Abstract
Angiotensin II (Ang II) exerts chronic stimulatory actions on tyrosine hydroxylase (TH), dopamine β-hydroxylase (DβH), and the norepinephrine transporter (NET), in part, by influencing the transcription of their genes. These neuromodulatory actions of Ang II involve Ras-Raf-MAP kinase signal transduction pathways (Lu, D., H. Yang, and M.K. Raizada. 1997. J. Cell Biol. 135:1609–1617). In this study, we present evidence to demonstrate participation of another signaling pathway in these neuronal actions of Ang II. It involves activation of protein kinase C (PKC)β subtype and phosphorylation and redistribution of myristoylated alanine-rich C kinase substrate (MARCKS) in neurites. Ang II caused a dramatic redistribution of MARCKS from neuronal varicosities to neurites. This was accompanied by a time-dependent stimulation of its phosphorylation, that was mediated by the angiotensin type 1 receptor subtype (AT1). Incubation of neurons with PKCβ subtype specific antisense oligonucleotide (AON) significantly attenuated both redistribution and phosphorylation of MARCKS. Furthermore, depletion of MARCKS by MARCKS-AON treatment of neurons resulted in a significant decrease in Ang II–stimulated accumulation of TH and DβH immunoreactivities and [3H]NE uptake activity in synaptosomes. In contrast, mRNA levels of TH, DβH, and NET were not influenced by MARKS-AON treatment. MARCKS pep148–165, which contains PKC phosphorylation sites, inhibited Ang II stimulation of MARCKS phosphorylation and reduced the amount of TH, DβH, and [3H]NE uptake in neuronal synaptosomes. These observations demonstrate that phosphorylation of MARCKS by PKCβ and its redistribution from varicosities to neurites is important in Ang II–induced synaptic accumulation of TH, DβH, and NE. They suggest that a coordinated stimulation of transcription of TH, DβH, and NET, mediated by Ras-Raf-MAP kinase followed by their transport mediated by PKCβ-MARCKS pathway are key in persistent stimulation of Ang II's neuromodulatory actions.
Keywords: MARCKS, brain neurons, AT1 receptors, neuromodulation, protein kinase C subtypes
Evidence has been accumulating that angiotensin II (Ang II)1 exerts diverse physiological actions in both peripheral and neural tissues. In the periphery, its actions include vasoconstriction, hypertrophy, cell multiplication, and tissue remodeling, whereas the central nervous system (CNS) actions of Ang II include neuroendocrine hormone secretion, regulation of sympathetic activation and dampening of baroreceptor function (Saavedra, 1992; Steckeling et al., 1992; Dzau, 1993; Timmermans et al., 1993; Naftilan, 1994; Raizada et al., 1994; Wright and Harding, 1994). Sympathetic activation of the CNS neurons by Ang II is associated with the stimulation of turnover, synthesis, and release of catecholamines (Steckeling et al., 1992; Gelband et al., 1998). All of these diverse physiological effects of Ang II are mediated by its interaction with the angiotensin type 1 receptor subtype (AT1 receptor), and that such diversity may stem from coupling of the receptor to various signal transduction pathways. For example, the vascular smooth muscle cell AT1 receptor is linked to the JAK-STAT signaling system that mediates Ang II's effects on DNA replication, hypertrophy, and extracellular matrix; whereas neuronal AT1 receptor is coupled to the Ras-Raf-MAP kinase signaling pathway, which regulates enhanced neuromodulatory actions of Ang II including stimulation of tyrosine hydroxylase (TH), dopamine β-hydroxylase (DβH), and norepinephrine transporter (NET) (Marrero et al., 1995; Lu et al., 1996, 1997; Yang et al., 1996; Yu et al., 1996; Gelband et al., 1998).
In addition to its coupling with JAK-STAT and Ras-Raf-MAP kinase pathways, the AT1 receptor is also coupled to the phospholipase C–phosphoinositide–protein kinase C (PKC) signaling system (Sumners and Raizada, 1993; Freeman et al., 1995). Studies have established that AT1 receptor stimulation increases inositol phosphate (IP) formation, stimulates PKC, and mobilizes Ca2+ from IP3-sensitive pools in both brain neurons and peripheral cells (Sumners and Raizada, 1993; Freeman et al., 1995). This pathway is particularly important in neurons because both Ca2+ mobilization and PKC activation are involved in the synthesis, release, and re-uptake of many neurotransmitters (Zhu and Ikeda, 1994; Patel et al., 1995). Since Ang II regulates neurotransmitter synthesis and release, it was of great importance for us to further delineate the mechanism of PKC involvement in Ang II–induced neuromodulation. In this study we focused our attention on myristoylated alanine-rich C-kinase substrate (MARCKS) since it is a major PKC substrate, exists in high concentrations in neurons, and has been implicated in cytoskeletal rearrangement, membrane trafficking, and neurotransmitter release (Wang et al., 1989; Aderem, 1995; Blackshear, 1993; Manenti et al., 1994). In spite of well-defined roles of MARCKS in cell motility and membrane trafficking, little is known about its involvement in neurotransmitter synthesis and release, including its role in enhanced neuromodulation. In addition, the role of MARCKS in any G protein–coupled receptor's signal transduction propagation is even less understood. In view of these gaps in our understanding, coupled with our observation that AT1 receptors stimulate PKC and neuromodulation, the objective in this investigation was to test the following hypothesis: Ang II interaction with the neuronal AT1 receptor stimulates phosphorylation of MARCKS by PKC. Phosphorylated MARCKS redistributes itself in a way to facilitate the transport of TH, DβH, and NET along the neurites to synaptic vesicles where they participate in increased synthesis, release, and re-uptake of norepinephrine (NE). Observations presented in this study provide evidence in support of this hypothesis.
Materials and Methods
1-d-old Wistar Kyoto (WKY) rats were obtained from our breeding colony, which originated from Harlan Sprague-Dawley (Indianapolis, IN). DME, plasma-derived horse serum (PDHS), and 1× crystal trypsin were from Central Biomedia (Irwin, MO). Phosphate-free DME was purchased from Life Technologies (Grand Island, NY). [32P]Orthophosphate (1 mCi = 37 MBq), [γ-32P]ATP (3,000 Ci/mmol), dl[3H]NE (10.4 Ci/mmol), and chemiluminescence assay reagents were from Dupont/NEN (Boston, MA). Nitrocellulose membrane was from Micron Separations, Inc. (Westboro, MA). Ang II and mAb to synaptophysin were purchased from Sigma Chemical Co. (St. Louis, MO). Polyclonal antibodies to TH and DβH were obtained from Chemicon (Temecula, CA). Rabbit polyclonal antibodies to various isoforms of PKC (α, β, γ) and agarose-conjugated protein A/G were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Specificity of these antibodies to respective PKC subtypes is well established by both immunocytochemical staining and by immunoblotting (Asotra and Macklin, 1994; Kim et al., 1994; Sylvia et al., 1996). Anti–rabbit and anti–mouse Fab fragments conjugated with rhodamine or fluorescein were from Boehringer Mannheim, Co. (Indianapolis, IN). All other reagents were purchased from Fisher Scientific (Pittsburgh, PA), and were the highest quality available.
Sense and antisense oligonucleotides to MARCKS and PKCα, PKCβ, and PKCγ were synthesized in the DNA synthesis facility of the Interdisciplinary Center for Biotechnology Research, University of Florida (Gainesville, FL). These sequences have been used successfully to downregulate MARCKS and PKC by other groups (Aigner and Caroni, 1993; Balboa et al., 1994). The sequences are listed as follows:
MARCKS: Sense: 5′-AAGAAGCCAGCATGGGTGCACAGTT-3′
Antisense: 5′-AACTGTGCACCCATGCTGGCTTCTT-3′
PKCα: Sense: 5′-GAACCATGGCTGACGTTTACC-3′
Antisense: 5′-GGTAAACGTCAGCCATGGTTC-3′
PKCβ: Sense: 5′-AAGATGGCTGACCCGGCTCGC-3′
Antisense: 5′-CGCAGCCGGGTGACCCGGCCGC-3′
PKCγ: Sense: 5′-CAAGATGGCTGACCCGGCCGC-3′
Antisense: 5′-GCGGCCGGGTCAGCCATCTTG-3′
A synthetic peptide corresponding to amino acids 148–165 of MARCKS (KRFSFKKSFKLSGFSFKK; pep148–165) and its mutant counterpart in which serines at positions 151, 155, 159, and 162, were replaced by alanine (KRFAFKKAFKLAGFAFKK; mut148–165) were synthesized by Genemed Biotechnologies (San Francisco, CA). Pep148–165 competes for PKC-mediated phosphorylation of MARCKS since three out of the four serines (151, 155, and 162) in this molecule are known PKC phosphorylation sites (Amess et al., 1992). In contrast, mut148–165 would not be a competitor, and thus serves as control for pep148–165.
Preparation of Neuronal Cultures
Hypothalamus–brainstem areas of 1-d-old WKY rat brains were dissected and brain cells were dissociated by trypsin as described previously (Raizada et al., 1984, 1993). Dissociated brain cells were plated in poly- l-lysine–precoated tissue culture dishes (2 × 107 cells/100-mm-diam dish or 3 × 106 cells/35-mm-diam dish) in DME containing 10% PDHS and neuronal culture established as previously described (Raizada et al., 1984, 1993). The cultures were allowed to grow for 15 d before their use in experiments. These cultures contain 85–90% neuronal cells and 10–15% astroglial cells (Raizada et al., 1984, 1993).
Determination of TH, DβH Immunoreactivities, and Specific [3H]NE Uptake in Synaptosomal Preparations of Neuronal Cells
Neuronal cells, established in 100-mm-diam tissue culture dishes, were subjected to various pretreatments followed by incubation with 100 nM Ang II for indicated time periods. Cells from 10 culture dishes were collected, cell pellets homogenized in 0.32 M sucrose, and then homogenates were used for synaptosomal preparation essentially as described elsewhere for neuronal cultures (Kishi et al., 1991). Purity of the synaptosomal fraction was established with the use of synaptophysin antibody as marker (Kishi et al., 1991). Synaptosomal preparations containing 100 μg protein were subjected to 4–15% SDS-PAGE, separated proteins were transferred to nitrocellulose membrane, and then blotted with the use of 1 μg/ml anti-TH or anti-DβH antibodies essentially as described previously (Lu et al., 1997). Antibodies bound to TH or DβH were identified by HRP-labeled anti–rabbit antibody and visualized by chemiluminescence as described previously (Yang et al., 1996; Lu et al., 1997). Bands corresponding to TH and DβH immunoreactivities were quantitated by SW5000 Gel Analyzer after ascertaining that densities of each immunoreactive band was within the linear range as described previously (Lu et al., 1996, 1997; Yang et al., 1996).
Specific [3H]NE uptake was measured by incubating synaptosomal preparations containing 1 mg protein with 1 nM [3H]NE (2 μCi) in the absence or presence of 1 μM maprotiline essentially as described previously (Lu et al., 1996). Specific uptake was calculated by subtracting the total [3H]NE uptake from that in the presence of maprotiline.
Western Blotting of PKCα, β, and γ Subtypes and MARCKS
Western blotting was used to identify and quantitate the PKCα, PKCβ, PKCγ, and MARCKS proteins essentially as described previously (Yang et al., 1996). Briefly, cell-free lysates were prepared and electrophoresed on 10% SDS-PAGE and then proteins were transferred to nitrocellulose membranes. Membranes were treated with 5% nonfat dry milk in TBST (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.05% Tween 20) for 1 h, followed by incubation with rabbit anti–PKC subtype antibodies (1 μg/ml) or rabbit anti-MARCKS antibody (1 μg/ml), and then incubated with HRP-labeled anti–rabbit antibody, and enhanced chemiluminescence assay reagents. Densities in each band was quantitated by SW5000 Gel Analyzer (Lu et al., 1996, 1997).
Immunofluorescent Staining of Neurons for MARCKS
Neuronal cells, established in 35-mm-diam culture dishes, were fixed in methanol at −10°C, and then incubated with rabbit anti-MARCKS antibody (1:200 diluted antiserum) in PBS containing 0.5% BSA overnight at 4°C. Specificity of this antibody has been previously established (Watson and Lenox, 1996). After removal of primary antibody, cells were incubated with FITC-conjugated anti–rabbit IgG (1 μg/ml), and then processed for confocal microscopy (Yang et al., 1996; Lu et al., 1997). For colocalization of MARCKS with synaptophysin, cells were first stained with anti-MARCKS antibody followed by incubation with mAb to synaptophysin (1 μg/ml in PBS) for 1 h at 37°C. Cells were stained with rhodamine-conjugated anti–mouse IgG (1 μg/ml) and subjected to confocal microscopy (Yang et al., 1996; Lu et al., 1997).
