Abstract
Hemidesmosomes (HDs) are stable anchoring structures that mediate the link between the intermediate filament cytoskeleton and the cell substratum. We investigated the contribution of various segments of the β4 integrin cytoplasmic domain in the formation of HDs in transient transfection studies using immortalized keratinocytes derived from an epidermolysis bullosa patient deficient in β4 expression. We found that the expression of wild-type β4 restored the ability of the β4-deficient cells to form HDs and that distinct domains in the NH2- and COOH-terminal regions of the β4 cytoplasmic domain are required for the localization of HD1/plectin and the bullous pemphigoid antigens 180 (BP180) and 230 (BP230) in these HDs. The tyrosine activation motif located in the connecting segment (CS) of the β4 cytoplasmic domain was dispensable for HD formation, although it may be involved in the efficient localization of BP180. Using the yeast two-hybrid system, we could demonstrate a direct interaction between β4 and BP180 which involves sequences within the COOH-terminal part of the CS and the third fibronectin type III (FNIII) repeat. Immunoprecipitation studies using COS-7 cells transfected with cDNAs for α6 and β4 and a mutant BP180 which lacks the collagenous extracellular domain confirmed the interaction of β4 with BP180. Nevertheless, β4 mutants which contained the BP180-binding region, but lacked sequences required for the localization of HD1/plectin, failed to localize BP180 in HDs. Additional yeast two- hybrid assays indicated that the 85 COOH-terminal residues of β4 can interact with the first NH2-terminal pair of FNIII repeats and the CS, suggesting that the cytoplasmic domain of β4 is folded back upon itself. Unfolding of the cytoplasmic domain may be part of a mechanism by which the interaction of β4 with other hemidesmosomal components, e.g., BP180, is regulated.
Keywords: hemidesmosome assembly, PA-JEB keratinocytes, protein–protein interaction, bullous pemphigoid antigens, α6β4 integrin
Hemidesmosomes (HDs)1 are specialized multiprotein complexes that mediate the adhesion of basal epithelial cells to the basement membrane in stratified and certain complex epithelia, and provide a link between the keratin intermediate filaments (IFs) and the extracellular matrix (Staehelin, 1974; Burgeson and Christiano, 1997). One of the transmembrane components in these complexes is the α6β4 integrin which is thought to play a key role in promoting the formation of HDs and in cell adhesion (for review see Borradori and Sonnenberg, 1996; Green and Jones, 1996). Support for this supposition is derived from several observations. Mutations in the genes for the β4 or the α6 integrin subunits cause junctional epidermolysis bullosa associated with pyloric atresia (PA-JEB), an inherited skin blistering disorder characterized by defective dermo-epidermal adhesion and the formation of only rudimentary HDs (Vidal et al., 1995; Brown et al., 1996; Niessen et al., 1996; Pulkkinen et al., 1997; Ruzzi et al., 1997). Similarly, targeted disruption of the genes for α6 or β4 results in widespread subepidermal blistering in neonatal mice, which are unable to form HDs (Dowling et al., 1996; Georges-Labouesse et al., 1996; Van der Neut et al., 1996).
α6β4 is a receptor for various laminin isoforms, including laminin-5 (Niessen et al., 1994; Rousselle and Aumailley, 1994), a major component of the epidermal basement membrane (Carter et al., 1991; Rousselle et al., 1991). α6β4-mediated adhesion and signaling are likely to be regulated by the cytoplasmic domain of β4 (Spinardi et al., 1993, 1995; Giancotti, 1996). This domain consists of ∼1,000 amino acid residues and contains two pairs of type III fibronectin (FNIII) repeats which are separated by a connecting segment (CS) (Hogervorst et al., 1990; Suzuki and Naitoh, 1990; Tamura et al., 1990). Recent studies have identified sequences within the second FNIII repeat and the CS that appear to be critical for the localization of α6β4 in HDs (Spinardi et al., 1993; Mainiero et al., 1995; Niessen et al., 1997a ). Furthermore, the β4 cytoplasmic domain appears to form a complex with the hemidesmosomal plaque component HD1/plectin and to regulate the subcellular localization of HD1/plectin (Niessen et al., 1997b ) and of the bullous pemphigoid antigen 180 (BP180) (Borradori et al., 1997). BP180, a collagenous protein, is the other known transmembrane hemidesmosomal constituent that is also likely to function as a cell-matrix receptor (Giudice et al., 1992; Li et al., 1993; Jonkman et al., 1995; McGrath et al., 1995). BP180 has been suggested to interact with the α6 integrin subunit, and this interaction may contribute to the stabilization of HDs (Hopkinson et al., 1995).
The cytoplasmic hemidesmosomal proteins include, in addition to HD1/plectin (Wiche et al., 1991; Hieda et al., 1992), the bullous pemphigoid antigen 230 (BP230) (Stanley et al., 1988; Sawamura et al., 1991), IFAP300 (Yang et al., 1985; Skalli et al., 1994), and the P200 protein (Kurpakus and Jones, 1991). IFAP300 and HD1/plectin appear to be related and may even be identical (Herrmann and Wiche, 1987; Baker et al., 1997). HD1/plectin and BP230 mediate the attachment of keratin IFs to the basal plasma membrane, since in patients with epidermolysis bullosa simplex with muscular dystrophy, who lack HD1/plectin, and in null mutant mice lacking either HD1/plectin or BP230 the attachment of IFs to HDs is strongly reduced (Guo et al., 1995; Gache et al., 1996; McLean et al., 1996; Smith et al., 1996; Andrä et al., 1997).
Recent cell transfection studies have defined regions in the β4 cytoplasmic domain that are important for the localization of α6β4 within HDs, based on the ability of β4 mutants to become incorporated into HDs or to disrupt them (Spinardi et al., 1993; Mainiero et al., 1995; Niessen et al., 1997a ). However, the value of these studies is limited because the cells used express β4 endogenously and form HDs, so that mutants could associate with preexisting HDs. Thus, it was not demonstrated whether the mutants are able to initiate HD formation. The availability of human β4-deficient keratinocytes thus provides a unique opportunity to investigate the role β4 and of specific domains in β4 in the formation of HDs and the recruitment of other hemidesmosomal components.
In this study, we have used immortalized keratinocytes derived from a patient with PA-JEB, who completely lacked expression of the β4 integrin subunit (Niessen et al., 1996). We report experiments aimed at defining: (a) the ability of these immortalized PA-JEB keratinocytes to assemble HD-like structures; (b) the feasibility to reverse their phenotype by reexpressing wild-type β4; (c) the potential of β4 mutants, lacking distinct regions of the β4 cytoplasmic domain or carrying mutations in the tyrosine activation motif (TAM), to induce the formation of HD-like structures by recruiting the hemidesmosomal components HD1/plectin, BP180, and BP230 to sites of cell–substrate contact; and (d) the interaction between the cytoplasmic domain of β4 and BP180.
Materials and Methods
Generation of Immortalized Cell Lines
HPV 16 immortalized normal human foreskin keratinocytes (NHK) have been described previously (Steenbergen et al., 1996). NHK morphologically resemble the parental cells, but they are slightly larger and flatter (Steenbergen et al., 1996). Also, the cells are less stratified even when they reach confluence.
Primary keratinocytes obtained from a patient with PA-JEB who completely lacked expression of the β4 integrin subunit (Niessen et al., 1996) were immortalized by transfection with full-length HPV 16 DNA (p1432; Münger et al., 1989). This resulted in the generation of a clonal culture that was expanded for further characterization. The cells were relatively large with a polygonal shape and morphologically resembled the parental cells. They grew with a doubling time of ∼36 h and showed normal stratification and differentiation as detected by electron microscopy upon culture postconfluent in HAMF12/DME (1:3) medium (data not shown). Ultrastructural analysis of the PA-JEB keratinocytes showed that in the absence of β4 only a few rudimentary HD-like structures are formed in some cells, as in PA-JEB patients (Vidal et al., 1995; Niessen et al., 1996; data not shown).
Cells and Antibodies
The two keratinocyte cell lines were grown in keratinocyte serum-free medium (SFM) (GIBCO-BRL, Paisley, UK) supplemented with 50 μg/ml bovine pituitary extract, 5 ng/ml epidermal growth factor, 100 U/ml penicillin, and 100 U/ml streptomycin. Alternatively, the cells were cultured in HAMF12/DME (1:3) medium containing 10% (vol/vol) FCS, 100 U/ml penicillin, 100 U/ml streptomycin, l-glutamine, 0.4 μg/ml hydrocortisone (Sigma Chemical Co., St. Louis, MO) and 1 μM isoproterenol (Sigma Chemical Co.). The African monkey kidney cell line COS-7 was cultured in DME (GIBCO-BRL) supplemented with 10% (vol/vol) FCS, 100 U/ml penicillin, and 100 U/ml streptomycin. The cells were grown at 37°C in a humidified, 5% CO2 atmosphere.
The following antibodies against human integrin subunits were used: the mouse mAbs P1E6 and P1H5, anti-α2 (Wayner and Carter, 1987); the mAb J143, anti-α3 (Kantor et al., 1987); the mAb Sam-1, anti-α5 (Keizer et al., 1987) and the NKI-M9, anti-αv (Von dem Borne et al., 1989) were obtained from C.G. Figdor (University of Nijmegen, Nijmegen, The Netherlands); the mouse mAb J8H, the rat mAb GoH3 and a rabbit polyclonal antiserum, anti-α6 have been described (Sonnenberg et al., 1987; Hogervorst et al., 1993; Delwel et al., 1994); the mouse mAb 113C, anti-β4, was prepared by A.M. Martínez de Velasco in our laboratory (unpublished results); the mouse mAb 4.3E1 against β4 (Hessle et al., 1984) was provided by E. Engvall (The Burnham Institute, La Jolla, CA); the mouse mAbs 450-10D and 450-9D, and the rat mAb 439-9B, anti-β4 (Kennel et al., 1989, 1990), were kindly provided by S.J. Kennel (Oak Ridge National Laboratory, Oak Ridge, TN); a rabbit antiserum (67p120) to recombinant human β4 cytoplasmic domain was prepared as previously described (Niessen et al., 1994); the rat mAb AIIB2, anti-β1 (Werb et al., 1989), was a gift from C.H. Damsky (University of California, San Francisco, CA); an the mAb TS2/16 against β1 was obtained from the American Type Culture Collection (Rockville, MD). A rabbit antiserum to rat IgG has been described previously (Sonnenberg et al., 1986). The mouse mAb VIIF9 against vinculin (Glukhova et al., 1990) was a generous gift from M.A. Glukhova (École Normale Supérieure, Paris, France). A rabbit antiserum directed against the COOH-terminal domain of BP230 (Tanaka et al., 1990) was kindly provided by J.R. Stanley (University of Pennsylvania, Philadelphia, PA). The mouse mAbs 1D1 and 233 against the intra- and extracellular portion of BP180, respectively (Nishizawa et al., 1993), and the mAb 121 directed against HD1 (Hieda et al., 1992) were kindly donated by K. Owaribe (Nagoya University, Nagoya, Japan). A rabbit antiserum against the cytoplasmic domain of BP180 was generously provided by L. Bruckner-Tuderman (University of Münster, Münster, Germany). The mouse mAb anti-FLAG™ M2 against the FLAG™ peptide (DYKDDDDK) was purchased (IBI, Eastman Kodak Co., New Haven, CT). Species-specific FITC-conjugated goat anti–mouse IgG (Zymed Laboratories, San Franscisco, CA), Texas red-conjugated goat anti–rat IgG (Rockland, Gilbertsville, PA), and Texas red-conjugated donkey anti–rabbit IgG (Amersham Int., Buckinghamshire, UK) were purchased, as were species-specific horseradish peroxidase-conjugated antibodies (Amersham Int.).
cDNA Constructs
The full-length β4A and β4B cDNA constructs, and the cDNA constructs encoding β4 with COOH-terminal truncations or internal deletions of the cytoplasmic domain have been described previously (Niessen et al., 1997a ,b). The cDNA plasmid encoding β4A with combined phenylalanine substitutions of the tyrosine activation motif (Mainiero et al., 1995) was kindly provided by F.G. Giancotti (New York University School of Medicine, New York). The construct was assembled into pcDNA3 (Stratagene, La Jolla, CA). The pRc-CMV expression construct encoding full-length α6A cDNA, as well as the pCI-Neo construct encoding the cytoplasmic domain of BP180 (clone B, BP180Δ521–1497) have been described previously (Borradori et al., 1997). Correctness of all constructs was verified by sequencing. The molecular weights of the different expressed β4 proteins correspond to that predicted based on the DNA sequences, as assessed by Western blot analysis of transiently transfected COS-7 cells (Niessen et al., 1997a ; data not shown).
