Abstract
We show that Bcl-2 expression in skeletal muscle cells identifies an early stage of the myogenic pathway, inhibits apoptosis, and promotes clonal expansion. Bcl-2 expression was limited to a small proportion of the mononucleate cells in muscle cell cultures, ranging from ∼1–4% of neonatal and adult mouse muscle cells to ∼5–15% of the cells from the C2C12 muscle cell line. In rapidly growing cultures, some of the Bcl-2–positive cells coexpressed markers of early stages of myogenesis, including desmin, MyoD, and Myf-5. In contrast, Bcl-2 was not expressed in multinucleate myotubes or in those mononucleate myoblasts that expressed markers of middle or late stages of myogenesis, such as myogenin, muscle regulatory factor 4 (MRF4), and myosin. The small subset of Bcl-2–positive C2C12 cells appeared to resist staurosporine-induced apoptosis. Furthermore, though myogenic cells from genetically Bcl-2–null mice formed myotubes normally, the muscle colonies produced by cloned Bcl-2–null cells contained only about half as many cells as the colonies produced by cells from wild-type mice. This result suggests that, during clonal expansion from a muscle progenitor cell, the number of progeny obtained is greater when Bcl-2 is expressed.
Keywords: apoptosis, Bcl-2, myoblast, myogenin, skeletal muscle
The formation of skeletal muscle fibers proceeds though several distinct stages. In developing vertebrates, the earliest stages of skeletal muscle formation occur in the somites, where muscle precursor cells arise. These precursor cells give rise to myoblasts that subsequently fuse with each other to form multinucleate myofibers (for reviews see Miller, 1992; Stockdale, 1992). At different stages of this myogenic pathway, cells express distinct sets of muscle-specific proteins. Desmin, Myf-5, and MyoD, for example, are expressed at relatively early stages of the myogenic pathway, whereas myogenin, myogenin regulatory factor 4 (MRF4),1 and myosin are expressed at later stages (George-Weinstein et al., 1993; Smith et al., 1993, 1994; Wang and Walsh, 1996). Coexpression of myogenin with p21 appears to mark a stage in the myogenic pathway in which the myoblasts are destined for fusion and terminal differentiation (Skapek et al., 1995; Wang and Walsh, 1996). The muscle-specific neural cell adhesion molecule (NCAM) isoform, β7-integrin, nestin, c-met, and M-cadherin are also expressed by myoblasts (George-Weinstein et al., 1993; Peck and Walsh, 1993; Irintchev et al., 1994; Kachinsky et al., 1994; Cornelison and Wold, 1997). Though c-met, M-cadherin, desmin, Myf-5, and MyoD are expressed at relatively early stages of myogenesis, these proteins are also found in myogenin-expressing myoblasts and/or myotubes at later stages of myogenesis (George-Weinstein et al., 1993; Smith et al., 1993; Kachinsky et al., 1994; Wang and Walsh, 1996; Cornelison and Wold, 1997). Molecular markers that are expressed by muscle cells at only the earliest stages of the myogenic pathway have been lacking.
Because cells in the early stages of myogenesis must be long-lived in vivo (Webster and Blau, 1990) and programmed cell death (apoptosis) can be a feature of normal myogenesis (McClearn et al., 1995) and muscle diseases (Matsuda et al., 1995; Tidball et al., 1995; Sandri et al., 1997; Tews and Goebel, 1997; Vachon et al., 1997), we have examined the expression and function of Bcl-2, an apoptosis-inhibiting protein (for reviews see Korsmeyer, 1995; Kroemer, 1997; Reed, 1997), in muscle cells. Bcl-2 is a well-characterized member of a family of proteins that regulate programmed cell death. When expressed in a number of nonmuscle cell types, Bcl-2 has the ability to suppress or delay apoptosis (Korsmeyer, 1995). In myogenic cells, however, the expression pattern and function of Bcl-2 had not been fully determined.
In this work, we show that only a small percentage of myogenic cells express Bcl-2, and that these Bcl-2–positive cells resist apoptosis and are at an early stage of a process leading from muscle progenitor cell to myotube. In addition, we show that cells from genotypically Bcl-2–null mice form smaller muscle colonies than cells from wild-type mice, indicating that Bcl-2 expression is required for normal growth and/or survival during clonal expansion of muscle cells. Our finding that Bcl-2 functions to protect muscle cells from apoptosis suggests that Bcl-2 expression might be important for long-term survival of muscle cells. Muscle cell apoptosis is found in several neuromuscular diseases, including dystrophin deficiency, merosin deficiency, and spinal muscular atrophy (Matsuda et al., 1995; Tidball et al., 1995; Sandri et al., 1997; Tews and Goebbel, 1997; Vachon et al., 1997). Perhaps muscle cell apoptosis could be ameliorated by altering Bcl-2 family expression. In addition, Bcl-2 expression identifies a new stage of the myogenic pathway, and in cultures that contain cells at multiple stages of the pathway, Bcl-2 expression can be used to distinguish mononucleate cells at an early stage from those at later stages.
Materials and Methods
Cells
C2C12 and Sol8 cells (Yaffe and Saxel, 1977; Blau et al., 1985; Montarras et al., 1991) were maintained in growth medium (DME with 15% fetal bovine serum, 2 mM l-glutamine, 10 mM Hepes, pH 7.4, and 100 U/ml penicillin) and induced to form myotubes in differentiation medium (growth medium with 2% horse serum in place of fetal bovine serum). Cells for initial primary cultures were obtained from >6-wk-old adult CD-1 or C57Bl/6 mice (Charles River Laboratories, Wilmington, MA). For additional cultures, Bcl-2 (+/−) B6,129-Bcl2tm1Sjk mice (Veis et al., 1993; Jackson Laboratories, Bar Harbor, ME) were interbred, and progeny were genotyped (Veis et al., 1993) and used for cell preparation. Apoptosis was induced by transfer to serum-free differentiation medium, with or without 0.5 μM staurosporine, and cell viability was measured by the bulk photometric MTT dye assay of mitochondrial function (Jacobsen et al., 1994).
