Abstract
Cumulative evidence indicates that osteoblasts and adipocytes share a common mesenchymal precursor and that bone morphogenetic proteins (BMPs) can induce both osteoblast and adipocyte differentiation of this precursor. In the present study, we investigated the roles of BMP receptors in differentiation along these separate lineages using a well-characterized clonal cell line, 2T3, derived from the mouse calvariae. BMP-2 induced 2T3 cells to differentiate into mature osteoblasts or adipocytes depending upon culture conditions. To test the specific roles of the type IA and IB BMP receptor components, truncated and constitutively active type IA and IB BMP receptor cDNAs were stably expressed in these cells. Overexpression of truncated type IB BMP receptor (trBMPR-IB) in 2T3 cells completely blocked BMP-2–induced osteoblast differentiation and mineralized bone matrix formation. Expression of trBMPR-IB also blocked mRNA expression of the osteoblast specific transcription factor, Osf2/ Cbfa1, and the osteoblast differentiation-related genes, alkaline phosphatase (ALP) and osteocalcin (OC). BMP-2–induced ALP activity could be rescued by transfection of wild-type (wt) BMPR-IB into 2T3 clones containing trBMPR-IB. Expression of a constitutively active BMPR-IB (caBMPR-IB) induced formation of mineralized bone matrix by 2T3 cells without addition of BMP-2. In contrast, overexpression of trBMPR-IA blocked adipocyte differentiation and expression of caBMPR-IA induced adipocyte formation in 2T3 cells. Expression of the adipocyte differentiation-related genes, adipsin and PPARγ, correlated with the distinct phenotypic changes found after overexpression of the appropriate mutant receptors. These results demonstrate that type IB and IA BMP receptors transmit different signals to bone-derived mesenchymal progenitors and play critical roles in both the specification and differentiation of osteoblasts and adipocytes.
Keywords: 2T3 cells, BMP signaling, commitment, mineralization, Osf2/cbfal
Pluripotent mesenchymal cells are able to differentiate into several distinct cell types, including osteoblasts and adipocytes (Taylor and Jones, 1979; Friedenstein et al., 1987), but the regulatory mechanisms responsible remain undefined. Bone morphogenic proteins (BMPs)1 were originally identified as peptides that induce bone and cartilage formation in ectopic extraskeletal sites in vivo (Wang et al., 1988, 1990; Wozney et al., 1988). Extensive studies have demonstrated that BMPs play important roles in bone formation and bone cell differentiation (Wozney et al., 1988; Harris et al., 1994, 1995; Chen et al., 1997), but the activities of the BMPs are not restricted to bone and cartilage formation. BMPs appear to be involved in most morphogenetic processes during development (Winnier et al., 1995; Hogan, 1996). BMP-2 and BMP-4 induce ventral mesoderm formation during embryogenesis and differentiation of cells with mesodermal origins (Winnier et al., 1995). Addition of recombinant BMP-2 and BMP-7 or transfection of BMP-2 and BMP-4 cDNA in a pluripotent mouse fibroblast cell line, C3H10T1/2, induces the cells to differentiate into three cell types: osteoblasts, chondroblasts, and adipocytes (Ahrens et al., 1993; Wang et al., 1993; Asahina et al., 1996). The receptor mediation for these effects of BMPs is not known.
Recently, several BMP (IA, IB, and II) and activin (I, II, and IIB) receptors have been cloned and characterized (Koenig et al., 1994; ten Dijke et al., 1994; Yamaji et al., 1994; Kawabata et al., 1995; Liu et al., 1995; Nohno et al., 1995; Rosenzweig et al., 1995; Ruberte et al., 1995; Yamashita et al., 1995). These receptors bind BMP ligands and transduce their signals. BMP and activin receptors belong to the TGFβ receptor family of serine/threonine kinases. Although both type I and type II BMP receptors bind BMP ligands, heteromers of type I and type II receptors are required for a signal to be transduced (Nohno et al., 1995; Ruberte et al., 1995). BMP-2 and BMP-4 bind weakly to both type IA and IB receptors (ten Dijke et al., 1994), but coexpression of type II BMP receptor with IA or IB BMP receptor increases binding affinity and dramatically enhances biological activity (Massagué, 1996). Type I BMP receptors with mutations in the glycine/serine juxtatransmembrane domain have been defined. They have the capacity to activate cells constitutively in the absence of a BMP ligand (Hoodless et al., 1996).
Previous studies have demonstrated that injection of truncated type IA or type II BMP receptor mRNA into Xenopus embryos blocks ventral mesoderm formation (Graff et al., 1994; Maeno et al., 1994; Suzuki et al., 1994; Ishikawa et al., 1995). Studies on viral mediated transfection of kinase defective and constitutively active BMPR-IA and BMPR-IB in chicken embryos demonstrated that these two receptors regulate distinct processes during chick limb development. BMPR-IB is required for the initial steps of chondrogenesis and mediates programmed cell death and BMPR-IA regulates the rate of chondrocyte differentiation (Zou and Niswander, 1995; Zou et al., 1997). In chicken embryos, chondrogenesis of limb mesenchymal cells and bone formation of the distal limb bud are markedly inhibited by dominant-negative type IB and type II BMP receptors, but not by dominant-negative BMPR-IA (Kawakami et al., 1996). The constitutively active BMPR-IB increases distal cartilage fusion and disrupts joint formation whereas the constitutively active BMPR-IA delays chondrocyte differentiation (Zou et al., 1997).
In situ hybridization and immunohistochemistry studies for expression of the two type I BMP receptors also support distinct roles of type IA and IB BMP receptors during embryonic development. For example, high levels of BMPR-IA are expressed in the rapidly growing loose mesenchymal cells surrounding the precartilaginous condensations (Yamaji et al., 1994; Dewulf et al., 1995) and BMPR-IA is also selectively expressed in the prehypertrophic zone in chick limbs (Zou et al., 1997). BMPR-IB is expressed in the early differentiating cartilage condensations (Yamaji et al., 1994; Dewulf et al., 1995). In 15-d-old mouse embryos, expression of BMPR-IB, but not of BMPR-IA, was observed in the cells of the perichondrium of developing cartilage. At 17.5 and 19.5 d, respectively, expression of both receptors was observed in chondrocytes and in osteoblasts. Thus, tissue-specific spatial and temporal expression of different types of BMPs and BMP receptors, as well as inhibitors, such as chordin and noggin, may determine a specific function and threshold level of BMP action (Holley et al., 1996; Piccolo et al., 1996; Zimmerman et al., 1996; Graff, 1997; Tonegawa et al., 1997).
In the present study, we investigated the roles of type IA and IB BMP receptors during osteoblast and adipocyte differentiation using 2T3 cells. 2T3 cells were derived from the calvaria of a transgenic mouse expressing T antigen driven by the BMP-2 promoter and are primarily specified to differentiate into mature osteoblasts (Ghosh-Choudhury et al., 1996). Overexpression of the truncated and constitutively active type IA and IB BMP receptors respecified 2T3 cells to differentiate into completely different phenotypes. Our results suggest that type IA and IB BMP receptors mediate different signals responsible for alternate paths of differentiation and suggest that the temporal acquisition or loss of a selective BMP receptor in unspecified precursor cells may be a key step for the commitment and specification of osteoblasts and adipocytes during differentiation processes.
Materials and Methods
Cell Culture
The 2T3 cell line was isolated and cloned from a transgenic mouse containing BMP-2 promoter driving the SV-40 T antigen transgene and has been characterized previously (Ghosh-Choudhury et al., 1996). These cells are BMP-2 responsive and undergo bone matrix formation in vitro. 2T3 cells were plated into T-150 flasks (Costar Corp., Cambridge, MA) at density of 2 × 106 cells per flask for RNA extraction or plated on 24-well plates (Costar Corp.) at density of 2 × 104 cells per well for bone matrix formation and adipocyte formation assays. For the mineralized bone matrix formation assay, the cells were cultured with α minimal essential medium (αMEM) supplemented with 10% FCS. When the cells reached confluency (day 0), the medium was changed to αMEM containing 5% FCS, 100 μg/ml ascorbic acid, and 5 mM β-glycerol phosphate, with or without recombinant BMP-2. The medium was changed every other day and fresh reagents were added. To assay adipocyte formation, the cells were cultured with αMEM supplemented with 5% rabbit serum, 0.5 mM of 3-isobutyl-1-methyl xanthine (IBMX), and 60 mM of indomethacin. Accumulation of lipid droplets in the cytoplasm was determined by Oil red O staining (Novikoff et al., 1980).
