Abstract
The β2 integrins and intercellular adhesion molecule-1 (ICAM-1) are important for monocyte migration through inflammatory endothelium. Here we demonstrate that the integrin αvβ3 is also a key player in this process. In an in vitro transendothelial migration assay, monocytes lacking β3 integrins revealed weak migratory ability, whereas monocytes expressing β3 integrins engaged in stronger migration. This migration could be partially blocked by antibodies against the integrin chains αL, β2, αv, or IAP, a protein functionally associated with αvβ3 integrin. Transfection of β3 integrin chain cDNA into monocytes lacking β3 integrins resulted in expression of the αvβ3 integrin and conferred on these cells an enhanced ability to transmigrate through cell monolayers expressing ICAM-1. These monocytes also engaged in αLβ2-dependent locomotion on recombinant ICAM-1 which was enhanced by αvβ3 integrin occupancy. Antibodies against IAP were able to revert this αvβ3 integrin-dependent cell locomotion to control levels. Finally, adhesion assays revealed that occupancy of αvβ3 integrin could decrease monocyte binding to ICAM-1.
In conclusion, we show that αvβ3 integrin modulates αLβ2 integrin-dependent monocyte adhesion to and migration on ICAM-1. This could represent a novel mechanism to promote monocyte motility on vascular ICAM-1 and initiate subsequent transendothelial migration.
Keywords: monocyte, αvβ3 integrin, αLβ2 integrin, migration, ICAM-1
M onocytes are among the first leukocytes to enter inflamed tissue where they play a vital role in the healing process. These cells, like other leukocytes, leave the circulation by crossing the vascular endothelium. The dynamic process of transendothelial migration (TEM)1 in vivo is a multistep mechanism. It includes initial tethering of leukocytes to the vessel wall, followed by rolling along the endothelium, tight adhesion to the endothelial surface, and ultimately movement of the leukocyte through the intercellular junctions into the underlying tissue (9, 20, 66). The selectin family of adhesion molecules and their ligands have been implicated in the initial tethering of leukocytes to the vessel wall through weak adhesions that permit leukocytes to roll in the direction of flow (40). Another class of adhesion molecules, the integrins, of which β1 and β2 are key players in TEM (1, 34, 65, 70), mediate arrest, tight adhesion, and spreading of leukocytes on the endothelium (2). Cellular activation precedes integrin-mediated adhesion and chemoattractants are potent activators in vivo (34, 68).
Monocytes express a selection of adhesion molecules including selectins, β1, β2, and αv integrins (28, 45). The β1 integrin α4β1 present on monocytes promotes arrest and adhesion to vascular cell adhesion molecule-1 (VCAM-1) on the vascular endothelium (1). The β2 integrins αLβ2 and αMβ2 (CD11a/CD18 and CD11b/CD18, respectively) also present on monocytes (60), bind to the endothelial ligand ICAM-1 (CD54) (17, 65), and mediate tight adhesion to the endothelium (70). However, this presents a paradox: if β2 integrins mediate tight adhesion of a leukocyte to ICAM-1, how does the cell initiate the motility necessary for subsequent diapedesis? The cell must be able to modulate adhesions at the cell surface in order to move forward. It was recently shown that αLβ2 on lymphocytes can downregulate α4β1 integrin activity and enhance cell motility on fibronectin (52). We have previously demonstrated that the αvβ3 integrin can regulate lymphocyte motility on VCAM-1 by modulating the function of α4β1 (32).
The αvβ3 integrin can bind to multiple ligands in an Arg-Gly-Asp–dependent manner (22, 23). The integrin per se mediates cell locomotion and is involved in cell migration on components of the extracellular matrix (ECM) (11, 41). It can also modulate the activity of other integrins, such as phagocytosis mediated by α5β1 (3) and adhesion through αMβ2 (33, 71). The αvβ3 integrin has been shown to be physically and functionally associated with integrin-associated protein (IAP, CD47) (7), a 50-kD membrane protein found on a variety of different cell types (55), as antibodies against IAP can block some αvβ3 integrin-mediated functions (7, 44). IAP on its own is a receptor for the carboxy-terminal domain of thrombospondin-1 (25), and anti-IAP antibodies can block TEM of leukocytes at a step subsequent to tight adhesion (12). It was recently shown that certain forms of platelet endothelial cell adhesion molecule (PECAM-1)/CD31 are heterotypic ligands for αvβ3 integrin (8, 51). Interestingly, several groups have shown that antibodies against PECAM-1 are also able to block TEM (49, 72). Therefore, there is some evidence to suggest that the αvβ3 integrin might be involved in TEM.
We looked specifically at the role of αvβ3 integrin in monocyte migration. A β3 integrin-deficient monocytic cell line displayed poor migratory ability compared with a β3 integrin-positive monocytic cell line in TEM assays. Antibodies against αv or IAP inhibited transmigration of β3-positive monocytes. Moreover, transfection of the β3 chain into β3-deficient cells with subsequent expression of β3 integrins conferred on these cells an enhanced ability to transmigrate. In the process of elucidating the mechanism of this enhanced transmigration, we found that β3 integrin-positive monocytic cells preferentially transmigrated through ICAM-1–expressing cell monolayers. Subsequent studies of monocyte locomotion on recombinant ICAM-1 and adhesion assays revealed a cross talk mechanism between αvβ3 integrin and αLβ2 integrin on monocytes which affects monocyte binding to and migration on ICAM-1.
Our results point to a role for the αvβ3 integrin in β2 integrin-dependent migration of monocytes on ICAM-1, which could be a mechanism that enables monocytes to overcome tight adhesion to endothelial ICAM-1 under inflammatory conditions and engage in subsequent TEM.
Materials and Methods
Cell Lines
J774.2 and WEHI-3 murine monocytic cell lines were obtained from American Type Culture Collection (Rockville, MD). The mouse endothelioma cell line e.end2 was from W. Risau (Max-Planck, Bad Neuheim, Germany). Untransfected L cells and L cells transfected with full-length CD31 were obtained from S. Albelda (The Wistar Institute, Philadelphia, PA) and have previously been described (14). The L cells transfected with ICAM-1 were obtained from C. Figdor (University Hospital, Nijmengen, The Netherlands). The THP-1 human monocytic cell line was obtained from the lab of A. Lanzavecchia (The Basel Institute for Immunology, Basel, Switzerland).
Medium and Reagents
J774.2, e.end2, untransfected L cells, and L cells transfected with CD31 were grown in DME media (GIBCO BRL, Paisley, Scotland) supplemented with 10% FCS (GIBCO BRL, Auckland, New Zealand), nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 mg/ml streptomycin (all from GIBCO BRL, Auckland) and 5 × 10−5 M 2-mercaptoethanol (Fluka, Buchs, Switzerland). WEHI-3 cells were grown in Iscove's modified Dulbecco's (IMDM) media (GIBCO BRL, Paisley) supplemented as above. Murine β3-transfected WEHI-3 cells were cultured in IMDM with 0.5 g/liter geneticin, (G418 sulfate from Calbiochem-Novabiochem Corp., La Jolla, CA). L cells transfected with ICAM-1 were cultured in IMDM with 1 g/liter of G418. The human monocytic cell line THP-1 was cultured in RPMI (GIBCO BRL, Paisley) with 10% FCS. Human unbilical vein endothelial cells (HUVEC) cells were obtained from U. Vischer (Centre Médical Universitaire, Geneva, Switzerland) at first or second passage.
The mouse soluble recombinant adhesion molecules ICAM-1, PECAM-1, and VCAM-1 have been previously described (51). Soluble recombinant human ICAM-1 was obtained from J.E. Meritt (Roche Products Ltd., Herts, UK). The mouse and human chemokine MCP-1 used in the transmigration assays were from R&D Systems, Inc. (Abingdon, UK). Mouse and human TNF-α and mouse laminin were all from GIBCO BRL (Paisley). Human plasma fibronectin and human plasma vitronectin were from Collaborative Research (Bedford, MA). BSA was from Sigma Chemical Co. (Buchs, Switzerland).
Other Reagents and Antibodies
For FACS® analysis the following antibodies were used: anti-β3, anti-αM, anti α4, anti-CD31 (all from PharMingen, San Diego, CA), anti-MHC class II, anti-αv, anti-IAP, anti-α6 (EA-1) (57) and anti-αL (see below). For TEM and migration assays on ICAM-1, only affinity-purified preservative-free antibodies were used. The anti-mouse antibodies were anti-αv integrin (RMV-7 from H. Yagita [Juntendo University, Tokyo, Japan]), anti-αL (FD441.8) (61), anti-α4 (PS/2) (48), anti-IAP (MIAP 301) (43), anti MHC class II (M5/114) ATCC TIB 120, and anti-α6 (GoH3) (53). The anti-human antibodies directed against the integrins β1 (JB1a), β2 (P489-A11), αvβ3 (LM609), αvβ5 (P1F6), and αv (CLB-706), and anti-MHC class I were all from Chemicon (Temecula, CA). Anti-human IAP (B6H12) has previously been described (7, 26). The anti-αL subunit function blocking antibody (mAb 38) was from the lab of N. Hogg (Imperial Cancer Research Fund, London, UK) (52).. For cross-linking experiments, the following secondary affinity-purified preservative-free polyclonal antibodies were used: Fc fragment-specific goat anti–rat IgG and Fc fragment-specific goat anti–mouse IgG (Chemicon). Rabbit antibodies against human fibronectin (Sigma Chemical Co., St. Louis, MO) or against human fibrinogen (Dako A/S, Copenhagen, Denmark), both cross-reactive with the mouse proteins, were used in the immunofluorescent studies. The secondary reagent was a FITC-labeled goat anti–rabbit antibody (Southern Biotechnologies, Birmingham, AL).
Isolation of Human Peripheral Blood Monocytes
Human blood from healthy donors was collected with heparin (Liquemin; Roche). Peripheral blood mononuclear cells were separated from whole blood by density gradient centrifugation using Ficoll-hypaque (Pharmacia Biotech., Inc., Dübendorf, Switzerland). Monocytes were then separated from the lymphocytes using a Percoll gradient (Pharmacia Biotech., Inc.). The isolated monocytes were used within 48 h for TEM assays or FACS® analysis, and cultured in RPMI medium with 10% FCS (Boehringer Mannheim, Mannheim, Germany).
