Abstract
Sphingosine-1-phosphate (SPP), a bioactive lipid, acts both intracellularly and extracellularly to cause pleiotropic biological responses. Recently, we identified SPP as a ligand for the G protein–coupled receptor Edg-1 (Lee, M.-J., J.R. Van Brocklyn, S. Thangada, C.H. Liu, A.R. Hand, R. Menzeleev, S. Spiegel, and T. Hla. 1998. Science. 279:1552–1555). Edg-1 binds SPP with remarkable specificity as only sphinganine-1-phosphate displaced radiolabeled SPP, while other sphingolipids did not. Binding of SPP to Edg-1 resulted in inhibition of forskolin-stimulated cAMP accumulation, in a pertussis toxin–sensitive manner. In contrast, two well-characterized biological responses of SPP, mitogenesis and prevention of apoptosis, were clearly unrelated to binding to Edg-1 and correlated with intracellular uptake. SPP also stimulated signal transduction pathways, including calcium mobilization, activation of phospholipase D, and tyrosine phosphorylation of p125FAK, independently of edg-1 expression. Moreover, DNA synthesis in Swiss 3T3 fibroblasts was significantly and specifically increased by microinjection of SPP. Finally, SPP suppresses apoptosis of HL-60 and pheochromocytoma PC12 cells, which do not have specific SPP binding or expression of Edg-1 mRNA. Conversely, sphinganine-1-phosphate, which binds to and signals via Edg-1, does not have any significant cytoprotective effect. Thus, SPP is a prototype for a novel class of lipid mediators that act both extracellularly as ligands for cell surface receptors and intracellularly as second messengers.
Keywords: sphingolipids, mitogenesis, G proteins, apoptosis, signal transduction
Sphingolipid metabolites, ceramide, sphingosine, and sphingosine-1-phosphate (SPP),1 are emerging as members of a new class of lipid second messengers (Kolesnick and Fuks, 1995; Spiegel and Milstien, 1995; Hannun, 1996). Ceramide, formed by receptor-coupled activation of sphingomyelinase, has been associated with cell growth arrest and is an important component of stress responses and apoptosis (Hannun, 1996), whereas SPP, a further metabolite of ceramide, is mitogenic in diverse cell types (Zhang et al., 1991; Bornfeldt et al., 1995; Gomez-Munoz et al., 1995; Pyne et al., 1996) and opposes ceramide-mediated apoptosis (Cuvillier et al., 1996; Edsall et al., 1997). Thus, we have proposed that the relative intracellular levels of these two sphingolipid metabolites is an important factor that determines whether cells will survive or die (Cuvillier et al., 1996). More recently, it has been demonstrated that this ceramide/SPP rheostat is an evolutionarily conserved stress regulatory mechanism influencing growth and survival of yeast (Mandala et al., 1997).
Various stimuli, including PDGF and serum (Olivera and Spiegel, 1993; Bornfeldt et al., 1995), NGF (Edsall et al., 1997), activation of protein kinase C (Mazurek et al., 1994; Buehrer et al., 1996), and cross-linking of the FcεRI receptor by antigens (Choi et al., 1996), increase cellular levels of SPP by activation of sphingosine kinase, the enzyme that catalyzes the phosphorylation of sphingosine. Competitive inhibitors of sphingosine kinase eliminate formation of SPP and selectively block cellular proliferation induced by PDGF and serum (Olivera and Spiegel, 1993; Rani et al., 1997), as well as FcεRI-mediated calcium signaling (Choi et al., 1996) and the cytoprotective effects of cytokines (Cuvillier et al., 1996) and NGF (Edsall et al., 1997), further supporting a role for endogenous SPP.
Although many studies indicate an intracellular site of action of SPP, some of its biological effects when added exogenously may be due to binding to cell surface receptors. SPP is stored in high concentrations and is released from human platelets upon activation by physiological stimuli (Yatomi et al., 1995), and it is present at high levels in serum (Yatomi et al., 1997b ). Previously, we have shown that pertussis toxin–sensitive G proteins are involved in some of the signaling pathways activated by SPP, suggesting that it might activate a receptor coupled to a Gi/Go-protein (Goodemote et al., 1995). In agreement, low concentrations of SPP activate Gi protein–gated inward rectifying K+-channels only when applied at the extracellular face of atrial myocytes (van Koppen et al., 1996). Moreover, nanomolar concentrations of SPP (EC50 = 2 nM) rapidly induced Rho-dependent neurite retraction and cell rounding of mouse N1E-115 neurons (Postma et al., 1996) and markedly inhibited melanoma cell motility by binding to an extracellular receptor (Yamamura et al., 1997).
Recently, we identified Edg-1 as the receptor for SPP and demonstrated that binding of SPP to Edg-1 induces morphogenetic differentiation via a Rho-dependent signaling pathway (Lee et al., 1998). edg-1 was cloned as an immediate-early gene induced by phorbol ester treatment of human umbilical vein endothelial cells (Hla and Maciag, 1990) and is thought to be involved in endothelial cell differentiation (Hla and Maciag, 1990; Lee et al., 1998). Here we report that SPP has dual actions. In addition to signaling through a cell surface Gi-coupled receptor, SPP acts intracellularly to regulate cellular proliferation and suppression of apoptosis.
Materials and Methods
Materials
SPP, dihydrosphingosine-1-phosphate (dihydro-SPP), sphingosine, N,N-dimethylsphingosine, and N-acetyl sphingosine (C2-ceramide) were purchased from BIOMOL Research Laboratory, Inc. (Plymouth Meeting, PA). N-octanoyl ceramide-1-phosphate (C8-ceramide-1-P) was from Calbiochem (La Jolla, CA). Other lipids were purchased from Avanti Polar Lipids (Birmingham, AL). SPP-phosphonate was kindly provided by Dr. Richard R. Schmidt (University of Konstanz, Konstanz, Germany). [methyl-3H]Thymidine (83 Ci/mmol) and [γ-32P]ATP (3,000 Ci/mmol) were purchased from Amersham Corp. (Arlington Heights, IL). Fura-2/ acetoxy-methyl ester (fura-2/AM) was from Molecular Probes, Inc. (Eugene, OR). Serum and medium were obtained from Biofluids, Inc. (Rockville, MD). Pertussis toxin was from List Biological Labs (Campbell, CA).
Cell Culture
Human embryonic kidney cells (HEK293, ATCC CRL-1573 [American Type Culture Collection, Rockville, MD]) stably transfected with epitope-tagged human edg-1 cDNA in the pcDNANeo expression vector or pcDNANeo control vector and NIH 3T3 fibroblasts (ATCC CRL-1658) stably transfected with pMexNeo vector or pMexNeo containing FLAG-tagged edg-1 were grown in DME containing 10% fetal bovine serum, 0.25 g/liter G418 sulfate (Biofluids, Inc.) as previously described (Lee et al., 1996, 1998). Swiss 3T3 cells (ATCC CCL-92) were subcultured at a density of 1.5 × 104 cells/cm2 in DME supplemented with 2 mM glutamine and 10% calf serum, refed with the same medium after 2 d, and used 5 d later when the cells were confluent and quiescent (Olivera and Spiegel, 1993). Rat pheochromocytoma PC12 cells (ATCC CRL-1721) were maintained in RPMI medium supplemented with 10% heat-inactivated horse serum, 5% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Edsall et al., 1997). Human promyelocytic HL-60 cells (ATCC CCL-240) were grown in RPMI 1640 containing 10% fetal bovine serum as previously described (Cuvillier et al., 1996).
Sphingosine-1-Phosphate Binding Assay
[32P]SPP was synthesized enzymatically using partially purified sphingosine kinase as previously described (Olivera et al., 1994). The specific activity of [32P]SPP was 1.28 × 106 cpm/pmol. Cells were incubated with 1 nM [32P]SPP (200,000 cpm) in 200 μl binding buffer (20 mM Tris-HCl, pH 7.4, 100 mM NaCl, 15 mM NaF, 2 mM deoxypyridoxine, 0.2 mM PMSF, 1 μg/ml aprotinin and leupeptin) for 30 min at 4°C. Unlabeled lipid competitors were added as 4 mg/ml fatty acid-free BSA complexes (Zhang et al., 1991). Cells were washed twice with 200 μl ice-cold binding buffer containing 0.4 mg/ml fatty acid-free BSA and resuspended in PBS, and bound [32P]SPP was quantitated by scintillation counting.
Measurement of Cyclic AMP Levels
Cells were incubated for 15 min at 37°C in DME containing the phosphodiesterase inhibitor IBMX (0.5 mM), in the absence or presence of 10 μM forskolin, and the indicated concentration of SPP or vehicle. Medium was then aspirated, cells were washed with PBS, and cAMP was extracted by sonication in 4 mM EDTA. After centrifugation, samples were boiled for 3 min and recentrifuged, and cAMP was measured using the Biotrak [3H]cAMP assay system (Amersham Corp.) according to the manufacturer's instructions.
Measurement of DNA Synthesis
Cells were grown to confluence in 24-well tissue culture plates, washed with serum-free DME containing 5 μg/ml transferrin and 20 μg/ml BSA, stimulated by the addition of the indicated concentration of SPP, and after 16 h, pulsed with 1.0 μCi of [3H]thymidine for 8 h. Incorporation of [3H]thymidine into trichloroacetic acid–insoluble material was measured as described (Olivera et al., 1992). Values are the means of triplicate determinations, and standard deviations were routinely less than 10% of the mean.