Northern Analysis
Total RNA was isolated from 2T3 cells using RNAzol B method (Tel-test Inc., Houston, TX). Enrichment of polyadenylated RNA was obtained using Stratagene oligo (dT) cellulose columns (Stratagene, San Diego, CA). 5 μg of poly (A+) RNA was denatured in 2.2 M of formaldehyde and 50% formamide and then run on a 1% agarose gel containing 2.2 M of formaldehyde. The gel was transferred to a Nytran filter (Schleicher & Schuell, Keene, NH) by capillary blotting with 20× SSC (1× SSC = 150 mM NaCl, 15 mM Na citrate, pH 7.0). The RNA was then cross-linked to the filter by UV irradiation (Stratalink; Stratagene). Prehybridization was carried out at 42°C in 5× SSC containing 50% formamide and 150 mg/ml of denatured salmon sperm DNA. After a 1–2-h prehybridization, the DNA probes were added to the hybridization solution at a concentration of 5 × 105 cpm/ml. Hybridization was carried out for 15 h at 42°C. The filters were then washed twice with 2× SSC and 0.1% SDS, once with 0.5% SSC and 0.1% SDS, and once with 0.1× SSC and 0.1% SDS at 56°C for 15 min each. The filters were dried at room temperature, exposed at −70°C overnight and then quantitated in cpm using an AMBIS image acquisition and analysis system (AMBIS, San Diego, CA). The type IA, IB, and type II BMP receptor probes are rat and human DNA fragments encoding the extracellular domain of BMP receptors (0.5 kb). The alkaline phosphatase (ALP) and osteocalcin (OC) probes are rat cDNA fragments (1.3 and 0.5 kb). The Osf2/Cbfa1, adipsin, and PPARγ cDNA probes are mouse cDNA fragments of 0.3, 0.5, and 1.8 kb, respectively.
Construction of Expression Plasmids
Truncated forms of type IA and IB BMP receptors were generated by the PCR method using rat BMPR-IA (CFK-23a) and BMPR-IB (CFK-43a) cDNAs as templates. The human homologue of CFK-23a is ALK-3 (EMBL/GenBank/DDBJ accession number Z22535) and mouse homologue of CFK43a is ALK-6 (accession number Z23143). The deletion mutants of BMPR-IA and BMPR-IB that encode 175 amino acids (aa) for trBMPR-IA, 146 aa for trBMPR-IB plus 9 aa for influenza virus hemagglutinin (HA) epitope at the COOH terminus, were constructed with four primers, as described below. The 5′ primers for trBMPR-IA and trBMPR-IB were 5′-ACGTGCGAATTG-GACAATGA-3′ and 5′-TGGCGCTGAGCTATGACAAG-3′. The 3′ primers for trBMPR-IA and trBMPR-IB were 5′-CTAAGCGTAGTCTGGGACGTCGTATGGGTAACAGAAGCAGCTGGAGAA-3′ and 5′-CTAAGCGTAGTCTGGGACGTCGTATGGGTAACAGAATAAAATAAT-3′, which encoded for the complementary sequences of FSSCFC (aa 170–175) for trBMPR-IA or IILFC (aa 142–146) for trBMPR-IB, and YPYDVPDYA for HA peptide and stop codon on each receptor. The amplified trBMPR-IA and trBMPR-IB PCR products were cloned into PCRII vector using TA cloning kit (Invitrogen, San Diego, CA). The trBMPR-IA and trBMPR-IB cDNAs in pCRII vector were digested with HindIII/XhoI, gel purified, and then subcloned into pcDNA3 vector (Invitrogen). The plasmids were sequenced to verify accuracy of the PCR amplification reaction. CaBMPR-IA (ALK3, Q233D), caBMPR-IB (ALK6, Q203D) and wild-type BMPR-IB (ALK-6) expression plasmids were kindly provided by J. Wrana (The Hospital for Sick Children, Toronto, Canada). These cDNA constructs were tagged with HA epitope at their COOH terminus.
Transfection of 2T3 Cells
TrBMPR-IA, trBMPR-IB, caBMPR-IA, and caBMPR-IB expression plasmids were stably transfected into 2T3 cells using lipofection method (Strauss, 1996). 48 h after transfection, the cells were replated on 150- × 25-mm culture dishes at density of 105 cells per dish. αMEM containing 400 μg/ml G418 (GIBCO BRL, Gaithersburg. MD) was added. Colonies grew after 2 wk and were cloned using cloning cylinders. In the rescue experiment, wtBMPR-IB cDNA was transiently transfected into 2T3 clones expressing trBMPR-IB. 2 d after transfection, BMP-2 was added to the cultures. The cells were incubated for an additional 1 d and then assayed for ALP activity using the Sigma ALP assay kit (Sigma Chemical Co., St. Louis, MO). The transfection efficiency was estimated to be 80% by tartrate-resistant acid phosphatase (TRAP) in situ staining (Reddy et al., 1993).
Western Analysis
The 2T3 cells transfected with empty vector, trBMPR-IA, trBMPR-IB, caBMPR-IA, caBMPR-IB, and wtBMPR-IB were lysed in lysis buffer (20 mM Hepes, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 1.5 mM MgCl2, and 1 mM EDTA) containing protease inhibitors and run on SDS-PAGE gels (Mini-PROTEIN II Ready gels; Bio-Rad Laboratories, Inc., Hercules, CA). The proteins were transblotted on a nitrocellulose membrane (Schleicher & Schuell) in transblotting buffer (20 mM Tris, 150 mM glycine, 20% methanol, pH 8.0) at 4°C for 16 h. The membrane was blocked with 5% BSA (Sigma Chemical Co.) in TBS for 2 h at room temperature and then incubated with rabbit anti-HA polyclonal antibody (1: 1,000; Babco, Richmond, CA) in TBS for 2 h at room temperature. The incubation with horseradish peroxidase-conjugated protein A (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) was performed at room temperature for 2 h. The membrane was then washed with TBS containing 0.1% Triton X-100 five times for 5 min and with TBS two times. Immunostaining was detected using the enhanced chemiluminescence system (Amersham Int., Buckinghamshire, UK).
Competitive Reverse Transcription-PCR Assay
In competitive reverse transcription (RT)-PCR assay, a DNA competitor containing the same primer template sequences as the target competes for primer binding and amplification (Feng et al., 1995). The competitor DNA fragment was generated by PCR using BMPR-IA cDNA as template. The upper and lower priming sites for detecting truncated and endogenous type IA and IB BMP receptors were added to this competitor by PCR. The upper primer for detecting truncated and endogenous type IA receptors was 5′-TTACTGGGAGCCTGTCTG-3′ and the upper primer for detecting truncated and endogenous type IB receptors was 5′-GCAACTCGGCCATAAGTG-3′. The lower primer for detecting truncated type IA and IB BMP receptors was 5′-CGTCGTATGGGTAACAG-3′. The lower primers for detecting endogenous type IA and IB receptors were 5′-GCCGAACCATCTGAATCT-3′ and 5′-AGCGGCCTTTTCCAATCT-3′. These primers have equal efficiency to amplify truncated and endogenous receptor transcripts.
First strand cDNA was synthesized from 10 μg of total RNA with an 18-nucleotide oligo dT primer using Superscript reverse transcriptase (GIBCO BRL) in a total volume of 50 μl. The cDNA was then used as a template for PCR competition assay. For competitive PCR, 1 μCi of [α- 32P]-dCTP was added to each PCR reaction. 1 μl of RT cDNA, 1 μl of upper and lower primers (10 mM) each, and 1 μl of competitor (0.001, 0.01, 0.1, 1, 10, and 100 fmol) were then added to the PCR tubes with 45 μl of PCR SuperMix (GIBCO BRL). After PCR amplification, a 10-μl sample of PCR products from each reaction was run on agarose gels, stained with ethidium bromide (50 μg/ml), photographed, dried, and then scanned using an AMBIS image acquisition and analysis system (AMBIS). The logarithm of the ratio of truncated or endogenous BMP receptors to competitor PCR products was plotted as a function of the logarithm of the known amount of competitor added to the PCR reaction after size correction. The molar concentration of truncated and endogenous BMP receptors was calculated from the linear regression curves based on the known amount of competitor added.
Mineralized Bone Matrix Formation Assay
Bone cell differentiation was monitored using a mineralized matrix formation assay as described by Bharagava et al. (1986). Von Kossa stain of mineralized bone matrix was performed as follows. The cell cultures were washed with PBS twice, fixed in phosphate-buffered formalin for 10 min and then washed with water, and serially dehydrated in 70, 95, and 100% ethanol, twice each, and then air dried. The plates were rehydrated from 100 to 95 to 80% ethanol/water before staining. The water was removed, a 2% silver nitrate solution was added, and then the plates were exposed to sunlight for 20 min after which the plates were rinsed with water. 5% sodium thiosulfate was added for 3 min and the plates were then rinsed with water. The modified van Gieson stain was then used as a counterstain after the von Kossa stain. The unmineralized collagen matrix can be recognized by the yellow-red van Gieson stain. The acid fuchsin solution (5 parts of 1% acid fuchsin, 95 parts of picric acid, and 0.25 part of 12 M HCl) was added for 5 min. The plates were washed with water and then with 2× 95% ethanol, 2× 100% ethanol and then dried for image analysis.
The area of von Kossa-stained matrix was quantified by automated image analysis using a video analysis program (Jandel Scientific, San Rafael, CA) linked to a video screen camera (CCD/RGB; Sony Corp., Park Ridge, NJ) and microscope (model BH2; Olympus Corp., Precision Instruments Division, Lake Success, NY) equipped with metallurgical lenses.
