Abstract
We have investigated the functions of troponin T (CeTnT-1) in Caenorhabditis elegans embryonic body wall muscle. TnT tethers troponin I (TnI) and troponin C (TnC) to the thin filament via tropomyosin (Tm), and TnT/Tm regulates the activation and inhibition of myosin-actin interaction in response to changes in intracellular [Ca2+]. Loss of CeTnT-1 function causes aberrant muscle trembling and tearing of muscle cells from their exoskeletal attachment sites (Myers, C.D., P.-Y. Goh, T. StC. Allen, E.A. Bucher, and T. Bogaert. 1996. J. Cell Biol. 132:1061–1077). We hypothesized that muscle tearing is a consequence of excessive force generation resulting from defective tethering of Tn complex proteins. Biochemical studies suggest that such defective tethering would result in either (a) Ca2+-independent activation, due to lack of Tn complex binding and consequent lack of inhibition, or (b) delayed reestablishment of TnI/TnC binding to the thin filament after Ca2+ activation and consequent abnormal duration of force. Analyses of animals doubly mutant for CeTnT-1 and for genes required for Ca2+ signaling support that CeTnT-1 phenotypes are dependent on Ca2+ signaling, thus supporting the second model and providing new in vivo evidence that full inhibition of thin filaments in low [Ca2+] does not require TnT.
Keywords: troponin T, Caenorhabditis elegans, troponin C/troponin I interactions, human heart disease, Ca2+-regulation
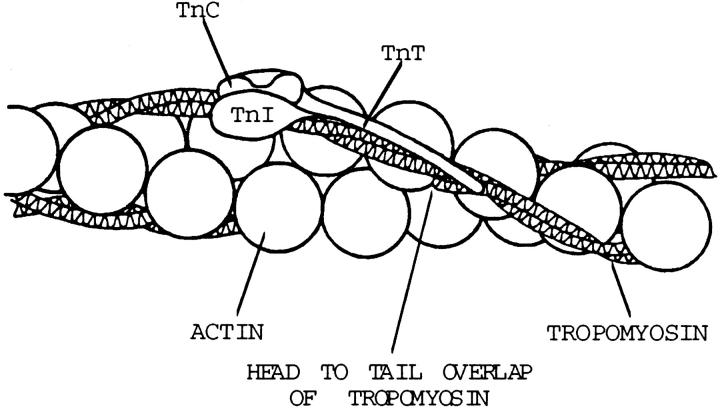
Contraction of striated muscle is regulated by a Ca2+-sensitive switch that is located on actin filaments and composed of four proteins: tropomyosin (Tm)1, troponin C (TnC), troponin I (TnI), and troponin T (TnT). At submicromolar concentrations of free Ca2+, the regulatory switch, simply referred to as the Tn–Tm complex, is thought to block attachment of myosin to actin, resulting in a relaxed muscle (22, 31, 38). At micromolar and higher concentrations, Ca2+ binds to TnC, thereby inducing a shift in position of the Tn–Tm complex that permits actin–myosin interaction and force production (22, 31, 38, 48). Knowledge of the functions of each of the four regulatory proteins, as well as of the interactions among them, is still rudimentary and derived primarily from biochemical studies of binary complex formation (for review see 36, 63, 78). These biochemical studies have led to a model in which TnT tethers TnC and TnI to Tm and hence to the thin filament (see Fig. 1). Biochemical and physiological studies (54, 62, 64) indicate that TnT modulates the Ca2+-sensitivity of force production, and genetic studies show that mutations of TnT can impair muscle assembly and compromise muscle function, as well as lead to lethality in nematodes (46) and humans (39, 61, 72, 73). Recent studies of human cardiomyopathies caused by TnT mutation also have revealed unexpected affects of TnT mutation on regulation of myosin–actin interaction, underscoring our limited understanding of TnT in vivo functions (39).
Figure 1.
Schematic diagram showing the relationships among the thin filament proteins. In the absence of Ca2+, the Tn–Tm complex sterically blocks actin-myosin interaction. In the presence of Ca2+, this steric hindrance is relieved (adapted from 13).
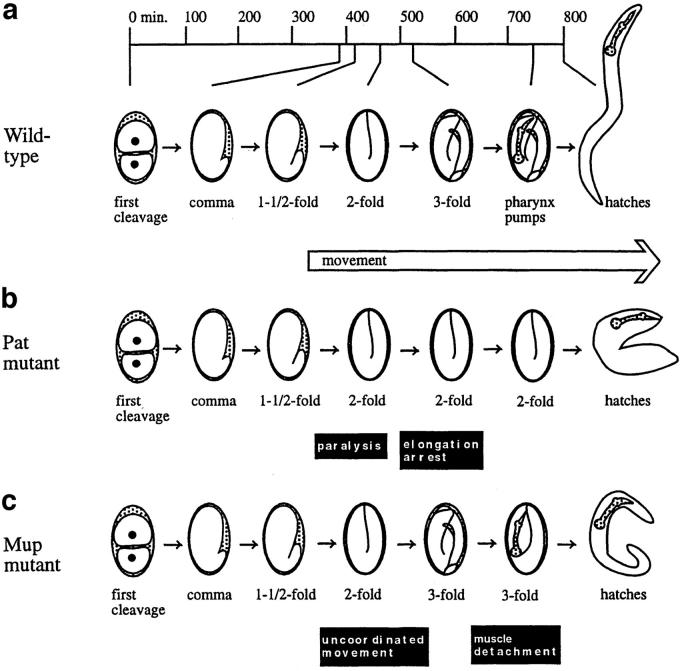
Here, we investigate further specific hypotheses regarding the in vivo defects in the regulation of muscle contraction caused by mutation of the TnT-1 gene by investigating the consequences of placing a TnT-1 mutation in combination with other mutations affecting the Ca2+-signal in the body wall muscles of the nematode Caenorhabditis elegans. Numerous discrete phenotypes affecting the striated body wall muscle in C. elegans are caused by mutations of genes encoding muscle proteins, including the Tm–Tn complex. The striated body wall muscle of the nematode is responsible for locomotion of the worm, and body wall muscle development and ultrastructure have been described in detail (for review see 42, 68). Body wall muscle differentiation occurs during the period of organismal morphogenesis, during which the embryo elongates from a ball of cells into its final tube shape (30, 51, 59). Based on gross morphology, the stages of elongation are descriptively referred to as bean, 1.5-fold (referring to embryo length in comparison with the length of the egg), twofold, and threefold (see Fig. 2). In the bean stage, before the onset of elongation, body wall muscle cells have already migrated to specific positions that define the future dorsal left and right, as well as ventral left and right, quadrants of the worm. At this stage, some muscle-specific protein expression can be detected. By the 1.5-fold stage, organization of myosin into A bands can be detected. By the 1.75-fold stage, A bands and the actin-containing I bands are well organized, and muscle is obviously generating force, since the embryo visibly moves within the egg shell. During the twofold stage, the movement becomes coordinated, and embryos begin to roll within the egg shell. Vigorous rolling continues during the threefold stage. At this stage there are 81 body wall muscle cells, which have two sarcomeres each and are aligned from the nose to tail in 4 quadrants. During larval stages, the number of body wall muscle cells increases to 95, and the number of sarcomeres within each of these cells increases to ∼10. Biochemical studies support the view that striated muscle in C. elegans, like that in many other invertebrates (37), requires both thick and thin filament activation (dual regulation) to achieve contraction (21).
Figure 2.
Schematic representation of embryonic development in WT and mutant (Mup and Pat) worms. Highlights of WT muscle development are indicated in a. Pat mutants fail to show muscle movement at the 1.75 stage and arrest development at the twofold stage of development. Animals bearing the mup-2(ts) mutation exhibit two temperature sensitive periods (tsp). The tsp relevant to this study is during the threefold stage of embryogenesis: embryos shifted to the restrictive temperature (25°C) exhibit the Mup phenotype (see Introduction). Embryos raised at the permissive temperature (15°C) are viable and appear WT at hatching. The mup-2(ts) animals, regardless of the temperature at which raised, and mup-2(up1) mutants usually exhibit the trembling phenotype (46).
Many mutations that affect the body wall muscle cause viable uncoordinated phenotypes such as slowed movement, paralysis, hypercontraction and uncontrolled twitching (8, 68, 71). Other muscle mutations cause embryonic paralysis and lethality that result in embryonic arrest at the twofold stage (3, 66, 69, 75), known as the Pat (paralyzed and arrested elongation at twofold) phenotype (see Fig. 2 and Tables I and II). This phenotype has revealed that muscle contraction is required for normal elongation of the embryo, and it has enabled the identification of a set of muscle-specific proteins that is required for proper assembly and/or function of muscle. For example, mutants for β-integrin and perlecan are defective in cell polarization and assembly of thick and thin filaments (75); mutants for vinculin lack thin filaments (3, 75); and mutants for myosin heavy chain A lack thick filaments (69, 75). Other mutations appear to affect not assembly, but rather the ability of muscle to contract (75). Among this last group are mutations in genes encoding TnC, Tm, and a voltage-sensitive Ca2+ channel (2, 34, 35, 75). In all of these mutants, abnormalities in muscle cell position arise, but they occur after arrest and likely reflect a secondary breakdown of muscle attachments to the extracellular matrix (ECM)/hypodermis (75). For simplicity, we will refer to these, collectively, as pat mutants, although in many cases the gene name is not pat.
Table I.
Mutations Used in This Study That Cause the Pat Phenotype
| pat alleles | Protein encoded | Cellular phenotype | Reference | |||
|---|---|---|---|---|---|---|
| unc-52(st549) | Perlecan | No muscle cell polarization, no thick or thin filaments | (53, 75) | |||
| deb-1(st555) | Vinculin | No thin filaments | (3, 75) | |||
| myo-3(st386) | Myosin heavy chain A (mhcA) | No thick filaments | (69, 75) | |||
| egl-19(st556) pka pat-5 * | α1 subunit of muscle voltage-gated | Thick and thin filaments present | (75) | |||
| Ca2+ channel (muscle) | ||||||
| pat-10(st568) | Troponin C (TnC-1) | Thick and thin filaments present | (35, 75) |
Previously known as pat-5(st556), subsequently found to be an allele of egl-19.
Table II.
