Abstract
In Caenorhabditis elegans, mutations in the lin-2 gene inactivate the LET-23 receptor tyrosine kinase/Ras/MAP kinase pathway required for vulval cell differentiation. One function of LIN-2 is to localize LET-23 to the basal membrane domain of vulval precursor cells. LIN-2 belongs to the membrane-associated guanylate kinase family of proteins. We have cloned and characterized the human homolog of LIN-2, termed hCASK, and Northern and Western blot analyses reveal that it is ubiquitously expressed. Indirect immunofluorescence localizes CASK to distinct lateral and/or basal plasma membrane domains in different epithelial cell types. We detect in a yeast two-hybrid screen that the PDZ domain of hCASK binds to the heparan sulfate proteoglycan syndecan-2. This interaction is confirmed using in vitro binding assays and immunofluorescent colocalization. Furthermore, we demonstrate that hCASK binds the actin-binding protein 4.1. Syndecans are known to bind extracellular matrix, and to form coreceptor complexes with receptor tyrosine kinases. We speculate that CASK mediates a link between the extracellular matrix and the actin cytoskeleton via its interaction with syndecan and with protein 4.1. Like other membrane-associated guanylate kinases, its multidomain structure enables it to act as a scaffold at the membrane, potentially recruiting multiple proteins and coordinating signal transduction.
Keywords: CASK, LIN-2, syndecan, protein 4.1, MAGUK
The cortical actin cytoskeleton is implicated in organizing specialized membrane domains and in coordinating membrane-signaling networks. Members of the membrane-associated guanylate kinase (MAGUK)1 family have recently been recognized as important organizing proteins in these cortical networks (reviewed in Fanning et al., 1996). MAGUKs are defined by a tripartite domain structure: a Src homology 3 (SH3) domain, a domain with homology to the enzyme guanylate kinase (GUK), and a PDZ domain. The latter domain is named for three MAGUK proteins: PSD-95, a 95-kD protein of the postsynaptic density; Dlg, the product of the Drosophila lethal(1)discs-large-1 tumor suppressor gene; and ZO-1, a vertebrate tight junction protein (Cho et al., 1992; Woods and Bryant, 1991; Willott et al., 1993).
Based on their multidomain structure and their specific membrane localization, MAGUKs are thought to act as scaffolds, organizing and coupling diverse extracellular signals at the plasma membrane to intracellular signal transduction pathways and the cortical cytoskeleton. For example, in neurons, the related proteins PSD-95 and hDlg, the human homolog of Drosophila Dlg, have been shown to bind via their PDZ domains to Shaker-type K+ channels and NMDA receptors (Kim et al., 1995; Kornau et al., 1995). They also interact with multiple intracellular proteins, some involved in signal transduction such as nitric oxide synthase (Brenman et al., 1996a ; Brenman et al., 1996b ) and the APC tumor suppressor (Matsumine et al., 1996). MAGUK proteins associate with the cortical actin cytoskeleton in different ways. For example, in erythrocytes, MAGUK protein p55 is part of a ternary complex linking the membrane protein glycophorin C to the spectrin/actin cytoskeleton via protein 4.1 (Marfatia et al., 1997; Marfatia et al., 1994), and in epithelial cells hDlg also binds protein 4.1 (Lue et al., 1994). The tight junction MAGUK ZO-1 binds to serine–threonine kinase ZAK (Balda et al., 1996), and links the transmembrane protein occludin to the cortical cytoskeleton by binding directly to f actin (Itoh et al., 1997). In epithelial cells, the C. elegans protein LIN-2 may share this functional paradigm, and is known to be required for activity of a receptor tyrosine kinase/Ras/MAP kinase signaling pathway (Hoskins et al., 1995). However, protein interactions of LIN-2 that underlie its potential role as a multidomain scaffolding protein have yet to be elucidated.
Like all MAGUKs, LIN-2 contains a PDZ, SH3, and a GUK domain. In addition, at its NH2 terminus, LIN-2 has a domain with homology to calcium/calmodulin-dependent protein kinase (CAMK; Hoskins et al., 1995). In C. elegans, the lin-2 gene is required for development of the vulva, the egg-laying orifice on the ventral surface of hermaphrodites (Ferguson and Horvitz, 1985). Genetic studies indicate that LIN-2 is responsible for localizing the LET-23 receptor tyrosine kinase (Aroian et al., 1990) to the basal domain of vulval precursor cells (Simske et al., 1996). Receptor mislocalization results in failure of Ras signal transduction, lack of cell differentiation, and subsequent vulvaless phenotype. These studies suggest either the receptor must be polarized in order to receive the activating ligand, or that LIN-2 may be involved in organizing signaling events downstream of the receptor. Presently, the role of LIN-2 in this pathway is not fully defined; however, it is known that LIN-2 does not bind directly to the LET-23 receptor (Simske et al., 1996). Recently, the rat homolog of LIN-2, termed CASK, was cloned by virtue of its ability to bind neurexins (Hata et al., 1996), a family of neuronal transmembrane proteins that localize near synapses and are thought to play a role in axon guidance or adhesion (Puschel and Betz, 1995). A Drosophila homolog of LIN-2, CAMGUK, has also been reported, but its function and mutant phenotype are as of yet undefined (Dimitratos et al., 1997).
To explore the structural and signaling role of CASK in vertebrate epithelial tissues, we have cloned the human homolog of CASK/LIN-2 (hCASK), and we show in a yeast two-hybrid screen that the PDZ domain of hCASK binds to the transmembrane protein syndecan-2. In vitro binding assays and immunofluorescent colocalization in multiple tissues confirm this interaction and suggest that hCASK is capable of binding to all four syndecan family members (syndecan-1,-2,-3, and -4). Syndecans are cell surface heparan sulfate proteoglycans that are thought to act both as extracellular matrix receptors (e.g., through binding fibronectin, laminin, and collagen types I, III, and V) and as low-affinity coreceptors with receptor tyrosine kinases such as bFGFR (reviewed in Carey, 1997). We demonstrate that in addition to binding syndecan, hCASK interacts with the actin/spectrin-binding protein 4.1. We propose that hCASK mediates a link between the extracellular matrix and the cortical actin cytoskeleton. These results are consistent with the present model of MAGUKs as scaffolding proteins that recruit or organize other proteins at the plasma membrane to coordinate signal transduction pathways within the cortical cytoskeleton.
