Abstract
Adducin is a membrane skeletal protein that binds to actin filaments (F-actin) and thereby promotes the association of spectrin with F-actin to form a spectrin-actin meshwork beneath plasma membranes such as ruffling membranes. Rho-associated kinase (Rho- kinase), which is activated by the small guanosine triphosphatase Rho, phosphorylates α-adducin and thereby enhances the F-actin–binding activity of α-adducin in vitro. Here we identified the sites of phosphorylation of α-adducin by Rho-kinase as Thr445 and Thr480. We prepared antibody that specifically recognized α-adducin phosphorylated at Thr445, and found by use of this antibody that Rho-kinase phosphorylated α-adducin at Thr445 in COS7 cells in a Rho-dependent manner. Phosphorylated α-adducin accumulated in the membrane ruffling area of Madin-Darby canine kidney (MDCK) epithelial cells and the leading edge of scattering cells during the action of tetradecanoylphorbol-13-acetate (TPA) or hepatocyte growth factor (HGF). The microinjection of Botulinum C3 ADP-ribosyl-transferase, dominant negative Rho-kinase, or α-adducinT445A,T480A (substitution of Thr445 and Thr480 by Ala) inhibited the TPA-induced membrane ruffling in MDCK cells and wound-induced migra- tion in NRK49F cells. α-AdducinT445D,T480D (substi- tution of Thr445 and Thr480 by Asp), but not α-adducinT445A,T480A, counteracted the inhibitory effect of the dominant negative Rho-kinase on the TPA-induced membrane ruffling in MDCK cells. Taken together, these results indicate that Rho-kinase phosphorylates α-adducin downstream of Rho in vivo, and that the phosphorylation of adducin by Rho-kinase plays a crucial role in the regulation of membrane ruffling and cell motility.
Keywords: Rho, Rho-kinase, adducin, membrane ruffling, cell motility
Membrane ruffling, a dynamically fluctuating movement of protrusion consisting of filopodia and lamellipodia, is rapidly induced in response to certain extracellular signals and is also induced in the leading edges of motile cells (Stossel, 1993; Lauffenburger and Horwitz, 1996; Mitchison and Cramer, 1996; Welch et al., 1997). Membrane ruffling is thought to be important for cell motility (Mitchison and Cramer, 1996). The small GTPase Rho regulates the formation of actin stress fibers and focal adhesions (Ridley and Hall, 1992, 1994; Hall, 1998), membrane ruffling (Nishiyama et al., 1994; Takaishi et al., 1995) and cell motility (Takaishi et al., 1993, 1994) in certain types of cells, cell morphology (Paterson et al., 1990), cell aggregation (Tominaga et al., 1993), smooth muscle contraction (Hirata et al., 1992; Gong et al., 1996), neurite retraction in neuronal cells (Nishiki et al., 1990; Jalink et al., 1994), the formation of microvilli (Shaw et al., 1998), and cytokinesis (Kishi et al., 1993; Mabuchi et al., 1993). Rho exerts its biological functions through interaction with specific targets (Van Aelst and D'Souza-Schorey, 1997). Several targets of Rho have been identified, including protein kinase N (PKN)1 (Amano et al., 1996b; Watanabe et al., 1996), Rho-kinase/ROK/ROCK (Leung et al., 1995; Ishizaki et al., 1996; Matsui et al., 1996), the myosin-binding subunit (MBS) of myosin phosphatase (Kimura et al., 1996), p140mDia (Watanabe et al., 1997), citron (Madaule et al., 1995), citron-kinase (Madaule et al., 1998), rhophilin, rhotekin (Reid et al., 1996), and kv1.2 (Cachero et al., 1998). Rho-kinase, which is activated by Rho, regulates the phosphorylation of myosin light chain (MLC) by the direct phosphorylation of MLC (Amano et al., 1996a) and by the inactivation of myosin phosphatase through the phosphorylation of MBS (Kimura et al., 1996). Rho-kinase regulates the formation of stress fibers and focal adhesions (Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997), smooth muscle contraction (Kureishi et al., 1997), and neurite retraction (Amano et al., 1998; Hirose et al., 1998; Katoh et al., 1998) through the phosphorylation of MLC. Rho-kinase also phosphorylates the ezrin/radixin/ moesin (ERM) family proteins (Matsui et al., 1998) and then regulates the formation of microvilli (Oshiro et al., 1998). However, it remains to be clarified how Rho regulates membrane ruffling and cell motility.
Recently, we identified adducin as an MBS-binding protein and a substrate of Rho-kinase in vitro (Kimura et al., 1998). Adducin is a membrane skeletal protein that binds to the fast growing ends (Kuhlman et al., 1996; Li et al., 1998) and the sides (Mische et al., 1987) of actin filaments (F-actin), and thereby promotes the association of spectrin with F-actin to form a spectrin-actin meshwork beneath plasma membranes (Gardner and Bennett, 1987; Bennett et al., 1988). Adducin is composed of either α and β, or α and γ subunits which are closely related in amino acid sequence and domain organization (Gardner and Bennett, 1986; Bennett et al., 1988; Dong et al., 1995). Each adducin subunit has three distinct domains: an amino-terminal head domain, a neck domain, and a carboxy-terminal tail domain (Joshi and Bennett, 1990; Joshi et al., 1991; Dong et al., 1995). The tail domain has a domain called the myristoylated alanine–rich C kinase substrate (MARCKS)-related domain, which contains a highly basic stretch and binds to calmodulin (Joshi et al., 1991; Dong et al., 1995; Matsuoka et al., 1996). Adducin is also a substrate for PKC and PKA (Palfrey and Waseem, 1985; Kaiser et al., 1989; Dong et al., 1995). The phosphorylation of adducin by PKA reduces the activity of adducin to associate with F-actin and spectrin–F-actin complexes and to recruit spectrin to F-actin (Matsuoka et al., 1996). The phosphorylation of adducin by PKC also inhibits activity of adducin in binding to F-actin and recruiting spectrin to F-actin (Matsuoka et al., 1998). On the other hand, the phosphorylation of adducin by Rho-kinase results in the interaction of adducin with F-actin in vitro (Kimura et al., 1998).
In this study, we identified the sites of phosphorylation of α-adducin by Rho-kinase as Thr445 and Thr480, and prepared antibody that specifically recognized α-adducin phosphorylated at Thr445 to elucidate the functions of α-adducin phosphorylated by Rho-kinase. We found that α-adducin was phosphorylated at Thr445 via the Rho/ Rho-kinase pathway in intact cells, and that the phosphorylation of α-adducin by Rho-kinase plays a crucial role in membrane ruffling and cell motility.
Materials and Methods
Materials and Chemicals
MDCK cells were kindly provided by Dr. S. Tsukita (Kyoto University, Kyoto, Japan). NRK49F cells were purchased from American Type Culture Collection. pEF-BOS-HA-RhoAV14, RhoAN19, Rac1V12, Cdc42V12, pEF-BOS-myc-Rho-kinase (full-length, 6–1388 amino acids [aa]), Rho-binding domain (RB; 941–1075 aa), RB/PH (TT) (941–1388 aa), and CAT (the catalytic domain of Rho-kinase, 6–553 aa) were constructed as described (Kuroda et al., 1996; Matsui et al., 1996; Amano et al., 1997, 1998). pcDSRα-PKCβ lacking C1 domain at positions 6–159 aa was constructed as described (Kaibuchi et al., 1989). pQE-rat γ-adducin fragment (319–671 aa; 35H fragment-4D) was kindly provided by Dr. S. Jaken (University of Vermont, Burlington, VT), and 6 X His-tagged γ-adducin fragment was produced and purified as described (Dong et al., 1995). The expression plasmid of C3 transferase (pGEX-C3) was kindly provided by Dr. A. Hall (University College London, London, UK), and C3 was produced and purified as described (Fukata et al., 1998). Glutathione-S-transferase (GST)-RB was produced and purified from Escherichia coli as described (Amano et al., 1997). GST-CAT was produced and purified from Sf9 cells as described previously (Matsuura et al., 1987; Amano et al., 1996a). Rac1N17 was produced and purified from E. coli as described (Kuroda et al., 1996). Anti-hemagglutinin (HA) monoclonal Ab (12CA5) was purchased from Boehringer Mannheim. [γ-32P]ATP was purchased from Amersham Corp. All materials used in the nucleic acid study were purchased from Takara Shuzo Corp. Other materials and chemicals were obtained from commercial sources.
Plasmid Constructs
The cDNA encoding human α-adducin (1–737 aa; Joshi et al., 1991) was inserted into the KpnI site of pEF-BOS-HA, into the KpnI site of pAcYM1-HA to obtain recombinant α-adducin by the use of a baculovirus system, and into the BamHI site of pGEX-2T. The cDNA encoding human β-adducin (1–726 aa; Joshi et al., 1991) was also inserted into the KpnI site of pAcYM1-HA. The cDNAs of α-adducinT445A,T480A (AA) and α-adducinT445D,T480D (DD), which were substituted for Thr at both 445 and 480 amino acid residues by Ala or Asp, were generated with the use of a site-directed mutagenesis kit (Stratagene), and subcloned into pEF-BOS-HA, pAcYM1-HA, and pGEX-2T. The cDNAs of α-adducinT445A and α-adducinT480A were also generated as above and subcloned into pGEX-2T.
Protein Purification
RB/PH (TT) (Amano et al., 1998) was expressed as a maltose-binding protein (MBP) fusion protein in E. coli and purified. GST-α-adducin, GST-α-adducin-AA, GST-α-adducinT445A, and GST-α-adducinT480A were produced and purified from E. coli. HA-α-adducin, HA-β-adducin, HA-α-adducin-AA, and HA-α-adducin-DD were produced in Sf9 cells. The cells expressing HA-α-adducin were suspended in homogenizing buffer (20 mM Tris-HCl, pH 8.0, 1 mM EDTA, 1 mM DTT, 10 μM A-PMSF, 10 μg/ml leupeptin). The suspension was sonicated and centrifuged at 100,000 g for 1 h at 4°C. The supernatant was applied onto a Mono Q column (Pharmacia LKB Biotechnology) which had been equilibrated with buffer A (20 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1 mM DTT). The column was then washed, and the proteins were eluted with a linear concentration gradient of NaCl (0–600 mM) in buffer A. HA-α-adducin was eluted with ∼200 mM NaCl and purified to near homogeneity. HA-β-adducin, HA-α-adducin-AA, and HA-α-adducin-DD were prepared as HA-α-adducin.
Determination of Phosphorylation Sites of α-Adducin by Rho-Kinase
HA-α-adducin (70 μg of protein) was phosphorylated with GST-CAT (120 ng of protein) in 2 ml of kinase buffer (50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT) containing 100 μM [γ-32P]ATP for 1 h at 30°C, and the reaction product was digested with Achromobacter protease-1 (AP-1) at 37°C for 20 h. The obtained peptides were applied onto a C18 reverse-phase column (4.6 × 250 mm; Shiseido, Japan) and eluted with a linear gradient of 0–48% acetonitrile for 100 min at a flow rate of 1.0 ml/min by HPLC (System Gold, Beckman). The radioactive peaks were separated and phosphoamino acid sequencing was carried out with a peptide sequencer (PPSQ-10; Shimadzu, Japan) as described (Bodwell et al., 1991). The fractions obtained from each Edoman degradation cycle were measured for 32P in a Beckman liquid scintillation counter.
Production of Site- and Phosphorylation State–specific Antibody for α-Adducin
The phosphopeptide Cys-Gln440-Gln-Arg-Glu-Lys-phospho-Thr-Arg-Trp- Leu-Asn-Ser450 was chemically synthesized as an antigen by Peptide Institute Inc. Rabbit polyclonal antibody (anti-pT445) was raised against the phosphopeptide and affinity purified as described (Inagaki et al., 1997). To examine the specificity of anti-pT445, equal amounts of HA-α-adducin (40 fmol) with various ratios between phosphorylated and nonphosphorylated proteins were subjected to SDS-PAGE. HA-α-adducin-AA (40 fmol) was incubated in kinase buffer containing GST-CAT and ATP, and subjected to SDS-PAGE. The amount of phosphates incorporated into HA-α-adducin was simultaneously determined by [γ-32P]ATP. Immunoblot analysis was then performed with anti-pT445, anti-pT445 which was preincubated with a 100-fold amount of antigen-phosphopeptide, and anti-HA Ab. For some experiments, HA-α-adducin, HA-β-adducin, or 6 X His-γ-adducin fragment was separately phosphorylated with GST-CAT, and 40 fmol (as 32P-incorporated amount) of phosphorylated proteins was subjected to immunoblot analysis with anti-pT445.
