Abstract
Membrane scaffolding complexes are key features of many cell types, serving as specialized links between the extracellular matrix and the actin cytoskeleton. An important scaffold in skeletal muscle is the dystrophin-associated protein complex. One of the proteins bound directly to dystrophin is syntrophin, a modular protein comprised entirely of interaction motifs, including PDZ (protein domain named for PSD-95, discs large, ZO-1) and pleckstrin homology (PH) domains. In skeletal muscle, the syntrophin PDZ domain recruits sodium channels and signaling molecules, such as neuronal nitric oxide synthase, to the dystrophin complex. In epithelia, we identified a variation of the dystrophin complex, in which syntrophin, and the dystrophin homologues, utrophin and dystrobrevin, are restricted to the basolateral membrane. We used exogenously expressed green fluorescent protein (GFP)-tagged fusion proteins to determine which domains of syntrophin are responsible for its polarized localization. GFP-tagged full-length syntrophin targeted to the basolateral membrane, but individual domains remained in the cytoplasm. In contrast, the second PH domain tandemly linked to a highly conserved, COOH-terminal region was sufficient for basolateral membrane targeting and association with utrophin. The results suggest an interaction between syntrophin and utrophin that leaves the PDZ domain of syntrophin available to recruit additional proteins to the epithelial basolateral membrane. The assembly of multiprotein signaling complexes at sites of membrane specialization may be a widespread function of dystrophin-related protein complexes.
Keywords: syntrophin, utrophin, dystrobrevin, Madin-Darby canine kidney, green fluorescent protein
The cytoskeleton plays an important role in establishing structural and functional specializations at sites of cell–cell contact. For instance, at the postsynaptic density, cytoskeletal proteins participate in a complex that localizes receptors and ion channels for efficient signal transduction (Cho et al., 1992; Kornau et al., 1995; Kim et al., 1996; Dong et al., 1997; Srivastava et al., 1998). In polarized epithelia, membrane specializations form tight junctions (TJs)1 which separate the apical and basolateral membranes, and function as barriers to diffusion across the epithelium (Gumbiner, 1987). Proteins such as ZO-1 are highly localized at the TJ (Stevenson et al., 1986) where they are thought to anchor transmembrane proteins, such as occludin (Furuse et al., 1994; Fanning et al., 1998). Similar multiprotein complexes have been described for the apical and basolateral membranes where transmembrane proteins, such as the cystic fibrosis transmembrane conductance regulator, and the proteoglycan, syndecan, are linked to the actin cytoskeleton (Cohen et al., 1998; Short et al., 1998). Finally, in skeletal muscle, the cytoskeletal protein, dystrophin, anchors transmembrane and signaling proteins at the neuromuscular junction (Colledge and Froehner, 1998). Although expressed in different cell types, these complexes share the common ability to serve as a scaffold on which transmembrane and peripheral membrane proteins assemble.
A scaffolding complex common to neurons, epithelia, and muscle is the dystrophin-associated protein complex (DAPC; Lidov et al., 1990; Kim et al., 1992; Ahn and Kunkel, 1993; Ervasti and Campbell, 1993; Schmitz et al., 1993; Montanaro et al., 1995; Durbeej et al., 1998). Dystrophin is a 427-kD flexible rod-like protein consisting of an NH2-terminal actin binding domain, 24 spectrin-like repeats, a cysteine-rich region, and a COOH-terminal domain (Koenig et al., 1988; Koenig and Kunkel, 1990; Ahn and Kunkel, 1993; Rybakaova et al., 1996). The DAPC has been studied most extensively in skeletal muscle, where mutations in the dystrophin gene result in the severe muscle-wasting of Duchenne and Becker muscular dystrophies (Straub and Campbell, 1997). Dystrophin belongs to a large family of proteins, including short forms of dystrophin and the dystrophin homologues, utrophin, dystrophin-related protein 2 (DRP2), and α- and β-dystrobrevins (Tinsley et al., 1992; Blake et al., 1996, 1998; Roberts et al., 1996; Sadoulet-Puccio et al., 1996; Peters et al., 1997b; Puca et al., 1998).
Two possible functions have been ascribed to dystrophin family members. First, dystrophin binds a transmembrane protein, β-dystroglycan, which is linked to extracellular laminin through α-dystroglycan (Ibraghimov-Beskrovnaya et al., 1992; Gee et al., 1993). Dystrophin also binds actin, thereby providing a link between the extracellular matrix (ECM) and the cytoskeleton (Ahn and Kunkel, 1993; Ervasti and Campbell, 1993; Rybakaova et al., 1996). This structural role may protect muscle from the shearing forces of contraction (Petrof et al., 1993; Pasternak et al., 1995). In addition, the dystrophin complex has emerged as a potential signaling complex, due in part to a family of modular adapter proteins, the syntrophins. The three isoforms of syntrophin (α1, β1, and β2) interact directly with dystrophin family members, are encoded by separate genes, and consist primarily of protein–protein interaction domains (Ahn et al., 1994, 1996; Adams et al., 1995). Syntrophins have two pleckstrin homology (PH) domains, which are often found in cytoskeletal or signaling proteins where they mediate protein–protein or protein– lipid interactions (Shaw, 1996). Syntrophins also contain a single PDZ domain, named for the three proteins in which they were first recognized (PSD-95, discs large, ZO-1). PDZ domains bind specific sequences on the COOH-terminal tails of ion channels and receptors, perhaps serving to cluster these proteins at specialized sites (reviewed in Sheng, 1996; Kornau et al., 1997). In addition, PDZ domains form homo- or heterodimers (Brenman et al., 1996; Srivastava et al., 1998; Xu et al., 1998). Using both mechanisms, syntrophin PDZ domains bind the extreme COOH-terminal tail of voltage-gated sodium channels (Gee et al., 1998; Schultz et al., 1998), or the PDZ domain of neuronal nitric oxide synthase (nNOS; Brenman et al., 1996), thereby recruiting these signaling proteins to the dystrophin complex.
The DAPC is often concentrated at sites of membrane specialization, such as central nervous system synapses (Lidov et al., 1990; Kim et al., 1992; Schmitz et al., 1993; Montanaro et al., 1995) and the neuromuscular junction (Ohlendieck et al., 1991b; Bewick et al., 1992; Peters et al., 1997a, 1998), where the complex may serve both structural and signaling functions. Interestingly, the precise composition of proteins in the DAPC varies by cell type or subcellular localization. For instance, at the crests of the neuromuscular postjunctional folds, rapsyn and utrophin are concentrated with nAChRs (Fertuck and Salpeter, 1974; Sealock et al., 1984; Ohlendieck et al., 1991b; Bewick et al., 1992), while in the troughs of the folds dystrophin, β2-syntrophin, and voltage-gated sodium channels predominate (Flucher and Daniels, 1989; Kramarcy, N., and R. Sealock, personal communication). In addition, forms of α-dystrobrevin generated by alternative splicing are distributed differentially at the neuromuscular junction (Balasubramian et al., 1998; Peters et al., 1998). Presumably, the functional properties of the DAPC are determined by its protein composition.
The expression of syntrophins, utrophin, and some dystrobrevin isoforms in tissues such as lung, kidney, testis, and intestine (Khurana et al., 1990; Love et al., 1991; Adams et al., 1993; Blake et al., 1996; Peters et al., 1997a,b) suggests that these proteins may also play a role in epithelial tissues. Expression of dystroglycans in epithelia in vivo (Durbeej et al., 1998) and the demonstration that antibodies that block laminin binding to dystroglycan disrupt epithelial cell differentiation (Durbeej et al., 1995) indicate that the dystrophin complex may play a critical functional role in epithelial cells. We have explored the localization and targeting of the DAPC using the MDCK epithelial cell line. Our data define a new variation of the DAPC that may be involved in the generation or maintenance of cell polarity and the formation of signaling complexes in epithelia.
Materials and Methods
Antibodies
Antisyntrophin Antibodies.
mAb SYN1351 recognizes all syntrophin isoforms (Froehner et al., 1987). Syntrophin isoform-specific polyclonal antibodies SYN17 (α1-syntrophin), SYN28 (β2-syntrophin), and SYN37 (β1-syntrophin; all used at 30 nM IgG) have been described previously (Peters et al., 1997a).
Antiutrophin Antibody.
mAb MANCHO-3 (a gift of G.E. Morris) is described elsewhere (Nguyen et al., 1991; Morris et al., 1998).
Antidystrobrevin Antibodies.
Antibody 13H1 (a gift of J.B. Cohen; Carr et al., 1989) recognizes both α- and β-dystrobrevins (Peters et al., 1997b).
Antidystrophin Antibodies.
Antibody NCL-DYS2 (epitope in the COOH terminus of dystrophin) is known to cross-react with the canine protein (Novacastra). mAb 1808 raised against Torpedo dystrophin recognizes the rod domain of dystrophin (Sealock et al., 1991).
Antidystroglycan Antibody.
Monoclonal anti–α-dystroglycan antibody, VIA4-1 (Upstate Biotechnology Inc.), is described elsewhere (Ohlendieck et al., 1991a).
Other Antibodies.
Monoclonal and polyclonal anti-GFP (green fluorescent protein) antibodies (Clontech Laboratories, Inc.), anti–β-catenin (Santa Cruz Biotechnology), anti–ZO-1 (Zymed Labs, Inc.), and anti-Na/K ATPase (Chemicon International, Inc.) were used according to manufacturers' specifications.
Transfections
The following mouse β2-syntrophin constructs were subcloned using common restriction sites into pEGFPC2 expression vector (Clontech Laboratories, Inc.): full-length (FL; aa 1–520); PH1 (aa 1–94 and 175–288); PDZ (aa 90–185); PH2 (aa 296–425); and syntrophin unique (SU; aa 421–520). α1-syntrophin PDZ (aa 75–171) and β1-syntrophin PDZ (aa 105–200) were also subcloned into the same vector. The PH2SU (aa 296–520) and PH1PDZ (aa 1–288) constructs were amplified by PCR and were subcloned using engineered restriction sites. All constructs were sequenced before use. Type II MDCK cells were transfected using lipofectamine (GIBCO BRL) according to manufacturer's recommendations. After 10– 14 d of growth in selection medium (DME + 5% FBS + 400 μg/ml G418), individual colonies were isolated and stable lines were established. Wild-type MDCK cells were maintained in DME + 5% FBS.
