Abstract
The architectural complexity of the hepatocyte canalicular surface has prevented examination of apical membrane dynamics with methods used for other epithelial cells. By adopting a pharmacological approach, we have documented for the first time the internalization of membrane proteins from the hepatic apical surface. Treatment of hepatocytes or WIF-B cells with phosphoinositide 3-kinase inhibitors, wortmannin or LY294002, led to accumulation of the apical plasma membrane proteins, 5′-nucleotidase and aminopeptidase N in lysosomal vacuoles. By monitoring the trafficking of antibody-labeled molecules, we determined that the apical proteins in vacuoles came from the apical plasma membrane. Neither newly synthesized nor transcytosing apical proteins accumulated in vacuoles. In wortmannin-treated cells, transcytosing apical proteins traversed the subapical compartment (SAC), suggesting that this intermediate in the basolateral-to-apical transcytotic pathway remained functional. Ultrastructural analysis confirmed these results. However, apically internalized proteins did not travel through SAC en route to lysosomal vacuoles, indicating that SAC is not an intermediate in the apical endocytic pathway. Basolateral membrane protein distributions did not change in treated cells, uncovering another difference in endocytosis from the two domains. Similar effects were observed in polarized MDCK cells, suggesting conserved patterns of phosphoinositide 3-kinase regulation among epithelial cells. These results confirm a long-held but unproven assumption that lysosomes are the final destination of apical membrane proteins in hepatocytes. Significantly, they also confirm our hypothesis that SAC is not an apical endosome.
Keywords: polarity, phosphoinositide 3-kinase, hepatocyte, endocytosis, subapical compartment
The plasma membrane of polarized epithelial cells is separated into discrete domains (apical and basolateral) that are characterized by distinct activities and protein compositions. The mechanisms that regulate the polarized expression of proteins at a given cell surface are currently actively being examined. We are investigating the cellular itineraries of resident apical plasma membrane proteins in hepatocytes. Our recent studies have been performed in the polarized hepatic cell line, WIF-B (Ihrke et al., 1993, 1998; Shanks et al., 1994). As observed in situ, WIF-B apical membrane proteins that have been studied to date are transported to the apical surface via an “indirect” route (Bartles et al., 1987; Schell et al., 1992; Bartles and Hubbard, 1988; Ihrke et al., 1998). The proteins are first transported from the trans-Golgi network to the basolateral plasma membrane, where they are retrieved by endocytosis and transcytosed to the apical cell surface. The transcytosing molecules traverse through similar intermediate compartments (early endosomes and the subapical compartment [SAC]1) in WIF-B cells as they do in situ (Barr and Hubbard, 1993; Barr et al., 1995; Ihrke et al., 1998). Many factors have been identified both in hepatocytes and WIF-B cells that are likely mediating transport along both the biosynthetic and transcytotic pathways. This “molecular machinery” includes the now familiar soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) complex proteins, rab GTPases, coat proteins and coat-nucleating factors, microtubules and associated motors, and annexins (for review, see Ihrke and Hubbard, 1995). Although the precise mechanisms by which these molecules exert their physiological activities are currently unknown, the framework is clearly set to direct future investigations.
Once hepatic apical plasma membrane proteins have reached their specific destination (the bile canalicular/apical plasma membrane), much less is known about the mechanisms that retain them or regulate their turnover. A major obstacle to our understanding of bile canalicular events is the ultrastructural complexity of the liver. The bile canaliculus is unique in its organization in the cell and within the intact organ. Unlike the lumenal domains of kidney or intestinal epithelia, the hepatocyte lumenal surfaces are branching networks between adjacent cells that are not easily accessible to experimental manipulation. Although WIF-B cells have emerged as an excellent in vitro polarized hepatic cell model, the bile canalicular domain is also sequestered from the external milieu and is not amenable for the types of experimentation performed in simpler, columnar epithelia.
Although structural constraints have hindered our ability to examine the dynamics of apical plasma membrane proteins, theoretically we know that apical membrane retrieval systems must exist. Only in recent years has the extent of vesicle delivery to the apical domain been appreciated. From studies of fluid phase transcytosis, it has been estimated that 600–850 transcytotic vesicles (100 nm in diameter) fuse with the apical surface every minute (Crawford, 1996). This means that the apical membrane surface area would double or triple every 20 min if not compensated (Crawford, 1996). Early models proposed that the detergent-like bile was responsible for the solubilization of both membrane lipids and proteins from the canalicular surface (for review, see Oude Elferink et al., 1995). By extension, this would help maintain the apical membrane steady state surface area. However, we reported that a maximum of only 20–30% of the loss of resident apical proteins can be accounted for by passive solubilization (Scott and Hubbard, 1992). Furthermore, recent work in transgenic animals deficient in the multidrug resistance 2 (mdr2) P-glycoprotein gene has also challenged the solubilization/release hypothesis (Smit et al., 1993; Groen et al., 1995; Crawford et al., 1997).
We further infer the presence of apical membrane retrieval mechanisms from studies examining the proteins present at that domain. For example, the polymeric IgA receptor is continuously and robustly synthesized and delivered to the apical domain by transcytosis in hepatocytes. Once delivered, the receptor is cleaved, releasing the secretory component into the bile and leaving a 30-kD tail fragment anchored in the membrane. Only a small proportion of the tail has been detected in bile (Solari et al., 1989), suggesting that other mechanisms are in place to rid the apical domain of the fragment.
The most obvious mechanism in eukaryotic cells for the retrieval and turnover of membrane proteins is endocytosis and delivery to lysosomes. This degradative organelle is clearly the destination of exogenous macromolecules endocytosed from the basolateral surface and of autophagocytosed bits of the hepatic cytoplasm. However, its role in the turnover of plasma membrane proteins from the apical surface has never been demonstrated, due primarily to the long half-lives of apical membrane proteins and the attendant difficulty in accumulating detectable levels before their degradation.
Although examination of hepatic apical membrane dynamics has been difficult, there are strategies available. One such strategy is to identify and localize molecules implicated in membrane traffic. For example, the presence of clathrin-coated profiles at the canalicular surface (Hubbard, A.L., unpublished observations) indicates that endocytic retrieval occurs at that domain and provides clues to possible mechanisms. Additionally, soluble N-ethylmaleimide–sensitive factor attachment protein receptor molecules (Fujita et al., 1998), rab GTPases, and annexins (for review, see Ihrke and Hubbard, 1995) have all been found at the apical domain in hepatocytes. The functional studies are needed to determine whether such molecules are regulating transport events to or from the apical surface.
Another strategy includes examining the effects of various pharmacological agents on canalicular membrane retrieval. For these studies, the preferred reagents should be specific and effective disrupters of endocytic transport steps. Both wortmannin and LY294002 fulfill these criteria. Wortmannin is a potent irreversible inhibitor (IC50, 3 nM) of all mammalian phosphoinositide 3-kinases (PI 3-kinases) tested to date and, at nanomolar concentrations, its effect is thought to be specific (Ui et al., 1995). LY294002, a reversible inhibitor, is also thought to be specific in its inhibition, but at higher (micromolar) concentrations (Vlahos et al., 1993). In mammalian cells, many endocytic transport steps have been shown to be sensitive to these agents, including transferrin receptor internalization and recycling (Li et al., 1995; Martys et al., 1996; Spiro et al., 1996), horseradish peroxidase and albumin uptake (Li et al., 1995; Brunskill et al., 1998), activated PDGF receptor transport from the cell surface to lysosomes (Joly et al., 1995), early endosome fusion (Jones and Clague, 1995), autophagic vacuole formation (Blommart et al., 1997), and basolateral-to-apical transcytosis of dimeric IgA, ricin, and horseradish peroxidase (Cardone and Mostov, 1995; Hansen et al., 1995; Folli et al., 1997).
Here we report that PI 3-kinase is also an important and selective regulator of apical plasma membrane protein dynamics in hepatic cells. In the presence of wortmannin or LY294002, a subset of resident apical plasma membrane proteins accumulated in intracellular lysosomal vacuoles in both liver hepatocytes and WIF-B cells. This is the first documentation of membrane protein internalization from the apical cell surface in hepatic cells. SAC, an intermediate of the basolateral-to-apical transcytotic pathway, remained intact in treated cells, but was not an intermediate of the apical endocytic pathway. The distributions of basolateral membrane proteins did not change in the presence of either inhibitor, indicating PI 3-kinases selectively regulate apical membrane proteins. The finding that MDCK cells responded similarly to treatment with the inhibitors suggests a general role for PI 3-kinase in apical membrane transport in polarized cells. These results not only reveal aspects of membrane protein dynamics previously not observed, but also highlight the functional diversity of the polarized cell surface.
Materials and Methods
Materials and Antibodies
Male Sprague-Dawley rats (125–150 g) were supplied by Charles River Breeding Laboratories. Wortmannin, cycloheximide, cytochalasin D, and nocodazole were purchased from Sigma Chemical Co. Wortmannin, cytochalasin D, and nocodazole were stored at −20°C as 10-, 1-, or 16.5-mM stock solutions, respectively, in DMSO. Cycloheximide was prepared as a 10-mg/ml stock solution in 5% ethanol and used directly. LY294002 was purchased from Calbiochem Corp. and stored at −20°C as a 10-mM solution in DMSO. Cell culture media and FBS were purchased from GIBCO BRL. Fluorescently labeled secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. Anti–β-tubulin antibodies were obtained from Sigma Chemical Co. Texas red–conjugated phalloidin was purchased from Molecular Probes Inc., and stored at −20°C as a 200-U/ml stock in methanol. The antibodies recognizing the 120-kD lysosomal glycoprotein (LGP-120), mannose 6-phosphate receptor (M6P-R), 5′nucleotidase (5′NT), and endogenous canine plasma membrane antigens (3F2 and G12) were kindly provided by W. Dunn (University of Florida, Gainesville, FL), Peter Nissley (National Institutes of Health, Bethesda, MD), Paul Luzio (Cambridge University, Cambridge, UK), and George Ojakian (State University of New York, Oswego, New York), respectively. Antibodies against aminopeptidase N (APN), CE9, HA4, asialoglycoprotein receptor (ASGP-R), HA321, syntaxin 3, and endolyn-78 were all prepared by the Hubbard laboratory and have been described elsewhere (Bartles et al., 1985; Scott and Hubbard, 1992; Barr and Hubbard, 1993; Ihrke et al., 1993).
Cell Culture
WIF-B cells were grown in a humidified 7% CO2 incubator at 37°C as described (Shanks et al., 1994). In brief, cells were grown in modified Ham's F12 medium, pH 7.0, supplemented with HAT (10 μM hypoxanthine, 40 nM aminoterpin, 1.6 μM thymidine) and 5% FBS. MDCK cells were grown at 37°C in a 5% CO2 humidified incubator as described (Weisz et al., 1992). For indirect immunofluorescence experiments, cells were seeded onto glass coverslips at 1.3 × 104 cells/cm2 and cultured for 8–12 d (WIF-B) or 4–5 d (MDCK) until they reached maximal density and polarity (Shanks et al., 1994).