Cell Culture
MDCK cells and NIH3T3 cells were maintained in DMEM containing 10% calf serum, streptomycin, and penicillin. COS7 cells were maintained in DMEM containing 10% FBS, streptomycin, and penicillin. NRK49F cells were maintained in DMEM containing 5% calf serum and 1% nonessential amino acids. To obtain MDCK cells stably expressing HA-α-adducin, MDCK cells were transfected with pEF-BOS-HA-α-adducin along with pSVIISRα vector containing the neomycin resistance gene using Lipofectamine (GIBCO BRL) as described (Kuroda et al., 1998), and neomycin-resistant clones were selected.
Detection of Thr445-phosphorylated α-Adducin in Intact Cells
The transfection of plasmids into COS7 cells was carried out by the standard DEAE-dextran method. COS7 cells were cotransfected with pEF-BOS-HA-α-adducin and pEF-BOS-vector, pEF-BOS encoding HA-Rac1V12, HA-Cdc42V12, HA-RhoAV14, HA-RhoAN19, myc-Rho-kinase (full-length), HA-RhoAV14 and myc-Rho-kinase (full-length), HA-RhoAV14 and myc-RB, HA-RhoAV14 and myc-RB/PH (TT), myc-CAT, or pcDSRα-PKCβ. Separately, COS7 cells were cotransfected with pEF-BOS-HA-α-adducin-AA and myc-CAT. After a 24-h incubation, the transfected cells were incubated in serum-free medium for 24 h. The cells were treated with 10% (wt/vol) trichloroacetic acid. The resulting precipitates were subjected to immunoblot analysis using anti-HA Ab and anti-pT445. MDCK cells stably expressing HA-α-adducin were incubated in the absence or the presence of tetradecanoylphorbol-13-acetate (TPA) for 15 min followed by incubation in the presence of 0.1 μM calyculin A for 10 min. The cells were then treated as COS7 cells and subjected to immunoblot analysis.
Immunofluorescence Analysis
MDCK cells were deprived of serum for 24 h and incubated in DMEM containing 200 nM TPA or 50 pM recombinant human hepatocyte growth factor (HGF; Calbiochem) for 15 min at 37°C. For cell scattering, the cells were not deprived of serum and incubated in DMEM containing 5% calf serum and 200 nM TPA for 2 h. The cells were fixed with 3.0% formaldehyde in PBS for 10 min and treated with PBS containing 0.2% Triton X-100 for 10 min. Phosphorylated adducin and F-actin were doubly stained with anti-pT445 and TRITC-phalloidin. For anti-pT445, the cells were then labeled with FITC-conjugated anti–rabbit Ig Ab. For a double immunofluorescence study, the clone stably expressing HA-α-adducin was stimulated as described above. Phosphorylated adducin and HA-α-adducin were doubly stained with anti-pT445 and anti-HA Ab. The cells were then labeled with FITC-conjugated anti–rabbit Ig Ab and Texas red–conjugated anti–mouse Ig Ab. The cells were examined using a Zeiss Axiophot microscope or a confocal microscope (Carl Zeiss).
Microinjection
MDCK cells were seeded at a density of 2.5 × 103 cells per 13-mm cover glass in 60-mm tissue culture dishes and incubated for 8 h. Then, the cells were deprived of serum for 24 h. For microinjection, proteins were concentrated and during the concentration the buffer was replaced by microinjection buffer (20 mM Tris-HCl, pH 7.4, 20 mM NaCl, 1 mM MgCl2, 0.1 mM EDTA, 5 mM 2-mercaptoethanol). The indicated proteins were microinjected along with a marker protein (rabbit or mouse IgG at 0.5 mg/ml) into the cytoplasm of cells. Using microcapillaries (tip diameter is 0.5 μm), ∼3 × 10−14 liter of each protein was microinjected by one injection. When C3 was microinjected at 0.1 mg/ml, the injected amount per cell was ∼3 × 10−15 g (the intracellular concentration was estimated to be ∼0.15 μM). When HA-α-adducin mutants were microinjected at 5.0 mg/ml, the injected amount per cell was ∼150 × 10−15 g (the intracellular concentration was estimated to be ∼2.0 μM, and it seemed to be much higher than the level of endogenous α-adducin). 30 min after the microinjection, the cells were incubated in DMEM containing 200 nM TPA for 15 min at 37°C. The cells were then fixed and stained with anti-pT445 or TRITC-phalloidin as described above.
Wound Healing Assay
Cell migration assay using a wound healing model was performed as described previously (Santos et al., 1997). The confluent monolayer of the cells was scraped with a white chip. Soon after wounding, the medium was changed and indicated recombinant proteins were microinjected along with a marker protein (rabbit IgG, 1.0 mg/ml) into the cytoplasm of only the cells along the wound edge as described above. The original wound edge was determined by taking photographs soon after the cells were wounded and microinjected. After 6 h, the cells were then stained with TRITC-phalloidin, and the cells that moved (>20 μm) from the original wound edge were counted by taking photographs of the same fields as the original photograph.
Other Procedures
SDS-PAGE was performed as described (Laemmli, 1970). Protein concentrations were determined with BSA as the reference protein as described (Bradford, 1976).
Results
Determination of the Sites of Phosphorylation of α-Adducin by Rho-Kinase
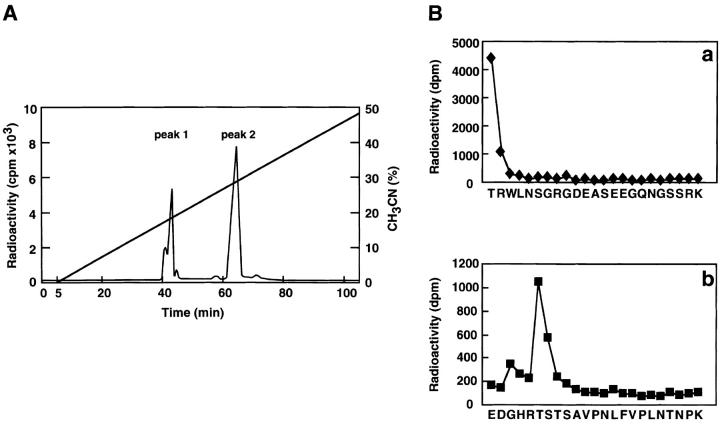
We first determined the sites of phosphorylation of human α-adducin by Rho-kinase as follows. HA-tagged α-adducin, which was phosphorylated by Rho-kinase in the presence of [γ-32P]ATP, was digested with AP-I and subjected to reverse-phase HPLC as described (Shirataki et al., 1993). Two major radioactive peaks (named peak 1 and 2, respectively) were obtained (Fig. 1 A) and their amino acid sequences were determined. The sequence obtained from peak 1 was TRWLNSGRGDEASEEGQNGSSPK, corresponding to residues 445–467 of human α-adducin, and Thr445 turned out to be the phosphorylation site for Rho-kinase (Fig. 1 B, panel a). The sequence obtained from peak 2 was EDGHRTSTSAVPNLFVPLNTNPK, corresponding to residues 475–497 of human α-adducin, and Thr480 turned out to be the phosphorylation site for Rho-kinase (Fig. 1 B, panel b). Both phosphorylation sites were in the neck domain of α-adducin, and were different from previously identified sites of phosphorylation by PKA (Ser408, Ser436, Ser481, and Ser726) and by PKC (Ser726) (Matsuoka et al., 1996) (Fig. 1 C). When GST-α-adducinT445A or GST-α-adducinT480A (substitution of Thr445 or Thr480 by Ala) was phosphorylated by Rho-kinase, GST-α-adducinT480A was more efficiently phosphorylated by Rho-kinase than was GST-α-adducinT445A (Fig. 1 D). This result is apparently inconsistent with the observation that the radioactivity of peak 1 containing Thr445 is lower than that of peak 2 containing Thr480 in the HPLC analysis (Fig. 1 A). This may be due to the different recovery of each peptide during separation by HPLC. Taken together, these results suggest that Thr445 is a preferential site of phosphorylation by Rho-kinase in vitro.
Figure 1.
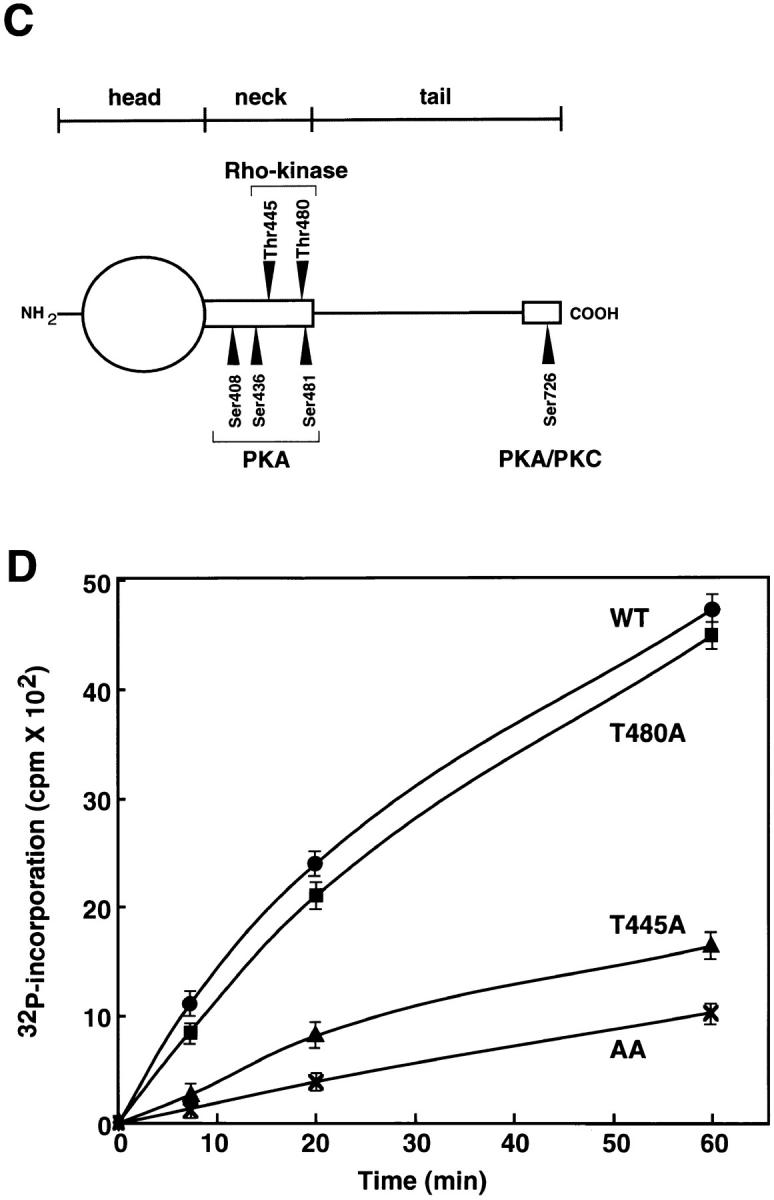
Determination of sites of phosphorylation of α-adducin by Rho-kinase. (A) Reverse-phase HPLC for AP-1 fragments of α-adducin phosphorylated by Rho-kinase was carried out under the conditions described in Materials and Methods. CH3CN, acetonitrile. (B) Amino acid sequences of phosphopeptides derived from α-adducin phosphorylated by Rho-kinase. The radioactive peaks 1 (panel a) and 2 (panel b) were separated. Phosphoamino acid sequencing of each peak was carried out with a peptide sequencer, and the fractions obtained from each Edoman degradation cycle were measured for 32P in a liquid scintillation counter. (C) Structure and phosphorylation sites of α-adducin. A model for α-adducin based on Matsuoka et al. (1996) is presented. The phosphorylation sites of α-adducin for PKA, PKC, and Rho-kinase are presented. (D) Preferential phosphorylation of α-adducin at Thr445 by Rho-kinase in vitro. GST-α-adducin (WT, circles), GST-α-adducin-AA (AA, X), GST-α-adducinT445A (T445A, triangles), and GST-α-adducinT480A (T480A, squares) were phosphorylated by GST-CAT for various periods. Data are means ± SEM of triplicate determinations.