Stable Transfection of the β3 Integrin Chain into WEHI-3 Cells
A 2.6-kb cDNA fragment containing the entire mouse β3 integrin coding region was excised from the pBluescript II KS− vector with BamHI and XhoI and then inserted into the pcDNA3 vector (Invitrogen, Leek, The Netherlands). WEHI-3 cells were transfected using the lipofectamine method. Briefly, 12 μg of DNA in a 50-μl volume was mixed with 30 μl of lipofectamine (GIBCO BRL, Basel, Switzerland) in a total volume of 100 μl with distilled water. After a 15-min incubation at room temperature, this was added dropwise to 5 × 106 WEHI-3 cells in 3 ml Opti-MEM (GIBCO BRL, Paisley). After 24 h at 37°C without serum, 3 ml of medium containing 20% FCS was added and cells were left for another 24 h at 37°C. Cells were then harvested and cultured in medium with 500 mg/ml geneticin and seeded at limiting dilution into 96-well plates. Colonies were picked 14 d later. Cell clones were expanded individually and clones expressing the β3 integrin chain were selected by FACS® analysis. These were then expanded further.
Flow Cytometry
Suspension and trypsinized adherent cells were collected and resuspended in Dulbecco's PBS with 1% BSA. Cells (105 per sample) were washed twice in this medium and then resuspended in DPBS/BSA with saturating amounts of mAbs. After a 30-min incubation at 4°C, cells were washed twice in DPBS/BSA and then resuspended in staining solution containing FITC-labeled goat anti–rat IgG (Jackson ImmunoResearch, Milan, Italy and Analytica, La Roche, Switzerland) for rat monoclonals, FITC-labeled goat anti–hamster IgG for hamster monoclonals, or FITC-labeled goat anti–rabbit IgG for antibodies raised in rabbit (Southern Biotechnologies, Birmingham, Alabama). After another 30-min incubation at 4°C, cells were washed twice, resuspended in the staining solution containing 0.1% propidium iodide, and then analyzed by flow cytometry (FACScan®; Becton Dickinson Co., Mountain View, CA). Control cell suspensions were incubated with secondary antibody alone.
Transendothelial Migration Assay
Transwell culture inserts of 24-well tissue culture plates (6.5-mm-diam polycarbonate membranes with 5 μm diameter pores [Costar Corp., Cambridge, MA]) were coated with 50 mg/ml laminin in Earle's balanced salt solution for 30 min. Excess laminin was removed from the inserts and e.end2 cells at 106/ml were seeded on the inserts in 100 μl of medium. Cells were allowed to grow to confluence on filters for 48 h. Cell confluence was checked by staining some filters with May-Grunwald-Wright- Giemsa solution (Fluka) followed by microscopic control. The cultures were then washed once in DME with 5% FCS and then preincubated in medium with or without 20 ng/ml of TNF-α for 5 h at 37°C, after which the cultures were washed twice with medium.
Cells of the J774.2 or WEHI-3 line were washed once in medium and then adjusted to 106 cells/ml. 100 μl of cell suspension were added per insert. Thereafter, 300 μl of medium were placed into the lower chambers of the Transwells with 125 ng/ml of monocyte chemoattractant protein-1 (MCP-1). The inserts were carefully placed into the lower chambers to avoid air bubbles forming at the interface between the underside of the insert and the medium. Migration was allowed to proceed for 4 h at 37°C. The assay was stopped by removing the medium from the upper well and rinsing the upper surface of the insert twice with 0.2% EDTA in PBS. The number of cells which had migrated into the lower chamber was determined by light microscopy at a magnification of 10. Alternatively for the human cell experiments, HUVECs were cultured for 48 h on filters precoated with 50 μg/ml fibronectin. The rest of the assay was as described before. However, freshly isolated human monocytes were allowed to transmigrate for 2 h.
For antibody-blocking studies, 300 μl of monocytic cells at 106 cells/ml were spun down and re-suspended in 100 μl of medium in an Eppendorf tube (Hamburg, Germany). The antibodies were added to a final concentration of 50 μg/ml. The tubes were then incubated on a shaker for 40 min at 4°C. Before the assay, cells were spun down, washed once in medium, and then resuspended in 300 μl of fresh medium. This was then added to three wells per condition. For each experiment, the number of cells which had transmigrated was expressed as the mean value of cells counted in three wells.
Transmigration through L Cells
Basically, the same method as for endothelial cells was used. Untransfected L cells, L cells expressing PECAM-1, or L cells expressing ICAM-1 were seeded at a concentration of 105 cells per well on laminin-precoated inserts and then allowed to grow to confluence for 48 h. Cultures were washed once in medium and monocytic cells were added in a 100-μl volume to the upper well. Chemokines were used at the same concentration as in the TEM assay. Cell confluence was checked as before. For this experiment, the mean value of transmigrated cells counted in three wells was expressed as a percentage of the total number of cells added per well.
Cell Migration Assay
Single wells of a 96-well plate (Serocluster; Costar Corp.) were coated with 2.4 μM of recombinant soluble adhesion molecules. This concentration has been shown to be the saturating concentration for a single well of a 96-well plate (32). A typical saturating coating consisted of either 100% mouse or human ICAM-1. In the case of mixes, the saturating coating consisted of 99% ICAM-1 and 1% CD31, obtained by mixing 2.376 μM of ICAM-1 and 0.024 μM of CD31. The total protein concentration remained at 2.4 μM. For the control experiments ∼1% vitronectin or ∼1% laminin was used. Wells were coated for 1 h with protein and then blocked for 1 h with 20% BSA, all at room temperature, after which wells were washed three times with serum-free medium. 105 cells in the exponential growth phase were washed once with 500 μl of serum-free medium, resuspended in 100 μl, added to a coated well, and then allowed to adhere at 37°C for 5 min. Nonadherent cells were washed away by gently adding 100 μl of medium to the well and exchanging this volume twice. The plate was then placed under an Axiovert 100 television microscope (Carl Zeiss AG, Jena, Germany) equipped with an incubator chamber. The temperature of the air and the microscope plate was maintained at 37°C by a TRZ 3700 unit and the CO2 level (10 or 5%) was controlled by a CTI controller 3700 (all from Carl Zeiss AG). Continuous recording of cell migration was performed using a 20× objective with video time-lapse equipment.
During incubation, antibodies were added manually in a volume of 10 μl using a Gilson pipette and a curved multiprecision tip (Sorenson Bioscience, Inc., Salt Lake City, Utah). Before the addition of antibody, cells were allowed to migrate on the substrate for 40 min in order to record locomotion in the absence of antibody. After antibody had been added, cell locomotion was recorded for another 40 min. To block molecules on the cell surface, anti-αL or anti-IAP antibodies were added at a final concentration of 50 μg/ml. To cross-link molecules, anti-αv, anti-β2, anti-α6 or anti-major histocompatibility complex (MHC) antibodies were used at a final concentration of 10 μg/ml, together with 10 μg/ml of secondary anti-Fc–specific antibody.
Data Analysis
After completion of the assay, the video was played 60 times faster on a Sony color video television. The displacement of individual cells was traced on transparent write-on films. A minimum of 10 tracks were followed for 30 min in each experiment. Migration distance was measured in centimeters for each track using a curvimeter. Results are expressed in μm/h by using the conversion factor 8 cm = 100 μm.
Immunofluorescent Studies
L cells expressing ICAM-1 were trypsinized and cultured on eight chamber glass slides (Nunc, Inc., Naperville, IL) for 24 h. Cells were fixed in ice-cold acetone for 7 min and then allowed to air dry. Wells were blocked with 10% FCS in PBS for 30 min after which primary antibody was added at a 1:50 dilution in PBS/BSA. This was left at room temperature for 30 min, after which the secondary FITC-labeled antibody was added and left for another 30 min. Each step was followed by a washing step in PBS and distilled water.
Cell Bead Attachment Assay
Ligand-coated beads were prepared as described previously (52). Briefly, 200 μl (108) of 3.2-μm polystyrene beads (Sigma Chemical Co.) were washed twice in distilled water followed by two further washes and resuspension in 0.1 M bicarbonate buffer, pH 9. ICAM-1 or fibronectin (control) was added to the beads at a final concentration of 10 μg/ml. To prepare BSA-coated control beads, they were incubated with 2% BSA. The beads were rotated for 1 h, washed once in PBS and blocked with 2% BSA for 2 h, all at room temperature. Finally, the beads were washed twice in 20 mM Hepes, 140 mM NaCl, and 2 mg/ml glucose, pH 7.4 (assay buffer).
Multiwell Lab-Tek chamber slides (Nunc, Inc.) were coated overnight at 4°C with the following molecules: recombinant ICAM-1; vitronectin; anti-αvβ3, anti-αvβ5, anti-α6, anti-β2, and anti-MHC class I antibodies; all at 50 μg/ml or BSA. The next day, the wells were washed twice with PBS and nonspecific binding sites were blocked with 2% BSA at room temperature for 2 h. THP-1 monocytic cells (150 μl of 2 × 106/ml) in assay buffer were added to the wells and allowed to settle on ice for 15 min. Freshly prepared ligand-coated beads were then added to the wells at a 100:1 bead/cell ratio in 50 μl of assay buffer. After 30 min at 37°C, unbound beads and cells were removed by washing the wells four times in prewarmed assay buffer. Bound cells were fixed with 1% formaldehyde in PBS for 20 min and the cells were then stained with haematoxylin for 10 min. 100 cells were counted under the microscope (40× oil immersion objective; Carl Zeiss AG, Jena, Germany) and the number of beads which had bound to these cells was determined (attachment index). For antibody-blocking studies, anti-αL (mAb38), anti-β1, or anti-α6 was added to the cells at a final concentration of 50 μg/ml and left for 15 min at 4°C before the addition of beads.
