Abstract
Insertion of newly synthesized proteins into or across the mitochondrial outer membrane is initiated by import receptors at the surface of the organelle. Typically, this interaction directs the precursor protein into a preprotein translocation pore, comprised of Tom40. Here, we show that a prominent β-barrel channel protein spanning the outer membrane, human voltage- dependent anion-selective channel (VDAC), bypasses the requirement for the Tom40 translocation pore during biogenesis. Insertion of VDAC into the outer membrane is unaffected by plugging the translocation pore with a partially translocated matrix preprotein, and mitochondria containing a temperature-sensitive mutant of Tom40 insert VDAC at the nonpermissive temperature. Synthetic liposomes harboring the cytosolic domain of the human import receptor Tom20 efficiently insert newly synthesized VDAC, resulting in transbilayer transport of ATP. Therefore, Tom20 transforms newly synthesized cytosolic VDAC into a transmembrane channel that is fully integrated into the lipid bilayer.
Keywords: voltage-dependent anion-selective channel, porin, Tom20, import, mitochondria
Sorting of newly synthesized proteins to specific organelles within the cell requires a mechanism to direct the protein to its correct location and to overcome the thermodynamic barrier imposed by the lipid bilayer during transmembrane translocation. Typically, recognition is provided by organelle-specific receptors and translocation is mediated by a transbilayer machinery that includes a pore complex through which the polypeptide can move (Schatz and Dobberstein, 1996). In the case of mitochondria, both the outer and inner membranes are competent for protein translocation and achieve this by distinct translocation machineries (Hauke and Schatz, 1997; Neupert, 1997; Pfanner and Meijer, 1997). A complex of receptors embedded in the outer membrane (Tom20, Tom22, Tom37, and Tom70) mediates recognition of all known proteins destined for internalization into the organelle. After binding to the receptor complex, the preprotein moves into a translocation apparatus comprised of the predicted channel, Tom40, and the ancillary proteins, Tom5, Tom6, and Tom7. Recent reconstitution of functional protein translocation in synthetic lipid bilayers faithfully recapitulates this process and suggests the existence of a transbilayer water-filled pore as the polypeptide translocator (Hill et al., 1998; Kunkele et al., 1998).
Mitochondrial preproteins that contain hydrophobic membrane-spanning segments are likely arrested and released from the translocation pore into the surrounding lipid bilayer during the translocation process. Such a mechanism is well documented in the ER (Rapoport et al., 1996). Indeed, hydrophobic sequences analogous to ER stop-transfer and signal-anchor sequences have been identified as topogenic sequences that trigger protein integration into the mitochondrial outer membrane (Nguyen et al., 1988; Li and Shore, 1992; McBride et al., 1992). In contrast, little is understood about β-barrel proteins, prototypically represented by the bacterial porins (Weiss et al., 1991), which lack uniformly hydrophobic domains, but whose overall amphiphilic character permits integration of almost the complete protein structure into the bilayer to form a transmembrane channel. Such a porin exists as an abundant 30-kD voltage-dependent anion-selective channel (VDAC)1 in the mitochondrial outer membrane of all eukaryotes examined (Colombini et al., 1996; Mannella, 1997). It is comprised of a 12- to 13-strand barrel (Colombini et al., 1987; Stanley et al., 1995) that provides the major pathway for transport of metabolites, including ATP, through the membrane (Rostovtseva and Colombini, 1997; Lee et al., 1998). Coordinated regulation of this outer membrane channel with those in the inner membrane may play an important role in mitochondria function and signaling, including events contributing to the mitochondrial involvement in apoptosis (Green and Reed, 1998).
Here, we have examined the import pathway of VDAC. Tom20 plays a direct role in targeting newly synthesized VDAC for integration into the mitochondrial outer membrane. Import is blocked by Tom20 gene deletion in vivo (Sollner et al., 1989) and by competing anti-Tom20 antibodies in vitro (Ramage et al., 1993; Goping et al., 1995). Moreover, a direct physical interaction between VDAC and Tom20 has been observed (Schleiff et al., 1997). Consistent with this, targeting sequences that specify protein translocation into or across the outer membrane effectively compete for VDAC insertion into the outer membrane (Millar and Shore, 1996). We now demonstrate, however, that VDAC bypasses the requirement for the Tom40 preprotein translocation pore. Rather, Tom20 alone is capable of catalyzing direct insertion of this β-barrel protein into the membrane lipid bilayer.
Materials and Methods
General
Previous articles (McBride et al., 1992, 1996; Millar et al., 1996; Goping et al., 1998) describe the routine procedures used in this study. These include isolation of rat heart mitochondria, transcription-translation of plasmids encoding VDAC and preornithine carbamyl transferase (pOCT), and import of 35S-labeled translation products into mitochondria in vitro. Additional details are provided in the figure legends.