DNA Transfections
For transfection, the keratinocytes were first grown in keratinocyte-SFM medium to 40–60% confluency in six-well tissue culture plates (Falcon; Becton Dickinson, Lincoln Park, NJ). The cells were transfected using the cationic lipid Lipofectin® (GIBCO-BRL). The DNA/Lipofectin® mixture was prepared using serum-free medium (OPTI-MEM®, GIBCO-BRL). The final concentration of plasmid DNA and Lipofectin® in serum-free transfection medium was 2.5 μg/ml and 10 μg/ml, respectively. 1 ml of transfection medium was added to each monolayer that had been previously washed with serum-free medium and cells were incubated with the transfection medium for 9–10 h at 37°C with 5% CO2. The transfection medium was then replaced with keratinocyte-SFM medium for 12 h and subsequently with HAMF12/DME (1:3) medium for an additional 24 h, after which gene expression was assessed.
COS-7 cells (1.2 × 106 cells/60 cm2) were transiently transfected using the DEAE-dextran method (Cullen, 1987) with 2 μg of DNA per construct and assayed for gene expression after 48 h.
Immunofluorescence Microscopy
Cells grown on glass coverslips in six-well tissue culture plates in HAMF12/DME (1:3) medium for 24 h were fixed with 1% formaldehyde in PBS for 10 min and permeabilized with 0.5% Triton X-100 for 5 min at room temperature. After rinsing with PBS and blocking with 2% (wt/vol) BSA in PBS for 30 min at 37°C, the cells were incubated with primary antibody for 30 min at 37°C and then washed three times with PBS. The cells were subsequently incubated with FITC-labeled anti-mouse IgG, Texas red-labeled anti-rabbit IgG, Texas red-labeled anti-rat IgG, or rabbit anti– rat IgG followed by Texas red-labeled donkey anti–rabbit IgG for 30 min at 37°C, respectively. For double labeling studies, cells were incubated with the respective antibodies as described previously (Niessen et al., 1997a ). The coverslips were subsequently washed, mounted in Vectashield (Vector Labs, Inc., Burlingame, CA), and then viewed under a Bio-Rad MRC-600 confocal scanning laser microscope (Richmond, CA).
Immunoprecipitation Studies and Immunoblotting
Keratinocytes cultured in keratinocyte-SFM medium were detached using 20 mM EDTA in PBS and washed three times with PBS. Cells were surface-labeled with 125I (Amersham Int.) by the lactoperoxidase/hydrogen peroxide method (Sonnenberg et al., 1987; Niessen et al., 1996). Thereafter, the cells were washed three times with PBS and lysed on ice with NP-40 lysis buffer (1% Nonidet P-40, 25mM Tris-HCl, pH 7.5, 4 mM EDTA, 100 mM NaCl, 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml soybean trypsin inhibitor). The lysates were then used for immunoprecipitation, as described previously (Sonnenberg et al., 1993; Niessen et al., 1996). Immune complexes were released from the beads by boiling for 5 min in nonreducing SDS sample buffer and resolved on a 5% SDS-PAGE gel.
Alternatively, keratinocytes were washed twice with PBS and incubated with DME without methionine and cysteine (ICN Biomedicals Inc., Costa Mesa, CA) for 1 h at 37°C. Cells were then labeled with 100 μCi/ml [35S]methionine/cysteine (Amersham Int.) for 4 h, washed, and then lysed with NP-40 lysis buffer and used for immunoprecipitation analysis as described above.
Transfected COS-7 cells were washed twice with PBS and scraped in 1 ml CHAPS lysis buffer (1% CHAPS, 25 mM Hepes, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM PMSF, 10 μg/ml leupeptin and 10 μg/ml soybean trypsin inhibitor). The lysates were clarified by centrifugation and incubated with antibodies previously bound to GammaBind plus Sepharose CL4B beads (Pharmacia LKB Biotech., Uppsala, Sweden). Immune complexes were washed three times with lysis buffer and two times with PBS. Immunoprecipitates were released from the beads by boiling for 5 min in nonreducing SDS sample buffer, resolved on an 8% SDS-PAGE gel, and blotted to polyvinylene difluoride membranes (Immobilon-P; Millipore Corp., Waters Chromatography, Bedford, MA). The immunoblots were blocked for 1 h in TBSTB (10 mM Tris, pH 8.0, 150 mM NaCl, 0.05% Tween-20, 2% [wt/vol] baby milk powder) and probed with primary antibodies in TBSTB for 1 h at room temperature. After extensive washing in TBSTB (with only 0.2% [wt/vol] baby milk powder), blots were incubated for 1 h with secondary horseradish peroxidase-conjugated antibodies diluted 1:5,000 in TBSTB. The blots were then washed again and developed using enhanced chemiluminescence (Amersham Int.).
Yeast Two-hybrid Assay
All yeast galactose metabolism regulatory gene 4 (GAL4) expression plasmids containing parts of the β4 or BP180 cytoplasmic domains that were used for the yeast two-hybrid assay are listed in Figs. 10 and 12. Numbers in superscript correspond to the β4 amino acid residues (numbered according to Niessen et al., 1997a ) that are encoded within the GAL4 activation domain (AD) or binding domain (BD) fusion proteins. The sequences encoding β4 were amplified by PCR from the full-length β4A and β4B cDNA constructs used for the transfection studies described above, using β4-specific sense and antisense primers containing restriction site tags. PCR products were cut with the appropriate restriction enzymes, correctly sized DNA fragments were isolated from agarose gels using the Easy-Pure™ kit (Biozym, Landgraaf, The Netherlands), and ligated into the yeast GAL4(AD) expression vector pACT2 (Harper, 1993; Clontech, Palo Alto, CA) cut with conforming restriction enzymes. This resulted in the in-frame fusion of each β4 coding sequence to the 3′ end of the GAL4 (768–881) transcriptional AD. For the experiments described in Fig. 12, several β4 sequences were recloned into the yeast GAL4 (BD) expression vector pAS2-1 (Durfee et al., 1993; Clontech) using restriction sites in the polylinkers of the vectors. This resulted in the in-frame fusion of β4- encoding sequences to the 3′ end of the GAL4 (1–147) DNA-BD.
Figure 10.
Survey of the sites of interaction between the cytoplasmic domains of β4 and BP180. Yeast strain PJ69-4A was cotransformed with pAS2-BP180 and one of each of the listed pACT2-β4 constructs, or with an empty pACT2. Transformation mixtures were spread on SC-LT and SC-LTHA plates and grown for 9 d at 30°C. Plating efficiency on SC-LTHA plates is expressed relative to that on SC-LT plates of the same transformation. ++, \>50%; ±, 5–25%; and −, 0% indicate relative efficiencies, respectively. Plates were scored after 4 and 9 d of growth. Plating efficiencies above 25% represent fast-growing colonies that could be scored after 4 d; plating efficiencies lower than 25% represent colonies that clearly grow more slowly and could only be scored after 9 d of growth. All efficiencies listed represent an average of multiple independent transformation experiments on at least three separate days. Cotransformation efficiencies (on SC-LT plates) for all plasmid combinations listed were always at least 104 cfu/μg, and the difference between the various β4 plasmids tested never was greater than twofold. Cotransformation of yeast PJ69-4A with empty pAS2-1 and pACT2 vectors never resulted in the growth of colonies on SC-LTHA plates, nor did cotransformation of the yeast strain with either the pAS2-BP180 plasmid and an empty pACT2 vector or any of the pACT2-β4 plasmids and an empty pAS2-1 vector, showing that none of the GAL4 fusion proteins encoded by these recombinant plasmids by themselves could cause activation of the His and Ade reporter genes.
Figure 12.
Intramolecular interactions in the β4 cytoplasmic domain. Yeast strain PJ69-4A was cotransformed with two different β4 constructs as listed. Other details are as in Fig. 10, except that the efficiencies represent an average of multiple independent transformation experiments on at least two separate days.
A cDNA clone containing nucleotides 1–1,398 encoding the entire cytoplasmic domain, i.e., the first 466 amino acid residues, of human BP180 (Hopkinson et al., 1992) was isolated from a λgt11 human keratinocyte library by PCR using BP180-specific sense and antisense primers containing restriction site tags, purified and cut with the appropriate restriction enzymes as described above, and then cloned into pAS2-1. This BP180 construct, pAS2-BP180(0), caused autonomous activation of the reporter genes in the yeast host strain, presumably because of the presence of multiple Gly and Cys residues in the BP180 protein sequence immediately preceding the putative transmembrane region. A subclone containing only the first 1,201 nucleotides of the BP180 sequence and lacking the nucleotides encoding the Gly and Cys repeats was isolated from pAS2-BP180(0) using the StuI site (at position 1,201 in the BP180 sequence) and recloned into pAS2-1, resulting in pAS2-BP180(C) which encodes the first 400 amino acids of the BP180 protein. This construct did not cause autonomous transactivation of the reporter genes in the yeast host strain. The BP180 and β4 coding sequences within the yeast expression constructs were confirmed by sequence analysis using the T7Sequencing kit (Pharmacia Biotech.).
Yeast strain PJ69-4A (gift of P. James, University of Wisconsin, Madison, WI), which contains the genetic markers trp1-901, leu2-3, his3-200, gal4Δ, gal80Δ, LYS2::GAL1-HIS3, and GAL2-ADE2 (James et al., 1996), was used as the host strain for the two-hybrid assay. It contains two tightly regulated and selectable GAL4-driven reporter genes, His and Ade, and is therefore suited for sensitive detection of protein interactions. Strain PJ69-4A was grown and transformed with plasmid DNA essentially as described (Gietz et al., 1995; James et al., 1996). PJ69-4A yeast cells were cotransformed with a pACT2 (-derived) plasmid as well as a pAS2-1 (-derived) plasmid, and aliquots of the same transformation were spread on plates containing SC-LT medium, yeast synthetic complete medium (SC) lacking only the vector markers Leu (for pACT2 and derivatives) and Trp (for pAS2-1 and derivatives), and on plates containing SC-LTHA medium, lacking Leu and Trp as well as the interaction markers His and Ade. Plates were scored after 4 and 9 d of growth, and the number of colonies on the SC-LT plate compared with that on the SC-LTHA plate. As a positive control for GAL4-driven activation of the Ade and His reporter genes of PJ69-4A, two combinations of vectors were used that enable growth on SC-LTHA plates. One combination was pCL1, full-length GAL4 (which is able to activate the two reporter genes on its own) in a pACT2-like vector (Fields and Song, 1989; Clontech) together with the empty pAS2-1 vector. In the other combination, two vectors, pTD1-1, SV-40 large T antigen in pACT2 (Li and Fields, 1993; Clontech), together with pVA3-1, a p53 subclone in pAS2-1 (Iwabuchi et al., 1993; Clontech), were used, that express proteins that are known to interact and thereby cause expression of the reporter genes.
