Abstract
By co-injecting fluorescent tubulin and vinculin into fish fibroblasts we have revealed a “cross talk” between microtubules and early sites of substrate contact. This mutuality was first indicated by the targeting of vinculin-rich foci by microtubules during their growth towards the cell periphery. In addition to passing directly over contact sites, the ends of single microtubules could be observed to target several contacts in succession or the same contact repetitively, with intermittent withdrawals. Targeting sometimes involved side-stepping, or the major re-routing of a microtubule, indicative of a guided, rather than a random process. The paths that microtubules followed into contacts were unrelated to the orientation of stress fiber assemblies and targeting occurred also in mouse fibroblasts that lacked a system of intermediate filaments. Further experiments with microtubule inhibitors showed that adhesion foci can: (a) capture microtubules and stabilize them against disassembly by nocodazole; and (b), act as preferred sites of microtubule polymerization, during either early recovery from nocodazole, or brief treatment with taxol. From these and other findings we speculate that microtubules are guided into substrate contact sites and through the motor-dependent delivery of signaling molecules serve to modulate their development. It is further proposed this modulation provides the route whereby microtubules exert their influence on cell shape and polarity.
Locomoting metazoan cells rely primarily on the dynamic reorganization of their actin cytoskeleton to move. But depending on the cell type, the persistence and directionality of movement is more or less dependent on an intact microtubule system. In the presence of microtubule inhibitors, fibroblasts typically lose the ability to polarize (Vasiliev and Gelfand, 1976) and leukocytes and macrophages may still move, but undergo random, instead of directional migration in a chemotactic gradient (Mareel and De Mets, 1984). The rate of spreading of freshly seeded fibroblasts on a glass substrate is also markedly reduced when the microtubule system is disassembled and the spreading process, involving lamellipodium protrusion, is uncoordinated around the cell periphery (Ivanova et al., 1976; De Brabander et al., 1977). According to these observations, Vasiliev and Gelfand (1976) attributed microtubules with a role in determining the stable and active (protrusive) regions of the cytoplasm, and thereby the polarity of a migrating cell. In this role, microtubules were not seen as providers of structural support, but as cellular highways for relaying components or signals which modulate the tension of the actin cytoskeleton (Vasiliev and Gelfand, 1976; De Brabander et al., 1977). More direct evidence in support of this view is now beginning to emerge.
In consequence of the seminal studies of Hall and colleagues (Ridley, 1996; Tapon and Hall, 1997) signaling pathways are currently being unveiled that mediate the assembly of the different subcompartments of the actin cytoskeleton. Thus, protrusion of lamellipodia and filopodia are signaled by the small G proteins rac and cdc42, respectively; and the assembly of stress fibers and focal contacts required for anchorage are signalled by rho. Significantly, the assembly and activity of both the protrusive and anchorage compartments of the actin cytoskeleton are influenced by microtubules, suggesting that they may well modulate these same signaling pathways. In this connection, drug-mediated microtubule disruption stimulates the growth of stress fibers and focal contacts (Lloyd et al., 1977), an effect that is particularly dramatic in starved cells (Bershadsky et. al. 1996; Enomoto, 1996), and which correlates with the activation of rho (Enomoto, 1996).
In the same vein, the decreased rate of spreading of cells in the presence of microtubule inhibitors correlates with slower rates of lamellipodia protrusion (Bershadsky et al., 1991), which is dependent on rac. A similar effect is seen in non-inhibited cells microinjected with a function-blocking kinesin antibody (Rodionov et al., 1993), suggesting perhaps that components transported by molecular motors may modulate or be involved in rac signaling. Notably, the translocation of fibroblasts and neuronal growth cones is also blocked by low concentrations of microtubule inhibitors that do not destroy microtubules, but only inhibit their growth dynamics (Liao et al., 1995; Tanaka et al., 1995). And an increase in the dynamic turnover of microtubules is seen in epithelial cells exposed to scatter factor (Wadsworth and Battaro, 1996), which induces ruffling activity via the activation of rac (Ridley et al., 1995). So the dynamic turnover of microtubules appears to be essential for them to perform at least some of their modulatory roles.
In a previous study from this laboratory (Rinnerthaler et al., 1988) a frequent association was noted between vinculin-containing contact sites at the base of fibroblast lamellipodia and the ends of microtubules that radiated towards the cell periphery. This finding was taken to suggest the involvement of microtubules in the regulation of contact site formation, thus providing a possible link between the microtubule and actin systems. But was this colocalization fortuitous, or do microtubules directly target contact sites? And if so, how can this targeting activity of microtubule ends into early contacts be reconciled with the amplification in contact number and size seen on microtubule disassembly? With the aim of answering some of these questions, we have investigated the interrelationship between microtubules and contact sites in living cells co-injected with fluorescent tubulin and vinculin. From these and additional experiments we show not only that microtubules directly and temporarily target contact sites, but that contact sites can capture microtubules, stabilize them against depolymerization and have the potential to nucleate microtubule assembly. The possible function of microtubule targeting in contact genesis and the involvement of molecular motors is discussed.
Materials and Methods
Cells
Three standard fibroblast cell lines were used in this study. Mouse embryo fibroblasts (Swiss 3T3) and rat embryo fibroblasts (REF-52) were maintained in DME with either 10% FBS (3T3) or 10% fetal calf serum (REF-52) at 37°C in the presence of 5% CO2. Goldfish fin fibroblasts (line CAR; American Type Culture Collection, Rockville, MD [No. CCL71]) were maintained in basal Eagle medium with HBSS and non-essential amino acids, and with 15% fetal calf serum at 25°C. Goldfish cells were plated onto coverslips coated with human serum fibronectin (Boehringer Mannheim GmbH, Vienna, Austria); for some experiments with REF-52 cells, both fibronectin and poly-l-lysine (p1274; Sigma Chemical Co., Vienna, Austria) coating was used. For poly-l-lysine coating, coverslips were incubated on a drop of aqueous 100 μg/ml poly-l-lysine for 30 min at room temperature, rinsed with water, and then dried. Fibronectin was coated onto poly-l-lysine–treated coverslips by incubation on a drop of 50 μg/ml fibronectin in PBS at 4°C overnight; after rinsing in PBS these coverslips were used without drying. 3T3 and REF-52 cells were otherwise grown on uncoated coverslips.