Materials and Methods
Cloning
A degenerate PCR strategy was used to amplify hCASK from a fetal lung cDNA library (CLONTECH Laboratories, Inc., Palo Alto, CA). The primers were made from the known p55 sequence; the sense primer corresponded to a sequence at the start of the PDZ domain, and the antisense primer corresponded to a region at the end of the SH3 domain. A 300-bp PCR product was isolated, subcloned into the vector pCRII (Invitrogen Corp., Carlsbad, CA), and sequenced. Internal forward and reverse PCR primers were then designed to its SH3 domain; each one was paired with a corresponding anchored primer to either the Sp6 or the T7 promoter regions of the vector. Two distinct overlapping PCR products were isolated, subcloned into the vector pCRII (Invitrogen Corp.), and sequenced. The first clone encompassed the calmodulin-binding domain and the SH3 domain. The second clone extended from the SH3 domain to the end of the guanylate kinase domain. These two clones were used as probes to screen an oligo dT–primed human fetal brain cDNA library constructed in lambda gt10 (Stratagene, La Jolla, CA), and a human liver library in lambda ZAP specifically primed with anti-sense ZO-1 oligonucleotides (CLONTECH Laboratories, Inc.). Clones encoding the 5′-most sequence were obtained by screening an oligo dT- primed human fetal brain cDNA library in lambda uni-ZAP (Stratagene) with a probe constructed by PCR amplification of residues 104–289 using a clone from the initial brain screen as a template. Hybridization probes were coupled to horseradish peroxidase and detected on x-ray film by a light emission–based system (Amersham Corp., Arlington Heights, IL). The hCASK cDNA sequence, Genbank accession no. AF032119, was determined by dideoxy sequencing of overlapping clones in both directions.
Northern Analysis
Northern blot derived from multiple human tissues containing 2 μg of poly(A)+ RNA per lane was obtained from Clontech. The blot was probed with a 1.073-kb cDNA fragment of hCASK (bases 777–1850) that was labeled by random priming as per manufacturer's instructions (Boehringer Mannheim Corp., Indianapolis, IN) using [α-32P] dCTP (Amersham Corp.). As a control, the same blot was washed and reprobed with a cDNA fragment of rat β actin (kindly provided by Dr. Prabhat Gosh, Yale University Department of Neurosurgery).
Antibodies
hCASK antibodies were raised in three rabbits to a recombinant glutathione-S-transferase (GST) fusion protein. The hCASK-GST vector was constructed by PCR using forward and reverse primers flanking residues 316–415 with an hCASK cDNA plasmid as template. The resulting PCR product, confirmed by dideoxy sequencing in both directions, was cloned into the vector pGEX-2T (Promega Corp.). The GST fusion protein was expressed in E. coli, and was purified on glutathione-Sepharose beads (Pharmacia Biotech, Inc., Piscataway, NJ). The rabbit serum with the highest titer was used for all data shown here. Antibodies are affinity- purified using antigen immobilized on a CNBr-activated Sepharose column (Pharmacia Biotech, Inc.). Rat anti-mouse syndecan-1 and syndecan-2 antibodies, 281-2 and F-90, respectively, were the generous gift of Dr. Merton Bernfield (Children's Hospital, Harvard Medical School).
Western Analysis
Rat tissue samples were prepared using dounce homogenization in 1 mM NaHCO3 in the presence of protease inhibitors, followed by addition of an SDS-based gel sample buffer (Fallon et al., 1993). Equal total protein amounts were loaded in each well (BCA Protein Assay; Pierce Chemical Co., Rockford, IL), and samples were resolved by SDS-PAGE on 10% acrylamide gels (Laemmli, 1970). Proteins were transferred to nitrocellulose (Towbin et al., 1979), and nonspecific binding was blocked with 10% nonfat dry milk in PBS for at least 1 h. Blots were probed with affinity-purified rabbit anti-hCASK antibodies, and detection was by enhanced chemiluminescence (Amersham Corp.).
Immunofluorescence
Tissue was harvested, fixed in 4% paraformaldehyde, and 5-μm serial sections were cut by conventional methods (Yale Critical Technologies Center). Paraffin was removed from sections by incubating 2 × 5 min in xylene, followed by 3 × 3 min in 100% ethanol. Endogenous peroxidase activity was blocked by incubating in methanol/hydrogen peroxide for 30 min. Sections were rinsed in TBS, and then hydrated (dH2O) for at least 5 min. Antigenicity was retrieved in a pressure cooker filled with dilute citric acid buffer (3.84 g Na citrate in 2 L dH2O, pH 6.0), as described in Norton et al. (1994). Sections were rinsed again in TBS and blocked for 1 h at room temperature in 10% goat serum. The sections were labeled with affinity-purified rabbit anti-hCASK or rat antisyndecan (281-2 or F-90) primary antibodies overnight at 4°C. Slides were washed 5× in TBS, 1× in 0.01% Triton/TBS, and 1× in TBS. Texas Red or FITC-conjugated anti-rabbit or Texas-Red anti-rat secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were added for 1 h at room temperature. Subsequently, sections were washed as described above and mounted with Vectashield™ (Vector Labs, Inc., Burlingame, CA). Labeling was visualized using a Microphot™ (Nikon, Inc., Melville, NY) with 20× and 40× objectives and images captured using either T-MAX 400 film or a Sensys cooled-CCD camera (Photometric, Tucson, AZ) and processed using Adobe Photoshop 3.0 or Image Pro Plus (Media Cybernetics, Silver Spring, MD), respectively.
Yeast Two-Hybrid
The yeast two-hybrid screen was performed in the laboratory of Dr. Morgan Sheng (Massachusetts General Hospital, Harvard Medical School). Yeast two-hybrid screens were done using the L40 yeast strain as described in Niethammer et al. (1996). The bait consisted of the PDZ domain of hCASK (residues 489–572) made by PCR using specific primers and subcloned in frame with the lexA DNA-binding domain into vector pBHA. The bait was used to screen a human liver cDNA library constructed in pGAD10 (Clontech). DNA from positive interacting clones, as assayed by beta-galactosidase staining, was isolated from yeast colonies and transformed into HB101 bacteria by electroporation, isolated from bacteria, and sequenced.
