Abstract
Cartilage fibrils contain collagen II as the major constituent, but the presence of additional components, minor collagens, and noncollagenous glycoproteins is thought to be crucial for modulating several fibril properties. We have examined the distribution of two fibril constituents—decorin and collagen IX—in samples of fibril fragments obtained after bovine cartilage homogenization. Decorin was preferentially associated with a population of thicker fibril fragments from adult articular cartilage, but was not present on the thinnest fibrils. The binding was specific for the gap regions of the fibrils, and depended on the decorin core protein. Collagen IX, by contrast, predominated in the population with the thinnest fibrils, and was scarce on wider fibrils. Double-labeling experiments demonstrated the coexistence of decorin and collagen IX in some fibrils of intermediate diameter, although most fibril fragments from adult cartilage were strongly positive for one component and lacked the other. Fibril fragments from fetal epiphyseal cartilage showed a different pattern, with decorin and collagen IX frequently colocalized on fragments of intermediate and large diameters. Hence, the presence of collagen IX was not exclusive for fibrils of small diameter. These results establish that articular cartilage fibrils are biochemically heterogeneous. Different populations of fibrils share collagen II, but have distinct compositions with respect to macromolecules defining their surface properties.
Keywords: cartilage, fibril, collagen IX, decorin, proteoglycan
The extracellular matrix of hyaline cartilage contains two major supramolecular elements: a framework formed by collagen II–rich fibrils, and a hydrated substance with a high content of the cartilage-specific proteoglycan aggrecan. The proteoglycan-rich gel has a high electrostatic charge density that gives rise to a high osmotic swelling pressure. Fibrils strongly resist tension, and the fibrillar network contains the swelling pressure. As a consequence, the tissue is provided with mechanical stiffness and elasticity, which is essential for its capacity to withstand shearing and compressive forces. These properties are required particularly in mature joint cartilage, where mobility is combined with tolerance for extremely high loads. The extracellular matrix of adult articular cartilage shows a complex pattern ultrastructurally. Fibril diameters and the preferential orientation of fibrils vary with the location within the tissue (Lane and Weiss, 1975; Poole et al., 1984; Hunziker and Herrmann, 1990). The organization of interfibrillar proteoglycans is variable, too (Poole et al., 1982). The precise tuning of the supramolecular structure is poorly understood. Most likely, less abundant macromolecules that participate in specific interactions are important modulators of matrix assembly.
Several studies have focused on fibrillar organization in distinct connective tissues. It is now well-established that fibrils in general are heteropolymers containing more than one collagen type (Birk et al., 1988; Mendler et al., 1989). Interactions between different collagens during fibrillogenesis may regulate the final shape and the molecular packing of the fibril. Furthermore, both collagenous and noncollagenous components at the fibril surface may mediate interactions with perifibrillar matrix constituents and neighboring fibrils. Therefore, fibril surface macromolecules probably play a key role in organizing the macromolecular aggregates and determining the biomechanical properties of the tissue.
Analyses of embryonic chicken sternal cartilage have demonstrated an intimate association of the cartilage-specific collagens II, IX, and XI in a D-periodic array (Vaughan et al., 1988; Mendler et al., 1989). Collagen II, representing ∼80% of the collagen content, is the bulk component. Collagen IX constitutes ∼10% of total collagen, and is arranged within the fibrils in antiparallel orientation with respect to collagen II (Wu et al., 1992). The COL3 and NC4 domains of collagen IX protrude into the extrafibrillar space (Vaughan et al., 1988). Reconstitution experiments with purified soluble collagens suggested that collagens II, IX, and XI were required in authentic proportions of 8:1:1 to form aggregates resembling the thin primary fibrils (∼20 nm) of immature cartilage (Eikenberry et al., 1992). However, collagen IX represents only 1–2% of the total collagen in adult bovine articular cartilage (Eyre et al., 1992). Since thin fibrils are much less abundant in mature cartilage, their existence may arguably depend on the presence of collagen IX. Furthermore, immunolocalization studies with adult pig articular cartilage showed a preferential occurrence of collagen IX in pericellular compartments where thin fibrils predominate (Wotton et al., 1988). By contrast, collagen IX is distributed homogeneously throughout chick embryo cartilages where uniformly thin fibrils of ∼20 nm in width are found (Irwin et al., 1985; Müller-Glauser et al., 1986).
Decorin is another molecule believed to occur at the fibril surface. This small proteoglycan is a member of a group of widely distributed matrix glycoproteins with a central domain composed of leucine-rich tandem repeats (reviewed by Iozzo and Murdoch, 1996; Iozzo 1997). It was localized by electron microscopy to the d and e bands in the gap zones of fibrils in collagen I-rich tissues like tendon, skin, and cornea. In addition, decorin strongly binds to collagens I and II in vitro. Within articular cartilage, decorin is present mainly in interterritorial compartments (Poole et al., 1986), but little is known about its supramolecular organization. Immunoelectron microscopy of human articular cartilage sections showed only weak labeling for decorin near the fibrils, but a striking association with globular structures in the extrafibrillar matrix (Miosge et al., 1994). Hence, the precise function of decorin in cartilage is still unclear. The molecule may play a role similar to collagen IX in modulating fibril morphology and in maintaining interfibrillar spacing.
To gain further insights into the possible involvement of decorin and collagen IX in controlling fibril architecture in cartilage, we examined the distribution of these two components among native fibril fragments released from bovine articular cartilage.
Materials and Methods
Tissue Preparation
Fresh tissues were obtained from a local slaughterhouse and were either used directly or frozen in liquid nitrogen and stored at −80°C. Adult articular cartilage was prepared from tarsometatarsal joints of ∼2-yr-old cows. Epiphyseal cartilage was dissected from the ends of developing bones (tibia, femur) of a bovine fetus of ∼30 wk gestational age.
Isolation of Cartilage Collagens
Collagens II, IX, and XI were extracted from fetal bovine cartilage by limited pepsin digestion, and were purified by differential salt precipitation essentially as previously described (Mayne et al., 1995). In brief, cartilage pieces were homogenized in 100 vol of 0.2 M NaCl, 0.5 M acetic acid, adjusted to pH 2.5 with 12 N HCl, and then digested with pepsin (100 μg/ml) for 48 h at 4°C. After centrifugation, the pellet was digested with pepsin once more to complete solubilization of collagens. The two extracts were combined and adjusted with NaOH to pH 8.0 to inactivate pepsin. Collagens II, XI, and IX were selectively precipitated from this solution by dialysis against 0.9, 1.2 and 2.0 M NaCl, respectively.
Antisera to Collagen IX and Decorin
Polyclonal antisera to the pepsin-resistant fragments HMW and LMW of bovine collagen IX were raised in transgenic mice lacking the entire collagen IX protein (Fässler et al., 1994; Hagg et al., 1997). Mice were immunized by intraperitoneal injections of ∼100 μg protein. Initial immunization was with Freund's complete adjuvant, and subsequent boosters after 3 and 6 wk were with Freund's incomplete adjuvant. Antisera were tested for reactivity and specificity by ELISA (Engvall and Perlmann, 1972) and immunoblotting. Decorin antibodies were those described elsewhere (Heinegård et al., 1985).
SDS-PAGE and Immunoblotting
Samples were prepared for electrophoresis by cold ethanol precipitation. The precipitates were dissolved in SDS-PAGE sample buffer and electrophoresed in 4.5–15% polyacrylamide gradient gels (Laemmli, 1970). Immunoblots were performed using wet electrophoretic transfer and detection with horseradish peroxidase–coupled antibody and 4-chloro-1-naphthol as substrate (Towbin et al., 1979).
Extraction of Fibril Fragments
Cartilage slices were homogenized with a Polytron™ (Kinematica, Littau, Switzerland) in 10 vol of 0.15 M NaCl, 2 mM sodium phosphate, pH 7.4 (PBS) containing a mixture of proteinase inhibitors (0.1 mM PMSF, 0.1 M 6-aminohexanoic acid, 5 mM benzamidine, 5 mM N-ethylmaleimide and 20 mM EDTA). The suspension was centrifuged at 27,000 g for 30 min, and the clear supernatant was collected. This procedure was repeated twice with fresh extraction buffer, and the three supernatants were combined.