Results
The αvβ3 Integrin Is Involved in Monocyte Transendothelial Migraton
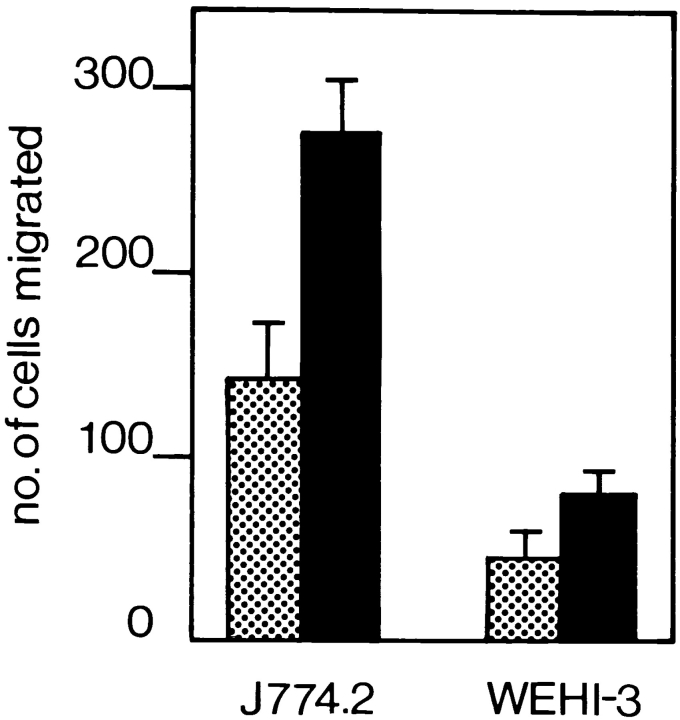
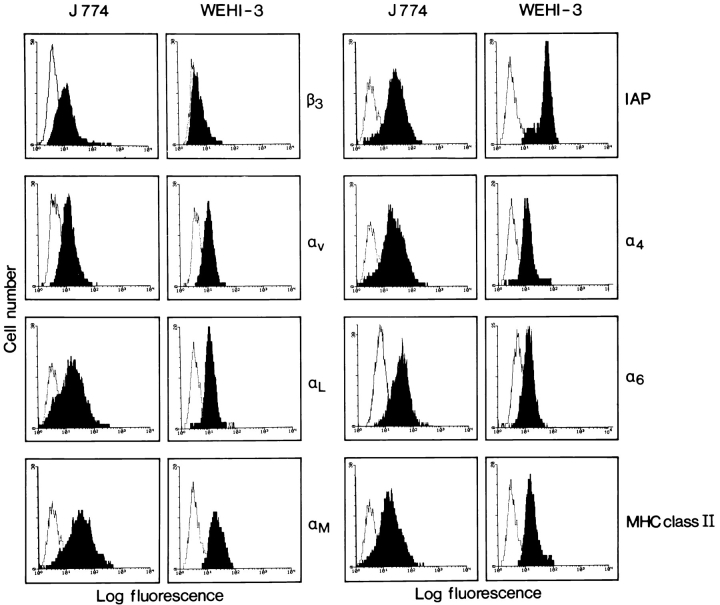
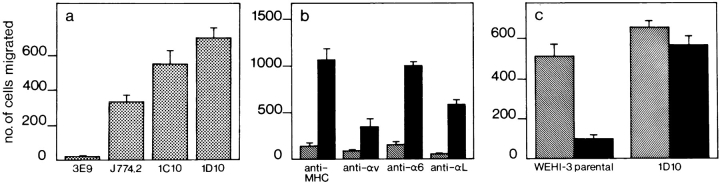
To study molecules involved in TEM we set up an in vitro assay. A murine endothelial cell line was grown to confluence on laminin-precoated polycarbonate filters with defined 5-μm-diam pores. Several murine monocytic cell lines were screened for their ability to transmigrate. In vivo, monocytes preferentially home to acute inflammatory tissue. Inflammation is accompanied by increased expression of both ICAM-1 and VCAM-1 on the endothelium, which are essential molecules for leukocyte TEM (47). We therefore treated the endothelial monolayer with the inflammatory cytokine TNF-α, which led to increased expression levels of ICAM-1 and VCAM-1 as determined by FACS® analysis (data not shown). As a soluble gradient of endogenous chemokine promotes the TEM of monocytes in vitro (54), we included the chemokine MCP-1 in our assay (69). An optimal concentration of 125 ng/ml was chosen because MCP-1 has been shown to be maximally chemotactic at around this concentration (56). As expected, transmigration of monocytes was more efficient through the TNF-α–activated endothelial monolayer. The J774.2 monocytic cell line was able to selectively migrate through the endothelial monolayer, but not through plain filters or filters coated with ECM molecules alone (Fig. 1 and data not shown). In comparison, the WEHI-3 monocytic cell line transmigrated threefold less efficiently through the endothelial monolayer. We performed FACS® analysis on the J774.2 and WEHI-3 cells to quantitate the expression levels of different adhesion molecules known to play a role in leukocyte migration. Although both J774.2 and WEHI-3 cells expressed αL, αM, α4, α6 and αv integrin chains, and IAP, β3 integrin chain expression was markedly low on WEHI-3 cells (Fig. 2). There was no differential expression of PECAM-1 on the two murine cell lines (data not shown).
Figure 1.
In vitro migration of monocytic cells across an endothelial cell layer. Murine monocytic J774.2 and WEHI-3 cells were used in the TEM assay as described in Materials and Methods. J774.2 cells do not transmigrate through either plain filters or ECM-coated filters (data not shown). Transmigration was allowed to proceed across a preestablished layer of unactivated (dotted bars) or TNF-α–activated (solid bars) e.end2 endothelioma cells for 4 h at 37°C. Cells that had passed through the endothelial cell layer into the lower chamber were counted. J774.2 cells transmigrated more efficiently than WEHI-3 cells through nonactivated or activated endothelium. The results are expressed as the arithmetic mean of the number of cells ± SE from three wells per condition. A representative experiment out of three is shown.
Figure 2.
Flow cytometry to determine expression levels of MHC class II, IAP, and the integrin chains α6, β3, αv, αL, αM, and α4 on the J774.2 and WEHI-3 murine monocytic cells. J774.2 cells expressed all the molecules tested, whereas β3 integrin chain expression was markedly low on WEHI-3 cells.
β3 integrins have not previously been described to play a role in monocyte TEM. However, previous experiments have shown that IAP plays a role in the TEM of some leukocyte subsets, whereas others have shown that IAP is necessary for some αvβ3-mediated functions (7). The adhesion molecule PECAM-1, found on circulating leukocytes and endothelial cells, is another molecule involved in TEM (49, 72). We previously demonstrated that some forms of PECAM-1 can interact with the αvβ3 integrin.
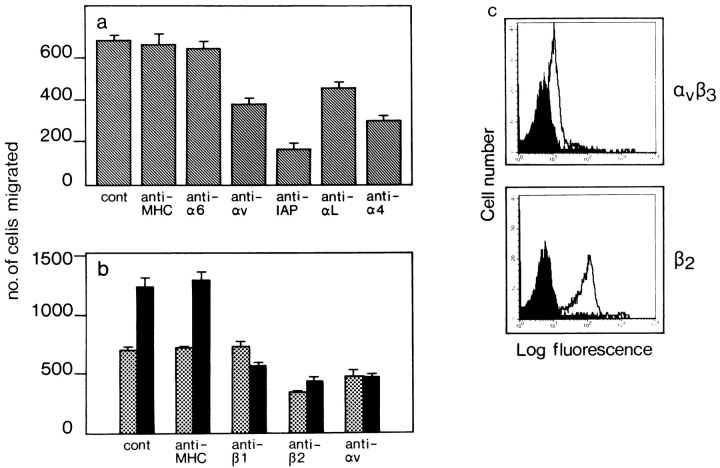
We investigated the effect of antibodies against the αv integrin chain in the in vitro assay. These antibodies were able to block TEM of J774.2 cells by 50% through TNF-α–activated endothelium as shown in Fig. 3 a. Antibodies against IAP, αL, and α4 integrins but not against α6 or MHC class II also blocked TEM under inflammatory conditions. Although these experiments reinstated the importance of IAP, αL, and α4 for monocyte TEM, they also indicated that αv integrins were involved in the process. In a subsequent TEM assay using primary HUVEC cell cultures as the endothelial monolayer, we tested the ability of human peripheral blood monocytes to transmigrate under normal and inflammatory conditions. Antibodies against β2 and αv integrins were able to inhibit TEM across nonactivated endothelium by 50%, whereas anti-β1 had no effect (Fig. 3 b). This was consistent with the fact that nonactivated endothelium does not express the α4β1 ligand VCAM-1. As a result of TNF-α treatment, HUVECs express VCAM-1, and ICAM-1 levels are increased (data not shown). This resulted in a twofold enhancement of TEM which could be inhibited by anti-β1, anti-β2, and anti-αv antibodies (Fig. 3 b). Control anti-MHC class I antibodies had no effect on TEM under either condition. Freshly isolated human monocytes express the αvβ3 integrin albeit at lower levels than β2 integrins (Fig. 3 c).
Figure 3.
Inhibition of murine and human monocyte TEM by antibodies against the αv integrin chain. Details of the TEM assay are described in Materials and Methods. (a) Effect of antibodies on TEM of murine J774.2 monocytic cells across a TNF-α–activated e.end2 endothelial monolayer. TEM in the absence of any antibody (cont) or in the presence of anti-MHC, anti-α6, anti-αv, anti-IAP, anti-αL or anti-α4. (b) Effect of different antibodies on TEM of freshly isolated human peripheral blood monocytes across a layer of nonactivated (dotted bars) or TNF-α–activated (solid bars) HUVEC cells. TEM was allowed to proceed at 37°C for 2 h. Antibodies against β2 or αv blocked TEM of cells under nonactivated conditions, whereas under activated conditions TEM increased and antibodies against β1, β2, or αv were able to block this. Results are the arithmetic means (± SE) of the number of cells from three wells per condition. A representative experiment out of three is shown. (c) FACS® analysis of freshly isolated human peripheral blood monocytes for expression of αvβ3 and β2 integrins. Human monocytes expressed αvβ3 albeit at lower levels than β2 integrins.
