Abstract
The composition of the plasma membrane domains of epithelial cells is maintained by biosynthetic pathways that can sort both proteins and lipids into transport vesicles destined for either the apical or basolateral surface. In MDCK cells, the influenza virus hemagglutinin is sorted in the trans-Golgi network into detergent-insoluble, glycosphingolipid-enriched membrane domains that are proposed to be necessary for sorting hemagglutinin to the apical cell surface. Site- directed mutagenesis of the hemagglutinin transmembrane domain was used to test this proposal. The region of the transmembrane domain required for apical transport included the residues most conserved among hemagglutinin subtypes. Several mutants were found to enter detergent-insoluble membranes but were not properly sorted. Replacement of transmembrane residues 520 and 521 with alanines converted the 2A520 mutant hemagglutinin into a basolateral protein. Depleting cell cholesterol reduced the ability of wild-type hemagglutinin to partition into detergent-insoluble membranes but had no effect on apical or basolateral sorting. In contrast, cholesterol depletion allowed random transport of the 2A520 mutant. The mutant appeared to lack sorting information but was prevented from reaching the apical surface when detergent-insoluble membranes were present. Apical sorting of hemagglutinin may require binding of either protein or lipids at the middle of the transmembrane domain and this normally occurs in detergent-insoluble membrane domains. Entry into these domains appears necessary, but not sufficient, for apical sorting.
Keywords: polarized epithelia, apical, sorting, protein traffic, lipid domain
To maintain surface membrane domains that differ in their composition and biological functions, polarized cells, such as epithelial cells and neurons, are able to sort membrane proteins and lipids into separate pathways that transport cargo from the Golgi complex to the plasma membrane. The recognition events that form the basis for sorting membrane components in the biosynthetic pathway are beginning to be understood (Matter and Mellman, 1994; Eaton and Simons, 1995), in particular for sorting transmembrane proteins into the pathway leading to the basolateral surface. A number of small peptide epitopes have been characterized that are capable of specifying transport to the basolateral surface of epithelial cells (Aroeti et al., 1993; Geffen et al; 1993; Prill et al., 1993; Thomas et al., 1993; Hunzicker and Fumey, 1994; Honing and Hunziker, 1995; Reich et al., 1996; Lin et al., 1997; Maisner et al., 1997; Odorizzi and Trowbridge 1997), or to the cell body of neurons (Dotti and Simons, 1990; Dotti et al., 1991; de Hoop et al., 1995; Haass et al., 1995; Le Gall et al., 1997). All of these signals are located in the cytoplasmic domains of transmembrane glycoproteins, but they are diverse enough to suggest that there is more than one class of signal, implying that there are multiple binding sites for basolateral signals in the sorting machinery.
In addition to basolateral signals, three types of signal for sorting proteins to the apical surface of epithelial cells, or to the axon of neurons, are known. Glycolipid anchors direct proteins to the apical surface of several types of epithelial cells (Brown et al., 1989; Lisanti et al., 1989), apparently by associating in the trans-Golgi network (TGN) with detergent-insoluble membrane domains enriched in glycosphingolipids and cholesterol (Simons and Ikonen, 1997). Oligosaccharides on some secreted proteins appear to specify apical transport (Scheiffele et al., 1997), although this mechanism does not apply to all secreted proteins (Ullrich et al., 1991; Ragno et al., 1992; Soole et al., 1992; Gonzalez et al., 1993; Yeaman et al., 1997) and has not been conclusively demonstrated for membrane bound proteins (Green et al., 1981; Geffen et al., 1993; Haller and Alper, 1993; Thomas et al., 1993; Stephens and Compans, 1986; Le Gall et al., 1997). The transmembrane segments of the influenza virus neuraminidase and the simian virus 5 hemagglutinin neuraminidase specify apical transport (Kundu et al., 1996; Huang et al., 1997). Like glycolipid anchors, the transmembrane domains of the influenza virus neuraminindase and hemagglutinin (HA)1 allow those proteins to associate with glycosphingolipid-enriched, detergent-resistant membrane domains (DIGs) (Kundu et al., 1996; Scheiffele 1997). The mechanism by which proteins partition into DIGs is not understood. Some apically sorted proteins are not found associated with DIGs (Tienari et al., 1996), and it is not known whether there is a separate mechanism for sorting proteins into a parallel pathway to the apical surface, or if these proteins associate with DIGs with an affinity too weak to be observed experimentally. Finally, it is clear that transport pathways with biochemical and pharmacological characteristics of the basolateral and apical pathways exist in nonpolarized cells (Skibbens et al., 1989; Musch et al., 1996, Yoshimori et al., 1996). It is possible that all cells maintain a repertoire of membrane transport mechanisms that are adapted to different uses in different cell types.
The influenza virus HA has been used extensively to study membrane traffic in polarized epithelial cells (Rodriguez-Boulan and Powell 1992; Compans, 1995). The feature of HA that is recognized by the cellular sorting machinery is not known, but does not require the HA cytoplasmic domain (Thomas and Roth, 1994) or carbohydrate (Roth et al., 1979; Green et al., 1981). Since the transmembrane segments (TM) of two other viral glycoproteins are sufficient to target reporter sequences to the apical surface of MDCK cells (Kundu et al., 1996; Huang et al., 1997), the most likely location for an apical sorting signal in HA is within the TM. To identify the apical sorting signal in the HA TM, we used site-directed mutagenesis to change blocks of two or four contiguous residues throughout the transmembrane sequence and studied the sorting of the mutant proteins. The pattern of intracellular transport of these mutants suggested that most, if not all HA, travels to the apical surface in DIGs and that entry into DIGs is necessary, but not sufficient, for sorting.
