Abstract
Stimulation of β-adrenergic receptors activates type I and II cyclic AMP–dependent protein kinase A, resulting in phosphorylation of various proteins in the heart. It has been proposed that PKA II compartmentalization by A-kinase–anchoring proteins (AKAPs) regulates cyclic AMP–dependent signaling in the cell. We investigated the expression and localization of AKAP100 in adult hearts. By immunoblotting, we identified AKAP100 in adult rat and human hearts, and showed that type I and II regulatory (RI and II) subunits of PKA are present in the rat heart. By immunofluorescence and confocal microscopy of rat cardiac myocytes and cryostat sections of rat left ventricle papillary muscles, we localized AKAP100 to the nucleus, sarcolemma, intercalated disc, and at the level of the Z-line. After double immunostaining of transverse cross-sections of the papillary muscles with AKAP100 plus α-actinin–specific antibodies or AKAP100 plus ryanodine receptor–specific antibodies, confocal images showed AKAP100 localization at the region of the transverse tubule/junctional sarcoplasmic reticulum. RI is distributed differently from RII in the myocytes. RII, but not RI, was colocalized with AKAP100 in the rat heart. Our studies suggest that AKAP100 tethers PKA II to multiple subcellular compartments for phosphorylation of different pools of substrate proteins in the heart.
Keywords: AKAP, PKA, RI, RII, heart
Stimulation of β-adrenergic receptors results in activation of the cAMP-dependent signaling pathway. cAMP-dependent protein kinase (PKA)1 is a tetrameric holoenzyme consisting of two catalytic (C) and two regulatory (R) subunits. When four molecules of cAMP bind to inactive PKA, the C subunits are released from the regulatory subunit-cAMP complex (Taylor et al., 1990). By phosphorylating substrate proteins, PKA regulates a broad range of cellular responses. Two classes of R subunit exist—RI and RII—that form type I and type II PKA (PKA I and II), respectively. PKA I is predominantly cytosolic, whereas up to 75% of PKA II is compartmentalized to specific cellular compartments via binding to a family of A-kinase–anchoring proteins (AKAPs; Rubin, 1994). Recent studies demonstrated that RI may also bind to AKAPs (Burton et al., 1997; Huang et al., 1997). AKAPs can bind other enzymes, thus forming a scaffold for a multi-enzyme complex. For example, AKAP79 can simultaneously bind PKA II, calcium- and calmodulin- dependent protein phosphatase-2B, and protein kinase C (PKC; Klauck et al., 1996), and AKAP250 contains binding sites for both RII and PKC (Nauert et al., 1997). It has been proposed that AKAPs serve as scaffolding proteins in PKA- and PKC-signaling pathways, permitting sequential activation of each enzyme and tethering of enzymes close to their substrates (Faux and Scott, 1996).
By using radioactively labeled RII in an overlay assay (Lohmann et al., 1984), several AKAPs have been identified in various tissues (Dell'Acqua and Scott, 1997). Generally, AKAPs were named according to the apparent molecular weight of the proteins on SDS-PAGE gels. AKAPs have been localized to different subcellular compartments; e.g., sAKAP84 to mitochondria, AKAP220 to peroxisomes, AKAP85 to Golgi, MAP2 in microtubules and AKAP95 in nucleus (Dell'Acqua and Scott, 1997). Distribution of AKAPs in multiple subcellular compartments has not been reported. Scott and coworkers recently proposed the PKA-targeting hypothesis whereby each AKAP tethers the PKA complex or other signaling enzymes to a particular organelle or location in the cell (Scott, 1997). According to this hypothesis, subcellular location of the AKAP/PKA II complex is determined by the targeting domain of the AKAP molecule.
In cardiac muscle cells, PKA has broad substrate specificity. Major PKA substrates in the heart are L-type Ca2+ channels (Tsien et al., 1986) in the sarcolemma, primarily in the transverse (T) tubules (Piper and Isenberg, 1989), ryanodine receptors (RyR) in the junctional sarcoplasmic reticulum (SR; Takasago et al., 1991; Takasago et al., 1989), phospholamban (Garvey et al., 1988), and Cl− channel in the SR (Kawano et al., 1992), troponin I in the thin filaments, and C-protein in the thick filaments (Garvey et al, 1988). Since each of these PKA substrates has a different subcellular location, AKAP-dependent PKA compartmentalization is likely to be an important regulatory mechanism for β-adrenergic signaling in the heart.
However, knowledge about AKAPs in the heart is very limited. In 1995, Scott's laboratory isolated an AKAP cDNA by screening a human brain cDNA library with radioactively labeled RII (McCartney et al., 1995). The identified AKAP cDNA encodes a 78-kD protein. Because antibodies against this AKAP recombinant recognized a protein of ∼100 kD in rat embryonic muscle cell lines, the AKAP was named AKAP100. AKAP100 mRNA was found to be abundant in human heart and skeletal muscle. In the rat embryonic cardiac H9c2 cell line, AKAP100 and RII colocalized to the perinuclear region (McCartney et al., 1995) at the same location as the signal sequence receptor, a marker of the rough ER (Krijnse-Locker et al., 1995). Embryonic hearts undergo significant changes during maturation, including a switch of protein isoform expression, development of the T-tubular system, and SR network and formation of sarcomeres. H9c2 cells do not have the organized sarcomere structures of differentiated adult cardiac myocytes (Hescheler et al., 1991); therefore, the embryonic cardiac cell line cannot be considered representative of the highly differentiated cardiac myocytes of the adult heart. The present study investigated the expression and localization of AKAP100 in adult hearts.
Materials and Methods
Antibodies
Polyclonal rabbit IgG antibodies against human AKAP100 recombinant protein and polyclonal goat IgG antibodies against mouse RII recombinant were obtained from Upstate Biotechnology Inc. (Lake Placid, NY). RI-specific monoclonal antibody (clone 18) was obtained from Transduction Laboratories (Lexington, KY). RyR-specific monoclonal antibody (clone C3-33) was obtained from Affinity Bioreagents (Golden, CO). The specificity of the anti-RyR antibody has been previously described (Carl et al., 1995). An α-actinin–specific monoclonal antibody (clone EA-53), obtained from Sigma Chemical Co. (St. Louis, MO), has been used to characterize α-actinin in rat cardiac myocytes (Sussman et al., 1994). A monoclonal antibody against the 6 histidine tag was obtained from CLONTECH Laboratories, Inc. (Palo Alto, CA). HRP-conjugated rabbit anti-mouse antibodies, HRP-goat anti–rabbit antibodies, and HRP-rabbit anti–goat antibodies were purchased from Sigma Chemical Co. FITC-conjugated donkey anti–mouse antibodies, FITC-donkey anti–goat antibodies, and lissamine rhodamine B sulfonyl chloride (LRSC)–conjugated donkey anti–rabbit antibodies were obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). FITC-phalloidin was purchased from Molecular Probes, Inc. (Eugene, OR).
Preparation of Rat Left Ventricular Myocytes
All procedures involving animals follow the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). The Animal Care Core Facility of the Cleveland Clinic Foundation is accredited by the American Association for the Accreditation of Laboratory Care.
Cardiac myocytes were isolated from the left ventricle of adult Sprague-Dawley rats (purchased from Taconic Farms, Inc. [Germantown, NY] at 20–30 wk of age) by collagenase digestion using a modified Langendorff perfusion according to methods previously described (McConnell et al., 1997). The isolated myocytes were resuspended in O2-saturated Hepes-buffered saline containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM MgCl2, 1.25 mM CaCl2, 11 mM glucose, 0.68 mM glutamine, 5 mM pyruvate, and 25 mM Hepes, pH 7.35, supplemented with 0.1 mM Eagle's minimum essential medium, basal medium Eagle's vitamin, and amino acid solution at room temperature. The viability of the isolated cardiac myocytes was typically 75–85%, as determined by rod-shaped morphology and lack of granulation or blebs. After isolation, the ventricular myocytes were immediately used either for extraction of myocardial proteins or immunolocalization.
Myocardial Protein Preparations
Expression of AKAP100 was determined in an adult rat cardiac myocyte extract. Preparation of the crude protein extract followed methods previously described (McCartney et al., 1995). In brief, the isolated rat left ventricular myocytes were collected from the Hepes-buffered saline suspension by centrifugation at 500 g for 2 min at room temperature. The myocytes were then resuspended in ice-cold Buffer A (50 mM Tris-HCl, pH 7.5, 0.1% Triton X-100, 0.05 mM DTT, 0.5 mM MgCl2, 0.125 mM EDTA, 5 μg/ml antipain, 10 μg/ml leupetin, 5 μg/ml pepstatin, and 43 μg/ml PMSF), and were sonicated for 30 s (three bursts of 10 s each) on ice followed by centrifugation at 12,000 g for 15 min at 4°C. The protein extract in the supernatant was separated from the pellets and subjected to immunoblot analysis. In addition, expression of AKAP100 was also determined in adult rat and human heart tissues. Human heart tissue was obtained from an unmatched organ donor (male, age 40) from LifeBanc of Northeast Ohio (Cleveland, Ohio). For preparation of heart homogenates, left ventricle from an adult Sprague-Dawley rat or 0.8 g of left ventricle from the human donor was homogenized in 3 ml of ice-cold Buffer A, followed by sonication as above. The homogenates were filtered by using four layers of cheesecloth, and the large tissue debris was removed. The homogenates were then subjected to immunoblot and immunoprecipitation analyses as described below. To characterize the specificity of anti-RI and anti-RII antibodies, soluble rat left ventricle extracts prepared according to methods previously described (Jahnsen et al., 1985) were used in the immunoblot analysis.
Immunoblot, Immunoprecipitation, and RII Overlay Assays
Immunoblot analysis was performed to determine expression of AKAP100 in adult hearts, and to characterize the antibody specificity. Myocardial proteins were separated on 10% polyacrylamide gels by SDS-PAGE under reducing conditions using the Bio-Rad electrophoresis system (Mini-Protean II, 8 × 7.3 cm; Bio-Rad Laboratories, Hercules, CA) or the Hoefer electrophoresis system (SE 600, 18 × 16 cm; Pharmacia Biotech, Inc., Piscataway, NJ) followed by electrical transfer. After blocking with nonfat dry milk, the nitrocellulose membrane was incubated with primary antibodies followed by incubation with HRP-conjugated secondary antibodies. The immunoblot was then developed by enhanced chemiluminescence (ECL) assay followed by autoradiography. Alternately, protein bands were visualized by color development using the 4-chloro-1-naphthol substrate. The protein concentration of the samples was determined by the Bio-Rad protein assay. Approximately 50 μg and 100 μg of myocardial proteins were loaded on each lane of the minigel and the large gel, respectively. To determine the apparent molecular weight of AKAP100, unstained Perfect Protein Markers™ from Novagen, Inc. (Madison, WI) were also run on the SDS-PAGE gels. These unstained protein markers consist of precisely sized recombinant proteins carrying an S•Tag™ peptide that can be visualized by using HRP-conjugated S-protein (Novagen, Inc. Madison, WI) in the immunoblot analysis.