Peptide-binding Assay
A hCASK PDZ-GST recombinant fusion protein was made by PCR amplification of the PDZ domain (residues 489–572) using hCASK cDNA as a template. The resulting PCR product was sequenced and subcloned into pGEX-2T vector (Promega Corp., Madison, WI). The fusion protein was expressed in Escherichia coli and purified on glutathione-Sepharose beads (Sigma Chemical Co., St. Louis, MO). A COOH-terminal syndecan-2 peptide (APTKEFYA-COOH) was synthesized and coupled to CNBr- activated Sepharose beads (Pharmacia). The beads with immobilized peptide were then incubated with recombinant GST-hCASK PDZ fusion protein (4 μg/ml) with various concentrations (0–200 μM) of the same syndecan-2 peptide or with homologous syndecan-1 (TKQEEFYA-COOH) peptide in TBS buffer containing 0.01% NP-40, BSA (1 mg/ml) and DTT (1 mM; as described in Songyang et al., 1996). Controls consisted of GST alone incubated with beads containing immobilized peptide, GST-hCASK PDZ fusion protein with uncoupled beads, and GST-PDZ fusion protein incubated with a mixture of beads coupled to syn-2 peptide and an excess of control dlg peptides in solution. The dlg control peptide (KKKKETDV-COOH) has optimal binding affinity for murine Dlg PDZ as determined by Songyang et al. (1996) using an oriented peptide library technique. All mixtures were incubated at 4°C for 45 min. Bound proteins were washed three times with TBS buffer, separated by SDS-PAGE, transferred to nitrocellulose, and visualized by protein immunoblotting with anti-GST antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Relative binding was quantified using a Gel-Pro scanner (Media Cybernetics, MD).
Protein 4.1 Blot Overlay and Sedimentation-binding Assay
Preparation of 125I-labeled NH2-terminal 30-kD domain of protein 4.1 and subsequent blot overlay and sedimentation-binding assays were performed as described in Marfatia et al. (1995). The hCASK GST fusion protein used in these assays contains most of the MAGUK region of hCASK, extending from residue 536 through the COOH terminus. This construct was made by subcloning a restriction enzyme DNA fragment of hCASK into the pGEX-2T vector (Promega Corp., Madison, WI). The GST-p55 construct used as a positive control in these experiments contains the full-length human p55 sequence, and has been described previously in Marfatia et al. (1994). Recombinant fusion proteins were expressed in E. coli and purified on glutathione-Sepharose beads (Sigma Chemical Co.).
Results
Cloning of Human CASK/Lin-2 and Initial Characterization
In C. elegans, LIN-2 is required to localize the LET-23 receptor tyrosine kinase to the basal membrane where it can respond to the LIN-3/EGF-like ligand and induce a Ras/ MAP kinase signaling pathway required for cell fate determination (Simske, 1996). The original goal of our work was to determine whether there was a functional vertebrate homolog of LIN-2. A degenerate PCR strategy followed by hybridization library screening was used to clone the full-length hCASK/LIN-2 (hCASK) cDNA from human liver, lung, and brain libraries (Materials and Methods). Sequence analysis of hCASK cDNA confirms that it is composed of an NH2-terminal calcium calmodulin- dependent protein kinase–like domain, PDZ and SH3 domains, a potential protein 4.1 binding motif, and a domain homologous to guanylate kinase. The high degree of overall identity with rat CASK and C. elegans LIN-2, 99% and 52% respectively, implies that this gene represents the human ortholog (Fig. 1). We note that the protein sequence of hCASK that lies C-terminal to the CAMK domain (i.e., the MAGUK region) is more similar to the erythrocyte protein p55 than to other MAGUK proteins.
Figure 1.
Comparative domain organization of hCASK-related proteins. Sequence homology of hCASK with rat CASK (Hata et al., 1996), C. elegans LIN-2 (Hoskins et al., 1995), human erythrocyte p55 (Ruff et al., 1991), and alpha subunit of rat brain CAMKII (Lin et al., 1987). hCASK protein contains a domain homologous to CAMKII, a conserved calmodulin-binding domain (CBD), a PDZ domain, an SH3 domain, a potential protein 4.1–binding motif (4.1), and a domain homologous to guanylate kinase (GUK). Percent amino acid identities within defined domains are noted in comparison with hCASK.
Northern analysis of human tissue poly(A)+ RNA demonstrates that hCASK is ubiquitously expressed (Fig. 2). All tissues examined have a 4.4-kb mRNA transcript, which is especially enriched in pancreas, brain, and heart relative to a β-actin mRNA loading control. Some tissues (heart, brain, muscle and pancreas) have a conspicuous 8.5-kb transcript. Others (particularly placenta and kidney) express a transcript of 3.1 kb. All three transcript sizes are large enough to encompass the full-length coding region (2765 bp).
Figure 2.

hCASK is ubiquitously expressed. Northern blot of human poly(A)+ mRNA probed with hCASK cDNA. The tissue source of the blotted RNA is indicated above each lane. Three differently sized transcripts are apparent at 8.5 kb, 4.4 kb, and 3.1 kb. All are large enough to encode full-length cDNA (2.765 kb). The position of size markers are indicated on the left (kb). Hybridization to 1.9-kb β-actin transcript is shown in the bottom panel as a loading control. Heart and skeletal muscle characteristically show a second β-actin mRNA form at 1.5 kb.
Western blot analysis reveals a predominant band of CASK reactivity at ∼112 kD in all rat and human tissues and cultured cells tested (Fig. 3). This size is consistent with the full-length coding region, with the 110-kD nematode protein (Hoskins et al., 1995), and with the previously reported 112-kD rat protein (Hata et al., 1996). Antibodies for these studies were raised in rabbits against a recombinant GST fusion protein that included 94 residues COOH-terminal to the putative calmodulin-binding domain of hCASK, an area that corresponds to the highly variable association domain of CAM kinases. The deduced amino acid sequence of 921 residues predicts a protein of 106 kD, which is 5% smaller than the apparent molecular mass demonstrated by SDS/PAGE. A minor band of 75 kD was sometimes detected by antibodies affinity-purified against GST-CASK. Immunoreactivity towards this 75-kD species could be selectively reduced by immunodepletion of anti-GST activity from the affinity-purified anti-CASK sera.
Figure 3.
Anti-hCASK antibodies recognize one band at 112 kD, consistent with full-length cDNA and the nematode homolog. Immunoblot of rat tissues probed with affinity-purified anti-hCASK antibodies. Whole tissue samples were normalized by total protein, and were resolved by SDS-PAGE (10%). Positions of molecular weight markers are shown.