Labeling of Neurons with [32P]Orthophosphate and Analysis of Phosphorylated MARCKS
Neuronal cells, established in 100-mm-diam culture dishes, were rinsed once with phosphate-free DME, followed by incubation in the phosphate-free DME containing 10% dialyzed PDHS for 4 h at 37°C. [32P]Orthophosphate (1 mCi/ml) was added and incubation was continued for an additional 20 h. After stimulation with 100 nM Ang II for desired time period, cultures were rinsed free of [32P]orthophosphate, cells were lysed in lysis buffer (25 mM Tris-HCl, pH 7.4, 25 mM NaCl, 1% Triton X-100, 1% deoxycholic acid, 1% SDS, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 10 mM sodium pyrophosphate, 0.5 mM EGTA, 1 mM PMSF, 10 μg/ml aprotinin, and 0.8 μg/ml leupeptin), and then lysates were subjected to an immunoprecipitation protocol to separate 32P-labeled MARCKS (Lu et al., 1997). Briefly, cell lysates were mixed with 1 μg/ml anti-MARCKS antiserum at 4°C and incubated overnight with gentle shaking (Watson and Lenox, 1996). This was followed by incubation with 10 μl agarose-conjugated anti–rabbit IgG for 4 h at 4°C. Immunoprecipitates were collected by centrifugation, washed six times with the lysis buffer, suspended in Laemmli's buffer, electrophoresed in 4–15% SDS-PAGE and subjected to autoradiography to detect 32P-labeled bands representing phosphorylated MARCKS (Yang et al., 1996; Lu et al., 1997).
Treatment of Neuronal Cells with Antisense Oligonucleotides or Sense Oligonucleotides to PKC Subtypes or MARCKS
Neuronal cultures, established in 35-mm-diam tissue culture dishes, were incubated with 2.5 μM antisense oligonucleotides (AON) or sense oligonucleotides (SON) to PKC subtypes (α, β, or γ) or MARCKS for various time periods as described elsewhere (Aigner and Caroni, 1993; Balboa et al., 1994; Yang et al., 1996). Cells were lysed, 50 μg protein from cell lysates were subjected to 4–15% SDS-PAGE, followed by Western blotting to quantitate PKCα, β, and γ subtypes or MARCKS as described above.
Osmotic Loading of Neurons with pep148–165 and mut148–165
Osmotic loading of neurons with pep148–165 or mut148–165 was carried out essentially as described previously by Ahmad et al. (1995). This method has recently been adapted by us for neuronal cell culture (Lu et al., 1998). In brief, neuronal cells were rinsed with PBS, pH 7.4, and incubated for 10 min with a loading solution (0.5 M sucrose, 10% polyethylene glycol 1000, 10% FBS, and 200 μg/ml pep148–165 or mut148–165 in DME buffered with 25 mM Hepes, pH 6.8). Cultures were rapidly rinsed with a hypotonic solution (6.5 vol H20: 3.5 vol DME, buffered with 25 mM Hepes, pH 6.8) (Reddy et al., 1991; Ahmad et al., 1995), incubated with DME containing 10% PDHS, and were subjected to the experimental protocol for the measurement of Ang II stimulation of synaptosomal TH, DβH, and [3H]NE uptake as described above.
Experimental Groups and Data Analysis
Each data point represented triplicate culture dishes and cells in these dishes were derived from multiple brains of 1-d-old WKY rats. Each experiment was replicated three times unless indicated otherwise. Densities of radioactive bands representing phosphorylated MARCKS or bands on immunoblots representing MARCKS, PKC subtypes, TH, or DβH were quantitated by SW5000 Gel Analyzer as described elsewhere (Yang et al., 1996; Lu et al., 1997). Observed densities (OD) of these bands were such that they were all in the linear range as previously established by calibrating protein concentrations with ODs. Data were normalized for equal loading with the use of equal amounts of proteins in each sample, and were presented as mean absorbance of at least three experiments' ± SE. Comparison between experimental data were made using one-way analysis of variance (ANOVA) and Dunnett's test with Statistica software (Statistica, Inc., Orlando, FL). [3H]NE uptake was done in triplicate dishes and data were presented as specific [3H]NE uptake and were mean ± SE of triplicate determinations.
Results
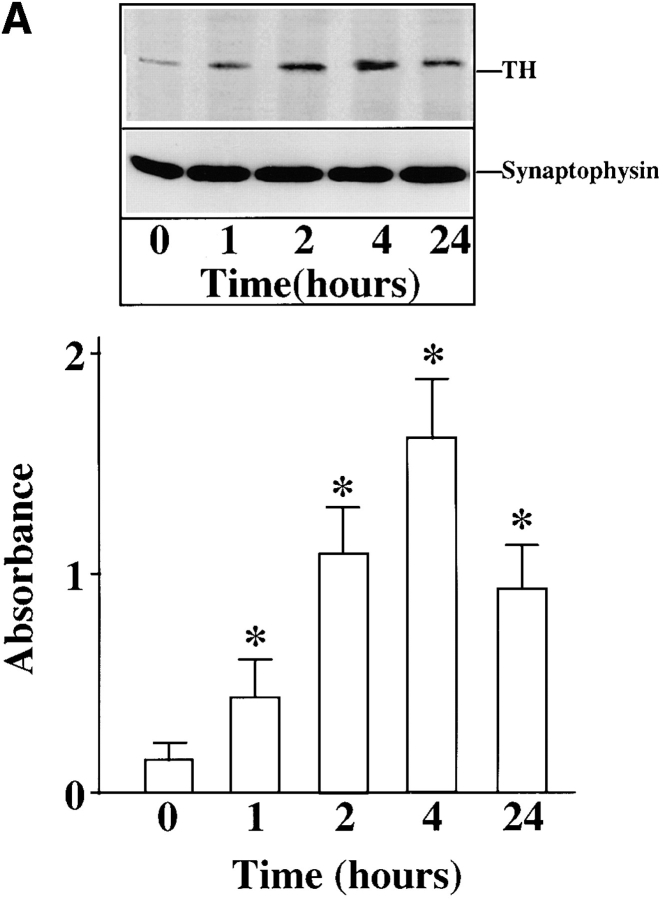
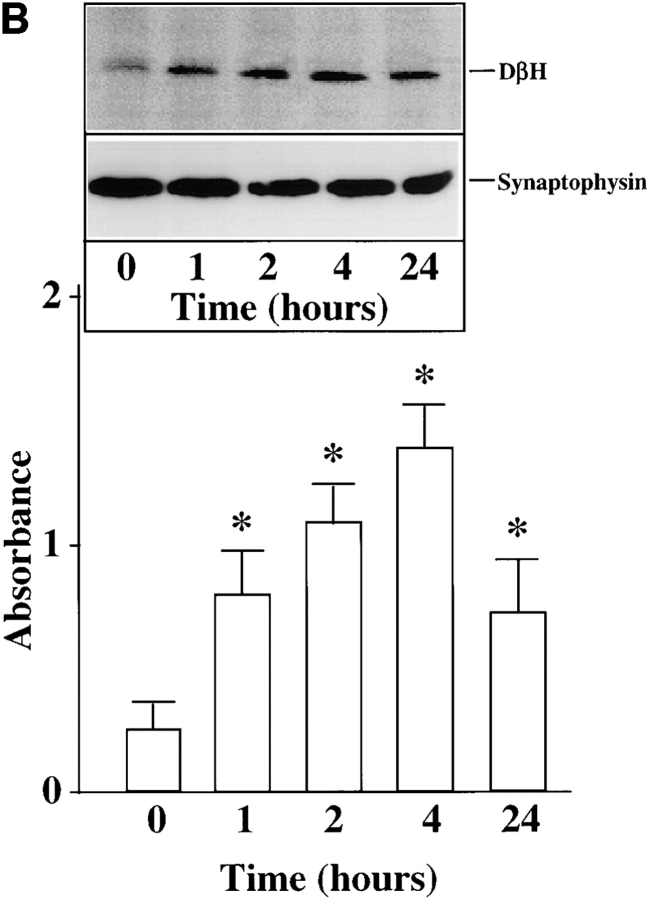
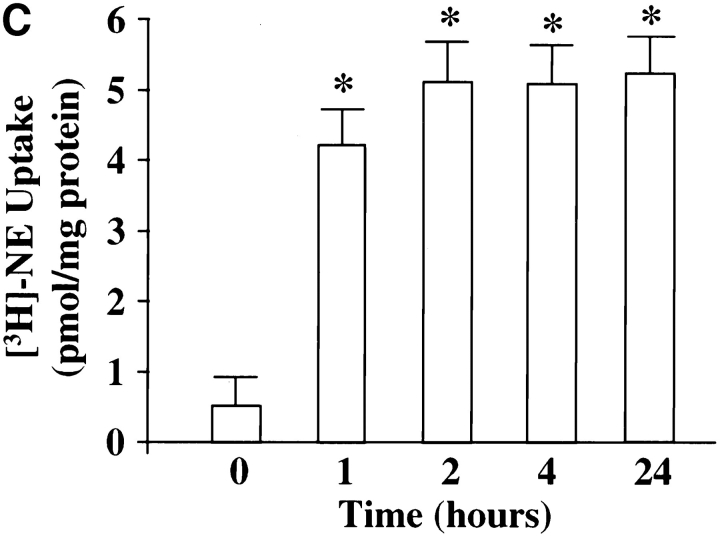
Chronic stimulation of NE neuromodulation by Ang II is mediated by activation of the neuronal AT1 receptor and is associated with increased expression of genes for TH, DβH, and NET in neuronal cultures (Lu et al., 1996; Yu et al., 1996; Gelband et al., 1998). These observations have led us to hypothesize that stimulation of NE synthesis, release, and uptake by Ang II is dependent upon the transcription of TH, DβH, and NET genes followed by transport of these activities to the synaptic terminals. Our first aim in this study was to determine if the transport process itself was influenced by Ang II. Synaptosomal preparations showed low but significant basal immunoreactive TH and DβH (Fig. 1, A and B). Similarly, [3H]NE uptake, a measure of NET activity, was also low (Fig. 1 C). Treatment with 100 nM Ang II resulted in a time-dependent increase in immunoreactive TH, DβH, and [3H]NE uptake by synaptosomes. Maximal stimulation of 8.8-, 7.4-, and 7.9-fold in TH, DβH, and [3H]NE uptake, respectively, was observed in 4 h, and stimulated levels were still evident 24 h after Ang II treatment. No significant change in the levels of immunoreactive synaptophysin was observed to demonstrate the specificity of Ang II's actions. The levels of TH and DβH immunoreactivities were compared in the whole cell homogenates and synaptosomal preparations of Ang II–treated neurons to further confirm the effects of Ang II on the transport process. Fig. 1, D and E show that Ang II caused a 2.7- and 3.9-fold increase in TH levels in whole cells and synaptosomes, respectively. Similarly, a three- and fivefold increase in DβH levels were seen in whole cells and synaptosomes. Comparison of data indicated that the level of TH and DβH stimulation was 30– 40% higher in synaptosomes compared with the whole cells.
Figure 1.