Dilution of primary and secondary antibodies followed supplier's instructions. To characterize human AKAP100 recombinant protein, the anti-AKAP100 polyclonal antibodies used in immunoblot analysis were preabsorbed by using nitrocellulose membrane bound with Escherichia coli bacterial antigens. In addition, E. coli bacterial lysates containing human AKAP100 recombinant were used to deplete AKAP100-specific IgGs. The depleted antibodies were then used in immunoblot analysis to characterize AKAP100 protein in adult hearts. As a complementary experiment, the polyclonal human AKAP100-specific IgG antibodies were affinity-purified by using Western blot strips immobilized with the 80-kD AKAP protein according to the method of Beall and Mitchell (Beall and Mitchell, 1986) as previously described (Yang et al., 1996). The purified antibodies were then used in a recombinant AKAP100 immunoblot assay.
The immunoprecipitation assay was performed to characterize the rat heart AKAP by using the anti-AKAP100 rabbit IgG antibodies and anti-RII goat IgGs antibodies according to methods previously described (Gray et al., 1997). Rat left ventricle homogenates (0.5 ml) were incubated with 10 μg of the primary IgG antibodies followed by incubation with 100 μl of Protein G Sepharose 4 Fast Flow™ gel (Pharmacia Biotech., Inc.) to precipitate the antigen–antibody complexes. In addition, the cAMP-agarose affinity copurification assay was also performed to identify the AKAP100-RII complexes in the rat heart. cAMP-agarose beads (200 μl) obtained from Sigma Chemical Co. were incubated with rat left ventricle homogenates (0.5 ml) as described (Hausken et al., 1996). The RII-AKAP complex–bound-Protein G Sepharose beads and cAMP-agarose were washed three times with 1 ml Buffer A. The immunoprecipitated proteins and copurified proteins were eluted by incubation with SDS-PAGE sample buffer (10 min at 95°C), and were then analyzed by immunoblotting with anti-AKAP100 antibodies.
The murine RIIα recombinant plasmid (RII-pET11d) was a generous gift of Dr. John D. Scott, (Vollum Institute, OR). The recombinant RII was used to transform E. coli bacteria strain BL21(DE3). The RII recombinant protein was expressed under 2 mM isopropyl-b-D-thiogalactopyranoside (IPTG) induction followed by affinity purification using cAMP-agarose as described (Dransfield et al., 1997). The purified recombinant RII was separated on SDS-PAGE gels, analyzed by Coomassie blue staining, and immunoblotted with RII-specific antibodies. Purified recombinant RII was labeled with [32P]ATP by in vitro phosphorylation using PKA catalytic subunits obtained from Sigma Chemical Co. as described (Bregman et al., 1989). The reaction was carried out at 30°C for 2 h. An RII overlay assay was then performed following methods previously described (Keryer et al., 1993) to determine the interaction between the RII and AKAP100 recombinants. The RII-binding proteins were detected by PhosphorImaging (Molecular Dynamics, Inc., Sunnyvale, CA). The inhibitory Ht 31 peptide (1 μM), synthesized according to the published sequence (Carr et al., 1992), was used to compete with the RII-AKAP100 binding in the RII overlay assay.
Cloning and Characterization of Human AKAP100 Recombinant
Human AKAP100 cDNA was generated by RT-PCR using human heart poly(A)+ RNA as templates and primers flanking the published AKAP100 coding region (McCartney et al., 1995). Total RNA was isolated from left ventricles of the unmatched organ donor by using TRIzol reagent (Gibco Laboratories, Grand Island, NY). Poly(A)+ RNA was purified from total RNA by oligo(dT)-cellulose chromatography (Qiagen, Inc., Valencia, CA). The primers were designed to create a BamHI site at the 5′-end (5′-CGGGATCCCGATGTCCTTTACTGGCCAGATGTC-3′) and a BglII site at the 3′-end (5′-GAAGATCTTCGGGGAGGGGGTACATTCTACCTATGC-3′). The amplified human AKAP100 cDNA (2 kb) was cloned into pCR2.1 vector (Invitrogen Corp., San Diego, CA). To confirm that the isolated cDNA was from AKAP100, the cDNA insert was sequenced in both directions by an automatic sequencer. The pCR2.1 vector-specific T7 promoter primer and M13 reverse primer were used for the initial sequencing, and subsequent primers were synthesized on the basis of the cDNA sequence. The oligonucleotides for sequencing were purchased from Gibco Laboratories. The DNA sequence was then aligned to the published AKAP100 cDNA using computer software (DNASTAR Inc., Madison, WI). The AKAP100 cDNA insert was then subcloned into BamHI and BglII sites of the expression vector pRSET C (Invitrogen Corp.). In this construct, the vector contributes a 6 histidine tag to the NH2-terminus of the recombinant, as described (Yang et al., 1996). An enterokinase cleavage site is located two amino acid residues upstream of the AKAP100 recombinant. The recombinant AKAP100 plasmid was then used to transform E. coli bacteria strain BL21(DE3). The AKAP100 recombinant protein was expressed under 2 mM IPTG induction for 2 h. The AKAP100 recombinant bacterial lysates were separated on a preparative SDS-PAGE gel followed by immunoblotting with anti-AKAP100 antibodies and anti-6 His tag monoclonal antibody. The AKAP100 recombinant was further characterized by using the RII overlay assay.
Cryostat Sections of Rat Papillary Muscles
Cryostat sections from Sprague-Dawley rat left ventricle papillary muscles were prepared as previously described (Carl et al., 1995). In brief, adult Sprague-Dawley rats were killed, and the left ventricle was washed with ice-cold relaxing solution (0.1 M KCl, 5 mM EGTA, 5 mM MgCl2, 3 mM 2,3-butanedione monoxime, 0.25 mM DTT, and 10 mM histidine at pH 6.8) as described (Gwathmey et al., 1991). Papillary muscles were dissected at approximately resting sarcomere length. After equilibration for 5 min in the relaxing solution, the tissue strips were mounted on cork specimen holders using gum tragacanth. The isolated papillary muscle was flash-frozen in liquid nitrogen–cooled isopentane. The papillary muscle was sectioned either longitudinally or transversely with a section thickness of 8 μm using a Microm cryostat (Carl Zeiss, Inc., Batavia, IL). Frozen sections were collected on Superfrost Plus glass slides (Baxter Diagnostics, Inc., Deerfield, IL).
Immunofluorescence Labeling
To determine the subcellular localization of AKAP100 in the heart, immunostaining and confocal laser scanning microscopy were performed on isolated adult rat ventricular myocytes and on cryostat sections from papillary muscles from hearts of Sprague-Dawley rats. Freshly isolated cardiac myocytes were adsorbed to Superfrost Plus glass slides for 20 min at room temperature. To enhance the attachment of the myocytes, the glass slides were pretreated with 0.1% poly-l-lysine obtained from Sigma Chemical Co., followed by extensive washing in distilled water. The myocytes or the cryostat sections were fixed on the slides with freshly prepared fixative solution (4% paraformaldehyde and 4% sucrose in PBS, pH 7.4) for 20 min at room temperature, followed by three PBS washes of 10 min each. Unreactive sites were blocked with 3% BSA in PBS for 1 h at room temperature. To enhance antibody penetration of the myocytes, Triton-X 100 at a final concentration of 0.6% was added to the blocking solution. Since there is no membrane barrier in the cells of the cryostat sections, Triton-X 100 was not used for blocking of these samples.
The myocytes or the cryostat sections were incubated with primary antibodies diluted in 3% BSA in PBS overnight at 4°C. The concentration of the primary antibodies was as follows: 29 μg/ml anti-AKAP100 antibodies, 20 μg/ml anti-RI antibodies, 25 μg/ml anti-RII antibody, 17 μg/ml anti-RyR antibody, and 34 μg/ml anti-α-actinin antibody. After intensive washing with PBS as described above, the myocytes were incubated with FITC- or LRSC-conjugated secondary antibodies for 1 h at room temperature. The concentration of the antibodies was 15 μg/ml LRSC-conjugated donkey anti–rabbit IgG, 26 μg/ml FITC-conjugated donkey anti– mouse IgG, and 15 μg/ml FITC-conjugated donkey anti–goat IgG. After washing off the unbound secondary antibodies, the myocytes or the cryostat sections were mounted in VectaShield mounting medium (Vector Labs, Inc., Burlingame, CA). For double immunostaining, the slides were sequentially stained with two individual primary antibodies, followed by simultaneous incubation with two secondary antibodies. Because phalloidin has high affinity and specificity for β-actin, FITC-phalloidin (1 U/ slide) was directly added to the blocking solution for immunostaining the thin filaments of the myocytes.
The immunostained slides were examined using a Leica TCS-NT confocal laser scanning microscope with an 100× objective lens (Leica Lasertechik GmbH, Heidelberg, Germany). The thickness (depth) of the optical sections was ∼400 nm. Confocal images of the samples were examined along the z-axis with a step-size of 0.5 μm. The digitized image was analyzed using the Leica TCS-NT computer software. For comparison of double-stained patterns, the images from FITC and TRITC channels were simultaneously acquired from the same area of the sample, and were superimposed. Since the binding affinity of the antibodies may affect the intensity of the staining, in order to obtain optimally superimposed images, the intensity of the signals from each channel was balanced before merging.
To determine the specificity of the AKAP100 and RII staining, cryostat sections of the rat left ventricles were immunostained with anti-AKAP100 antibodies or anti-RII antibodies in the presence of 200 μl of recombinant AKAP100 or RII bacterial lysates, respectively. In addition, as negative controls, the myocytes and the cryostat sections were incubated with secondary antibodies only. Negative control slides were observed for each experiment.
All confocal images shown in this study represent results from at least three independent experiments. Negative controls stained only with secondary antibodies were observed at the same brightness and contrast settings as the stained slides in all experiments. No specific staining was observed in the negative controls (data not shown).