Recombinant Cytosolic Domain of Human Tom20
Recombinant glutathione S-transferase (GST)–human Tom20Δ1-29 (formerly named GST-Δ30hTom20), lacking the NH2-terminal transmembrane segment of hTom20, was expressed in TOPP2 cells and purified (Schleiff et al., 1997). Protein immobilized on glutathione-Sepharose 4B was washed and suspended in 20 mM NaPO4, pH 7.4, 150 mM NaCl, 1 mM dithiothreitol, 0.5 mM CaCl2, and incubated with 5 μg/ml thrombin for 18 h at 4°C, generating hTom20Δ1-29 with an extra gly residue at the NH2 terminus derived from the thrombin cleavage site. The mixture was passed through a Mono S HR 5/5 column and protein was eluted with a linear gradient (buffer A, 10 mM MES, pH 5.0; buffer B, 10 mM 3-cyclohexylamino-1-propane sulfonic acid, pH 10, 500 mM NaCl). Peak fractions containing hTom20Δ1-29 were resolved on a Superdex 200 column in 10 mM Hepes, pH 7.0, and 100 mM NaCl. Fractions containing the purified protein were dialyzed against the same buffer containing 5 mM DTT and the protein concentrated to 1.5 mg/ml.
Plasmid encoding GST-hTom20Δ1-29 was manipulated by standard recombinant DNA procedures to substitute cys at hTom20 codon position 100 with ser and to introduce ser-cys after gly at the thrombin cleavage site. The thrombin cleavage product, hTom20Δ1-29/N-GSC/C100S, was generated and purified as above.
Liposomes
Approximately 100 nm large unilamellar vesicles (LUVs) (lipid composition: PC, phosphotidylcholine; PE, phosphotidylethanolamine; PI, phosphotidylinositol; PS, phosphotidylserine; PC/PE/PI/PS at molar ratios of 55:28:13:3 or PC/PE/PI/PS/PE-bmps at molar ratios of 54.5:27.5:13:3:1) were prepared in 170 mM sucrose, 20 mM Tris acetate, pH 7.0, and 2 mM CaCl2 (Shahinian and Silvius, 1995). LUVs containing β-maleimidopropionic acid N-hydroxysuccinimide ester of PE (PE-bmps) were incubated for 12 h at 4°C with hTom20Δ1-29/N-GSC/C100S (10-fold molar excess relative to PE-bmps; coupling efficiency, 85–95%) followed by two 30 min incubations with excess LUVs generated in medium lacking sucrose (100 mM NaCl, 20 mM Tris acetate, pH 7.0, and 2 mM CaCl2) to competitively remove unincorporated hTom20. Sucrose-loaded LUVs containing the covalently attached cytosolic domain of hTom20 were recovered by centrifugation at 50,000 × g for 60 min in a Beckman 75Ti rotor. They were used in protein import reactions (Goping et al., 1998) in place of mitochondria.
Results and Discussion
Standard protein import was conducted by combining rat heart mitochondria with human VDAC synthesized in reticulocyte lysate. In this system, the preprotein translocation pore can be jammed by partially importing a chimeric protein containing a matrix-targeting signal fused to dihydrofolate reductase (DHFR). Unfolding of the DHFR moiety on the cytosolic side of the outer membrane is prevented by the high-affinity DHFR active site inhibitor, methotrexate (MTX; Eilers and Schatz, 1986; Vestweber et al., 1989). In the presence of MTX, excess pODHFR (Sheffield et al., 1990), a chimeric protein consisting of the matrix targeting signal of pOCT fused to DHFR and purified from expressing bacteria, blocked import and processing of pOCT (Fig. 1 A, lower panel, lanes 3 and 4), whose processed form otherwise acquires resistance to external trypsin (Fig. 1 A, lower panel, lanes 8 and 9). In addition to inhibition of pOCT import, pODHFR/MTX inhibited outer membrane insertion of yTom70(1-29)DHFR (Fig. 1 B), also designated pOMD29, a chimeric protein comprised of the transmembrane signal-anchor domain of yeast Tom70 fused to DHFR, whose insertion into the outer membrane of heart mitochondria in vitro has been documented previously (Li and Shore, 1992; McBride et al., 1992). yTom70(1-29)DHFR is inserted into the outer membrane in the Nin-Ccyto orientation (Li and Shore, 1992; McBride et al., 1992) and therefore, its import in the absence of competing pODHFR was unaffected by MTX (not shown).
Figure 1.