Northern Hybridization
Total RNA from various cell lines was extracted by the acid guanidine thiocyanate/phenol/chloroform method (Chomczynski and Sacchi, 1987). RNA (10 μg) was electrophoresed on 1% agarose/formaldehyde gels and transferred to Zeta-probe membranes (Bio-Rad Laboratories, Hercules, CA). Mouse edg-1 cDNA probe was labeled with [α-32P]dCTP using a random primer labeling kit (Amersham Corp.). Membranes were hybridized as described previously (Liu and Hla, 1997) and exposed to x-ray film for autoradiography.
Measurement of Uptake and Metabolism of SPP
Cells were washed with PBS, incubated in DME at 4 or 37°C for 30 min in the presence of 10 μM [32P]SPP (64,000 cpm/nmol) (Van Veldhoven and Mannaerts, 1994), washed with DME containing 0.4 mg/ml fatty acid-free BSA, and then incubated in DME for the indicated time. After washing with cold DME, cells were trypsinized, and cellular lipids were extracted as described (Olivera and Spiegel, 1993). Extracts were applied to silica gel 60 G plates, which were developed with chloroform/methanol/25 mM NaH2PO4 (60:35:8, vol/vol), and then exposed to a Phosphor Screen (Molecular Dynamics, Sunnyvale, CA) for 2 h. Radioactive bands were quantified using a Storm densitometer with the ImageQuant program (Molecular Dynamics). Radioactivity in aliquots from each sample was also quantitated by liquid scintillation counting for measurement of SPP uptake. Sphingosine production was measured as described (Lavie et al., 1994).
Determination of Tyrosine Phosphorylation of p125FAK
HEK293 cells were treated with SPP added as a complex with 4 mg/ml BSA for the indicated times, washed twice with PBS, and then lysed by addition of 0.5 ml lysis buffer (50 mM Hepes, pH 7.9, 1% Triton X-100, 100 mM NaCl, 10 mM EDTA, 4 mM sodium pyrophosphate, 10 mM NaF, 1 mM PMSF, 2 mM Na3VO4, and 2 μg/ml aprotinin and leupeptin). p125FAK was immunoprecipitated from samples containing equal amounts of protein with anti-p125FAK monoclonal antibody (Upstate Biotechnology, Inc., Lake Placid, NY). Immunoprecipitates were washed three times with lysis buffer and separated by 7% SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Bio-Rad Laboratories) and probed with monoclonal antiphosphotyrosine 4G10 (Upstate Biotechnology, Inc.). Bands were visualized with Super Signal chemiluminescent reagent (Pierce Chemical Co., Rockford, IL).
Measurement of Cytoplasmic Free Calcium Concentrations
Cells were grown on 35-mm plastic tissue culture dishes and loaded with the fluorescent calcium-sensitive dye, fura-2/AM (5 μM), for 45 min at 37°C in DME supplemented with 60 μg/ml BSA. Subsequently, cells were washed with Locke's buffer (137 mM NaCl, 2.7 mM KCl, 0.5 mM MgCl2, 0.93 mM CaCl2, 6.5 mM Na2HPO4, 1.5 mM KH2PO4, 1 mg/ml glucose, 1 mg/ml BSA, pH 7.4) and mounted in a 35-mm holder maintained at 30°C. Changes in fura-2 fluorescence were monitored in single cells by dual excitation imaging using an Attofluor Digital Fluorescence Microscopy System (Atto Instruments Inc., Rockville, MD). [Ca2+]i was determined from the ratio of fura-2 fluorescence emission after excitation at wavelengths of 334 and 380 nm (Mattie et al., 1994).
Phosphatidic Acid Determination
Cells were washed and incubated in DME containing [32P]orthophosphate (100 μCi/ml) for 24 h. Cells were then treated with SPP or vehicle alone for the indicated times, the medium was rapidly removed, and lipids were extracted (Zhang et al., 1990). Phosphatidic acid was analyzed by thin-layer chromatography using the organic phase of the mixture of isooctane/ethyl acetate/acetic acid/water (50:110:20:100) (Zhang et al., 1990). In this system, phosphatidic acid (R f = 0.1) was separated from other phospholipids (R f = 0). Lipid standards were visualized with molybdenum blue (Sigma Chemical Co., St. Louis, MO). Phosphatidic acid was quantified using a Storm densitometer with the ImageQuant program (Molecular Dynamics).
Staining of Apoptotic Nuclei
Cells were cultured in serum-free medium in the absence or presence of the indicated sphingolipids for 15 h, washed in PBS, and fixed in 3.7% formaldehyde for 10 min. After washing with PBS, fixed cells were then incubated with bisbenzimide trihydrochloride (24 μg/ml in 30% glycerol/ PBS; Hoechst #33258, Calbiochem) for 10 min. Stained cells were examined with a Zeiss Photoscope II fluorescent microscope (Thornwood, NY). Apoptotic cells were distinguished by condensed, fragmented nuclear regions. A minimum of 2,000 cells was scored.
Quantitation of DNA Fragmentation
DNA fragmentation was determined in cells prelabeled with [3H]thymidine (1 μCi/ml) for 24 h (Duke and Cohen, 1992). Cells were washed gently twice with serum-free medium and then incubated in the same medium with the indicated sphingolipids for 5 h at 37°C. Cells were harvested and lysed in TTE (10 mM Tris, pH 7.4, 10 mM EDTA, 0.2% Triton X-100), and fragmented DNA was separated from intact chromatin by centrifugation. Pellets were suspended in TTE, and TCA was added to a final concentration of 12.5%. [3H]Thymidine incorporated into both intact and fragmented DNA was determined by liquid scintillation counting (Duke and Cohen, 1992). Percent fragmented DNA = 100 × [fragmented / (fragmented + intact chromatin)].
Microinjection and Immunostaining
Swiss 3T3 fibroblasts were grown on CELLocate gridded glass coverslips (Eppendorf, Hamburg, Germany) and rendered quiescent by starvation for 24 h in serum-free DME supplemented with transferrin (4 μg/ml) and fatty acid-free BSA (20 μg/ml). Cells were microinjected on a heated stage using an Eppendorf Micromanipulator 5171 and Transjector 5246. 400 serum-starved cells were microinjected cytoplasmically with SPP (∼10 fl of 500 μM, estimated cytoplasmic concentration = 5 μM) together with rabbit IgG (RIgG, 5 mg/ml) as a tracking agent using sterile Femtotips (Eppendorf) at 100 hPa for 0.2 s. Control cells were microinjected with rabbit IgG alone. Immediately after microinjection, cells were incubated in fresh serum-free DME with bromodeoxyuridine (BrdU; 10 mM) for 24 h at 37°C. Cells were then fixed in acidic ethanol (70%, in 50 mmol/liter glycine buffer, pH 2.0) and stained with mouse monoclonal anti-BrdU antibody and anti–mouse Ig-fluorescein according to the manufacturer's instructions (BrdU Labeling and Detection Kit; Boehringer Mannheim GmbH, Mannheim Germany). Cells were stained for RIgG with a 1:200 dilution of anti-RIgG Texas red–conjugated secondary antibody to localize injected cells. After the coverslips were mounted on slides using the Molecular Probes Anti-Fade kit (Eugene, OR), cells were analyzed with a fluorescent microscope (model Photoscope II; Carl Zeiss, Inc.). Cell counts of nuclear BrdU labeling were determined by fluorescence microscopy using a triple band pass filter.
Results
Specificity of SPP Binding to Edg-1
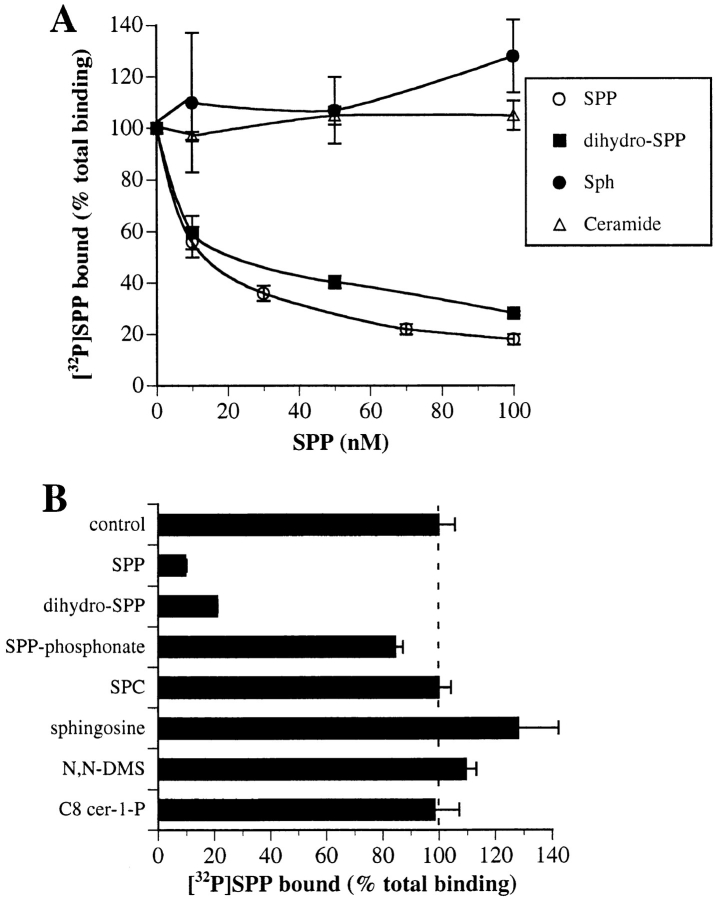
We recently identified the G protein–coupled receptor Edg-1 as a receptor for SPP (Lee et al., 1998). In an attempt to identify other potential agonists or antagonist of Edg-1, we examined the effects of structurally related lipids on binding of [32P]SPP to human embryonic kidney 293 fibroblasts stably expressing FLAG epitope–tagged Edg-1 (HEK293edg-1). Only sphinganine-1-phosphate (dihydro-SPP), which lacks the double bond at the 4 position, blocked binding in a dose-dependent manner nearly as potently as did unlabeled SPP (K i = 15 nM) (Fig. 1 A). In contrast, other related lipids, including sphingosine, the nonhydrolyzable analogue, SPP-phosphonate (Tarnowski et al., 1997), a short chain SPP analogue (C8-SPP), N,N-dimethylsphingosine, C2-ceramide, sphingosylphosphorylcholine (SPC), and N-octanoyl ceramide-1-phosphate (C8-cer-1-P), did not compete with SPP for binding to Edg-1 (Fig. 1 B).
