Abstract
The binding of Fas ligand to Fas recruits caspase 8 to Fas via an adaptor, FADD/MORT1, and activates a caspase cascade leading to apoptosis. Here, we describe a human Jurkat-derived cell line (JB-6) that is deficient in caspase 8. This cell line was resistant to the apoptosis triggered by Fas engagement. However, the multimerization of Fas-associated protein with death domain, through the use of a dimerizing system, killed the JB-6 cells. This killing process was not accompanied by the activation of caspases or DNA fragmentation. The dying cells showed neither condensation nor fragmentation of cells and nuclei, but the cells and nuclei swelled in a manner similar to that seen in necrosis. These results suggested that Fas-associated protein with death domain can kill the cells via two pathways, one mediated by caspases and another that does not involve them.
Keywords: apoptosis, caspase, Fas, Fas-associated protein with death domain, necrosis
Two categories of cell death have been defined: apoptosis and necrosis (Kerr et al., 1972). The apoptotic process is accompanied by condensation and fragmentation of cells and nuclei, as well as by degradation of the chromosomal DNA into nucleosomal units (Wyllie 1980; Wyllie et al., 1980). The necrotic process is characterized by swelling and disintegration of the cells and nuclei. Most apoptotic processes are mediated by a family of cysteine proteases called caspases (Cohen, 1997; Nicholson and Thornberry, 1997). Caspases are activated by various apoptotic stimuli and cleave cellular substrates such as poly(ADP-ribose) polymerase (PARP),1 lamin, actin, p27, and inhibitor of caspase-activated DNase, which is responsible to cause morphological changes of nuclei, and degradation of chromosomal DNA. The molecular mechanism of the necrotic cell death process, however, is not well understood.
Fas ligand (FasL) is a member of the tumor necrosis factor (TNF) family. By binding to Fas, its receptor, FasL induces apoptosis (Baker and Reddy, 1996; Nagata, 1997). This apoptotic process proceeds by the binding of an adaptor molecule, Fas-associated protein with death domain (FADD), to the death domain of the Fas cytoplasmic region. This binding causes recruitment of pro-caspase 8 to the Fas receptor to establish the death-inducing signaling complex (DISC) (Kischkel et al., 1995). Pro-caspase 8 is processed at the DISC to the mature active enzyme, and activates other caspases, such as caspase 3, in the downstream of the caspase cascade. In this report, we describe a Jurkat-derived cell line that does not express caspase 8. This cell line was resistant to Fas-induced cell death. However, multimerization of FADD in this cell line killed the cells. This killing process proceeded without caspase activation, and was accompanied neither by apoptotic morphological changes of the cells nor by DNA fragmentation. An analysis of the dying cells under the electron microscopy indicated that this process was accompanied by necrotic morphological changes.