CASK Localizes to Specific Membrane Domains in Different Epithelial Cell Types
Localization of C. elegans LIN-2 has not been determined; however, indirect evidence implies that it is localized to the basal domain of prevulval epithelial cells (Simske et al., 1996). To determine whether CASK also shows specific membrane domain localization, we performed indirect immunofluorescence microscopy on paraffin-embedded rat tissue sections using affinity-purified anti-hCASK antibodies. CASK was observed to localize to specific but different membrane domains in different epithelial cell types. For example, in choroid plexus epithelial cells and in hepatocytes, CASK is predominantly at the basal surface (Fig. 4 a and Fig. 6 a). In contrast, in small and large intestinal epithelial cells, the staining is concentrated in the basal region of the lateral plasma membrane (Fig. 4 b and Fig. 6 c). In pancreatic acinar cells and in renal distal tubule cells (not shown), staining is localized equally along the basal and lateral membranes (Fig. 6 e). CASK was never observed at the apical membrane of any cell type tested. Specificity for CASK was demonstrated by the ability of immobilized GST–CASK fusion protein to deplete the antiserum of all anti-CASK reactivity, and to abolish the observed cell immunostaining. Analogous immunodepletion of the antiserum with immobilized GST had no effect on the observed immunolocalization of CASK (data not shown).
Figure 4.
CASK localizes to distinct membrane domains in different epithelial cell types. Rat tissue sections were stained with affinity-purified anti-hCASK antibodies. (a) In choroid plexus epithelial cells, CASK localizes along the basal membrane; bar, 100 μm. (b) In colonic epithelial cells, CASK is enriched at the base of the lateral plasma membrane; bar, 40 μm.
Figure 6.

CASK and syndecan-1 colocalize in situ in epithelial tissues. Mouse tissue sections were stained with affinity-purified rabbit anti-hCASK and monoclonal rat anti-syndecan-1 antibodies and visualized with Texas red–conjugated anti-rabbit and FITC-conjugated anti-rat secondary antibodies, respectively. (a, b) In hepatocytes, both CASK and syndecan-1 are located along the basal sinusoidal membrane. (c, d) In small intestinal enterocytes, the two proteins are localized at the lateral plasma membrane. CASK also stains a subset of the vessels that syndecan-1 stains in the lamina propria. (e, f) In the pancreas, CASK and syndecan-1 colocalize on both the basal and lateral membrane domains of acinar cells; bar, 40 μm.
The PDZ Domain of hCASK Binds to the COOH Terminus of Syndecans
The restricted subcellular localization of CASK is similar to that of other PDZ-containing proteins, some of which have been shown to bind to COOH termini of transmembrane proteins in a sequence-specific fashion (reviewed in Fanning and Anderson, 1996). In neurons, rat CASK has been shown to bind to the COOH terminus of the neuron-specific cell surface protein neurexin; this binding is presumed but not proven to require the CASK PDZ domain (Hata et al., 1996). To identify a binding partner for hCASK in polarized epithelial cells, we performed a yeast two-hybrid screen of a human liver cDNA library using the PDZ domain of hCASK as bait. One positive interacting clone was isolated, which encoded residue 107 through the cytoplasmic COOH terminus (residue 201) of the cell surface heparan sulfate proteoglycan syndecan-2 (Marynen et al., 1989).
To verify the yeast two-hybrid result and to test whether it is based on a typical PDZ domain tail interaction requiring binding to the COOH terminus of the target protein, we performed in vitro binding studies with a syndecan-2 peptide and a GST-hCASK PDZ fusion protein. A syndecan peptide representing the last eight residues (Fig. 5 a) was coupled to CNBr-activated Sepharose beads. The beads with immobilized peptide were then incubated with hCASK recombinant GST-PDZ fusion protein in the presence of various concentrations of soluble peptide (Fig. 5 b). hCASK bound specifically to the syndecan-2 peptide based on several criteria. First, GST alone did not bind to beads containing immobilized peptide. Second, GST-PDZ fusion protein did not bind to uncoupled beads. Third, binding of GST-PDZ fusion protein to immobilized syndecan-2 was successfully competed off with increasing amounts of soluble peptide. 50% maximal displacement of hCASK PDZ domain occurred at a range of 40–70 μM peptide, an apparent inhibition constant consistent with other biologically relevant PDZ domain interactions (Songyang et al., 1997). Binding was not affected by the presence of a control peptide representing a sequence that has optimal binding affinity for the murine Dlg PDZ domain, as previously determined by the oriented peptide library technique (Songyang et al., 1997).
Figure 5.
The PDZ domain of hCASK binds to the COOH-terminal sequence of syndecans. (a) Alignment of the cytoplasmic domains of rat syndecan family members demonstrate the high degree of homology among the four proteins (reviewed in Bernfield et al., 1992). In particular, note the conservation of the last four residues (in bold), which in other proteins are known to determine PDZ tail-binding specificity. (b) Direct binding study with syn-2 peptide and hCASK PDZ fusion protein. Syn-2 peptide representing the last eight residues was coupled to CNBr- activated Sepharose beads. The beads with immobilized peptide were incubated with GST-PDZ recombinant fusion protein with increasing concentrations of syn-2 or syn-1 peptides (0–200 μM) in solution. Controls consisted of GST alone incubated with peptides immobilized on beads; GST-PDZ fusion protein with peptide-free Sepharose beads (Beads alone); and GST-PDZ fusion protein incubated with beads coupled to syn-2 peptide and 50 μM control dlg peptide in solution (Ctr). The dlg control peptide (KKKKETDV-COOH) has optimal binding affinity for murine Dlg PDZ domain (Songyang et al., 1997).
To investigate whether hCASK is capable of binding to syndecan-1 (Saunders et al., 1989; Mali et al., 1990), the GST-hCASK PDZ fusion protein was incubated with increasing amounts of a soluble peptide representing the last eight amino acids of syndecan-1, and the syndecan-2 peptide coupled to beads. Identical competition characteristics were observed for the syndecan-1 peptide as for the syndecan-2 peptide (Fig. 5 b). These experiments suggest that the last four residues (EFYA) shared by syndecans 1 and 2 are sufficient for hCASK PDZ binding. Furthermore, because all four syndecan gene products end in the same last four residues, we predict that hCASK has the ability to bind to syndecans 3 (Carey et al., 1992) and 4 (Kojima et al., 1992) as well (Fig. 5 a).
CASK and Syndecan Colocalize In Situ in Epithelial Tissues
Yeast two-hybrid results and peptide-binding studies reveal that hCASK is capable of binding to syndecans 1 and 2. To determine whether they colocalize in vivo, indirect immunofluoresence microscopy was performed on rat tissues using anti-hCASK, anti-syndecan-1, and anti-syndecan-2 antibodies (Materials and Methods). CASK, syndecan-1, and syndecan-2 (not shown) colocalize to the basal sinusoidal surface of hepatocytes in liver (Fig. 6, a and b). In contrast, in small intestine where syndecan-1 expression predominates over the other syndecan proteins, both CASK and syndecan-1 decorate the lateral membrane of enterocytes with a striking and similar concentration of staining in the most basal aspect of the lateral membrane. However, in the lamina propria, an area rich in blood vessels and lymphatics, syndecan appears to stain all vessels, whereas CASK is less conspicuous or absent (Fig. 6, c and d). In pancreas, CASK and syndecan-1 are located with similar levels on both the basal and lateral membranes of acinar cells (Fig. 6, e and f). Furthermore, they both localize only to the lateral membrane of cells lining pancreatic ducts (not shown).