Strains Used in This Study
| Strain | Genotype | |
|---|---|---|
| RW6010 | unc-52(st549)/unc-52(st549) II; mnDp34(II;f) | |
| RW3538 | myo-3(st386) +/+ sqt-3(e24) V | |
| RW3562 | + unc-44(e362) deb-1(st555) +/unc-82(e1223) ++ unc-24(e138) IV | |
| RW3563 | + egl-19(st556) +/unc-82(st1323) + unc-24(e138) IV | |
| RW3691 | pat-10(st568) +/+ dpy-5(e61) I | |
| EE67 | him-8(e2489)/him-8(e2489) IV; mup-2(e2346ts)/ mup-2(e2346ts) X |
We and others have observed a second muscle-related embryonic phenotype, known as Mup, since mutations in mup genes cause defects in muscle positions (16, 17, 23, 46). The mup-2 gene in C. elegans encodes the major embryonic isoform of TnT, which we have designated as CeTnT-1 (46). Two mutations in mup-2 have been isolated in genetic screens: one terminates translation near the NH2 terminus and is believed to be a null allele, while the other, a heat-sensitive allele, eliminates 64 residues from the COOH terminus. All animals mutant for mup-2 exhibit as a terminal phenotype detached body wall muscles at the dorsal mid-anterior and dorsal mid-posterior regions, corresponding to the points at which the embryo bends during the threefold stage to accommodate the constraints of the egg shell. This terminal phenotype presumably reflects loss of previously formed connections between muscle cells and the extracellular matrix and hypodermis. The mutant embryos that manage to hatch before dying show a characteristic croissant-shape due to the muscle displacements. Before the muscle cells detach, the embryos do not show the vigorous rolling within the shell typical of WT embryos, but rather exhibit uncoordinated, slow twitching (46).
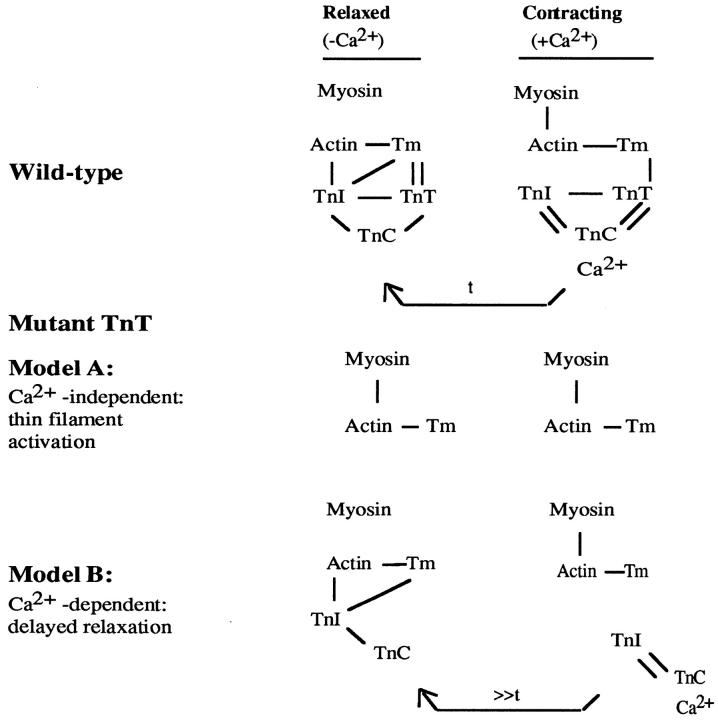
We reasoned that the tearing of attached muscle cells from the dorsal surfaces of the embryo is caused by aberrant muscular activity and that mutations of CeTnT-1 directly affect the regulation of muscle contraction, either by permanently disinhibiting the thin filament regardless of the concentration of free Ca2+ or by hindering relaxation (resulting in prolonged force) once Ca2+-dependent muscular activity has occurred (46). If the former hypothesis were true, we would predict that the binding of Ca2+ to the thin filament should not be required for the development of the Mup phenotype (Fig. 3, Model A). If the latter hypothesis were true, then development of the Mup phenotype should require Ca2+-dependent disinhibition of the thin filament in order to elicit muscular activity (Fig. 3, Model B). Biochemical research is equivocal regarding these predictions (also see Discussion): studies of ATPase activities of reconstituted actomyosin systems would predict that the first hypothesis would be true (for review see 27), whereas biochemical studies of protein interactions would largely predict that the latter hypothesis would be true (see 36). We have undertaken to distinguish these two possibilities, and test in vivo the biochemical predictions, by analyzing for epistasis in doubly mutant strains: a TnT-1 mutation was placed in combination with mutations in selected members of the pat class, including those affecting Ca2+-signaling in C. elegans embryonic body wall muscle. Epistasis occurs when the phenotype of a mutation in one gene is masked by a mutation in another gene. By this analysis we can determine whether the Mup phenotype is Ca2+-independent and, hence, epistatic to mutations that prevent the binding of Ca2+ to the thin filament and render the muscle flaccidly paralyzed.
Figure 3.
Model for associations of muscle proteins. This figure presents a simplified view of how the thin filament proteins function based on biochemical studies (adapted from 36). TnI is proposed to be capable of interacting with TnC independent of TnT. The relationship of these interactions in vivo is uncertain although there are two simple hypotheses. The first hypothesis, consistent with biochemical studies of reconstituted thin filaments, would be that TnI in vivo cannot associate with the thin filament in the absence of TnT (Model A). The second hypothesis, consistent with biochemical studies of troponin subunit interactions, would be that TnI and TnC, in vivo, can interact with actin and inhibit contraction under conditions of low Ca2+ in the absence of the TnT tethering function, albeit less efficiently, i.e., it would take longer (>>t) to reestablish the inhibited state (Model B, delayed relaxation). If the first hypothesis were true, one would predict that the Mup phenotype should be Ca2+-independent, since TnI and TnC would never associate with the thin filament, and hence the filament would be constantly activated. Our data are consistent with Model B.
Here we report the results of these genetic experiments which demonstrate that worms that are homozygously mutant both for TnT-1 and for TnC functions are paralyzed and arrest at twofold, which is the phenotype of TnC mutants, rather than the hypercontracted Mup phenotype of TnT-1 mutants. In addition, we find that the TnC mutation enhances the defects caused by mutation of TnT-1. These findings: (a) do not support the hypothesis that mutations of CeTnT-1 permanently activate the thin filament regardless of the concentration of free Ca2+ (Model A); (b) provide support for the hypothesis that, in the absence of TnT tethering functions, TnI and TnC confer Ca2+-dependent, although aberrant, regulation of muscular activity, consistent with the biochemical studies of protein interactions (Model B); and (c) are compatible with a model in which the Mup phenotype arises due to delayed relaxation of muscle contraction and in which prolonged force production causes detachment of muscles from dorsal surfaces in mup-2 mutants. These studies offer for the first time in vivo support for the biochemical predictions that full inhibition of the thin filament, as well as Ca2+-dependent disinhibition, can occur in the absence of TnT tethering functions. The findings are discussed in the context of previous biochemical studies of thin filament protein interactions and are related to in vivo dysfunctions caused by TnT mutations, including those that cause human cardiomyopathy.
Materials and Methods
Strains
Strains were maintained and standard crosses performed according to Brenner (1974). The WT strain used was N2 Bristol. Strains bearing the mup-2(e2346ts) allele were maintained at 15°C. Other strains were maintained at either 15 or 20°C. The specific temperatures used for experiments are indicated in the text.
Genes, mutations, and chromosomal rearrangements used in this work were the following: LG I: pat-10(st568) (75), dpy-5(e61) (8), unc-54(e1213) (70), LG II: unc-52(st549) (75), mnDp34(II;f) (25); LG III: unc-36(e251) (8), unc-32(e189) (8); LG IV: unc-82(e1223 and st1323) (71), unc-44(e362) (8), deb-1(st555) (3, 75), egl-19(st556) (previously known as pat-5(st556) (75), unc-24(e138) (8), him-8(e1489) (28), unc-22(ct37) (5); LG V: myo-3 (st386) (69, 75), sqt-3(e24) (8), LG X: mup-2(e2346ts and up1) (46), unc-6 (e78) (8), stDf1 (6). Tables I and II describe the pat genes and strains used.
Analysis of the Mup and Pat Phenotypes
Generation and analysis of mup-2 strains for the data in Table III were as previously described (46). Strains were analyzed by transferring single hermaphrodites daily and scoring the progeny ∼48 h later. For the data reported in Tables III and V, individuals were not picked, but the Mup and Pat phenotypes were assessed only for those embryos which had been released, without manipulation, from the egg shell (Table III): progeny exhibiting an obvious Mup or Pat phenotype were counted, and unhatched progeny that could be either Mup or Pat were simply scored as unhatched eggs.
Table III.
Quantitation of Phenotypes in Control Strains
| Parental genotype | % WT | % Mup | % Pat | % E | Broods | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control phenotypes at 25°C | ||||||||||
| + mup-2(up1)/lon-2 + | 79 | 5 | 0 | 16 | 6 | |||||
| + mup-2(ts)/lon-2 + | 74 | 21 | 0 | 5 | 9 | |||||
| unc-52/unc-52; mnDp34 | 67 | 0 | 3 | 30 | 5 | |||||
| myo-3 +/+ sqt-3 | 66 | 0 | 7 | 26 | 4 | |||||
| + unc-44 deb-1 +/unc-82 ++ unc-24 | 81 | 0 | 3 | 16 | 6 | |||||
| + egl-19 +/unc-82 + unc-24 | 87 | 0 | 1 | 12 | 6 | |||||
| pat-10 +/+ dpy-5 | 88 | 0 | 2 | 10 | 7 | |||||
| Control phenotypes at 15°C | ||||||||||
| mup-2(ts)/mup-2(ts) | 95 | .08 | 0 | 3 | 5 | |||||
| unc-52/unc-52; mnDp34 | 71 | 0 | 1.5 | 27 | 5 | |||||
| myo-3 +/+ sqt-3 | 8 | 0 | 6 | 10 | 8 | |||||
| + unc-44 deb-1 +/unc-82 ++ unc-24 | 85 | 0 | 4 | 11 | 5 | |||||
| + egl-19 +/unc-82 + unc-24 | 82 | 0 | 1 | 17 | 5 | |||||
| pat-10 +/+ dpy-5 | 79 | 0 | 2 | 19 | 5 |
WT refers to any animal that was not Mup, Pat or unhatched (E) regardless of genotype. Mups and Pats were scored as those animals that had hatched, without manipulation, from the egg shell. The specific alleles used are designated in Tables I and II. The skewed percentage of WT (>75% expected, relative to number of embryos) and Pats scored (25% expected) is likely due to disintegration of arrested mutants several days after egg laying.
Table V.