Results
Specificity of the Anti-RI and Anti-RII Antibodies
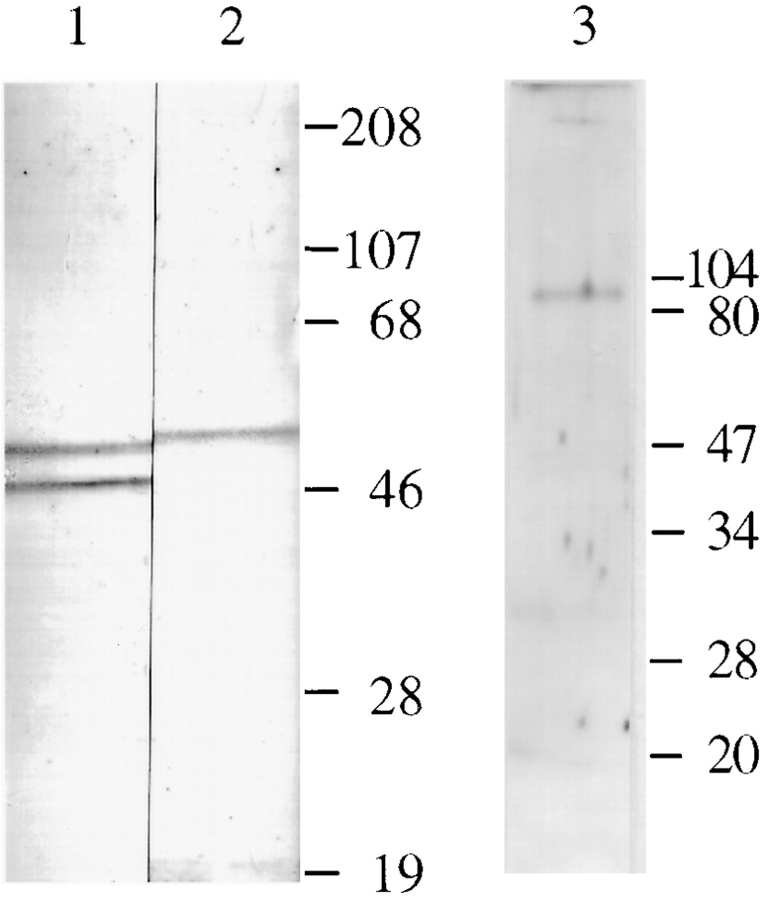
Specificity of the RI- and RII-specific antibodies in adult Sprague-Dawley rat heart was determined by immunoblot analysis. The anti-RI antibody specific for the RIα and RIβ isoforms recognized two protein bands with apparent molecular masses of 49 and 53, respectively, in the rat heart (Fig. 1, lane 1). Consistent with previous findings of RIα and RIβ isoforms in rat testes and brain (Skalhegg et al., 1992; DeManno et al., 1994), and according to the migration pattern of the doublets on the RI immunoblot (Fig. 1, lane 1), the 49-kD and 53-kD protein bands should represent the α and β isoforms of the RI subunits. By immunoblotting, the RII-specific antibodies recognized one protein band of ∼54 kD in the rat heart extracts (Fig. 1 A, lane 2). Our results are consistent with previous studies (Jahnsen et al., 1985; De Camilli et al., 1986) that showed only one RII isoform present in the rat heart.
Figure 1.
Immunoblot analysis of RI, RII, and AKAP100 in adult hearts. Soluble fraction of the rat left ventricle extracts (lanes 1 and 2, from a preparative gel) and crude protein extracts from isolated rat left ventricular myocytes (lane 3, 50 μg/lane) were separated on 10% SDS-PAGE gels by using the mini gel system (Bio-Rad Laboratories, Hercules, CA). The immunoblots were incubated with RI-specific monoclonal antibody (lane 1; 0.25 μg/ml), RII-specific goat antibodies (lane 2; 10 μg/ml) and AKAP100-specific rabbit antibodies (lane 3; 3.5 μg/ml), respectively. The protein bands were visualized by color development as described in the text (lanes 1 and 2) or by ECL (lane 3). Molecular weight markers are in kD.
Identification of AKAP100 in Adult Hearts
It has previously been reported that human AKAP100– specific antibodies bind to a protein with an apparent molecular mass of 100 in the rat embryonic cardiac H9c2 cell line and skeletal L6P cell line (McCartney et al., 1995). By immunoblotting, we showed that the anti-AKAP100 rabbit antibodies bound to a single protein band of ∼80 kD in rat ventricular myocyte extracts (Fig. 1 A, lane 3). Expression of AKAP100 in rat heart tissue was further examined in homogenates of left ventricles from adult Sprague-Dawley rats. As shown in Fig. 2 A, the anti-AKAP100 antibodies also react to one protein in the rat heart homogenates (lane 3), with a mobility on the gel consistent with the protein identified in the myocyte extract (Fig. 1, lane 3). According to the unstained molecular weight markers on the same gel (Fig. 2 A, lane 1), the size of the protein recognized by the anti-AKAP100 antibodies is estimated to be 80 kD (lane 3). On the same immunoblot, the AKAP100-specific antibodies bound to a slightly larger protein (∼82 kD) in homogenates obtained from the left ventricle of a human donor heart (Fig. 2 A, lane 2). These results were reproducible in four different Sprague-Dawley rats, one failing, and one nonfailing human heart (data not shown). Because AKAP100 proteins appear to be very labile in the tissues, fresh tissue homogenates were used for these studies.
Figure 2.
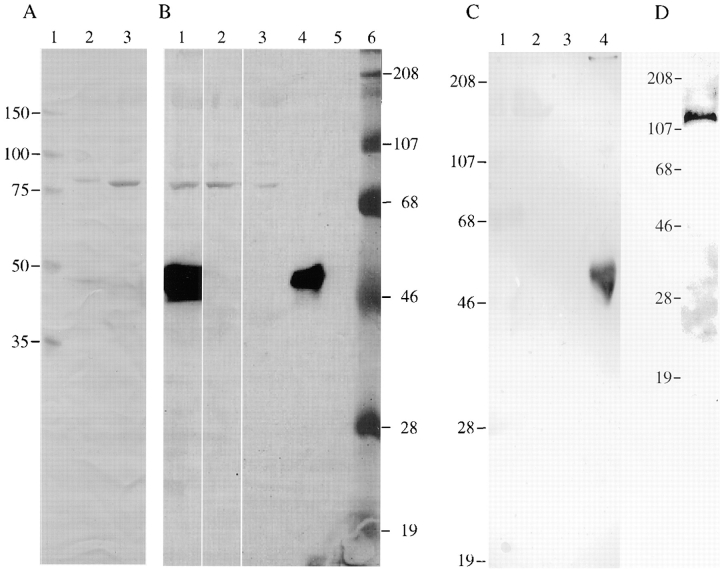
Immunoblot, immunoprecipitation, and cAMP-agarose copurification analyses of AKAP100 in the rat heart. In A, human (lane 2) and rat (lane 3) left ventricle homogenates were separated on a 10% SDS-PAGE gel (100 μg/lane) followed by immunoblotting with anti-AKAP100 antibodies. Unstained molecular weight markers were loaded on lane 1. In B, rat left ventricle homogenates (0.5 ml) were incubated with 10 μg of AKAP100-specific rabbit IgGs (lane 1), 10 μg of RII-specific goat IgGs (lane 2), and 200 μl of cAMP-agarose (lane 3). The AKAP100-IgG complexes were precipitated by incubation with Protein G Sepharose beads. The precipitated proteins were solubilized from the agarose or sepharose beads with SDS-PAGE sample buffer followed by immunoblotting with anti-AKAP100 antibodies. As controls for the assay, 2 μg of rabbit IgGs and goat IgGs were loaded on lanes 4 and 5, respectively. Prestained molecular weight markers are loaded on lane 6. In C, human (lane 1) and rat (lane 2) left ventricle homogenates and human AKAP100 recombinant bacterial lysates (lane 3) were separated on a 10% SDS-PAGE gel (100 μg/lane) followed by immunoblotting with anti-AKAP100 antibodies that have been previously preabsorbed by AKAP100 recombinant bacterial lysates. As a control, 2 μg of rabbit IgGs were loaded on lane 4. In D, AKAP100 recombinant bacterial lysates (50 μg/lane) were separated on a 10% SDS-PAGE gel followed by immunoblotting with affinity-purified anti-AKAP100 IgGs as described in the text. The Hoefer (A–C) and Bio-Rad (D) SDS-PAGE systems were used for these studies. A and B represent different lanes from the same gel. The protein bands in A–C were visualized by color development, whereas the immunoblot shown in D was developed by ECL. Molecular weight markers are in kD.
An immunoprecipitation assay was performed to further characterize AKAP100 in the rat heart. By immunoblotting, the anti-AKAP100 antibodies recognized one 80-kD protein precipitated by the AKAP100-specific antibodies (Fig. 2 B, lane 1). No higher molecular weight protein was recognized by the anti-AKAP100 antibodies in the precipitates (Fig. 2 B, lane 1). We further demonstrated that the 80-kD protein is an RII-binding protein. By immunoblotting, the anti-AKAP100 antibodies recognized an 80-kD protein immunoprecipitated by the RII-specific antibodies (Fig. 2 B, lane 2). These results were verified in the cAMP-agarose copurification assay. The anti-AKAP100 antibodies specifically react to an 80-kD protein in the cAMP-agarose copurified RII-AKAP complexes (Fig. 2 B, lane 3). These studies demonstrate that the 80-kD rat heart protein recognized by the anti-AKAP100 antibodies is an RII-binding protein.
Characterization of the Human AKAP100 Recombinant Protein
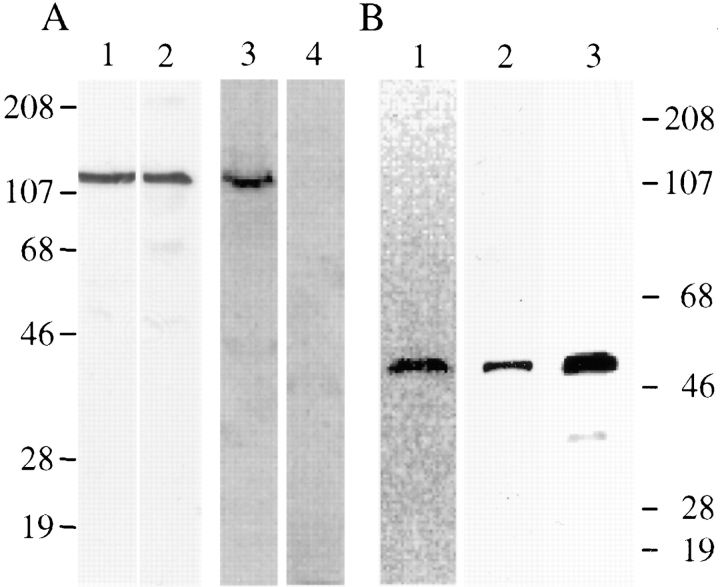
To further characterize the AKAP100 proteins in the adult hearts, and to determine the specificity of the anti-AKAP100 antibodies, we have cloned the full-length AKAP100 cDNA from the human heart by RT-PCR. One base differs between our sequence and the published AKAP100 cDNA sequence. A guanine-adenine transition at position 1978 results in a conservative amino acid substitution of aspartic acid for asparagine. Our results suggest that AKAP100 cDNA is highly conserved among individuals. The human AKAP100 cDNA was then subcloned into an expression vector for generating AKAP100 recombinant protein. By immunoblotting (Fig. 3 A), one protein band of ∼120 kD was recognized by both anti-6 His tag monoclonal antibody (lane 1) and anti-AKAP100 polyclonal IgGs (lane 2). Antibodies with different specificity recognized a protein of the same size on a preparative immunoblot, suggesting that a full-length AKAP100 recombinant was generated. To further characterize the AKAP100 recombinant, we have generated and purified the recombinant RII subunits by cAMP-agarose chromatography. The eluate from the cAMP-agarose column was separated by SDS-PAGE followed by Coomassie blue staining and immunoblotting. As shown in Fig. 3 B, one 52-kD protein band was identified on the Coomassie blue– stained gel (lane 1), and was recognized by the anti-RII antibodies on the immunoblot (lane 2), suggesting that recombinant RII was purified. The purified RII recombinant was phosphorylated in vitro (Fig. 3 B, lane 3). The 32P-labeled RII subunits bound to the AKAP100 recombinant in an RII overlay assay (Fig. 3 A, lane 3). The RII-AKAP100 binding was inhibited by incubation with 1 μM of the Ht31 inhibitory peptide (Fig. 3 A, lane 4). We have thus obtained a functional AKAP100 recombinant.