Quantitation of Collagens in Cartilage Tissue and Extracted Fibrils
The cartilage-specific collagens II, IX, and XI present in samples of adult and fetal cartilage tissue were solubilized by two consecutive digestions with pepsin for 48 h at 4°C. After neutralization of the digests, collagens were precipitated at 4.5 M NaCl. The extraction residue was hydrolyzed in 6 M HCl and analyzed for contents of hydroxyproline (Stegeman and Stalder, 1967) to confirm that solubilization of collagens was complete, and that hydroxyproline was absent in the residue. PBS extracts containing fibril fragments were first titrated to pH 2.5 with HCl, and were then similarly digested with pepsin. The salt-precipitated collagens were separated by SDS-PAGE, parallel with standards of pure collagens II, IX, and XI. After completion of electrophoresis, gels were stained with Coomassie Blue, and a densitometric analysis was performed by using an Omni Media XRS Scanner (MWG-Biotech, Ebersberg, Germany) and the Whole Band Analyzer image program (Bio Image™ v3.2; MWG-Biotech). Quantitation relied on measurement of the α1(II) band for collagen II, the LMW band for collagen IX, and the α1(XI) band for collagen XI. The standard curves that were used to calculate the amounts of the individual collagens were linear within the selected collagen concentration range.
Immunoelectron Microscopy
Aliquots of fibril fragment extracts were spotted onto sheets of Parafilm. Copper grids covered with formvar and coated with carbon were floated on the drops for 2 min to allow adsorption of fibrils, subsequently washed with PBS, and treated for 30 min with 2% (wt/vol) dried skim milk in PBS. The adsorbed material was allowed to react for 2 h with antibodies to collagen IX and/or decorin diluted in PBS containing 0.2% dry milk. Next, after washing five times for 2 min with PBS, the grids were put on drops of 0.2% milk solution containing colloidal gold particles (12 or 18 nm) coated with goat antibodies to rabbit or mouse immunoglobulins (Dianova, Hamburg, Germany). For double-labeling experiments, a mixture of gold particles of two different sizes was used. Finally, the grids were washed with PBS and negatively stained with 2% uranyl acetate for 5 min. Control experiments were done with the first antibody omitted or with preimmune serum. Electron micrographs were taken at 80 kV with a CM 10 electron microscope (Philips Electron Optics, Mahwah, NJ).
To examine fibrils after proteolytic treatment, EM grids with adsorbed fibril fragments were floated on droplets containing either trypsin at 0.1 mg/ml in TBS, pH 8.0, or pepsin at 0.5 mg/ml in 0.2 M NaCl, 0.5 M acetic acid, pH 2.5. Digestion was allowed to proceed at room temperature for various time periods, and was stopped by washing several times with PBS. In other experiments, the effects of the dissociative agent urea was studied. Portions of 8 M urea were added to aliquots of fibril extracts to give final urea concentrations ranging from 0.25–6.0 M. The fibril fragments were then allowed to adsorb onto EM grids, followed by extensive washing. Immunolabeling and electron microscopy was done as described above.
For quantitation of fibril dimensions and label intensity, electron micrographs were calibrated on the basis of the D = 67-nm banding pattern of the fibrils. Diameters of fibril fragments were measured with a Peak scale magnifying glass (10×; Plano, Marburg, Germany) on micrographs of a final magnification of at least 8 × 104-fold. Gold particles were considered to label fibrils if they were projected on a fibril or within a 15-nm distance from the fibril boundary.
Rotary Shadowing
Rotary shadowing was performed essentially as described by Tyler and Branton (1980). Fibril fragments were washed several times in 8 M Urea, 0.15 M NaCl, 50 mM Tris-HCl, pH 7.4, followed by extensive dialysis against 0.2 M ammonium bicarbonate at 4°C. Aliquots were combined with an equal volume of glycerol and immediately sprayed onto freshly cleaved mica. After drying under vacuum (10−5 mm Hg), the mica sheets were shadowed with platinum/carbon at an angle of 4.5°, and then with a supporting film of carbon. The Pt/C films were floated on water and picked up on 400-mesh copper grids followed by electron microscopy as above.
Immunohistochemistry
A 5-mm punch biopsy of the tarsometatarsal joint cartilage of a 2-yr-old cow was fixed in 4% paraformaldehyde in PBS for 2 h, and was then subjected to routine paraffin embedding. Sections (3 μm) were prepared and mounted on poly-l-lysine–coated slides. Next, sections were deparaffinized and, in case of decorin staining, treated for 30 min at 37°C with chondroitinase ABC (Seikagaku America, Inc., Rockville, MD) at 0.5 U/ml, or, in case of collagen IX staining, with a mixture of chondroitinase ABC at 0.5 U/ml and hyaluronidase (Fluka AG, Buchs, Switzerland) at 0.4 U/ml. After washing with PBS containing 0.05% Tween20 (PBS-Tween), nonspecific binding was blocked with 10% normal goat serum in PBS-Tween. After several washes in PBS-Tween, sections were incubated for 90 min at 37°C with primary antibodies in PBS-Tween, washed again, incubated with a peroxidase-labeled goat anti-rabbit or anti-mouse IgG antibody (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD), and developed with diaminobenzidine and H2O2. Finally, the sections were counterstained with Giemsa reagent (Sigma Chemical Co., St. Louis, MO). Negative controls were done with normal rabbit and mouse serum instead of primary antibodies.
Results
Fibril Fragment Extraction
Previous ultrastructural localization of collagens IX and XI used fibril fragments released by high-speed homogenization from embryonic chicken sternal cartilage. It was noted that for immunoelectron microscopic examination, these sheared fibrils have important advantages over fixed and embedded tissue sections. Problems due to epitope masking and poor antibody penetration are less likely to occur, and the distinction between fibrils and extrafibrillar substance is clearer. In the present study we used this approach to characterize fibrils from load-bearing mammalian articular cartilage and fetal epiphyseal cartilage. To enable localization of macromolecules associated with the fibril surface via noncovalent interactions, some modifications of the method were necessary. An extraction buffer of approximately physiological ionic strength rather than high salt buffer was used in order to prevent dissociation. Furthermore, purification of the fibril fragments through ultracentrifugation was avoided, since pelleting of the material induced aggregation, with redistribution of components between fibril- associated and soluble pools as a possible consequence.
The extracted fibril fragments were characterized by SDS-PAGE analysis after pepsin digestion, and by fibril diameter measurement. The total amounts of fibrils were determined as the aggregated amounts of collagens II, IX, and XI. The extract from adult bovine articular cartilage contained <2% of total fibrils (Table I). To address the question whether the molecular composition of fibrils in the extract was representative of those in the tissue, the amounts of the individual collagen types II, IX, and XI were compared. The proportions of collagens IX and XI in extracted fibrils were more than twice those in the entire tissue (Table I). This result implied that thinner fibrils, presumably rich in collagens IX and XI, were preferentially extracted. In support of this notion, electron microscopy revealed that thinner fibrils were overrepresented in the extracts (Fig. 1, top) compared with tissue sections (Hedlund et al., 1993). Fibril fragments of diameters larger than 50 nm were quite scarce in extracts of articular cartilage, but predominated in the tissue. It also was observed that the thicker fragments were comparably short. Thus, it appears that the thicker cartilage fibrils are more firmly integrated within the tissue, and hence are less extractable. Extracts of fetal epiphyseal cartilage contained about 1.5 times more fibril fragments than those of adult articular cartilage, consistent with the fact that thin fibrils are more abundant in the fetal tissue (Table II). The low overall yield of fibril fragments indicated, however, that fibrils in immature cartilage already are firmly anchored.
Table I.