Results
Altered Distribution of Hemidesmosomal Components in Immortalized PA-JEB Keratinocytes
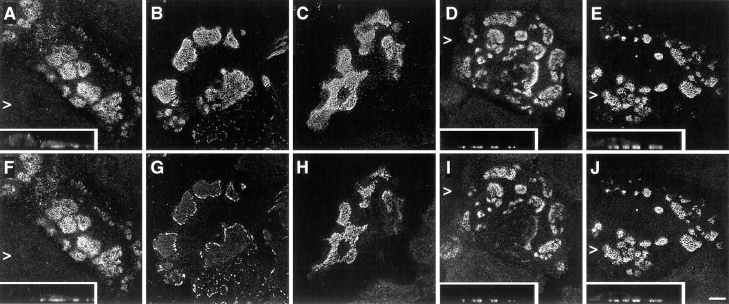
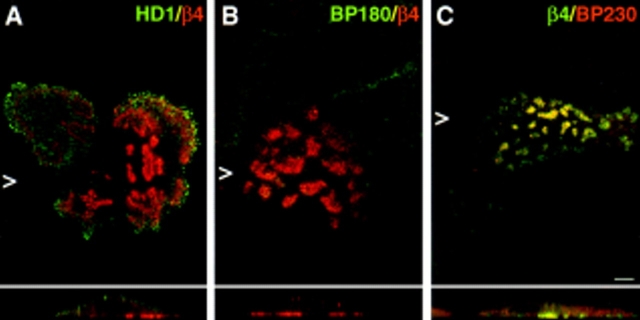
We investigated the distribution of hemidesmosomal proteins in preconfluent monolayers of the immortalized NHK and PA-JEB cell lines by confocal immunofluorescence microscopy. In NHK, the α6β4 integrin and HD1/ plectin were concentrated at cell–substrate contact sites in structures appearing as dots and large patches (Fig. 1, A, B, and E). This staining pattern is characteristic for HD-like structures (Marchisio et al., 1991, 1993) or stable anchoring contacts (Carter et al., 1990) of cultured keratinocytes. In addition, the localization pattern of BP230 and BP180 was similar to that of α6β4 and HD1/plectin, although the staining was less extensive in most cells (Fig. 1, C and D). In the PA-JEB cells, no β4 staining was found using mAbs directed against either the extra- or the intracellular domain of β4 (Fig. 1 G; data not shown). α6 (Fig. 1 F) was codistributed with vinculin in short linear arrays at the basal cell surface at the ends of actin stress fibers (data not shown), consistent with its localization in focal contacts in the parental PA-JEB cells (Niessen et al., 1996). In only a few cells (less than 1%) BP230 and BP180 were concentrated in HD-like structures at the basal cell surface (Fig. 1, H and I). However, whereas HD1/plectin was found in some HD-like structures in the primary PA-JEB keratinocytes (Niessen et al., 1996), it was completely absent from such structures in the immortalized cells (Fig. 1 J). These findings show that the formation of HD-like structures is impaired in the immortalized PA-JEB keratinocytes.
Figure 1.
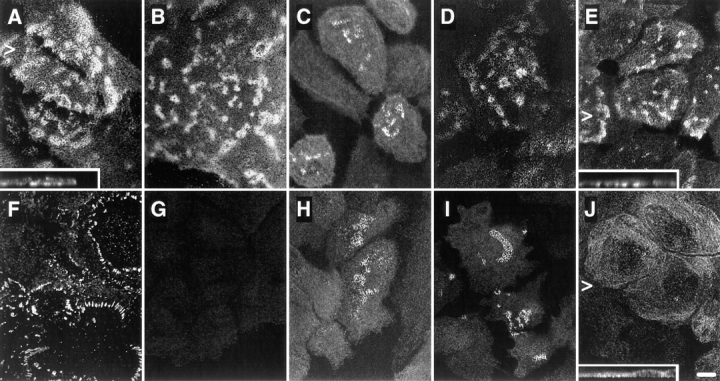
Immunolocalization of hemidesmosomal components in the NHK (A–E) and PA-JEB cell lines (F–J) by confocal laser microscopy. Cells were grown on glass coverslips in HAMF12/DME (1:3) medium, fixed, and immunolabeled using the rat mAb GoH3 directed against α6 (A and F), the mAb 450-9D against β4 (B and G), a rabbit anti-BP230 antiserum (C and H), the mAb 233 against BP180 (D and I), and the mAb 121 against HD1 (E and J). In NHK, the hemidesmosomal components are concentrated at sites of cell– substrate contact in patches characteristic for HD-like structures. In PA-JEB keratinocytes, only BP230 (H) and BP180 (I) are found concentrated in the rare HD-like structures in fewer than 1% of the cells. α6 is colocalized with vinculin (data not shown) in dots and streaks representing focal adhesions (F), whereas HD1/plectin is found diffusely distributed throughout the cell (J). No β4 reactivity is observed in PA-JEB cells (G). Sections were focused at the cell–substrate interface. Arrowheads, positions from which the perpendicular sections, shown in the insets, were taken. Bar, 10 μm.
Coimmunoprecipitation of α6 with β1 in PA-JEB Keratinocytes
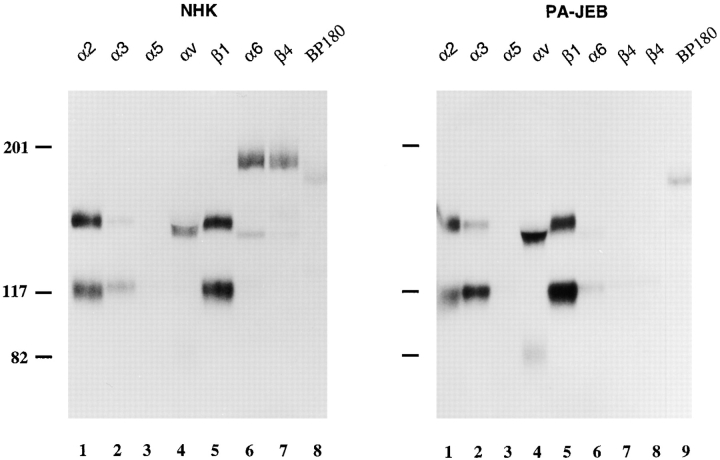
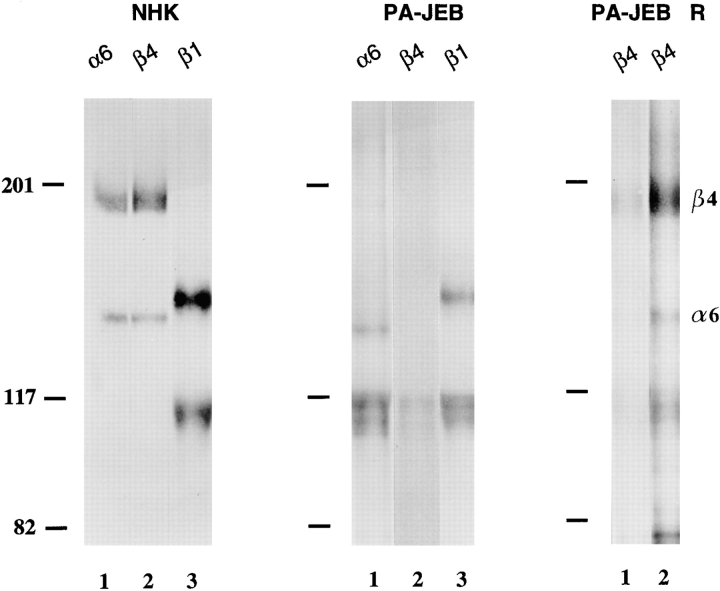
The expression profile of integrins was determined by immunoprecipitation of 125I surface-labeled PA-JEB keratinocytes and NHK (Fig. 2). The mAb against α6 precipitated this subunit associated with β4 from NHK (Fig. 2, left), whereas α6 and β1, but not β4, were precipitated from the PA-JEB keratinocytes (Fig. 2, right). β1 was coprecipitated together with α2, α3, α5, and α6 from the PA-JEB keratinocytes, but only with α2, α3, and α5 from the NHK. These results demonstrate that although NHK express α6β4, PA-JEB keratinocytes express α6β1 on their surface. In addition, no β4 was detected by flow cytometry on the surface of PA-JEB keratinocytes, whereas the expression level of α6 was substantially decreased as compared to that on NHK (data not shown). The αv subunit was precipitated from both cell lines (Fig. 2) and was associated with both β3 and β5 subunits (data not shown).
Figure 2.
Immunoprecipitation of integrin complexes and BP180 from NHK and PA-JEB keratinocytes. Lysates of 125I-labeled NHK (left) and PA-JEB keratinocytes (right) were immunoprecipitated with the mAbs P1E6 (against α2, lane 1), J143 (α3, lane 2), Sam-1 (α5, lane 3), NKI-M9 (αv, lane 4), J8H (α6, lane 5), TS2/16 (β1, lane 6), 450-9D (β4, lane 7), 439-9B (β4, lane 8, right) and 1D1 (BP180, lane 8, left and lane 9, right, respectively). The antibody against α6 precipitated this subunit associated with β4 from NHK, whereas from the PA-JEB cells α6 and β1 were precipitated, but not β4. The faint band which migrates just above β1 and seen in the lanes containing the anti-β4 immunoprecipitates from PA-JEB cells, represents a nonspecific product. β1 is found in association with α2, α3, α5, and α6 in PA-JEB cells, but only with α2, α3, and α5 in NHK. Precipitation of β1 with α5 is evident after prolonged exposure (data not shown). Samples were analyzed on a SDS-polyacrylamide (5%) gel under nonreducing conditions. The positions of molecular weight standards (in kD) are indicated on the left.
Reexpressed β4 in PA-JEB Keratinocytes Is Associated with α6 and Induces the Formation of HD-like Structures
To restore expression of β4, the PA-JEB keratinocytes were transiently transfected with cDNA encoding wild-type β4 (Fig. 3 A). Immunoprecipitation analysis of lysates of radiolabeled transfected PA-JEB keratinocytes is shown in Fig. 4. A mAb against β4 precipitated β4 and α6 from transfected PA-JEB keratinocytes (Fig. 4, right), but not from untransfected cells (Fig. 4, middle). Thus, β4 is expressed and forms a heterodimer with endogenous α6 in the transfected cells, as in NHK (Fig. 4, left).
Figure 3.
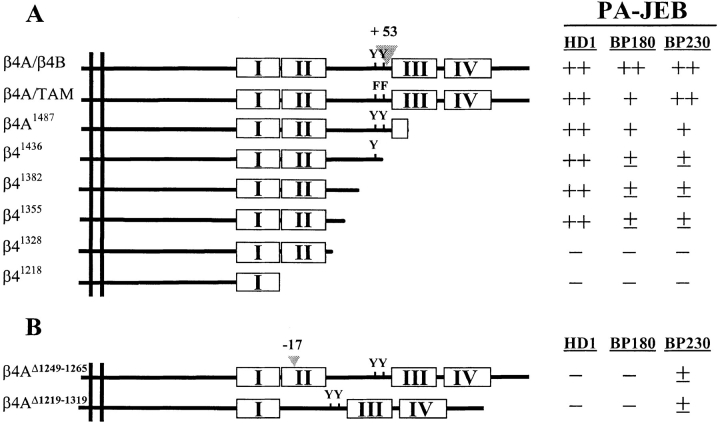
Expression of full-length, TAM-mutated, COOH-terminally truncated (A), and internal deletion mutant (B) β4 cDNAs in PA-JEB keratinocytes. Schematic representation of the cDNA constructs encoding wild-type and mutant forms of β4 carrying deletions of the cytoplasmic domain. Boxes, FNIII repeats in which the number of the repeat is shown; triangle, the β4B-specific insert of 53 amino acids in the CS (A), or the 17-amino acid deletion in the second FNIII repeat as described for a PA-JEB patient (Vidal et al., 1995) (B). Y > F mutations of the TAM are represented by F. The immunolocalizations of the hemidesmosomal components HD1/plectin, BP180, and BP230 were investigated upon transfection of the β4 constructs in PA-JEB cells. Colocalization of HD1/plectin, BP180, and BP230, respectively, with α6β4 at the basal cell surface in HD-like structures is observed in ++, 75–100%; +, 25–75%; ±, 1–25%; −, 0%, of the β4-transfected cells.
Figure 4.
Immunoprecipitation analysis of PA-JEB keratinocytes transfected with cDNA encoding wild-type β4 (PA-JEB R). Lysates of [35S]methionine/cysteine-labeled NHK and PA-JEB cells were immunoprecipitated with the mAb J8H against α6 (lane 1), the anti-β4 mAb 450-9D (lane 2), and the mAb TS2/16 against β1 (lane 3). Antibodies against α6 precipitate α6 and β4 from NHK, and α6 together with β1 from PA-JEB cells. Antibodies against β4 precipitate this subunit together with α6 from NHK, but not from PA-JEB cells. In contrast, from PA-JEB cells transfected with cDNA for β4 (PA-JEB R) antibodies against β4 (lane 1) precipitate β4 together with α6. Coprecipitation of α6 is evident after prolonged exposure (lane 2). Samples were analyzed on a SDS-polyacrylamide (5%) gel under nonreducing conditions. The positions of molecular weight standards (in kD) are indicated on the left and those of the β4 and α6 integrin subunits are indicated on the right.