Immunofluorescence Analysis of MDCK Cells
Cells were grown to confluence on glass coverslips (5–7 d), fixed in 2% paraformaldehyde for 15 min, and either analyzed for GFP expression immediately, or prepared further for immunofluorescence. Cells were permeabilized for 15 min in PBS/0.5% Triton X-100, blocked in PBS/1% fish gelatin/0.8% BSA for 30 min, and incubated with primary antibodies at the appropriate dilution for 1 h at room temperature or overnight at 4°C. After washing in PBS/0.5% Triton X-100, cells were incubated with Alexa 488–conjugated secondary antibody (Molecular Probes, Inc.) and either Texas red–conjugated (Jackson ImmunoResearch Laboratories, Inc.) or Alexa 594–conjugated (Molecular Probes, Inc.) secondary antibody for 1 h at room temperature. Cells were washed with PBS/0.5% Triton X-100 and mounted onto slides in glycerol with n-propyl gallate to minimize fading (Giloh and Sedat, 1982). Staining was analyzed using confocal microscopy (Leica TCS-NT). Results were similar when MDCK cells were grown for 5–7 d on transwell filters (Corning Costar).
Coimmunoprecipitations
Wild-type or GFP-expressing MDCK cell lines were grown on 100-mm plates for 5–7 d. Cells were rinsed in ice-cold homogenization buffer (HB; 10 mM sodium phosphate, 0.4 M NaCl, 5 mM EDTA, pH 7.8) and lysed for 30 min on ice in HB/1% Triton X-100 (750 μl/plate) with protease inhibitors (2 mM PMSF, 1 μM bestatin, and 1 μg/ml each of aprotinin, leupeptin, antipain, and pepstatin A). Insoluble proteins were pelleted at 39,000 g for 30 min. The soluble extracts were then incubated with 1 μg of specific antibody or control IgG for 1 h at 4°C. Protein A– or G–agarose (Sigma Chemical Co.; 25 μl of 50% slurry) was added and incubated overnight at 4°C. Beads were washed extensively in HB containing 1 M NaCl/ 1% Triton X-100. Proteins eluted in SDS sample buffer were separated by SDS-PAGE and transferred to nitrocellulose for immunoblotting as described previously (Peters et al., 1997a). In brief, after blocking in TBS/ 0.1% Tween 20 (TBST) with 5% milk for 1 h at room temperature, nitrocellulose was incubated with primary antibody diluted in TBST/1% milk for 1 h at room temperature. Blots were washed 3 times for 15 min each in TBST, and were then incubated with an HRP-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc.) for 1 h at room temperature. Blots were washed as before and signal was detected using enhanced chemiluminescence (Pierce Chemical Co.) and exposed to film. Nitrocellulose blots were occasionally reprobed after stripping with stripping buffer (Chemicon International, Inc.).
Results
Syntrophin, Utrophin, and Dystrobrevin Are Expressed in Polarized Epithelial Cells
Syntrophins are found in many tissues in which epithelia are a major cell type, including lung and kidney (Adams et al., 1993; Peters et al., 1997a). However, these tissues contain a mixture of epithelial and nonepithelial cells. To study the expression and distribution of syntrophins in polarized epithelial cells, we used MDCK cells. Expression of components of the DAPC was determined by immunoblotting of Triton X-100 soluble and insoluble fractions from MDCK cells. An mAb that recognizes all three syntrophin isoforms (SYN1351) detected a single band of 60 kD (the size expected for syntrophins; Fig. 1). Most of this protein was found in the Triton X-100 soluble fraction. Utrophin, a dystrophin homologue, was also found (Fig. 1) and, like syntrophins, was detected primarily in the Triton X-100 soluble fraction. Syntrophin and utrophin were also found in two additional polarized epithelial cell lines: HBE, a human bronchial epithelia cell line; and LLCPK, a pig renal epithelia cell line (data not shown).
Figure 1.
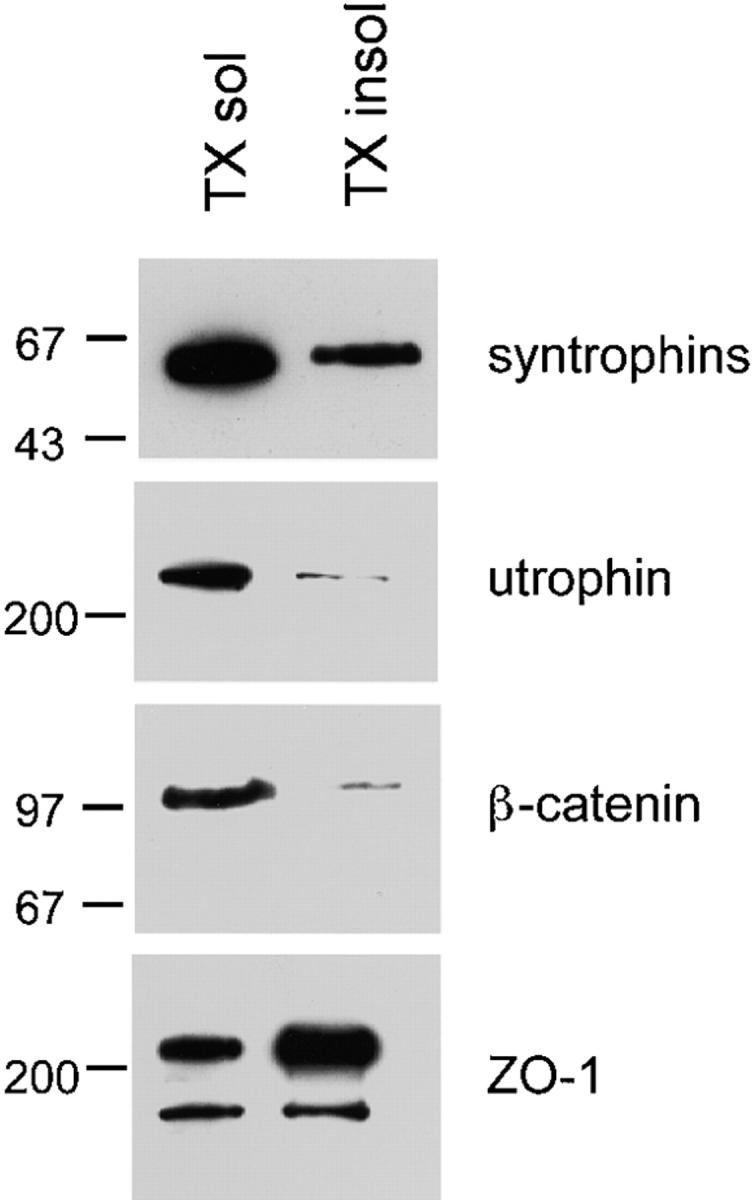
Syntrophins and utrophin are expressed in MDCK cells. MDCK cells were solubilized in HB containing 1% Triton X-100. Equal volumes of Triton X-100 soluble and insoluble fractions were separated by SDS-PAGE and immunoblotted with panspecific syntrophin antibody SYN1351, antiutrophin, anti–β-catenin, or anti–ZO-1 antibodies. Syntrophin and utrophin are found primarily in the Triton X-100 soluble fraction. Positions of molecular mass markers are shown in kD on the left.
The expression of syntrophin isoforms differs among tissue types. For instance, skeletal muscle contains all three syntrophin isoforms, while β1-syntrophin is the predominant form in liver (Peters et al., 1997a). To identify the syntrophin isoforms expressed in polarized epithelia, samples enriched for syntrophins by immunoprecipitation with SYN1351 were analyzed by immunoblotting with antibodies specific for each of the three syntrophin isoforms. β2-Syntrophin was the only syntrophin isoform consistently detected (Fig. 2), although in some experiments small amounts of α1-syntrophin were also found. Given its abundance in the epithelial cell lines tested and in tissues rich in epithelial cells, β2-syntrophin appears to be the predominant form of syntrophin in epithelia.
Figure 2.
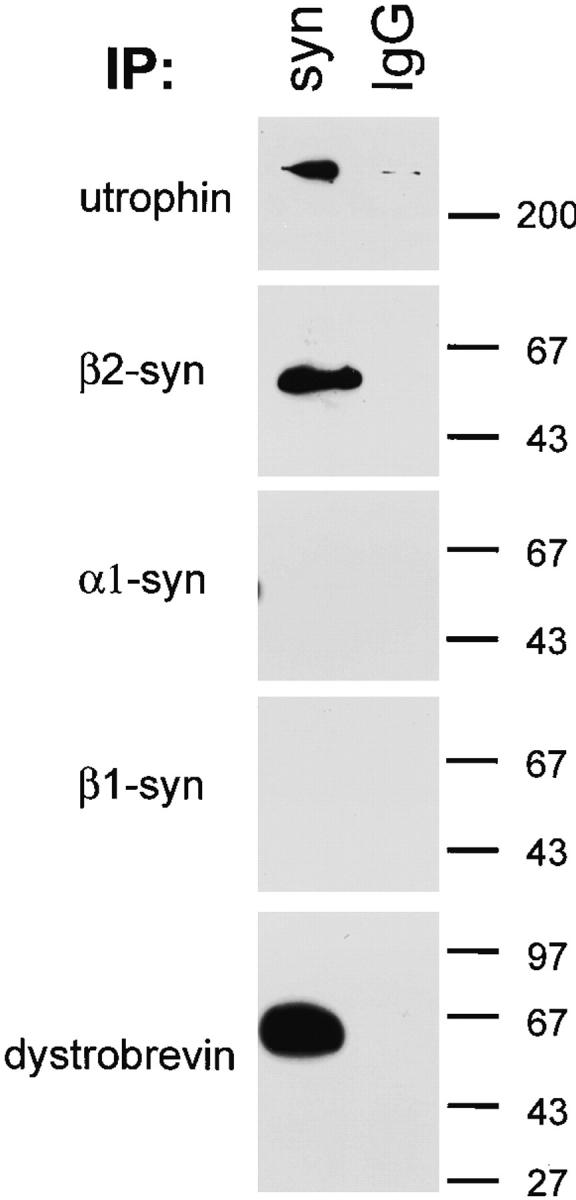
β2-Syntrophin, utrophin, and dystrobrevin exist in a stable complex in MDCK cells. Triton X-100 soluble fractions from MDCK cells were enriched for syntrophins by immunoprecipitation with SYN1351 (panspecific syntrophin antibody) or mouse IgG as a control. Enriched fractions were immunoblotted with antiutrophin, antidystrobrevin, or antibodies specific for each syntrophin isoform. β2-Syntrophin is the predominant isoform of syntrophin in MDCK cells and is found in a stable complex with utrophin and a form of dystrobrevin.