Materials and Methods
Establishment of Stable Transformants
Plasmid MF2FE carries a DNA fragment coding for the FK506 binding protein (FKBP)–Fas chimeric molecule that is consisting of the cytoplasmic region of Fas, two tandem repeats of FKBP12, the myristilation-targeting peptide, and the influenza hemagglutinin (HA) epitope (see Fig. 2 A). The coding region of FKBP-Fas was excised from pMF2FE (Spencer et al., 1996) (provided by Dr. M. Gilman in ARIAD, Cambridge, MA), and inserted in pEF-BOS expression vector to generate pEF-FKBP-Fas. The expression vector for FKBP-FADD (pEF-FKBP-FADD) was constructed by replacing the Fas cytoplasmic region of pEF-FKBP-Fas by the entire coding region of FADD/MORT1 (Boldin et al., 1995; Chinnaiyan et al., 1995). To construct the expression vector for human caspase 8, the entire coding region of human caspase 8 (Boldin et al., 1996; Muzio et al., 1996) was fused in frame with the sequence of FLAG epitope, and inserted in pEF-BOS vector (Mizushima and Nagata, 1990) to generate pEF-CAS8.
Figure 2.
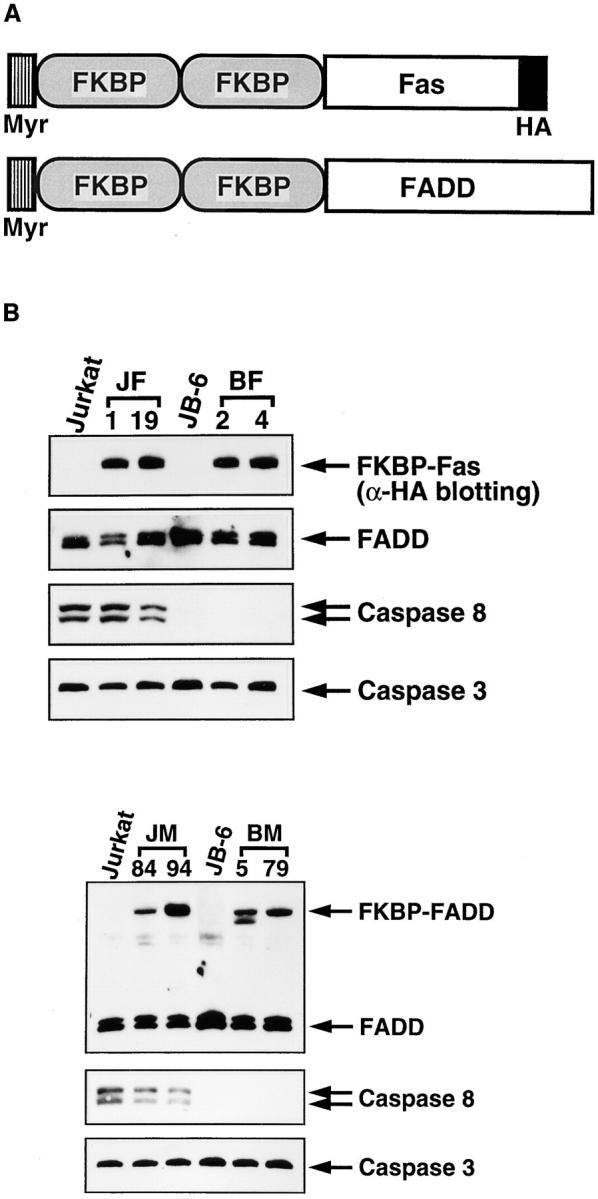
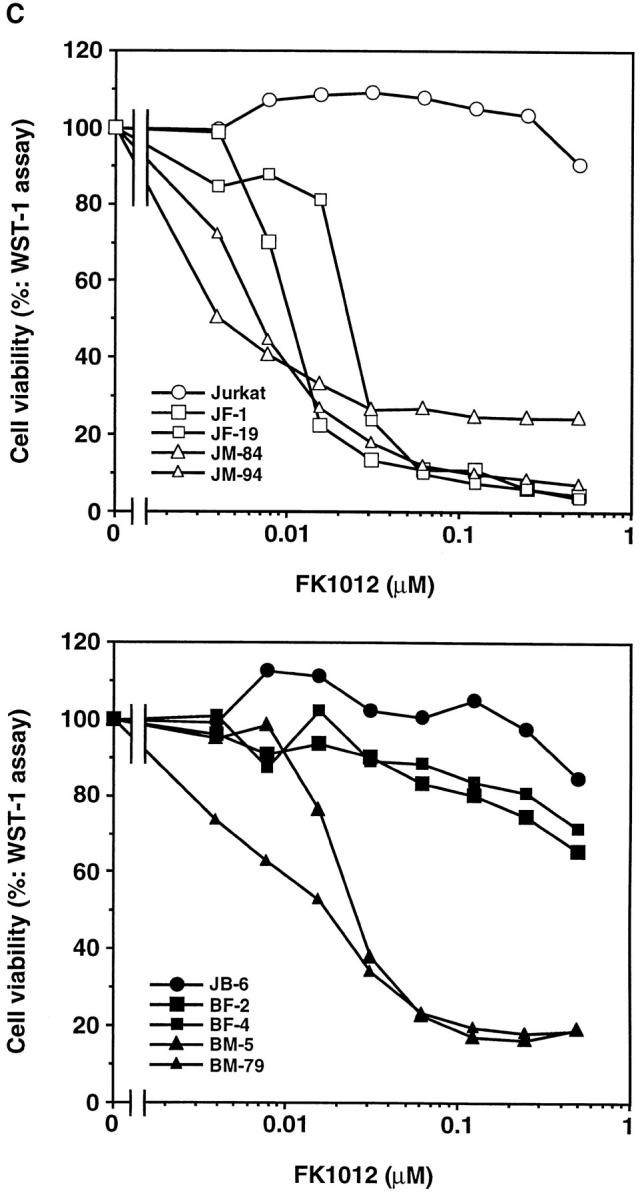
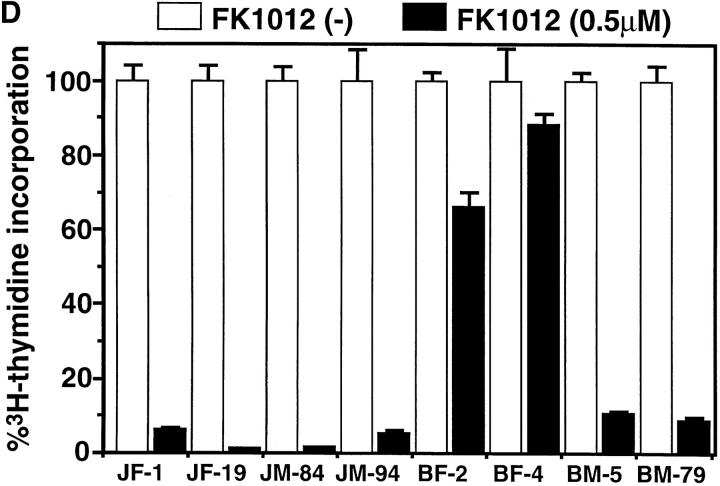
Killing of cells by oligomerization of Fas and FADD. (A) A schematic representation of the chimeric molecules, FKBP-Fas and FKBP-FADD. FKBP, Fas, and FADD represent a domain of human FKBP12 from the amino acid position from 2 to 107, the cytoplasmic domain of Fas (residues 179–319), and the entire coding region of FADD, respectively. Myr and HA indicate the myristilation targeting peptide and influenza hemagglutinin epitope, respectively. (B) Expression of the chimeric molecules, FADD, and caspases in Jurkat-derived cell lines. JF-1 and -19 clones are Jurkat cells transformed with FKBP-Fas, while JM-84 and -94 are Jurkat cells transformed with FKBP-FADD. The JB-6 cells were also transformed with FKBP-Fas (BF-2 and BF-4) or FKBP-FADD (BM-5 and BM-79). The cytosolic extracts (derived from 2.5 × 105 cells/lane) prepared from the indicated cells were separated on SDS-PAGE and analyzed by Western blotting with antibodies against HA, FADD, caspase 8, and caspase 3. Arrows indicate the positions of respective molecules. (C) Effect of FK1012 on the cell viability. Cells were incubated at 37°C for 6 h with the indicated concentrations of FK1012, and the cell viability was determined by the WST-1 method. Results are expressed as percentage of that obtained without FK1012. The experiments were done in triplicate, and the average values are plotted. (D) Effect of FK1012 on the 3H-thymidine incorporation. The indicated transformant clones (3 × 104 cells/100 μl) were incubated at 37°C for 20 h in the presence of 0.5 μM FK1012. The cells were then pulsed with 3H-thymidine, and the radioactivity incorporated into the cells were determined as described in Materials and Methods. Results are expressed as percentage of that obtained without FK1012. The experiments were done in triplicate, and the average values are shown with the standard deviation indicated by bars.