Transmission electron microscopy was used to analyze the cell and matrix structures. The cells were washed with PBS twice and fixed in 10 mM of Na cacodylate buffer, pH 7.2, containing 2% glutaraldehyde overnight at 4°C. The cells were then postfixed with 1% osmium tetroxide in 0.1 M Na cacodylate buffer for 1 h at room temperature. The specimens were dehydrated in an ethanol series, embedded in Polybed 812 resin (Polyscience, Warrington, PA), polymerized for 72 h at 60°C, sectioned with a Sorvall MT 5000 ultramicrotome (Sorvall, Inc., Norwalk, CT), and then viewed in a JEOL 1200 EX transmission electron microscope (JEOL USA, Inc., Peabody, MA).
Results
BMP-2 Induces 2T3 Cells to Differentiate into Mature Osteoblasts and Adipocytes
Previously, we characterized the osteoblast differentiation properties of 2T3 cells (Ghosh-Choudhury et al., 1996). BMP-2 induced 2T3 cells to differentiate into mature osteoblasts, indicating that 2T3 cells are precursors for the osteoblast lineage. However, since other cells with this potential also have the capability of differentiating along the adipocyte lineage, we cultured 2T3 cells in an adipocyte-promoting medium containing a PPARγ ligand, indomethacin (Lehmann et al., 1997). We found that 2T3 cells have a weak potential to differentiate into adipocytes if cultured in this adipocyte-promoting medium and BMP-2–enhanced adipocyte formation. This observation demonstrates that 2T3 cells can also differentiate to a limited extent into mature adipocytes if placed in the appropriate environment (refer to Materials and Methods).
Expression of BMP Receptors in 2T3 Cells
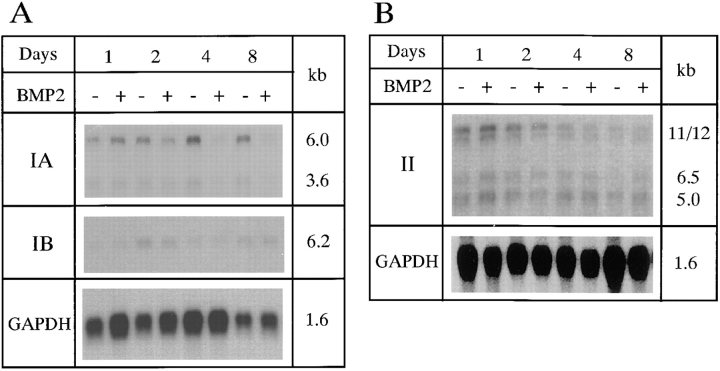
We examined expression of type IA, IB, and type II BMP receptors in 2T3 cells. Expression of all three BMP receptors was detected in 2T3 cells by Northern analysis. Two transcripts for BMPR-IA, a single transcript for BMPR-IB, and four transcripts for BMPR-II were detected using DNA probes encoding the extracellular domain of the receptors. Expression levels of BMPR-IA were higher than those of BMPR-IB in 2T3 cells as assayed with probes of the same specific activity on duplicate RNA samples (Fig. 1). Addition of BMP-2 into 2T3 cell cultures decreased expression of BMPR-IA during osteoblast differentiation but has no significant effects on BMPR-IB expression (Fig. 1 A). Expression of the larger 11- and 12-kb BMPR-II transcripts decreased with time (Fig. 1 B). Type II activin receptor expression was also detected in 2T3 cells by RT-PCR (data not shown).
Figure 1.
Type IA, IB, and type II BMP receptor mRNA expression in 2T3 cells. 2T3 cells were cultured under conditions in which mineralized bone matrix forms. Day 0 is defined as the day the cells reach confluence and the medium is changed to αMEM supplemented with 5% FCS, 100 μg/ml ascorbic acid, and 5 mM β-glycerol phosphate with or without 40 ng/ml of BMP-2. (A) Two transcripts (3.6 and 6.0 kb) of BMPR-IA, one transcript (6.2 kb) of BMPR-IB, and (B) four transcripts (5.0, 6.5, 11, and 12 kb) of BMPR-II were detected in 2T3 cells.
Expression of Truncated BMP Receptors in 2T3 Cells
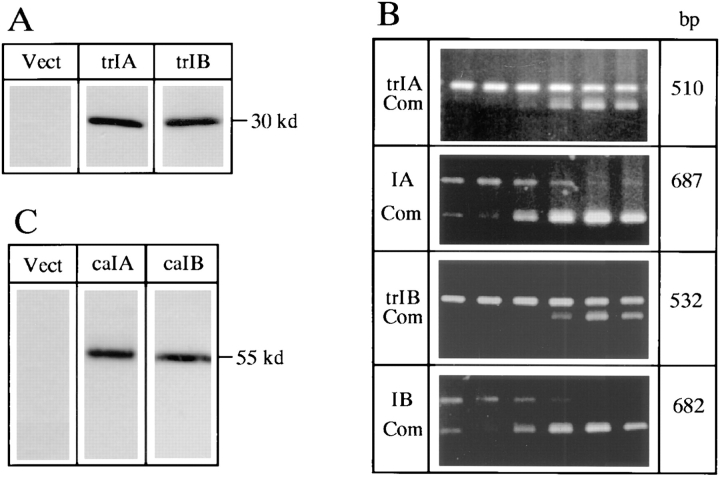
To test the function of type IA and IB BMP receptors in 2T3 cells, trBMPR-IA and trBMPR-IB cDNAs in the expression vector were independently and stably transfected in 2T3 cells. The DNA sequence encoding HA peptide was placed on the 3′ end of the receptor cDNA just after the transmembrane domain. As a consequence, the kinase domain of the receptor was deleted. The protein expression of truncated type IA and IB BMP receptors in three independent clones for each mutant was detected by Western analysis using anti-HA peptide antibody (Fig. 2 A). Expression levels of truncated and endogenous type IA and IB BMP receptor mRNAs were quantitated by competitive RT-PCR (Fig. 2 B). The primers for detecting mRNAs of truncated and endogenous BMPR-IA or BMPR-IB did not amplify their counterparts. The mRNA levels of truncated type IA and IB BMP receptors in three different clones were over 10-fold higher than those of endogenous type IA and IB BMP receptors.
Figure 2.
Expression of truncated type IA and IB and constitutively active type IA and IB BMP receptors in 2T3 cells. (A) Expression of trBMPR-IA (trIA) and trBMPR-IB (trIB) protein (∼30 kD) in 2T3 cells was detected by Western analysis using anti-HA antibody. (B) Quantitation of expression levels of the truncated and endogenous BMPR-IA (trIA and IA) and BMPR-IB (trIB and IB) transcripts in 2T3 clones expressing trBMPR-IA and trBMPR-IB. The generation of a competitor and RT-PCR conditions were described in Materials and Methods. Increasing concentrations of DNA competitor (Com) at concentrations of 0.001, 0.01, 0.1, 1, 10, and 100 fmol (left to right) were added to each PCR reaction. The intensity of PCR products was quantitated by an AMBIS image acquisition system. (C) Expression of caBMPR-IA (caIA) and caBMPR-IB (caIB) protein (∼55 kD) in 2T3 cells was detected by Western analysis using anti-HA antibody.
Effects of Truncated BMPR-IB on Bone Matrix Formation
During long-term cultures, 2T3 cells undergo a process of proliferation followed by a low level of differentiation as measured by mineralized bone matrix formation. The cells are highly dependent on exogenous BMP-2 addition for extensive differentiation and formation of mineralized bone matrix. The mineralized bone matrix has the characteristics of normal woven bone and is similar to the mineralized matrix produced by primary cultures of fetal rat calvarial (FRC) osteoblasts as determined by von Kossa staining, electron microscopy analysis (Ghosh-Choudhury et al., 1996) and Fourier transform infrared spectroscopy (FTIR) analysis (our unpublished data).