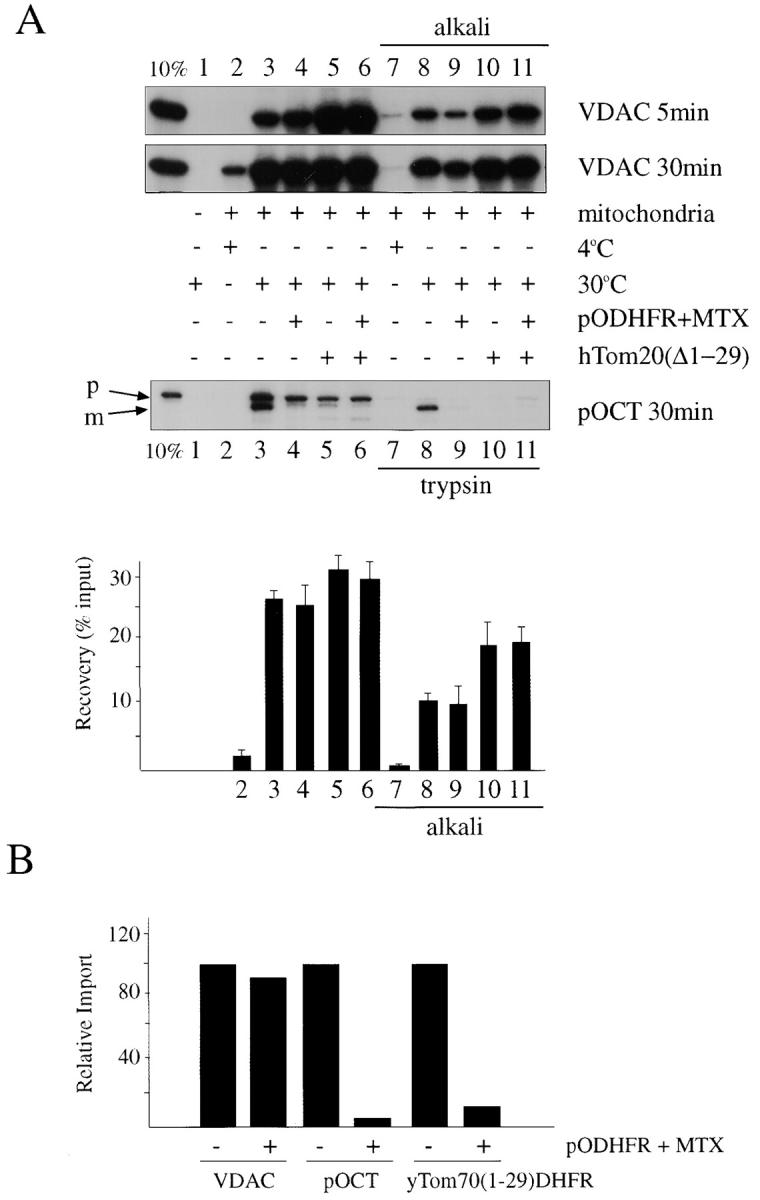
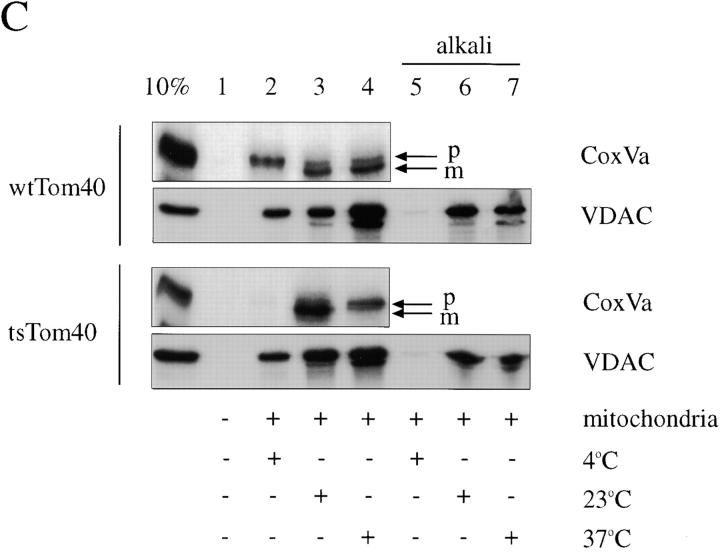
Soluble cytosolic domain of hTom20 stimulates insertion of VDAC into the outer membrane of rat heart mitochondria in vitro by a pathway that is independent of the preprotein translocation pore. (A) 35S-labeled transcription-translation products of human VDAC and rat pOCT in reticulocyte lysate were subjected to standard protein import reactions in the presence of purified rat heart mitochondria for the indicated times at 4°C (lanes 2 and 7) or 30°C (lanes 1, 3–6, and 8–11) in the absence (lane 1) or presence (lanes 2–11) of mitochondria. Also included in the reactions were 15 μg purified pODHFR (Sheffield et al., 1990) and 2 mM MTX (lanes 4, 6, 9, and 11), or 15 μg purified cytosolic domain of hTom20, hTom20Δ1-29 (lanes 5, 6, 10, and 11). At the end of the reactions, the mitochondria were either treated with trypsin (pOCT, lanes 7–11; McBride et al., 1992) or extracted with 0.1 M NaCO3, pH 11.5 (alkali, VDAC lanes 8–11; Goping et al., 1998). Reaction products were resolved by 10% SDS-PAGE and visualized by fluorography. Lane 1, 10% of input [35S]VDAC or [35S]pOCT. p, Precursor form of OCT; m, mature form of OCT. The bar graph quantifies the radioactive bands from import reactions of VDAC at 30 min, using a Power MacIntosh 7200/120 and NIH image v1.61 image analysis software. Shown are the averages from four separate experiments with standard deviations. The bar numbers refer to the lane numbers in the VDAC (30 min) fluorogram. (B) Import (30 min) of [35S]VDAC, [35S]pOCT, and [35S]yTom70(1-29)DHFR (previously called pOMD29; McBride et al., 1992) was conducted in the presence or absence of pODHFR + MTX. Alkaline-resistant VDAC and yTom70(1-29)DHFR and processed pOCT were analyzed and quantified as in A. (C) Same as A, except that import of [35S]VDAC or yeast [35S]pCox Va was conducted using mitochondria isolated (Daum et al., 1982) from wild-type Saccharomyces cerevisiae (strain D273-10B) or from a yeast strain (KKY3.3) harboring a temperature sensitive mutation in Tom40 (Kassenbrock et al., 1995), and incubated at the nonpermissive (37°C) or permissive (23°C) temperatures for Tom40 for 60 min before conducting import reactions. Import was for 30 min at 4, 23, or 37°C, as indicated. p, Precursor form of Cox VA; m, mature form of Cox Va.