Myogenic cells from hindlimb muscle of adult and newborn mice were isolated by trypsinization of tissue (Smith et al., 1993) followed by purification on Percoll gradients. Highly enriched myogenic cell populations containing few nonmyogenic cells were collected from the 35–50% Percoll interface of either three-step (35, 50, and 70% Percoll) or two-step (35 and 50% Percoll) gradients as described (Bischoff and Heintz, 1994). In some experiments, nonfractionated cells were also used. Cells were cultured for up to 8 d on ECL Matrix (Upstate Biotechnology, Lake Placid, NY) in DME with 15% horse serum, 3% chicken embryo extract, 2 mM l-glutamine, 10 mM Hepes, pH 7.4, 100 U/ml penicillin, and 1 mM pyruvate. For high-density cultures, cells were seeded at 3,000–5,000 cells/cm2, and for clonal cultures, cells were seeded at 5–17 cells/cm2 on the day of isolation. For comparison of Bcl-2–null and wild-type muscle cells, a two-step plating procedure was used to ensure accuracy of viable cell plating density. Cells were plated at high density on the day of isolation and then, within 24 h, were trypsinized from plates. Viable cells (identified by trypan blue exclusion) were counted and reseeded at high density (320 cells/ cm2) or clonal density (1.7 cells/cm2). Clonal density plates were fixed with paraformaldehyde (see below) after 8 d of growth, immunostained for desmin expression, and counted to determine the number of nuclei per colony and fusion index. Cell genotypes were determined only after counting. Cell proliferation in high-density cultures was monitored over 4 d by scoring cell density using an inverted phase microscope with calibrated field areas. Bulk population doubling time was estimated between 12 and 60 h after replating during the logarithmic phase of rapid cell growth. Statistical analysis was by the appropriate unpaired, two-tailed t test or nonparametric Mann-Whitney test using InStat (version 1.12; Graphpad Software, San Diego, CA).
Immunostaining
The hamster mAb, 3F11 (from C. Milliman and S.J. Korsmeyer), is specific for mouse Bcl-2 (Krajewski et al., 1993) and was used at 25 μg/ml. Mouse mAbs to myosin heavy chain (F59) and myogenin (F5D), and rabbit antisera specific for MyoD, Myf-5, and MRF4 were used as before (Miller, 1990; Smith et al., 1993; Block et al., 1996). A mouse mAb to desmin (Cappel Laboratories, Malvern, PA) was used at 1:40 dilution. Paraformaldehye-fixed and Triton X-100–permeabilized cultures (Smith et al., 1993) were incubated overnight at room temperature with both a rabbit antiserum and the Bcl-2 mAb, washed four times for 20 min each with 0.1% Triton X-100 in PBS, and incubated for 1.5 h at room temperature with a combination of Texas red– or Cy3-conjugated anti–rabbit IgG (Jackson ImmunoResearch, West Grove, PA) and fluorescein-conjugated anti–hamster IgG (Vector Laboratories, Burlingame, CA) at 1.0 μg/ml. For staining of muscle colonies, cells were stained with a 1:1,000 dilution of rabbit anti–desmin (Cappel Laboratories) using a horseradish peroxidase–based detection system (Vectastain Elite kit; Vector Laboratories) with diaminobenzidine substrate (Smith et al., 1994).
To double stain for Bcl-2 and antigens detected by mouse mAbs, cultures were incubated sequentially with (a) the mouse mAb, (b) a lissamine rhodamine–conjugated Fab fragment of goat anti–mouse IgG (Jackson ImmunoResearch) at 10 μg/ml, (c) the hamster anti–Bcl-2 mAb, and (d) fluorescein-conjugated anti–hamster IgG at 1.0 μg/ml. Except where noted, ≥300 Bcl-2–expressing cells in at least two independent cultures were examined. To double stain for bromodeoxyuridine (BrdU) and Bcl-2, cultures that had been incubated with 10μM BrdU were fixed at room temperature for 15 min in 2% paraformaldehyde, permeabilized in 0.1% Triton X-100 for 15 min, and blocked overnight at 4°C (Smith et al., 1993). Fixed cultures were rinsed twice with distilled H2O, incubated in 4 N HCl for 10 min at room temperature, washed twice for 5 min each in PBS, blocked for 1 h at room temperature, and incubated with anti-BrdU (mAb G3G4; Developmental Studies Hybridoma Bank, Baltimore, MD) at 37°C for 1 h at room temperature. After washing, cultures were incubated with Lissamine rhodamine–conjugated goat anti–mouse (Fab fragment; Jackson ImmunoResearch) at 1 μg/ml for 30 min at 37°C, blocked overnight, and incubated sequentially with anti–Bcl-2 mAb and fluorescein-conjugated secondary antibody as above.
Immunoblotting
Cells were scraped into 1 ml of cold PBS and centrifuged at 12,000 rpm for 15 s. Cell pellets or adult mouse thymuses were immediately lysed in ∼2 vol of SDS-PAGE sample buffer, boiled for 4 min, and analyzed by SDS-PAGE in 15% gels (Kachinsky et al., 1994). After SDS-PAGE, proteins were electroblotted to a polyvinyl difluoride membrane for 1.5 h at 75 V. The transfers were dried for 30 min in a vacuum chamber and incubated for 1 h at room temperature with Bcl-2 mAb at 25 μg/ml in Tris-buffered saline with 0.3% Triton X-100 and 1% nonfat dried milk. Antibody binding was visualized using a horseradish peroxidase secondary antibody system (ABC-Elite; Vector Laboratories) with chemiluminescent substrate (ECL; Amersham Corp., Arlington Heights, IL).