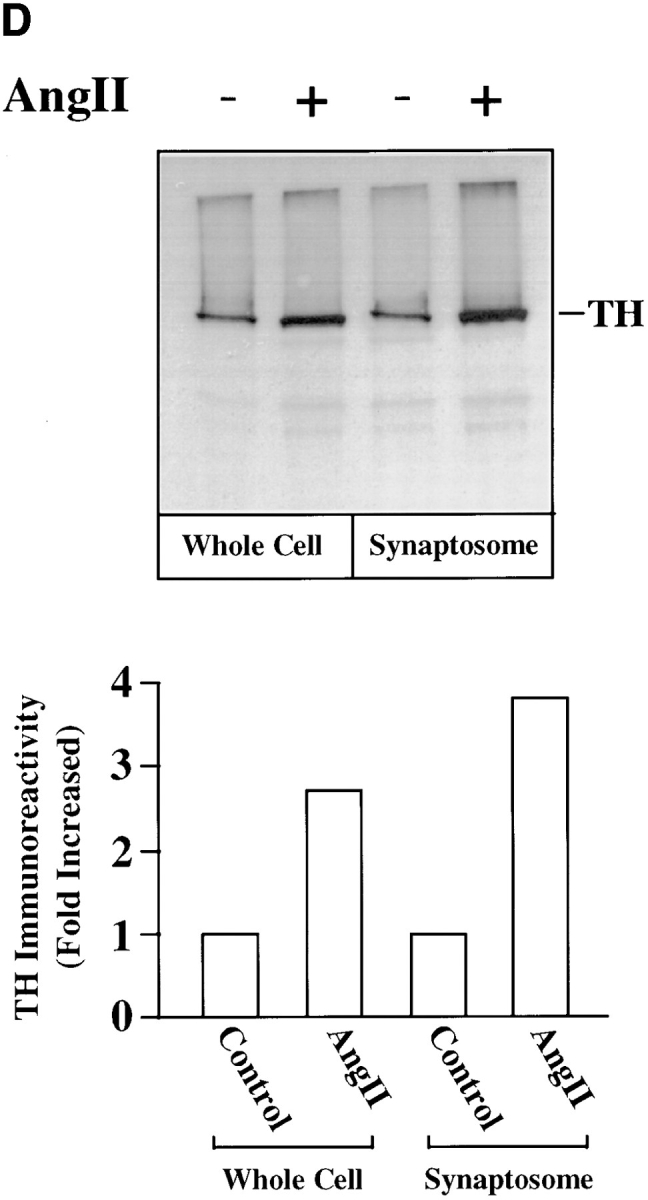
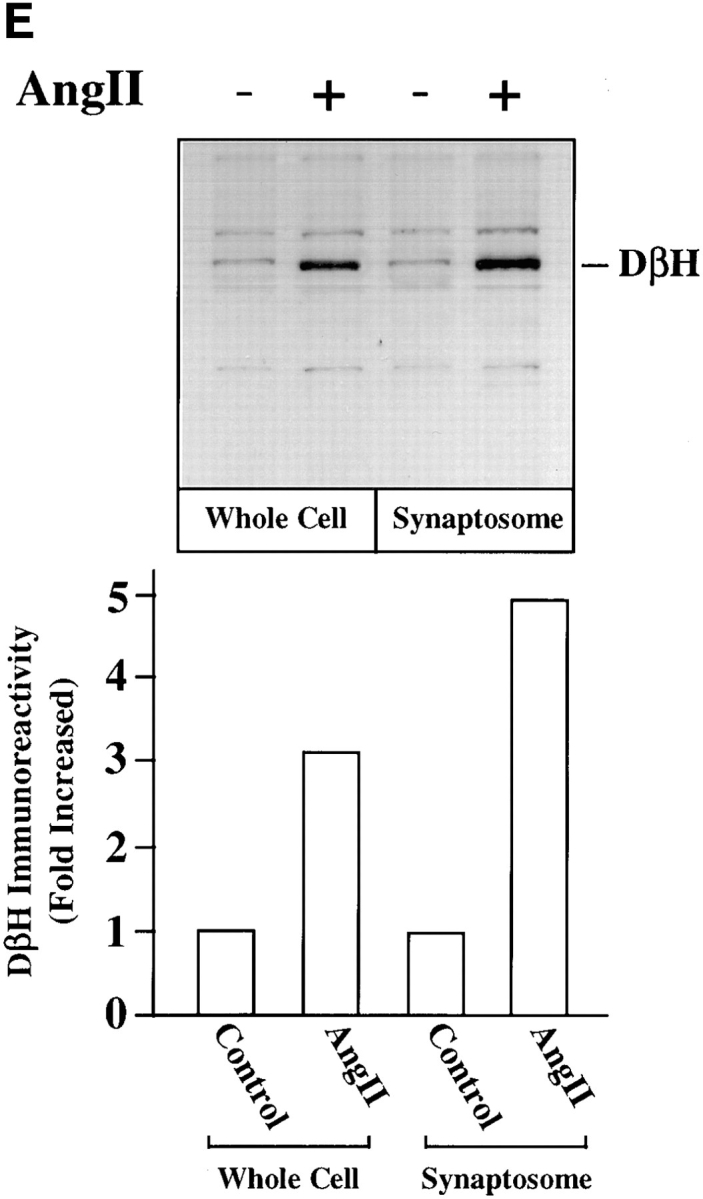
Effects of Ang II on TH, DβH immunoreactivities, and [3H]NE uptake in synaptosomes and whole cells of brain neurons. (A–C) Neuronal cultures were incubated with 100 nM Ang II for indicated time periods, synaptosomes were prepared and subjected to quantitation of TH (A) and DβH (B) immunoreactivities, and [3H]NE uptake (C) activities essentially as described in Materials and Methods. A comparable amount of proteins in each sample were also electrophoresed and subjected to Western blotting with the use of antibodies to synaptophysin. Top in A and B are representative autoradiograms. Bottom in A and B are mean data from three experiments mean ± SE. Data in C are mean ± SE (n = 3). *, significantly different (P < 0.01) from zero time. (D and E) TH and DβH levels in whole cells and synaptosomes. After treatment with Ang II for 4 h essentially as described above, whole neuronal cells and synaptosomal preparation from them were subjected to TH (D) and DβH (E) Western blotting. Equal amounts of proteins (20 μg) were used for electrophoresis. Top in D and E are representative autodiagrams. Bottom, mean data from two experiments.
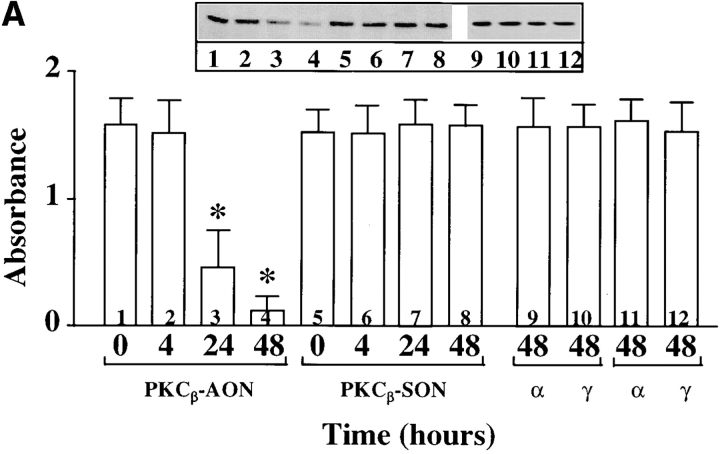
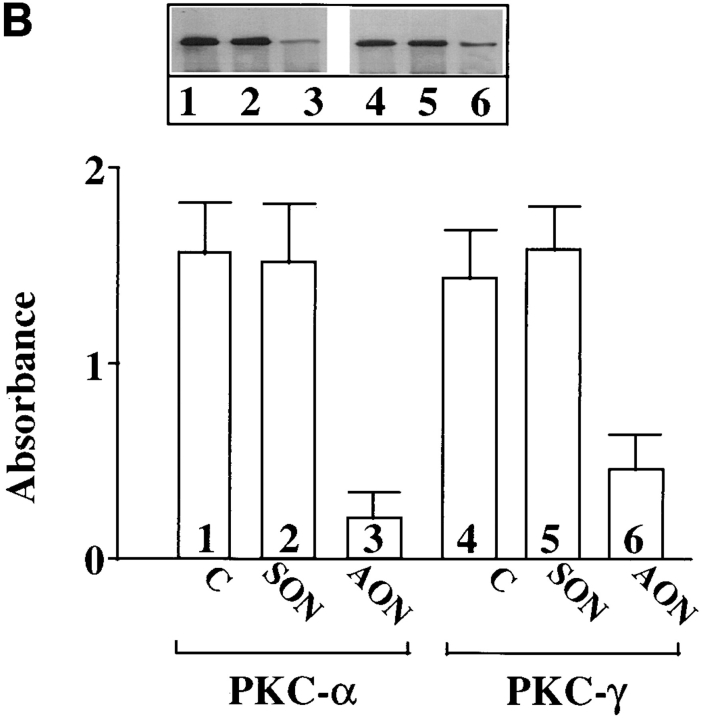
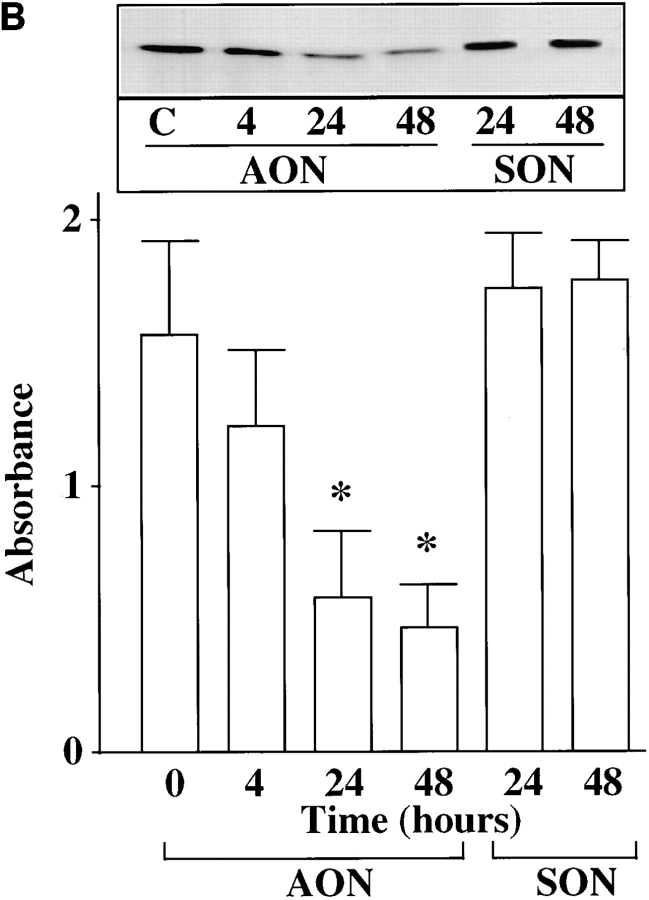
Ang II interacts with the AT1 receptor subtype to regulate NE neuromodulatory effects (Gelband et al., 1998). In view of the observations that the AT1 receptor belongs to G protein–coupled receptor superfamily that is coupled to PLC-PKC signaling pathway, and that Ang II stimulates PKC in neurons in a calcium-dependent manner (Sumners and Raizada, 1993; Sumners et al., 1995; Gelband et al., 1998), our next aim was to determine which calcium- dependent PKC subtype was involved in Ang II stimulation of TH, DβH transport, and NE uptake. Fig. 2 shows that preincubation of neurons with AON to PKCβ caused a time-dependent decrease in PKCβ immunoreactivity. Maximal decrease of 86% was observed with 2.5 μM PKCβ AON in 48 h. In contrast, PKCβ SON showed no such decrease in PKCβ immunoreactivity during the same time period; also in contrast, both PKCβ AON and SON failed to influence immunoreactive levels of PKCα and PKCγ. However, AON to PKCα and PKCγ caused a maximum of 73% decrease in their respective PKC subtype immunoreactivities in 48 h (Fig. 2 B). Depletion of PKCβ by its AON was associated with a significant attenuation of Ang II–induced increase in TH and DβH immunoreactivities and [3H]NE uptake in synaptosomes (Fig. 3, A–C). This attenuation was specific since PKCβ SON or PKCα and PKCγ AONs showed no significant effects on Ang II stimulation of TH, DβH, and NE uptake. An example of the lack of effect of PKCα and PKCγ AONs on TH immunoreactivity is shown in Fig. 4.
Figure 2.
(A) Effect of PKCβ AON on PKCα, PKCβ, and PKCγ immunoreactivities in brain neurons. Neuronal cultures were incubated with 2.5 μM PKCβ AON or SON for indicated time periods. Cells were lysed and lysates were used for Western blot to determine levels of PKCβ, PKCα, and PKCγ as described in the Materials and Methods. Top, a representative autoradiogram. Bottom, data from three experiments mean ± SE. *, significantly different from control (P < 0.05). (B) Effects of PKCα and PKCγ AON and SON on PKCα and PKCγ immunoreactivities in brain neurons. Neuronal cultures were treated with 2.5 μM PKCα or PKCγ AON or SON for 48 h at 37°C essentially as described for PKCβ above. Levels of PKCα and PKCγ was determined by Western blot. Top, representative autoradiogram. Bottom, mean ± SE (n = 3).
Figure 3.
Effect of PKCβ AON and SON on Ang II stimulation of transport of TH, DβH, and [3H]NE uptake activities in the synaptosomes of brain neurons. Neuronal cultures were pretreated with 2.5 μM PKCβ AON or SON for 48 h, essentially as described in Fig. 2. This was followed by incubation of cells with 100 nM Ang II for 4 h. Synaptosomes were prepared and TH (A), DβH (B), and [3H]NE (C) uptake activities were determined as described in Materials and Methods. Top in A and B show representative autoradiograms. Bottom, mean data from three experiments ± SE. Data in C are mean ± SE (n = 3). *, significantly different (P < 0.05) from control. **, significantly different (P < 0.01) from Ang II–treated neurons.
Figure 4.
