Abstract
Maintenance of mitochondrial DNA (mtDNA) during cell division is required for progeny to be respiratory competent. Maintenance involves the replication, repair, assembly, segregation, and partitioning of the mitochondrial nucleoid. MGM101 has been identified as a gene essential for mtDNA maintenance in S. cerevisiae, but its role is unknown. Using liquid chromatography coupled with tandem mass spectrometry, we identified Mgm101p as a component of highly enriched nucleoids, suggesting that it plays a nucleoid-specific role in maintenance. Subcellular fractionation, indirect immunofluorescence and GFP tagging show that Mgm101p is exclusively associated with the mitochondrial nucleoid structure in cells. Furthermore, DNA affinity chromatography of nucleoid extracts indicates that Mgm101p binds to DNA, suggesting that its nucleoid localization is in part due to this activity. Phenotypic analysis of cells containing a temperature sensitive mgm101 allele suggests that Mgm101p is not involved in mtDNA packaging, segregation, partitioning or required for ongoing mtDNA replication. We examined Mgm101p's role in mtDNA repair. As compared with wild-type cells, mgm101 cells were more sensitive to mtDNA damage induced by UV irradiation and were hypersensitive to mtDNA damage induced by gamma rays and H2O2 treatment. Thus, we propose that Mgm101p performs an essential function in the repair of oxidatively damaged mtDNA that is required for the maintenance of the mitochondrial genome.
Keywords: mtDNA, nucleoid, maintenance, DNA repair, yeast
An increasing number of human diseases are being identified that are associated with mitochondrial DNA (mtDNA)1-linked mutations (Brown and Wallace, 1994; Sherratt et al., 1997). To understand the etiology of these diseases, the details of the basic molecular mechanisms underlying the maintenance of mtDNA must first be understood. The accurate maintenance of mtDNA requires several distinct processes. As is the case for nuclear DNA, mtDNA must be replicated and packaged into nucleoids. The nucleoids then must be segregated from one another and partitioned into daughter cells. The repair of damaged mtDNA is also likely to be important for mtDNA maintenance, especially considering the highly oxidative environment of the organelle. Defects in any one of these processes might result, at a minimum, in nonfunctional mtDNA, and may cause the complete loss of mtDNA, a lethal event in humans. The budding yeast Saccharomyces cerevisiae has been used to analyze these processes in more detail. In addition to being tractable to genetic, biochemical and cytological approaches, yeast are facultative anaerobes. Thus, S. cerevisiae cells that lose mtDNA can still be propagated and studied, when a fermentable carbon source such as glucose is provided.
In S. cerevisiae, there are an average of 50–100 of the 80-kb mitochondrial genome copies, depending on growth conditions and ploidy (Willamson, 1976). The genome is known to encode seven proteins that include subunits of energy transducing inner membrane complexes, one mitochondrial ribosomal protein, several minor proteins involved in gene splicing and conversion, ribosomal and transfer RNAs, and one RNA involved in tRNA processing (Pon and Schatz, 1991). In vivo, mtDNA is organized into punctate nucleoid structures that are distributed in a somewhat regular pattern within the mitochondrial reticulum. Each nucleoid has been estimated to contain ∼4–5 copies of mtDNA and is packaged in a manner that makes it more resistant to digestion by exogenously added DNases (Williamson, 1976; Miyakawa et al., 1987, 1995; Newman et al., 1996). These structures have been shown to be the unit of inheritance for mtDNA (Lockshon et al., 1995).
Genetic studies in yeast have suggested that mtDNA partitioning into daughter cells is a nonrandom process (Coen et al., 1970; Birky, 1978; Strausberg and Perlman, 1978; Zinn et al., 1987; Azpiroz and Butow, 1993). More recently, this model has been substantiated by direct observation and comparison of the behavior of mtDNA and mitochondrial proteins in yeast zygotes formed by mating rho+ (containing mtDNA) cells (Azpiroz and Butow, 1993; Nunnari et al., 1997; Okamoto et al., 1998). Specifically, it has been observed, that while haploid-derived mitochondrial proteins are able to freely diffuse within yeast zygotic mitochondrial reticulum, haploid mtDNA remains segregated in the zygote (Nunnari et al., 1997). Although haploid-derived mtDNA does not move throughout the zygote, it preferentially moves into the first zygotic bud (Nunnari et al., 1997). Similar conclusions have been recently drawn from cytological experiments using zygotes formed by mating rho+ to rho° (lacking mtDNA) cells (Okamoto et al., 1998). These observations suggest that mtDNA is linked to putative partitioning machinery that insures accurate inheritance of mtDNA. To date, components of this proposed partitioning apparatus remain unidentified. Interestingly, a subset of mutations that cause abnormal mitochondrial morphology such as mdm10, mmm1, and fzo1 also result in mtDNA loss, suggesting that these components may be important for mtDNA inheritance (Burgess et al., 1994; Sogo and Yaffe, 1994; Hermann et al., 1998). No yeast proteins have been identified with convincing sequence similarity to eubacterial nucleoid segregation or division proteins, suggesting that novel mechanisms and components for these processes have arisen for mitochondria in eucaryotes.
In contrast to the partitioning apparatus, components directly involved in the replication, repair, and recombination of mtDNA have been described. Replication of mtDNA in S. cerevisiae is mediated by Mip1p, a pol-γ DNA polymerase that has been shown to possess both polymerizing and 3′ to 5′ exonuclease activity (Foury, 1989). This exonuclease activity, together with Msh1p, a MutS homologue required for mitochondrial DNA mismatch repair, mediates proofreading and thus the high fidelity replication of mtDNA (Vanderstraeten et al., 1998). Additional proteins likely to be involved in the replication, recombination, and repair of mtDNA include Rim1p, the mitochondrial homologue of eubacterial SSB, Pif1p, a DNA helicase, Abf2p, a DNA binding protein in the HMG family, (Lahaye et al., 1991; Diffley and Stillman, 1992; Kao et al., 1993), Mgt1p, a cruciform-cutting endonuclease present in the mitochondria and the genetic locus, MHR1, which corresponds to an unknown gene identified in a genetic screen for mtDNA recombination-deficient mutants (Ling et al., 1995; Lockshon et al., 1995). Although many genes important for mtDNA metabolism have been described, surprisingly little is known about the molecular mechanisms involved in mtDNA replication, recombination and repair.
MGM101 (for mitochondrial genome maintenance) was isolated in a genetic screen for mutants that caused temperature-sensitive loss of mtDNA and a null mutation in MGM101 indicates that it is essential for the maintenance of mtDNA (Chen et al., 1993). However, the functional role that Mgm101p plays in mtDNA maintenance is unknown. Sequence analysis of Mgm101p indicates it is a novel, highly basic 30-kD protein (Chen et al., 1993). In addition to the S. cerevisiae MGM101, genes encoding the K. lactis and S. pombe MGM101 homologues have been identified (Chen et al., 1993; Clark-Walker and Chen, 1996; see Fig. 1). These gene products have an overall high degree of identity and similarity (Corpet, 1988). The most conserved region is contained in the COOH-terminal half of Mgm101p, where the majority of the basic residues are found, suggesting that this region is important for Mgm101p function. Deletion of a portion of the COOH terminus of S. cerevisiae Mgm101p causes a complete loss of function (Chen et al., 1993). The high degree of conservation between the evolutionarily divergent S. cerevisiae and S. pombe suggests that a metazoan Mgm101p homologue may exist. Given the likely importance of this novel protein in mtDNA maintenance, our goal was to characterize Mgm101p and, through the phenotypic analysis of a temperature-sensitive mgm101 allele, determine its essential role in the maintenance of the mitochondrial genome.
Figure 1.
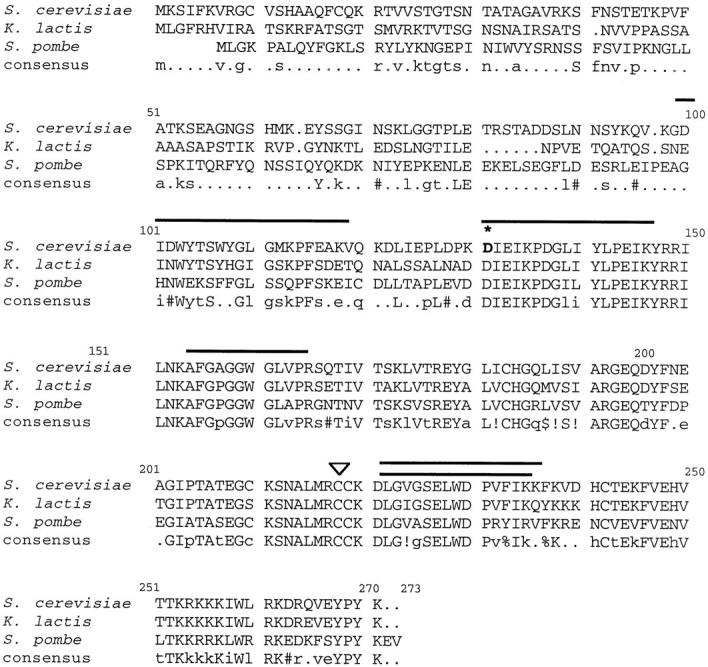
Mgm101p is conserved. The two known Mgm101p homologues from K. lactis and S. Pombe were identified using the BLAST search algorithm (Altschul et al., 1997). Alignments were obtained using the MultAlin program (Corpet, 1988). Amino acid identities between S. cerevisae, K. lactis, and S. pombe proteins are indicated by capital letters and identity between any two is indicated by small case letters. Amino acid similarities are indicated by the following symbols: ! is anyone of IV, $ is anyone of LM, % is anyone of FY, and # is anyone of NDQEBZ. Bold overlines indicate peptides identified by liquid chromatography tandem mass-spectrometric analysis of trypsin-digested nucleoid proteins (see Materials and Methods). The asterisk and arrowhead mark the position of the mutation found in mgm101-2 (see Results) and the position of the marker insertion in strain CS6-1D (Chen et al., 1993), respectively.
Materials and Methods
Yeast Genetic Techniques
Yeast strains used in this study are listed in Table I. Media preparations and genetic techniques were performed as described (Guthrie and Fink, 1991). Either glucose (D at 2%) or glycerol (G at 2%) was used as a carbon source as specified.
Table I.
Yeast Strains
| Strain | Genotype | Source | ||
|---|---|---|---|---|
| W303 | ade2-1, leu2-3, his3-11,15, trp1-1, ura3-1, can1-100, Mata or Matα | R. Rothstein | ||
| CS6-1D | leu2, ura3, his4, ade2, ade3, MGM101::URA3, Mata | Chen et al., 1993 | ||
| JNY131 | same as W303, except mgm101-2, Mata, or Matα | This study | ||
| W303TK | same as W303, except can1::GPD-HSVTK, Mata | Nunnari et al., 1997 | ||
| JNY131TK | same as JNY131, except can1::GPD-HSVTK, Mata | This study |
Isolation and Characterization of mgm101-2 Cells
W303 haploid cells were mutagenized using either EMS, nitrosoguanidine or UV light to 10% viability and screened for temperature sensitive growth on YPG. Cells from these colonies were grown at 37°C on YPD for ∼100 generations and were screened for loss of mtDNA using DAPI and fluorescence microscopy.
