Abstract
Members of the synaptobrevin/VAMP family of v-SNAREs are thought to be essential for vesicle docking and exocytosis in both lower and higher eukaryotes. Here, we describe yeast mutants that appear to bypass the known v-SNARE requirement in secretion. Recessive mutations in either VBM1 or VBM2, which encode related ER-localized membrane proteins, allow yeast to grow normally and secrete in the absence of Snc v-SNAREs. These mutants show selective alterations in protein transport, resulting in the differential trafficking and secretion of certain protein cargo. Yet, processing of the vacuolar marker, carboxypeptidase Y, and the secreted protein, invertase, appear normal in these mutants indicating that general protein trafficking early in the pathway is unaffected. Interestingly, VBM1 and VBM2 are allelic to ELO3 and ELO2, two genes that have been shown recently to mediate the elongation of very long chain fatty acids and subsequent ceramide and inositol sphingolipid synthesis. Thus, the v-SNARE requirement in constitutive exocytosis is abrogated by mutations in early components of the secretory pathway that act at the level of lipid synthesis to affect the ability of secretory vesicles to sort and deliver protein cargo.
Keywords: Snc proteins, SNAREs, synaptobrevin, sphingolipids, ceramides, exocytosis
Protein transport between organelles relies upon carrier vesicles that bud from one compartment and fuse selectively with another. This process is thought to be fundamental to both the functioning of the secretory pathway, as well as its overall organization. Therefore, organelle-specific trafficking of vesicles requires specialized proteins that regulate their transport, docking, and fusion. Many of these proteins have been identified and studies from both simple and higher eukaryotes suggest that their modes of action have remained conserved in evolution (Bennett and Scheller, 1993; Ferro-Novick and Jahn, 1994; Rothman, 1994).
The current model for how transport vesicles are thought to recognize their acceptor compartments relies upon genetic and biochemical studies that reveal that specific membrane-localized receptors (termed SNAREs)1 are present on both donor and acceptor compartments (Söllner et al., 1993a ,b and reviewed in Rothman and Warren, 1994). Complex formation between SNAREs of the donor (or vesicular) compartment (v-SNAREs) with those of the acceptor (or target) compartment (t-SNAREs) is thought to be necessary and sufficient to confer vesicle docking. Initially, formation of the SNARE complex was proposed to be a prerequisite for assembly of a prefusion particle that included the soluble N-ethylmaleimide-sensitive fusion protein (NSF) and its attachment proteins (SNAPs), that are recruited from the cytosol. Subsequent hydrolysis of ATP by NSF was found to result in disassembly of the SNARE complex in vitro and was suggested to lead directly to membrane fusion (Söllner et al., 1993a ,b and reviewed in Rothman and Warren, 1994). As compartment-specific v- and t-SNAREs have been found at nearly every level of the secretory pathway, the universality of this model has been generally accepted. Yet, later studies on vacuolar membrane fusion in vitro have shown that NSF/ SNAP may act before SNARE assembly (Mayer et al., 1996) and mediate upstream events that result in priming of the t-SNARE component involved in homotypic fusion (Xu et al., 1998). Moreover, it was demonstrated that together v- and t-SNAREs are necessary and sufficient for conferring both membrane association and bilayer fusion, using a liposome-based in vitro assay (Weber et al., 1998). Thus, the requirements for bilayer fusion, as originally proposed (Söllner et al., 1993a ,b) may differ at the organellar level. This idea is supported by additional studies that show that organelle fusion is t-SNARE-dependent and occurs in the absence of a v-SNARE partner (Nichols et al., 1997; Patel et al., 1998).
Previously, we have shown that yeast possess two homologues of the synaptobrevin/VAMP family of v-SNAREs, named Snc1 and Snc2 (Gerst et al., 1992; Protopopov et al., 1993). These proteins are components of post-Golgi exocytic vesicles and are required for normal cellular secretion and viability (Protopopov et al., 1993). Like their vertebrate homologues, Snc proteins form complexes with t-SNAREs from the plasma membrane, including Sec9 (SNAP-25 homologue; Brennwald et al., 1994) and the Sso proteins (syntaxin 1A, B homologues; Aalto et al., 1993). Snc proteins coprecipitate with these t-SNAREs from cell lysates (Brennwald et al., 1994; Couve and Gerst, 1994) and the SNC genes interact genetically with genes whose products contribute to formation of the exocytic SNARE complex, including: SEC9, SSO1/2, and SEC17 (α-SNAP homologue; Couve and Gerst, 1994; Gerst, 1997). Along with a recent study that demonstrates formation of a Snc1-Sso1-Sec9 ternary complex in vitro (Rossi et al., 1997), this suggests that the neuronal and yeast exocytic SNARE complexes are highly similar. Thus, the Snc1/2, Sso1/2, and Sec9 proteins are archetypal v- and t-SNARE elements that have evolved from conferring constitutive exocytosis in lower eukaryotes to mediate both constitutive and stimulus-coupled secretion in higher organisms.
Earlier, we described null mutations in the SNC genes as resulting in a conditional lethal phenotype. snc yeast are temperature sensitive, unable to grow on amino acid–rich medium, fail to undergo cell wall separation after cytokinesis, accumulate post-Golgi secretory vesicles, and are defective in invertase secretion (Protopopov et al., 1993). Yet, such cells are viable and grow slowly on synthetic minimal medium and at temperatures ≤30°C. Somehow, snc cells maintain a level of secretion competence, despite the complete absence of these v-SNAREs. Thus, an unknown mechanism must allow yeast lacking exocytic v-SNAREs to deliver lipids and proteins to the cell surface to maintain cell growth and division.
Here, we have isolated spontaneous revertants of snc cells and show that recessive mutations in two genes, designated as VBM1 and VBM2, allow cells to become temperature-resistant, grow normally, and to secrete selected cargo molecules efficiently in a Snc v-SNARE-independent fashion. Interestingly, cells bearing these mutations, designated here as v-SNARE bypass mutants (VBMs), also show a selective recoupling of the exocytic pathway, such that some protein cargo is trafficked efficiently in one mutant class, while accumulating in another. This selectivity suggests that the late secretory pathway in yeast is bifurcated, as first proposed by Harsay and Bretscher (1995), but also that the alternate routes to the cell surface may be distinctly modulated. As VBM1 and VBM2 are allelic to ELO3 and ELO2, two genes with overlapping functions that are involved in long chain fatty acid (LCFA) elongation and sphingolipid synthesis, we suggest that ceramide and sphingolipid metabolism may prove to be an important determinant in protein sorting and the trafficking of secretory vesicles in yeast.
Materials and Methods
Media and Genetic Manipulations
Yeast were grown in media containing 2% glucose or 3.5% galactose. Amino acid–rich yeast extract/peptone/dextrose medium (YPD), synthetic minimal medium (SC), and SC drop-out medium, lacking an essential amino acid or nucleotide base, were used. Media were prepared similar to that described by Rose et al. (1990). Standard methods were used for the introduction of DNA into yeast, the preparation of genomic DNA, and for tetrad dissection (Rose et al., 1990). Growth assays were performed as described (Gerst et al., 1991).
Plasmids
Vectors included: YEp13M4, a 2μ plasmid that bears a LEU2 selectable marker and pAD4Δ, a similar plasmid that contains the ADH1 constitutive promoter. Centromeric vectors included: pSE358, which bears a TRP1 marker; pRS315, which bears a LEU2 marker; and pAD11, which bears the ADE2 and HIS3 markers. Previously described plasmids included: pADH-SNC1 (Gerst et al., 1992) and pTGAL-SNC1 (Protopopov et al., 1993).
Constructs created included: pTADH-SNC1, which contains a BamHI fragment bearing the ADH1-SNC1 construct from pADH-SNC1 cloned into pSE358. Constructs for the expression of VBM1/SUR4 and VBM2/ GNS1 were created by PCR. Oligonucleotides designed to prime upstream and downstream to the various genes were used in the PCR reaction. All PCR-generated fragments bore encoded SalI and SacI sites that were used for subcloning into 2μ vectors, pAD4Δ and YEp13M4 to give: pADH-SUR4/VBM1 and YEpGNS1/VBM2; into pRS315 to give YCpSUR4/VBM1; and into pSE358 to give YCpGNS1/VBM2. A frame-shift mutation in SUR4/VBM1 was created using a mutant forward oligonucleotide that encodes a termination signal after the third codon. This was cloned into pAD4Δ to yield pADH-SUR4FS. Tagged VBM genes were created using reverse primers that encoded the HA epitope. These PCR products were subcloned into pRS315 to give YCpVBM1HA and YCpVBM2HA, and into pAD4Δ to yield pADH-VBM1HA. Disruption constructs for SUR4/VBM1 and GNS1/VBM2 were created by inserting TRP1 and LEU2 markers either into the StyI and BlpI sites of SUR4, or the EcoRV site of GNS1, via blunt-end ligations, to give pVBM1T, pVBM2T, pVBM1L, and pVBM2L, respectively. Plasmid pAHGAL-SNC2, which contains SNC2 under control of the GAL promoter, was created by subcloning the appropriate EcoRV-SacI fragment of pGAL-SNC2 (Protopopov et al., 1993) into the SmaI-SacI sites of pAD11. A HIS3 disruption construct for SNC2, pSNC2H, was created by subcloning a BamHI fragment of HIS3 into the BglII site of a 3-kb genomic fragment bearing SNC2, carried in plasmid p2H (Couve and Gerst, 1994). The snc2:: HIS3 fragment was released by digestion with BspHI and used in transformations. All constructs were verified by restriction analysis.
Immunofluorescence and Immunoblot Analysis
Cell lysates were prepared as described (Couve and Gerst, 1994). Lysates and vesicle preparations were transferred to nitrocellulose membranes for detection. For immunofluorescence, fixed yeast spheroplasts were permeabilized with 0.5% SDS, incubated with antibodies, and visualized by confocal microscopy. Affinity-purified antisera included: anti-Dpm1 (Molecular Probes), anti-HA (12CA5), anti-Mnn1 (gift of S. Emr, Howard Hughes Medical Institute, University of California, San Diego, CA), and anti-Sso (gift of P. Brennwald, Cornell University Medical College, New York, NY). FM4-64 labeling was carried out, essentially as described (Vida and Emr, 1995).
Yeast Strains
Wild-type strains included: W303-1a (Mata can1 his3 leu2 lys2 trp1 ura3 ade2), W303-1b (Mat α can1 his3 leu2 lys2 trp1 ura3 ade2), and SP1 (Mata leu2 ura3 trp1 ade8 can1 his3). Other strains included: NY778 (Mat α leu2-3,112 ura3-52 sec6-4), NY782 (Mata leu2-3,112 ura3-52 sec9-4; Walworth et al., 1992), H603 (Mata can1 leu2 trp1 ura3 ade2 sso1Δ::HIS3 sso2-1; Gerst, 1997), and JG8 T15:85 (Mata his3 leu2 trp1 snc1::URA3 snc2:: ADE8 pGAL-SNC1 or pTGAL-SNC1; Protopopov et al., 1993). A Mat α strain of JG8 T15:85, JG8 T15:85α, was generated by mating-type switching. Mutant strains analyzed in this study included: vbm1-1 (JG8R-A14; Mata his3 leu2 trp1 snc1::URA3 snc2::ADE8 vbm1-1), vbm2-1 (JG8R- α39; Mat α his3 leu2 trp1 snc1::URA3 snc2::ADE8 vbm2-1) and vbm2-2 (JG8R-α10; Mat α his3 leu2 trp1 snc1::URA3 snc2::ADE8 vbm2-2). VBM disruption strains in the snc background included: DD1 (Mata his3 leu2 snc1::URA3 snc2::ADE8 vbm1::TRP1) and SS1 (Mata his3 leu2 snc1:: URA3 snc2::ADE8 vbm2::LEU2), which were created by transformation of JG8 cells with the appropriate disruption constructs. A snc sso1Δ sso2-1 strain (JG9-S2) was created by transformation of a sso1::HIS3 sso2-1 haploid segregant (derived from a cross between H603 and W303-1b) with plasmid pAHGAL-SNC2, followed by the sequential disruption of SNC1 and SNC2. Cells were maintained continually on galactose-containing medium to prevent lethality. Next, the disruption of VBM1 or VBM2 in JG9-S2 cells was accomplished by transformation using the appropriately digested pVBM1T and pVBM2T plasmids. Strains: JG9-S2V1 (Mata ade2 snc1::URA3 snc2::LEU2 vbm1::TRP1 sso1::HIS3 sso2-1) and JG9-S2V2 (Mata ade2 snc1::URA3 snc2::LEU2 vbm2::TRP1 sso1::HIS3 sso2-1) were obtained in this fashion. VBM disruptions in the sec6-4 and wild-type backgrounds yielded strains: DD2 (Mat α ura3-52 sec6-4 vbm1::LEU2), SS2 (Mat α ura3-52 sec6-4 vbm2::LEU2), and SS3 (Mata ura3 trp1 ade8 can1 his3 vbm2::LEU2). Disruptions were accomplished by transformation of the sec6-4 and SP1 strains using a fragment obtained from digestions of the pVBM1L and pVBM2L plasmids. All disruptions were verified by Southern or PCR analyses.