RNA Analyses
For reverse transcriptase (RT)-PCR, 5 μg of total RNA from C2C12 cells or 0.2 μg poly (A)+ RNA from adult mouse brain were reverse transcribed using oligo (dT) primers. 1/5 of each cDNA product was subjected to PCR (GeneAmp; Perkin-Elmer Corp, Norwalk, CT). For mouse Bcl-2, the upstream primer was 5′-AGCCCTGTGCCACCATGTGTC-3′, and the downstream primer was 5′-GGCAGGTTTGTCGACCTCACT-3′. The primers are complementary to sequences in two Bcl-2 exons that are separated by a large intron in genomic DNA. The amplified cDNA is 480 bp and includes sequences corresponding to the COOH terminal 153 amino acids encoded by the ∼7.5-kb Bcl-2 mRNA (Negrini et al., 1987). PCR conditions were: 94°C, 5 min; 30 cycles of 94°C, 1 min, 55°C, 1 min, 72°C 1 min; and 72°C, 10 min. Samples (1/5) of each product were analyzed by Southern blotting using an 865-bp HindIII-EcoRI fragment of mouse Bcl-2 cDNA (plasmid 3027, S. Korsmeyer) as probe. Northern blots of total RNA (10 μg/lane) from growing and differentiated C2C12 cells were also probed with this cDNA. Hybridizations were as described for RNA blots (Dominov and Miller, 1996) with final washes in 0.2× SSC, 0.1% SDS, 65°C.
Nomenclature
As in previous work (Miller and Stockdale, 1986; Smith and Miller, 1992), myoblasts are considered to be mononucleate cells that express one or more MRFs, but not skeletal muscle myosin heavy chain (MHC); myocytes are mononucleate cells that express skeletal muscle MHC(s); and myotubes (or myofibers) are multinucleate, nonmitotic cells that express skeletal muscle MHC(s). The myogenic pathway is the process by which mononucleate muscle progenitor cells progress through the myoblast stage to form myotubes.
Results
When we immunostained cells of the C2C12 and Sol8 mouse muscle cell lines with an mAb specific for Bcl-2, we found that a small subset of the mononucleate cells showed the punctate cytoplasmic staining expected (Krajewski et al., 1993) for Bcl-2 (Fig. 1, A and B, and not shown). In contrast, none of the multinucleate myotubes showed Bcl-2 staining (Fig. 1, A and B). The percentage of Bcl-2–positive cells ranged from ∼5–20% for C2C12 cells and from ∼3–5% for Sol8 cells. Similarly, Bcl-2 was expressed by a small percentage of mononucleate but not multinucleate cells in primary cultures of adult mouse muscle cells (Fig. 1, C and D). In primary cell cultures, we found by two methods that the Bcl-2–expressing cells were myogenic. First, desmin was coexpressed with most Bcl-2–positive cells in mouse primary muscle cell cultures, including the Bcl-2–positive cell in Fig. 1 C (not shown), confirming that these cells were myogenic (George-Weinstein et al., 1993). Second, Bcl-2 was expressed by ∼2–5% of the mononucleate cells in clonal, myotube-containing muscle colonies formed by the progeny of single adult mouse muscle cells (not shown).
Figure 1.
In myogenic cell cultures, Bcl-2 was expressed by a small subset of the mononucleate cells (closed arrows) but not in myotubes (open arrows). Bcl-2 immunostaining (A and C) and phase contrast (B and D) views of C2C12 (A and B) and mouse primary (C and D) myogenic cell cultures. Immunoblots (E) and RT-PCR (F) showed that Bcl-2 protein (∼26 kD) and mRNA (∼7.5 kb) were present in C2C12 myogenic cells and in adult mouse thymus cells. 30, location of 30-kD marker; RT, reverse transcriptase; GM, growth medium; DM, differentiation medium; H2O, no RNA control. Bar, 20 μm.
Immunoblotting, Northern blotting, and RT-PCR confirmed that Bcl-2 mRNA and protein were expressed in myogenic cell cultures. On immunoblots probed with anti– Bcl-2 mAbs, lysates of both mouse thymus, which expresses high levels of Bcl-2 (Haldar et al., 1996), and C2C12 cells showed identical bands of ∼26 kD, as expected (Haldar et al., 1996) for Bcl-2 protein (Fig. 1 E). On Northern blots, we detected a transcript of ∼7.5 kb, the size expected (Negrini et al., 1987) for Bcl-2 mRNA, in both growing and differentiated C2C12 cells (not shown). When we examined mRNAs from both adult mouse brain, in which Bcl-2 is expressed (Negrini et al., 1987), and C2C12 cells, we found that RT-PCR with Bcl-2–specific primers produced a single DNA band that was the expected size (480 bp) and hybridized to a Bcl-2 probe (Fig. 1 F).
Upon comparing the expression patterns of Bcl-2 and several muscle-specific proteins, we found that Bcl-2–positive C2C12 cells are at a very early stage of myogenic differentiation. Specifically, Bcl-2 was not coexpressed with proteins that mark the middle and late stages of the myogenic pathway. Upon examining ≥300 Bcl-2–positive C2C12 cells for each marker, we found no individual cells in which Bcl-2 was coexpressed with myosin, myogenin, MRF4, or nestin (Fig. 2, A–D, and not shown). In primary cultures, there was also no coexpression of Bcl-2 with myogenin or myosin (not shown). Myosin and MRF4 mark late stages of C2C12 myogenesis and are largely restricted to myotubes, whereas myogenin and nestin mark middle stages of myogenesis and are found in many mononucleate myoblasts, as well as in all myotubes (Kachinsky et al., 1994; Wang and Walsh, 1996).
Figure 2.

In growing cultures, the mononucleate C2C12 cells that express Bcl-2 (A, C, E, and G) did not coexpress myosin (B) or myogenin (D). About 80% of the Bcl-2–positive cells in growing cultures did not express MyoD (F), whereas ∼20% of the Bcl-2–positive cells did express MyoD (E and F insets). Desmin (H), as noted in the text, was coexpressed with many, but not all, Bcl-2–positive cells. Closed, downward pointing arrows indicate cells that expressed Bcl-2, but not the other tested protein. Open, downward-pointing arrows indicate cells that coexpressed Bcl-2 and the tested protein. Small, upward-pointing arrows indicate cells that expressed the tested antigen, but not Bcl-2. Bar, 20 μm.