In contrast to pOCT and yTom70(1-29)DHFR, import and membrane insertion of VDAC in this system, assessed by the temperature-sensitive acquisition of resistance to alkaline extraction (Fujiki et al., 1982; Goping et al., 1998; Fig. 1 A, upper panel, compare lanes 7 and 8), was relatively unaffected by pODHFR/MTX (Fig. 1 A, upper panel, lanes 8 and 9). The slight reduction in VDAC import at 5 min effected by pODHFR/MTX may relate to the fact that preproteins like pODHFR can stimulate recruitment of Tom20 into the translocation complex, thereby competitively reducing the amount of free Tom20 in the membrane that is available for interaction with VDAC (Rapaport et al., 1998). The failure of partially translocated pODHFR/MTX to block membrane insertion of VDAC suggested that VDAC may bypass the requirement for the Tom40 translocation pore during import.
To address this question further, import of VDAC was examined using isolated yeast mitochondria containing a temperature-sensitive mutant of Tom40 (Kassenbrock et al., 1995). In contrast to control mitochondria, temperature-sensitive Tom40 mitochondria were incapable of importing the matrix preprotein form of cytochrome oxidase subunit Va (COX Va) at the nonpermissive temperature (37°C), as judged by the failure of pCOX Va to be processed by the mitochondria at 37°C (Fig. 1 C, lane 4), while processing of pCOX Va was observed at the permissive temperature of 23°C. In contrast to pCOX Va, membrane insertion of VDAC occurred at both 23 and 37°C (Fig. 1 C). The slight decrease in membrane insertion of VDAC at 37°C compared with 23°C was similar for both wild-type and temperature-sensitive Tom40 mitochondria (Fig. 1 C), suggesting that this difference was related to events other than the temperature-sensitive phenotype of Tom40.
The cytosolic domain of hTom20, hTom20Δ1-29, when included as a soluble entity in excess in the import reaction, inhibited import of pOCT (Fig. 1 A, lower panel, compare lanes 8 and 10), presumably because it sequestered the preprotein through direct protein interaction (Schleiff et al., 1997) and prevented transfer of the preprotein to the translocation machinery. hTom20Δ1-29 can also physically interact with VDAC (Schleiff et al., 1997). In distinct contrast to pOCT, however, hTom20Δ1-29 did not interfere with insertion of VDAC into the outer membrane. In fact, it had a slight stimulatory effect (Fig. 1 A, upper panel, compare lanes 8 and 10), suggesting that potential interactions between VDAC and hTom20Δ1-29 in the import reaction must be readily reversible. Moreover, the difference in response of pOCT and VDAC to hTom20Δ1-29 in the import reaction suggested a potential fundamental difference in the import pathways of the two proteins. However, the results also imply that a complex of VDAC and soluble hTom20Δ1-29 can make contact with the mitochondrial surface in vitro, a suggestion that is compatible with the observed binding of the cytosolic domain of hTom20 to lipid surfaces in vitro (Schleiff and Turnbull, 1998; Fig. 2 A).
Figure 2.