In skeletal muscle, syntrophins associate directly with dystrophin family members (Kramarcy et al., 1994; Yang et al., 1994; Ahn and Kunkel, 1995; Dwyer and Froehner, 1995; Ahn et al., 1996). This also appears to be the case in MDCK cells. Utrophin and a form of dystrobrevin were specifically enriched in SYN1351 preparations, but were absent from control preparations (Fig. 2). These results indicate that syntrophin, utrophin, and dystrobrevin exist in a stable complex in polarized epithelial cells.
Syntrophin and Utrophin Are Expressed on the Basolateral Membrane of Polarized Epithelia
Polarized epithelial cells have two distinct membrane domains that differ greatly in lipid and protein content (Simons and Fuller, 1985; Rodriguez-Boulan and Nelson, 1989). To determine the localization of the syntrophin/ utrophin complex, we compared the distribution of syntrophin with ZO-1, β-catenin, and Na/K ATPase, markers of distinct membrane specializations in polarized MDCK cells. The distribution of syntrophin (Fig. 3 A) was very similar to that of Na/K ATPase, which is largely basolateral. Syntrophin outlined the cell on its basal and lateral surfaces, but was absent from the apical membrane.
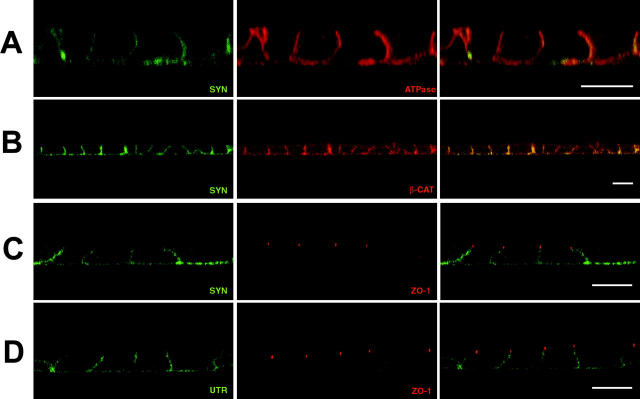
Figure 3.
The syntrophin/utrophin complex is restricted to the basolateral membrane of polarized epithelia. MDCK cells were grown to confluence on glass coverslips for immunofluorescence analysis. Staining was analyzed using confocal microscopy and is shown as XZ scans. (A) Syntrophin is restricted to the basolateral membrane where it colocalizes with the largely basolateral protein, Na/K ATPase. (B) Syntrophin colocalizes with the β-catenin on the lateral membrane. β-Catenin is also found in cytosolic pools. (C) Syntrophin distribution extends up to, but not past, TJs (labeled by anti–ZO-1). (D) The distribution of utrophin is indistinguishable from that of syntrophin. Utrophin does not extend past TJs (labeled by anti–ZO-1). Bars, 10 μm.
The lateral membrane of polarized epithelial cells contains two specialized structures, the TJ and the adherens junction. The TJ is located at the intersection of the apical and basolateral membrane, where it acts to regulate diffusion across the epithelium and as a barrier between the apical and basolateral membrane (reviewed in Gumbiner, 1987). The adherens junction is a cadherin-based specialization believed to be involved in adhesion of epithelial cells (Gumbiner, 1996; reviewed in Yap et al., 1997). In addition to providing useful markers for subdomains within the lateral membrane, the TJ and adherens junction are key structures of epithelial cells where numerous PDZ-containing proteins have been shown to be important (Stevenson et al., 1986; Beatch et al., 1996; reviewed in Kim, 1997; Mandai et al., 1999; Haskins et al., 1998; Izumi et al., 1998; Satoh et al., 1998). Therefore, we were interested in the relationship between the syntrophin complex and these junctions. β-Catenin is found enriched at the adherens junction and in a cytosolic pool (Hinck et al., 1994; Nathke et al., 1994). As shown in Fig. 3 B, syntrophin and β-catenin are colocalized on the lateral membrane. Anti– ZO-1 specifically labeled TJs (Stevenson et al., 1986), and appeared to represent the upper limit of syntrophin expression (Fig. 3 C). The distribution of utrophin was indistinguishable from that of syntrophin, having the same limits of expression when double labeled with anti–ZO-1 antibodies (Fig. 3 D).
GFP-tagged β2-Syntrophin Targets to the Basolateral Membrane of MDCK Cells
While PDZ-containing proteins have been shown to be important at synapses and cell–cell junctions (reviewed in Kim, 1997), only the targeting of Dlg/SAP97 (Lue et al., 1996; Hough et al., 1997; Wu et al., 1998) and ZO-1 (Fanning et al., 1998) has been studied extensively. As a first step in determining which domains of syntrophin are responsible for its localization on the basolateral membrane, we studied the distribution of exogenously expressed GFP fusion proteins of syntrophin (Fig. 4). MDCK cells were transfected with a plasmid encoding GFP fused to the NH2 terminus of full-length β2-syntrophin (GFP-FL) and multiple stable cell lines were established. GFP-FL was found exclusively on the basolateral membrane, in a distribution indistinguishable from that of endogenous syntrophin (Fig. 5 A). In contrast, GFP alone was found only in the cytoplasm.
Figure 4.
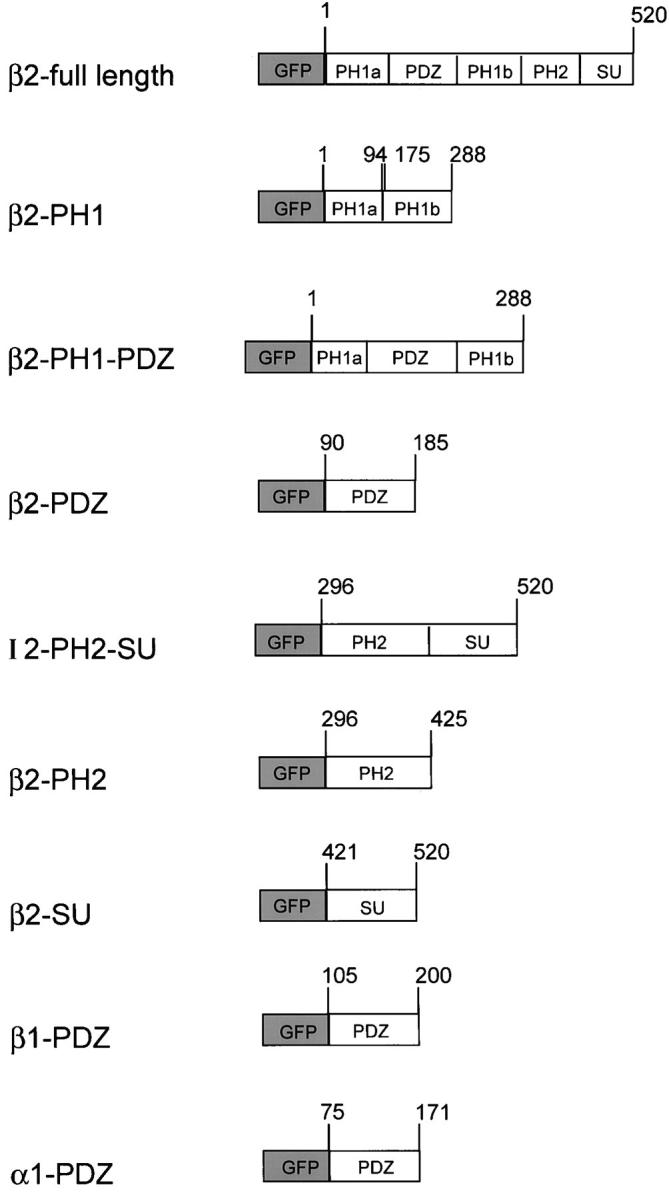
GFP fusion protein constructs. GFP was fused to the NH2 terminus of full-length or individual domains of syntrophin. Numbers indicate the amino acid residues of syntrophin, beginning with the initiator methionine. The PH1a and PH1b domains, normally separated by the PDZ domain, were assembled into a continuous fusion protein.
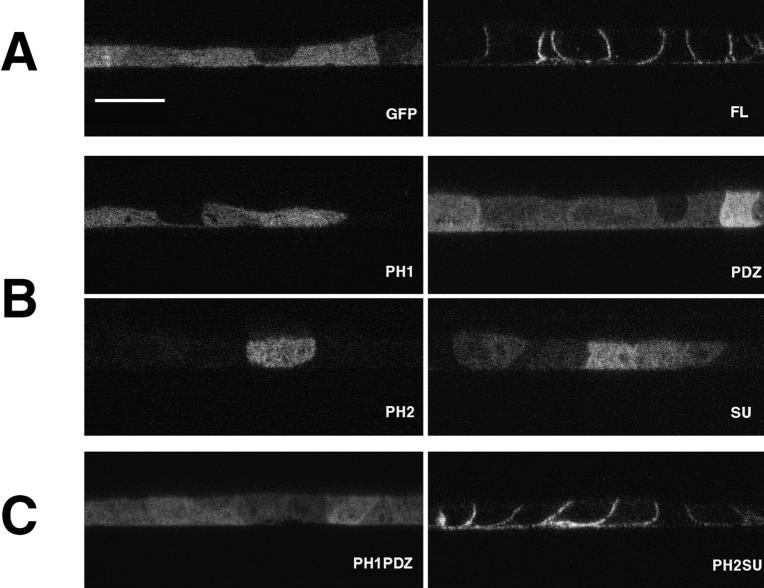
Figure 5.