Next we examined possible coexpression of Bcl-2 with three markers of earlier stages of the myogenic pathway: desmin, Myf-5, and MyoD (George-Weinstein et al., 1993; Smith et al., 1993). As noted above, Bcl-2 was expressed by only ∼10% of the mononucleate C2C12 cells, and we first examined this small subset of Bcl-2–positive cells for coexpression of desmin. We found that desmin was expressed by ∼85% of the Bcl-2–positive C2C12 cells as rapidly growing cultures neared confluence in growth medium (Fig. 2, G and H, and Table I), but by only ∼15% of the Bcl-2–positive cells in more quiescent cultures after 3–4 d in differentiation medium (Table I). As noted previously, desmin also was coexpressed with most Bcl-2–positive cells in mouse primary muscle cell cultures, including the Bcl-2–positive cell in Fig. 1 C (not shown), confirming that these cells were myogenic (George-Weinstein et al., 1993). In double staining experiments, >99% of growing C2C12 cells expressed desmin, Bcl-2, or both proteins. In particular, desmin was expressed by the ∼90% of all mononucleate cells that were Bcl-2 negative. (The remaining ∼10% of the mononucleate cells were Bcl-2 positive, with the patterns of Bcl-2 and desmin coexpression noted above and in Table I.)
Table I.
Percentage of Bcl-2–positive C2C12 Cells That Coexpressed Additional Muscle-specific Proteins
| Protein examined | Culture condition* | Number of Bcl-2+ cells examined‡ | Percentage (number) ofBcl-2+ cells that coexpressed the examined protein |
|||
|---|---|---|---|---|---|---|
| Desmin | GM | 314 | 85.6% (269) | |||
| 1 d DM | 300 | 69.7% (209) | ||||
| 3 d DM | 302 | 13.9% (42) | ||||
| 4 d DM | 300 | 14.7% (44) | ||||
| Myf-5 | GM | 303 | 21.5% (65) | |||
| 1 d DM | 298 | 5.4% (16) | ||||
| 2 d DM | 317 | 5.7% (18) | ||||
| 4 d DM | 320 | 0.0% (0) | ||||
| MyoD | GM | 380 | 18.9% (72) | |||
| 1 d DM | 300 | 3.0% (9) | ||||
| 2 d DM | 290 | 4.8% (14) | ||||
| 4 d DM | 305 | 0.0% (0) | ||||
| Myogenin | All | ≥300 | 0.0% (0) | |||
| MRF4 | All | ≥300 | 0.0% (0) | |||
| Myosin | All | ≥300 | 0.0% (0) |
As indicated, cells were stained either when they were near confluence in growth medium (GM) or during myotube formation after 1 d, 2–3 d, or 4 d in differentiation medium (DM).
Cultures were double immunostained and scanned for Bcl-2–positive cells. Each Bcl-2–positive cell was examined for coexpression of the tested protein. As described in the text, Bcl-2–positive cells amounted to ∼10% of the total number of mononucleate cells at all culture stages.
MyoD and Myf-5 were expressed by a much smaller percentage of the Bcl-2–positive cells than desmin. As C2C12 cultures approached confluence in growth medium, only ∼20% of the Bcl-2–positive cells expressed MyoD or Myf-5 (Fig. 2, E and F insets, and Table I), whereas ∼80% of the Bcl-2–expressing cells did not express either MyoD or Myf-5 (Fig. 2, E and F, and Table I). When cultures were switched to differentiation medium, the percentage of Bcl-2–positive cells that coexpressed either MyoD or Myf-5 decreased rapidly until, after 4 d in low serum medium, neither MyoD nor Myf-5 was expressed in any of the Bcl-2–positive cells (Table I). As with desmin, most of the C2C12 cells that expressed MyoD or Myf-5 were Bcl-2 negative. As C2C12 cells reached confluence in growth medium, for example, we found that ∼50% of the mononucleate cells expressed Myf-5 or MyoD but not Bcl-2, whereas only ∼2% coexpressed Myf-5 or MyoD with Bcl-2. In clonal cultures of primary cells, ∼50% of the Bcl-2–positive cells also expressed Myf-5 (not shown).
As measured by BrdU incorporation, C2C12 cells that were Bcl-2 positive were about as likely to be in S-phase as cells that were Bcl-2 negative. In one experiment, for example, we incubated nearly confluent C2C12 cells for 1 d in growth medium supplemented with 10 μM BrdU and then double immunostained the cells for BrdU and Bcl-2. BrdU was incorporated by ∼24% (35 of 148 observed) of the Bcl-2–positive cells and by ∼36% (207 of 574) of the Bcl-2–negative cells.
We next examined how Bcl-2 expression, cell viability, and differentiation capability were altered by treatments that induce apoptosis. Because serum-free medium and staurosporine (a protein kinase inhibitor) induce apoptosis in many types of cells (Jacobsen et al., 1994, 1996), including Sol8 cells (Mampuru et al., 1996), we switched growing C2C12 cells into one of three media: differentiation medium with 2% horse serum, serum-free medium, or serum-free medium with 0.5 μM staurosporine. At 1–2 d after the switch, the number of viable cells, measured by mitochondrial function (Jacobsen et al., 1994), had decreased in serum-free cultures but had increased in serum-containing cultures (Fig. 3 a). Pyknotic nuclei, which indicate apoptotic cells (Korsmeyer, 1995; Jacobsen et al., 1994), were abundant in serum-free cultures after 1–2 d but rare in serum-containing cultures (not shown). The percentage of C2C12 cells that expressed Bcl-2 remained at <20% in serum-containing cultures but was significantly (P < 0.01) increased to 50–80% after 2 d in serum-free culture with or without staurosporine (Fig. 3 b). For example, after treatment with staurosporine and serum-free medium for 2 d, the number of viable cells was reduced to 10% of the starting number, whereas the percentage of Bcl-2–positive cells increased about fourfold to 75% from an initial 16% (Fig. 3). Thus, expression of Bcl-2 is associated with resistance to apoptosis. Some of the C2C12 cells that survived serum-free medium and staurosporine were able to proliferate and carry out all stages of myogenesis, including myotube and clonal muscle colony formation, when returned to serum-containing media (not shown).
Figure 3.

(a) The number of viable C2C12 cells, as measured by MTT assay of mitochondrial function (plotted on a log scale), was greatly reduced in serum-free medium (SF) or serum-free medium with 0.5 μM staurosporine (STS) compared with growth medium (GM) or low serum differentiation medium (2% HS). Means ± SD were from quadruplicate wells in three separate experiments. (b) The percentage of C2C12 cells that expressed Bcl-2 (linear scale) was greater after incubation in serum-free medium with (STS) or without (SF) staurosporine than in control cultures that were maintained in serum-containing media (GM or 2% HS, as in a).
