Abstract
The extracellular matrix exerts a stringent control on the proliferation of normal cells, suggesting the existence of a mitogenic signaling pathway activated by integrins, but not significantly by growth factor receptors. Herein, we provide evidence that integrins cause a significant and protracted activation of Jun NH2-terminal kinase (JNK), while several growth factors cause more modest or no activation of this enzyme. Integrin-mediated stimulation of JNK required the association of focal adhesion kinase (FAK) with a Src kinase and p130CAS, the phosphorylation of p130CAS, and subsequently, the recruitment of Crk. Ras and PI-3K were not required. FAK–JNK signaling was necessary for proper progression through the G1 phase of the cell cycle. These findings establish a role for FAK in both the activation of JNK and the control of the cell cycle, and identify a physiological stimulus for JNK signaling that is consistent with the role of Jun in both proliferation and transformation.
Keywords: integrins, focal adhesion kinase, Jun NH2-terminal kinase, Jun, cell cycle
Normal cells require adhesion to extracellular matrix components to proliferate in vitro, suggesting that integrins activate signaling pathways that are necessary for cell cycle progression (reviewed in Giancotti, 1997). In principle, integrins could cooperate with growth factor receptors to produce a synergistic stimulation of one or more mitogenic signaling pathways. Indeed, the results of several studies support this model (reviewed in Ruoslahti and Reed, 1994; Clark and Brugge, 1995; Parsons, 1996; Clark and Hynes, 1997; Schwartz, 1997; Yamada and Geiger, 1997; Howe et al., 1998; Schlaepfer and Hunter, 1998). However, such a mechanism does not readily explain the strict adhesion requirement for growth displayed by normal cells. In addition, or instead, integrins could activate a signaling pathway that is not significantly activated by growth factors, but is necessary for cell proliferation. The identification of such a pathway would provide a more complete understanding of the anchorage-dependent growth than is currently available.
Integrins activate common, as well as subgroup-specific, signaling pathways. A subset of integrins, which includes α1β1, α5β1, αvβ3, and α6β4, is coupled to the Ras-extracellular signal-regulated kinase (ERK)1 signaling pathway by the adaptor protein Shc (Mainiero et al., 1995; Wary et al., 1996). Shc binds directly to the uniquely large cytoplasmic domain of β4 when this integrin undergoes tyrosine phosphorylation (Mainiero et al., 1995; 1997). In contrast, the recruitment of Shc by β1 and αv integrins is indirect, requiring the interaction of the integrin α subunit with the membrane adaptor caveolin-1 and associated tyrosine kinase, Fyn (Wary et al., 1998). Biochemical and genetic evidence suggest that integrins recruit Shc independently of focal adhesion kinase (FAK), and that this event is necessary and sufficient to activate the Ras-ERK pathway. In primary cells, inhibition of integrin-mediated Shc signaling results in cell cycle arrest, despite the presence of growth factors, suggesting that a combined stimulation of Ras by Shc-linked integrins and growth factor receptors is required for progression through the G1 phase of the cell cycle (Wary et al., 1996, 1998).
Certain integrins appear to associate preferentially with specific growth factor receptors and contribute to their activation (Miyamoto et al., 1996; Schneller et al., 1997; Moro et al., 1998; Soldi et al., 1999). For example, αvβ3 combines with the platelet-derived growth factor (PDGF) receptor. Hence, fibroblasts show enhanced proliferation in response to PDGF when attaching to the αvβ3 ligand vitronectin than they do on the β1 integrin ligand collagen (Schneller et al., 1997). The selective association of integrins with growth factor receptors represents a second potential mechanism of integrin-specific growth control.
Whereas the aforementioned pathways are activated only by certain integrins, the tyrosine kinase FAK is activated by most integrins (Parsons, 1996). Activated FAK undergoes autophosphorylation at Tyr 397 and thereby binds to the SH2 domain of the Src-family kinase Src or Fyn (Schaller et al., 1994; Schlaepfer et al., 1994). The Src-family kinase then phosphorylates a number of FAK-associated proteins, including p130CAS and paxillin, which contain multiple docking sites for the adaptor proteins Crk and Nck (Schaller and Parsons, 1995; Richardson and Parsons, 1996; Vuori et al., 1996; Schlaepfer et al., 1997). In addition, Src phosphorylates FAK at a tyrosine residue able to recruit Grb2 (Schlaepfer et al., 1994). It is possible that FAK contributes to the activation of the Ras-ERK cascade by these (Schlaepfer et al., 1997) and potentially other mechanisms (Chen et al., 1996; King et al., 1997; Lin et al., 1997; Renshaw et al., 1997). Although previous studies have provided direct evidence for a role of FAK in cell migration (Ilic et al., 1995; Fincham and Frame, 1998; Cary et al., 1998; Klemke et al., 1998) and protection from apoptotic cell death (Frisch et al., 1996b; Ilic et al., 1998), it is unclear whether FAK also regulates cell proliferation, and if so, by what mechanism.
Integrin-mediated adhesion activates not only ERK, but also Jun NH2-terminal kinase (JNK; Miyamoto et al., 1995; Mainiero et al., 1997; MacKenna et al., 1998). JNK is the final element of a mitogen-activated protein kinase (MAPK) cascade known to be activated by stress stimuli, such as UV radiation, hyperosmolar conditions, and inflammatory cytokines (Ip and Davis, 1998). Upon activation, JNK enters the nucleus, and phosphorylates and activates the transcription factors c-Jun and activating transcription factor 2 (ATF2), thereby regulating AP-1–dependent transcription (Karin et al., 1997). Because there is evidence that c-Jun is required for cell proliferation (Riabowol et al., 1992; Johnson et al., 1993), we sought to examine the mechanism by which integrins activate JNK and test the hypothesis that activation of this signaling pathway contributes to the control of cell cycle progression.
Our results indicate that integrins cause a significant and protracted activation of JNK, while growth factors appear to be unable to do so. By using various dominant-negative signaling molecules, we provide evidence that the activation of JNK by integrins is mediated by FAK and is necessary for cell cycle progression.
Materials and Methods
Antibodies and Extracellular Matrix Proteins
The mAb M2 to FLAG tag was purchased from Eastman-Kodak and the anti-CD2 mAb RPA-2.10 from PharMingen. The anti-H–Ras mAb R02120 (clone 18) and anti-p130CAS mAb P27820 (clone 21) were from Transduction Laboratories. The origin and specificity of the affinity-purified rabbit antibodies to ERK2, phospho-ERK, Src, and GST and of the anti–5-bromodeoxyuridine (anti-BrdU) mAb were described previously (Wary et al., 1996; 1998). The mAb 3C2 reacting with the gag portion of v-Crk was also described previously (Potts et al., 1987). Human fibronectin was from GIBCO BRL and poly-l-lysine from Sigma Chemical Co.