Immunofluorescence Microscopy
To examine the effects of wortmannin or LY294002 on the steady state distributions of various proteins, cells were incubated at 37°C up to 3 h in their respective serum-free culture medium buffered with either 20 mM Hepes, pH 7.0 (for WIF-B cells), or 44 mM NaHCO3, pH 7.0 (for MDCK cells), in the presence or absence of either agent (see Results or figure legends for details). After treatment, cells were rinsed briefly in PBS and placed on ice, fixed with chilled PBS containing 4% paraformaldehyde for 1 min, and permeabilized with methanol (also chilled) for 10 min (Ihrke et al., 1993). Cells were rehydrated in PBS by three washes of 5 min each. Cells were further processed for single- or double-labeled indirect immunofluorescence according to previously published methods (Ihrke et al., 1993) with the following primary antibodies: anti–HA321, –LGP-120 and –APN (rabbit polyclonals, 1:100, 1:200, and 1:300, respectively), anti– 5′NT, –endolyn and –HA4 (mouse monoclonal ascites, 1:300, 1:500, and 1:100, respectively). MDCK cells were processed for indirect immunofluorescence using anti–3F2 and –G12 (hybridoma culture supernatants, 1:10). The secondary antibodies (FITC or Cy3 goat anti–rabbit or anti–mouse) were used at 5–10 μg/ml.
To assess the effects of microtubule, actin, or protein synthesis disruption on redistribution, cells were pretreated for 1 h at 37°C with nocodazole (33 μM), cytochalasin D (1 μM), or cycloheximide (25 μg/ml), respectively. Cells were incubated an additional 3 h at 37°C in the presence of wortmannin, and the continued presence of either nocodazole, cytochalasin D, or cycloheximide. The treatments were stopped by fixation and the cells were processed for indirect immunofluorescence. Anti–β-tubulin antibodies (mouse monoclonal) were diluted to 1:500. Texas red–conjugated phalloidin was diluted to 5 U/ml.
Isolated Liver Perfusion
Livers from fasted (12–18 h) rats (125–200 g) were surgically removed and perfused in a recirculating balanced salt solution as described (Dunn et al., 1983) in the absence or presence of 2 μM wortmannin and 50 μM leupeptin. Biopsies (0.25–1.0 g) were removed at the indicated times and fixed by immersion in Krebs buffer containing 4% PFA for at least 1 h at room temperature. The intact liver was fixed by perfusion with 200–300 ml of the same fixative. After fixation, the biopsies and intact liver were processed as described (Dunn et al., 1983) and semithin sections (0.5 μm) cut with a Reichert Jung Ultracut E microtome. The liver sections were processed for immunofluorescence according to previously published methods (Hubbard et al., 1985) using the following primary antibodies: anti–APN, –HA321 (rabbit polyclonals, 1:300 and 1:100, respectively), anti–HA4 and –endolyn (mouse monoclonal ascites, 1:100 and 1:300, respectively). The secondary antibodies (FITC or Cy3 goat anti–rabbit or anti–mouse) were used at 5–10 μg/ml.
Transcytosis Assay
The transcytosis assays were performed as described (Ihrke et al., 1998). WIF-B cells were placed in Hepes-buffered, serum-free medium and treated at 37°C with the agents as specified in Results or the figure legends. The cells were placed on ice for 5 min to cool to 4°C. Antigens present at the basolateral cell surface were labeled (those also present at the apical plasma membrane are excluded from labeling) at 4°C for 15 min with anti–APN (rabbit polyclonal, 1:50) or purified mouse monoclonal antibodies against 5′NT (purified IgG fraction, 20–50 μg/ml) or CE9 (mouse monoclonal ascites, 1:25). Total IgG was recovered from mouse ascites using EZ Sep (Pharmacia LKB Biotechnology Inc.) according to the instructions from the manufacturer. All antibodies were diluted in buffered, serum-free medium containing 2 mg/ml BSA in the absence or presence of the various agents. After labeling, the cells were washed extensively in buffered, serum-free medium containing 2 mg/ml BSA to get rid of excess and nonspecifically associated antibodies. Cells were then returned to 37°C and incubated for the desired times of chase in the absence or presence of the different agents (see Results or figure legends). Treatments were stopped by fixation and permeabilization as described above. The trafficked antibodies were labeled with Cy3- or FITC-conjugated goat anti–rabbit or anti–mouse secondary antibodies (5–10 μg/ml) and visualized by indirect immunofluorescence.
Electron Microscopy
The transcytosis assay was performed as described above with anti–5′NT antibodies (20–50 μg/ml) directly conjugated to 5-nM gold particles (Slot and Slot, 1985). The cells were fixed and processed in situ (on the glass coverslip) using standard Epon embedding techniques (Ihrke et al., 1998). Ultrathin sections were cut parallel to the growth substrate, stained with lead citrate, visualized, and photographed with an EM10 transmission electron microscope (Carl Zeiss, Inc.).
Imaging
The cells and perfused liver sections were visualized by epifluorescence (Axioplan Universal Microscope; Carl Zeiss, Inc.). Pictures were taken and processed using standard photographic techniques. Scanned images of the photographic prints were compiled, and figures prepared using Adobe Photoshop and Microsoft PowerPoint software. For Fig. 4, WIF-B immunofluorescence was analyzed using a laser scanning confocal microscope (LSCM 410; Carl Zeiss, Inc.).
Figure 4.
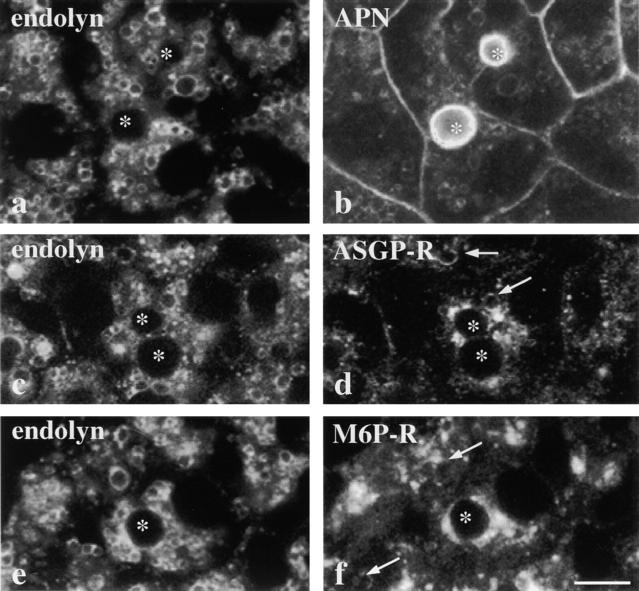
The apical plasma membrane proteins accumulate in vacuoles that also contain resident lysosomal membrane proteins. Cells were treated for 180 min with 100 nM wortmannin, fixed, permeabilized, and processed for indirect immunofluorescence. Confocal images of cells double labeled for endolyn (a) and APN (b), endolyn (c) and ASGP-R (d), or endolyn (e) and M6P-R (f) are shown. *Bile canaliculi. Arrows are pointing to vacuoles that contain ASGP-R (d) or M6P-R (f). The APN image was overexposed to enhance vacuolar visibility. Bar, 10 μm.
Results
PI 3-Kinase Inhibitors Alter the Steady State Distributions of Apical Plasma Membrane Proteins in WIF-B Cells
Our initial approach to examine the effects of the selective inhibitors, wortmannin and LY294002, on apical plasma membrane dynamics was to determine the steady state distributions of apical membrane proteins in WIF-B cells in treated cells. As observed for other cell types, these agents led to extensive vacuolation (compare Fig. 1 a with c, e, and g). By indirect immunofluorescence, we observed that the apical membrane proteins, 5′NT and APN, accumulated in vacuoles after treatment with 100 nM wortmannin or 200 μM LY294002 (compare Fig. 1 b with d, f, and h). Similar patterns of redistribution were observed for dipeptidyl peptidase IV, another apical membrane protein (data not shown). Vacuole size in cells treated with LY294002 was consistently smaller than observed in wortmannin-treated cells for all markers examined. This may reflect differences in the inhibitory mechanisms of these agents or the decreased potency of LY294002 (Vlahos et al., 1993; Ui et al., 1995).
Figure 1.
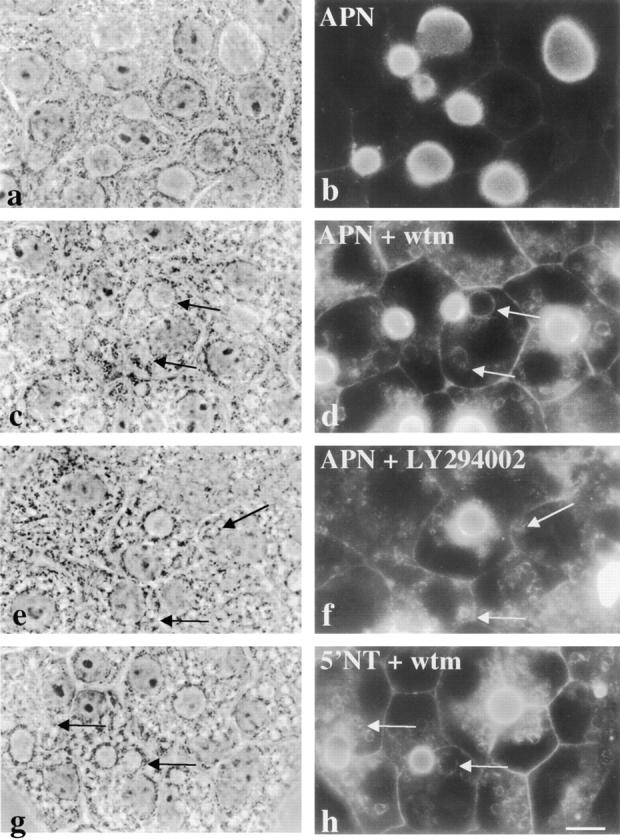
Apical plasma membrane proteins accumulate in vacuoles in the presence of wortmannin (wtm) or LY294002. WIF-B cells were incubated for 180 min in the absence (a and b) or presence (c, d, g, and h) of 100 nM wortmannin or 200 μM LY294002 (e and f). Cells were fixed, permeabilized and stained for APN (b, d, and f) or 5′NT (h). Arrows point to vacuoles containing intracellular apical proteins. Fluorescent images were intentionally overexposed so that vacuolar staining was more visible. The control staining in b was overexposed to the same extent as treated cells. 5′NT staining in control cells is indistinguishable from that of APN shown in a. Bar, 10 μm.
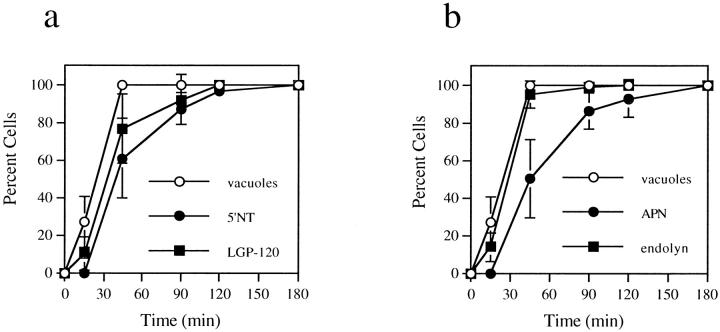
Vacuole formation and accumulation of the apical proteins into vacuoles were both time dependent, with vacuole formation preceding redistribution (Fig. 2, a and b). By phase microscopy, vacuoles were observed within 15 min of treatment and, by 45 min, all cells contained numerous, large vacuoles (Fig. 2, a and b). In contrast, apical proteins were first faintly detected in vacuoles after 45 min of treatment and did not reach their peak redistribution until after 120–180 min, when all cells were positive for vacuole staining (Fig. 2, a and b). The vacuoles containing apical proteins increased in diameter as wortmannin treatment was prolonged. At 45 min, apical protein-positive vacuoles were ∼1.9 ± 0.5 μm in diameter, whereas by 180 min, the diameter had increased to an average of 3.1 ± 0.9 μm (Table I). Both the increase in vacuole size and gradual accumulation of apical protein staining suggest the inhibitors allowed forward traffic into vacuoles, but blocked transport from them. Alternatively, the inhibitors may have altered the relative kinetics of membrane traffic to and from the vacuole.