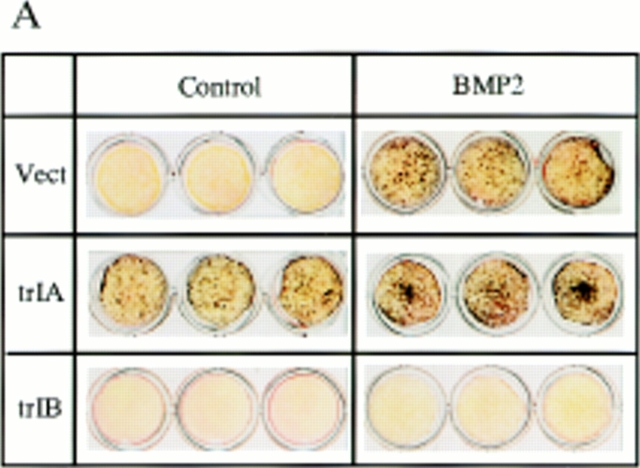
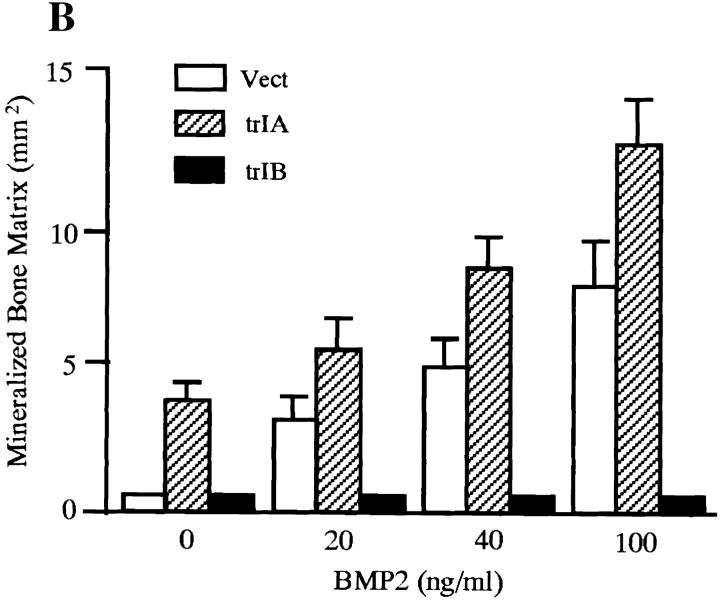
Overexpression of trBMPR-IB in 2T3 cells completely blocked BMP-2–induced mineralized bone matrix formation at all concentrations of BMP-2 (20–100 ng/ml) that induced bone matrix formation in control 2T3 clones (Fig. 3, A and B). Time-course studies confirmed these results. The 2T3 clones overexpressing trBMPR-IB formed multilayers but failed to form mineralized bone matrix during the entire culture period (data not shown). In parallel experiments, overexpression of the trBMPR-IA did not block BMP-2–induced mineralized bone matrix formation in 2T3 cells. Instead, it enhanced bone matrix formation in the absence of BMP-2 (Fig. 3, A and B). These observations suggest that BMPR-IB is primarily responsible for BMP-2–induced osteoblast differentiation. The growth rates of trBMPR-IB–expressing 2T3 clones were similar to those of control 2T3 clones (data not shown), suggesting that impairment of mineralized bone matrix formation is not due to defects in cell growth or multilayering.
Figure 3.
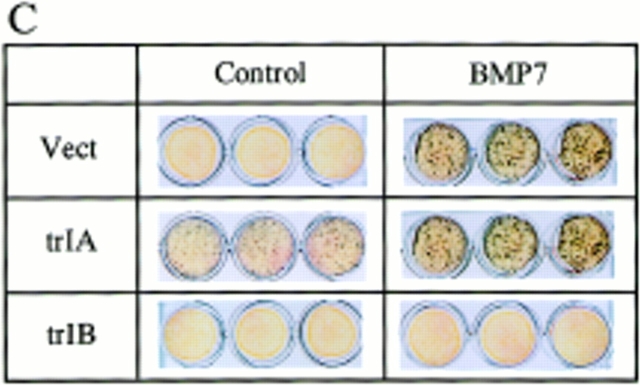
Von Kossa stain of 2T3 clones containing the empty vector, trBMPR-IA, trBMPR-IB and caBMPR-IB. (A) Effects of overexpression of trBMPR-IA (trIA) and trBMPR-IB (trIB) on BMP-2–induced mineralized bone matrix formation. The cells were cultured for 12 d in the presence or absence of 100 ng/ml of BMP-2. After incubation, the cells were fixed with 10% formalin and stained by von Kossa method. (B) The mineralized bone matrix formation of 2T3 cells containing the empty vector (Vect); trIA and trIB were quantitated by computer image analyzer. The data represent the mean ± SE for three samples. (C) Effects of overexpression of trBMPR-IA (trIA) and trBMPR-IB (trIB) on BMP-7– induced mineralized bone matrix formation. The cells were cultured for 12 d in the presence or absence of 200 ng/ml of BMP-7. (D) Effects of overexpression of caBMPR-IB (caIB) on mineralized bone matrix formation. The cells were cultured for 12 d and stained by von Kossa method.
To determine if other BMP family members stimulate osteoblast differentiation through the same BMP receptor, we examined the effect of BMP-7 (OP-1) on mineralized bone matrix formation in control and trBMPR-IB–expressing 2T3 clones. BMP-7 induced mineralized bone matrix formation in a dose-dependent manner (50–200 ng/ml) in control 2T3 clones. Overexpression of trBMPR-IB completely blocked BMP-7–induced mineralized bone matrix formation in these trBMPR-IB clones (Fig. 3 C). Overexpression of trBMPR-IA in 2T3 cells did not block BMP-7–induced mineralized bone matrix formation (Fig. 3 C). These observations suggest that BMP-7 and BMP-2 act through the same BMP receptors to stimulate osteoblast differentiation.
To determine if other growth and differentiation factors that mediate osteoblast differentiation were affected by overexpression of trBMPR-IB, we examined the effect of retinoic acid on ALP activity in control and trBMPR-IB–expressing 2T3 clones. Retinoic acid stimulated ALP activity in both cells at similar potency (data not shown), suggesting that some differentiation functions of these cells remains intact although BMPR-IB signaling is blocked.
Analysis of the Mineralized Bone Matrix Structure
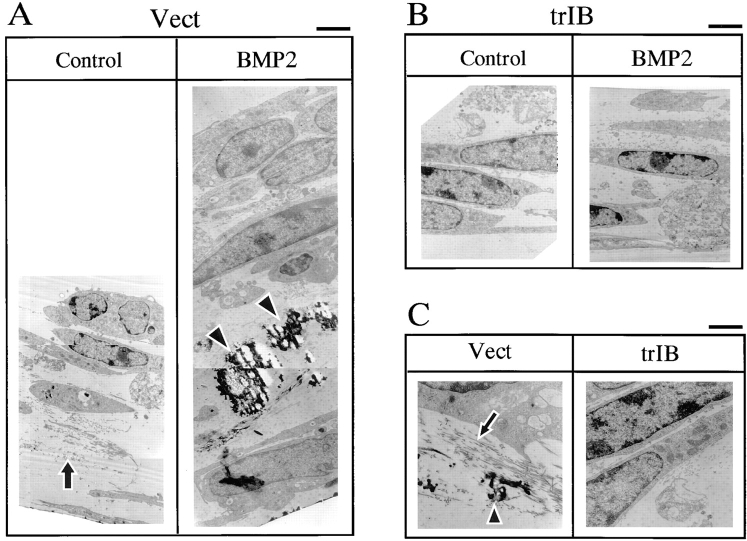
The mineralized bone matrix structure was analyzed by the transmission electron microscopy. Similar to the primary FRC cell cultures, low levels of the mineralized matrix were associated with unmineralized collagen fibrils between the cell layers in control 2T3 clones (Fig. 4, A and C). Addition of BMP-2 to control 2T3 clones resulted in massive increases in mature mineralized matrix in which the collagen fibers were integrated into the mineral structure (Fig. 4, A and C). In cultures of 2T3 clones overexpressing trBMPR-IB, multiple cell layers were formed but no mineralized matrix was found between the cell layers even in the presence of BMP-2 (Fig. 4, B and C). There was a large decrease in the collagen fibers formed between the cell layers. The number of cells and of cell layers was similar between control and trBMPR-IB–expressing 2T3 clones but the thickness of the culture of trBMPR-IB clones was highly reduced due to the lack of extensive collagen fibril containing matrix (Fig. 4, A and B). These results indicate that the mineralization observed by von Kossa staining is matrix-associated mineralization and that overexpression of trBMPR-IB inhibits osteoblast terminal differentiation in 2T3 cells.
Figure 4.
Transmission electron microscopic analysis of the mineralized bone matrix structure of 2T3 cells. (A and B) Empty vector control (Vect) and trBMPR-IB (trIB)–expressing 2T3 clones were cultured for 12 d in the presence or absence of 100 ng/ml of BMP-2 and fixed with Na cacodylate and glutaraldehyde buffer for electron microscopy analysis. (C) High magnification of collagen fibers and mineralized bone matrix of 2T3 cells containing the empty vector (Vect) and trBMPR-IB (trIB). Arrows, unmineralized collagen fibers; arrowheads, mature mineralized matrix. Bars: (A and B) 2 μm; (C) 1 μm.
Effects of Truncated BMPR-IB on Osf2/Cbfa1 Expression
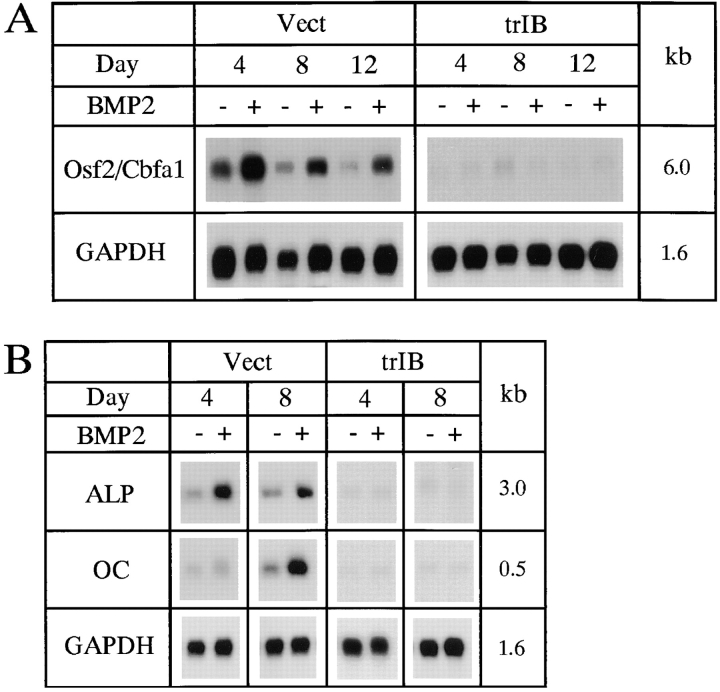
Osf2/Cbfa1 is an osteoblast-specific transcription factor. Osf2/Cbfa1 mRNA was expressed in 2T3 cells and BMP-2 enhanced Osf2/Cbfa1 mRNA expression (Fig. 5 A). In trBMPR-IB–expressing 2T3 clones, Osf2/Cbfa1 mRNA expression was significantly decreased (Fig. 5 A) but in trBMPR-IA–expressing 2T3 clones, Osf2/Cbfa1 mRNA expression and BMP-2 induction remain intact (data not shown). These results suggest that BMP-2 stimulates Osf2/ Cbfa1 expression through the BMPR-IB and further demonstrates the essential roles of the BMPR-IB in osteoblast differentiation.