Thin-sectioning and Electron Microscopy
The fixation, thin-sectioning and electron microscopy of yeast was performed as described previously (Zelicof et al., 1996). Uranyl acetate staining of membranes from density gradients was performed by first diluting the three fractions of a given peak of enzymatic activity in the gradient buffer (Harsay and Bretscher, 1995) lacking Nycodenz, followed by the addition of glutaraldehyde to a final concentration of 3%. After 2–4 h with shaking, the membranes were pelleted at 100,000 g and resuspended in 20 μl of buffer. Next, grids bearing collodium support film were incubated with the membranes for 1 min, followed by staining with 1% uranyl acetate (1 min). Membranes were visualized by electron microscopy.
Secretion of Media Proteins and Pulse–Chase Analysis
The secretion of proteins into the medium was measured using the method of Gaynor and Emr (1997). Intracellular protein processing was monitored by pulse–chase analysis using 35[S]methionine (Amersham Inc., Princeton, NJ), as described (Couve et al., 1995). Anti-CPY (gift of S. Emr), anti-invertase (gift of E. Bibi, Weizmann Institute of Science, Rehovot, Israel), and anti-Gas1 (gift of R. Schekman, University of California, Berkeley, CA) antibodies were used in the immunoprecipitation reactions. Autoradiography was performed using a fluorescence enhancer.
Vesicle Preparations and Density Gradient Centrifugation
Vesicle preparations and density gradient centrifugation were performed, as described by Harsay and Bretscher (1995). sec6 or sec9 cells were temperature-shifted to 37°C for 1–2 h before harvesting, while snc vbm and snc cells were maintained at 26°C. snc cells were shifted to phosphate-depleted synthetic medium to induce acid phosphatase, unlike sec6 cells that were shifted to phosphate-depleted YPD. In other experiments, sec6 or sec9 cells were incubated for 2 h in low glucose (0.05%) YPD, before temperature-shifting, to induce invertase expression.
15–30% Nycodenz gradients were prepared and collected using a Buchler Autodensi-Flow IIc gradient builder. Fractions from the gradient (∼700 μl) were aliquoted and frozen at −70°C, until use. Samples were taken to determine protein concentration, using the Bradford protein dye binding assay (Bio-Rad), and density.
Enzymatic Assays
Acid phosphatase and exoglucanase were assayed by the methods of Van Rijn et al. (1972) and Santos et al. (1979), respectively, as described in Harsay and Bretscher (1995). Activity is expressed in arbitrary units based upon the absorbance measured at 415 nm. ATPase activity was assayed by using method of Bowman and Slayman (1979), with some modifications of its application described in Harsay and Bretscher (1995). First, substrate concentration was modified to 2 mM ATP, as higher concentrations result in an elevated background. Second, blanks were measured to determine the rate of endogenous ATP hydrolysis and were subtracted from values obtained with samples from the gradient. Detection of inorganic phosphate was assayed by the method of Ames (1966). Optical density was measured after 10 min and the activity expressed in arbitrary units, based upon absorption at 820 nm. Invertase activity was assayed by the method of Goldstein and Lampen (1975) and is expressed either in arbitrary units, based upon absorption at 540 nm, or in units, where 1 unit = 1 μmol glucose released/min/100 mg of dry cells.
Metabolic Labeling of Sphingolipids
Yeast cells grown to log phase in SC medium were pulsed for 30 min with 3H-myo-inositol (1 μCi/OD unit), followed by a chase for 90 min. Afterwards, yeast were lysed and equivalent amounts of protein were extracted with CHCl3:CH3OH:H2O (10:10:3). The organic phase was removed and split, whereby half was untreated, while the rest was treated with alkali (0.2 N KOH in CH3OH) to hydrolyze the phosphoinositides and then neutralized with acetic acid. Both sets of extracts were dried, resuspended in CHCl3:CH3OH (1:1), and subjected to thin-layer chromatography on Silica gel 60 plates (Merck, Darmstadt, Germany), using CHCl3:CH3OH: 0.25% KOH (55:45:10) as solvent to separate the inositol-labeled lipids. A Fuji BAS1000 phosphorimager and BAS-TR2040S screen was used in the imaging and quantitation of the labeled lipids.
Results
Isolation of v-SNARE Bypass Mutants
Because snc null mutations greatly reduce cellular secretion and viability, we examined whether other yeast proteins could recouple exocytosis and restore full viability in the absence of Snc protein. We screened multi-copy gene libraries in snc yeast and isolated colonies that were temperature-resistant and able to grow on YPD media in a plasmid-dependent manner. Subsequent analysis of the ∼70 clones isolated revealed that all encoded either SNC1 or SNC2 (data not shown). Thus, it seems unlikely that other yeast proteins can substitute for the Snc v-SNAREs and confer normal constitutive secretion. In addition, similar experiments using v-SNAREs involved in ER–Golgi transport, Bet1 and Bos1 (Dascher et al., 1991; Shim et al., 1991; Newman et al., 1992), as well as rat VAMP1 or VAMP2 (Baumert et al., 1989; Elferink et al., 1989) failed to show suppression of the snc phenotypes (Gerst, 1997 and unpublished data).
To isolate chromosomal suppressors for the snc null phenotype, we plated snc yeast (which carry a galactose-inducible SNC1 gene and are maintained normally on galactose-containing medium) onto YPD plates and allowed for the occurrence of spontaneous revertants. YPD-resistant cells arose at a frequency of >1:105, were morphologically indistinguishable from wild-type cells, and were temperature resistant (data not shown). We examined 100 revertants from both mating types and determined their genetic disposition. Nearly all (98%) mutants appeared to have undergone recessive mutation, as back-crossing to the parental snc cells resulted in failure of the diploids to grow on YPD. Next, by Western analysis, we examined whether Snc protein expression had been restored. We found that 45% of the revertants expressed Snc1, indicating that they underwent mutation to restore protein expression from the galactose-inducible promoter. Nevertheless, over half the cells did not express Snc protein and were able to lose the plasmid-borne copy of GAL-SNC1 without any loss in cellular viability. Such cells appear to bypass the v-SNARE requirement in exocytosis and were designated as VBMs.
Complementation analysis showed that the recessive mutations fall into two groups, representing two genes designated here as VBM1 and VBM2. Out of 50 mutants examined, roughly one-quarter were found to be vbm1, whereas the rest were vbm2. The VBM phenotype was found to segregate 2:2 in meiotic segregants derived from crosses with snc cells (data not shown).
Differential Secretion of Invertase by vbm1 and vbm2 Cells
Since v-SNARE bypass mutants grow like wild-type yeast, we examined their ability to secrete proteins. We first examined their ability to secrete invertase, an enzyme used as a standard for studying secretion competence, and whose secretion is essentially blocked in snc cells (Protopopov et al., 1993; Gerst, 1997). We found that snc vbm1 cells were unable to secrete invertase at 37°C, unlike either wild-type or snc vbm2 cells, but much like sec6 cells shifted to nonpermissive conditions (Fig. 1 A, left). Cells bearing temperature-sensitive mutations in late-acting sec genes (i.e., SEC6) are known to be deficient in invertase secretion when shifted to the nonpermissive temperature (e.g., 37°C; Novick et al., 1980; Walworth et al., 1988). In contrast, snc vbm2 cells secreted ∼50% of newly synthesized invertase, which was more similar to the wild-type SP1 cells that secreted 75% (Fig. 1 A).
Figure 1.
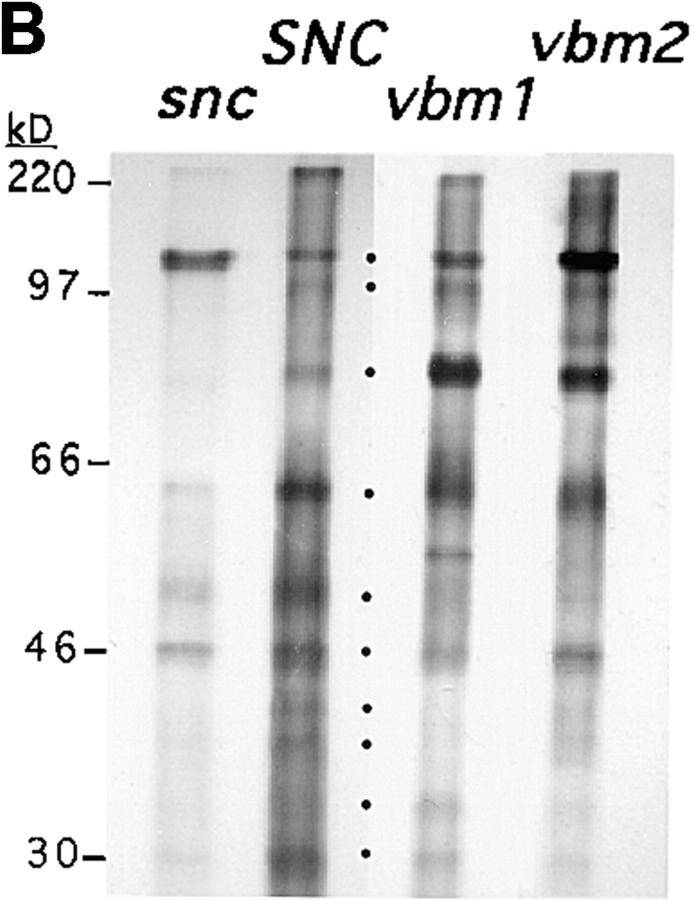
Secretion of invertase and media proteins from v-SNARE bypass mutants. (A) Invertase secretion was followed in wild-type (WT), sec6, snc, snc vbm1, and snc vbm2 cells that were maintained at 26°C or temperature-shifted to 37°C for 2 h (to induce vesiculation in sec6 cells). Values for cells derepressed on low glucose medium are shown. NS represents the nonsecreted enzyme activity, measured from lysed spheroplasts. S indicates the secreted enzyme, measured from untreated cells. Values are given in units (1 unit = 1 μmol glucose released/min/100 mg of dry cells). Cells in the panel marked 37°C express native levels of Snc protein, where appropriate. Cells in the panel marked 37°C, pTADH-SNC1 include snc vbm cells that bear a single-copy plasmid that expresses SNC1 constitutively. (B) Secreted media proteins were precipitated from snc, wild-type (SNC), vbm1, or vbm2 cells that were pulse–chase labeled with [35S]methionine at 30°C. Molecular mass is given in kilodaltons (kD). The filled-in circles designate those proteins routinely identified from the culture medium of wild-type cells.
Interestingly, we found that snc vbm1 cells were capable of secreting some invertase at lower temperatures (e.g., 26°C; Fig. 1 A, right). We found that snc vbm1 cells secreted ∼40% of newly synthesized invertase in several experiments (Fig. 1 A and data not shown). Under these same conditions, though, snc vbm2 cells were found to secrete invertase more efficiently and secreted ∼70% of newly synthesized invertase (Fig. 1 A and data not shown). snc control cells were found to synthesize and secrete only small amounts of enzyme. Thus, while snc vbm1 and snc vbm2 cells grow in an identical fashion, they secrete invertase differentially and in a temperature-dependent fashion.