For investigations of focal complexes, a porcine fetal testicular cell line (CRL 1746; American Type Culture Collection) was used. The vimentin knockout cell line was obtained as a subclone of SV-40 transformed fibroblasts derived originally by Holwell et al. (1997) from a vimentin knockout mouse (Colucci-Guyon et al., 1994) and generously donated by P. Traub (Max-Planck Institute, Heidelberg, Germany). These cells were plated onto coverslips coated with 50 μg/ml human fibronectin (Boehringer Mannheim GmbH) to enhance spreading.
Microtubule Antagonists
Nocodazole (Sigma Chemical Co.) was added to culture medium from a 5 mg/ml stock solution in DMSO. Tests of the relative stability of microtubules were performed using doses of 1.5 μg/ml for 10–20 min or 2.5 μg/ml for 5–10 min. Complete depolymerization of microtubules was achieved using a concentration of 2.5 μg/ml for 3 h. In one series of microtubule nucleation experiments, a low concentration (0.05 μm) of taxol (paclitaxel; Sigma Chemical Co.) was added for 1 h to the culture medium. Taxol was stored as an 8.54 mg/ml (10 mM) stock solution in DMSO.
Microscopy of Living Cells
Cells were observed at 37°C (3T3, Ref-52) or room temperature (CAR) on an inverted microscope (Axiovert 135TV; Carl Zeiss, Vienna, Austria) equipped for epifluorescence and phase-contrast microscopy, using 40×/ NA 1.3 Plan-Neofluar or 100×/NA 1.4 Plan-Apochomat objectives, and up to 2.5 optovar intermediate magnification. Data were acquired and stored as 16-bit digital sequences using a back-illuminated, cooled CCD camera (Princeton Research Instruments, Inc., Princeton, NY) driven by IPLabs software (both from Visitron Systems, Eichenau, Germany).
Immunofluorescence
Cells were fixed for 15 min in a mixture of 3% paraformaldehyde, 0.2% glutaraldehyde, and 0.1% Triton X-100 in cytoskeleton buffer (CB; 10 mM MES, 150 mM NaCl, 5 mM EGTA, 5 mM glucose, 5 mM MgCl2, pH 6.1), and then rinsed in the same buffer. Free aldehyde groups were blocked by incubation with 0.5 mg/ml NaBH4 in CB at 0°C for 5 min. The procedures for antibody labeling, washing, and mounting were essentially as described by Herzog et al. (1994). As primary antibodies, we used the following: mouse monoclonal anti-vinculin IgG clone hVIN-1 (Sigma Chemical Co.); mouse monoclonal anti-paxillin IgG (Transduction Laboratories, Lexington, KY); mouse monoclonal anti–Tyr-tubulin IgM clone 27C2 kindly provided by J. Wehland (GBF, Braunschweig, Germany); and Cy3 phalloidin kindly provided by H. Faulstich (Max-Planck Institute). Secondary antibodies and reagents were: goat anti–mouse IgG Cy3-conjugated (Jackson Immunoresearch Laboratories, Inc., West Grove, PA); goat anti–mouse Igs biotinylated (DAKO, Wien, Austria); Goat anti– mouse IgM (μ-chain specific) FITC-conjugated (Sigma Chemical Co.); FITC-streptavidin (DAKO); AMCA–avidin D (Vector Labs., Inc., Burlingame, CA). Preparations were observed and data recorded as described for microscopy of living cells.
Microinjection
Ready-made, sterile Femtotips II (Eppendorf, Hamburg, Germany) were used for microinjection in conjunction with a Leitz Micromanipulator M (Leitz, Vienna, Austria) and an Eppendorf Microinjector 5242 (Eppendorf). The injection pressure was adjusted to 20–30 hPa in the back pressure mode to give a continuous outflow from the needle.
Proteins for Microinjection
Rhodamine-conjugated rat tubulin was kindly provided by R. Tournebize and T. Hyman (European Molecular Biology Laboratory, Heidelberg, Germany) and stored at −70°C in aliquots of 5 μl (∼20 mg/ml) in BRB80 buffer (80 mM potassium Pipes, pH 6.8, 1 mM MgCl2, 1 mM EGTA). For microinjection, aliquots were diluted 1:4 with Tris-acetate injection buffer (2 mM Tris-acetate, pH 7.0, 50 mM KCl, 0.1 mM DTE), and then used on the same day. Turkey vinculin, kindly provided by M. Gimona, was conjugated with Cy2 (Amersham Corp., Arlington Heights, IL) or TAMRA (carboxytetramethylrhodamin; Molecular Probes, Inc., Eugene, OR) according to the manufacturer's instructions. The coupled protein (1 mg/ml) was stored in Tris acetate injection buffer at −70°C in the presence of sucrose (2 mg sucrose per 1 mg protein) and was dialyzed against injection buffer before use.
Fluorescent proteins were injected separately, first vinculin, followed by tubulin 5–10 min later. Mixing of probes led to a decreased incorporation of vinculin at contact sites. Control experiments showed that microinjected Rh-vinculin localized to all contact sites labeled with antibodies applied to the same cells after fixation. Injected cells could be traced and observed for periods of up to 3 h after injection.
Analysis of Targeting in Advancing Lamellae and of Multiple Targeting
Six cells were chosen in which there was a net advance of the cell front over the recorded period. A rectangle, 4.5-μm wide and extending across the full width of the cell was then overlaid on the first frame of the video just behind and parallel to the cell front and maintained in the same position for the whole sequence. The percentage of contacts in this rectangle targeted by microtubules was then recorded for all frames in the sequence. The time between frames was either 17 (cells 2, 3, 4, and 6) or 22 s (cells 1 and 5). The total number of contacts per rectangle per cell ranged from 18 to 32. For each cell, a pattern of “dummy contacts” was also created by flipping the rectangle of real contacts by 180 degrees around a line perpendicular to the center of its long axis. The analysis was then repeated with this dummy contact pattern.