Jurkat is a human leukemic T lymphoma cell line, and JB-6 is a Jurkat-derived cell line that overexpresses human Bcl-2. To establish stable transformants expressing FKBP-Fas, FKBP-FADD, or human caspase 8, Jurkat and JB-6 cells (107) were cotransfected with 50 μg of the expression vectors (pEF-FKBP-Fas, pEF-FKBP-FADD, or pEF-CAS8) with 1 μg of the hygromycin-resistant gene (pMiwhyg) by electroporation using a Gene Pulser (Bio-Rad Laboratories, Hercules, CA) as described previously (Suda et al., 1993). The hygromycin-resistant clones were selected by culturing cells in the presence of 800 μg/ml of hygromycin. The transformant clones expressing FKBP-Fas, FKBP-FADD, or caspase 8 were then identified by Western blotting using anti–HA, anti–FADD antibody, or anti–FLAG antibody, respectively. JF and JM clones were Jurkat-derived cells expressing FKBP-Fas and FKBP-FADD, respectively. Whereas, BC, BF, and BM clones were JB-6-derived cells expressing human caspase 8, FKBP-Fas, and FKBP-FADD, respectively.
Cell Viability Assay
The cell viability was determined by the WST-1 assay (Kawahara et al., 1998b ) or 3H-thymidine incorporation. In brief, 5 × 104 cells were stimulated with various concentrations of FK1012 for 6 h in 100 μl. The WST-1 reagent, 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)2[H] tetrazolium monosodium salt (Dojin Laboratories, Kumamoto, Japan), and 1-methoxy-5-methylphenazinium methylsulfate were added to the cells at the final concentrations of 5 and 0.2 mM, respectively, and incubated for 1 h. The cell viability was determined by measuring the difference between the absorbance at 450 and 620 nm using an ELISA autoreader. For 3H-thymidine incorporation assay, 3 × 104 cells were incubated with 0.5 μM of FK1012 for 20 h in 100 μl, and then pulsed with 1 μCi of 3H-thymidine (6.7 Ci/mmol; Dupont-NEN, Boston, MA) for 4 h before harvest.
Assay for Caspase Activity
The caspase activity in the cell lysates was determined as described (Enari et al., 1996). In brief, the cell lysates (S-30 fraction, 10 μg) were incubated for 30 min at 30°C in 0.5 ml of the assay buffer (100 mM HEPES-KOH, pH 7.5, 10% sucrose, 0.1% CHAPS, 10 mM DTT, and 0.1 mg/ml ovalbumin) containing 1 μM MCA-DEVDAPK(dnp) (Peptide Institute, Osaka, Japan). The fluorescence was measured at an excitation wavelength of 325 nm and an emission wavelength of 395 nm using a spectrofluorometer (RF-1500; Shimadzu, Tokyo, Japan).
Western Blotting and DNA Fragmentation
Cells were incubated on ice for 30 min in the lysis buffer (20 mM Tris-HCl, pH 7.5, 140 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1 mM p-amidinophenyl methanesulfonyl fluoride hydrochloride, 50 mM NaF, 1 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10% glycerol). After removing insoluble materials by centrifugation, the supernatant was recovered as cytosolic extracts. In some cases, the crude lysates were prepared by heating the cells at 85°C for 30 min in 100 μl of lysis buffer (2.5% SDS, 120 mM Tris-HCl, pH 6.8, 200 μM DTT, 20% glycerol). The lysate was then mixed with 100 μl of 2× Laemmli's sample buffer.
Western blotting was performed as previously described (Kawahara et al., 1998a ). Anti–human caspase 8 (clone 5F7) antibody was purchased from Medical and Biological Laboratories (Nagoya, Japan). Anti–Lamin B1 and anti–α-tubulin antibodies were from Oncogene Science (Cambridge, MA), and anti–human FADD and anti–human caspase 3 antibodies were from Transduction Laboratories (Lexington, KY). Anti–HA and anti– human Bcl-2 antibodies were from Boehringer Mannheim Biochemicals (Indianapolis, IN) and from PharMingen (San Diego, CA), respectively. Anti–human PARP antibody (clone C-2-10) was provided by Dr. G.G. Poirier (Centre Hospitalier de l'Université Laval Research Center, Quebec, Canada). Proteins recognized by the primary antibody were visualized using the enhanced chemiluminescence system (Renaissance; Dupont-NEN) after reacting with the horseradish peroxidase–conjugated anti–mouse IgG (DAKO Corp., Santa Barbara, CA). DNA fragmentation assay was performed as described previously (Enari et al., 1995).
Results
Requirement of Caspase 8 for Fas-induced Apoptosis
During establishment of Jurkat cell transformants overexpressing Bcl-2, we obtained a clone (JB-6) that was completely resistant to Fas-induced cell death (Fig. 1 A). This cell line, JB-6, expressed Fas as abundantly as the parental Jurkat cells (Fig. 1 B); however, it expressed very little, if any, caspase 8 (Fig. 1 B). Other signaling molecules, such as FADD and caspase 3, were expressed in JB-6 cells as abundantly as in Jurkat cells. The expression level of caspase 10 (Mch 4, FLICE 2), which is another caspase containing the death effector domain (Fernandes-Alnemri et al., 1996; Vincenz and Dixit, 1997), was also comparable between Jurkat and JB-6 cells (data not shown). These results suggested that the resistance of this cell line to Fas-induced apoptosis was likely due to the lack of caspase 8. Accordingly, when an expression vector for human caspase 8 was introduced into the JB-6 cell line, the transformants (BC clones) regained the sensitivity to Fas-induced apoptosis (Fig. 1, A and B).
Figure 1.