Complementation analysis using a recessive truncated loss of function allele of MGM101 (strain CS6-1D, kindly provided by G.D. Clark-Walker) revealed that one mutant from this screen was allelic to MGM101 [JNY131(mgm101-2)], The Australian National University. Sporulation and tetrad analysis of this cross confirmed the mutation was at the MGM101 locus.
To characterize JNY131(mgm101-2), cultures of W303 or JNY131 (mgm101-2) were grown at permissive temperature (22°C) to log phase in YPG, washed into YPD and were incubated at either permissive or nonpermissive temperatures. At various time intervals, aliquots were taken and cells were analyzed for respiratory competence, the presence of mtDNA and mitochondrial morphology. To analyze for respiratory competence, cells were plated onto YPD plates and colony color was assessed and quantitated. Because all strains analyzed contained the ade2 mutation, on YPD, white and sectored colonies were classified as respiratory deficient and red colonies were classified as respiratory competent. To visualize mtDNA, cells were fixed either in 70% ethanol containing 10 ng/ml DAPI or with 3.7% formaldehyde for 1 h, followed by spheroplasting and incubation in PBS containing 1 μg/ml DAPI. Fixed, stained cells were washed into PBS and imaged using standard epi-fluorescence microscopy. To analyze mitochondrial morphology, aliquots of cells were also processed for indirect immunofluorescence and imaged using confocal microscopy (see below).
Sequence Analysis of mgm101-2
The mutation in mgm101-2 was determined by double-stranded DNA sequence analysis. Yeast genomic DNA from strains JNY131 and W303 was prepared as described (Guthrie and Fink, 1991) and used as template for polymerase chain reaction (PCR) with the following sets of primers corresponding to the MGM101 gene: 5′-CGGAATTCATGAAAAGCATTTTCAAGG-3′ and 5′-GCTCTAGACTATTTATAAGGATATTCAAC-3′, 5′-GGAATTCCTCCTTGGACAACACTTTCGT-3′ and 5′-CGG- GATCCCGCAACTTCTTTTGGATACCAG-3′ (BRL-Custom Primers Inc.). The PCR products from five independent reactions with a given set of primers were pooled, gel purified, and sequenced using the above listed primers by the Division of Biological Sciences Automated DNA Sequencing Facility at University of California, Davis.
Fusion Protein Expression and Antibody Production
For the production of anti-Mgm101p and anti-Abf2p antibodies, maltose binding protein (MBP) fusions were created. MGM101 and ABF2 were cloned into pMAL-c2 (New England Biolabs, Inc.) using an in-frame 5′ EcoRI site and a 3′ XbaI site introduced using PCR. Fusion proteins were expressed in Escherichia coli (DH5 α) at 25°C and purified using amylose affinity chromatography following the methods outlined by New England Biolabs, Inc. Rabbit polyclonal antibodies were raised against fusion proteins by injecting 1 mg of purified protein subcutaneously every 2 wk. Anti-Mgm101p antibody was affinity purified as described (Pringle et al., 1991).
Preparation of Mitochondria
Highly enriched yeast mitochondria were prepared essentially as described (Glick and Pon, 1995) with the following modifications. Cultures of either W303 or JNY131 were grown in YPG media to log phase. Before harvesting, yeast cell walls were removed using yeast lytic enzyme (ICN). Isolated crude mitochondrial pellets from lysed cells were resuspended in 50% w/v optiprep (Nycomed), 20 mM HEPES, pH 7.4, osmotically balanced with sorbitol to 600 milli-osmolal. This mixture was used as the bottom layer of optiprep step gradients formed in SW28 centrifuge tubes (Beckman); the layers atop it had densities of 1.1 g/ml and 1.16 g/ml, and were similarly osmotically balanced with sorbitol. Gradients were spun at 80,000 g for a minimum of 3 h in an SW28 rotor; mitochondria floated to the interface between the 1.1 and 1.16 g/ml. This interface was collected and diluted approximately fivefold with mitochondria isolation buffer (MIB: 0.6 M sorbitol, 20 mM Hepes, pH 7.4) and spun at 10,000 g for 10 min to pellet the mitochondria. This pellet was resuspended, centrifuged at 3,000 g for 5 min to pellet aggregated material and the supernate was centrifuged again at 10,000 g for 10 min and the pellet was resuspended in a minimal volume of MIB.
Isolation of Mitochondrial Nucleoids and Fractions Enriched in mtDNA binding Proteins
Mitochondrial nucleoids were prepared using a modification of a technique described by Miyakawa et al. (1987). Mitochondrial lysate was prepared by diluting purified mitochondria to a protein concentration of 2.5 mg/ml with nucleoid extraction buffer (NXB: 20 mM Hepes, pH 7.4, 100 mM sucrose, 20 mM KCl, 1% NP-40, 1 mM spermidine, 100 ng/ml DAPI, 1 mM PMSF, 0.5 mM DTT) followed by an incubation on ice for 1 h. This lysate was layered onto a 37.5%/60%/80% sucrose step gradient. In these gradients, the 37.5% layer contained NXB, and the 60% layer contained 20 mM Hepes, pH 7.4, 20 mM KCl, 1% Mega-8 (Calbiochem), 1 mM spermidine, 100 ng/ml DAPI, and 0.5 mM DTT. Gradients were spun at 100,000 g for 90 min; mitochondrial DNA-containing structures banded at the 37.5%/ 60% interface as assessed by fluorescence microscopy of DAPI stainable structures and enrichment of both DNA assayed using the picogreen reagent (Molecular Probes) and the nucleoid-associated protein Abf2p (see below). This material was collected and diluted twofold with NXB containing 2% NP-40 and rebanded over another 37.5%/60%/80% step sucrose gradient.
LC/MS/MS Analysis of Nucleoid-associated Proteins
To extract nucleoid-associated proteins from DNA, an equal volume of NXB containing 2 M KCl was added to the diluted nucleoid fraction. This mixture was incubated for 1–2 h on ice to produce a high salt extract enriched for nucleoid-associated proteins. This mixture was layered atop a 30% sucrose cushion containing NXB with 1 M KCl, and centrifuged at 100,000 g for 90 min. The top fraction, not including the top/30% interface, was collected. Protein in this fraction was precipitated by the addition of trichloroacetic acid to 15% wt/vol followed by centrifugation at 20,000 g for 30 min. The resulting pellet was rinsed twice with cold 80% acetone and then resuspended in freshly prepared 8 M urea, 100 mM ammonium bicarbonate, pH 8.0, at room temperature. After 12 h at 4°C, the mixture was centrifuged at 20,000 g for 30 min. The protein concentration in the supernatant from this spin, termed HSE, was determined using the Nano-orange reagent kit (Molecular Probes). DTT was added to HSE to 4 mM, and the mixture was incubated for 30 min at 37°C then diluted fourfold with 50 mM Tris-HCl, pH 8.0, 20 mM KCl, 1 mM CaCl2 containing 1 μg of modified trypsin (Boehringer) for every 25 μg of protein. This mixture was incubated for 12 h at 37°C and then frozen in liquid nitrogen.
Micro-column high performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry was used to identify the mixture of proteins present in the HSE fraction. The solution was loaded onto a 100 μm by 200 μm fused silica capillary (Polymetrics, Inc.) that was packed to a length of ∼15 cm with 10 μm POROS 10 R2 reverse phase material (Perseptives Biosystems; Kennedy and Jorgenson, 1989). The fritted end of the column was inserted into the needle of the Finnigan TSQ-700 electrospray ion source. The sample was directly loaded onto the micro column by helium pressurization of the sample in a stainless steel bomb (Kennedy and Jorgenson, 1989).
Solvent flow from a dual syringe pump (Applied Biosystems) was split 100:1 pre-column to deliver a final flow rate of 1 to 1.5 μm/min through the column. By using a mobile phase consisting of 0.5% acetic acid (solvent A) and 80:20 acetonitrile/water containing 0.5% acetic acid (solvent B) liquid chromatography was performed by ramping from 0% B to 60% B over 90 min. Electrospray ionization was carried out at a voltage of 4.6 kV and tandem mass spectra were acquired automatically during the entire gradient run (Link et al., 1997). Each tandem mass spectrum was searched, using the SEQUEST program (Yates et al., 1996) against the yeast orfs protein database obtained from the Saccharomyces Genome Database (Stanford University). Sequences for bovine trypsin and human keratin were included to facilitate identification of potential contaminants. Each high scoring peptide sequence, and the corresponding tandem mass spectrum, was manually inspected to insure the match was correct.
DNA Cellulose Chromatography of Nucleoid-associated Proteins
Nucleoid-associated proteins were isolated from mitochondria and extracted from mitochondrial DNA according to the methods described above. Nucleoid-extracted proteins were dialyzed for 3 h at 4°C against a buffer containing 25 mM Tris, pH 7.5, 100 mM NaCl, 1 mM EDTA, 1 mM beta-mercaptoethanol. After dialysis, the extract was fractionated using native DNA cellulose chromatography as described (Alberts and Herrick, 1971).
Rapid Preparation of Extracts for Western Blotting
Cultures were grown either in YPD or YPG to early log phase. 5 OD600 units were harvested by centrifugation and cells were washed once in distilled water. Cell pellets were resuspended in 75 μl SDS-PAGE sample buffer (2% SDS, 80 mM Tris-HCl, pH 6.8, 10% glycerol (vol/vol), 5% 2-mercaptoethanol, 1 mM phenylmethyl sulfonyl fluoride, 3 mM benzamidine). The mixture was boiled for 10 min at 100°C, and centrifuged for 15 min at 13,000 g. 5 μl of the supernatant was used for SDS-PAGE and immunoblot analysis.
SDS-PAGE and Western Blotting
Proteins were analyzed using 17.5% SDS-PAGE, prepared according to (Anderson et al., 1973). For Western blotting, anti-Mgm101p, anti-Abf2p, and anti-Por1p (Molecular Probes) antibodies were used at dilutions of 1:500, 1:1,000, and 1:500, respectively. Primary antibodies were incubated for a minimum of 1 h at 25°C, followed by incubation for a minimum of 30 min with secondary antibody at a dilution of 1:1000 (goat anti–rabbit HRP antibody and goat anti–mouse HRP antibody; Southern Biotechnology Associates, Inc.). Western blots were developed using ECL reagents (Amersham).
Indirect Immunofluorescence
To analyze mitochondrial morphology in strain JNY131, cells were shifted to nonpermissive temperature (37°C) and samples were taken at various time intervals and processed for indirect immunofluorescence. To localize Mgm101p, W303 was transformed with a 2μ plasmid containing MGM101 (kindly provided by G.D. Clark-Walker) and was grown in YPG at 30°C to log phase. At least 5 A600 units of cells were fixed and processed for indirect immunofluorescence as described (Pringle et al., 1991). Primary antibody incubation was for 1 h at room temperature with the following antibody dilutions into PBS/4% BSA: anti-Por1p at 1 μg/ml and affinity-purified anti-Mgm101p at 1:50. Fluorescein-conjugated anti-mouse secondary antibody or Texas red–conjugated anti-rabbit (Kappel) at a dilution of 1:100 in PBS/4% BSA was incubated with the cells at room temperature for 1 h, followed by three washes with PBS/4% BSA and one with PBS containing 1 μg/ml of DAPI. Mounting media (FITC-Guard; Testgog) was placed on fixed cells and coverslips were sealed with nail polish. All fluorescent samples were analyzed using a Leica confocal microscope and a 100× 1.4 NA objective. Figures were prepared using Adobe Photoshop and Adobe Illustrator.
