27632 for 30 min, and then stimulated with LPA for 2 min. The cells were lysed and analyzed as above. The content of MLC in the cells did not change during LPA stimulation or Y-27632 treatment.
Effects of p160ROCK Activation and Inhibition on Assemblies of Intermediate Filaments and Microtubules in N1E-115 Cells
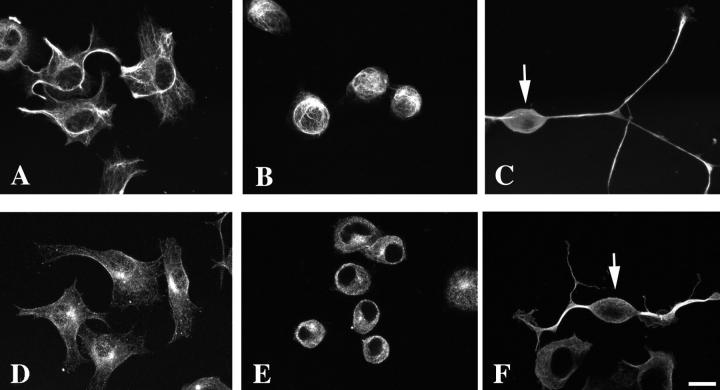
The above findings obtained by the pharmacological and molecular biological manipulations of p160ROCK activity strongly suggested that the agonist-induced neurite retraction and, to some extent, cell rounding was mediated by p160ROCK at least partly through generation of the actomyosin-driven contractility. Our data also suggest that the neurite outgrowth in N1E-115 cells might be tonically suppressed by the Rho–p160ROCK pathway activated in the presence of serum, and could be reinitiated by inhibiting this pathway. Because neuritic processes are known to contain specifically organized intermediate filaments and microtubules as major cytoskeletons in addition to the actin microfilaments, we wondered whether the activation and inhibition of p160ROCK could respectively induce disassemblies and assemblies of the intermediate filaments and microtubules in the neurite-like processes, in parallel to the regulation of the actin cytoskeleton characterized above. To address this point, we stained the serum-starved neurite-extending cells, the serum-induced round cells, and the cells expressing the KD-IA mutant of p160ROCK with anti-peripherin and anti-tubulin antibodies. In the serum-starved cells, peripherin, a major intermediate filament in N1E-115 cells, was well organized into a network in the flattened cell bodies and part of it extended into the neurites as thick bundles (Fig. 8). The peripherin cytoskeleton became disorganized and collapsed into a swirled form in the round cells in the presence of serum (Fig. 8 B). In contrast, a marked staining for peripherin which originated from the cell bodies and extended into the base of the growth cones was present in the neurites even in the presence of serum when KD-IA was expressed (Fig. 8 C). Similarly, serum-dependent disorganization and ROCK inhibition-induced reassembly was observed for microtubule organization. Strong tubulin staining was found in neurites of the cells expressing KD-IA (Fig. 8 F), whereas it formed a fine network in serum-starved cells (Fig. 8 D) and dismantled in the presence of serum (Fig. 8 E). These results clearly showed that p160ROCK is critically involved in the activity-dependent disassembly and reorganization of the intermediate filaments and microtubules in the neurites of N1E-115 cells, and suggested that inhibition of p160ROCK might be necessary to promote the assemblies of the two neuritic cytoskeletons.
Figure 8.
Peripherin and tubulin staining of serum-starved, serum-fed, and KD-IA–transfected N1E-115 cells. N1E-115 cells were cultured in the absence (A and D) or presence (B and E) of serum, or were transfected with the KD-IA mutant of p160ROCK and cultured in DME containing 10% serum (C and F). The cells were stained with either anti-peripherin (A, B, and C) or anti-tubulin (D, E, and F) antibodies. In C and F, the cells were also stained with either anti-myc antibody (C), or anti-ROCK antibody (F), and the cells expressing KD-IA protein were identified (indicated by arrows). Images built from optical sections by a confocal imaging system are shown. Bar, 20 μm.