Figure 2.
Vacuole formation precedes appearance of apical membrane proteins in vacuoles. WIF-B cells were treated for the indicated times with 100 nM wortmannin, fixed, permeabilized, and double labeled for APN and endolyn (a) or 5′NT and LGP-120 (b). Random phase and fluorescent images were collected for each time point. From phase images, the number of cells containing vacuoles was counted. Vacuoles were defined as the large, spherical intracellular structures induced by wortmannin treatment. From fluorescent images, cells containing vacuoles that stained positive for resident apical membrane (APN or 5′NT) or resident lysosomal membrane proteins (endolyn or LGP-120) were counted. The plotted values are averages from three experiments ± SD.
Table I.
The Diameter of Vacuoles that Contain Resident Apical Plasma Membrane Proteins Increases with Prolonged Wortmannin Treatment
| Time | Vacuole size | |
|---|---|---|
| min | μm | |
| 0 | 0.0 ± 0.0 | |
| 15 | 0.0 ± 0.0 | |
| 45 | 1.9 ± 0.5 | |
| 90 | 2.2 ± 0.6 | |
| 120 | 2.7 ± 0.8 | |
| 180 | 3.1 ± 0.9 |
WIF-B cells were treated with 100 nM wortmannin for the indicated times at 37°C. The cells were fixed, permeabilized, and processed for indirect immunofluorescent detection of APN or 5′NT. The diameters of vacuoles containing the apical proteins were measured. For each time point, fluorescent images were digitally captured (350× magnification) from 8–10 random fields and vacuoles counted. In general, two to three vacuoles per cell were measured and, for each time point, 104 vacuoles were analyzed. Only polarized cells were included in the analysis. Approximate numbers of vacuoles per cell remained the same during wortmannin treatment. Values are expressed as the mean ± SD. Measurements were performed on three experiments.
The wortmannin concentration used in this and many other published studies was 100 nM. At higher concentrations, wortmannin can inhibit other enzymes, including myosin light chain kinase and PI 4-kinase (Ui et al., 1995). To determine whether the effects described here were due to specific inhibition of PI 3-kinase isoforms, we incubated WIF-B cells with varying concentrations of wortmannin. The extents and rates of accumulation of the apical proteins and vacuole formation were similar in cells treated with wortmannin concentrations ranging from 10 nM to 6 μM (data not shown). Also, increased times of treatment with either agent (up to 10 h) did not lead to visibly greater extents of accumulation (see Discussion).
Not all apical membrane proteins accumulated in vacuoles. Although the cells were heavily vacuolated after 180 min of treatment, both HA4 (Fig. 3 a) and syntaxin 3 (data not shown) maintained their apical distributions. This suggests that wortmannin and LY294002 were selectively perturbing protein trafficking rather than generally disrupting apical membrane integrity. Interestingly, the distributions of the resident basolateral plasma membrane proteins HA321 (Fig. 3 b) and CE9 (data not shown) did not change in the presence of either inhibitor, indicating the effect was specific to proteins at the apical surface. In addition, the staining patterns of the tight junction component, ZO-1, were not changed in the presence of wortmannin (data not shown), suggesting tight junctions remained intact during treatment. Together, these data indicate that plasma membrane polarity was not compromised in treated cells.
Figure 3.
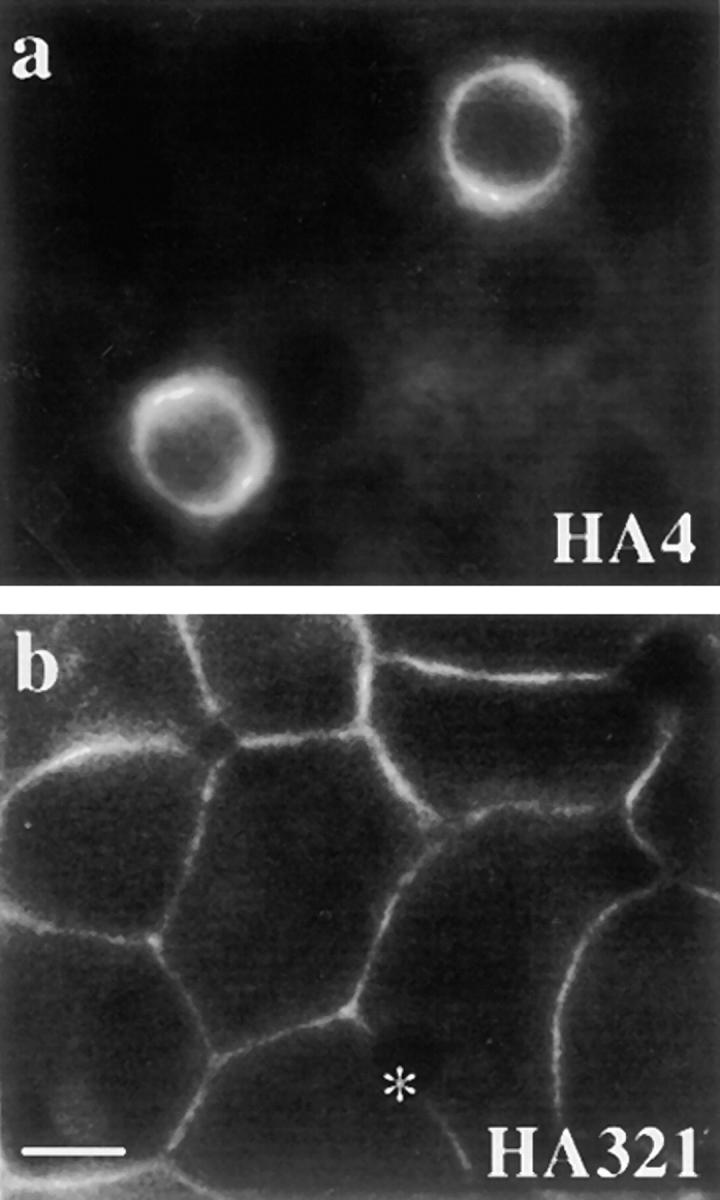
Plasma membrane polarity is maintained in the presence of wortmannin. WIF-B cells were treated for 180 min with 100 nM wortmannin. The cells were fixed, permeabilized, and processed for indirect immunofluorescence. HA4 staining (a) was detected at the apical membrane with no intracellular vacuolar staining and HA321 staining (b) detected only at the basolateral. Phase images of these cells were indistinguishable from those shown in Fig. 1, d, f, and h. *Bile canalicular space in b. Overexposure of images as in Fig. 1 revealed no vacuolar staining. Bar, 10 μm.
Identification of the Vacuoles as Lysosomal
We next determined what other molecules were present in the vacuoles containing apical proteins. The distributions of TGN-38, p115, and albumin (a major hepatic secretory protein) were unaltered by wortmannin treatment (data not shown). This is in agreement with findings from other cell types that wortmannin does not affect organelles or transport steps of the biosynthetic pathway (Brown et al., 1995; Hansen et al., 1995; Martys et al., 1996; Reaves et al., 1996; Shpetner et al., 1996). However, disruption of endocytic compartments has been reported in nonpolarized cells. In particular, the formation of large intracellular vacuoles derived from early and late endosomes and lysosomes has been observed (Brown et al., 1995; Reaves et al., 1996; Shepherd et al., 1996; Shpetner et al., 1996; Spiro et al., 1996; Fernandez-Borja et al., 1999). We examined whether wortmannin induced similar morphological changes in WIF-B cells. For these experiments, cells were treated for 180 min with 100 nM wortmannin and the steady state distributions of three recycling membrane proteins, ASGP-R (an early endosomal membrane protein), M6P-R (a late endosomal membrane protein) and endolyn-78 (a lysosomal membrane protein), were examined by confocal microscopy. The most notable vacuolation was observed when cells were stained for endolyn-78 (Fig. 4, a, c, and e) or LGP-120 (data not shown). Unlike reports from other cell types, few vacuoles contained early or late endosomal markers (here, ASGP-R or M6P-R; Fig. 4, d and f). Rather, these markers were primarily found in intracellular structures that did not significantly overlap with the endolyn-78 positive structures. These results indicate that wortmannin was disrupting mainly lysosomal compartments in WIF-B cells.
Comparison of APN and endolyn-78 staining patterns revealed striking colocalization (Fig. 4, a and b). After 180 min of wortmannin treatment, all cells contained vacuoles that were positive for both APN and endolyn-78 (Fig. 4, a and b). Similar results were obtained when 5′NT distributions were compared with those of another resident lysosomal membrane protein, LGP-120 (data not shown). The kinetics of appearance of endolyn or LGP-120 into vacuoles generally paralleled those of vacuole formation itself and preceded the appearance of apical plasma membrane proteins (Fig. 2, a and b). By 45 min, the majority of cells examined contained vacuoles that were positive for endolyn-78 and LGP-120 and, by 120 min, redistribution was complete. The appearance of LGP-120 in vacuoles consistently trailed the appearance of endolyn-78. Whether this reflects differences in their cellular itineraries or kinetics (Ihrke et al., 1998) is not known. From these data, we conclude that the apical plasma membrane proteins accumulated in lysosomally derived vacuoles in the presence of PI 3-kinase inhibitors.
The Apical Plasma Membrane Proteins Observed in Vacuoles Originate from the Apical Cell Surface
The apical proteins found in vacuoles could have originated from three possible sources: from the biosynthetic pathway, the transcytotic pathway, or from the apical plasma membrane itself. To rule out a biosynthetic origin, WIF-B cells were pretreated in the absence or presence of 25 μg/ml cycloheximide for 60 min to inhibit protein synthesis. Cells were further incubated for 180 min with 100 nM wortmannin in the continued absence or presence of cycloheximide. Cells treated with wortmannin alone or with cycloheximide and wortmannin achieved the same levels of vacuole formation (100% positive cells) and 5′NT vacuole redistribution (92 and 93% positive cells, respectively). Cells treated with cycloheximide alone were not observed to vacuolate, nor did apical plasma membrane protein distributions change. Similar results were obtained for APN, further indicating that newly synthesized apical proteins were not the source of apical molecules accumulating in vacuoles.
We next investigated the possibility that the apical membrane proteins observed in vacuoles originated from the transcytotic pathway. For these experiments, WIF-B cells were pretreated in the absence or presence of 100 nM wortmannin for 15 min at 37°C. Transcytosis of apical proteins was examined after their labeling with specific antibodies at 4°C. Since the antibodies were excluded from the apical plasma membrane by tight junctions, only those molecules en route from the basolateral plasma membrane were labeled (Ihrke et al., 1998; and see Fig. 5, a and d). After extensive washing, the cells were warmed to 37°C and the antibody–antigen complexes chased for the indicated times. The cells were fixed and permeabilized and the trafficked antibody–antigen complexes were visualized with secondary antibodies.