Production and Characterization of the Site- and Phosphorylation State–specific Antibody for α-Adducin
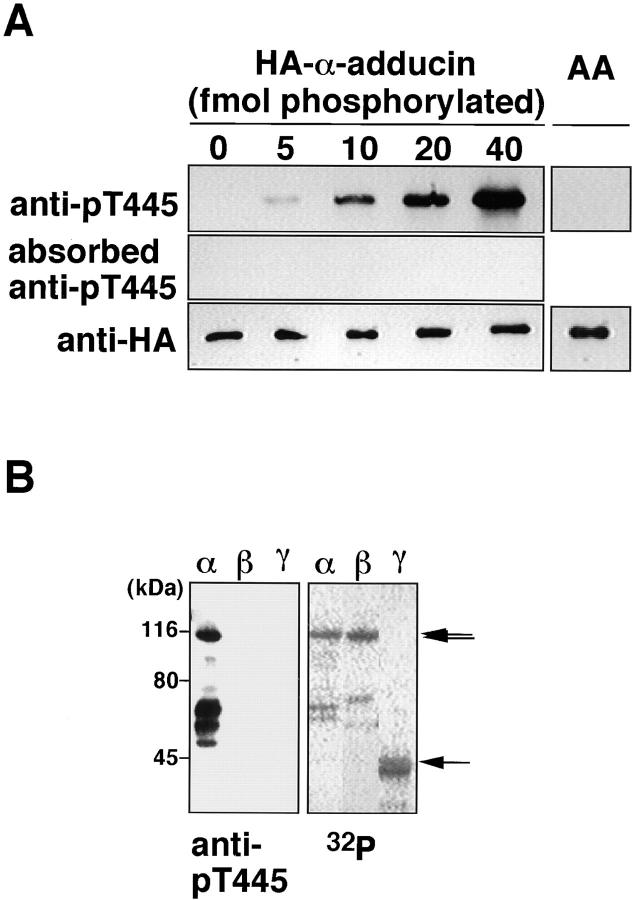
An antibody that recognizes the phosphorylated state of a substrate at a specific site is a useful tool with which to evaluate site-specific phosphorylation in vivo and to visualize the cellular distribution of the protein phosphorylated at a specific site (Inagaki et al., 1997). We prepared and affinity purified the polyclonal antibody that recognized the Thr445-phosphorylated α-adducin (anti-pT445), against the synthetic phosphopeptide Cys-Gln-Gln-Arg-Glu-Lys-phospho-Thr445-Arg-Trp-Leu-Asn-Ser. An immunoblot analysis revealed that anti-pT445 specifically bound to HA-α-adducin phosphorylated by Rho-kinase in a dose-dependent manner, but not to nonphosphorylated HA-α-adducin or HA-α-adducinT445A,T480A (substitution of Thr445 and Thr480 by Ala; HA-α-adducin-AA) incubated with Rho-kinase and ATP (Fig. 2 A). HA-α-adducin, which was phosphorylated by PKC or PKN at levels similar to that by Rho-kinase, was not recognized by anti-pT445 (data not shown). The binding of anti-pT445 to α-adducin phosphorylated by Rho-kinase was neutralized by preincubation of the antibody with the antigen phosphopeptide. Rho-kinase phosphorylated β- and γ-adducin in vitro as well (Fig. 2 B). Neither β- nor γ-adducin phosphorylated by Rho-kinase was recognized by anti-pT445 (Fig. 2 B). These results indicate that anti-pT445 specifically recognizes only α-adducin phosphorylated at Thr445.
Figure 2.
Specificity of anti-pT445. (A) HA-α-adducin (40 fmol), containing the indicated amounts of HA-α-adducin phosphorylated by Rho-kinase, and HA-α-adducin-AA (40 fmol) phosphorylated by Rho-kinase were subjected to immunoblot analysis with anti-pT445, anti-pT445 preincubated with a 100-fold amount of antigen-phosphopeptide, and anti-HA antibody. The amount of phosphates incorporated into HA-α-adducin was simultaneously determined by [γ-32P]ATP. (B) Anti-pT445 reacts with only α-adducin phosphorylated by Rho-kinase. HA-α-adducin, HA-β-adducin, or 6 X His-γ-adducin fragment (319–671 aa) was separately phosphorylated with GST-CAT, resolved by SDS-PAGE, and visualized by an image analyzer (right). 40 fmol (as 32P-incorporated amount) of phosphorylated proteins was subjected to immunoblot analysis with anti-pT445 (left). From the top, arrows indicate the positions of the intact HA-α-adducin, HA-β-adducin, or 6 X His-γ-adducin, respectively. Anti-pT445 also bound to the degradation products of HA-α-adducin. The results are representative of three independent experiments.
Rho/Rho-Kinase–dependent Phosphorylation of α-Adducin in COS7 Cells
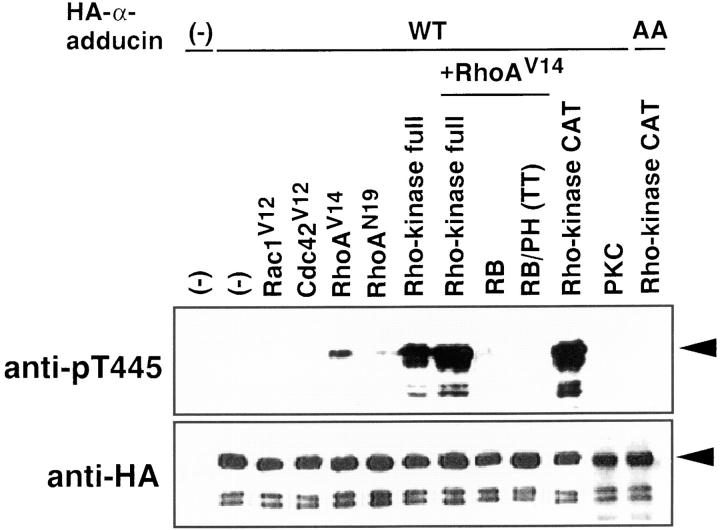
We examined whether α-adducin was phosphorylated via the Rho/Rho-kinase pathway in COS7 cells by immunoblot analysis with anti-pT445. cDNA of HA-α-adducin was cotransfected with plasmids carrying the cDNA of dominant active Rho family members, or dominant active or negative Rho-kinase (Fig. 3). HA-α-adducin was almost equally expressed in all the HA-α-adducin–transfected cells. α-Adducin phosphorylated at Thr445 was undetectable in serum-starved COS7 cells expressing HA-α-adducin alone. The expression of dominant active RhoA (RhoAV14), a RhoA mutant that is defective in GTPase activity and thought to be constitutively the GTP-bound form in the cells, induced a small increment of the α-adducin phosphorylation at Thr445, whereas the expression of dominant negative RhoA (RhoAN19), a RhoA mutant that preferentially binds GDP rather than GTP and is thought to be constitutively the GDP-bound form in the cells, had minimal effects. Although the exact reason for the minimal increase in phosphorylated α-adducin in COS7 cells expressing RhoAN19 is unknown, it is likely that the overexpression of GDP-bound RhoAN19 induced the phosphorylation of α-adducin to a minimal extent because GDP · Rho also activates Rho-kinase in vitro to a small extent as described (Matsui et al., 1996). Dominant active Rac1 (Rac1V12) or dominant active Cdc42 (Cdc42V12) had no effects. Under the conditions, RhoAV14, RhoAN19, Rac1V12, and Cdc42V12 were almost equally expressed (data not shown). The expression of Rho-kinase induced the α-adducin phosphorylation to some extent. By the coexpression of RhoAV14 and Rho-kinase, the α-adducin phosphorylation was further enhanced. Rho-kinase is composed of catalytic (CAT), coiled-coil (COIL), Rho-binding (RB), and pleckstrin-homology (PH) domains (Matsui et al., 1996). RB (941–1075 aa) binds to Rho and inhibits the Rho-dependent Rho-kinase activity in vitro and in vivo (Amano et al., 1997). RB/PH (TT) (941–1388 aa), which is the carboxy-terminal portion of Rho-kinase and encompasses the RB and PH domains, has point mutations in the RB domain and does not bind to Rho (Fujisawa et al., 1996; Leung et al., 1996; Amano et al., 1998). RB/PH (TT) inhibits the lysophosphatidic acid (LPA)-induced stress fiber formation in fibroblasts and neurite retraction in neuroblastoma cells (Amano et al., 1998), suggesting that RB/PH (TT) serves as the dominant negative form of Rho-kinase. Recently, we found that RB/ PH (TT) interacted with the catalytic domain of Rho-kinase and thereby inhibited the Rho-kinase activity in vitro without titrating out Rho (Amano, M., K. Chihara, N. Nakamura, T. Kaneko, Y. Matsuura, and K. Kaibuchi, manuscript submitted for publication). We further found that RB/PH (TT) had no effect on catalytic activity of PKN or myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK), which is identified as a target of Cdc42 and is homologous to Rho-kinase within the kinase domain (Leung et al., 1998), in vitro (Amano, M., K. Chihara, N. Nakamura, T. Kaneko, Y. Matsuura, and K. Kaibuchi, manuscript submitted for publication). RB/PH (TT) inhibited the Rho-kinase–induced stress fiber, but not MRCK-induced stress fiber (Leung et al., 1998) in fibroblasts (Amano, M., K. Chihara, N. Nakamura, T. Kaneko, Y. Matsuura, and K. Kaibuchi, manuscript submitted for publication). These observations indicate that RB/PH (TT) more specifically inhibits the Rho-kinase activity in intact cells. α-Adducin phosphorylation at Thr445 by RhoAV14 was suppressed by RB and RB/PH (TT) (Fig. 3). Constitutively active Rho-kinase (CAT) greatly induced the α-adducin phosphorylation (Fig. 3), whereas constitutively active MRCK did not (data not shown). When the dominant active form of PKC, which activates the TPA-responsive element linked to the chloramphenicol acetyltransferase reporter gene in the absence of phorbol ester (Kaibuchi et al., 1989), was expressed, the phosphorylation of α-adducin was not detected by anti-pT445 (Fig. 3). HA-α-adducin-AA coexpressed with CAT was not recognized by anti-pT445 (Fig. 3). These results indicate that α-adducin was specifically phosphorylated at Thr445 via the Rho/ Rho-kinase pathway in intact cells.
Figure 3.
The phosphorylation of α-adducin via the Rho/Rho-kinase pathway in vivo. pEF-BOS-HA-α-adducin (WT) (3 μg) was cotransfected into COS7 cells with pEF-BOS vector (6 μg), pEF-BOS encoding HA-Rac1V12 (3 μg), HA-Cdc42V12 (3 μg), HA-RhoAV14 (3 μg), HA-RhoAN19 (3 μg), myc-Rho-kinase (full-length) (3 μg), HA-RhoAV14 (3 μg) and myc-Rho-kinase (full-length) (3 μg), HA-RhoAV14 (3 μg) and myc-RB (6 μg), HA-RhoAV14 (3 μg) and myc-RB/PH (TT) (6 μg), and myc-CAT (3 μg) or pcDSRα-PKC (6 μg). pEF-BOS-HA-α-adducin-AA (3 μg) was also cotransfected with myc-CAT (3 μg). As a negative control, pEF-BOS vector (12 μg) alone was transfected. After a 24-h incubation, the transfected cells were incubated in serum-free medium for 24 h. The lysates of the cells were subjected to immunoblot analysis with indicated antibodies. The amounts of the sample in lanes of HA-α-adducin or HA-α-adducin-AA cotransfected with CAT of the upper panel were 30% of the others. Arrowheads indicate the positions of the intact band of HA-α-adducin. The anti-pT445–immunoreactive bands just below the intact HA-α-adducin correspond to endogenous α-adducin. The additional bands which anti-pT445 recognized were the degradation products, because these proteins were also detected by anti-HA antibody. The ratios of phosphorylation at Thr445 to expressed HA-α-adducin were quantitated, and the proportions relative to the ratio of CAT-induced phosphorylation of HA-α-adducin were indicated in brackets as follows: HA-α-adducin alone, or with Rac1V12 or Cdc42V12 (0%); with RhoAV14 (4%); with RhoAN19 (0.3%); with Rho-kinase (20%); with RhoAV14 and Rho-kinase (32%); with RhoAV14 and RB (0.1%); with RhoAV14 and RB/PH (TT) (0%); with CAT (100%); with PKC (0%); and HA-α-adducin-AA with CAT (0%). The results are representative of three independent experiments.