Phenotypes Observed at 25°C
| Parental genotype | % WT | % Mup | % Pat | % E | Pulse-lay No. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mup-2(ts)/mup-2(ts) | 0 | 80 | 0 | 20 | 4 | |||||
| unc-52/+; mup-2(ts)/mup-2(ts) | 0 | 61 | 21 | 18 | 9 | |||||
| myo-3/+; mup-2(ts)/mup-2(ts) | 0 | 53 | 24 | 23 | 8 | |||||
| deb-1/+; mup-2(ts)/mup-2(ts) | 0 | 55 | 24 | 21 | 5 | |||||
| egl-19/+; mup-2(ts)/mup-2(ts) | 0 | 67 | 11 | 22 | 25 | |||||
| pat-10/+; mup-2(ts)/mup-2(ts) | 0 | 63 | 17 | 20 | 27 |
To assist quantitative analysis of the phenotypes, we picked progeny to the edge of the plate. This manipulation of the embryos stimulated release of many unhatched Mup and Pat mutants from the egg shell. Unhatched eggs (E) could be either Mup or Pat mutants.
Generation of Strains Homozygous for mup-2(e2346ts) and Heterozygous for the Different pat Alleles
The pat-bearing mutant strains (Table II) were mated to him-8(e2489); mup-2(e2346ts) males at 20°C. (For ease of description, the remainder of the strategy is written for pat-10.) The construction of double mutants was feasible due to the availability of the conditional mup-2(e2346ts) allele (abbreviated as mup-2(ts) from hereon) and the ability of mup-2(ts) males to mate efficiently at either permissive or restrictive temperatures. Approximately 12 individual WT hermaphrodite cross progeny were picked singly to plates and allowed to lay eggs at 15°C. In the case of the pat-10 construction, which began with the strain pat-10+/+ dpy-5, WT hermaphrodite cross-progeny would be of the genotype either (a) pat-10/+; mup-2(ts)/+ or (b) dpy-5/+; mup-2(ts)/+. Hermaphrodites that segregated Pats (pat-10 phenotype) but not Dpys (dpy-5 phenotype) were identified. Viable segregants on this plate were expected to have one of the following genotypes: (a) pat-10/+; mup-2(ts)/+, (b) pat-10/+; mup-2(ts)/mup-2(ts), (c) pat-10/+; +/+, (d) +/+; mup-2(ts)/+, (e) +/+; mup-2(ts)/mup-2(ts), (f) +/+; +/+. Approximately 25 individual WT hermaphrodites from these plates were picked singly to plates and allowed to lay eggs. The hermaphrodites were transferred singly to new plates and the original plate shifted to 25°C to identify parent hermaphrodites that were mup-2(ts)/ mup-2(ts) homozygotes based on segregating 100% Mup progeny. For lines that were confirmed to be mup-2(ts)/mup-2(ts) homozygotes, the sibling plate at 15°C was inspected for the Pat phenotype to identify animals whose parent must have been pat-10/+; mup-2(ts)/mup-2(ts). The strain was maintained by picking single hermaphrodites to plates at 15°C and inspecting for Pat segregants at each generation. Generation of all but one of the other strains homozygous for mup-2(ts) and heterozygous for the different pat gene alleles was carried out essentially as indicated above with the specific strains indicated in Table II, using markers that were followed in a manner similar to that described for dpy-5. Construction of unc-52(st549); mup-2(ts) was initiated by mating him-8(e2489);mup-2(e2346ts) males to unc-52(st549); mnDp34(III;f) hermaphrodites. In this last case, all cross progeny were heterozygotes, unlike the cross progeny that arose in the corresponding step in the other constructions described above.
Analysis of pat-x; mup-2(ts) Double Mutants
Epistasis was determined by undertaking temperature shift experiments of strains, as described in Table IV, and then examining the shifted strains in two ways. First, the terminal phenotype at 25°C was established by determining with the dissecting microscope (total magnification: 500×) whether mutants arrested at either the twofold or threefold stage. The phenotypes were scored at 48 h after the temperature shift because many Mups and Pats do not hatch at all, while others emerge from the shell only several days after the normal hatching time. To facilitate more quantitative assessment of the epistatic phenotype, we moved progeny to the edge of the plate. This manipulation of the embryos stimulated release of many unhatched Mup and Pat mutants from the egg shell. For the controls, in contrast, individuals were not picked, but the Mup and Pat phenotypes were scored only for those embryos which had been spontaneously released from the egg shell (see above, and Table III). The WT category includes any viable animal that was not Mup, Pat, or an unhatched egg, regardless of the genotype.
Table IV.
Temperature Shift Analysis of Double Mutant Strains
| Individual pat -x /+ ; mup- 2(ts)/mup-2 (ts) hermaphrodite | ||||||
|---|---|---|---|---|---|---|
| ↓ | ||||||
| 6-h pulse lay at 15°C Individual hermaphrodites transferred from “pulse lay” plates to fresh plates at 15°C. The pulse lay plates (eggs) are transferred to 25°C and scored 48 h later. | ||||||
| ↓ | ||||||
| Expected genotypes | +; mup-2(ts) +; mup-2(ts) | pat-x; mup-2(ts) +; mup-2(ts) | pat-x; mup-2(ts) pat-x; mup-2(ts) | |||
| Expected phenotypes @ 15°C | WT | WT* | Pat | |||
| Expected phenotypes @ 25°C | Mup | Mup | ? (Mup or Pat) | |||
The strains and strategies used for making the double mutant strains are presented in the Materials and Methods. mup-2(ts) represents the mup-2(e2346ts) allele. The mup-2(ts) mutants are viable at the permissive temperature of 15°C. Lines that were heterozygous for a lethal pat mutation and homozygous for the mup-2(ts) mutation were identified and maintained (see Materials and Methods). Segregants of a parent having the genotype pat/+; mup-2(ts)/mup-2(ts) were scored for the double mutant pat/pat; mup-2(ts)/mup-2(ts) phenotype at the permissive and restrictive temperatures for the mup-2(ts) mutation in the shift strategy as shown. Expected and observed phenotypes are indicated. For all genetic double combinations, Pats were observed at the restrictive temperature of 25°C (Table V).
We unexpectedly observed a high incidence of Mups at 15°C in the pat-10–bearing lines.
The second way of analyzing epistasis entailed videomicroscopy of selected temperature-shifted embryos. Videomicroscopy was done as previously described (46). Since the pat alleles used in the experiments are characterized by severe paralysis and absence of muscular activity, the detection of any muscular activity by videomicroscopy would demonstrate epistasis. Epistasis detected in this manner would not require embryonic development to the threefold stage, as is needed in the first method.
Phalloidin Staining of Worms
Individual worms were picked into S-buffer (0.1 M NaCl, 0.05 M potassium phosphate [pH 6], 1 ml/liter cholesterol [5 mg/ml in EtOH]) on microscope slides. The worms were rinsed with S-buffer, and the S-buffer was removed using a drawn out pasteur pipette, leaving only a few microliters of buffer behind. Embryos were then immediately fixed with cold (−20°C) acetone. Fixed worms were stored at room temperature and then stained with phalloidin as previously described (46). Stained worms were visualized by epifluorescence with a Reichert Jung Polyvar II microscope.
Results
The Phenotypes Caused by mup-2 and pat Gene Mutations Are Discrete
To examine the epistasis relationships between mup-2 and specific pat genes, we made strains that were doubly mutant for mup-2(ts) and selected muscle gene mutations causing the Pat phenotype, which are listed in Table I. As required for a successful analysis of epistasis, the Mup and Pat phenotypes are distinct and specific. Like others (75), we observed no Mup animals segregating from these pat-bearing strains at either the restrictive (25°C, Table III) or permissive temperatures (15°C, Table III) used in these studies: the segregants arrest elongation at the twofold stage and failed to show twitching of body wall muscle cells (Table III, Pat). Likewise, segregants from different mup-2–bearing strains, including the mup-2(up1) putative null allele and mup-2(ts)/Df (Table III), achieved at least the threefold stage of development, which requires body wall muscle function (75). Additionally, genetic studies (Table III and see 46) demonstrated that the mup-2 (e2346ts) mutation behaves like the putative null allele at the restrictive temperature; therefore, Mup is the null phenotype.
Analysis of Strains Mutant for pat Mutations That Affect Body Wall Muscle Assembly and for mup-2
To determine the requirements for development of a Mup phenotype, we generated doubly mutant strains with the mup-2(ts) allele of TnT-1 in combination with mutant alleles of selected pat genes (Tables I and II): the unc-52(st549) mutation of perlecan, which blocks assembly of thick and thin filaments, as well as proper placement of these filaments in the muscle cell; the deb-1(st555) mutation of vinculin, which disrupts thin filament assembly; and the myo-3(st386) mutation of myosin heavy chain A, which blocks thick filament assembly, but permits assembly and proper localization of thin filaments. Table IV gives an overview of the experimental protocol for analysis of the segregants, as well as a description of expected genotypes and phenotypes. All doubly mutant strains (including mutants described in section below) were examined at 25°C, the restrictive temperature for mup-2(ts). Table V lists the percentage of Pat segregants observed at 25°C for each of the doubly mutant strains. If mup-2(ts) were epistatic to any of the tested pat alleles, the percentage of Pat segregants should have been either zero or close to zero. Alternatively, if mup-2(ts) were not epistatic, 25% of the segregants should be Pat. Since not all Mup and Pat mutants escape from the egg shell, we could not score the exact percentage of Pat segregants; however, in all cases (see description of results below), the observed number of Pat segregants in double mutants was not significantly different from the expected value (P > 0.10).
We first tested the hypothesis that mutations in genes that affect muscle assembly are epistatic to mup-2(ts). We expected this would be the case because these alleles severely disrupt assembly of the sarcomere: sarcomere assembly is necessary for both embryonic development (elongation past twofold) and the twitching observed with the Mup phenotype. Indeed, the data shown in Table V demonstrate that mup-2(ts) is epistatic to the tested alleles of unc-52, deb-1, and myo-3. These data also provide important controls for quantitation of Pat mutants at 25°C.
Analysis of Strains Mutant for mup-2(ts) and pat Mutations That Affect Muscle Contraction
We sought to determine whether the Mup phenotype is Ca2+-independent, as would be suggested by biochemical studies of ATPase activities of reconstituted, regulated actomyosin from vertebrate muscle (for review see 27), but not by analyses of thin filament protein interactions (see 36). The availability of the egl-19 and pat-10 mutations, which in contrast to unc-52, deb-1, and myo-3 mutations do not disrupt sarcomeric structures, but rather compromise activation of muscle contraction, allowed us to test this hypothesis. Thus, we generated doubly mutant strains with the mup-2(ts) allele of TnT-1 in combination with: (a) the egl-19(st556) mutation of the α1 subunit of a sarcolemmal voltage-gated Ca2+ channel, which allows proper assembly of sarcomeric structures, but causes flaccid paralysis, presumably by eliminating the influx of Ca2+ during the muscle action potential; and (b) pat-10(st568), which causes flaccid paralysis and affects not the actual Ca2+ signal, but rather TnC, the Ca2+ receptor.