The subcellular distribution of the newly synthesized β4 in transfected PA-JEB keratinocytes was assessed by confocal immunofluorescence microscopy. In transfected cells, β4 (i.e., β4A or β4B with a 53 amino acid insertion in the CS) was concentrated in HD-like structures at the basal side of the cell (Fig. 5, A–E), where it is colocalized with α6, BP180 and BP230 (Fig. 5, F, I, and J). In addition, HD1/plectin was no longer diffusely distributed in the cytoplasm, but colocalized with α6β4 at cell–substrate contact sites (Fig. 5, C and H). Staining for vinculin revealed the presence of focal contacts organized at the periphery of the HD-like clusters (Fig. 5, B and G). We conclude that expression of β4 restores the capacity of PA-JEB keratinocytes to form HD-like structures.
Figure 5.
Expression of wild-type β4 in PA-JEB keratinocytes induces the formation of HD-like structures. PA-JEB cells were transfected with cDNA encoding β4A. After 36 h, cells were fixed, permeabilized, and subjected to double labeling immunofluorescence for β4 (A–E) and α6 (F), vinculin (G), HD1/plectin (H), BP180 (I), and BP230 (J). Upon transfection, expression of β4 results in the formation of HD-like structures, in which β4 is concentrated at sites of cell–substrate contact and codistributed with α6, HD1/plectin, BP180, and BP230 (insets are the perpendicular sections). In cells expressing β4, α6 is now found in HD-like structures (F), and no longer concentrated in focal adhesions at the outer periphery of these structures, as shown by staining for vinculin (G). Bar, 10 μm.
The β4 TAM Is Not Essential for the Formation of HD-like Structures
It has been suggested that phosphorylation of the TAM, which consists of two closely spaced tyrosine residues located at position 1,422 and 1,440 within the CS of the β4 cytoplasmic domain, is critical for the incorporation of α6β4 in HDs and HD assembly (Mainiero et al., 1995). Therefore, we investigated whether expression of β4 with a mutated TAM affected the formation of HD-like structures. β4 with phenylalanine substitutions in the TAM was concentrated at the basal side of the cells (Fig. 6, A, C, E, and G) together with α6, HD1/plectin, BP180, and BP230 (Fig. 6, B, D, F, and H, respectively) in a pattern indistinguishable from that seen upon transfection with wild-type β4 cDNA (Fig. 5). However, compared to wild-type β4, TAM-mutated β4A appeared to have a reduced ability to induce redistribution of BP180 to the basal side of the cell, because in ∼30% of the transfected cells the distribution of BP180 remained diffuse throughout the cell. Thus, although the β4A TAM may influence the association of BP180 with HDs, it is largely dispensable for the assembly of these structures.
Figure 6.
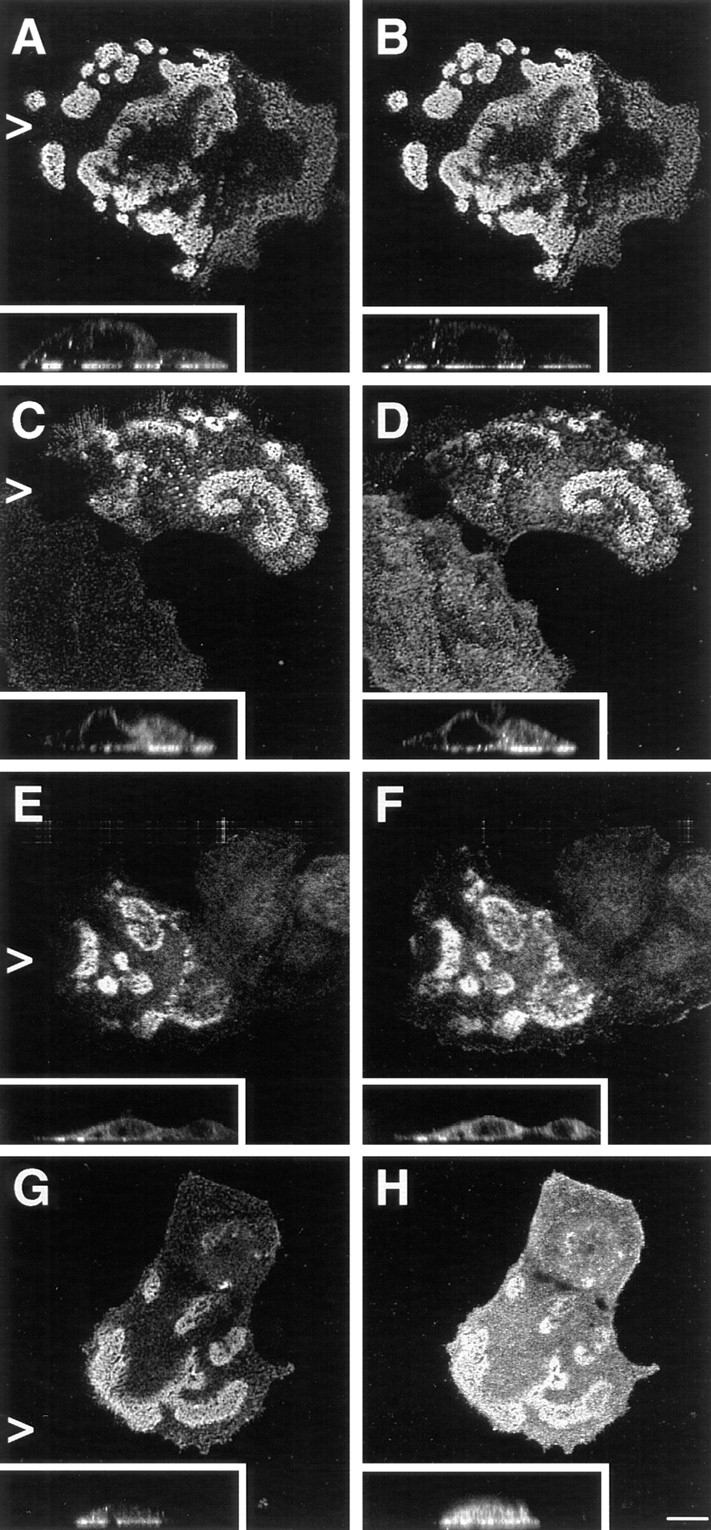
Expression of a β4 TAM mutant induces the assembly of HD-like structures in transfected PA-JEB keratinocytes. PA-JEB cells transfected with TAM-mutated β4A cDNA were double stained for β4 (A, C, E, and G) and α6 (B), HD1/plectin (D), BP180 (F), and BP230 (H). As shown in the perpendicular sections, a β4 molecule with phenylalanine substitutions at the TAM becomes localized together with α6 at the basal cell side and recruits HD1/plectin, BP180, and BP230 to sites of cell–substrate contact. The redistribution of BP180 was, however, slightly impaired. Bar, 10 μm.
Identification of Sequences within the β4 Cytoplasmic Domain Involved in the Recruitment of HD1/Plectin, BP180, and BP230 to HD-like Structures
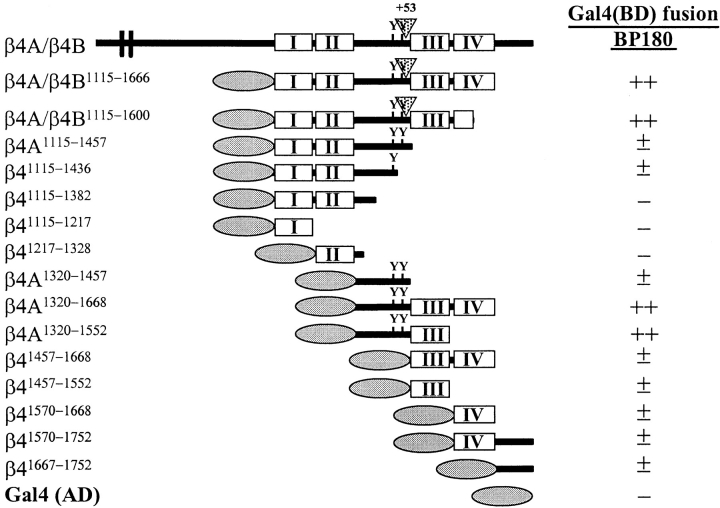
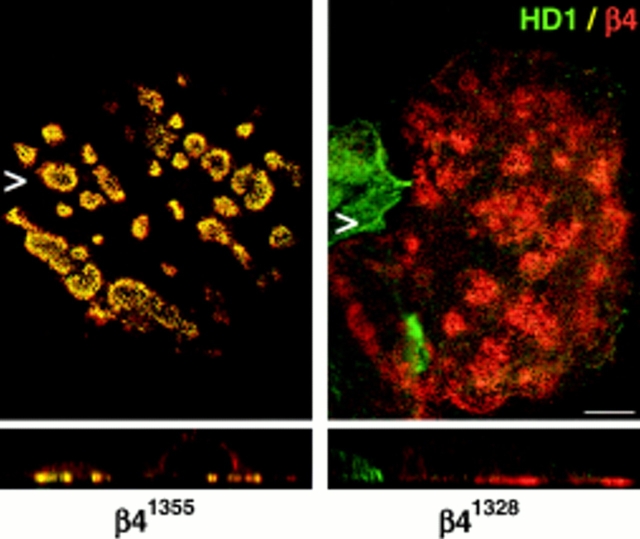
Previous studies have shown that the sequences within the β4 cytoplasmic domain that are responsible for inducing the redistribution of HD1/plectin in COS-7 cells and mouse embryonic fibroblasts are also sufficient to incorporate β4 mutants into existing HDs in 804G rat bladder carcinoma cells (Niessen et al., 1997a ; Sánchez-Aparicio et al., 1997). To identify the specific regions required for HD formation, we assessed the distribution of HD1/plectin, BP180, and BP230 in PA-JEB cells transfected with mutant β4 cDNAs. We found that the NH2-terminal, but not the COOH-terminal half of the β4 cytoplasmic domain, is involved in the recruitment of HD1/plectin to areas of cell–substrate contact in PA-JEB cells (refer to Fig. 3 A). Transfection of PA-JEB cells with the various cDNAs encoding COOH-terminal deletion mutants of β4 showed that the segment comprising the first pair of FNIII repeats and a stretch of 27 amino acids in the CS contains sequences that critically affect the distribution of HD1/plectin (Fig. 3 A and Fig. 7).
Figure 7.
A segment comprising the first pair of FNIII repeats and a 27-amino acid stretch of the CS is essential for the localization of HD1/plectin at the basal cell surface. Representatives of double immunofluoresence analyses of PA-JEB cells transfected with cDNA encoding COOH-terminal deletion mutants of β4 as depicted in Fig. 3 are shown. PA-JEB cells transfected with cDNA coding for β41,355 or β41,328 were immunolabeled with antibodies against β4 (red) and HD1 (green). Although β41,355 still induces the redistribution of HD1/plectin to the basal surface of the cell (left), β41,328, lacking an additional 27 amino acids of the CS does not affect the distribution of HD1/plectin (right). Noteworthy, the distribution pattern of α6β4 in the absence of HD1/plectin is comparable to that of α6β4 together with HD1/plectin and indistinguishable by confocal microscopy. Bar, 10 μm.