Enhanced TEM of WEHI-3 Cells Transfected with Full-length β3 cDNA
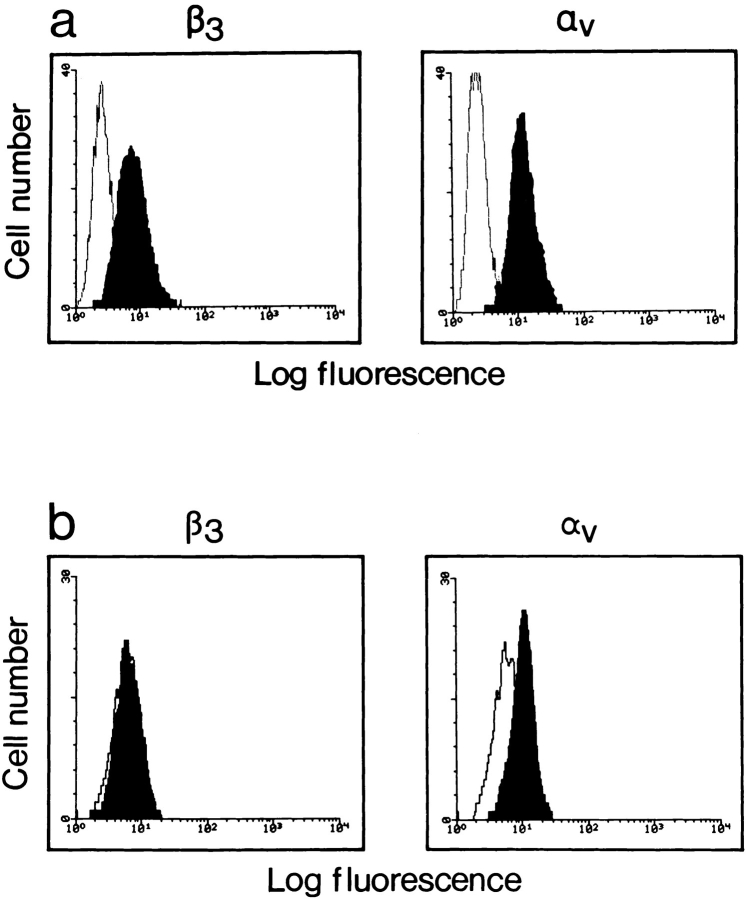
To clarify the importance of the αvβ3 integrin in TEM, β3-deficient WEHI-3 cells were transfected with full-length mouse β3 integrin cDNA. WEHI-3 clone 1D10 expressed β3 integrin chains and also showed increased expression levels of αv as compared with clone 3E9 which did not express β3 integrin chains (Fig. 4). A further WEHI-3 clone (1C10) expressing similar levels of the β3 integrin chain to clone 1D10 (data not shown), was also selected for subsequent TEM assays. The WEHI-3 β3+ clones 1D10 and 1C10 exhibited an enhanced ability to transmigrate under inflammatory conditions as compared with the β3− clone 3E9, and also surpassed J774.2 cells (Fig. 5 a). Furthermore, antibody-blocking studies on clone 1D10 and clone 3E9 cells showed that anti-αL and anti-αv integrin antibodies could inhibit TEM of clone 1D10 cells (Fig. 5 b). An antibody against α6 integrin or MHC class II, had no effect on TEM.
Figure 4.
FACS® analysis of the expression levels of the αv and β3 integrin chains on WEHI-3 cells that had been transfected with full-length mouse β3 cDNA. (a) Clone 1D10 expressed the β3 integrin chain. (b) Clone 3E9 did not express the β3 integrin chain.
Figure 5.
Migration of J774.2 or β3 chain transfected WEHI-3 monocytic cells across TNF-α–activated e.end2 endothelial monolayers. (a) Comparison of TEM of J774.2 and WEHI-3 clones. Clones 1D10 and 1C10 express β3 integrins. Clone 3E9 is a β3 nonexpressing clone. (b) Effect of antibodies against MHC class II, αv, α6, or αL integrins on TEM of WEHI-3 β3+ clone 1D10 cells (solid bars) and WEHI-3 β3− clone 3E9 cells (hatched bars). TEM of clone 1D10 was inhibited by anti-αv or anti-αL integrin antibodies. (c) Comparison of the transmigratory capacity of the WEHI-3 parental cell line with clone 1D10 through laminin-coated filters or activated endothelium. Both the WEHI-3 parental cell line and clone 1D10 transmigrated at comparable levels through laminin-coated filters (hatched bars). However, only clone 1D10 cells were able to transmigrate through activated endothelium (solid bars). Details of the assays are described in Materials and Methods. Results are the arithmetic mean (± SE) of cells from three wells per condition. A representative experiment out of three is shown.
To compare the transmigratory capacity of the WEHI-3 parental cell line with clone 1D10, we compared their ability to transmigrate through an inflammatory endothelium versus plain laminin-coated filters. WEHI-3 and clone 1D10 cells transmigrated at comparable levels through laminin-coated filters. However, only clone 1D10 cells transmigrated significantly through inflammatory endothelium (Fig. 5 c). These experiments demonstrated the importance of the αvβ3 integrin in monocyte TEM.
Transmigration Is Increased through L Cells Expressing ICAM-1
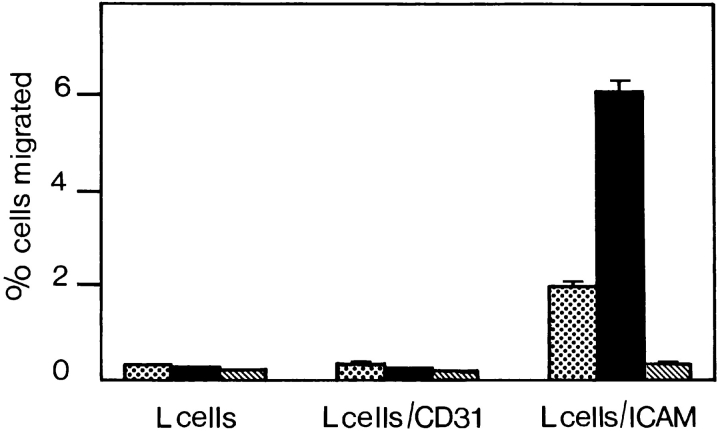
Expression of the αvβ3 integrin is known to be required for cell migration on ECM substrates (11, 22, 58, 59). To examine how the αvβ3 integrin increases cell migration in our studies, we used a modified transmigration assay. Fibroblast type L cells, which express the αvβ3 ligand fibronectin (data not shown) were used in lieu of the endothelial monolayers and grown to confluence on nucleopore filters. Neither J774.2 nor WEHI-3 β3+ clone 1D10 cells were able to transmigrate efficiently through these L cells (Fig. 6). We then used L cells expressing PECAM-1. Again, we could not detect significant transmigration of monocytic cells. In contrast, when L cells expressing ICAM-1 were used in the assay, we found that both J774.2 and WEHI-3 β3+ cells were able to transmigrate very efficiently, though ICAM-1 is not a known ligand for αvβ3 (2% of added J774.2 cells and 6% of added WEHI-3 β3+ cells transmigrated). L cells transfected with ICAM-1 also expressed fibronectin but not fibrinogen (Fig. 7), the latter can act as a bridging molecule between ICAM-1 and the αMβ2 integrin (38). Therefore, it was conceivable that the binding of fibronectin to αvβ3 potentiated the transmigration of monocytes across ICAM-1. However, this implied the existence of a cross talk mechanism between αvβ3 integrin and β2 integrins on the monocyte.
Figure 6.
Transmigration of J774.2 and β3 chain transfected WEHI-3 cells across layers of untransfected L cells, L cells transfected with CD31, or L cells transfected with ICAM-1. Transmigration required the expression of the β3 integrin chain on monocytes (J774.2, dotted bars and WEHI-3 β3+, solid bars) as well as the expression of ICAM-1 on L cells. Under no circumstance did transmigration of WEHI-3 β3− cells occur efficiently (hatched bars). Details of the assay are described in Materials and Methods. Cell migration is expressed as a percentage of the total number of cells added per well. The data represent the arithmetic mean (± SE) of cells from three wells per condition. A representative experiment out of three is shown.
Figure 7.
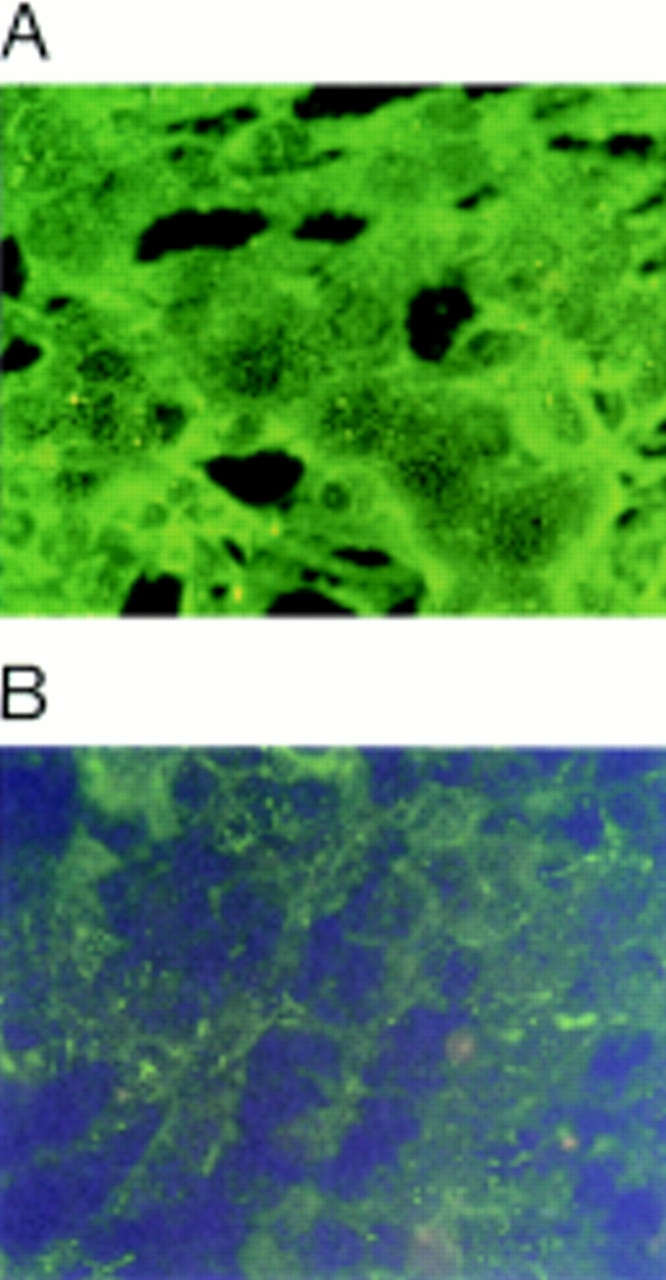
Immunofluorescent studies on L cells transfected with ICAM-1 for expression of fibronectin and fibrinogen. L cells were cultured for 24 h and tested for fibronectin or fibrinogen expression as described in Materials and Methods. L cells transfected with ICAM-1 expressed fibronectin (A) but not fibrinogen (B).