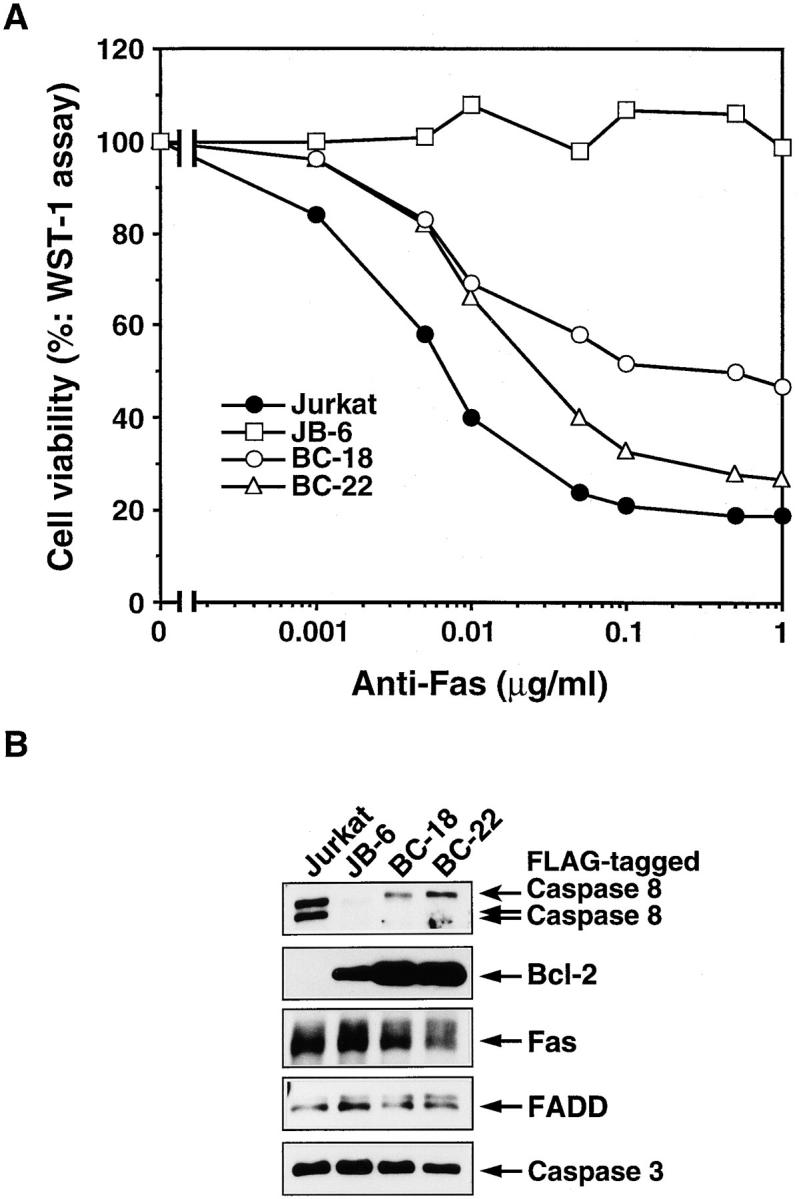
Resistance of human Jurkat cell variants to the Fas-induced apoptosis. (A) The Fas-induced apoptosis of human Jurkat and its variant cell lines. Human Jurkat, its variant (JB-6) lacking caspase 8, and JB-6 transformants (BC-18 and BC-22) expressing the FLAG-tagged caspase 8 were incubated for 15 h with the indicated concentrations of the anti–Fas antibody (CH11; lot 170). The cell viability was determined by the WST-1 assay as described in Materials and Methods, and is expressed as the percentage of that observed without the anti–Fas antibody treatment. (B) Expression of caspase 8 in Jurkat-derived JB-6 cells. The crude cell lysates were prepared from Jurkat, JB-6, BC-18, and BC-22 by lysing them with a high concentration of SDS. The lysates (derived from 5 × 104 cells/lane) were separated by electrophoresis on 10% or 10/20% polyacrylamide gel, followed by Western blotting analysis using antibodies against caspase 8, Bcl-2, Fas, FADD, and caspase 3. The corresponding bands are indicated by arrows on the right.
Oligomerization of FADD Kills the Cells Lacking Caspase 8
It is assumed that the engagement of Fas causes oligomerization of FADD, which leads to activation of caspase 8 (Boldin et al., 1996; Muzio et al., 1996). To examine whether the oligomerization of FADD causes cell death, two copies of FKBP (FK506-binding protein) were joined to the death domain of human Fas (Itoh and Nagata, 1993) or to full-length human FADD (Boldin et al., 1995; Chinnaiyan et al., 1995; Fig. 2 A), and introduced into Jurkat cells or JB-6 cells. As shown in Fig. 2 B, the Jurkat-derived transformant clones JF-1 and -19, and the JB-6–derived BF-2 and -4 clones all expressed FKBP-Fas of 44 kD, which was recognized by the anti–HA antibody. In addition, the Jurkat-derived JM-84 and -94 clones, as well as the JB-6-derived BM-5 and -79 clones, expressed a FKBP-FADD of 50 kD, detected by an anti–human FADD antibody. The expression level of the FKBP-FADD was comparable with that of the endogenous FADD, which is 32 kD (Fig. 2 B). As found in the parental JB-6 cells, all transformant clones derived from JB-6 were deficient in expressing caspase 8. However, the expression levels of the endogenous Fas, FADD, and caspase 3 were comparable among all transformants (Fig. 2 B, and data not shown).
The transformants were then treated for 6 h with increasing concentrations of FK1012, which should induce FKBP dimerization (Spencer et al., 1993, 1996), and thus oligomerization of FKBP-Fas or FKBP-FADD. As shown in Fig. 2 C, FK1012 did not show any toxicity to the parental Jurkat or JB-6 cells at a concentration of 0.5 μM. When Jurkat cell transformants expressing FKBP-Fas or FKBP-FADD were treated with FK1012, viability (as assayed by the WST-1 method) was lost, indicating that the oligomerized FKBP-Fas or FKBP-FADD could kill the cells. The JB-6 transformants expressing FKBP-Fas were resistant to the FK1012-induced cell death, and >65% of the cells were still alive after treatment with 0.5 μM of FK1012 for 6 h. These results were consistent with the observation that JB-6 cells were resistant to the anti–Fas antibody-induced cell death as described above (Fig. 1 A). On the other hand, the JB-6 transformants expressing FKBP-FADD were efficiently killed by FK1012 (Fig. 2 C). The sensitivity of these cells to FK1012 was comparable to that found with Jurkat cell transformants expressing FKBP-FADD. The cell viability was also assayed by 3H-thymidine uptake. As shown in Fig. 2 D, the cells were incubated for 20 h with 0.5 μM of FK1012, and then pulsed for 4 h with 3H-thymidine. The Jurkat cell transformants (JF and JM clones) expressing FKBP-Fas or FKBP-FADD lost the ability to incorporate thymidine. The JB-6 transformants expressing FKBP-Fas (BF clones) survived after treatment with FK1012, while the BM clones expressing FKBP-FADD were efficiently killed by the same treatment.