Figure 1.
Effects of SPP analogues and other lipids on specific binding of [32P]SPP to Edg-1. Competition of SPP binding by related lipids. HEK293–edg-1 cells were incubated in the presence of 1 nM [32P]SPP with increasing concentrations of unlabeled SPP, dihydro-SPP, sphingosine, or C2-ceramide (A) or in the presence of 100 nM of the indicated lipids (B), and binding was measured as described in Materials and Methods. Specific binding of SPP to HEK293–edg-1 (total binding minus binding in the presence of 100-fold excess unlabeled SPP) was 32 fmol/105 cells. Results are means ± standard deviations of triplicate determinations.
SPP Decreases cAMP Levels via the Gi-coupled Receptor Edg-1
Biochemical evidence and the yeast two-hybrid system indicate that Edg-1 is capable of interaction with Giα1 and Giα3 (Lee et al., 1996). Moreover, Edg-2 and the cannabinoid receptors, which are related to the edg family, are known to be linked to Gi signaling pathways leading to decreased levels of cAMP (Howlett, 1995; Hecht et al., 1996). Therefore, functional coupling of the Edg-1 receptor overexpressed in HEK293 cells in response to its ligand SPP was investigated. The effect of SPP on cAMP accumulation was examined after stimulation of adenylate cyclase with forskolin in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) to ensure that changes in cAMP levels were not mediated by effects on phosphodiesterase activity. Binding of SPP to Edg-1 markedly inhibited forskolin-stimulated cAMP accumulation in a dose-dependent manner (Fig. 2 A), which correlated closely with binding, as the K d of Edg-1 for SPP is 8 nM (Lee et al., 1998). Although lower concentrations of SPP had no effect on cAMP accumulation induced by forskolin in vector-transfected cells (HEK293-vector) devoid of Edg-1 expression and specific SPP binding, much higher concentrations slightly reduced cAMP levels, albeit to a lesser extent than in cells that express Edg-1 (Fig. 2 A). Thus, the additional decrease in cAMP seen at higher SPP concentrations may be due to non–receptor-mediated effects. Similarly to SPP, dihydro-SPP, which also binds to Edg-1, decreased cAMP levels (Fig. 2 B). In contrast, other structurally related lipids that do not bind to Edg-1, including SPC, sphingosine, C8-SPP, C8-cer-1-P, and particularly SPP-phosphonate, were without effect (Fig. 2 B). Moreover, pretreatment with N,N-dimethylsphingosine (10 μM), at a concentration that markedly inhibits sphingosine kinase and production of endogenous SPP, had no effect on the decreased cAMP accumulation induced by binding of SPP to Edg-1 (data not shown).
Figure 2.
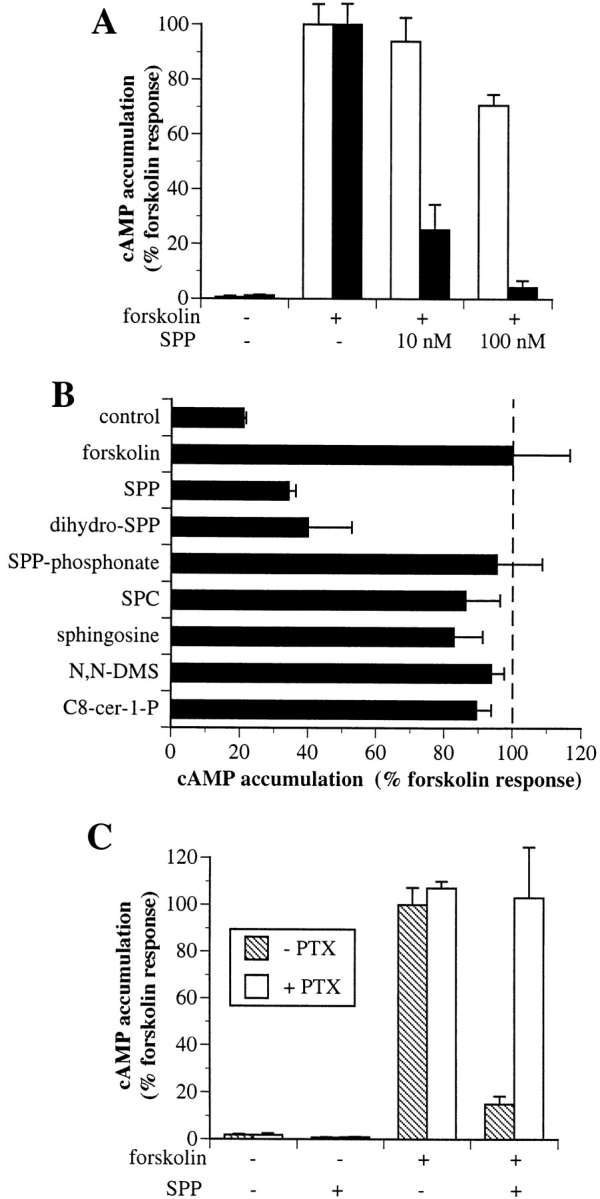
SPP decreases cAMP levels through the Edg-1 Gi-coupled receptor. (A) HEK293-vector (open bars) or HEK293–edg-1 cells (filled bars) were stimulated with 10 μM forskolin in the presence of 0.5 mM IBMX and the indicated concentrations of SPP for 15 min, and levels of cAMP were then measured. The levels of cAMP in untreated and forskolin-treated cells were 20 ± 1.7 and 420 ± 30 pmol/106 cells and 10 ± 1.5 and 820 ± 60 pmol/ 106 cells for vector and edg-1–overexpressing cells, respectively. Results are means ± SD of triplicate determinations. (B) Effects of SPP analogues on cAMP accumulation. HEK293–edg-1 cells were treated with lipids or SPP analogues (100 nM), and forskolin-stimulated cAMP accumulation was determined as described in A. (C) Pertussis toxin inhibits the effect of SPP on cAMP accumulation. Cells were pretreated in the absence (filled bars) or presence (open bars) of 200 ng/ml pertussis toxin (PTX) for 3 h and then were treated for 15 min with forskolin (10 μM) and/or SPP (100 nM), or combinations, as indicated, and cAMP accumulation was measured.
The SPP-induced cAMP decrease in HEK293-edg-1 cells was completely blocked by pretreatment with pertussis toxin, which ADP ribosylates and inactivates Gi and Go proteins (Fig. 2 C). Therefore, binding of SPP to the serpentine receptor Edg-1 on the cell surface activates a pertussis toxin–sensitive Gi protein.
SPP Stimulates Non–receptor-mediated Signal Transduction Pathways
Recently, it was shown that the decrease in cAMP resulting from binding of the endogenous ligand anandamide to the CB-1 receptor resulted in tyrosine phosphorylation of focal adhesion kinase (p125FAK) (Derkinderen et al., 1996). Moreover, we previously showed that SPP enhances phosphorylation of p125FAK in Swiss 3T3 cells (Wang et al., 1997). Surprisingly, we found that SPP-induced tyrosine phosphorylation of p125FAK was independent of Edg-1 receptor activation, as the response was greater in cells lacking the receptor and was even decreased in cells overexpressing edg-1 (Fig. 3 A). Furthermore, tyrosine phosphorylation of p125FAK was increased even more at concentrations of SPP higher than those that saturate Edg-1 binding (Lee et al., 1998).
Figure 3.