Inhibition of p160ROCK Is Sufficient to Induce Neurite-like Processes in Swiss 3T3 Cells by Promoting Assemblies of Microtubules and Intermediate Filaments
Since inhibition of p160ROCK led to the assemblies of the intermediate filaments and microtubules in neuritic processes, we next asked if this mechanism operated in morphological remodeling of other types of cells. To address this question, we used Swiss 3T3 cells, a naive system which does not form neurite-like processes under normal culture conditions. Inactivation of Rho by botulinum C3 exoenzyme in Swiss 3T3 cells caused round cell bodies with long thin beaded processes (for examples see Figs. 10 E and 11 E). These processes have often been regarded as retraction fibers, although they extend over the original size of cells in many cases. To address this issue, we monitored the morphology of Swiss cells injected with C3 exoenzyme by video microscopy. As shown in Fig. 9, cell processes generated by C3 exoenzyme treatment changed their morphology in time and some of them actively elongated, indicating that new organization of cytoskeletons is responsible for this process generation. Because these processes morphologically resembled neurites of neuronal cells, we reasoned that a mechanism analogous to the neurite outgrowth in N1E-115 cells might be implicated in the control of cell shapes in Swiss 3T3 cells. Indeed, treatment with Y-27632 for more than 2 h induced the C3-like morphological phenotype in Swiss 3T3 cells even in the presence of serum, although the morphological phenotype was much weaker with shorter processes bearing less branching (Figs. 10 G and 11 G). Actin stress fibers disappeared in these cells, confirming the complete loss of the ROCK activity (Fig. 10, E and G and Fig. 11, E and G). Swiss 3T3 cells were then subjected to staining for tubulin and vimentin, a major intermediate filament in these cells. Sharp and strong staining of vimentin and tubulin were observed in the processes of both C3 exoenzyme- and Y-27632– treated cells (Fig. 10, F and H and Fig, 11 F and H). Furthermore, similar cell morphology and patterns of vimentin and tubulin staining were observed in the serum-starved cells as well, though to a smaller extent (Fig. 10, C and D and Fig. 11, C and D). These results indicate the intriguing possibility that inhibition of the Rho–ROCK pathway per se may be sufficient to initiate the rearrangement of microtubules and intermediate filaments required to trigger new process formation in nonneuronal cells such as in Swiss 3T3 cells, thus suggesting that a common mechanism may govern the reorganization of different cytoskeletons in neuronal cells and fibroblasts. Elucidation of the molecular mechanism linking p160ROCK and the reorganization of intermediate filaments and microtubules awaits further studies.
Figure 10.

Vimentin staining of Swiss 3T3 cells. Swiss 3T3 cells were cultured in the presence (A and B) or absence (C and D) of serum, or with 30 μg/ml C3 exoenzyme for 72 h (E and F) or 10 μM Y-27632 for 2 h (G and H) in the presence of serum, and were stained with OregonGreen phalloidin (A, C, E, and G) or with anti-vimentin antibody (B, D, F, and H). Images built up from optical sections by a confocal imaging system are shown. Bar, 20 μm.
Figure 11.

Tubulin staining of Swiss 3T3 cells. Swiss 3T3 cells were cultured in the presence (A and B) or absence (C and D) of serum, or with C3 exoenzyme (E and F) or Y-27632 (G and H) in the presence of serum, and were stained with OregonGreen phalloidin (A, C, E, and G) or with anti-tubulin antibody (B, D, F, and H). Images built from optical sections by a confocal imaging system are shown. Bar, 20 μm.