Cell Lines, Constructs, and Transfections
293 human embryonic kidney cells were cultured in DME 10% FCS on gelatin-coated plates. NIH-3T3 mouse fibroblasts were cultured in DME 10% calf serum (CS). Fibroblasts from Src−/− and Fyn−/− embryos were obtained from Philippe Soriano (Fred Hutchinson Cancer Research Center, Seattle, WA) and cultured in DME 10% CS. Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics and cultured on gelatin-coated dishes in human endothelial serum-free medium (SFM; GIBCO BRL) supplemented with 20% FCS (GIBCO BRL), 10 ng/ml EGF, 20 ng/ml bFGF, and 1 μg/ml heparin (all from Intergen).
The reporter plasmid pcoll TRE-tk-Luc, in which the expression of luciferase is driven by a single copy of the collagen gene 12-O-tetradecanoylphorbol-13-aretate (TPA)-responsive element (TRE) linked to the Hepes simplex virus thymidine kinase minimal promoter, was described previously (Galien et al., 1994). Vectors encoding the FLAG-tagged version of JNK 1, glutatione S-transferase (GST)-Jun, dominant-negative Ras (N17), and HA-tagged β-galactosidase were described previously (Mainiero et al., 1997). The CMV promotor-based pCDM8 vectors encoding CD2-FAK (wild-type), CD2-FAK K454R (kinase dead), and CD2-FAK Y397F were described previously (Chan et al., 1994). A kinase dead version of chicken c-Src was obtained from Sara Courtneidge (EMBL, Heidelberg, Germany) and subcloned in the cytomegalovirus (CMV) promotor-based vector pRK5. The pEBG vectors expressing GST-tagged MKK4 (wild-type) and MKK4 K129R (kinase dead) from the human elongation factor 1-α promoter were described previously (Su et al., 1997). The Moloney Leukemia Virus (MLV)-LTR based pMEXneo vectors encoding v-Crk (wild-type), v-Crk R273N (SH2 mutant), and v-Crk D386DRHAD (SH3 insertional mutant) were described previously (Altun-Gultekin et al., 1998). The pEBG vectors encoding GST-tagged rat p130CAS (short form) and its substrate region deleted form (SD, Δ213– 514) were also described (Mayer et al., 1995). The TAM-67 transactivation domain mutant form of c-Jun (Jun Δ3–122) was expressed from pCMV and previously characterized (Brown et al., 1993). The dominant-negative version of paxillin used in this study carries three phenylalanine permutations at tyrosine 31, 118, and 187 and is unable to bind to Crk. The MLV–LTR-based expression vector pΔraf-22w encodes an activated version of c-Raf 1 lacking an NH2-terminal segment of 305 amino acids (Stanton et al., 1989). The vector encoding activated Ras, pDCR-Ha-ras (G12V), was kindly provided by John Westwick (Signal Pharmaceuticals).
NIH-3T3 cells were transiently transfected with Lipofectamine according to the manufacturer's instructions (GIBCO BRL). 293 cells were plated at 6 × 106 per 15-cm diam dish for 8 h and then transfected overnight with various amounts of plasmid by the calcium phosphate method. All transfections were normalized to the same total amount of DNA with empty vector. Cells were allowed to recover for 12 h before growth factor starvation.
Biochemical Methods
To monitor the activation of JNK and ERK during G1, HUVECs were synchronized in G0 by a 24 h incubation in human endothelial SFM containing 0.2% FCS. They were then detached with 0.02% EDTA, collected in SFM containing 0.2% heat-inactivated BSA, washed in the same medium, and kept in suspension at a density of 106/ml for 15 min at room temperature to recover. Aliquots consisting of 1.5 × 107 cells were plated on 15-cm diam dishes coated with 20 μg/ml fibronectin and postcoated with 0.2% heat-inactivated BSA in SFM supplemented with ITS+1 (Sigma Chemical Co.), EGF (10 ng/ml), bFGF (20 ng/ml), and heparin (1 μg/ml) for the indicated times. Cells from an identical aliquot were pelleted and lysed in suspension as a control. Before biochemical analysis, NIH-3T3 cells were serum starved for 18 h and 293 cells for 24 h in DME containing 0.2% CS or FCS, respectively. After detachment with 0.02% EDTA, cells were collected in DME containing 0.2% heat-inactivated BSA, washed in the same medium, and kept in suspension at a density of 106/ml for 15 min at room temperature to recover. Aliquots consisting of 1.5 × 107 cells were plated on 15-cm diam dishes, coated with 20 μg/ml fibronectin and postcoated with 0.2% heat-inactivated BSA for the indicated times. Cells from an identical aliquot were pelleted and lysed in suspension as a control. NIH-3T3 cells were treated with growth factors as indicated.
To analyze the activation of JNK, cells were extracted for 30 min on ice with 0.5 ml/dish of modified Triton lysis buffer (25 mM Hepes, pH 7.5, 300 mM NaCl, 0.1% Triton X-100, 0.2 mM EDTA, 20 mM β-glycerophosphate, 1.5 mM MgCl2, and 0.5 mM DTT) containing phosphatase and protease inhibitors. Aliquots containing 0.5 mg of total proteins were brought to 0.8 ml with modified Triton lysis buffer and diluted to 1.2 ml with HBB buffer (20 mM Hepes, pH 7.7, 50 mM NaCl, 0.05% Triton X-100, 0.1 mM EDTA, 20 mM β-glycerophosphate, 2.5 mM MgCl2, and 10 mM DTT) supplemented with phosphatase and protease inhibitors. Endogenous JNK was precipitated with 5 μg of GST-Jun fusion protein coupled to glutathione agarose beads (Hibi et al., 1993). The beads were washed four times in HBB buffer, twice in kinase buffer (20 mM Hepes pH 7.5, 20 mM β-glycerophosphate, 10 mM MgCl2, and 10 mM DTT), and incubated with 35 μl of kinase buffer containing 10 μCi of γ[32P]ATP (ICN) and 20 μM cold ATP. Recombinant FLAG-tagged JNK 1 was immunoprecipitated with the anti-Flag mAb M2. The beads were washed as above and incubated with 35 μl of kinase buffer containing 5 μg of GST-Jun, 10 μCi of γ[32P]ATP, and 20 μM cold ATP. After 30 min of incubation at 30°C, the samples were boiled in sample buffer and separated by SDS-PAGE.
Immunoprecipitation and immunoblotting were performed essentially as described previously (Mainiero et al., 1995). Secondary reagents for immunoblotting included peroxidase-conjugated protein A and affinity-purified rabbit anti–goat IgGs.
To measure transcription from the collagen promotor TRE, NIH-3T3 cells were transiently transfected with the reporter plasmid pcoll TRE-tk-Luc. After 24 h of growth factor starvation, the cells were detached, kept in suspension for 30 min, and then solubilized or plated on dishes coated with 20 μg/ml fibronectin for the indicated times in the absence or presence of 20 ng/ml PDGF. Luciferase activity in cell lysates was estimated as described previously (Mainiero et al., 1997).