We also determined whether SNC gene expression from a single-copy plasmid could restore invertase secretion from snc vbm1 cells at 37°C (Fig. 1 A, middle). As expected, Snc1 expression conferred secretion competence to snc vbm1 cells and also improved secretion from snc vbm2 cells. Under these conditions, both snc vbm1 and snc vbm2 cell types secreted ∼75% of newly synthesized invertase. Therefore, reexpression of the exocytic v-SNARE restored invertase secretion from snc vbm cells to wild-type levels.
Restored Secretion of Media Proteins by vbm Mutations
Whereas some secreted proteins remain within the periplasm, many are released into the growth medium (Robinson et al., 1988 and Gaynor and Emr, 1997). To determine whether the secretion of these proteins occurs efficiently in snc and snc vbm cells, we examined the secretion of media proteins at 26 and 37°C, using pulse–chase labeling (Fig. 1 B and data not shown). The results shown in Fig. 1 B are from a representative experiment performed at 26°C. Identical results were obtained from strains shifted to 37°C (1–2 h). After gel electrophoresis and autoradiography, we found that wild-type SP1 cells released at least 9–10 labeled proteins into the media, as shown previously for another wild-type strain (Gaynor and Emr, 1997). In contrast, snc cells secreted reduced amounts of these proteins (e.g., 20–30% of SP1 cells, on average) and showed differential patterning (Fig. 1 B). The highest molecular weight band, which corresponds to HSP150 (Gaynor and Emr, 1997), was elevated between two- and fivefold, depending upon the experiment. In contrast, some bands (i.e., at 33, 37, and 39 kD) were barely detectable or were missing altogether (e.g., at ≥100 kD) from the culture medium of snc cells.
Mutations in the VBM genes restored the secretion of proteins into the medium from snc cells. Both snc vbm1 and snc vbm2 cells had a secretion profile basically similar to that of wild-type cells and secreted a similar amount of protein into the medium, as deduced by autoradiography (Fig. 1 B) and scintillation counting (data not shown). Levels of the different proteins, as well as the number of bands, did vary somewhat from experiment to experiment. The reason for these differences is unclear. Identical results were obtained at 37°C (data not shown), indicating that the restoration in media protein secretion from snc cells is temperature-independent.
Intracellular Processing of CPY and Invertase Is Normal, but That of the GPI-anchored Protein, Gas1, Is Retarded in snc vbm Cells
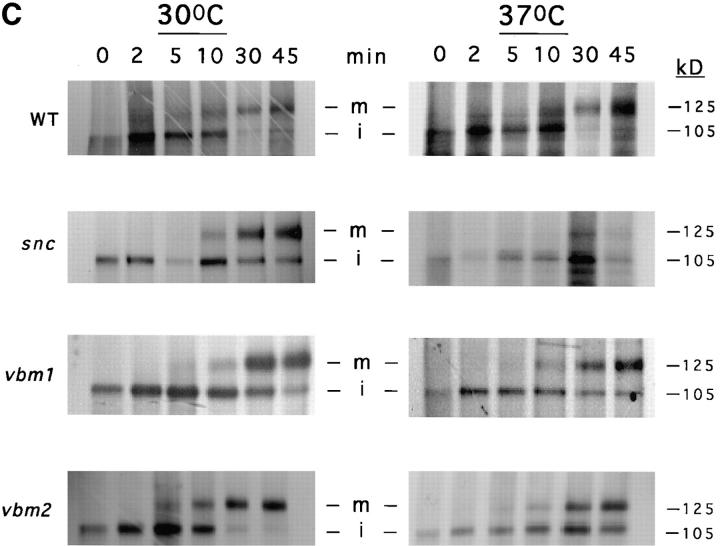
Because snc vbm cells secrete invertase differentially, we examined the intracellular trafficking of other proteins by 35S-methionine labeling and pulse–chase analysis at 30 and 37°C. First, we found that carboxypeptidase Y (CPY), a vacuolar hydrolase that reaches maturation upon transport to the vacuole, is not secreted from snc vbm cells and is found solely in its mature form in yeast grown to mid-log phase, as determined by Western analysis (data not shown). Pulse–chase labeling experiments also revealed that CPY underwent normal processing to its mature (m) form in snc vbm cells at both 30 and 37°C, like wild-type yeast (Fig. 2 A). We note that in snc cells, unlike wild-type or snc vbm cells, this processing is incomplete within 45 min at 30°C and that some protein remains in the p2 form. This is further exacerbated at restrictive conditions (37°C), which result in an even greater proportion of CPY in its immature p1 and p2 forms. Thus, mutations in the VBM genes restore the normal rate of processing of CPY to snc cells.
Figure 2.
Processing of CPY, invertase, and Gas1 in snc, snc vbm, and wild-type yeast cells. The intracellular processing of carboxypeptidase Y (A), invertase (B), and Gas1 (C) was followed in wild-type (WT), snc, snc vbm1, and snc vbm2 cells that were maintained at 30°C or temperature-shifted to 37°C (1 h), by pulse–chase analysis. Cells were labeled with [35S]methionine (pulse) followed by the addition of fresh medium containing unlabeled excess methionine and cysteine (chase). Samples were removed at various times; cellular proteins were extracted, immunoprecipitated using specific antibodies, electrophoresed, and autoradiographed as described (Couve et al., 1995). Molecular masses are given in kilodaltons (kD).
Other pulse–chase experiments demonstrated that the secreted enzyme, invertase, is processed to its glycosylated higher molecular weight forms in snc vbm cells, as shown for both snc and wild-type yeast at 30 and 37°C (Fig. 2 B). Thus, both trafficking of CPY to the vacuole and the processing of invertase in the VBM cells are similar to that found in wild-type yeast, suggesting that protein trafficking along early stages of the pathway is unaffected. In addition, we also examined uptake of fluorescent dyes (i.e., FM4-64) that are internalized via the endocytic pathway and label the yeast vacuole. We found no qualitative defects in dye uptake and vacuolar labeling in either snc vbm cell type, suggesting that the endocytic pathway is not significantly affected in these mutants.
In contrast, pulse–chase analysis did show that the kinetics of maturation for the GPI-anchored protein, Gas1, were slowed in snc vbm cells, in comparison to wild-type cells, in particular at 37°C (Fig. 2 C). Whereas Gas1 was found in its mature form by 30 min in wild-type cells, this was not true in either snc or snc vbm cells, which still showed Gas1 in its immature form after 45 min. This difference was more prominent at high temperature (37°C) and showed that ∼40–50% of the protein had not undergone maturation. Thus, unlike CPY or invertase, the trafficking of the GPI-anchored protein, Gas1, is not restored by mutations in the vbm genes.
Differential Accumulation of Secretory Vesicles in snc vbm Cells
As invertase is secreted efficiently by snc vbm2 cells, but not snc vbm1 cells, we examined both VBMs by electron microscopy. Unlike wild-type cells, VBMs were found to accumulate 50–100-nm transport vesicles, the approximate size of post-Golgi secretory vesicles (Fig. 3). This is similar to that shown previously for snc cells (Protopopov et al., 1993), however, snc vbm1 cells accumulate vesicles primarily in the bud (Fig. 3 a), while snc vbm2 cells accumulate vesicles in both mother and bud (Fig. 3 c). The distribution of vesicles in snc vbm2 cells is similar to that seen with snc cells (Protopopov et al., 1993), although snc cells accumulate greater numbers of vesicles (data not shown). Aside from the accumulation of vesicles seen in the snc vbm mutants, no additional morphological abnormalities were observed.
Figure 3.
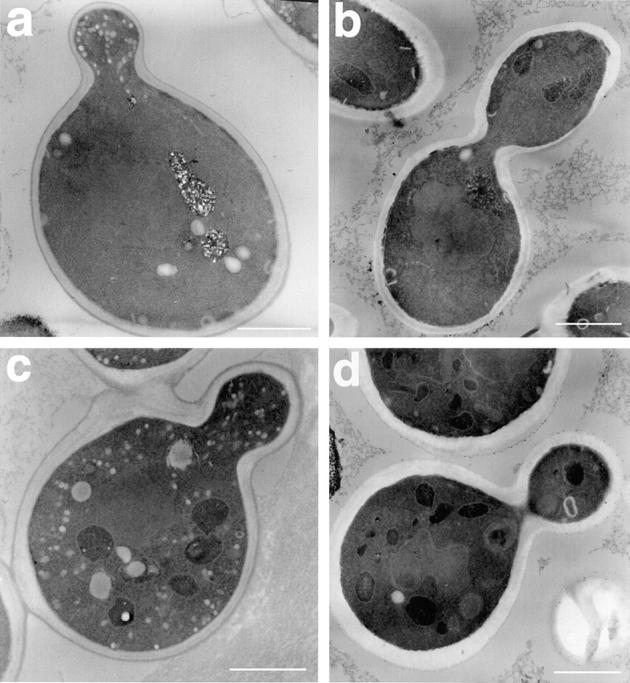
Accumulation of vesicles in v-SNARE bypass mutants. snc vbm cells were harvested in log phase, fixed, and subjected to thin-sectioning and electron microscopy. Representative cells are shown. a shows a snc vbm1 cell, whereas b shows a snc vbm1 cell that expresses SNC1 constitutively from a single-copy plasmid. c shows a snc vbm2 cell, whereas d shows a snc vbm2 cell that expresses SNC1 from a single-copy plasmid. Bars, 1 μm.
Next we determined whether the restoration of SNC gene expression could abolish vesicle accumulation in VBM cells. As predicted from the invertase secretion experiments (Fig. 1 A), restoration of SNC1 expression blocked vesicles from accumulating in either snc vbm1 or snc vbm2 cells (Fig. 3, b and d, respectively), as evidenced by electron microscopy. Thus, the reexpression of Snc protein in snc vbm cells recouples both invertase secretion and vesicle transport.
Because snc vbm cells accumulate vesicles and secrete invertase in a differential manner, one possible explanation is that the VBM genes mediate trafficking of two different types of carrier vesicles. If so, crosses between vbm1 and vbm2 mutants should yield segregants that have both trafficking pathways coupled. To test this, we crossed snc vbm1 and snc vbm2 cells, sporulated the diploids (in the presence of a plasmid that expresses SNC1, which is required for sporulation), and dissected the resulting tetrads.
The results from tetrad analysis demonstrate that segregation of the invertase secretion-deficient phenotype was 2:2, while that for the growth on YPD and the resistance to temperature was 3:1, in the absence of Snc1 protein expression (data not shown). Thin-section microscopy of the segregants obtained from a representative tetrad is shown in Fig. 4. Phenotypic analysis and complementation revealed that one segregant was snc null (a); one was snc vbm1 (d); one was snc vbm2 (c); whereas one was identical to wild-type cells and was snc vbm1 vbm2 (b). Specifically, the segregants shown in a, c, and d had the same phenotypes described previously, while that in b was found able to grow on YPD and at 37°C, secreted invertase normally (data not shown), and did not accumulate secretory vesicles. Thus, the presence of both vbm mutations in the snc null background appears to restore these cells to the wild-type state.
Figure 4.