Specific Phosphorylation of α-Adducin at Thr445 in the TPA-induced Membrane Ruffling Areas
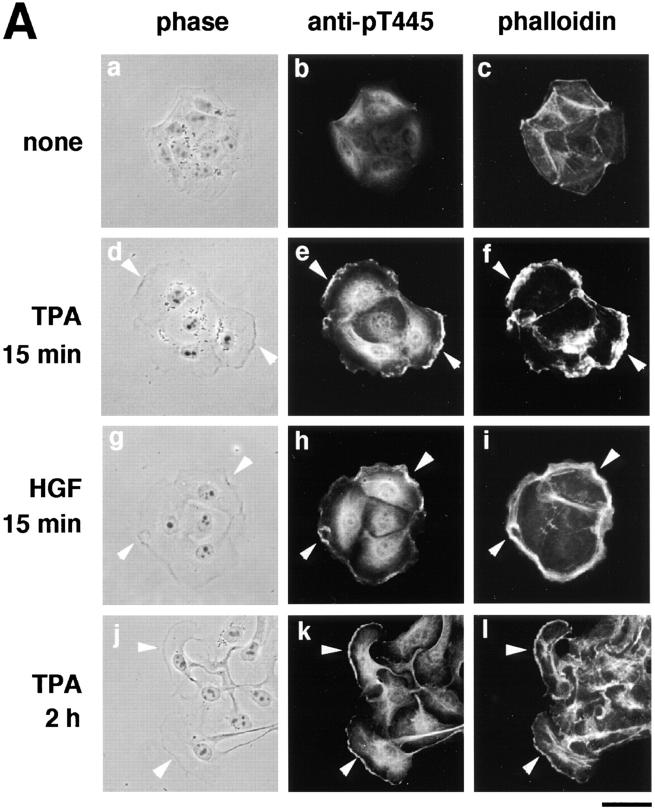
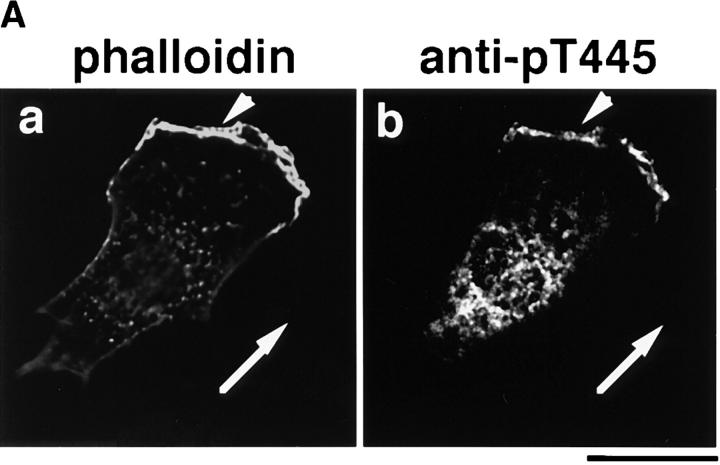
Both Rho and Rac are thought to be necessary for the HGF- and TPA-induced membrane ruffling and HGF-induced cell motility in MDCK epithelial cells, although neither RhoAV14 nor Rac1V12 by itself induces membrane ruffling or cell motility (Takaishi et al., 1993, 1994, 1995; Nishiyama et al., 1994; Ridley et al., 1995). We examined the distribution of Thr445-phosphorylated α-adducin in the TPA- or HGF-stimulated MDCK cells. We have found by immunoblotting with isoform-specific antibodies that 120-kD α-adducin and 90-kD γ-adducin (alternatively spliced form of 80-kD γ-adducin) were expressed almost equally in MDCK cells. We have also found that 110-kD β-adducin was expressed to a lesser extent in MDCK cells. Therefore, it is likely that anti-pT445 recognizes only phospho-Thr445 in α-adducin in MDCK cells. The addition of TPA induced membrane ruffling at the outer edge of cell colonies (Fig. 4 A) as described (Nishiyama et al., 1994; Takaishi et al., 1995). The immunoreactivity of anti-pT445 accumulated in the membrane ruffling areas, where F-actin (Fig. 4 A) and spectrin (data not shown) also accumulated. Phosphorylated α-adducin was also detected in the perinuclear region. Similar results were obtained when the cells were stimulated with HGF instead of TPA (Fig. 4 A). The immunoreactivity with anti-pT445 was abolished by preincubation of the antibody with the antigen phosphopeptide (data not shown). In the nonstimulated MDCK cells, phosphorylated α-adducin was diffusely present in the cytoplasm, but not in the free ends of the plasma membrane. Between 2 and 16 h after the addition of TPA, the cells dissociated from each other and scattered, with polarized morphology and membrane ruffling in the leading edge as described (Takaishi et al., 1994; Ridley et al., 1995). Phosphorylated α-adducin also accumulated in the leading edge of TPA-induced scattering cells (Fig. 4 A). To compare the localization of phosphorylated α-adducin and total α-adducin, a double-label immunofluorescence study was performed in MDCK cells stably expressing HA-α-adducin. Immunoreactivity showing the distribution of HA-α-adducin (red) was detected strongly in the cell–cell contact sites (Fig. 4 B, asterisk), weakly in the membrane ruffling areas (Fig. 4 B, arrow), and diffusely in the cytoplasm, as described (Kaiser et al., 1989; Marrs et al., 1993). Phosphorylated α-adducin (green) was enriched in the membrane ruffling areas in the TPA-stimulated MDCK cells (Fig. 4 B, arrow). The merged image of red and green immunofluorescence revealed the enrichment of phosphorylated α-adducin in the membrane ruffling areas and a small increment of phosphorylated α-adducin in the cytoplasm in the TPA-stimulated cells (Fig. 4 B, panel f, greenish image). Phosphorylated α-adducin did not accumulate at the cell–cell contact sites (Fig. 4 B, panels c and f, reddish images indicated by the asterisks). Similar results were obtained when the cells were stimulated with HGF. To confirm that α-adducin is specifically phosphorylated at Thr445 during the action of TPA, immunoblot analysis with anti-pT445 was performed. The addition of TPA to the MDCK cells stably expressing HA-α-adducin increased the phosphorylation level of HA-α-adducin at Thr445 (Fig. 4 C).
Figure 4.
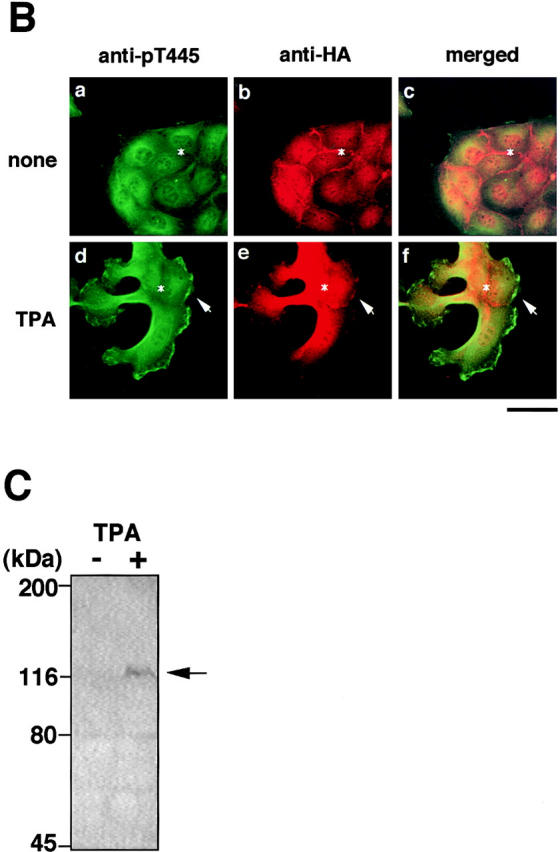
The localization of Thr445-phosphorylated α-adducin in the TPA- or HGF-induced membrane ruffling. (A) The serum-deprived MDCK cells were stimulated with 200 nM TPA (panels d–f) or 50 pM HGF (panels g–i) for 15 min or with 200 nM TPA for 2 h (panels j–l). Nonstimulated (panels a–c) and stimulated cells were doubly labeled with anti-pT445 (panels b, e, h, and k) and TRITC-phalloidin (panels c, f, i, and l). Phase-contrast images are shown (panels a, d, g, and j). Arrowheads indicate the induced membrane ruffling areas or the leading edges. Bar, 25 μm. (B) The enrichment of phosphorylated α-adducin in the membrane ruffling areas. MDCK cells stably expressing HA-α-adducin were established. Nonstimulated (panels a–c) or TPA-stimulated (panels d–f) MDCK cells stably expressing HA-α-adducin were doubly stained with anti-pT445 (green; panels a and d) and anti-HA (red; panels b and e). c and f show merged images of a and b, and d and e, respectively. Arrows indicate the induced membrane ruffling areas. Asterisks indicate the cell–cell contact sites. Bar, 25 μm. (C) The TPA-induced Thr445 phosphorylation in α-adducin. MDCK cells stably expressing HA-α-adducin were stimulated by vehicle (−) or TPA (+) for 15 min followed by incubation in the presence of calyculin A, and then lysed and subjected to immunoblot analysis with anti-pT445. An arrow indicates the phosphorylated HA-α-adducin. These results are representative of three independent experiments.
The Inhibition of the TPA-induced Membrane Ruffling by the Dominant Negative Rho-Kinase and α-Adducin Mutant
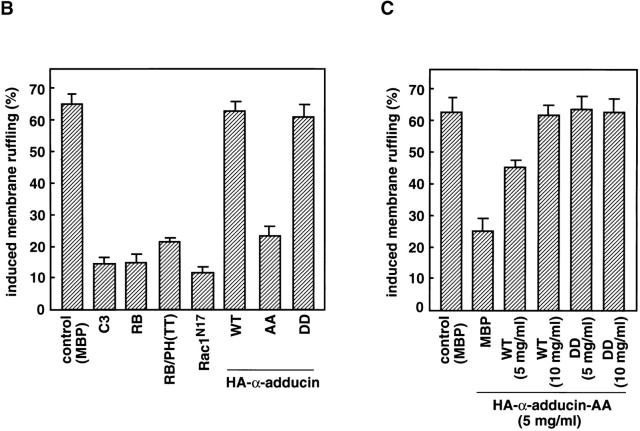
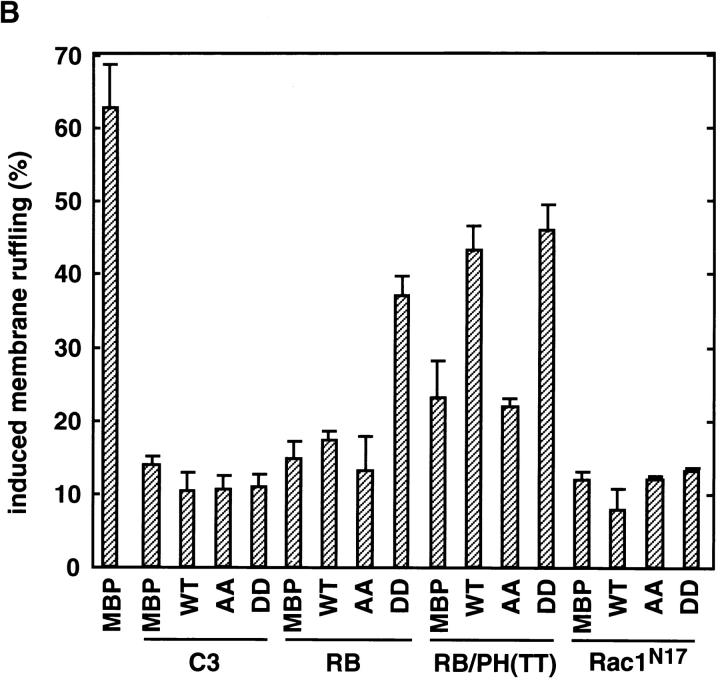
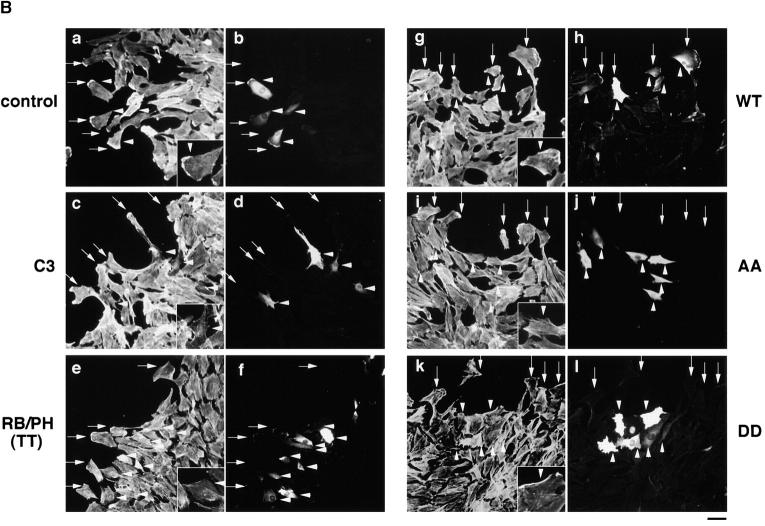
We next examined whether the Rho/Rho-kinase pathway was required for the TPA-induced membrane ruffling. The microinjection of C3, which is thought to interfere with endogenous Rho functions, RB, or RB/PH (TT) inhibited the TPA-induced membrane ruffling and the accumulation of phosphorylated adducin in the free end of the plasma membrane (Fig. 5, A and B); membrane ruffling was induced in 64% of TPA-stimulated cells in the outer edges of cell colonies, but in only 14% of cells injected with C3, 15% of cells injected with RB, and 22% of cells injected with RB/PH (TT). Dominant negative Rac (Rac1N17) also inhibited the TPA-induced membrane ruffling to a similar extent (Ridley et al., 1995). These results indicate that the Rho-kinase function is required for the TPA-induced membrane ruffling in MDCK cells.