SPP stimulates non–Edg-1 receptor-mediated signal transduction pathways. (A) SPP stimulates tyrosine phosphorylation of p125FAK independently of Edg-1 expression. HEK293– edg-1 and vector-transfected HEK293 cells were treated with the indicated concentrations of SPP for various times, and cell lysates were immunoprecipitated with anti-p125FAK mAb and analyzed by Western blotting with anti-Tyr(P) antibody. The arrow indicates the migration p125FAK. (B) SPP-induced changes in intracellular free calcium. Vector and edg-1 stably transfected HEK293 cells were loaded with fura-2/AM, washed, and incubated at 37°C in Locke's buffer. At the indicated times, SPP (100 nM or 10 μM) was added, and [Ca2+]i was determined by fura-2 imaging (Mattie et al., 1994). Subsequent addition of ionomycin always caused increased [Ca2+]i. (C) SPP-induced phosphatidic acid accumulation. Vector and edg-1 stably transfected HEK293 cells and NIH 3T3 fibroblasts were prelabeled with [32P]i for 24 h and then stimulated with vehicle (control) or the indicated concentrations of SPP for 1 h. Lipids were extracted and separated by thin-layer chromatography, and [32P]phosphatidic acid was measured (Zhang et al., 1990). Data are expressed as fold- increases of [32P]phosphatidic acid. The incorporations of [32P] into phosphatidic acid in untreated cells were 1,500 ± 50 and 1,000 ± 100 cpm, and 2,500 ± 600 and 3,200 ± 200 cpm for vector and edg-1 stably transfected HEK293 cells and NIH 3T3 fibroblasts, respectively, determined from an aliquot of the phospholipid extract containing 150,000 cpm.
In many cell types, SPP has been shown to activate several signal transduction pathways (Desai et al., 1992; Chao et al., 1994; Ghosh et al., 1994; Mattie et al., 1994; Natarajan et al., 1994; Bornfeldt et al., 1995; Goodemote et al., 1995; Choi et al., 1996; Blakesly et al., 1997; Fatatis and Miller, 1997; Okajima et al., 1997), particularly mobilization of calcium and activation of phospholipase D, which in certain cell types are activated by low concentrations of SPP. Thus, it was of interest to determine whether these responses were dependent or independent of activation of the cell surface receptor Edg-1. As illustrated in Fig. 3 B, low concentrations of SPP (100 nM) had no effect on calcium mobilization in either vector-transfected or edg-1– overexpressing HEK293 cells, whereas treatment with 10 μM SPP led to identical increases in [Ca2+]i, suggesting that this response to SPP is not mediated by Edg-1. The calcium responses were also identical in vector- and edg-1– transfected NIH 3T3 cells (data not shown). In agreement, only high, mitogenic concentrations of SPP release calcium from internal sources in Swiss 3T3 fibroblasts (Mattie et al., 1994). Similarly, mobilization of calcium from internal sources by cannabinoid agonists was also shown to be independent of activation of the cannabinoid receptor (Felder et al., 1992). The effects of SPP on activation of phospholipase D were also Edg-1 receptor independent and were even attenuated by edg-1 overexpression in NIH 3T3 fibroblasts (Fig. 3 C). Thus, several mitogenic signal transduction pathways that are thought to be involved in SPP-induced proliferation are clearly Edg-1 receptor independent.
SPP Regulates Cell Proliferation and Survival Independently of Edg-1 Expression
Because the most well-established biological response to SPP is the stimulation of cell growth (Zhang et al., 1991; Olivera and Spiegel, 1993; Su et al., 1994; Gomez-Munoz et al., 1995; Goodemote et al., 1995; Wu et al., 1995; Pyne et al., 1996; Blakesly et al., 1997; Rani et al., 1997), we examined the effect of SPP on DNA synthesis in several cell lines expressing different levels of edg-1: SPP is a powerful mitogen for Swiss 3T3 fibroblasts (Zhang et al., 1991; Desai et al., 1992; Su et al., 1994; Rani et al., 1997; Wang et al., 1997) and is also mitogenic for NIH 3T3 fibroblasts, albeit less potent (Blakesly et al., 1997). In agreement with previous studies (Zhang et al., 1991; Desai et al., 1992; Su et al., 1994; Rani et al., 1997; Wang et al., 1997), 10 μM SPP induced marked stimulation of DNA synthesis in Swiss 3T3 fibroblasts (Fig 4 A). SPP was less mitogenic for control, vector-transfected NIH 3T3 fibroblasts (NIH 3T3/pMEX.2), and least mitogenic for edg-1–transfected NIH 3T3 cells (NIH 3T3/pMFE1.2), which express human Edg-1.
Figure 4.

SPP-stimulated DNA synthesis does not correlate with Edg-1 expression. (A) Swiss 3T3 fibroblasts (filled bars), vector-transfected NIH 3T3 cells (hatched bars), NIH 3T3 cells stably transfected with edg-1 (open bars), HEK293-vector (stippled bars), and HEK293–edg-1 cells (gray bars) were grown to confluence in 24-well tissue culture plates, washed with serum-free DME containing 5 μg/ml transferrin and 20 μg/ml BSA, and then stimulated with the indicated concentration of SPP. After 16 h, cells were pulsed with 1.0 μCi of [3H]thymidine for 8 h, and incorporation of [3H]thymidine into trichloroacetic acid–insoluble material was measured. Values are the means of triplicate determinations, and standard deviations were routinely less than 10% of the mean. (B) Northern analysis of Edg-1 expression was performed on total RNA from the indicated cells as described in Materials and Methods. A probe corresponding to the mouse edg-1 gene (Liu and Hla, 1997) was used to detect the low level of edg-1 mRNA present in Swiss 3T3 cells. For analysis of HEK293–edg-1 and HEK293-vector cells, a probe corresponding to the human edg-1 gene was used (Hla and Maciag, 1990). (C) Specific [32P]SPP binding to various cell types. Nonspecific (open bars) and total binding (filled bars) of 1 nM [32P]SPP to Swiss 3T3 fibroblasts, vector-transfected NIH 3T3 cells, NIH 3T3 cells stably transfected with edg-1, vector-transfected HEK293 cells, and edg-1-transfected HEK293 were measured as described in Materials and Methods.
Northern analysis of HEK293–edg-1 and HEK293-vector cells using a human edg-1 cDNA probe revealed, as expected, a prominent band in HEK293–edg-1 cells and no band in HEK293-vector cells (Fig. 4 B). As edg-1 expression was expected to be low in mouse fibroblasts, a mouse edg-1 probe that detects mouse edg-1 more sensitively than the human probe was used to analyze NIH 3T3 and Swiss 3T3 cells. Swiss 3T3 cells express the lowest levels of endogenous edg-1 mRNA of the three fibroblast lines tested (Fig. 4 B), which was confirmed by reverse transcriptase PCR analysis (data not shown). NIH 3T3 cells stably transfected with human edg-1 show the presence of both mouse and human edg-1 (2.9 and 1.8 kb, respectively; Fig. 4 B). In agreement, Swiss 3T3 cells showed a low level of specific binding (Fig. 4 C), whereas NIH–edg-1 cells have much higher specific [32P]SPP binding (Fig. 4 C). Thus, it seems that there is no correlation between edg-1 expression and the mitogenic response to SPP (compare Fig. 4 A, and B and C). In agreement, SPP was not mitogenic for HEK293 cells regardless of whether or not they expressed Edg-1 (Fig. 4 A).
Although low levels of SPP bound specifically to Swiss 3T3 fibroblasts at 4°C, no specific binding could be detected at any concentration of [32P]SPP (up to 10 μM) in Swiss 3T3 fibroblasts at 37°C. However, there was a large, cell-associated fraction of [32P]SPP (278 pmol per 106 cells) detected when Swiss 3T3 fibroblasts were incubated with 10 μM [32P]SPP at 37°C. This is due to uptake, as there was extensive intracellular metabolism catalyzed by SPP lyase and SPP phosphatase, both located in the endoplasmic reticulum (Van Veldhoven and Mannaerts, 1994; Mandala et al., 1997), and 57% of the cell-associated SPP was metabolized within 1 h while after 15 h, only 18% of the SPP still remained intact (data not shown).
To further substantiate the notion that the mitogenic actions of SPP observed at high concentrations was due to intracellular actions rather than binding to a cell surface receptor, we took advantage of our previous observation that mitogenesis induced by SPP only required a short exposure time (Wang et al., 1997). In agreement, exposure of Swiss 3T3 fibroblasts to SPP for 30 min at 37°C, followed by washing and replacement of the media, resulted in 50% of maximum DNA synthesis induced by 24 h of treatment (Fig. 5 A). In contrast, when Swiss 3T3 fibroblasts were incubated with SPP for 30 min at 4°C, to allow binding but significantly less uptake (Fig. 5 B), there was much less stimulation of DNA synthesis, suggesting that SPP must be taken up by cells to produce a mitogenic effect. Furthermore, in another approach to increase uptake and delivery of SPP into cells, SPP was mixed with the cationic lipid, lipofectamine, which forms liposomes that are readily internalized and has been widely used to deliver DNA, RNA, and protein (Felgner et al., 1987). In this liposome form, the dose-response for stimulation of DNA synthesis by SPP was shifted to much lower concentrations (Fig. 5 C) with a corresponding increase in [32P]SPP uptake (Fig. 5 D). Collectively, these results strongly suggest that the mitogenic effects of SPP are independent of receptor- mediated actions. In agreement, the nonhydrolyzable SPP-phosphonate, which does not bind to Edg-1 or stimulate the Gi signaling pathway, is as potent a mitogenic agent for Swiss 3T3 fibroblasts as is SPP (data not shown).
Figure 5.