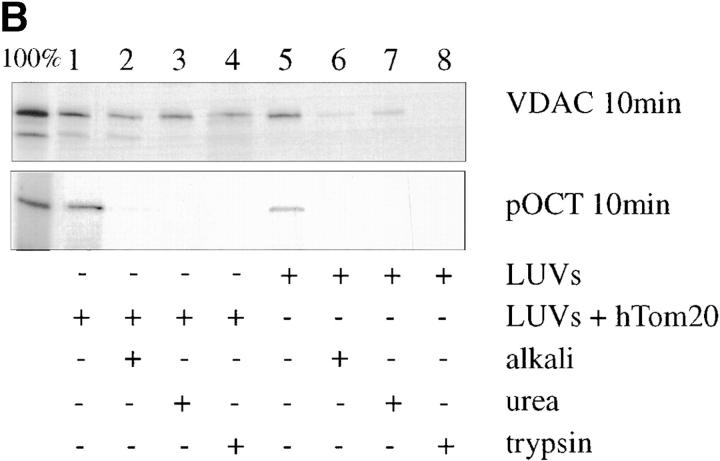
hTom20 catalyzes insertion of VDAC into synthetic lipid bilayers. (A) Schematic illustration of covalent coupling of cytosolic domain of hTom20 (hTom-20Δ1-29/N-GSC/C100S; see Materials and Methods) to the PE-pmbs incorporated into preformed LUVs. (B) Standard protein import reactions contained sucrose-loaded LUVs (diameter ∼100 nm; 0.02 mM lipid; 1.0 mol% PE-pmbs), with or without covalently attached hTom20 (∼90 nM), and were incubated for 10 min at room temperature with [35S]VDAC or [35S]pOCT. After a 20-fold dilution with reaction medium, the LUVs were recovered by centrifugation at 170,000 × g in an airfuge. Recovery of sucrose-loaded LUVs after centrifugation in all reactions was >95%, as judged by incorporation of 0.1 mol% rhodamine-labeled PE and detection by fluorescence. The pellets were subjected to extraction with 0.1 M NaCO3, pH 11.5 (alkali; Goping et al. 1998), or with 8 M urea, 40 mM Hepes, pH 7.0 (urea). Alternatively, reactions were cooled to 4°C and incubated with trypsin (1.0 μg) for 20 min, followed by incubation for 20 min with 10 μg soybean trypsin inhibitor before dilution and recovery of LUVs. Final liposomal pellets were subjected to 10% SDS-PAGE and the products were visualized by fluorography. Total input VDAC and pOCT is designated 100%. (C) Import reactions were conducted with [35S]VDAC or [35S]pOCT in the presence of sucrose-loaded LUVs (0.07 mM lipid) with or without attached cytosolic domain of hTom20. After 10 min, a 10-fold molar excess of plain lipid vesicles lacking sucrose was added for the indicated time periods, the sucrose-loaded vesicles were isolated by centrifugation as described above, and the associated [35S]VDAC or [35S]pOCT was quantified by scintillation counting (expressed as percent of total input). Shown are the averages of three determinations with standard deviations for each time point.
To assess the possibility that hTom20 can mediate direct insertion of VDAC into a membrane lipid bilayer, synthetic LUVs were created in which the cytosolic domain of hTom20 was linked to the bilayer surface via covalent attachment to PE-pmbs (Fig. 2 A). To that end, the protein was modified to contain a unique cys at the NH2 terminus of hTom20Δ1-29; the other cys located at codon position 100 was converted to ser. These changes did not influence the ability of the receptor to interact with either VDAC or pOCT in vitro (data not shown). The liposomes had a phospholipid composition similar to that of total mitochondrial outer membrane in rat liver (de Kroon et al., 1997). Under the conditions used for mitochondrial protein import reactions with VDAC synthesized in reticulocyte lysate such liposomes were found to efficiently insert the protein, as determined by acquired resistance to extraction at alkaline pH or with urea (Fig. 2 B, upper panel, lanes 2 and 3). Moreover, the resulting import product was resistant to treatment with trypsin (lane 4), reflective of VDAC in native membranes (Colombini et al., 1996; Mannella, 1997). LUVs bearing the hTom20 cytosolic domain did not translocate pOCT, as judged by the failure of pOCT to acquire resistance to trypsin (Fig. 2 B, lower panel), nor did they insert yTom70(1-29)DHFR, as judged by its extractability from liposomes with 7 M urea (not shown). Conversely, LUVs lacking the hTom20 cytosolic domain did not insert VDAC (Fig. 2 B, upper panel, lanes 6–8). Furthermore, VDAC that associated with LUVs lacking the hTom20 cytosolic domain exhibited facile interliposomal transfer (Fig. 2 C), indicating that this binding by VDAC was merely peripheral. As expected, VDAC that was inserted into the LUV lipid bilayer by the hTom20 cytosolic domain did not transfer to subsequently added protein-free LUVs. In contrast, pOCT that bound to LUVs containing the hTom20 cytosolic domain could subsequently transfer to protein-free LUVs, but slower than pOCT that bound to LUVs lacking hTom20 (∼t 1/2, 15 versus 1 min; Fig. 2 C). This finding is consistent with the ability of hTom20 to physically interact with pOCT (Schleiff et al., 1997). Moreover, the relatively slow dissociation of pOCT from the hTom20 cytosolic domain explains the ability of excess hTom20Δ1-29 to inhibit import of pOCT into mitochondria in vitro (Fig. 1 A).