Materials and Methods
Cell Lines Expressing Mutant HAs
Mutations were introduced into HAs by megaprimer mutagenesis (Sarkar and Sommer 1990; Thomas and Roth, 1994). The PCR product was digested with XbaI and BamHI and used to replace an XbaI to BamHI fragment in an expression vector pCB6-HA-Y543 xba− that encoded the 133 carboxyl-terminal amino acids of Japan HA. Each PCR fragment was sequenced to confirm the identity of the mutation and that second-site mutations had not occurred. MDCK subline D5 cells (Brewer and Roth, 1995) were transfected with HA mutants in the expression vector pCB6 (Brewer, 1994) and selected for survival in G418. The uncloned, drug- resistant cell population was used for experiments to avoid any effects from clonal variation.
Folding Assay for HA TM Mutants
MDCK cell monolayers were grown, treated with 10 mM sodium butyrate, and then labeled with 1.14 μCi/ml Trans35Slabel (ICN Biomedicals, Inc., Irvine, CA) as described (Lin et al., 1997). To measure rates of entry to the Golgi complex, monolayers expressing each mutant HA that had been labeled with radioactive amino acids were chased with prewarmed nonradioactive medium at 37°C for either 0, 10, 20, 30, or 60 min. The cells were lysed and immunoprecipitated with rabbit anti-HA antiserum. The immunoprecipitates were analyzed by PAGE and quantified by scanning with a PhosphorImager (Molecular Dynamics, Inc., Sunnyvale, CA). The proportion of each immunoprecipitated protein that had shifted to the slower migrating, Golgi-modified form was measured as a fraction of the total labeled HA in the immunoprecipitate.
To measure rates of arrival at the cell surface, cells were pulse-labeled as described above and chased at 37°C in DME containing 10 μg/ml of trypsin for 0, 30, 60, 120, or 180 min. Trypsin cleaves HA arriving at the cell surface into two disulfide-bonded fragments, HA1 and HA2. The cells were shifted to ice and treated with 100μg/ml of soybean trypsin inhibitor (STI) for 15 min, and then HAs were immunoprecipitated, separated by PAGE, and the amount of HA0, HA1, and HA2 was quantified. For each sample, the percentage of HA at the cell surface was calculated as ([HA1 + HA2]/ total HA) × 100%. For the 4A511 protein, the chase medium did not contain trypsin and arrival at the cell surface was measured by biotinylation at 4°C as previously described (Brewer and Roth, 1991).
Assays for Polarized Transport and Insolubility in Triton X-100
These assays were essentially as previously described (Skibbens et al., 1989; Lin et al., 1997). For assays of polarized transport, confluent MDCK monolayers expressing each mutant HA were grown for 5 d on filter culture inserts. Cells were treated with DME containing 10 mM sodium butyrate for 15 h to increase HA expression and were pulse labeled as described (Lin et al., 1997). For experiments in which cholesterol was depleted from the cells before the chase, cells were grown for 4 d in DME containing 10% fetal bovine serum. 16 h before the chase, cells were treated with DME containing 10% lipoprotein-deficient newborn calf serum and 50 μM of compactin (Hua et al., 1996). 50 μM of compactin was also present in media used for labeling with radioactive amino acids. After pulse labeling, HAs were chased to the cell surface in serum-free DME. For each HA, one sample had trypsin present in the apical chase medium, one had trypsin in the basolateral chase medium, and one had no trypsin. Excess STI was always included in the medium on the side of the filter opposite to that containing trypsin. At the end of the chase, samples were treated with STI and the HAs were immunoprecipitated and analyzed as described for the cell surface assay. The percentage of HA at the apical surface was calculated by dividing the fraction of labeled HA that was cleaved by trypsin at the apical surface by the fraction of labeled HA that was cleaved at both apical and basolateral surfaces (the surface population) × 100%. Each value was corrected for the fraction of HA1 + HA2 in samples chased in the absence of trypsin, which was usually <10%.
For assays of insolubility in Triton X-100, MDCK cells were grown for 2 d on plastic culture dishes. Cells were then treated for 15 h with sodium butyrate, were labeled with 1.14 μCi/ml Trans35Slabel for 15 min at 37°C, and then chased in normal medium for 80 min. Cells were extracted on ice for 30 min in 50 mM Tris-HCl, pH 7.5, containing 150 mM NaCl, 2 mM EDTA, 2 mM DTT, 1× Protein Inhibitor Set (Boehringer Mannheim Biochemicals, Inc., Indianapolis, IN), 100 μg/ml STI, and 1% Triton X-100. Cells were scraped into 1.5-ml microfuge tubes and were centrifuged at 12,000 g for 5 min (model Microfuge R; Beckman Coulter, Fullerton, CA). Samples were separated into supernatants and pellets. Pellets were solubilized with 50 mM Tris-HCl, pH 8.8, containing 5 mM EDTA and 1% SDS and passed several times through a 27-gauge needle. The pellet fraction was diluted with extraction buffer and supernatant fractions with SDS so that the final concentration of SDS in each was 0.17%. HA was recovered from each fraction by immunoprecipitation with anti-HA rabbit antiserum. Immunoprecipitates were analyzed by PAGE and quantified by PhosphorImager analysis.