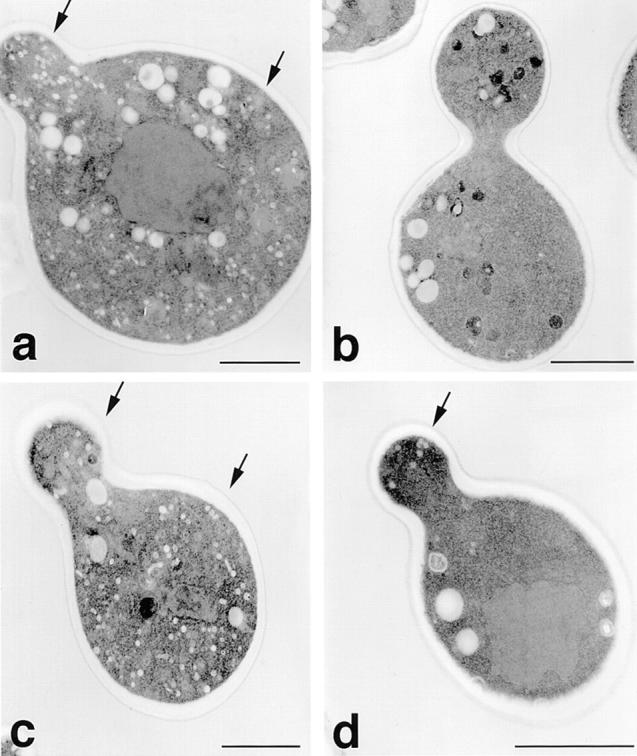
Accumulation of vesicles in segregants derived from vbm1 × vbm2 crosses. Haploid segregants derived crosses between snc vbm1 and snc vbm2 cells, obtained from tetrad analysis, were processed for electron microscopy. Segregants derived from one tetrad are shown. (a–d) Representative cells of each segregant. a is snc null, b is snc vbm1 vbm2, c is snc vbm2, and d is snc vbm1, based upon phenotypic and complementation analyses. Bars, 1 μm.
snc and sec6 Cells Accumulate the Same Two Types of Exocytic Vesicles
Snc proteins appear to mediate the trafficking of two distinct types of secretory vesicles, one containing invertase that is likely to accumulate in the bud and one lacking invertase that accumulates both in the mother and bud. Recent work by Harsay and Bretscher (1995) proposed, in fact, that yeast have two types of secretory vesicles: one of high density, which contains invertase and acid phosphatase; and one of lower density that contains the plasma membrane H+-ATPase, Pma1. These two different vesicle populations were found to accumulate in a temperature-shifted sec6 mutant and were separated physically by density gradient centrifugation.
To determine whether snc cells also accumulate two different types of vesicles, as predicted by our analysis of the snc vbm mutants, we performed density gradient centrifugation of membrane preparations (100,000 g spins) from sec6 cells that had been shifted to nonpermissive temperatures for 1–2 h and snc cells, which accumulate vesicles constitutively. Fractions collected from the gradient were analyzed for acid phosphatase, invertase, ATPase, and exoglucanase activities, using standard assays. We found that sec6 cells accumulate acid phosphatase, invertase (data not shown), and exoglucanase in the high density secretory vesicles (HDSVs), as first described by Harsay and Bretscher (1995; Fig. 5 A). Additionally, we were able to detect ATPase activity in the low density secretory vesicles (LDSVs). In contrast to the previous study, we found that the majority of exoglucanase activity purified with the HDSV population, instead of being distributed amongst both populations. Identical results were also obtained from sec9-4 cells (data not shown).
Figure 5.
Nycodenz gradient separation of secretory vesicles from sec6 and snc cells. Secretory vesicles were obtained from temperature-shifted (37°C) and nonshifted sec6 cells (A) and from snc cells (B) maintained continuously at 28°C, as described under Materials and Methods. Vesicles were fractionated by density gradient centrifugation over 15–30% Nycodenz gradients. Density, protein concentration, and the activities of various enzymes in each fraction were assayed and plotted. The results of representative experiments are shown. Acid phosphatase and exoglucanase activities are expressed in arbitrary units based upon the absorbance measured at 415 nm. ATPase activity is expressed in arbitrary units based upon the absorbance measured at 820 nm.
Fractionation of secretory vesicles from snc cells yielded similar results to those obtained with sec6 (Fig. 5 B). Two distinct populations of vesicles having different densities and biochemical contents were identified, and corresponded exactly with those obtained from sec6 cells. Thus, the disruption of vesicle trafficking is similar between the sec6 and snc mutants, showing that the blocks in secretion competence are similar. This demonstrates that the Snc v-SNAREs mediate the trafficking of both vesicle populations, as suggested by previous studies (Protopopov et al., 1993; Couve and Gerst, 1994; Gerst, 1997; this study, see Figs. 1 A and 3). Furthermore, Western blot analysis of the fractions obtained from density gradient centrifugation reveal that Snc1 and Snc2 both reside on the LDSVs and HDSVs prepared from either sec6 or sec9 cells (data not shown).
v-SNARE Bypass Mutants Accumulate One Population of Vesicles
Because snc null cells accumulate two distinct populations of vesicles, it was necessary to determine whether the snc vbm cells accumulate only one vesicle type each, as might be predicted by the invertase secretion and electron microscopy data. We made vesicle preparations from snc vbm1 or snc vbm2 cells and separated them on Nycodenz density gradients as described above (Fig. 6). In addition, samples from the different fractions were stained with uranyl acetate and visualized by electron microscopy to determine the nature of the membranes that accumulate within the VBM mutants.
Figure 6.

Nycodenz gradient separation of secretory vesicles from vbm1 and vbm2 cells. Secretory vesicles from snc vbm2 (A) and snc vbm1 (B) mutants grown continually at 28°C were isolated using Nycodenz density gradient centrifugation. Density, protein concentration, and the activities of various enzymes in each fraction were assayed. The results of representative experiments are shown. Acid phosphatase and exoglucanase activities are expressed in arbitrary units based upon the absorbance measured at 415 nm. ATPase activity is expressed in arbitrary units based upon the absorbance measured at 820 nm. Invertase activity is expressed in arbitrary units, based upon absorption at 540 nm. C Shows uranyl acetate-stained membrane from the peak enzymatic fractions derived from either vbm1 or vbm2 cells. It should be noted that these vesicles assume a more spherical shape (e.g., as seen right corner of the vbm2 panel) when examined at lower concentrations of material (data not shown). Crowding during the concentration and visualization procedures appears to distort the appearance of these membranes. Bar, 100 nm.
We found that only one population of vesicles could be identified in vbm2 cells. This population fractionated at the lower density and was found to contain the membranal H+-ATPase activity, as well as, low amounts of exoglucanase activity (Fig. 6 A). In contrast, no significant level of acid phosphatase, exoglucanase, and invertase (not shown) activities were detected at the density corresponding to the HDSVs (Fig. 6 A). We did note, however, that the single LDSV population eluted at a slightly lower average density than that found previously in sec9 or snc cells (e.g., 1.149 vs. 1.155 g/ml Nycodenz, respectively). Uranyl acetate staining of the membranes that fractionated with the ATPase activity revealed that they consist of a relatively uniform population of 90–120-nm vesicles (Fig. 6 C), as predicted earlier by the thin-section data (Figs. 3 and 4).
Similarly, only one population of vesicles could be identified in vbm1 cells. However, this population fractionated at a significantly lower average density than that typical for HDSVs (e.g., 1.152 vs. 1.177 g/ml), and was found to contain high levels of both invertase and exoglucanase activities, along with detectable ATPase activity (Fig. 6 B). The membranes that accumulated in these fractions were also shown by uranyl acetate staining to consist of a relatively uniform population of 90–120-nm vesicles (Fig. 6 C), as predicted above (Figs. 3 and 4), and did not show the broken membranes and microsomal structures (≥200 nm) found in the early fractions of the gradient (data not shown) and that bear typical ER and Golgi markers (see below). These results seem to imply that each VBM accumulates only one type of secretory vesicle, but may also suggest that the density and cargo content of the vesicles may be altered as a result of mutations in the VBM genes.
Cloning of the VBM Genes
To clone the VBM genes, we first dissected tetrads obtained from crosses between the individual snc vbm mutants and wild-type cells. We were unable to find any haploid segregants that had sensitivity to either temperature or YPD. Thus, the presence of vbm mutations in a wild-type background did not confer an obvious phenotype. It is unlikely, then, that the VBM genes would have been identified in the original sec mutant screen (Novick et al., 1980). Similarly, haploid segregants obtained from crosses of snc vbm1 and snc vbm2 cells also revealed no synthetic phenotype and resulted in a 3:1 segregation of the suppressor activity (described above).
We cloned VBM1 by screening snc vbm1 cells with genomic libraries carried on single-copy plasmids, in order to isolate plasmids that conferred the snc phenotype (inability to grow at 37°C or on YPD). Out of 30,000 transformants, one was temperature- and YPD-sensitive in a plasmid-dependent manner (Fig. 7 A). Sequence analysis revealed that this plasmid contained sequence from chromosome XII and restriction mapping of the complementing activity showed that it corresponded to the SUR4/ APA1/ELO3 gene (these data are available from GenBank/EMBL/DDBJ under accession number X78326; Desfarges et al., 1993; Garcia-Arranz et al., 1994; Silve et al., 1996; Oh et al., 1997). This was verified by creating a frame-shift mutation in SUR4/VBM1 that terminates transcription and testing the ability of the mutated gene to complement the snc vbm1 mutation. This mutant was found to be unable to confer complementation (Fig. 7 A). Finally, the targeted disruption of SUR4/VBM1 in snc cells was found to confer resistance to both temperature and YPD (Fig. 7 A).
Figure 7.
Complementation of the vbm mutations by SUR4 and GNS1. (A) snc vbm1 cells (JG8R-A14) were transformed with single-copy plasmids expressing: genomic DNA from chromosome XII (YCpORF369-373); and VBM1 under the control of either its native promoter (YCpSUR4) or a constitutive promoter (YCpADH-SUR4). Cells were also transformed with 2 μ plasmids expressing VBM1 (pADH-VBM1) and VBM1 bearing a frame-shift mutation (pADH-SUR4FS), under the control of a constitutive promoter. In addition, snc vbm1 cells bearing a control plasmid, snc cells, and snc cells bearing a disruption in VBM1/SUR4 (snc sur4::TRP1) are shown. For A and B, all cells contained a galactose-inducible SNC1 gene and were examined for their ability to grow on YPD or on SC at 25 and 37°C. (B) snc vbm2 cells (JG8R-A10) were transformed with plasmids expressing: HA-taggedVBM2/GNS1 from a single-copy plasmid (YCpGNS1HA); VBM2/GNS1 from a multi-copy plasmid (YEpGNS1), or a control plasmid. In addition, snc cells and snc cells bearing a disruption in VBM2/GNS1 (snc gns1::LEU2) are shown.
Vbm1/Sur4/Apa1/Elo3 is a protein of 345 amino acids that has five putative transmembrane domains (Garcia-Arranz et al., 1994; Silve et al., 1996). Cells bearing mutations in APA1 were isolated as mutants in which Pma1 levels were greatly reduced (Garcia-Arranz et al., 1994), whereas SUR4 was identified as a gene, which when inactivated, allows yeast to become resistant to mutations in the RVS161 gene (Desfarges et al., 1993) and to inhibitors of the ergosterol biosynthetic pathway (Silve et al., 1996). The mechanism by which mutations in SUR4 suppresses the toxicity of these drugs is unknown. More recently, the ELO3 gene was identified as encoding a protein that participates in the elongation of LCFAs, leading to ceramide and sphingolipid synthesis (Oh et al., 1997).
Because the yeast genome encodes two proteins with structural homology to Vbm1/Fen1/Gns1/Elo2 (these data are available from GenBank/EMBL/DDBJ under accession number X59720; El-Sherbeini and Clemas, 1995; Oh et al., 1997) and Elo1 (accession number Z49471; Toke and Martin, 1996), we tested whether either could complement the vbm2 mutation. We found that FEN1/GNS1/ ELO2 expression from either single or multi-copy plasmids was able to complement the vbm2 mutation (Fig. 7 B), suggesting that it is allelic to VBM2. This was verified by the gene-targeted disruption of FEN1/GNS1/ELO2 in snc cells, which conferred both temperature-resistance and growth on YPD (Fig. 7 B). As the ELO2 gene product was also demonstrated to participate in LCFA elongation leading to sphingolipid synthesis (Oh et al., 1997), this result was not entirely unexpected.