Necrotic Death Caused by FADD
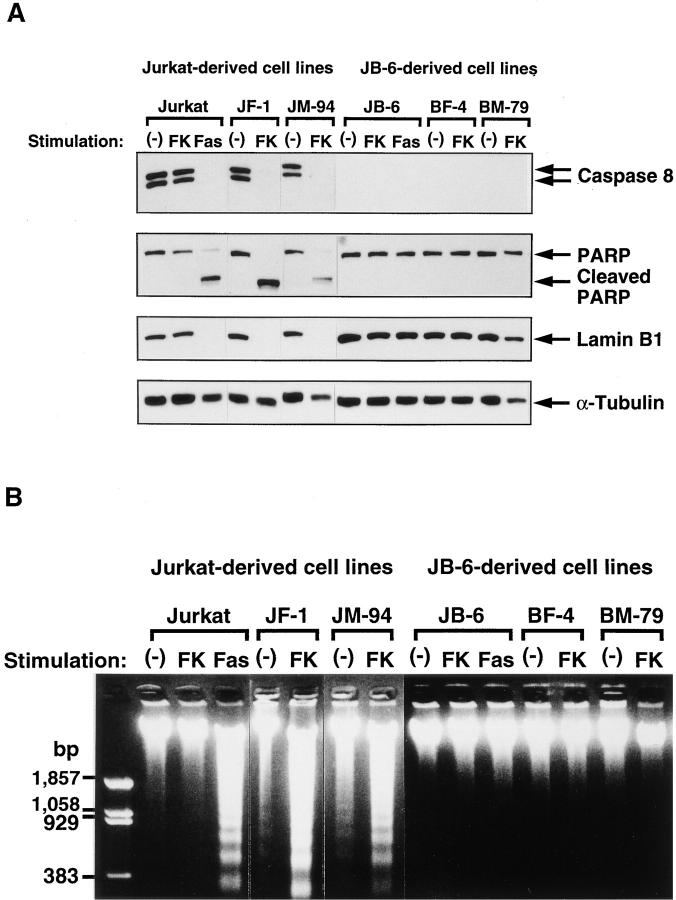
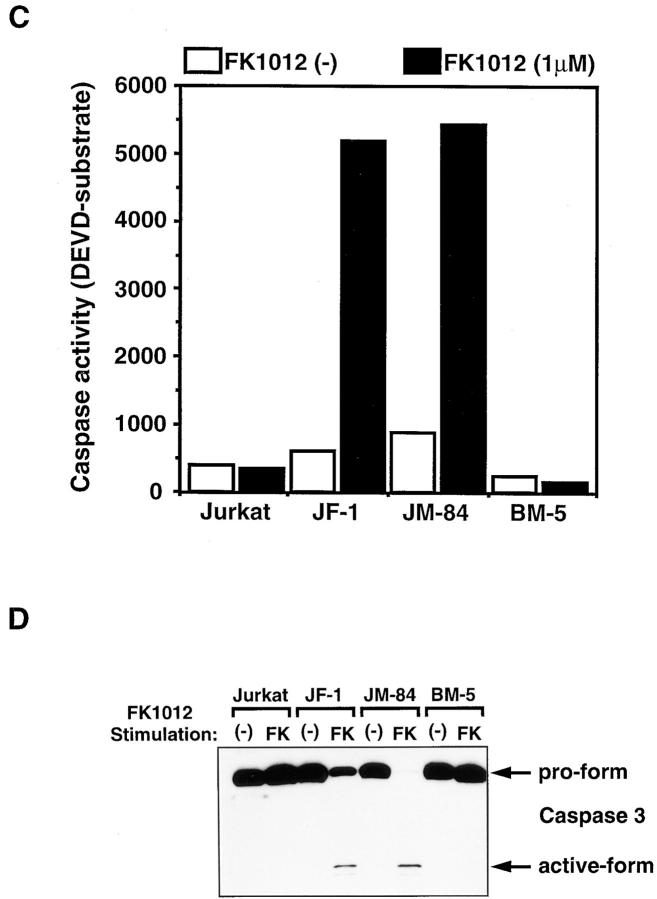
The above results indicated that the killing of cells by oligomerization of Fas requires caspase 8. However, FADD, which mediates the Fas signal to caspase 8, can kill cells without caspase 8. To examine the molecular mechanism behind the FADD-induced cell death, activation of caspase 3 and DNA fragmentation were analyzed in Jurkat and JB-6 transformant clones. As shown in Fig. 3, A and D, when the Jurkat cell transformants were treated with FK1012, the proforms of caspases 8 and 3 disappeared within 6 h. This processing of caspases was accompanied by a strong activation of caspase 3–like protease, the cleavage of PARP and lamin B1, and the degradation of chromosomal DNA (Fig. 3). On the other hand, the JB-6 cell transformants expressing FKBP-Fas did not show any proteolysis of the nuclear proteins lamin B1 and PARP, nor any DNA fragmentation, as predicted by their unresponsiveness to FK1012 treatment. The transformants expressing FKBP-FADD also showed neither caspase 3 activation nor DNA degradation by the treatment with FK1012, despite massive cell death. These results indicated that oligomerization of FADD can kill the cells without activating caspases or causing DNA fragmentation. The dying cells were then examined using transmission electron microscopy. As shown in Fig. 4, death of Jurkat cells induced by oligomerization of FKBP-Fas or FKBP-FADD was accompanied by apoptotic morphological changes such as chromatin condensation, cell shrinkage, and apoptotic bodies. On the other hand, the caspase 8–deficient BM cells dying as result of FADD oligomerization showed neither condensation of cells nor fragmentation of chromatin. Rather, the cells swelled without forming apoptotic bodies. These results indicated that FADD can kill cells without inducing apoptosis in BM cells.
Figure 3.