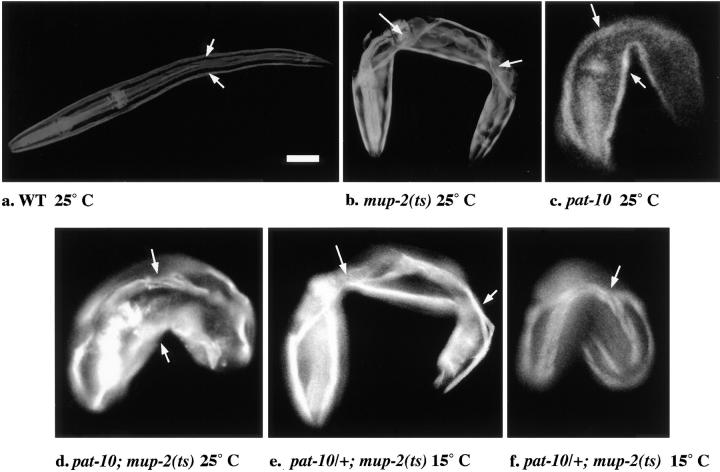
The data shown in Table V demonstrate that egl-19 and pat-10 mutations, like unc-52, deb-1, and myo-3, are epistatic to the mup-2(ts) mutation. Representative mutants and controls are shown in Fig. 4 (a–d). Analysis of both mutations was essential since epistasis of the egl-19(st556) Ca2+ channel mutation to mup-2(ts) does not definitively indicate whether activation (disinhibition) of thin filaments is needed: it is believed that activation of muscle contraction in dually regulated muscles, such as in the C. elegans body wall, requires activation (disinhibition) of both thick and thin filaments (67). Thus, the likely consequences of the egl-19(st556) mutation upon thick filament activation (disinhibition) would preclude observing any independence of the Mup phenotype from thin filament activation (disinhibition). In contrast, the epistasis test of pat-10 with mup-2 enables this assessment since the thick filaments would be activated in this case, and the thin filament activation can be examined independently.
Figure 4.
Rhodamine-phalloidin analysis of Mup and Pat mutant phenotypes. (a) WT L1-stage worm. Arrows demarcate dorsal and ventral body wall muscle quadrants. (b) mup-2(ts) Mup individual. Arrows demarcate displaced dorsal body wall muscle. (c) pat-10 Pat individual. Arrows demarcate body wall muscle staining. (d) Pat individual shifted to 25°C before the embryonic tsp for mup-2(ts) of the genotype pat-10/pat-10; mup-2(ts)/mup-2(ts). Arrows demarcate body wall muscle staining. (e and f) Mup individuals observed at 15°C segregating from a parent of the genotype pat-10/+; mup-2(ts)/mup-2(ts) and inferred to be of the same genotype (our analysis supports that 50% of the worms of this genotype are Mup at 15°C). Arrows demarcate displaced body wall muscle. Bar, 10 μm.
Time-Lapse Video Analysis of pat-10; mup-2(ts) Mutants
It could be argued that epistasis of mup-2(ts) to pat-10 occurred, but not to the extent needed for elongation of the double mutants to the threefold stage. In other words, the flaccid paralysis of pat-10(st568) might have been ameliorated by the presence of mup-2(ts), but the resulting muscular activity might have been insufficient to drive elongation to the threefold stage (i.e., a lat phenotype: late paralysis and arrested elongation at twofold; 75). Thus, we examined by time-lapse videomicroscopy with differential interference contrast optics whether pat-10; mup-2(ts) doubly mutant worms evidenced muscular activity in addition to the arrest at elongation scored in the above experiments. Matching the result obtained with control pat homozygotes, all 10 segregants recorded that arrested at twofold (genotype: pat-10/pat-10; mup-2(ts)/mup-2(ts)) also failed to show visible twitching of body wall muscles (data not shown). Thus, we conclude that the double mutants are indeed paralyzed.
Analysis of Phenotypes for Doubly Mutant Strains at 15°C, the Permissive Temperature for mup-2(ts)
During the course of constructing the double mutant combinations of mup-2(ts) with different pat alleles, we observed an increased incidence at 15°C of segregants with a Mup phenotype, i.e., improper muscle positioning and croissant-shaped body. The mup-2(ts) animals raised at 15°C are WT, and only rarely (<1% of progeny) is a Mup animal observed. Table VI lists the percentages of WT, twofold arrested, threefold arrested, and unhatched segregants at 15°C. Strains in which mup-2(ts) is combined with unc-52(st549), myo-3(st386), deb-1(st555) or egl-19 (st556) showed a slightly higher percentage of Mups segregating at 15°C (2–4% in comparison with <1%; Table VI, but this percentage is not statistically significant (P > 0.50). In contrast, a striking percentage of Mup animals (25%) segregated in the strains bearing pat-10(st568) TnC mutation, and the percentage of WT was lower than expected (P < 0.005). As discussed further below, these Mup animals represent animals of the genotype pat-10/+; mup-2(ts)/mup-2(ts).
Table VI.
Phenotypes Observed at 15°C
| Parental genotype | % WT | % Mup | % Pat | % E | Broods | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mup-2(ts)/mup-2(ts) | 96 | 0.8 | 0 | 3 | 5 | |||||
| unc-52/+; mup-2(ts)/mup-2(ts) | 66 | 4 | 1 | 29 | 4 | |||||
| myo-3/+; mup-2(ts)/mup-2(ts) | 72 | 3 | 5 | 20 | 5 | |||||
| deb-1/+; mup-2(ts)/mup-2(ts) | 73 | 2 | 2 | 21 | 5 | |||||
| egl-19/+; mup-2(ts)/mup-2(ts) | 75 | 2 | 2 | 20 | 5 | |||||
| pat-10/+; mup-2(ts)/mup-2(ts) | 49 | 27 | 1 | 22 | 5 |
WT refers to any animal that was not Mup, Pat, or unhatched (E) regardless of genotype. Mups and Pats were scored as those animals that had hatched, without manipulation, from the egg shell.
To ascertain whether the Mup-like characteristics of these segregants at 15°C truly resembled those of mup-2(ts) mutants, we examined the structure and positioning of muscle cells. Fixed worms were stained with rhodamine-conjugated phalloidin, which binds filamentous actin, for immunofluorescent visualization (Fig. 4). The muscle detachment of mup-2 mutants (b as compared with WT in a) is distinct from that seen with other members of the mup gene class, because the detachment occurs only from the dorsal surfaces at the major bends of the embryos. Similar to the mup-2(ts) mutants raised at the restrictive temperature of 25°C, the Mups segregating from strains pat-10/+; mup-2(ts)/mup-2(ts) at the permissive temperature of 15°C (e and f) have defects in muscle attachment comparable to those seen with mup-2(ts) alone.
The appearance of Mup-like animals segregating from parents of the genotype pat-10/+; mup-2(ts)/mup-2(ts) at 15°C could be caused either by incomplete suppression of a Pat phenotype by mup-2(ts) or by enhancement of the Mup phenotype by pat-10(st568). In the former case, the Mup-like progeny would have the genotype pat-10/pat-10; mup-2(ts)/mup-2(ts); in the latter, the genotype would be pat-10/+; mup-2(ts)/mup-2(ts). We favor the view that the Mup animals were heterozygous for the pat mutation for several reasons. First, because suppression of the Pat phenotype by mup-2(ts) failed to occur at 25°C, suppression at 15°C would require that the Mup phenotype arose at 15°C by a mechanism different from that at 25°C. Second, the increase in percentage of Mups correlated with a diminution in percentage of WT, not Pat, segregants. Note that the percentage of embryos failing to hatch at 15°C, presumed to be Pat, was comparable to that found for pat-10 controls, further suggesting that the increase in Mups occurred without a decrease in the Pat population (Table VI).
One possibility for the appearance at 15°C of Mup-like worms is that the truncated TnT-1 intensifies subtle muscle sarcomere assembly defects caused by a single mutant copy of the pat-10 gene mutation; however, if this were true, we would have expected an increase the incidence of Pat-like animals in addition to or instead of Mup-like ones, since one class of mutation causing the Pat phenotype disrupts sarcomeric assembly (Tables I and II). An explanation, which we favor, is that a single mutant copy of pat-10 might further the detrimental effects of the aberrant muscular activity associated with mup-2(ts)/mup-2(ts) at 15°C (see Discussion). We conclude that the Mup phenotype caused by the mup-2(ts) TnT-1 mutation is exacerbated by heterozygosity of pat-10(st568) TnC at what is ordinarily a permissive temperature for mup-2(ts).
Discussion
A range of biochemical studies has suggested three potential roles for TnT in the regulation of muscle contraction: (a) at low concentrations of free Ca2+, to strengthen the inhibition of the thin filament; (b) at high levels of free Ca2+, to tether TnI/TnC+(Ca2+) to the thin filament; and (c) also at high levels of free Ca2+, to activate a disinhibited thin filament, leading to greater actin-activated myosin MgATPase in the presence of TnT than in its absence. Whereas results of biochemical research have been equivocal about the absolute necessity for TnT in regulation, the discovery of a lethal heart disease linked to mutations in human cardiac TnT suggests that the functions of TnT are indispensable. This view is bolstered by the phenotype of C. elegans homozygous for severe loss-of-function alleles of TnT-1, because the mutants display aberrant muscle contractions and develop lethal muscle displacements. When first reported, the phenotype of the TnT-1 loss-of-function mutations was reasoned to be caused by one of two mechanisms. First, the phenotype could stem from loss of inhibition of the thin filament at low free Ca2+ concentration (i.e., loss of role 1, Model A, Fig. 3), leading to constant application of forces to the muscle-hypodermal-cuticle connections and ultimately to collapse of the dorsal muscle quadrants. Alternatively, breakage of the anchoring junctions and collapse of the dorsal quadrants could stem from abnormally prolonged contractions caused by tardiness of untethered TnI/TnC to restore inhibition of the thin filament after cessation of the Ca2+ signal (i.e., loss of role 2, Fig. 3, Model B).
The availability of a heat-sensitive, loss-of-function allele for TnT-1, as well as a range of mutations in other muscle proteins, allowed us to determine with C. elegans the conditions necessary in vivo for development of the aberrant muscle contractions and lethal muscle displacements linked to loss of TnT function. The in vivo experiments specifically tested whether the mup-2(ts) TnT-1 mutation is epistatic to representatives of the pat class of mutation, all of which cause flaccid paralysis. If, for example, loss of TnT function disinhibits the thin filament in the absence of Ca2+ signals, then the Mup mutation should be epistatic to those Pat mutations blocking Ca2+-dependent disinhibition. The results demonstrated that mup-2(ts) is not epistatic to representatives of three classes of pat mutation, including those that block Ca2+-dependent disinhibition. Additionally, the results showed enhancement of the mutant phenotype of mup-2(ts) by heterozygosity for the TnC pat-10 mutation. As discussed below, these observations support the view that loss of TnT function in C. elegans leads to Ca2+-dependent contraction of abnormal duration, rather than to loss of inhibition and production of Ca2+-independent force. Moreover, the results suggest that breakage of the anchoring junctions between muscle and hypodermis-cuticle relates not to the extent of peak force achieved during contraction, but rather to perturbations in the duration of contraction.