Creation of GFP-tagged Mgm101p
A GFP fusion to the NH2 terminus of MGM101 was constructed by introducing 5′ and 3′ BamHI sites (in frame with the initiator ATG codon and after the termination codon) in MGM101 codon by PCR with Vent polymerase (New England Biolabs). A mitochondrially targeted (signal corresponding to bases 1–60 of COXIV) form of GFP downstream of the ADH promoter in plasmid pOK29 (kindly provided by Rob Jensen, Johns Hopkins, Baltimore, MD) was modified by introducing a BamHI site into the 3′ end of GFP(S65T) and eliminating the termination codon, resulting in pmitoNGFP. Modified MGM101 was ligated into pmitoNGFP, resulting in a gene encoding mitochondrially targeted NH2-terminal GFP fusion of Mgm101p expressed from the ADH promotor. Yeast cells expressing mito-GFPMgm101p were imaged vitally without fixation after incubation with 1 mg/ml DAPI for 30 min using a Leica confocal microscope and a 100× 1.4 NA objective.
BrdU Incorporation and Detection
Thymidine kinase gene from Herpes virus was introduced into JNY131(mgm101-2) and W303 by crossing and sporulation. These strains, JNY131TK and W303TK, were cultured overnight in YPG at 25°C to 0.2/ml OD600 units. Cells were washed twice and resuspended in 37°C YPD at same OD600 unit/ml, and placed in 37°C. At fixed time points, a 5-ml aliquot (1 OD600 unit) of each culture was removed, pelleted, and BrdU was incorporated into DNA by resuspension in 2 ml of YPD media containing 5 mg/ml sulfanilimide, 10 μg/ml amethopterin, and 0.5 mg/ml BrdU, and then incubated at 37°C for 30 min. BrdU incorporation into mtDNA was detected by indirect immunofluorescence as previously described (Nunnari et al., 1997).
UV, Gamma Ray, and H2O2 Exposure
W303 and JNY131(mgm101-2) strains were grown at 25°C overnight in YPG to 0.2–0.3 OD600 unit/ml. For UV-irradiation, both JNY131 (mgm101-2) and W303 cells were washed into YPD and preincubated at 37°C for 2 h before irradiation. Cells were then plated onto YPD plates and exposed to UV radiation (Stratalinker; Stratagene) at doses ranging from 0–200 J/m2. For gamma-irradiation, overnight cultures of cells were washed twice, resuspended in 0.9% NaCl at 2 × 106 cells/ml, placed on ice and exposed to a Cs source for various times corresponding to 0–600 grays. For H2O2 treatment, overnight cultures of cells were washed once in YPD, resuspended in YPD containing 0–20 mM H2O2 and incubated at 25°C for 1 h. Cells were then harvested and washed twice in 20 mM potassium phosphate, pH 7.4. Immediately after exposure, cells were plated on YPD plates. After exposure to each mutagen, colonies were formed at a semi-permissive temperature of 34°C (4-d incubation) and respiratory competency was determined by colony color as described.
Results
Independent Genetic and Biochemical Approaches Indicate That Mgm101p's Function Is Nucleoid-specific
In an effort to understand the mechanism underlying mitochondrial nucleoid organization and function, we have started to identify the proteins found in highly enriched (∼70-fold from total extracts) preparations of nucleoids using the technique of liquid chromatography coupled with tandem mass spectrometry (see Materials and Methods; Yates et al., 1996). Using this technique, many individual proteins can be identified from a heterogeneous mixture of proteins, making it ideal for analyzing constituents of biological complexes (Yates et al., 1996). Tandem mass spectrometry analysis of trypsin-digested nucleoid-enriched fractions consistently identified several peptides contained in the protein encoded by the MGM101 gene (Fig. 1, see overlines). These biochemical data suggest that Mgm101p plays a direct role in mtDNA maintenance through an association with the mitochondrial nucleoid.
We have also isolated a temperature sensitive allele of MGM101, JNY131(mgm101-2), in a genetic screen for mutants that are unable to maintain mtDNA (see Materials and Methods; J. Wagner, E.D. Wong, and J. Nunnari, unpublished observations). To determine the specific mutation in mgm101-2, we sequenced the mutated gene. Sequencing of the mgm101-2 allele revealed a single point mutation, resulting in a change of the conserved Asp131 residue to Asn (Fig. 1, asterisk). This residue is invariant in Mgm101p among all species and is found at the beginning of the highly conserved COOH-terminal region of the protein. Interestingly, this alteration is a relatively conservative change in Mgm101p's primary structure. The fact that it causes the catastrophic loss of mtDNA under nonpermissive conditions reinforces the importance of the COOH-terminal region in Mgm101p function.
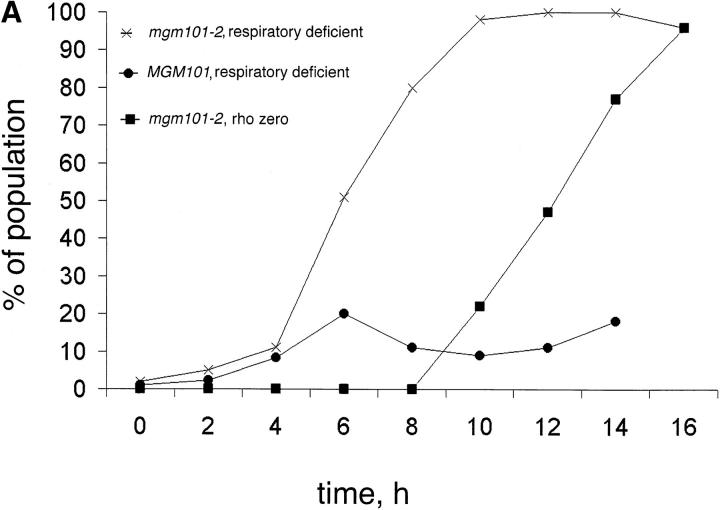
To gain more insight into the role of MGM101 in mtDNA maintenance, we have characterized the kinetics of mtDNA loss in JNY131(mgm101-2). Loss of respiratory competence was determined by plating JNY131(mgm101-2) cells onto YPD media at fixed time points after shifting to nonpermissive temperature. After exposure to nonpermissive conditions, colonies were formed at permissive temperature and respiratory competence was assessed by examining colony color. Respiratory competent cells containing an ade2 mutation accumulate a red pigment when adenine is limiting in the medium. Thus, on YPD plates, red colonies are classified as respiratory competent and white and sectored colonies are classified as respiratory deficient. This classification was confirmed by analyzing the growth of colonies replica plated on medium containing the nonfermentable carbon source, glycerol. Under permissive conditions, no difference in the generation of respiratory incompetent colonies was observed in JNY131 (mgm101-2) and W303, indicating that mgm101-2 allele is functional under these conditions (Fig. 2 A, t = 0 h and Table II). After shifting to nonpermissive temperature, however, JNY131(mgm101-2) cells began to lose respiratory competence after 4 h (two doublings). With continued exposure to nonpermissive temperature, the number of nonrespiratory colonies increased until the entire population of JNY131(mgm101-2) cells were respiratory deficient at 12 h (Fig. 2 A and Table II). In contrast, a substantially lower percentage (overall 10%) of W303 cells became respiratory deficient at nonpermissive conditions (Fig. 2 A). The relatively rapid loss of respiratory competence in the JNY131(mgm101-2) strain under nonpermissive conditions is similar to that observed previously for mgm101-1 cells (Chen et al., 1993) and reflects the importance of Mgm101p in mtDNA maintenance.
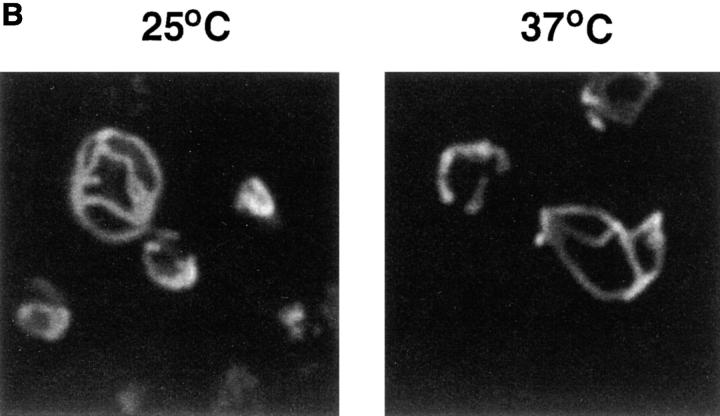
Figure 2.
Respiratory function is lost in mgm101-2 cells under nonpermissive conditions, but mitochondrial morphology is unaffected. W303 and JNY131(mgm101-2) cells were grown to log phase in YPG, cells were harvested, split, and washed into YPD medium at 24 or 37°C. Parallel cultures of JNY131(mgm101-2) and W303 were grown in log phase in YPD at 24 and 37°C and at fixed time points, samples were collected and analyzed for respiratory competence, and for the presence for mtDNA by DAPI staining of fixed cells (A, at indicated times) and by indirect immunofluorescence using anti-Por1p (B, 12 h) as described in Materials and Methods.
Table II.
Correlation of mgm101-2 Phenotypes
| Time, hrs at 37°C | Respiratory deficient | Rho° | Unlabeled by DAPI | Unlabeled by BrdU | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2/91 (2%) | N/D | 0/50 (0%) | 1/50 (2%) | ||||
| 2 | 4/66 (6%) | N/D | 0/50 (0%) | 3/50 (6%) | ||||
| 4 | 10/89 (11%) | N/D | 2/50 (4%) | 9/50 (18%) | ||||
| 6 | 56/110 (51%) | N/D | 23/50 (46%) | 12/50 (24%) | ||||
| 8 | 90/113 (80%) | 0/12 (0%) | 0/50 (100%) | 16/50 (32%) | ||||
| 10 | 137/141 (97%) | 4/18 (22%) | N/D | 21/50 (42%) | ||||
| 12 | 68/68 (100%) | 7/15 (47%) | N/D | 30/50 (60%) | ||||
| 14 | (100%) | 13/17 (77%) | N/D | N/D | ||||
| 16 | (96%) | 23/24 (96%) | N/D | N/D |
To determine the nature of the nonrespiring phenotype in JNY131(mgm101-2), cells from nonrespiring colonies were crossed to wild-type cells lacking mtDNA. Diploids resulting from this cross were also respiratory deficient, indicating that loss of respiratory function was the result of a mutation (rho−) or complete loss (rho°) of mtDNA. To distinguish between rho− and rho° cells, mtDNA was examined directly in cells from nonrespiratory JNY131 (mgm101-2) colonies by staining with the DNA-specific fluorescent probe, DAPI. Interestingly, after a nonpermissive exposure time that resulted in 100% nonrespiring colonies, only ∼50% of the nonrespiring colonies consisted of rho° cells (Fig. 2 A and Table II). The remaining half of the colonies consisted of rho− cells that still possessed mitochondrial nucleoids. However, after a more prolonged exposure of JNY131(mgm101-2) cells to nonpermissive temperature (16 h), 100% of nonrespiring colonies consisted of cells that were rho° (Fig. 2 A and Table II), consistent with the colony phenotype observed in the screen that identified JNY131(mgm101-2) (see Materials and Methods). These observations indicate that a transition occurs where nonfunctional mtDNA (rho−) is formed in JNY131(mgm101-2) cells before the complete loss of mtDNA (rho°). This phenotype is consistent with an early deficiency in JNY131(mgm101-2) cells that causes the degradation of mtDNA or results in a block in mtDNA replication.