Figure 5.
Osf2/Cbfa1, alkaline phosphatase (ALP) and osteocalcin (OC) mRNA expression in 2T3 cells. 2T3 cells containing the empty vector (Vect) or trBMPR-IB (trIB) were cultured for 8 d in αMEM containing 5% FCS, 100 μg/ml ascorbic acid, and 5 mM β-glycerol phosphate with or without 40 ng/ml of BMP-2. (A and B) The Osf2/Cbfa1 (6.0 kb), ALP (3.0 kb), and OC (0.5 kb) mRNA expression was detected by Northern analysis.
Effects of Truncated BMPR-IB on ALP and OC Expression
Expression of several osteoblast differentiation-related genes was examined in this study including ALP and OC. Like FRC osteoblasts, ALP mRNA was expressed in the early phase of differentiation and OC mRNA was expressed at initiation of the mineralization phase of differentiation in 2T3 cells. BMP-2 enhanced ALP and OC mRNA expression in control 2T3 clones. In trBMPR-IB–expressing 2T3 clones, ALP and OC mRNAs were significantly reduced in the absence or presence of BMP-2 (Fig. 5 B). Overexpression of trBMPR-IB also inhibited ALP activity and blocked BMP-2 induction of ALP in 2T3 cells (Fig. 6 B). These results are consistent with the studies reported above showing that trBMPR-IB inhibited mineralized bone matrix formation.
Figure 6.
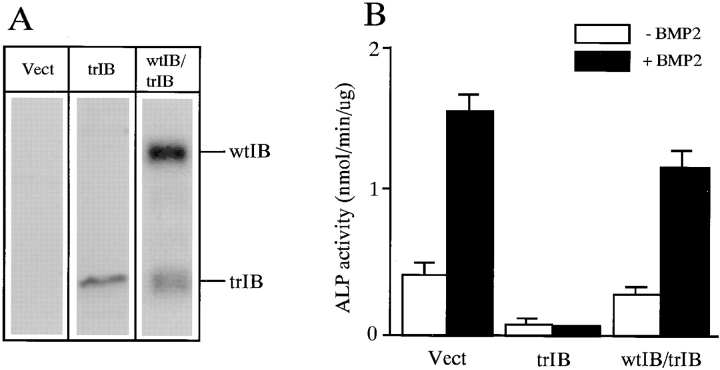
Effects of BMP-2 on ALP activity in 2T3 cells. 2T3 clones expressing trBMPR-IB was transiently transfected with wtBMPR-IB. (A) Expression of wtBMPR-IB (wtIB) and trBMPR-IB (trIB) in 2T3 clones expressing trBMPR-IB was detected by Western analysis. (B) ALP activity and BMP-2 (100 ng/ml) response was assayed in control, trBMPR-IB and wtBMPR-IB/ trBMPR-IB clones. The unit of ALP activity is nanomoles of p-nitrophenol formed per microgram of protein per minute. Data are the mean ± SE for three samples.
Rescue of the Phenotype of Truncated BMPR-IB Clones
To further test the specificity of the effects of trBMPR-IB, we transfected wtBMPR-IB into 2T3 clones containing trBMPR-IB. Expression of wtBMPR-IB was detected by Western analysis using anti-HA antibody (Fig. 6 A). The wild-type and truncated BMPR-IB can be readily distinguished by size. The expression levels of wtBMPR-IB were higher than those of trBMPR-IB (Fig. 6 A). Expression of wtBMPR-IB in 2T3 clones containing trBMPR-IB rescued suppressed ALP activity and more importantly, the BMP-2 responsiveness (Fig. 6 B). These results suggest that trBMPR-IB in 2T3 clones specifically blocks BMPR-IB signaling.
Effects of Constitutively Active BMPR-IB on Bone Matrix Formation
To confirm the role of BMPR-IB in osteoblast differentiation, we stably transfected caBMPR-IB (ALK6, Q203D) in 2T3 cells. This caBMPR-IB has been shown to increase Smad1 phosphorylation without a BMP ligand in transient transfection assays (Hoodless et al., 1996). CaBMPR-IB expression in three independent clones was detected by Western analysis using anti-HA antibody (refer to Fig. 2 C). Expression of caBMPR-IB in these clones induced mineralized bone matrix formation without addition of BMP-2 (refer to Fig. 3 D). These results support the concept that BMPR-IB signaling is required for osteoblast differentiation.
Respecification of 2T3 Cells by Inhibition of BMPR-IB
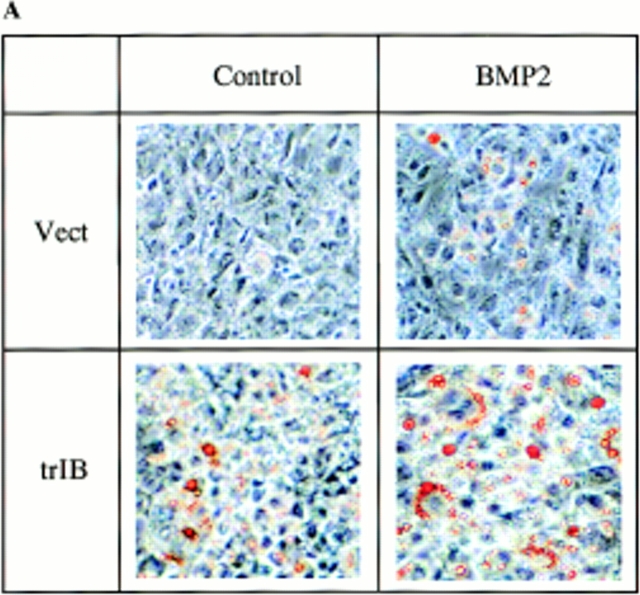
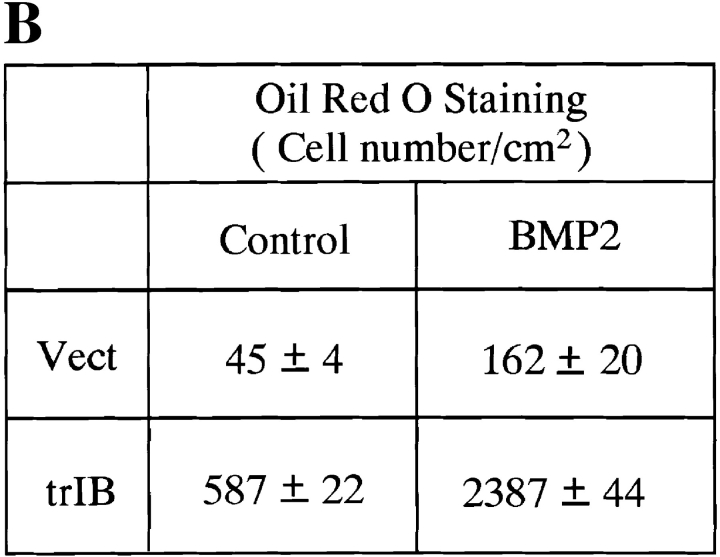
Parent 2T3 cells, when cultured in the osteoblast differentiation medium, differentiate into mature osteoblasts producing an extensive mineralized bone matrix in the presence of BMP-2. Under these conditions, no adipocytes were observed either in the absence or presence of BMP-2. When control 2T3 clones were cultured in an adipocyte-promoting medium containing 60 mM of indomethacin, a PPARγ ligand, a few mature adipocytes were observed by Oil red O staining. A measurable increase in adipocyte number was found in control 2T3 clones when 100 ng/ml of BMP-2 was added to the culture media (Fig. 7, A and B). However, when trBMPR-IB–expressing 2T3 clones were cultured in this same adipocyte-supporting media, a 12-fold increase in the number of mature adipocytes was observed and BMP-2 (100 ng/ml) further enhanced mature adipocyte formation (Fig. 7, A and B). Microscopic examination of the cells demonstrated that trBMPR-IB– expressing 2T3 clones had dramatic changes in morphology from day 3 onward. The trBMPR-IB expressing clones changed from a typical spindle-shaped osteoblast structure to polygonal cells with perinuclear accumulations of fat droplets in the cytoplasm. Highly differentiated adipocytes with large lipid vacuoles were identified by Oil red O staining at day 8 (Fig. 7, A and B). No adipocyte formation was observed in 2T3 clones containing caBMPR-IB with or without BMP-2 in this adipogenic media (data not shown). These results demonstrate that BMPR-IB may be essential not only for osteoblast differentiation but also for the commitment and specification to the osteoblast lineage.