Contents of Cartilage-specific Collagen Types in Adult Bovine Articular Cartilage and in Extracted Fibril Fragments
| Collagen type | Entire tissue | Extract | ||||||
|---|---|---|---|---|---|---|---|---|
| Amounts | Relative | Amounts | Relative | |||||
| mg/g tissue | % | mg/g tissue | % | |||||
| II | 129.0 ± 6.4 | 96.3 ± 4.8 | 2.092 ± 0.109 | 89.2 ± 4.5 | ||||
| IX | 1.42 ± 0.44 | 1.1 ± 0.3 | 0.060 ± 0.004 | 2.6 ± 0.2 | ||||
| XI | 3.55 ± 0.67 | 2.6 ± 0.5 | 0.191 ± 0.009 | 8.2 ± 0.4 | ||||
| Total | 134.0 | 2.34 | ||||||
Collagens II, IX, and XI were solubilized by pepsin digestion, electrophoretically separated, and quantified by densitometric analysis of Coomassie-stained gels. Samples were pooled tissue pieces representing the superficial, middle, and deep zones of articular cartilage from three adult cows. In case of the entire tissue, the sample was homogenized and divided into three identical portions that were then separately analyzed. Similarly, the extract was divided into three identical portions before quantitation. Data represent mean of triplicate samples ± SD.
Figure 1.
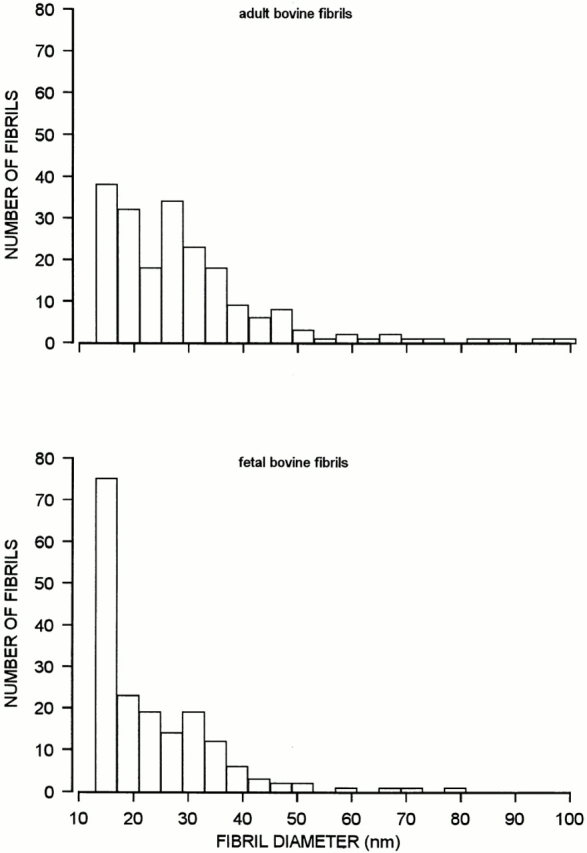
Histograms showing the diameter distribution of fibril fragments from bovine adult articular cartilage (top) and fetal cartilage (bottom), as determined by electron microscopy after negative staining. The fibril extracts were those described in the footnotes to Tables I and II. The overall number of measured fibrils were 202 and 181, respectively.
Table II.
Contents of Cartilage-specific Collagen Types in Fetal Bovine Cartilage and in Extracted Fibril Fragments
| Collagen type | Entire tissue | Extract | ||||||
|---|---|---|---|---|---|---|---|---|
| Amounts | Relative | Amounts | Relative | |||||
| mg/g tissue | % | mg/g tissue | % | |||||
| II | 86.2 ± 7.2 | 87.0 ± 7.3 | 2.244 ± 0.066 | 82.8 ± 2.4 | ||||
| IX | 5.96 ± 0.82 | 6.0 ± 0.8 | 0.202 ± 0.007 | 7.5 ± 0.3 | ||||
| XI | 6.92 ± 0.97 | 7.0 ± 1.0 | 0.260 ± 0.014 | 9.6 ± 0.5 | ||||
| Total | 99.1 | 2.71 | ||||||
Collagens II, IX, and XI were solubilized by pepsin digestion, electrophoretically separated, and quantified by densitometric analysis of Coomassie-stained gels. Samples were pooled slices of fetal bovine cartilage from the ends of developing tibia and femur. In the case of the entire tissue, the sample was homogenized, and divided into three identical portions that were then separately analyzed. Similarly, the extract was divided into three identical portions before quantitation. Data represent mean of triplicate samples ± SD.
Localization of Decorin on the Surface of Cartilage Fibrils
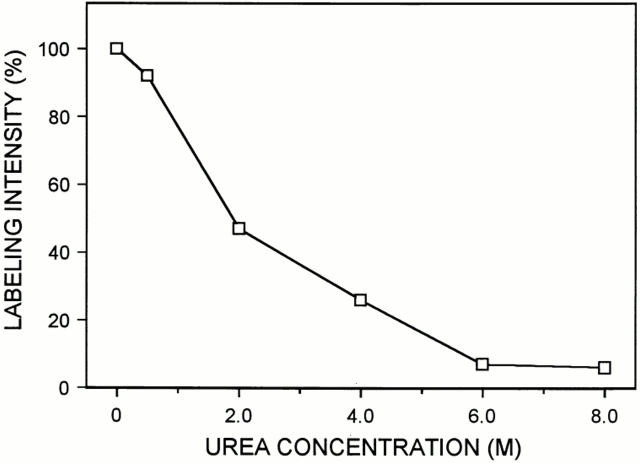
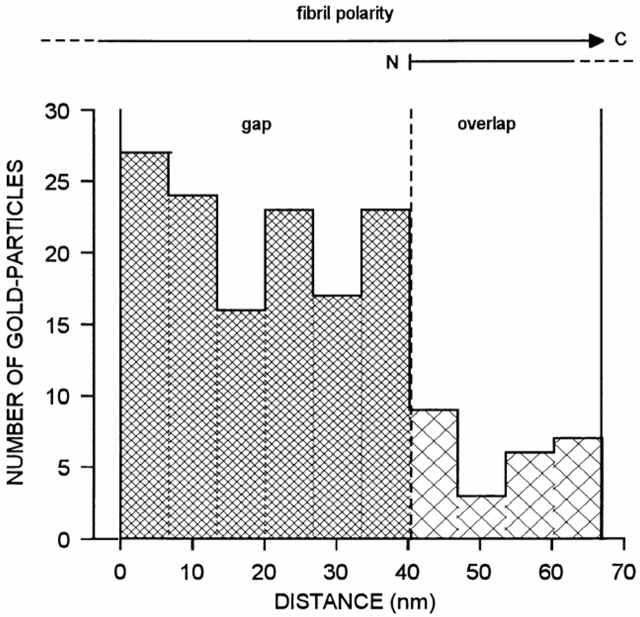
The distribution of decorin along the fibril fragments was determined by using decorin-specific antibodies and antibody-directed gold staining. Electron microscopy revealed that some fibril fragments were labeled, whereas others were negative (Fig. 2). Gold particles were also deposited abundantly outside of the fibrils, indicating that a significant proportion of the cartilage decorin was not fibril-associated. Most of this extrafibrillar decorin appeared to be included in macromolecular aggregates, because it showed high sedimentation rates upon ultracentrifugation (data not shown). However, no corresponding supramolecular structure could be discerned after negative staining. The nonfibrillar decorin aggregates were not further studied here. As decorin was observed to occur associated with some of the fibrils, the opportunity was offered to investigate further the binding of decorin within these native fibrils. The effect of increased ionic strength was examined by suspending samples of fibrils in buffers containing NaCl at concentrations ranging from 0.25–2 M. All these samples demonstrated strong labeling of fibrils (not shown). By contrast, decorin was displaced from the fibrils by urea treatment in a dose-dependent fashion (Fig. 3). Decorin was dissociated from the collagenous backbones at urea concentrations that were expected to denature its core protein. These observations suggest that decorin is bound to the fibril surface through a noncovalent protein-dependent interaction, well in agreement with previous data on binding of decorin to isolated collagens in vitro (Brown and Vogel, 1989; Hedbom and Heinegård, 1993). The distribution of gold particles along the fibrils was nonrandom. About 85% of the markers were localized to the gap zone within the D-period (Fig. 4). In previous immunolocalization studies, a similar association of decorin was observed with collagen I–rich fibrils in skin and tendon (Pringle and Dodd, 1990; Fleischmajer et al., 1991).