Monocyte Migration on Recombinant Molecules
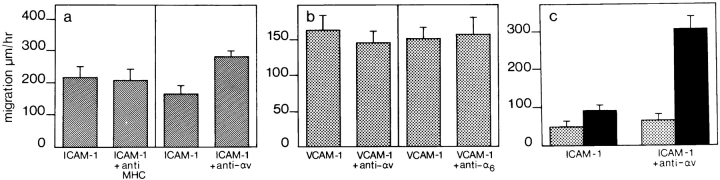
To investigate a potential cross talk mechanism, we analyzed the migratory behavior of WEHI-3 β3+ cells on recombinant ICAM-1. Recombinant ICAM-1 was coated on plastic at a concentration of 2.4 μM, which has been determined to be the saturating protein concentration for these assays (32). Furthermore, this concentration is consistent with the expression levels of ICAM-1 on cytokine activated e.end2 monolayers as determined by ELISA (data not shown). Monocytes are able to migrate on recombinant ICAM-1 and this migration could be decreased by antibodies against the αL integrin chain but not with antibodies against MHC class II molecules (Fig. 8 a). In the first 10 min after the addition of anti-αL, the cells begin to lose their adherent morphology and start to round up. They reduce their velocity of migration and reach a stationary phase 50 min after the addition of antibody. Cell migration was recorded during the first 40 min of the experiment.
Figure 8.
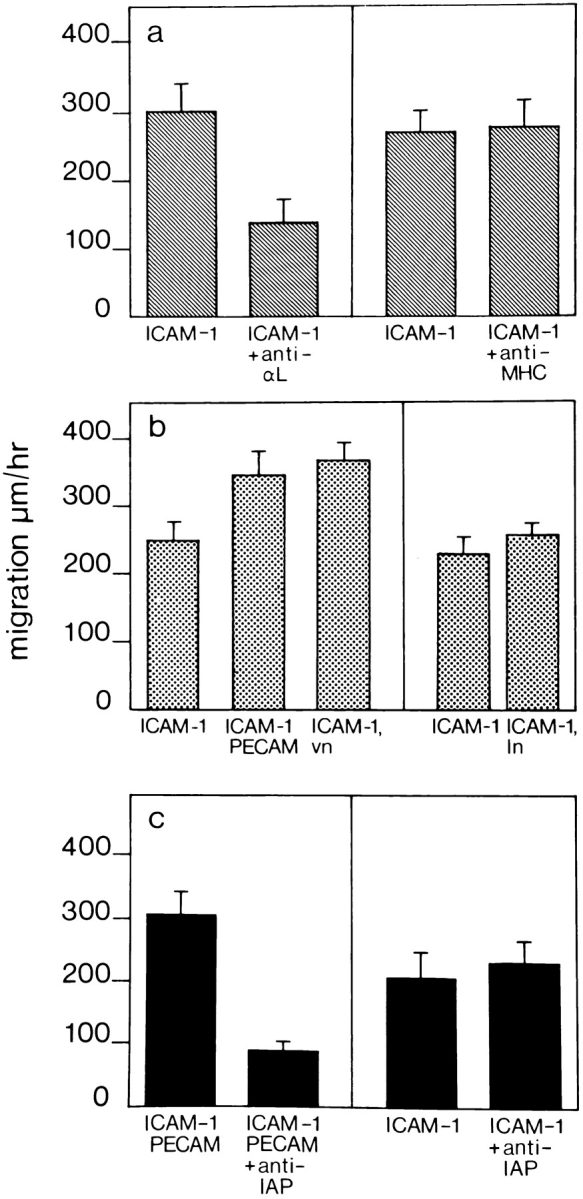
WEHI-3 β3+ monocyte migration on recombinant ICAM-1. Recombinant ICAM-1 was coated at 2.4 μM in a single well of a 96-well plate. (a) The locomotion of monocytic cells without the addition of antibody (ICAM-1) or in the presence of anti-αL (ICAM-1 + anti-αL) or anti-MHC class II (ICAM-1 + anti-MHC) antibodies, was assessed by time-lapse video microscopy. (b) A small concentration of PECAM-1 (ICAM-1, PECAM) or vitronectin (ICAM-1, vn), ligands for αvβ3 integrin, coated together with ICAM-1 significantly increased the speed of locomotion of monocytes compared with migration on ICAM-1 alone (ICAM-1). In contrast, coating ICAM-1 with laminin (ICAM-1, ln) did not affect monocyte locomotion. (c) Migration on a mixture of ICAM-1 and PECAM-1 could be decreased by anti-IAP (ICAM-1, PECAM + anti-IAP). However, migration on ICAM-1 alone was not affected by anti-IAP antibodies (ICAM-1 + anti-IAP). The experiments were performed as described in Materials and Methods. The data represent the mean speed of locomotion (± SE), determined independently of ten different migrating cells for each condition. A representative experiment of three is shown in each case.
When a low concentration of PECAM-1 or vitronectin, also a ligand for αvβ3, was coated together with ICAM-1, the speed of cell locomotion increased (Fig. 8 b). In contrast, coating the same concentration of laminin together with ICAM-1 had no effect (Fig. 8 b). Subsequently, cell locomotion on a mixture of ICAM-1 and PECAM-1 could be inhibited by antibodies against IAP, the protein associated with some functions of the αvβ3 integrin. In contrast, anti-IAP did not decrease the speed of monocyte locomotion on ICAM-1 alone (Fig. 8 c). In these experiments, whereas ICAM-1 coating was at 99% saturation, coating of PECAM-1, vitronectin, or laminin was at 1% saturation. The 1% saturation of different proteins alone does not support cell adhesion or migration on surfaces (data not shown).
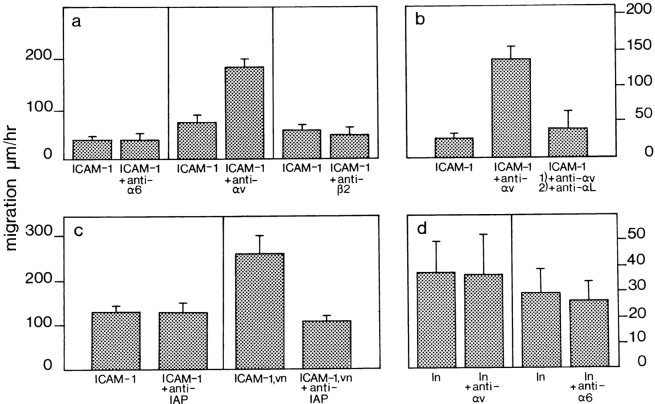
In addition, we observed a 1.4-fold increase in cell migration on ICAM-1 alone when αv was cross-linked on the cell surface by antibodies (Fig. 9 a). Cross-linking antibodies against MHC class II had no effect. To determine whether the effect of αv cross-linking on monocytes was specific for β2 integrins, or if it could also influence the activity of other integrins important in TEM such as α4β1, we looked at monocyte migration on recombinant VCAM-1. As can be seen in Fig. 9 b, cross-linking αv on monocytes migrating on VCAM-1 failed to increase their speed of locomotion and cross-linking the α6 integrin chain as a control also had no effect. Surface molecule cross-linking was done in the presence of a low concentration of primary antibody (10 μg/ml) plus a secondary anti-Fc antibody (10 μg/ml), to ensure capping of the integrin/MHC on the cells for lateral migration. This is contrary to the effect of antibodies used in the TEM-blocking studies. There, 50 μg/ml of primary antibody alone was used, to ensure blocking and not capping of cell surface molecules.
Figure 9.
Migration of WEHI-3 β3+ monocytic cells on recombinant ICAM-1 is affected by cross-linking the αv integrin chain. (a) Cross-linking antibodies against αv (ICAM-1 + anti-αv) increased the speed of cell locomotion on ICAM-1 from values in the absence of αv cross-linking (ICAM-1), whereas cross-linking MHC class II (ICAM-1 + anti-MHC) on the cells had no effect. (b) Monocyte migration on saturating concentrations of VCAM-1 was not enhanced by cross-linking αv (VCAM-1 + anti-αv) or α6 (VCAM-1 + anti-α6) integrins. (c) Comparison of locomotion of β3+ clone 1D10 (solid bars) and β3− clone 3E9 (dotted bars) monocytic cells on recombinant ICAM-1 before (ICAM-1) and after (ICAM-1 + anti-αv) cross-linking of the αv integrin chain. Locomotion of clone 3E9 cells was not affected by αv integrin cross-linking. The experimental procedure is described in Materials and Methods. The data represent the mean speed of locomotion (± SE) determined independently, of ten different migrating cells for each condition. A representative experiment of three is shown.
Finally, we compared locomotion of cells of the WEHI-3 β3+ clone 1D10 with cells of the WEHI-3 β3− clone 3E9, on recombinant ICAM-1. Clone 1D10 and clone 3E9 cells migrated at comparable levels on ICAM-1 alone. However, after αv cross-linking, locomotion was enhanced only with cells of clone 1D10 (Fig. 9 c). Clone 3E9 cells did not respond to cross-linking of the αv integrin chain.
These experiments were repeated with the human monocytic cell line THP-1, which expresses αLβ2 and αvβ3 integrins (tested by FACS®, data not shown). From Fig. 10 a, it is clear that locomotion of THP-1 monocytic cells on recombinant ICAM-1 was increased 2.5-fold upon cross-linking of the αv integrin chain, but cross-linking the α6 or β2 integrin chains had no effect. (The anti-mouse α6 antibody recognizes α6 integrins on THP-1 cells as detected by FACS®; data not shown). In a further experiment, the effect of blocking αLβ2 on monocytic cells after enhancing their migration on ICAM-1 by cross-linking αv, was assessed by adding an anti-αL antibody. As can be seen in Fig. 10 b, cell motility on ICAM-1 returned to control levels after addition of anti-αL, an indication that modulation of cell migration on ICAM-1 by αv is dependent on the function of the αLβ2 integrin. THP-1 cell migration was also enhanced twofold on a mixture of ICAM-1/vitronectin which could be decreased by antibodies against IAP. Again, anti-IAP had no effect on monocyte migration on ICAM-1 alone (Fig. 10 c). Finally, as a control for integrin cross talk on monocytic cells, we looked at the effect of cross-linking αv on THP-1 cells migrating on laminin to determine whether αv integrins could influence α6 integrins. As can be seen from Fig. 10 d, background migration on laminin was low. However, there was no increase in cell locomotion of monocytes on laminin after cross-linking the αv integrin chain.
Figure 10.