Figure 5.
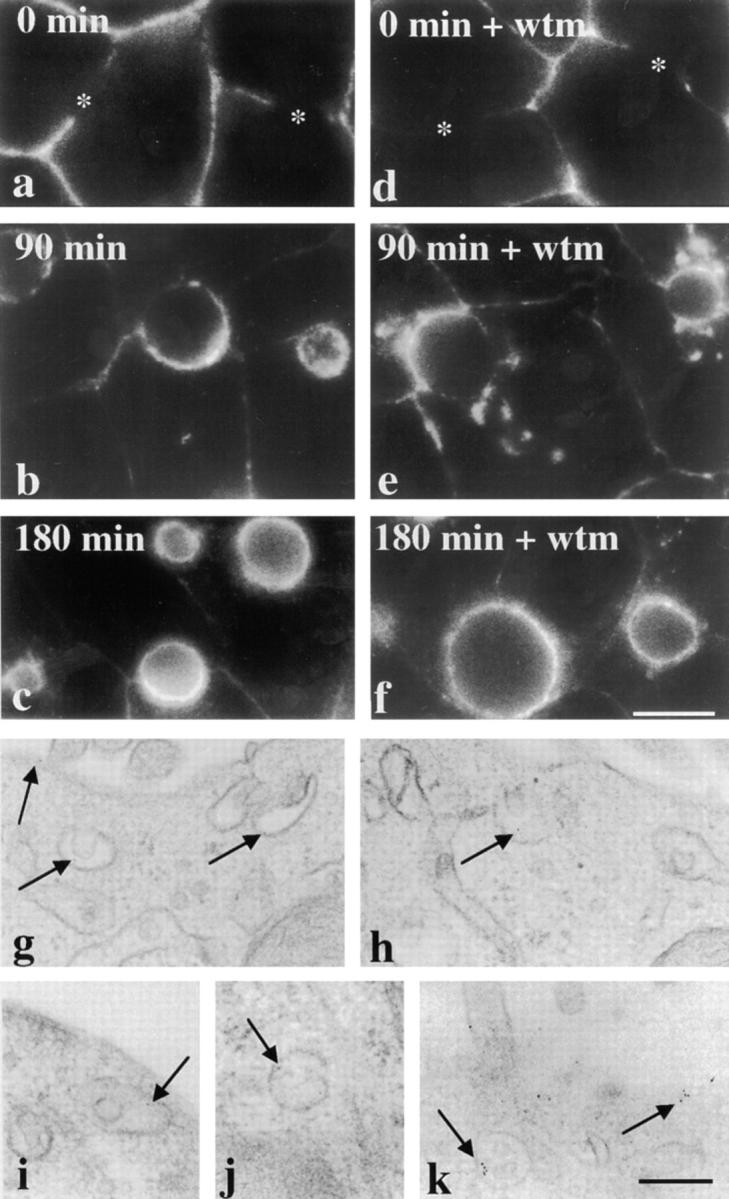
Transcytosing apical plasma membrane proteins bypass vacuoles. (a–f) Cells were pretreated for 15 min in the absence or presence of 100 nM wortmannin (wtm) at 37°C. The cells were chilled to 4°C and labeled with anti–5′NT antibodies for 15 min. After extensive washing, the cells were either fixed and permeabilized directly (0 min of chase; a and d) or placed at 37°C and the antibodies chased for 90 (b and e) or 180 (c and f) min in the continued absence or presence of 100 nM wortmannin. After chase, the cells were fixed, permeabilized, and the trafficked antibodies detected with cy3-conjugated secondary antibodies. The control experiment is shown in a–c and wortmannin-treated cells are shown in d–f. Asterisks in a and d point to unlabeled bile canalicular domains at 0 min of chase in both control and treated cells. Bar, 10 μm. (g–k) Cells were pretreated and labeled as described above, except anti–5′NT antibodies were directly conjugated to 5-nm gold particles. The antibodies were chased for 90 min in the continued absence (g and h) or presence (i–k) of 100 nM wortmannin and processed for electron microscopic visualization (see Methods). Arrows are pointing to gold particles located at the apical cell surface or in the SAC in both control and treated cells. Bar, 250 nm.
After 90 min in control cells, the majority of 5′NT staining was observed at or near the apical plasma membrane and, after 180 min, nearly all transcytosing 5′NT was at the apical surface signaling its successful delivery (Fig. 5, a–c, and see the ultrastructural analysis, g and h). Similarly, in wortmannin-treated cells, 5′NT was also successfully delivered to the apical plasma membrane by 180 min. However, we observed more intracellular accumulation of the trafficked molecule after 90 min (Fig. 5 e). Similar results were obtained for APN and HA4 in both control and wortmannin- or LY294002-treated cells (data not shown). We also monitored the dynamics of the resident basolateral protein, CE9, using the antibody-labeling and trafficking approach. In wortmannin-treated cells, CE9 maintained its basolateral distributions (data not shown), further indicating that PI 3-kinases differentially regulate resident proteins at each domain.
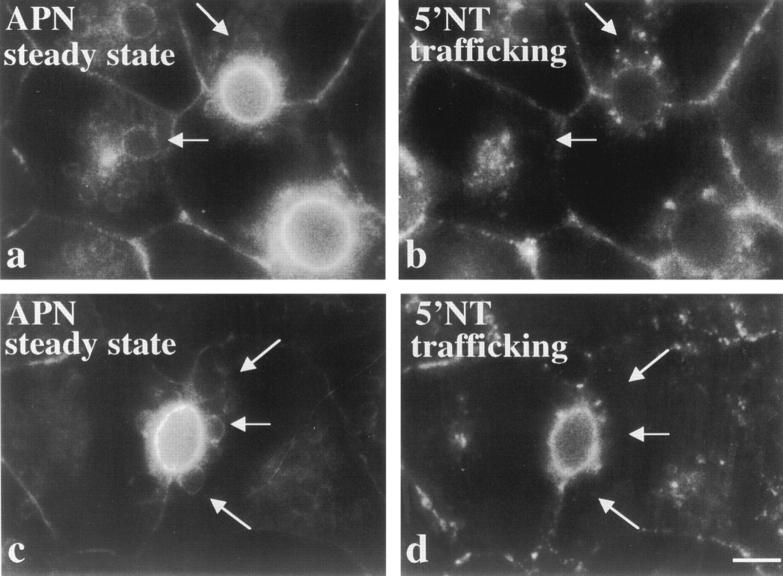
Because only a 15-min wortmannin pretreatment was used in these experiments, we needed to rule out the possibility that transcytosing molecules were transported past a potential point of diversion before vacuoles were fully formed. Therefore, we pretreated cells for 120 min with wortmannin before antibody labeling of live cells. As shown in Fig. 6, transcytosis remained fully functional. We observed no further impairment of 5′NT delivery to the apical surface and no increased intracellular staining relative to cells pretreated for only 15 min. Importantly, we also determined the steady state distributions of APN in the same cells to determine if transcytosing 5′NT molecules appeared in vacuoles. Comparison of the staining patterns in Fig. 6, a and b, and c and d, reveals that the structures containing trafficking 5′NT were distinct from vacuoles. Some 5′NT molecules were found in discrete structures surrounding the APN-positive vacuoles, but the patterns never overlapped.
Figure 6.
Longer pretreatment of cells with wortmannin does not lead to the vacuolar accumulation of transcytosing apical plasma membrane proteins. WIF-B cells were pretreated with 100 nM wortmannin for 120 min at 37°C, subsequently chilled to 4°C, and labeled with anti–5′NT antibodies. After extensive washing the cells were returned to 37°C and the antibodies chased for 60 (a and b) or 120 (c and d) min. Anti–5′NT antibodies were detected with cy3-conjugated secondary antibodies (b and d). The corresponding steady state APN distributions were determined in the same cells (a and c). Arrows point to vacuoles that contain APN in a and c and the corresponding areas in b and d. Note the lack of colocalization between the steady state APN labeling and the trafficking 5′NT molecules. Bar, 10 μm.
These results revealed that basolateral-to-apical transcytosing molecules in wortmannin-treated cells did not accumulate in vacuoles, but seemed to traverse similar membrane compartments (albeit displaced or delayed) as in control cells. Therefore, we turned to ultrastructural analysis of antibody-antigen complexes in wortmannin-treated cells to further examine the compartments through which the molecules were traveling. In particular, we were interested in determining whether 5′NT traveled through the SAC, a known intermediate of the transcytotic pathway in hepatocytes (Barr and Hubbard, 1993; Barr et al., 1995; Ihrke et al., 1998). As shown in Fig. 5, gold-conjugated anti–5′NT antibodies were detected at the apical surface in both control and treated cells after 90 min of trafficking. As predicted, gold particles were also observed just adjacent to the apical cell surface in the SAC. Examination of the SAC in control and treated cells revealed no significant differences in its placement, size, or number (Table II). These data indicate that the SAC remained functional during treatment and further show that the intracellular structures containing trafficking 5′NT in wortmannin-treated cells were not an altered SAC. The identity of the compartment containing trafficking 5′NT in treated cells is not known, but may likely be an altered early endosomal compartment, another known intermediate of the transcytotic pathway in hepatocytes (Barr and Hubbard, 1993; Barr et al., 1995; Ihrke et al., 1998). Preliminary studies revealed that these structures stained positive for ASGP-R (data not shown). Also, when 5′NT and ASGP-R were trafficked together and visualized, both molecules traversed a similar compartment (data not shown), presumably an early endosome.
Table II.
Wortmannin Does Not Alter the Morphology or Function of the SAC
| Time | Diameter of SAC containing transcytosing 5′NT | Gold positive structures per 10 μm apical plasma membrane | ||||||
|---|---|---|---|---|---|---|---|---|
| −wtm | +wtm | −wtm | +wtm | |||||
| min | μm | μm | ||||||
| 0 | — | — | — | — | ||||
| 90 | 0.17 ± 0.08 | 0.16 ± 0.03 | 0.9 | 1.1 | ||||
| 180 | 0.18 ± 0.1 | 0.17 ± 0.1 | 0.8 | 1.0 | ||||
Cells were labeled with gold-conjugated anti–5′NT antibodies for 15 min at 4°C. After extensive washing, the antibodies were chased for the indicated times in the presence or absence of wortmannin. The cells were fixed, processed, and analyzed as described in Materials and Methods. The diameters of gold-positive structures within 1 μm of the bile canalicular membrane were measured. If a structure was asymmetric in shape, the longest axis was measured. In general, the gold-positive structures were amorphous or cup-shaped and small (<200 nm). After 0 min of chase, no intracellular gold was identified. To calculate the number of gold-positive structures per 10-μm length of apical plasma membrane, data from three experiments were pooled and are presented as a single value. Measurements of organelle diameter were performed on the same three experiments and values are expressed as the mean ± SD. For each condition, between 12 and 33 bile canaliculi and between 135 and 476 μm of apical plasma membrane were examined.