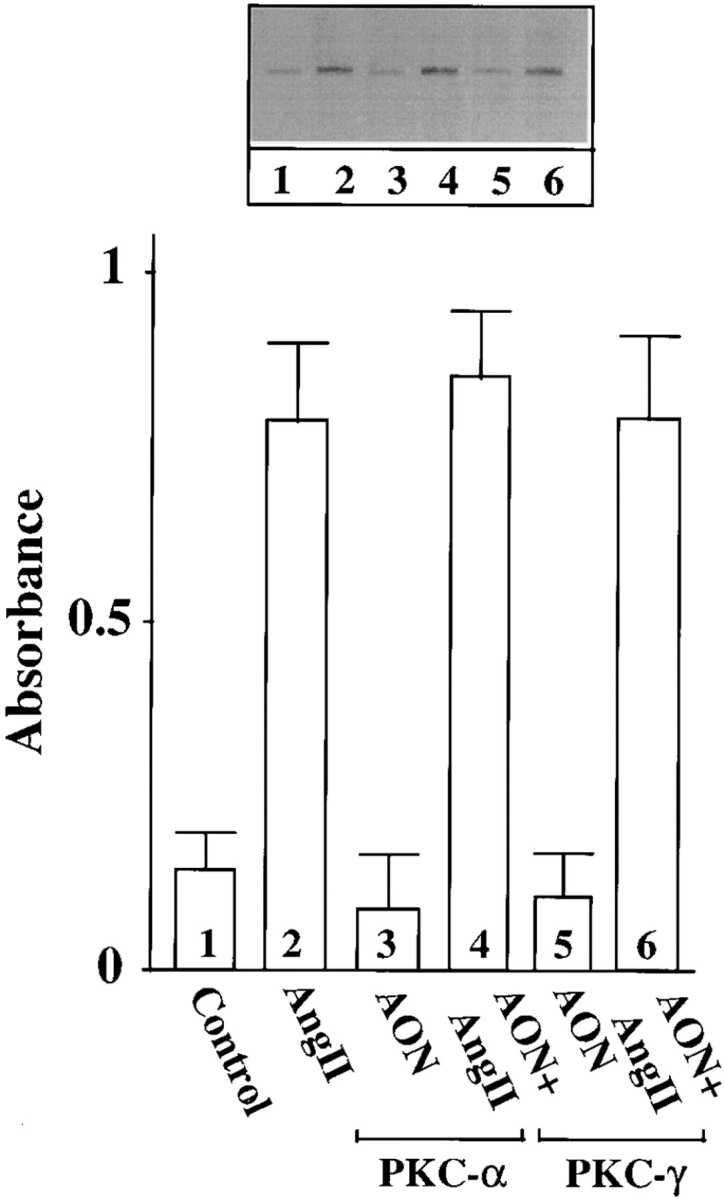
Effects of PKCα and PKCg AONs on Ang II stimulation of the transport of TH in the synaptosomes of brain neurons. Neuronal cultures were pretreated with 2.5 μM PKCα AON or PKCγ AON for 48 h at 37°C. This was followed by incubation of cells with 100 nM Ang II for 4 h. Synaptosomes were prepared and TH immunoreactivity was determined essentially as described in the Materials and Methods. Top, representative autoradiogram. Bottom, mean of two independent experiments.
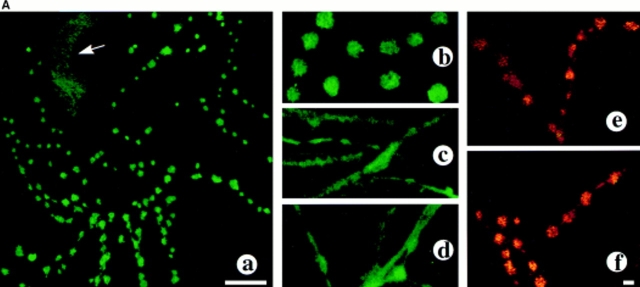
Next, we studied the involvement of MARCKS in Ang II stimulation of NE neuromodulation. The rationale was based on the evidence that MARCKS is a substrate for PKC, is present in high concentrations in neurons, and is proposed to be involved in regulation of neurotransmitter release (Zhu and Ikeda, 1994; Patel et al., 1995). Fig. 5 A shows representative images of immunocytochemical distribution of MARCKS and its colocalization with TH and synaptophysin in the neurons. MARCKS immunoreactivity was predominately localized in vesicular structures of neurites with little distribution in the cell soma (Fig. 5 A, a and b). This distribution overlapped with the distribution of immunoreactive synaptophysin in the neurites. Treatment with Ang II resulted in a dramatic redistribution of MARCKS (Fig. 5 A, c and d). MARCKS immunoreactivity began to diffuse out of the varicosities and within 30 min it was completely diffused throughout the neurites.
Figure 5.
Effects of Ang II on neuronal MARCKS. (A) Effect of Ang II on immunocytochemical localization of MARCKS. Neuronal cultures were treated without (a and b) or with 100 nM Ang II for 0.5 h (c) and 4 h (d–f) followed by immunocytochemical analysis of MARCKS immunoreactivity (a–d). Samples in e and f were double stained with the use of antibodies to MARCKS and synaptophysin (e) or MARCKS and TH (f) essentially as described previously (Lu et al., 1997). Confocal microscopy was carried out as described in Methods. Green color represents MARCKS and TH staining while yellow color presents co-staining of MARCKS with either synaptophysins (e) or with TH (f). a, depicts beaded localization of MARCKS in neuronal vercosities. Little distribution was seen in the cell soma (arrow). b, higher magnification of varicosities. c–f, treatment with Ang II resulted in redistribution of MARCKS and by 4 h it was uniformly distributed throughout neurites. (B) Effect of Ang II on MARCKS phosphorylation. Neuronal culture, pre-labeled with [32P]orthophosphate and incubated with 100 nM Ang II for indicated time periods. 32P-labeled MARCKS was immunoprecipitated by MARCKS-specific antibody and subjected to SDS-PAGE followed by autoradiography as described in the Materials and Methods. Top, a representative autoradiogram. Bottom, mean data from three experiments ± SE. *, significantly different (P < 0.05) from time zero. (C) Effect of Ang receptor antagonists on MARCKS phosphorylation. Experimental conditions were essentially as described above in Fig. 4 B, except cultures were incubated without (1, 3, 5) or with 100 nM Ang II (2, 4, 6) in the presence of either 10 μM losartan (3 and 4) or 10 μM PD123,319 (5 and 6). *, significantly different (P < 0.05) from control. **, significantly different (P < 0.05) from Ang II–treated neurons. Bars in A: (a) 4 μm; (b–e) 4 μm.
MARCKS immunoreactivity was colocalized with synaptophysin (Fig. 5 A, e) and with TH (Fig. 5 A, f) in Ang II–treated neurons. This further supports our contention that AT1 receptor–induced MARCKS redistribution occurs in noradrenergic neurons. A hypothalamic–brainstem neuronal cells in primary culture from 1-d-old rat contain 20–40% TH-positive neurons (Sumners and Raizada, 1993; Raizada et al., 1994). This coupled with our estimation that ∼30% of neurons express AT1 receptor, would indicate that significant number of noradrenergic neurons would be Ang II responsive. In fact, our immunocytochemical observations on Ang II–induced redistribution of MARCKS depicted in Fig. 5 support this view.
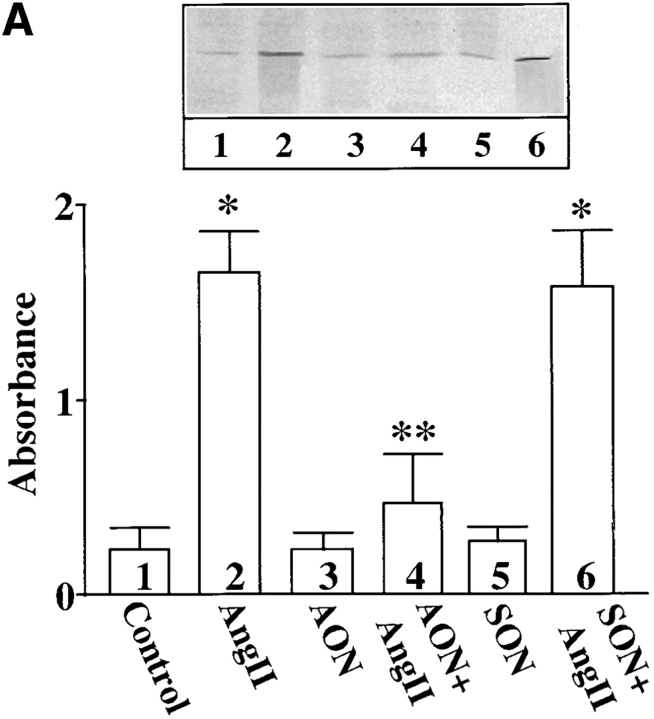
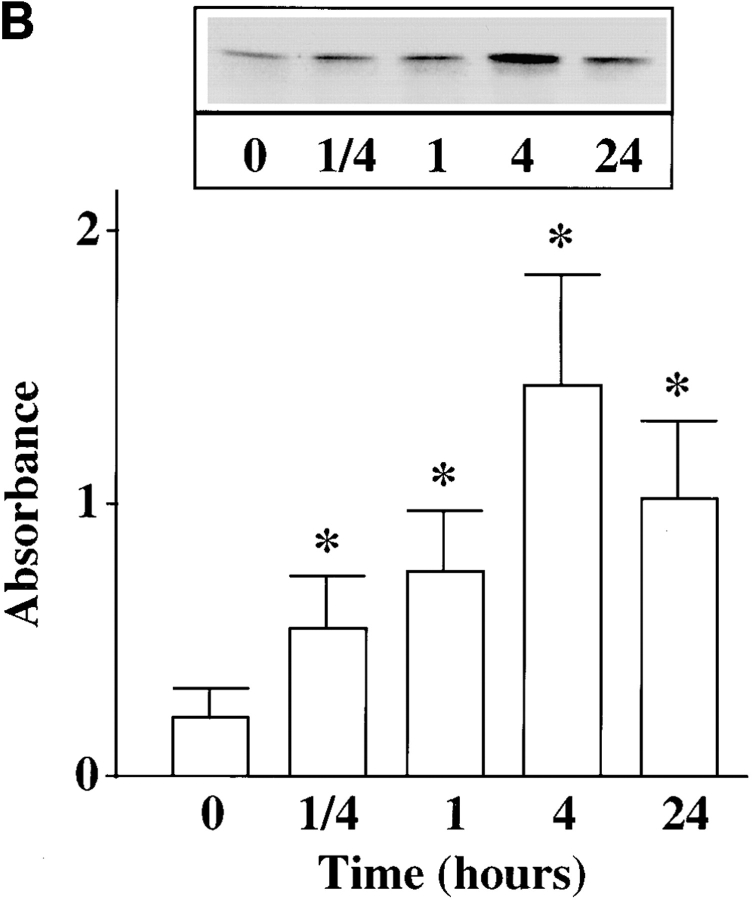
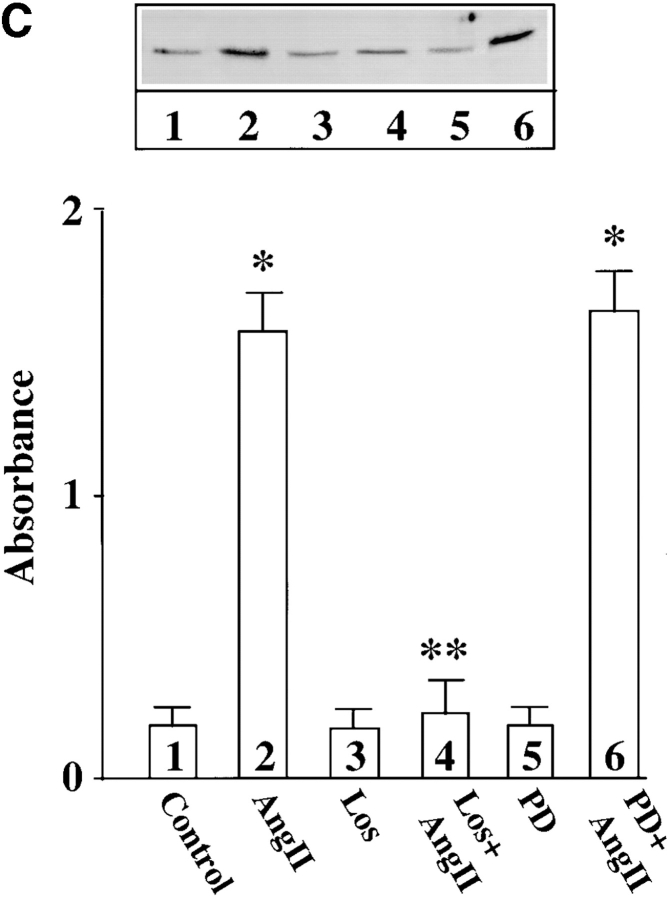
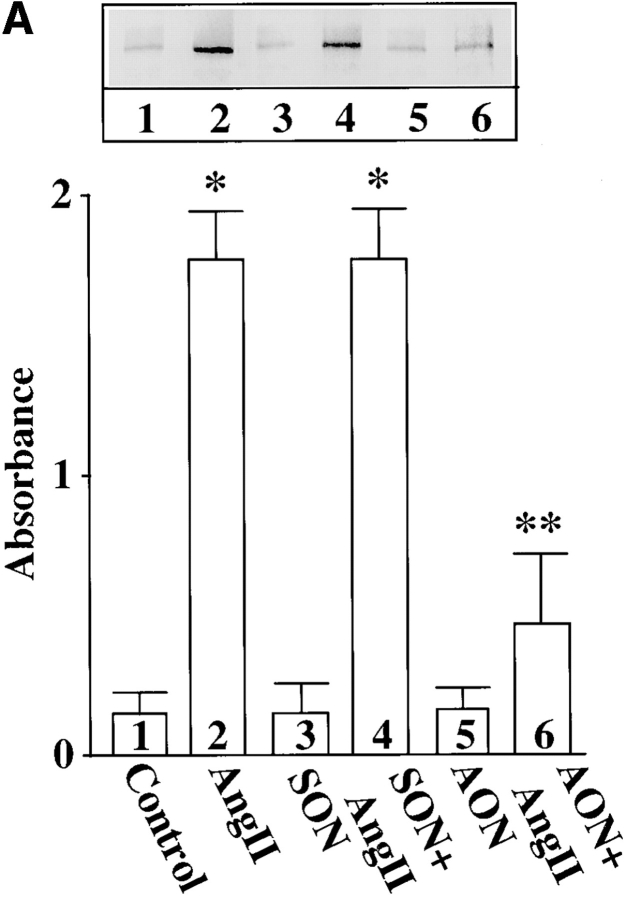
In addition to its redistribution, Ang II also stimulated the phosphorylation of MARCKS. Fig. 5 B shows a time-dependent increase in the incorporation of 32P in immunoprecipitated MARCKS. Low but significant phosphorylation of MARCKS was observed in control, untreated neurons. A twofold stimulation of phosphorylation was seen as early as 15 min with 100 nM Ang II and maximal stimulation of 4.7-fold was observed in 4 h. The stimulation was completely blocked by 10 μM losartan, an AT1 receptor subtype–specific antagonist and not by 10 μM PD123319, an AT2 receptor subtype–specific antagonist (Fig. 5 C). Neuronal cultures were preincubated with PKCβ AON for 48 h to specifically deplete them of PKCβ subtype. Ang II failed to stimulate phosphorylation of MARCKS in these PKCβ-depleted neurons (Fig. 6), further supporting our view that PKCβ subtype is involved in this effect.