The analysis of multiple targeting of single contacts was carried out in the thin regions of spread cells and for a time period of 10 min. A dummy contact pattern was created as above and the analysis repeated. Data was collected for a total of 276 real contacts and 267 dummy contacts, in five cells. The two groups were compared using a Mann-Whitney Rank Sum test confirming a statistically significant difference (P < 0.0001). Statistical analysis was carried out using SigmaStat version 2.0 (Jandel Corp., San Rafael, CA).
Analysis of Microtubule Capture and of the Excursion of Microtubule Ends
Capture of microtubules was assayed in cells treated with 1.5 μg/ml nocodazole by measuring the length of those microtubules whose ends could be traced throughout the sequence. Zero time was taken as the time of nocodazole addition and the end point of each microtubule in the last frame of the sequence was registered as a length of 1 pixel. Lengths were traced using IPLabs segment tools and the frame separation was as above. The dynamic excursion of microtubule ends was measured from sequences recorded using an interval of 6 s between frames. All plots were made using KaleidaGraph version 2.3.1 (Synergy Software, Inc., Reading, PA).
Results
Microtubules Target Peripheral, Substrate Contact Sites
Microtubules and vinculin-containing contact sites have characteristic and very different morphologies. It was therefore possible to follow them simultaneously in living goldfish fibroblasts when both were labeled with a rhodamine conjugate. Experiments were also performed with rhodamine tubulin and Cy-2 vinculin with essentially the same result; however, the spatial interrelationships could then only be ascertained after image superposition and correction for any shifts resulting from filter changes. The video sequences shown are hence those taken in a single fluorescence channel, for which direct and immediate correlations could be made.
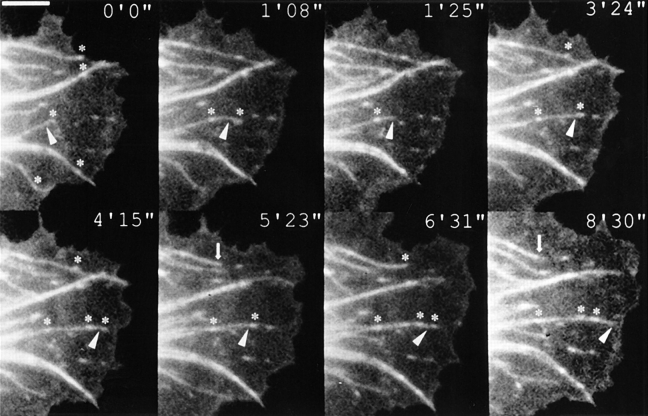
In line with earlier studies (Sammak and Borisy, 1988) we observed that peripheral microtubules grew radially towards expanding, lamella regions of the cell periphery. For cells co-injected with fluorescent vinculin a striking correlation was seen between the paths followed by microtubules and the position of newly formed contact sites (Fig. 1). In this example of a moving cell front, all microtubules passed through or terminated in vinculin containing contact sites (highlighted with neighboring asterisks). The microtubule marked by an arrowhead successively targeted four contact sites during its extension to the cell periphery. To do this, a sidestep was necessary at time 1 min 8 s. Another microtubule at the top of the figure, retracted from its contact target (marked by the upper asterisk at 4′15′′ in Fig. 1) at 5 min 23 s, targeted it again at 6 min 31 s, and then retracted once more at 8 min 30 s.
Figure 1.
Microtubule targeting of focal adhesions. Figure shows selected frames from a video sequence of a goldfish fibroblast co-injected with rhodamine tubulin and TAMRA vinculin. Some of the focal contacts crossed by microtubules are indicated by asterisks. The ends of two microtubules (arrowhead and arrow) are highlighted. One (arrowhead) targeted successive adhesion sites on its way to the cell periphery, and the other (arrow, 5′23′′–8′30′′) targeted and withdrew from a focal adhesion. For further details, see text. The inset times are given in minutes and seconds in this and subsequent figures of video sequences. Bar, 5 μm.
A quantitative analysis of this targeting activity in the advancing lamella regions of six cells is shown in Fig. 2. The way the analysis was carried out is illustrated in Fig. 2 B (see also Materials and Methods). Briefly, the percentage of contacts targeted by microtubules was determined for a given set of contacts confined within a rectangle overlaid just behind the cell front in the first video frame. The targeting of the set of real contacts was compared with that of an equivalent set of dummy contacts created by flipping the real contact pattern 180 degrees (Fig. 2 B). As shown (Fig. 2 A), at least 75% of the real contacts were targeted during the video sequence (7–20 min) as compared with a score of ∼25% for the dummy contacts. These data and the following data indicated that targeting was a directed and not a random process.
Figure 2.
Progressive targeting in advancing lamellae. (A) Plots for six cells of the percentage, over time, of vinculin-containing contacts targeted by microtubules in a pre-selected, rectangular area positioned close to the cell front in the first frame of the video sequence. Solid lines show data for real contacts and broken lines for dummy contacts (see B and text for further details). (B) Graphic illustration of targeting analysis. A rectangle 4.5 μm in width was placed behind the cell front in the first video frame to define the area of analysis of contact targeting. The dummy contact pattern was created by rotating the real contact pattern by 180 degrees about a line through the center and perpendicular to the long axis of the rectangle.
Repetitive Targeting, Multi-directional Targeting, and Re-routing
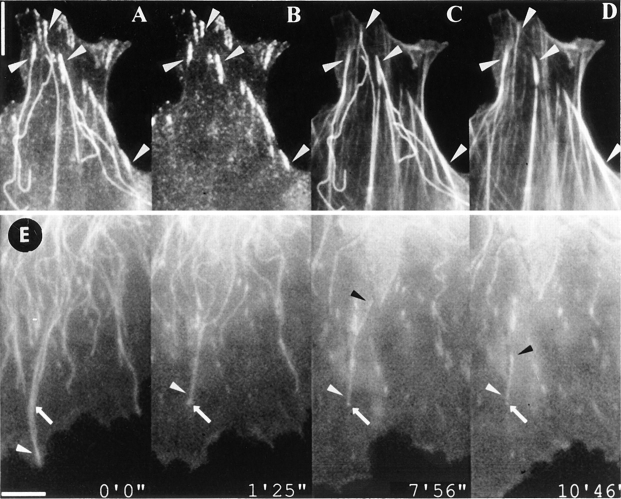
The specificity of the targeting interactions was underlined by the variety of ways in which microtubules reached contact sites or became diverted from one contact to another. The targeting of two widely separated contacts by the same microtubule was notable in this connection and two examples are shown in Fig. 3, A and B. The same examples include the not uncommon instance of repetitive targeting of the same contact.