Basolateral targeting of GFP-tagged syntrophin requires the PH2SU domains. Stable MDCK cell lines expressing GFP-tagged syntrophin fusion proteins were grown to confluence on glass coverslips and were analyzed by confocal microscopy. At least three separate clones were analyzed for each construct, and similar results were obtained when cells were grown on transwell filters. Images are shown as XZ scans. (A) GFP-FL was restricted to the basolateral membrane, in a pattern indistinguishable from endogenous syntrophin. (B) The PH1, PDZ, PH2, and SU fusion proteins remain cytoplasmic and were not enriched at the basolateral membrane. (C) While GFP-PH1PDZ remained cytoplasmic, GFP-PH2SU was restricted to the basolateral membrane, in a pattern indistinguishable from endogenous syntrophin or GFP-FL. Bar, 10 μm.
Syntrophins contain four distinct domains: two PH domains, a PDZ domain, and an SU domain (Adams et al., 1995; Ahn and Kunkel, 1996). Any of these domains could be involved in targeting to the basolateral membrane. Since PH domains in other proteins have been shown to be capable of binding phospholipids (Shaw et al., 1996), the PH domain(s) of syntrophin may recruit syntrophin to the basolateral membrane through binding to specific types of lipids. This model is especially attractive since the apical and basolateral membranes differ in lipid content (Simmons and Fuller, 1985; Rodriguez-Boulan and Nelson, 1989). Alternatively, the PDZ domain could direct syntrophin to the basolateral surface by binding a ligand that is restricted to the basolateral membrane. This could be a general mechanism by which PDZ-containing proteins are recruited to specialized sites. Finally, the SU domain, which is unrelated to any known protein domain, may be responsible for targeting syntrophin to specific subcellular compartments.
To examine these possibilities, we established stable MDCK cell lines expressing individual β2-syntrophin domains fused with GFP (Fig. 4) and compared the distribution of the GFP fusion proteins with endogenous syntrophin. The PH1, PDZ, PH2, and SU domains of β2-syntrophin all failed to accumulate at the basolateral membrane (Fig. 5 B). The PDZ domains of α1-syntrophin and β1-syntrophin also failed to target (data not shown). Each individual domain was distributed diffusely throughout the cell and was identical to the distribution of GFP alone (Fig. 5 B). In the PH1 and PH2 cell lines, labeled cells were very sparse. We counted >200 positive cells in three independent cell lines for each construct. In all cases, the GFP-tagged domain was diffusely distributed throughout the cell.
The failure of individual domains to localize preferentially to the basolateral membrane may be due to their inability to bind a partner, perhaps utrophin, which resides on this membrane. The COOH-terminal 37 kD of β1-syntrophin, consisting of part of the PH1 domain, along with the PH2 and SU domains, is sufficient to bind to dystrophin family members in vitro (Ahn and Kunkel, 1995). Individual domains of syntrophin may not retain their ability to bind utrophin, and therefore cannot be recruited to or retained at the basolateral membrane. To test this hypothesis, we made two additional GFP constructs which consisted of tandemly linked PH2 and SU domains (PH2SU), or the linked PH1 and PDZ domains of β2-syntrophin (Fig. 4). In multiple stable cell lines, GFP-PH2SU was found on the basolateral membrane (Fig. 5 C), in a distribution indistinguishable from endogenous syntrophin or expressed GFP-FL. In contrast, GFP-PH1PDZ failed to accumulate at the basolateral membrane (Fig. 5 C), indicating that the PH2SU construct is necessary and sufficient for basolateral sorting. Recently, the targeting of some GFP-tagged proteins was shown to be substratum dependent (Moyer et al., 1998). However, we obtained similar syntrophin domain targeting results whether transfected MDCK cells were plated on glass coverslips or on transwell filters (data not shown).
Syntrophin Domain Interactions with Utrophin
To test biochemically whether GFP-PH2SU retains the ability to bind utrophin while individual syntrophin domains and PH1PDZ do not, we immunoprecipitated each GFP fusion protein and determined whether utrophin was specifically coisolated. Detergent extracts from cell lines expressing GFP, or fusion proteins of full-length β2-syntrophin, PH2SU, PH1PDZ, or individual syntrophin domains were subjected to immunoprecipitation with anti-GFP or control antibody. To confirm the size and expression level of each fusion protein, immunoprecipitates were analyzed by immunoblotting with a monoclonal anti-GFP antibody. Sample loadings were then adjusted to obtain comparable amounts of each fusion protein (Fig. 6). The top halves of the same blots were incubated with a monoclonal antiutrophin antibody. As expected, utrophin was specifically coimmunoprecipitated with GFP-FL, indicating that fusion with GFP did not interfere with the interaction between syntrophin and utrophin (Fig. 6 A). Utrophin also coimmunoprecipitated with the PH2SU tandemly linked domains, but not with PH1PDZ or individual syntrophin domains (Fig. 6 A). This ability of the GFP-PH2SU fusion protein to bind utrophin may underlie its localization on the basolateral membrane.
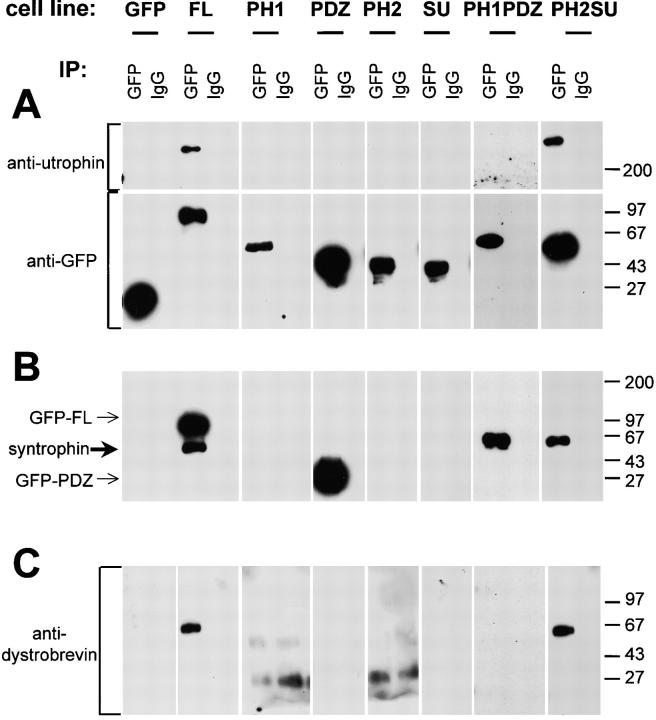
Figure 6.
Interaction of syntrophin domains with utrophin and dystrobrevin. Triton X-100 soluble extracts from transfected cell lines were incubated with either a polyclonal GFP antibody or control rabbit IgG. Immunoprecipitated proteins were immunoblotted with specific antibodies. (A) GFP-tagged syntrophin constructs, revealed by immunoblotting with a GFP mAb, are the expected sizes and are present in approximately equal amounts. Utrophin was coimmunoprecipitated with GFP-FL and GFP-PH2SU fusion proteins, but not with individual domains. (B) Endogenous syntrophin (thick arrow) was coimmunoprecipitated with GFP-FL and GFP-PH2SU only. Since the epitope recognized by the syntrophin antibody SYN1351 is in the PDZ domain of syntrophin, GFP-FL, GFP-PH1PDZ, and GFP-PDZ fusion proteins were also labeled (thin arrows). GFP-PH1PDZ comigrates with syntrophin, precluding any conclusions regarding the copurification of endogenous syntrophin with this construct. (C) A 65-kD dystrobrevin isoform was coimmunoprecipitated with GFP-FL and GFP-PH2SU, but not with individual domains or PH1PDZ. Positions of molecular mass markers are shown in kD on the right.
A current model of the stoichiometry of the DAPC in muscle predicts one utrophin, one dystrobrevin, and two syntrophins per complex: one syntrophin binds directly to utrophin or dystrophin and another binds to a dystrobrevin family member (Fig. 7; Peters et al., 1997a; Sadoulet-Puccio et al., 1997). To test whether two syntrophins are present in epithelial syntrophin/utrophin complexes, we determined whether endogenous syntrophin copurified with exogenously expressed GFP-syntrophin (Fig. 6 B). We used an antibody directed against the syntrophin PDZ domain (SYN1351) to detect endogenous syntrophin in samples immunoprecipitated with GFP antibodies. Although SYN1351 also detects GFP-FL, GFP-PH1PDZ, and GFP-PDZ constructs (Fig. 6 B, thin arrows), they are well separated from the endogenous syntrophin on our immunoblots (except in the case of the PH1PDZ construct, which is the same size as endogenous syntrophin). In these immunoprecipitation experiments we find that endogenous syntrophin (Fig. 6 B, thick arrow) copurifies with GFP-FL and GFP-PH2SU, but not with GFP-PH1, GFP-PDZ, GFP-PH2, or GFP-SU fusion proteins. Although we cannot determine whether endogenous syntrophin copurifies with GFP-PH1PDZ, we believe it does not since it fails to copurify utrophin and dystrobrevin. To accommodate two syntrophin binding sites, a dystrobrevin must also be present in the complex. When blots were probed with a panspecific dystrobrevin antibody, we observed a 65-kD dystrobrevin isoform in samples immunoprecipitated from GFP-FL and GFP-PH2SU cell lines (Fig. 6 C). Dystrobrevin was not detected when GFP antibody was used to immunopurify individual GFP-tagged syntrophin domains or the PH1PDZ fusion protein. The failure of the PDZ fusion proteins to associate with endogenous syntrophin suggests that PDZ homodimerization does not occur with syntrophins as it does with other PDZ proteins (Srivastava et al., 1998; Xu et al., 1998).
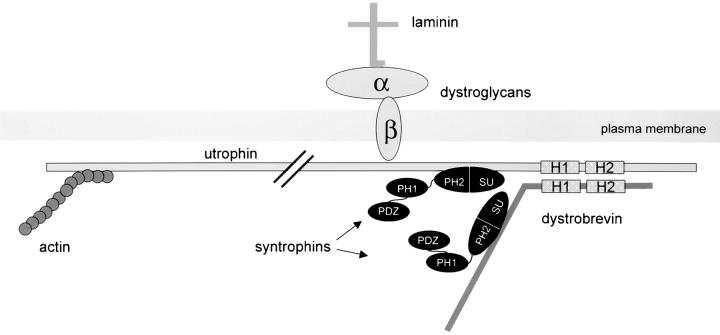
Figure 7.