Results
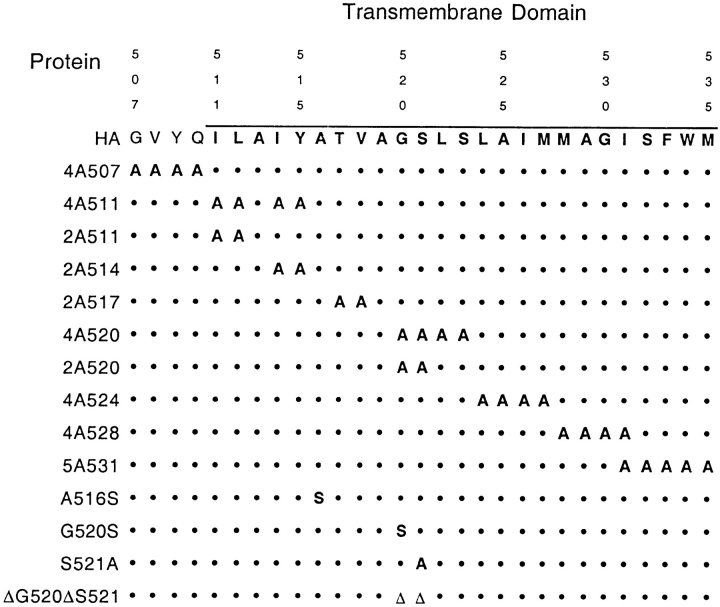
To investigate a possible role for the HA TM in apical transport of HA in MDCK cells, a cDNA for HA was mutated to produce a series of HAs in which blocks of four or five contiguous amino acids were converted to alanines at different positions throughout the entire TM (Fig. 1). In addition, since it has been proposed that interactions between lipid head groups might stabilize the association of lipids in DIGs (Simons and Ikonen, 1997), four amino acids preceding the TM were also changed to alanine. A highly polarized MDCK clonal cell line, D5, used previously by us (Thomas and Roth, 1994; Lin et al., 1997), was transfected with expression plasmids containing the mutant cDNAs and uncloned, polarized MDCK cell populations were selected that stably expressed mutant HAs. Since the HA TM can influence the folding and trimerization of the protein (Lazarovits et al., 1990), and trimerization is required for efficient export of HA from the ER (Copeland et al., 1986; Gething et al., 1986), the rate at which each protein reached the Golgi complex or cell surface was measured by pulse-chase experiments (Table I). Only one of the mutant proteins, HA 2A514, (Fig. 1), was found to be severely defective in transport to the Golgi complex. The other proteins were exported from the ER to the Golgi complex at rates that ranged from 50 to 100% of the rate of the wild-type HA.
Figure 1.
Mutations in the HA TM. Amino acids of the A/Japan H2 HA TM are given in single letter code beginning with the last four residues of the external domain and ending with the residue predicted to be the last of the TM. •, no change from the wild-type sequence; ▵, deletion of the residue at that position.
Table I.
Effect of Mutations in the HA TM on Kinetics of Transport through the Secretory Pathway
| Protein | Percent in Golgi after 30 min | Percent at surface after 60 min | Percent at surface after 180 min | |||
|---|---|---|---|---|---|---|
| HA | 73 | 48 | 87 | |||
| 4A507 | 42 | 47 | 71 | |||
| 4A511 | 70 | 32 | 47 | |||
| 2A511 | 61 | 57 | 89 | |||
| 2A514 | 5 | 10 | 7 | |||
| 2A517 | 44 | 61 | 80 | |||
| 4A520 | 64 | 53 | 70 | |||
| 2A520 | 61 | 47 | 70 | |||
| 4A524 | 37 | 45 | 87 | |||
| 4A528 | 54 | 84 | 90 | |||
| 5A531 | 45 | 84 | 97 | |||
| A516S | 42 | 77 | 97 | |||
| G520S | 68 | 72 | 97 | |||
| S521A | 68 | 71 | 96 | |||
| ΔG520ΔS521 | 65 | 53 | 73 |
Results are shown from a representative pulse-chase experiment on MDCK cells permanently expressing various HA mutants. Arrival at the Golgi was monitored by the appearance of terminally glycosylated HA, which migrates more slowly during PAGE. Arrival at the cell surface was measured as the percentage of HA that was converted into its HA1 and HA2 subunits by trypsin added to the chase medium. An exception was the 4A511 mutant, which was degraded by trypsin. Arrival of this protein to the cell surface was measured by biotinylation.
Properly folded HA is cleaved by extracellular trypsin at a single site, producing mature, biologically active HA composed of disulfide-linked HA1 and HA2 subunits (Wiley and Skehel, 1987). When pulse-labeled mutant HAs were chased in medium containing trypsin, only HA 4A511 was degraded by the trypsin, indicating that it was not properly folded. The other mutant proteins were cleaved into HA1 and HA2 and reached the cell surface as fast or faster than wild-type HA, and to a comparable extent, with the exception of HA 2A514 which was retained in the ER.