Analysis of Cell Cycle Progression
NIH-3T3 cells were transiently transfected with vector encoding β-galactosidase in combination with various doses of the indicated constructs. The cells were allowed to recover in complete medium, synchronized in G0 by growth factor deprivation, and plated at low density on microtiter wells coated with 10 μg/ml poly-l-lysine or 20 μg/ml fibronectin in defined medium (DME supplemented with 6.25 μg/ml insulin, 6.25 μg/ml transferrin, 0.625 ng/ml selenous acid, 1.25 mg/ml BSA, and 5.35 μg/ml linoleic acid) supplemented with 20 ng/ml PDGF and 10 μM BrdU. After 16 h, the cells were fixed and stained with X-gal followed by anti-BrdU mAb and AP-conjugated anti-mouse IgGs. The percentage of X-gal positive cells that had incorporated BrdU was evaluated microscopically after light counterstaining with hematoxylin.
Results
Integrins Activate JNK and TRE-dependent Transcription
Preliminary experiments were conducted to examine if JNK was physiologically activated in primary cells progressing through the G1 phase of the cell cycle. HUVECs were synchronized in G0 by growth factor deprivation and detached to simulate the cell rounding physiologically occurring at mitosis. They were then plated on fibronectin in the presence of growth factors to allow entry into and progression through G1.
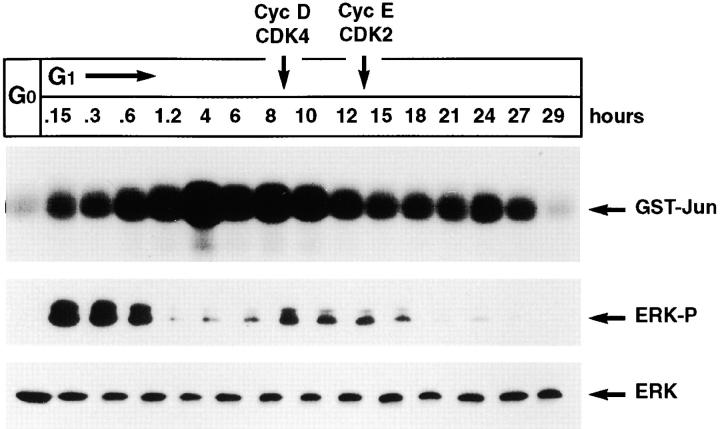
In vitro kinase assays with the NH2-terminal fragment of c-Jun as a substrate showed that JNK is activated to a significant level during mid-G1, before the activation of the D-type cyclin-dependent kinase CDK4 (Fig. 1). Peak JNK activity was observed 4 h after entry into G1. In contrast, the activation of ERK was biphasic with a first peak 10 to 20 min after entry into G1 and a second minor peak 8 h later (Fig. 1). Since unstimulated cells are known to contain detectable levels of c-Jun, but not c-Fos (Karin, 1995), the rapid activation of ERK at the onset of G1 may serve to induce serum response element (SRE)-dependent transcription of the c-Fos gene before JNK-mediated transcriptional activation of c-Jun. The AP-1 transcription factor Fos/Jun may then promote the expression of genes necessary for G1 progression. The extent and timing of JNK activation during the cell cycle are thus consistent with a potential role in the control of G1 progression.
Figure 1.
Activation of JNK and ERK during the G1 phase of the cell cycle in primary cells. HUVECs were synchronized in G0, detached, and then were lysed (G0) or replated on fibronectin for the indicated times in SFM supplemented with growth factors and then lysed. JNK was precipitated from extracts containing equal amounts of total proteins by using GST-Jun beads and subjected to an in vitro kinase assay. The position of phosphorylated GST-Jun is indicated. Total lysates containing the same amount of proteins were subjected to immunoblotting with antiphospho-ERK (ERK-P). The blot was stripped and reprobed with antibodies to ERK 1 to demonstrate equal loading (ERK). Peak activation of Cyclin D/CDK 4 and Cyclin E/CDK 2 occurs at the indicated times in HUVECs under these experimental conditions (F.G. Giancotti, unpublished results).
We next compared the ability of integrins and growth factor receptors to activate JNK in NIH-3T3 fibroblasts. Preliminary experiments showed that the activity of JNK was significantly higher in growth factor deprived, stably adherent cells than in cells that had been detached and immediately lysed (Fig. 2 A). In accordance with the previous observation that several growth factors cause a relatively modest activation of JNK (Kyriakis et al., 1994; Minden et al., 1994), exposure to mitogenic concentrations of PDGF, bFGF, and insulin increased the activity of JNK only to a limited extent in growth factor starved, stably adherent NIH-3T3 cells. In contrast, PDGF, bFGF, and to a minor extent, insulin, caused a significant activation of ERK under the same conditions (Fig. 2 A).
Figure 2.

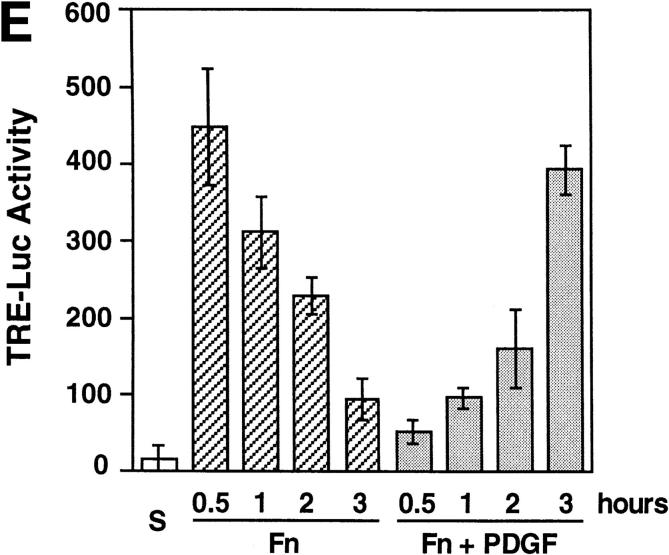
Adhesion to fibronectin induces activation of JNK and TRE-dependent transcription. (A) After serum starvation, NIH-3T3 cells were treated while adherent with 20 ng/ml of PDGF, 20 ng/ml bFGF, or 6.25 μg/ml insulin for 10 min, or left untreated and either detached (S) or adherent (Starved). JNK was precipitated from extracts containing equal amounts of total proteins and subjected to an in vitro kinase assay (GST-Jun). Total lysates containing the same amount of proteins were subjected to immunoblotting with antiphospho-ERK (Phospho-ERK). (B) Serum starved NIH-3T3 cells were detached and either left in suspension (S) or plated on fibronectin (Fn) in SFM for the indicated times. Alternatively, they were left adherent and treated with the indicated concentrations of TNF-α for 15 min. JNK was precipitated from extracts containing equal amounts of total proteins and subjected to an in vitro kinase assay. (C) Suspended cells were treated with the indicated concentrations of PDGF for 5 min and subjected to JNK assay as above. (D) Suspended cells were lysed (S) or plated on fibronectin for the indicated times in SFM supplemented with or without 20 ng/ml PDGF during the last 5 min of adhesion (Fn+PDGF) before extraction. JNK assay was performed as above. (E) NIH-3T3 cells were transfected with pcoll TRE-tk-Luc. After serum starvation, the cells were detached and left in suspension (S) or plated on fibronectin (Fn) for the indicated times in SFM supplemented with or without 20 ng/ ml PDGF. Cell lysates containing equal amounts of total proteins were subjected to luciferase assay. Values are expressed in arbitrary units. After cycloheximide-induced blockage of protein synthesis, luciferase activity decays with a half-life of <1 h in serum starved NIH-3T3 cells replated on fibronectin (data not shown).