To directly determine whether the resident apical plasma membrane proteins in vacuoles originated from the apical cell surface, we examined the trafficking of antigen–antibody complexes that were first delivered to the apical surface. For this analysis, APN or 5′NT molecules were labeled at the basolateral surface at 4°C, chased to the apical membrane for several hours at 37°C, chased an additional 2 h in the presence or absence of wortmannin, and labeled with secondary antibodies for immunofluorescent detection. As shown in Fig. 7, b and h, nearly all the labeled APN or 5′NT molecules were at the apical surface in control cells. After the final 2 h chase in the presence of wortmannin, APN and 5′NT staining was observed in vacuoles (Fig. 7, d, f, and j). These data indicate that the apical membrane proteins found in vacuoles originate from the apical surface. This is the first direct evidence for internalization of membrane proteins from the hepatic apical cell surface.
Figure 7.
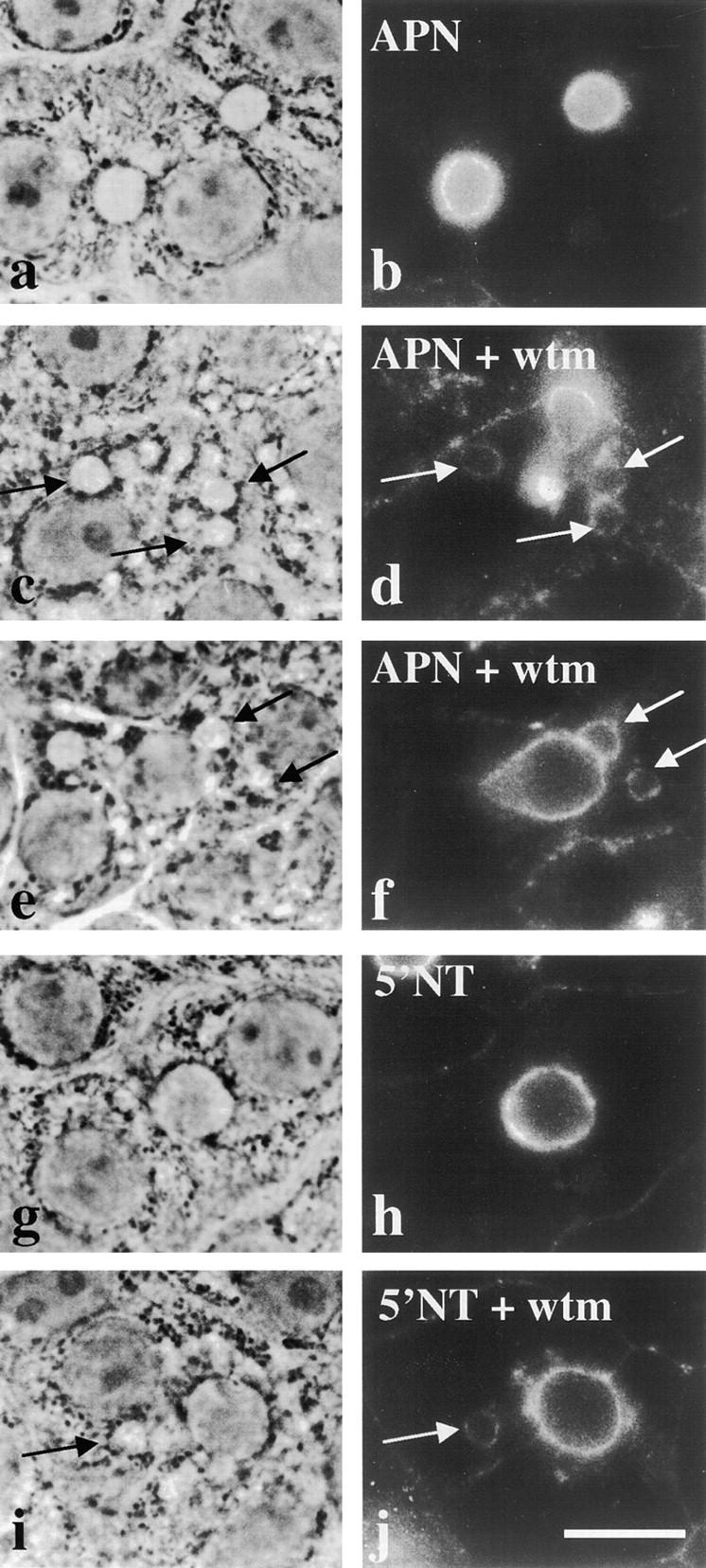
The apical proteins accumulated in vacuoles originate from the apical cell surface. WIF-B cells were chilled to 4°C and labeled with anti–APN (a–f) or anti–5′NT antibodies (g–h). After extensive washing, the cells were returned to 37°C and the antibodies allowed to chase for 5 h (in the case of APN antibodies) or 3 h (for 5′NT antibodies). Cells were then incubated in the absence (a, b, g, and h) or presence (c, d, i, and j) of 100 nM wortmannin (wtm) for an additional 2 h. Cells were fixed, permeabilized, and labeled with secondary antibodies. The corresponding phase images are shown in the left-hand column. In b and h, no vacuolar staining is observed in untreated cells. Arrows are pointing to vacuoles that stained positive for apical plasma membrane proteins after the additional incubation with wortmannin in c–f and i–j. Bar, 10 μm.
Characteristics of Intracellular Accumulation of Apical Plasma Membrane Proteins
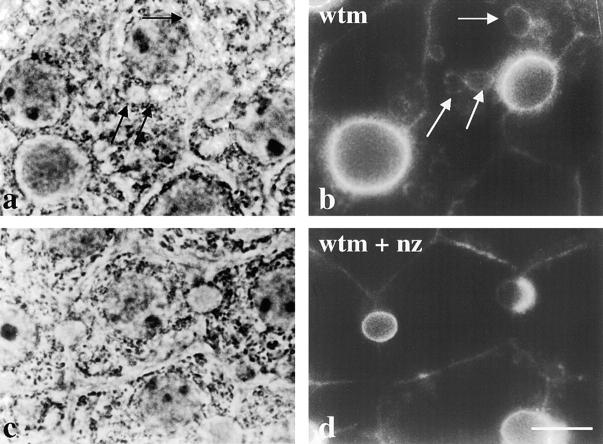
To further characterize the mechanism(s) by which resident apical membrane proteins were internalized and accumulated intracellularly, we examined the effect of cytoskeletal disruption on redistribution. Microtubules were depolymerized by addition of 33 μM nocodazole for 1 h at 37°C (as assayed by β-tubulin staining, data not shown). The cells were further incubated for 180 min with wortmannin in the continued absence or presence of nocodazole. As shown in Fig. 8, the addition of nocodazole prevented vacuole formation, further implying that vacuolar compartments were enlarged due to accumulated cargo and membrane received from vesicular intermediates. Consistent with this conclusion, microtubule disruption also prevented any detectable intracellular accumulation of APN (Fig. 8 d). Similar experiments were performed using 1 μM cytochalasin D to disrupt the actin cytoskeletal network. Although actin filaments were depolymerized, no changes in the kinetics of vacuole formation or apical protein redistribution were observed (data not shown).
Figure 8.
Vacuolar formation and apical redistribution are microtubule-dependent processes. WIF-B cells were treated for 60 min in the absence (a and b) or presence (c and d) of 33 μM nocodazole (nz) to depolymerize microtubules. Cells were then incubated with 100 nM wortmannin (wtm) in the continued absence (a and b) or presence (c and d) of nocodazole. Cells were fixed, permeabilized, and processed for indirect immunofluorescent detection of APN steady state distributions. The corresponding phase images are shown. Arrows in a and b point to vacuoles that are positive for APN staining. In c and d, vacuole formation and redistribution of APN are inhibited by nocodazole treatment. Bar, 10 μm.
Apical Plasma Membrane Proteins also Redistribute into Vacuoles in Hepatocytes In Situ and MDCK Cells
Because the effects of PI 3-kinase inhibitors on protein trafficking patterns and cellular morphology have been examined only in cultured cells, it was important to rule out the possibility of a tissue culture–induced effect by the inhibitors. Therefore, we treated hepatocytes in isolated perfused livers with 2 μM wortmannin for 180 min. We observed hepatocyte vacuolation with positive staining for APN (Fig. 9 b') and 5′NT (data not shown). As observed for WIF-B cells, the vacuoles also contained the resident lysosomal membrane proteins, endolyn-78 (Fig. 9 b') and LGP-120 (data not shown). HA321 and HA4 distributions did not change in treated livers, indicating that similar wortmannin-sensitive transport steps were operating in intact hepatocytes as in WIF-B cells (Fig. 9, d and f). These results indicate that regulation of apical internalization by wortmannin-sensitive molecules occurs in the intact liver.
Figure 9.
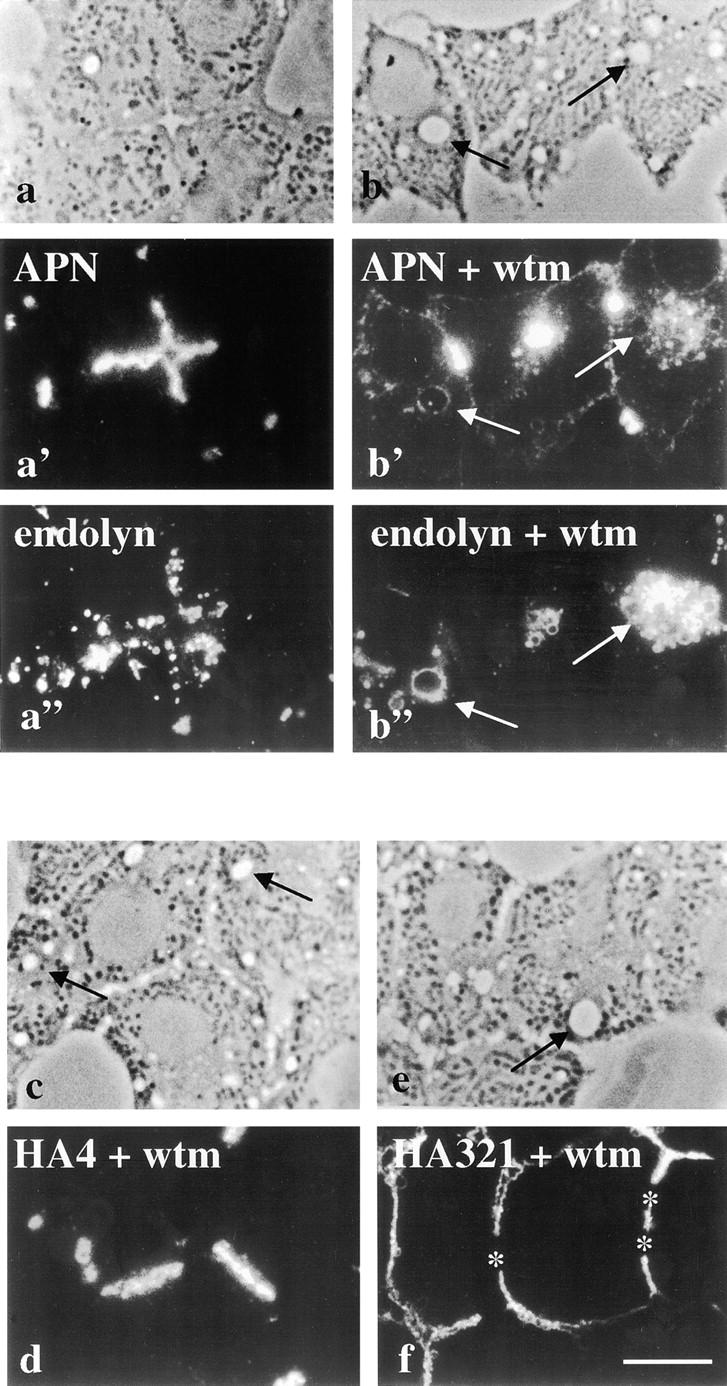
Apical plasma membrane proteins redistribute to lysosomally derived vacuoles in intact liver hepatocytes. Intact livers were perfused in the absence or presence of 2 μM wortmannin (wtm) and 50 μM leupeptin for 180 min at 37°C. Biopsies were removed and fixed by immersion. Semi-thin sections (0.5 μm) were cut and processed for indirect immunofluorescence. Control perfused liver sections (a–a”) and wortmannin-perfused sections (b–b”) are shown. APN staining is shown (a' and b') with corresponding double-labeled images for endolyn (a” and b”). Phase images for both conditions are shown at the top of each column. HA4 (c) and HA321 (f) staining patterns are shown as single labels with corresponding phase images. Arrows in a–a” and b–b” point to vacuoles that are visible by phase and are positive for both APN and endolyn staining. Arrows in c and e point to vacuoles with no detectable HA4 or HA321 staining. The image showing APN staining in treated hepatocytes (b') was overexposed to enhance vacuolar staining visualization. Bar, 10 μm.