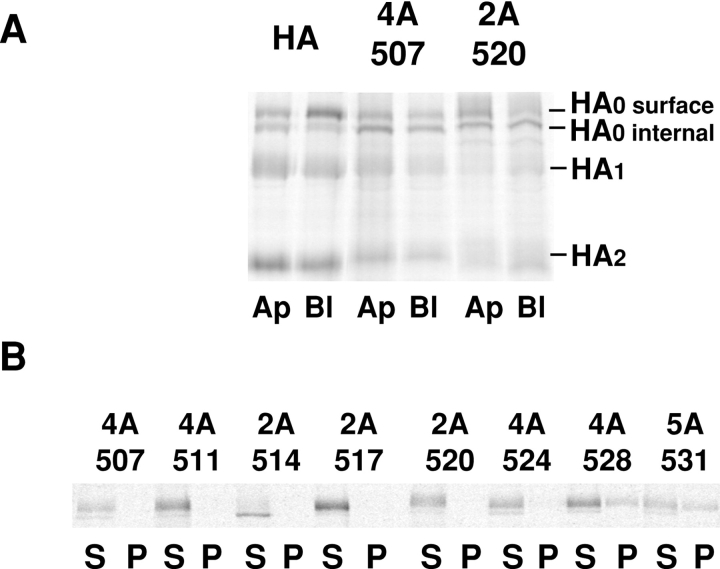
MDCK cells expressing each of the HA mutants or the wild-type HA were grown as confluent monolayers on filter culture inserts, and the ability of the cells to sort HA into the apical pathway was determined by pulse-chase protocols (Matlin and Simons, 1984; Brewer and Roth, 1991). An image of a typical experiment is shown in Fig. 2 A. We estimate from labeling experiments such as this that expression of the various mutant HAs differed by no more than fourfold and in each case was relatively low, at least 50-fold less than in MDCK cells infected with influenza virus (data not shown). No correlation between sorting of HAs and expression level in these cell lines was observed. The results of many experiments measuring delivery of HAs to the apical and basolateral surfaces of MDCK monolayers grown on filter inserts are presented in Table II. Changing the last five amino acids of the HA TM sequence to alanines (5A531) had no effect on delivery of the protein to the apical surface. Changing sequences of four or five amino acids to alanines on the other side of the transmembrane domain, either before (4A507) or immediately after the point where the chain is predicted to enter the outer bilayer (2A511, 4A511) caused the protein to be delivered equally to the apical and basolateral surface. However, mutation of residues predicted to lie near the base of the outer leaflet of the bilayer had a much more dramatic effect. Some of these proteins were preferentially sorted to the basolateral surface. This is seen most clearly with the 2A520 protein, which was sorted more efficiently to the basolateral surface than the wild-type HA was sorted to the apical. Thus, there is a critically important region near the center of the HA TM that is necessary for the protein to enter into apical transport pathways (Table II) and mutation of this region did not affect the folding of the external domain of HA (Table I). Mutation of residues immediately beyond position 520, which would be predicted to lie within the inner leaflet of the bilayer, also prevented apical sorting but were less severe.
Figure 2.
Mutations in the HA TM affect transport to the apical cell surface and solubility in Triton X-100. (A) MDCK monolayers expressing the HA types shown were grown on filter culture inserts for 5 d and then subjected to a pulse-chase protocol in which trypsin was present during the chase in either the apical (Ap) or basolateral (Bl) compartment. After the chase, HAs were immunoprecipitated, separated by PAGE, and then analyzed by a PhosphorImager. A representative image comparing HA and two mutants is shown. (B) MDCK cells expressing the mutants shown were pulse-labeled and chased for 40 min, then the cells were lysed in 1% Triton X-100 on ice. The cell lysate was centrifuged in a microfuge and separated into supernatant (S) and pellet (P) fractions. HA was immunoprecipitated from each fraction and analyzed as in A.
Table II.
Effect of Mutations in the HA Transmembrane Domain on the Delivery of the Protein to the Apical Surface of MDCK Cells
| Protein | Average percent apical | Number of experiments | Percent insoluble in Triton X-100 | Number of experiments | ||||
|---|---|---|---|---|---|---|---|---|
| HA | 73 ± 3 | 3 | 38 ± 9 | 3 | ||||
| 4A507 | 51 ± 2 | 5 | 2, 1 | 2 | ||||
| 4A511 | 46 ± 6 | 3 | 3, 3 | 2 | ||||
| 2A511 | 45 ± 4 | 3 | 3 ± 2 | 3 | ||||
| 2A517 | 61 ± 2 | 4 | 2 ± 1 | 3 | ||||
| 4A520 | 31 ± 6 | 4 | 4, 2 | 2 | ||||
| 2A520 | 19 ± 8 | 5 | 2, 2 | 2 | ||||
| 4A524 | 30 | 2 | 18, 9 | 2 | ||||
| 4A528 | 42 ± 2 | 3 | 26, 18 | 2 | ||||
| 5A531 | 77 ± 8 | 5 | 37 ± 3 | 3 | ||||
| A516S | 50 ± 7 | 4 | 38 ± 3 | 4 | ||||
| G520S | 51 ± 10 | 4 | 25, 22 | 2 | ||||
| S521A | 33 ± 2 | 3 | 3, 3 | 2 | ||||
| ΔG520ΔS521 | 32 ± 7 | 3 | 7, 5 | 2 |
Results are shown from pulse-chase experiments on MDCK cells permanently expressing various HA mutants. Arrival at the apical cell surface was measured by adding trypsin to the chase medium on either the apical or basolateral side of the cell to convert HA into its HA1 and HA2 subunits. The percentage of HA arriving at the apical surface was calculated by dividing fraction of HA cleaved to HA1 and HA2 when trypsin was present at the apical side of the monolayer by the fraction cleaved by trypsin present on both sides of the monolayer. Values are averages from three or more independent experiments and are presented with standard deviations. The fraction of each mutant that was insoluble when cells were lysed in Triton X-100 was determined in parallel experiments. For three or more experiments the average value with standard deviation is presented. Individual values are shown for pairs of experiments.