Figure 7.
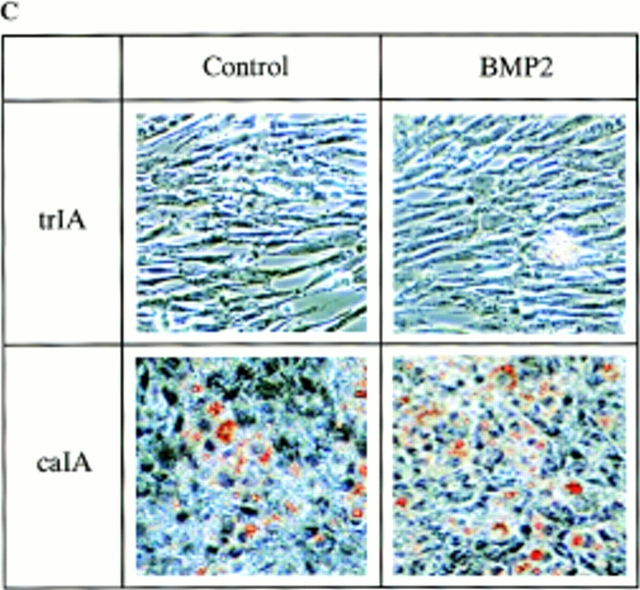
Adipocyte formation in 2T3 cells containing different mutant BMP receptors. (A) 2T3 cells containing the empty vector (Vect) and trBMPR-IB (trIB) were cultured for 8 d in αMEM containing 5% rabbit serum, 0.5 mM IBMX, and 60 mM indomethacin in the presence or absence of 100 ng/ml of BMP-2. After incubation, the cells were fixed with 10% formalin and stained with 0.2% Oil red O solution. (B) Oil red O–positive staining adipocytes in control and trBMPR-IB–expressing clones were quantitated using a phase-contrast microscope. The data represents the mean ± SE for three samples. (C) Adipocyte formation in 2T3 cells expressing trBMPR-IA (trIA) and caBMPR-IA (caIA) in the presence or absence of 100 ng/ml of BMP-2. After 8 d of incubation, the cells were stained by the Oil red O staining method.
Effects of BMPR-IA on Adipocyte Formation
Since addition of BMP-2 further enhanced adipocyte formation in trBMPR-IB–expressing clones, we then examined if BMPR-IA is involved in this BMP-2–stimulated adipocyte formation. We transfected trBMPR-IA and caBMPR-IA cDNAs into 2T3 cells. Expression of these mutant receptors was detected by Western analysis using anti-HA antibody (refer to Fig. 2, A and C). 2T3 clones containing trBMPR-IA show no tendency to form adipocytes in the same adipocyte-promoting medium, even in the presence of BMP-2. The cells have a typical fibroblastic morphology (Fig. 7 C). Expression of caBMPR-IA in 2T3 cells induced adipocyte formation even without addition of BMP-2 (Fig. 7 C). These results suggest that BMPR-IA plays important roles in adipocyte differentiation.
Expression of Adipocyte Differentiation-related Genes
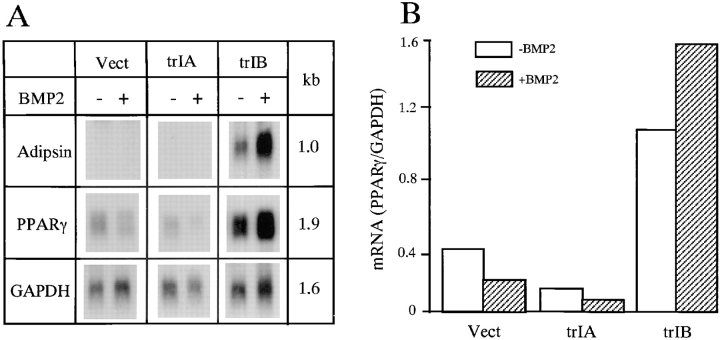
Expression of the mature adipocyte marker genes, adipsin and PPARγ, was examined by Northern analysis. The cells were cultured in the adipocyte-promoting media. Adipsin mRNA expression was only detected in 2T3 clones overexpressing trBMPR-IB at day 8. It was not detected in control or trBMPR-IA clones, either in the absence or presence of BMP-2 (Fig. 8 A). PPARγ mRNA expression was detected during the entire culture period in 2T3 clones containing empty vector, trBMPR-IA, and trBMPR-IB. However, in trBMPR-IB–expressing 2T3 clones, PPARγ mRNA expression was about threefold higher than control 2T3 clones. Addition of BMP-2 further enhanced both adipsin and PPARγ mRNA expression in trBMPR-IB–expressing clones (Fig. 8, A and B). In trBMPR-IA–expressing 2T3 clones, PPARγ mRNA expression was lower compared with control 2T3 clones and BMP-2 further suppressed PPARγ mRNA expression (Fig. 8, A and B). These results support the observation at the molecular level that the specification of 2T3 cells has been reprogrammed by selectively blocking a subtype of BMP receptor family.
Figure 8.
Adipocyte marker gene expression in 2T3 cells. 2T3 cells containing the empty vector (Vect), trBMPR-IA (trIA), and trBMPR-IB (trIB) were cultured for 8 d in αMEM containing 5% rabbit serum, 0.5 mM IBMX, and 60 mM of indomethacin with or without 40 ng/ml of BMP-2. (A) Adipsin (1.0 kb) and PPARγ (1.9 kb) mRNA expression were detected by Northern analysis. (B) The levels of PPARγ mRNA expression was quantitated and normalized to GAPDH levels.
Discussion
In this study, we examined the roles of type IA and IB BMP receptors in the commitment and differentiation of clonal 2T3 cells. The osteoblast differentiation properties of 2T3 cells have been characterized extensively in our laboratory. BMP-2, BMP-4, and BMP-6, and type IA, IB, and type II BMP receptors and type II activin receptor are expressed in 2T3 cells. BMP-2 stimulates BMP-2 and BMP-4 mRNA expression and suppresses BMPR-IA mRNA expression during osteoblast differentiation. BMP-2 and BMP-7 stimulate ALP activity, osteoblast differentiation-related gene expression, and mineralized bone matrix formation in 2T3 cells. 2T3 cells have retained the osteoblast differentiation properties up to passage 50. In the present study, we used passage 15 of 2T3 cells for the transfection and differentiation studies. To rule out the possibility of clonal variation, we characterized three independent clones for each stable transfection of mutant BMP receptors. The structure and mineral composition of the mineralized bone matrix produced by 2T3 cells have been characterized by electron microscopy analysis and FTIR. They are similar to the normal woven bone and the mineralized bone matrix produced by primary cultures of FRC osteoblasts. These features make 2T3 cells an ideal model for osteoblast differentiation and mineralization studies since many osteosarcoma cell lines have incomplete phenotypes and lack the mineralization capacity for terminal differentiation evaluation.
Osteoblast Differentiation in 2T3 Cells
Parent 2T3 cells form an extensive mineralized bone matrix in the presence of BMP-2. 2T3 clones containing truncated BMPR-IB fail to form mineralized bone matrix, even with addition of high concentrations of BMP-2. Bone cell differentiation is completely blocked but growth and multilayering is unaffected. The lack of an extensive collagen-fibril matrix was found in truncated BMPR-IB–expressing clones. Expression of the osteoblast specific transcription factor, Osf2/Cbfa1, and osteoblast differentiation-related genes, ALP and OC, was significantly inhibited. The basal ALP activity and BMP-2 induction were also inhibited. These observations demonstrate that BMPR-IB is required for osteoblast differentiation. Expression of a constitutively active BMPR-IB induced osteoblast differentiation without a ligand, further supporting this conclusion.
Adipocyte Differentiation in 2T3 Cells
Parent 2T3 cells can be induced to differentiate into a small number of mature adipocytes in the adipocyte-promoting medium and BMP-2 enhances adipocyte formation in these cells. By expressing truncated BMPR-IA in 2T3 cells, the capacity of 2T3 cells to form mature adipocytes is blocked, even in the presence of BMP-2. The expression profile of adipocyte marker genes, adipsin and PPARγ, correlated with these phenotypic changes. Basal levels of PPARγ expression in 2T3 clones containing truncated BMPR-IA are lower compared with control 2T3 clones and suppressed by BMP-2 as in control 2T3 clones. 2T3 clones overexpressing constitutively active BMPR-IA form mature adipocytes without addition of BMP-2. The results demonstrate that adipocyte differentiation stimulated by BMP-2 is mediated through BMPR-IA signaling. The results also suggest that BMP-2 may stimulate adipocyte differentiation partly by enhancing expression of PPARγ, a key molecule for adipocyte differentiation. It has been reported that just overexpression of PPARγ cDNA in NIH-3T3 fibroblasts and activation of PPARγ receptor by PPARγ ligands, prostaglandin J2 metabolites, in pluripotent precursor C3H10T1/2 cells can induce these cell lines into mature adipocytes (Kliewer et al., 1995; Brun et al., 1996). This is further supported by the fact that 2T3 clones containing truncated BMPR-IB spontaneously form adipocytes and BMP-2 further enhances adipocyte differentiation process. Adipsin mRNA is expressed in 2T3 clones containing truncated BMPR-IB and not in empty vector or parent 2T3 clones. Adipsin mRNA is also increased with BMP-2 addition in 2T3 clones containing truncated BMPR-IB.