Figure 6.
Effect of PKCβ AON on Ang II–induced MARCKS phosphorylation. Neuronal cultures were pre-treated with 2.5 μM SON or AON for PKCβ as described in legend to Fig. 2. After 24 h, 1 mCi/ml [32P]orthophosphate was added to the cultures and incubation was continued for an additional 20 h. 32P-labeled cells were incubated with 100 nM Ang II for 4 h. Immunoprecipitation of 32P-labeled MARCKS, followed by its quantitation, was carried out as described in Materials and Methods. Top, a representative autoradiogram. Bottom, mean ± SE (n = 3). *, significantly different (P < 0.05) from control. **, significantly different (P < 0.05) from Ang II–treated neurons.
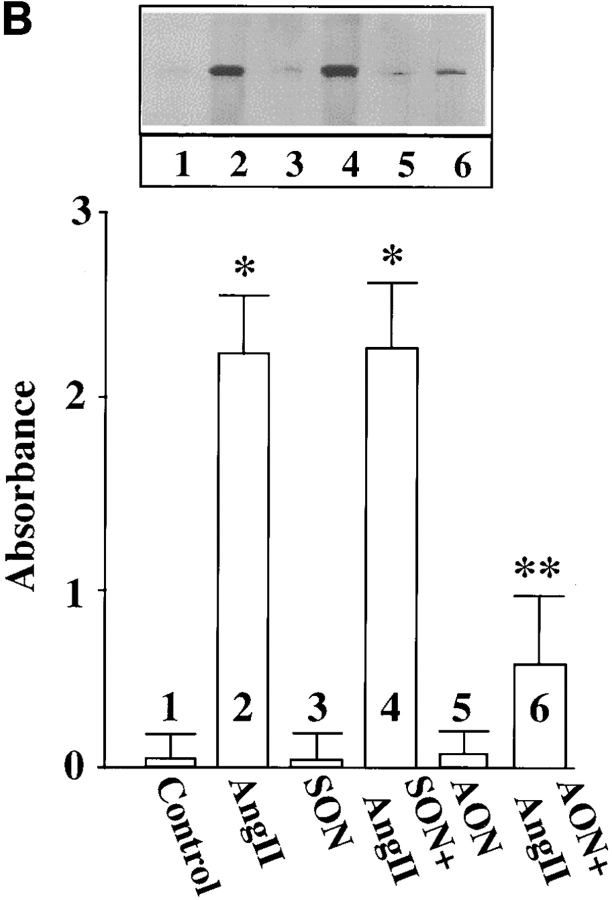
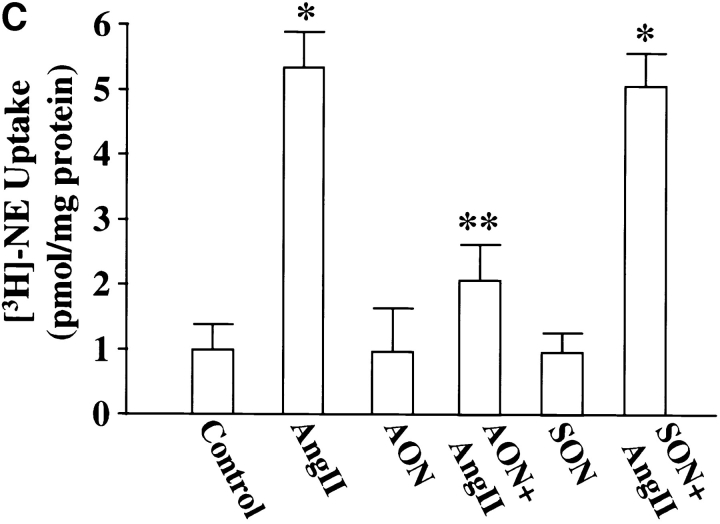
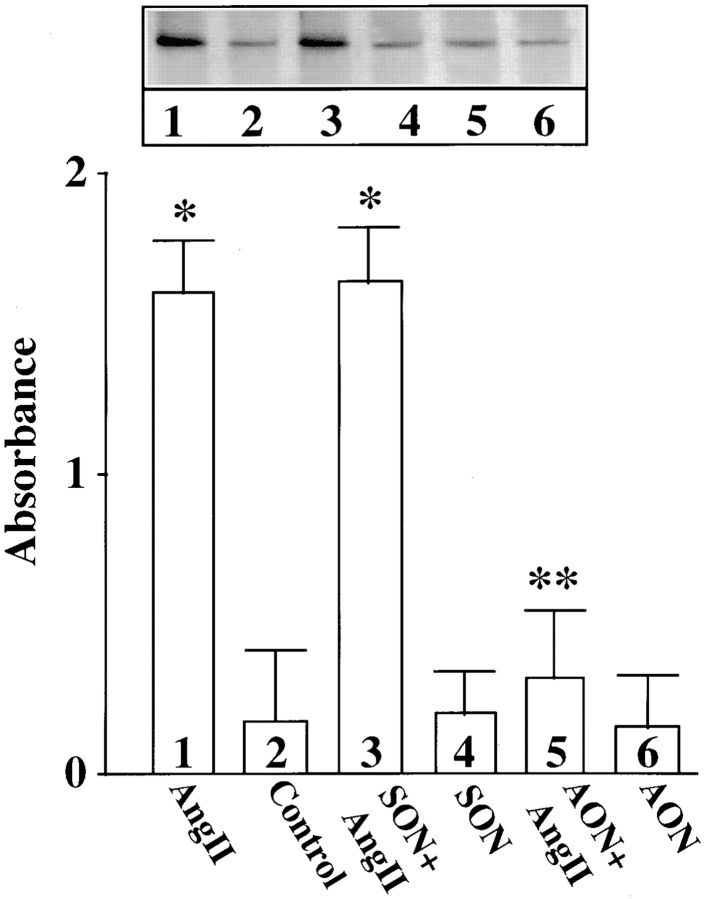
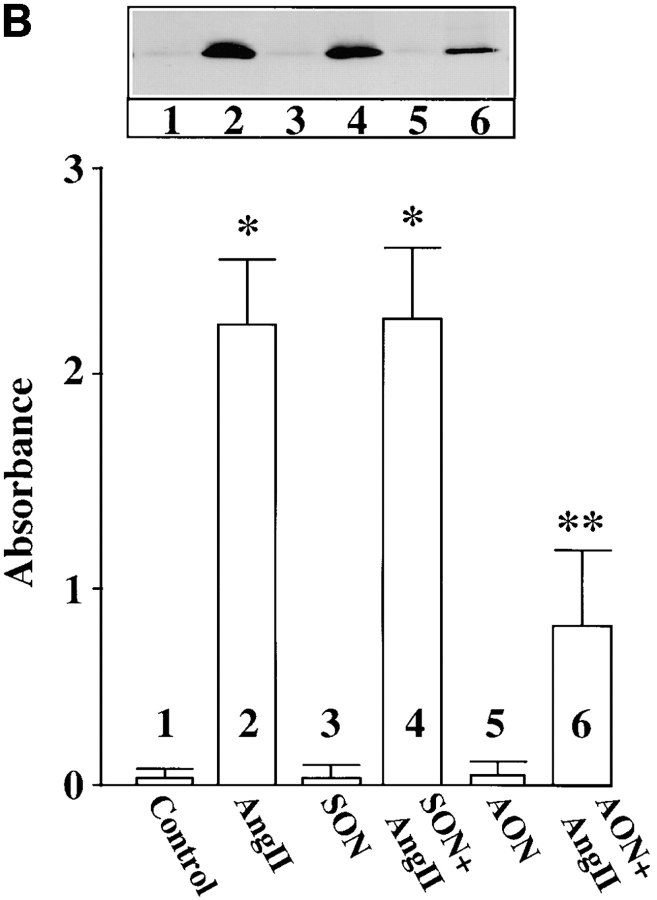
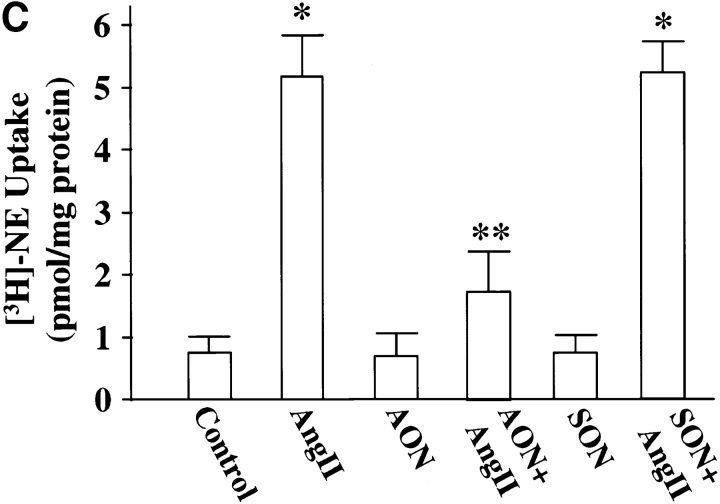
Cultures were treated with MARCKS AON to deplete neurons of MARCKS immunoreactivity to determine the role of MARCKS in Ang II–induced NE neuromodulation. Typical distribution of MARCKS immunoreactivity in varicosities was seen in untreated, control neurons (Fig. 7 A, a). Pre-incubation of neurons for 48 h with AON to MARCKS significantly reduced intensity of MARCKS staining (Fig. 7 A, b). MARCKS SON showed no effect under these conditions (Fig. 7 A, c). Quantitation of immunoreactive MARCKS by Western blotting revealed that MARCKS AON caused a time-dependent decrease of this protein and a maximal decrease of 77% was observed in 48 h (Fig. 7 B). MARCKS SON treatment did not reduce immunoreactive MARCKS. Neurons were pre-treated with MARCKS AON or SON for 48 h, and then incubated with 100 nM Ang II for 4 h and levels of TH, DβH, and [3H]NE uptake in synaptosomes were analyzed. Fig. 8 shows that depletion of MARCKS resulted in a 65–70% attenuation in the ability of Ang II to stimulate TH and DβH immunoreactivities and [3H]NE uptake. This effect on NET, TH, DβH appeared to be posttranscriptional because MARCKS AON did not effect Ang II stimulation of mRNAs for these proteins (Fig. 9). Direct effect of Ang II on the release of [3H]NE from neuronal synaptosomes was measured to determine if MARCKS is also involved in the release of catecholamines. Neurons were treated with MARCKS AON. Synaptosomes were prepared, pre-loaded with [3H]NE and used for Ang II–stimulated release experiments (Wakade et al., 1995). Basal release of [3H]NE was comparable in control, MARCKS SON– and MARCKS AON–treated neurons. Ang II caused a 3.7-fold increase in specific [3H]NE release from synaptosomes of control neurons. This accounted for ∼29% of total synaptosomal [3H]NE. MARCKS AON treatment did not alter this level of [3H]NE release. This indicated that, in spite of a decrease in the intracellular levels of MARCKS, Ang II–stimulated release of [3H]NE was not affected.
Figure 7.