Figure 5.
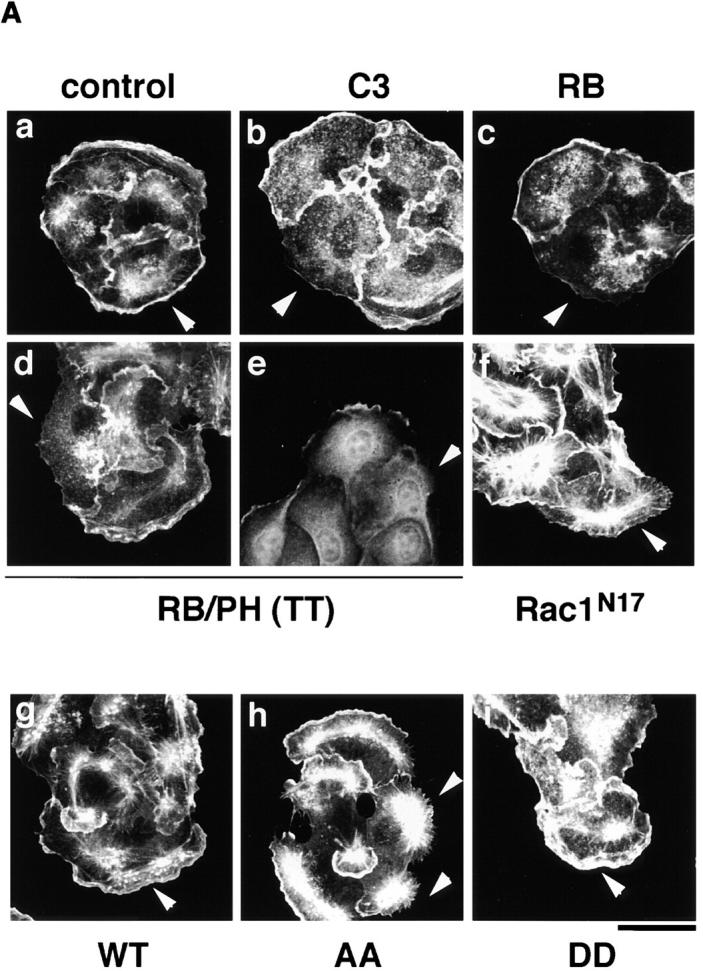
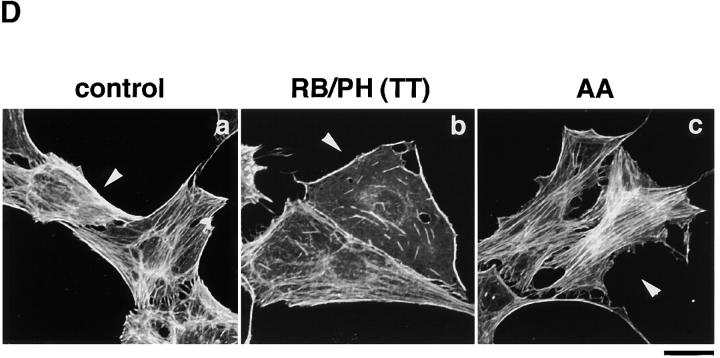
The inhibition of the TPA-induced membrane ruffling by the mutants of Rho-kinase and α-adducin. (A) The serum-deprived MDCK cells were microinjected with MBP (2 mg/ ml; panel a), C3 (0.1 mg/ml; panel b), GST-RB (2 mg/ml; panel c), MBP-RB/ PH (TT) (2 mg/ml; panels d and e), or Rac1N17 (0.2 mg/ml; panel f), HA-α-adducin (WT) (5 mg/ml; panel g), HA-α-adducin-AA (5 mg/ml; panel h), or HA-α-adducin-DD (5 mg/ml; panel i) along with a marker protein (rabbit or mouse IgG, 0.5 mg/ml). After a 30-min incubation, the cells were stimulated with 200 nM TPA for 15 min. F-actin (panels a–d and f–i) and Thr445-phosphorylated α-adducin (panel e) were visualized. Arrowheads indicate the microinjected cells. Bar, 25 μm. (B) The ratios of the membrane ruffling–induced cells to the cells injected with proteins described in A are indicated. Data are means ± SEM of triplicate determinations. (C) Specificity of the effect of HA-α-adducin-AA on the TPA-induced membrane ruffling. HA-α-adducin-AA (5 mg/ml) was microinjected along with indicated proteins. The ratios of the membrane ruffling–induced cells to the injected cells are indicated. Data are means ± SEM of triplicate determinations. (D) HA-α-adducin-AA had no effect on stress fiber formation. The serum-deprived NIH3T3 cells were microinjected with MBP (2 mg/ml; panel a), MBP-RB/PH (TT) (2 mg/ml; panel b), or HA-α-adducin-AA (5 mg/ml; panel c). After a 30-min incubation, the cells were stimulated with 50 ng/ml LPA for 20 min. F-actin was visualized. Arrowheads indicate the microinjected cells. Bar, 10 μm. These results are representative of three independent experiments.
To determine whether the phosphorylation of adducin by Rho-kinase is necessary or sufficient for the membrane ruffling, we produced and used cDNAs encoding α-adducin mutants; HA-α-adducinT445A,T480A (HA-α-adducin-AA, substitution of Thr residues by Ala), which was not phosphorylated by Rho-kinase and expected to serve as the dominant negative form, and HA-α-adducinT445D,T480D (HA-α-adducin-DD, substitution of Thr residues by Asp), which was expected to mimic phosphorylated α-adducin and to serve as the constitutively active form (Sweeney et al., 1994). The microinjection of HA-α-adducin, HA-α-adducin-AA, or HA-α-adducin-DD by itself did not induce the membrane ruffling in MDCK cells (data not shown). This observation is consistent with the notion that RhoAV14 alone does not induce the membrane ruffling, and that a lot of processes are required for the induction of membrane ruffling. HA-α-adducin or HA-α-adducin-DD was translocated to the membrane ruffling area (data not shown) and did not inhibit the TPA-induced membrane ruffling, whereas HA-α-adducin-AA inhibited the ruffling; membrane ruffling was induced in ∼60% of TPA-stimulated cells injected with HA-α-adducin or HA-α-adducin-DD, but in only 24% of cells injected with HA-α-adducin-AA (Fig. 5, A and B). Although the inhibitory mechanism of HA-α-adducin-AA is not known, HA-α-adducin-AA may substitute for endogenous α-adducin to oligomerize with endogenous β- or γ-adducin, and thereby stay as an inactive complex to inhibit functions of endogenous adducins. Essentially similar results as to the effects of RB/PH (TT) or HA-adducin-AA on membrane ruffling were obtained when MDCK cells were stimulated with HGF instead of TPA (data not shown). To confirm that HA-α-adducin-AA specifically inhibits the function of endogenous α-adducin but not those of other substrates of Rho-kinase such as MLC or the ERM family proteins, we examined whether the inhibitory effect of HA-α-adducin-AA on the membrane ruffling in MDCK cells was reversed by coinjection of HA-α-adducin or HA-α-adducin-DD. As shown in Fig. 5 C, HA-α-adducin reversed the inhibitory effect of HA-α-adducin-AA in a dose-dependent manner. HA-α-adducin-DD more efficiently reversed the inhibitory effect. These results indicate that HA-α-adducin-AA specifically inhibits the function of endogenous α-adducin but not those of other substrates. When HA-α-adducin-AA was microinjected into NIH3T3 cells, HA-α-adducin-AA did not affect the LPA-induced formation of stress fibers, which is mediated by MLC phosphorylation through the Rho/Rho-kinase pathway (Chihara et al., 1997; Amano et al., 1998), whereas RB/PH (TT) inhibited the LPA-induced formation of stress fibers as described (Amano et al., 1998; Fig. 5 D). HA-α-adducin-AA did not affect the LPA-induced formation of microvilli, which is mediated by ERM phosphorylation through the Rho/Rho-kinase pathway, under the conditions in which RB/PH (TT) inhibited the LPA-induced formation of microvilli (Oshiro et al., 1998; data not shown). Taken together, these results suggest that HA-α-adducin-AA specifically interferes with the pathway mediated through the α-adducin phosphorylated by Rho-kinase in intact cells.
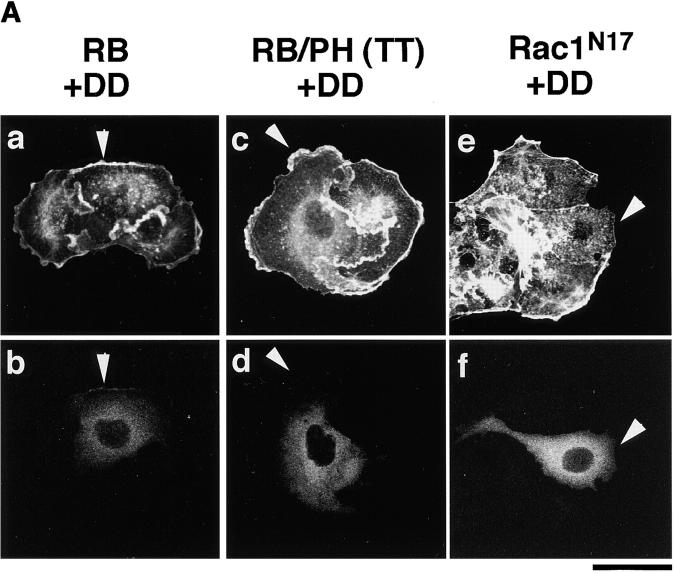
When HA-α-adducin-DD, but not HA-α-adducin-AA, was comicroinjected with RB or RB/PH (TT), the inhibitory effect of RB or RB/PH (TT) on the TPA-induced membrane ruffling was counteracted (Fig. 6, A and B). Under these conditions, HA-α-adducin also counteracted the inhibitory effect of RB/PH (TT), but not that of RB (Fig. 6 B). This difference is likely due to the distinct mode of action between RB and RB/PH (TT). RB/PH (TT) interacts with the catalytic domain of Rho-kinase and inhibits the activity of Rho-kinase in a manner competitive with peptide substrates of Rho-kinase such as RRRLSSLRA without titrating out Rho (Amano, M., K. Chihara, N. Nakamura, T. Kaneko, Y. Matsuura, and K. Kaibuchi, manuscript submitted for publication). The excess of HA-α-adducin over RB/PH (TT) in the cells may neutralize a competitive inhibition of Rho-kinase by RB/PH (TT), and then HA-α-adducin can be phosphorylated by the activated Rho-kinase. Consistently, when RB/PH (TT) was microinjected at higher concentrations, HA-α-adducin could not counteract the inhibitory effect of RB/PH (TT), whereas HA-α-adducin-DD was still capable of counteracting (data not shown). In contrast to RB/PH (TT), RB binds to the effector domain of Rho, and is expected to inhibit the interaction of Rho-kinase with Rho and thereby the activation of Rho-kinase by Rho (Amano et al., 1997). Thus, HA-α-adducin may not overcome the inhibitory effect of RB in MDCK cells. On the other hand, because HA-α-adducin-DD can mimic α-adducin phosphorylated at Thr445 and Thr480 in vivo, HA-α-adducin-DD may bypass Rho-kinase to induce membrane ruffling during the action of TPA irrespective of the Rho-kinase activity. HA-α-adducin-DD counteracted neither the inhibitory effect of C3 nor that of Rac1N17 (Fig. 6, A and B). GTPγS-bound RhoAI41, which is insensitive to C3, could completely overcome the inhibitory effect of C3 as described (Nishiyama et al., 1994; data not shown). Taken together, these results indicate that HA-α-adducin-DD serves as the constitutively active form, and that α-adducin is a crucial substrate of Rho-kinase downstream of Rho for membrane ruffling.