Figure 2.
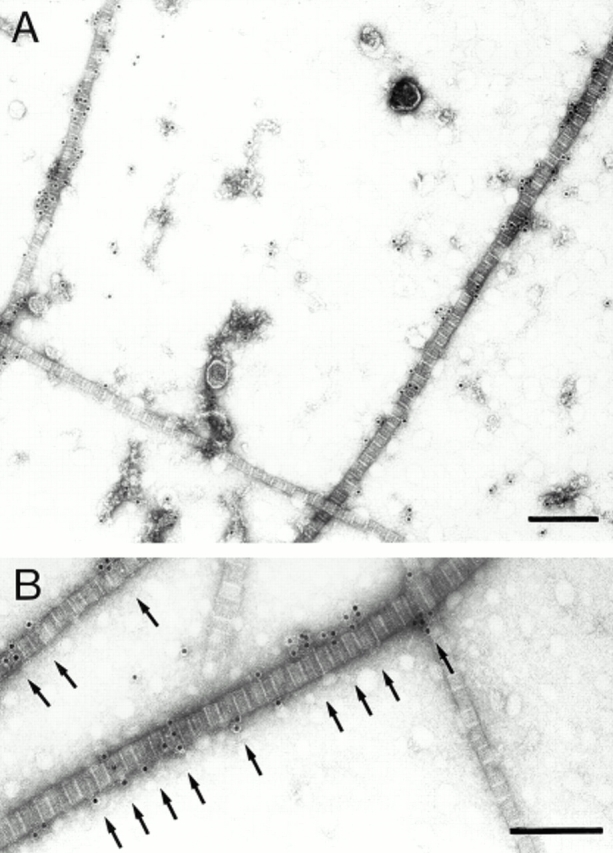
Ultrastructural immunolocalization of decorin in adult bovine articular cartilage extracts. (A) Fibril fragments having similar morphological appearance are either unlabeled or strongly labeled with gold probes (12 nm) for decorin. Note also the specific decorin labeling in structures apart from fibrils. (B) Electron micrographs at higher magnification indicate the preferential localization of colloidal gold to the gap regions (arrows). Bars, 200 nm.
Figure 3.
Effect of urea treatment on the association of decorin within fibril fragments extracted from adult bovine articular cartilage. For each urea concentration, several electron micrographs were taken, gold particles associated with fibrils were counted, and the average number of probes per D-period was calculated. The results are shown as relative labeling intensities, with the density of markers in the sample not treated with urea set to 100%.
Figure 4.
Frequency distribution of decorin-directed gold particles along fibril fragments. Distances were measured between the NH2-terminal starting point of the gap region and the center of each gold particle within the respective D-period. For the evaluation, D-periods were considered only if the polarity of the collagen molecules within the fibril could be clearly identified.
Ultrastructural Localization of Collagen IX
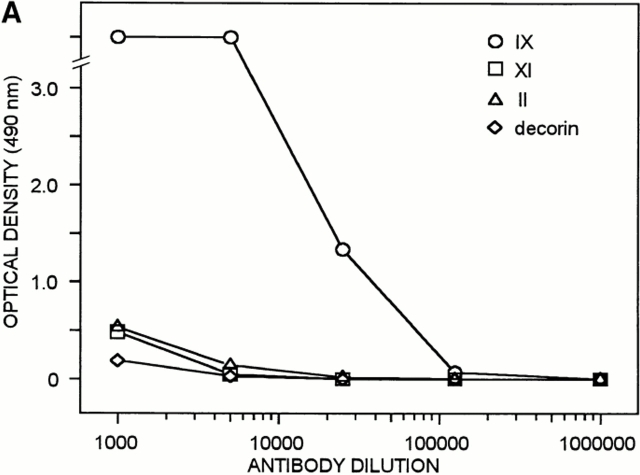
Antibodies were raised against bovine collagen IX, and reacted specifically when tested in ELISA and immunoblot assay (Fig. 5). Electron microscopy showed that the collagen IX antibodies preferentially labeled the thinnest fibrils of adult cartilage (Fig. 6). However, the absence of labeling among thicker fibrils could have been an effect of immunochemical masking of collagen IX. Therefore, attempts were made to disclose additional collagen IX epitopes by partial disruption of the fibrils, e.g., by treatment with 8 M urea or limited proteolysis with pepsin or trypsin. In none of these experiments the collagen IX immunoreactivity was increased, demonstrating that masking of collagen IX epitopes was unlikely (data not shown). As a complement to the immunolocalization approach, rotary shadowing was used to provide a more direct visualization of structures on the fibril surface. Electron microscopy after rotary shadowing showed portions of collagen IX projecting from the surface of thin fibrils (Fig. 6, inset). In agreement with previous results from studies of chick cartilage (Vaughan et al., 1988), the COL3 domain was seen as an ∼35-nm long rod, and the globular NC4 domain as a knob. Such projections were abundant among the thinnest fibril fragments from adult cartilage, whereas the thickest fibrils again appeared to be devoid of collagen IX.
Figure 5.
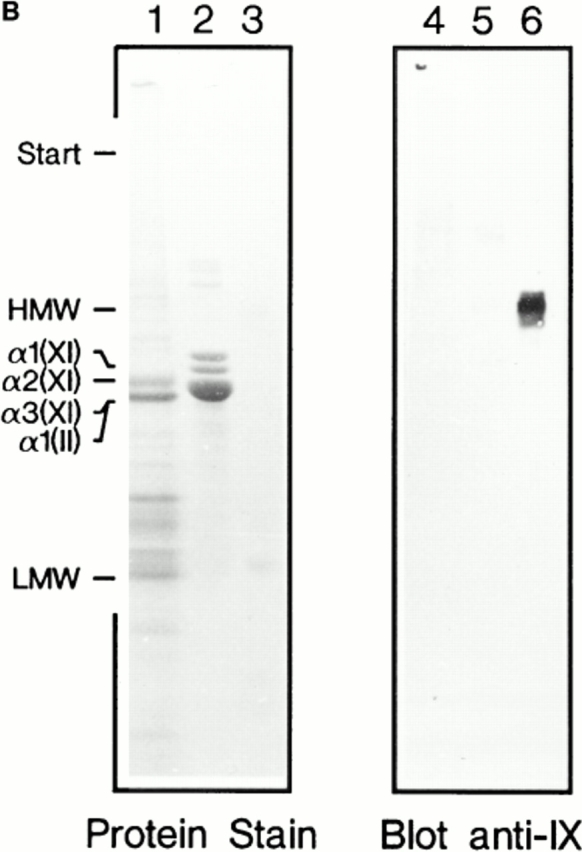
Characterization of polyclonal mouse antiserum against bovine collagen IX by ELISA and immunoblotting. (A) The antigens tested by ELISA were collagen IX (○), collagen XI (□), collagen II (▵), and decorin (⋄). (B) Noncollagenous proteins were extracted from chondroitinase ABC–digested pieces of bovine articular cartilage with boiling SDS-PAGE sample buffer containing 2% β-mercaptoethanol. Aliquots were electrophoresed on 4.5–15% polyacrylamide gels (lanes 1 and 4). Portions of a sample containing purified collagens II and XI were loaded in lanes 2 and 5. Pepsin-resistant collagen IX, i.e., the antigen, was applied in lanes 3 and 6. Proteins in the gel were stained with Coomassie Blue (lanes 1–3) or transferred to nitrocellulose and subjected to immunoblotting (lanes 4–6).
Figure 6.