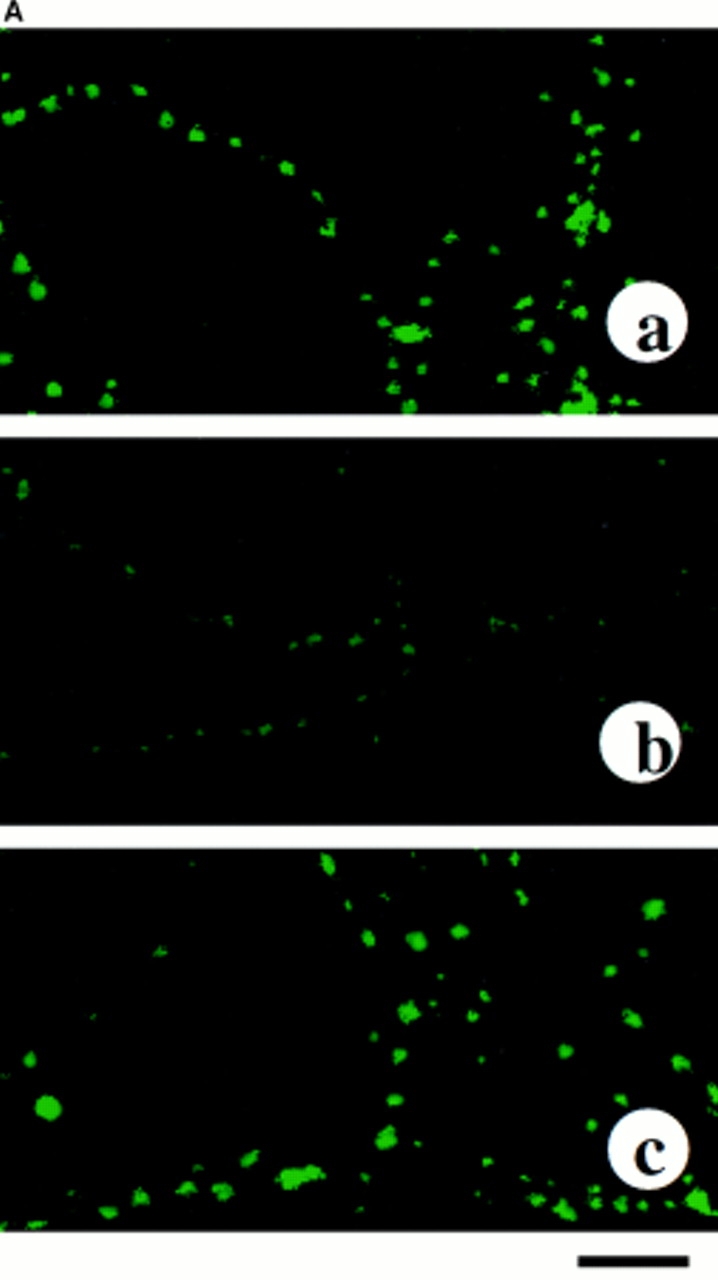
Effect of MARCKS AON and SON treatments on neuronal MARCKS. (A) Neuronal cultures were treated without (a) or with 2.5 μM MARCKS AON (b) or 2.5 μM MARCKS SON (c) for 48 h. MARCKS immunoreactivity was determined by the use of confocal microscopy. (B) After treatment with MARCKS AON or SON for indicated time periods, levels of MARCKS were determined by Western blotting as described in Materials and Methods. Top, a representative autoradiogram. Bottom, mean data ± SE (n = 3). *, significantly different (P < 0.01) from control. Bar, 4 μm.
Figure 8.
Ang II stimulation of TH and DβH immunoreactivities, and [3H]NE uptake in MARCKS-depleted neurons. Neuronal cultures were pretreated with 2.5 μM MARCKS SON or AON for 48 h essentially as described in the legend to Fig. 7. Synaptosomal preparation was used to quantitate immunoreactive TH (A) or DβH (B) or specific [3H]NE uptake (C) as described in Materials and Methods. Top in A and B are representative autoradiograms. Bottom in A and B represented mean ± SE (n = 3). Data in C are mean ± SE (n = 3). *, significantly different (P < 0.01) from control. **, significantly different (P < 0.01) from Ang II–treated neurons.
Figure 9.
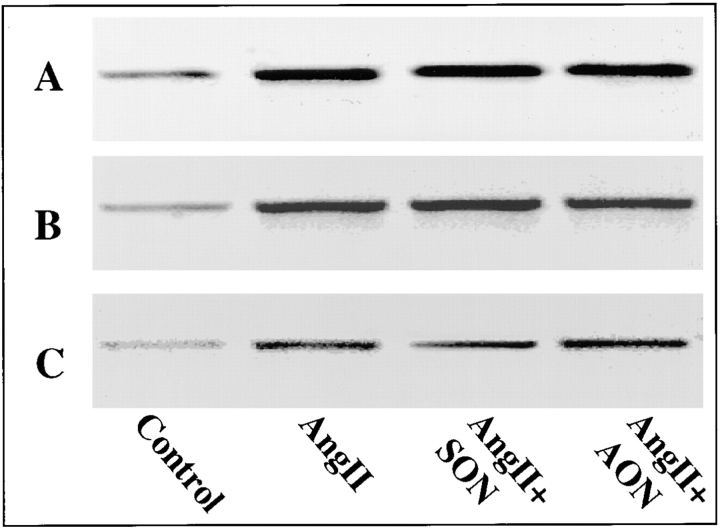
Effects of MARCKS AON treatment on TH, DβH, and NET mRNA levels in neurons. Neuronal cultures were pretreated with 2.5 μM MARCKS AON or SON for 48 h. After incubation without or with 100 nM Ang II for 4 h, total RNA was isolated and mRNA levels for TH (A), DβH (B), and NET (C) were measured by reverse transcription PCR essentially as established by us previously (Lu et al., 1996; Yu et al., 1996).
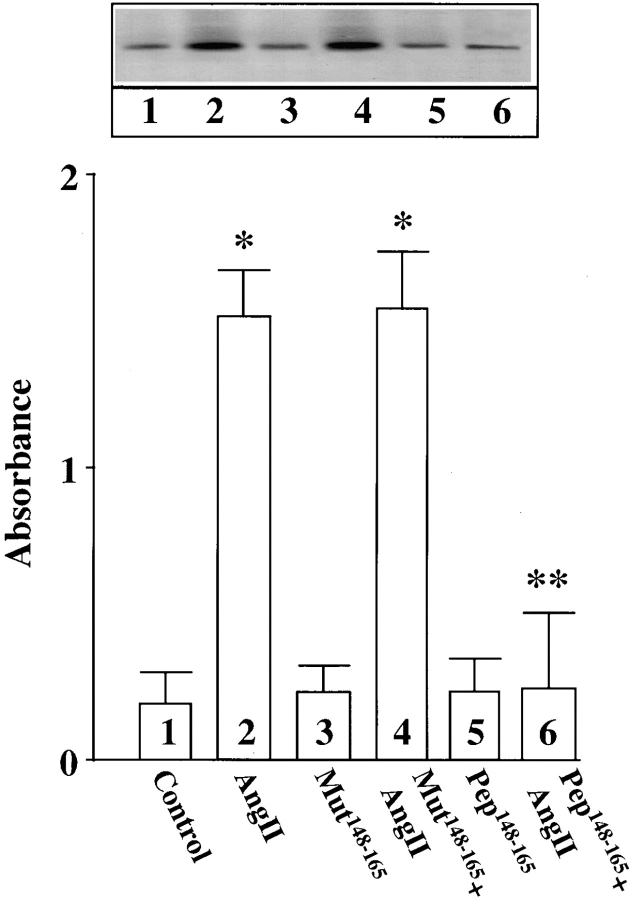
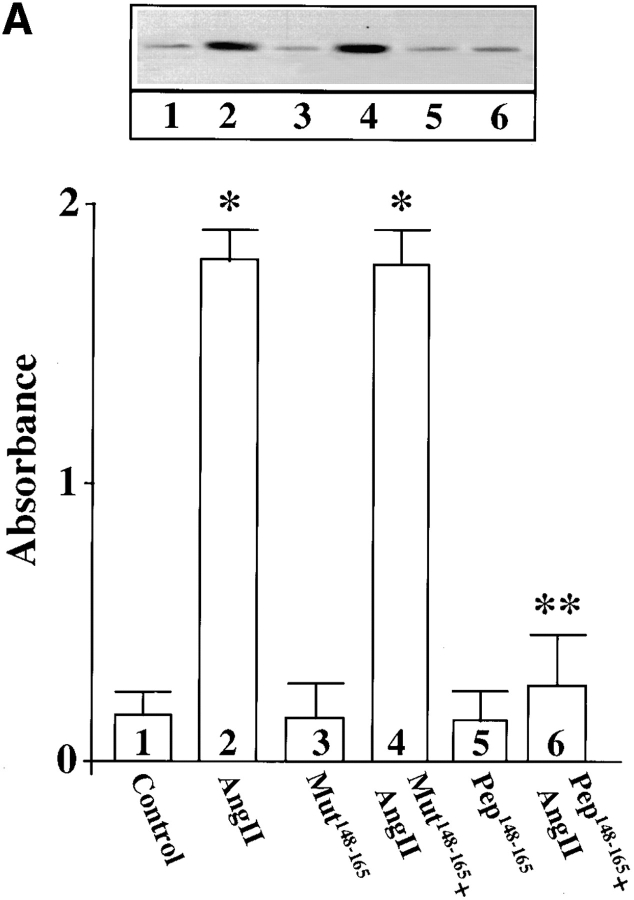
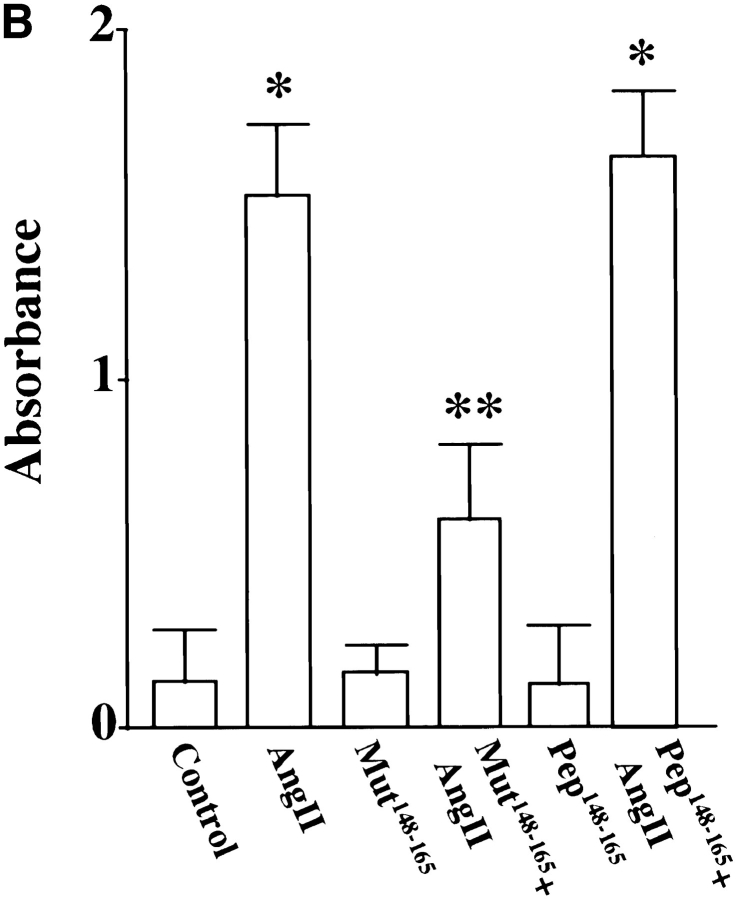
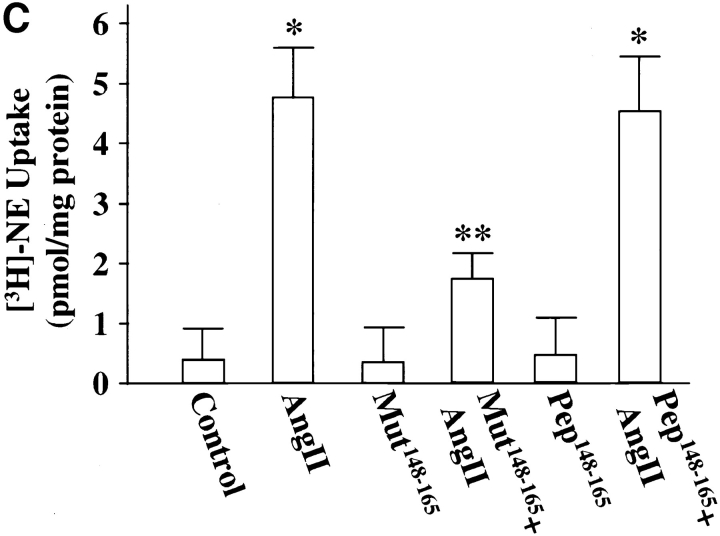
Finally, neuronal cultures were osmotically loaded with pep148–165 or mut148–165 followed by stimulation with Ang II to further determine the involvement of MARCKS in the transport of TH, DβH, and NET. The rationale behind this experiment was based on the hypothesis that pep148–165 would compete with the native MARCKS for phosphorylation by PKCβ, and as a result Ang II–mediated phosphorylation of MARCKS would be decreased. Fig. 10 confirms this prediction and shows that Ang II–induced phosphorylation of MARCKS was decreased by 81% in neurons pre-loaded with pep148–165. Pre-loading with mut148–165 did not affect Ang II stimulation of MARCKS phosphorylation. Fig. 11 shows that pep148–165 treatment resulted in 83–87% decrease in the ability of Ang II to stimulate TH (Fig. 11 A), DβH (Fig. 11 B), and [3H]NE uptake (Fig. 11 C) in synaptosomes. mut148–165 peptide showed no such inhibitory effect. These data confirm that phosphorylation of MARCKS by PKCβ is an important step in the transport of TH, DβH, and NET from cell soma to synaptic terminal.