To investigate the individual contributions for apical sorting of residues G520 and S521 of the A/Japan/305 HA, each was mutated in separate cDNAs. In addition, both residues were deleted from a third cDNA. Mutation of S521 to alanine produced as severe a defect as deleting both G520 and S521, and caused the S521A protein to be delivered predominately to the basolateral surface. The G520S mutation was less severe, and caused essentially random transport.
Scheiffele and colleagues (1997) have shown that the ability of the HA to associate stably with DIGs in fibroblasts is sensitive to changes in the sequences of the portion of the TM that span the outer leaflet of the bilayer. The sequences that would span the inner leaflet were found to be relatively unimportant for this property. We confirmed these observations in MDCK cells expressing the HA TM mutants (Figure 2 B and Table II). Consistent with the hypothesis that association with detergent-insoluble membrane domains is required for proper sorting of HA into an apical transport pathway, we did not observe proper sorting of any mutant protein that was soluble in Triton X-100. However, mutants 5A528, A516S, and G520S were as insoluble in Triton X-100 as was wild-type HA, but were not sorted properly to the apical surface. Thus, association of HA with DIGs appears to be necessary, but not sufficient, for apical sorting.
The observation that the 2A520 mutant was transported predominately to the basolateral surface of MDCK cells could be explained either by exclusion of most of the mutant protein from apical transport pathways, forcing it into the basolateral pathway, or by creation of a positive basolateral signal that allowed active basolateral sorting. There has been no previous evidence for either a cryptic basolateral sorting signal in HA or for the existence of basolateral sorting signals in TM domains. However, there is evidence that efficient basolateral sorting of the Na+/K+ ATPase requires an intact apical pathway containing DIGs, which presumably excludes the Na+/K+ ATPase and forces it into the basolateral pathway (Mays et al., 1995). If basolateral sorting of the 2A520 mutant occurred by a similar mechanism, then treatments that inhibited the formation of DIGs might allow HA 2A520 access into apically directed vesicles, and the protein should be exported randomly. DIGs require cholesterol to form (Schroeder et al., 1994; Scheiffele et al., 1997). Thus, MDCK cells expressing either the HA 2A520 mutant, wild-type HA, or HA+8 D549H, which contains a dominant basolateral signal (Lin et al., 1997), were grown as monolayers and depleted of cholesterol. Under the conditions used, the ability of HA to partition into membranes insoluble in Triton X-100 was decreased by 50%. The delivery of each of these HAs to the plasma membranes of cells depleted in cholesterol was measured (Table III). Depletion of cholesterol had no effect on the apical transport of wild-type HA, indicating that the apical pathway functioned under these conditions. However, twice as much HA 2A520 reached the apical surface in cholesterol-depleted cells as did when cholesterol levels were normal, resulting in randomized transport of HA 2A520. This suggests that the presence of DIGs in cells having normal levels of cholesterol excluded this mutant from apical pathways. Cholesterol depletion had no effect on the basolateral pathway, as shown by continued basolateral transport of HA+8 D549H.
Table III.
Depletion of Cellular Cholesterol Allows Expression of HA 2A520 to Become More Apical but Has No Effect on the Distribution of Wild-Type HA or HA+8 D549H, a Basolaterally Expressed HA Mutant*
| Protein | Cholesterol depletion | Percent apical | Trials | |||
|---|---|---|---|---|---|---|
| Wild-type HA | − | 70 ± 5 | 3 | |||
| Wild-type HA | + | 69 ± 6 | 5 | |||
| HA 2A520 | − | 20 ± 7 | 3 | |||
| HA 2A520 | + | 49 ± 12 | 4 | |||
| HA+8 D549H | − | 14 ± 4 | 3 | |||
| HA+8 D549H | + | 15 ± 6 | 4 |
Cholesterol was depleted from MDCK cell monolayers expressing each of the HA proteins by treatment overnight in cholesterol-free medium containing 50 μM of compactin according to published methods (Hua et al., 1996). Distribution of HAs at the cell surface was measured as described for Table II. Values are presented ± SD.