To determine whether vacuolar accumulation of apical membrane proteins is a phenomenon specific to hepatocytes, we examined the effects of wortmannin and LY294002 on the distributions of membrane proteins in MDCK cells. As shown in Fig. 10, wortmannin induced the formation of large vacuoles that also stained positive for the endogenous resident apical membrane protein, 3F2 (compare Fig. 10, c with d). The distribution of the basolateral resident membrane protein, G12, remained unaltered by wortmannin treatment (data not shown). The kinetics of vacuolar formation and apical protein redistribution in MDCK cells were similar to those observed in WIF-B cells. Within 30 min, all cells contained numerous large vacuoles (Fig. 10 e). Vacuolar 3F2 staining was observed after 30 min of treatment in ∼50% of cells and, by 120 min, nearly all cells examined contained vacuoles positive for 3F2 (Fig. 10 e). These results indicate that PI 3-kinase is regulating the dynamics of resident plasma membrane proteins in MDCK cells and suggest that similar processes may be operating in other polarized epithelial cell types.
Figure 10.
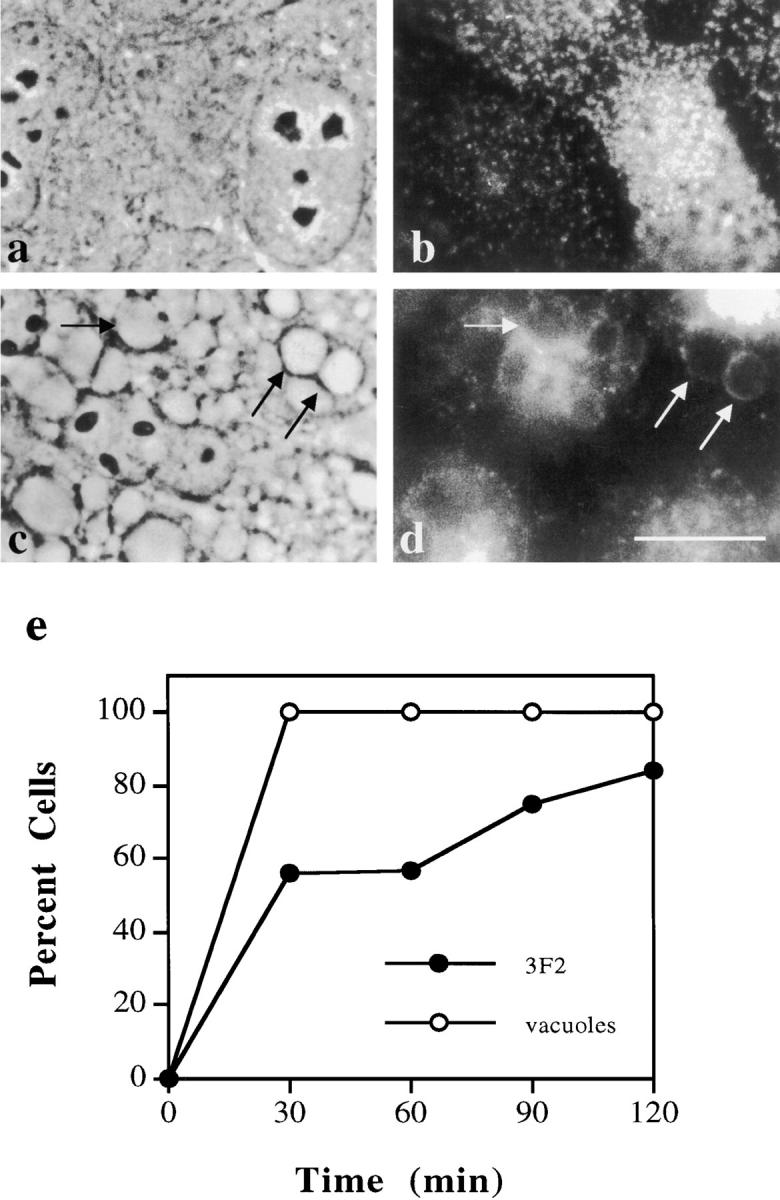
Apical plasma membrane proteins in MDCK cells accumulate in vacuoles in the presence of wortmannin. (a–d) MDCK cells were treated in the absence (a and b) or presence (c and d) of 2 μM wortmannin and 50 μM leupeptin for 120 min at 37°C. Cells were fixed, permeabilized, and processed for indirect immunofluorescent detection of the apical plasma membrane protein, 3F2 (b and d). The corresponding phase images are shown (a and c). The nuclear focal plane is shown in a and the cell surface focal plane is shown in b. Arrows point to vacuoles that contain 3F2 in wortmannin-treated cells. Bar, 10 μm. (e) Vacuole formation and appearance of positive 3F2 vacuole staining was measured as described in Fig. 2. The average of two experiments is plotted for each time point.
Discussion
Using a pharmacological approach, we have documented for the first time the internalization of apical membrane proteins from the canalicular surface of hepatocytes. When the PI 3-kinase inhibitors, wortmannin and LY294002, were applied to WIF-B cells or intact liver hepatocytes, large lysosomal vacuoles formed that contained apical plasma membrane proteins. Residents of other organelles, including the basolateral plasma membrane and early or late endosomes were completely or largely absent from these structures. The source of the apical membrane proteins was the apical plasma membrane itself.
Our study has several important implications. First, our finding that PI 3-kinase inhibitors affect apical endocytosis differently from basolateral endocytosis in both MDCK and hepatic cells adds to the list of differences between endocytic properties of the two plasma membrane domains in epithelial cells. Secondly and unexpectedly, the SAC, which we identified both in vivo and in WIF-B cells as an intermediate in the basolateral-to-apical transcytosis of apical proteins, is not involved in apical endocytosis. We know this because the plasma membrane proteins found in vacuoles did not traverse the SAC. Furthermore, PI 3-kinase inhibitors have little or no effect on SAC's basolateral-to-apical function or morphology. Thus, the results of this study support our earlier hypothesis that apical plasma membrane proteins present in SAC are delivered exclusively to the apical membrane even though other SAC cargo (i.e., endolyn-78) can be sorted off to other destinations (Ihrke et al., 1998). Finally, since our results show that the life cycles of selected resident apical membrane proteins include delivery to lysosomes, we propose that degradation in this organelle is the normal fate of such membrane proteins.
Identification of the PI 3-Kinase Isoform Involved
Mammalian cells, including hepatocytes, encode at least three different classes of PI 3-kinase isoforms (for review, see Carpenter and Cantley, 1996; Shepherd et al., 1996; Toker and Cantley, 1997; Vanhaesebroeck et al., 1997). Class I includes the catalytic 110-kD subunits complexed with other accessory proteins. Class II PI 3-kinases include higher molecular weight kinases that contain C2 domains, and class III kinases share the highest sequence similarity with the sole isoform identified in yeast, Vps34p. All mammalian PI 3-kinase isoforms are sensitive to wortmannin and LY294002 at the concentrations used in the studies described here and elsewhere (Shepherd et al., 1996; Vanhaesebroeck et al., 1997; Corvera and Czech, 1998). This has led to ambiguity in distinguishing the roles that specific PI 3-kinases play in membrane transport.
Despite the present uncertainties in a pharmacological approach, a picture emerging from studies in multiple cell types identifies the lysosomal system as the principal target of PI 3-kinase regulation. Yeast strains deficient in the sole PI 3-kinase isoform, Vps34p, missort lysosomal hydrolases from the trans-Golgi network to the extracellular medium (Herman and Emr, 1990; Herman et al., 1992). Recently, a mammalian homologue of this enzyme has been implicated in both transferrin recycling and delivery of activated PDGF receptor to lysosomes, pointing to its role in endosomal sorting/retrieval (Siddhanta et al., 1998). However, others have reported that a p110p85 isoform (Class I), traditionally viewed as a mediator of signal transduction (Carpenter and Cantley, 1996; Toker et al., 1997; Vanhaesebroeck et al., 1997), also influences transferrin recycling and PDGF receptor delivery to lysosomes (Siddhanta et al., 1998; Joly et al., 1994). Equally intriguing is the report that Vps34p, and by inference membrane transport, may play a role in mitogenesis (Siddhanta et al., 1998). Clearly, we need specific probes to dissect these complex and interacting pathways.
Our understanding of how PI 3-kinases regulate vesicle trafficking in mammalian cells is also in its infancy. Several proteins with roles in membrane transport have been shown to bind PI 3-kinase catalytic products (phosphatidylinositol 3 phosphate (PtdIns(3)P), PtdIns(3,4)P2, and PtdIns(3,4,5)P3) in vitro including dynamin, the clathrin adaptor protein, AP2, GRP1, cytohesin-1, EEA1 and ARNO (De Camilli et al., 1996; Klarlund et al., 1997; Patki et al., 1997; Corvera and Czech, 1998). One possibility is that the inhibition of the formation of specific lipid species by wortmannin in turn inhibits the recruitment of these factors to cellular membranes where they function in membrane transport. In hepatocytes, the roles these proteins play (if any) in apical membrane internalization have not been identified. Alternatively, the lipids themselves may contribute to alterations in membrane physical properties that allow membrane transport to occur (e.g., fluidity, fusibility). Further experimentation is clearly needed to discriminate between these possibilities.
Apical and Basolateral Endocytosis Differ in Yet Another Way
Our finding that PI 3-kinase inhibitors differentially affect the behaviors of apical and basolateral membrane proteins in both WIF-B and MDCK cells is consistent with other pharmacological studies of endocytosis in polarized epithelial cells. For example, in MDCK cells, addition of calmodulin antagonists (W7 and trifluoperazine) or phorbol esters activated apical endocytosis of ricin, while no changes were seen at the basolateral domain (Llorente et al., 1996; Shurety et al., 1996). In Caco2 and MDCK cells, addition of cytochalasin D inhibited apical uptake of fluid phase markers and no changes were observed from the basolateral domain (Gottlieb et al., 1993; Jackman et al., 1994; Shurety et al., 1996). These differences, as well as those observed with PI 3-kinase inhibitors, may reflect the specialized functions and/or environments of each membrane domain.