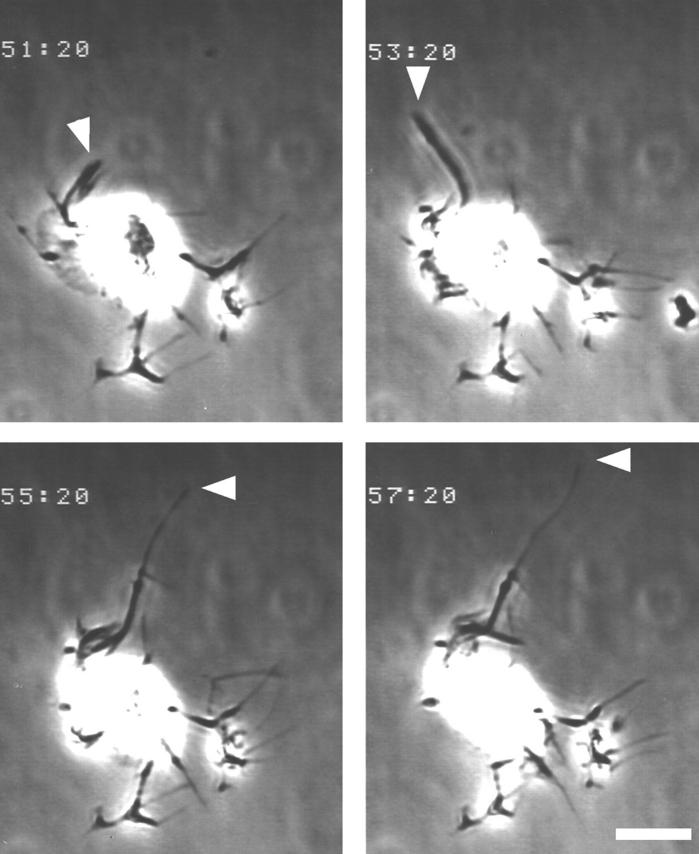
Figure 9.
Video-microscopy of Swiss 3T3 fibroblast microinjected with C3 exoenzyme. Swiss cells were maintained on a glass coverslip in Hepes-buffered DME containing 10% FBS and microinjected with C3 exoenzyme. Morphology of the injected cells was monitored by time-lapse video-microscopy. Four images taken every two min are shown. Time after the injection is indicated in the top left corner of each image. Note cell processes change in their morphology in a time-dependent manner; arrowhead, time-dependent elongation of one process. Bar, 20 μm.
Discussion
p160ROCK Mediates Agonist-induced Neurite Retraction through Increase in Actomyosin-based Contractility
The neuronal cell line N1E-115 is well known to become flattened and to extend neurites upon serum starvation. They also retract neurites and become round in response to agonists including serum and LPA. A role for the small GTPase Rho was suggested by many studies including ours. However, the downstream effector molecule involved in this Rho-mediated process has remained elusive. Here we investigated the possibility that the ROCK/ROK family of protein serine/threonine kinases including p160ROCK and its isozyme, ROKα/Rho-kinase/ROCK-II, may be one of the critical Rho effectors. In this study we have demonstrated that Y-27632, a specific inhibitor of these kinases competitive for their ATP-binding site (Uehata et al., 1997), inhibited neurite retraction induced by LPA and thrombin. The concentration dependency of the compound for inhibition of the neurite retraction was similar to that previously determined for the inhibition of agonist-induced smooth muscle contraction by this compound, and was consistent with its inhibitory potency on p160ROCK. Furthermore, we have found that the overexpression of wild-type p160ROCK evoked marked cell rounding even under the serum-starved conditions, whereas the expression of the KD-IA mutant, a dominant-negative form of p160ROCK, completely abolished neurite retraction induced by serum and induced new neurites in these cells. These results strongly suggest that p160ROCK acts downstream of Rho and that its kinase activity is necessary and sufficient to mediate the agonist-induced neurite retraction in N1E-115 cells. Recently, a similar neurite-retracting activity of ROKα was shown in PC-12 pheochromocytoma cells (Katoh et al., 1998).