Figure 3.

Re-routing of microtubules from one contact to another and multiple targeting. Selected video frames of three lamella regions of fish fibroblasts co-injected with fluorescent tubulin and vinculin, as for Fig. 1. Times are given in minutes and seconds. (A) Two contacts on the cell edge (open and closed arrowhead) were targeted by microtubules 1, 2, and 3. Microtubule 2 targeted one contact three times (open arrowhead at 0′44′′, 2′12′′, and 4′24′′), and the other contact once (closed arrowhead, 3′40′′). This involved a dramatic re-routing from one contact to the other and back (2′34′′–4′24′′). (B) Two peripheral contacts (open and closed arrowhead) situated on either side of a retracted cell edge were targeted by the same microtubule. This involved shrinkage to a more proximal contact (asterisk at 2′50′′) followed by growth into the second contact in a direction normal to the first. (C) Example of one contact (solid arrowhead) that was targeted successively by three microtubules (1, 2, and 3) that approached from different directions. Bar, 10 μm.
In the first example, one microtubule (Fig. 3 A, marked 2 at 0′44′′) targeted one contact (open arrowhead) three times, at 0 min 44 s, 2 min 12 s, and 4 min 24 s, and during this period managed to target also a second contact, 3 μm removed from the first (solid arrowhead). This involved a complicated exercise in shortening (Fig. 3 A, 2′34′′), turning, and growth into the second contact (3′40′′), followed by shortening (4′02′′), and growth again into the first (4′24′′). The neighboring microtubules 1 and 3 also targeted the same peripheral contact sites. In the second example (Fig. 3 B), a microtubule targeted two peripheral contacts situated either side of a retracting cell edge. The first contact (Fig. 3 B, solid arrowhead) was targeted at 1 min 25 s, and this was followed by shrinkage to one of the more proximal contact sites that the microtubule had passed through on its way to the cell periphery (asterisk, 2′50′′). The microtubule then grew into the second peripheral contact (Fig. 3 B, open arrowhead, 4′49′′) in a direction approximately normal to the first, targeting this contact twice (at 4′49′′ and 5′57′′).
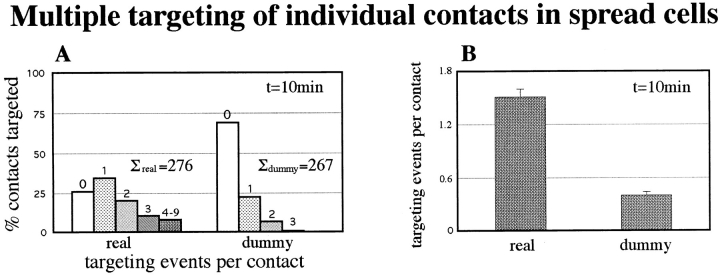
Single contact sites could also be targeted by several microtubules approaching from different directions, indicating again the lack of randomness of the phenomenon. In the example shown in Fig. 3 C, one contact (solid arrowhead) was targeted successively by three microtubules (Fig. 3 C, 1, 2, and 3) whose directions of approach subtended a total angle of around 90 degrees. Fig. 4 shows a quantitative analysis of this multiple targeting activity for individual contacts in the lamella regions of spread cells. The number of times real contacts were targeted was compared with the scores for dummy contacts generated in the same cytoplasmic area, as above. For a total of 270 contacts assayed, the real contacts were targeted up to nine times in the selected time frame of 10 min, as compared with a maximum of three times for the dummy contacts (Fig. 4 A). Further, the number of untargeted dummy contacts was three times higher than the number of real contacts not targeted in the 10-min time frame. The same data, collected in Fig. 4 B, show that real contacts had a probability of being targeted by 1.5 (± 0.09) microtubules and dummy contacts by 0.4 (± 0.04) microtubules.
Figure 4.
Multiple targeting of individual contacts in spread cells. (A) Histogram of the number of targeting events of individual contacts by microtubules in a period of 10 min. The data is presented for real contacts (total 276) and for dummy contacts (total 267). (B) Data in A summarized in terms of the average number of targeting events per contact. See text for further details.
Influence of Contact Association on Dynamic Instability
By tracing the excursions of microtubule ends (seven microtubules in four cells) as they approached and passed over vinculin containing sites, we could define three types of behavior (for this analysis a period of 6 s between frames was used over a total time of up to 4 min). In the first case, fluctuations in microtubule length appeared to be unaffected by the proximity of a contact site (11 out of 30 contacts). In the second, targeting was followed by shrinkage of the microtubule, which could be followed by regrowth into the same contact in a repetitive fashion (7 out of 30 contacts). In the third case, microtubules paused in (up to 20 s; 10 out of 30) or remained associated with a contact site (2 out of 30), the latter situation being most commonly observed with large contacts at the cell periphery.
Contact Sites Stabilize Targeting Microtubules Against Depolymerization by Nocodazole
Experiments in which 3T3 cells were treated with nocodazole at limiting concentrations (0.03–0.2 μg/ml, 30 min) or for short times (2.5 μg/ml, 5 min; or 1.5 μg/ml, 10 min) demonstrated that microtubules whose ends colocalized with vinculin-containing contact sites were conferred with additional resistance to depolymerization (Fig. 5, A–D). The type of contact site showing this property was predominantly associated with the ends of stress fibers close to the cell front.
Figure 5.