Differential effects of wortmannin administration were also seen among apical plasma membrane proteins. APN and 5′NT (as reported here) and dipeptidyl peptidase IV and polymeric IgA receptor (Tuma, P.L., data not shown) all accumulated in lysosomal vacuoles in treated cells, whereas HA4 and syntaxin 3 maintained their apical distributions. No overt sequence or structural motifs are apparent that group the proteins in the two classes, nor are the half-lives of the proteins significantly different. Differences have been determined in the kinetics of delivery of newly synthesized proteins to the apical surface (Bartles et al., 1987; Schell et al., 1992). In particular, HA4 was found to be the slowest molecule with only 15–20% detected at the apical membrane after 2.5 h. Whether slower delivery also implies slower rates of internalization from the apical membrane and, by extension, decreased vacuolar accumulation, is not known. Further examination of these molecules in terms of lipid binding properties and specific internalization mechanisms (e.g., vesicle types, signals) are required before we understand the reasons for the selective regulation by PI 3-kinases.
Although wortmannin and LY294002 have been shown to impair transcytosis of dIgA and ricin in MDCK cells (Cardone and Mostov, 1995; Hansen et al., 1995), the effect of these agents on cellular morphology or the distributions of apical membrane proteins had not been examined before this report. Thus, our finding that MDCK cells mirror WIF-B cells in the regulation by PI 3-kinases leads us to predict that many of the apical regulatory mechanisms described for other polarized epithelial cells may be operating at the hepatocyte canalicular domain.
Apical Plasma Membrane Proteins Are Degraded in Lysosomes
Based on the results of this study, we propose that apical membrane proteins are degraded in lysosomes as part of their normal life cycle. While such a fate might have been expected, this study is the first to document accumulation of resident apical membrane proteins in a lysosomal compartment of polarized hepatic cells. These results are consistent with limited examples from polarized intestinal cells (Caco-2; Matter et al., 1990). Our proposal raises several questions. For example, why are the levels of apical proteins in the vacuoles so low? From fluorescent micrographs, we estimate that <5% of the apical proteins are found there after 120 min treatment with wortmannin. The subtlety of the effect is probably not due to degradation of a portion of the population since addition of lysosomal proteinase inhibitors, such as leupeptin, together with longer exposure of cells to wortmannin, did not significantly increase the signal. Rather, we favor an explanation that takes into account the apical proteins' relatively long half-lives, which are between 3 and 6 d in vivo (Scott and Hubbard, 1992). That is, if such long-lived molecules remain at the apical membrane until they are retrieved for degradation, only 1–3% of the total APN or dipeptidyl peptidase IV would be sorted to lysosomes in 120 min, a value consistent with our estimations. Despite the good agreement, we cannot rule out the formal possibility that wortmannin induced endocytosis at the apical domain. Interestingly, this model assumes that the apical proteins do not recycle through an intracellular compartment(s) as part of their normal life cycle. Alternatively, the recycling compartment is not sensitive to wortmannin.
Another question raised by our results is why there is any expression of apical proteins if the vacuoles are degradative. The ability to detect apical proteins in the vacuoles suggests that they have decreased degradative potential, consistent with their identification in other cells as prelysosomal intermediates. In treated WIF-B cells, cathepsin D (an acid hydrolase) staining was not observed in vacuoles, but in smaller, punctate structures, implying that mature lysosomes are not altered by wortmannin (Tuma, P.L., data not shown). Reports from NRK and human melanoma (Mel JuSo) cells have documented that dense core (and presumably end-stage) lysosomes were not changed by wortmannin with regard to their morphology (Brown et al., 1995; Reaves et al., 1996; Spiro et al., 1996; Fernandez-Borja et al., 1999) or their ability to receive internalized proteins and lipid dyes (Reaves et al., 1996; Shpetner et al., 1996; Fernandez-Borja et al., 1999). In addition, previous studies have shown that acid hydrolases are missorted to the constitutive secretory pathway in wortmannin-treated cells (Brown et al., 1995; Davidson, 1995; Martys et al., 1996). Thus, similar missorting may occur in hepatic cells such that the vacuoles have decreased hydrolytic activity.
A final puzzle focuses on the vacuoles themselves. If entry of apical membrane into this prelysosomal compartment continues but subsequent events are blocked by PI 3-kinase inhibition, resulting in an enlarged vacuole, what is the “normal” mechanism for maintaining the steady state dimensions of the compartment in untreated cells? A tempting hypothesis is that membrane microdomains invaginate into the luminal space and pinch off to form multivesicular endosomes/lysosomes. This scenario would accomplish both degradation of membrane components and maintenance of compartment size. However, there is limited experimental evidence for such an idea (Haigler et al., 1979; van Deurs et al., 1993; Futter et al., 1996).
SAC Is Not an Intermediate in the Delivery of Resident Apical Proteins to Lysosomes
Our recent studies of plasma membrane biogenesis have focused on the “indirect” route taken by newly synthesized apical membrane proteins and identified SAC as a subapical compartment in that route (Barr and Hubbard, 1993; Barr et al., 1995; Ihrke et al., 1998). It is important to emphasize that transcytosing apical proteins moved through the SAC and were successfully delivered to the apical surface in wortmannin-treated cells. Ultrastructural analysis confirmed these results and further revealed that the morphology, placement, and number of the subapical organelles were not changed by wortmannin. Thus, the function of the SAC as an intermediate in the transcytotic pathway was not changed. Most importantly, in the presence of wortmannin, the resident apical proteins did not travel through the SAC on their way to lysosomal vacuoles. This indicates that the SAC is not an intermediate of the apical endocytic pathway and is consistent with work in hepatocytes that biochemically identified the SAC and found it contained no recycling resident populations of apical membrane proteins (Barr and Hubbard, 1993; Barr et al., 1995). Previously, we proposed that the SAC is a “one-way” sorting station to the apical domain and the results presented here continue to support our hypothesis.
What is the entry point for those apical membrane residents that were subsequently found in the lysosomal vacuoles? Endosomal structures have been identified in other polarized cells that receive proteins internalized from the apical surface (Bomsel et al., 1989; Hughson and Hopkins, 1990; Apodaca et al., 1994; Barroso and Sztul, 1994). Morphologically, these compartments resemble the SAC in hepatic cells (Parton et al., 1989; Apodaca et al., 1994; Knight et al., 1995; Odorizzi et al., 1996). Since we are limited in our ability to access the apical surface, we have been unable to directly test whether the hepatic SAC acts similarly. However, there are recent reports of a subapically located structure in HepG2 cells receiving fluorescently labeled glycosphingolipids (C6-NBD-glucosylceramide and C6-NBD-sphingomyelin) that have been first accumulated at the apical surface (van IJzendoorn et al., 1997; van IJzendoorn and Hoekstra, 1998). Whether this compartment is identical to the SAC or if it receives apically internalized proteins is not currently known. Future experiments are needed to distinguish among the various possibilities and to identify the putative intracellular compartment that receives incoming cargo from the apical membrane.
Acknowledgments
We thank W. Dunn, P. Nissley, P. Luzio, and G. Ojakian for generously providing antibodies. We also thank the members of the Hubbard laboratory, Carolyn Machamer, and Anne Kenworthy for critically reading the manuscript and for many helpful comments.
This work was supported by the National Institutes of Health grants GM29185 and DK44375 awarded to A.L. Hubbard and fellowship DK09620 awarded to P.L. Tuma.
Abbreviations used in this paper
- 5′NT
5′nucleotidase
- APN
aminopeptidase N
- ASGP-R
asialoglycoprotein receptor
- LGP-120
120-kD lysosomal glycoprotein
- M6P-R
mannose 6-phosphate receptor
- PI
phosphoinositide
- SAC
subapical compartment
References
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr VA, Hubbard AL. Newly synthesized hepatocyte plasma membrane proteins are transported in transcytotic vesicles in the bile duct-ligated rat. Gastroenterology. 1993;105:554–571. doi: 10.1016/0016-5085(93)90734-t. [DOI] [PubMed] [Google Scholar]
- Barr VA, Scott LJ, Hubbard AL. Immunoadsorption of hepatic vesicles carrying newly synthesized dipeptidyl peptidase IV and polymeric IgA receptor. J Biol Chem. 1995;270:27834–27844. doi: 10.1074/jbc.270.46.27834. [DOI] [PubMed] [Google Scholar]
- Barroso M, Sztul E. Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G-sensitive passage through the apical endosome. J Cell Biol. 1994;124:83–100. doi: 10.1083/jcb.124.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles JR, Braiterman LT, Hubbard AL. Biochemical characterization of domain-specific glycoproteins of the rat hepatocyte plasma membrane. J Biol Chem. 1985;260:12792–12802. [PubMed] [Google Scholar]
- Bartles JR, Feracci HM, Stieger B, Hubbard AL. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathway taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987;105:1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles JR, Hubbard AL. Plasma membrane sorting in epithelial cells: do secretory pathways hold the key? . Trends Biochem Sci. 1988;13:181–184. doi: 10.1016/0968-0004(88)90147-8. [DOI] [PubMed] [Google Scholar]
- Blommart EFC, Krause U, Schellens JPM, Vreeling-Sindelarova H, Meijer AJ. The phosphatidyl 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated hepatocytes. Eur J Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Prydz K, Parton RG, Gruenberg J, Simons K. Endocytosis in filter-grown Madin-Darby canine kidney cells. J Cell Biol. 1989;109:3243–3258. doi: 10.1083/jcb.109.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WJ, DeWald DB, Emr SD, Plutner H, Balch WE. Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J Cell Biol. 1995;130:781–796. doi: 10.1083/jcb.130.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunskill NJ, Stuart J, Tobin AB, Walls J, Nahorski S. Receptor-mediated endocytosis of albumin by kidney proximal tubes is regulated by phosphatidylinositide 3-kinase. J Clin Invest. 1998;101:2140–2150. doi: 10.1172/JCI1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone M, Mostov K. Wortmannin inhibits transcytosis of dimeric IgA by the polymeric immunoglobulin receptor. FEBS Lett. 1995;376:74–76. doi: 10.1016/0014-5793(95)01251-8. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Cantley LC. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- Corvera S, Czech MP. Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol. 1998;8:442–446. doi: 10.1016/s0962-8924(98)01366-x. [DOI] [PubMed] [Google Scholar]