Discussion
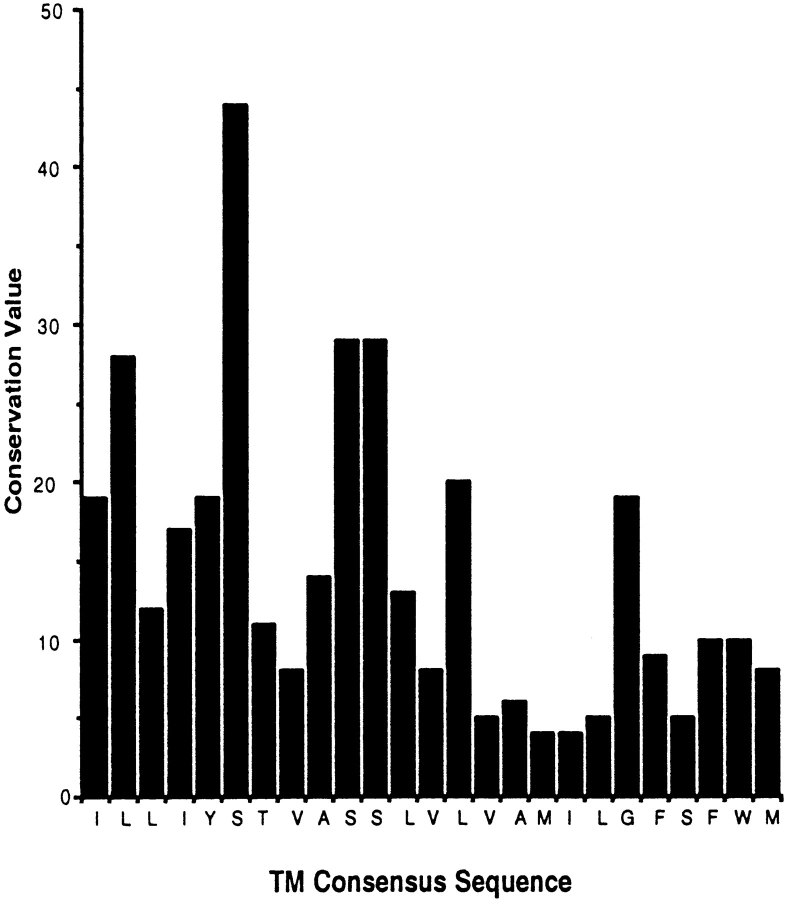
The effects of mutations in the transmembrane domain of the A/Japan HA are summarized in Fig. 3. Residues I514 and Y515 were critical for proper folding of this HA. Although there was some reduction in transport rates observed for mutants with changes in other positions, the folding and trimerization of HA was generally tolerant of mutation of other TM residues to alanine. Mutations in the region of HA predicted to be just outside and within the outer leaflet of the bilayer caused a loss of apical sorting and loss of insolubility in Triton X-100. These residues would be in contact with lipids of DIGs. The effects of the mutations increased as the region mutated approached two TM residues at positions 520 and 521 that are highly conserved among influenza virus HA types (Fig. 4), and decreased as the region mutated approached the portion of the TM that would reside in the inner leaflet of the bilayer. The pattern observed is consistent with HA having an important recognition feature within the membrane that contains S521 as the most important element.
Figure 3.
Effects of changing regions of the HA TM to alanine. The thickness of the bar beneath the HA TM sequence is proportional to the severity of the inhibition of apical transport. *, positions of the sequence most conserved among influenza A and B HAs.
Figure 4.
Conservation of amino acids in HA TMs. Transmembrane sequences from 36 HA proteins selected from all 14 influenza A subtypes and including three type B HAs were compared. For each position in the sequence of the TM, a conservation value was calculated by dividing the percentage of sequences having the most common amino acid by the number of different amino acids found at that position. For each position in the TM sequence, the conservation value and the most common amino acid are displayed. Left, amino terminus of the sequence. Amino acids that would reside in the outer leaflet of the membrane bilayer are more conserved than those predicted to reside within the inner leaflet.
The observations that certain HA mutants not only lost the ability to be sorted, but were actually sorted to the basolateral surface as efficiently as the wild-type HA was sorted apically, could have two independent causes. Either HA contains weak basolateral sorting information that is normally recessive to apical sorting information in the TM, which the mutant lacks, or HA must be able to enter DIGs to gain access to the apical surface, and the mutant cannot enter DIGs. Our data support the latter interpretation. Decreasing cholesterol content in cells to the extent that twofold less HA partitioned into DIGs had no effect on sorting of wild-type HA, or a mutant of HA with a strong basolateral sorting signal. However, transport of the 2A520 mutant became random under the same conditions. These results suggest that the 2A520 mutant had no basolateral sorting information, but in the presence of DIGs was excluded from the apical pathway.
Under the conditions of our experiments, lowering cholesterol levels such that half as much wild-type HA was recovered in a cell fraction insoluble in Triton X-100 did not decrease sorting of HA to the apical surface. Recently, Keller and Simons (1998) reported that depleting cholesterol levels by 60–70% caused missorting of HA to the basolateral surface of MDCK cells infected with influenza virus. However, in our cell lines, HA expression is at least 50-fold lower than in cells infected with influenza virus and it is possible that sufficient apical sorting capacity remained intact under our experimental conditions to accommodate the available HA cargo. Nonetheless, the fact that only half as much wild-type HA was found in the detergent-insoluble fraction but apical sorting was unchanged means that the sorting event is not identical to the partitioning event, as measured by this assay. In cholesterol-depleted cells, either the apical sorting machinery remained with the residual DIGs and was sufficient to sort HAs, or the machinery was able to interact with the apical signal in the TM of HAs outside of DIGs. The later possibility is supported by the observation that mutant A516S was as insoluble in Triton X-100 as was wild-type HA, but was not sorted apically. Thus, entry into detergent insoluble membranes is not sufficient for apical sorting of HA.