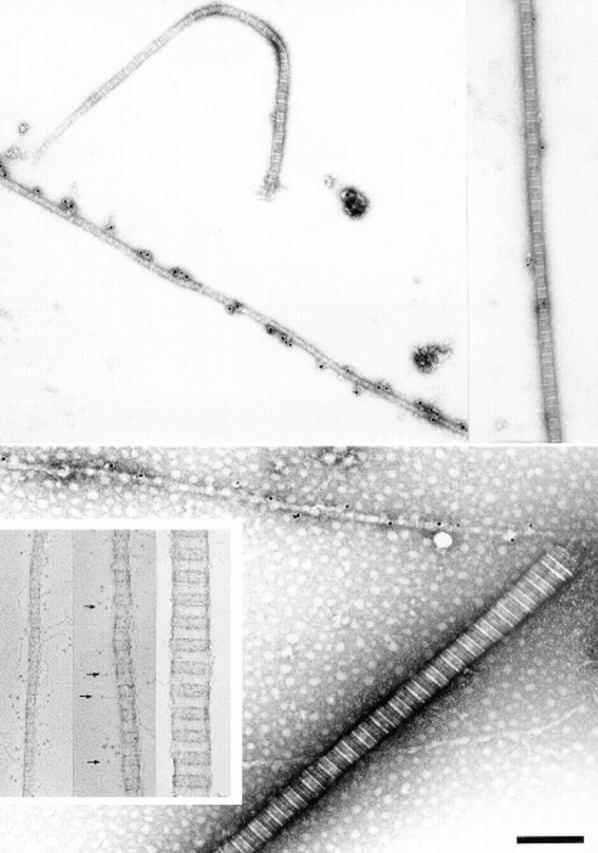
Ultrastructural localization of collagen IX in adult bovine articular cartilage extracts. Typical immunolabeling patterns are shown. A strongly labeled 20-nm fibril is seen together with a unlabeled 30-nm-fibril (top left). A 40-nm fibril demonstrates extremely weak labeling (top right). A thick 80-nm fibril is completely unlabeled, whereas the adjoining thin fibril is densely populated by gold particles (bottom). Rotary shadowing images of three selected fibril fragments are shown in the inset panel. Collagen IX projections (arrows) are seen with a rigid stalk (COL3) and a terminal globule (NC4). A typical thin fibril (∼20 nm) displays abundant collagen IX projections (left of inset), a fibril of intermediate thickness shows fewer of these projections (middle of inset), and the thick fibril fragment appears to be devoid of collagen IX (right of inset). Bar, 200 nm.
Thin fibrils are particularly abundant in immature cartilage. Consequently, extracts of fetal epiphyseal cartilage contained mainly thin fibril fragments (Fig. 1, bottom). These were characteristically collagen IX–positive. The occasional thicker fibril fragments from fetal cartilage were usually labeled as well (see below), in contrast to thicker fibrils from adult cartilage, consistent with the higher relative amount of collagen IX in fetal cartilage (Tables I and II).
Comparison of Fibril Populations Containing Decorin and Collagen IX
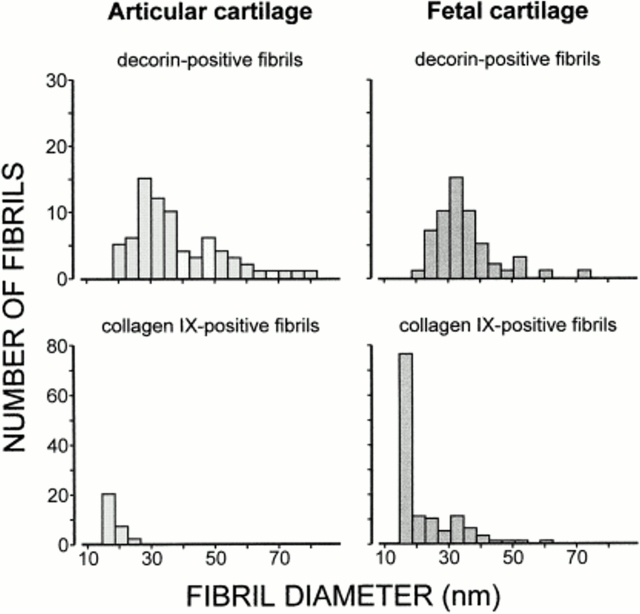
The observation that fibril fragments could be distinguished by the presence or absence of decorin and collagen IX, respectively, suggested that separate fibril populations exist in adult articular cartilage. To better characterize such populations, a systematic analysis of diameter distributions was performed in a large number of electron micrographs. The diameters of decorin-positive fibrils varied within a broad range (Fig. 7, top left). However, it was obvious that fibril fragments with diameters <19 nm were excluded from this population, and that slightly thicker fibrils (19–26 nm) were underrepresented. Similar results were obtained with samples from fetal cartilage (Fig. 7, top right). The decorin-positive fibril fragments of fetal cartilage showed a narrower diameter distribution than those of adult cartilage, but this could be a consequence of the general scarcity of thick fibril fragments in the fetal material. Labeling for collagen IX showed the opposite characteristic. In samples from adult articular cartilage, only fibrils within the diameter range of 15–26 nm were collagen IX–positive (Fig. 7, bottom left). By contrast, in samples from fetal cartilage with predominantly thin fibrils, collagen IX was detected along fibrils of all diameters (Fig. 7, bottom right).
Figure 7.
Distribution of diameters of extracted fibril fragments from adult articular cartilage and fetal bovine cartilage stained for decorin or collagen IX. Only fibrils with an average density of ≥5 gold particles per 10 D-periods were considered in this analysis. Thereby, labeling above background was ensured in all cases.
The results shown in Fig. 7 do not exclude the possibility that fibrils of intermediate size, labeled for decorin and collagen IX, respectively, were derived from distinct populations. However, double-labeling experiments revealed that decorin and collagen IX coexisted in many fibrils of intermediate diameters (Fig. 8). In particular, fetal cartilage fibril fragments were frequently double-labeled, except those with diameters <19 nm (Fig. 9).
Figure 8.
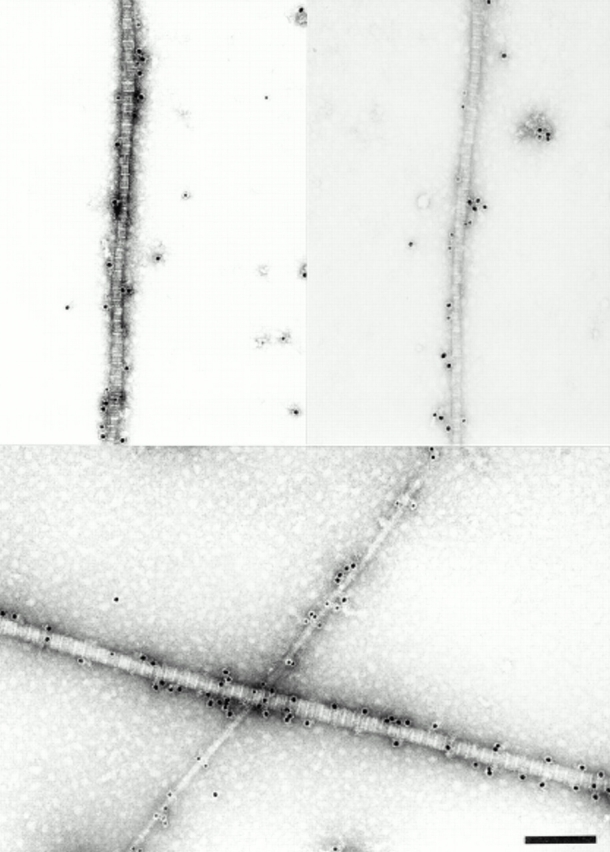
Combined immunolocalization of decorin and collagen IX on fibril fragments from adult bovine articular cartilage. Rabbit anti-decorin antibodies were detected by 18-nm gold probes and mouse anti-collagen IX antibodies by 12-nm probes. Two representative examples of fibrils with diameters between 30 and 40 nm are shown (top). These fibrils, although weakly labeled (<5 gold particles per 10 D-periods), demonstrate colocalization of the two fibril constituents. A typical thin fibril carries dense label with collagen IX-probes only, whereas the thick 60-nm fibril is labeled exclusively for decorin (bottom). Bar, 200 nm.
Figure 9.