The in vivo interaction between CASK and syndecan is strongly supported by their peculiar pattern of colocalization on the lateral, basal, or basolateral membrane domain depending on the epithelial cell type. Despite evidence for colocalization and specific in vitro binding, we were unable to coimmunoprecipitate a CASK–syndecan complex from detergent-solubilized cells. This result is consistent with results reported for CASK–neurexin and for other PDZ domain–transmembrane protein interactions in which binding had been established by other methods (Hata et al., 1996; Niethammer et al., 1996). A possible explanation for the inability to coimmunoprecipitate transmembrane proteins and their PDZ-containing binding partners may be that conditions necessary for extracting both proteins do not maintain the interaction.
hCASK Binds to the Actin/Spectrin-Binding Protein 4.1
MAGUK proteins p55 and hDlg have been shown to bind the actin-binding protein 4.1 through a conserved lysine-rich sequence motif located between the SH3 and the GUK domains (Marfatia et al., 1995; Lue et al., 1994). Sequence comparisons reveal that hCASK shares striking homology with p55 and hDlg in this putative 4.1 binding region. The reciprocal binding site on protein 4.1 for p55 resides within a 30-kD domain produced by chymotrypsin digestion of native protein 4.1 (Marfatia et al., 1994). To determine whether hCASK can bind to protein 4.1, we performed in vitro binding assays similar to those used to establish the interaction between the NH2-terminal 30-kD domain of erythrocyte protein 4.1, and both p55 and hDlg (Marfatia et al., 1994; Lue et al., 1994).
In a blot overlay assay, a 125I-labeled 30-kD fragment of protein 4.1 was incubated with fusion proteins GST-hCASK, GST-p55, and GST alone immobilized on nitrocellulose (Fig. 7 a). GST–hCASK encodes a fragment of hCASK from the middle of the PDZ domain through the COOH terminus, and includes the putative protein 4.1 binding motif. The radiolabeled protein 4.1 fragment bound to GST-hCASK and to GST-p55, but not to GST alone. In addition, radiolabeled protein 4.1 bound to a different recombinant GST-hCASK fusion protein that also contains the putative 4.1 binding site (data not shown).
Figure 7.
hCASK binds to the actin/spectrin-binding protein 4.1. (a) [125I]-labeled 30-kD fragment of protein 4.1 was incubated with GST-hCASK, GST-p55, and GST alone immobilized on nitrocellulose. Radiolabeled protein 4.1 bound GST-hCASK and GST-p55, but not GST alone. (Left) SDS-PAGE of expressed fusion proteins used for the experiment. (b)125I-labeled 30-kD fragment of protein 4.1 was incubated with GST-hCASK and GST alone coupled to glutathione Sepharose beads. Significantly more radiolabeled protein 4.1 bound to GST-hCASK than to GST alone, and the binding was effectively competed by incubation with an excess of nonradiolabeled protein 4.1. Experiments were performed in triplicate.
The interaction of hCASK with protein 4.1 was further investigated by incubating a 125I-labeled 30-kD fragment of protein 4.1 with glutathione–Sepharose beads bound to the fusion protein GST-hCASK or to GST alone (Fig. 7 b). Significantly more radiolabeled protein 4.1 bound to GST-hCASK than to GST alone, and the binding was effectively competed by incubation in the presence of a 10× molar excess of nonradiolabeled protein 4.1.
Discussion
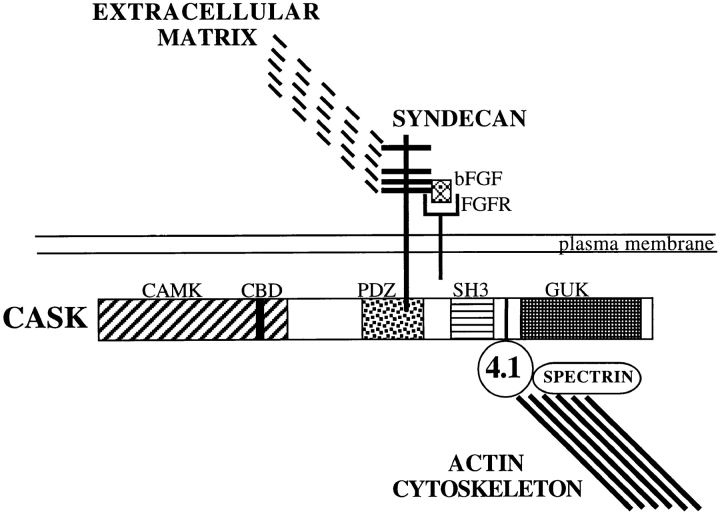
Our studies extend the genetic description of C. elegans LIN-2 to its potential role in vertebrate epithelial cells, and provide several new insights about its possible function. We demonstrate that CASK is ubiquitously expressed and is localized predominantly to either the basal, lateral or basolateral plasma membrane domains in different epithelial cell types. In addition, we show that hCASK binds to the heparan sulfate proteoglycan syndecan-2 via its PDZ domain, and to the actin/spectrin-binding protein 4.1, presumably through a previously characterized motif located between the SH3 and GUK domains (Marfatia et al., 1995; Lue et al., 1994). Based on these associations, we speculate that in epithelial cells CASK mediates a direct link between the extracellular matrix and the cortical actin cytoskeleton through syndecan and protein 4.1, respectively (Fig. 8). These interactions may have functional significance for membrane signaling and assembly of cell polarity.
Figure 8.
A hypothetical model of hCASK mediating a direct link between extracellular matrix and actin cytoskeleton by binding to syndecan and protein 4.1. hCASK binds to syndecan via its PDZ domain, and to protein 4.1 presumably through a sequence motif located between the SH3 and the GUK domains, as previously demonstrated for p55 and hDlg. Syndecans have been shown to bind extracellular matrix components and to form noncovalent coreceptor complexes with receptor tyrosine kinases, such as bFGFR. hCASK, in close proximity to the receptor tyrosine kinase, could act on the cytoplasmic surface to recruit proteins used in signaling events downstream of the bFGF receptor, or alternatively, could itself be a substrate for the receptor.