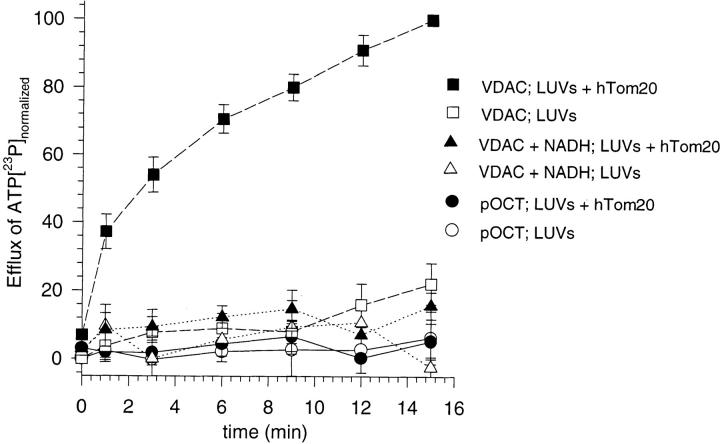
Finally, VDAC that had been inserted into LUVs by the hTom20 cytosolic domain was examined for its ability to transport a physiological substrate of this channel protein, ATP (Rostovtseva and Colombini, 1997; Lee et al., 1998). LUVs were loaded with [32P]ATP before the import reaction, and the subsequent release of ATP was measured at various times after the initiation of the reaction. Insertion of a single functional VDAC channel into the vesicle would be predicted to release encapsulated ATP. Release of radioactive ATP commenced immediately upon initiation of VDAC insertion into liposomes (Fig. 3). Egress of ATP was dependent on functional VDAC, inhibited by a known antagonist, NADH (Zizi et al., 1994), and required the presence of the hTom20 cytosolic domain on the liposome surface to integrate VDAC into the lipid bilayer. In contrast, the control protein pOCT did not stimulate ATP export from LUVs alone or LUVs with hTom20 (Fig. 3).
Figure 3.
hTom20-mediated insertion of VDAC into synthetic lipid membranes stimulates efflux of ATP. Sucrose-loaded LUVs with or without covalently attached hTom20 and containing 1.8 mM [32P]ATP (20 Ci/mol) were incubated in a standard protein import reaction (Goping et al., 1998) with VDAC or pOCT transcription-translation products in the presence or absence of 1 mM NADH. At the indicated times, 20 vol of import reaction medium was added, the LUVs were collected by centrifugation, and radioactivity in the supernatant was determined as in Fig. 2 B. Shown are the average of the normalized results of three determinations with standard deviations (maximum radioactivity = 2,100 cpm). The range of total encapsulated ATP released from LUVs-Tom20 by VDAC at 15 min was 5–10%.
The first indication that a β-barrel protein like VDAC might not require a complex preprotein translocation machinery for insertion into a membrane lipid bilayer came with the observation that purified detergent-solubilized VDAC can spontaneously integrate into planar lipid bilayers as a functional entity. Moreover, in this system insertion is cooperative as a result of self-mediated stimulation (Xu and Colombini, 1996). Although the lag period for this insertion was relatively long, the results suggest an inherent ability of the molecule to partition into a lipid bilayer, but presumably this is because the detergent-extracted entity is in a favorable state for lipid bilayer integration. However, in normal physiology, other considerations apply: the question of membrane specificity; the problem of competing interactions of VDAC with other proteins, specific or otherwise; the necessity to maintain a conformation of soluble VDAC after release from the ribosome that is compatible with subsequent membrane insertion; and the requirement for a mechanism to catalyze VDAC insertion upon contact with the mitochondrial outer membrane. In the import reaction documented here, the appropriate translocation-competent conformation for VDAC may be supplied by chaperones/factors present in reticulocyte lysate (Smith et al., 1994). The only other minimum requirement was provided by hTom20 on the surface of the membrane. While it is impossible to rule out any involvement of the Tom40 translocation pore, its contribution to VDAC membrane insertion was not detected in the assays described here, whereas its influence on import of matrix pOCT and pCOX Va, and on membrane insertion of outer membrane Tom70(1-29)DHFR was pronounced. In addition to its role as a receptor in directing newly synthesized VDAC exclusively to the mitochondrial outer membrane within the context of a whole cell, our results suggest that hTom20 is capable of catalyzing direct insertion of the protein into the lipid bilayer. The receptor may accomplish this simply by bringing VDAC into close proximity to the bilayer and/or by triggering release of presumptive chaperones or other factors from VDAC that otherwise maintain the protein water-soluble. In either scenario, VDAC may spontaneously insert into the proximate bilayer. Alternatively, or in addition, hTom20 may play a direct role in guiding the conformational change that allows the transbilayer β-barrel channel to form.