Figure 3.
Characterization of human AKAP100 and RII recombinant proteins. In A, human AKAP100 recombinant bacterial lysates were analyzed by immunblotting (lanes 1 and 2, from a preparative gel) and RII overlay (lanes 3 and 4, 100 μg/lane) assays. Lanes 1 and 2 were incubated with anti-6 His tag antibody and anti-AKAP100 antibodies, respectively. The protein bands were visualized by ECL. The RII overlay assay was performed as described in the text with (lane 4) or without (lane 3) the inhibitory peptide Ht31. The 32P-labeled RII binding protein was detected by PhosphorImaging (Molecular Dynamics, Inc.). In B, purified RII recombinant protein was analyzed by Coomassie blue staining (lane 1, 50 μg/lane) and immunoblotting (lane 2, 10 μg/lane) with anti-RII antibodies. The protein band was visualized by ECL. Lane 3 was loaded with 10 μl of 32P-labeled RII, which was detected by PhosphorImaging. Molecular weights are in kD.
The molecular weight of our AKAP100 recombinant protein (∼120 kD) is similar to the size of the AKAP100 recombinant previously reported (McCartney et al., 1995), but obviously larger than the 78-kD full-length AKAP100 predicted by the cDNA sequence (McCartney et al., 1995) and larger than the 80–82 kD proteins that we identified by using anti-AKAP100 antibodies in homogenates from adult rat and human hearts. Immunoblotting of enterokinase- digested AKAP100 recombinant demonstrated that the NH2-terminal histidine tag significantly retards migration of the recombinant on the SDS-PAGE gel (data not shown).
Specificity of the Anti-AKAP100 Antibodies
The anti-AKAP100 antibodies obtained from Upstate Biotechnology were generated against human AKAP100 recombinant (McCartney et al., 1995). McCartney and colleagues have shown that AKAP100-specific antibodies cross-react with AKAP100 in extracts from rat embryonic skeletal and cardiac cell lines (McCartney et al., 1995). Since we showed that the anti-AKAP100 antibodies recognized smaller proteins in the adult hearts when compared with one in the cell lines, we examined specificity of the anti-AKAP100 antibodies in the rat heart. As shown in Fig. 2 C, incubation of the anti-AKAP100 antibodies with AKAP100 recombinant bacterial lysates depleted the IgGs that recognized the 82-kD human heart protein (lane 1), the 80-kD rat heart protein (lane 2), and the AKAP100 recombinant (lane 3). In a complementary experiment, rabbit IgGs affinity-purified by using the 80-kD adult rat heart AKAP, recognized the 120-kD AKAP100 recombinant on the immunoblot (Fig. 2 D). These studies demonstrate that (a) the 80-kD rat heart protein is of AKAP100 origin; and (b) the anti-AKAP100 antibodies are specific for the AKAP in the rat heart.
Immunolocalization of AKAP100 in Longitudinal Sections of the Adult Rat Cardiac Myocytes
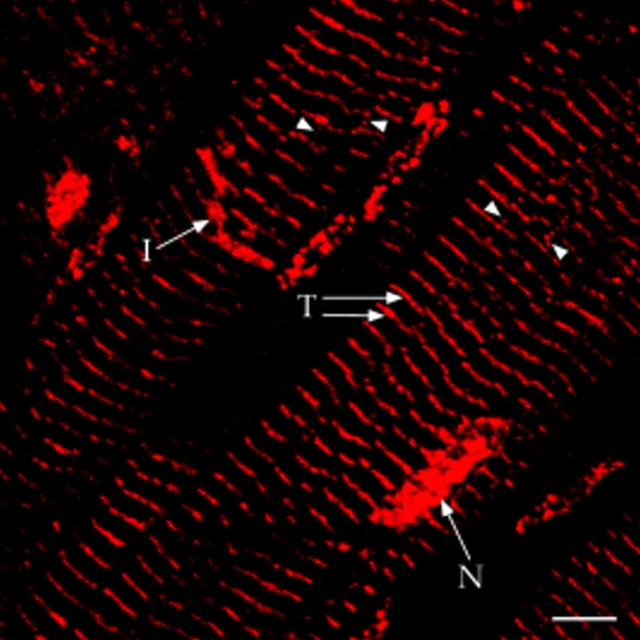
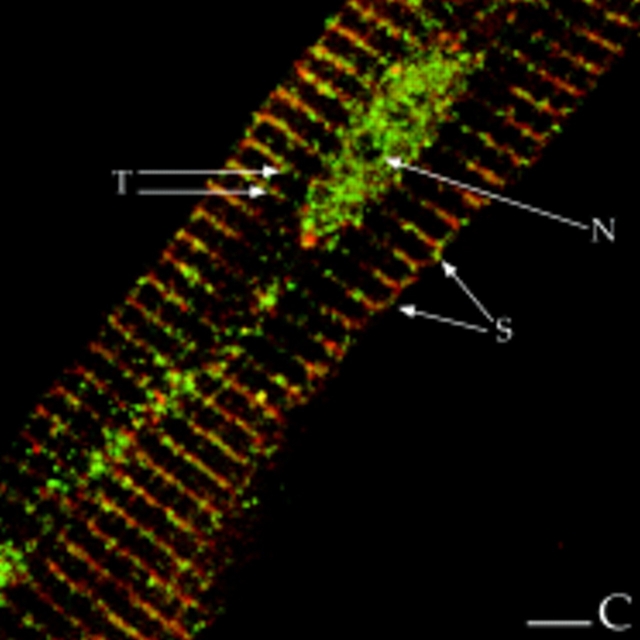
AKAP100 was localized in adult rat hearts using indirect immunofluorescence and laser scanning microscopy in cryostat sections of rapidly frozen rat papillary muscles. In longitudinal sections, AKAP100 staining was evident in the nucleus, cytoplasm, and at the intercalated disc of the myocytes (Fig. 4). In the cytoplasm, AKAP100 staining was observed predominantly as transverse bands, showing a periodic striated pattern at ∼2-μm intervals (Fig. 4). Occasionally, AKAP100 staining was also observed as longitudinal connections between the transverse bands (Figs. 4 and 7 C, arrowheads). To verify our findings in rat papillary muscles, we also determined the localization of AKAP100 in freshly isolated left ventricular myocytes from adult rat hearts. In longitudinal sections of the myocytes, the AKAP100 staining was consistent with the AKAP100 staining in the papillary muscles (data not shown). The same AKAP100 staining pattern was also seen in the subsequent studies described below.
Figure 4.
Immunolocalization of AKAP100 in longitudinal sections of the rat papillary muscle. Longitudinal sections of rat papillary muscles were immunostained with AKAP100-specific rabbit antibodies and examined by confocal microscopy. Localization of the primary antibodies was detected with LRSC-conjugated donkey anti–rabbit IgG antibodies. The transverse periodic staining (T), the nuclear staining (N), and the intercalated disc staining (I) are indicated. Arrowheads label the AKAP100 staining between the transverse staining. Bar, 5 μm.
Figure 7.
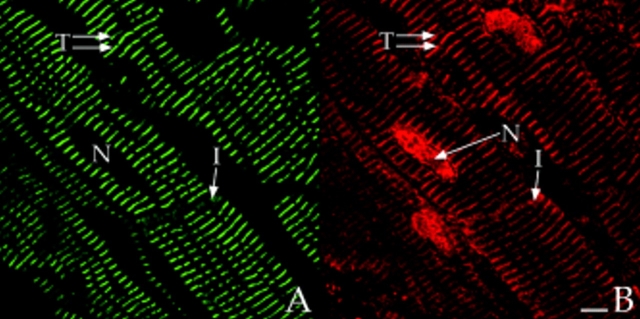
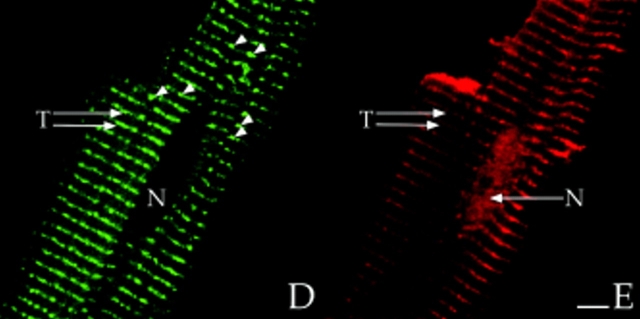
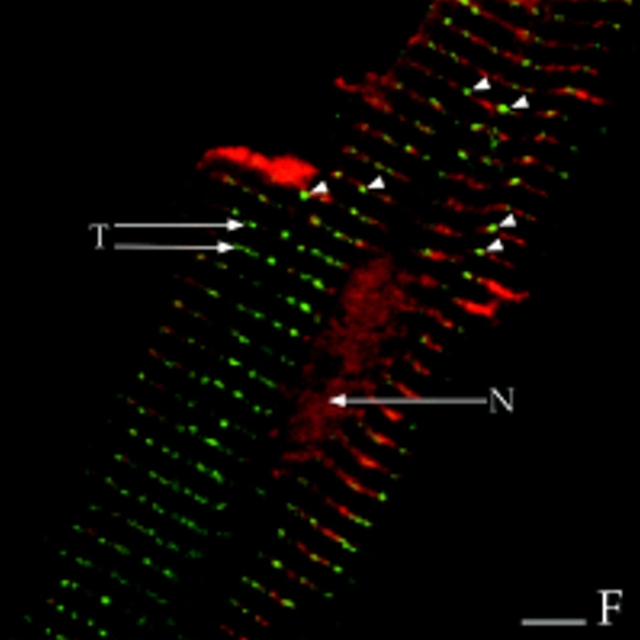
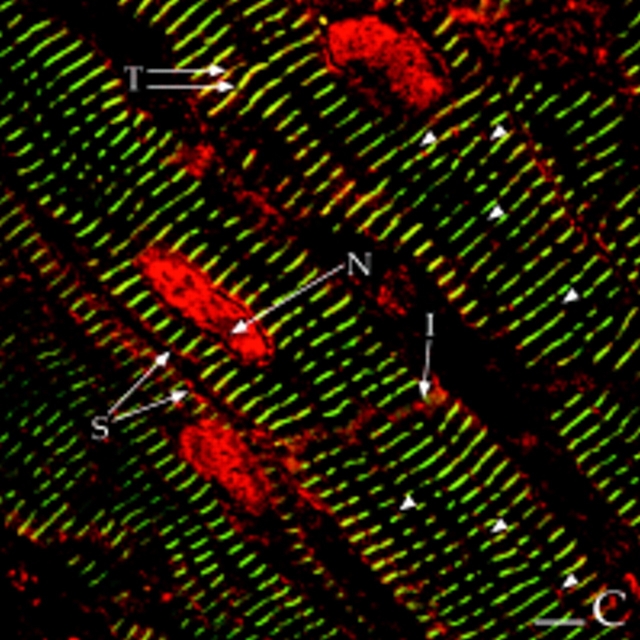
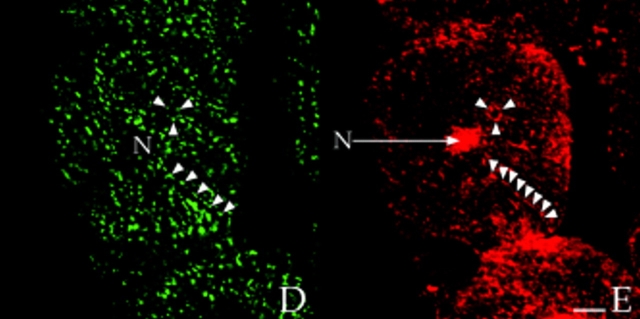
Colocalization of AKAP100 with α-actinin and RyR in longitudinal sections of cardiac myocytes. Longitudinal cryostat sections of rat papillary muscles (A–C) or freshly isolated adult rat left ventricular myocytes (D–F) were double-immunostained with either α-actinin–specific (A) and AKAP100-specific (B) antibodies or RyR-specific (D) and AKAP100-specific (E) antibodies. The anti-AKAP100 rabbit antibodies were detected with LRSC-conjugated donkey anti–rabbit IgG antibodies. Localization of the RyR- and α-actinin-specific monoclonal antibodies were visualized by using FITC-conjugated donkey anti–mouse IgG antibodies. C is the superimposed image of A and B; F combines from the images in D and E. Transverse periodic staining (T), sarcolemmal staining (S), nuclear staining (N), and intercalated disc staining (I) are indicated. In C, arrowheads indicate the longitudinal AKAP100 staining localized between the transverse staining, whereas in D and F, arrowheads label the punctate RyR staining. The nucleus without staining is labeled with an N. Bar, 5 μm.