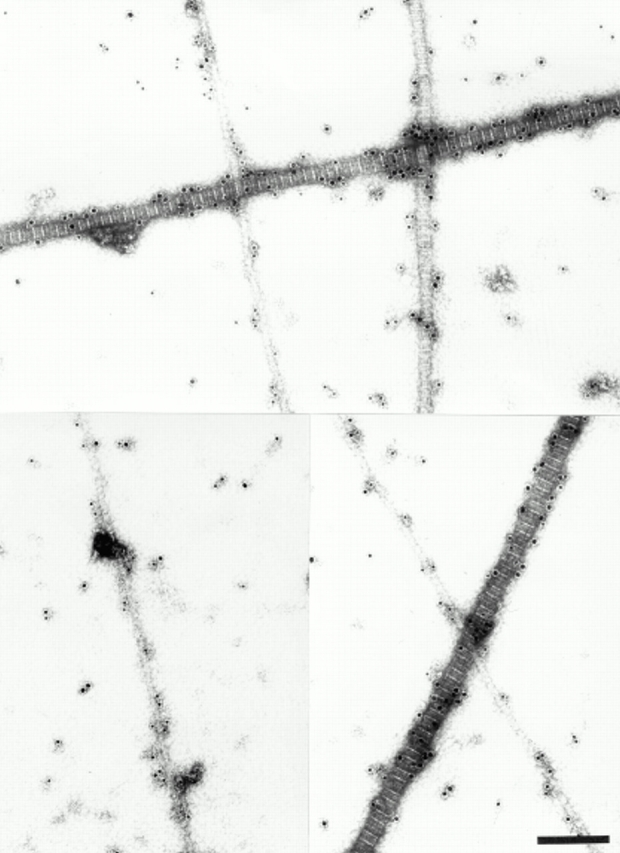
Combined immunolocalization of decorin and collagen IX in fetal bovine cartilage extracts. Rabbit anti-decorin antibodies were detected by 18-nm gold probes and mouse anti-collagen IX antibodies by 12-nm probes. Note that fibrils of all diameters are labeled with collagen IX-probes. The coexistence of decorin and collagen IX is obvious for fibrils thicker than 20 nm. Bar, 200 nm.
Immunohistochemical Localization of Decorin and Collagen IX in Articular Cartilage
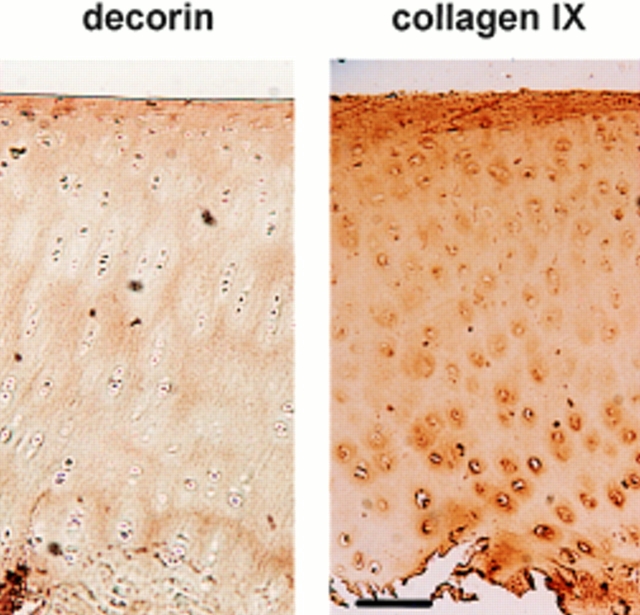
To compare the distribution of decorin with that of collagen IX in adult cartilage tissue, immunohistochemistry was used. The staining patterns demonstrated clearly that the two matrix components have different spatial distribution within articular cartilage in vivo (Fig. 10). In the deep zone, representing about two thirds of the tissue, decorin was detectable mainly in the interterritorial matrix. Collagen IX, on the other hand, was found mainly in the territorial regions that surround the chondrocytes. Strong staining of both matrix components was apparent in the superficial zone that represents ∼10% of the cartilage.
Figure 10.
Localization of decorin and collagen IX in adult bovine articular cartilage. Sections of tarsometatarsal joint cartilage were incubated with antiserum against decorin (left) and collagen IX (right). Bound antibodies were detected by indirect immunoperoxidase staining. Decorin and collagen IX show differential distribution with partial overlapping. Both appear enriched in the most superficial cartilage. In the zone representing the deeper two-thirds of the tissue, strong staining for collagen IX is restricted to the territorial matrix surrounding the chondrocytes. Staining for decorin in the deep zone, by contrast, is strongest in the interterritorial matrix. Bar, 70 μm.
Discussion
In this study, we clearly show that the collagen-containing fibrils of mammalian hyaline cartilage are biochemically heterogeneous, even if they are morphologically similar. Decorin and collagen IX are differentially distributed among the fibrils. The majority of fibril fragments in homogenates of adult bovine articular cartilage contained one of these two constituents, but not the other. Furthermore, it was notable that a few of the fibril fragments contained both decorin and collagen IX. Such coexistence of the two molecules was conspicuous in samples from fetal epiphyseal cartilage, where collagen IX was present in fibrils of all diameters. Thus, a second conclusion is that decorin and collagen IX sometimes occur adjacent to each other.
The interaction between decorin and the collagenous scaffold of fibrils so far has been addressed by ultrastructural immunolocalization, and by in vitro binding studies. In addition, the physiological role of decorin has been studied in mice with decorin deficiency. Ultrastructural immunolocalization in placenta (Ruoslahti, 1988), pericardium (Simionescu et al., 1989), tendon (Pringle and Dodd, 1990), skin (Fleischmajer et al., 1991), and articular cartilage (Miosge et al., 1994) revealed staining close to or at striated fibrils, suggesting that decorin was a component of collagen-containing fibrils. In parallel, decorin strongly bound to monomeric and fibrillar collagens I and II in vitro with apparent dissociation constants of 10−8–10−9 M (Hedbom and Heinegård 1989; Schönherr et al., 1995; Svensson et al., 1995). However, there was no direct proof for tight binding of decorin to collagen in tissues. The case of cartilage was particularly ambiguous. Localization of decorin in human knee joint cartilage by immunoelectron microscopy showed label preferentially on globular structures between the fibrils, while label directly associated with fibrils was sparse (Miosge et al., 1994). In addition, targeted disruption of the decorin gene in mice, although resulting in conspicuously abnormal fibril morphology in skin and tendon, did not affect skeletal development (Danielson et al., 1997). This result implies that cartilage ultrastructure may have been normal in these animals. Our results here show unambiguously that decorin is a cartilage fibril component. Moreover, decorin binding is mediated by noncovalent protein-dependent interactions. Interestingly, the thinnest fibrils were devoid of decorin, whereas the surface of the fibril fragments with diameters larger than 26 nm was frequently labeled for the proteoglycan. In support of these results, immunohistochemical detection of decorin in adult articular cartilage here (Fig. 10) and in previous studies (Poole et al., 1986; Miosge et al., 1994) indicates that the proteoglycan mainly occurs in the superficial zone and in the deep zone interterritorial compartments, while it seems to be absent from the pericellular compartments where thin unbanded fibrils predominate. Thus, decorin-positive and decorin-negative fibrils appear to be accumulated in separate regions of mature mammalian articular cartilage. Since the matrix of the superficial zone contains arrays of essentially parallel fibrils, and the same holds true for the interterritorial regions of the deep zone, it is tempting to speculate that decorin is needed for maintenance of correct fibril-to-fibril spacing. A comparable role has been suggested for decorin in the organization of parallel collagen I–containing fibrils in cornea and tendon (Scott, 1992).
In a model structure presented by Weber et al. (1996), the decorin core protein has an arch shape similar to the porcine ribonuclease inhibitor (Kobe and Deisenhofer, 1993). The inner concave surface accommodates a single triple- helical collagen molecule. The specific binding sites for decorin are located in the gap zone of the fibril D-period (Fig. 11). In decorin, parts of the central domain and probably also the COOH-terminal portion of the core protein are involved in the collagen association. As a consequence, the glycosaminoglycan side chain located near the NH2-terminus would be relatively free to align in various directions, and this polyanionic chain would qualify well for interactions with extrafibrillar structures.
Figure 11.