Our previous study (Jalink et al., 1994) indicated the generation of tension along the neurites in N1E-115 cells exposed to LPA. In this study, we have shown that LPA application increased the level of phosphorylated MLC, with a time course overlapping with that of the LPA-induced morphological changes. Furthermore, Y-27632 inhibited both the phosphorylation and morphological changes with a similar dose-response relationship. Since MLC phosphorylation is a critical step in generating an actomyosin-based contractile force, our results suggest an essential role for p160ROCK in triggering a Rho-dependent contraction in N1E-115 cells, thus presumably leading to neurite retraction.
The contraction of the actomyosin system in smooth muscle and nonmuscle cells is thought to be regulated by two mechanisms: (a) one is dependent on increase in free Ca2+ ion in the cell and is mediated by MLC kinase and (b) the other is a Ca2+ sensitization mechanism. Several lines of evidence now indicate a role for the ROCK/ROK kinase family in the latter mechanism. Kimura et al. (1996) reported that Rho-kinase phosphorylates the myosin binding subunit of myosin phosphatase in vitro and thereby inhibits its activity. Amano et al. (1996) further suggested the possibility that Rho-kinase may also directly phosphorylate MLC. Finally, Uehata et al. (1997) demonstrated that Y-27632, which blocks the ROCK/ROK kinases in vitro, inhibited smooth muscle contraction in vivo by specifically acting on the Ca2+-sensitization mechanism. Our present work extends these findings and provides a common basis for establishing an essential role for p160ROCK in catalyzing the Rho-dependent regulation of actomysion-based contractility, in neuronal as well as in nonneuronal cell types.
The Rho–ROCK Pathway Regulates Reorganization of the Microtubules and the Intermediate Filaments
It is well known that the intermediate filaments and the microtubules play important roles in the formation of neurites or similar processes. Microtubule depolymerizating drugs can cause neurite collapse in many types of cultured neuronal cells (Daniels, 1975; Solomon and Magendantz, 1981; Joshi et al., 1985). In PC-12 cells, exposure to tubulin mRNA antisense oligonucleotides led to tubulin depletion and inhibition of neurite outgrowth (Teichman-Weinberg et al., 1988). As for the intermediate filaments, a similar antisense mRNA strategy was used to demonstrate the requirement for glial fibrillary acidic protein (GFAP) in the formation of stable processes in human astrocytoma cells (Weinstein et al., 1991).
The present study has demonstrated that in N1E-115 cells, inhibition of ROCK in the presence of serum, by overexpressing a dominant-negative form of p160ROCK, generated an outgrowth of neuritic processes, which were filled with thick fibers immunopositive for tubulin and peripherin (refer to Fig. 8). We also showed that in Swiss 3T3 cells, treatments with C3 exoenzyme or Y-27632, by themselves, induced extension of long neurite-like processes, positively stained for structures containing both tubulin and vimentin, a predominant intermediate filament in this cell type. These results suggest that the ROCK/ROK protein kinase family may be critically involved in the regulated reorganization of the intermediate filaments and the microtubules, not only in neuronal but also in nonneuronal cells. Our findings are consistent with Paterson et al. (1990) who showed that the microinjection of V14Rho caused the collapse of the vimentin structure in Swiss 3T3 cells. Thus, the morphological phenotypes and signal transduction pathways regulated by p160ROCK may be quite similar in neuronal cells such as N1E-115 cells and nonneuronal cells such as Swiss 3T3 cells. However, the ultimate morphology of two types of cells appears different; marked cell rounding in neuronal cells and flattened cell bodies with stress fibers in nonneuronal cells. This is likely due to the difference in the integrin-based focal adhesions of these cells. How p160ROCK is involved in the differential regulation of these structures in the two types of cells remains to be investigated.