Human THP-1 cell migration on ICAM-1 can be regulated by cross-linking αv. Recombinant ICAM-1 was coated at a saturating concentration of 2.4 μM as before. (a) Cross-linking antibodies against αv (ICAM-1 + anti-αv) increased cell locomotion from control values for ICAM-1 alone (ICAM-1), whereas cross-linking antibodies against α6 (ICAM-1 + anti-α6) or β2 (ICAM-1 + anti-β2) integrins had no effect. (b) The enhanced migration on ICAM-1 after cross-linking the αv integrin (ICAM-1 + anti-αv) could be decreased by antibodies against the αL integrin chain (ICAM-1 + anti-αv, + anti-αL). (c) Locomotion of THP-1 on a mixture of ICAM-1/vitronectin (ICAM-1, vn) was decreased by an antibody against IAP (ICAM-1, vn + anti-IAP), whereas locomotion on ICAM-1 alone (ICAM-1) was not affected by this antibody (ICAM-1 + anti-IAP). (d) THP-1 cell migration on laminin (ln) was not enhanced by cross-linking αv (ln + anti-αv) or cross-linking α6 (ln + anti-α6) integrins. These experiments were performed as described in Materials and Methods. The data represent the mean speed of locomotion (± SE), determined independently of ten different migrating cells for each condition. A representative experiment of three is shown.
Effect of αvβ3 Integrin Occupancy on ICAM-1 Binding
To determine the effect of αvβ3 occupancy on the function of β2 integrins, we investigated the ability of THP-1 monocytic cells to bind beads coated with ICAM-1 upon adherence to immobilized BSA, anti-MHC class I, ICAM-1, vitronectin, or antibodies against the integrins αvβ5 (THP-1 cells express αvβ5; data not shown), αvβ3, α6, and β2. The data summarized in Fig. 11 a show that monocytes adherent on anti-MHC class I, anti-αvβ5, anti-α6, and anti-β2 antibodies or ICAM-1, bound ICAM-1–coated beads at comparable levels. However, if the cells were allowed to interact with an anti-αvβ3 antibody or vitronectin, significantly fewer ICAM-1–coated beads were bound. The epitope for the anti-αvβ3 antibody used here (LM609), is near the Arg-Gly-Asp binding site of the integrin (4). Therefore, binding of the antibody to the integrin could mimic integrin occupancy. As a control, the ability of THP-1 cells to bind fibronectin-coated beads under similar conditions was assessed. Cells immobilized on anti-αvβ3 or vitronectin bound similar numbers of fibronectin coated beads as compared with cells immobilized on other substrates (Fig. 11 b). Hardly any monocytes adhered to BSA (data not shown) and there was only negligible binding of BSA-coated beads to monocytes immobilized on the different substrates (Fig. 11 b).
Figure 11.
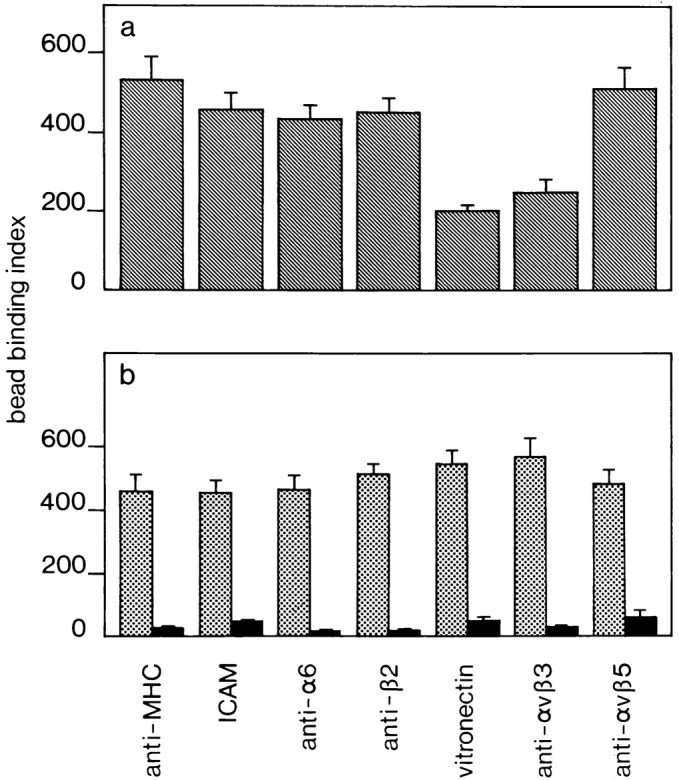
Inhibition of the binding of ICAM-1–coated beads to THP-1 cells immobilized on vitronectin or anti-αvβ3 antibody. (a) Cells were immobilized on plastic coated with anti-MHC class I, ICAM-1, anti-α6, anti-β2, vitronectin, anti-αvβ3, or anti-αvβ5 and incubated with ICAM-1–coated beads. Cells immobilized on anti-αvβ3 or vitronectin bound less ICAM-1–coated beads. (b) Cells adhered to the above substrates were incubated with fibronectin- (dotted bars) or BSA-coated beads (solid bars). THP-1 cells immobilized on different substrates displayed no differential ability to bind either fibronectin- or BSA-coated beads. The bead binding assays were performed as described in Materials and Methods. Data are expressed as a binding index, that is the number of beads bound to 100 cells. Data represent the mean of ten high power fields ± SE. A representative experiment of three is shown.
Last but not least, we tested whether anti-αL integrin antibodies could block the binding of ICAM-1–coated beads to THP-1 cells adherent on ICAM-1. As can be seen from Fig. 12 b, addition of 50 μg/ml of this antibody dramatically reduced binding of ICAM-1–coated beads to the cells. On the other hand, addition of a control antibody against α6 integrin had no effect (Fig. 12 a). Moreover, an anti-β1 integrin antibody reduced binding of fibronectin coated beads to THP-1 cells immobilized on ICAM-1 (Fig. 12 d), whereas the anti-α6 antibody again had no effect (Fig. 12 c).
Figure 12.
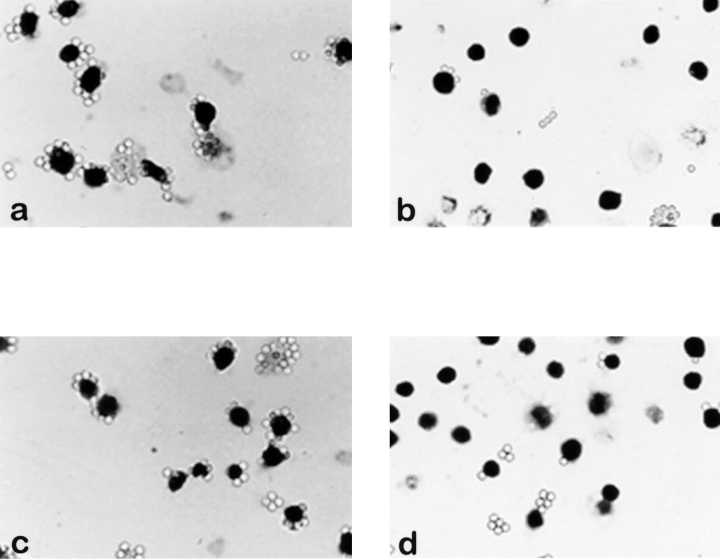
Photomicrographs showing the attachment of ICAM-1– or fibronectin-coated beads to THP-1 cells immobilized on ICAM-1. THP-1 cells that had adhered to ICAM-1 were treated with (a) anti-α6 or (b) anti-αL integrin antibodies at 50 μg/ml before incubation with ICAM-1–coated beads. Less ICAM-1– coated beads bound to THP-1 cells in the presence of anti-αL. Cells adherent to ICAM-1 were treated with anti-α6 (c) or anti-β1 (d) integrin antibodies at 50 μg/ml before incubation with fibronectin-coated beads. Fibronectin-coated bead binding was reduced in the presence of anti-β1 antibodies.
Discussion
Although much is known about the rolling and tight adhesion steps before TEM, little is known about the events that lead to transition from tight adhesion to migration of a leukocyte on the apical surface of the endothelium and subsequent diapedesis between the endothelial cells to the basal side of the blood vessel wall. The β1 and β2 integrins mediate tight adhesion of the leukocyte to inflammatory vascular endothelium. However, induction of TEM requires a dynamic regulation of adhesion of these integrins to their respective ligands. Our results indicate that occupancy of αvβ3 integrin on monocytes can modulate β2 integrin-dependent adhesion to and migration on ICAM-1. This could be a mechanism which enables monocytes to overcome tight adhesion to endothelial ICAM-1 under inflammatory conditions and engage in subsequent TEM.
J774.2 monocytic cells expressing the αvβ3 integrin transmigrated through TNF-α–activated endothelium, whereas WEHI-3 cells deficient in this integrin were hampered in the process. TEM of J774.2 cells could be partially blocked under inflammatory conditions by antibodies against IAP, α4β1, αLβ2 and αv integrins. TEM assays carried out with primary human monocytes reinstated that β2 and αv integrins are important in this process. Transfection of β3 integrin chain cDNA into WEHI-3 cells resulted in expression of the αvβ3 integrin on the cell surface. These cells were then able to engage in enhanced TEM through TNF-α–activated endothelium which could be inhibited by antibodies against αL or αv. Although these experiments demonstrate the importance of the αvβ3 integrin in monocyte TEM, they do not reveal how the integrin is involved in the process. The integrin αvβ3 can mediate cell spreading and migration on immobilized vitronectin (41, 42), and is a molecule involved in tumor metastasis (63). The integrin is also upregulated on proliferating endothelial cells (24), and initiates a Ca+2-dependent signaling pathway that leads to endothelial cell migration and the process of angiogenesis (6, 42). To study how the αvβ3 integrin is involved in TEM, we modified the transmigration assay by using L cells instead of e.end2 cells. Neither e.end2 cells nor L cells form tight junctions, but grow to confluence on laminin-coated filters in 48 h. L cells express fibronectin, an αvβ3 integrin ligand. However, transmigration of αvβ3 integrin-positive monocytic cells was low through untransfected L cells or L cells expressing PECAM-1. This demonstrated that simply the presence of αvβ3 ligands could not ensure efficient transmigration. Surprisingly, however, β3+ monocytic cells were able to transmigrate effectively through ICAM-1–expressing L cells which also expressed fibronectin. ICAM-1 is not a known ligand for αvβ3 but can bind fibrinogen which in turn can interact with αMβ2 on leukocytes (15, 39). This αMβ2-fibrinogen–ICAM-1 association is able to mediate leukocyte TEM (38). However, since our L cells did not express fibrinogen, we ruled out this mechanism. We speculated instead that perhaps the binding of αvβ3 to fibronectin was enhancing β2 integrin-mediated migration of the monocytes on ICAM-1.