Human CASK demonstrates a high degree of overall identity with rat CASK, Drosophila CAMGUK, and C. elegans LIN-2. It is composed of an NH2-terminal CAMK, a PDZ domain, an SH3 domain, a protein 4.1-binding motif, and a domain homologous to GUK. Sequence alignments with the alpha subunit of rat brain CAMKII (Lin et al., 1987) suggest that hCASK is likely to bind calmodulin, but is unlikely to be an active enzyme. The latter conclusion is based on substitution within hCASK of several residues that are conserved among all enzymatically active CAMKs, including two within the putative ATP binding site (Colbran and Soderling, 1990; Hanks et al., 1988). Hata et al. (1996) have shown that recombinant fragments of CASK containing its putative calmodulin-binding site bind calmodulin efficiently in a calcium-dependent manner. However, they found no evidence for CAMK activity using recombinant proteins, and did not observe autophosphorylation of CAMK domains expressed in bacteria, mammalian cells, or insect cells (Hata et al., 1996). In native CAMKII, calmodulin binding releases autoinhibition on the catalytic domain (reviewed in Braun and Schulman, 1995). By analogy, calmodulin binding may regulate CASK by inducing a conformational change, although in this case it would not be expected to induce kinase activity.
Sequence comparisons of hCASK with porcine and yeast GUK enzymes suggest that hCASK has the potential to be an active guanylate kinase enzyme, although this activity has not been tested (Zschocke et al., 1993; Stehle and Schulz, 1992). p55 is the only other MAGUK that conserves or conservatively substitutes all of the residues known to be essential for GUK enzymatic activity. Active GUK domains might contribute to signaling by affecting guanine nucleotide levels and activity of small GTP-binding proteins, or by possessing effector function themselves when bound to guanine nucleotides. Recently, a novel protein family called GKAP (guanylate kinase–associated protein) or SAPAP (SAP90/PSD-95–associated protein) has been shown to bind to the GUK domain of PSD-95/ SAP90 and related synaptic proteins (Kim et al., 1997; Takeuchi et al., 1997), although their sequences are novel and functions unknown. None of these newly identified proteins bind the GUK domain of CASK, leaving open the possibility that additional GKAP/SAPAP-like proteins remain to be discovered.
In this study we demonstrate that CASK is ubiquitously expressed and shows distinct localization patterns predominantly at the basal, lateral, or basolateral membrane domains in different epithelial cell types. CASK appears to be excluded from the apical membrane compartment. Despite its apparently distinct localization patterns, CASK is always associated with a membrane domain that faces bloodborne growth factors and hormones. This fact suggests a role for CASK analogous to that of C. elegans LIN-2, which is thought to localize to the basal membrane domain of vulval precursor epithelial cells in association with the LET-23 growth factor receptor.
To identify a binding partner for hCASK in polarized epithelial cells, we performed a yeast two-hybrid screen of a human liver library using the PDZ domain as bait. We found that the hCASK PDZ domain binds to the transmembrane protein syndecan-2. X-ray crystallography and mutational analysis had previously demonstrated that the COOH-terminal residues of the cytoplasmic tail of PDZ-binding transmembrane proteins confer the specificity of the PDZ domain-tail interaction (Doyle et al., 1996; Kim et al., 1995). Using an oriented peptide library technique, Songyang et al. (1997) assigned PDZ domains into classes according to their peptide-binding specificities. hCASK, like p55 and Tiam-1, falls in the so-called class II, selecting peptides with hydrophobic or aromatic side chains at position –2 relative to the carboxy terminus. The physical basis for this specificity is consistent with the recent x-ray crystalographic structure of the hCASK PDZ domain (Daniels et al., 1998). Inspection of known PDZ-binding motifs reveals that E at position –3 is very common (Doyle et al., 1996). Syndecan-2, which terminates in the sequence EFYA, as well as neurexin, which ends with residues EYYV, are both predicted to bind to the PDZ domain of CASK. It is possible that CASK has other PDZ-binding partners in addition to syndecan and neurexin, that terminate with hydrophobic or aromatic residues.
In vitro binding studies reported here demonstrate that the PDZ domain of CASK binds specifically to the COOH-terminal EFYA motif present in both syndecans 1 and 2. The apparent K i in our binding assay with syndecan peptides consisting of the terminal eight residues is in the range of 40–70 μM, consistent with previously reported PDZ interactions (Songyang et al., 1996). Although we were unable to coimmunoprecipitate a complex of CASK and syndecan from native cells, this result has been the experience for other PDZ interactions for which there is either genetic or functional proof of an interaction.
In addition to these in vitro binding studies, we demonstrate that CASK and syndecans 1 and 2 share the same membrane domain–restricted distribution in different epithelial cells. Although we lack antibodies to investigate a possible colocalization with syndecans 3 and 4, the COOH-terminal sequence identity among all four syndecan gene products suggests that CASK can interact with all syndecans. This possibility is of interest because while all contain a highly conserved cytoplasmic domain, syndecans have divergent extracellular domains, cellular expression patterns, and presumed functions (Carey, 1997). For example, syndecan-1, expressed almost exclusively in epithelial cells of mature tissue, is thought to act as a matrix receptor, regulate cell morphology, and participate in a coreceptor complex with bFGF receptor. In contrast, syndecan-4, expressed by both epithelial and fibroblastic cells, is enriched and codistributed with integrins in focal contacts, raising the possibility that syndecan-4 forms a coreceptor complex with integrin receptors (Woods and Couchman, 1994).
In this study we show that CASK, like p55 and hDlg, binds to the NH2-terminal 30-kD domain of erythrocyte protein 4.1. This domain is highly conserved among 4.1- related proteins, raising the possibility that MAGUK proteins can bind to other 4.1 family members such as ezrin, radixin, moesin, merlin, or talin. All of these proteins have been implicated in organizing actin in the cortical cytoskeleton (reviewed in Tsukita and Yonemura, 1997). Perhaps an interaction with MAGUK proteins offers a mechanism for tethering transmembrane proteins into the cortical actin network. This connection would serve to localize proteins to the proper cell membrane domain and bring them into proximity with other proteins in the same signaling pathway. For example, CASK could stabilize syndecan at the basolateral surface where it binds extracellular matrix and acts as a coreceptor for ligands such as bFGF. CASK might then recruit other proteins used in signaling events downstream of the bFGF receptor. Although direct interactions between CASK and signaling proteins have not yet been demonstrated, other MAGUKs clearly have such associations. For example, hDlg binds via its proline-rich NH2-terminal domain to the SH3 domain of the nonreceptor tyrosine kinase p56 lck in lymphocytes (Hanada et al., 1997). It also binds the tumor suppressor APC in epithelial cells and neurons (Matsumine et al., 1996). The synaptic proteins PSD-95 and PSD-93 bind neuronal nitric oxide synthase (Brenman et al., 1996a and Brenman et al., 1996b ). Finally, the tight junction MAGUK ZO-1 binds through its SH3 domain to a novel serine threonine kinase, ZO-1–associated kinase, or ZAK (Balda et al., 1996).