The study by Oh et al. (1997), demonstrated that Elo2/3 act downstream of Elo1 and catalyze the elongation of C22-containing fatty acids to LCFAs of 24 and 26 carbons, respectively. LCFAs are required for the subsequent synthesis of ceramide and the three types of inositol-containing sphingolipids in yeast: inositol-phosphorylceramide (IPC), mannose inositol-phosphorylceramide (MIPC), and mannose (inositolphosphoryl)2-ceramide (M(IP)2C) (reviewed by Lester and Dickson, 1993). Interestingly, Elo2/3 were shown to confer partially overlapping functions, as ceramide and inositol sphingolipid synthesis were not completely abolished by the disruption of either gene and had little effect upon cell growth. In contrast, disruption of both genes resulted in a loss of sphingolipid synthesis and had a lethal phenotype. Thus, the VBM1 and VBM2 genes identified in this study encode structural and functional homologues involved in fatty acid and sphingolipid synthesis.
Vbm1/Elo3 and Vbm2/Elo2 Localize to the ER
A likely site of action for Vbm1/2 would be on the separate vesicle populations, however, LCFA, ceramide, and sphingolipid synthesis have been proposed to occur principally at the level of the ER (reviewed by Lester and Dickson, 1993). Thus, it was necessary to determine where Vbm1/Elo3 and Vbm2/Elo2 reside in yeast and whether they are present on secretory vesicles. Elo1, a homologous protein involved early in LCFA synthesis, was previously noted to have a KKXX motif at the −6 position from the COOH terminus, which was proposed to act as a possible ER-retention signal (Toke and Martin, 1996). Interestingly, Vbm1/Elo3 has three consecutive putative KKXX motifs beginning at positions −37, −33, and −29 from the COOH terminus. This motif is lacking in Vbm2/Elo2, however, several of the lysines are positionally conserved and a string of basic residues (HRRKR) is present.
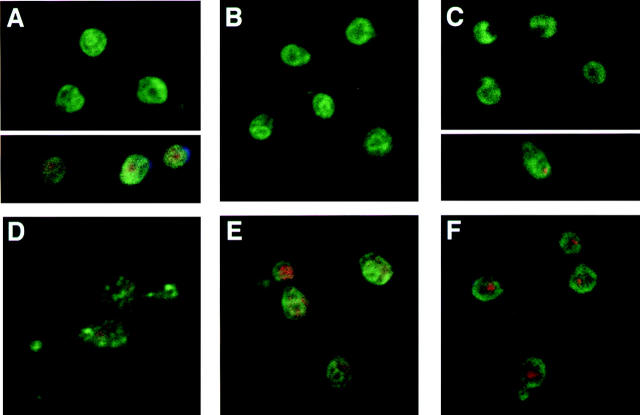
To test whether the Vbm proteins could be ER- or Golgi-localized, or whether they are present on secretory vesicles, we created tagged forms of the proteins, Vbm1HA and Vbm2HA, which bear an HA epitope at their COOH termini and which complement snc vbm1 and snc vbm2 cells, respectively (Fig. 7 B and data not shown). By immunofluorescence, we found that both Vbm1HA and Vbm2HA localize to areas in the cell that correspond to the ER, when expressed from either single-copy or multi-copy vectors (Fig. 8 A and data not shown). The labeling of Vbm1HA (Fig. 8 A) and Vbm2HA (Fig. 8 B) was peripheral to the nucleus, yielding ring-like structures that corresponded to that of a typical ER resident protein, Dpm1 (Fig. 8 C). Moreover, the labeling of Vbm1 or 2 was neither similar to, nor could colocalize extensively with, the Golgi marker Mnn1 (Fig. 8, D and E, respectively). Finally, Vbm proteins did not show a staining pattern corresponding to Sso t-SNAREs (Fig. 8 F), which serve as markers of the plasma membrane.
Figure 8.
Localization of Vbm proteins by indirect immunofluorescence and confocal microscopy. sec9-4 cells expressing Vbm1-HA from a multi-copy plasmid (a, c, d, and e) and Vbm2-HA from a single-copy plasmid (b) were grown under permissive conditions and fixed for immunofluorescence. Cells were labeled with preadsorbed, affinity-purified anti-HA (1:1,000; a, b, and e), anti-Dpm1 (3.3 μg/ml; c), anti-Mnn1 (1:10; d and e), or anti-Sso (1:100; f) antisera. FITC-conjugated goat anti–mouse (a, b, c, and e) and anti–rabbit (d and f), as well as rhodamine-conjugated goat anti–rabbit (e) secondary antibodies were used. Nuclear staining with propidium iodide is shown in a (beneath white line), d, and f. Cells were visualized by confocal microscopy.
Subcellular fractionation also supports the probability of ER localization. Fractionated temperature-shifted sec9-4 cell preparations (which yield both LDSV and HDSV populations), show that neither Vbm1HA nor Vbm2HA copurified to a large extent with the vesicles, but eluted primarily in the initial fractions of the gradient (data not shown). This corresponded to the localization of various resident ER and Golgi proteins: Kar2 (Harsay and Bretscher, 1995), Wbp1, Sec22 (an ER-Golgi v-SNARE), Mnn1, and Emp47 (data not shown). In contrast, the Snc v-SNAREs and plasma membrane proteins, Sso1/2 and Gas1, and the secreted protein, Hsp150, were found to fractionate along the entire length of the gradient, and were enriched in the later fractions (data not shown).
The results from these complimentary strategies suggest a probable ER and, perhaps, early Golgi localization for the Vbm proteins. Given this, and our inability to detect significant amounts of the proteins on the secretory vesicles, suggests that their actions upon vesicle docking and fusion are probably exerted in a distal fashion.
v-SNARE Bypass Mutants Show Altered Levels of PI and Sphingolipids
We next determined whether the vbm mutations isolated in our genetic screen also affect the synthesis of sphingolipid. To do so, we labeled yeast with 3H-myo-inositol, extracted the cellular lipid, and separated the inositol-containing lipids (phosphoinositides and sphingolipids) by thin-layer chromatography. The results from a representative experiment are shown in Fig. 9.
Figure 9.
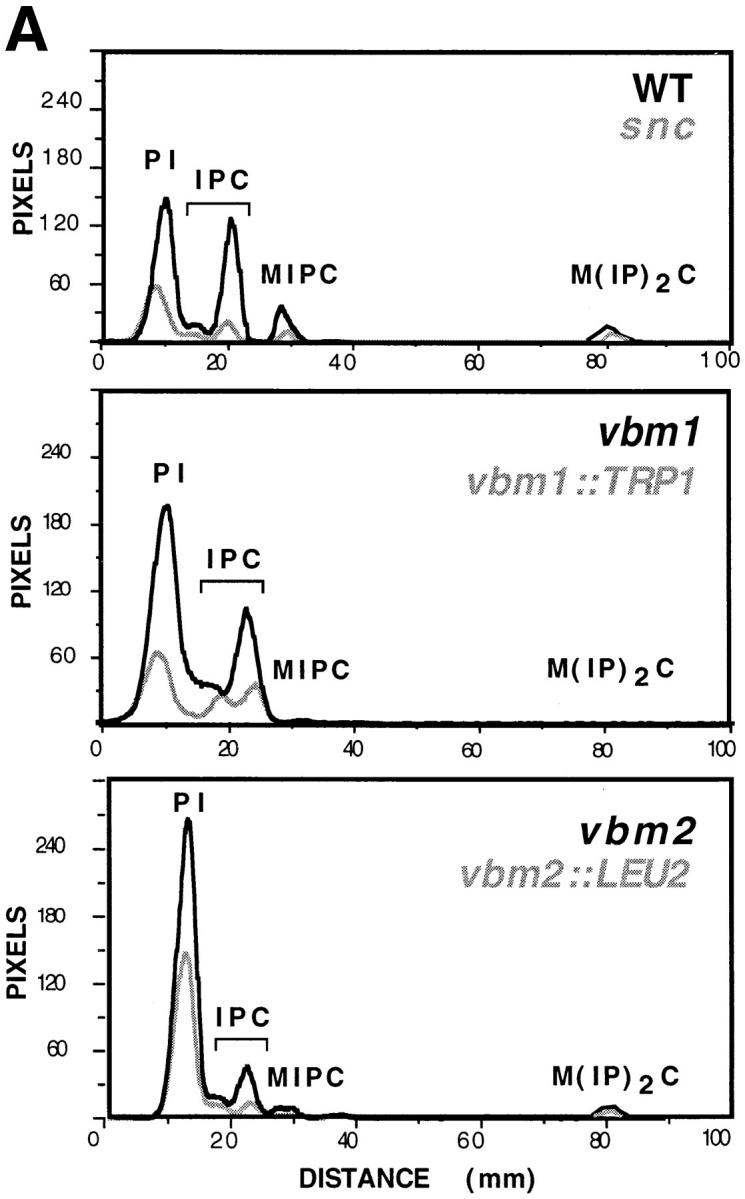
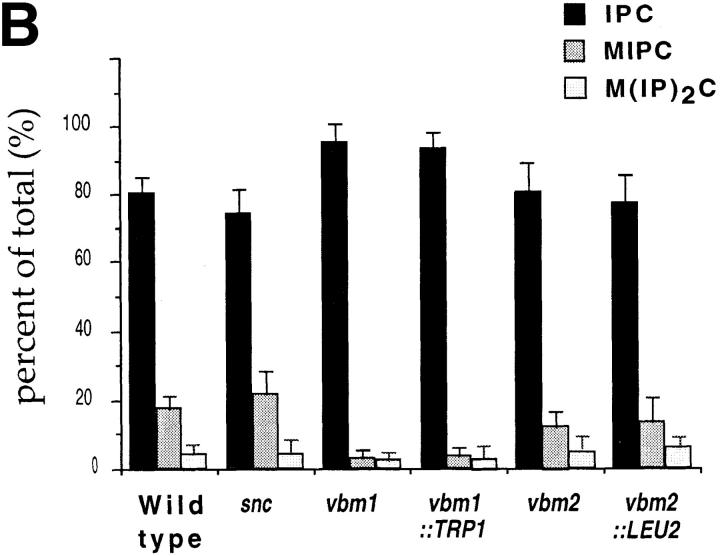
Sphingolipid synthesis is deficient in v-SNARE bypass mutants. Yeast strains including: SP1 wild-type cells; JG8 snc null cells; JG8R-A14 snc vbm1 cells; JG8R-α10 snc vbm2 cells; DD1 snc vbm1:: TRP1 cells; and SS1 snc vbm2::LEU2 cells were labeled with [3H]myo-inositol, as described under Materials and Methods. Aliquots from extracted cells were separated by thin-layer chromatography and visualized by phosphorimager analysis. A Shows the quantitative tracing of a representative experiment, where PI indicates phosphatidylinositides; IPC indicates inositolphosphorylceramide; MIPC indicates mannose inositolphosphorylceramide; and M(IP)2C indicates mannose (inositolphosphoryl)2-ceramide. B Shows the relative percentages of sphingolipid within a given strain, as determined from three or four similar experiments. Error bars indicate the standard error of the mean.
First, the level of phosphoinositide (PI) synthesized in snc cells was <40% of wild-type cells (Fig. 9 A). This probably reflects the reduced cellular metabolism and growth rate of snc cells (Protopopov et al., 1993; Gerst, 1997; data not shown). In contrast, snc cells bearing recessive mutations in the VBM genes, which were isolated in the VBM screen, showed elevated levels of PI. PI levels in vbm1 and vbm2 cells were 40 and 80% higher, respectively, than those of wild-type cells and at least three- to fourfold higher than those of snc null cells. This initially suggested that PI levels might influence the viability of snc yeast. However, we found that snc yeast bearing targeted disruptions in the VBM genes, while phenotypically identical to the vbm recessive mutants (Fig. 7, A and B), did not have greatly elevated levels of PI (Fig. 9 A and data not shown). In four similar experiments, we found that the levels of PI tended to be twofold over the levels of snc yeast, but 20% less than that of wild-type cells (data not shown). These differences indicated that the vbm mutations isolated by genetic screening behave somewhat differently than the targeted gene knockouts and may not fully inactivate Vbm function.