Since the COOH-terminal half of the β4 cytoplasmic domain has previously been shown to be responsible for the localization of BP180 in transfected COS-7 cells (Borradori et al., 1997), we investigated whether β4 mutants with increasing COOH-terminal truncations were able to recruit BP180 and BP230 into newly formed HD-like structures. In contrast to its effect on the localization of HD1/plectin, truncation after amino acid 1,487 (β4A1,487) already reduced the ability of the mutated β4 molecules to localize BP180 and BP230 together with α6β4 and HD1/ plectin at the basal side of the cells (Fig. 3 A and Fig. 8). Progressive COOH-terminal truncations up to amino acid 1355 (β41,355) resulted in a gradual increase in the percentage of β4-transfected cells in which BP180 and BP230 remained diffusely distributed throughout the cell (refer to Fig. 3 A). Furthermore, in cells expressing β4 that was truncated after amino acid 1,328 (β41,328), in which HD1/ plectin was no longer concentrated together with α6β4 at the basal side of the cell, BP180 and BP230 were also diffusely distributed throughout the cell. These results suggest that sequences within the CS and the second pair of FNIII repeats of β4 are involved in targeting BP180 and BP230 into HD-like structures. In addition, the presence of HD1/plectin at the basal cell surface appears to be crucial for these translocation events as well (see also below).
Figure 8.
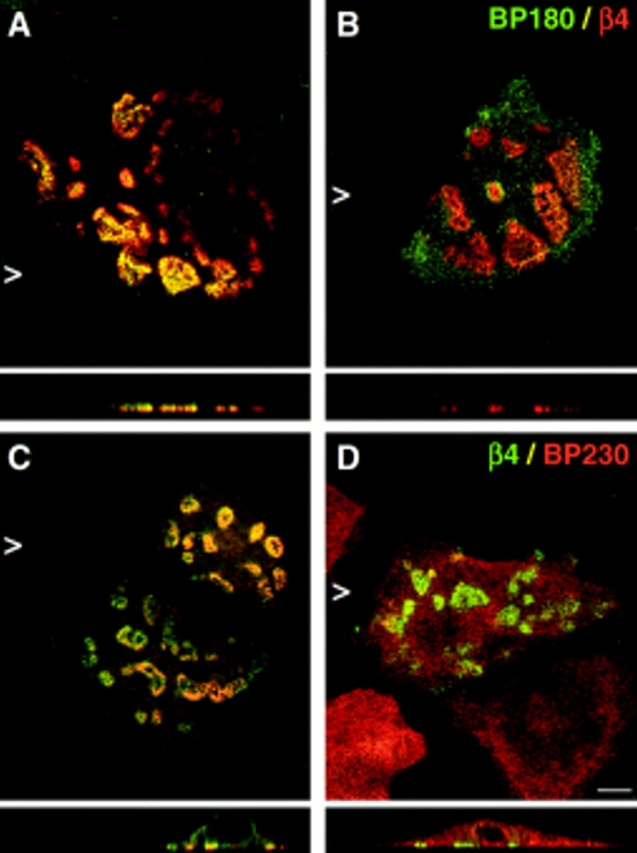
Distribution of BP180 and BP230 in PA-JEB keratinocytes is affected by COOH-terminal truncations of β4. PA-JEB cells transfected with cDNA encoding mutant forms of β4 were double-stained for β4 (red) and BP180 (green) (A and B) or for β4 (green) and BP230 (red) (C and D). Shown are representatives of transfections with β41,436 cDNA. Truncation of the second pair of FNIII repeats already impairs the recruitment of BP180 (B) and BP230 (D) to the basal cell surface, although cells showing colocalization of the BP antigens with β4 can readily be found (A and C). Increasing COOH-terminal truncations further impair the localization of BP180 and BP230 at the basal cell side (refer to Fig. 3 A). In cells transfected with β41,328 cDNA, which also do not show basal localization of HD1/plectin, BP180 and BP230 remain diffusely distributed throughout the cell (data not shown). Bar, 10 μm.
Interaction between β4 and BP180 in COS-7 Cells
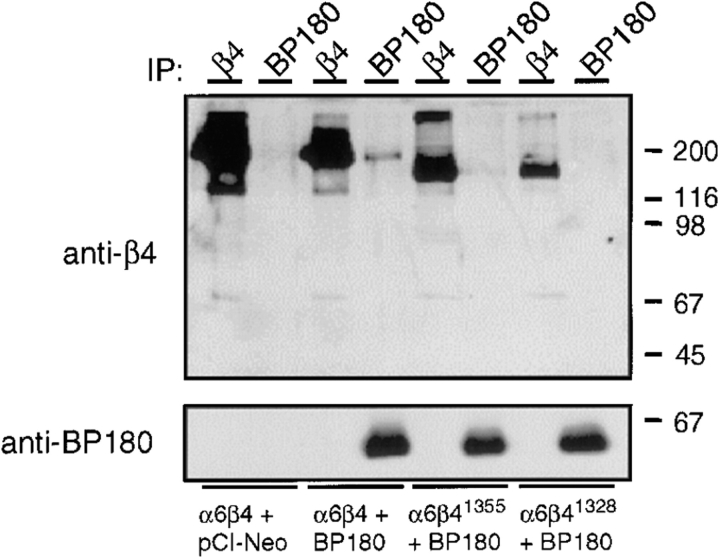
To test whether β4 interacts with BP180 in mammalian cells, we have performed immunoprecipitation and immunoblotting experiments using lysates from COS-7 cells that were transfected with cDNAs encoding α6A and FLAG-tagged BP180 (clone B, BP180Δ521–1,497) together with various β4 mutants. As shown in Fig. 9, β4 is present in anti-FLAG™ M2 immunoprecipitates from lysates of cells transfected with cDNAs for α6A, β4A, and BP180 (Fig. 9 , top, lane 4). Furthermore, in agreement with our localization studies, small amounts of β41,355 mutant protein were co-precipitated by anti-FLAG™ M2 antibodies (Fig. 9, top, lane 6). As expected, the β41,328 mutant protein was not detectable in these immunoprecipitates (Fig. 9, top, lane 8), although equal amounts of mutant BP180 molecules were precipitated by the anti-FLAG™ M2 antibodies from the cells transfected with the corresponding cDNAs (Fig. 9, bottom). Together, these data reveal that β4 and BP180 are present in immune complexes and support the results obtained with the localization studies in PA-JEB cells.
Figure 9.
Coimmunoprecipi-tation of β4 and BP180. Lysates of COS-7 cells cotransfected with cDNAs for α6A and wild-type β4A, β41,355, or β41,328 as well as an empty pCI-Neo vector or a pCI-Neo construct encoding the BP180 cytoplasmic domain were subjected to immunoprecipitation with a mixture (1:1:1) of three anti-β4 mAbs, 4.3E1, 113C and 450-9D, respectively, or with the mAb FLAG™ M2. Samples were resolved on a SDS-polyacrylamide (8%) gel under nonreducing conditions. Shown is an immunoblot analysis developed with the rabbit polyclonal anti-serum against β4 (top) and the mAb FLAG™ M2 to detect BP180 (bottom) among the immunoprecipitated proteins. When samples immunoprecipitated with the anti-β4 mAbs were subjected to immunoblotting with the mAb FLAG™ M2 (bottom) or a polyclonal anti-BP180 antiserum (data not shown), the mutant form of BP180 was not detectable in the anti-β4 immunoprecipitates. The positions of molecular weight standards (in kD) are indicated on the right.
Direct Interaction between the Cytoplasmic Domains of β4 and BP180
To investigate whether the cytoplasmic domains of β4 and BP180 can directly bind to each other, and to determine which site on β4 is involved in this interaction, a yeast two-hybrid assay (Fields and Song, 1989) was performed (Fig. 10). Yeast strain PJ69-4A was cotransformed with the GAL4(AD) pACT2 and the GAL4(BD) pAS2-1 vectors or derivatives thereof. For several pACT2-β4 plasmids, cotransformation of the yeast strain together with the pAS2-BP180 plasmid (and only then) supported growth of colonies on SC-LTHA plates, showing that the His and Ade reporter genes in these yeast cells were activated by a direct interaction between the BP180- and β4-GAL4 fusion proteins. The highest plating efficiency on SC-LTHA plates was observed with the β41,115–1,666 construct (both the β4A and the β4B splice variant). The number and growth rate of the colonies on SC-LTHA plates were comparable to those on SC-LT plates, indicating that both reporter genes were efficiently expressed as a result of a strong interaction between β4 and BP180 (refer to Materials and Methods). In fact, the plating efficiency was comparable to that of the pCL1/pAS2-1 and pTD1-1/pVA3-1 positive controls.
The site of interaction was mapped using β4 with COOH-terminal deletions. Removal of the COOH terminus together with the second part of the fourth FNIII repeat, β41,115–1,666 (again for both the β4A and β4B splice variants), resulted in only a slight decrease in the plating efficiency and growth rate. However, COOH-terminal deletions up to the end of the CS (β4A1,115–1,457) resulted in a dramatic reduction in binding. Truncation up to the last 21 amino acids of the CS (β41,115–1,436) had no effect on the binding, but there was no binding at all when 54 additional residues were deleted (β41,115–1,382). These results suggest that the main binding sites for BP180 on β4 reside in the segment comprising the COOH-terminal half of the CS and the third FNIII repeat.
To confirm this finding, fragments of the β4 cytoplasmic domain were used. As expected, deletion of the first two FNIII repeats (β4A1,320–1,668) did not result in decreased binding to BP180, as compared to β4A1,115–1,666. The same was found for binding of BP180 to β4A1,320–1,552, compared to β41,115–1,600. Accordingly, neither the first (β41,115–1,217) nor the second (β41,217–1,328) FNIII repeat can bind to BP180. Efficient binding to BP180 was only observed when both the CS and the third FNIII repeat were present (β4A1,320–1,552). The third or fourth FNIII repeat or the COOH terminus alone (β41,457–1,552, β41,570–1,668, and β41,667– 1,752, respectively) showed only weak binding. The results obtained with the yeast two-hybrid assay are in good agreement with those obtained with the cell transfection studies (as shown above), and conclusively prove that β4 interacts directly with BP180.
A Role for HD1/Plectin in the Localization of BP180
The presence of small amounts of β41,355 protein, which does not contain the BP180 binding site(s), in the BP180 immunoprecipitate may have been due to the presence of HD1/plectin in these immune complexes. To determine whether HD1/plectin play a role in the recruitment of BP180 into HDs, we assessed the localization of BP180 in PA-JEB cells transfected with cDNAs encoding β4 mutants that lack sequences essential for the recruitment of HD1/plectin (β4AΔ1,219–1,319 and β4AΔ1,249–1,265; Fig. 3 B). Neither of the β4 mutants induced a redistribution of HD1/plectin, despite the fact that they were able to cluster with α6 at the basal side of the cells (Fig. 11 A). Remarkably, in the absence of HD1/plectin, but in the presence of the binding sites for BP180 on β4, BP180 was not recruited to the basal cell surface but remained diffusely distributed (Fig. 11 B). Thus, in addition to β4, HD1/plectin is essential for the recruitment of BP180 into HD-like structures.
Figure 11.
Basal localization of HD1/plectin together with β4 is essential for the recruitment of BP180. PA-JEB cells transfected with cDNA encoding internal deletion mutants of β4 were double-stained for (A) β4 (red) and HD1/plectin (green), (B) β4 (red) and BP180 (green), and (C) β4 (green) and BP230 (red). Shown are representatives of transfections with β4AΔ1,249–1,265. Deletion of 17 amino acids in the second FNIII repeat or its complete deletion prevent the recruitment of HD1/plectin (A). As a consequence, α6β4 clustered at the basal cell surface is no longer capable of recruiting BP180 to these HD1/plectin-lacking clusters although the binding sites for BP180 are still present (B). In a few cases (i.e., <25% of β4-transfected cells) these β4 mutants were able to recruit BP230 in an HD1/plectin- and BP180-independent manner to the basal cell surface (C). Bar, 10 μm.
It is noteworthy that in a few cases (i.e., <25% of transfected cells) these β4 mutants recruited BP230 in an HD1/ plectin- and BP180-independent manner to the basal cell surface (Fig. 11 C). These results together with the results obtained with the COOH-terminal truncation mutants suggest a BP180-independent, (in)direct interaction between β4 and BP230.