Hypothetical model of the syntrophin/utrophin complex in MDCK cells. Utrophin binds cytoskeletal actin through its NH2-terminal domain and is linked to laminin in the ECM via α- and β-dystroglycans. Utrophin and dystrobrevin associate through coiled-coil interactions (H1 and H2). Syntrophin binding sites on utrophin and dystrobrevin recruit two syntrophins to the complex. The interaction of syntrophin with utrophin and dystrobrevin occurs via the PH2 and SU domains of syntrophin, leaving the PH1 and PDZ domains free to bind additional proteins.
Discussion
In this study, we have characterized an epithelial utrophin-associated protein complex expressed in MDCK cells. Our data demonstrate that this complex is restricted to the basolateral cell surface of epithelial cells, and includes β2-syntrophin and an isoform of dystrobrevin. We find that utrophin and dystrobrevin copurify with syntrophin, providing strong evidence that these proteins are associated in MDCK cells as they are in other tissues (Kramarcy et al., 1994; Ahn and Kunkel, 1995; Dwyer and Froehner, 1995; Yang et al., 1995).
The dystrobrevins are divided into two families, α and β, which undergo extensive alternative splicing (Blake et al., 1996, 1998; Sadoulet-Puccio et al., 1996; Peters et al., 1997b; Puca et al., 1998). The molecular mass of dystrobrevin found in MDCK cells is ∼65 kD (Figs. 2 and 6 C) and, thus, could correspond to either α-dystrobrevin-2 or β-dystrobrevin. Although our isoform-specific antibodies are not reactive with the canine proteins, therefore preventing a direct test of this question, we believe that this MDCK protein is likely β-dystrobrevin for two reasons. First, β-dystrobrevin is enriched in epithelial tissues (Peters et al., 1997b; Blake et al., 1998), while α-dystrobrevin-2 is restricted to brain, skeletal, and cardiac muscle (Peters et al., 1997b). In addition, α-dystrobrevin-2 associates preferentially with dystrophin (Peters et al., 1998), and therefore is unlikely to be found in a utrophin complex. Taken together, these data suggest that β-dystrobrevin is the isoform present in MDCK cells.
While multiple syntrophins are often expressed in the same tissue, a single isoform often predominates. For instance, α1-syntrophin is the major form in skeletal and cardiac muscle, β1-syntrophin in liver and smooth muscle, and β2-syntrophin in epithelial-rich tissues like kidney and lung (Peters et al., 1997a). In tissues where β2-syntrophin is not the dominant form, such as brain and muscle, it is often restricted to sites of membrane specialization, such as the neuromuscular junction (Peters et al., 1994, 1997a) and retinal synapses (Peters, M.F., C. Houlihan, and S.C. Froehner, unpublished results). Thus, β2-syntrophin may play a unique role at membrane specializations. Here, we find that β2-syntrophin is the dominant syntrophin isoform expressed in MDCK cells. The predominance of β2-syntrophin could be explained by the failure of the α1-syntrophin and two β1-syntrophin isoform specific antibodies to recognize canine syntrophins. However, the abundance of β2-syntrophin in epithelial-rich rodent tissues and in a human bronchial epithelial cell line (data not shown), in which our antibodies are known to be reactive, suggests that β2-syntrophin is the major isoform in epithelia.
The syntrophin/utrophin complex of MDCK cells probably includes the dystroglycans. We find α-dystroglycan on the basolateral membrane of MDCK cells (data not shown) consistent with report of dystroglycans on the basal surface of epithelial cells in vivo (Durbeej et al., 1998). These data support a model (Fig. 7) in which the syntrophin/utrophin complex serves as a link between the ECM and the actin cytoskeleton in epithelia, as it does in skeletal muscle.
There have been reports of a dystrophin short form, Dp140, on the basal surface of tubule epithelial cells in kidney (Durbeej et al., 1997; Lidov and Kunkel, 1998). While we did detect some dystrophin by immunofluorescence in MDCK cells (data not shown), it was not found at the basolateral membrane. Thus, dystrophin may be important in certain epithelia, but we do not believe it is part of the basolateral complex in MDCK cells.
Interaction of Syntrophin Domains with Utrophin
The region on dystrophin that binds syntrophins has been mapped to a short segment encoded by exon 74 (and to homologous regions in utrophin and dystrobrevin; Yang et al., 1994; Ahn and Kunkel, 1995; Suzuki et al., 1995). However, identification of the region of syntrophin responsible for binding to dystrophin family members has not been reported. Binding studies using in vitro translated proteins showed that a 37-kD fragment containing the COOH terminus of β1-syntrophin is sufficient for association with dystrophin family members (Ahn and Kunkel, 1995). We find that individual PH2 or SU domains fail to bind utrophin, but when these domains are tandemly linked (as they are in the native protein) binding to utrophin is restored. These data suggest that the combined PH2 and SU domains function as a unit. Perhaps the binding site for utrophin is formed by noncontiguous partial binding sites in the PH2 and SU domains. Alternatively, the region of syntrophin responsible for binding utrophin may bridge sequences in the PH2 and SU domains. Although the individual constructs used for expression of PH2 and SU domains overlapped by four amino acids, it is possible that proper folding of this bridge region requires a longer polypeptide. Finally, it is possible that the utrophin binding site is contained within a single domain, but that both domains are required for proper folding of the interacting site. While our data do not discriminate between these possibilities, they do provide in vivo evidence that an intact PH2SU domain is necessary and sufficient for syntrophin binding to utrophin.
Two Syntrophins per Complex
In muscle, the current model of the DAPC includes dystrophin or utrophin directly associated with a dystrobrevin family member through a coiled-coil interaction (Peters et al., 1997a; Sadoulet-Puccio et al., 1997). The presence of syntrophin binding sites in both dystrophin or utrophin, and dystrobrevin allows for the binding of two syntrophins per complex (Fig. 7). Support for this model comes from the demonstration that dystrophin or utrophin complexes contain pairs of syntrophin isoforms (Peters et al., 1997a) and a stoichiometry of two syntrophins per complex (Yoshida and Ozawa, 1990; Ervasti and Campbell, 1991).
In this study, we tested the hypothesis that two syntrophins are contained within the epithelial syntrophin/ utrophin complex by expressing GFP-tagged syntrophin (GFP-FL) in MDCK cells. In experiments in which GFP-tagged syntrophin was immunoprecipitated with anti-GFP, we detected not only utrophin and dystrobrevin, but also endogenous syntrophin. A single syntrophin binding site on utrophin and dystrobrevin likely accounts for the two syntrophins in the purified complex. The amount of endogenous syntrophin is approximately half that of the GFP-tagged syntrophin, suggesting that some purified complexes contain two GFP-tagged syntrophins, while others contain one GFP-tagged syntrophin and one endogenous syntrophin. Whether GFP-tagged syntrophin displaces endogenous syntrophin or instead binds to unoccupied sites on utrophin and dystrobrevin is unclear. Alternatively, our results could also be explained by dimerization of syntrophins within the complex. Although evidence for syntrophin dimerization has been reported (Yang et al., 1994; Madhavan and Jarrett, 1995), alternative explanations for these findings have been suggested (Ahn et al., 1996; Peters et al., 1997). Our data indicate that if syntrophin dimerization does occur, it must be via the PH2 and SU domains, since none of the other constructs were able to copurify endogenous syntrophin.
Targeting of the Syntrophin/Utrophin Complex in Polarized Epithelia
Epithelial cells contain distinct apical and basolateral cell surfaces with unique protein and lipid compositions (Simon and Fuller, 1985; Rodriguez-Boulan and Nelson, 1989). The epithelial syntrophin/utrophin complex is restricted to the basolateral cell surface. As a first step in understanding the sorting of the syntrophin/utrophin complex to the basolateral membrane of epithelial cells, we investigated the targeting of syntrophin in MDCK cells. Our results show that the tandemly linked PH2 and SU domains are necessary and sufficient for directing GFP-tagged syntrophin to the basolateral membrane (Fig. 5 C). The PH domain and additional COOH-terminal sequences are also required to target cytohesion-1 and Bruton's tyrosine kinase to the plasma membrane (Nagel et al., 1998). However, it is not simply the presence of a PH domain which confers membrane targeting of syntrophin since the PH1PDZ construct failed to accumulate at the basolateral membrane. Interestingly, replacement of the cytohesion-1 PH domain with the PH domain of the β1-adrenergic receptor abolished membrane targeting (Nagel et al., 1998).
The amino acid residues responsible for targeting syntrophin to the basolateral membrane may also be necessary for binding to utrophin. Alternatively, syntrophin may contain two independent regions, one responsible for binding to utrophin and a second involved in binding structures at the basolateral membrane. Site-directed mutagenesis of the PH2SU domain may allow for the discrimination of utrophin-binding and basolateral targeting functions within this region of syntrophin.
In epithelia, the signals responsible for polarized sorting are best characterized for transmembrane proteins. For instance, several integral membrane proteins that target to the basolateral surface in epithelial cells contain di-leucine or tyrosine-based basolateral sorting sequences (Ktistakis et al., 1990; Thomas et al., 1993; Hunziker and Fumey, 1994; Matter et al., 1994; Sheikh and Isacke, 1996; Simonsen et al., 1998). Therefore, we examined the sequence of β-dystroglycan for potential targeting sequences and found a conserved sequence, EDQATFI (amino acids 784–790 in mouse and human) in the COOH terminus of β-dystroglycan, that is very similar to the consensus sequence for the di-leucine basolateral sorting motif, DDQxxLI (Matter et al., 1994; Simonsen et al., 1998). In β-dystroglycan, this motif is predicted to be cytoplasmic and does not overlap with the dystrophin binding site (Jung et al., 1995). The cytoplasmic targeting motifs found in basolateral proteins are often followed by small clusters of acidic residues (Matter et al., 1994). The presence of the sequence DELDD downstream of the putative di-leucine motif in β-dystroglycan supports a role for this motif in basolateral sorting. It will be interesting to examine the importance of this sequence in the targeting of β-dystroglycan and other components of the syntrophin/utrophin complex in epithelial cells.