Figure 6.
Effects of α-adducin mutants on the membrane ruffling in the cells introduced with the dominant negative Rho-kinase. MBP, HA-α-adducin (WT), HA-α-adducin-AA, or HA-α-adducin-DD (10 mg/ml) was comicroinjected with C3 (0.1 mg/ml), GST-RB (2 mg/ ml), MBP-RB/PH (TT) (2 mg/ml), or Rac1N17 (0.2 mg/ml) along with a marker protein (rabbit IgG, 0.5 mg/ml) into the serum-deprived MDCK cells. After a 30-min incubation, the cells were stimulated with 200 nM TPA for 15 min. (A) The cells into which HA-α-adducin-DD was comicroinjected with GST-RB (panels a and b), MBP-RB/PH (TT) (panels c and d), and Rac1N17 (panels e and f) are shown. The cells were doubly labeled with TRITC-phalloidin (panels a, c, and e) and FITC anti–rabbit Ig Ab (panels b, d, and f). Arrowheads indicate the microinjected cells. Bar, 25 μm. These results are representative of three independent experiments. (B) The ratios of the membrane ruffling–induced cells to injected cells are indicated. Data are means ± SEM of triplicate determinations.
Adducin Phosphorylation via the Rho/Rho-Kinase Pathway and Cell Motility
Membrane ruffling is thought to be an essential event in cell motility (Mitchison and Cramer, 1996). We then investigated whether the α-adducin phosphorylation by Rho-kinase is involved in cell motility. C3 has been shown to inhibit the wound-induced migration of IEC-6 cells (Santos et al., 1997). We performed a wound healing assay by the use of NRK49F cells to investigate roles of Rho-kinase and adducin in cell motility, since it was difficult to estimate the precise distance moved in MDCK cells and to evaluate cell motility induced by TPA or HGF, and NRK49F cells were more suitable for a wound healing assay than were MDCK cells. Linear wounds were made in a confluent monolayer of NRK49F cells and ∼200 cells along the wound edge were microinjected with C3, RB/PH (TT), or the α-adducin mutants. 6 h after wounding, the cells that moved (>20 μm) from the original wound edge were counted. We also evaluated the wound-induced migration by a time-lapse recording. When MBP was injected as a control protein, 63% of the total injected cells showed wound-oriented migration, and ∼60% of the total injected cells had membrane rufflings (Fig. 7, B and C). About 90% of the migrating injected cells had membrane rufflings in their leading edges (Fig. 7 B). We found that α-adducin phosphorylated at Thr445 accumulated at the membrane ruffling areas in the leading edge and the perinuclear region in migrating NRK49F cells (Fig. 7 A), as shown in MDCK cells. C3 and RB/PH (TT) inhibited the migration to the wound; only 24% of cells injected with C3 and 17% of cells injected with RB/PH (TT) migrated to the wound (Fig. 7, B and C). The microinjection of HA-α-adducin-AA also inhibited the migration; only 21% of cells injected with HA-α-adducin-AA migrated to the wound (Fig. 7, B and C). When C3, RB/PH (TT), or α-adducin-AA was injected, the percentages of the cells with membrane ruffling in the total injected cells (∼20%) were roughly the same as those of the migrating cells in the total injected cells (17–24%), and the stationary injected cells had few membrane rufflings (Fig. 7 B). Whatever proteins were injected, the majority (∼90%) of migrating injected cells had membrane ruffling in the leading edge. These results suggest that the inhibition of cell migration by HA-α-adducin-AA, C3, or RB/PH (TT) correlates with the inhibition of membrane ruffling. In the HA-α-adducin– injected cells, the wound-oriented migration and membrane ruffling were not inhibited (Fig. 7, B and C). HA-α-adducin-DD also inhibited the migration; 32% of cells injected with HA-α-adducin-DD migrated to the wound (Fig. 7, B and C). However, the percentage of the cells with membrane ruffling in the total injected cells (∼60%) was much bigger than that of the migrating cells in the total injected cells (32%). The majority of migrating cells injected with α-adducin-DD had membrane rufflings in the leading edge, and about one-half of the stationary cells injected with α-adducin-DD had membrane rufflings (Fig. 7 B, panel k). These results indicate that α-adducin-DD did not inhibit the membrane ruffling but did inhibit cell migration, suggesting that cycling between the phosphorylated and dephosphorylated states of α-adducin appears to be necessary for cell migration, but not for membrane ruffling. Consistently, RhoAV14 or CAT as well as Rac1N17 inhibited the wound-induced migration (data not shown).
Figure 7.
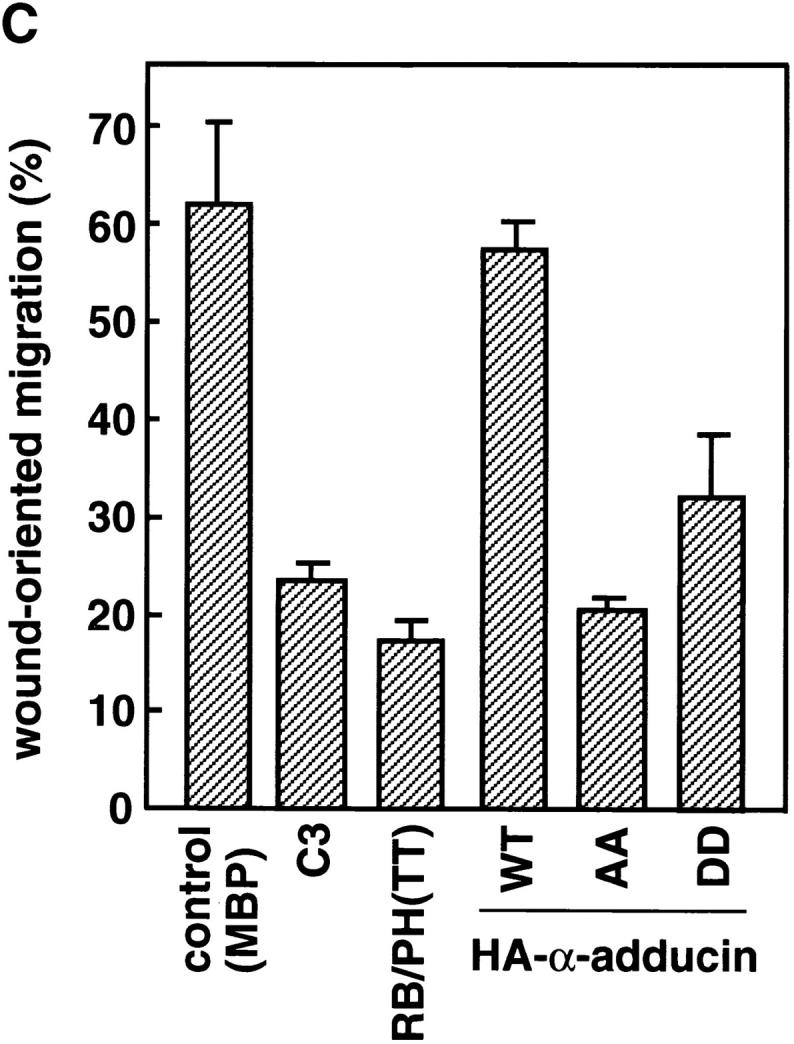
Effects of the mutants of Rho-kinase and α-adducin on cell migration in a wound healing assay. (A) The localization of Thr445-phosphorylated α-adducin in the migrating NRK49F cell. NRK49F cells which migrated in response to the wounding were stained with TRITC-phalloidin (panel a) and anti-pT445 (panel b). Arrows indicate the direction of migration. Arrowheads indicate the membrane ruffling in the leading edge. Bar, 20 μm. (B) A confluent monolayer of NRK49F cells was linearly wounded with a white chip. Soon after wounding, MBP (5 mg/ml; panels a and b), C3 (0.1 mg/ml; panels c and d), MBP-RB/PH (TT) (5 mg/ml; panels e and f), HA-α-adducin (WT) (5 mg/ml; panels g and h), HA-α-adducin-AA (5 mg/ml; panels i and j), or HA-α-adducin-DD (5 mg/ml; panels k and l) was microinjected along with a marker protein (rabbit IgG, 1.0 mg/ml) into the cytoplasm of only the cells along the wound edge. The original wound edge was determined by taking photographs soon after the cells were wounded and microinjected, and then the cells that moved (>20 μm) from the original wound edge were counted by taking photographs of the same fields after 6 h. The cells were doubly labeled by staining with TRITC-phalloidin (panels a, c, e, g, i, and k) and FITC anti–rabbit Ig Ab (panels b, d, f, h, j, and l). Arrows indicate the positions of the migrating wound edge cells. Arrowheads indicate the injected cells. Morphologies of the injected cells that showed the typical phenotypes were shown in insets. Bar, 20 μm. These results are representative of three independent experiments. (C) The ratios of migrating cells to the total injected cells are indicated. Data are means ± SEM of triplicate determinations.
Discussion
Phosphorylation Sites of α-Adducin by Rho-Kinase and Site- and Phosphorylation State–specific Antibodies
In this study, we identified the sites of phosphorylation of α-adducin by Rho-kinase as Thr445 and Thr480 in the neck domain. α-Adducin is phosphorylated at Ser408, Ser436, and Ser481 in the neck domain by PKA, and at Ser726 in the MARCKS-related domain by PKC and PKA (Matsuoka et al., 1996). Among the in vitro phosphorylation sites of α-adducin, Thr445 and Thr480 are unique for Rho-kinase. We raised and used a site- and phosphorylation state–specific antibody for α-adducin. Anti-pT445 recognized HA-α-adducin phosphorylated by Rho-kinase, but not by PKC or PKN. Anti-pT445 recognized neither nonphosphorylated HA-α-adducin nor HA-α-adducinT445A,T480A incubated with Rho-kinase and ATP (Fig. 2 A). These results indicate that anti-pT445 is a useful tool with which to detect the specific phosphorylation of α-adducin by Rho-kinase both in vitro and in vivo, distinguishing from the phosphorylation of α-adducin by PKC. Although Rho-kinase phosphorylated β- and γ-adducin in vitro (Fig. 2 B), the phosphorylation sites have not been determined. Since the surrounding amino acid sequences of Thr437 in rat γ-adducin (QQREKTRWLNS) are completely identical to those of Thr445 in α-adducin (selected as antigen phosphopeptide for anti-pT445), the result that anti-pT445 did not cross-react with rat γ-adducin phosphorylated by Rho-kinase (Fig. 2 B) revealed that Rho-kinase does not primarily phosphorylate γ-adducin at Thr437. The surrounding amino acid sequences of Thr431 in β-adducin (QQKEKTRWLNT) are a little bit different from those of Thr445 in α-adducin. At this stage, we do not know whether Rho-kinase phosphorylates β-adducin at Thr431. Adducin is thought to be comprised of α- and β-, or α- and γ-adducin in vivo (Bennett et al., 1988; Dong et al., 1995). We found that in MDCK and NRK49F cells a heterodimer of adducin which consists of α- and γ-adducin was mainly expressed (data not shown). Therefore, it is likely that anti-pT445 recognizes only phospho-Thr445 in α-adducin but not in β- and γ-adducin in both cells (see below). Further analysis is necessary to understand how the phosphorylation of β- or γ-adducin is regulated through Rho-kinase in vitro and in vivo.