Because mtDNA loss is also observed in a sub-group of mutants that affect mitochondrial morphology, such as mgm1, mdm10, mdm12, and fzo1, mitochondrial structure in JNY131(mgm101-2) cells was assessed by indirect immunofluorescence using antibodies directed against Por1p, a mitochondrial outer membrane protein (Burgess et al., 1994; Hermann et al., 1998; Sogo and Yaffe, 1994). Mitochondrial morphology was unchanged and was indistinguishable from the cortical, reticular structure of wild-type mitochondria in S. cerevisiae. Transmission of the mitochondrial organelle also was unaffected, even after exposure to nonpermissive temperature for 12 h (Fig 2 B). This indicates that loss of respiratory function and mtDNA in JNY131(mgm101-2) is not associated with mitochondrial morphology and transmission defects. Together with the biochemical identification of Mgm101p as a component of enriched nucleoid preparations, these data suggest that Mgm101p plays a more direct role in mtDNA maintenance.
Mgm101p Is a Nucleoid-associated Protein
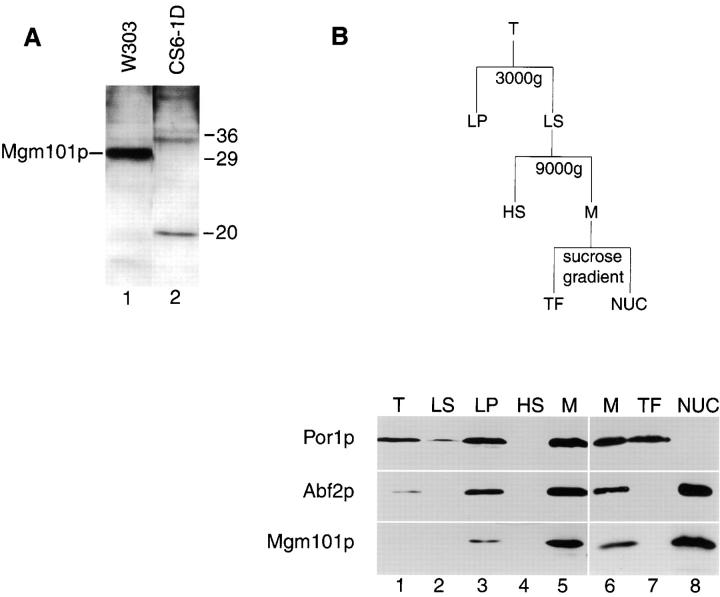
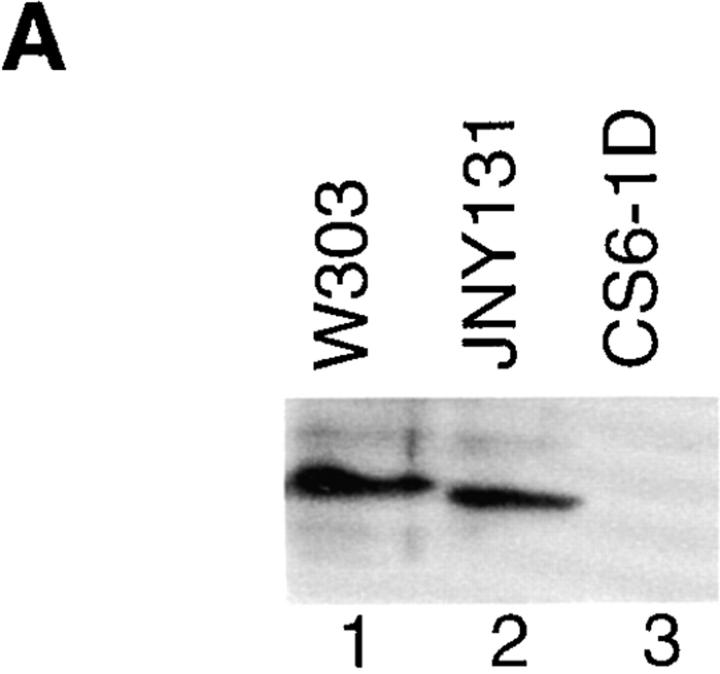
Both genetic and biochemical analyses suggest that Mgm- 101p is localized to the mitochondrial nucleoid. To determine the subcellular localization of Mgm101p, we raised polyclonal antibodies against a MBP-Mgm101p fusion protein. Western blot analysis of W303 whole cell extracts with anti-Mgm101p antibodies detected a band at 30 kD, the predicted molecular mass of Mgm101p (Fig. 3 A, lane 1). In cell extracts made from CS6-1D, which contains a recessive inactive truncated copy of MGM101, a band at ∼20 kD was detected, corresponding to the predicted Mgm- 101p product formed by the chromosomal disruption in this strain (Fig. 1, open triangle, Fig. 3 A, lane 2).
Figure 3.
Mgm101p cofractionates with mitochondrial nucleoids. (A) Cultures of strain W303(pep4) and CS6-1D were grown in YPD. Total cell extract was prepared by lysing cells using the rapid boiling method (see Materials and Methods). A constant amount of total cell extract (0.2 OD600 units) from W303(pep4) (lane 1) and CS6-1D (lane 2) was analyzed by SDS-PAGE and immunoblotted with anti-Mgm101p antibody. (B) Cultures of strain W303(pep4) were grown in YPG. Cells were spheroplasted, lysed and fractionated as described in Materials and Methods. A constant amount (50 μg) of the total cell extract (T, lane 1), the 3,000 g supernate (LS, lane 2) and pellet fractions (LP, lane 3) and the 9,000 g supernate (lane 4) and pellet fractions (M, lane 5) was analyzed by SDS-PAGE and immunoblotted with anti-Por1p, anti-Abf2p, and anti-Mgm101p antibodies. Mitochondria were lysed and mitochondrial nucleoids were enriched by sucrose gradient centrifugation according to Materials and Methods. A constant amount (50 μg) of the M fraction (M, lane 6), the top fraction from the sucrose gradient (TF, lane 7) and the 60/80% interface fraction containing mitochondrial nucleoids (NUC, lane 8) was analyzed as described above.
To determine whether Mgm101p was localized to the mitochondria, we fractionated whole cell extracts from W303(pep4) cells by differential centrifugation. Samples from each fraction were analyzed by SDS-PAGE and immunoblotted with anti-Por1p, a mitochondrial outer membrane protein, anti-Abf2p, a known mitochondrial nucleoid-associated protein, and anti-Mgm101p (Fig. 3 B). After low speed centrifugation (3000 g) of total cell lysate (Fig. 3 B, lane 1), mitochondria were recovered in both the supernatant (Fig. 3 B, lane 2) and pellet (Fig. 3 B, lane 3), as indicated by the detection of Por1p and Abf2p. The presence of mitochondria in the low speed pellet was probably due to incomplete cell lysis. A similar fractionation pattern was observed for Mgm101p. When the low speed supernatant was centrifuged at 9,000 g, Por1p and Abf2p again cofractionated and were recovered in the high speed pellet (Fig. 3 B, M, lane 5), consistent with the mitochondrial localization of these proteins. Mgm101p was also recovered and enriched in the pellet fraction, indicating that it is a mitochondrial protein (Fig. 3 B, M, lane 5).
To determine whether Mgm101p was associated with mitochondrial nucleoids, the mitochondrial-enriched pellet (Fig. 3 B, M, lane 5) was subjected to equilibrium density centrifugation and detergent extraction (Fig. 3 B, M, lane 6). Mitochondrial nucleoids were purified further by fractionation of the mitochondrial detergent extract on two successive sucrose density gradients (Fig. 3 B, lanes 7 and 8). Samples from the second sucrose gradient were subjected to SDS-PAGE and immunoblotted with antibodies against Por1p, Abf2p, and Mgm101p. As indicated by the fractionation behavior of the nucleoid marker Abf2p, mitochondrial nucleoids migrated to the 37.5%/ 60% sucrose interface, consistent with previously published results (Fig. 3 G, NUC, lane 8; Newman et al., 1996). In contrast, Por1p did not migrate into the sucrose gradient, as indicated by its presence in the gradient's top fraction, confirming its nonnucleoid association and demonstrating the observed enrichment of nucleoid-associated proteins at the 37.5%/60% sucrose interface (Fig. 3 B, TF, lane 7). Mgm101p cofractionated with Abf2p and was quantitatively recovered in the fraction representing the 37.5%/60% interface, suggesting that it is tightly associated with nucleoids (Fig. 3 B, TF, lane 8).
To confirm the biochemical fractionation data, we localized Mgm101p by tagging the protein at the NH2 and the COOH termini with GFP. Fusion of mito-GFP to the NH2 terminus of Mgm101p (mito-GFP-Mgm101p, see Materials and Methods) created a protein that when expressed from a constitutive promoter (ADH) in JNY131(mgm-101-2) cells could partially complement the temperature-sensitive growth phenotype on glycerol, indicating that it was functional. In addition, DAPI staining of nonrespiring JNY131(mgm101-2) cells expressing mito-GFP-Mgm101p revealed that they were rho−, in contrast to the rho° phenotype that results from mgm101-2. This also indicates that mito-GFP-Mgm101p is able to partially rescue the defect in JNY131(mgm101-2) cells. Expression of a COOH-terminal fusion of GFP to Mgm101p failed to complement growth of JNY131(mgm101-2) on nonfermentable carbon sources. However, JNY131(mgm101-2) cells expressing Mgm101p-GFP were also 100% rho− under nonpermissive conditions. This indicates that Mgm101-GFP also retains some function. Interestingly, overexpression of either mito-GFP-Mgm101p or Mgm101p-GFP, but not wild-type Mgm101p, in W303 cells resulted in a significant increase in the formation of nonrespiratory cells (∼50-fold increase), suggesting that fusion of GFP at either terminus interferes with some process required for mtDNA maintenance.
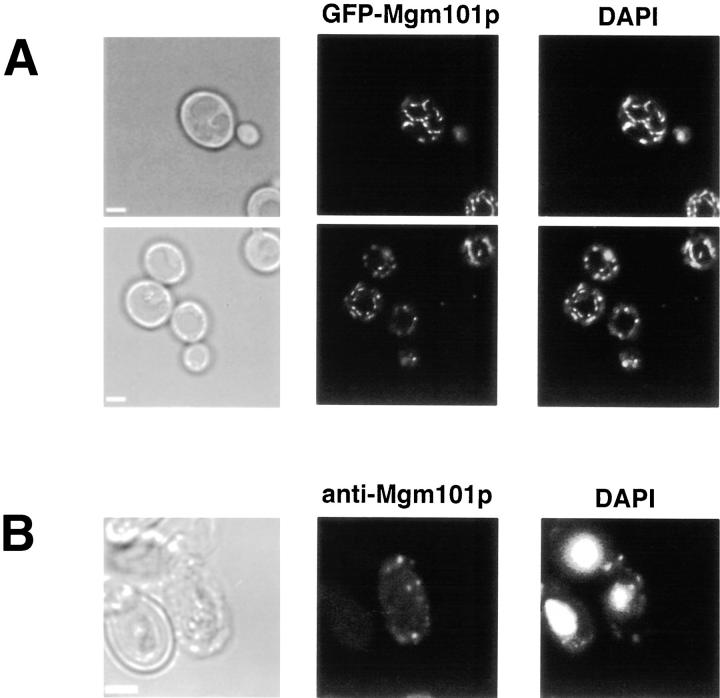
Visualization of either live wild-type or JNY131(mgm-101-2) cells expressing GFP-Mgm101p (Fig. 4 A) and Mgm101-GFP (data not shown) revealed that GFP was localized to punctate structures at the cortex of the cell that completely overlap with vitally DAPI-labeled mtDNA. Thus, in vivo, Mgm101p/GFP fusion proteins are localized to the mitochondrial nucleoids.