Colocalization of RII and AKAP100 in Adult Rat Heart
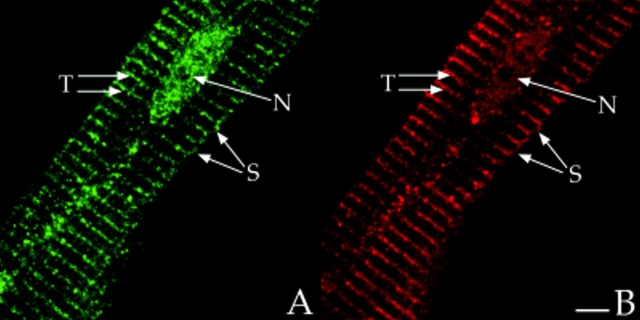
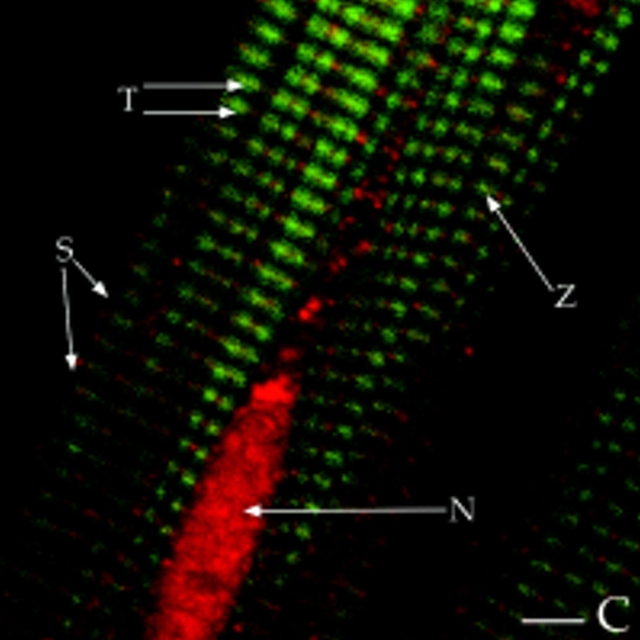
Scott and coworkers observed that RII colocalized with AKAP100 in the perinuclear region in rat H9c2 cells (McCartney et al., 1995). However, we identified AKAP100 in four distinct subcellular compartments of the adult rat cardiac myocyte, suggesting that localization of RII in the adult heart is very different from that in the embryonic H9c2 cells. We also localized RI and RII subunits in freshly isolated adult rat left ventricular myocytes. In longitudinal sections of the myocytes, RII staining showed a periodic transverse pattern in the cytoplasm (Fig. 5 A), very similar to the AKAP100 staining, though not identical (Fig. 5 B). Similar results were obtained in immunostained transverse cryostat sections (Fig. 9, A and B). In the superimposed image of RII/AKAP100 staining, the majority of the AKAP100 and RII staining overlapped (Fig. 5 C). Colocalization of RII-AKAP100 is also evident in the nucleus, sarcolemma (Fig. 5, A and B), and at the intercalated disc (data not shown) of the myocytes. Thus, colocalized RII and AKAP100 are found in several distinct subcellular compartments in the adult rat heart. As shown in Fig. 5 C, we also identified some nonoverlapping RII and AKAP100 staining, which may suggest that RII could be compartmentalized by AKAPs other than AKAP100 in the cell. No AKAP100 and RII staining was identified in the presence of an excess amount of recombinant AKAP100 and RII, respectively (data not shown). These competition studies demonstrate that the AKAP100 and RII staining shown in Figs. 4 and 5 is specific for AKAP100 and RII in the cardiac myocytes.
Figure 5.
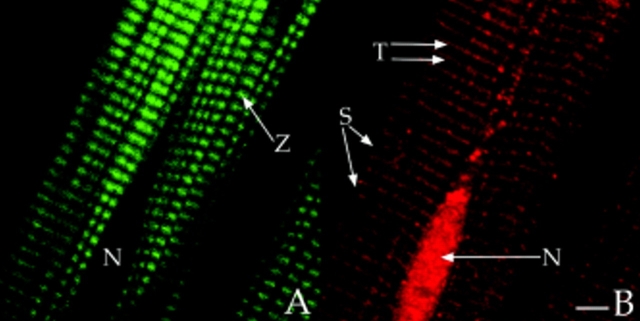
Colocalization of RII with AKAP100 and immunolocalization of RI in cardiac myocytes. Freshly isolated adult rat left ventricular myocytes were double-stained with RII- (A) and AKAP100-specific antibodies (B), or with RI-specific antibody (D). The anti-AKAP100 rabbit antibodies were detected with LRSC-conjugated donkey anti–rabbit IgG antibodies. Localization of RI-specific monoclonal antibody and RII-specific goat antibodies were visualized by using FITC-conjugated donkey anti–mouse IgG antibodies and FITC-conjugated donkey anti–goat IgG antibodies, respectively. C is the superimposed image of A and B. The transverse periodic staining (T), sarcolemmal staining (S), and the nuclear staining (N) are indicated. In D, the longitudinal staining pattern is labeled with arrowheads, and the nucleus without staining is labeled with an N. Bar, 5 μm.
Figure 9.
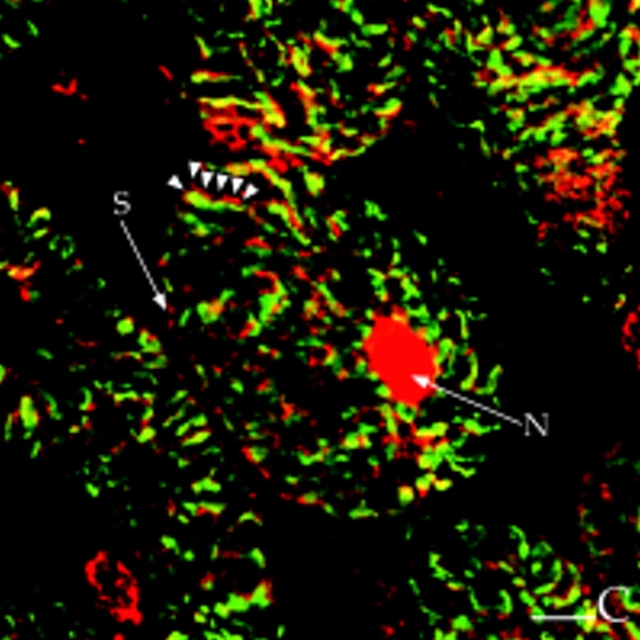
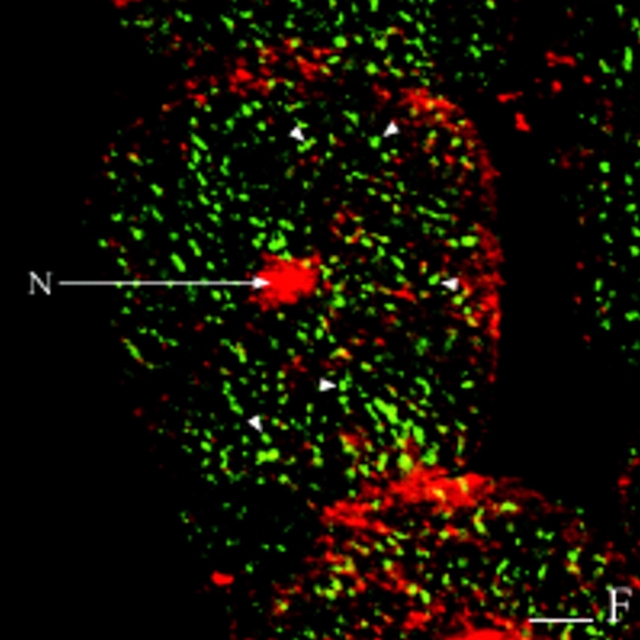
Colocalization of RII with AKAP100 in cross-sections of cardiac myocytes. Transverse cryostat sections of rat papillary muscles were double-immunostained with RII-specific (A) and AKAP100-specific (B) antibodies. The primary antibodies were detected as described in Fig. 5. The arrows label the nuclear staining (N) for RII and for AKAP100. The arrowheads indicates the similar RII and AKAP100 network staining patterns. Bar, 5 μm.
In contrast, RI staining was observed only in the cytoplasm, with no RI staining in the nucleus (Fig. 5 D). In the cytoplasm, the RI staining had a diffuse longitudinal pattern with no evidence of periodic cross-striations (Fig. 5 D, arrowheads). This longitudinal RI staining pattern suggests that RI may be distributed in a region close to the myofibrils. Our studies demonstrate that RI is not colocalized with AKAP100, and that RI and RII are most likely located in distinct subcellular compartments in the adult rat heart.