To further examine the relationship between Bcl-2 expression and myogenic cells, we compared Bcl-2 expression in early passage and late passage C2C12 cells. At early passage, almost all cells of lines such as C2C12 are able to express myogenin and form myotubes, whereas after repeated passage, differentiation-defective cells accumulate, and the percentage of cells that form myotubes decreases (c.f., Wright, 1984; Clegg and Hauschka, 1987; Rastinejad and Blau, 1993). We compared an early passage C2C12 line, in which ∼90% of the cells expressed myogenin and fused into myotubes, with a late passage line, in which only ∼60% of the cells expressed myogenin and fused into myotubes. In one experiment, we examined parallel cultures of these two lines as they neared confluence in growth medium, and we found that Bcl-2 was expressed by 6.9% (178 of 1,903) of the newly cloned cells, but by only 4.2% (56 of 1,339) of the multiply passaged cells. Similarly, after 3 d of differentiation, Bcl-2 was expressed by 8.1% (116 of 1,437) of the mononucleate newly cloned cells, but by only 3.1% (26 of 845) of the mononucleate multiply passaged cells. Thus, a higher percentage of Bcl-2–positive cells was associated with a higher percentage of myogenic cells.
We next cloned C2C12 cells and determined the size and Bcl-2 expression patterns of the resulting muscle colonies. C2C12 cells were allowed 4 d to form muscle colonies, at which time the colonies had a wide variation in size, ranging from 2 to 184 nuclei per colony. Of 67 colonies examined by immunofluorescence, 33 did not contain Bcl-2–positive cells and 34 did. The colonies without Bcl-2–positive cells had an average ± SE of 44.4 ± 6.7 nuclei and were significantly (P < 0.02) smaller than the colonies with Bcl-2–positive cells, which had an average of 70.2 ± 7.5 nuclei. At this early stage of colony formation, multinucleate cells had not formed.
Finally, to further study the relationship between Bcl-2 expression and myogenesis, we examined myogenesis by genotypically Bcl-2–null cells and wild-type cells. We found that Bcl-2–null cells produce myotubes but form smaller muscle colonies than wild-type cells. We compared cells obtained from the limbs of newborn Bcl-2 (−/−) mice with those obtained from wild-type littermates. In high-density cultures, Bcl-2–null and wild-type cells had similar log phase rates of cell proliferation in growth medium with population doubling times of ∼10 h and showed no differences in myotube formation (not shown). In contrast, clonal analyses showed a distinct difference between Bcl-2–null and wild-type cells.
For clonal analyses, cells were cultured at clonal density and allowed 8 d to form muscle colonies. Independent clonal cultures were established from three Bcl-2–null newborns and four wild-type newborns from two litters. Cultures were stained for desmin to distinguish desmin-positive muscle colonies from desmin-negative nonmuscle colonies. Muscle colonies were examined to determine both the total number of nuclei in the colony and the percentage of nuclei in myotubes.
Muscle colonies formed from Bcl-2 (−/−) cells contained an average ± SE of 112.6 ± 9.7 nuclei (n = 178), whereas muscle colonies formed from wild-type cells contained an average ± SE of 202.8 ± 11.8 nuclei (n = 274), a highly significant (P < 0.0001) difference. Both Bcl-2–null and wild-type cells produced colonies with a wide range of nuclear number, though Bcl-2–null cells produced relatively more small colonies and relatively fewer large colonies than wild-type cells (Fig. 4). In contrast to the differences in colony size, fusion indices and cloning efficiencies were similar for Bcl-2–null and wild-type cells. The average percentage of nuclei within myotubes was 24.9 ± 1.3% for cells without Bcl-2 and 22.3 ± 0.9% for wild-type cells, and the percentage of cloned cells that formed muscle colonies ranged, in different experiments, from ∼20 to 40% for both Bcl-2–null and wild-type cells.
Figure 4.
When cloned, Bcl-2–null cells produce smaller muscle colonies than wild-type cells. Clonal cultures were established from individual newborn mice (n = 3 for Bcl-2–null, and n = 4 for wild-type), and the number of nuclei in each resulting muscle colony was determined. Colony sizes from each individual were grouped into one of four bins (≤100, 101–250, 251–500, or >500 nuclei) and graphed with the four points obtained from each individual in the same relative position (e.g., the left-most bar in each of the four bins was from a single individual). Most of the muscle colonies produced from Bcl-2–null cells contained fewer than 100 nuclei (top). In contrast, the size distribution of muscle colonies produced from wild-type cells was shifted to larger sizes, so that most wild-type colonies contained more than 100 nuclei (bottom).
Discussion
In skeletal muscle cells, our results show that Bcl-2 expression is a marker for a small subset of cells that are at an early step in the pathway from muscle progenitor cell to myotube. Furthermore, Bcl-2 expression inhibits apoptosis of these cells and is needed for formation of normal-sized muscle colonies. The results suggest that Bcl-2 expression may be important for long-term survival of muscle progenitor cells and for the regeneration of large numbers of muscle cells. In addition, Bcl-2 expression identifies a new stage in the myogenic cell lineage and provides the first molecular marker that is expressed by muscle cells only at the early stages of the progression from progenitor cell to myotube. Thus, when analyzing mixed groups of myogenic cells, it appears that Bcl-2 expression can be used to directly distinguish mononucleate cells at early (Bcl-2–positive) and later (Bcl-2–negative) stages of the pathway.
The observed patterns of muscle gene expression suggest that, as Bcl-2–positive muscle cells and their progeny progress towards myotube formation, Bcl-2 expression stops as first Myf-5 and MyoD and then later markers of terminal differentiation are expressed (Fig. 5). This series of events, in which mononucleate muscle cells progress from an early stage when none of the MRFs is expressed to later stages in which the MRFs are expressed in a defined sequence, has also been demonstrated in mass cultures and by direct analyses of individual muscle satellite cells (Smith et al., 1993; Cornelison and Wold, 1997). This proposed sequence of gene expression in early stage muscle cells and myoblasts is supported by our immunostaining and cloning studies. At all stages of culture, including in newly formed muscle colonies, there were Bcl-2–positive cells that did not express detectable amounts of any of the tested muscle-specific genes, including the MRFs, as well as Bcl-2–positive cells that coexpressed desmin, MyoD, or Myf-5. However, the percentage of the cells that coexpressed Bcl-2 with desmin, MyoD, or Myf-5 was much higher in rapidly growing, low-density cultures (when myogenin-expressing myoblasts would be produced at a rapid rate) than in more quiescent, differentiating cultures (when myogenin-expressing cells would be produced slowly).