Stabilization of microtubules at focal adhesions. (A–D) Figure shows a 3T3 fibroblast that was fixed and triple labeled for actin (D), paxillin (B), and tubulin (A and C) after treatment with 1.5 μg/ml nocodazole for 10 min. All peripheral microtubules disassembled, except those whose ends targeted focal adhesions (arrowheads). (E) Video sequence showing the stabilization of a shrinking microtubule at a focal adhesion. Goldfish fibroblast co- injected with vinculin and tubulin. Frames are taken from a video sequence for which nocodazole (1.5 μg/ ml) was added at time 0. One of a pair of microtubules that extended to the periphery at the beginning of the sequence (white arrowhead) was prevented from shrinking beyond an adhesion site over which it passed (arrow). Eventually, it shrank into this adhesion site via depolymerization at its minus end (black arrowhead). Bars, 5 μm.
The stabilization effect was more dramatically apparent in video sequences of fibroblasts co-injected with tubulin and vinculin (Fig. 5 E). The times indicate the period after addition of nocodazole (1.5 μg/ml). Whereas most microtubules in this example shrank rapidly towards the cell body, one (Fig. 5 E, white arrowheads) was stabilized by a contact site over which it had passed (arrow). The tethered, plus end of the microtubule was protected from depolymerization by the contact and eventually the minus end (Fig. 5 E, black arrowhead) shrank towards (time 10′46′′) and eventually, into the contact site (not shown). This behavior was quantitated in five different cells for microtubules whose ends could be traced throughout the video sequences. From a total of 47 microtubules analyzed, 18 shrank without pause, 18 were transitorily stabilized, with a pause at a contact of 40–300-s duration before finally shrinking and 11 were stabilized and not released by the end of the sequence, remaining in association with a contact site for up to 12 min.
Contact Sites Can Capture and Stabilize Microtubules
Further examples of nocodazole-treated cells showed that contact sites can capture shrinking microtubules that were initially remote from a contact and temporarily prevent their further depolymerization. One such example (Fig. 6) shows a sequence of events before (−) and after (+) addition of nocodazole (1.5 μg/ml). In this series, a microtubule (Fig. 6, arrowhead) oriented along the cell periphery began to shrink and at the same time moved inward, so that it lay over a contact site (arrow, +1′42′′). Shrinking continued rapidly down to the contact site (Fig. 6, +2′16′′), but then abruptly stopped for a further 3 min. Finally (Fig. 6, +5′23′′), the microtubule was released and shrank rapidly into the main cell body. The same type of behavior was observed for microtubule fragments that were spontaneously produced in cells not treated with nocodazole. Such fragments were seen to be captured at a contact site and were then observed to grow with one end tethered to the contact (not shown).
Figure 6.
Capture and stabilization of a microtubule at a focal adhesion that was remote from the contact site at the time of addition of nocodazole. Conditions as for Fig. 4, except that negative and positive times signify before and after nocodazole addition, respectively. Before nocodazole treatment, the microtubule marked with an arrowhead grew and moved laterally and became positioned over a focal adhesion (arrow, 0′44′′). Nocodazole caused rapid shrinkage down to the contact (+1′42′′ –2′16′′), where the end then remained stable for a further 3 min (2′16′′ –5′06′′) before finally shrinking into the cell body (5′23′′– 6′31′′). Bar, 5 μm.
Decreased Stabilization of Microtubules in Cells Lacking Focal Contacts
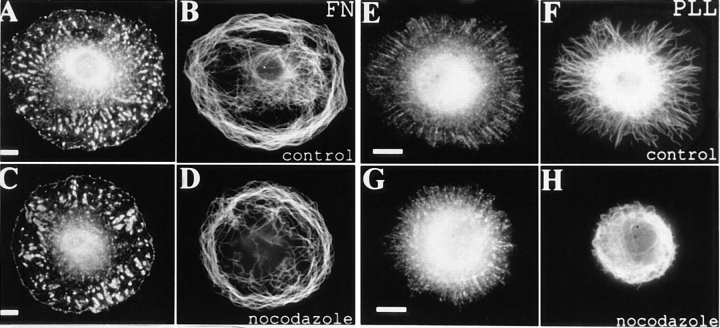
It has formerly been shown that spreading 3T3 cells require extracellular matrix molecules such as fibronectin, to form focal contacts: in the absence of matrix and on polylysine, spreading occurs, but no contact foci are detected (Hotchin and Hall, 1995). The same result is found with REF 52 fibroblasts. On fibronectin substrates, these cells form particularly prominent focal adhesions (Chrzanowska-Wodnicka and Burridge, 1996; Fig. 7 A) whereas on polylysine only a speckled labeling of vinculin, beneath wide lamellipodia is observed (Fig. 7 E). In these two situations the microtubule distribution is markedly different (Fig. 7, B and F): on fibronectin, many microtubules lie parallel to the cell periphery, whereas on polylysine they are radially distributed. In each case however, microtubules remain individual and are not bundled.
Figure 7.
General stabilization of microtubules by focal adhesions in REF-52 fibroblasts. Cells spreading on fibronectin show numerous focal adhesions (A) as compared with a finely punctate vinculin label on polylysine (E). The corresponding microtubule distributions are shown in B and F. After brief nocodazole treatment (1.5 μg/ ml, 10 min) peripheral microtubules in cells plated on fibronectin remain essentially unaffected (D) whereas those in cells spread on polylysine shrink rapidly into the cell body (H). Bar, 10 μm.
The relative stability of microtubules under these two substrate conditions was tested by exposure of cells to 1.5 μg/ml nocodazole for 10 min. As shown in Fig. 7 D, these conditions had little effect on the peripheral microtubules of cells grown on fibronectin, but caused the rapid disassembly of those in cells attached to polylysine (compare Fig. 7, F and H).
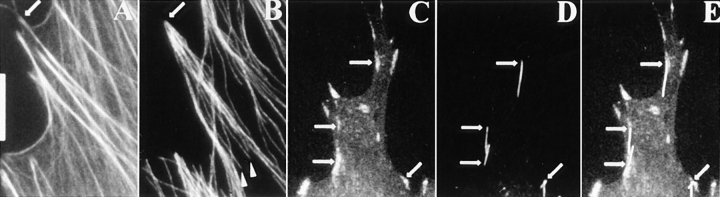
Nobes and Hall (1995) have described a punctate type of contact, termed focal complexes, at the periphery of 3T3 cells stimulated with rac that are not associated with actin stress fiber bundles. Similar complexes are pronounced in cells of a porcine testicular cell line (Fig. 8 A). These peripheral, focal complexes are targeted by microtubules, but are incompetent (in contrast to focal contacts in the same cell) to prevent depolymerization of microtubules in the presence of nocodazole (Fig. 8 B).