AKAP100 Is Localized at the Level of the Z-line in Cardiac Muscle Cell
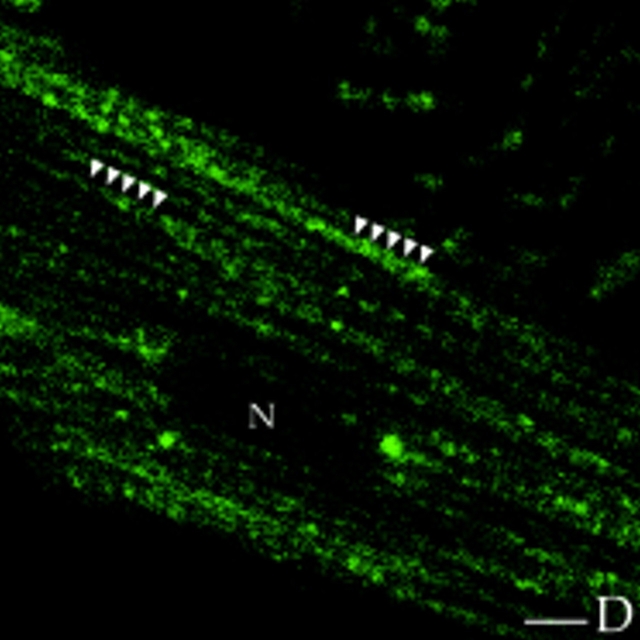
To align the transverse periodic AKAP100 staining to specific sarcomeric components, we immunostained freshly isolated adult rat left ventricular myocytes with FITC-phalloidin and AKAP100-specific antibodies. Phalloidin has a high affinity for F-actin in thin filaments. As expected, in the phalloidin-stained image, the longitudinally orientated thin filaments are visualized as periodic thick bands (Fig. 6 A). The unstained region of the myofibrils represents the H-zone at the center of the A-band, the area of the sarcomere where there are no thin filaments (Fig. 6 A). There was no phalloidin staining in either the nucleus or the interfibrillar space (Fig. 6 A). In the cytoplasm, the transverse periodic AKAP100 staining is shown as continuous lines crossing the myofibrils (Fig. 6 B). In the superimposed image of the phalloidin/AKAP100 staining, the AKAP100 staining aligns with the center of the phalloidin-stained thin filaments (Fig. 6 C), suggesting that AKAP100 is localized at the level of the Z-line. In Fig. 6 C, AKAP100 staining is also identified in the nucleus and in the sarcolemma where there is no evidence of phalloidin staining. These studies suggest that the periodic AKAP100 staining is at the level of the Z-line, but that AKAP100 may not be directly associated with the myofibrils in the heart.
Figure 6.
Localization of AKAP100 in the FITC-phalloidin–stained cardiac myocytes. Freshly isolated adult rat left ventricular myocytes were incubated with FITC-phalloidin (A) followed by immunostaining with AKAP100-specific rabbit antibodies (B). Localization of the anti-AKAP100 antibodies was detected with LRSC-conjugated donkey anti–rabbit IgG antibodies. C is the superimposed image of A and B. In A, the location of the Z-line at the center of phalloidin staining is indicated (Z), and the nucleus without staining is labeled with an N. In A and C, transverse periodic staining (T), sarcolemmal staining (S), and nuclear staining (N) are shown. Bar, 5 μm.
Colocalization of AKAP100 with α-Actinin and RyR in Longitudinal Sections of Cardiac Myocytes
α-Actinin is a major component of the Z-line of myofilaments (Tokuyasy et al., 1981). It can therefore serve as a marker to help determine the localization of AKAP100 in cardiac myocytes. We colocalized AKAP100 and α-actinin in cryostat sections of papillary muscle. In longitudinal sections, α-actinin staining showed a periodic transverse pattern at 2-μm intervals in the cytoplasm of the myocytes (Fig. 7 A). The α-actinin–specific antibody also detected the protein at the intercalated disc (Fig. 7 A). No α-actinin staining was observed in the nucleus or sarcolemma (Fig. 7, A and C). In the same sections, AKAP100 staining (Fig. 7 B) exhibited similar cytoplasmic staining patterns to α-actinin (Fig. 4 A). Superimposed images of the α-actinin (Fig. 7 A) and AKAP100 staining (Fig. 7 B) showed that the α-actinin and AKAP100 transverse periodic staining and intercalated disc staining overlapped (Fig. 7 C). In the superimposed image, we also identified some longitudinal AKAP100 staining (Fig. 7 C, arrowheads) similar to the longitudinal staining in Fig. 4. This longitudinal AKAP100 staining pattern resembled the distribution of the longitudinal components of the T-tubule network (Forbes et al., 1984). These results further confirm that AKAP100 is localized at the level of the Z-line in the nucleus, in the sarcolemma, and at the intercalated disc in the adult heart.
In adult cardiac myocytes, RyR is located in the junctional SR, apposed to the T-tubule located at the level of the Z-line (Opie, 1991; Takagishi et al., 1997). We therefore used RyR as an additional marker to determine the location of AKAP100 within the cardiac muscle cells. In longitudinal sections of left ventricular myocytes, RyR-specific staining was punctate and exhibited a periodic (∼2 μm) transverse pattern (Fig. 7 D). No RyR staining was evident in the nucleus (Fig. 7 D). These results are consistent with previously described RyR localization in the junctional SR in cardiac muscle (Jorgensen et al., 1993). AKAP100 staining (Fig. 7 E) was very similar to the RyR staining pattern (Fig. 7 D). In the RyR/AKAP100 double-stained cells, superimposed images demonstrated that the periodic transverse patterns of AKAP100 (Fig. 7 E) and RyR (Fig. 7 D) predominantly overlapped (Fig. 7 F), although RyR staining was punctate (Fig. 7 D, arrowheads), and AKAP100 staining (Fig. 7 E) exhibited a continuous transverse pattern. Some of the discrete punctate RyR staining did not colocalize with the AKAP100 staining (Fig. 7 F, arrowheads). These results suggest that AKAP100 may be localized in the region of the T-tubule/junctional SR close to the RyR.
AKAP100 is in the T-tubule/Junctional SR in the Cardiac Myocytes
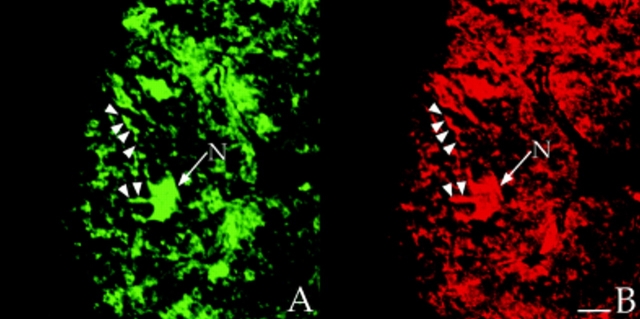
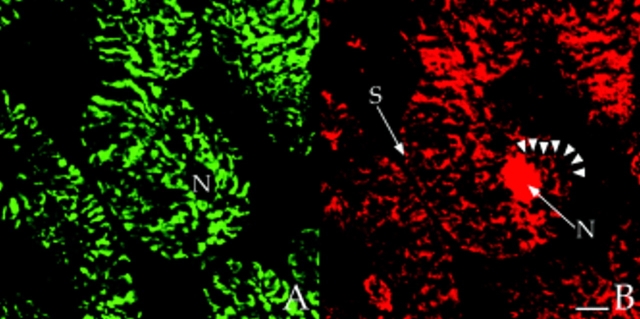
In longitudinal orientations, the staining patterns for AKAP100 essentially overlapped with those of α-actinin and RyR (Fig. 7), suggesting that all three proteins are located at the level of the Z-line. However, because of superposition of subcellular structures in the 400 nm–thick optical sections, a longitudinal orientation does not permit clear differentiation between a T-tubule/junctional SR network or the Z-disc as the location of AKAP100. To distinguish between these two possibilities, cross-sections of rat papillary muscle were double-immunostained with AKAP100-specific antibodies plus α-actinin–specific, or RyR-specific antibodies. α-Actinin staining was observed in the cytoplasm, but not in the nucleus or sarcolemma (Fig. 8 A). The stained regions represent the Z-discs of the myofibrils, whereas the unstained regions are intramyofibrillar space or myofibrils sectioned at regions other than at the level of the Z-disc. As in longitudinal sections, AKAP100 staining was observed in the cytoplasm, nucleus, and at the sarcolemma (Fig. 8 B). In the cytoplasm we identified a unique transverse AKAP100 staining pattern originating at the sarcolemma, consistent with the location of the T-tubule network (Fig. 8 B, arrowheads). In superimposed images of the α-actinin/AKAP100 double staining, the majority of the AKAP100 staining was distinct from and in the region surrounding the α-actinin staining (Fig. 8 C, arrowheads). These findings indicate that AKAP100 is not a major component of the Z-disc; therefore, it is likely that AKAP100 is localized to the region of the T-tubule/junctional SR.
Figure 8.
Colocalization of AKAP100 with α-actinin and RyR in cross-sections of cardiac myocytes. Cross-sections of the rat papillary muscle were double-immunostained with either α-actinin–specific (A) and AKAP100-specific (B) antibodies or RyR-specific (D) and AKAP100-specific (E) antibodies. The primary antibodies were detected as described in Fig. 5. C is the superimposed image of A and B; F is combined from the images in D and E. The myocytes are labeled as in Fig. 5. In B, arrowheads indicate the network pattern of the AKAP100 staining, which is not seen in the α-actinin staining (A). In C, AKAP100 staining localized in the boundary of the α-actinin staining is indicated by arrowheads. In D and E, arrowheads indicate the similar RyR and AKAP100 network staining. In F, arrowheads indicate the punctate RyR staining that does not overlap with the AKAP100 staining. Bar, 5 μm.
The T-tubule and junctional SR are found in a network around and between the myofibrils (Opie, 1991). In transverse sections of papillary muscles, the punctate RyR staining exhibited a network pattern (Fig. 8 D), and the AKAP100 staining showed a similar pattern (Fig. 8 E). This network pattern of the AKAP100 staining is consistent with the network pattern identified in the α-actinin/ AKAP100 double-staining (Fig. 8 B). Compared with the punctate RyR staining (Fig. 8 D, arrowheads), the AKAP100 staining was more continuous (Fig. 8 E, arrowheads). In the superimposed image of the AKAP100/RyR double-stained cross-sections, some of the punctate RyR stainings were obviously distinct from the AKAP100 staining (Fig. 8 F, arrowheads). Since AKAP100 does not colocalize with α-actinin, the similar network pattern of the RyR and AKAP100 staining suggests the AKAP100 is localized to the region of the T-tubule/junctional SR.
Finally, we also investigated colocalization of RII and AKAP100 in the transverse cryostat sections of the rat papillary muscles. As we expected, both RII and AKAP100 staining exhibit very similar network patterns (Fig. 9, A and B), which are consistent with (a) the AKAP100 staining in the region of the T-tubule/junctional SR network identified in Fig. 8, and (b) the RII-AKAP100 colocalization identified in the longitudinal sections of the cardiac myocytes (Fig. 5, A–C).