Role of Syntrophin/Utrophin Complex in Epithelia
At this time, we can only speculate as to the function of the basolateral syntrophin/utrophin complex in polarized epithelia. The syntrophin/utrophin complex, through its ability to link ECM proteins to actin, may serve a structural role in epithelia. In skeletal muscle, the link between the ECM and the cytoskeleton is thought to maintain cell membrane integrity during contraction (Petrof et al., 1993; Pasternak et al., 1995). Some epithelia must also withstand the forces of contraction (i.e., in the gastrointestinal tract). Thus, a link between the ECM and actin provided by the syntrophin/utrophin complex, may maintain membrane integrity in epithelia. The syndecan/CASK complex also links the ECM to the actin cytoskeleton (Cohen et al., 1998), and may play a role similar to the syntrophin/utrophin complex in epithelia.
The syntrophin/utrophin complex may also function to recruit proteins to the basolateral surface of epithelial cells. Syntrophins are modular adapter proteins made up almost exclusively of protein–protein interaction domains (Ahn et al., 1994, 1996; Adams et al., 1995). Our results indicate that the PH1 and PDZ domains do not play a role in targeting syntrophin to the basolateral membrane. In contrast, the PDZ domains of Dlg/SAP97 are necessary for efficient subcellular targeting (Lue et al., 1996; Hough et al., 1997; Wu et al., 1998), indicating that modular adapter proteins may be targeted via different mechanisms. The PH1 and PDZ domains of syntrophin are also unnecessary for the interaction of syntrophin with utrophin. Therefore, these domains are free to interact with additional proteins to generate a large multiprotein complex (Fig. 7). Binding partners for the PH1 domain of syntrophin have not been identified, but PH domains in other proteins are capable of binding proteins such as protein kinase C, and the β and γ subunits of G proteins (Touhara et al., 1994; Yao et al., 1994).
The ability of the PDZ domain of syntrophin to bind nNOS in muscle (Brenman et al., 1996) suggests that one function of syntrophin is to recruit signaling proteins to the membrane. Interestingly, the presence of two syntrophins per complex (Fig. 7) may allow two different proteins to be brought in close apposition, allowing for one to modulate the other. For instance, in skeletal muscle, syntrophin PDZ domains also bind voltage-gated sodium channels (Brenman et al., 1996; Gee et al., 1998; Schultz et al., 1998). NO modulates the activity of certain sodium channels (Li et al., 1998), a process that may occur with high efficiency and specificity if nNOS and sodium channels reside in the same complex. The recruitment of two proteins into the DAPC in muscle has potential functional consequences: the formation of similar complexes containing signaling molecules, and effector proteins may also occur in epithelia. Thus, it will be important to identify binding partners for the PH1 and PDZ domains to gain further understanding of the function of the syntrophin/utrophin complex in epithelia.
PDZ domain-containing proteins are a common feature of many scaffolding complexes (Cho et al., 1992; Kornau et al., 1995; Kim et al., 1996; Dong et al., 1997; Cohen et al., 1998; Short et al., 1998; Srivastava et al., 1998; Xu et al., 1998). Through their interactions with the COOH-terminal tails of receptors and ion channels, PDZ domains are critical in the assembly of multiprotein complexes. Many scaffolding proteins contain multiple PDZ domains, which may tether multiple copies of the same ligand at a particular subcellular location. More often, the PDZ domains within a single protein have distinct binding specificities, allowing different proteins to be recruited to the same subcellular location. An elegant illustration of the efficiency of such complexes comes from studies of INAD (inactivation no after potential D) where a single type of scaffold links all (or most) proteins needed for Drosophila phototransduction (reviewed in Montell, 1998). Interestingly, homodimerization of INAD molecules generates a complicated network of proteins at the membrane (Xu et al., 1998). Furthermore, the potential for homodimerization of proteins which contain 6–13 PDZ domains (Dong et al., 1997; Srivastava et al., 1998; Ullmer et al., 1998), raises the complexity of scaffolding complexes to almost incomprehensible heights.
In epithelia, the asymmetric localization of proteins and lipids results in functional differences between the apical and basolateral membranes required for epithelial cell function. MDCK cells sort secretory and membrane-associated proteins to apical and basolateral surfaces by several different mechanisms (reviewed in Simons and Wandinger-Ness, 1990; Caplan, 1997). Some proteins are packaged upon exit from the TGN into separate apical or basolateral transport vesicles (Wadinger-Ness et al., 1990). Other proteins are targeted exclusively to the basolateral domain but do not remain there; instead they are internalized into endosomes and targeted via the transcytotic pathway to the apical cell surface. Finally, some proteins are transported in a nonpolarized manner to both cell surfaces, but are selectively stabilized at one surface. For example, the Na/K ATPase is stabilized at the basolateral cell surface by association with the actin cytoskeleton and ankyrin (Nelson and Veshnock, 1987; Jordan et al., 1995; Thevananther et al., 1997). Syntrophin may play a similar role and act to specifically anchor transmembrane proteins by high affinity protein–protein interactions via the PDZ or PH domains. However, since PDZ proteins are present on both apical and basolateral cell surfaces, and at the TJs, additional factors that define binding specificities must be involved.
In addition to a targeting role once a polarized monolayer is formed, the epithelial syntrophin/utrophin complex may be involved in the development of polarity in epithelial cells. Drubin and Nelson (1996) proposed that extracellular cues, such as cell–cell adhesion, define discrete areas of membrane as sites of submembranous cytoskeletal assembly. Once assembled, the cytoskeleton serves as a docking site for specific proteins, leading to further specialization of this region of membrane. In MDCK cells, E-cadherin–mediated adhesion defines the site for recruitment of the sec6/sec8 complex (Grindstaff et al., 1998). This protein complex then serves as a docking site for additional proteins. By serving as a link between the ECM and the submembranous cytoskeleton, the syntrophin/utrophin complex may also assist in defining basal membranes during morphogenesis. Laminin, a component of the basement membrane which binds dystroglycan (Ibraghimov-Beskrovnaya et al., 1992; Gee et al., 1993), plays an important role in the differentiation of epithelia in vivo and in vitro (Klein et al., 1988; Ekblom et al., 1990; Durbeej et al., 1995). Antibodies that block the binding of laminin to dystroglycan in organ cultures inhibit epithelial differentiation (Durbeej et al., 1995). Perhaps the binding of laminin serves to recruit the syntrophin/utrophin complex to the basal membrane, where it participates in the specialization of this cell surface. In addition, the heparan sulfate proteoglycan, agrin, is found in the basement membrane of epithelial tissues, where it binds dystroglycan (Gesemann et al., 1998; Raats et al., 1998). It is important to note that antibodies that disrupt the laminin–dystroglycan interaction may also block agrin binding (Ervasti and Campbell, 1993; Campanelli et al., 1994; Gee et al., 1994), suggesting that agrin may also be involved in epithelial morphogenesis via the syntrophin/utrophin complex. The use of additional cell culture model systems and transgenic or knockout animals will help in defining functions for the epithelial syntrophin/utrophin complex.
Acknowledgments
We thank Peter Mohler for advice on culturing and transfecting epithelial cells, Stuart Krall for assistance with tissue culture, Dr. Michael Chua for assistance with confocal microscopy, Dr. Raghavan Madhavan for pointing out the potential targeting sequence in β-dystroglycan, and Dr. Marcie Colledge and members of the Froehner and Milgram labs for helpful discussion. We also thank the following individuals for providing reagents: Dr. Stephen Gee for plasmids encoding syntrophin domains, Dr. G.E. Morris for donating the antiutrophin antibody, and Dr. J.B. Cohen for providing the antidystrobrevin antibody.
This work was supported by National Institutes of Health grant R29DK50744 and Cystic Fibrosis Foundation grant MILGRA9710 to S.L. Milgram, and National Institutes of Health grant NS33145 to S.C. Froehner.