Figure 4.
Mgm101p is localized to mitochondrial nucleoids in vivo. (A) W303 cells expressing mito-GFP-Mgm101p were grown in YPG, stained vitally with DAPI, and directly imaged as described in Materials and Methods. (B) W303 cells overexpressing Mgm101p were grown in YPG and were processed for indirect immunofluorescence with anti-Mgm101p and imaged as described in Materials and Methods. Bar, 2 μm.
Localization of Mgm101p in cells was also determined using indirect immunofluorescence with anti-Mgm101p antibodies. No staining above background was observed with anti-Mgm101p in wild-type cells (data not shown). However, in cells overexpressing Mgm101p, anti-Mgm-101p labeled punctate structures at the cortex of the cell (Fig. 4 B). These punctate structures were also labeled with DAPI, indicating that they contained mtDNA. As stated above, overexpression of Mgm101p in wild-type cells had no observable effect on mtDNA maintenance or inheritance as compared with wild-type. It is important to note that because we could only detect Mgm101p when overexpressed in cells, we cannot rule out the possibility that, when present at wild-type levels, Mgm101p may localize to only a subset of nucleoids. However, these data are in agreement with data obtained from both the biochemical fractionation behavior of Mgm101p and the localization of the Mgm101p/GFP fusions and indicate that Mgm101p is localized specifically and uniquely to the mitochondrial nucleoid.
Mgm101p Binds DNA
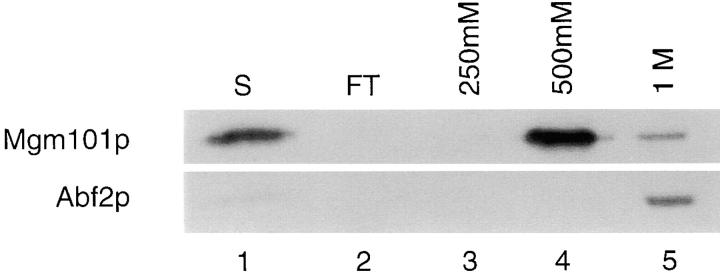
The COOH-terminal region of Mgm101p is highly basic suggesting that Mgm101p has the ability to bind DNA. To determine if Mgm101p has DNA binding activity, nucleoids were isolated from W303 and associated proteins were extracted with high salt containing buffer, separated from mtDNA by sucrose gradient centrifugation and subjected to DNA-cellulose chromatography. Both nucleoid proteins Abf2p and Mgm101p were quantitatively recovered in this high salt nucleoid extract, indicating that their association with this structure is salt-sensitive. Given that this may reflect their association with mtDNA, the salt concentration in the extract was lowered to 200 mM before DNA-cellulose chromatography. Under these conditions, both Abf2p and Mgm101p were soluble and sedimented as monomers as determined by sucrose gradient sedimentation (data not shown). Abf2p bound to the DNA-cellulose column and could be eluted only at high salt concentrations (1 M), consistent with its known high affinity interaction with DNA (Fig. 5, compare lanes 1 and 5). Mgm101p also bound and eluted quantitatively from the DNA cellulose at relatively high salt concentrations (500 mM, Fig. 5, lane 4). This tight association of Mgm- 101p with DNA cellulose suggests that Mgm101p specifically interacts with DNA. Furthermore, both Mgm101p and Abf2p were significantly enriched, 60- and 100-fold, respectively, in DNA-cellulose column eluates. Taken together these data suggest that the localization of Mgm101p to the nucleoid is at least in part due to direct interaction with mtDNA.
Figure 5.
Mgm101p binds DNA. Mitochondrial nucleoids were isolated as described in Materials and Methods. Nucleoid-associated proteins were dissociated and separated from mitochondrial DNA by incubation for 2 h in 1 M KCl followed by centrifugation where the salt extract was layered over a 30% sucrose cushion. The supernate containing high salt extracted proteins was dialyzed into Tris-EDTA buffer, pH 7.5, 200 mM NaCl (S, lane 1) and adsorbed to a native DNA cellulose column. Proteins bound to the column were eluted with 250 mM, 500 mM, and 1 M NaCl. The dialyzed high salt extract (S, lane 1), flow through (FT, lane 2), and fractions from 250 mM, 500 mM, and 1 M elutions (lanes 3–5) were analyzed by SDS-PAGE and immunoblotting.
Mgm101p Content Decreases in mgm101-2 Cells at Nonpermissive Temperature
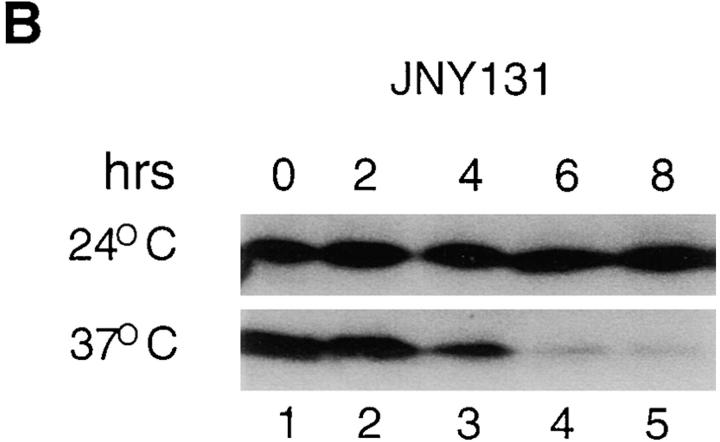
To gain more insight into MGM101 function, we examined Mgm101p under both permissive and nonpermissive conditions in JNY131(mgm101-2). Total extracts from JNY131(mgm101-2) and W303 cultures grown under both conditions were analyzed by SDS-PAGE and immunoblotting with anti-Mgm101p (Fig. 6, A and B). In extracts from JNY131(mgm101-2) cultured under permissive conditions, a band was recognized specifically by anti-Mgm101p that migrated faster than Mgm101p (Fig. 6 A, compare lanes 1 and 2). This faster migrating product is unlikely to be an in vitro isolation artifact because the identical migration behavior was observed when Mgm101-D131Np was examined from denaturing immunoprecipitation of Mgm101p from S35-Met pulse-labeled cells (data not shown). The faster migration of Mgm101p observed under permissive conditions is thus likely the result of an in vivo proteolytic modification that may contribute to the phenotype observed at nonpermissive temperature (see below).
Figure 6.
Mgm101p content in mgm101-2 cells decreases under nonpermissive conditions. A. Cultures of strain W303(pep4), JNY131(mgm101-2), and CS6-1D were grown in YPD. Total cell extracts were prepared by the rapid boiling method as described in Materials and Methods. 0.33 OD600 units of W303, pep4 (lane 1), JNY131 (lane 2) and CS6-1D (lane 3) was analyzed by SDS-PAGE and immunoblotted with anti-Mgm101p antibody. B. JNY131(mgm101-2) cells were grown to log phase in YPG and cells were harvested, split and washed into YPD medium at 24°C or YPD medium prewarmed at 37°C. These two cultures of JNY131(mgm101-2) were grown in YPD at 24°C and 37°C, respectively, and samples were collected every 2 h. Total cell extracts were prepared and analyzed as described in Part A.
As a function of time at nonpermissive temperature, the amount of Mgm101-D131Np observed was reduced dramatically in JNY131(mgm101-2) cells (Fig. 6 B, lanes 1–5). Specifically, reduction of Mgm101-D131Np content occurred after 4 h and Mgm101-D131Np was not detectable above background after 6 h of exposure to nonpermissive temperature. In contrast, the steady state level of Mgm- 101p was unaffected under these conditions (data not shown). This observed thermolability of Mgm101-D131Np slightly precedes and correlates with loss of respiratory function and mtDNA in JNY131(mgm101-2) observed at nonpermissive temperature (Fig. 2 A) and therefore is likely the primary cause of these phenotypes.
Gross Alterations in Packaging, Segregation, and Partitioning of mtDNA Are Not Observed in mgm101-2
We investigated the function of Mgm101p in mtDNA maintenance by characterizing changes in nucleoid morphology in JNY131(mgm101-2) during loss of mtDNA under nonpermissive conditions. Failure of nucleoid segregation, as observed in ΔMGT1 cells, causes the accumulation of fewer and brighter-stained nucleoid structures (Lockshon et al., 1995). Defects in mtDNA packaging, as observed in ΔABF2 cells, cause nucleoid staining to become more diffuse (Newman et al., 1996). We reasoned that such analysis would help distinguish between these possible explanations of loss of mtDNA in JNY131(mgm101-2).
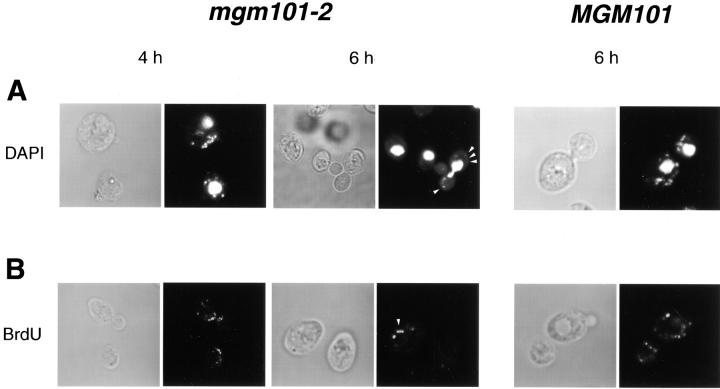
We visualized nucleoids using DAPI in fixed JNY-131(mgm101-2) and W303 cells collected at fixed time points after exposure to nonpermissive temperature. Up to 4 h of exposure to nonpermissive temperature, the majority of JNY131(mgm101-2) cells contained mitochondrial nucleoids that were indistinguishable from nucleoids observed in W303 cells (Fig. 7 A, 4 h and Table II). This is consistent with the fact that the majority of JNY131 (mgm101-2) cells retained respiratory competence during this time (Fig. 2 A and Table II). In JNY131(mgm101-2) cells exposed to greater than 4 h of nonpermissive temperature mitochondrial nucleoid morphology remained punctate, but the staining of the nucleoids with DAPI became less intense and fewer nucleoids were detected (Fig. 7 A, 6 h, see arrows and Table II). No staining of nucleoids with DAPI was detected after 8 h of exposure time (Table II). In contrast, no change in nucleoid intensity was observed in either JNY131(mgm101-2) cells cultured under permissive conditions or W303 cells exposed to nonpermissive temperature, indicating that the effect was specific to the temperature-sensitive mgm101-2 allele (Fig. 7 A). This phenotype correlates with and is likely the result of the temperature-dependent decrease in Mgm101-D131Np levels observed in JNY131(mgm101-2) at nonpermissive temperature. The lack of significant change in nucleoid morphology in JNY131(mgm101-2) suggests that segregation and packaging of mtDNA are not significantly affected. Furthermore, nucleoid partitioning into daughter buds of JNY131(mgm101-2) cells was also unaffected (Fig. 7 A, 6 h, see arrows).