Discussion
We have shown for the first time the expression of AKAP100 in adult rat and human hearts. Our studies consistently demonstrate that the apparent molecular weights of AKAP100 from adult rat and human hearts (Fig. 1) are very similar to the 78-kD protein predicted by the AKAP100 cDNA sequence, but less than the 100-kD protein previously reported in the H9c2 cell line. No higher molecular weight protein was identified in the anti-AKAP100 antibody-precipitated rat heart homogenates (Fig. 2 B). By RII immunoprecipitation and cAMP-agarose copurification assays, we have specifically shown that the 80-kD rat heart protein is an RII-binding protein (Fig. 2 B). We have further characterized the 80-kD rat AKAP by using recombinant human AKAP100. Results of the recombinant AKAP100 depletion and the affinity purification of anti-AKAP100 antibodies studies (Fig. 2, C and D) demonstrate that the 80-kD rat heart protein is of AKAP100 origin. This finding was further supported by our enterokinase digestion studies. An ∼30-kD histidine tag was generated from the ∼120-kD fusion protein by the enzyme digestion (data not shown). Our studies therefore suggest that the size of AKAP100 in the mature adult heart is significantly <100 kD.
Developmental regulation of cardiac proteins, e.g., troponin I and troponin T, is an important characteristic of maturation of the mammalian heart (Murphy, 1996). In addition, posttranscriptional modification has previously been reported for certain AKAPs, e.g., AKAP82 (Johnson et al., 1997a ). Johnson and colleagues also demonstrated that a 26-kD NH2-terminal fragment from pro-AKAP82 (109 kD) is cleaved off during spermiogenesis, and AKAP80 is observed as an 82-kD protein in epididymal sperm (Johnson et al., 1997a ). From these studies, it is therefore reasonable to speculate that similar modification could occur in AKAP100.
The molecular weights of the human and rat AKAP100 homologues are slightly different (Fig. 2 A). By Northern blotting, human AKAP100 cDNA weakly hybridized to rat heart mRNA (data not shown). Therefore, there may be species differences in the amino acid sequence of AKAP100 between rat and human. It has been shown that AKAP is a family of heterogeneous proteins. Other than the amphipathic helix motif responsible for the RII-AKAP interaction, there is no overall similarity in nucleotide sequence among the identified AKAPs. Studies have shown that AKAP75, AKAP79, and AKAP150 are bovine, human, and rat homolgues, respectively (Rubin, 1994). The similarities and differences between the rat and human AKAP100 homolgues will be elucidated once the nucleotide sequence of the rat AKAP100 is determined.
By immunoblotting, we show that the 49-kD RIα, 53-kD RIβ, and the 54-kD RIIα subunit are present in adult rat hearts (Fig. 1). These findings are consistent with previous demonstrations of RI and RII studies in the rat (Skalhegg et al., 1992; DeManno et al., 1994; Jahnsen et al., 1985; De Camilli et al., 1986). By immunostaining and confocal microscopy, we identify RI- and RII-specific staining in rat cardiac myocytes, thus confirming expression of both RI and RII subunits in the adult heart.
In contrast to previous findings in the H9c2 cardiac cell line where AKAP100 was associated with RII in a perinuclear location (McCartney et al., 1995), we have shown that RII colocalizes with AKAP100 in multiple subcellular compartments in adult hearts (Fig. 3). Because the RII and AKAP100 staining patterns are very similar (Fig. 3), our results also suggest that AKAP100 is likely to be the major PKA II anchor in the heart.
The cytoplasmic RI staining and lack of RI nuclear staining observed in our studies are consistent with previous findings by Meinkoth and colleagues (Meinkoth et al., 1990). These authors demonstrated that RI subunits serve as anchors that sequester C subunits in the cytoplasm until the holoenzyme dissociates in response to increased cAMP, and the dissociated C subunits are then transported into the nucleus. Our results suggest that RI may be associated with myofilaments (Fig. 5 D). It has recently been shown that RI may bind to AKAPs with low affinity (Burton et al., 1997), and that D-AKAP1 can simultaneously bind RI and RII (Huang et al., 1997). It is therefore possible that RI is compartmentalized in rat cardiac myocytes by mechanisms yet to be determined. One of the major findings from our studies is that we have identified that RI and RII subunits are distributed differently in the cardiac myocytes, implying that PKA I and II may have different functions in the heart.
In addition to AKAP100 localization in the cytoplasm, we also localized AKAP100 to the nuclei of cardiac myocytes. Nuclear localization of AKAP100 may be a feature of the differentiated cardiac myocyte, but not the embryonic cardiac H9c2 cell line. Previous studies have shown nuclear distributions for AKAPs (Coghlan et al., 1994; Zhang et al., 1996). Our results suggest that AKAP100 may be another nuclear AKAP, although distinct from AKAP95, since AKAP100 is also found in the cytoplasm. Our studies therefore demonstrate the diversity of AKAP100-dependent PKA II compartmentalization, and suggest that AKAP100 may have different regulatory functions at different sites in the adult heart.
By double immunostaining of cardiac myocytes with AKAP100-specific antibodies and FITC-phalloidin (Fig. 6), and also by colocalization of AKAP100 with α-actinin and with RyR (Fig. 7), we have shown that AKAP100 is localized in the region of the T-tubule/junctional SR in adult ventricular myocytes (Fig. 8). L-type Ca2+ channels in the sarcolemma and T-tubule play an important role in excitation–contraction coupling in striated muscle cells (Opie, 1991). Recent studies demonstrated that disruption of RII-AKAP binding with an inhibitory peptide, Ht31, prevents time- and voltage-dependent modulation of the skeletal muscle L-type Ca2+ channel expressed in a kidney cell line (Johnson et al., 1997b ). In HEK293 cells transfected with AKAP79, AKAP-dependent regulation of cardiac L-type Ca2+ channel was observed (Gao et al., 1997). By immunoprecipitation and RII overlay, a 15-kD AKAP15 was found to be associated with the L-type Ca2+ channel in skeletal muscle (Gray et al., 1997). Since the L-type Ca2+ channel is a major PKA substrate in the heart (Tsien et al., 1986), AKAP100 could potentially function in tethering PKA II to this channel, or in the vicinity of the channel, in cardiac myocytes.
In cryostat sections of papillary muscles, we also observed AKAP100 staining in the sarcolemma and at the intercalated disc of cardiac myocytes. The intercalated disc is an area of intercellular junctions that facilitates intercellular adhesion and communication. Careful ultrastructural studies of cardiac myocytes at the EM level have revealed that the sarcolemma can extend from the intercalated disc of one myocyte into the T-tubule network of the adjacent cell (Meddoff and Page, 1968). The T-tubule is an invagination of the myocyte membrane, and thus the sarcolemma, the intercalated disc, and the T-tubule network can be considered to be an interconnected membrane system, perhaps explaining the distribution of AKAP100 in each of these subcellular compartments. In addition, it has been shown that the Kv1.5 K+ channel is localized at the intercalated disc of cardiac myocytes (Mays et al., 1995). Activity of certain K+ channels is regulated by PKA phosphorylation (Chen and Yu, 1994; Chuang et al., 1991), and thus localization of AKAP100 at the intercalated disc may imply PKA regulation of the K+ channel in the cardiac muscle cell.
It was initially believed that each AKAP contains two main classes of binding sites: a conserved anchoring motif that binds RII, and a targeting domain that directs subcellular localization of the AKAP/PKA II complex (Dell'Acqua and Scott, 1997; Rubin, 1994). According to the location of known AKAPs, a targeting hypothesis was proposed (Dell'Acqua and Scott, 1997; Faux and Scott, 1996). It suggests that the targeting domain is an essential feature of each AKAP as it confers specificity by tethering the anchored PKA complex to a particular organelle or location in the cell. However, with the exception of a report of putative targeting domains on AKAP75 (Glantz et al., 1993), the portion of AKAP molecules responsible for targeting remains to be identified.
We have shown that AKAP100 is located in multiple subcellular compartments in cardiac myocytes, and also that RII colocalizes with AKAP100 in the cardiac muscle cells. Our findings therefore suggest that AKAP100 tethers PKA II to several distinct subcellular compartments rather than targeting PKA II to a single site. In order for the AKAP100/PKA II complex to localize to multiple sites in the cell, we propose that the AKAP100/PKA II complex may associate with an unidentified molecule(s) common to a number of different regions in the cell.
Acknowledgments
We thank Russell W. Desnoyer, James Lang, and Ambrose J. To for technical assistance, Dr. Robert Stewart and other members of the cardiac transplant team of the Department of Thoracic and Cardiovascular Surgery, members of the Department of Pathology of the Cleveland Clinic Foundation, LifeBanc of Northeast Ohio for providing human heart tissue, and Drs. Dianne M. Perez and Li Zhang, Department of Molecular Cardiology, Cleveland Clinic Foundation, for critical review of the manuscript.
This work was supported by National Institutes of Health RO1 HL56256 (to M. Bond), and by National Institutes of Health cardiovascular training grant HL07653 (to J.C. Yang).