Acknowledgments
We thank Rania Leventis for help preparing liposomes and Nancy Martin for providing yeast strain KKY3.3.
Abbreviations used in this paper
- DHFR
dihydrofolate reductase
- GST
glutathione S-transferase
- hTom20
human Tom20
- LUVs
large unilamellar liposomes
- MTX
methotrexate
- pCOX Va
precytochrome oxidase subunit Va
- PE
phosphatidylethanolamine
- PE-pmbs
β-maleimidopropionic acid N-hydroxysuccinimide ester of PE
- pOCT
preornithine carbamyl transferase
- VDAC
voltage-dependent anion-selective channel
Footnotes
This work was financed by operating grants from the Medical Research Council and National Cancer Institute of Canada.
References
- Colombini M, Yeung CL, Tung J, Konig T. The mitochondrial outer membrane channel, VDAC, is regulated by a synthetic polyanion. Biochim Biophys Acta. 1987;905:279–286. doi: 10.1016/0005-2736(87)90456-1. [DOI] [PubMed] [Google Scholar]
- Colombini M, Blachly DE, Forte M. VDAC, a channel in the outer mitochondrial membrane. Ion Channels. 1996;4:169–202. doi: 10.1007/978-1-4899-1775-1_5. [DOI] [PubMed] [Google Scholar]
- Daum G, Bohni PC, Schatz G. Import of proteins into mitochondria. Cytochrome b2and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982;257:13028–13033. [PubMed] [Google Scholar]
- de Kroon A, Dolis D, Mayer A, Lill R, de Kruijff KB. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane. Biochim Biophys Acta. 1997;1325:108–116. doi: 10.1016/s0005-2736(96)00240-4. [DOI] [PubMed] [Google Scholar]
- Eilers M, Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986;322:228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Fowler S, Shio H, Hubbard AL, Lazarow P. Polypeptide and phospholipid composition of the membrane of rat liver peroxisomes: comparison with endoplasmic reticulum and mitochondrial membrane. J Cell Biol. 1982;93:103–110. doi: 10.1083/jcb.93.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goping IS, Millar DG, Shore GC. Identification of the human mitochondrial protein import receptor, huMas20p. FEBS Lett. 1995;373:45–50. doi: 10.1016/0014-5793(95)01010-c. [DOI] [PubMed] [Google Scholar]
- Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC. Regulated targeting of BAX to mitochondria. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Haucke V, Schatz G. Import of proteins into mitochondria and chloroplasts. Trends Cell Biol. 1997;7:103–106. doi: 10.1016/S0962-8924(96)10052-0. [DOI] [PubMed] [Google Scholar]
- Hill K, Model K, Ryan MT, Dietmeimer K, Martin F, Wagner R, Pfanner N. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature. 1998;395:516–521. doi: 10.1038/26780. [DOI] [PubMed] [Google Scholar]
- Kassenbrock CK, Gao GJ, Groom KR, Sulo P, Douglas MG, Martin NC. RPM2, independently of its mitochondrial RNAse Pfunction, suppresses an ISP42 mutant defective in mitochondrial import and is essential for normal growth. Mol Cell Biol. 1995;15:4763–4770. doi: 10.1128/mcb.15.9.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkele KP, Heins S, Dembowski M, Nargang FE, Benz R, Thieffry M, Walz J, Lill R, Nussberger S, Neupert W. The preprotein translocation channel of the outer membrane of mitochondria. Cell. 1998;93:1009–1019. doi: 10.1016/s0092-8674(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Lee AC, Xu X, Blachly DE, Forte M, Colombini M. The role of yeast VDAC genes on the permeability of the mitochondrial outer membrane. J Membr Biol. 1998;161:173–181. doi: 10.1007/s002329900324. [DOI] [PubMed] [Google Scholar]
- Li JM, Shore GC. Reversal of the orientation of an integral protein of the mitochondrial outer membrane. Science. 1992;256:1815–1817. doi: 10.1126/science.1615327. [DOI] [PubMed] [Google Scholar]
- Mannella CA. On the structure and gating mechanism of the mitochondrial channel, VDAC. J Bioenerg Biomembr. 1997;29:525–531. doi: 10.1023/a:1022489832594. [DOI] [PubMed] [Google Scholar]