To further examine the relative contribution of integrins and growth factor receptors to the activation of JNK, NIH-3T3 cells were either plated on fibronectin in the absence of growth factors or treated with various doses of PDGF while in suspension. As shown in Fig. 2 B, adhesion to fibronectin induced a rapid, strong, and protracted activation of JNK. By contrast, JNK activity increased only slowly and modestly over time in suspension, perhaps in response to the activation of a stress pathway, as observed by others (Frisch et al., 1996a; Khwaja and Downward, 1997). The activation of JNK caused by integrin ligation was comparable in intensity to that observed in cells treated with 5 to 10 ng/ml TNF-α (Fig. 2 B), a known activator of JNK (Kyriakis et al., 1994; Minden et al., 1994). Exposure to a wide range of PDGF concentrations caused little or no activation of JNK in suspended cells (Fig. 2 C). Whereas exposure to 1 μg/ml lysophosphatidic acid (LPA), which is known to activate FAK (Chrzanowska-Wodnicka and Burridge, 1994), amplified the activation of JNK caused by integrin ligation by ∼70% (data not shown), treatment with PDGF did not exert this effect. However, PDGF changed the time course of JNK activation in cells adhering to fibronectin. Specifically, while adhesion to fibronectin caused maximal stimulation of JNK in ∼10 min, simultaneous exposure to PDGF significantly delayed the peak of activation of the kinase (Fig. 2 D). This effect of PDGF may be related to its ability, when used at mitogenic concentrations as were used here, to transiently disrupt the cytoskeleton and thus delay integrin-mediated activation of FAK (Rankin and Rozengurt, 1994). Taken together, these observations indicate that JNK is activated by integrins, but only to a limited extent by PDGF, bFGF, and insulin. Exposure to growth factors may, however, contribute to sustain the activation of JNK caused by integrin ligation.
Phosphorylation of c-Jun by JNK is required for transcriptional activation of the dimeric transcription factor AP-1 and for the oncogenic cooperation between c-Jun and Ha-Ras (Smeal et al., 1991). To examine if the activation of JNK caused by integrin ligation could contribute to immediate early gene expression by promoting AP-1 dependent transcription from TRE, NIH-3T3 cells were transiently transfected with a vector encoding the luciferase gene under the transcriptional control of a TRE and plated on fibronectin in the presence or absence of PDGF. As shown in Fig. 2, D and E, adhesion to fibronectin promoted TRE-dependent transcription with kinetics that closely followed that of JNK activation. Simultaneous exposure to PDGF caused a delay in the transcriptional response to fibronectin, as observed for the activation of JNK. The induction of TRE-dependent transcription by integrins required the transcriptional activity of c-Jun because it was suppressed by the TAM-67 dominant-negative form of the transcription factor (93.3% inhibition). Integrin-mediated activation of ERK and SRE-dependent transcription of Fos (Wary et al., 1996; Mainiero et al., 1997) can increase the levels of AP-1 available for phosphorylation by JNK. However, TRE-dependent transcription could not have occurred in the absence of JNK-mediated phosphorylation of c-Jun.
Integrin-mediated Activation of JNK Requires FAK, Src, p130CAS, Crk, and MKK
The mechanism by which integrins activate JNK was examined by introducing dominant-negative versions of various signaling components into human embryonic kidney 293 cells. Since preliminary experiments suggested that the ability to activate JNK is shared by all integrins, irrespective of whether they are able or not to recruit Shc (data not shown), we decided to examine the role of FAK in this process. Inactivating mutations were introduced at either the Src SH2-binding site or the ATP-binding site of CD2-FAK, a chimeric, membrane-anchored form of FAK that localizes efficiently to focal adhesions (Chan et al., 1994; Frisch et al., 1996b). We reasoned that membrane attachment would promote the interaction of these FAK mutants with focal adhesion components and thereby facilitate a dominant-negative effect.
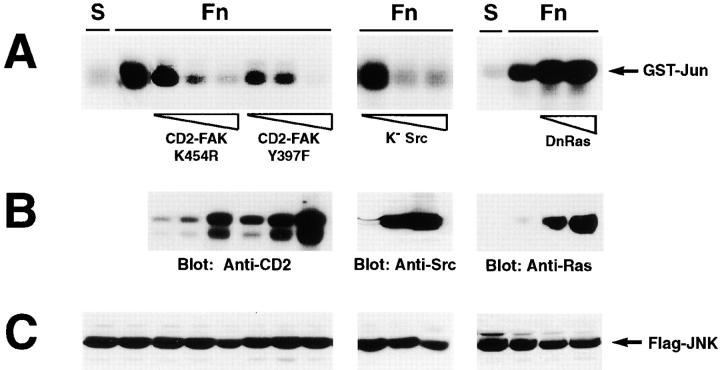
As shown in Fig. 3, both CD2-FAK mutants exerted a dominant-negative effect on fibronectin-mediated activation of JNK, indicating that this process requires the kinase activity of FAK and its association with an Src family kinase. Accordingly, integrin-mediated activation of JNK was also suppressed by a kinase dead version of Src. In addition, we observed that the activation of JNK by integrins is partially defective in Src−/− and in Fyn−/− fibroblasts, in accordance with the notion that FAK can combine with both kinases (data not shown). Integrin-mediated activation of JNK was also blocked by a kinase inactive version of MKK4, one of the major enzymes that binds to and phosphorylates JNK (Fig. 4). In contrast, it was not inhibited by expression of dominant-negative Ras (Fig. 3) or by exposure to the PI-3K inhibitors Wortmannin (100 nM) and LY294002 (50 μM) (data not shown). These results indicate that the FAK/Src complex links integrins to MKK4 or a related enzyme, and thereby JNK. In addition, they suggest that Ras and its substrate, PI-3K, which can activate Rac and thus JNK (Rodriguez-Viciana et al., 1994; Nobes et al., 1995; Klippel et al., 1996), do not contribute to this process.