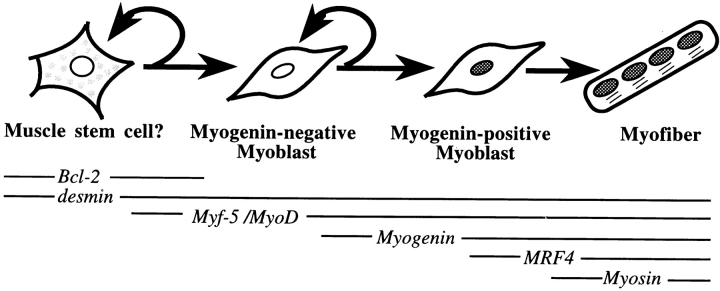
Figure 5.
Proposed stages in the myogenic pathway of satellite cells, including the expression patterns of Bcl-2 and additional marker genes. Bcl-2 expression is proposed to precede expression of the MRFs and myosin. Note also that Bcl-2 expression stops before myogenin expression, whereas expression of each of the additional markers is known to continue into the myofiber stage of differentiation.
Our results raise the possibility that Bcl-2–positive cells may include or be identical with muscle stem cells. Bcl-2–positive muscle cells can divide and appear to produce myogenin-positive myoblasts as progeny, but they do not themselves terminally differentiate. These properties are expected of muscle stem cells (i.e., self-renewing cells that produce myoblasts as progeny but do not themselves terminally differentiate). The existence of muscle stem cells has been inferred from cloning studies in vitro (Baroffio et al., 1996), where the descendants of cloned stem cells include the new stem cells, myoblasts, and myotubes that constitute large muscle colonies in vitro. Muscle stem cells are likely identical to muscle colony–forming cells (Hauschka et al., 1978; Rutz et al., 1982), as both are defined by the ability, when cloned, to self-renew and to generate progeny that form myotubes. Estimates of stem cell proportion range from 1–2% of adult human muscle cells (Baroffio et al., 1996) to up to 28% of embryonic chicken muscle cells (Rutz et al., 1982). The percentage of mononucleate muscle cells that expressed Bcl-2 was thus in the same range as the percentage of stem cells inferred from cloning studies. Anatomical studies (Polakowska et al., 1994; Krajewski et al., 1995; Merritt et al., 1995) suggest that stem cells of some epithelia express Bcl-2. Additional experiments are needed to purify the Bcl-2–positive cells and determine if they include or are identical with muscle stem cells.
The mononucleate C2C12 cells that expressed Bcl-2 appeared to resist apoptosis because the cells that survived induction of apoptosis by serum-free medium and staurosporine were largely those that expressed Bcl-2. Thus, expression of Bcl-2 from the endogenous promoter appeared to protect the small subset of Bcl-2–expressing muscle cells from apoptosis, whereas the Bcl-2–negative muscle cells were relatively more susceptible. This result is consistent with the apoptosis-inhibiting function of endogenous or ectopic Bcl-2 in numerous other cell types, including motor neurons and cardiac myocytes (Korsmeyer, 1995; Michaelidis et al., 1996; Kroemer, 1997; Kirschenbaum and de Moissac, 1997). Furthermore, ectopic Bcl-2 expression can protect merosin-deficient myotubes from apoptosis (Vachon et al., 1997). Because Bcl-2 expression appears sufficient to increase the resistance of myoblasts and myotubes to staurosporine-induced apoptosis, we conclude that other anti–apoptotic Bcl-2 family members that might normally be expressed after Bcl-2 expression ceases in myogenic cells are insufficient to prevent death by this signal. Our results, and those of Vachon et al. (1997), raise the possibility that diseases with signs of muscle cell apoptosis, such as merosin deficiency, dystrophin deficiency, and spinal muscular atrophy (Matsuda et al., 1995; Tidball et al., 1995; Vachon et al., 1997), could be ameliorated by altering Bcl-2 family expression.
The full extent to which muscle cell function is affected by lack of Bcl-2 remains to be determined. Bcl-2–null knock-out mice form muscles, but they grow more slowly after birth than wild-type littermates and have multiple abnormalities in nonmuscle tissues, including motor neurons (Veis et al., 1993; Nakayama et al., 1994; Kamada et al., 1995; Michaelidis et al., 1996). As shown here, Bcl-2–null cells can also produce myotubes in culture, though our colony formation assays show that Bcl-2–null muscle cells produce smaller muscle colonies than wild-type cells. Because Bcl-2–null cells form muscle colonies in vitro and myofibers in vivo, it appears that Bcl-2 is not absolutely required for the formation or function of cells with the qualities expected of muscle stem cells. Rather, Bcl-2 expression appears to quantitatively promote clonal expansion, likely by regulating the survival and/or proliferation of muscle progenitor cells. A role for Bcl-2 in clonal expansion is consistent with the finding that Bcl-2 is restricted to muscle cells that are at an early stage of the myogenic pathway because it is early-stage cells, before myogenin and p21 expression (Wang and Walsh, 1996), that are likely to be progenitors of muscle colonies.
Muscle cells and tissues express several members of the Bcl-2 family in distinct patterns (Krajewski et al., 1994, 1996; Ibi et al., 1996; Sandri et al., 1997), so one or more members of the Bcl-2 family may be important at all stages of myogenesis. Adult human, type IIB fast muscle fibers appear to express Bcl-2 (Ibi et al., 1996). Because we did not find Bcl-2 in myotubes formed in culture (including human myotubes; Dunn, J.J., and J.B. Miller, unpublished observation), this fiber type–specific expression of Bcl-2 may be innervation dependent. In addition, further studies are needed to determine if Bcl-2 expression identifies cells at early stages of myogenesis in each of the different lineages of myogenic cells found during development (Miller, 1992; Stockdale, 1992).