In Vivo Phosphorylation of α-Adducin via the Rho/Rho-Kinase Pathway
Using anti-pT445, we found that Thr445 phosphorylation of α-adducin occurred in a Rho/Rho-kinase-dependent manner in COS7 cells (Fig. 3). We further found that α-adducin was phosphorylated at Thr445 in the TPA-stimulated MDCK cells (Fig. 4 C). TPA also drives the activation of PKC. Indeed, TPA induces the phosphorylation of α-adducin at Ser726 in renal proximal tubule epithelial cells and MDCK cells (Fowler et al., 1998; Matsuoka et al., 1998). However, it is unlikely that PKC directly phosphorylates α-adducin at Thr445 in the TPA-stimulated cells, because anti-pT445 did not recognize α-adducin which was phosphorylated by PKC in vitro and was coexpressed with the dominant active PKC in COS7 cells. Rac and Cdc42 are also involved in TPA-induced phenomena such as membrane ruffling (Ridley et al., 1995). It is possible that the protein kinases activated by Rac or Cdc42 phosphorylate α-adducin. However, we found that neither Rac1V12 nor Cdc42V12 induced the Thr445 phosphorylation of α-adducin in COS7 cells, and that the dominant active MRCK, which is identified as the target of Cdc42 (Leung et al., 1998) and Rac1 (Nakamura, N., M. Amano, Y. Fukata, N. Oshiro, T. Leung, L. Lim, and K. Kaibuchi, manuscript in preparation), induced the phosphorylation of MLC but not of α-adducin at Thr445. Taken together, these results indicate that in intact cells α-adducin was specifically phosphorylated at Thr445 via the Rho/Rho-kinase pathway during the action of TPA. Although an in vitro experiment using GST-α-adducinT445A and GST-α-adducinT480A (Fig. 1 D) suggests that Thr445 is a preferential site of phosphorylation by Rho-kinase in vitro, at this stage it is unknown which of Thr445 or Thr480 is a preferential site in intact cells.
Involvement of the Phosphorylation of α-Adducin via the Rho/Rho-Kinase Pathway in Membrane Ruffling and Cell Motility
Membrane ruffling, which is induced by several growth factors and is observed in the leading edges of motile cells, is thought to play an essential role in cell motility. The regulatory mechanism of the membrane ruffling is less well characterized. Not only Rac but also Rho is thought to regulate the membrane ruffling downstream of extracellular signals in certain types of cells, because C3 inhibits the TPA- or HGF-induced membrane ruffling in epithelial cells such as MDCK and KB cells (Nishiyama et al., 1994; Takaishi et al., 1995). Here, we found that Thr445-phosphorylated α-adducin accumulated at the TPA-induced membrane ruffling areas and the leading edges of migrating cells (Figs. 4 and 7 A). We furthermore found that RB and RB/PH (TT) as well as C3 inhibited the TPA-induced membrane ruffling, that α-adducin-AA also inhibited the membrane ruffling as the dominant negative α-adducin (Fig. 5, A and B), and that α-adducin-DD specifically counteracted the inhibitory effect of RB and RB/PH (TT) on the membrane ruffling (Fig. 6). These results indicate that α-adducin is a crucial substrate of Rho-kinase downstream of Rho for membrane ruffling. The fact that α-adducin-DD counteracted the inhibitory effect of RB but not that of C3 (Fig. 6 B) is probably due to difference between the inhibitory mechanisms by RB and C3. RB is the fragment of Rho-kinase which consists of the RB domain of Rho-kinase. RB binds to the effector domain of Rho, and then inhibits the interaction of Rho with Rho-kinase (Amano et al., 1997). Recent studies revealed that the various effector mutants of RhoAV14, in which interaction with some effectors is maintained but with others is lost, make it possible to dissect the diverged pathways downstream of Rho. For example, RhoAY42C abolishes interaction with PKN but maintains interaction with ROCK-I (an isoform of Rho-kinase) and RhoAF39L,C20R maintains interaction with PKN but abolishes interaction with ROCK-I (Sahai et al., 1998). RhoAY42C induces stress fiber formation, whereas RhoAF39L,C20R does not induce stress fiber formation, suggesting that ROCK-I but not PKN is required for stress fiber formation. These observations indicate that there are distinct interfaces in the effector domain of Rho for the different classes of effector proteins. Although at this time we cannot completely rule out the possibility that RB inhibits interaction of Rho with the other Rho targets such as PKN and p140mDia, RB may inhibit preferentially the interaction of Rho with Rho-kinase rather than with other Rho targets. On the other hand, C3 interferes with the whole functions of Rho. In fact, C3 inhibits the activity of PKN as well as Rho-kinase in Swiss 3T3 cells (Amano et al., 1996) and the Rho-dependent recruitment of p140mDia to the dynamic membrane structures in Swiss 3T3 cells (Watanabe et al., 1997). C3 may inhibit the membrane ruffling by impairing the whole functions of Rho, whereas RB may inhibit the membrane ruffling by mainly (or specifically) impairing the function of Rho-kinase.
We also found that not only C3 and RB/PH (TT) but also α-adducin-AA inhibited cell motility as well as membrane ruffling in NRK fibroblasts (Fig. 7, B and C). This suggests that α-adducin is one of the substrates for Rho-kinase involved in cell motility, probably regulating the membrane ruffling in the leading edges of motile cells. As for the substrates of Rho-kinase, MLC is another major substrate which is thought to play an important role in cell motility. Indeed, injection of anti-MLC kinase antibody diminishes the cell motility of macrophages (Wilson et al., 1991). Moreover, phosphorylated MLC is enriched in both the leading edges and rear ends of motile fibroblasts and epithelial cells (Matsumura et al., 1998), suggesting that a force derived from myosin-actin filament driven by the MLC phosphorylation contributes to cell motility. Thus, Rho, acting through Rho-kinase, appears to regulate cell motility through the spatial and temporal regulation of phosphorylation of certain substrates including adducin and MLC.
It should be noted that in Swiss 3T3 or NIH3T3 fibroblasts, in which the dominant active Rac by itself is sufficient to induce the membrane ruffling (Hall, 1998), α-adducin phosphorylated at Thr445 was not observed in the dominant active Rac-induced ruffling areas, and neither C3, RB/PH (TT), nor HA-α-adducin-AA inhibited the dominant active Rac-induced ruffling (data not shown). On the other hand, we found that in KB epithelial cells as well as MDCK epithelial cells, α-adducin phosphorylated at Thr445 accumulated in the TPA-induced membrane ruffling areas, and that C3 and RB/PH (TT) inhibited the TPA-induced membrane ruffling (data not shown). When RB/PH (TT) or α-adducin-AA was injected to MDCK cells, the HGF-induced membrane ruffling was also inhibited as the TPA-induced membrane ruffling (data not shown). The regulatory system of the membrane ruffling may vary among cell types. It is conceivable that the membrane ruffling in a certain type of cell such as MDCK epithelial cells may require not only the Rac-mediated pathway but also the phosphorylation of adducin by the Rho/ Rho-kinase pathway, whereas that in other types of cells such as Swiss 3T3 fibroblasts may require only the Rac-mediated pathway. Further studies are necessary to determine what causes the differences between the two types of cell lines.
Regulation of α-Adducin Function through Phosphorylation by Rho-Kinase and PKC
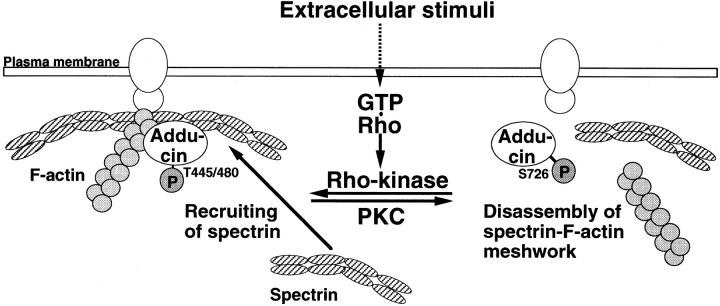
These results indicate that the phosphorylation of α-adducin by the Rho/Rho-kinase pathway plays a crucial role in the regulation of membrane ruffling and cell motility. What is the function of the α-adducin phosphorylation in membrane ruffling and cell motility? Membrane ruffles contain complicated cytoskeletal structures composed of F-actin and other F-actin–associated proteins (Mitchison and Cramer, 1996). Spectrin, which binds to F-actin beneath plasma membrane and forms a membrane cortical meshwork, also accumulates at the leading edges of motile cells (Burridge et al., 1982; Sormunen and Lehto, 1995). This lattice-like meshwork is dynamically reconstructed and required for the formation of membrane ruffling (Lauffenburger and Horwitz, 1996). Since the phosphorylation of α-adducin by Rho-kinase is known to promote the binding of α-adducin to F-actin (Kimura et al., 1998) and this in turn induces the recruitment of spectrin to F-actin (Gardner and Bennett, 1987; Bennett et al., 1988), it is possible that the formation of a spectrin-F-actin meshwork is dynamically regulated in the active membrane ruffling by the Rho/Rho-kinase pathway (Fig. 8). It is also possible that Rho-kinase modulates the F-actin–capping activity (Li et al., 1998) or cross-linking activity (Mische et al., 1987) of adducin and then regulates actin dynamics in the membrane ruffling areas. Adducin is also a substrate of PKC, which is activated in response to TPA or HGF, and the phosphorylation of α-adducin by PKC inhibits the activity of adducin in promoting the formation of a spectrin– actin complex in vitro (Matsuoka et al., 1998). TPA induces the phosphorylation of α-adducin at Ser726 and the redistribution of α-adducin phosphorylated at Ser726 from the cell membrane to cytoplasm accompanied by that of spectrin in renal proximal tubule epithelial and MDCK cells (Fowler et al., 1998; Matsuoka et al., 1998). Interestingly, α-adducin phosphorylated at Ser726 was also observed in the membrane ruffling areas (Matsuoka, Y., and V. Bennett, manuscript in preparation). It is conceivable that α-adducin phosphorylated by PKC dissociates from a spectrin-F-actin meshwork during membrane ruffling. Thus, it is tempting to speculate that the adducin function may be cyclically regulated through the phosphorylation by Rho-kinase and PKC during the action of TPA or HGF, and the alternative phosphorylation contributes to the turnover of a spectrin-F-actin meshwork for membrane ruffling. In this regard, it is interesting that α-adducin-DD inhibits the wound-induced migration of NRK49F cells but not their membrane ruffling. This suggests that the “on” and “off” states of α-adducin are necessary for cell migration (Fig. 8).
Figure 8.
Model for the role of adducin phosphorylated by the Rho/Rho-kinase pathway in cell motility. A spectrin-F-actin meshwork is linked to the plasma membrane by association with membrane accessory proteins such as band 4.1. Upon the activation of Rho-kinase by Rho during the action of extracellular stimuli, α-adducin is phosphorylated at Thr445 and Thr480. Phosphorylated α-adducin binds to F-actin, and then facilitates the recruitment of spectrin to F-actin. This may promote the formation of a spectrin-F-actin meshwork beneath plasma membrane for membrane ruffling. On the other hand, PKC phosphorylates α-adducin at Ser726, and then inhibits the activity of adducin in recruiting spectrin to F-actin. This may in turn induce the disassembly of a spectrin-F-actin meshwork. Transition between the phosphorylation states of α-adducin by Rho-kinase and PKC may regulate the remodeling of membrane ruffling, which is important for cell migration.
Acknowledgments
We thank Dr. S. Jaken for providing pQE-35H fragment (4D) and antiserum against γ-adducin, Dr. A. Hall for providing pGEX-C3, and Dr. S. Tsukita for providing MDCK cells. We are also grateful to A. Takemura for secretarial assistance.