Figure 3.
Role of the FAK/Src complex in integrin-mediated activation of JNK. 293 cells were transiently transfected with 3 μg of vector encoding FLAG-tagged JNK alone or in combination with 3, 9, and 27 μg of plasmids encoding CD2-FAK K454R (catalytically inactive), CD2-FAK Y397F (unable to bind to the SH2 domain of Src family kinases), and K− Src (kinase dead), or with 9 and 27 μg of vector encoding DnRas (N17, dominant-negative). The cells were serum starved for 24 h, detached, and lysed immediately (S) or plated on fibronectin for 20 min (Fn). FLAG-tagged JNK was immunoprecipitated from extracts containing equal amounts of total proteins and subjected to an in vitro kinase assay with GST-Jun as a substrate (A). Total lysates containing the same amount of proteins were subjected to immunoblotting with antibodies to CD2, Src, and Ras to verify that the expression of the various dominant-negative proteins was proportional to the amount of DNA transfected (B). Immunoblotting with anti-FLAG antibodies was used to verify equal expression of FLAG-JNK in all samples (C).
Figure 4.
Role of p130CAS and Crk in integrin-mediated activation of JNK. 293 cells were transiently transfected with 3 μg of vector encoding FLAG-tagged JNK alone or in combination with 3.75, 7.5, and 15 μg of plasmid encoding a mutant form of p130CAS carrying a deletion of the entire substrate region (SD-CAS), or with 3, 9, and 27 μg of plasmids encoding SH2− v-Crk (SH2 mutant), SH3− v-Crk (SH3 mutant), and GST-DnMKK4 (kinase inactive). The cells were serum starved for 24 h, detached, and solubilized immediately (S) or plated on fibronectin for 20 min (Fn). FLAG-tagged JNK was immunoprecipitated from extracts containing equal amounts of total proteins and subjected to an in vitro kinase assay with GST-Jun as a substrate (A). Total lysates containing the same amount of proteins were subjected to immunoblotting with antibodies to p130CAS, gag, and GST to verify that the expression of the various dominant-negative proteins was proportional to the amount of DNA transfected (B). Immunoblotting with anti-FLAG antibodies was used to verify equal expression of FLAG-JNK in all samples (C).
To examine the mechanism by which the FAK/Src complex activates JNK, we focused on the role of the docking/ adaptor proteins p130CAS and paxillin, which bind to the FAK/Src complex and become heavily phosphorylated on tyrosine residues upon integrin engagement (Schaller and Parson, 1995; Richardson and Parsons, 1996; Vuori et al., 1996; Schlaepfer et al., 1997). The activation of JNK by integrins was inhibited to a significant extent by a mutant form of p130CAS carrying a deletion of the entire substrate region (SD-CAS; Fig. 4). In contrast, a mutant form of paxillin carrying phenylalanine substitutions at three tyrosine phosphorylation sites, including all the Crk binding sites, inhibited this event modestly and only when expressed at relatively high levels (data not shown). These results indicate that p130CAS plays a major role, and paxillin perhaps a minor one, in integrin-mediated JNK activation.
9 out of 15 tyrosine phosphorylation sites within the substrate region of p130CAS conform to the consensus motif for binding to the SH2 domain of Crk (Sakai et al., 1994). In addition to the SH2 domain, Crk contains either one or two SH3 domains able to interact with downstream target-effectors (Mayer et al., 1988; Matsuda et al., 1992; ten Hoeve et al., 1993). Previous studies have indicated that the adaptor function of Crk is regulated positively by recruitment to the plasma membrane and negatively when tyrosine 222 becomes phosphorylated and associates intramolecularly with the SH2 domain (Mayer and Hanafusa, 1990; Feller et al., 1994).
We reasoned that an SH3 mutant form of the viral version of Crk, which is anchored to the membrane via its gag sequences and truncated before tyrosine 222, would have interacted efficiently, via its intact SH2 domain, with p130CAS, but not with downstream target effectors. As shown in Fig. 4, expression of this mutant form of Crk effectively suppressed JNK activation in cells plated on fibronectin. Conversely, the introduction of a control construct with a mutated SH2 domain stimulated the activation of JNK, suggesting that the recruitment of Crk to the plasma membrane and its interaction with downstream target(s) via the SH3 domain are sufficient to activate JNK (Fig. 4). These observations indicate that the FAK/Src/ p130CAS complex activates JNK by recruiting Crk to the plasma membrane. They are also in agreement with previous studies implicating Crk in the activation of JNK (Tanaka et al., 1997).
Integrin-mediated JNK Signaling Controls Cell Cycle Progression
To examine whether FAK signaling to JNK was required for cell proliferation, NIH-3T3 fibroblasts were transiently transfected with various amounts of vectors encoding wild-type and mutant versions of the signaling components of this pathway, in combination with the marker β-galactosidase. The cells were synchronized in G0 and plated on fibronectin in the presence of PDGF. Entry of the transfected cells into S phase was evaluated by double staining with X-gal and anti-BrdU antibodies.
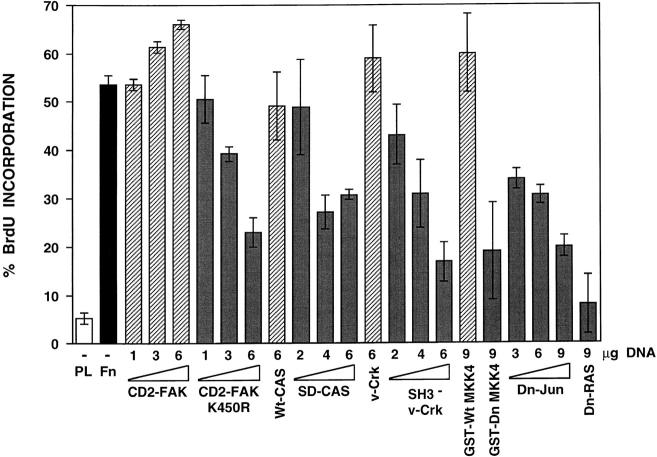
While CD2-FAK, which is constitutively active (Chan et al., 1994), promoted entry into the S phase to a limited extent, its kinase dead version suppressed it (Fig. 5). In both cases, the effects observed were dose dependent. In addition, whereas wild-type p130CAS did not affect progression through G1, a mutant version carrying a deletion of the substrate region, which includes all the Crk binding sites, partially inhibited transit through G1 (Fig. 5). The incomplete effect of this mutant may be due to residual, integrin-induced binding of Crk to paxillin (Schaller and Parsons, 1995). In accordance with this hypothesis, dominant-negative Crk suppressed entry into S phase as effectively as the kinase dead version of CD2-FAK. Finally, cell cycle progression was also suppressed by dominant-negative versions of MKK4 and Jun (Fig. 5). These findings suggest that integrin-mediated activation of the FAK– JNK pathway is necessary for progression through the G1 phase of the cell cycle.