The syndecan–CASK–protein 4.1 interaction may explain the results of previous studies demonstrating a regulated association between syndecan and the actin cytoskeleton. In stably transfected Schwann cells, syndecan-1 has been seen to colocalize transiently with actin filaments during cell spreading. Similarly, antibody-mediated clustering of syndecan molecules on the cell surface also induces actin colocalization (reviewed in Carey, 1997). Mutational analysis has implicated the involvement of a tyrosine residue within the syndecan-1 cytoplasmic domain in this connection (Carey et al., 1996); however, the potential role of CASK as a link has not yet been explored.
Our results suggest several novel roles for CASK in epithelial cells. Activation of the bFGF receptor tyrosine kinase is optimal when the bFGF ligand is presented as a ternary complex bound to the heparan sulfate moieties of syndecan (Rapraeger et al., 1991; Yayon et al., 1991). If CASK is involved in bFGF signaling it may in this context facilitate receptor tyrosine kinase (RTK) function by restricting syndecan to the basolateral cell membrane with the receptor. A previously published study supports a role for CASK in proper syndecan localization (Miettinen et al., 1994). When wild-type syndecan was transfected into Madin-Darby canine kidney cells, it appeared on the basal and lateral membrane domains. A truncated syndecan missing the last twelve amino acids of the cytoplasmic tail mislocalized to both the apical and basolateral cell surfaces. These data may be explained by the inability of truncated syndecan to bind the PDZ domain of CASK. CASK might be necessary for syndecan targeting to the correct membrane domains; alternatively, CASK may be stabilizing syndecan at the membrane by linking it to the actin cytoskeleton.
In vertebrate epithelial cells and in nematode vulval precursor cells, indirect evidence suggests that CASK and LIN-2, respectively, are each in close proximity to a receptor tyrosine kinase. LIN-2 is responsible for localizing the LET-23 receptor to the basal cell surface through a mechanism that does not involve direct binding to the RTK (Simske et al., 1996). There is genetic evidence in C. elegans for involvement of other proteins in intracellular LET-23 binding (Simske et al., 1996). However, by analogy to the proposed CASK–syndecan–bFGFR interaction in mammalian cells, it is possible that LIN-2 binds to the C. elegans syndecan homolog, perhaps forming a coreceptor complex with the LET-23 RTK. Interestingly, C. elegans syndecan terminates with the same four residues as the vertebrate syndecan EFYA (Carey, 1997), suggesting that the LIN-2 PDZ domain is capable of binding to its COOH-terminal tail. Whatever the mechanism of association between CASK/LIN-2 and the cytoplasmic domain of RTKs, this proximity raises the possibility of a role in signal transduction. For example, such a role might be to recruit other signaling molecules, to act itself as a substrate for the RTK, or to regulate the availability of other substrates. There is presently no direct evidence for this model; however, CASK/LIN-2 has several domains that might serve such purposes. These hypotheses provide directions for future experiments concerning the role of CASK in vertebrate epithelial cell signaling.
Acknowledgments
The authors thank Drs. Alan Fanning, Z. Songyang, and Chrisina Van Itallie for technical advice; Dr. Morgan Sheng and Scott Naisbitt for their instrumental role in the yeast two-hybrid screen, Dr. Sara Kolla for providing a radiolabeled β-actin cDNA, and Dr. Merton Bernfield for generously providing the syndecan antibodies. This research was funded by a Program Project grant from the National Cancer Institute (NCI CA 66263, director P.J. Bryant) to J.M. Anderson, A.H. Chishti, and D.F. Woods, and by core facilities of the Yale Liver Center (National Institutes of Health DK38979).
Abbreviations used in this paper
- CAMK
calcium/calmodulin-dependent protein kinase
- GST
glutathione-S-transferase
- GUK
guanylate kinase
- MAGUK
membrane-associated guanylate kinase
- RTK
receptor tyrosine kinase
- SH3
Src homology 3
Footnotes
Address all correspondence to James M. Anderson, 1080 LMP, Department of Internal Medicine, Yale University School of Medicine, 333 Cedar Street, 1080 LMP, P.O. Box 208019, New Haven, CT 06520-8019. Tel: 203-785-7312. Fax: 203-785-7273; E-mail: james.anderson@yale.edu
References
- Aroia RV, Koga M, Mendel JE, Ohshima Y, Sternberg PW. The let-23 gene necessary for Caenorhabditis elegansvulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990;348:693–699. doi: 10.1038/348693a0. [DOI] [PubMed] [Google Scholar]
- Balda MS, Anderson JM, Matter K. The SH3 domain of the tight junction protein ZO-1 binds to a serine protein kinase that phosphorylates a region COOH-terminal to this domain. FEBS Lett. 1996;399:326–332. doi: 10.1016/s0014-5793(96)01352-x. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Ann Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Ann Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide Synthase with the postsynaptic density protein PSD95 and α1-syntrophin mediated by PDZ domains. Cell. 1996a;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Christopherson KS, Craven SE, McGee AW, Bredt DS. Cloning and characterization of postsynaptic density 93, a nitric oxide synthase interacting protein. J Neurosci. 1996b;16:7407–7415. doi: 10.1523/JNEUROSCI.16-23-07407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DJ. Syndecans: multifunctional cell-surface co-receptors. Biochem J. 1997;327:1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DJ, Evans DM, Stahl RC, Asundi VK, Conner KJ, Garbes P, Cizmeci-Smith G. Molecular cloning and characterization of N-syndecan, a novel transmembrane heparan sulfate proteoglycan. J Cell Biol. 1992;117:191–201. doi: 10.1083/jcb.117.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DJ, Bendt KM, Stahl RC. The cytoplasmic domain of syndecan-1 is required for cytoskeleton association but not detergent insolubility. J Biol Chem. 1996;271:15253–15260. doi: 10.1074/jbc.271.25.15253. [DOI] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophiladiscs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Soderling TR. Calcium/calmodulin-dependent protein kinase II. Curr Topics Cell Regul. 1990;31:181–221. doi: 10.1016/b978-0-12-152831-7.50007-x. [DOI] [PubMed] [Google Scholar]
- Daniels DL, Cohen AR, Anderson JM, Brünger AT. Crystal structure of the hCASK PDZ domain reveals the structural basis Class II PDZ domain target recognition. Nat Struct Biol. 1998;5:317–324. doi: 10.1038/nsb0498-317. [DOI] [PubMed] [Google Scholar]