SPP-stimulated DNA synthesis correlates with uptake of SPP. (A) Swiss 3T3 fibroblasts, cultured in serum-free DME containing 4 μg/ml insulin, 4 μg/ml transferrin, and 20 μg/ml BSA, were treated with the indicated concentrations of SPP at 4 (filled bars) or 37°C (open bars) for 30 min, followed by washing and replacement in the same medium without SPP. Cells were then incubated for 16 h at 37°C, and [3H]thymidine incorporation was measured. (B) In duplicate cultures, uptake was measured after 30 min incubation with 10 μM [32P]SPP. (C) Swiss 3T3 fibroblasts cultured in serum-free DME were treated with the indicated concentrations of SPP added as a complex with 4 mg/ml BSA (open symbols) or with 5 μl/ml lipofectamine (filled symbols) for 30 min followed by washing and replacement with DME containing 4 μg/ml insulin, 4 μg/ml transferrin, and 20 μg/ml BSA, and DNA synthesis was measured 16 h later. (D) In duplicate cultures, uptake of [32P]SPP was measured in the absence (open bars) or presence (filled bars) of lipofectamine.
To conclusively demonstrate that SPP stimulates DNA synthesis intracellularly, we microinjected SPP into the cytoplasm of quiescent Swiss 3T3 fibroblasts together with IgG to identify microinjected cells and examined the incorporation of BrdU into nascent DNA 24 h later. Using double immunofluorescence to visualize injected cells and BrdU incorporation, it is evident that DNA synthesis is increased after microinjection of SPP, as 6% of injected cells were positive for BrdU incorporation compared with 1% of adjacent, noninjected cells in the same field (Fig. 6). It should be noted that after exogenous treatment of Swiss 3T3 fibroblasts with optimum mitogenic concentrations of SPP or vehicle alone, 10 and 1% of the cells showed BrdU staining, respectively. Microinjection of rabbit IgG alone had no significant effect on DNA synthesis. Moreover, in contrast to the mitogenic effect of SPP, C8-ceramide-1-P, which has no effect on [3H]thymidine incorporation when added exogenously, also had no significant effect on BrdU incorporation when microinjected into quiescent Swiss 3T3 fibroblasts. It is noteworthy that similarly to the synergistic effect between insulin and SPP on [3H]thymidine incorporation (Zhang et al., 1991), microinjected SPP also potentiated the stimulation of BrdU incorporation by insulin (Fig. 6 H). Thus, induction of DNA synthesis by SPP does not require interaction with a cell surface receptor to activate the cellular machinery needed for proliferation. In addition, DNA synthesis stimulated by microinjected SPP was insensitive to pertussis toxin treatment, whereas 70% of that caused by exogenous SPP was blocked by pertussis toxin (Fig. 6 H), in agreement with our previous results (Goodemote et al., 1995).
Figure 6.
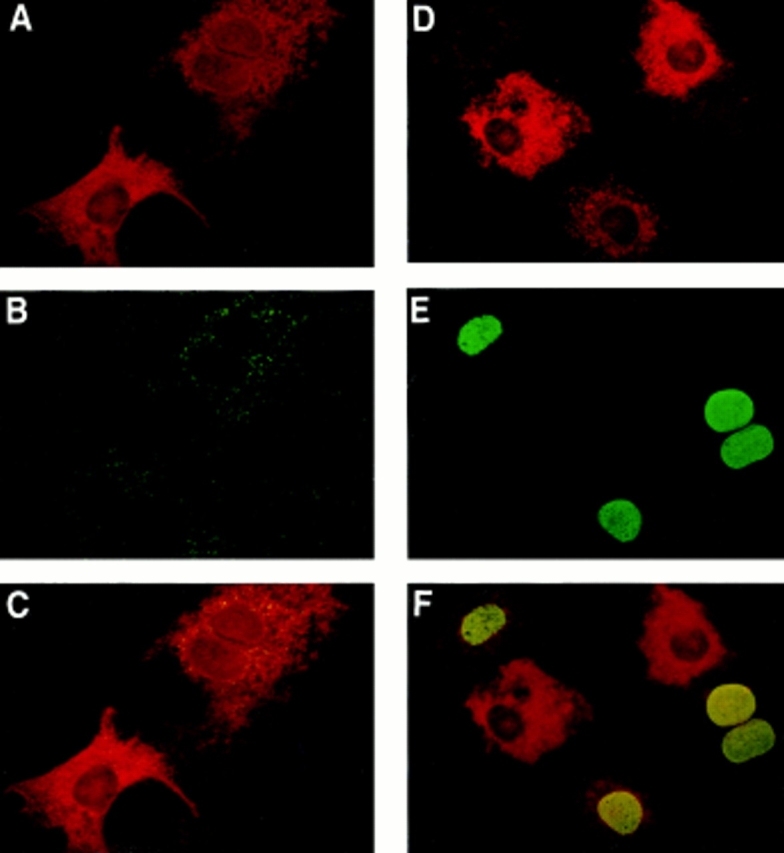

Microinjected SPP stimulates DNA synthesis. Serum-starved Swiss 3T3 cells were microinjected with rabbit IgG together with vehicle (A–C) or SPP (D–F). Cells were visualized by Texas red fluorescence (red), demonstrating immunoglobulin G localization after microinjection (A and D). BrdU incorporation into injected and uninjected cells on the same coverslips was visualized by green FITC fluorescence (B and E). C and F are superimposed images visualized using a triple band pass filter. Yellow-orange color indicates colocalization of microinjected SPP with BrdU staining. In G, stimulation of DNA synthesis in cells injected (open bars) with IgG in the absence or presence of SPP or C8-cer-1-P, as well as in adjacent uninjected cells (filled bars), was assessed by BrdU incorporation. Values (means ± SD) are the percentage of cells positive for BrdU staining and correspond to the average of three measurements in which 100 cells were scored. At least 400 cells were microinjected and scored in each experiment. For comparison, BrdU staining of Swiss 3T3 fibroblasts treated with vehicle (cross-hatched bar) or with 10 μM exogenous SPP (dotted bar) was also determined. (H) Swiss 3T3 cells were serum-starved 24 h in the presence of 5 μg/ml insulin and then treated with or without 200 ng/ml pertussis toxin for 3 h. Cells were then microinjected with rabbit IgG together with vehicle or SPP, or treated with 10 μM exogenous SPP and BrdU incorporation was assessed. In the experiment where SPP was microinjected, 14.5% of adjacent, uninjected cells were positive for BrdU incorporation.
To examine whether the cytoprotective effects of SPP can also be separated from its binding to Edg-1, we used two cell lines, HL-60 and PC12 cells, in which SPP is known to have a strong survival effect (Cuvillier et al., 1996; Edsall et al., 1997). In agreement with the lack of detectable edg-1 mRNA in HL-60 or PC12 cells (Fig. 4 B), no specific binding of SPP could be detected in either cell line (Fig. 7 A). SPP-phosphonate, which lacks the oxygen atom at the 1 position and does not compete for binding to Edg-1 (Fig. 1 B), was at least as potent as SPP in suppressing ceramide-mediated apoptosis in HL-60 cells (Fig. 7 B) and was as potent as SPP in prevention of DNA fragmentation due to serum deprivation in PC12 cells (Fig. 7 C). These protective effects were specific, because dihydro-SPP, which lacks the trans double bond present in SPP and binds to Edg-1 (Fig. 1), did not significantly prevent apoptosis either in HL-60 or in PC12 cells.
Figure 7.