Figure 5.
Integrin-mediated activation of JNK is required for progression through the G1 phase of the cell cycle. NIH-3T3 fibroblasts were transiently transfected with 1 μg of vector encoding β-galactosidase alone or in combination with the indicated amounts of plasmids encoding CD2-FAK, CD2-FAK K454R, GST-CAS (wild-type), GST-SD-CAS (substrate region deleted), v-Crk, SH3− v-Crk, GST-Wt MKK4 (wild-type), GST-Dn MKK4, Dn-Jun (Δ3-122), or Dn-RAS (N17). After synchronization in G0 by serum deprivation, the cells transfected only with the β-galactosidase plasmid were plated on coverslips coated with poly-l-lysine (PL, white bar) or fibronectin (Fn, black bar), while those cotransfected with vectors encoding wild-type and constitutively active proteins (stippled bars) or corresponding dominant-negative versions (gray bars) were plated only on fibronectin. After 16 h of incubation in defined medium supplemented with PDGF and BrdU, the cells were fixed and stained with anti-BrdU mAb. The number of transfected cells entering S phase was evaluated as described in Materials and Methods. Under these conditions, none of the cells plated on fibronectin in the absence of PDGF enter into S phase. The diagram shows the mean value and standard deviation from triplicate samples.
Discussion
Although the details of FAK's interaction with a number of cytoskeletal and signaling components are known, the biological function of this kinase is incompletely understood. Our results suggest that FAK mediates activation of JNK and c-Jun in response to integrin ligation, and by doing so, regulates progression through the G1 phase of the cell cycle.
What is the mechanism by which FAK activates JNK? Upon activation, FAK undergoes autophosphorylation at tyrosine 397 and combines with the SH2 domain of Src or Fyn (Parsons, 1996). The most prominent substrates of the FAK/Src complex are the docking adaptor proteins p130CAS and paxillin (Schaller and Parsons, 1995; Richardson and Parsons, 1996; Vuori et al., 1996). Both contain tyrosine phosphorylation sites conforming to the consensus for binding to the adaptor protein Crk. However, while paxillin has only two such sites and does not appear to associate efficiently with Crk in response to integrin ligation (Schaller and Parsons, 1995), p130CAS contains nine Crk-binding motifs and associates well with Crk in cells adhering to fibronectin (Vuori et al., 1996). Our results indicate that the expression of dominant-negative versions of FAK, Src, p130CAS, and Crk suppress the activation of JNK by integrins. Together with complementary results of a recent study (Dolfi et al., 1998), these findings provide evidence that integrin-mediated activation of JNK requires the association of FAK with Src (or Fyn) and p130CAS, and the recruitment of Crk. It is unlikely that the coupling of Ras to Rac mediated by PI-3K (Rodriguez-Viciana et al., 1994; Nobes et al., 1995; Klippel et al., 1996) contributes in a significant manner to the activation of JNK by integrins because a dominant-negative form of Ras and specific inhibitors of PI-3K did not interfere with activation of this pathway. Thus, it appears that the β1 and αv integrins activate JNK and ERK via two separate pathways (see Fig. 6 for a model). By contrast, the α6β4 integrin, which is presumably unable to activate FAK because it does not contain the sequences required for its recruitment, is coupled to JNK signaling via the Ras–PI-3K–Rac pathway (Mainiero et al., 1997).
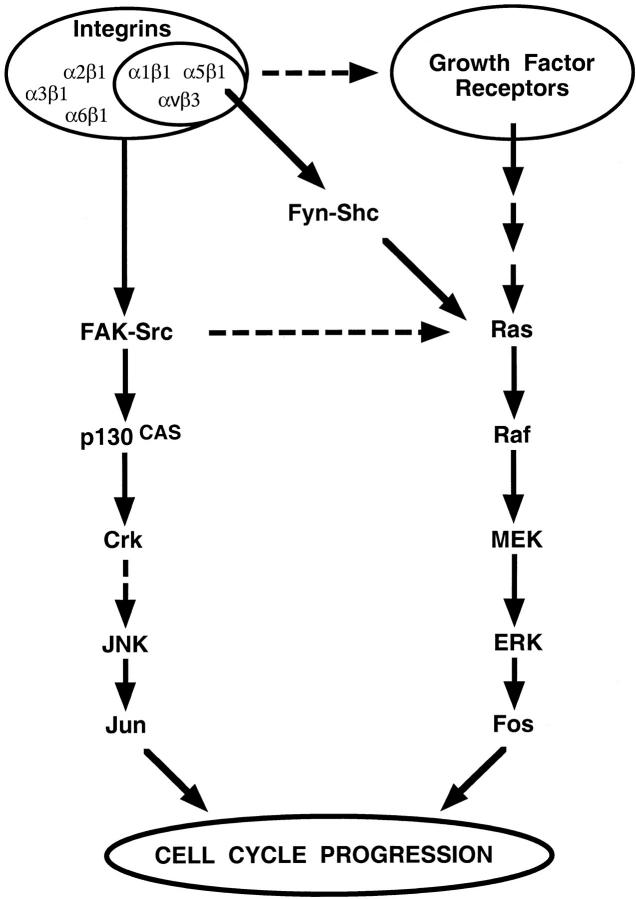
Figure 6.
Model of anchorage-dependent cell growth. Shc-linked integrins and growth factors receptors cooperate to activate the Ras-ERK cascade and promote the transcription of c-Fos. All β1 and αv integrins stimulate the FAK–JNK pathway in the absence of a significant input from growth factor receptors and induce transcription of c-Jun, as well as activation of newly formed Fos/ Jun dimers.
The mechanism by which Crk activates JNK in response to integrin ligation remains to be examined. Crk is known to interact via one of its SH3 domains with the exchange factor C3G (Tanaka et al., 1994) and previous studies have indicated that C3G is required for activation of JNK by the viral oncoprotein v-Crk (Tanaka et al., 1997). Interestingly, the activation of JNK by v-Crk and C3G appears to require the sequential action of the mixed lineage kinases MLK3 and DLK, but not the activity of Rho family GTPases or PAK proteins (Tanaka and Hanafusa, 1998). In addition, or instead, Crk may activate Rac, and thereby JNK, by promoting the association of C3G with DOCK 180 or mSOS (Dolfi et al., 1998).
The identity of genes regulated by JNK is largely unknown, but they must include genes important for cell proliferation. The evidence for this is several fold: first, deregulated expression of c-Jun or its mutated viral version v-Jun is sufficient to cause neoplastic transformation of primary avian and mammalian fibroblasts (Vogt, 1994); second, primary fibroblasts derived from c-Jun−/− mice display a severe proliferation defect (Johnson et al., 1993); and third, several oncoproteins, including v-Src, activated Ras, v-Crk, Bcr-Abl, and Met, potently activate JNK and there is evidence to suggest that this activation is required to cause neoplastic transformation (Derijard et al., 1994; Minden et al., 1995; Raitano et al., 1995; Johnson et al., 1996; Rodrigues et al., 1997; Tanaka et al., 1997).