Biochemical characterization of the cell death induced by FKBP-Fas or FKBP-FADD. (A) Proteolysis of caspase 8 and nuclear proteins. The indicated cells were incubated at 37°C for 6 h without (−) or with 0.5 μM FK1012 (FK) or 0.5 μg/ml anti–Fas antibody (CH11) (Fas). The cells were lysed with 2.5% SDS, and the crude lysates were subjected to the Western blotting analysis with the antibodies against caspase 8, PARP, lamin B1, and α-tubulin. Arrows indicate the positions of respective molecules. (B) DNA degradation. The indicated cells were incubated at 37°C for 6 h without (−) or with 0.5 μM FK1012 (FK) or 0.5 μg/ml anti– Fas antibody (CH11) (Fas). Total DNA was extracted from the cells and electrophoresed on a 1.5% agarose gel in the presence of 1.0 μg/ml ethidium bromide. (C) Activation of caspase 3–like protease activity. The indicated cells were stimulated for 4 h with or without 1 μM FK1012. The caspase 3–like protease activity was then determined with 10 μg of the cell lysates (S-30 fraction) and 1 μM fluorogenic substrate, MCA-DEVDAPK(dnp), as described in Materials and Methods. The caspase activity is expressed as an arbitrary unit. 1 ng of the recombinant caspase 3 gave 220 arbitrary units under the same conditions. (D) Processing of caspase 3. The indicated cells were incubated at 37°C for 4 h with 1 μM FK1012. The cell lysates (S-30 fraction, 20 μg/lane) were then subjected to the Western blotting analysis using anti– caspase 3 antibody. The proform of 32 kD and active processed form of 17 kD are indicated by arrows.
Figure 4.
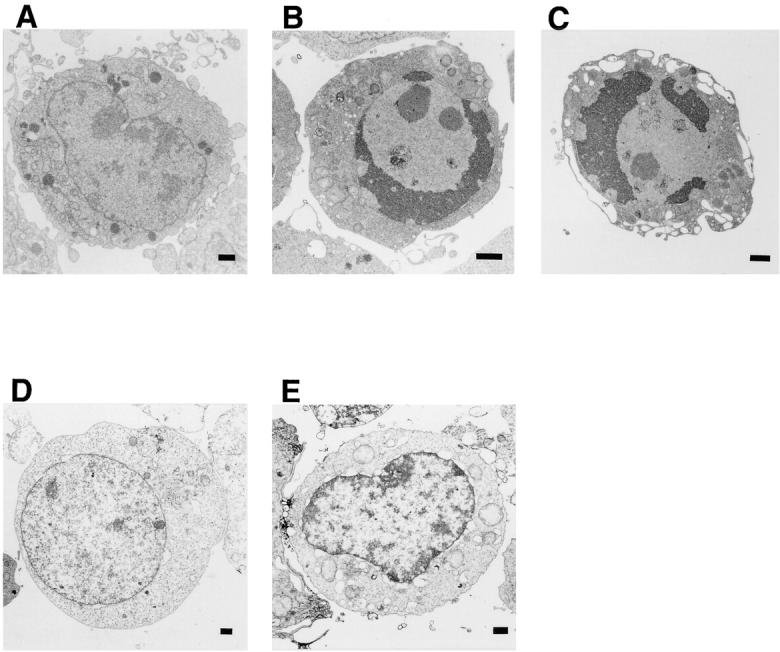
Electron microgram of Jurkat-derived cells undergoing cell death. The Jurkat cell transformant expressing FKBP-Fas (clone JF-1, B) or FKBP-FADD (clone JM-94, C), and JB-6 transformant expressing FKBP-FADD (clone BM-79, D) were incubated at 37°C for 4 h with 0.5 μM FK1012, and examined by electron transmission microscopy using Hitachi H7100. In E, the clone JM-94 cells were pretreated at 37°C for 1 h in the presence of 500 μM Z-VAD-fmk, and then incubated at 37°C for 4 h with 0.5 μM FK1012. The untreated Jurkat cells (A) were also examined under electron microscope as a control. The untreated other cell lines showed a similar morphology as Jurkat cells. Bars, 1 μm.
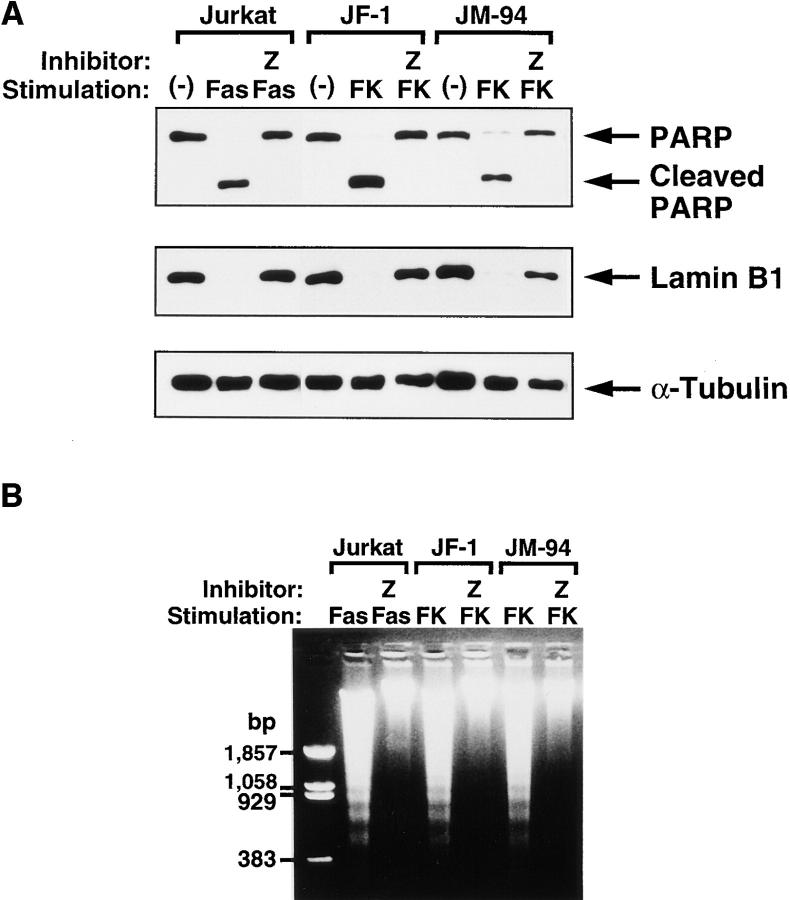
We then examined the effect of a broad caspase inhibitor, Z-VAD-fmk, on the cell death in Jurkat cells. As shown in Fig. 5, A and B, Z-VAD-fmk completely blocked the proteolysis of nuclear proteins and DNA fragmentation that occur during FKBP-Fas-induced cell death. Accordingly, the cell death induced by FKBP-Fas was largely prevented by Z-VAD-fmk (Fig. 5 C). In contrast, Z-VAD-fmk did not inhibit the FKBP-FADD-mediated cell death, although it inhibited proteolysis of nuclear proteins and DNA degradation. The dying Jurkat cells under electron microscope showed a necrotic morphological change (Fig. 4 E). These results suggested that FADD can kill the Jurkat cells via two pathways. One is an apoptotic pathway in which activation of a caspase cascade leads to fragmentation of chromosomal DNA, and the other is a nonapoptotic pathway that does not involve caspases, but is accompanied by necrotic morphological changes of the cells.