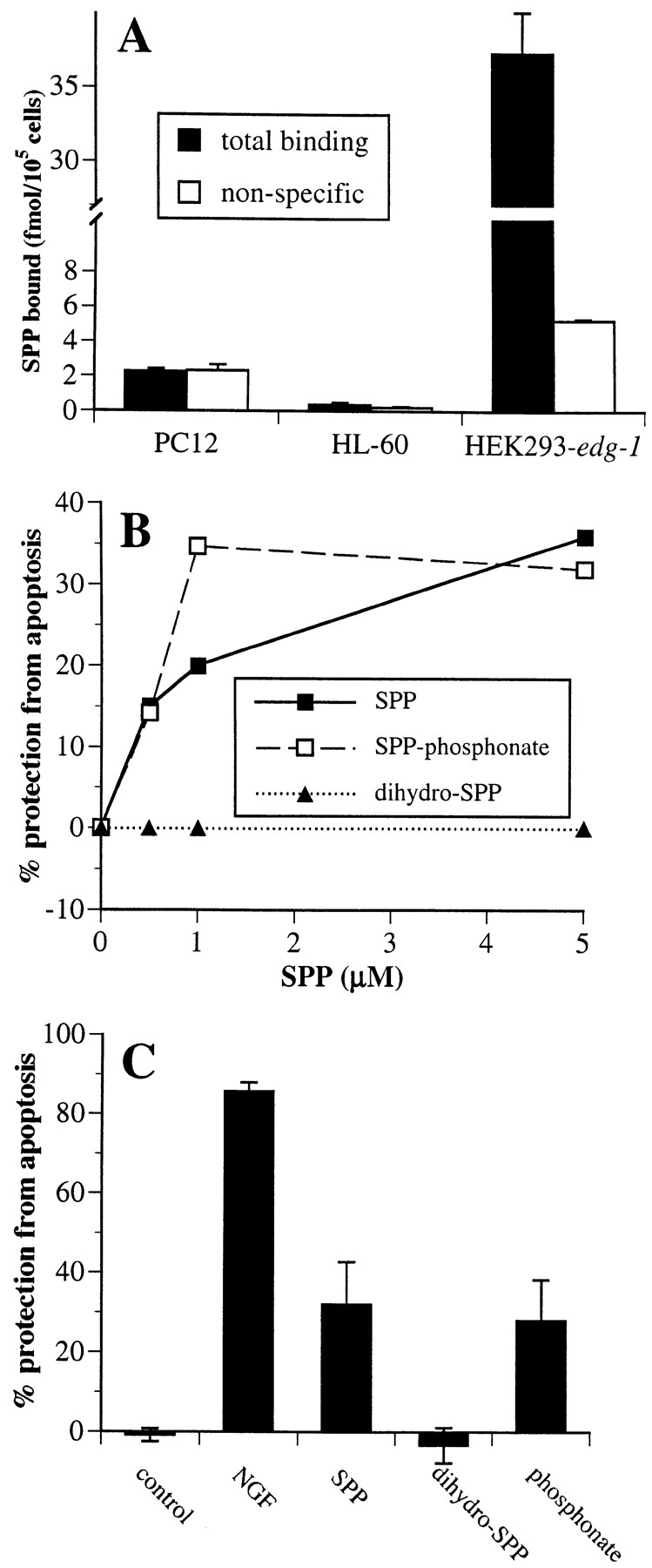
Effects of SPP and analogues on suppression of apoptosis. (A) Lack of specific [32P]SPP binding to HL-60 and PC12 cells. Nonspecific (open bars) and total binding (filled bars) of 1 nM [32P]SPP to HL-60 and PC12 cells was measured. (B) Inhibition of sphingomyelinase-induced DNA fragmentation by SPP in HL-60 cells. HL-60 cells were incubated with [3H]thymidine (1 μCi/ml) for 24 h to label DNA, washed, and then treated without or with 100 mU/ml Staphylococcus aureus sphingomyelinase in the presence of vehicle or with the indicated concentrations of SPP (filled squares), SPP-phosphonate (open squares), or dihydro-SPP (filled triangles), added as BSA complexes. After 5 h, DNA fragmentation was determined from the ratio of unfragmented/fragmented DNA (Cuvillier et al., 1996). Data are expressed as percent inhibition of DNA fragmentation. Percent protection from apoptosis = 100 × [(fragmentation induced by SMase) − (fragmentation induced by SMase in the presence of cytoprotective agents)] / [(fragmentation induced by SMase) − (fragmentation of untreated controls)]. (C) SPP and its hydrolysis-resistant analogue suppress apoptosis in PC12 cells induced by trophic factor withdrawal. PC12 cells were incubated in serum-free medium in the absence or presence of 5 μM SPP, dihydro-SPP, SPP-phosphonate, or NGF (100 ng/ml) for 15 h, fixed in 3.7% formaldehyde, and then stained with bisbenzimide trihydrochloride (24 μg/ml in 50% glycerol/PBS; Hoechst #33258, Calbiochem) (Edsall et al., 1997). Cells with chromatin condensation or segmentation of nuclei into three or more fragments were considered to be apoptotic. A minimum of 2,000 cells in each field was scored. Values are means ± SD of triplicate determinations. Percent protection from apoptosis = 100 × [(number of cells with fragmented nuclei) / (number of cells with fragmented nuclei + number of intact cells)].
Discussion
Apparently contradictory reports describe intracellular and extracellular actions of SPP in diverse cell types (Spiegel and Milstien, 1995; Spiegel et al., 1996; Moolenaar et al., 1997). This study demonstrates that SPP indeed has dual actions, acting as a second messenger to stimulate cell growth and prevent apoptosis, and as a first messenger through its cell surface receptor Edg-1 (Lee et al., 1998). Similar to the effects of the ligands for other closely related G protein–coupled receptors, such as CB-1 (Bouaboula et al., 1995; Felder et al., 1995) and Edg-2 (Hecht et al., 1996), binding of SPP to Edg-1 markedly inhibits cAMP accumulation (Fig. 2) and activates the mitogen-activated protein (MAP) kinase, ERK-2 (Lee et al., 1998), in a pertussis toxin–sensitive manner. Although the biological function of Edg-1 is not completely understood, it was cloned as an immediate early gene involved in endothelial differentiation (Hla and Maciag, 1990). Binding of SPP to Edg-1 also induces morphogenetic differentiation of HEK293–edg-1 cells and P-cadherin expression by a Rho-dependent mechanism (Lee et al., 1998). Thus, SPP might play a role in angiogenesis since the K d for SPP binding to Edg-1 is 8 nM (Lee et al., 1998) and SPP is a serum-borne component, where its concentration is 480 nM (Yatomi et al., 1997b ). However, because edg-1 expression has been detected in many tissues, including brain, spleen, heart, lung, placenta, muscle, liver, uterus, and kidney (Liu and Hla, 1997), both Edg-1 and SPP might regulate diverse signaling events.
Specific binding of [3H]SPP has also been detected in platelets (Yatomi et al., 1997a ) and in F10 melanoma cells (Yamamura et al., 1997). However, the SPP receptors in these cells have characteristics distinct from Edg-1. In both cases, the affinity for SPP (K d > 110 nM) was much lower than the affinity of Edg-1 for SPP (K d = 8 nM), and lysophosphatidic acid (LPA) was a better competitor than SPP, suggesting that these receptors can bind both LPA and SPP (Yatomi et al., 1997a ). Similarly, it was found that a K+ conductance was activated equally well by SPP and SPC in guinea pig atrial myocytes with heterologous desensitization (Bünemann et al., 1996). However, Edg-1 is highly selective as a high-affinity receptor for SPP because SPC and several other closely related lipids have no effect on either SPP binding or forskolin-stimulated cAMP accumulation. Two receptors closely related to Edg-1, Edg-3 and H218, were shown to confer responsiveness to SPP of a serum response element-driven reporter gene when expressed in Jurkat cells and to allow SPP-stimulated 45Ca2+ efflux in Xenopus oocytes (An et al., 1997). However, these authors did not present direct binding data; therefore, it is not clear at present whether Edg-3 and H218 are indeed SPP receptors. Although Edg-1 was ineffective in these assays, it may not link to the signaling pathways investigated. In agreement, we have shown here that SPP-stimulated increased [Ca2+]i is not mediated by Edg-1.
Several lines of evidence clearly indicate that mitogenesis and prevention of apoptosis induced by SPP are not mediated through Edg-1. First, the mitogenic action of SPP correlates with intracellular uptake rather than with cell surface binding of SPP or with edg-1 expression. Indeed, SPP is most potently mitogenic for Swiss 3T3 cells, which have only low specific SPP binding and edg-1 expression. In addition, the mitogenic effect of SPP requires micromolar concentrations, whereas putative receptor-mediated effects of SPP (Bünemann et al., 1996; Postma et al., 1996; van Koppen et al., 1996) have been shown to occur in the same nanomolar concentration range as binding to Edg-1. Second, although we have shown that binding of SPP to Edg-1 leads to decreases in cAMP levels, inhibition of adenylate cyclase may not be associated with regulation of mitogenesis. In Swiss 3T3 cells, cAMP has been shown unequivocally to act as a positive effector of proliferation (Rozengurt, 1986), and it is thus unlikely that SPP-induced decreases in cAMP play a significant role in the induction of proliferation. However, in several other cell types, including Rat-1, airway smooth muscle, and human foreskin fibroblasts, cAMP is a negative effector of mitogenesis, and the relevance of reductions in cAMP levels to mitogenic effects of SPP in these cell types is presently unclear (Pyne and Pyne, 1996). Third, N,N-dimethylsphingosine inhibits sphingosine kinase (Yatomi et al., 1996) and formation of endogenous SPP, selectively blocks cellular proliferation induced by PDGF and serum (Olivera and Spiegel, 1993; Rani et al., 1997) and the cytoprotective effects of cytokines (Cuvillier et al., 1996) and NGF (Edsall et al., 1997), but had no effect on SPP binding, nor did it affect SPP-mediated decreased cAMP levels. Thus, effects of this sphingosine kinase inhibitor are not mediated through inhibition of Edg-1 signaling, and it provides a useful tool for discriminating between intra- and extracellular actions. Additional evidence that the mitogenic effect of SPP is not mediated through a cell surface receptor comes from the use of the polyanionic drug suramin, a broad-specificity inhibitor of ligand receptor interactions (Postma et al., 1996; Wang et al., 1997). Suramin has no effect on SPP-induced p125FAK phosphorylation and mitogenesis, but it inhibits responses to LPA and SPC (Wang et al., 1997). The most compelling evidence that the mitogenic effect of SPP is mediated intracellularly is that microinjection of SPP causes increased DNA synthesis. Moreover, because uninjected cells adjacent to SPP microinjected cells showed no significant increase in BrdU incorporation, it is unlikely that SPP is leaking from microinjected cells or from the pipet and stimulating other cells in autocrine fashion. Since microinjection effectively bypasses cell surface receptors to introduce SPP directly into cells, these data conclusively demonstrate that binding to cell surface receptors is not required for SPP-induced mitogenesis, consistent with a role for Edg-1 in morphogenetic differentiative (Lee et al., 1998), rather than mitogenic, signaling pathways.