Despite the clear requirement for c-Jun transcriptional activity in cell proliferation, it has been difficult to identify a physiological, nonstress stimulus for JNK consistent with its role in the regulation of AP-1 transcription. With the notable exception of EGF, mitogenic neuropeptides, and muscarinic receptor ligands, which indeed activate FAK or the related kinase PYK-2 (Zachary et al., 1992; Coso et al., 1995; Tokiwa et al., 1996; Yu et al., 1996; Cadwallader et al., 1997; Higashita et al., 1997; Logan et al., 1997; Slack, 1998), most growth factors cause a relatively modest activation of JNK (Kyriakis et al., 1994; Minden et al., 1994). Our results indicating that integrin ligation causes a significant activation of JNK and TRE-dependent transcription provide a physiological stimulus for JNK signaling that is consistent with its role in the control of cell proliferation.
Our present results imply that FAK, which appears to be activated by all β1 and αv integrins, is required during G1 progression because of its ability to activate JNK. Recently, it has been shown that a mutant form of FAK, which is truncated at the COOH terminus and thus unable to localize to focal adhesions, interferes with both fibronectin-induced activation of ERK and progression through the cell cycle (Zhao et al., 1998). On the basis of these results, it has been argued that FAK regulates cell proliferation by stimulating ERK. However, an alternative explanation is that this truncated form of FAK acts as a cytoplasmic sink for all Src-family kinases and thus disrupts not only FAK, but also Shc signaling. In accordance with this hypothesis, two more specific dominant-negative forms of FAK, FRNK and FAK-Y397F, inhibit ERK activation to a much lesser degree, but interfere with cell proliferation nonetheless (Zhao et al., 1998; see also Gilmore and Romer, 1996). Although additional mechanisms cannot be excluded, our observation that dominant-negative forms of FAK, p130CAS, Crk, MKK4, and Jun all inhibit entry into the S phase provides evidence that FAK regulates cell proliferation by activating JNK.
If FAK is required for cell cycle progression, why then do the FAK−/− cells not display an obvious proliferation defect (Ilic et al., 1995)? There are two potential explanations. First, it is now apparent that the FAK−/− cells originally examined by Ilic and colleagues also lack a functional form of the cell cycle regulator p53 (Furuta et al., 1995). Some of the cell lines generated more recently do have wild-type p53, but are transformed by the polyoma middle T antigen (Ilic et al., 1998). It is possible that the lack of p53 or presence of middle T antigen bypasses the requirement for FAK during cell proliferation. In addition, it recently has been shown that FAK−/− cells have elevated levels of PYK-2, which may compensate, at least in part, for the lack of FAK (Sieg et al., 1998).
The mitogenic signaling pathway linking integrins to JNK is likely to be deregulated in, and to contribute to, the transformation of at least some neoplastic cells. Previous studies have provided evidence that FAK is overexpressed in invasive carcinomas (Owens et al., 1995) and that the constitutively active CD2-FAK induces anchorage-independent growth in MDCK cells (Frisch et al., 1996b). In addition, the viral version of Src is a potent oncogene capable of transforming a variety of cells types, and there is strong genetic evidence that p130CAS is a necessary substrate of v-Src-induced transformation (Honda et al., 1998). In accordance with these findings, we have observed that CD2-FAK and p130CAS cooperate with activated Raf to induce anchorage-independent growth in NIH-3T3 cells (F. Liu and F.G. Giancotti, unpublished results). Finally, v-Crk and v-Jun are potent oncogenes (Mayer et al., 1988; Vogt, 1994). Taken together, these observations suggest that the FAK–JNK pathway can contribute to neoplastic transformation.
Previous studies have provided evidence that α6β4 and a subset of β1 and αv integrins activate the Ras-ERK signaling cascade by recruiting Shc (Mainiero et al., 1995; 1997; Wary et al., 1996; 1998). Mitogens and Shc-linked integrins synergize to promote transcription from the Fos SRE. Accordingly, ligation of integrins linked to Shc enables these cells to progress through the G1 phase of the cell cycle in response to mitogens, whereas adhesion mediated by other integrins results in growth arrest, despite the presence of mitogens. These mechanisms also appear to operate in vivo, as mice lacking the integrin α1 subunit or the cytoplasmic domain of β4 display cell cycle defects consistent with the lack of Shc signaling (Murgia et al., 1998; Pozzi et al., 1998). These data suggest that integrin-mediated Shc signaling is necessary for cell cycle progression.
The results of this and previous studies support the model that integrins control cell cycle progression primarily by regulating immediate early gene expression (Fig. 6). While Shc-linked integrins and growth factor receptors cooperate to activate the Ras-ERK cascade and promote SRE-dependent transcription of c-Fos, all β1 and αv integrins appear to be able to stimulate the FAK–JNK pathway in the absence of a significant contribution from growth factor receptors. It is likely that, in response to integrin ligation, JNK not only acts on preexisting Jun/ATF2 and ATF2/ATF2 dimers, thereby promoting the CRE-dependent transcription of c-Jun, but also activates the Fos/Jun dimers formed in response to the coordinated action of both integrins and growth factor receptors. The existence of a signaling pathway activated by all integrins within a signaling network coordinately regulated by a subset of integrins and growth factor receptors ensures that the control of cell proliferation exerted by the extracellular matrix is both stringent and integrin-specific.
Acknowledgments
We are indebted to B. Binetruy, S. Courtneidge, E. Skolnik, K. Vuori, and J. Westwick for constructs. We thank P. Soriano for the Src−/− and Fyn−/− 3T3 fibroblasts. We are grateful to members of the Giancotti laboratory for discussions and comments on the manuscript.
This work was supported by National Institutes of Health grants R01 CA78901 (to F.G. Giancotti) and P30 CA08748 (to the Memorial Sloan-Kettering Cancer Center). F.G. Giancotti is an Established Investigator of the American Heart Association.
Abbreviations used in this paper
- ATF2
activating transcrition factor 2
- BrdU
5-bromodeoxyuridine
- CS
calf serum
- CMV
cytomegalovirus
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- GST
glutathione S-transferase
- HUVECs
human umbilical vein endothelial cells
- JNK
Jun NH2-terminal kinase
- PDGF
platelet-derived growth factor
- SD
substrate region deleted
- SFM
serum-free medium
- SRE
serum response element
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TRE
TPA-responsive element
Footnotes
Maja Oktay's present address is Department of Pathology, Yale University School of Medicine, New Haven, CT 06520.