The levels of sphingolipid synthesized in snc cells were also reduced vis a vis wild-type cells. Reductions in IPC, MIPC, and M(IP)2C levels by ∼60% were detected (Fig. 9 A), although no significant change in the ratio of IPC to MIPC or M(IP)2C, was seen relative to wild-type cells (the ratios in both cell types being ∼4:1 and 25:1, respectively; n = 4 experiments; Fig. 9 B). This reduction in sphingolipid synthesis also corresponds with the reduced metabolism and growth of the snc null strain.
Mutation in, or disruption of, either ELO/VBM gene was found to affect the levels of sphingolipid in both wild-type and snc yeast (Oh et al., 1997 and this study). As first shown by Oh et al. (1997), the levels of inositol sphingolipids are reduced significantly by the disruption of either ELO2 or ELO3 in wild-type cells, although the amount of IPC remained at wild-type levels or higher in elo3Δ cells. In the snc background, we also found that the levels of IPC were elevated as a result of either vbm1 mutation (>400%) or vbm1::TRP1 deletion (+130%; Fig. 9 A), and constituted >90% of the bulk sphingolipid present (n = 4 experiments; Fig. 9 B). In contrast, the amount of MIPC and M(IP)2C was almost undetectable (<5% of that found in wild-type and snc cells; Fig. 9 A). The IPC:MIPC ratio in vbm1 and vbm1::TRP1 cells was found to be 52:1 and 31:1, respectively, in this experiment. Thus, mutations in VBM1/ELO3 appear to result in the accumulation of IPC in the cell, probably at the expense of the mannosylated sphingolipids, MIPC and M(IP)2C. This, along with the work of Oh et al. (1997), suggests that the C26 LCFAs may be necessary for the generation of mannosylated sphingolipids.
Similar to that shown by Oh et al. (1997) for the disruption of ELO2, we also found that mannosylated sphingolipids, MIPC and M(IP)2C, were present in lipid extracts from vbm2 and vbm2::LEU2 cells. Their levels were <40% of wild-type levels, but were fourfold higher (on average) than those of the vbm1 and vbm1::TRP1 strains (n = 3 experiments; Fig. 9 B). Correspondingly, there was a decrease in the level of IPC by >60 and 85% in the vbm2 and vbm2::LEU2 strains, respectively, relative to wild-type cells (Fig. 9 A). The IPC:MIPC ratio in vbm2 and vbm2:: LEU2 cells was 7:1 and 6:1, respectively; n = 4 experiments.
These experiments suggest that snc vbm1 and snc vbm2 mutants have elevated levels of PI and reductions in certain sphingolipids, relative to wild-type cells. The effects upon sphingolipid levels are highly similar to those shown previously for the disruption of ELO2 or 3 in wild-type cells and also by us for their disruption in sec6-4 and SP1 wild-type cells (Sundarababu, S., and J.E. Gerst, unpublished results). More importantly, they imply that the ability of the recessive mutations to confer the v-SNARE bypass effect is related to the role of the Vbm proteins in LCFA/sphingolipid synthesis and not via an unrelated function.
Deletion of Either VBM1 or VBM2 in Wild-type or sec6 Cells Does Not Significantly Alter Protein Trafficking
Because mutations in the VBM/ELO genes affect protein trafficking in cells lacking the Snc v-SNAREs, it was of importance to determine whether they also affect protein trafficking and secretion in either wild-type or sec yeast. We first examined whether disruption of the VBM/ELO genes affected cell growth. According to the study of Oh et al. (1997), these disruptions reduced the rate of cell growth somewhat. In contrast, we were unable to see any significant difference in the growth rates of either wild-type or sec6 cells bearing disruptions in either VBM1/ELO3 or VBM2/ELO2, nor was the temperature sensitivity in the sec6 background affected (data not shown). Some minor morphological changes were noted and will be described elsewhere.
To test whether protein trafficking in these cells is affected, we examined the secretion of invertase and media proteins, as well as, the processing of CPY, invertase, and Gas1 therein. We found that invertase secretion from cells bearing VBM1/ELO3 or VBM2/ELO2 disruptions in either the wild-type or sec6 background was identical to control wild-type or sec6 cells, respectively (data not shown). In temperature-shifted cells (37°C), no blockage or alteration in the intracellular accumulation of invertase was detected. Likewise, disruption of VBM1/ELO3 or VBM2/ ELO2 in the wild-type or sec6 backgrounds did not alter the secretion of proteins into the medium, as shown by metabolic labeling studies (data not shown). Finally, the processing of CPY and invertase in these cells was identical to that shown for wild-type cells (Fig. 2, A and B), whereas that of Gas1 was delayed, as shown above for snc vbm cells (data to be presented elsewhere). Thus, disruption of the individual VBM genes in either a wild-type or sec6 background had no deleterious effects upon general protein processing and secretion.
At restrictive conditions (37°C), secretory vesicles were found to accumulate in sec6 cells bearing disruptions in either VBM1/ELO3 or VBM2/ELO2, as assessed by electron microscopy (Shinder, V., and J.E. Gerst, data not shown). Density gradients performed using membranes derived from temperature-shifted sec6 vbm1::LEU2 and sec6 vbm2::LEU2 cells did not reveal significant changes in the biochemical identity and distribution of the LDSV and HDSV populations (data not shown). Thus, we suggest that disruption of VBM1 or VBM2 in a wild-type background does not alter vesicle biogenesis or protein secretion.
In contrast, we found that disruption of either VBM1/ ELO3 or VBM2/ELO2 in the snc null background did ameliorate protein secretion and processing in the same manner as described above for the snc vbm cells isolated in the genetic screen (data to be presented elsewhere). This result was predicted from the genetic complementation and inositol labeling experiments shown above (Figs. 7 and 9, respectively).
v-SNARE Bypass Mutants Do Not Bypass the t-SNARE Requirement in Exocytosis
To determine whether VBM cells bypass the known t-SNARE requirement in secretion, we disrupted either VBM1 or VBM2 in yeast lacking both Snc v-SNAREs and the Sso1 t-SNARE (Aalto et al., 1993), but bearing a temperature-sensitive mutation in the other syntaxin homologue, Sso2 (Aalto et al., 1993). Previously, we found that cells bearing both snc null mutations and a temperature-sensitive mutation either in SEC9 (sec9-4) or SEC17 (sec17-1) are inviable, indicating that a fully functional Sec9 t-SNARE and Sec17 are required in yeast lacking the Snc v-SNAREs (Couve and Gerst, 1994; Gerst, 1997). For this study, we found that combined snc null and sso1Δ sso2-1 mutations also lead to inviability (synthetic lethality), which is suppressed by the expression of SSO1, SSO2, or SNC2 (Fig. 10). Thus, results obtained here with the mutant Sso2 t-SNARE are identical to those shown previously for the mutant Sec9 t-SNARE and Sec17 α-SNAP homologue.
Figure 10.
t-SNARE dependence of v-SNARE bypass mutants. snc sso1Δ sso2-1 cells transformed with a control plasmid (control) or with plasmids expressing SSO1 or SSO2 under control of the ADH1 promoter (ADH-SSO1 and ADH1-SSO2) were tested for viability on synthetic medium containing galactose (SC GAL) or glucose (SC GLU), or on amino acid–rich medium (YPD). snc sso1Δ sso2-1 cells bearing a disruption of VBM1 (snc sso1Δ sso2-1 vbm1::TRP1) were tested alongside. All cells carry the pAHGAL-SNC2 plasmid, which expresses SNC2 under the control of a galactose-inducible promoter, and maintains the cellular viability of the snc sso1Δ sso2-1 strain on galactose-containing medium. Cells were grown on SC GAL at room temperature before replica plating onto SC GAL, SC GLU, and YPD for 2 d at 26°C.
We conjectured that if vesicle trafficking in snc vbm cells is conferred in a t-SNARE-independent fashion, then the Sso t-SNAREs should be dispensable for function. To test this hypothesis, either VBM1 or VBM2 were disrupted in sncΔ sso1Δ sso2-1 cells, which bear a plasmid that expresses SNC2 from a galactose-inducible promoter and which were maintained on galactose. Next we examined the viability of these yeast by replica plating patches of cells onto glucose-containing plates (Fig. 10). We found that sncΔ sso1Δ sso2-1 vbm1Δ (Fig. 10) and sncΔ sso1Δ sso2-1 vbm2Δ (not shown) cells remained inviable in the absence of SNC2 gene expression. Thus, an intact and fully functional Sso protein is indispensable in snc vbm cells, implying that vesicle docking and fusion therein is t-SNARE dependent.
Discussion
Are v-SNAREs Essential for Exocytosis?
According to the SNARE hypothesis, interactions between v-SNAREs on vesicles and t-SNAREs on target membranes result in SNARE complex formation, allowing for membrane fusion to occur (reviewed in Rothman and Warren, 1994). Therefore, mutations that result in the inactivation, or loss of expression, of either v- or t-SNAREs should block membrane fusion at their specific sites of action. In support of this tenet, it was shown that deletions in yeast genes encoding the individual v- and t-SNAREs involved in ER-Golgi transport (i.e., Bet1, Bos1, and Sed5), as well as, the t-SNAREs involved in exocytosis (i.e., Sec9, Sso1 and Sso2 together) result in lethality (Dascher et al., 1991; Shim et al., 1991; Newman et al., 1992; Hardwick et al., 1992; Brennwald et al., 1994; Aalto et al., 1993). Studies on higher organisms also tend to support this idea. For example, the targeted expression of tetanus toxin in Drosophila embryos results in the loss of evoked neurotransmitter release, presumably resulting from the inactivation of one of the synaptobrevin isoforms (n-syb; Sweeny et al., 1995). The presence in these embryos of syb-a, an ubiquitously expressed isoform, as well as synaptotagmin, a synaptic vesicle protein that bears v-SNARE-like qualities (Schiavo et al., 1995, 1997) may prevent defects in embryogenesis from occurring.
In contrast to the idea that v-SNAREs are essential, we have characterized yeast that bypass the v-SNARE requirement in constitutive secretion. Yeast lacking the SNC genes, which encode redundant v-SNAREs of the exocytic pathway, were originally shown to be viable under certain conditions (Protopopov et al., 1993). This suggested that they are required only for efficient exocytosis, or that an unknown v-SNARE is present on secretory vesicles. The latter possibility seems remote for several reasons. First, other than SNC1 or SNC2, no other v-SNARE was isolated in multicopy suppression screens of the snc phenotype. Second, overexpression of other yeast v-SNAREs could not suppress the defects in snc yeast. Third, deletion of the SNC genes in certain wild-type strains was found to be lethal (Gerst, J.E., unpublished data). The differences between strain backgrounds are unclear, however, a similar phenomenon was demonstrated for the CHC1 gene, which encodes clathrin heavy chain (Payne and Schekman, 1985; Lemmon and Jones, 1987; Munn et al., 1991). Since snc null cells show a conditional lethal phenotype and are restored to full viability by recessive (and inactivating) mutations, it seems likely that the exocytic v-SNAREs are not essential for secretion, but do allow for more efficient vesicle docking and fusion.
This idea is supported by studies on vacuolar membrane fusion that show that the v-SNARE (Nyv1) is dispensable, in part, for the formation of vacuoles in vivo and membrane fusion in vitro (Nichols et al., 1997). It is also supported by a recent study that shows that the Ufe1 t-SNARE is sufficient to confer ER membrane fusion (Patel et al., 1998). In this study, we find that mutations in the VBM genes allow for the bypass of the v-SNARE requirement in exocytosis. However, they do not confer a bypass of the t-SNARE requirement, as a fully functional Sso protein is required for viability (Fig. 10). This suggests that a protein with SNARE-like functions may be present on secretory vesicles and can partner efficiently with Sec9 and Sso, in the absence of Snc protein. Based upon the studies of Nichols et al. (1997) and Patel et al. (1998), the likely candidates for such a function are the Sso t-SNAREs themselves, although this remains to be formally proven. Alternatively, missorting of a v-SNARE originating from an earlier compartment might also be capable of conferring fusion, provided it could interact productively with the Sso proteins.