We used time-lapse video microscopy studies to test this hypothesis. Both murine and human monocytic cells engage in αLβ2-dependent migration on recombinant ICAM-1. Cocoating a ligand for αvβ3 or cross-linking the αv integrin to mimic αvβ3 integrin occupancy increased the speed of monocyte locomotion on ICAM-1. The αv chain can associate with other β chains such as β1, β5, and β8 (16, 31, 68). However, cross-linking the αv integrin chain on β3 integrin-deficient WEHI-3 monocytic cells failed to enhance their locomotion on ICAM-1, indicating that β3 is the essential partner chain for αv in αv-mediated monocyte motility on ICAM-1. The αvβ3 integrin is functionally associated with IAP (7), since IAP has been shown to be necessary for some β3 integrin-dependent functions (43). The effect of anti-IAP antibodies on the migration of monocytes on the mix of ICAM-1 and PECAM-1 or ICAM-1 and vitronectin suggests that IAP is also involved in αvβ3 integrin- mediated locomotion on ICAM-1. However, as anti-IAP antibodies were able to block the TEM of monocytes to a greater degree than anti-αv antibodies alone, it is likely that IAP may also have an αvβ3-independent function in leukocyte TEM.
We previously showed that cross-linking the αv integrin chain on T lymphocytes regulates α4β1 function and cell migration on VCAM-1 (32). However, in the present study cross-linking αv on monocytes migrating on VCAM-1 did not affect their speed of locomotion, indicating that the activity of α4β1 on monocytes is not influenced by occupancy of the αvβ3 integrin. We also examined whether αv integrins could influence α6 integrins to rule out a nonspecific cross talk mechanism between αv integrins and other integrins on the cell. Cross-linking αv on THP-1 cells migrating on laminin had no effect on their speed of locomotion. Finally, to prove that the increase in monocyte locomotion on ICAM-1 is not an artifact of integrin cross-linking, we cross-linked MHC class II on murine monocytic cells and chains from two other integrins on THP-1 cells. Cross-linking of MHC, α6, or β2 failed to have any effect on the migration of monocytic cells on ICAM-1. Thus, cross-linking specifically the αv integrin chain on monocytic cells enhanced their migration on ICAM-1, and although the αvβ3 integrin modulated β2 integrin function, it did not modulate the function of either α4β1 or α6 integrins on these cells.
The β2 integrins αMβ2 and αLβ2 are both expressed on the monocytic cells used in our assays. Does αvβ3 modulate one or both of these integrins? The focus of our present study was the αLβ2 integrin. ICAM-1 has five tandemly repeated Ig-like domains (21, 27), and whereas the binding site for αLβ2 is on the first two Ig-like domains (67), the binding site for αMβ2 is on the third Ig-like domain (18). The recombinant murine ICAM-1 used in our experiments consists of just the first two Ig-like domains which lack the αMβ2 binding site but supports murine monocyte migration, which could be decreased by anti-αL antibodies. Furthermore, anti-αL antibodies also decreased the enhanced migration of human THP-1 cells on ICAM-1 brought about by cross-linking the αv integrin chain. Therefore, we concluded that αLβ2 is a candidate β2 integrin that responds to occupancy of αvβ3. This does not rule out that αvβ3 integrin occupancy may also affect the activity of αMβ2 in vivo.
The integrin αLβ2 forms tight interactions with endothelial ICAM-1. Cell adhesion is regulated both by the affinity of the extracellular regions of integrins for their ligands and by intracellular integrin–cytoskeletal associations (29). The strength of adhesion between cell surface receptors and the substrate is therefore a key factor in the migration process (30). Previous studies have indicated an inverse correlation between adhesion and cell migration (19). Studies on the αIIbβ3 integrin revealed that high- affinity states of the receptor results in a decrease in the migration rate of the cell (29), or locking β1 integrins in a state of high avidity by using activating β1 mAb inhibits leukocyte extravasation (35). Thus, tight adhesion of receptors to their substrates is detrimental for cell locomotion. Therefore, it seemed likely that if αvβ3 occupancy could modulate monocyte locomotion on ICAM-1, this occupancy must lead to a deadhesion between αLβ2 and ICAM-1. Monocytes adherent on anti-αvβ3 or vitronectin were less efficient in binding ICAM-1–coated beads than monocytes adherent on ICAM-1, anti-αvβ5 or other control substrates. Furthermore, monocytes adherent to anti-αvβ3 or vitronectin do not display differential ability to bind fibronectin-coated beads. This demonstrated that occupancy of αvβ3 integrin on the monocyte can decrease the cell's binding capacity to ICAM-1. ICAM-1–coated bead binding to THP-1 cells could be blocked with antibodies against αL. But occupancy of αvβ3 did not reduce ICAM-1–coated bead binding to the same extent as the anti-αL antibodies. However, if αvβ3 occupancy reduced the interactions between αLβ2 and ICAM-1 totally, the cell would not be able to migrate on the surface of the endothelium, but would detach instead from the vessel wall. Modulation of integrin function is therefore a key concept for cell locomotion. A cell can continuously move forward only if there is a dynamic regulation of integrin mediated adhesion and deadhesion. Chemokines can differentially regulate the avidity of α4β1 integrins by rapidly activating and deactivating them on monocytes and eosinophils (73, 74). No doubt this mechanism contributes to monocyte motility on VCAM-1. We previously showed that the αvβ3 integrin can modulate the activity of α4β1 on T lymphocytes and enhance their migration on VCAM-1 (32). Now we demonstrate that αvβ3 can modulate the function of αLβ2 integrins on monocytes and favor their migration on ICAM-1.
How do integrins communicate with each other? Integrins lack intrinsic enzymatic activity to trigger signaling, but several groups have shown that integrin cytoplasmic tails can bind to structural cytoskeletal proteins which in turn interact with components of the intracellular signaling machinery en route to other cell surface receptors (36, 62). Integrins can also interact directly to form cis-acting complexes on the cell surface. The β2 integrins serve as signaling partners for leukocyte receptors in this way. The urokinase plasminogen activator receptor (CD87) and αMβ2 form a functional unit on monocytic cells (64). Interestingly, it has recently been shown that the urokinase plasminogen activator receptor (uPAR) is necessary for αLβ2-mediated leukocyte migration under inflammatory conditions, and monocyte recruitment to sites of inflammation is impaired in the absence of uPAR (46). The urokinase receptor can also associate with β1 and β3 integrins on tumor cells adherent on vitronectin which may regulate tumor cell migration (75). Further work will address the mechanism by which the αvβ3 integrin regulates αLβ2 function on the same cell in monocyte transmigration.
Peripheral blood monocytes express αvβ3 albeit at lower levels than β2 integrins. This level is probably sufficient to mediate the signal required to initiate cell motility on ICAM-1. However, cell motility on the ECM requires high expression levels of the integrin (5, 76). The cytokine granulocyte macrophage colony-stimulating factor (GM-CSF) can upregulate the expression levels of αvβ3 on monocytes (13), and is produced by inflammatory endothelium (50). Interestingly, it has been shown that mice transgenic for the GM-CSF gene develop accumulations of macrophages in tissues (37). Therefore, it is likely that levels of αvβ3 on monocytes immobilized to inflammatory endothelium in vivo is upregulated by the influence of GM-CSF released by the endothelium, which in turn would promote monocyte locomotion on the endothelium and the underlying ECM.
Previous studies have emphasized the requirement for an integrin hierarchy to facilitate the coordinated migration of leukocytes across the endothelium into tissues. The α4β1 integrin is involved in the arrest and initial adhesion of rolling leukocytes to inflammatory endothelium via VCAM-1. Subsequently, αLβ2 mediates tight adhesion of the leukocyte to vascular ICAM-1 after cellular activation (10). The αLβ2 integrin is then able to downregulate α4β1 and cell adhesion to VCAM-1 (52). In the next step of the integrin hierarchy, the αvβ3 integrin downregulates αLβ2 activity, modulating leukocyte adhesion to ICAM-1, and enabling the cell to migrate effectively across the endothelium.
In summary, we show that the αvβ3 integrin is involved in the transition between tight adhesion of monocytes to the vascular endothelium and subsequent diapedesis. This may be an important mechanism not only for the TEM of monocytes but also for other leukocyte subsets that use β2 integrins during transendothelial diapedesis.
Acknowledgments
We would like to thank P. Hammel, G. Wiedle, J.-P. Dangy, B. Ecabert, M. Dessing, S. Meyer, and S. Cooper (all from Basel Institute for Immunology, Basel, Switzerland, except Wiedle from Céntre Medicale Universitaire, Geneva, Switzerland) for their technical assistance. We would also like to thank K. Campbell and R. Torres (both from Basel Institute for Immunology) for reviewing and improving the manuscript, and H. Stahlberger, H. Spalinger, and B. Pfeiffer (all three from Basel Institute for Immunology) for artwork and photography. Finally, we thank U. Vischer for the HUVEC cultures, B. Sinha (both from Basel Institute for Immunology) for his help with experimental protocols, K. Willimann (Theador Kocher Institute, Bern, Switzerland) for reagents, and B. Englehardt (Max Planck Institute, Bad Nauheim, Germany) for advice with the transmigration assays.
This work was supported in part by grants from the Swiss National Science Foundation (3100-049241.96) and the Recherche Suisse contre le Cancer (KFS 412-1-97). The Basel Institute for Immunology was founded and is supported by F. Hoffmann-La Roche AG, CH-4005 Basel, Switzerland.