Figure 10.
Effect of MARCKS pep148–165 and mut148–165 on Ang II stimulation of phosphorylation of MARCKS. Neuronal cultures were prelabeled with [32P]orthophosphate and subjected to osmotic loading of MARCKS pep148–165 and mut148–165 essentially as described in Materials and Methods. After incubation with 100 nM Ang II for 4 h, radiolabeled MARCKS was immunoprecipitated and subjected to quantitation as described in Materials and Methods. Top, a representative autoradiogram. Bottom, mean absorbance data ± SE (n = 3). *, significantly different (P < 0.05) from control. **, significantly different (P < 0.05) from Ang II–treated neurons.
Figure 11.
Effect of MARCKS peptide, pep148–165, on Ang II stimulation of TH and DβH immunoreactivities and [3H]NE uptake in neurons. pep148–165 or its mut148–165 was osmotically loaded, followed by incubation of the cells with 100 nM Ang II for 4 h as described in Materials and Methods. Synaptosomal preparations were used to measure the TH (A) and DβH (B) proteins, and [3H]NE uptake (C) as described in legend to Fig. 1. A and B, (top) represent autoradiograms. Bottom in A and B represent mean data ± SE (n = 3). Data in C are mean ± SE (n = 3). *, significantly different (P < 0.01) from the control. **, significantly different (P < 0.01) from Ang II–treated neurons.
Discussion
The most significant observation of this study is our demonstration that Ang II stimulates redistribution and phosphorylation of MARCKS in a PKCβ-dependent process, and that this phosphorylation is involved in the transport of TH, DβH, and NET in hypothalamic/brainstem neurons. This is of particular interest since MARCKS has recently been shown to be highly expressed in limbic regions of the brain that retain neuroplastic properties beyond early development (McNamara and Lenox, 1997). Thus, MARCKS may play an important role in the mediation of fast axonal transport of catecholamine-synthesizing enzymes and NET to synaptic terminals. As a result, an increased turnover and release of NE is achieved under chronic stimulation of brain neurons by Ang II.
Ang II has been previously shown to stimulate catecholamines synthesis, turnover, and release both acutely and chronically (MacLean et al., 1990; Stadler et al., 1992; Sumners and Raizada 1993; Gelband et al., 1998). These effects are mediated by activation of the AT1 receptor subtype. Acute stimulation, which has been termed evoked response involves posttranscriptional processes (Lu et al., 1996), whereas a chronic or enhanced response of Ang II is associated with both translocation of pre-existing intracellular NET and transcription of NET, TH, and DβH genes. (Lu et al., 1996; Yu et al., 1996; Gelband et al., 1998). This may explain the observation that Ang II stimulation of NET activity occurs more rapidly as compared with its effect on TH and DβH (Fig. 1). Our studies have also established that the enhanced regulation of Ang II– induced NE neuromodulation requires activation of Ras-Raf-MAP kinase signal transduction pathway (Yang et al., 1996; Lu et al., 1997; Gelband et al., 1998). Activation of MAP kinase leads to nuclear translocation of MAP kinase and other signaling molecules such as Fos, STAT3, and Jun, which participate in transcriptional control of TH, DβH, and NET (Gelband et al., 1998). In this study we have presented evidence for an additional signaling pathway that we believe is equally important in enhanced response of NE neuromodulation by Ang II. In this scheme, we propose that Ang II stimulates transport of TH, DβH, and NET along the neurites for them to be available for increased synthesis, release, and re-uptake of NE at the synaptic level. This stimulation involves activation of PKCβ: (a) Ang II stimulates PKC, which is involved in axonal transport (Komoly et al., 1991); (b) treatment of neurons with AON to PKCβ, which selectively depletes neurons of this subtype, causes attenuation of Ang II stimulation of TH, DβH, and NET transport. This effect is selective for PKCβ subtype since AONs to other major calcium-dependent PKC subtypes such as PKCα and PKCγ had no such effect. It is also pertinent to point out that Ang II–induced MARCKS phosphorylation and redistribution appears to be a relatively slower response compared with its relatively rapid effect on PKC. Although, we have suggested that PKCβ stimulation is directly involved in Ang II–induced MARCKS phosphorylation and redistribution. Alternate possibilities can not be ruled out at the present time. For example, it is possible that Ang II influences PKCβ-dependent neuronal activity. A change in this activity could activate an entire set of distinct neurons to stimulate redistrubution of MARCKS, TH, and DβH. However, our observations that AT1 receptors are present on nonadrenergic neurons and that the AT1 receptor blockade attenuates NET activity in these neurons (Lu et al., 1996) argue against such an indirect effect.
Phosphorylation of MARCKS is instrumental in its redistribution. MARCKS possesses a basic phosphorylation site domain (PSD). Phosphorylation of this PSD domain prevents the electrostatic interaction of the effector region of the MARCKS to the plasma membrane (George and Blackshear, 1992; Taniguchi and Manenti, 1993; Kim et al., 1994). Whereas there is little evidence for a preferential phosphorylation of MARCKS by the PKCβ subtype, there is considerable evidence for its presence and involvement in synaptosomal activities (Guadagno et al., 1992; Heemskerk et al., 1993; Sheu et al., 1995; Cabell et al., 1996; Seki et al., 1996). Present data are novel since they provide evidence that PKCβ subtype is involved in Ang II stimulation of MARCKS phosphorylation, redistribution, and in NE neuromodulation. The evidence for this includes the following: (a) Ang II stimulation of phosphorylation of MARCKS is blocked by depletion of PKCβ by AON treatment. No such attenuation was observed with AON to PKCα or PKCγ; (b) Ang II–induced redistribution of immunoreactive MARCKS from varicosities is also blocked by PKCβ-AON treatment; (c) Presence of PKC substrate domain is well documented in MARCKS and a peptide containing this domain blocks Ang II stimulation of MARCKS phosphorylation.
Finally, phosphorylation of MARCKS appears to be key in its redistribution and increased accumulation of TH, DβH, and NET in synaptic vesicles: (a) blocking the phosphorylation of MARCKS by pep148–165 inhibits Ang II– induced redistribution of MARCKS in neurites. As a result, MARCKS immunoreactivity remains localized in the varicosities (unpublished data); (b) AON to MARCKS, that significantly reduces endogenous levels of neuronal MARCKS, attenuates Ang II–induced accumulation of TH, DβH, and NET in synaptosomes; (c) Ang II induces redistribution of MARCKS. Such a PKC-activated redistribution has been proposed in membrane trafficking and cycling of MARCKS between plasma membrane and lysosomes in other systems (Allen and Aderem, 1995); and (d) blocking the phosphorylation of MARCKS by osmotic loading of the peptide148–165 attenuates Ang II enhancement of TH and DβH in synaptosomes. Collectively, these observations strongly support the notion that PKCβ-MARCKS are facilitating signaling pathway in enhanced regulation of NE neuromodulation by Ang II.
These observations are important in that they provide the first evidence that two distinct signaling pathways induced by AT1 receptor activation, converge downstream to participate in the enhanced stimulation of neuromodulation. Such long-term enhancement of noradrenergic neurotransmission has been shown to involve an increase in critical proteins such as TH and DβH and their transport to synaptic sites. Studies over the years have provided data in support of the close association of both TH and DβH transport and the association of organelles in the process (Coyle and Wooten, 1972; Dahlstrom et al., 1982). Whereas it has been clear that microtubule-dependent movement is essential to transport associated with a variety of intracellular organelles, it is only more recently that a role for actin microfilaments in this process has been elucidated (Morris and Hollenbeck, 1995). In fact, there is extensive interaction between microtubules and the actin cytoskeleton, and it has been suggested that the specialized properties of these two cytoskeletal filament systems may act in a coordinated manner to achieve regional and even compartmentalized net transport of organelles and associated proteins for proper physiological regulation (Goldstein and Vale, 1992; Morris and Hollenbeck, 1995). MARCKS is a member of a small family of proteins that bind calmodulin in the presence of calcium and bind and cross-link actin in a mutually exclusive fashion. PKC-mediated phosphorylation prevents both calmodulin binding and actin cross-linking and shuttles MARCKS out of the plasma membrane (Wang et al., 1989; Rosen et al., 1990; Thelen et al., 1991). Recent studies have demonstrated that MARCKS is transported from plasma membrane to lysosomes upon PKC-mediated phosphorylation, and recycling of MARCKS to the plasma membrane appears to depend upon interaction with intact microtubules (Allen and Aderem, 1995). By virtue of the fact that MARCKS' intracellular location and properties are responsive to cell signaling events, there is significant evidence that MARCKS may play an important role in translating extracellular signal–mediated cytoskeletal restructuring associated with cell movement, phagocytosis, and neurosecretion (Aderem, 1995; Blackshear, 1993; Wang et al., 1989). Both calcium flux and PKC activation are in a position to prevent MARCKS-mediated actin cross-linking, thus altering the dynamic interaction of microtubules and actin filaments, leading to a reduction in membrane rigidity and facilitation of transport of macromolecules in the neurites (Hartwig et al., 1992). It is also possible that calmodulin, released from MARCKS phosphorylation, is involved in accelerating the transport process. This proposal is consistent with the demonstrated involvement of calmodulin in Ang II stimulation of TH in particular, and its role in axonal transport and neuromodulation in general (Graff et al., 1989; Hammerschlag, 1994; Rivera et al., 1995). Thus both the release of calmodulin and the inability of phosphorylated MARCKS to form actin crossbridges may be involved in Ang II–induced chronic neuromodulation. Studies are underway to delineate these processes as they relate to Ang II–induced phosphorylation of MARCKS and transport of TH, DβH, and NET.
Abbreviations used in this paper
- Ang II
angiotensin II
- AON
antisense oligonucleotide
- AT1 receptor
angiotensin type 1 receptor subtype
- DβH
dopamine β hydroxylase
- MARCKS
myristoylated alanine-rich C kinase substrate
- NE
norepinephrine
- NET
norepinephrine transporter
- PDHS
plasma-derived horse serum
- PKC
protein kinase C
- PSD
basic phosphorylation site domain in MARCKS
- SON
sense oligonucleotide
- TH
tyrosine hydroxylase
- WKY
Wistar Kyoto
Footnotes
Address all correspondence to Dr. Mohan K. Raizada, Professor and Associate Dean for Graduate Education, Department of Physiology, College of Medicine, University of Florida, PO Box 100274, Gainesville, FL 32610. Tel.: (352) 392–3791. Fax: (352) 846-0270. E-mail: mraizada@dean.med.ufl.edu
R.H. Lenox's present address is Department of Psychiatry, University of Pennsylvania, Philadelphia, PA.