At present, we do not know whether the ROCK effect on intermediate filaments is a direct or indirect consequence of phosphorylation events mediated by ROCK/ ROK kinases. Phosphorylation of the intermediate filaments has been shown to be required for their interaction with other cytoskeletal components and has been implicated in neurite extension and functions (Nixon et al., 1987; Aletta et al., 1989). Kosako et al. (1997) reported that Rho-kinase, an isozyme of p160ROCK, phosphorylated a specific residue in GFAP, that had been previously associated with the disassembly of the GFAP filaments during cytokinesis in astrocytes. Thus, it is possible that activated p160ROCK directly phosphorylates different types of intermediate filament proteins, even in the cell types that we examined, namely N1E-115 and Swiss 3T3 cells. Our preliminary analysis, however, did not detect enhanced phosphorylation of peripherin after LPA stimulation of N1E-115 cells, although we could not exclude the possibility that residual p160ROCK activity could phosphorylate a specific residue of this protein (Hirose, M., T. Ishizaki, and S. Narumiya, unpublished observations). On the other hand, the disassembly of intermediate filaments as well as microtubules may well be a secondary consequence of the regulation of the actomyosin system, in which the tension generated by the ROCK-induced increase in the myosin-based contractility may generate a yet unknown secondary negative signaling event, which then acts on the cytoskeletons and leads to their disruption. As discussed below, many lines of evidence suggest that a positive regulation of the neurite outgrowth triggered by inactivation of Rho may be exerted by other members of the Rho family GTPases, such as Cdc42 and Rac. Thus, Rho-dependent regulation of the assembly and disassembly of the intermediate filaments might in fact be controlled via a reciprocal activation/inactivation processes between Rho and Cdc42/Rac.
The present study has also shown that activation and inactivation of p160ROCK regulates the disassembly and assembly of the microtubules. Previous reports demonstrated that microtubule-depolymerizing agents induced stress fiber formation in a C3 exoenzyme-sensitive manner, thereby suggesting a signaling pathway from disruption of microtubules to Rho (Danowski et al., 1989; Enomoto, 1996; Zhang et al. 1997). However, no pathway from Rho to the microtubules has been indicated. Our study represents the first formal demonstration of a significant role for a Rho-associated molecule, p160ROCK, in this process. The parallelism in the p160ROCK-dependence seen in both intermediate filaments and microtubules indicate that Rho may simultaneously regulate the assembly and disassembly of both cytoskeletal components through a similar mechanism. In any event, the present study raises an interesting possibility that the regulatory role of Rho GTPases on the cytoskeleton may extend to the reorganization of the intermediate filaments and microtubules, in addition to the well-known roles in actin cytoskeleton. Current efforts in our laboratories are directed towards the identification of the ROCK substrates implicated during neurite retraction process, in order to sort out the distinct signaling pathways involved in these multiple regulatory processes.
Signaling Pathways in Neurite Remodeling: Interaction of the Rho–ROCK Pathway with Other Pathways
The present study has demonstrated a critical role for the Rho-ROCK pathway not only in the agonist-induced neurite retraction, but also in the tonic inhibition of neurite outgrowth in N1E-115 cells in the presence of serum. Suppression of this inhibitory pathway releases the cells from their constraint and enables them to sprout and extend new neurites. This role of the Rho–ROCK pathway as a tonic negative regulator in cell process formation appears not to be limited to N1E-115 cells, because previous studies demonstrated that inactivation of Rho by botulinum C3 exoenzyme could also induce long neurites in PC12 cells (Nishiki et al., 1990; Tigyi et al., 1996a ) as well as in neuroblastoma NG108-15 cells (Moolenaar, 1994). Kozma et al. (1997) found that this apparently stimulatory effect of C3 exoenzyme on neurite outgrowth in N1E-115 cells could be inhibited by expressing dominant-negative forms of Cdc42 and Rac. Conversely, activation of Cdc42 and Rac stimulated neurite outgrowth by inducing filopodia and lamellipodia in the growth cones. Furthermore, coinjection of Rho with Cdc42 blocked the Cdc42-induced formation of filopodia, suggesting that Rho signaling downregulates Cdc42 signaling in N1E-115 cells in a dominant fashion (Kozma et al., 1997). Similarly, the overexpression of the guanine nucleotide exchange factor for Rac, Tiam1, induced neurite-like processes, which could be antagonized by expression of constitutively active Rho (van Leeuwen et al., 1997). Taken together, these findings indicate that p160ROCK, as a downstream effector of Rho, may negatively regulate the Cdc42 and Rac pathway, both of which play a positive role in growth cone expansion. Consistent with this idea, we found that expression of N17Cdc42 and N17Rac, dominant-negative forms of Cdc42 and Rac respectively, prevented the neurite growth induced by the dominant-negative form of p160ROCK (refer to Fig. 6).