The pat-10(st568) TnC Phenotype Is Epistatic to the mup-2(ts) TnT Phenotype
To determine the conditions necessary for development of aberrant muscular activity and improper muscle cell positioning in mup-2(ts) animals, our experiments tested whether the mup-2(ts) mutation of CeTnT-1 can mask the severe, flaccid paralysis of representatives of the following three classes of pat mutation: (a) those disrupting integrity of muscle-hypodermal linkages (unc-52 perlecan); (b) those preventing assembly of thin and thick filaments (deb-1 vinculin and myo-3 myosin-A); and (c) those impairing binding of Ca2+ to thin filaments (egl-19 Ca2+ channel and pat-10 TnC) (Table I). As we expected, the aberrant muscular activity of mup-2(ts) requires assembly of thin and thick filaments, as well as their proper anchorage to the hypodermal cells, and the analysis of these first two classes provided important controls for examination of mutations that affect the regulation of muscle contraction.
The mup-2(ts) mutation of CeTnT-1 was likewise unable to overcome the flaccid paralysis caused by mutation of a sarcolemmal Ca2+ channel, egl-19. Assembly of sarcomeric structures in egl-19 animals appears WT (75); hence, paralysis presumably results from elimination of Ca2+ currents needed for contraction. These currents might be responsible for inducing release of Ca2+ from the sarcoplasmic reticulum or for directly triggering regulatory proteins (e.g., TnC, calmodulin, and myosin light chains). The results indicate that the trembling and improper muscle cell positioning of mup-2(ts) require normal Ca2+ dynamics within muscle.
The results with the mutation of the sarcolemmal Ca2+ channel egl-19 leave unclear, however, whether mup-2(ts) can disinhibit the thin filament in the absence of Ca2+. It is believed that the body wall muscles of C. elegans have both thin and thick filament regulatory systems (21) and that tension development in dually regulated muscle cells requires disinhibition/activation of both thin and thick filament systems (37, 67). In this regard, failure of the mup-2(ts) mutation to ameliorate the limp paralysis caused by mutation of TnC pat-10(st568) suggests the inability of the truncated TnT-1 to disinhibit the thin filament in the absence of Ca2+. In the pat-10; mup-2(ts) double mutants at 25°C, Ca2+ signals and activation of thick filaments in the muscle cells should occur without hindrance; only the binding of Ca2+ to TnC and the subsequent disinhibition of the thin filaments should be impaired by the pat-10 mutation. The results thus lead to the view that the aberrant contractions observed in mup-2(ts) homozygotes, while Ca2+-dependent, deviate from normal contractions by having an impaired relaxation after cessation of the Ca2+ signal. This is proposed as Model B in Fig. 3 and, as discussed in detail below, is our favored interpretation of the genetic data.
Evaluation of pat-10(st568) TnC Epistasis to mup-2(ts) TnT and Biochemical Models of Thin Filament Regulation
Biochemical studies of the interactions in vitro among the troponin subunits and tropomyosin from rabbit skeletal muscle (for review see 36, 63) offer a mechanism by which loss of TnT function could impair relaxation (Fig. 3, Model B); these studies support a model of regulation first proposed in 1973 (27). At submicromolar concentrations of free Ca2+, the inhibitory subunit of troponin, TnI, shows numerous interactions with actin, tropomyosin, and TnT; however, as the free Ca2+ concentration rises to micromolar levels, the interactions between TnI and actin/tropomyosin diminish greatly or disappear, resulting in only an indirect connection mediated by TnT (Fig. 3, Wild-type). The biochemical model of interactions receives support from low resolution crystallographic analyses of tropomyosin/troponin cocrystals (10, 74), revealing two regions of contact between the troponin complex and tropomyosin. One contact involves interaction of Tm with the extended NH2-terminal half of TnT (Fig. 1), and this contact is believed on the basis of biochemical work to persist as Ca2+ concentration rises. The second contact is believed to be abolished by the binding of Ca2+ to TnC; this contact involves the COOH-terminal half of TnT and TnI/TnC. Because the aberrant muscular activity in mup-2(ts) mutants cannot occur without Ca2+-dependent thin-filament disinhibition, we suggest that the abnormality stems from an inability of TnI/TnC, in the absence of TnT function, to remain tethered to the disinhibited thin filament during periods of high free [Ca2+]. Without tethering, TnI/TnC would diffuse away from its binding site on the thin filament, and thus the re-establishment of inhibition and relaxed levels of tension after cessation of the Ca2+ signal would be delayed (Fig. 3, Model B).
Model B (Fig. 3) appears well-supported by the studies of troponin subunit interactions and by the genetic work presented here; however, the biochemical data considered in full are equivocal. In contrast to analyses of troponin subunit interactions, biochemical studies of actomyosin MgATPase using thin filaments reconstituted from purified proteins (actin, Tm, TnT, TnI, and TnC) have highlighted an essential role of TnT in ensuring maximal inhibition of MgATPase activity at low free Ca2+ concentrations (20, 27, 33, 41). The basal, non-Ca2+-activated level of ATPase activity in the absence of TnT significantly exceeds that in its presence. The loss of inhibition is reflected in an affinity of TnI/TnC for actin-Tm detectably less than that of whole troponin (26, 33, 41, 50). The weakened binding and inhibition exhibited by TnI/TnC have been attributed to a neutralization by TnC of TnI activity in the absence of TnT. Model A depicts this role of TnT in countering the neutralization action of TnC. The biochemical observations giving rise to Model A suggest that loss of TnT function in a muscle should cause Ca2+-independent actomyosin ATPase and hence contraction. We thus also considered whether our data could support this prediction; however, contrary to this prediction, our results showed that the aberrant muscular activity in mup-2(ts) worms, in which TnT-1 activity is severely reduced or totally abolished, depends upon Ca2+-mediated disinhibition of the thin filament.
At least three explanations can be considered for the maintenance in vivo of thin filament inhibition in the absence of functional TnT in our epistasis experiments, despite the necessity for TnT in biochemical experiments with thin filaments reconstituted with physiologic concentrations of TnI and TnC (Model A). These possibilities are considered in turn below, with the third being potentially problematic with respect to ruling out Model A with the epistasis data alone.
One possible explanation is that the need for TnT in effecting inhibition may be specific to reconstituted thin filaments. Native and stoichiometrically reconstituted bovine cardiac thin filaments differ in the extent of cooperativity manifested in activation of MgATPase, as well as in Ca2+ sensitivity (65). Interestingly, filaments reconstituted in the presence of excess troponin subunit concentrations achieved the behavior of native thin filaments.
The second explanation is that invertebrate TnT, unlike vertebrate TnT, may lack a role in ensuring maximal inhibition of the thin filament in relaxed muscles. Invertebrate TnT is substantially greater in mass than is vertebrate TnT due to a long COOH-terminal extension, but shares the same major motifs, including a highly conserved NH2 terminus, a pattern of charged residues in a region predicted to interact with Tm, and a domain believed to form a coiled-coil with TnI (46, 57). The extension on the invertebrate isoform has no known function, but because it is rich in acidic and basic residues, the extension might play a role in protein–protein interactions (9). We conjecture that the extra residues ensure the integrity of TnT within the troponin complex, because the mup-2(ts) mutation, which eliminates about half of the extension of CeTnT-1, behaves as a severe loss-of-function allele at elevated temperatures, comparable in strength to a putative null allele. On the basis of these arguments, we would suggest that the invertebrate extension of TnT, rather than negating a role of one of the conserved motifs (e.g., ensuring inhibition by TnI/TnC at low free Ca2+ concentrations), imparts stability to the protein in its folding and its association with the thin filament.
A third possibility is that pat-10 mutant TnC may fail to bind to thin filaments. TnI, in the absence of TnT and TnC, binds well to actin-Tm (26, 49) and exerts a level of inhibition comparable to that of whole troponin (12, 20, 27). The pat-10 mutant TnC differs from WT TnC in two ways: Asp64, located in the second of four putative Ca2+-binding motifs, is altered in the mutant TnC to Asn; Trp153 is changed to a stop codon, leading to severe truncation of the last (helix G) of the predicted helices in the protein (35). The truncation likely weakens the interaction between TnI and TnC. Crystallographic analyses (58) of vertebrate TnC reveal that the homologous helix establishes a number of hydrophobic interactions thought to stabilize the structure of the COOH half of TnC, and it is this half of vertebrate TnC that is thought important in maintaining association of TnC with the thin filament at low free Ca2+ concentrations (77). Supporting the view of weakened interactions between the mutant TnC and the thin filament is an experiment with proteolytic fragments of rabbit skeletal TnC, which showed the abolition of interaction with TnI for a fragment (residues 101–153) lacking the terminal six residues, but not for a fragment (101– 159) having these residues (19). Thus, if the mutant TnC were unable to bind to thin filaments, our observation of flaccid paralysis in embryos homozygous for both pat-10(st568) and mup-2(ts) would be consistent with assays of ATPase activity of regulated actomyosin depleted of TnT and TnC, since TnC neutralization of TnI inhibition would not occur in our particular double mutant combination and, consequently, filaments would not appear “activated” in our genetic assay.
Enhancement of the Mup Phenotype in pat-10/+; mup-2(ts)/mup-2(ts) Animals at the Permissive Temperatures Supports Model B
Given the probable lack of association between pat-10 mutant TnC and the thin filament, the failure of mup-2(ts) to be epistatic to pat-10(st568) at 25°C cannot alone be taken as evidence that the TnT mutation fails to cause Ca2+-independent contractions in mup-2(ts) homozygotes; however, the observed enhancement for the Mup phenotype in pat-10/+; mup-2(ts)/mup-2(ts) animals at the permissive temperatures provides important evidence for Model B, rather than Model A. Relevant to this view are our interpretations (a) that the collapse of dorsal muscle quadrants characteristic of the Mup phenotype (Fig. 4) is a consequence of the abnormal contractions also associated with the phenotype and (b) that the increase of Mups at the permissive temperatures of animals having the genotype pat-10/+; mup-2(ts)/mup-2(ts) is caused by enhancement of the mup-2(ts) contraction defects by one mutant allele of pat-10 (as described in Results).