VBM1 and 2 Encode ER-localized Membrane Proteins That Act upon LCFA, Ceramide, and Sphingolipid Synthesis
In our search for genes that restore secretion and normal cell growth in snc cells, we isolated revertants that bear mutations in genes designated here as VBM1 and VBM2. These genes were characterized by another group (designated therein as ELO2 and 3) and were shown to encode proteins involved in the elongation of LCFAs and subsequent synthesis of inositol-containing sphingolipids (Oh et al., 1997). We have shown that these Vbm/Elo proteins localize to the ER (Fig. 8), the proposed site of ceramide and sphingolipid synthesis, and that their inactivation leads to the v-SNARE-bypass phenotype (Fig. 7). Thus, we demonstrate a genetic relationship between genes involved in ceramide/sphingolipid metabolism and the regulation of cellular secretion.
Earlier studies implicated these same genes in the regulation of membrane trafficking. For example, mutations in SUR4 were shown originally to suppress defects that result from the disruption of the RVS161/END6 gene in yeast (Crouzet et al., 1991; Desfarges et al., 1993; Munn et al., 1995). The gene product from RVS161/END6 is involved in endocytosis and is homologous to amphiphysin (David et al., 1994; Sivadon et al., 1995), a vesicle-associated protein that participates in synaptic vesicle endocytosis in mammals (Schupiakov et al., 1997). How mutations in SUR4/VBM1/ELO3 restore the viability of rvs161 mutants remains unknown, but it suggests a possible role for Sur4/ Vbm1/Elo3 in the endocytic trafficking of vesicles. Additional support for the idea that ceramide and sphingolipid synthesis may play a role in endocytosis is the finding that another end mutant, end8, is allelic to the lcb1 gene, that encodes the serine palmitoyltransferase activity that contributes to sphinganine and ceramide synthesis (Sütterlin et al., 1997). Thus, other studies using yeast have implicated these lipids in essential trafficking events.
The precise mechanism by which inactivating mutations in the VBM/ELO genes restore cellular secretion from snc cells remains unclear. Since Vbm1/2 localize to the ER and not to secretory vesicles, and were shown to act upon LCFA and sphingolipid synthesis (Oh et al., 1997 and this study), they appear to mediate the docking and fusion of secretory vesicles in a distal fashion. It is not clear whether such regulation exists in wild-type cells or is revealed only in the context of the snc null background, where the fusion competence of vesicles is challenged. Our examination of VBM1/ELO3 or VBM2/ELO2 gene disruption in wild-type or sec6 cells indicates, thus far, that the secretion of invertase and media proteins is not significantly affected, and does not block biogenesis of the two types of vesicles (data not shown).
On the basis of these results, we suggest that reduced functioning of the Vbm/Elo proteins is necessary and sufficient to lead to the v-SNARE bypass phenotype and a restoration in secretion (Fig. 1). This occurs without adversely affecting the transport of vacuolar and certain secreted proteins early in the pathway (Fig. 2). Moreover, electron microscopy (Figs. 3 and 4) and vesicle purification studies (Figs. 5 and 6), go so far as to suggest that each vbm mutation might preferentially recouple transport of one of the secretory vesicle populations found in yeast lacking the Snc v-SNAREs. This idea is supported by initial studies of VBM/ELO gene disruption in sec6 cells, which do not show defects in vesicle biogenesis. Should this be borne out by further examination, then alterations in cellular LCFA and, perhaps, sphingolipid composition can be said to have a direct and profound effect upon the fusion competence of the two classes of secretory vesicles.
Labeling experiments performed with [3H]inositol demonstrate that snc vbm cells have reduced levels of certain sphingolipids (Fig. 9). This was shown originally for the disruption of ELO2 or 3 in wild-type yeast (Oh et al., 1997 and data not shown). Thus, mutations in the VBM/ELO genes appear to yield the same effect upon sphingolipid synthesis, irrespective of whether the SNC genes are present or not. The study by Oh et al. (1997) also found that fatty acid chain length was altered as a result of mutation in either ELO gene. In elo2Δ cells, saturated and hydroxy C26-fatty acids were present, whereas in elo3Δ, cells saturated and hydroxy C22-fatty acids were prominent. Because the effects of VBM/ELO mutation upon sphingolipid synthesis are independent of strain background, we expect that chain length is affected similarly in snc vbm cells. Thus changes, or a series of changes, in fatty acid chain length, ceramide and sphingolipid composition, may result in the enhanced trafficking of one type of vesicle (and its cargo), relative to the other, in snc vbm cells. While unresolved at present, the possible levels at which chain length and sphingolipid composition might alter protein trafficking include: (a) the sorting of protein cargo into vesicles at either early or late stages of the secretory pathway (e.g., from the ER or trans Golgi); (b) regulation of protein–protein interactions in the sphingolipid-containing bilayers; and (c) regulation of the physical characteristics of the lipid bilayers, such as to either enhance or impede fusion.
That sphingolipids participate in the selective sorting of cargo to yield vesicles that differ in protein composition has interesting parallels with higher eukaryotes. In mammalian cells, for example, sphingolipids have been suggested to form separate entities involved in the apical trafficking of proteins (see review by Simons and Ikonen, 1997). In yeast, others have demonstrated that sphingolipid synthesis is required for the sorting of GPI-anchored proteins from the ER to the Golgi (Horvath et al., 1994; Skrzypek et al., 1997). This may explain why the processing of Gas1, but not CPY or invertase, is slowed in the snc vbm mutants, which are deficient in sphingolipid synthesis (Fig. 2). It may also explain, in part, why the densities and contents of the vesicle types in snc vbm cells differ somewhat from those found in sec6 or snc cells. These differences could, potentially, result in vesicles having an altered ability to recruit protein cargo. This alone might lead to a reduction in fusion competence, if a SNARE or a protein that regulates SNARE assembly was not recruited efficiently.
An alternative explanation is that the vbm mutations block biogenesis of one of the vesicle types. Our view is that this possibility is less likely. First, a distinct vesicle population accumulates in either snc vbm mutant (Figs. 1, 3, 4, and 6), thus, another population must undergo recoupled transport in order to restore normal growth to snc cells. Second, invertase undergoes maturation in vbm1 cells (Fig. 2), which would not be expected if it were accumulating within the Golgi complex. Third, both populations of vesicles were found to exist in sec6 yeast that bore disruptions in either VBM1 or VBM2 (data not shown).
There are other possible explanations, including the idea that LCFAs and their products, ceramides and sphingolipids, alter the sorting or functioning of proteins involved in vesicle docking and fusion (i.e., SNAREs). These effects could be exerted at the structural level and alter the physical properties of membranal proteins. For example, fatty acid chain length might significantly affect membrane thickness, thus, altering protein sorting and, perhaps, even function. As evidence for the former, it has been shown that the length of membrane-spanning domains can be an important determinant for organelle retention (Munro, 1995; Rayner and Pelham, 1997). According to the lipid-based sorting model (reviewed in Munro, 1998), a lowered capacity for sphingolipid synthesis might result in membrane thinning and, as a result, alter sorting of proteins destined for the plasma membrane, as well as those normally retained by the Golgi complex. Similarly, we cannot rule out the possibility that ceramides act upon signaling processes that effect vesicle transport and fusion. For example, ceramides have been shown to play a role in viral entry and membrane fusion (Nieva et al., 1994), while ceramide analogues have been shown to reduce fluid-phase and receptor-mediated uptake of markers in fibroblasts (Chen et al., 1993) and in yeast (Riezman et al., 1998), suggesting a probable role in endocytosis. Moreover, work in progress in our laboratory suggests at least a partial role for ceramides in mediating the VBM effect (Marash, M., and J. Gerst, unpublished results). Finally, a role for LCFAs in the nuclear transport of mRNA and maintenance of the nuclear envelope, as well as, in mitochondrial inheritance has been suggested (reviewed in Schneiter and Kohlwein, 1997). Therein, it was speculated that LCFAs themselves might modulate membrane rigidity and curvature. Thus, more work will be necessary in order to define the different possible roles that LCFAs and their metabolites play in membrane trafficking.
Alternate Paths to the Cell Surface in Yeast
Work by Harsay and Bretscher (1995) and us suggest that the secretory pathway in yeast is bifurcated and that at least two separate routes to the cell surface are used. Here, we show that the VBM genes may be involved in the regulation of the two constitutive routes, perhaps by helping to define the conditions for protein sorting along the alternate paths. Thus, the existence of two types of secretory vesicles, which differ significantly both in nature and cargo molecule content, implies that a high degree of specificity is operant in the trafficking of proteins to the cell surface. These two populations: HDSVs, which are rich in soluble secreted enzymes, and LDSVs, which contain a membranal H+-ATPase, reflect the distinct sorting determinants (either proteinaceous or lipid) for the individual cargo molecules. Alternatively, we should consider the idea that these vesicles may originate from distinct post-Golgi compartments (i.e., one from the trans-Golgi and the other from an intermediate or endosomal compartment). Thus, they may represent two pools of trafficked proteins, one purely of biosynthetic origin, while the other contributing components recycled from the plasma membrane.
The results from these studies suggest that preferential routes are used by individual proteins, e.g., invertase, which accumulates in HDSVs and in the vesicles found in snc vbm1 cells. Yet, this may not be true for membrane proteins that are required for essential functions. Presumably, the viability of both snc vbm1 and snc vbm2 cells implies that proteins essential for growth (i.e., amino acid transporters) reach the cell surface via either route, while nonessential cargo may be trafficked more specifically. Given the robust condition of either vbm mutant, this hypothesis would seem reasonable and is supported by the finding that proteins are secreted into the medium equally well by either mutant (Fig. 1 B).
Although much remains unanswered, our results suggest that protein delivery to the cell surface is far more regulated and specific than previously described. This added level of regulation may have evolved during bifurcation of the late secretory pathway early in eukaryote evolution and may have given rise to the multiple trafficking paths (i.e., apical/basolateral and constitutive/stimulus-coupled) found in multicellular organisms.
Acknowledgments
The authors are grateful to Zvulun Elazar, Scott Emr, Tony Futterman, Erin Gaynor, Edina Harsay, Charles Martin, Eyal Schejter, and Ben-Zion Shilo for helpful advice; Patrick Brennwald, Tamara Doering, Scott Emr, Erin Gaynor, Todd Graham, Peter Novick, Howard Riezmann, Hans Ronne, David Stillman, Randy Schekman, Talila Volk, and Michael Wigler for reagents. Special thanks to Andres Couve, Su-Oh Ham, Vered Lavi, Vardit Lustgarten, Michael Marash, and Vera Shinder for technical help.
Abbreviations used in this paper
- HDSV
high density secretory vesicles
- IPC
inositol-phosphorylceramide
- LCFA
long chain fatty acid
- LDSV
low density secretory vesicles
- MIPC
mannose inositol-phosphorylceramide
- M(IP)2C
mannose (inositolphosphoryl)2-ceramide
- NEM
N-ethylmaleimide
- NSF
NEM-sensitive fusion protein
- PI
phosphoinositide
- SC
synthetic minimal medium
- SNAP
soluble NSF attachment protein
- SNARE
SNAP receptor
- VAMP
vesicle-associated membrane protein
- VBM
v-SNARE bypass mutants
- YPD
yeast extract/peptone/dextrose
Footnotes
J.E. Gerst is the recipient of an Allon Fellowship, grants from the Forchheimer Center for Molecular Genetics; the Israel Science Foundation (Dorot Science Fellowship); and the Minerva Foundation, Germany. J.E. Gerst is holder of the Henry Kaplan Chair in Cancer Research.