A simple explanation for our observations is that apical sorting machinery is normally sequestered in DIGs. We propose that sequences including S521 form the binding site for an integral membrane protein associated with DIGs. This binding event is required for sorting HA to the apical surface. The behavior of the HA TM mutants is consistent with a multistep process for sorting proteins into the apical pathway. In the first step, only proteins capable of entering membrane domains enriched in glycosphingolipids are able to come into contact with apical sorting machinery associated with those domains. However, only those proteins having the ability to bind tightly to elements of the sorting apparatus will be efficiently concentrated within the DIGs and be effectively sorted to the apical surface. The degree to which transmembrane proteins lacking sorting signals would reach the apical surface would depend upon their partitioning coefficient with DIGs, and upon the availability of empty space within the nascent apical transport vesicle. The latter would depend upon the concentration of cargo with apical sorting signals. Basolateral sorting might also involve multiple steps. All evidence suggests that the basolateral sorting machinery resides outside of DIGs. The probability that a protein would be transported basolaterally would depend upon the relative affinity of that protein for the basolateral sorting machinery, its partitioning coefficient with DIGs, and available space within the basolateral vesicles. Our observations with the HA mutant 2A520 indicate that, under certain conditions, the inability to partition into DIGs may be sufficient to force a protein into the basolateral pathway. Perhaps the converse is true, that efficient partitioning into DIGs might keep proteins from entering the basolateral pathway by default. It is possible that proteins with glycosphingolipid membrane anchors are sorted apically in such a manner, without interacting with the machinery that sorts apical transmembrane proteins.
Acknowledgments
We thank R. Rawson and M. Brown (both from University of Texas Southwestern, Dallas, TX) for help with the cholesterol-depletion experiments.
This work was supported in part by a grant from the National Institutes of Health (GM 37547).
Abbreviations used in this paper
- DIG
glycosphingolipid-enriched detergent-resistant membrane domain
- HA
hemagglutinin
- STI
soybean trypsin inhibitor
- TM
transmembrane
Footnotes
Address all correspondence to Michael G. Roth, Department of Biochemistry, University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd., Dallas, TX 75235-9038. Tel.: (214) 648-3276. Fax: (214) 648-8856. E-mail: mroth@biochem.swmed.edu
References
- Aroeti B, Kosen PA, Kuntz ID, Cohen FE, Mostov KE. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J Cell Biol. 1993;123:1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer CB. Cytomegalovirus plasmid vectors for permanent lines of polarized epithelial cells. Methods Cell Biol. 1994;43:233–245. doi: 10.1016/s0091-679x(08)60606-8. [DOI] [PubMed] [Google Scholar]
- Brewer CB, Roth MG. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991;114:413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer CB, Roth MG. Polarized exocytosis in MDCK cells is regulated by phosphorylation. J Cell Sci. 1995;108:789–796. doi: 10.1242/jcs.108.2.789. [DOI] [PubMed] [Google Scholar]
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Compans R. Virus entry and release in polarized epithelial cells. Curr Top Microbiol Immunol. 1995;202:209–219. doi: 10.1007/978-3-642-79657-9_14. [DOI] [PubMed] [Google Scholar]
- Copeland CS, Doms RW, Bolzau EM, Webster RG, Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986;103:1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoop M, von Poser C, Lange C, Ikonen E, Hunziker W, Dotti CG. Intracellular routing of wild-type and mutated polymeric immunoglobulin receptor in hippocampal neurons in culture. J Cell Biol. 1995;130:1447–1459. doi: 10.1083/jcb.130.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Parton RG, Simons K. Polarized sorting of glypiated proteins in hippocampal neurons. Nature. 1991;349:158–161. doi: 10.1038/349158a0. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Eaton S, Simons K. Apical, basal, and lateral cues for epithelial polarization. Cell. 1995;82:5–8. doi: 10.1016/0092-8674(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Geffen I, Fuhrer C, Leitinger B, Weiss M, Huggel K, Griffiths G, Spiess M. Related signals for endocytosis and basolateral sorting of the asialoglycoprotein receptor. J Biol Chem. 1993;268:20772–20777. [PubMed] [Google Scholar]
- Gething MJ, McCammon K, Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986;46:939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Nicovani S, Juica F. Apical secretion of hepatitis B surface antigen from transfected Madin-Darby canine kidney cells. J Biol Chem. 1993;268:6662–6667. [PubMed] [Google Scholar]
- Green RF, Meiss HK, Rodriguez-Boulan E. Glycosylation does not determine segregation of viral envelope proteins in the plasma membrane of epithelial cells. J Cell Biol. 1981;89:230–239. doi: 10.1083/jcb.89.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Koo EH, Capell A, Teplow DB, Selkoe DJ. Polarized sorting of beta-amyloid precursor protein and its proteolytic products in MDCK cells is regulated by two independent signals. J Cell Biol. 1995;128:537–547. doi: 10.1083/jcb.128.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller C, Alper SL. Nonpolarized surface distribution and delivery of human CD7 in polarized MDCK cells. Am J Physiol. 1993;265:C1069–C1079. doi: 10.1152/ajpcell.1993.265.4.C1069. [DOI] [PubMed] [Google Scholar]