Commitment of Precursor Cells
Studies of stromal cell transplantation in vivo (Friedenstein et al., 1987) and analysis of different mouse or rat clonal cell populations in vitro (Bennett et al., 1991; Beresford et al., 1992) provide evidence that multipotential mesenchymal cells can differentiate into different cell types including osteoblasts, chondroblasts, and adipocytes. These mesenchymal cells have the capacity to undergo the commitment process to give rise to progeny with more limited or monopotential differentiation capacity. The mechanism of the commitment and the specification of osteoblasts and adipocytes is not fully understood. BMPs appear to play regulatory roles during osteoblast and adipocyte differentiation processes (Aubin et al., 1995). Type IA BMP receptor expression was detected in preadipocyte BMS2 cells (Gimble et al., 1995), osteoblast MC3T3-E1 cells (Heffel et al., 1997), and myoblast C2C12 cells (Namiki et al., 1997). In the present study, we found that selectively blocking BMPR-IB in 2T3 cells respecified cells to differentiate into adipocytes instead of osteoblasts. These results suggest that expression of BMPR-IB in mesenchymal precursor cells is required for commitment of the osteoblast lineage. Overexpression of truncated BMPR-IA in 2T3 cells blocked adipocyte differentiation and promoted osteoblast differentiation. These results suggest that high levels of BMPR-IA signaling are involved in adipocyte differentiation. The results of overexpression of constitutively active type IA or IB BMP receptors in 2T3 cells further support these findings.
The Specificity of Phenotypes of Mutant Receptor Clones
The kinase domains and kinase activities of type I BMP receptors are dispensable for receptor complex formation but are required to transmit a signal to the nucleus (Massagué, 1996). The truncated type I and type II BMP receptors without kinase domain or with mutations in the kinase domain have been shown to block BMP signaling efficiently in vivo and in vitro (Graff et al., 1994; Maeno et al., 1994; Suzuki et al., 1994). Our observations indicate that truncated type IB and IA BMP receptors act as a dominant-negative mutants to block osteoblast or adipocyte differentiation induced by BMPs.
The specificity of type IA and IB BMP receptor signaling in osteoblast and adipocyte differentiation has been supported by several lines of evidence in this study. First, expression of truncated BMPR-IB in 2T3 cells completely blocked osteoblast differentiation and mineralized bone matrix formation. This was not seen in 2T3 clones overexpressing truncated BMPR-IA. Similarly, expression of truncated BMPR-IA in 2T3 cells blocked adipocyte differentiation and expression of truncated BMPR-IB induced adipocyte differentiation. Second, constitutively active BMPR-IB and BMPR-IA induced mineralized bone matrix formation and adipocyte formation, respectively, without addition of BMP-2. These results support the findings that truncated BMPR-IB or truncated BMPR-IA blocked osteoblast or adipocyte differentiation in 2T3 cells, respectively. Finally, we have been able to rescue the ALP activity and BMP-2 responsiveness by overexpression of wild-type BMPR-IB in 2T3 clones containing truncated BMPR-IB.
Signaling Pathways of BMPR-IA and BMPR-IB
One of the earliest downstream signals of BMP-2 action is the activation and nuclear translocation of the mothers-against-dpp (MAD) family members or Smad proteins (Hoodless et al., 1996; Nishimura et al., 1998). When Smad1 is translocated to the nucleus it can supply a transcriptional activation domain and DNA-binding domain which then, in turn, interact with other DNA-binding transcription factors, resulting in activating or repressing specific genes (Liu et al., 1996). One possibility is that BMPR-IA and BMPR-IB can activate different signaling molecules or different transcription factors after forming a complex with different type II receptors. For example, activin is known to signal cells through Smad2 protein. Smad2 can then interact with a specific target cell wing-helix-loop-helix transcription factor, FAST1 (Chen et al., 1996). Thus, BMP-2 may signal through a BMP type II or activin type II receptor complexed with a specific BMP type I receptor. These different complexes can generate different signaling pathways responsible for alternate paths of differentiation. More detailed studies are required to address this issue.
Recent studies on activin receptor signaling also suggest that different subtypes of activin receptors transduce alternate biological signals. Truncated type IB activin receptor blocked activin-induced transcriptional activity in both Chinese hamster ovary and K562 human erythroleukemic cells, whereas truncated type I (IA) activin receptor had no effect in either cell line (Tsuchida et al., 1995). Our results now demonstrate functional differences of the BMP receptor subtypes in their capacity to induce multipotent cells to differentiate along osteoblast or adipocyte lineages.
Downstream Target Genes of Type IA and IB BMP Receptor Signaling
Osf2/Cbfa1 is an osteoblast-specific transcription factor. A total lack of bone and retention of the partially calcified cartilagenous skeleton were found in Osf2/Cbfa1 knockout mice. Both membranous bone of the skull and endochondral bone in the rest of the skeleton were absent in Osf2/Cbfa1 knockout mice. Mutation of Osf2/Cbfa1 gene is found in humans with cleidocranial dysplasia (Ducy et al., 1997; Komori et al., 1997; Mundlos et al., 1997; Otto et al., 1997). These findings indicate that Osf2/Cbfa1 plays essential roles in bone formation. Osf2/Cbfa1 is a downstream molecule of BMP action since BMP-7 induced Osf2/Cbfa1 mRNA expression in C3H10T1/2 cells. In the present study, we demonstrated that expression of Osf2/Cbfa1 was enhanced by BMP-2 and blocked by truncated BMPR-IB in 2T3 cells. These results suggest that BMP-2 stimulates Osf2/Cbfa1 expression through BMPR-IB.
Similar to Osf2/Cbfa1 in osteoblast differentiation, PPARγ is a critical molecule for adipocyte differentiation. Expression of truncated BMPR-IA suppresses PPARγ mRNA expression and expression of truncated BMPR-IB enhances PPARγ mRNA expression, suggesting that PPARγ may be one of the important downstream target genes for BMPR-IA signaling during adipocyte differentiation.
In summary, we have demonstrated that type IB and IA BMP receptors play essential roles for commitment and differentiation of osteoblasts and adipocytes. Commitment of osteoblasts and adipocytes can be respecified by selectively blocking or activating type IB or IA BMP receptor signalling. This finding suggests that the temporal acquisition or loss of a subtype of BMP receptors in precursor cells may be a key step for commitment and differentiation of osteoblasts and adipocytes.
Acknowledgments
This work was supported by grants from the National Institutes of Health to G.R. Mundy and S.E. Harris (P01-AR 39529) and S.E. Harris (R01-AR 44728).
Abbreviations used in this paper
- αMEM
α minimal essential medium
- aa
amino acids
- ALP
alkaline phosphatase
- BMP
bone morphogenic protein
- caBMPR-IB
constitutively active type IB BMP receptor
- FRC
fetal rat calvarial
- HA
hemagglutinin
- IBMX
3-isobutyl-1-methyl xanthine
- OC
osteocalcin
- RT
reverse transcription
- trBMPR-IB
truncated type IB BMP receptor
- wt
wild-type
Footnotes
We would like to acknowledge M. Dallas and D. Guerro (University of Texas Health Science Center at San Antonio, TX) for the quantitation of mineralized bone matrix formation and assistance with the electron microscopy. We thank J. Wrana (The Hospital for Sick Children, Toronto, Canada) for BMPR-IB and constitutively active BMPR-IA and BMPR-IB cDNA constructs and J. Massagué (Memorial Sloan-Kettering Cancer Center, New York) and B. Spiegelman (Harvard Medical School, Boston, MA) for BMPR-II and PPARγ cDNA probe. We are grateful to N. Garrett for her help with the preparation of this manuscript. We also wish to thank L. Bonewald (both from University of Texas Health Science Center at San Antonio) for her comments on the manuscript.