In addition to these signaling cross-talks at the GTPases level, functional interaction between different kinase pathways may also have to be considered. It has been known for some time that activation of the cAMP pathway induces neurite outgrowth in various neuronal cells including PC12 cells (for example see Gunning et al., 1981). Recently, Tigyi et al. (1996b) has reported that LPA-induced neurite retraction is inhibited by the elevation of cAMP levels in PC12 cells. This effect was not seen in a mutant PC-12 clone deficient in protein kinase A. These results suggest that the Rho–ROCK pathway may be antagonized by the cAMP signaling pathway during ligand-induced neurite remodelling and that inhibition of neurite retraction by cAMP may be exerted by an A kinase–dependent inhibition of the Rho–ROCK pathway. Whether the cyclic AMP-PKA effect acts on Rho, ROCK, a ROCK substrate, or further downstream remains to be determined. The possible interaction of these signaling pathways is depicted in Fig 12.
Figure 12.
Model of signal transduction and cross-talks of the Rho–ROCK pathway in the neurite remodeling. p160ROCK is activated downstream of Rho in response to agonist stimulation, and in turn induces the actomyosin-based contractility and the disassembly of the microtubules and the intermediate filaments, leading to the neurite retraction. p160ROCK also transmits a negative signal to the Cdc42/Rac pathways and suppresses the neurite outgrowth by tonically inhibiting their actions. The activation of the cAMP–A kinase pathway is likely to inhibit the Rho–ROCK pathway and to release the suppression of neurite outgrowth. Disassembly of intermediate filaments may be caused by direct phosphorylation by p160ROCK at specific amino acid residue(s) of this cytoskeletal proteins as shown for GFAP (Kosako et al., 1997) or may well be a secondary consequence of ROCK's effect on the actomyosin system.
In summary, the present study has demonstrated that Rho-dependent p160ROCK activation is not only necessary but also sufficient in mediating the agonist-induced neurite retraction in N1E-115 cells. This study also demonstrated that activation of the Rho–ROCK pathway exerts tonic inhibition of neurite sprouting. How this signaling pathway is regulated by physiological cues during axonal pathfinding remains to be elucidated.
Acknowledgments
The authors thank K. Itoh and K. Yoshioka (Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan) for advice on MLC phosphorylation, P. Madaule (Kyoto University, Kyoto, Japan) for discussion, and K. Nonomura and T. Arai for technical and secretarial assistance, respectively. M. Hirose is on leave from the Department of Obstetrics and Gynecology, Shiga University of Medical Science, and thanks its chairman Y. Noda for encouragement.
This work was supported in part by a Grant-in-aid for Specially Promoted Research (08102007) from the Ministry of Education, Science, and Culture of Japan, and grants from Human Frontier Science Program, from the Japanese Foundation on Metabolism and Diseases, and from the Kyoto University Academic Research Award.
Abbreviations used in this paper
- GFAP
glial fibrillary acidic protein
- LPA
lysophosphatidic acid
- MLC
myosin light chain
- p160ROCK
p160 Rho-associated coiled coil-forming protein kinase
Note Added in Proof
An independent report also suggested an involvement of Rho-kinase–mediated MLC phosphorylation in LPA-induced neurite retraction in N1E-115 cells. (Amano, M., K. Chihara, N. Nakamura, Y. Fukata, T. Yano, M. Shibata, M. Ikebe, and K. Kaibuchi. 1998. Genes Cells 3:177–188).