SPP prevents apoptosis induced by trophic factor withdrawal or by ceramide elevations in PC12 and HL-60 cells, both of which do not express edg-1 or have detectable SPP cell surface binding. In addition, metabolically stable SPP-phosphonate, which does not bind to Edg-1, protects these cells from apoptosis as potently as does SPP. Conversely, dihydro-SPP, which binds to and signals via Edg-1, does not have any significant cytoprotective effect. Thus, similarly to its mitogenic effect, the survival effect of SPP is independent of Edg-1 and is probably mediated intracellularly. In agreement, unfertilized oocytes treated with the anticancer drug doxorubicin undergo apoptosis accompanied by increased ceramide levels (Perez et al., 1997). Doxorubicin-induced apoptosis was completely blocked by microinjection of SPP, indicating that oocyte destruction caused by chemotherapy can be prevented by manipulation of intracellular levels of SPP (Perez et al., 1997).
SPP has also been shown to activate several signal transduction pathways, most of which have been associated with mitogenic signaling and, as shown here, are unrelated to Edg-1–mediated pathways. SPP increased [Ca2+]i to the same level in HEK293-vector cells and HEK293 cells overexpressing Edg-1. Interestingly, it has been shown that cannabinoids can also increase [Ca2+]i independently of receptor expression (Felder et al., 1992). Moreover, similar to SPP (Mattie et al., 1994), calcium mobilization was independent of inositol trisphosphate formation (Felder et al., 1992). Our results indicate that these responses to SPP are not mediated by Edg-1 and suggest that SPP may activate these pathways by acting intracellularly. However, we cannot exclude the possibility that other SPP receptors exist, which may lead to activation of these pathways. The fact that tyrosine phosphorylation of p125FAK and activation of phospholipase D by SPP was attenuated in HEK293–edg-1 cells suggests that Edg-1 may in some cases oppose the mitogenic signaling of SPP. This is a reasonable proposal since Edg-1 is thought to be involved in endothelial cell differentiation (Hla and Maciag, 1990) and mediates morphogenetic differentiation of HEK293–edg-1 cells treated with SPP (Lee et al., 1998). However, we cannot exclude the possibility that in certain cell types, binding of SPP to Edg-1 might be a contributing factor involved in cellular proliferation. Transient activation of MAP kinase, in contrast to sustained and robust activation, might lead to cellular proliferation rather than differentiation (Marshall, 1995). Binding of SPP to Edg-1 in Cos-7 cells transiently transfected with edg-1 stimulates MAP kinase in a pertussis toxin–sensitive manner (Lee et al., 1998). This might explain the observation that SPP activates MAP kinase and DNA synthesis in Swiss 3T3 fibroblasts by both pertussis toxin– sensitive and –insensitive pathways (Goodemote et al., 1995; Wu et al., 1995). It is noteworthy that in these cells, 62% of SPP-stimulated DNA synthesis is insensitive to pertussis toxin. (Stimulation by SPP is 8.4- and 5.2-fold in the absence or presence of 100 ng/ml pertussis toxin, respectively.) SPP-stimulated DNA synthesis was also partially insensitive to pertussis toxin in the presence of insulin (Fig. 6 H). This correlates with our microinjection experiments, which showed that microinjected SPP gave 60% of the response of exogenous SPP (Fig. 6 G) and 41% of the response to exogenous SPP in the presence of insulin (Fig. 6 H). In agreement, pertussis toxin decreased DNA synthesis stimulated by exogenous SPP to approximately the level of that seen in response to microinjected SPP.
While ceramide acts as a mediator of stress responses, causing cell cycle arrest and/or apoptosis (Hannun, 1996), SPP counteracts these effects (Cuvillier et al., 1996; Edsall et al., 1997). Thus, we have suggested that the balance of cellular levels of SPP and ceramide is a critical factor that determines cell fate (Cuvillier et al., 1996). Similarly, heat stress in Saccharomyces cerevisiae causes the accumulation of phytosphingosine, dihydrosphingosine, and ceramide (Dickson et al., 1997; Jenkins et al., 1997) and activates transcription of the global stress response element, indicating a role for sphingolipid metabolites as stress signals (Dickson et al., 1997). In contrast, deletion of long chain sphingoid base phosphate phosphatase leads to the accumulation of phosphorylated long chain sphingoid bases and reduced ceramide levels, and concomitant enhancement of survival upon severe heat shock (Mandala et al., 1997). Thus, it seems that the ceramide/SPP rheostat is an evolutionarily conserved stress regulatory mechanism. Therefore, in addition to its role as a ligand for Edg-1, the cellular level of SPP is important for regulation of cell proliferation and survival. Collectively, this data indicates that SPP is a prototype for a novel class of lipid mediators that act both extracellularly as ligands for cell surface receptors and intracellularly as second messengers.
Acknowledgments
We thank Dr. R.R. Schmidt for providing sphingolipid analogues, and Dr. Susette C. Mueller, director of the Lombardi Cancer Center Microscopy/ Imaging shared resources (supported by U.S. Public Health Service Grant 1P30-CA-51008), for providing use of microinjection and microscopy facilities.
This work was supported by Research Grants from the National Institutes of Health (GM43880) to S. Spiegel and (DK45659) to T. Hla.
Abbreviations used in this paper
- BrdU
bromodeoxyuridine
- C2-ceramide
N-acetyl sphingosine
- C8-cer-1-P
N-octanoyl ceramide-1-phosphate
- fura-2/AM
fura-2/acetoxy-methyl ester
- IBMX
3-isobutyl-1-methylxanthine
- LPA
lysophosphatidic acid
- MAP
mitogen-activating protein
- RIgG
rabbit IgG
- p125FAK
p125 focal adhesion kinase
- SPC
sphingosylphosphorylcholine
- SPP
sphingosine-1-phosphate
Footnotes
J.R. Van Brocklyn, M.-J. Lee, T. Hla, and S. Spiegel contributed equally to this study.
Address all correspondence to Dr. Sarah Spiegel, Department of Biochemistry and Molecular Biology, Georgetown University Medical Center, 357 Basic Science Building, 3900 Reservoir Road NW, Washington, DC 20007. Tel.: (202) 687-1432; Fax: (202) 687-7186. E-mail: spiegel@biochem1.basic-sci.georgetown.edu
References
- An S, Bleu T, Huang W, Hallmark OG, Coughling SR, Goetzel EJ. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–282. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- Blakesly VA, Beitner-Johnson D, Van Brocklyn JR, Rani S, Shen-Orr Z, Stannard BS, Spiegel S, LeRoith D. Sphingosine 1-phosphate stimulates tyrosine phosphorylation of Crk. J Biol Chem. 1997;272:16211–16215. doi: 10.1074/jbc.272.26.16211. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE, Graves LM, Raines EW, Igarashi Y, Wayman G, Yamamura S, Yatomi Y, Sidhu JS, Krebs EG, Hakomori S, Ross R. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol. 1995;130:193–206. doi: 10.1083/jcb.130.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrié B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehrer BM, Bardes ES, Bell RM. Protein kinase C-dependent regulation of human erythroleukemia (HEL) cell sphingosine kinase activity. Biochim Biophys Acta. 1996;1303:233–242. doi: 10.1016/0005-2760(96)00092-6. [DOI] [PubMed] [Google Scholar]
- Bünemann M, Liliom K, Brandts BK, Pott L, Tseng J-L, Desidirio DM, Sun G, Miller D, Tigyi G. A novel membrane receptor with high affinity for lysosphingomyelin and sphingosine 1-phosphate in atrial myocytes. EMBO (Eur Mol Biol Organ) J. 1996;15:5527–5533. [PMC free article] [PubMed] [Google Scholar]
- Chao CP, Laulederkind SJF, Ballou LR. Sphingosine-mediated phosphatidylinositol metabolism and calcium mobilization. J Biol Chem. 1994;269:5849–5856. [PubMed] [Google Scholar]
- Choi OH, Kim J-H, Kinet J-P. Calcium mobilization via sphingosine kinase in signalling by the FcεRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Derkinderen P, Toutant M, Burgaya F, Le Bert M, Siciliano JC, de Franciscis V, Gelman M, Girault JA. Regulation of a neuronal form of focal adhesion kinase by anandamide. Science. 1996;273:1719–1722. doi: 10.1126/science.273.5282.1719. [DOI] [PubMed] [Google Scholar]
- Desai NN, Zhang H, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a metabolite of sphingosine, increases phosphatidic acid levels by phospholipase D activation. J Biol Chem. 1992;267:23122–23128. [PubMed] [Google Scholar]
- Dickson RC, Nagiec EE, Skrzypek M, Tillman P, Wells GB, Lester RL. Sphingolipids are potential heat stress signals in Saccharomyces. J Biol Chem. 1997;272:30196–30200. doi: 10.1074/jbc.272.48.30196. [DOI] [PubMed] [Google Scholar]
- Duke, R.C., and J.J. Cohen. 1992. Morphological and biochemical assays of apoptosis. In Current Protocols in Immunology, Suppl. 3. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, and W. Strober, editors. Green/ Wiley, New York. 3.17.1–3.17.16.
- Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci. 1997;17:6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatatis A, Miller RJ. Platelet-derived growth factor (PDGF)- induced Ca2+signaling in the CG4 oligodendroglial cell line and in transformed oligodendrocytes expressing the β-PDGF receptor. J Biol Chem. 1997;272:4351–4358. doi: 10.1074/jbc.272.7.4351. [DOI] [PubMed] [Google Scholar]
- Felder CC, Veluz JS, Williams HL, Briley EM, Matsuda LA. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol Pharmacol. 1992;42:838–845. [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh TK, Bian J, Gill DL. Sphingosine 1-phosphate generated in the endoplasmic reticulum membrane activates release of stored calcium. J Biol Chem. 1994;269:22628–22635. [PubMed] [Google Scholar]
- Gomez-Munoz A, Waggoner DW, O'Brien L, Brindley DN. Interaction of ceramides, sphingosine, and sphingosine 1-phosphate in regulating DNA synthesis and phospholipase D activity. J Biol Chem. 1995;270:26318–26325. doi: 10.1074/jbc.270.44.26318. [DOI] [PubMed] [Google Scholar]
- Goodemote KA, Mattie ME, Berger A, Spiegel S. Involvement of a pertussis toxin sensitive G protein in the mitogenic signaling pathways of sphingosine 1-phosphate. J Biol Chem. 1995;270:10272–10277. doi: 10.1074/jbc.270.17.10272. [DOI] [PubMed] [Google Scholar]
- Hannun Y. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- Howlett AC. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- Jenkins GM, Richards A, Wahl T, Mao C, Obeid L, Hannun Y. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. . J Biol Chem. 1997;272:32566–32572. doi: 10.1074/jbc.272.51.32566. [DOI] [PubMed] [Google Scholar]
- Kolesnick R, Fuks Z. Ceramide: a signal for apoptosis or mitogenesis? . J Exp Med. 1995;181:1949–1952. doi: 10.1084/jem.181.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie Y, Blusztajn JK, Liscovitch M. Formation of endogenous free sphingoid bases in cells induced by changing medium conditions. Biochim Biophys Acta. 1994;1220:323–328. doi: 10.1016/0167-4889(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Lee M-J, Evans M, Hla T. The inducible G protein-coupled receptor edg-1 signals via the Gi/mitogen-activated protein kinase pathway. J Biol Chem. 1996;271:11272–11282. doi: 10.1074/jbc.271.19.11272. [DOI] [PubMed] [Google Scholar]
- Lee M-J, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor Edg-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Liu CH, Hla T. The mouse gene for the inducible G-protein-coupled receptor edg-1. . Genomics. 1997;43:15–24. doi: 10.1006/geno.1997.4759. [DOI] [PubMed] [Google Scholar]
- Mandala S, Thornton R, Tu Z, Kurtz M, Nickels J, Broach J, Menzeleev R, Spiegel S. Sphingoid base 1-phosphate phosphatase, a key regulator of sphingolipid metabolism and stress response. Proc Nat Acad Sci USA. 1997;95:150–155. doi: 10.1073/pnas.95.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Mattie M, Brooker G, Spiegel S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J Biol Chem. 1994;269:3181–3188. [PubMed] [Google Scholar]
- Mazurek N, Megidish T, Hakomori S-I, Igarashi Y. Regulatory effect of phorbol esters on sphingosine kinase in BALB/C 3T3 fibroblasts (variant A31): demonstration of cell type-specific response. Biochem Biophys Res Commun. 1994;198:1–9. doi: 10.1006/bbrc.1994.1001. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH, Kranenburg O, Postma FR, Zondag GCM. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr Opin Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Jayaram HN, Scribner WM, Garcia JGN. Activation of endothelial cell phospholipase D by sphingosine and sphingosine-1-phosphate. Am J Respir Cell Mol Biol. 1994;11:221–229. doi: 10.1165/ajrcmb.11.2.8049083. [DOI] [PubMed] [Google Scholar]
- Okajima F, Tomura H, Sho K, Kimura T, Sato K, Im DS, Akbar M, Kondo Y. Sphingosine 1-phosphate stimulates hydrogen peroxide generation through activation of phospholipase C-Ca2+system in FRTL-5 thyroid cells: possible involvement of guanosine triphosphate-binding proteins in the lipid signaling. Endocrinology. 1997;138:220–229. doi: 10.1210/endo.138.1.4883. [DOI] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine-1-phosphate as a second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Olivera A, Buckley NE, Spiegel S. Sphingomyelinase and cell-permeable ceramide analogs stimulate cellular proliferation in quiescent Swiss 3T3 fibroblasts. J Biol Chem. 1992;267:26121–26127. [PubMed] [Google Scholar]
- Olivera A, Rosenthal J, Spiegel S. Sphingosine kinase from Swiss 3T3 fibroblasts: a convenient assay for the measurement of intracellular levels of free sphingoid bases. Anal Biochem. 1994;223:306–312. doi: 10.1006/abio.1994.1589. [DOI] [PubMed] [Google Scholar]
- Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 1997;3:1228–1232. doi: 10.1038/nm1197-1228. [DOI] [PubMed] [Google Scholar]
- Postma FR, Jalink K, Hengeveld T, Moolenaar WH. Sphingosine-1-phosphate rapidly induces rho-dependent neurite retraction: action through a specific cell surface receptor. EMBO (Eur Mol Biol Organ) J. 1996;15:2388–2392. [PMC free article] [PubMed] [Google Scholar]
- Pyne S, Pyne NJ. The differential regulation of cyclic AMP by sphingomyelin-derived lipids and the modulation of sphingolipid-stimulated extracellular signal regulated kinase-2 in airway smooth muscle. Biochem J. 1996;315:917–923. doi: 10.1042/bj3150917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne S, Chapman J, Steele L, Pyne NJ. Sphingomyelin-derived lipids differentially regulate the extracellular signal-regulated kinase 2 (ERK-2) and c-Jun N-terminal kinase (JNK) signal cascades in airway smooth muscle cells. Eur J Biochem. 1996;237:819–826. doi: 10.1111/j.1432-1033.1996.0819p.x. [DOI] [PubMed] [Google Scholar]
- Rani CS, Berger A, Wu J, Sturgill TW, Beitner-Johnson D, LeRoith D, Varticovski L, Spiegel S. Divergence in signal transduction pathways of PDGF and EGF receptors: involvement of sphingosine-1-phosphate in PDGF but not EGF signaling. J Biol Chem. 1997;272:10777–10783. doi: 10.1074/jbc.272.16.10777. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986;234:161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Foster D, Kolesnick R. Signal transduction through lipid second messengers. Curr Opin Cell Biol. 1996;8:159–167. doi: 10.1016/s0955-0674(96)80061-5. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingolipid metabolites: members of a new class of lipid second messengers. J Membr Biol. 1995;146:225–237. doi: 10.1007/BF00233943. [DOI] [PubMed] [Google Scholar]
- Su Y, Rosenthal D, Smulson M, Spiegel S. Sphingosine 1-phosphate, a novel signaling molecule, stimulates DNA binding activity of AP-1 in quiescent Swiss 3T3 fibroblasts. J Biol Chem. 1994;269:16512–16517. [PubMed] [Google Scholar]
- Tarnowski A, Bär T, Schmidt RR. Efficient synthesis of sphingosine-1-phosphonate and homo-sphingosine-1-phosphonate. Bioorg Med Chem Lett. 1997;7:573–576. [Google Scholar]
- van Koppen CJ, Meyer zu Heringdorf D, Laser KT, Zhang C, Jakobs KH, Bünnemann M, Pott L. Activation of a high affinity Giprotein-coupled plasma membrane receptor by sphingosine-1-phosphate. J Biol Chem. 1996;271:2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven PP, Mannaerts GP. Sphinganine 1-phosphate metabolism in cultured skin fibroblasts: evidence for the existence of a sphingosine phosphatase. Biochem J. 1994;299:597–601. doi: 10.1042/bj2990597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Nobes CD, Hall A, Spiegel S. Sphingosine 1-phosphate stimulates Rho-mediated tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 fibroblasts. Biochem J. 1997;324:481–488. doi: 10.1042/bj3240481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Spiegel S, Sturgill TW. Sphingosine 1-phosphate rapidly activates the mitogen-activated protein kinase pathway by a G protein-dependent mechanism. J Biol Chem. 1995;270:11484–11488. doi: 10.1074/jbc.270.19.11484. [DOI] [PubMed] [Google Scholar]
- Yamamura S, Yatomi Y, Ruan F, Sweeney EA, Hakomori S, Igarashi Y. Sphingosine 1-phosphate regulates melanoma cell motility through a receptor-coupled extracellular action and in a pertussis toxin- insensitive manner. Biochemistry. 1997;36:10751–10759. doi: 10.1021/bi970926s. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Megidish T, Toyokuni T, Hakomori S, Igarashi Y. N,N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry. 1996;35:626–633. doi: 10.1021/bi9515533. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Yamamura S, Ruan F, Igarashi Y. Sphingosine 1-phosphate induces platelet activation through an extracellular action and shares a platelet surface receptor with lysophosphatidic acid. J Biol Chem. 1997a;272:5291–5297. doi: 10.1074/jbc.272.8.5291. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997b;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- Zhang H, Desai NN, Murphey JM, Spiegel S. Increases in phosphatidic acid levels accompany sphingosine-stimulated proliferation of quiescent Swiss 3T3 cells. J Biol Chem. 1990;265:21309–21316. [PubMed] [Google Scholar]
- Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]