Figure 8.
(A and B) Targeting, but not stabilization at focal complexes. Images of porcine testicular cells labeled for tubulin and vinculin: A, control cell; B, cell treated with 1.5 μg/ml nocodazole for 20 min. The focal complexes characteristically found on the edges of these cells are targeted by microtubules (A), but they do not stabilize microtubules against depolymerization by nocodazole (B). (C and D) Targeting and stabilization occurs in the absence of intermediate filaments. Fibroblasts of a mouse vimentin knockout cell line labeled for tubulin and vinculin. C, control cell showing targeting of microtubules to contact sites; D, cell treated with 2.5 μg/ml nocodazole for 10 min showing stabilization of microtubules at focal contacts. Bar, 10 μm.
Microtubule Guidance to Contacts Involves Neither Stress Fiber Bundles Nor Intermediate Filaments
From images of cells triple labeled for vinculin, actin, and tubulin it was evident that the path a microtubule look into a contact site bore no relation to that of the actin filament bundles which terminated in the same site (Fig. 9).
Figure 9.
Microtubules are not guided by stress fibers to contact sites. Goldfish fibroblast triple labeled for actin, vinculin, and tubulin. A, actin pattern revealed with phalloidin; B, overlay of tubulin and vinculin; C, actin pattern overlaid with graphic renditions of microtubules that target contact sites (arrowheads). The courses taken by microtubules and stress fibers to contact sites are unrelated. Bar, 10 μm.
To assess the possible role of intermediate filaments in guiding microtubules to contact sites we analyzed an embryo fibroblast cell line derived from a vimentin knockout mouse (Colucci-Guyon et al., 1994). In these knockout cells, as well as in vimentin positive controls (not shown), microtubules were observed to target contact sites (Fig. 8 C) and were also stabilized by contact sites in the presence of nocodazole (Fig. 8 D). In addition, video sequences of vimentin-negative cells co-injected with vinculin and tubulin displayed the same dynamic targeting of contact sites as already described above.
Focal Contacts Can Act as Microtubule Nucleation Sites
Under conditions of forced microtubule assembly (in the presence of taxol) or during recovery from microtubule disassembly by nocodazole, focal adhesions were found to be preferred sites of microtubule nucleation (Fig. 10). Fig. 10, A and B show the periphery of a 3T3 cell that had been exposed to taxol (0.05 μm) for 60 min. The growth of non-centrosomal microtubules was centered around the peripheral termini of stress fiber bundles, corresponding to focal adhesion sites. And following the washout of nocodazole with fresh medium, after complete depolymerization of the microtubule network, the first seeds of microtubule assembly were invariably associated with focal adhesions (Fig. 10, C–E).
Figure 10.
Nucleation of microtubule growth at focal adhesions, in 3T3 fibroblasts. A and B show a 3T3 fibroblast labeled for actin and tubulin that had been exposed to 0.05 μm taxol for 1 h before fixation. Arrowheads indicate the ends of some of the microtubules that had been nucleated at the stress fiber terminus (arrow). (C–E) Part of a REF-52 fibroblast after short term (4 min) recovery from complete disassembly of microtubules by nocodazole. Non-centrosomal microtubule segments (D) are specifically associated with peripheral focal adhesions (C and E), marked with arrows. In E, the microtubule segments (D) have been graphically superimposed on the vinculin image to show the correspondence between the two patterns. Bar, 10 μm.
Discussion
By following simultaneously the assembly of microtubules and the formation of substrate adhesion sites we have now been able to reveal a dramatic and specific interaction between the two. Since microtubules can side-step to target a contact, or make excursions from one contact to another and back again, as well as target a single contact repetitively, we are not dealing with a stochastic process but one of deliberate nature and intention. This was also confirmed by statistical analysis of contact targeting in moving and spread cells. Whereas most of our observations have been made with fish fibroblasts, targeting also has been observed in fibroblasts from rat, mouse, and chicken (our unpublished observations) indicating that it is a general phenomenon.
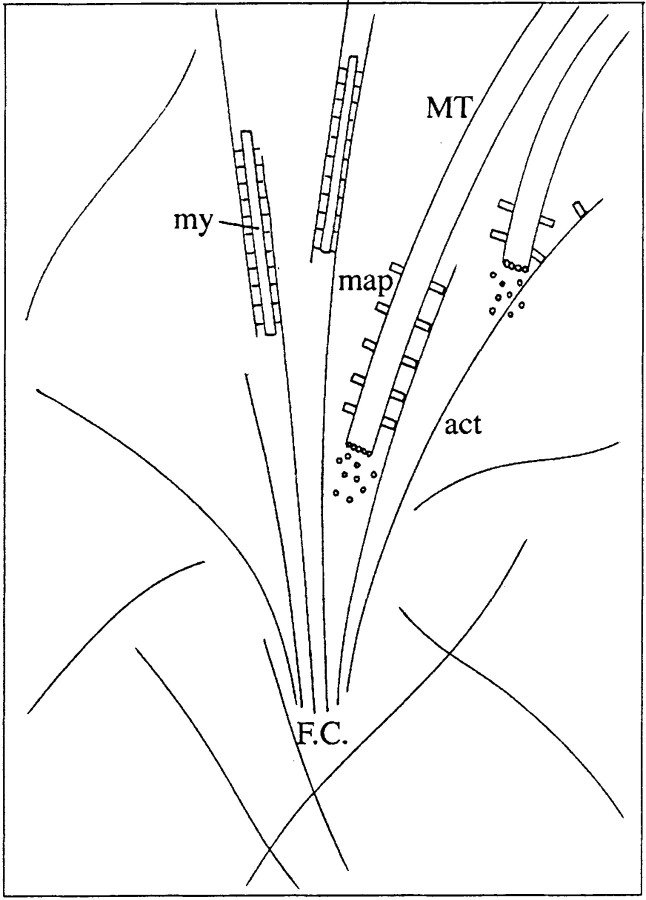
In some functional respects, the parallels between focal adhesions and kinetochores (Mitchison, 1990; Hyman, 1995) cannot be overlooked: they are each targeted by microtubule plus ends, they can capture microtubules and have the potential to nucleate microtubule assembly. For kinetochores, specific microtubule motors resident at these chromosomal foci have been implicated in capturing and tethering microtubules (Hyman, 1995). As we have shown, neither stress fibers nor intermediate filaments provide the cues for guiding microtubules into contacts and since contact structures are covered with actin filament arrays, presumably impermeable to microtubules, components within the contact are unlikely to be involved in either guidance or capture. A possible way in which microtubules could be guided to contacts is depicted in Fig. 11. In this scheme, one or a few actin filaments that splay out from early contact sites tether approaching microtubules and orient their growth. There are already ample examples of proteins that can cross-link actin filaments and microtubules (Gavin, 1997) and such components, or motors could align a microtubule in parallel with a tethering actin filament and direct it into the contact site. Shrinkage of the microtubule away from the tethering filament(s) would allow it to adopt another route, before being tethered once again by filaments from the same or another, early contact. Further work will be required to establish the nature of this guidance process.