- Crawford AR, Smith AJ, Hatch VC, Oude RPJ, Elferink, Borst P, Crawford JM. Hepatic secretion of phospholipid vesicles in the mouse critically depends on mdr2 or MDR3 P-glycoprotein expression. J Clin Invest. 1997;100:2562–2567. doi: 10.1172/JCI119799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JM. Role of vesicle-mediated transporter pathways in hepatocellular bile secretion. Semin Liver Dis. 1996;16:169–189. doi: 10.1055/s-2007-1007230. [DOI] [PubMed] [Google Scholar]
- Davidson HW. Wortmannin causes mistargeting of procathespsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J Cell Biol. 1995;130:797–805. doi: 10.1083/jcb.130.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Emr SD, McPherson PS, Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1538. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- Dunn WA, Wall DA, Hubbard AL. Use of isolated perfused liver in studies of receptor mediated endocytosis. Methods Enzymol. 1983;98:225–241. doi: 10.1016/0076-6879(83)98151-x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, Dusseljee S, Neefijies J. Multivesicular body morphogenesis requires phosphatidylinositol 3-kinase activity. Curr Biol. 1999;9:55–58. doi: 10.1016/s0960-9822(99)80048-7. [DOI] [PubMed] [Google Scholar]
- Folli F, Alvaro D, Gigliozzi A, Bassotti C, Kahn CR, Pontiroli AE, Capocaccia L, Jezequel AM, Benedetti A. Regulation of endocytic-transcytotic pathways and bile secretion by phosphatidylinositol 3-kinase in rats. Gastroenterology. 1997;113:954–965. doi: 10.1016/s0016-5085(97)70192-6. [DOI] [PubMed] [Google Scholar]
- Fujita H, Tuma PL, Finnegan CM, Locco L, Hubbard AL. Endogenous syntaxins 2, 3, and 4 exhibit distinct but overlapping patterns of expression at the hepatocyte plasma membrane. Biochem J. 1998;329:527–538. doi: 10.1042/bj3290527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb TA, Ivanov IE, Adesnik M, Sabatini DA. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen AK, Van Wijland MJA, Fredricks WM, Smit JJM, Schinkel AH, Oude RJP, Elferink Regulation of protein secretion into bile: studies in mice with a disrupted mdr2 P-glycoprotein gene. Gastroenterology. 1995;109:1997–2006. doi: 10.1016/0016-5085(95)90768-8. [DOI] [PubMed] [Google Scholar]
- Haigler HT, McKanna JA, Cotien S. Direct visualization of the binding and internalization of epidermal growth factor in human carcinoma cells A431. J Cell Biol. 1979;81:382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SH, Olsson A, Casanova JE. Wortmannin, an inhibitor of phosphoinositide 3-kinase, inhibits transcytosis in polarized epithelial cells. J Biol Chem. 1995;270:28425–28432. doi: 10.1074/jbc.270.47.28425. [DOI] [PubMed] [Google Scholar]
- Herman PK, Emr SD. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. . Mol Cell Biol. 1990;10:6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman PK, Stack JH, Emr SD. An essential role for a protein and lipid kinase complex in secretory protein sorting. Trends Cell Biol. 1992;2:363–368. doi: 10.1016/0962-8924(92)90048-r. [DOI] [PubMed] [Google Scholar]
- Hughson HEJ, Hopkins CR. Endocytic pathways in polarized Caco-2 cells: identification of an endosomal compartment accessible from both apical and basolateral surfaces. J Cell Biol. 1990;110:337–348. doi: 10.1083/jcb.110.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard AL, Bartles JR, Braiterman LT. Identification of rat hepatocyte plasma membrane proteins using monoclonal antibodies. J Cell Biol. 1985;100:1115–1125. doi: 10.1083/jcb.100.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrke, G., and A.L. Hubbard. 1995. Control of vesicle traffic in hepatocytes. In Progress in Liver Diseases. J.L. Boyer and R.K. Ockner, editors. W.B. Saunders Co., Philadelphia, PA. 63–99. [PubMed]
- Ihrke G, Neufeld EB, Meads T, Shanks MR, Cassio D, Laurent M, Schroer TA, Pagano RE, Hubbard AL. WIF-B cells: an in vitro model for studies of hepatocyte polarity. J Cell Biol. 1993;123:1761–1775. doi: 10.1083/jcb.123.6.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrke I, Martin GV, Shanks MR, Schrader M, Schroer TA, Hubbard AL. Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes. J Cell Biol. 1998;141:115–133. doi: 10.1083/jcb.141.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman MR, Shurety W, Ellis JA, Luzio JP. Inhibition of apical but not basolateral endocytosis of ricin and folate in Caco-2 cells by cytochalasin D. J Cell Sci. 1994;107:2547–2556. doi: 10.1242/jcs.107.9.2547. [DOI] [PubMed] [Google Scholar]
- Joly M, Kazlauskas A, Fay FS, Corvera S. Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase binding sites. Science. 1994;263:684–688. doi: 10.1126/science.8303278. [DOI] [PubMed] [Google Scholar]
- Joly M, Kazlauskas A, Corvera S. Phosphatidylinositol 3-kinase activity is required at a postendocytic step in platelet-derived growth factor receptor trafficking. J Biol Chem. 1995;270:13225–13230. doi: 10.1074/jbc.270.22.13225. [DOI] [PubMed] [Google Scholar]
- Jones AT, Clague MJ. Phosphatidylinositol 3-kinase activity is required for early endosome fusion. Biochem J. 1995;311:31–34. doi: 10.1042/bj3110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarlund JK, Guilherme A, Holik JJ, Virbasius JV, Chawla A, Czech MP. Signaling of phosphoinositide-3,4,5-trisphosphate through proteins containing plekstrin and Sec7 homology domains. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- Knight A, Hughson E, Hopkins CR, Cutler DF. Membrane protein trafficking through the common apical endosome compartment of polarized Caco-2 cells. Mol Biol Cell. 1995;6:597–610. doi: 10.1091/mbc.6.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, D'Souza-Schorey C, Barbieri MA, Roberts RL, Klippel A, Williams LT, Stahl PD. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc Natl Acad Sci USA. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente A, Garred O, Holm OPK, Eker P, Jacobsen J, van Deurs B, Sandvig K. Effect of calmodulin antagonists on endocytosis and intracellular transport of ricin in polarized MDCK cells. Exp Cell Res. 1996;227:298–308. doi: 10.1006/excr.1996.0279. [DOI] [PubMed] [Google Scholar]
- Martys JL, Wjasow C, Gangi DM, Kielian MC, McGraw TE, Backer JM. Wortmannin-sensitive trafficking pathways in Chinese hamster ovary cells. J Biol Chem. 1996;271:10953–1096. doi: 10.1074/jbc.271.18.10953. [DOI] [PubMed] [Google Scholar]
- Matter K, Stieger B, Klumperman J, Ginsel L, Hauri H-P. Endocytosis, recycling, and lysosomal delivery of brush border hydrolases in cultured human intestinal cells (CaCo-2) J Biol Chem. 1990;265:3503–3512. [PubMed] [Google Scholar]
- Odorizzi G, Pearse A, Domingo D, Trowbridge IS, Hopkins CR. Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism. J Cell Biol. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Elferink, R.P.J., D.K.F. Meijer, F. Kuipers, P.L.M. Jansen, A.K. Groen, and G.M.M. Groothuis. Hepatobiliary secretion of organic compounds; molecular mechanisms of membrane transport. Biochim Biophys Acta. 1995;1241:215–268. doi: 10.1016/0304-4157(95)00006-d. [DOI] [PubMed] [Google Scholar]
- Parton RG, Prydz K, Bomsel M, Simons K, Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby canine kidney cell in late endosomes. J Cell Biol. 1989;109:3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki V, Virbasius J, Lane WS, Toh B, Shpetner HS, Corvera S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1997;94:7326–7330. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves BJ, Bright NA, Mullock BM, Luzio JP. The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggest a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Maurice M, Stieger B, Hubbard AL. 5′Nucleotidase is sorted to the apical domain of hepatocytes via an indirect route. J Cell Biol. 1992;119:1173–1182. doi: 10.1083/jcb.119.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Hubbard AL. Dynamics of four rat liver plasma membrane proteins and polymeric IgA receptor. Rates of synthesis and selective loss into the bile. J Biol Chem. 1992;267:6099–6106. [PubMed] [Google Scholar]
- Shanks MS, Cassio D, Lecoq O, Hubbard AL. An improved rat hepatoma hybrid cell line. Generation and comparison with its hepatoma relatives and hepatocytes in vivo. J Cell Sci. 1994;107:813–825. doi: 10.1242/jcs.107.4.813. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Reaves BJ, Davidson HW. Phosphoinositide 3-kinases and membrane traffic. Trends Cell Biol. 1996;6:92–97. doi: 10.1016/0962-8924(96)80998-6. [DOI] [PubMed] [Google Scholar]
- Shpetner H, Joly M, Hartley D, Corvera S. Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J Cell Biol. 1996;132:595–605. doi: 10.1083/jcb.132.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurety W, Bright NA, Luzio JP. The effects of cytochalasin D and phorbol myristate acetate on the apical endocytosis of ricin in polarised Caco-2 cells. J Cell Sci. 1996;109:2927–2935. doi: 10.1242/jcs.109.12.2927. [DOI] [PubMed] [Google Scholar]
- Siddhanta U, McIlroy J, Shah A, Zhang Y, Backer JM. Distinct roles for p110a and hVPS34 phosphatidylinositol 3′-kinases in vesicular trafficking, regulation of the actin cytoskeleton, and mitogenesis. J Cell Biol. 1998;143:1647–1659. doi: 10.1083/jcb.143.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Slot HJ. A new method for preparing gold probes for multiple labeling cytochemistry. Eur J Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- Smit JJM, Schinkel AH, Oude RPJ, Elferink, Groen AK, Wagenaar E, van Deemter L, Mol CAAM, Ottenhoff R, van der Lugt NMT, van Roon MA, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Solari R, Schraerer E, Tallichet C, Braiterman LT, Hubbard AL, Kraehenbul J-P. Cellular location of the cleavage event of the polymeric immunoglobulin receptor and fate of its anchoring domain in the rat hepatocyte. Biochem J. 1989;257:759–768. doi: 10.1042/bj2570759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro DJ, Boll W, Kirchhausen T, Wessling-Resnick M. Wortmannin alters the transferrin receptor endocytic pathway in vivo and in vitro. Mol Biol Cell. 1996;7:355–367. doi: 10.1091/mbc.7.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A, Cantley LC. Signaling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signaling protein, phosphoinositide 3-kinase. TIBS (Trends Biochem Sci) 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- van Deurs B, Holm PL, Layser K, Sanvig K, Hansen SH. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur J Cell Biol. 1993;61:208–224. [PubMed] [Google Scholar]
- van IJzendoorn S, Hoekstra D. (Glyco)sphingolipids are sorted in subapical compartments in HepG2 cells: a role for non–Golgi-related intracellular sites in the polarized distribution of (glyco)sphingolipids. J Cell Biol. 1998;142:683–696. doi: 10.1083/jcb.142.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn SCD, Zegars MMP, Kok JW, Hoekstra D. Segregation of glucosylceramide and sphingomyelin occurs in the apical to basolateral transcytotic route in HepG2 cells. J Cell Biol. 1997;137:347–357. doi: 10.1083/jcb.137.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. TIBS (Trends Biochem Sci) 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- Vlahos SJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidyl 3-kinase, 2-(4-morpholinyl)-8-4H-1-benzopyran-4-1 (LY42009) J Biol Chem. 1993;269:5241–5248. [PubMed] [Google Scholar]
- Weisz OA, Machamer CE, Hubbard AL. Rat liver dipeptidylpeptidase IV contains competing apical and basolateral targeting information. J Biol Chem. 1992;267:22282–22288. [PubMed] [Google Scholar]