Footnotes
M. Hirose and T. Ishizaki contributed equally to this work.
References
- Amano M, Itoh M, Kimura K, Fukata Y, Chihara K, Nakano T, Mastuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Mastuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Aletta JM, Shelanski ML, Greene LA. Phosphorylation of the peripherin 58-kD, a neuronal intermediate filament protein. Regulation by nerve growth factor and other agents. J Biol Chem. 1989;264:4619–4627. [PubMed] [Google Scholar]
- Bently D, O'Connor TP. Cytoskeletal events in growth cone steering. Curr Opin Neurobiol. 1994;4:43–48. doi: 10.1016/0959-4388(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Daniels M. The role of microtubules in the growth and stabilization of nerve fibers. Annu NY Acad Sci. 1975;253:535–544. doi: 10.1111/j.1749-6632.1975.tb19227.x. [DOI] [PubMed] [Google Scholar]
- Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93:255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- Enomoto T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct Funct. 1996;21:317–326. doi: 10.1247/csf.21.317. [DOI] [PubMed] [Google Scholar]
- Fujita A, Saito Y, Ishizaki T, Maekawa M, Fujisawa K, Ushikubi F, Narumiya S. Integrin-dependent translocation of p160ROCK to cytoskeletal complex in thrombin-stimulated human platelets. Biochem J. 1997;328:769–775. doi: 10.1042/bj3280769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning PW, Landerth GE, Bothwell MA, Shooter EM. Differential and synergistic actions of nerve growth factor and cyclic AMP in PC12 cells. J Cell Biol. 1981;89:240–245. doi: 10.1083/jcb.89.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamastu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO (Eur Mol Biol Organ) J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS (Fed Eur Biochem Soc) Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Jalink K, Moolenaar WH. Thrombin receptor activation causes rapid neural cell rounding and neurite retaction independent of classic messengers. J Cell Biol. 1992;118:411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity of thrombin action. Cell Growth Diff. 1993;4:247–255. [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and cell rounding by ADP-ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi HC, Chu D, Buxbaum RE, Heidemann SR. Tension and compression in the cytoskeleton in PC12 neurites. J Cell Biol. 1985;101:697–705. doi: 10.1083/jcb.101.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Aoki J, Ichikawa A, Negishi M. p160 RhoA-binidng kinase ROKα induces neurite retration. J Biol Chem. 1998;273:2489–2492. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- Keynes RJ, Cook GMW. Repulsive guidance cues. Curr Opin Neurobiol. 1995;5:75–82. doi: 10.1016/0959-4388(95)80090-5. [DOI] [PubMed] [Google Scholar]
- Kimura K, Itoh M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamastu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;269:221–223. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kosako H, Amano M, Yanagida M, Tanabe K, Nishi Y, Kaibuchi K, Inagaki M. Phosphorylation of glial fibrillary acidic protein at the same sites by cleavage furrow kinase and Rho-associated kinase. J Biol Chem. 1997;272:10333–10336. doi: 10.1074/jbc.272.16.10333. [DOI] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of periphral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPase and neuronal growth cone remodeling: relationship between increased complexity induced by Cdc42Hs, Rac1 and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenbrug OW, Poland M, Gebbink M, Oomen L, Moolenaar WH. Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differetial localization of RhoA. J Cell Sci. 1997;110:2417–2427. doi: 10.1242/jcs.110.19.2417. [DOI] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membrane. J Biol Chem. 1995;268:3813–3816. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Thompson CA, Foescher P. Cytoskeletal reorganization underlying growth cone motility. Curr Opin Neurobiol. 