Figure 11.
Possible mode of guidance of microtubules to contact sites. An early contact site (F.C.) is depicted with splaying actin filaments (act), some of which are already associating with myosin filaments (my) during the initial stages of stress fiber assembly. A microtubule (MT; 1) decorated with associated molecules with potential actin binding activity (map) appears in the vicinity of the contact. Signaling components in the region of the contact activate the cross-linking activity of the map, leading to the parallel alignment of the actin filament and microtubule (2) and guidance into the contact.
A switch from microtubule growth to shrinkage was often connected with the targeting of peripheral contacts or very early contacts, whereas stabilization was observed only in larger, presumably more mature contacts. Stabilization may thus require more prominent focal contact structures or additional components that are specifically associated with them. To explain the stabilization of microtubules at contact sites we suggest that regulatory factors localize to focal adhesions that do not bind to microtubules, but only modify MAPs or other components, already attached to microtubules, to activate their stabilizing activity. These factors could be downstream from rho in the signaling pathway. The microtubule-nucleating activity of focal contacts could be explained in similar terms.
During revision of the present work, a paper by Cook et al. (1998) appeared, showing that activation of rho causes stabilization of microtubules in starved cells. Our data demonstrate the mechanism of this stabilization; namely, the activation of rho leads to contact formation and then microtubules can be captured and stabilized at rho-induced focal contacts. The stabilization of microtubules in cytochalasin-treated starved cells (Cook et al., 1998) on rho activation is also consistent with the present findings, since under these conditions focal contacts can still form (Nobes and Hall, 1995; our unpublished observations).
As already noted (see Introduction) the disassembly of microtubules in normal (Lloyd et al., 1977) or starved cells (Bershadsky et al., 1996; Enomoto, 1996) leads to the stimulation of contact formation, associated with the activation of rho (Enomoto, 1996), tyrosine kinases, and contractility (Danowski, 1989; Bershadsky et al., 1996; Chrzanowska-Wodnicka and Burridge, 1996). The same stimulation was not seen when microtubules were stabilized by taxol, but taxol does induce a rearrangement of contact sites in fibroblasts to one reminiscent of epithelial cells (Pletjushkina et al., 1994), underlining again a link between microtubule and contact dynamics. Contact enhancement by microtubule disassembly has been attributed to a tension-controlling effect of microtubules on the actin system (Bershadsky et al., 1996) and more specifically, to the association of signal molecules with the microtubule cytoskeleton (Enomoto, 1996). Enomoto (1996) has suggested that microtubules bind signaling factors that are released either in a controlled way under normal conditions, or globally when microtubules are disrupted. Our findings give reason to speculate that microtubules may in fact deliver signaling molecules in a site-directed manner to contact sites, that act to regulate their maturation.
The idea that microtubules play a role in the organization of the actin cytoskeleton of fibroblasts and hence on their form and polarity is one that has been continually reiterated (Vasiliev and Gelfand 1976; Bershadsky and Vasiliev, 1986). More recently, Waterman-Storer and Salmon (1997) have confirmed the penetration of microtubules into the active lamellipodia of motile cells, where they have been previously localized (Lindberg et al., 1981; Small and Rinnerthaler, 1985) and have again emphasized the dynamic interaction between the microtubule and actin systems. Our present results indicate that the dynamics of microtubules and contacts appear to be interdependent phenomena. Thus, the dynamics of individual microtubules could be strongly modified over focal contact sites, seen either as a switch from growth to shrinkage or the stabilization of a microtubule end in the focal contact. This mutual interdependence of dynamic behavior suggests that feedback mechanisms may operate during microtubule–focal contact interactions, which could serve to modulate the dosing of molecular regulators. In this respect, the common dependence of both microtubule polymerization and the activation of rho proteins by GTP should not be overlooked.
In conclusion, our results indicate that it is the anchorage machinery of the cell that serves as the interface between the actin and microtubule cytoskeletons. Microtubules appear to exert their effect on cell form by influencing the lifetime and stability of the contacts that a cell makes with its substrate and we contend that this constitutes the basis of a general underlying mechanism for the determination of cell polarity and guidance. Ongoing efforts are aimed at correlating the contact-targeting activity of microtubules with reorganizations of the actin cytoskeleton and towards establishing whether or not molecular motors in conjunction with signaling molecules play a role in this process.
Acknowledgments
The authors thank Drs T. Hyman and R. Tournebize for generous gifts of rhodamine tubulin and Profs. J. Wehland and H. Faulstich for antibodies and phalloidin, and Prof. P. Traub for the vimentin negative cell line. We also thank Dr. K. Anderson for invaluable help with video microscopy, Dr. M. Gimona for providing the vinculin probes, Ms. M. Schmittner for photography, Ms. B. Mies for assistance, and Ms. E. Eppacher for typing. Acknowledgments are also due to the referees and editors of this work for their constructive criticism of earlier versions.