Figure 7.
Mitochondrial nucleoid morphology and mtDNA replication are unaffected in mgm101-2 cells. W303TK and JNY131TK cells were cultured overnight at 25°C in YPG, washed and resuspended in YPD at 37°C. Aliquots of cells were taken at fixed time points after incubation at 37°C and either (A) fixed and labeled with DAPI or (B) labeled with BrdU. Representative samples at various time points are shown.
Although segregation, packaging and partitioning of mtDNA do not seem to be grossly affected in JNY131 (mgm101-2) cells, we did observe a dramatic decrease in the intensity of DAPI nucleoid staining over time at nonpermissive temperature. Interestingly, DAPI staining of nucleoids became undetectable before complete mtDNA loss in JNY131(mgm101-2) as assessed by examining mtDNA in cells from nonrespiratory JNY131(mgm101-2) colonies (Fig. 2 A and Table II), consistent with our interpretation that mgm101-2 may cause mtDNA loss as a result of a block in mtDNA replication or by causing mtDNA degradation.
Mgm101p Is Not Required for the Maintenance of mtDNA Replication
To determine whether loss of mtDNA in JNY131 (mgm-101-2) was the result of a block in mtDNA replication, we monitored the incorporation of the thymidine analogue, 5-bromodeoxyuridine (BrdU) into mtDNA by indirect immunofluorescence as previously described (Nunnari et al., 1997). Towards this goal, we constructed JNY131 (mgm101-2) and W303 strains that contained a chromosomal copy of an exogenous thymidine kinase (JNY131TK and W303TK, respectively). Mitochondrial DNA replication was monitored in JNY131TK and W303TK cells grown under both permissive and nonpermissive conditions by pulse labeling with BrdU. Punctate staining in both JNY131TK and W303TK cells was detected at the cell cortex by indirect immunofluorescence of labeled cells with anti-BrdU antibodies (Fig. 7 B). This punctate staining was not detected in either W303 rho° cells or W303 that had not been labeled with BrdU, indicating that it was specifically the result of incorporation of BrdU into mtDNA (Nunnari et al., 1997 and data not shown). Under permissive conditions, no significant differences in BrdU incorporation into mtDNA between JNY131TK and W303TK were observed (data not shown).
Exposure of cells to nonpermissive temperature up to 4 h did not cause a significant reduction in the number of JNY131TK cells containing BrdU-labeled mtDNA or the intensity of mtDNA labeling as compared with W303 cells (Fig. 7 B, 4 h and Table II). This nonpermissive exposure time correlates with the onset of loss of respiratory function and the decrease in nucleoid staining intensity observed with DAPI in JNY131(mgm101-2) (Fig. 7 A and Table II). Thus, the lack of an observable change in BrdU incorporation into mtDNA in JNY131(mgm101-2) before these phenotypes suggests that a block in mtDNA replication in JNY131TK is not the cause of these defects. Even after exposure times where Mgm101-D131Np content was greatly reduced by Western analysis, we observed BrdU-labeled mtDNA in JNY131(mgm101-2) cells of equal intensities (measured by pixel intensity, n = 20) to BrdU-labeled mtDNA in W303 cells (Fig 7 B, 6 h, see arrows and Table II). However, the percentage of JNY131TK cells that contained any detectable BrdU-labeled mtDNA decreased with time (Table II). Not surprisingly, this unlabeled population correlates with the JNY131(mgm101-2) respiratory deficient colonies that contained rho° cells (Fig. 2 A and Table II), indicating that lack of BrdU-labeled mtDNA in this population of cells was a secondary consequence of mtDNA loss. Taken together, these data suggest that under nonpermissive conditions, loss of respiratory function and mtDNA in JNY131 is not caused by a block in ongoing mtDNA replication.
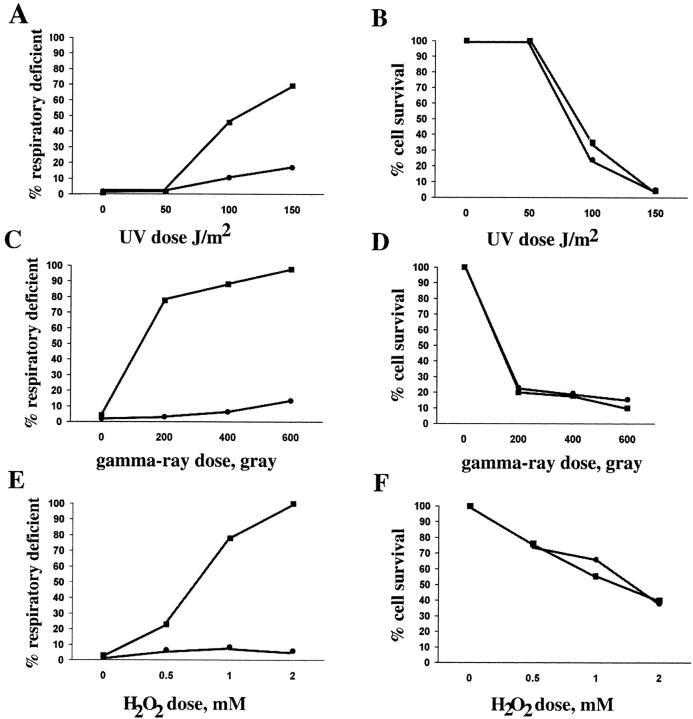
mgm101-2 Cells Are Sensitive to UV-induced mtDNA Mutations and Hypersensitive to Both Gamma Ray and H2O2-induced mtDNA Mutations
Given that ongoing mtDNA replication is unaffected in JNY131(mgm101-2), we reasoned that the decrease in nucleoid staining by DAPI observed in JNY131(mgm101-2) might be the result of mtDNA degradation. Consistent with this interpretation is that, after exposure to nonpermissive temperature, JNY131(mgm101-2) cells recovered rho− mtDNA under permissive conditions before complete mtDNA loss (Fig. 2 A). Observations made in other systems, such as E. coli, indicate that damaged DNA is susceptible to DNA degradation. Thus, one explanation for these phenotypes is that JNY131(mgm101-2) cells are deficient in the repair of damaged mtDNA.
To determine if Mgm101p plays a role in mtDNA repair, we examined the sensitivity of mtDNA to DNA damage in JNY131(mgm101-2) and W303 strains by monitoring the percent formation of respiratory deficient colonies. To examine the role of Mgm101p in the repair of UV-damaged mtDNA, we compromised MGM101 function by pretreating JNY131(mgm101-2) cells for 2 h at nonpermissive temperature before irradiation. After UV irradiating JNY131(mgm101-2) and W303 cells at various doses, colonies were formed at the semi-permissive temperature of 34°C. Under these conditions, in the absence of irradiation, we observed only a slight increase (2%) in the percentage of nonrespiratory colonies formed by JNY131 (mgm101-2) cells as compared with W303 cells (Fig. 8 A, time 0). Upon UV-irradiation, however, a significant dose-dependent increase in the percentage of nonrespiratory colonies was observed in JNY131(mgm101-2) as compared with W303 (Fig. 8 A), indicating a defect in UV-induced mtDNA repair in JNY131(mgm101-2) cells. In contrast to the sensitivity of JNY131(mgm101-2) cells to mtDNA damage, nuclear DNA damage, as assessed by DNA damage-induced cell death, is unaffected (Fig. 8 A), indicating that Mgm101p functions specifically in the repair of mtDNA and not nuclear DNA. Both pretreatment at nonpermissive temperature and incubation at the semi-permissive temperature of 34°C were necessary to observe significant mgm101-2-dependent sensitivity to UV-induced mtDNA damage, consistent with the fact that Mgm101-D131Np is functional under permissive conditions.
Figure 8.
mgm101-2 cells are defective for the repair of oxidatively damaged mtDNA. (A and B) UV irradiation of W303 and JNY131(mgm101-2) cells was performed at indicated doses after cells were cultured in log phase at 37°C in YPD for 2 h and plated onto YPD media at 25°C. (C and D) Gamma ray treatment was performed at 4°C on cultures of W303 and JNY131(mgm101-2) cells that were grown overnight at 25°C in YPG, washed into saline solution and sonicated. (E and F) H2O2 treatment was performed at 25°C on cultures of W303 and JNY131 (mgm101-2) cells that were grown overnight in YPG, washed into YPD and treated with H2O2 at the indicated concentrations. Respiratory deficiency (A, C, and E) and cell survival (B, D, and F) were assessed as described in Materials and Methods. W303 cells are represented by circles and JNY131(mgm101-2) cells are represented by squares.
To determine if the mtDNA repair defect in JNY131 (mgm101-2) cells was specific to UV-induced types of DNA damage, such as the formation of pyrimidine dimers, we examined the sensitivity of JNY131(mgm101-2) to gamma ray–induced mtDNA damage. In contrast to UV-irradiation, no preincubation at nonpermissive temperature was required before gamma ray treatment to observe a significant mgm101-2-dependent sensitivity to mtDNA damage. However, after exposure, both JNY131(mgm101-2) and W303 colonies were formed at the semi-permissive temperature of 34°C. Similar to what was observed in the case of UV treatment, gamma ray irradiation of JNY131 (mgm101-2) caused an increase in mtDNA-specific damage as compared with that observed for W303 cells (Fig. 8, C and D). However, two significant differences were observed between the two mutagenic treatments. First, W303 cells were more sensitive to UV than gamma ray–induced mtDNA damage (compare Fig. 8, A and C). Given that we observed a significant induction of gamma ray–induced damage in JNY131(mgm101-2) cells, the resistance of wild-type cells to gamma ray–induced mtDNA damage suggests that they repair the types of damage caused by this treatment more efficiently than damage caused by UV treatment. Most importantly, JNY131(mgm101-2) cells were more sensitive to gamma ray–induced mtDNA damage than to UV-induced mtDNA damage (compare Fig. 8, A and C). This observation suggests that Mgm101p functions primarily in the repair of mtDNA damage selectively caused by gamma ray irradiation.
Ionizing radiation in the form of gamma rays results in a range of DNA damage, but the predominant form is oxidative damage to bases and oxidative damage to sugars, which results in single strand breaks in the DNA (Ward, 1988). The severe defect of JNY131(mgm101-2) cells in the repair of ionizing-induced mtDNA damage is consistent with a model in which MGM101 functions in the repair of oxidative damage. To test this model, we examined the sensitivity of JNY131(mgm101-2) cells to oxidative mtDNA damage caused by the oxidant H2O2. As shown in Fig. 8, E and F, JNY131(mgm101-2) cells were hypersensitive to H2O2-induced mtDNA damage as compared with wild-type. As in the case of gamma ray–induced damage, no preincubation at nonpermissive temperature was required before treatment to observe the mgm101-2-dependent hypersensitivity to mtDNA damage, but after treatment colonies were formed at the semi-permissive temperature of 34°C. The hypersensitivity to both gamma ray– and H2O2-induced mtDNA damage in JNY131(mgm101-2) cells indicates that MGM101 function is required selectively for the repair of oxidatively damaged mtDNA. Indeed, this repair defect in JNY131(mgm101-2) cells may be the cause of the catastrophic loss of mtDNA in JNY131(mgm101-2) cells at 37°C that have not been treated with DNA damaging agents.