Address all correspondence to S.E. Harris, Medicine/Endocrinology, 7703 Floyd Curl Drive, San Antonio, TX 78284-7877. Tel.: (210) 567-4900. Fax: (210) 567-6693. E-mail: harris@uthscsa.edu
References
- Ahrens M, Ankenbauer T, Schroder D, Hollnagel A, Mayer H, Gross G. Expression of human bone morphogenetic protein-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12:871–880. doi: 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastics, osteoblastics and/or adipocyte differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995;17:77S–83S. doi: 10.1016/8756-3282(95)00183-e. [DOI] [PubMed] [Google Scholar]
- Bennett JH, Joyner CJ, Triffitt JT, Owen ME. Adipocytic cells cultured from marrow have osteogenic potential. J Cell Sci. 1991;99:131–139. doi: 10.1242/jcs.99.1.131. [DOI] [PubMed] [Google Scholar]
- Beresford JN, Bennett JH, Delving C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102:341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- Bharagava U, Bar-Lev M, Bellows CG, Aubin JE. Ultrastructural analysis of bone nodules formed in vitro by isolated fetal rat calvaria cells. Bone. 1986;9:155–163. doi: 10.1016/8756-3282(88)90005-1. [DOI] [PubMed] [Google Scholar]
- Brun RP, Tontonoz P, Forman BM, Ellis R, Chen J, Evans RM, Spiegelman BM. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 1996;10:974–984. doi: 10.1101/gad.10.8.974. [DOI] [PubMed] [Google Scholar]
- Chen D, Harris MA, Rossini G, Dunstan CR, Dallas SL, Feng JQ, Mundy GR, Harris SE. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4 and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int. 1997;60:283–290. doi: 10.1007/s002239900230. [DOI] [PubMed] [Google Scholar]
- Chen X, Rubock MJ, Whitman M. A transcriptional partner for MAD proteins in TGF-β signaling. Nature. 1996;383:691–696. doi: 10.1038/383691a0. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Spiegle KV, Miyazono K, Huylebroeck D. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/ Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Chen D, Cooney AJ, Tsai MJ, Harris MA, Tsai SY, Feng M, Mundy GR, Harris SE. The mouse bone morphogenetic protein-4 gene: Analysis of promoter utilization in fetal rat calvarial osteoblasts and regulation by COUP-TFI orphan receptor. J Biol Chem. 1995;270:28364–28373. doi: 10.1074/jbc.270.47.28364. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan PK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Ghosh-Choudhury N, Windle JJ, Koop BA, Harris MA, Guerrero DL, Wozney JM, Mundy GR, Harris SE. Immortalized murine osteoblasts derived from BMP2-T-antigen expressing transgenic mice. Endocrinology. 1996;137:331–339. doi: 10.1210/endo.137.1.8536632. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Morgan C, Kelly K, Wu X, Dandapani V, Wang C, Rosen V. Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. J Cell Biochem. 1995;58:393–402. doi: 10.1002/jcb.240580312. [DOI] [PubMed] [Google Scholar]
- Graff JM. Embryonic patterning: to BMP or not to BMP, that is the question. Cell. 1997;89:171–174. doi: 10.1016/s0092-8674(00)80196-8. [DOI] [PubMed] [Google Scholar]
- Graff JM, Thies RS, Song JJ, Ceteste AJ, Melton DA. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994;79:169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Harris SE, Sabatini M, Harris MA, Feng JQ, Wozney JM, Mundy GR. Expression of bone morphogenetic protein messenger RNA in prolonged cultures of fetal rat calvarial cells. J Bone Miner Res. 1994;9:389–394. doi: 10.1002/jbmr.5650090314. [DOI] [PubMed] [Google Scholar]
- Harris SE, Feng JQ, Harris MA, Ghosh-Choudhury N, Dallas MR, Wozney JM, Mundy GR. Recombinant bone morphogenetic protein 2 accelerates bone cell differentiation and stimulates BMP-2 mRNA expression and BMP-2 promoter activity in primary fetal rat calvarial osteoblast cultures. Mol Cell Differ. 1995;3:137–155. [Google Scholar]
- Heffel DF, Iida-Klein A, Yamaguchi DT, Miller TA. Type I BMP-receptor mRNA expression in MC3T3-E1 cells over time. J Bone Miner Res. 1997;12(Suppl.):S312. [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O'Connor MB, De Robertis EM, Ferguson EL. The Xenopus dorsalizing factor noggin ventralizes Drosophilaembryos by preventing DPP from activating its receptor. Cell. 1996;86:607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Haerry T, Abdollah S, Stapleton M, O'Connor MB, Attisano L, Wrana JL. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Yoshioka H, Ohuchi H, Noji S, Nohno T. Truncated type II receptor for BMP-4 induces secondary axial structures in Xenopus embryos. Biochem Biophys Res Commun. 1995;216:26–33. doi: 10.1006/bbrc.1995.2587. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Chytil A, Moses HL. Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-β receptor. J Biol Chem. 1995;270:5625–5630. doi: 10.1074/jbc.270.10.5625. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Ishikawa T, Shimabara M, Tanda N, Enomoto-Iwamoto M, Iwamoto M, Kuwana T, Ueki A, Noji S, Nohno T. BMP signaling during bone pattern determination in the developing limb. Development (Camb) 1996;122:3557–3566. doi: 10.1242/dev.122.11.3557. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Koenig BB, Cook JS, Wolsing DH, Ting J, Tiesman JP, Correa PE, Olson CA, Pecquet AL, Ventura F, Grant RA, et al. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol Cell Biol. 1994;14:5961–5974. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao Y, Inada M, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Lehmann LM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors α and γ are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- Liu F, Ventura F, Doody J, Massagué J. Human type II receptor for bone morphogenetic proteins (BMPs): Extension of the two-kinase receptor model to the BMPs. Mol Cell Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Hata A, Baker JC, Doody J, Carcamo J, Harland RM, Massagué J. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature. 1996;381:620–623. doi: 10.1038/381620a0. [DOI] [PubMed] [Google Scholar]
- Maeno M, Ong RC, Suzuki A, Ueno N, Kung HF. A truncated bone morphogenetic protein 4 receptor alters the fate of ventral mesoderm to dorsal mesoderm: Roles of animal pole tissue in the development of ventral mesoderm. Proc Natl Acad Sci USA. 1994;91:10260–10264. doi: 10.1073/pnas.91.22.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. TGFβ signaling: receptors, transducers and Mad proteins. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JHM, et al. Mutations involving the transcription factor Cbfa1 cause cleidocranial dysplasia. Cell. 1997;89:773–779. doi: 10.1016/s0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- Namiki M, Akiyama S, Katagiri T, Suzuki A, Ueno N, Yamaji N, Rosen V, Wozney JM, Suda T. A kinase domain-truncated type I receptor blocks bone morphogenetic protein-2-induced signal transduction in C2C12 myoblasts. J Biol Chem. 1997;272:22046–22052. doi: 10.1074/jbc.272.35.22046. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Kato Y, Chen D, Harris SE, Mundy GR, Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem. 1998;273:1872–1879. doi: 10.1074/jbc.273.4.1872. [DOI] [PubMed] [Google Scholar]
- Nohno T, Ishikawa T, Saito T, Hosokawa K, Noji S, Wolsing DH, Rosenbaum JS. Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors. J Biol Chem. 1995;270:22522–22526. doi: 10.1074/jbc.270.38.22522. [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Novikoff PM, Rosen OM, Rubin CS. Organelle relationships in cultured 3T3-L1 preadipocytes. J Cell Biol. 1980;87:180–196. doi: 10.1083/jcb.87.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denze A, Gilmour KC, Rosewell IR, Stamp GWH, Beddington RSP, Mundlos S, Olsen BR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SV, Takahashi S, Haipek C, Chirgwin JM, Roodman GD. Tartrate-resistant acid phosphatase gene expression as a facile reporter gene for screening transfection efficiency in mammalian cell cultures. Biotechniques. 1993;15:444–447. [PubMed] [Google Scholar]
- Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin C, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberte E, Marty T, Nellen D, Affolter M, Basler K. An absolute requirement for both the type II and type I receptors, punt and thick veins, for Dpp signaling in vivo. Cell. 1995;80:889–897. doi: 10.1016/0092-8674(95)90292-9. [DOI] [PubMed] [Google Scholar]
- Strauss WM. Transfection of mammalian cells via lipofection. Methods Mol Biol. 1996;54:307–327. doi: 10.1385/0-89603-313-9:307. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Thies RS, Yamaji N, Song JJ, Wozney JM, Murakami K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci USA. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SM, Jones PA. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979;17:771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- Tonegawa A, Funayama N, Ueno N, Takahashi Y. Mesodermal subdivision along the mediolateral axis in chicken controlled by different concentrations of BMP-4. Development (Camb) 1997;124:1975–1984. doi: 10.1242/dev.124.10.1975. [DOI] [PubMed] [Google Scholar]
- Tsuchida K, Vaughan JM, Wiater E, Gaddy-Kurten D, Vale WW. Inactivation of activin-dependent transcription by kinase-deficient activin receptors. Endocrinology. 1995;136:5493–5503. doi: 10.1210/endo.136.12.7588300. [DOI] [PubMed] [Google Scholar]
- Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9:57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- Wang, E.A., V. Rosen, J.S. D'Alessandro, M. Bandy, P. Cordes, T. Harada, D.I. Israel, R.M. Hewick, K.M. Kerns, P. Lapan, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc. Natl. Acad. Sci. USA. 87:2220–2224. [DOI] [PMC free article] [PubMed]
- Wang EA, Rosen V, Cordes P, Hewick RM, Kriz MJ, Luxenberg DP, Sibley BS, Wozney JM. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci USA. 1988;85:9484–9488. doi: 10.1073/pnas.85.24.9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BLM. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz R, Hewick R, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Yamaji N, Celeste AJ, Thies RS, Song JJ, Bernier SM, Goltzman D, Lyons KM, Nove J, Rosen V, Wozney JM. A mammalian serine/threonine kinase receptor specifically binds BMP-2 and BMP-4. Biochem Biophys Res Comm. 1994;205:1944–1951. doi: 10.1006/bbrc.1994.2898. [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1995;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]
- Zou H, Wieser R, Massagué J, Niswander L. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997;11:2191–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]