References
- Aderem A. The MARCKS family of protein kinase-C substrates. Biochem Soc Trans. 1995;23:587–591. doi: 10.1042/bst0230587. [DOI] [PubMed] [Google Scholar]
- Ahmad F, Li PM, Meyerovitch J, Goldstein BJ. Osmotic loading of neutralizing antibodies demonstrates a role for protein-tyrosine phosphatase 1B in negative regulation of the insulin action pathway. J Biol Chem. 1995;270:20503–20508. doi: 10.1074/jbc.270.35.20503. [DOI] [PubMed] [Google Scholar]
- Aigner L, Caroni P. Depletion of 43-kD growth-associated protein in primary sensory neurons leads to diminished formation and spreading of growth cones. J Cell Biol. 1993;123:417–429. doi: 10.1083/jcb.123.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LA, Aderem A. Protein kinase C regulates MARCKS cycling between the plasma membrane and lysosomes in fibroblasts. EMBO (Eur Mol Biol Organ) J. 1995;14:1109–1120. doi: 10.1002/j.1460-2075.1995.tb07094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amess B, Manjarrez-Hernandex HA, Howell SA, Learmonth M, Aitken A. Multisite phosphorylation of the 80 kDa (MARCKS) protein kinase C substrate in C3H/10T1/Z fibroblasts, quantitative analysis of individual sites by solid-phase microsequencing. FEBS (Fed Eur Biochem Soc) Lett. 1992;297:285–291. doi: 10.1016/0014-5793(92)80557-w. [DOI] [PubMed] [Google Scholar]
- Asotra K, Macklin WB. Developmental expression of protein kinase C isozymes in oligodendrocytes and their differential modulation by 4 beta-phorbol-12,3-dibutyrate. J Neurosci Res. 1994;39:273–289. doi: 10.1002/jnr.490390305. [DOI] [PubMed] [Google Scholar]
- Balboa MA, Firestein BL, Godson C, Bell KS, Insel PA. Protein kinase C alpha mediates phospholipase D activation by nucleotides and phorbol ester in Madin-Darby canine kidney cells. Stimulation of phospholipase D is independent of activation of polyphosphoinositide-specific phospholipase C and phospholipase A2. J Biol Chem. 1994;269:10511–10516. [PubMed] [Google Scholar]
- Blackshear, P.J. 1993. The MARCKS family of cellular protein kinase C substrates. J. Biol. Chem. 268:1501–1504. [PubMed]
- Cabell CH, Verghese GM, Ranki NB, Burns DJ, Blackshear PJ. MARCKS phosphorylation by individual protein kinase C isozymes in insect Sf9 cells. Proc Assoc Am Physicians. 1996;108:37–46. [PubMed] [Google Scholar]
- Coyle JT, Wooten GF. Rapid axonal transport of tyrosine hydroxylase and dopamine-hydroxylase. Brain Res. 1972;44:701–704. doi: 10.1016/0006-8993(72)90340-x. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Booj S, Goldstein M, Larsson PA. Cytofluorometric scanning: a tool for studying axonal transport in monoaminergic neurons. Brain Res Bull. 1982;9:61–68. doi: 10.1016/0361-9230(82)90121-6. [DOI] [PubMed] [Google Scholar]
- Dzau VJ. Vascular renin-angiotensin system and vascular protection. J Cardiovasc Pharmacol. 1993;22(Suppl.):1–9. doi: 10.1097/00005344-199322005-00002. [DOI] [PubMed] [Google Scholar]
- Freeman EJ, Chisolm GM, Tallant EA. Role of calcium and protein kinase C in the activation of phospholipase D by angiotensin II in vascular smooth muscle cells. Arch Biochem Biophys. 1995;319:84–92. doi: 10.1006/abbi.1995.1269. [DOI] [PubMed] [Google Scholar]
- Gelband CH, Sumners C, Lu D, Raizada MK. Angiotensin receptors and neuromodulation: implications of functional coupling. Reg Peptides. 1998;73:141–147. doi: 10.1016/s0167-0115(97)11050-3. [DOI] [PubMed] [Google Scholar]
- George DJ, Blackshear PJ. Membrane association of the myristoylated alanine-rich C kinase substrate (MARCKS) protein appears to involve myristate-dependent binding in the absence of a myristoyl protein receptor. J Biol Chem. 1992;267:24879–24885. [PubMed] [Google Scholar]
- Goldstein LS, Vale RD. Cell biology. New cytoskeletal liaisons. Nature. 1992;359:193–194. doi: 10.1038/359193a0. [DOI] [PubMed] [Google Scholar]
- Graff JM, Young TN, Johnson JD, Blackshear PJ. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989;264:21818–21823. [PubMed] [Google Scholar]
- Guadagno SN, Borner C, Weinstein IB. Altered regulation of a major substrate of protein kinase C in rat 6 fibroblasts overproducing PKC beta I. J Biol Chem. 1992;67:2697–2707. [PubMed] [Google Scholar]
- Hammerschlag R. Is the intrasomal phase of fast axonal transport driven by oscillations of intracellular calcium? . Neurochem Res. 1994;19:1431–1437. doi: 10.1007/BF00972472. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium calmodulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Heemskerk FM, Chen HC, Huang FL. Protein kinase C phosphorylates Ser152, Ser156 and Ser163 but not Ser160 of MARCKS in rat brain. Biochem Biophys Res Commun. 1993;190:236–241. doi: 10.1006/bbrc.1993.1036. [DOI] [PubMed] [Google Scholar]
- Kim J, Shishido T, Jiang X, Aderem A, McLaughlin S. Phosphorylation, high ionic strength, and calmodulin reverse the binding of MARCKS to phospholipid vesicles. J Biol Chem. 1994;269:28214–28219. [PubMed] [Google Scholar]
- Kishi M, Ohkuma S, Ma FH, Kuriyama K. Pharmacological characteristics of choline transport system in mouse cerebral cortical neurons in primary culture. Jpn J Pharmacol. 1991;55:223–232. doi: 10.1254/jjp.55.223. [DOI] [PubMed] [Google Scholar]
- Komoly S, Liu Y, Webster HD, Chan KF. Distribution of protein kinase C isozymes in rat optic nerves. J Neurosci Res. 1991;29:379–389. doi: 10.1002/jnr.490290313. [DOI] [PubMed] [Google Scholar]
- Lu D, Yu K, Paddy MR, Rowland NE, Raizada MK. Regulation of norepinephrine transport system by angiotensin II in neuronal cultures of normotensive and spontaneously hypertensive rat brains. Endocrinology. 1996;137:763–772. doi: 10.1210/endo.137.2.8593828. [DOI] [PubMed] [Google Scholar]
- Lu D, Yang H, Raizada MK. Angiotensin II regulation of neuromodulation: Downstream signaling mechanism from activation of mitogen-activated protein kinase. J Cell Biol. 1997;135:1609–1617. doi: 10.1083/jcb.135.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Yang H, Raizada MK. Angiotensin II-induced nuclear targeting of AT1receptor in brain neurons. Endocrinology. 1998;439:365–375. doi: 10.1210/endo.139.1.5679. [DOI] [PubMed] [Google Scholar]
- Manenti S, Sorokine O, Van Dorsselaer A, Taniguchi H. Demyristoylation of the major substrate of protein kinase C (MARCKS) by the cytoplasmic fraction of brain synaptosomes. J Biol Chem. 1994;269:8309–8313. [PubMed] [Google Scholar]
- Marrero MB, Schieffer B, Paxton WG, Heerdt L, Berk BC, Delafontaine P, Bernstein KE. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1receptor. Nature. 1995;375:247–250. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- MacLean MR, Raizada MK, Sumners C. The influence of angiotensin II on catecholamine synthesis in neuronal cultures from rat brain. Biochem Biophys Res Commun. 1990;167:492–497. doi: 10.1016/0006-291x(90)92050-a. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Lenox RH. Comparative distribution of myristoylated alanin-rich C kinase substrates (MARCKS) and F1/GAP-43 gene expression in the adult rat brain. J Comp Neurol. 1997;379:48–71. [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol. 1995;131:1315–1326. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftilan AJ. Role of the tissue renin-angiotensin system in vascular remodeling and smooth muscle cell growth. Curr Opin Nephrol Hypertens. 1994;3:218–227. doi: 10.1097/00041552-199403000-00014. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Hung A, Jacques-Berg W, Kiss J, Rodriguez J. Effects of protein kinase C modulation on NMDA receptor mediated regulation of neurotransmitter enzyme and cfos protein in cultured neurons. Neurochem Res. 1995;20:561–569. doi: 10.1007/BF01694537. [DOI] [PubMed] [Google Scholar]
- Raizada MK, Muther TF, Sumners C. Increased angiotensin II receptors in neuronal cultures from hypertensive rat brain. Am J Physiol. 1984;247:364–372. doi: 10.1152/ajpcell.1984.247.5.C364. [DOI] [PubMed] [Google Scholar]
- Raizada MK, Lu D, Tang W, Kurian P, Sumners C. Increased angiotensin II type-1 receptor gene expression in neuronal cultures from spontaneously hypertensive rats. Endocrinology. 1993;132:1715–1722. doi: 10.1210/endo.132.4.8462471. [DOI] [PubMed] [Google Scholar]
- Raizada, M.K., D. Lu, and C. Sumners. 1994. AT1 receptors and angiotensin actions in the brain and neuronal cultures of normotensive and hypertensive rats. In Current Concepts. M. Mukhopadhyay, and M.K. Raizada, editors. Plenum Press, New York. 331–348. [DOI] [PubMed]
- Rivera DT, Langford GM, Weiss G, Nelson DJ. Calmodulin regulates fast axonal transport of squid axoplasm organelles. Brain Res Bull. 1995;37:47–52. doi: 10.1016/0361-9230(94)00256-8. [DOI] [PubMed] [Google Scholar]
- Reddy R, Zhou F, Huang L, Carbone F, Bevan M, Rouse BT. pH sensitive liposomes provide an efficient means of sensitizing target cells to class I restricted CTL recognition of a soluble protein. J Immunol Methods. 1991;141:157–163. doi: 10.1016/0022-1759(91)90142-3. [DOI] [PubMed] [Google Scholar]
- Rosen A, Keenan KF, Thelen M, Nairn AC, Aderem A. Activation of protein kinase C results in the displacement of its myristoylated, alanine-rich substrate from punctate structures in macrophage filopodia. J Exp Med. 1990;172:1211–1215. doi: 10.1084/jem.172.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM. Brain and pituitary angiotensin. Endocrine Rev. 1992;13:329–380. doi: 10.1210/edrv-13-2-329. [DOI] [PubMed] [Google Scholar]
- Seki K, Sheu FS, Huang KP. Binding of myristoylated alanine-rich protein kinase C substrate to phosphoinositides attenuates the phosphorylation by protein kinase C. Arch Biochem Biophys. 1996;326:193–201. doi: 10.1006/abbi.1996.0065. [DOI] [PubMed] [Google Scholar]
- Sheu FS, Huang FL, Huang KP. Differential responses of protein kinase C substrates (MRCKS, neuromodulin, and neurogranin) phosphorylation to calmodulin and S100. Arch Biochem Biophys. 1995;316:335–342. doi: 10.1006/abbi.1995.1045. [DOI] [PubMed] [Google Scholar]
- Stadler T, Veltmar A, Qadri F, Unger T. Angiotensin II evokes noradrenaline release from the paraventricular nucleus in conscious rats. Brain Res. 1992;569:117–122. doi: 10.1016/0006-8993(92)90377-l. [DOI] [PubMed] [Google Scholar]
- Steckeling V, Lebrun C, Quadri F, Veltman A, Unger T. Role of brain angiotensin in cardiovascular regulation. J Cardiovasc Pharmacol. 1992;19(Suppl.):73–79. doi: 10.1097/00005344-199219006-00012. [DOI] [PubMed] [Google Scholar]
- Sumners, C., and M.K. Raizada. 1993. Angiotensin II receptors subtypes in neuronal cells. In Cellular and Molecular Biology of the Renin-Angiotensin System. M.K. Raizada, M.I. Phillips, and C. Sumners, editors. CRC Press, Boca Raton, FL. 379–411.
- Sumners C, Raizada MK, Kang J, Lu D, Posner P. Receptor mediated effects of angiotensin II on neurons. Front Neuroendocrinol. 1995;15:203–230. doi: 10.1006/frne.1994.1009. [DOI] [PubMed] [Google Scholar]
- Sylvia VL, Schwartz Z, Ellis EB, Helm SH, Gomez R, Dean DD, Boyan BD. Nongenomic regulation of protein kinase C isoforms by the vitamin D metabolites 1 alpha, 25-(OH)2D3 and 24R,25-(OH)2D3. J Cell Physiol. 1996;167:380–393. doi: 10.1002/(SICI)1097-4652(199606)167:3<380::AID-JCP2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Manenti S. Interaction of myristolated alanine-rich protein kinase C substrate (MARCKS) with membrane phospholipids. J Biol Chem. 1993;268:9960–9963. [PubMed] [Google Scholar]
- Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991;351:320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Timmermans PBMWM, Wang PC, Chiu AT, Herblin MF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JAM, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- Wakade AR, Przywakra DA, Bhare SV, Mashalkar V, Wakade TD. Cardiac cells control transmitter release and calcium homeostasis in sympathetic neurons cultured from embryonic chicks. J Physiol (Lond) 1995;488:587–600. doi: 10.1113/jphysiol.1995.sp020992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JK, Walaas SI, Sihra TS, Aderem A, Greengard P. Phosphorylation and associated translocation of the 87-kDa protein, a major protein kinase C substrate, in isolated nerve terminal. Proc Natl Acad Sci USA. 1989;86:2253–2256. doi: 10.1073/pnas.86.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, D.G., and R.H. Lenox. 1996. Chronic lithium-induced down-regulation of MARCKS in immortalized hippocampal cells: potentiation by muscarinic receptor activation. J. Neurochem. 67:767–777. [DOI] [PubMed]
- Wright JW, Harding JW. Brain angiotensin receptor subtypes in the control of physiological and behavioral responses. Neurosci Biobehavioral Rev. 1994;18:21–53. doi: 10.1016/0149-7634(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu D, Yu K, Raizada MK. Regulation of neuromodulatory actions of angiotensin II in the brain neurons by the Ras-dependent mitogen-activated protein kinase pathway. J Neurosci. 1996;16:4047–4058. doi: 10.1523/JNEUROSCI.16-13-04047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Lu D, Rowland NE, Raizada MK. Angiotensin II regulation of tyrosine hydroxylase gene expression in the neuronal cultures of normotensive and spontaneously hypertensive rats. Endocrinology. 1996;137:2503–2513. doi: 10.1210/endo.137.8.8754788. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ikeda SR. Modulation of Ca(2+)-channel currents by protein kinase C in adult rat sympathetic neurons. J Neurophysiol. 1994;72:1549–1560. doi: 10.1152/jn.1994.72.4.1549. [DOI] [PubMed] [Google Scholar]