Abbreviations used in this paper
- DAPC
dystrophin-associated protein complex
- ECM
extracellular matrix
- GFP
green fluorescent protein
- GFP-FL
GFP fused to NH2 terminus of full-length β2-syntrophin
- HB
homogenization buffer
- nNOS
neuronal nitric oxide synthase
- PDZ
protein domain named for PSD-95, discs large, ZO-1
- PH
pleckstrin homology
- SU
syntrophin unique
- TJ
tight junction
References
- Adams ME, Butler MH, Dwyer TM, Peters MF, Murnane AA, Froehner SC. Two forms of mouse syntrophin, a 58-kD dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron. 1993;11:531–540. doi: 10.1016/0896-6273(93)90157-m. [DOI] [PubMed] [Google Scholar]
- Adams ME, Dwyer TM, Dowler LL, White RA, Froehner SC. Mouse α1-and β2-syntrophin gene structure, chromosome localization, and homology with a discs large domain. J Biol Chem. 1995;270:25859–25865. doi: 10.1074/jbc.270.43.25859. [DOI] [PubMed] [Google Scholar]
- Ahn AH, Kunkel LM. The structural and functional diversity of dystrophin. Nat Genet. 1993;3:81–93. doi: 10.1038/ng0493-283. [DOI] [PubMed] [Google Scholar]
- Ahn AH, Kunkel LM. Syntrophin binds to an alternatively spliced exon of dystrophin. J Cell Biol. 1995;128:363–371. doi: 10.1083/jcb.128.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn AH, Yoshida M, Anderson MS, Feener CA, Selig S, Hagiwara Y, Ozawa E, Kunkel LM. Cloning of human basic A1, a distinct 59-kD dystrophin-associated protein encoded on chromosome 8q23-24. Proc Natl Acad Sci USA. 1994;91:4446–4450. doi: 10.1073/pnas.91.10.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn A, Feener HCA, Gussoni E, Yoshida M, Ozawa E, Kunkel LM. The three human syntrophin genes are expressed in diverse tissues, have distinct chromosomal locations, and each bind to dystrophin and its relatives. J Biol Chem. 1996;271:2724–2730. doi: 10.1074/jbc.271.5.2724. [DOI] [PubMed] [Google Scholar]
- Balasubramian S, Fung ET, Huganir RL. Characterization of the tyrosine phosphorylation and distribution of dystrobrevin isoforms. FEBS Lett. 1998;432:133–140. doi: 10.1016/s0014-5793(98)00804-7. [DOI] [PubMed] [Google Scholar]
- Beatch M, Jesaitis LA, Gallin WJ, Goodenough DA, Stevenson BR. The tight junction protein ZO-2 contains three PDZ (PSD-95/Discs-Large/ZO-1) domains and an alternatively spliced region. J Biol Chem. 1996;271:25723–25726. doi: 10.1074/jbc.271.42.25723. [DOI] [PubMed] [Google Scholar]
- Bewick GS, Nicholson LBV, Young C, O'Donnell E, Slater CR. Different distributions of dystrophin and related proteins at nerve muscle junctions. Neuroreport. 1992;3:857–860. doi: 10.1097/00001756-199210000-00009. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Nawrotzki R, Peters MF, Froehner SC, Davies KE. Isoform diversity of dystrobrevin, the murine 87-kD postsynaptic protein. J Biol Chem. 1996;271:7802–7810. doi: 10.1074/jbc.271.13.7802. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Nawrotzki R, Loh NY, Górecki DC, Davies KE. β-dystrobrevin, a member of the dystrophin-related protein family. Proc Natl Acad Sci USA. 1998;95:241–246. doi: 10.1073/pnas.95.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Campanelli JT, Roberds SL, Campbell KP, Scheller RH. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell. 1994;77:663–674. doi: 10.1016/0092-8674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Caplan MJ. Membrane polarity in epithelial cell: protein sorting and establishment of polarized domains. Am J Physiol. 1997;272:F425–F429. doi: 10.1152/ajprenal.1997.272.4.F425. [DOI] [PubMed] [Google Scholar]
- Carr C, Fischbach GD, Cohen JB. A novel 87,000 M r protein associated with acetylcholine receptors in Torpedoelectric organ and vertebrate skeletal muscle. J Cell Biol. 1989;109:1753–1764. doi: 10.1083/jcb.109.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Cohen AR, Wood DF, Marfatia SM, Walther Z, Chrishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Froehner SC. Signals mediating ion channel clustering at the neuromuscular junction. Curr Opin Neurobiol. 1998;8:357–363. doi: 10.1016/s0959-4388(98)80061-5. [DOI] [PubMed] [Google Scholar]
- Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Larsson E, Ibraghimov-Beskrovnaya O, Roberds SL, Campbell KP, Ekblom P. Nonmuscle α-dystroglycan is involved in epithelial development. J Cell Biol. 1995;130:79–91. doi: 10.1083/jcb.130.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M, Jung D, Hjalt T, Campbell KP, Ekblom P. Transient expression of Dp140, a product of the Duchenne muscular dystrophy locus, during kidney tubulogenesis. Dev Biol. 1997;181:156–167. doi: 10.1006/dbio.1996.8430. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem. 1998;46:449–457. doi: 10.1177/002215549804600404. [DOI] [PubMed] [Google Scholar]
- Dwyer TM, Froehner SC. Direct binding of Torpedosyntrophin to dystrophin and the 87-kD dystrophin homologue. FEBS Lett. 1995;375:91–94. doi: 10.1016/0014-5793(95)01176-f. [DOI] [PubMed] [Google Scholar]
- Ekblom M, Klein G, Mugrauer G, Fecker L, Deutzmann R, Timpl R, Ekblom P. Transient and locally restricted expression of laminin A chain mRNA by developing epithelial cells during kidney organogenesis. Cell. 1990;60:337–346. doi: 10.1016/0092-8674(90)90748-4. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane specialization of the dystrophin–glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. A role for the dystrophin–glycoprotein complex as a transmembrane link between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Fertuck HC, Salpeter MM. Localization of acetylcholine receptor by 125I-labeled α-bungarotoxin binding in mouse motor endplates. Proc Natl Acad Sci USA. 1974;71:1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher BE, Daniels MP. Distribution of Na+channels and ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43-kD protein. Neuron. 1989;3:163–175. doi: 10.1016/0896-6273(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Froehner SC, Murnane AA, Tobler M, Peng HB, Sealock R. A postsynaptic M r 58,000 (58K) protein concentrated at acetylcholine receptor-rich sites in Torpedoelectroplaques and skeletal muscle. J Cell Biol. 1987;104:1633–1646. doi: 10.1083/jcb.104.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee SH, Blacher RW, Douville PJ, Provost PR, Yurchenco PD, Carbonetto S. Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparan binding domain of laminin. J Biol Chem. 1993;268:14972–14978. [PubMed] [Google Scholar]
- Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. Dystroglycan-α, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994;77:675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Gee SH, Madhavan R, Levinson SR, Caldwell JH, Sealock R, Froehner SC. Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J Neurosci. 1998;18:128–137. doi: 10.1523/JNEUROSCI.18-01-00128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesemann M, Brancacio A, Schumacher B, Ruegg MA. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J Biol Chem. 1998;273:600–605. doi: 10.1074/jbc.273.1.600. [DOI] [PubMed] [Google Scholar]
- Giloh H, Sedat JW. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982;217:1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu S, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell–cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol. 1987;253:C749–C758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough CD, Woods DF, Park S, Bryant PJ. Organizing a functional junctional complex requires specific domains of the DrosophilaMAGUK discs large. Genes Dev. 1997;11:3242–3253. doi: 10.1101/gad.11.23.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO (Eur Mol Biol Organ) J. 1994;13:2963–2967. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis eleganspolarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C, Puschel B, Koob R, Drenckhahn D. Identification of a binding motif for ankyrin on the alpha-subunit of Na+,K(+) ATPase. J Biol Chem. 1995;270:29971–29975. doi: 10.1074/jbc.270.50.29971. [DOI] [PubMed] [Google Scholar]
- Jung D, Yang B, Meyer J, Chamberlain JS, Campbell KP. Identification and characterization of the dystrophin anchoring site on β-dystroglycan. J Biol Chem. 1995;270:27305–27310. doi: 10.1074/jbc.270.45.27305. [DOI] [PubMed] [Google Scholar]
- Khurana TS, Hoffman EP, Kunkel LM. Identification of a chromosome 6-encoded dystrophin-related protein. J Biol Chem. 1990;265:16717–16720. [PubMed] [Google Scholar]
- Kim E, Cho KO, Rothschild A, Sheng M. Heteromultimerization and NMDA receptor-clustering activity of Chapsyn-110, a member of the PSD-95 family of proteins. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- Kim SK. Polarized signaling: basolateral receptor localization in epithelial cells by PDZ-containing proteins. Curr Opin Cell Biol. 1997;9:853–859. doi: 10.1016/s0955-0674(97)80088-9. [DOI] [PubMed] [Google Scholar]
- Kim TW, Wu K, Xu JL, Black IB. Detection of dystrophin in the postsynaptic density of rat brain and deficiency in a mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 1992;89:11642–11644. doi: 10.1073/pnas.89.23.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Koenig M, Kunkel LM. Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J Biol Chem. 1990;265:4560–4566. [PubMed] [Google Scholar]
- Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Kornau H, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic protein PSD-95. Science. 1995;269:1737–1739. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Kornau H, Seeburg PH, Kennedy MB. Interaction of ion channel and receptors with PDZ domain proteins. Curr Opin Neurobiol. 1997;7:368–373. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- Kramarcy NR, Vidal A, Froehner SC, Sealock R. Association of utrophin and multiple dystrophin short forms with the mammalian M r58,000 dystrophin-associated protein (syntrophin) J Biol Chem. 1994;269:2870–2876. [PubMed] [Google Scholar]
- Ktistakis NT, Thomas D, Roth MG. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J Cell Biol. 1990;111:1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chapleau MW, Bates JN, Bielefeldt K, Lee H-C, Abboud AF. Nitric oxide as an autocrine regulator of sodium currents in baroreceptor neurons. Neuron. 1998;20:1039–1049. doi: 10.1016/s0896-6273(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Lidov HG, Kunkel LM. Dystrophin and Dp140 in the adult rodent kidney. Lab Invest. 1998;78:1543–1551. [PubMed] [Google Scholar]
- Lidov HG, Beyers TJ, Watkins SC, Kunkel LM. Association of dystrophin to postsynaptic regions of the central nervous system cortical neurons. Nature. 1990;348:725–728. doi: 10.1038/348725a0. [DOI] [PubMed] [Google Scholar]