- McBride HM, Millar DG, Li JM, Shore GC. A signal-anchor sequence selective for the mitochondrial outer membrane. J Cell Biol. 1992;119:1451–1457. doi: 10.1083/jcb.119.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride HM, Goping IS, Shore GC. The human mitochondrial import receptor, hTom20p, prevents a cryptic matrix targeting sequence from gaining access to the protein translocation machinery. J Cell Biol. 1996;134:370–313. doi: 10.1083/jcb.134.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar DG, Shore GC. Signal anchor sequence insertion into the outer mitochondrial membrane. Comparison with porin and the matrix targeting pathway. J Biol Chem. 1996;271:25823–25829. doi: 10.1074/jbc.271.42.25823. [DOI] [PubMed] [Google Scholar]
- Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Bell AW, Shore GC. Protein sorting between mitochondrial membranes specified by position of the stop-transfer domain. J Cell Biol. 1988;106:1499–1505. doi: 10.1083/jcb.106.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N, Meijer M. The Tom and Tim machine. Curr Biol. 1997;7:R100–R103. doi: 10.1016/s0960-9822(06)00048-0. [DOI] [PubMed] [Google Scholar]
- Ramage L, Junne T, Hahne K, Lithgow T, Schatz G. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO (Eur Mol Biol Organ) J. 1993;12:4115–4123. doi: 10.1002/j.1460-2075.1993.tb06095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D, Kunkele KP, Dembowski M, Ahting U, Narang FE, Neupert W, Lill R. Dynamics of the Tom complex of mitochondria during binding and translocation of preproteins. Mol Cell Biol. 1998;18:5256–5262. doi: 10.1128/mcb.18.9.5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T, Jungnickel AB, Kutay U. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu Rev Biochem. 1996;65:271–303. doi: 10.1146/annurev.bi.65.070196.001415. [DOI] [PubMed] [Google Scholar]
- Rostovtseva T, Colombini M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys J. 1997;72:1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- Schleiff E, Turnbull JL. Functional and structural properties of the mitochondrial outer membrane receptor Tom20. Biochemistry. 1998;37:13043–13051. doi: 10.1021/bi9807456. [DOI] [PubMed] [Google Scholar]
- Schleiff E, Shore GC, Goping IS. Interactions of the human mitochondrial protein import receptor, hTom20, with precursor proteins in vitro reveal pleiotropic specificities and different receptor domain requirements. J Biol Chem. 1997;272:17784–17789. doi: 10.1074/jbc.272.28.17784. [DOI] [PubMed] [Google Scholar]
- Shahinian S, Silvius JR. A novel strategy affords high-yield coupling of antibody Fab′ fragments to liposomes. Biochim Biophys Acta. 1995;1239:157–167. doi: 10.1016/0005-2736(95)00145-s. [DOI] [PubMed] [Google Scholar]
- Sheffield WP, Shore GC, Randall SK. Mitochondrial precursor protein. Effects of 70-kilodalton heat shock protein on polypeptide folding, aggregation, and import competence. J Biol Chem. 1990;265:11069–11076. [PubMed] [Google Scholar]
- Smith M, Hicks S, Baker K, McCauley R. Rupture of the mitochondrial outer membrane impairs porin assembly. J Biol Chem. 1994;269:28460–28464. [PubMed] [Google Scholar]
- Sollner T, Griffiths G, Pfaller R, Pfanner N, Neupert W. MOM19, an import receptor for mitochondrial precursor proteins. Cell. 1989;59:1061–1070. doi: 10.1016/0092-8674(89)90762-9. [DOI] [PubMed] [Google Scholar]
- Stanley SJ, Dias A, D'Arcangelis D, Mannella CA. Peptide-specific antibodies as probes of the topography of the voltage gated channel of the mitochondrial outer membrane of Neurospora crassa. . J Biol Chem. 1995;270:16694–16700. doi: 10.1074/jbc.270.28.16694. [DOI] [PubMed] [Google Scholar]
- Vestweber D, Brunner J, Baker A, Schatz G. A 42K outer membrane protein is a component of the yeast mitochondrial protein import site. Nature. 1989;341:205–209. doi: 10.1038/341205a0. [DOI] [PubMed] [Google Scholar]
- Weiss MS, Abele U, Weckesser J, Welte W, Schiltz E, Schultz GE. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991;254:1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- Xu X, Colombini M. Self-catalyzed insertion of proteins into phospholipid membranes. J Biol Chem. 1996;271:23675–23682. doi: 10.1074/jbc.271.39.23675. [DOI] [PubMed] [Google Scholar]
- Zizi M, Forte M, Blachly DE, Colombini M. NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. J Biol Chem. 1994;269:1614–1616. [PubMed] [Google Scholar]