Discussion
The mitochondrial nucleoid is a complex nucleoprotein structure that functions not only to package and store the genetic material, but also to organize the metabolic and segregational activities associated with its maintenance and inheritance. It is surprising that, with a few exceptions, both the constituents and the mechanisms involved in the assembly of this structure are relatively uncharacterized to date (Cho et al., 1988; Van Dyck et al., 1992; Newman et al., 1996; Okamoto et al., 1998). The MGM101 gene was previously shown to be required for the maintenance of mtDNA, but its precise role remained unidentified (Chen et al., 1993).
MGM101 Has a Nucleoid-specific Function
Our analysis of Mgm101p and the mgm101-2 mutant strain indicates that MGM101's function is specific to the nucleoid structure and mtDNA. Loss of MGM101 function initially causes cells to rapidly lose respiratory function due to irreversible mutations in mtDNA (accumulation of rho− genomes) and ultimately causes the complete and catastrophic loss of mtDNA. This loss of mtDNA in mgm101-2 cells is not associated with any changes in the structure or organization of the mitochondrial organelle. This is in contrast to another group of mutants that cause mtDNA loss as a result of a primary defect in the maintenance of mitochondrial morphology (Burgess et al., 1994; Sogo and Yaffe, 1994; Hermann et al., 1998). Using a number of independent approaches, we have also observed that Mgm101p is exclusively localized to nucleoids in cells. The nucleoid-specific localization of Mgm101p is probably due to its ability to bind tightly and specifically to DNA, suggesting that Mgm101p's function in mtDNA maintenance requires this DNA binding activity. Consistent with this is the fact that deletion of a small portion of the COOH terminus composed of 23% of positively charged residues results in loss of MGM101 function (Chen et al., 1993).
MGM101 Is Not Involved in mtDNA Packaging, Segregation, or Partitioning
What is the nature of Mgm101p's nucleoid-specific function? In vivo, nucleoid-specific functions required for mtDNA maintenance include the replication, repair, assembly, segregation, and partitioning of the mitochondrial nucleoid. From our analyses of mgm101-2 cells, it is unlikely that Mgm101p is required for mtDNA packaging or segregation because in mgm101-2 cells, nucleoid morphology remained punctate and only a decrease in DAPI staining intensity of nucleoids under nonpermissive conditions was observed. It is important to note that our analysis of nucleoid morphology using DAPI might not have identified a subtle change in packaging or segregation of mtDNA. However, one would not expect a subtle change to cause the catastrophic loss of mtDNA observed in mgm101-2 cells. In addition, alterations in both the packaging and segregation of mtDNA have been previously observed using this technique. In cells lacking ABF2, a nuclear gene that encodes a mtDNA packaging protein, nucleoids lose their punctate structure and instead possess a more diffuse morphology within the organelle (Newman et al., 1996). In cells lacking MGT1, a gene that encodes a cruciform-cutting endonuclease, fewer nucleoids are observed and each contains a greater amount of mtDNA, as assessed by DAPI staining intensity (Lockshon et al., 1995). Furthermore, loss of MGM101 function did not cause a defect in the partitioning of nucleoids to daughter buds. To date, no mutants have been found that block the movement of mtDNA within the organelle. The observed decrease in nucleoid staining intensity with DAPI during loss of MGM101 function suggests instead that MGM101 is involved in another, more central process required for mtDNA maintenance, such as DNA metabolism.
MGM101 Is Not Involved in mtDNA Replication
One key metabolic function required for mtDNA maintenance is replication. The decrease in nucleoid staining intensity with DAPI we observed in mgm101-2 cells is consistent with a decrease in nucleoid mtDNA content. We reasoned that this decrease could be the result of a block in mtDNA replication in mgm101-2 cells. Surprisingly, mtDNA replication, as monitored by BrdU incorporation in mgm101-2 cells did not appear to require MGM101 function. In contrast, we observed a rapid block (within 1 h at nonpermissive temperature) in mtDNA replication in a strain containing a temperature sensitive allele of MIP1, the gene encoding mtDNA polymerase, that exhibited loss of respiratory function with kinetics similar to mgm101-2 cells (Meeusen, S., and J. Nunnari, unpublished data). This observation indicates that the sensitivity of our assay for mtDNA replication is sufficient to detect defects in mtDNA replication. Based on our analysis, however, we cannot rule out a potential role of Mgm101p in the initiation of mtDNA replication. For example, one might not expect to observe a decrease in BrdU incorporation if mtDNA were replicating via a rolling circle mechanism and initiation occurred before the loss of MGM101 function. Although unlikely, such a scenario might explain why we observe wild-type BrdU labeling intensity of mtDNA several generations after the complete loss of Mgm101-D131Np.
MGM101 Is Required for the Repair of Oxidatively Damaged mtDNA
Based on our data, the most likely model is that MGM101 functions in the repair of oxidatively damaged mtDNA. Most consistent with this interpretation, is that mtDNA in mgm101-2 cells is hypersensitive to damage caused by gamma ray irradiation and H2O2. These two agents induce DNA damage predominantly in the form of oxidative damage to bases and to sugars (Ward, 1985, 1988). In contrast, mgm101-2 cells were less sensitive to damage caused by UV treatment that induces oxidative DNA damage to a lesser extent (Ward, 1988). In addition, at the time of DNA damage by gamma ray and H2O2 treatments, Mgm101-D131Np was functional in mgm101-2 cells, indicating that Mgm101p functions in the repair of damaged mtDNA and not in the protection of mtDNA from damage. In light of the probable role of MGM101 in mtDNA repair, the decrease in nucleoid DAPI staining intensity observed in mgm101-2 cells likely results from a transition where damaged mtDNA is degraded and ultimately lost, a phenomenon observed in other systems in response to unrepaired, damaged DNA (Michel et al., 1997). Our data also indicate that MGM101 functions exclusively in the repair of DNA contained in the mitochondrial organelle.
It is interesting to note that mtDNA in wild-type cells is more resistant to treatments that produce oxidative damage, suggesting that yeast cells possess a greater ability to repair this type of mtDNA lesion (Foury, 1982). Thus, the repair pathway for oxidatively damaged mtDNA might be one of the major repair pathways in the mitochondrial organelle. Indeed, given the highly oxidative environment of the mitochondrial organelle it is possible that MGM101 functions exclusively in oxidative damage repair and that this function is essential for mtDNA maintenance. Alternatively, it is also possible that MGM101 function is not restricted to mtDNA repair and that its requirement for mtDNA maintenance reflects its role in a more fundamental DNA metabolic activity.
What type of mtDNA repair pathway is Mgm101p involved in? As is the case for nuclear DNA, several different repair pathways function in the mitochondria. In yeast, there are at least two known repair pathways for mtDNA. One direct pathway is responsible for the repair of UV-induced pyrimidine dimers and is dependent on the PHR1 gene, which encodes a photolyase that functions in both the nucleus and the mitochondria (Sancar, 1985; Branda and Isaya, 1995). Given that MGM101 functions selectively in the repair of oxidatively damaged mtDNA, it is unlikely that it is involved in the direct PHR1-dependent photoreactivation repair pathway. Furthermore, the effects of UV-induced mtDNA damage in mgm101-2 cells were assessed in the dark and the PHR1 pathway is known to be dependent on light for activation (Sancar, 1985). A mtDNA mismatch excision repair pathway has also been characterized in yeast and is dependent on the MSH1 gene, which encodes an ATPase that is homologous to eubacterial MutS mismatch repair protein (Chi and Kolodner, 1994a,b). It is also unlikely that MGM101 is involved specifically in this pathway, because, while loss of MGM-101 function causes the complete loss of mtDNA, loss of MSH1 function results only in the accumulation of mutated copies of the mitochondrial genome, a substantially less severe phenotype (Chi and Kolodner, 1994). Thus, if MGM101 is involved in mismatch repair, it is likely to be required additionally for a process more central to mtDNA maintenance.
Modification of nucleotide bases is the predominant form of DNA damage by oxidants (Ward, 1985, 1988). Thus, it is possible that Mgm101p functions in base excision and/or nucleotide excision repair pathways, the major pathways responsible for the repair of this type of DNA lesion. Mitochondrial base excision repair pathways have been characterized in other organisms, making it likely that such pathways will also exist in yeast mitochondria (Pinz and Bogenhagen, 1998). However, to date, there is no evidence to suggest that mitochondria from any organism possess nucleotide excision repair pathways. Indeed, from a number of studies it has been suggested that mitochondria lack mechanisms to repair more bulky DNA lesions (Foury, 1982). Oxidative damage to sugar moieties in DNA also result in single strand breaks (Ward, 1985, 1988). If multiple single strand breaks occur in a localized area, the accurate repair of such a lesion would require a recombinational process (Ward et al., 1985). Thus, it is also possible that Mgm101p functions in such a repair pathway. Recombination-mediated repair pathways have not been described in detail in mitochondria (Foury and Kolodynski, 1983; Ling et al., 1995). However, it is known that mtDNA is highly recombinogenic (Thomas and Wilkie, 1968), suggesting that damaged mtDNA can be repaired in a manner dependent on this process.
What molecular role does Mgm101p perform in mitochondrial DNA repair and maintenance? Clues to the molecular function of mitochondrial proteins can often be gained by searching for similarity among their origins in eubacterial proteins. However, it is interesting to note that structural homologues of mitochondrial proteins involved in DNA metabolism are also found in such evolutionarily diverse systems as eukaryotes and phage, suggesting that mtDNA metabolism has evolved significantly from its eubacterial ancestors. Mgm101p falls into a potentially novel class of evolutionarily distinct proteins. Although Mgm- 101p shows a high degree of conservation with other putative Mgm101p homologues in organisms as diverse as S. pombe, it exhibits no significant homology to any known proteins, nor does it possess any obvious enzymatic domains or motifs. Based on our findings that Mgm101p is essential for both mtDNA maintenance and repair and is able to bind directly to DNA, it seems probable that Mgm101p is directly involved in mtDNA metabolism.
Acknowledgments
We thank Wolf Heyer, Frank McNally, Ted Powers, Lesilee Rose, and members of the Nunnari lab for critical reading of the manuscript and members of the UC Davis DNA Repair and Recombination Club for discussions.
This work was supported by the National Science Foundation (NSF MCB-9724143) to J. Nunnari and National Institutes of Health (Signal Transduction 61-8529 and Center Grant 61-8268) and NSF (Science and Technology 61-3356) to J.R.Y. III. S.L.M. is supported by a NIH (GM-07377) Training Grant.
Abbreviations used in this paper
- MBP
maltose binding protein
- mtDNA
mitochondrial DNA
Footnotes
Eric Weiss' present address is Department of Molecular and Cellular Biology, University of California, Berkeley, CA 94720.