It should now be possible to use Bcl-2 promoter expression as a marker to isolate enriched populations of early muscle cells for molecular and functional characterization. It will be important, for example, to identify additional markers for these cells, to devise assays to measure their possible stem cell function, to study mechanisms of early muscle cell–specific gene expression, and to identify molecules that influence early muscle cell division and progress toward terminal differentiation. Furthermore, myogenic cells have been used, with uneven success (for review see Brown and Miller, 1996), as delivery vehicles for gene therapy. It should soon prove possible to examine whether success might be improved by using myogenic cells that are enriched for those at an early stage of the myogenic pathway.
Acknowledgments
We thank C. Milliman and S.J. Korsmeyer (Washington University, St. Louis, MO) for the anti–Bcl-2 mAb and the Bcl-2 cDNA, F.M. Boyce (Massachusetts General Hospital) for advice and pRSVneo, N. Rosenthal (Massachusetts General Hospital) for C2C12 cells, Caitlin Houlihan for cloning C2C12 cells, and Alejandro Perez for help with early experiments.
This work was supported by a grant to J.A. Dominov from the W.R. Hearst Foundation and by grants to J.B. Miller from The National Institute of Dental Research, The National Institute of Arthritis and Musculoskeletal and Skin Diseases, and The Muscular Dystrophy Association.
Abbreviations used in this paper
- BrdU
bromodeoxyuridine
- MHC
myosin heavy chain
- MRF
muscle regulatory factor
- RT-PCR
reverse transcriptase PCR
Footnotes
J.A. Dominov and J.J. Dunn contributed equally to this work.
Address all correspondence to Dr. Jeffrey B. Miller, Myogenesis Research Laboratory, Massachusetts General Hospital, 149 13th Street, Charlestown, MA 02129. Tel.: (617) 726-5754. Fax: (617) 726-8543. E-mail: Miller@helix.mgh.harvard.edu
References
- Baroffio A, Hamann M, Bernheim L, Bochatonpiallat ML, Gabbiani G, Bader CR. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation (Camb) 1996;60:47–57. doi: 10.1046/j.1432-0436.1996.6010047.x. [DOI] [PubMed] [Google Scholar]
- Bischoff R, Heintz C. Enhancement of skeletal muscle regeneration. Dev Dyn. 1994;201:41–54. doi: 10.1002/aja.1002010105. [DOI] [PubMed] [Google Scholar]
- Blau HM, Pavlath GK, Hardeman EC, Chiu C-P, Silberstein L, Webster SB, Miller SC, Webster C. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Block NE, Zhu Z, Kachinsky AM, Dominov JA, Miller JB. Acceleration of somitic myogenesis in embryos of myogenin promoter-MRF4 transgenic mice. Dev Dyn. 1996;207:382–394. doi: 10.1002/(SICI)1097-0177(199612)207:4<382::AID-AJA3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Brown RH, Miller JB. Progress, problems and prospects for gene therapy in muscle. Curr Opin Rheum. 1996;8:539–543. doi: 10.1097/00002281-199611000-00007. [DOI] [PubMed] [Google Scholar]
- Clegg CH, Hauschka SD. Heterokaryon analysis of muscle differentiation: regulation of the postmitotic state. J Cell Biol. 1987;105:937–947. doi: 10.1083/jcb.105.2.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison DDW, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Dominov JA, Miller JB. POU homeodomain genes in myogenesis. Dev Genet. 1996;19:108–118. doi: 10.1002/(SICI)1520-6408(1996)19:2<108::AID-DVG2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M, Foster RF, Gerhart JV, Kaufman SJ. In vitro and in vivo expression of α7 integrin and desmin define the primary and secondary myogenic lineages. Dev Biol. 1993;156:209–229. doi: 10.1006/dbio.1993.1071. [DOI] [PubMed] [Google Scholar]
- Haldar S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996;56:1253–1255. [PubMed] [Google Scholar]
- Hauschka, S.D., T.A. Linkhart, C. Clegg, and G. Merrill. 1979. Clonal studies of human and mouse muscle. In Muscle Regeneration. A. Mauro, editor. Raven Press Ltd., New York. 311–322.
- Ibi T, Sahashi K, Jing L, Zhang G, Mitsuma T. Immunostaining of anti–Bcl-2 antibody in diseased human muscles [Japanese] Clin Neurol. 1996;36:735–740. [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994;199:326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Jacobsen MD, Burne JF, Raff MC. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO (Eur Mol Biol Organ) J. 1994;13:1899–1910. doi: 10.1002/j.1460-2075.1994.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen MD, Weil M, Raff MC. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachinsky AM, Dominov JA, Miller JB. Myogenesis and the intermediate filament protein, nestin. Dev Biol. 1994;165:216–228. doi: 10.1006/dbio.1994.1248. [DOI] [PubMed] [Google Scholar]
- Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahasi T, Noda T, Kitamura Y, Kondoh H, Tsujimoto Y. bcl-2deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 1995;55:354–359. [PubMed] [Google Scholar]
- Kirschenbaum LA, de Moissac D. The bcl-2 gene product prevents programmed cell death of ventricular myocytes. Circulation. 1997;96:1580–1585. doi: 10.1161/01.cir.96.5.1580. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ. Regulators of cell death. Trends Genet. 1995;11:101–105. doi: 10.1016/S0168-9525(00)89010-1. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Tanaka A, Takayama S, Schibler MJ, Fenton W, Reed JC. Investigations of the subcellular distribution of the Bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Shabaik A, Wang H-G, Irie S, Fong L, Reed JC. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 1994;54:5501–5507. [PubMed] [Google Scholar]
- Krajewski S, Bodrug S, Krajewska M, Shabaik A, Gascoyne R, Berean K, Reed JC. Immunohistochemical analysis of Mcl-1 protein in human tissues. Am J Pathol. 1995;146:1309–1319. [PMC free article] [PubMed] [Google Scholar]
- Kroemer, G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat. Med. 3:614–620. [DOI] [PubMed]