Intramolecular Interactions in the β4 Cytoplasmic Domain
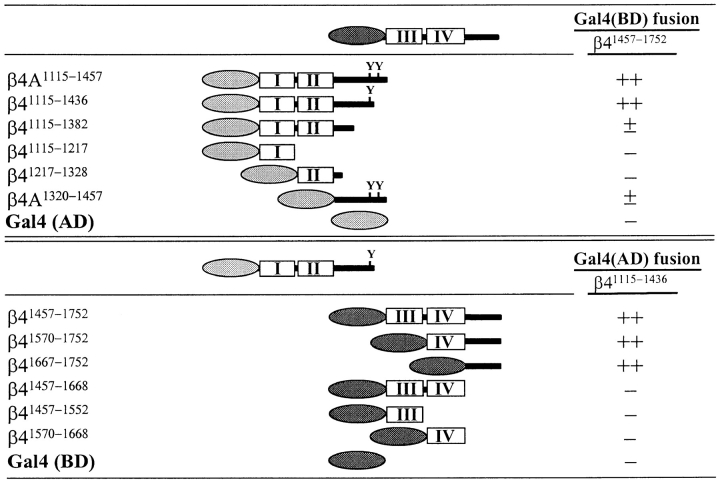
The observation that the β4 internal deletion mutants which lack the HD1/plectin binding site(s), but possess the binding sites for BP180 failed to recruit BP180, prompted us to investigate whether the NH2- and the COOH-terminal parts of the β4 cytoplasmic domain interact with each other. Such an intramolecular interaction might prevent the interaction with BP180 and proffer an explanation for the essential role of HD1/plectin in the interaction of β4 with BP180. The potential intramolecular interaction was tested in a yeast two-hybrid assay in which the GAL4 activation and DNA-binding domains were fused to various parts of the β4 cytoplasmic domain (Fig. 12).
A β41,457–1,752 cDNA was cloned into the pAS2-1(BD) vector and cotransformed to yeast in combination with an empty pACT2 vector (to check for autonomous transactivation) or with a pACT2-derivative containing diverse β4 inserts. Strong binding was observed with β4(AD) constructs β4A1,115–1,457 and β41,115–1,436 that contained the first two FNIII repeats together with a large part of the connecting region (Fig. 12, top). Binding was strongly, but not completely reduced by deletion of the first and second FNIII repeat (β4A1,320–1,457). No binding could be demonstrated with either the first or the second FNIII repeat alone (β41,115–1,217 and β41,217–1,328, respectively). To determine which part of the β4 COOH terminus binds to the more NH2-terminal part (β41,115–1,436), the smaller COOH-terminal β4 subclones as shown in Fig. 10 were excised from the pACT2(AD) vector and recloned in the pAS2-1(BD) vector. Subsequently, the resulting β4(BD) constructs were cotransformed to yeast in combination with an empty pACT2 vector (to check for autonomous transactivation) or with a pACT2-derivative containing the β41,115–1,436 insert (Fig. 12 bottom). The results show that only the COOH-terminal part of the β4 cytoplasmic domain strongly interacted with the β41,115–1,436 sequence, whereas the third and fourth FNIII repeat had no apparent role in this intramolecular association. These observations together with the results of the cell biological studies described in the previous paragraph suggest that the β4 protein can fold back upon itself, with the COOH-terminal 85 amino acids binding to the HD1/plectin-binding region located further NH2-terminal.
Discussion
The β4 Integrin Subunit Is Critical for the Formation of HD-like Structures
We have established an immortalized keratinocyte cell line from a PA-JEB patient lacking β4 expression (Niessen et al., 1996). Only a few of these cells have low numbers of disorganized HD-like structures that contain the hemidesmosomal components BP180 and BP230, but not α6 or HD1/plectin. Whereas α6 is concentrated in focal contacts, HD1/plectin is diffusely distributed in the cytoplasm. Our results demonstrate that transfection of these PA-JEB keratinocytes with cDNA encoding wild-type β4 results in the reexpression of the protein that is correctly associated with α6 and is clustered in patches at the basal cell surface. Most strikingly, restored expression of β4 results in the formation of organized HD-like structures that contain, in addition to the α6β4 integrin, the hemidesmosomal elements HD1/plectin, BP180 and BP230. These HD-like structures are encircled by focal contacts in a pattern indistinguishable from that observed in cultured normal human keratinocytes (Carter et al., 1990; Marchisio et al., 1990). These findings demonstrate that expression of β4 affects the subcellular localization of various hemidesmosomal components, suggesting that it recruits these components to HD-like structures.
Previous electron microscopical studies have shown that PA-JEB patients exhibit hypoplastic HDs with poorly developed attachment plaques and subbasal dense plates (Vidal et al., 1995; Niessen et al., 1996). Our results provide additional support for a crucial role of α6β4 in the formation and organization of HDs. It is noteworthy that, in contrast to our findings in vitro, HD1/plectin, BP180, and BP230 appeared to be correctly localized at sites of cell–substrate contact in the skin of this PA-JEB patient (Niessen et al., 1996). The different localization of these proteins in vitro is puzzling, but may reflect the inability of the immortalized PA-JEB cells to synthesize the ligand(s) for BP180 which normally induces its correct polarization at the basal cell surface via a ligand–receptor interaction.
Signaling via the β4 TAM Is Dispensable for the Formation of HD-like Structures
Mutagenesis experiments have suggested that phosphorylation of the β4 TAM is required for the formation of HDs in 804G rat bladder carcinoma cells, since a β4 molecule with phenylalanine substitutions in the TAM was not incorporated in HDs (Mainiero et al., 1995). However, using the same cells we could not reproduce these results (Niessen et al., 1997a ). Consistent with and in extension to that observation, we here show that a β4 molecule with a mutated TAM is able to initiate the assembly of well-organized HD-like structures containing HD1/plectin, BP180, and BP230 in PA-JEB keratinocytes. Nevertheless, in some cells expressing the β4 TAM mutant, BP180 remained diffusely distributed. This observation is in agreement with recently published results suggesting that mutations in the β4 TAM affect the localization of BP180 in COS-7 cells (Borradori et al., 1997). Together, our findings do not support the supposition that the TAM is critical for the formation of HDs.
Multiple Interactions between β4, HD1/Plectin, and BP180
Sequences within β4, encompassing the second FNIII repeat and a stretch of 27 amino acids of the CS, have previously been shown to be required for the localization of α6β4 in HDs of 804G cells that endogenously express β4 (Niessen et al., 1997a ). These sequences are also important for the localization of HD1/plectin into junctional complexes containing α6β4 (Niessen et al., 1997a ; Sánchez-Aparicio et al., 1997). Our present study indicates that this same region is critical for initiating the formation of HD-like structures in PA-JEB keratinocytes. In contrast to the abrupt loss of HD1/plectin recruitment when β4 is truncated after amino acid 1328 (β41,328), increasing COOH-terminal truncations of β4 appeared to gradually decrease the capacity of these mutants to redistribute BP180 and BP230 in PA-JEB cells.
We show here that the effect of β4 on the subcellular localization of BP180 in both transfected PA-JEB keratinocytes (this study) and COS-7 cells (Borradori et al., 1997) is due to its direct binding to BP180. Using the yeast two-hybrid system, we have identified major binding sites for BP180 in a segment comprising the COOH-terminal half of the CS and the third FNIII repeat. The interaction with BP180 of either the CS or the third FNIII repeat alone was only weak. In accordance with these results, β4 mutants with truncations in this region exhibited a reduced capacity to recruit BP180, as assessed by immunofluorescence analysis. Surprisingly, β4 mutants that lacked the sequences critical for localization of HD1/plectin (i.e., the first pair of FNIII repeats and 27 amino acids of the CS) were no longer able to recruit BP180, even though they contained the BP180 binding site(s). These findings suggest that the recruitment of BP180 into HDs requires the interaction of BP180 with both β4 and HD1/plectin. Three observations support this hypothesis. First, preliminary studies using the yeast two-hybrid system demonstrate a direct interaction between HD1/plectin and BP180 (Aho, S., and J. Uitto. 1997. J. Invest. Dermatol. 108:546a). Second, BP180 is not localized in HD-like structures at the basal cell side in keratinocytes derived from an epidermolysis bullosa simplex with muscular dystrophy patient lacking HD1/plectin (Gache et al., 1996). Finally, our immunoprecipitation analysis of transfected COS-7 cells showed the presence of the mutant β41,355 protein, containing the HD1/plectin-binding region but lacking the binding sites for BP180, in the BP180 immunoprecipitate. In contrast, coimmunoprecipitation of a β41,328 mutant which is no longer able to recruit HD1/plectin, was not observed. Although the presence of HD1/plectin in these immune complexes could not be assessed due to the lack of a suitable antibody which efficiently detects monkey HD1/plectin on immunoblots, these observations provide indirect evidence for an association of BP180 with both β4 and HD1/ plectin.
The finding that the efficient localization of BP180 into HDs seemed to depend on its interaction with at least two different hemidesmosomal components is not without precedent. The linkage of keratin filaments to the hemidesmosomal plaque involves the contribution of the two IF-binding proteins, HD1/plectin and BP230 (Foisner et al., 1988; Yang et al., 1996). Deletion of one of these molecules is not compensated by the remaining components and disturbs the anchorage of the keratin filaments to the hemidesmosomal plaque (Guo et al., 1995; Gache et al., 1996; McLean et al., 1996; Smith et al., 1996; Andrä et al., 1997). Thus, it is likely that proper assembly of HDs requires multiple interactions between the various components.
Recently, it was suggested that the incorporation of BP180 into HDs is facilitated by a direct interaction with the α6 integrin subunit (Hopkinson et al., 1995). Although it is not excluded that such an interaction may contribute to the stabilization of HDs, our results do not support this contention and they further show that the localization of BP180 clearly depends on distinct cytoplasmic regions of β4 rather than α6. Even when α6 and the various β4 mutants were clustered at the basal cell surface, BP180 remained diffusely distributed. In addition, we recently found that chimeric proteins consisting of the extracellular and transmembrane domains of the interleukin 2 receptor and the cytoplasmic domain of β4 recruit BP180 to the basal cell surface in a laminin-5– and α6-independent manner (Nievers et al., 1998).
The translocation of BP180 to the preexisting complex consisting of β4 and HD1/plectin at the basal cell surface may constitute an intermediate step in the nucleation of HD-like structures and precede the localization of BP230 (this study; Gagnoux-Palacios et al., 1997). Indeed, restored synthesis of BP180 in keratinocytes, derived from a patient suffering from generalized atrophic benign epidermolysis bullosa associated with a deficiency for BP180, affected the subcellular localization of BP230. BP230 was no longer diffusely distributed in the cytoplasm, but was found together with BP180 at the basal surface of the transfected cells (Borradori et al., 1998). However, it is possible that additional pathways exists regulating the distribution of BP230, as inferred from the observation that expression of β4 with a mutated TAM, appeared to impair the recruitment of BP180 but not of BP230, into HD-like structures. Furthermore, a BP180-independent redistribution of BP230 was observed in ∼10–20% of the cells transfected with the β4 internal deletion mutants. The partial redistribution which is seen of BP180 and BP230 upon transfection of some β4 mutants may also be due to compensatory mechanisms present in some cells but absent in others. This would mean that other proteins critical for hemidesmosome assembly are yet to be detected.
Are the β4 Binding Sites for BP180 Masked by an Intramolecular Interaction?
As has been described for vinculin (Johnson and Craig, 1995; Gilmore and Burridge, 1996) and the ERM protein family (Bretscher et al., 1997), conformational activation of proteins appears to be a mechanism by which the assembly of cell surface structures can be regulated. The results of our transfection studies and the observation by others that BP180 is not localized at the basal cell surface in HD1/plectin-deficient keratinocytes (Gache et al., 1996) raise the intriguing possibility that HD1/plectin contributes to the formation of HDs not only by providing an interaction site for BP180 (as discussed above), but also by affecting the capacity of β4 to bind to BP180. What are the mechanisms by which HD1/plectin might regulate the binding of β4 with BP180? The results of the two-hybrid assay suggest the possibility of an intramolecular interaction in the β4 cytoplasmic domain, since a segment of 85 COOH-terminal residues can bind directly to a more NH2-terminally located region. Strikingly, this region encompasses the sequences essential for the recruitment of HD1/ plectin; i.e., the first pair of FNIII repeats and part of the CS (this study; Niessen et al., 1997a ). Hence, it is possible that the intramolecular interaction is disrupted by a high-affinity interaction of HD1/plectin with β4 and that binding of BP180 to β4 is thus facilitated. Alternatively, the intramolecular interaction between the NH2 and COOH regions of the β4 cytoplasmic domain is regulated by phosphorylation. Binding of HD1/plectin may then help to maintain an unfolded structure of the β4 molecule, thereby rendering the binding site for BP180 accessible for interaction. Strong phosphorylation of the COOH-terminal segment of β4 has indeed been found in various epithelial cell lines (Falcioni et al., 1989; Kennel et al., 1989). Tyrosine phosphorylation of the TAM (Mainiero et al., 1995, 1996) may play only a secondary role in this process as mutation of the tyrosine residues in this motif had only a minor effect on the localization of BP180 (this study; Borradori et al., 1997).