Figure 5.
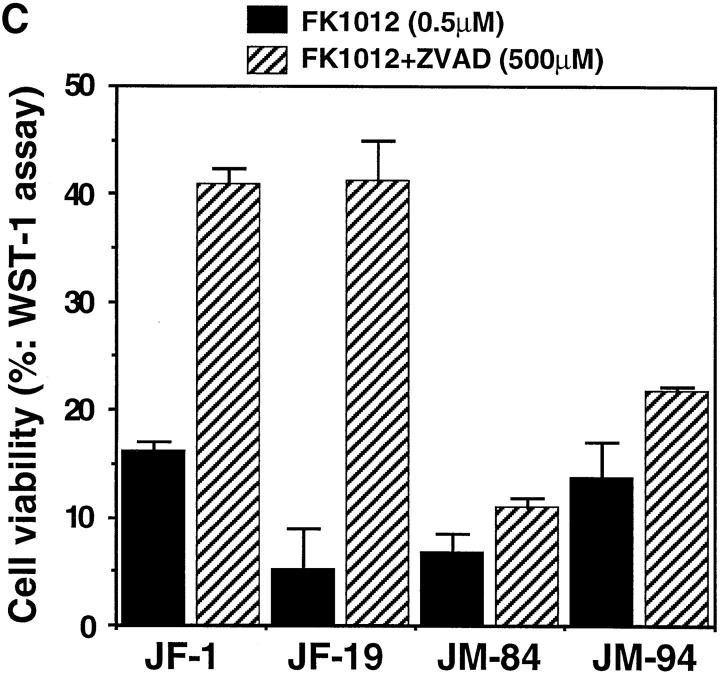
Effect of a caspase inhibitor on the cell death induced by FKBP-Fas or FKBP-FADD. (A) Effect of Z-VAD-fmk on Fas or FADD-induced proteolysis of nuclear proteins. Jurkat and its transformants expressing FKBP-Fas (clone, JF-1) or FKBP-FADD (clone, JM-94) were preincubated at 37°C for 1 h in the presence or absence of 500 μM Z-VAD-fmk (Z). The cells were left untreated (−), or treated at 37°C for 6 h with 0.5 μg/ml anti–Fas antibody (Fas) or 0.5 μM FK1012 (FK). The crude lysates were prepared by lysing the cells with 2.5% SDS, and analyzed by Western blotting with antibodies against PARP, lamin B1, and α-tubulin. The positions for intact and cleaved PARP, as well as for the intact lamin B1 and α-tubulin, are indicated by arrows on the right. The each lane contains the crude lysates derived from 2.5 × 104 cells. (B) Effect of Z-VAD-fmk on FK1012-induced DNA degradation. After preincubation with 500 μM Z-VAD-fmk, the indicated cells were incubated at 37°C for 6 h in the presence of 0.5 μg/ml anti–Fas antibody (Fas) or 0.5 μM FK1012 (FK). Total DNA was then electrophoresed on 1.5% agarose gel. (C) Effect of Z-VAD-fmk on the cell death induced by FKBP-Fas or FKBP-FADD. The Jurkat cell transformants expressing FKBP-Fas (JF-1 and JF-19) or FKBP-FADD (JM-84 and JM-94) were preincubated at 37°C for 1 h with (hatched bars) or without (closed bars) 500 μM Z-VAD-fmk, and then treated at 37°C for 6 h with 0.5 μM FK1012. The cell viability was determined by the WST-1 assay, as described in Materials and Methods, and is expressed as a percentage of that obtained without FK1012 treatment.
Discussion
In this report, we have shown that a derivative of human Jurkat cells that does not express caspase 8 was resistant to the apoptosis induced by the activation of endogenous Fas or oligomerization of the Fas death domain. These results indicate that caspase 8 is indispensable for the Fas-mediated apoptotic cell death process, which agrees with the recent results that embryonal fibroblasts from caspase 8 null mice are resistant to the Fas-induced apoptosis (Varfolomeev et al., 1998). On the other hand, oligomerization of FADD, an adaptor molecule that normally mediates the Fas signal to caspase 8, could kill cells that were deficient in caspase 8. This pathway does not use caspases to kill the cells, and seems to operate even in the cells (the parental Jurkat cells) that express caspase 8, because a broad caspase inhibitor could not prevent the cell death induced by oligomerization of FADD.