- Mampuru, L., S.-J. Chen, J. Kalenick, M.E. Bradley, and T. -C. Lee. 1996. Analysis of events associated with serum deprivation-induced apoptosis in C3H/ Sol8 muscle satellite cells. Exp. Cell Res. 226:372–380. [DOI] [PubMed]
- Matsuda R, Nishikawa A, Tanaka H. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans Blue: evidence for apoptosis in dystrophin-deficient muscle. Biochem J. 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- McClearn D, Medville R, Noden D. Muscle cell death during the development of head and neck muscles in the chick embryo. Dev Dyn. 1995;202:365–377. doi: 10.1002/aja.1002020406. [DOI] [PubMed] [Google Scholar]
- Merritt AJ, Potten CS, Watson AJ, Loh DY, Nakayama K, Nakayama K, Hickman JA. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995;108:2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- Michaelidis TM, Sendtner M, Cooper JD, Airaksinen MS, Holtmann B, Meyer M, Thoenen H. Inactivation of Bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal development. Neuron. 1996;17:75–89. doi: 10.1016/s0896-6273(00)80282-2. [DOI] [PubMed] [Google Scholar]
- Miller JB. Myogenic programs of mouse muscle cell lines: expression of myosin heavy chain isoforms, MyoD1, and myogenin. J Cell Biol. 1990;111:1149–1160. doi: 10.1083/jcb.111.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JB. Myoblast diversity in skeletal myogenesis: how much and to what end? . Cell. 1992;69:1–3. doi: 10.1016/0092-8674(92)90111-o. [DOI] [PubMed] [Google Scholar]
- Miller JB, Stockdale FE. Developmental regulation of the multiple myogenic cell lineages of the avian embryo. J Cell Biol. 1986;103:2197–2208. doi: 10.1083/jcb.103.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D, Chelly J, Bober E, Arnold H, Ott MO, Gros F, Pinset C. Developmental patterns in the expression of Myf5, MyoD, myogenin, and MRF4 during myogenesis. New Biol. 1991;3:592–600. [PubMed] [Google Scholar]
- Nakayama K, Nakayama K-I, Negishi I, Kuida I, Sawa H, Loh DY. Targeted disruption of Bcl-2aβ in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Nat Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini M, Silini E, Kozak C, Tsujimoto Y, Croce CM. Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell. 1987;49:455–463. doi: 10.1016/0092-8674(87)90448-x. [DOI] [PubMed] [Google Scholar]
- Peck D, Walsh FS. Differential effects of over-expressed neural cell adhesion molecule isoforms on myoblast fusion. J Cell Biol. 1993;123:1587–1595. doi: 10.1083/jcb.123.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakowska RR, Piacentini M, Bartlett R, Goldsmith LA, Haake AR. Apoptosis in human skin development: morphogenesis, periderm, and stem cells. Dev Dyn. 1994;199:176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- Rastinejad F, Blau HM. Genetic complementation reveals a novel regulatory role for 3′ untranslated regions in growth and differentiation. Cell. 1993;72:903–917. doi: 10.1016/0092-8674(93)90579-f. [DOI] [PubMed] [Google Scholar]
- Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- Rutz R, Haney C, Hauschka SD. Spatial analysis of limb bud myogenesis: a proximodistal gradient of muscle colony-forming cells in chick embryo leg buds. Dev Biol. 1982;90:399–411. doi: 10.1016/0012-1606(82)90389-x. [DOI] [PubMed] [Google Scholar]
- Sandri M, Podhorska-okolow M, Geromel V, Rizzi C, Arslan P, Franceshci C, Carraro U. Exercise induces myonuclear ubiquitination and apoptosis in dystrophin-deficient muscle of mice. J Neuropathol Exp Neurol. 1997;56:45–57. doi: 10.1097/00005072-199701000-00005. [DOI] [PubMed] [Google Scholar]
- Skapek SX, Rhee J, Spicer DB, Lassar AB. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- Smith TH, Miller JB. Distinct myogenic programs of embryonic and fetal mouse muscle cells: expression of the perinatal myosin heavy chain isoform in vitro. Dev Biol. 1992;149:16–26. doi: 10.1016/0012-1606(92)90260-n. [DOI] [PubMed] [Google Scholar]
- Smith TH, Block NE, Rhodes SJ, Konieczny SF, Miller JB. A unique pattern of expression of the four muscle regulatory factors distinguishes somitic from embryonic, fetal, and newborn mouse myogenic cells. Development (Camb) 1993;117:1125–1133. doi: 10.1242/dev.117.3.1125. [DOI] [PubMed] [Google Scholar]
- Smith TH, Kachinsky AM, Miller JB. Somite subdomains, muscle cell origins, and the four muscle regulatory factor proteins. J Cell Biol. 1994;127:95–105. doi: 10.1083/jcb.127.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale FE. Myogenic cell lineages. Dev Biol. 1992;154:284–298. doi: 10.1016/0012-1606(92)90068-r. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH. Apoptosis-related proteins in skeletal muscle fibers of spinal muscular atrophy. J Neuropath Exp Neurol. 1997;56:150–156. doi: 10.1097/00005072-199702000-00005. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Albrecht DE, Lokensgard BE, Spencer MJ. Apoptosis precedes necrosis of dystrophin-deficient muscle. J Cell Sci. 1995;108:2197–2204. doi: 10.1242/jcs.108.6.2197. [DOI] [PubMed] [Google Scholar]
- Vachon PH, Xu H, Liu L, Loechel F, Hayashi Y, Arahata K, Reed JC, Wewer UM, Engvall E. Integrins (α7β1) in muscle function and survival. Disrupted expression in merosin-deficient congenital muscular dystrophy. J Clin Invest. 1997;100:1870–1881. doi: 10.1172/JCI119716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Wang J, Walsh K. Resistance to apoptosis conferred by cdk inhibitors during myocyte differentiation. Science. 1996;273:359–361. doi: 10.1126/science.273.5273.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C, Blau HM. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet. 1990;16:557–565. doi: 10.1007/BF01233096. [DOI] [PubMed] [Google Scholar]
- Wright WE. Control of differentiation in heterokaryons and hybrids involving differentiation-defective myoblast variants. J Cell Biol. 1984;98:436–443. doi: 10.1083/jcb.98.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]