Abbreviations used in this paper
- ECM
extracellular matrix
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HUVEC
human umbilical vein endothelial cells
- IAP
integrin-associated protein
- ICAM-1
intercellular adhesion molecule-1
- IMDM
Iscove's modified Dulbecco's medium
- MCP-1
monocyte chemoattractant protein-1
- MHC
major histocompatibility complex
- PECAM-1
platelet endothelial cell adhesion molecule-1
- TEM
transendothelial migration
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
References
- 1.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 2.Berlin C, Bargatze RF, Campbell JJ, von Adrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 3.Blystone SD, Graham IL, Lindberg FP, Brown EJ. Integrin αvβ3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor α5β1. J Cell Biol. 1994;127:1129–1137. doi: 10.1083/jcb.127.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), αvβ3 integrin and GPIbα. J Exp Med. 1998;187:329–339. doi: 10.1084/jem.187.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brando C, Shevach EM. Engagement of vitronectin receptor (αvβ3) on murine T cells stimulates tyrosine phosphorylation of a 115-kDa protein. J Immunol. 1995;154:2005–2011. [PubMed] [Google Scholar]
- 6.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 7.Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley CD, Doyonnas R, Newton JP, Blystone SD, Brown EJ, Watt SM, Simmons DL. Identification of αvβ3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- 9.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 10.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 11.Clyman RI, Mauray F, Kramer RH. Integrins have different roles in the adhesion and migration of vascular smooth muscle cells on extracellular matrix. Exp Cell Res. 1992;200:272–284. doi: 10.1016/0014-4827(92)90173-6. [DOI] [PubMed] [Google Scholar]
- 12.Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin-associated protein (CD47) Proc Natl Acad Sci USA. 1995;92:3978–3982. doi: 10.1073/pnas.92.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Nichilo MO, Burns GF. Granulocyte-macrophage and macrophage colony-stimulating factors differentially regulate αv integrin expression on cultured human macrophages. Proc Natl Acad Sci USA. 1993;90:2517–2521. doi: 10.1073/pnas.90.6.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLisser HM, Chilkotowsky J, Yan H-C, Daise ML, Buck CA, Albelda SM. Deletions in the cytoplasmic domain of platelet- endothelial cell adhesion molecule-1 (PECAM-1, CD31) result in changes in ligand binding properties. J Cell Biol. 1994;124:195–203. doi: 10.1083/jcb.124.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond MS, Springer TA. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993;120:545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond MS, Springer TA. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–516. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 17.Diamond MS, Staunton DE, de Fougerolles AR, Stacker SA, Garcia AJ, Hibbs ML, Springer TA. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18) J Cell Biol. 1990;111:3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- 19.Dunlevy JR, Couchman JR. Controlled induction of focal adhesion disassembly and migration in primary fibroblasts. J Cell Sci. 1993;105:489–500. doi: 10.1242/jcs.105.2.489. [DOI] [PubMed] [Google Scholar]
- 20.Dunon D, Piali L, Imhof BA. To stick or not to stick: the new leukocyte homing paradigm. Curr Opin Cell Biol. 1996;8:714–723. doi: 10.1016/s0955-0674(96)80114-1. [DOI] [PubMed] [Google Scholar]
- 21.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL-1 and interferon-γ: tissue distribution, biochemistry and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 22.Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 23.Felding-Habermann B, Silletti S, Mei F, Siu C-H, Yip PM, Brooks PC, Cheresh DA, O'Toole TE, Ginsberg MH, Montgomery AMP. A single immunoglobulin-like domain of the human neural cell adhesion molecule L1 supports adhesion by multiple vascular and platelet integrins. J Cell Biol. 1997;139:1567–1581. doi: 10.1083/jcb.139.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freidlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct αv integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 25.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 26.Gresham HD, Goodwin JL, Allen PM, Anderson DC, Brown EJ. A novel member of the integrin receptor family mediates Arg-Gly-Asp-stimulated neutrophil phagocytosis. J Cell Biol. 1989;108:1935–1943. doi: 10.1083/jcb.108.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horley KJ, Carpenito C, Baker B, Tekei F. Molecular cloning of murine intercellular adhesion molecule (ICAM-1) EMBO (Eur Mol Biol Organ) J. 1989;8:2889–2896. doi: 10.1002/j.1460-2075.1989.tb08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang S, Endo RI, Nemerow GR. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 31.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 32.Imhof BA, Weerasinghe D, Brown EJ, Lindberg F, Hammel P, Piali L, Dessing M, Gisler R. Crosstalk between αvβ3 and α4β1 integrin regulates lymphocyte migration on vascular cell adhesion molecule 1. Eur J Immunol. 1997;27:3242–3252. doi: 10.1002/eji.1830271223. [DOI] [PubMed] [Google Scholar]
- 33.Ishibashi Y, Claus S, Relman DA. Bordetella pertussisfilamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/ CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Issekutz AC, Issekutz TB. Monocyte migration to arthritis in the rat utilizes both CD11/CD18 and very late antigen 4 integrin mechanisms. J Exp Med. 1995;181:1197–1203. doi: 10.1084/jem.181.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuijpers TW, Mul EPJ, Blom M, Kovach NL, Gaeta CA, Tollefson V, Elices MJ, Harlan JM. Freezing adhesion molecules in a state of high-avidity binding blocks eosinophil migration. J Exp Med. 1993;178:279–284. doi: 10.1084/jem.178.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lafrenie RM, Yamada KM. Integrin-dependent signal transduction. J Cell Biochem. 1996;61:543–553. doi: 10.1002/(sici)1097-4644(19960616)61:4<543::aid-jcb7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 37.Lang RA, Metcalf D, Cuthbertson RA, Lyons I, Stanley E, Kelso A, Kannourakis G, Williamson DJ, Klintworth GK, Gonda TJ, Dunn AR. Transgenic mice expressing a hematopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987;51:675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- 38.Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC. Regulation of leukocyte-endothelium interaction and leukocyte transendothelial migration by intercellular adhesion molecule 1-fibrinogen recognition. Proc Natl Acad Sci USA. 1995;92:1505–1509. doi: 10.1073/pnas.92.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Languino LR, Plescia J, Duperray A, Brian AA, Plow EF, Geltosky JE, Altieri DC. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1 dependent Pathway. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 41.Leavesley DI, Ferguson GD, Wayner EA, Cheresh DA. Requirement of the integrin β3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leavesley DI, Schwartz MA, Rosenfeld M, Cheresh DA. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993;121:163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 44.Lindberg FP, Gresham HD, Reinhold MI, Brown EJ. Integrin-associated protein immunoglobulin domain is necessary for efficient vitronectin bead binding. J Cell Biol. 1996;134:1313–1322. doi: 10.1083/jcb.134.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luscinskas FW, Kansas GS, Ding H, Pizcueta P, Schleiffenbaum BE, Tedder TF, Gimbrone MA. Monocyte rolling, arrest, and spreading on IL-4–activated vascular endothelium under flow is mediated via sequential action of L-selectin, β1 integrins, and β2 integrins. J Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.May, A.E., S.M. Kanse, L.R. Lund, R.H. Gisler, B.A. Imhof, and K.T. Preissner. 1998. Urokinase receptor (CD87) regulates leukocyte recruitment via β2 integrins in vivo. J. Exp. Med. In press. [DOI] [PMC free article] [PubMed]
- 47.Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1 and other ligands on endothelium. J Immunol. 1995;154:4099–4112. [PubMed] [Google Scholar]
- 48.Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991;173:599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicola NA. Hemopoietic cell growth factors and their receptors. Annu Rev Biochem. 1989;58:45–77. doi: 10.1146/annurev.bi.58.070189.000401. [DOI] [PubMed] [Google Scholar]
- 51.Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porter JC, Hogg N. Integrin cross talk: activation of lymphocyte function-associated antigen-1 on human T cells alters α4β1- and α5β1-mediated function. J Cell Biol. 1997;138:1437–1447. doi: 10.1083/jcb.138.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price AA, Cumberbatch M, Kimber I, Ager A. Alpha 6 integrins are required for Langerhans cell migration from the epidermis. J Exp Med. 1997;186:1725–1735. doi: 10.1084/jem.186.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Randolph GW, Furie MB. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J Immunol. 1995;155:3610–3618. [PubMed] [Google Scholar]
- 55.Rosales C, Gresham HD, Brown EJ. Expression of the 50k Da integrin-associated protein on myeloid cells and erythrocytes. J Immunol. 1992;149:2759–2764. [PubMed] [Google Scholar]
- 56.Roth SJ, Woldemar M, Carr, Springer TA. C-C chemokines, but not the C-X-C chemokines interleukin-8 and interferon γ inducible protein-10, stimulate transendothelial chemotaxis of T lymphocytes. Eur J Immunol. 1995;25:3482–3488. doi: 10.1002/eji.1830251241. [DOI] [PubMed] [Google Scholar]
- 57.Ruiz P, Wiles MV, Imhof BA. α6 integrins participate in pro-T cell homing to the thymus. Eur J Immunol. 1995;25:2034–2041. doi: 10.1002/eji.1830250735. [DOI] [PubMed] [Google Scholar]
- 58.Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- 59.Salcedo R, Patarroyo M. Constitutive alpha V beta 3 integrin-mediated adhesion of human lymphoid B cells to vitronectin substrate. Cell Immunol. 1995;160:165–172. doi: 10.1016/0008-8749(95)80023-c. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez-Madrid F, Nagy JA, Robbins E, Simon P, Springer TA. A human leukocyte differentiation antigen family with distinct α-subunits and a common β-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983;158:1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanchez-Madrid F, Simon P, Thompson S, Springer TA. Mapping of antigenic and functional epitopes on the α- and β- subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983;158:586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seftor RE, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJ. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA. 1992;89:1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simon DI, Rao NK, Xu H, Wei Y, Majdic O, Ronne E, Kobzic L, Chapman HA. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood. 1996;88:3185–3194. [PubMed] [Google Scholar]
- 65.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 66.Springer TA. Traffic signals for lymphocyte recirculaton and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 67.Staunton DE, Dustin ML, Erickson HP, Springer TA. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990;61:243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- 68.Stewart M, Thiel M, Hogg N. Leukocyte integrins. Curr Opin Cell Biol. 1995;7:690–696. doi: 10.1016/0955-0674(95)80111-1. [DOI] [PubMed] [Google Scholar]
- 69.Uguccioni M, D'Apuzzo M, Loetscher M, Dewald B, Baggiolini M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1a and MIP-1b on human monocytes. Eur J Immunol. 1995;25:64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- 70.van Kooyk Y, van de Wiel-van Kemenade E, Weder P, Huijbens RJF, Figdor CC. Lymphocyte function-associated antigen 1 dominates very late antigen 4 in binding of activated T cells to endothelium. J Exp Med. 1993;177:185–190. doi: 10.1084/jem.177.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Strijp JAG, Russell DG, Tuomanen E, Brown EJ, Wright SD. Ligand specificity of purified complement receptor type 3 (CD11b/CD18, αMβ2): indirect effects of an Arg-Gly-Asp sequence. J Immunol. 1993;151:3324–3336. [PubMed] [Google Scholar]
- 72.Vaporciyan AA, DeLisser HM, Yan HC, Mandiguren II, Thom SR, Jones ML, Ward PA, Albelda SM. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitement in vivo. Science. 1993;262:1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- 73.Weber C, Alon R, Moser B, Springer TA. Sequential regulation of α4β1 and a5β1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weber C, Kitayama J, Springer TA. Differential regulation of β1 and β2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA. 1996;93:10939–10944. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xue W, Mizukami I, Todd RF, III, Petty HR. Urokinase-type plasminogen activator receptors associate with β1 and β3 integrins of fibrosarcoma cells: dependence on extracellular matrix components. Cancer Res. 1997;57:1682–1689. [PubMed] [Google Scholar]
- 76.Zutter MM. Immunolocalization of integrin receptors in normal lymphoid tissues. Blood. 1991;77:2231–2236. [PubMed] [Google Scholar]