Abbreviations used in this paper
- AKAP
A-kinase anchoring protein
- C
PKA catalytic subunit
- ECL
enhanced chemiluminescence
- LRSC
lissamine rhodamine B sulfonyl chloride
- PKA
cAMP-dependent protein kinase A
- PKC
protein kinase C
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- T-tubule
transverse tubule
Footnotes
Address all correspondence to Meredith Bond, Ph.D., Department of Molecular Cardiology, FF10, The Lerner Research Institute, The Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195. Tel.: 216-444-3734; Fax: 216-445-6062; e-mail: bondm@cesmtp.ccf.org
References
- Beall JA, Mitchell GF. Identification of a particular antigen from a parasite cDNA library using antibodies affinity purified from selected portions of Western blot. J Immunol Methods. 1986;86:217–223. doi: 10.1016/0022-1759(86)90456-4. [DOI] [PubMed] [Google Scholar]
- Bregman DB, Bhattacharyya N, Rubin CS. High affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II-B. J Biol Chem. 1989;264:4648–4656. [PubMed] [Google Scholar]
- Burton KA, Johnson BD, Hausken ZE, Westenbroek RE, Idzerda RL, Scheuer T, Scott JD, Catterall WA, McKnight GS. Type II regulatory subunits are not required for the anchoring-dependent modulation of Ca21 channel activity by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1997;94:11067–11072. doi: 10.1073/pnas.94.20.11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl SL, Felix K, Caswell AH, Brandt NR, Ball WJ, Jr, Vaghy PL, Meissner G, Ferguson DG. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J Cell Biol. 1995;129:673–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DW, Hausken ZE, Fraser IDC, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein. J Biol Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- Chen Y, Yu L. Differential regulation by cAMP-dependent protein kinase and protein kinase C of the m opioid receptor coupling to a G protein-activated K1 channel. J Biol Chem. 1994;269:7839–7842. [PubMed] [Google Scholar]
- Chung S, Reinhart PH, Martin BL, Brautigan D, Levitan IB. Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science. 1991;253:560–562. doi: 10.1126/science.1857986. [DOI] [PubMed] [Google Scholar]
- Coghlan VM, Langeberg LK, Fernandez A, Lamb NJ, Scott JD. Cloning and characterization of AKAP 95, a nuclear protein that associates with the regulatory subunit of type II cAMP-dependent protein kinase. J Biol Chem. 1994;269:7658–7665. [PubMed] [Google Scholar]
- DeCamilli P, Moretti M, Donini SD, Walter U, Lohmann SM. Heterogeneous distribution of the cAMP receptor protein RII in the nervous system: evidence for its intracellular accumulation on microtubules, microtubule-organizing centers, and in the area of the golgi complex. J Cell Biol. 1986;103:189–203. doi: 10.1083/jcb.103.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua ML, Scott JD. Protein kinase A anchoring. J Biol Chem. 1997;272:12881–12884. doi: 10.1074/jbc.272.20.12881. [DOI] [PubMed] [Google Scholar]
- DeManno DA, Jackiw V, Brooks EJ, Hunzicker-Dunn M. Characterization of recombinant RIb and evaluation of the presence of RIb protein in rat brain and testicular extracts. Biochim Biophys Acta. 1994;1222:501–510. doi: 10.1016/0167-4889(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO (Eur Mol Biol Organ) J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux MC, Scott JD. Molecular glue: kinase anchoring and scaffold proteins. Cell. 1996;85:9–12. doi: 10.1016/s0092-8674(00)81075-2. [DOI] [PubMed] [Google Scholar]
- Faux MC, Scott JD. More on target with protein phosphorylation: conferring specificity by location. Trends Biochem Sci. 1996;21:312–315. [PubMed] [Google Scholar]
- Forbes MS, Hawkey LA, Sperelakis N. The transverse-axial tubular system (TATS) of mouse myocardium: its morphology in the developing and adult animal. Am J Anat. 1984;170:143–162. doi: 10.1002/aja.1001700203. [DOI] [PubMed] [Google Scholar]
- Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca21 channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Garvey JL, Kranias EG, Solaro RJ. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. Biochem J. 1988;249:709–714. doi: 10.1042/bj2490709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz SB, Li Y, Rubin CS. Characterization of distinct tethering and intracellular targeting domains in AKAP75, a protein that links cAMP-dependent protein kinase IIb to the cytoskeleton. J Biol Chem. 1993;268:12796–12804. [PubMed] [Google Scholar]
- Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- Gwathmey JK, Hajjar RJ, Solaro RJ. Contractile deactivation and uncoupling of crossbridges. Effects of 2,3-butanedione monoxime on mammalian myocardium. Circ Res. 1991;69:1280–1292. doi: 10.1161/01.res.69.5.1280. [DOI] [PubMed] [Google Scholar]
- Hausken ZE, Dell'Acqua ML, Coghlan VM, Scott JD. Mutational analysis of the A-kinase anchoring protein (AKAP)-binding site on RII. J Biol Chem. 1996;271:29016–29022. doi: 10.1074/jbc.271.46.29016. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- Huang LJ, Durick K, Weiner JA, Chun J, Taylor SS. Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J Biol Chem. 1997;272:8057–8064. doi: 10.1074/jbc.272.12.8057. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. 1996. Guide for the Care and Use of Laboratory Animals. National Academy Press, Washington, D.C. 118 pp.
- Jahnsen T, Lohmann SM, Walter U, Hedin L, Richards JS. Purification and characterization of hormone-regulated isoforms of the regulatory subunit of type II cAMP-dependent protein kinase from rat ovaries. J Biol Chem. 1985;260:15980–15987. [PubMed] [Google Scholar]
- Johnson LR, Foster JA, Haig-Ladewig L, VanScoy H, Rubin CS, Moss SB, Gerton GL. Assembly of AKAP82, a protein kinase A anchor protein, into the fibrous sheath of mouse sperm. Dev Biol. 1997a;192:340–350. doi: 10.1006/dbio.1997.8767. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Zheng W, Korach KS, Scheuer T, Catterall WA, Rubanyi GM. Increased expression of the cardiac L-type calcium channel in estrogen receptor-deficient mice. J Gen Physiol. 1997b;110:135–140. doi: 10.1085/jgp.110.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen AO, Shen AC, Arnold W, McPherson PS, Campbell KP. The Ca21-release channel/ryanodine receptor is localized in junctional and corbular sarcoplasmic reticulum in cardiac muscle. J Cell Biol. 1993;120:969–980. doi: 10.1083/jcb.120.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano S, Nakamura F, Tanaka T, Hiraoka M. Cardiac sarcoplasmic reticulum chloride channels regulated by protein kinase A. Circ Res. 1992;71:585–589. doi: 10.1161/01.res.71.3.585. [DOI] [PubMed] [Google Scholar]
- Keryer G, Rios RM, Landmark BF, Skalhegg B, Lohmann SM, Bornens M. A high-affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II in the centrosome of human cells. Exp Cell Res. 1993;204:230–240. doi: 10.1006/excr.1993.1029. [DOI] [PubMed] [Google Scholar]
- Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- Krijnse-Locker J, Parton RG, Fuller SD, Griffiths G, Dotti CG. The organization of the endoplasmic reticulum and the intermediate compartment in cultured rat hippocampal neurons. Mol Biol Cell. 1995;6:1315–1332. doi: 10.1091/mbc.6.10.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann SM, DeCamilli P, Einig I, Walter U. High-affinity binding of the regulatory subunit (RII) of cAMP-dependent protein kinase to microtubule-associated and other cellular proteins. Proc Natl Acad Sci USA. 1984;81:6723–6727. doi: 10.1073/pnas.81.21.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays DJ, Foose JM, Philipson LH, Tamkun MM. Localization of the Kv1.5 K1 channel protein in explanted cardiac tissue. J Clin Invest. 1995;96:282–292. doi: 10.1172/JCI118032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney S, Little BM, Langeberg LK, Scott JD. Cloning and characterization of A-kinase anchor protein 100 (AKAP100). A protein that targets A-kinase to the sarcoplasmic reticulum. J Biol Chem. 1995;270:9327–9333. doi: 10.1074/jbc.270.16.9327. [DOI] [PubMed] [Google Scholar]
- McConnell BK, Moravec CS, Morano I, Bond M. Troponin I phosphorylation in spontaneously hypertensive rat heart: effect of b-adrenergic stimulation. Am J Physiol. 1997;273:H1440–H1451. doi: 10.1152/ajpheart.1997.273.3.H1440. [DOI] [PubMed] [Google Scholar]
- Meddoff DA, Page E. Extensions of the cardiac plasma membrane from the intercalated disk of one cell into the transverse tubules of the adjacent cell. J Ultrastruct Res. 1968;24:508–521. doi: 10.1016/s0022-5320(68)80051-6. [DOI] [PubMed] [Google Scholar]
- Meinkoth JL, Ying J, Taylor SS, Feramisco JR. Dynamics of the distribution of cyclic AMP-dependent protein kinase in living cells. Proc Natl Acad Sci USA. 1990;87:9595–9599. doi: 10.1073/pnas.87.24.9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AM. Contractile protein phenotypic variation during development. Cardiovasc Res. 1996;31:E25–E33. [PubMed] [Google Scholar]
- Nauert JB, Klauck TM, Langeberg LK, Scott JD. Gravin, an autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffold protein. Curr Biol. 1997;7:52–62. doi: 10.1016/s0960-9822(06)00027-3. [DOI] [PubMed] [Google Scholar]
- Opie, L.H. 1991. The heart: physiology and metabolism. 2nd ed. Raven Press, Ltd. New York.
- Piper, H.M., and G. Isenberg. 1989. Isolated adult cardiomyocytes. Vol. 2. Hans Michael Piper and Gerrit Isenberg, editors. CRC Press LLC, Boca Raton, FL. 29–73.
- Rubin CS. A kinase anchor proteins and the intracellular targeting of signals carried by cyclic AMP. Biochim Biophys Acta. 1994;1224:467–479. [PubMed] [Google Scholar]
- Scott JD. Dissection of protein kinase and phosphatase targeting interactions. Soc Gen Physiol Ser. 1997;52:227–239. [PubMed] [Google Scholar]
- Skalhegg BS, Landmark B, Foss KB, Lohmann SM, Hansson V, Lea T, Jahnsen T. Identification, purification, and characterization of subunits of cAMP-dependent protein kinase in human testis. J Biol Chem. 1992;267:5374–5379. [PubMed] [Google Scholar]
- Skalhegg BS, Tasken K, Hansson V, Huitfeldt HS, Jahnsen T, Lea T. Location of cAMP-dependent protein kinase type I with the TCR-CD3 complex. Science. 1994;263:84–87. doi: 10.1126/science.8272870. [DOI] [PubMed] [Google Scholar]
- Sussman MA, Sakhi S, Barrientos P, Ito M, Kedes L. Tropomodulin in rat cardiac muscle: Localization of protein is independent of messenger RNA distribution during myofibrillar development. Circ Res. 1994;75:221–232. doi: 10.1161/01.res.75.2.221. [DOI] [PubMed] [Google Scholar]
- Takagishi Y, Rothery S, Issberner J, Levi A, Severs NJ. Spatial distribution of dihydropyridine receptors in the plasma membrane of guinea pig cardiac myocytes investigated by correlative confocal microscopy and label-fracture electron microscopy. J Electron Microsc. 1997;46:165–170. doi: 10.1093/oxfordjournals.jmicro.a023504. [DOI] [PubMed] [Google Scholar]
- Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem. 1991;109:163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- Takasago T, Imagawa T, Shigekawa M. Phosphorylation of the cardiac ryanodine receptor by cAMP-dependent protein kinase. J Biochem. 1989;106:872–877. doi: 10.1093/oxfordjournals.jbchem.a122945. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: Framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Tokuyasu KT, Dutton AH, Geiger B, Singer SJ. Ultrastructure of chicken cardiac muscle as studied by double immunolabeling in electron microscopy. Proc Natl Acad Sci USA. 1981;78:7619–7623. doi: 10.1073/pnas.78.12.7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986;18:691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Yang JC, Blanton RE, King CL, Fugjioka H, Aikawa M, Sam-Yellowe TY. Seroprevalence and specificity of human responses to the Plasmodium falciparumrhoptry protein Rhop-3 using a C-terminal recombinant protein. Infect Immun. 1996;64:3584–3591. doi: 10.1128/iai.64.9.3584-3591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Carr DW, Lerea KM, Scott JD, Newman SA. Nuclear localization of type II cAMP-dependent protein kinase during limb cartilage differentiation is associated with a novel developmentally regulated A-kinase anchoring protein. Dev Biol. 1996;176:51–61. doi: 10.1006/dbio.1996.9995. [DOI] [PubMed] [Google Scholar]