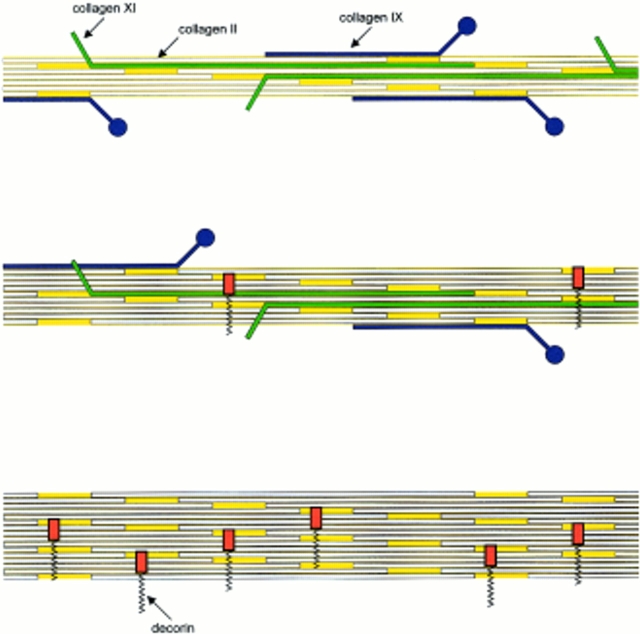
Schematic representation of the biochemical heterogeneity of D-periodic fibrils in mature articular cartilage. The thinnest fibrils (17–20 nm) are enriched in collagens IX and XI. Fibrils of intermediate size have lower contents of collagen IX, and the small proteoglycan decorin can occur as an additional component. The coexistence of collagen IX and decorin on the fibril surface is a notable feature. The thickest fibrils lack collagens IX and XI, but they frequently contain decorin. The suggested distribution of collagen IX and decorin is based on our data, whereas the distribution of collagen XI is drawn by analogy to that observed in human rib and fetal calf cartilage (Keene et al., 1995).
The suprastructure of collagen IX is known mainly from studies with avian embryonic cartilage. Fibrils in this tissue have a small and uniform diameter, are randomly oriented, and contain collagen IX homogeneously. Fibrils with the same characteristics are also found in mature mammalian articular cartilage, but are largely confined to its pericellular compartments. Immunohistochemical examination of pig articular cartilage showed strong staining of collagen IX in the territorial matrix surrounding the chondrocytes (Duance at al., 1982; Poole et al., 1988). At the ultrastructural level, collagen IX was preferentially colocalized with thin unbanded fibrils, while the large banded fibrils of the interterritorial matrix were only weakly labeled (Wotton et al., 1988). However, possible immunochemical masking may have complicated the interpretation of these results. Here we show that collagen IX in extracts from adult mammalian articular cartilage is specifically associated with the thinnest fibrils. Furthermore, the specific localization of collagen IX in the proximity of the chondrocytes was confirmed by immunohistochemistry. Hence, in fully developed load-bearing cartilage, collagen IX is a typical component of the fine fibrillar network of pericellular regions. A function of collagen IX, therefore, might be to limit lateral growth of fibrils through binding to the fibril surface. However, some important observations are inconsistent with this hypothesis. Cartilage of collagen IX–deficient mice contained fibrils that were morphologically indistinguishable from those of control mice (Hagg et al., 1997). Furthermore, the present study indicates that collagen IX is also abundant in some of the thicker fibrils from fetal bovine cartilage, and similar results have been obtained previously with human fetal cartilage (Bruckner et al., 1988). An alternative role of collagen IX may be modulation of the fibril surface for appropriate physical contacts with the environment. According to this view, collagen IX is required for long-term biomechanical stability of cartilage, but is not essential for ordered assembly of fibril components during cartilage development (Olsen, 1995). The observation here that the occurrence of collagen IX was restricted to thin fibrils in adult but not in fetal cartilage, suggests that collagen IX becomes separated from thicker fibrils when cartilage undergoes maturation. It can yet only be speculated on how such a separation arises. It should be noted, however, that if the thicker fibrils that lack collagen IX are derived from collagen IX–rich fibrils, a considerable degree of proteolytic processing and rebuilding has to take place.
Comparable to the cases of decorin and collagen IX, different fibril populations may be defined based on their content of collagen XI, another minor component of cartilage fibrils. An examination of the ultrastructural localization of collagen XI in the growth plate of human rib and fetal bovine epiphyseal cartilage demonstrated that this molecule was associated predominantly with fibrils <∼25 nm in diameter (Keene et al., 1995). The long triple helical domain of collagen XI appears to be buried within the fibril body, and not exposed at the fibril surface (Mendler et al., 1989). Therefore, if the data were correctly interpreted, a hypothetical remodeling of thin fibrils to generate the thicker ones must involve a dramatic biochemical modification that includes excision of collagen XI.
The different fibril populations identified in mature articular cartilage are schematically illustrated in Fig. 11. They share collagen II as the quantitatively major constituent, but are distinct in their contents of decorin and collagen IX. A strong correlation between the fibril diameter and the occurrence of decorin and minor collagens is apparent. The mechanisms by which these populations arise in the cartilage matrix are still unknown. Theoretically, the noticed biochemical differences between large and small diameter fibrils would be consistent with a model of fibril formation in which the different populations arise independently. However, this view is difficult to reconcile with the fact that only minute amounts of diffusible intact collagen monomers are present in the extracellular matrix of hyaline cartilage (Mayne et al., 1995). Furthermore, in cartilage there is an absence of short-lived collagenous aggregates that could represent intermediate stages of fibrillogenesis. Such precursors of fibril assembly have been observed in developing soft connective tissues (Birk et al., 1995). Consequently, it has been concluded that the large continuous fibrils characteristic of the mature tendon are generated by postdepositional fusion of fibril segments. For hyaline cartilage, available data suggest that the small-diameter fibrils are the precursors in the formation of the larger diameter fibrils. By contrast to the nascent fibrils of embryonic tendon, these early collagenous aggregates in the cartilage matrix soon become highly cross-linked. Their constituent collagen molecules, therefore, are not readily dissociated and redistributed. Obviously these thin fibrils represent lasting functional units and cannot be regarded solely as intermediates in cartilage maturation.
As stated already, the model in which the thicker fibrils are formed by reorganization and growth of thin fibrils requires extensive biochemical modification of the precursors. A high degree of proteolytic processing is necessary for adequate removal of collagens IX and XI. These processes obviously must take place at extracellular sites located far away from the chondrocyte. Therefore, a cellular control of this development would probably be indirect, and could involve secretion of inhibitors that prevent corresponding reactions in the matrix close to the cells. The view that morphogenesis of articular cartilage depends on exact tuning of degradative enzyme activities at defined locations within the extracellular matrix may help to explain pathological mechanisms involved in degenerative joint disease and some chondrodysplasias. However, this concept is based on several assumptions that are not yet proven by experimental data. Future studies will show to what extent these theoretic considerations apply to real facts.
Acknowledgments
We thank Dr. Horst Robenek for generously providing EM facilities, and Dr. David Troyer for expertise and kind advice in the use of EM techniques.
This work was supported by research grants from Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 310, B12.
Footnotes
Address all correspondence to Erik Hedbom, Institute of Physiological Chemistry and Pathobiochemistry, Waldeyerstrasse 15, D-48149 Münster, Germany. Tel: 49-251-8355570; Fax: 49-251-8355596.
References
- Birk DE, Fitch JM, Babiarz JP, Linsenmayer TF. Collagen type I and type V are present in the same fibrils in the avian corneal stroma. J Cell Biol. 1988;106:999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev Dyn. 1995;202:229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- Brown DC, Vogel KG. Characteristics of the in vitro interaction of a small proteoglycan (PGII) of bovine tendon with type I collagen. Matrix. 1989;9:468–478. doi: 10.1016/s0934-8832(11)80016-8. [DOI] [PubMed] [Google Scholar]
- Bruckner P, Mendler M, Steinmann B, Huber S, Winterhalter KH. The structure of human collagen type IX and its organization in fetal and infant cartilage fibrils. J Biol Chem. 1988;263:16911–16917. [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duance VC, Shimokomaki M, Bailey AJ. Immunofluorescence localization of type M collagen in articular cartilage. Biosci Rep. 1982;2:223–227. doi: 10.1007/BF01136720. [DOI] [PubMed] [Google Scholar]
- Eikenberry, E.F., M. Mendler, R. Bürgin, K.H. Winterhalter, and P. Bruckner. 1992. Fibrillar organization in cartilage. In Articular Cartilage and Osteoarthritis. K.E. Kuettner, R. Schleyerbach, J.G. Peyron, and V.C. Hascall, editors. Raven Press, Ltd., New York. 133–149.
- Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, ELISA. J Immunol. 1972;109:129–135. [PubMed] [Google Scholar]
- Eyre, D.R., J.J. Wu, and P. Woods. 1992. Cartilage-specific collagens—structural studies. In Articular Cartilage and Osteoarthritis. K.E. Kuettner, R. Schleyerbach, J.G. Peyron, and V.C. Hascall, editors. Raven Press, Ltd., New York. 119–131.
- Fässler R, Schnegelsberg PNJ, Dausman J, Shinya T, Muragaki Y, McCarthy MT, Olsen BR, Jaenisch R. Mice lacking α1(IX) collagen develop noninflammatory degenerative joint disease. Proc Natl Acad Sci USA. 1994;91:5070–5074. doi: 10.1073/pnas.91.11.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R, Fisher LW, MacDonald ED, Jacobs L, Perlish JS, Termine JD. Decorin interacts with fibrillar collagen of embryonic and adult human skin. J Struct Biol. 1991;106:82–90. doi: 10.1016/1047-8477(91)90065-5. [DOI] [PubMed] [Google Scholar]
- Hagg R, Hedbom E, Möllers U, Aszódi A, Fässler R, Bruckner P. Absence of the α1(IX) chain leads to functional knock-out of the entire collagen IX protein in mice. J Biol Chem. 1997;272:20650–20654. doi: 10.1074/jbc.272.33.20650. [DOI] [PubMed] [Google Scholar]
- Hedbom E, Heinegård D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989;264:6898–6905. [PubMed] [Google Scholar]
- Hedbom E, Heinegård D. Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J Biol Chem. 1993;268:27307–27312. [PubMed] [Google Scholar]
- Hedlund H, Mengarelli-Widholm S, Reinholt FP, Svensson O. Stereologic studies on collagen in bovine articular cartilage. APMIS. 1993;101:133–140. doi: 10.1111/j.1699-0463.1993.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Heinegård D, Björne-Persson A, Cöster L, Franzen A, Gardell S, Malmström A, Paulsson M, Sandfalk R, Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues from distinct subgroups. Biochem J. 1985;230:181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker, E.B., and W. Herrmann. 1990. Ultrastructure of cartilage. In Ultrastructure of skeletal tissues. E. Bonucci and P.M. Motta, editors. Kluwer Academic Publishers, Norwell, MA. 79–109.
- Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- Irwin MH, Silvers SH, Mayne R. Monoclonal antibody against chicken type IX collagen: Preparation, characterization, and recognition of the intact form of type IX collagen secreted by chondrocytes. J Cell Biol. 1985;101:814–823. doi: 10.1083/jcb.101.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene DR, Oxford JT, Morris NP. Ultrastructural localization of collagen types II, IX, and XI in the growth plate of human rib and fetal bovine epiphyseal cartilage: Type XI collagen is restricted to thin fibrils. J Histochem Cytochem. 1995;43:967–979. doi: 10.1177/43.10.7560887. [DOI] [PubMed] [Google Scholar]
- Kobe B, Deisenhofer J. Crystal structure of porcine ribonuclease inhibitor, a protein with leucine-rich repeats. Nature. 1993;366:751–756. doi: 10.1038/366751a0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane JM, Weiss C. Review of articular cartilage collagen research. Arthritis Rheum. 1975;18:553–562. doi: 10.1002/art.1780180605. [DOI] [PubMed] [Google Scholar]
- Mayne, R., M. van der Rest, P. Bruckner, and T. Schmid. 1995. Approaches for isolating and characterizing the collagens of cartilage (types II, IX, X, and XI) and the type IX-related collagens of other tissues. In Extracellular Matrix. A Practical Approach. M.A. Haralson and J.R. Hassell, editors. Oxford University Press, Oxford, United Kingdom. 73–97.
- Mendler M, Eich-Bender SG, Vaughan L, Winterhalter KH, Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989;108:191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miosge N, Flachsbart K, Götz W, Schultz W, Kresse H, Herken R. Light and electron microscopical immunohistochemical localization of the small proteoglycan core proteins decorin and biglycan in human knee joint cartilage. Histochem J. 1994;26:939–945. [PubMed] [Google Scholar]
- Müller-Glauser W, Humbel B, Glatt M, Sträuli P, Winterhalter KH, Bruckner P. On the role of type IX collagen in the extracellular matrix of cartilage: type IX collagen is localized to intersections of collagen fibrils. J Cell Biol. 1986;102:1931–1939. doi: 10.1083/jcb.102.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen BR. New insights into the function of collagens from genetic analysis. Curr Opin Cell Biol. 1995;7:720–727. doi: 10.1016/0955-0674(95)80115-4. [DOI] [PubMed] [Google Scholar]
- Poole AR, Pidoux I, Reiner A, Rosenberg L. An immunoelectron microscopy study of the organization of proteoglycan monomer, link protein, and collagen in the matrix of articular cartilage. J Cell Biol. 1982;93:921–937. doi: 10.1083/jcb.93.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AR, Webber C, Pidoux I, Choi H, Rosenberg LC. Localization of a dermatan sulfate proteoglycan (DS-PGII) in cartilage and the presence of immunologically related species in other tissues. J Histochem Cytochem. 1986;34:619–625. doi: 10.1177/34.5.3701029. [DOI] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW. Morphological and functional interrelationships of articular cartilage matrices. J Anat. 1984;138:113–138. [PMC free article] [PubMed] [Google Scholar]
- Poole CA, Wotton SF, Duance VC. Localization of type IX collagen in chondrons isolated from porcine articular cartilage and rat chondrosarcoma. Histochem J. 1988;20:567–574. doi: 10.1007/BF01002611. [DOI] [PubMed] [Google Scholar]
- Pringle GA, Dodd CM. Immunoelectron microscopic localization of the core protein of decorin near the D-bands and E-bands of tendon collagen fibrils by use of monoclonal antibodies. J Histochem Cytochem. 1990;38:1405–1411. doi: 10.1177/38.10.1698203. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Ann Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Schönherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of biglycan with type I collagen. J Biol Chem. 1995;270:2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- Scott JE. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992;6:2639–2645. [PubMed] [Google Scholar]
- Simionescu D, Iozzo RV, Kefalides NA. Bovine pericardial proteoglycan: biochemical, immunochemical and ultrastructural studies. Matrix. 1989;9:301–310. doi: 10.1016/s0934-8832(89)80006-x. [DOI] [PubMed] [Google Scholar]
- Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Svensson L, Heinegård D, Oldberg Å. Decorin-binding sites for collagen type I are mainly located in leucine-rich repeats 4-5. J Biol Chem. 1995;270:20712–20716. doi: 10.1074/jbc.270.35.20712. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets; procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler JM, Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980;71:95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Vaughan L, Mendler M, Huber S, Bruckner P, Winterhalter KH, Irwin MI, Mayne R. D-periodic distribution of collagen IX along cartilage fibrils. J Cell Biol. 1988;106:991–997. doi: 10.1083/jcb.106.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and inplications for collagen fibrillogenesis. J Biol Chem. 1996;271:31767–31770. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- Wotton SF, Duance VC, Fryer PR. Type IX collagen: a possible function in articular cartilage. FEBS Lett. 1988;234:79–82. doi: 10.1016/0014-5793(88)81307-3. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Woods PE, Eyre DR. Identification of cross-linking sites in bovine cartilage type IX collagen reveals an antiparallel type II-type IX molecular relationship and type IX to type IX bonding. J Biol Chem. 1992;267:23007–23014. [PubMed] [Google Scholar]