According to the predictions of Model A, if the mup-2(ts) mutation were indeed able to disinhibit actin-Tm-TnI-TnC, but not actin-Tm-TnI (due to strong inhibition by TnI), then a reduction of TnC levels in mup-2(ts) animals should antagonize the effects of the mup-2(ts) mutation, promote inhibition of the thin filament, and ameliorate the Mup phenotype. Observations of mutant strains, however, showed that heterozygosity for the pat-10 TnC mutation in mup-2(ts) homozygotes exacerbated the deleterious effects of the TnT mutation and caused the appearance of phenotypically Mup animals at 15°C, a normally permissive temperature. Moreover, since no segregant from the pat-10/+; mup-2(ts)/mup-2(ts) strain was WT at 25°C, there was no indication of amelioration of the Mup phenotype at the restrictive temperature.
Unlike Model A, Model B readily accommodates the observation of an enhancement of the Mup phenotype by heterozygosity for pat-10(st568) mutant TnC in mup-2(ts) animals at 15°C. The two sequence alterations that define the pat-10(st568) allele render mutant muscle flaccidly paralyzed. In addition to a premature stop codon that likely affects the interaction of the mutant protein with the thin filament, pat-10 mutant TnC has an alteration of Asp64 to Asn (35). The aspartate, which is strictly conserved in functional Ca2+-binding loops, provides one oxygen atom necessary for coordination of Ca2+ and a second needed for stability of the loop, and even the conservative change to asparagine is predicted to reduce the Ca2+-affinity of the loop dramatically (58). If reduction of TnC in pat-10(st568)/+ (normally a recessive mutation) in the mup-2(ts) backgroundwere to render sections of individual thin filaments in affected muscle Ca2+-insensitive, through incomplete saturation of the thin filament with functional TnC, then the cooperativity inherent in muscle regulation should be diminished, leading to contractions of abnormal duration (prolonged). For example, creation of Ca2+-insensitive regions along thin filaments in rabbit psoas cells, achieved by partial extraction of TnC, lessens the steep dependence of active tension upon free Ca2+ (7, 45). We envisage that the contractile deficits arising from haploidy for pat-10(st568), while too subtle to be detected in a WT genetic background, complement and intensify the defects caused by mup-2(ts) to the extent that collapse of dorsal quadrants occurs at even 15°C.
There is precedence for genetic interactions among mutations in muscle regulatory proteins (4, 14, 29). Recessive mutations of the held-up TnI and upheld TnT gene loci in Drosophila cause defects in flight muscle function and wing posture in specific double heterozygous mutant allele combinations (29). These specific interactions were interpreted to support strict quantitative, as well as qualitative relationships, among certain muscle gene products. Although the Drosophila studies do not make possible the discrimination among possible mechanisms to explain the genetic interactions, they are consistent with our interpretation that reduction of WT TnC content in combination with specific TnT mutations may sufficiently disrupt contractile activity to increase the appearance of mutant phenotypes.
Based upon the above reasoning, we conclude that enhancement of the Mup phenotype in pat-10/+; mup-2(ts)/ mup-2(ts) animals at the permissive temperatures, in combination with the inability to elicit Ca2+-independent contractions in pat-10/pat-10; mup-2(ts)/mup-2(ts) double homozygotes at 25°C, argues strongly that loss of TnT function does not disinhibit the thin filament and, moreover, that the aberrant muscular activity in mup-2(ts) homozygotes stems from compromised relaxation in Ca2+-activated cells, rather than from Ca2+-independent contractions in unexcited cells.
The Cellular Basis for the Mup Phenotype
Animals homozygous for mup-2(ts), when raised at 25°C, display a collapse of dorsal muscle quadrants from the mid-anterior and mid-posterior cuticle during the late threefold stage of embryogenesis. Construction of strains and testing for epistasis exploited the conditional viability of worms homozygous for the mup-2(ts) allele. Genetic analyses previously established that mup-2(ts) mutants behave as null for TnT-1 function in embryos raised at the restrictive temperature of 25°C: the embryos suffer from seemingly incessant trembling of the body wall muscles and develop defects in muscle positioning toward late embryogenesis. The temperature-sensitive period during the threefold stage of embryogenesis most likely reflects the time when muscle detaches and thus when damage to the embryo becomes irreparable (46). Embryos raised at 15°C show similar trembling of the body wall muscles, but these animals develop and maintain proper positioning of their muscles, as well as remain alive into adulthood. The aberrant muscular activity observed in the mutants is viewed as ultimately weakening the muscle-hypodermal-cuticular linkages at 25°C, and because of the folded shape of the elongating C. elegans embryo, the dorsal muscle quadrants, not the ventral ones, should collapse from the cuticle. It is notable that muscle collapse occurs at the time of cuticle synthesis, a period during which remodeling of attachments to the exoskeleton occurs and thus during which the attachments may be inherently weakened, in comparison with linkages at other developmental time points (also see 15, 46). Abnormal contractions could lead to tearing of the dorsal quadrants away from the cuticle in at least two ways, namely by excessive peak forces during individual contractions or through altered duration of normal or subnormal forces.
If the mup-2(ts) mutation were to promote abnormal forces, the anchoring junctions linking muscle to cuticle could conceivably be mechanically broken. That collapse of the muscle quadrants occurs only at elevated temperatures, while the aberrant muscular activity is visible at both cold and warm temperatures, could reflect the generation of higher forces by muscle at the higher temperatures. It is well known that active tension in vertebrate striated muscle (for review see 76) depends upon temperature. For example, the maximal Ca2+-activated tension in rabbit psoas cells rises 30% when the temperature is increased from 15 to 25°C (18). Our results, however, with mup-2(ts) animals heterozygous for the pat-10 TnC mutation are not compatible with the idea of excessive peak forces causing the collapse of muscle quadrants. Diminution of TnC function in vertebrate and invertebrate striated muscle leads to a clear reduction in the level of active tension that can be generated (1, 45, 78). Because haploidy for pat-10 mutant TnC enhanced, rather than suppressed, the Mup phenotype, leading to the appearance of Mup animals at 15°C, a normally permissive temperature, the results indicate that collapse of the muscle quadrants is not triggered by supranormal forces. Additionally, the mup-2(ts) mutation, in causing a severe loss of TnT function, would be predicted on the basis of biochemical research to promote lower, not greater, forces. Greaser and Gergely (1971) observed that the Ca2+-regulated MgATPase of regulated, rabbit actomyosin in vitro is maximal when TnT is present. Elimination of TnT from myosin-actin-Tm-troponin reduces the Ca2+-regulated ATPase to a level achieved by myosin-actin-Tm.
Rather than supranormal forces, an abnormal duration or frequency of contractile activity might trigger the collapse of muscle quadrants. This idea is consistent with our earlier discussion of the biochemical data related to Model B of Fig. 3 and with emerging information on the role of adhesion complexes as mechanotransducers. In a wide variety of cell types in other organisms, the expression of genes, especially those coding for cytoskeletal, extracellular matrix, and cell adhesion molecules, is sensitive to the extent, duration, and frequency of mechanical stresses (for review see 47). For example, the expression by chick fibroblasts of the gene for the extracellular matrix protein tenascin-C (11) and the expression of genes for actin and myosin in rat skeletal muscle (40) are modulated by stresses and strains imposed upon the cells. Moreover, mechanical stresses applied to integrins enhance the localization of mRNA and ribosomes at focal adhesions of human umbilical endothelial cells (11), as well as increase the phosphorylation of cytoskeletally anchored proteins and mitogen-activated protein kinases in an osteosarcoma cell line (55). It is thus possible that changes in the duration or frequency of force generation by mup-2(ts) muscles are sufficient to compromise the integrity of anchoring junctions via effects on protein synthesis or signaling cascades. The heat-sensitivity of the breakage of anchoring junctions could stem from a greater loss of TnT function at elevated temperatures. The free energy of stabilization of folded proteins, which generally amounts to only 30–60 kJ/mol, typically has an optimum between 10° and 40°C (for review see 32, 56). If fewer truncated TnT-1 proteins were able to fold properly at 25°C than at 15°C, then the effects of the mup-2(ts) mutation on duration and frequency of contractions would be more apparent at the elevated temperature.
In Vivo Studies Supporting That Mutation of TnT Can Alter Contractile Activity
Although one of the proposed functions of TnT is to tether the Tn complex to the thin filament, the extent of the roles of TnT in contraction is poorly understood. Physiological studies have provided evidence for regulation of Ca2+-responsiveness during contraction, and recent studies of human cardiomyopathy (HCM) mutations have suggested unexpected functions for TnT in the regulation of force generation and actin-myosin cross-bridge mechanics, and thus in the regulation of the contraction/relaxation cycle (39). The HCM missense mutation I79N of human cardiac TnT lies within the Tm binding domain, but biochemical studies have failed to reveal any effects of this mutation on the affinity of TnT for Tm, on the Tn-induced binding of Tm to actin, on the cooperative binding of myosin subfragment 1 to the thin filament, or on the Ca2+-dependence of the thin filament-myosin subfragment 1 ATPase activity. However, the mutation resulted in 50% faster thin filament movement over a surface coated with heavy meromyosin, as detected by in vitro motility assays. The increased sliding speed suggests an unexpected role for TnT in myosin-actin kinetics and the contraction/relaxation cycle that cannot be easily related to previous biochemical work on TnT. This increased sliding speed would predict increased shortening velocity in vivo, in comparison with WT muscle, and lower overall force generation since the duration of the power stroke is shorter. Studies with vertebrate muscle cells expressing mutant I79N TnT support this hypothesis (60).
Studies of specific missense alleles of the Drosophila TnT gene, heldup, also have led to the interpretation that certain defects in muscle structure and development could be caused by abnormalities in interactions of actin and myosin due to mutant TnT: in these missense mutant animals, myofibrils degenerate as muscle matures, and removal of myosin heavy chain prevents these structural abnormalities (14). Studies of these TnT mutants indicated that actin-myosin interactions directly damage myofibrils, possibly due to unstable thin filaments, but the findings did not distinguish further the possible mechanisms by which the TnT mutations could be acting.
The observations reported here greatly extend the previous in vivo analyses of TnT function afforded by the studies of the Drosophila TnT gene. In contrast to the Drosophila work, our double-mutant analysis with C. elegans sought to test two mechanistic hypotheses for the development of dysfunction in muscle with mutant TnT: (a) that loss of TnT function would promote abnormal, Ca2+-independent tension development; or (b) that loss of TnT function would compromise the ability of the cell to regain inhibition after cessation of the Ca2+-signal. We found that the C. elegans TnT-1 mutant phenotype is Ca2+-dependent and requires disinhibition of the thin filament. These observations, in combination with the enhancement of the Mup phenotype at a permissive temperature in mup-2(ts) TnT homozygotes that are heterozygous for the TnC mutation, support a model in which loss of TnT function promotes an abnormal duration of contraction.