In conclusion, the results of our study indicate that immortalized PA-JEB keratinocytes lacking β4 expression are not capable of forming organized HD-like structures. Transfection of these cells with β4 cDNA resulted in restored synthesis of this protein, which was correctly associated with α6 and induced the recruitment of various hemidesmosomal components to the basal side of the cell into junctional complexes typical for HD-like structures. Mutation of the TAM within the β4 cytoplasmic domain did not prevent the assembly of these structures. Distinct domains within the cytoplasmic tail of β4 were shown to regulate the localization of HD1/plectin and both BP180 and BP230 into HDs. A direct interaction between the cytoplasmic domains of β4 and BP180 is involved in this process. The observed interaction of the 85 COOH-terminal residues with a more NH2-terminally located region suggests that the cytoplasmic domain of β4 can undergo an intramolecular interaction. Finally, we propose that the localization of BP180 may be regulated by the complex formation of β4 and HD1/plectin that changes the β4 conformation such that it can directly interact with BP180.
Acknowledgments
We thank L. Oomen for excellent assistance with the confocal laser microscope and assembly of the figures, N. Ong for photographic work, and J. Calafat and H. Janssen (all four from The Netherlands Cancer Institute, Amsterdam, The Netherlands) for electron microscopy. We are grateful to M. Jonkman (Gronnigen University Hospital, Gronnigen, The Netherlands) for diagnosing the PA-JEB patient and providing us with skin from this patient. We thank P.M. Howley (Harvard University School of Medicine, Boston, MA) for providing the p1432 plasmid and E.M.H. van der Raay-Helmer (Free University of Amsterdam, Amsterdam, The Netherlands) for help with immortalizing the keratinocytes from the primary culture. We acknowledge A.M. Martínez de Velasco for generation of the mouse anti-β4 mAb 113C. We thank L. Bruckner-Tuderman, C.H. Damsky, C.G. Figdor, M.A. Glukhova, S.J. Kennel, K. Owaribe, and J.R. Stanley for their generous gifts of antibodies, and F.G. Giancotti for the TAM-mutated β4 construct. The authors would like to thank P. James for providing yeast strain PJ69-4A and advice. We acknowledge E. Roos, C.P. Engelfriet, and L. Fontao (all three from The Netherlands Cancer Institute except Engelfriet from Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands) for critical reading of the manuscript.
This work was supported by grants from the Dutch Cancer Society (NKI 96-1305 and NKI 95-979), the Biomedical and Health program (BIOMED, BMH4-CT97-2062), and the Dystrophic Epidermolysis Bullosa Research Association (DEBRA Foundation, Crowthorne, UK). L. Borradori was supported by the Roche Foundation (Basel, Switzerland) and a grant from the Swiss National Foundation of Scientific Research (32-51083.97).
Abbreviations used in this paper
- AD
activation domain
- BD
binding domain
- BP180
bullous pemphigoid antigen 180
- BP230
bullous pemphigoid antigen 230
- CS
connecting segment
- FNIII
type III fibronectin repeat
- GAL4
galactose metabolism regulatory gene 4
- HDs
hemidesmosomes
- IF
intermediate filament
- NHK
normal human foreskin keratinocytes
- PA-JEB
junctional epidermolysis bullosa associated with pyloric atresia
- SC
synthetic complete medium
- TAM
tyrosine activation motif
Note Added in Proof
Two recent publications have appeared in which a direct interaction between β4 and plectin (Rezniczek, G.A., J.M. de Pereda, S. Reipert, and G. Wiche. 1998. J. Cell Biol. 141:209–225) and between β4 and BP180 (Aho, S., and J. Uitto. Biochem. Biophys. Res. Commun. 243:694–699) has been demonstrated.
Footnotes
R.Q.J. Schaapveld, Luca Borradori, and D. Geerts contributed equally to this work.
Carien M. Niessen's present address is Cellular Biochemistry & Biophysics Program, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York 10021.
References
- Andrä K, Lassmann H, Bittner R, Shorny S, Fässler R, Propst F, Wiche G. Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev. 1997;11:3143–3156. doi: 10.1101/gad.11.23.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SE, Skalli O, Goldman RD, Jones JCR. Laminin-5 and modulation of keratin cytoskeleton arrangement in FG pancreatic carcinoma cells: involvement of IFAP300/HD1 and evidence that laminin-5/cell interactions correlate with a dephosphorylation of α6A integrin. Cell Motil Cytoskeleton. 1997;36:271–286. doi: 10.1002/(SICI)1097-0169(1997)37:3<271::AID-CM9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8:647–656. doi: 10.1016/s0955-0674(96)80106-2. [DOI] [PubMed] [Google Scholar]
- Borradori L, Koch PJ, Niessen CM, Erkeland S, van Leusden MR, Sonnenberg A. The localization of bullous pemphigoid antigen 180 (BP180) in hemidesmosomes is mediated by its cytoplasmic domain and seems to be regulated by the β4 integrin subunit. J Cell Biol. 1997;136:1333–1347. doi: 10.1083/jcb.136.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori L, Chavanas S, Schaapveld RQJ, Gagnoux-Palacios L, Calafat J, Meneguzzi G, Sonnenberg A. Role of the bullous pemphigoid antigen 180 (BP180) in the assembly of hemidesmosomes and cell adhesion. Reexpression of BP180 in generalized atrophic benign epidermolysis bullosa keratinocytes. Exp Cell Res. 1998;239:463–476. doi: 10.1006/excr.1997.3923. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Reczek D, Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface strutures. J Cell Sci. 1997;110:3011–3018. doi: 10.1242/jcs.110.24.3011. [DOI] [PubMed] [Google Scholar]
- Brown TA, Gil SG, Sybert VP, Lestringant GG, Tadini G, Caputo R, Carter WG. Defective integrin α6β4 expression in the skin of patients with junctional epidermolysis bullosa and pyloric atresia. J Invest Dermatol. 1996;107:385–391. doi: 10.1111/1523-1747.ep12363370. [DOI] [PubMed] [Google Scholar]
- Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997;9:651–658. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins α3β1 in focal adhesions and α6β4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for α3β1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Cullen BR. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–703. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Delwel GO, de Melker AA, Hogervorst F, Jaspars L, Fles DLA, Kuikman I, Lindblom A, Paulsson M, Timpl R, Sonnenberg A. Distinct and overlapping ligand specificities of the α3Aβ1 and α6Aβ1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–215. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J, Yu Q-C, Fuchs E. β4 integrin is required for hemidesmosome formation, cell adhesion, and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee T, Becherer K, Chen PL, Yeh SH, Yang Y, Kilbburn AE, Lee WH, Elledge SJ. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Falcioni R, Perrotti N, Piaggio G, Kennel SK, Sacchi A. Insulin-induced phosphorylation of the β4 integrin subunit expressed on murine metastatic carcinoma cells. Mol Carcinogen. 1989;2:361–368. doi: 10.1002/mc.2940020611. [DOI] [PubMed] [Google Scholar]
- Fields S, Song O. A novel system to detect protein-protein interactions. Nature. 1989;340:245–247. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Foisner R, Leichtfried FE, Herrmann H, Small JV, Lawson D, Wiche G. Cytoskeleton-associated plectin: in situ localization, in vitro reconstitution, and binding to immobilized intermediate filament proteins. J Cell Biol. 1988;106:723–733. doi: 10.1083/jcb.106.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gache YS, Chavanas S, Lacour JP, Wiche G, Owaribe K, Meneguzzi G, Ortonne JP. Defective expression in plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J Clin Invest. 1996;97:2289–2298. doi: 10.1172/JCI118671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnoux-Palacios L, Gache Y, Ortonne JP, Meneguzzi G. Hemidesmosome assembly assessed by expression of a wild-type integrin β4 cDNA in junctional epidermolysis bullosa keratinocytes. Lab Invest. 1997;77:459–468. [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, LeMeur M. Absence of the α6 integrin leads to epidermolysis bullosa and neonatal death in mice. Nature Gen. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Signal transduction by the α6β4 integrin: charting the path between laminin binding and nuclear events. J Cell Sci. 1996;109:1165–1172. doi: 10.1242/jcs.109.6.1165. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature. 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243–250. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- Glukhova MA, Frid MG, Koteliansky VE. Developmental changes in expression of contractile and cytoskeletal proteins in human aortic smooth muscle. J Biol Chem. 1990;265:13042–13046. [PubMed] [Google Scholar]
- Green KJ, Jones JCR. Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB (Fed Am Soc Exp Biol) J. 1996;10:871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Dowling J, Yu Q-C, Wollman R, Perman R, Fuchs E. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Harper JW, Adami G, Wei N, Keyomarski K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Wiche G. Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin. J Biol Chem. 1987;262:1320–1325. [PubMed] [Google Scholar]
- Hessle H, Sakai LY, Hollister DW, Engvall E. Basement membrane diversity detected by monoclonal antibodies. Differentiation. 1984;26:49–54. doi: 10.1111/j.1432-0436.1984.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Hieda Y, Nishizawa Y, Uematsu J, Owaribe K. Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. J Cell Biol. 1992;116:1497–1506. doi: 10.1083/jcb.116.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst F, Kuikman I, Kr AEG, von dem Borne, Sonnenberg A. Cloning and sequence analysis of β4 cDNA: an integrin subunit that contains a unique 118 kD cytoplasmic domain. EMBO (Eur Mol Biol Organ) J. 1990;9:765–770. doi: 10.1002/j.1460-2075.1990.tb08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst F, Admiraal LG, Niessen CM, Kuikman I, Daams H, Janssen H, Sonnenberg A. Biochemical characterization and tissue distribution of the A and B variants of the integrin α6 subunit. J Cell Biol. 1993;121:179–191. doi: 10.1083/jcb.121.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson SB, Riddelle KS, Jones JCR. Cytoplasmic domain of the 180-kD bullous pemphigoid antigen, a hemidesmosomal component: molecular and cell biologic characterization. J Invest Dermatol. 1992;99:264–270. doi: 10.1111/1523-1747.ep12616615. [DOI] [PubMed] [Google Scholar]
- Hopkinson SB, Baker SE, Jones JCR. Molecular genetic studies of a human epidermal autoantigen (the 180-kD bullous pemphigoid antigen/ BP180): identification of functionally important sequences within the BP180 molecule and evidence for an interaction between BP180 and α6 integrin. J Cell Biol. 1995;130:117–125. doi: 10.1083/jcb.130.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi K, Li B, Bartel P, Fields S. Use of the two-hybrid system to identify the domain of p53 involved in oligomerization. Oncogene. 1993;8:1693–1696. [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RP, Craig SW. F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995;373:261–264. doi: 10.1038/373261a0. [DOI] [PubMed] [Google Scholar]
- Jonkman MF, de Jong MCJM, Heeres K, Pas HH, van der Meer JB, Owaribe K, Martínez de Velasco AM, Niessen CM, Sonnenberg A. 180-kD bullous pemphigoid antigen (BP180) is deficient in generalized atrophic benign epidermolysis bullosa. J Clin Invest. 1995;95:1345–1352. doi: 10.1172/JCI117785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor RRS, Mattes MJ, Lloyd KO, Old LJ, Albino AP. Biochemical analysis of two cell surface glycoprotein complexes, very common antigen 1 and very common antigen 2. J Biol Chem. 1987;262:15158–15165. [PubMed] [Google Scholar]