1994;4:640–647. doi: 10.1016/0959-4388(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: DrosophilaDrac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- Luo L, Hensch TK, Ackeman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO (Eur Mol Biol Organ) J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Matsumura F, Ono S, Yamakita Y, Totsukawa G, Yamashiro S. Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis of cultured cells. J Cell Biol. 1998;140:119–129. doi: 10.1083/jcb.140.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoolenaarW.H. LPA: a novel lipid mediator with diverse biological actions. Trends Cell Biol. 1994;4:213–219. doi: 10.1016/0962-8924(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS (Fed Eur Biochem Soc) Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Ishizaki T, Watanabe N. Rho effectors and reorganization of actin cytoskeleton. FEBS (Fed Eur Biochem Soc) Lett. 1997;410:68–72. doi: 10.1016/s0014-5793(97)00317-7. [DOI] [PubMed] [Google Scholar]
- Nishiki T, Narumiya S, Morii N, Yamamoto M, Fujiwara M, Kamata Y, Sakaguchi G, Kozaki S. ADP-ribosylation of the rho/rac proteins induces growth inhibition, neurite outgrowth and acetylcholine esterase in cultured PC-12 cells. Biochem Biophys Res Commun. 1990;167:265–272. doi: 10.1016/0006-291x(90)91760-p. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Lewis SE, Marotta CA. Posttranslational modification of neurofilament proteins by phosphate during axoplasmic transport in retinal ganglion cell neurons. J Neurosci. 1987;7:1145–1158. doi: 10.1523/JNEUROSCI.07-04-01145.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multi-molecular focal complex associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Paterson HF, Self AJ, Garrett MD, Just I, Aktories K, Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma FR, Jalink K, Hengeveld T, Moolenaar WH. Sphingosine-1-phosphate rapidly induces Rho-dependent neurite retraction: action through a specific cell surface receptor. EMBO (Eur Mol Biol Organ) J. 1996;15:2388–2395. [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Solomon F, Magendantz M. Cytochalasin separates microtubule disassembly from loss of asymmetrical morphology. J Cell Biol. 1981;89:157–161. doi: 10.1083/jcb.89.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe D, Macdonald SG, Cadwallander K, Symons M, Hanckock JF. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- Suidan HS, Stone SR, Hemmings BA, Monard D. Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron. 1992;8:363–375. doi: 10.1016/0896-6273(92)90302-t. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Sabry J. Making the connection: Cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Teichman-Weinberg A, Littauer UZ, Ginzburg I. The inhibition of neurite outgrowth in PC12 cells by tubulin antisense oligodeoxyribonucleotides. Gene. 1988;72:297–307. doi: 10.1016/0378-1119(88)90155-2. [DOI] [PubMed] [Google Scholar]
- Tigyi G, Fischer DJ, Sebok A, Yang C, Dyer DL, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+signaling and Rho. J Neurochem. 1996a;66:537–547. doi: 10.1046/j.1471-4159.1996.66020537.x. [DOI] [PubMed] [Google Scholar]
- Tigyi G, Fischer DJ, Sebok A, Marshall F, Dyer DL, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: neurite-positive effects of cyclic AMP signaling. J Neurochem. 1996b;66:549–558. doi: 10.1046/j.1471-4159.1996.66020549.x. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F, Kalin HET, van der Kammen RA, Michiels F, Kranenburg OW, Collard JG. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; Opposing roles for the small GTPase Rac and Rho. J Cell Biol. 1997;39:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO (Eur Mol Biol Organ) J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein DE, Shelanski ML, Liem RKH. Suppression by antisense mRNA demonstrates a requirement for the glial fibrillary acidic protein in the formation of stable astrocytic processes in response to neurons. J Cell Biol. 1991;112:1205–1213. doi: 10.1083/jcb.112.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–1425. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipkin ID, Kindt RM, Kenyon CJ. Role of new Rho family member in cell migration and axon guidance in C. elegans. . Cell. 1997;90:883–894. doi: 10.1016/s0092-8674(00)80353-0. [DOI] [PubMed] [Google Scholar]