That an abnormal duration of contraction could cause the aberrant muscular activity observed in mup-2(ts) mutants at permissive and restrictive temperatures is further supported by genetic and physiological studies of mutations of the C. elegans unc-22 gene, which encodes Twitchin, a Titin homologue (5). The unc-22 mutations cause a twitching phenotype similar to that observed in both the mup-2(e2346ts) and mup-2(up1) TnT-1 mutants. Genetic suppression studies indicated that Twitchin, which is associated with the thick filament (43), interacts with myosin (44), and physiological studies with Aplysia muscle showed that WT twitchin inhibits the rate of muscle relaxation (24, 52). The observation that unc-22 Twitchin and mup-2 CeTnT-1 mutants have similar twitching phenotypes, suggests that both mutants alter the time-course of contraction/relaxation cycles, and specifically the time course of reestablishing inhibition, in affected muscles.
The availability of a wide range of muscle-affecting mutations in C. elegans, as well as of a conditional loss-of-function allele for TnT-1, permitted our in vivo test of whether loss of TnT function in C. elegans causes Ca2+-independent force production, as might be predicted on the basis of certain biochemical experiments of Tn subunit interactions and function in vitro. Our results: (a) support that the C. elegans TnT-1 mutation does not cause disinhibition of the thin filament; (b) provide the first in vivo evidence for the sufficiency of TnI/TnC to establish full inhibition of the thin filament in the absence of TnT function; and (c) are compatible with a model in which loss of TnT function increases the duration of the contraction by permitting untethered TnI/TnC to diffuse away from its proper binding site on the thin filament during periods of elevated free Ca2+ concentration (Model B). Importantly, these results are consistent with the detection of aberrant regulation of contractile activity in in vitro studies with human cardiac TnT harboring mutations linked to human cardiomyopathy (60) and further support that altering the thin-filament associations through mutations of TnT (missense or null) can alter the quality of force generation. Thus, our studies provide a basis for using C. elegans as a model genetic system in the analysis of in vivo functions and interactions of TnT, as well as allow comparison with work with TnT dysfunction in human cardiomyopathies.
Acknowledgments
We thank J. Sanger for equipment and assistance with the time-lapse video tape analysis; J. Ward, S. Kim, and R. Deehan for assistance with strains; and M. Stiffler for assistance with figures. We thank B. Williams for generously providing all of the alleles used that cause the Pat phenotype; R. Lee and L. Avery for information regarding the molecular identity of egl-19 (previously known as pat-5) before publication and; and H. Kagawa, B. Williams, and R. Waterston for information regarding the molecular identity of pat-10 before publication. Some strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health Center for Research Resources (NCRR). E.A. Bucher especially thanks C.P. Emerson and H.L. Sweeney for many stimulating discussions regarding TnT function and dysfunction.
This work was supported by a Grant-in-Aid from the American Heart Association and by a grant from the National Institutes of Health to E.A. Bucher. T. StC. Allen was supported by a Daland Fellowship from the American Philosophical Society and by grants from the University Research Foundation of the University of Pennsylvania and the Templeton Medical Research Fund.
Abbreviations used in this paper
- ECM
extracellular matrix
- Mup
muscle position defective
- Pat
paralyzed and arrested elongation at twofold
- Tm
tropomyosin
- TnC
troponin C
- TnI
troponin I
- WT
wild-type
Footnotes
The first two authors contributed equally to this work.
Address all correspondence to Elizabeth A. Bucher, University of Pennsylvania, Department of Cell and Developmental Biology, Pennsylvania Muscle Institute, School of Medicine, Philadelphia, PA 19104-6058. Tel.: (215) 898-2136. Fax: (215) 898-9871. E-mail: bucher@mail.med.upenn.edu
References
- 1.Ashley C, Lea T, Hoar P, Kerrick W, Strang P, Potter J. Functional characterization of the two isoforms of troponin C from the arthropod Balanus nubilus. J Muscle Res Cell Motil. 1991;12:532–542. doi: 10.1007/BF01738441. [DOI] [PubMed] [Google Scholar]
- 2.Avery L. The genetics of feeding in Caenorhabditis elegans. . Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barstead RJ, Waterston RH. Vinculin is essential for muscle function in the nematode. J Cell Biol. 1991;114:715–724. doi: 10.1083/jcb.114.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beall CJ, Sepanski MA, Fyrberg EA. Genetic dissection of Drosophilamyofibril formation: effects of actin and myosin heavy chain null alleles. Genes Dev. 1989;3:131–140. doi: 10.1101/gad.3.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Benian GM, Kiff JE, Neckelmann N, Moerman DG, Waterston RH. The sequence of twitchin: an unusually large protein implicated in regulation of myosin activity in C. elegans. . Nature. 1989;342:45–50. doi: 10.1038/342045a0. [DOI] [PubMed] [Google Scholar]
- 6.Bolten SL, Powell-Abel P, Fischhoff DA, Waterston RH. The sup-7(st5) X gene of Caenorhabditis elegansencodes a tRNATrpUAG amber suppressor. Proc Natl Acad Sci USA. 1984;81:6784–6788. doi: 10.1073/pnas.81.21.6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt PW, Diamond MS, Schachat FH. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. J Mol Biol. 1984;180:379–384. doi: 10.1016/s0022-2836(84)80010-8. [DOI] [PubMed] [Google Scholar]
- 8.Brenner S. The genetics of Caenorhabditis elegans. . Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullard B, Leonard K, Larkins A, Butcher G, Karlik C, Fyrberg E. Troponin of asynchronous flight muscle. J Mol Biol. 1988;204:621–637. doi: 10.1016/0022-2836(88)90360-9. [DOI] [PubMed] [Google Scholar]
- 10.Cabral-Lilly D, Tobacman LS, Mehegan JP, Cohen C. Molecular polarity in tropomyosin-troponin T co-crystals. Biophys J. 1997;73:1763–1770. doi: 10.1016/S0006-3495(97)78206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiquet-Ehrismann R, Tannheimer M, Koch M, Brunner A, Spring J, Martin D, Baumgartner S, Chiquet M. Tenascin-C expression by fibroblasts is elevated in stressed collagen gels. J Cell Biol. 1994;127:2093–2101. doi: 10.1083/jcb.127.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farah CS, Miyamoto CA, Ramos CHI, da Silva ACR, Quaggio RB. Structural and regulatory functions of the NH2- and COOH-terminal regions of skeletal muscle troponin I. J Biol Chem. 1994;269:5230–5240. [PubMed] [Google Scholar]
- 13.Flicker PF, Phillips GNJ, Cohen C. Troponin and its interactions with tropomyosin. An electron microscope study. J Mol Biol. 1982;162:495–501. doi: 10.1016/0022-2836(82)90540-x. [DOI] [PubMed] [Google Scholar]
- 14.Fyrberg E, Fyrberg CC, Beall C, Saville DL. Drosophila melanogastertroponin-T mutations engender three distinct syndromes of myofibrillar abnormalities. J Mol Biol. 1990;216:657–675. doi: 10.1016/0022-2836(90)90390-8. [DOI] [PubMed] [Google Scholar]
- 15.Gatewood BK, Bucher EA. The mup-4 locus in Caenorhabditis elegansis essential for hypodermal integrity, organismal morphogenesis and embryonic body wall muscle position. Genetics. 1997;146:165–183. doi: 10.1093/genetics/146.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goh, P.Y. 1992. Genetics of muscle pattern formation in C. elegans. National University of Singapore. 111 pp.
- 17.Goh PY, Bogaert T. Positioning and maintenance of embryonic body wall muscle attachments in C. elegansrequires the mup-1 gene. Development. 1991;111:667–681. doi: 10.1242/dev.111.3.667. [DOI] [PubMed] [Google Scholar]
- 18.Goldman YE, McCray JA, Ranatunga KW. Transient tension changes initiated by laser temperature jumps in rabbit psoas muscle fibres. J Physiol. 1987;392:71–95. doi: 10.1113/jphysiol.1987.sp016770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabarek Z, Drabikowski W, Leavis PC, Rosenfeld SS, Gergely J. Proteolytic fragments of troponin C. Interactions with the other troponin subunits and biological activity. J Biol Chem. 1981;256:121–127. [PubMed] [Google Scholar]
- 20.Greaser ML, Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971;246:4226–4233. [PubMed] [Google Scholar]
- 21.Harris HE, Tso MY, Epstein HF. Actin and myosin-linked calcium regulation in the nematode Caenorhabditis elegans. Biochemical and structural properties of native filaments and purified proteins. Biochemistry. 1977;16:859–865. doi: 10.1021/bi00624a008. [DOI] [PubMed] [Google Scholar]
- 22.Haselgrove JC. X-ray evidence for a conformational change in the actin containing filaments of vertebrate striated muscle. Cold Spring Harbor Symp Quant Biol. 1972;37:341–352. [Google Scholar]
- 23.Hedgecock EM, Culotti JG, Hall DH, Stern BD. Genetics of cell and axon migrations in Caenorhabditis elegans. . Development. 1987;100:365–382. doi: 10.1242/dev.100.3.365. [DOI] [PubMed] [Google Scholar]
- 24.Heierhorst J, Probst WC, Vilim FW, Buku A, Weiss KR. cAMP-dependent phosphorylation of Aplysia twitchin may mediate modulation of muscle contractions by neuropeptide cotransmitters. Proc Natl Acad Sci USA. 1994;91:8487–8491. doi: 10.1073/pnas.91.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman RK, Madl JE, Kari CK. Duplications in Caenohabditis elegans. . Genetics. 1979;92:419–435. doi: 10.1093/genetics/92.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hitchcock SE. Regulation of muscle contraction: bindings of troponin and its components to actin and tropomyosin. Eur J Biochem. 1975;52:255–263. doi: 10.1111/j.1432-1033.1975.tb03993.x. [DOI] [PubMed] [Google Scholar]
- 27.Hitchcock SE, Huxley HE, Szent-Gyorgyi AG. Calcium sensitive binding of troponin to actin-tropomyosin: a two-site model for troponin action. J Mol Biol. 1973;80:825–836. doi: 10.1016/0022-2836(73)90212-x. [DOI] [PubMed] [Google Scholar]