- Dimitratros SD, Woods DF, Bryant PJ. Camguk, Lin-2 and CASK: novel membrane-associated guanylate kinase homologs that also contain CaM kinase domains. Mech Dev. 1997;63:127–130. doi: 10.1016/s0925-4773(97)00668-0. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: Molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Lapierre LA, Brecher AR, Van Itallie CM, Anderson JM. Protein interactions in the tight junction: the role of MAGUK proteins in regulating tight junction organization and function. Curr Top Membr. 1996;43:211–235. [Google Scholar]
- Fanning AS, Anderson JM. Protein-protein interactions: PDZ domain networks. Curr Biol. 1996;6:1385–1388. doi: 10.1016/s0960-9822(96)00737-3. [DOI] [PubMed] [Google Scholar]
- Fallon MB, Mennone A, Anderson JM. Altered expression and localization of the tight junction ZO-1 after common bile duct ligation. Am J Physiol. 1993;264:C1439–C1447. doi: 10.1152/ajpcell.1993.264.6.C1439. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Horvitz HR. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. . Genetics. 1985;110:17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T, Lin L, Chandy KG, Oh SS, Chishti AH. Human homolog of the Drosophila discs large tumor suppressor binds to p56lck tyrosine kinase and shaker type Kv1.3 potassium channel in T lymphocytes. J Biol Chem. 1997;272:26899–26904. doi: 10.1074/jbc.272.43.26899. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hata Y, Butz S, Sudhof TC. CASK: A novel dlg/PSD95 homolog with an N-terminal CaM kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins R, Hajnal AF, Harp SA, Kim SK. The C. elegansvulval induction gene lin-2 encodes a member of the MAGUK family of cell junction proteins. Development. 1995;122:97–111. doi: 10.1242/dev.122.1.97. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based adhesion through its direct binding to (catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Naisbitt S, Hsueh Y, Rao A, Rothschild A, Craig AM, Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+channels by direct interaction with the PSD-95/ SAP90 family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kojima T, Shworak NW, Rosenberg RD. Molecular cloning and expression of two distinct cDNA-encoding heparan sulfate proteins from a rat endothelial cell line. J Biol Chem. 1992;267:4870–4877. [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interactions between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kapiloff MS, Durgerian S, Tatemoto K, Russo AF, Hanson P, Schulman H, Rosenfeld MG. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci USA. 1987;84:5962–5966. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue RA, Marfatia SM, Branton D, Chishti AH. Cloning and characterization of hdlg: the human homologue of the Drosophiladiscs large tumor suppressor binds to protein 4.1. Proc Natl Acad Sci USA. 1994;91:9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali M, Jaakkola P, Arvilommis AM, Jalkanen M. Sequence of human syndecan indicates a novel gene gamily of integral membrane proteoglycans. J Biol Chem. 1990;265:6884–6898. [PubMed] [Google Scholar]
- Marfatia SM, Morais-Cabral JH, Kim AC, Byron O, Chishti AH. The PDZ domain of human erythrocyte p55 mediates its binding to the cytoplasmic carboxyl terminus of glycophorin C. J Biol Chem. 1997;272:24191–24197. doi: 10.1074/jbc.272.39.24191. [DOI] [PubMed] [Google Scholar]
- Marfatia SM, Lue RA, Branton D, Chishti AH. Identification of the protein 4.1 binding interface on glycophorin C and p55, a homologue of the Drosophiladiscs-large tumor suppressor protein. J Biol Chem. 1995;270:715–719. doi: 10.1074/jbc.270.2.715. [DOI] [PubMed] [Google Scholar]
- Marfatia SM, Lue RA, Branton D, Chishti AH. In vitro binding studies suggest a membrane-associated complex between erythroid p55, protein 4.1, and glycophorin C. J Biol Chem. 1994;269:8631–8634. [PubMed] [Google Scholar]
- Marynen P, Zhang J, Cassiman JJ, Van den Berghe H, David G. Partial primary structure of the 48- and 90-kD core proteins of cell surface associated heparan sulfate proteoglycans of lung fibroblasts. J Biol Chem. 1989;264:7017–7024. [PubMed] [Google Scholar]
- Matsumine A, Ogai A, Senda T, Okumura N, Satoh K, Baeg G, Kawahara T, Kobayashi S, Okada M, Toyoshima K, Akiyama T. Binding of APC to the human homolog of the Drosophiladiscs large tumor suppressor protein. Science. 1996;272:1020–1023. doi: 10.1126/science.272.5264.1020. [DOI] [PubMed] [Google Scholar]
- Miettinen HM, Edwards SN, Jalkanen M. Analysis of transport and targeting of syndecan-1: effect of cytoplasmic tail deletions. Mol Biol Cell. 1994;5:1325–1339. doi: 10.1091/mbc.5.12.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C-terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton A, Jordan S, Yeomans P. Brief high-temperature denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed specimens. J Pathol. 1994;173:371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- Puschel AW, Betz H. Neurexins are differentially expressed in the embryonic nervous system of mice. J Neurosci. 1995;15:2849–2856. doi: 10.1523/JNEUROSCI.15-04-02849.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Ruff P, Speicher DW, Husain-Chishti A. Molecular identification of a major palmitoylated erythrocyte membrane protein containing the src homology 3 motif. Proc Natl Acad Sci USA. 1991;88:6595–6599. doi: 10.1073/pnas.88.15.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S, Jalkanen M, O'Farrell S, Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simske JS, Harp SA, Kim SK. Let-23 receptor localization by the cell junction protein Lin-7 during C. elegansvulval induction. Cell. 1996;85:195–204. doi: 10.1016/s0092-8674(00)81096-x. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxy-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Stehle T, Schulz GE. Refined structure of the complex between guanylate kinase and its substrate GMP at 2.0 A resolution. J Mol Biol. 1992;224:1127–1141. doi: 10.1016/0022-2836(92)90474-x. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. SAPAPs: a family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staelin T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Nat Acad Sci USA. 1979;76:44350–44354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Yonemura S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- Willott E, Balsa MS, Fanning AS, Jameson B, Van Itallie CM, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci USA. 1993;90:7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecan-4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell. 1994;5:183–192. doi: 10.1091/mbc.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophilaencodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Zschocke PD, Schlitz E, Schulz GE. Purification and sequence determination of guanylate kinase from pig brain. Eur J Biochem. 1993;213:263–269. doi: 10.1111/j.1432-1033.1993.tb17757.x. [DOI] [PubMed] [Google Scholar]