- Keizer GD, te Velde AA, Schwarting R, Figdor CG, de Vries JE. Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol. 1987;17:1317–1322. doi: 10.1002/eji.1830170915. [DOI] [PubMed] [Google Scholar]
- Kennel SJ, Foote LJ, Falcioni R, Sonnenberg A, Stringer CD, Crouse C, Hemler ME. Analysis of the tumor-associated antigen TSP-180. Identity with the α6β4 in the integrin superfamily. J Biol Chem. 1989;264:15515–15521. [PubMed] [Google Scholar]
- Kennel SJ, Epler RG, Lankford TK, Foote LJ, Dickas V, Canamucio M, Cavalierie R, Cosimelli M, Venturo I, Falcioni R, Sacchi A. Second generation monoclonal antibodies to the human integrin α6β4. Hybridoma. 1990;9:243–255. doi: 10.1089/hyb.1990.9.243. [DOI] [PubMed] [Google Scholar]
- Kurpakus MA, Jones JCR. A novel hemidesmosomal plaque component: tissue distribution and incorporation into assembling hemidesmosomes in an in vitro model. Exp Cell Res. 1991;194:139–146. doi: 10.1016/0014-4827(91)90143-i. [DOI] [PubMed] [Google Scholar]
- Li B, Fields S. Identification of mutations in p53 that affect its binding to SV40 T antigen by using the yeast two-hybrid system. FASEB (Fed Am Soc Exp Biol) J. 1993;7:957–963. doi: 10.1096/fasebj.7.10.8344494. [DOI] [PubMed] [Google Scholar]
- Li K, Tamai K, Tan EML, Uitto J. Cloning of type XVII collagen. Complementary and genomic DNA sequences of mouse 180-kilodalton bullous pemphigoid antigen (BPAG2) predict an interrupted collagenous domain, a transmembrane segment, and unusual features in the 5′-end of the gene and the 3′-untranslated region of the mRNA. J Biol Chem. 1993;268:8825–8834. [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J, Giancotti FG. Signal transduction by the α6β4 integrin: distinct β4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO (Eur Mol Biol Organ) J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio PC, Bondanza S, Cremona O, Cancedda R, De Luca M. Polarized expression of integrin receptors (α6β4, α2β1, α3β1, and αωβ5) and their relationship with the cytoskeletal and basement membrane matrix in cultured human keratinocytes. J Cell Biol. 1991;112:761–773. doi: 10.1083/jcb.112.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio PC, Cremona O, Savoia P, Pellegrin G, Ortonne JP, Verrando P, Burgeson RE, Cancedda R, De Luca M. The basement membrane protein BM-600/nicein codistributes with kalinin and the α6β4 integrin in human cultured keratinocytes. Exp Cell Res. 1993;205:205–211. doi: 10.1006/excr.1993.1078. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Gatalica B, Christiano AM, Li K, Owaribe K, McMillan JR, Eady RA, Uitto J. Mutations in the 180-kD bullous pemphigoid antigen (BPAG2), a hemidesmosomal transmembrane collagen (COL17A1), in generalized atrophic benign epidermolysis bullosa. Nature Genet. 1995;11:83–86. doi: 10.1038/ng0995-83. [DOI] [PubMed] [Google Scholar]
- McLean WHI, Pulkkinen L, Smith FJD, Rugg EL, Lane EB, Bullrich F, Burgeson RE, Amano S, Hudson DL, Owaribe K, McGrath JA, McMillan JR, Eady RAJ, Leigh IM, Christiano AM, Uitto J. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev. 1996;10:1724–1735. doi: 10.1101/gad.10.14.1724. [DOI] [PubMed] [Google Scholar]
- Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM, Hogervorst F, Jaspars LH, de Melker AA, Delwel GO, Hulsman EHM, Kuikman I, Sonnenberg A. The α6β4 integrin is a receptor for both laminin and kalinin. Exp Cell Res. 1994;211:360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- Niessen CM, van der Raaij-Helmer LMH, Hulsman EHM, van der Neut R, Jonkman MF, Sonnenberg A. Deficiency of the integrin β4 subunit in junctional epidermolysis bullosa with pyloric atresia: consequences for hemidesmosome formation and adhesion properties. J Cell Sci. 1996;109:1695–1706. doi: 10.1242/jcs.109.7.1695. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Hulsman EHM, Oomen LCJM, Kuikman I, Sonnenberg A. A minimal region on the integrin β4 subunit that is critical to its localization in hemidesmosomes regulates the distribution of HD1/plectin in COS-7 cells. J Cell Sci. 1997a;110:1705–1716. doi: 10.1242/jcs.110.15.1705. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Hulsman EHM, Rots ES, Sánchez-Aparicio P, Sonnenberg A. Integrin α6β4 forms a complex with the cytoskeletal protein HD1 and induces its redistribution in transfected COS-7 cells. Mol Biol Cell. 1997b;8:555–566. doi: 10.1091/mbc.8.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievers MG, Schaapveld RQJ, Oomen LCJM, Fontao L, Geerts D, Sonnenberg A. Ligand-independent role of the β4 integrin subunit in the formation of hemidesmosomes. J Cell Sci. 1998;111:1659–1672. doi: 10.1242/jcs.111.12.1659. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y, Uematsu J, Owaribe K. HD4, a 180 kDa bullous pemphigoid antigen, is a major transmembrane glycoprotein of the hemidesmosome. J Biochem (Tokyo) 1993;113:493–501. doi: 10.1093/oxfordjournals.jbchem.a124072. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L, Kimonis VE, Xu Y, Spanou EN, McLean WHI, Uitto J. Homozygous α6 integrin mutation in junctional epidermolysis bullosa with congenital duodenal atresia. Hum Mol Genet. 1997;6:669–674. doi: 10.1093/hmg/6.5.669. [DOI] [PubMed] [Google Scholar]
- Rousselle P, Lundstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Aumailley M. Kalinin is more efficient than laminin in promoting adhesion of primary keratinocytes and some other epithelial cells and has a different requirement for integrin receptors. J Cell Biol. 1994;125:205–214. doi: 10.1083/jcb.125.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzi L, Gagnoux-Palacios L, Pinola M, Belli S, Meneguzzi G, D'Alessio M, Zambruno G. A homozygous mutation in the integrin α6 gene in junctional epidermolysis bullosa with pyloric atresia. J Clin Invest. 1997;99:2826–2831. doi: 10.1172/JCI119474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Aparicio P, Martínez de Velasco AM, Niessen CM, Borradori L, Kuikman I, Hulsman EHM, Fässler R, Owaribe K, Sonnenberg A. The subcellular distribution of the high molecular mass protein, HD1, is determined by the cytoplasmic domain of the integrin β4 subunit. J Cell Sci. 1997;110:169–178. doi: 10.1242/jcs.110.2.169. [DOI] [PubMed] [Google Scholar]
- Sawamura D, Li K, Chu ML, Uitto J. Human bullous pemphigoid antigen (BPAG1). Amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J Biol Chem. 1991;266:17784–17790. [PubMed] [Google Scholar]
- Skalli O, Jones JCR, Gagescu R, Goldman RD. IFAP 300 is common to desmosomes and hemidesmosomes and is a possible linker of intermediate filaments to these junctions. J Cell Biol. 1994;125:159–170. doi: 10.1083/jcb.125.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FJD, Eady RAJ, Leigh IM, McMillan JR, Rugg EL, Kelsell DP, Bryant SP, Spurr NK, Geddes JF, Kirtschig G, Milana G, de Bono AG, Owaribe K, Wiche G, Pulkkinen L, Uitto J, McLean WHI, Lane EB. Plectin deficiency results in muscular dystrophy with epidermolysis bullosa. Nature Genet. 1996;13:450–457. doi: 10.1038/ng0896-450. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A, Daams H, Calafat J, Hilgers J. In vitro differentiation and progression of mouse mammary tumor cells. Cancer Res. 1986;46:5913–5922. [PubMed] [Google Scholar]
- Sonnenberg A, Janssen H, Hogervorst F, Calafat J, Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987;262:10376–10383. [PubMed] [Google Scholar]
- Sonnenberg A, de Melker AA, Martínez de Velasco AM, Janssen H, Calafat J, Niessen CM. Formation of hemidesmosomes in cells of a transformed murine mammary tumor cell line and mechanisms involved in adherence of these cells to laminin and kalinin. J Cell Sci. 1993;106:1083–1102. doi: 10.1242/jcs.106.4.1083. [DOI] [PubMed] [Google Scholar]
- Spinardi L, Ren Y-L, Sanders R, Giancotti FG. The β4 subunit cytoplasmic domain mediates the interaction of α6β4 integrin with the cytoskeleton of hemidesmosomes. Mol Biol Cell. 1993;4:871–884. doi: 10.1091/mbc.4.9.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinardi L, Einheber S, Cullen T, Milner TA, Giancotti FG. A recombinant tail-less integrin β4 subunit disrupts hemidesmosomes, but does not suppress α6β4-mediated cell adhesion to laminins. J Cell Biol. 1995;129:473–487. doi: 10.1083/jcb.129.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–278. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Stanley JR, Tanaka T, Mueller S, Klaus-Kovtun V, Roop D. Isolation of cDNA for bullous pemphigoid antigen by use of patients' autoantibodies. J Clin Invest. 1988;82:1864–1870. doi: 10.1172/JCI113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen RDM, Walboomers JMM, Meijer CJLM, van der Raaij-Helmer EMH, Parker JN, Chow LT, Broker TR, Snijders PJF. Transition of human papillomavirus type 16 and 18 transfected human foreskin keratinocytes towards immortality: activation of telomerase and allele losses at 3p, 10p, 11q and/or 18q. Oncogene. 1996;13:1249–1257. [PubMed] [Google Scholar]
- Suzuki S, Naitoh Y. Amino acid sequence of a novel integrin β4 subunit and primary expression of the mRNA in epithelial cells. EMBO (Eur Mol Biol Organ) J. 1990;9:757–763. doi: 10.1002/j.1460-2075.1990.tb08170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R, Rozzo C, Starr L, Chambers J, Reichardt LF, Cooper HM, Quaranta V. Epithelial integrin α6β4: complete primary structure of α6 and variant forms of β4. J Cell Biol. 1990;111:1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Korman NJ, Shimizu H, Eady RAJ, Klaus-Kovtun V, Cehrs K, Stanley JR. Production of rabbit antibodies against carboxy-terminal epitopes encoded by bullous pemphigoid cDNA. J Invest Dermatol. 1990;94:617–623. doi: 10.1111/1523-1747.ep12876200. [DOI] [PubMed] [Google Scholar]
- Van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nature Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Vidal F, Aberdam D, Miquel C, Christiano AM, Pulkkinen L, Uitto J, Ortonne JP, Meneguzzi G. Integrin β4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nature Genet. 1995;10:229–234. doi: 10.1038/ng0695-229. [DOI] [PubMed] [Google Scholar]
- Von dem Borne, A.E.G. Kr., P.W. Modderman, L.G. Admiraal, H.K. Nieuwenhuis. 1989. In Leukocyte Typing IV. W. Knapp, B. Dörken, W.R. Gilks, E.P. Rieber, R.E. Schmidt, H. Stein, and A.E.G. Kr. von dem Borne, editors. Oxford University Press, New York. 951 pp.
- Wayner EA, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cell possessing unique α and common β subunits. J Cell Biol. 1987;105:1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G, Becker B, Luber K, Weitzer G, Castanon MJ, Hauptmann R, Stratowa C, Stewart M. Cloning and sequencing of rat plectin indicates a 466-kD polypeptide chain with a three domain structure based on a central α-helical coiled-coil. J Cell Biol. 1991;114:83–99. doi: 10.1083/jcb.114.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H-Y, Lieska N, Goldman AE, Goldman RD. A 300,000 mol-wt intermediate filament-associated protein in baby hamster kidney (BHK-21) cells. J Cell Biol. 1985;100:620–631. doi: 10.1083/jcb.100.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Dowling J, Yu QC, Kouklis P, Cleveland DW, Fuchs E. An essential cytoskeletal linker protein connecting actin microfilaments to intermediate filaments. Cell. 1996;86:655–665. doi: 10.1016/s0092-8674(00)80138-5. [DOI] [PubMed] [Google Scholar]