These studies were supported in part by grants from the Austrian Science Research Council and the Austrian National Bank.
Note Added in Proof
Quick time movies of the video sequences may be downloaded from the following page on the World Wide Web: http://www.imolbio.oeaw.ac.at/jbc.htm
Footnotes
Address all correspondence to J. Victor Small, Austrian Academy of Sciences, Institute of Molecular Biology, Department of Cell Biology, A-5020 Salzburg, Billrothstrasse 11, Austria. Tel.: ++43-662-63961. Fax: ++43-662-63961-40. E-mail: jvsmall@edvz.sbg.ac.at
References
- Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Vaisberg EA, Vasiliev JM. Pseudopodial activity at the active edge of migrating fibroblast is decreased after drug-induced microtubule depolymerization. Cell Motil Cytoskeleton. 1991;19:152–158. doi: 10.1002/cm.970190303. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A.D., and J.M. Vasiliev. 1986. Cytoskeleton. Plenum Press, New York/London. 298 pp.
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pounin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Cook TA, Nagasaki T, Gundersen GG. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J Cell Biol. 1998;141:175–185. doi: 10.1083/jcb.141.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93:255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- De Brabander M, De Mey J, Van de Veire R, Aerts F, Geuens G. Microtubules in mammalian cell shape and surface modulation: an alternative hypothesis. Cell Biol Intl Reports. 1977;1:453–461. doi: 10.1016/0309-1651(77)90080-7. [DOI] [PubMed] [Google Scholar]
- Enomoto T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct Funct. 1996;21:317–326. doi: 10.1247/csf.21.317. [DOI] [PubMed] [Google Scholar]
- Gavin RH. Microtubule-microfilament synergy in the cytoskeleton. Intern Rev Cytol. 1997;173:207–242. doi: 10.1016/s0074-7696(08)62478-x. [DOI] [PubMed] [Google Scholar]
- Herzog, M., A. Draeger, E. Ehler, and J.V. Small. 1994. Immunofluorescence microscopy of the cytoskeleton: Double and triple immunofluorescence. In Cell Biology: A Laboratory Handbook. J.E. Celis, editor. Academic Press, San Diego, pp. 355–360.
- Holwell TA, Schweitzer SC, Evans RM. Tetracycline regulated expression of vimentin in fibroblasts derived from vimentin null mice. J Cell Sci. 1997;110:1947–1956. doi: 10.1242/jcs.110.16.1947. [DOI] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA. Kinetochores get a grip. Curr Biol. 1995;5:483–484. doi: 10.1016/s0960-9822(95)00097-2. [DOI] [PubMed] [Google Scholar]
- Ivanova OY, Margolis LB, Vasiliev JM, Gelfand IM. Effect of colcemid on the spreading of fibroblasts in culture. Exp Cell Res. 1976;101:207–219. doi: 10.1016/0014-4827(76)90431-6. [DOI] [PubMed] [Google Scholar]
- Liao G, Nagasaki T, Gundersen GG. Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level: implications for the role of dynamics microtubules in cell locomotion. J Cell Sci. 1995;108:3473–3483. doi: 10.1242/jcs.108.11.3473. [DOI] [PubMed] [Google Scholar]
- Lindberg U, Höglund AS, Karlsson R. On the ultrastructural organization of the microfilament system and the possible role of profilactin. Biochimie (Paris) 1981;63:307–323. doi: 10.1016/s0300-9084(81)80119-8. [DOI] [PubMed] [Google Scholar]
- Lloyd CW, Smith CG, Woods A, Rees DA. Mechanism of cellular adhesion. II. The interplay between adhesion, the cytoskeleton and morphology in substrate-attached cells. Exp Cell Res. 1977;110:427–437. doi: 10.1016/0014-4827(77)90309-3. [DOI] [PubMed] [Google Scholar]
- Mareel MM, De Mets M. Effect of microtubule inhibitors on invasion and on related activities of tumor cells. Intern Rev Cytology. 1984;90:125–168. doi: 10.1016/s0074-7696(08)61489-8. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ. The kinetochore in captivity. Nature. 1990;348:14–15. doi: 10.1038/348014a0. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Pletjushkina OJ, Ivanova OJ, Kaverina IN, Vasiliev JM. Taxol-treated fibroblasts acquire an epithelioid shape and a circular pattern of actin bundles. Exp Cell Res. 1994;212:201–208. doi: 10.1006/excr.1994.1135. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Rho: theme and variations. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paolo MC, Hall A. Regulation of scatter factory/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnerthaler G, Geiger B, Small JV. Contact formation during fibroblast locomotion: Involvement of membrane ruffles and microtubules. J Cell Biol. 1988;106:747–760. doi: 10.1083/jcb.106.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov VI, Gyoeva FK, Tanaka E, Bershadsky AD, Vasiliev JM, Gelfand VI. Microtubule-dependent control of cell shape and pseudopodial activity is inhibited by the antibody to kinesin motor domain. J Cell Biol. 1993;123:1811–1820. doi: 10.1083/jcb.123.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammak PJ, Borisy GG. Direct observation of microtubule dynamics in living cells. Nature. 1988;332:724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Small JV, Rinnerthaler G. Cytostructural dynamics of contact formation during fibroblast locomotion in vitro. Exp Biol Med. 1985;10:54–68. [Google Scholar]
- Tanaka E, Ho T, Kirschner MW. The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol. 1995;128:139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Vasiliev, J.M., and I.M. Gelfand. 1976. Effects of colcemid on morphogenetic processes and locomotion of fibroblasts. In Cell Motility. R. Goldman, T. Pollard, and J. Rosenbaum, editors. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 279–304.
- Wadsworth P, Bottaro DP. Microtubule dynamic turnover is suppressed during polarization and stimulated in hepatocyte growth factor scattered Madin-Darby canine kidney epithelial cells. Cell Motil Cytoskeleton. 1996;35:225–236. doi: 10.1002/(SICI)1097-0169(1996)35:3<225::AID-CM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J Cell Biol. 1997;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]