References
- Alberts B, Herrick G. DNA-cellulose chromatography. Methods Enzymol. 1971;21:198–217. [Google Scholar]
- Altschul SF, Madden AA, Schaffer T, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CW, Baum PR, Gesteland RF. Processing of adenovirus-2 induced proteins. J Virol. 1973;12:241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz R, Butow RA. Patterns of mitochondrial sorting in yeast zygotes. Mol Biol Cell. 1993;4:21–36. doi: 10.1091/mbc.4.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky CW. Transmission genetics of mitochondria and chloroplasts. Annu Rev Genet. 1978;12:471. doi: 10.1146/annurev.ge.12.120178.002351. [DOI] [PubMed] [Google Scholar]
- Branda SS, Isaya G. Prediction and identification of new natural substrates of the yeast mitochondrial intermediate peptidase. J Biol Chem. 1995;270:27366–27373. doi: 10.1074/jbc.270.45.27366. [DOI] [PubMed] [Google Scholar]
- Brown MD, Wallace DC. Molecular basis of mitochondrial DNA disease. J Bioenerg Biomembr. 1994;26:273–289. doi: 10.1007/BF00763099. [DOI] [PubMed] [Google Scholar]
- Burgess SM, Delannoy M, Jensen RE. MMM1 encodes a mitochondrial outer membrane protein essential for establishing and maintaining the structure of yeast mitochondria. J Cell Biol. 1994;126:1375–1391. doi: 10.1083/jcb.126.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-J, Guan M-X, Clark-Walker G. MGM101, a nuclear gene involved in maintenance of the mitochondrial genome in Saccharomyces cerevisiae. . Nucleic Acids Res. 1993;21:3473–3477. doi: 10.1093/nar/21.15.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi N-W, Kolodner R. The effect of DNA mismatches on the ATPase activity of MSH1, a protein in yeast mitochondria that recognizes DNA mismatches. J Biol Chem. 1994a;269:29993–29997. [PubMed] [Google Scholar]
- Chi NW, Kolodner RD. Purification and characterization of MSH1, a yeast mitochondrial protein that binds to DNA mismatches. J Biol Chem. 1994b;269:29984–29992. [PubMed] [Google Scholar]
- Cho JH, Ha SJ, Kao LR, Megraw TL, Chae C-B. A novel DNA-binding protein bound to the mitochondrial inner membrane restores the null mutation of mitochondrial histone Abf2p in Saccharomyces cerevisiae. . Mol Cell Biol. 1998;18:5712–5723. doi: 10.1128/mcb.18.10.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker GD, Chen XJ. A vital function for mitochondrial DNA in the petite-negative yeast Kluyveromyces lactis. . Mol Gen Genet. 1996;252:746–750. doi: 10.1007/BF02173982. [DOI] [PubMed] [Google Scholar]
- Coen D, Deutsch J, Netter P, Petrochilo E, Slonimski P. Mitochondrial genetics. I. Methodology and phenomenology. Symp Soc Exp Biol. 1970;24:449–496. [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley JF, Stillman B. DNA binding properties of an HMG1-related protein from yeast mitochondria. J Biol Chem. 1992;267:3368–3374. [PubMed] [Google Scholar]
- Foury F. Repair of mitochondrial DNA in Saccharomyces cerevisiae. . J Biol Chem. 1982;257:781–787. [PubMed] [Google Scholar]
- Foury F. Cloning and sequencing of the nuclear gene MIP1 encoding the catalytic subunit of the yeast mitochondrial DNA polymerase. J Biol Chem. 1989;264:20552–20560. [PubMed] [Google Scholar]
- Foury F, Kolodynski J. pif mutation blocks recombination between mitochondrial p+ and p− genomes having tandemly arrayed repeat units in Saccharomyces cerevisiae. . Proc Natl Acad Sci USA. 1983;80:5345–5349. doi: 10.1073/pnas.80.17.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Pon LA. Isolation of highly purified mitochondria from Saccharomyces cerevisiae. . Methods Enzymol. 1995;260:213–223. doi: 10.1016/0076-6879(95)60139-2. [DOI] [PubMed] [Google Scholar]
- Hermann GJ, Thatcher JW, Mills JP, Hales KG, Fuller MT, Nunnari J, Shaw JM. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. The . J Cell Biol. 1998;143:359–374. doi: 10.1083/jcb.143.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao L, Megraw T, Chae C. Essential role of the HMG domain in the function of yeast mitochondrial histone HM: functional complementation of HM by the nuclear nonhistone protein NHP6A. Proc Natl Acad Sci USA. 1993;90:5598–5602. doi: 10.1073/pnas.90.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RT, Jorgenson JW. Quantitative analysis of individual neurons by open tubular liquid chromatography with voltammetric detection. Anal Chem. 1989;56:1128. doi: 10.1021/ac00180a012. [DOI] [PubMed] [Google Scholar]
- Lahaye A, Stahl H, Thines-Sempoux D, Foury F. PIF1: a DNA helicase in yeast mitochondria. EMBO (Eur Mol Biol Organ) J. 1991;10:997–1007. doi: 10.1002/j.1460-2075.1991.tb08034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling F, Makishima F, Morishima N, Shibata T. A nuclear mutation defective in mitochondrial recombination in yeast. EMBO (Eur Mol Biol Organ) J. 1995;14:4090–4101. doi: 10.1002/j.1460-2075.1995.tb00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link AJ, Hays LG, Carmack EB, Yates JR., III Identifying the major proteome components of Haemophilus influenzaetype-strain NCTC 8143. Electrophoresis. 1997;18:1314–1334. doi: 10.1002/elps.1150180808. [DOI] [PubMed] [Google Scholar]
- Lockshon D, Zweifel SG, Freeman-Cook LL, Lorimer HE, Brewer BJ, Fangman WL. A role for recombination junctions in the segregation of mitochondrial DNA in yeast. Cell. 1995;81:947–955. doi: 10.1016/0092-8674(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Michel B, Ehrlich SD, Uzest M. DNA double-strand breaks caused by replication arrest. EMBO (Eur Mol Biol Organ) J. 1997;16:430–438. doi: 10.1093/emboj/16.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa I, Fumoto S, Kuroiwa T, Sando N. Characterization of DNA-binding proteins involved in the assembly of mitochondrial nucleoids in the yeast Saccharomyces cerevisiae. . Plant Cell Physiol. 1995;36:1179–1188. [PubMed] [Google Scholar]
- Miyakawa I, Sando N, Kawano S, Nakamura S, Kuroiwa T. Isolation of morphologically intact mitochondrial nucleoids from the yeast, Saccharomyces cerevisiae. . J Cell Sci. 1987;88:431–439. doi: 10.1242/jcs.88.4.431. [DOI] [PubMed] [Google Scholar]
- Newman SM, Zelenaya-Troitskaya O, Perlman PS, Butow RA. Analysis of mitochondrial DNA nucleoids in wild-type and a mutant strain of Saccharomyces cerevisiaethat lacks mitochondrial HMG box protein Abf2p. Nucleic Acids Res. 1996;24:386–398. doi: 10.1093/nar/24.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J, Marshall W, Straight A, Murray A, Sedat JW, Walter P. Mitochondrial transmission during mating in S. cerevisiaeis determined by mitochondrial fusion and fission and the intramitochondrial segregation of mtDNA. Mol Biol Cell. 1997;8:1233–1242. doi: 10.1091/mbc.8.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Perlman PS, Butow RA. The sorting of mitochondrial DNA and mitochondrial protein in zygotes: preferential transmission of mitochondrial DNA to the medial bud. J Cell Biol. 1998;142:613–623. doi: 10.1083/jcb.142.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinz KG, Bogenhagen DF. Efficient repair of a basic sites in DNA by mitochondrial enzymes. Mol Cell Biol. 1998;18:1257–1265. doi: 10.1128/mcb.18.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon, L., and G. Schatz, G. 1991. Biogenesis of yeast mitochondria. In The Molecular and Cellular Biology of the Yeast Saccharomyces. J. Broach, J. Pringle, and E. Jones, editors. Cold Spring Harbor Press, Cold Spring Harbor, NY. 333–406.
- Pringle JR, Adams AE, Drubin DG, Haarer BK. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- Sancar GB. Sequence of the Saccharomyces cerevisiae PHR1 gene and homolog of the PHR1 photolyase to E. coliphotolyase. Nucleic Acids Res. 1985;13:8231–8246. doi: 10.1093/nar/13.22.8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sherratt EJ, Thomas AW, Alcolado JC. Mitochondrial DNA defects: a widening clinical spectrum of disorders. Clin Sci (Lond) 1997;92:225–235. doi: 10.1042/cs0920225. [DOI] [PubMed] [Google Scholar]
- Sogo LF, Yaffe MP. Regulation of mitochondrial morphology and inheritance by Mdm10p, a protein of the mitochondrial outer membrane. J Cell Biol. 1994;126:1361–1373. doi: 10.1083/jcb.126.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausberg RL, Perlman PS. The effect of zygotic bud position on the transmission of mitochondrial genes in Saccharomyces cerevisiae. . Mol Gen Genet. 1978;163:131–144. doi: 10.1007/BF00267404. [DOI] [PubMed] [Google Scholar]
- Thomas DY, Wilkie D. Recombination of mitochondrial drug resistance factors in Saccharomyces cerevisiae. . Biochem Biophys Res Commun. 1968;30:368. doi: 10.1016/0006-291x(68)90753-5. [DOI] [PubMed] [Google Scholar]
- Van Dyck E, Foury F, Stillman B, Brill S. A single-stranded DNA binding protein required for mitochondrial DNA replication in S. cerevisiae is homologous to E. coliSSB. EMBO (Eur Mol Biol Organ) J. 1992;11:3421–3430. doi: 10.1002/j.1460-2075.1992.tb05421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderstraeten S, Van Den Brule S, Hu J, Foury F. The role of 3′-5′ exonucleolytic proofreading and mismatch repair in yeast mitochondrial DNA error avoidance. J Biol Chem. 1998;273:23690–23697. doi: 10.1074/jbc.273.37.23690. [DOI] [PubMed] [Google Scholar]
- Ward JF. Biochemistry of DNA lesions. Radiat Res. 1985;104:S103–S111. [PubMed] [Google Scholar]
- Ward JF. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and repairability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Ward JF, Blakely WF, Joner EJ. Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat Res. 1985;103:383–392. [PubMed] [Google Scholar]
- Williamson, D.H. 1976. Packaging and recombination of mitochondrial DNA in vegetatively growing cells. In Genetics, Biogenesis and Bioenergetics of Mitochondria. W. Bandlow, R.J. Schweyen, D.Y. Thomas, K. Wolf and F. Kaudewitz, editors. Walter de Gruyter, Berlin. 99–115.
- Yates J. Protein structure analysis by mass spectrometry. Methods Enzymol. 1996;271:351–377. doi: 10.1016/s0076-6879(96)71017-0. [DOI] [PubMed] [Google Scholar]
- Yates J, McCormack AL, Eng J. Mining genomes with MS. Anal Chem. 1996;68:534A–540A. doi: 10.1021/ac962050l. [DOI] [PubMed] [Google Scholar]
- Zinn AR, Pohlman JK, Perlman PS, Butow RA. Kinetic and segregational analysis of mitochondrial DNA recombination in yeast. Plasmid. 1987;17:248–256. doi: 10.1016/0147-619x(87)90033-3. [DOI] [PubMed] [Google Scholar]