- Love DR, Morris GE, Ellis JM, Fairbrother U, Marsden RF, Bloomfield JF, Edwards YH, Slater CP, Parry DJ, Davies KE. Tissue distribution of the dystrophin-related gene product and expression in the mdx and dymouse. Proc Natl Acad Sci USA. 1991;88:3243–3247. doi: 10.1073/pnas.88.8.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue RA, Brandin E, Chan EP, Branton D. Two independent domains of hDLG are sufficient for subcellular targeting: the PDZ1-2 conformational unit and an alternatively spliced domain. J Cell Biol. 1996;135:1125–1137. doi: 10.1083/jcb.135.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan R, Jarrett HW. Interactions between dystrophin glycoprotein complex proteins. Biochemistry. 1995;34:12204–12209. doi: 10.1021/bi00038a014. [DOI] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Takahashi K, Nishioka H, Mizoguchi A, Takai Y. Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell–cell and cell–matrix adherens junctions. J Cell Biol. 1999;144:1001–1018. doi: 10.1083/jcb.144.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Yamamoto EM, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanaro F, Carbonetto S, Campbell KP, Lindenbaum M. Dystroglycan expression in the wild type and mdxmouse neural retina: synaptic colocalization with dystrophin, dystrophin-related protein, but not laminin. J Neurosci Res. 1995;45:528–538. doi: 10.1002/jnr.490420411. [DOI] [PubMed] [Google Scholar]
- Montell C. TRP trapped in fly signaling web. Curr Opin Neurobiol. 1998;8:389–397. doi: 10.1016/s0959-4388(98)80066-4. [DOI] [PubMed] [Google Scholar]
- Morris GE, Sedgwick SG, Ellis JM, Chamberlain JS, Man NT. An epitope structure for the C-terminal domain of dystrophin and utrophin. Biochemistry. 1998;37:11117–11127. doi: 10.1021/bi9805137. [DOI] [PubMed] [Google Scholar]
- Moyer BD, Loffing J, Schwiebert EM, Loffing-Cueni D, Halpin PA, Karlson KH, Ismailov II, Guggino WB, Langford GM, Stanton BA. Membrane trafficking of the cystic fibrosis gene product, cystic fibrosis transmembrane conductance regulator, tagged with green fluorescent protein in Madin-Darby canine kidney cells. J Biol Chem. 1998;273:21759–21768. doi: 10.1074/jbc.273.34.21759. [DOI] [PubMed] [Google Scholar]
- Nagel W, Schilcher P, Zeitlmann L, Kolanus W. The PH domain and the polybasic c domain of cytohesin-1 cooperate specifically in plasma membrane association and cellular function. Mol Biol Cell. 1998;9:1981–1994. doi: 10.1091/mbc.9.8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol. 1994;125:1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+/K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987;328:533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- Nguyen TM, Ellis JM, Love DR, Davies KE, Gatter KC, Dickson G, Morris GE. Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: presence at neuromuscular junctions, in the sarcolemma of dystrophic muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J Cell Biol. 1991;115:1695–1700. doi: 10.1083/jcb.115.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K, Ervasti JM, Snook JB, Campbell KP. Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J Cell Biol. 1991a;112:135–148. doi: 10.1083/jcb.112.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K, Ervasti JM, Matsumura K, Kahl SD, Leveille CJ, Campbell KP. Dystrophin-related protein is localized to the neuromuscular junctions of adult skeletal muscle. Neuron. 1991b;7:499–508. doi: 10.1016/0896-6273(91)90301-f. [DOI] [PubMed] [Google Scholar]
- Pasternak C, Wong S, Elson EL. Mechanical function of dystrophin in muscle cells. J Cell Biol. 1995;128:355–361. doi: 10.1083/jcb.128.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MF, Kramarcy NR, Sealock R, Froehner SC. β2-syntrophin: localization at the neuromuscular junction in skeletal muscle. Neuroreport. 1994;5:1577–1580. [PubMed] [Google Scholar]
- Peters MF, Adams MA, Froehner SC. Differential association of syntrophin pairs with the dystrophin complex. J Cell Biol. 1997a;138:81–93. doi: 10.1083/jcb.138.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MF, O'Brien KF, Sadoulet-Puccio HM, Kunkel LM, Adams MA, Froehner SC. β-Dystrobrevin, a new member of the dystrophin family. Identification, cloning and protein associations. J Biol Chem. 1997b;272:31561–31569. doi: 10.1074/jbc.272.50.31561. [DOI] [PubMed] [Google Scholar]
- Peters MF, Sadoulet-Puccio HM, Grady RM, Kramarcy NR, Kunkel LM, Sanes JR, Sealock R, Froehner SC. Differential membrane localization and intermolecular associations of α-dystrobrevin isoforms in skeletal muscle. J Cell Biol. 1998;142:1269–1278. doi: 10.1083/jcb.142.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puca AA, Nigro V, Piluso G, Belsito A, Sampaplo S, Quaderi N, Rossi E, Di Iorio G, Ballabio A, Franco B. Identification and characterization of a novel member of the dystrobrevin gene family. FEBS Lett. 1998;425:7–13. doi: 10.1016/s0014-5793(98)00097-0. [DOI] [PubMed] [Google Scholar]
- Raats CJ, Bakker MA, Hoch W, Tamboer WP, Groffen AJ, van der Heuvel LP, Berden JH, van den Born J. Differential expression of agrin in renal basement membranes as revealed by domain-specific antibodies. J Biol Chem. 1998;273:17832–17838. doi: 10.1074/jbc.273.28.17832. [DOI] [PubMed] [Google Scholar]
- Roberts RG, Freeman TC, Kendall E, Vetrie DL, Dixon AK, Shaw-Smith C, Bone Q, Bobrow M. Characterization of DRP2, a novel human dystrophin homologue. Nat Genet. 1996;13:223–226. doi: 10.1038/ng0696-223. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rybakaova IN, Amann KJ, Ervasti JM. A new model for the interaction of dystrophin with F-actin. J Cell Biol. 1996;135:661–671. doi: 10.1083/jcb.135.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoulet-Puccio HM, Khurana TS, Cohen JB, Kunkel LM. Cloning and characterization of the human homologue of a dystrophin associated phosphoprotein found at the Torpedoelectric postsynaptic membrane. Hum Mol Genet. 1996;5:489–496. doi: 10.1093/hmg/5.4.489. [DOI] [PubMed] [Google Scholar]
- Sadoulet-Puccio HM, Rajala M, Kunkel LM. Dystrobrevin and dystrophin: an interaction through coiled-coil motifs. Proc Natl Acad Sci USA. 1997;94:12413–12418. doi: 10.1073/pnas.94.23.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, et al. Neurabin-II/spinophilin. An actin filament-binding protein with one PDZ domain localized at cadherin-based cell–cell adhesion sites. J Biol Chem. 1998;273:3470–3475. doi: 10.1074/jbc.273.6.3470. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Hooenbach M, Drenckhahn D. Colocalization of retinal dystrophin and actin in postsynaptic dendrites of rod and cone photoreceptor synapses. Histochemistry. 1993;100:473–479. doi: 10.1007/BF00267828. [DOI] [PubMed] [Google Scholar]
- Schultz J, Hoffmuller U, Krause G, Ashurst J, Macias MJ, Schmieder P, Schneideer-Mergener J, Oshkinat H. Specific interactions between the syntrophin PDZ domain and voltage-gated sodium channels. Nature Struct Biol. 1998;5:19–24. doi: 10.1038/nsb0198-19. [DOI] [PubMed] [Google Scholar]
- Sealock R, Wray BE, Froehner SC. Ultrastructural localization of the M r 43,000 protein and the acetylcholine receptor in Torpedopostsynaptic membranes using monoclonal antibodies. J Cell Biol. 1984;98:2239–2244. doi: 10.1083/jcb.98.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealock R, Butler MH, Kramarcy NR, Gao K-X, Murnane AA, Douville K, Froehner SC. Localization of dystrophin relative to acetylcholine receptor domains in electric tissue and adult and cultured skeletal muscle. J Cell Biol. 1991;113:1133–1144. doi: 10.1083/jcb.113.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. The pleckstrin homology domain: an intriguing multifunctional protein module. Bioessays. 1996;18:35–45. doi: 10.1002/bies.950180109. [DOI] [PubMed] [Google Scholar]
- Sheikh H, Isacke CM. A di-hydrophobic Leu-Val motif regulates the basolateral localization of CD44 in polarized Madin-Darby canine kidney epithelial cells. J Biol Chem. 1996;271:12185–12190. doi: 10.1074/jbc.271.21.12185. [DOI] [PubMed] [Google Scholar]
- Sheng M. PDZs and receptor/channel clustering: rounding up the latest suspects. Neuron. 1996;17:575–578. doi: 10.1016/s0896-6273(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Short DB, Trotter KW, Reczek SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- Simons K, Fuller SD. Cell surface polarity in epithelia. Ann Rev Biochem. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Bremnes B, Nordeng TW, Bakke O. The leucine-based motif DDQxxLI is recognized both for internalization and basolateral sorting of invariant chain in MDCK cells. Eur J Cell Biol. 1998;76:25–32. doi: 10.1016/S0171-9335(98)80014-9. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a wide variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V, Campbell KP. Muscular dystrophies and the dystrophin– glycoprotein complex. Curr Opin Neurol. 1997;10:168–175. doi: 10.1097/00019052-199704000-00016. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yoshida M, Ozawa E. Mammalian α1- and β1-syntrophin bind to the alternatively splice-prone region of the dystrophin COOH terminus. J Cell Biol. 1995;128:373–381. doi: 10.1083/jcb.128.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevananther S, Kolli AH, Devarajan P. Identification of a novel ankyrin isoform (AnkG190) in kidney and lung that associates with the plasma membrane and binds alpha-Na, K-ATPase. J Biol Chem. 1997;273:23952–23958. doi: 10.1074/jbc.273.37.23952. [DOI] [PubMed] [Google Scholar]
- Thomas DC, Brewer CB, Roth MG. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- Tinsley JM, Blake DJ, Roche A, Fairbrother U, Riss J, Byth BC, Knight AE, Kendrick-Jones J, Suthers GK, Love DR, et al. Primary structure of a dystrophin-related protein. Nature. 1992;360:591–593. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- Touhara K, Inglese J, Pritcher JA, Shaw G, Lefkowitz RJ. Binding of G protein beta gamma-subunits to pleckstrin homology domains. J Biol Chem. 1994;269:10217–10220. [PubMed] [Google Scholar]
- Ullmer C, Schmuck K, Figge A, Lubbert H. Cloning and characterization of MUPP1, a novel PDZ domain protein. FEBS Lett. 1998;424:63–68. doi: 10.1016/s0014-5793(98)00141-0. [DOI] [PubMed] [Google Scholar]
- Wandinger-Ness A, Bennett MK, Antony C, Simons K. Distinct transport vesicles mediate the delivery of the plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990;111:987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Reuver SM, Kuhlendahl S, Chung WJ, Garner CC. Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J Cell Sci. 1998;111:2365–2376. doi: 10.1242/jcs.111.16.2365. [DOI] [PubMed] [Google Scholar]
- Xu XS, Choudhury A, Li X, Montell C. Coordination of an array of signaling proteins through homo- and heteromeric interactions between PDZ domains and target proteins. J Cell Biol. 1998;142:545–555. doi: 10.1083/jcb.142.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Jung D, Rafael JA, Chamberlain JS, Campbell KP. Identification of α-syntrophin binding to syntrophin triplet, dystrophin, and utrophin. J Biol Chem. 1994;270:4975–4978. doi: 10.1074/jbc.270.10.4975. [DOI] [PubMed] [Google Scholar]
- Yao L, Kawakami Y, Kawakami T. The pleckstrin homology domain of Bruton tyrosine kinase interacts with protein kinase C. Proc Natl Acad Sci USA. 1994;91:9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Ozawa E. Glycoprotein complex anchoring dystrophin to the sarcolemma. J Biochem. 1990;108:748–752. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]