This work was supported by a grant from Research for the Future of Japan Society of the Promotion of Science, by the Human Frontier Science Program, and by a grant from Kirin Brewery Company Limited.
Abbreviations used in this paper
- α-adducin-AA
α-adducin with substitution of Thr445 and Thr480 by Ala
- α-adducin-DD
α-adducin with substitution of Thr445 and Thr480 by Asp
- CAT
catalytic domain
- ERM
ezrin/radixin/moesin
- GST
glutathione-S-transferase
- HA
hemagglutinin
- HGF
hepatocyte growth factor
- LPA
lysophosphatidic acid
- MBP
maltose-binding protein
- MBS
myosin-binding subunit
- MLC
myosin light chain
- PH
pleckstrin-homology domain
- PK
protein kinase
- RB
Rho-binding domain
- TPA
tetradecanoylphorbol-13-acetate
Note Added in Proof:
After we submitted this paper, it was reported that the GTPase cycle of Rho was important for cell migration in a wound healing assay (Nobes, C.D., and A. Hall. 1999. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144: 1235–1244).
References
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996a;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as a serine-threonine kinase, PKN. Science. 1996b;271:648–650. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Bennett V, Gardner K, Steiner JP. Brain adducin: a protein kinase C substrate that may mediate site-directed assembly at the spectrin-actin junction. J Biol Chem. 1988;263:5860–5869. [PubMed] [Google Scholar]
- Bodwell JE, Orti E, Coull JM, Pappin DJ, Smith LI, Swift F. Identification of phosphorylated sites in the mouse glucocorticoid receptor. J Biol Chem. 1991;66:7549–7555. [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burridge K, Kelly T, Mangeat P. Nonerythrocyte spectrins: actin-membrane attachment proteins occurring in many cell types. J Cell Biol. 1982;95:478–486. doi: 10.1083/jcb.95.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachero TG, Morielli AD, Peralta EG. The small GTP-binding protein RhoA regulates a delayed rectifier potassium channel. Cell. 1998;93:1077–1085. doi: 10.1016/s0092-8674(00)81212-x. [DOI] [PubMed] [Google Scholar]
- Chihara K, Amano M, Nakamura N, Yano T, Shibata M, Tokui T, Ichikawa H, Ikebe R, Ikebe M, Kaibuchi K. Cytoskeletal rearrangements and transcriptional activation of c-fosserum response element by Rho-kinase. J Biol Chem. 1997;272:25121–25127. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- Dong L, Chapline C, Mousseau B, Fowler L, Ramsay K, Stevens JL, Jaken S. 35H, a sequence isolated as a protein kinase C binding protein, is a novel member of the adducin family. J Biol Chem. 1995;270:25534–25540. doi: 10.1074/jbc.270.43.25534. [DOI] [PubMed] [Google Scholar]
- Fowler L, Dong L, Bowes RC, III, van de Water B, Stevens JL, Jaken S. Transformation-sensitive changes in expression, localization, and phosphorylation of adducins in renal proximal tubule epithelial cells. Cell Growth Differ. 1998;9:177–184. [PubMed] [Google Scholar]
- Fujisawa K, Fujita A, Ishizaki T, Saito Y, Narumiya S. Identification of the Rho-binding domain of p160ROCK, a Rho-associated coiled-coil containing protein kinase. J Biol Chem. 1996;271:23022–23028. doi: 10.1074/jbc.271.38.23022. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Kimura K, Oshiro N, Saya H, Matsuura Y, Kaibuchi K. Association of the myosin-binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J Cell Biol. 1998;141:409–418. doi: 10.1083/jcb.141.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner K, Bennett V. A new erythrocyte membrane-associated protein with calmodulin binding activity. Identification and purification. J Biol Chem. 1986;261:1339–1348. [PubMed] [Google Scholar]
- Gardner K, Bennett V. Modulation of spectrin-actin assembly by erythrocyte adducin. Nature. 1987;328:359–362. doi: 10.1038/328359a0. [DOI] [PubMed] [Google Scholar]
- Gong MC, Iizuka K, Nixon G, Browne JP, Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV, Somlyo AP. Role of guanine nucleotide-binding proteins, ras-family or trimeric proteins or both, in Ca2+sensitization of smooth muscle. Proc Natl Acad Sci USA. 1996;93:1340–1345. doi: 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hirata K, Kikuchi A, Sasaki T, Kuroda S, Kaibuchi K, Matsuura Y, Seki H, Saida K, Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem. 1992;267:8719–8722. [PubMed] [Google Scholar]
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH, Matsumura F, Maekawa M, Bito H, Narumiya S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)- regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141:1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Inagaki N, Takahashi T, Takai Y. Phosphorylation-dependent control of structures of intermediate filaments: a novel approach using site- and phosphorylation state-specific antibodies. J Biochem. 1997;121:407–414. doi: 10.1093/oxfordjournals.jbchem.a021603. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kD Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO (Eur Mol Biol Organ) J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R, Bennett V. Mapping the domain structure of human erythrocyte adducin. J Biol Chem. 1990;265:13130–13136. [PubMed] [Google Scholar]
- Joshi R, Gilligan DM, Otto E, McLaughlin T, Bennett V. Primary structure and domain organization of human alpha and beta adducin. J Cell Biol. 1991;115:665–675. doi: 10.1083/jcb.115.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K, Fukumoto Y, Oku N, Takai Y, Arai K, Muramatsu M. Molecular genetic analysis of the regulatory and catalytic domains of protein kinase C. J Biol Chem. 1989;264:13489–13496. [PubMed] [Google Scholar]
- Kaiser HW, O'Keefe E, Bennett V. Adducin: Ca2+-dependent association with sites of cell–cell contact. J Cell Biol. 1989;109:557–569. doi: 10.1083/jcb.109.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Aoki J, Ichikawa A, Negishi M. p160 RhoA-binding kinase ROKα induces neurite retraction. J Biol Chem. 1998;273:2489–2492. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kimura K, Fukata Y, Matsuoka Y, Bennett V, Matsuura Y, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of the association of adducin with actin filaments by rho-associated kinase (Rho-kinase) and myosin phosphatase. J Biol Chem. 1998;273:5542–5548. doi: 10.1074/jbc.273.10.5542. [DOI] [PubMed] [Google Scholar]
- Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopusembryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman PA, Hughes CA, Bennett V, Fowler VM. A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J Biol Chem. 1996;271:7986–7991. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Okubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Kaibuchi K. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Tan I, Manser E, Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Biol. 1998;18:130–140. doi: 10.1128/mcb.18.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Matsuoka Y, Bennett V. Adducin preferentially recruits spectrin to the fast growing ends of actin filaments in a complex requiring the MARCKS-related domain and a newly defined oligomerization domain. J Biol Chem. 1998;273:19329–19338. doi: 10.1074/jbc.273.30.19329. [DOI] [PubMed] [Google Scholar]
- Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. A rho-like protein is involved in the organization of the contractile ring in dividing sand dollar eggs. Zygote. 1993;1:325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- Madaule P, Furuyashiki T, Reid T, Ishizaki T, Watanabe G, Morii N, Narumiya S. A novel partner for the GTP-bound forms of rho and rac. FEBS Lett. 1995;377:243–248. doi: 10.1016/0014-5793(95)01351-2. [DOI] [PubMed] [Google Scholar]
- Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394:491–494. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- Marrs JA, Napolitano EW, Murphy-Erdosh C, Mays RW, Reichardt LF, Nelson WJ. Distinguishing roles of the membrane-cytoskeleton and cadherin mediated cell–cell adhesion in generating different Na(+),K(+)-ATPase distributions in polarized epithelia. J Cell Biol. 1993;123:149–164. doi: 10.1083/jcb.123.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO (Eur Mol Biol Organ) J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F, Ono S, Yamakita Y, Totsukawa G, Yamashiro S. Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis of cultured cells. J Cell Biol. 1998;140:119–129. doi: 10.1083/jcb.140.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Hughes CA, Bennett V. Adducin regulation. Definition of the calmodulin-binding domain and sites of phosphorylation by protein kinases A and C. J Biol Chem. 1996;271:25157–25166. doi: 10.1074/jbc.271.41.25157. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol. 1998;142:485–497. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura Y, Possee RD, Overton HA, Bishop DH. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J En Virol. 1987;68:1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Mische SM, Mooseker MS, Morrow JS. Erythrocyte adducin: a calmodulin-regulated actin-bundling protein that stimulates spectrin-actin binding. J Cell Biol. 1987;105:2837–2845. doi: 10.1083/jcb.105.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Nishiki T, Narumiya S, Morii N, Yamamoto M, Fujiwara M, Kamata Y, Sakaguchi G, Kozaki S. ADP-ribosylation of the rho/rac proteins induces growth inhibition, neurite outgrowth and acetylcholine esterase in cultured PC-12 cells. Biochem Biophys Res Commun. 1990;167:265–272. doi: 10.1016/0006-291x(90)91760-p. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Sasaki T, Takaishi K, Kato M, Yaku H, Araki K, Matsuura Y, Takai Y. rac p21 is involved in insulin-induced membrane ruffling and rho p21 is involved in hepatocyte growth factor- and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced membrane ruffling in KB cells. Mol Cell Biol. 1994;14:2447–2456. doi: 10.1128/mcb.14.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro N, Fukata Y, Kaibuchi K. Phosphorylation of moesin by Rho-associated kinase (Rho-kinase) plays a crucial role in the formation of microvilli-like structures. J Biol Chem. 1998;273:34663–34666. doi: 10.1074/jbc.273.52.34663. [DOI] [PubMed] [Google Scholar]
- Palfrey HC, Waseem A. Protein kinase C in the human erythrocyte. Translocation to the plasma membrane and phosphorylation of bands 4.1 and 4.9 and other membrane proteins. J Biol Chem. 1985;260:16021–16029. [PubMed] [Google Scholar]
- Paterson HF, Self AJ, Garrett MD, Just I, Aktories K, Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T, Furuyashiki T, Ishizaki T, Watanabe G, Watanabe N, Fujisawa K, Morii N, Madaule P, Narumiya S. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J Biol Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO (Eur Mol Biol Organ) J. 1994;13:2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/ hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO (Eur Mol Biol Organ) J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos MF, McCormack SA, Guo Z, Okolicany J, Zheng Y, Johnson LR, Tigyi G. Rho proteins play a critical role in cell migration during the early phase of mucosal restitution. J Clin Invest. 1997;100:216–225. doi: 10.1172/JCI119515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Henry M, Solomon F, Jacks T. RhoA-dependent phosphorylation and relocalization of ERM proteins into apical membrane/actin protrusions in fibroblasts. Mol Biol Cell. 1998;9:403–419. doi: 10.1091/mbc.9.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirataki H, Kaibuchi K, Sakoda T, Kishida S, Yamaguchi T, Wada K, Miyazaki M, Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993;13:2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormunen R, Lehto VP. Spectrin in the leading lamella of cultured chicken fibroblasts. Eur J Cell Biol. 1995;68:387–397. [PubMed] [Google Scholar]
- Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Yang Z, Zhi G, Stull JT, Trybus KM. Charge replacement near the phosphorylatable serine of the myosin regulatory light chain mimics aspects of phosphorylation. Proc Natl Acad Sci USA. 1994;91:1490–1494. doi: 10.1073/pnas.91.4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Kikuchi A, Kuroda S, Kotani K, Sasaki T, Takai Y. Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol. 1993;13:72–79. doi: 10.1128/mcb.13.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeichi M, Takai Y. Involvement of Rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene. 1994;9:273–279. [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kameyama T, Tsukita S, Tsukita S, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- Tominaga T, Sugie K, Hirata M, Morii N, Fukata J, Uchida A, Imura H, Narumiya S. Inhibition of PMA-induced, LFA-1–dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993;120:1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka A, Narumiya S. Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase Rho. Science. 1996;271:645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO (Eur Mol Biol Organ) J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MD, Mallavarapu A, Rosenblatt J, Mitchison TJ. Actin dynamics in vivo. . Curr Opin Cell Biol. 1997;9:54–61. doi: 10.1016/s0955-0674(97)80152-4. [DOI] [PubMed] [Google Scholar]
- Wilson AK, Gorgas G, Claypool WD, de Lanerolle P. An increase or a decrease in myosin II phosphorylation inhibits macrophage motility. J Cell Biol. 1991;114:277–283. doi: 10.1083/jcb.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]