The Fas-induced cell death is so far thought to transduce only apoptotic signal. On the other hand, TNF was shown to transduce not only an apoptotic signal, but also a necrotic signal. In particular, treatment of mouse L929 cells with TNF produces oxidants and kills the cells by inducing necrotic morphological changes of the cells (Vercammen et al., 1998a ). This process was shown to be enhanced by caspase inhibitors. More recently, Vercammen et al. (1998b) established mouse L929 cells overexpressing human Fas. When these cells were treated with the agonistic anti–Fas antibody, they underwent apoptosis. But, caspase inhibitors could not prevent the cell death process, and the cells died by necrosis. This result is apparently in contrast to our observation that the Fas engagement alone cannot kill the caspase 8–deficient human Jurkat cells. However, the sensitivity of the cells to necrosis may depend on the strength of the death signal evoked by Fas or FADD, the expression level of the downstream signal transducer for necrosis, and/or the balance between apoptotic and necrotic signals. In L929 cells, a downstream molecule(s) leading to necrosis is more abundant than in Jurkat cells, and the necrotic signal can be easily seen with the weak signal from the Fas receptor in the presence of caspase inhibitors, while its strong activation by direct oligomerization of FADD may be necessary to activate the necrotic pathway in Jurkat cells. FADD can also be activated by the TNF–TNF type I receptor system through an adaptor molecule called TRADD (TNFR1-associated death domain protein; Chinnaiyan et al., 1996; Hsu et al., 1996). It will be interesting to determine whether the TNF-induced necrosis in L929 cells is mediated by FADD or not. In addition, the TNF-induced necrosis in L929 cells can be inhibited by butylated hydroxyanisole, an anti–oxidant, suggesting an involvement of oxidants in this cell death process (Vercammen et al., 1998a ). Whether or not a similar oxidant(s) is activated during necrosis by oligomerization of FADD in Jurkat cells remains to be determined.
The FADD-mediated apoptosis occurs through recruitment of pro-caspase 8 to the FADD death effector domain, which leads to processing of caspase 8 and the activation of the downstream caspases, such as caspase 3, and a DNase (CAD; Nagata, 1997; Enari et al., 1998; Sakahira et al., 1998). It would be interesting to examine whether or not the same region of FADD is responsible for the necrotic signal transduction and what kinds of molecules are activated by FADD. The JB-6 cells abundantly express Bcl-2, yet oligomerization of FADD killed the JB-6 cells, suggesting that the FADD-induced necrotic cell death cannot be inhibited by Bcl-2. This agrees with no inhibitory effect of Bcl-2 on the TNF-induced cytotoxicity in mouse L929 cells (Vanhaesebroeck et al., 1993). In any case, the establishment of a system for studying necrosis in the absence of caspase activation will contribute to our understanding of the molecular mechanisms underlying necrosis.
Acknowledgments
We thank M. Gilman and H. Berger for the plasmid carrying FKBP and FK1012, V.M. Dixit for human caspase 8 (FLICE) cDNA, and S. Kumagai for secretarial assistance.
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Science and Culture in Japan, and by Special Coordination Funds of the Science and Technology Agency of the Japanese Government. A. Kawahara was supported by a postdoctoral fellowship in Cancer Research of the Japan Society for the Promotion of Science.
Abbreviations used in this paper
- FADD
Fas-associated protein with death domain
- FKBP
FK506 binding protein
- HA
hemagglutinin
- PARP
poly(ADP-ribose) polymerase
- TNF
tumor necrosis factor
Footnotes
Dr. Kawahara's present address is Laboratory of Molecular Genetics, National Institute of Child Health and Human Development, NIH, Bethesda, MD 20892.
Address all correspondence to Shigekazu Nagata, Osaka University Medical School, B-3, Department of Genetics, 2-2 Yamada-oka, Suita, Osaka 565-0871, Japan. Tel.: 81-6-879-3310. Fax: 81-6-879-3319. E-mail: nagata@genetic.med.osaka-u.ac.jp
References
- Baker SJ, Reddy EP. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12:1–9. [PubMed] [Google Scholar]
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1– and TNF receptor–induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/ APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. FADD/ MORT1 is a common mediator of CD95(Fas/APO-1) and tumor necrosis factor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M, Hase A, Nagata S. Apoptosis by a cytosolic extract from Fas-activated cells. EMBO (Eur Mol Biol Organ) J. 1995;14:5201–5208. doi: 10.1002/j.1460-2075.1995.tb00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Armstrong R, Krebs J, Srinivasula SM, Wang L, Bullrich F, Fritz L, Trapani J, Tomaselli K, Litwack G, Alnemri E. In vitroactivation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H, Shu H-B, Pan M-G, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transcription pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Itoh N, Nagata S. A novel protein domain required for apoptosis: mutational analysis of human Fas antigen. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- Kawahara A, Enari M, Talanian RV, Wong WW, Nagata S. Fas-induced DNA fragmentation and proteolysis of nuclear proteins. Genes Cells. 1998a;3:297–306. doi: 10.1046/j.1365-2443.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Kawahara, A., T. Kobayashi, and S. Nagata. 1998b Inhibition of Fas-induced apoptosis by Bcl-2. Oncogene. In press. [DOI] [PubMed]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins from a death-inducing signaling complex (DISC) with the receptor. EMBO (Eur Mol Biol Organ) J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S, Nagata S. pEF-BOS: a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- Spencer DM, Belshaw PJ, Chen L, Ho SN, Randazzo F, Crabtree GR, Schreiber SL. Functional analysis of Fas signaling in vivo using synthetic inducers of dimerization. Curr Biol. 1996;6:839–847. doi: 10.1016/s0960-9822(02)00607-3. [DOI] [PubMed] [Google Scholar]
- Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand: a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Reed J, De Valck D, Grooten J, Miyashita T, Tanaka S, Beyaert R, Van Roy F, Fiers W. Effect of Bcl-2 proto-oncogene expression on cellular sensitivity to tumor necrosis factor-mediated cytototoxicity. Oncogene. 1993;8:1075–1081. [PubMed] [Google Scholar]
- Varfolomeev EE, Schuchmann M, Luria V, Chiannikulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, et al. Target disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998a;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998b;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenz C, Dixit V. Fas-associated death domain protein interleukin-1β-converting enzyme 2 (FLICE), an ICE/Ced-3 homologue, is proximally involved in CD95- and p55-mediated death signaling. J Biol Chem. 1997;272:6578–6583. doi: 10.1074/jbc.272.10.6578. [DOI] [PubMed] [Google Scholar]
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]