- 28.Hodgkin H, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode Caenorhabditis elegans. . Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homyk RJ, Emerson CP., Jr Functional interactions between unlinked muscle genes within haploinsufficient regions of the Drosophilagenome. Genetics. 1988;119:105–121. doi: 10.1093/genetics/119.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hresko MC, Williams BD, Waterston RH. Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. . J Cell Biol. 1994;124:491–506. doi: 10.1083/jcb.124.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huxley HE. Structural changes in the actin and myosin containing filaments during contraction. Cold Spring Harbor Symp Quanti Biol. 1972;37:361–376. [Google Scholar]
- 32.Jaenicke R. Protein stability and molecular adaptation to extreme conditions. Eur J Biochem. 1991;202:715–728. doi: 10.1111/j.1432-1033.1991.tb16426.x. [DOI] [PubMed] [Google Scholar]
- 33.Jha PK, Leavis PC, Sarkar S. Interaction of deletion mutants of troponins I and T: COOH-terminal truncation of troponin T abolishes troponin I binding and reduces Ca2+sensitivity of the reconstituted regulatory system. Biochemistry. 1996;35:16573–16580. doi: 10.1021/bi9622433. [DOI] [PubMed] [Google Scholar]
- 34.Kagawa H, Sugimoto K, Matsumoto H, Inoue T, Imadzu H, Takuwa K, Sakube Y. Genome structure, mapping and expression of the tropomyosin gene tmy-1 of Caenorhabditis elegans. . J Mol Biol. 1995;251:603–613. doi: 10.1006/jmbi.1995.0459. [DOI] [PubMed] [Google Scholar]
- 35.Kagawa H, Takuwa K, Sakube Y. Mutations and expressions of the tropomyosin gene and the troponin C gene of Caenorhabditis elegans. . Cell Struct Funct. 1997;22:213–218. doi: 10.1247/csf.22.213. [DOI] [PubMed] [Google Scholar]
- 36.Leavis PC, Gergely J. Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. CRC Crit Rev Biochem. 1984;16:235–305. doi: 10.3109/10409238409108717. [DOI] [PubMed] [Google Scholar]
- 37.Lehman W, Szent-Georgyi AG. Regulation of muscle contraction. Distribution of actin control and myosin control in the animal kingdom. J Gen Physiol. 1975;66:1–30. doi: 10.1085/jgp.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehman W, Craig R, Vibert P. Ca2+-induced tropomyosin movement in Limulusthin filaments revealed by three-dimensional reconstruction. Nature. 1994;368:65–67. doi: 10.1038/368065a0. [DOI] [PubMed] [Google Scholar]
- 39.Lin D, Bobkova A, Homsher E, Tobacman LS. Altered cardiac troponin T in vitro function in the presence of a mutation implicated in familial hypertrophic cardiomyopathy. J Clin Invest. 1996;97:2842–2848. doi: 10.1172/JCI118740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loughna PT, Izumo S, Goldspink G, Nadal-Ginard B. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Poc Natl Acad Sci USA. 1988;85:339–343. doi: 10.1242/dev.109.1.217. [DOI] [PubMed] [Google Scholar]
- 41.Malnik B, Farah CS, Reinach FC. Regulatory properties of the NH2- and COOH-terminal domains of troponin T. J Biol Chem. 1998;273:10594–10601. doi: 10.1074/jbc.273.17.10594. [DOI] [PubMed] [Google Scholar]
- 42.Moerman, D., and A. Fire. 1997. Muscle: structure, function and development. In C. Elegans II. D. Riddle, T. Blumenthal, and J. Priess, editors. Cold Spring Harbor Laboratory Press, New York. 417–470. [PubMed]
- 43.Moerman DG, Benian GM, Barstead RJ, Schreifer L, Waterston RH. Identification and intracellular localization of the unc-22 gene product of C. elegans. . Genes Dev. 1988;2:93–105. doi: 10.1101/gad.2.1.93. [DOI] [PubMed] [Google Scholar]
- 44.Moerman DG, Plurad S, Waterston RH. Mutations in the unc-54 myosin heavy chain gene of Caenorhabditis elegansthat alter contractility but not muscle structure. Cell. 1982;29:773–781. doi: 10.1016/0092-8674(82)90439-1. [DOI] [PubMed] [Google Scholar]
- 45.Moss RL, Giulian GG, Greaser ML. The effects of partial extraction of TnC upon the tension-pCa relationship in rabbit skinned skeletal muscle fibers. J Gen Physiol. 1985;86:585–600. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myers CD, Goh PY, Allen TS, Bucher EA, Bogaert T. Developmental genetic analysis of troponin T mutations in striated and nonstriated muscle cells of Caenorhabditis elegans. . J Cell Biol. 1996;132:1061–1077. doi: 10.1083/jcb.132.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oluwole BO, Du W, Mills I, Sumpio BE. Gene regulation by mechanical forces. Endothelium. 1997;5:85–93. doi: 10.3109/10623329709079866. [DOI] [PubMed] [Google Scholar]
- 48.Parry DAD, Squire JM. Structural role of tropomyosin in muscle regulation: analysis of the X-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973;75:33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- 49.Potter JD, Gergely J. Troponin, tropomyosin, and actin interactions in the Ca2+ regulation of muscle contraction. Biochemistry. 1974;13:2697–2703. doi: 10.1021/bi00710a007. [DOI] [PubMed] [Google Scholar]
- 50.Potter JD, Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrilar ATPase. J Biol Chem. 1975;250:4628–4633. [PubMed] [Google Scholar]
- 51.Priess JR, Hirsh DI. Caenorhabditis elegansmorphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev Biol. 1986;117:156–173. doi: 10.1016/0012-1606(86)90358-1. [DOI] [PubMed] [Google Scholar]
- 52.Probst WC, Cropper EC, Heierhorst J, Hooper SL, Jaffe H, Vilim F, Beushausen S, Kupfermann I, Weiss KR. cAMP-dependent phosphorylation of Aplysia twitchin may mediate modulation of muscle contractions by neuropeptide cotransmitters. Proc Natl Acad Sci USA. 1994;91:8487–8491. doi: 10.1073/pnas.91.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogalski TM, Gilchrist EJ, Mullen GP, Moerman DG. Mutations in the unc-52 gene responsible for body wall muscle defects in adult Caenorhabditis elegansare located in alternatively spliced exons. Genetics. 1995;139:159–169. doi: 10.1093/genetics/139.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schachat FH, Diamond MS, Brandt PW. Effect of different troponin T-tropomyosin combinations on thin filament activation. J Mol Biol. 1987;198:551–554. doi: 10.1016/0022-2836(87)90300-7. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt C, Pommerenke H, Durr T, Nebe B, Rychly J. Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored proteins. J Biol Chem. 1998;273:5081–5085. doi: 10.1074/jbc.273.9.5081. [DOI] [PubMed] [Google Scholar]
- 56.Somero GN. Temperature and proteins: little things can mean a lot. News Phys Sci. 1996;11:72–77. [Google Scholar]
- 57.Stefancsik R, Jha PK, Sarkar S. Identification and mutagenesis of a highly conserved domain in troponin T responsible for troponin I binding: Potential role for coiled coil interaction. Proc Natl Acad Sci USA. 1998;95:957–962. doi: 10.1073/pnas.95.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strynadka NC, James MN. Crystal structures of the helix-loop-helix calcium-binding proteins. Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- 59.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. . Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 60.Sweeney, H.L., H.S. Feng, Z. Yang, and H. Watkins. 1998. Functional analyses of troponin T mutations that cause hypertrophic cardiomyopathy: new insights into disease pathogenesis and troponin function. Proc. Natl. Acad. Sci. USA. In press. [DOI] [PMC free article] [PubMed]
- 61.Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 62.Tobacman LS. Structure-function studies of the amino-terminal region of bovine cardiac troponin T. J Biol Chem. 1988;263:2668–2672. [PubMed] [Google Scholar]
- 63.Tobacman LS. Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol. 1996;58:447–481. doi: 10.1146/annurev.ph.58.030196.002311. [DOI] [PubMed] [Google Scholar]
- 64.Tobacman LS, Lee R. Isolation and functional comparison of bovine cardiac troponin T isoforms. J Biol Chem. 1987;262:4059–4064. [PubMed] [Google Scholar]
- 65.Tobacman LS, Sawyer D. Calcium binds cooperatively to the regulatory sites of the cardiac thin filament. J Biol Chem. 1990;265:9931–9939. [PubMed] [Google Scholar]
- 66.Venolia L, Waterston RH. The unc-45 gene of Caenorhabditis elegansis an essential muscle-affecting gene with maternal expression. Genetics. 1990;126:345–353. doi: 10.1093/genetics/126.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang F, Martin BM, Sellers JR. Regulation of actomyosin interactions in Limulus muscle proteins. J Biol Chem. 1993;268:3776–3780. [PubMed] [Google Scholar]
- 68.Waterston, R.H. 1988. Muscle. In The Nematode Caenorhabditis elegans. W.B. Wood, editor. Cold Spring Harbor Laboratory, Cold Spring Harbor. 281–335.
- 69.Waterston RH. The minor myosin heavy chain, mhcA, of Caenorhabditis elegansis necessary for the initiation of thick filament assembly. EMBO (Eur Mol Biol Organ) J. 1989;8:3429–3436. doi: 10.1002/j.1460-2075.1989.tb08507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waterston RH, Thomson JN, Brenner S. Mutants with altered muscle structure of Caenorhabditis elegans. . Dev Biol. 1980;77:271–302. doi: 10.1016/0012-1606(80)90475-3. [DOI] [PubMed] [Google Scholar]
- 71.Waterston RH, Smith KC, Moeerman DG. Genetic fine structure analysis of the myosin heavy chain gene unc-54 of Caenorhabditis elegans. . J Mol Biol. 1982;158:1–15. doi: 10.1016/0022-2836(82)90447-8. [DOI] [PubMed] [Google Scholar]
- 72.Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, et al. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332:1058–1064. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 73.Watkins H, Seidman CE, Seidman JG, Feng HS, Sweeney HL. Expression and functional assessment of a truncated cardiac troponin T that causes hypertrophic cardiomyopathy. J Clin Invest. 1996;98:1–6. doi: 10.1172/JCI119063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White SP, Cohen C, Phillips GN., Jr Structure of co-crystals of tropomyosin and troponin. Nature. 1987;325:826–828. doi: 10.1038/325826a0. [DOI] [PubMed] [Google Scholar]
- 75.Williams BD, Waterston RH. Genes critical for muscle development and function in Caenorhabditis elegansidentified through lethal mutations. J Cell Biol. 1994;124:475–490. doi: 10.1083/jcb.124.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woledge, R.C., N.A. Curtin, and E. Homsher. 1985. Energetic aspects of muscle contraction. Monographs of the Physiological Society. Vol. 41. Academic Press, London. [PubMed]
- 77.Zot HG, Potter JD. A structural role for the Ca2+-Mg2+sites on troponin C in the regulation of muscle contraction. Preparation and properties of troponin C depleted myofibrils. J Biol Chem. 1982;257:7678–7683. [PubMed] [Google Scholar]
- 78.Zot AS, Potter JD. Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annu Rev Biophysics Biophys Chem. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]