D. David and S. Sundarababu contributed equally to this study.
Address all correspondence to Jeffrey E. Gerst, Department of Molecular Genetics, Weizmann Institute of Science, Rehovot 76100, Israel. Tel.: 972 8 9342106. Fax: 972 8 9344108. E-mail: lvjeff@weizmann.weizmann.ac.il
References
- Aalto MK, Ronne H, Keränen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO (Eur Mol Biol Organ) J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN. Assay of inorganic phosphate, total phosphate, and phosphatases. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- Baumert M, Maycox PR, Navone F, DeCamilli P, Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicle of rat brain. EMBO (Eur Mol Biol Organ) J. 1989;8:379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Scheller RH. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman BJ, Slayman CW. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. . J Biol Chem. 1979;254:2928–2934. [PubMed] [Google Scholar]
- Brennwald P, Kerns B, Champion K, Keränen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be an effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Chen CS, Rosenwald AG, Pagano RE. Ceramide as a modulator of endocytosis. J Biol Chem. 1993;270:13291–13297. doi: 10.1074/jbc.270.22.13291. [DOI] [PubMed] [Google Scholar]
- Couve A, Gerst JE. Yeast Snc proteins complex with Sec9: functional interactions between putative SNARE proteins. J Biol Chem. 1994;269:23391–23394. [PubMed] [Google Scholar]
- Couve A, Protopopov V, Gerst JE. Yeast synaptobrevin homologs are modified post-translationally by palmitate addition. Proc Natl Acad Sci USA. 1995;92:5987–5991. doi: 10.1073/pnas.92.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet M, Urdaci M, Dulau L, Aigle M. Yeast mutant affected for viability upon nutrient starvation: characterization and cloning of the RVS161gene. Yeast. 1991;7:727–743. doi: 10.1002/yea.320070708. [DOI] [PubMed] [Google Scholar]
- Dascher C, Ossig R, Gallwitz D, Schmitt HD. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C, Solimena M, De Camilli P. Autoimmunity in Stiff-Man Syndrome with breast cancer is targeted to the C-terminal region of human amphiphysin, a protein similar to the yeast proteins, Rvs167 and Rvs161. FEBS Letts. 1994;351:73–79. doi: 10.1016/0014-5793(94)00826-4. [DOI] [PubMed] [Google Scholar]
- Desfarges L, Durrens P, Juguelin H, Cassagne C, Bonneu M, Aigle M. Yeast mutants affected in viability upon starvation have a modified lipid composition. Yeast. 1993;9:267–277. doi: 10.1002/yea.320090306. [DOI] [PubMed] [Google Scholar]
- El-Sherbeini M, Clemas JA. Cloning and characterization of GNS1: a Saccharomyces cerevisiaegene involved in synthesis of 1,3-β-glucan in vitro. J Bacteriol. 1995;177:3227–3234. doi: 10.1128/jb.177.11.3227-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink LA, Trimble WS, Scheller RH. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989;264:11061–11064. [PubMed] [Google Scholar]
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- García-Arranz M, Maldonado AM, Mazón MJ, Portillo F. Transcriptional control of yeast plasma membrane H+-ATPase by glucose. J Biol Chem. 1994;269:18076–18082. [PubMed] [Google Scholar]
- Gaynor EC, Emr SD. COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst JE, Ferguson K, Vojtek A, Wigler M, Field J. CAP is a bifunctional component of the Saccharomyces cerevisiaeadenylyl cyclase complex. Mol Cell Biol. 1991;11:1248–1257. doi: 10.1128/mcb.11.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst JE, Rodgers L, Riggs M, Wigler M. SNC1, a yeast homolog of the synaptic vesicle-associated membrane protein/synaptobrevin gene family: genetic interactions with the RAS and CAPgenes. Proc Natl Acad Sci USA. 1992;89:4338–4342. doi: 10.1073/pnas.89.10.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst JE. Conserved α-helical segments on yeast homologs of the synaptobrevin/VAMP family of v-SNAREs mediate exocytic function. J Biol Chem. 1997;272:16591–16598. doi: 10.1074/jbc.272.26.16591. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Lampen JO. β-d-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Pelham HRB. SED5encodes a 39-KD integral membrane protein required for vesicular transport between the ER and Golgi complex. J Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Sutterlin C, Manning-Krieg W, Movva NR, Riezman H. Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO (Eur Mol Biol Organ) J. 1994;13:3687–3695. doi: 10.1002/j.1460-2075.1994.tb06678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon SK, Jones EW. Clathrin requirement for the normal growth of yeast. Science. 1987;238:504–509. doi: 10.1126/science.3116672. [DOI] [PubMed] [Google Scholar]
- Lester RL, Dickson RC. Sphingolipids with inositolphosphate-containing head groups. Adv Lipid Res. 1993;26:253–274. [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion in yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Munn AL, Silveira L, Elgort M, Payne GS. Viability of clathrin heavy chain-deficient Saccharomyces cerevisiaeis compromised by mutations at numerous loci: implications for the suppressor hypothesis. Mol Cell Biol. 1991;11:3868–3878. doi: 10.1128/mcb.11.8.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn AL, Stevenson BJ, Geli MI, Riezman H. end5, end6, end7: mutations that cause actin delocalization and block the internalization step in endocytosis in Saccharomyces cerevisiae. . Mol Biol Cell. 1995;6:1721–1742. doi: 10.1091/mbc.6.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. An investigation of the role of transmembrane domains in Golgi protein retention. EMBO (Eur Mol Biol Organ) J. 1995;14:4695–4704. doi: 10.1002/j.1460-2075.1995.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Localization of proteins to the Golgi apparatus. Trends Cell Biol. 1998;8:11–15. doi: 10.1016/S0962-8924(97)01197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AP, Groesch M, Ferro-Novick S. Bos1p, a membrane protein required for ER to Golgi transport in yeast, copurifies with the carrier vesicles and with Bet1p and the ER membrane. EMBO (Eur Mol Biol Organ) J. 1992;11:3609–3617. doi: 10.1002/j.1460-2075.1992.tb05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HRB, Wickner WT, Haas A. Homotypic fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Nieva JL, Bron R, Corver J, Wilschut J. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO (Eur Mol Biol Organ) J. 1994;13:2797–2804. doi: 10.1002/j.1460-2075.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Oh C-S, Toke DA, Mandala S, Martin CE. ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1gene, function in fatty acid elongation and are required for sphingolipid formation. J Biol Chem. 1997;272:17376–17384. doi: 10.1074/jbc.272.28.17376. [DOI] [PubMed] [Google Scholar]
- Patel SK, Indig FE, Olivieri N, Levine ND, Latterich M. Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membrane fusion. Cell. 1998;92:611–620. doi: 10.1016/s0092-8674(00)81129-0. [DOI] [PubMed] [Google Scholar]
- Payne GS, Schekman R. A test of clathrin function in protein secretion and cell growth. Science. 1985;230:1009–1014. doi: 10.1126/science.2865811. [DOI] [PubMed] [Google Scholar]
- Protopopov V, Govindan B, Novick P, Gerst JE. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. . Cell. 1993;74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Rayner JC, Pelham HR. Transmembrane domain-dependent sorting of proteins to the ER and plasma membrane in yeast. EMBO (Eur Mol Biol Organ) J. 1997;16:1832–1841. doi: 10.1093/emboj/16.8.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H, Woodman PG, van-Meer G, Marsh M. Molecular mechanisms of endocytosis. Cell. 1997;91:731–738. doi: 10.1016/s0092-8674(00)80461-4. [DOI] [PubMed] [Google Scholar]
- Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, M.D., F. Winston, and P. Hieter. 1990. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 198 pp.
- Rossi G, Salminen A, Rice L, Brünger AT, Brennwald P. Analysis of a yeast SNARE complex reveals remarkable similarity to the neuronal SNARE complex and a novel function for the C-terminus of the SNAP-25 homolog, Sec9. J Biol Chem. 1997;272:16610–16623. doi: 10.1074/jbc.272.26.16610. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Santos T, del Rey F, Conde J, Villanueva JR, Nombela C. Saccharomyces cerevisiaemutant defective in exo-1,3-β-glucanase production. J Bacteriol. 1979;139:333–338. doi: 10.1128/jb.139.2.333-338.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Gmachl MJ, Stenbeck G, Söllner T, Rothman JE. A possible docking and fusion particle for synaptic transmission. Nature. 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Stenbeck G, Rothman JE, Söllner T. Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc Natl Acad Sci USA. 1997;94:997–1001. doi: 10.1073/pnas.94.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiter R, Kohlwein SD. Organelle structure, function, and inheritance in yeast: A role for fatty acid synthesis. Cell. 1997;88:431–434. doi: 10.1016/s0092-8674(00)81882-6. [DOI] [PubMed] [Google Scholar]
- Shim J, Newman AP, Ferro-Novick S. The BOS1gene encodes an essential 27-kD putative membrane protein that is required for vesicular transport from the ER to the Golgi complex in yeast. J Cell Biol. 1991;113:55–64. doi: 10.1083/jcb.113.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L. Synaptic vesicle endocytosis is impaired by disruption of the dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Silve S, Leplatois P, Josse A, Dupuy P-H, Lanau C, Kaghad M, Dhers C, Picard C, Rahier A, Taton M, et al. The immunosuppressant SR 31747 blocks cell proliferation by inhibiting a steroid isomerase in Saccharomyces cerevisiae. . Mol Cell Biol. 1996;16:2719–2727. doi: 10.1128/mcb.16.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Sivadon P, Bauer F, Aigle M, Crouzet M. Actin cytoskeleton and budding pattern are altered in the yeast rvs161mutant: the Rvs161 protein shares common domains with the brain protein amphiphysin. Mol Gen Genet. 1995;245:485–495. doi: 10.1007/BF00290452. [DOI] [PubMed] [Google Scholar]
- Skrzypek M, Lester RL, Dickson RC. Suppressor gene analysis reveals an essential role for sphingolipids in transport of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae. . J Bacteriol. 1997;179:1513–1520. doi: 10.1128/jb.179.5.1513-1520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993a;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to the sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993b;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Sütterlin C, Doering TL, Schimmöler F, Schröder S, Riezman H. Specific requirements for the ER to Golgi transport of GPI-anchored proteins. J Cell Sci. 1997;110:2703–2714. doi: 10.1242/jcs.110.21.2703. [DOI] [PubMed] [Google Scholar]
- Sweeny ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophilaspecifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Toke DA, Martin CE. Isolation and characterization of a gene affecting fatty acid elongation in Saccharomyces cerevisiae. . J Biol Chem. 1996;271:18413–18422. doi: 10.1074/jbc.271.31.18413. [DOI] [PubMed] [Google Scholar]
- Van Rijn HJM, Boer P, Steyn-Parvé EP. Biosynthesis of acid phosphatase of baker's yeast. Factors influencing its production by protoplasts and characterization of the secreted enyzyme. Biochim Biophys Acta. 1972;268:431–441. doi: 10.1016/0005-2744(72)90339-7. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Novick PJ. Purification and characterization of constitutive secretory vesicles from yeast. J Cell Biol. 1988;105:163–174. doi: 10.1083/jcb.105.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Brennwald P, Kabcenell AK, Garrett M, Novick P. Hydrolysis of GTP by Sec4 plays an important role in vesicular transport and is stimulated by a GTPase-activating protein in Saccharomyces cerevisiae. . Mol Cell Biol. 1992;12:2017–2028. doi: 10.1128/mcb.12.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wu W-I, Lin Y-P, Wang E, Merrill AH, Jr, Carman GM. Regulation of phosphatidate phosphatase activity from the yeast Saccharomyces cerevisiaeby sphingoid bases. J Biol Chem. 1993;268:13830–13837. [PubMed] [Google Scholar]
- Xu Z, Sato K, Wickner W. LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell. 1998;93:1125–1134. doi: 10.1016/s0092-8674(00)81457-9. [DOI] [PubMed] [Google Scholar]
- Zelicof A, Protopopov V, David D, Lin X-Y, Lustgarten V, Gerst JE. Two separate functions are encoded by the carboxyl-terminal domains of the yeast cyclase-associated protein and its mammalian homologs: dimerization and actin-binding. J Biol Chem. 1996;271:18243–18252. doi: 10.1074/jbc.271.30.18243. [DOI] [PubMed] [Google Scholar]