- Honing S, Hunziker W. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J Cell Biol. 1995;128:321–332. doi: 10.1083/jcb.128.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Sakai J, Brown MS, Goldstein JL. Regulated cleavage of sterol regulatory element binding proteins requires sequences on both sides of the endoplasmic reticulum membrane. J Biol Chem. 1996;271:10379–10384. doi: 10.1074/jbc.271.17.10379. [DOI] [PubMed] [Google Scholar]
- Huang XF, Compans RW, Chen S, Lamb RA, Arvan P. Polarized apical targeting directed by the signal/anchor region of simian virus 5 hemagglutinin-neuraminidase. J Biol Chem. 1997;272:27598–27604. doi: 10.1074/jbc.272.44.27598. [DOI] [PubMed] [Google Scholar]
- Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO (Eur Mol Biol Organ) J. 1994;13:2963–2967. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu A, Avalos RT, Sanderson CM, Nayak DP. Transmembrane domain of influenza virus neuraminidase, a type II protein, possesses an apical sorting signal in polarized MDCK cells. J Virol. 1996;70:6508–6515. doi: 10.1128/jvi.70.9.6508-6515.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovits J, Shia SP, Ktistakis N, Lee MS, Bird C, Roth MG. The effects of foreign transmembrane domains on the biosynthesis of the influenza virus hemagglutinin. J Biol Chem. 1990;265:4760–4767. [PubMed] [Google Scholar]
- Le Gall AH, Powell SK, Yeaman CA, Rodriguez-Boulan E. The neural cell adhesion molecule expresses a tyrosine-independent basolateral sorting signal. J Biol Chem. 1997;272:4559–4567. doi: 10.1074/jbc.272.7.4559. [DOI] [PubMed] [Google Scholar]
- Lin S, Naim HY, Roth MG. Tyrosine-dependent basolateral sorting signals are distinct from tyrosine-dependent internalization signals. J Biol Chem. 1997;272:26300–26305. doi: 10.1074/jbc.272.42.26300. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Caras IW, Davitz MA, Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989;109:2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisner A, Zimmer G, Liszewski MK, Lublin DM, Atkinson JP, Herrler G. Membrane cofactor protein (CD46) is a basolateral protein that is not endocytosed. Importance of the tetrapeptide FTSL at the carboxyl terminus. J Biol Chem. 1997;272:20793–20799. doi: 10.1074/jbc.272.33.20793. [DOI] [PubMed] [Google Scholar]
- Matlin KS, Simons K. Sorting of an apical plasma membrane glycoprotein occurs before it reaches the cell surface in cultured epithelial cells. J Cell Biol. 1984;99:2131–2139. doi: 10.1083/jcb.99.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Mays RW, Siemers KA, Fritz BA, Lowe AW, van Meer G, Nelson WJ. Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells. J Cell Biol. 1995;130:1105–1115. doi: 10.1083/jcb.130.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch A, Xu HX, Shields D, Rodriguez-Boulan E. Transport of vesicular stomatitis virus G protein to the cell surface is signal mediated in polarized and nonpolarized cells. J Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J Cell Biol. 1997;137:1255–1264. doi: 10.1083/jcb.137.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prill V, Lehmann L, von Figura K, Peters C. The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO (Eur Mol Biol Organ) J. 1993;12:2181–2193. doi: 10.1002/j.1460-2075.1993.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragno P, Estreicher A, Gos A, Wohlwend A, Belin D, Vassalli JD. Polarized secretion of urokinase-type plasminogen activator by epithelial cells. Exp Cell Res. 1992;203:236–243. doi: 10.1016/0014-4827(92)90060-l. [DOI] [PubMed] [Google Scholar]
- Reich V, Mostov K, Aroeti B. The basolateral sorting signal of the polymeric immunoglobulin receptor contains two functional domains. J Cell Sci. 1996;109:2133–2139. doi: 10.1242/jcs.109.8.2133. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Roth MG, Fitzpatrick JP, Compans RW. Polarity of influenza and vesicular stomatitis virus maturation in MDCK. Proc Natl Acad Sci USA. 1979;76:6430–6434. doi: 10.1073/pnas.76.12.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G, Sommer SS. The “megaprimer” method of site-directed mutagenesis. Biotechniques. 1993;8:404–407. [PubMed] [Google Scholar]
- Scheiffele P, Roth MG, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO (Eur Mol Biol Organ) J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-linked proteins: GPI-linked proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci USA. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Skibbens JE, Roth MG, Matlin KS. Differential extractability of influenza virus hemagglutinin during intracellular transport in polarized epithelial cells and nonpolar fibroblasts. J Cell Biol. 1989;108:821–832. doi: 10.1083/jcb.108.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soole KL, Hall J, Jepson MA, Hazlewood GP, Gilbert HJ, Hirst BH. Constitutive secretion of a bacterial enzyme by polarized epithelial cells. J Cell Sci. 1992;102:495–504. doi: 10.1242/jcs.102.3.495. [DOI] [PubMed] [Google Scholar]
- Stephens EB, Compans RW. Nonpolarized expression of a secreted murine leukemia virus glycoprotein in polarized epithelial cells. Cell. 1986;47:1053–1059. doi: 10.1016/0092-8674(86)90820-2. [DOI] [PubMed] [Google Scholar]
- Thomas DC, Brewer CB, Roth MG. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- Thomas DC, Roth MG. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem. 1994;269:15732–15739. [PubMed] [Google Scholar]
- Tienari PJ, De Strooper B, Ikonen E, Simons M, Weidemann A, Czech C, Hartmann T, Ida N, Multhaup G, Masters CL, et al. The beta-amyloid domain is essential for axonal sorting of amyloid precursor protein. EMBO (Eur Mol Biol Organ) J. 1996;15:5218–5229. [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Mann K, Haase W, Koch-Brandt C. Biosynthesis and secretion of an osteopontin-related 20-kDa polypeptide in the Madin-Darby canine kidney cell line. J Biol Chem. 1991;266:3518–3525. [PubMed] [Google Scholar]
- Wiley DC, Skehel JJ. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK Cells. J Cell Biol. 1997;139:929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]