References
- Altun-Gultekin ZF, Chandriani S, Bougeret S, Ishizaki T, Narumiya S, de Graaf P, van Bergen P en Henegouwen, H. Hanafusa, J.A. Wagner, and R.B. Birge. Activation of Rho-dependent cell spreading and focal adhesion biogenesis by the v-Crk adaptor protein. Mol Cell Biol. 1998;18:3044–3058. doi: 10.1128/mcb.18.5.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993;8:877–886. [PubMed] [Google Scholar]
- Cadwallader K, Beltman J, McCormick F, Cook S. Differential regulation of extracellular signal-regulated protein kinase 1 and Jun N-terminal kinase 1 by Ca2+and protein kinase C in endothelin-stimulated Rat-1 cells. Biochem J. 1997;321:795–804. doi: 10.1042/bj3210795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary LA, Cho D, Han, Polte TR, Hanks SK, Guan JL. Identification of p130Casas a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P-Y, Kanner SB, Whitney G, Aruffo A. A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125FAK . J Biol Chem. 1994;269:20567–20574. [PubMed] [Google Scholar]
- Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton in response to serum or LPA stimulation. J Cell Sci. 1994;107:3643–3654. doi: 10.1242/jcs.107.12.3643. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrin signaling: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Clark EA, Hynes RO. Meeting Report: 1997 keystone symposium on signal transduction by cell adhesion receptors. Biochim Biophys Acta. 1997;1333:R9–R16. doi: 10.1016/s0304-419x(97)00028-0. [DOI] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Kalinec G, Kyriakis JM, Wodgett J, Gutkind JS. Transforming G protein-coupled receptors potently activate JNK (SAPK): evidence for a divergence from the tyrosine kinase signaling pathway. J Biol Chem. 1995;270:5620–5624. doi: 10.1074/jbc.270.10.5620. [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu I, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-jun activation domain. Cell. 1994;76:1025–1035. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Dolfi F, Garcia-Guzman M, Ojaniemi M, Nakamura H, Matsuda M, Vuori K. The adaptor protein Crk connects multiple cellular stimuli to the JNK signaling pathway. Proc Natl Acad Sci USA. 1998;95:15394–15399. doi: 10.1073/pnas.95.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller SM, Knudsen B, Hanafusa H. c-Abl kinase regulates the protein binding activity of c-Crk. EMBO (Eur Mol Biol Organ) J. 1994;13:7295–7302. doi: 10.1002/j.1460-2075.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, Frame MC. The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO (Eur Mol Biol Organ) J. 1998;17:81–92. doi: 10.1093/emboj/17.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Kelaita D, Sicks S. A role for Jun N-terminal kinase in anoikis; suppression by Bcl-2 and crmA. J Cell Biol. 1996a;135:1377–1382. doi: 10.1083/jcb.135.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chan-Hui P-Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996b;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- Galien R, Emanoil-Ravier R, Mercier G. Differential effects of c-jun and CREB on c-AMP response element activation by Ha-ras. Oncogene. 1994;9:1101–1108. [PubMed] [Google Scholar]
- Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Higashita R, Li L, Van Putten V, Yamamura Y, Zarinetchi F, Heasley L, Nemeroff RA. Gα16 mimics vasoconstrictor action to induce smooth muscle alpha-actin in vascular smooth muscle cells through a Jun-NH2-terminal kinase-dependent pathway. J Biol Chem. 1997;272:25845–25850. doi: 10.1074/jbc.272.41.25845. [DOI] [PubMed] [Google Scholar]
- Honda H, Oda H, Nakamoto T, Honda Z-I, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130CAS . Nature Genetics. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ilic D, Almeida EAC, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal Kinase (JNK): from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Johnson RS, van Lingen P, Papaioannou VE, Spiegelman BM. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- Johnson RS, Spiegelman BM, Hanahan D, Wisdom R. Cellular transformation and malignancy induced by ras require c-jun. Mol Cell Biol. 1996;16:4504–4511. doi: 10.1128/mcb.16.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z-G, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Downward J. Lack of correlation between activation of Jun-NH2-terminal kinase and induction of apoptosis after detachment of epithelial cells. J Cell Biol. 1997;139:1017–1023. doi: 10.1083/jcb.139.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/Mitogen-Activated Protein Kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippel A, Reinhard C, Kavanaugh WM, Apell G, Escobedo M-A, Williams LT. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lin TH, Chen Q, Howe A, Juliano RL. Cell anchorage permits efficient signal transduction between Ras and its downstream kinases. J Biol Chem. 1997;272:8849–8852. [PubMed] [Google Scholar]
- Logan SK, Falasca M, Hu P, Schlessinger J. Phosphatidylinositol 3-kinase mediates epidermal growth factor-induced activation of the c-Jun N-terminal kinase signaling pathway. Mol Cell Biol. 1997;17:5784–5790. doi: 10.1128/mcb.17.10.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenna DA, Dolfi F, Vuori K, Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest. 1998;101:301–310. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J, Giancotti FG. Signal transduction by the α6β4 integrin: distinct β4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO (Eur Mol Biol Organ) J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero F, Murgia C, Wary KK, Curatola AM, Pepe A, Blumenberg M, Westwick JK, Der CJ, Giancotti FG. The coupling of α6β4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO (Eur Mol Biol Organ) J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Tanaka S, Nagata S, Kojima A, Kurata T, Shibuya M. Two species of human CRK cDNA encode proteins with distinct biological activities. Mol Cell Biol. 1992;12:3482–3489. doi: 10.1128/mcb.12.8.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer BJ, Hanafusa H. Mutagenic analysis of the v-crk oncogene. Requirement of SH2 and SH3 domains and correlation between increased phosphotyrosine and transformation. J Virol. 1990;64:3581–3589. doi: 10.1128/jvi.64.8.3581-3589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer BJ, Hamaguchi M, Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988;332:272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- Mayer BJ, Hirai H, Sakai R. Evidence that SH2 domains promote processive phosphorylation by protein-tyrosine kinases. Curr Biol. 1995;5:296–305. doi: 10.1016/s0960-9822(95)00060-1. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, Smeal T, Derijard B, Cobb M, Davis RJ, Karin M. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden A, Lin A, Claret F-X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO (Eur Mol Biol Organ) J. 1998;17:6622–6632. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia C, Blaikie P, Kim N, Dans M, Petrie HT, Giancotti FG. Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin β4 cytoplasmic domain. EMBO (Eur Mol Biol Organ) J. 1998;17:3940–3951. doi: 10.1093/emboj/17.14.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptor. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- Parsons JT. Integrin-mediated signaling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr Opin Cell Biol. 1996;8:146–152. doi: 10.1016/s0955-0674(96)80059-7. [DOI] [PubMed] [Google Scholar]
- Potts WM, Olsen M, Boettiger D, Vogt VM. Epitope mapping of monoclonal antibodies to gag protein p19 of avian sarcoma and leukemia viruses. J Gen Virol. 1987;68:3177–3182. doi: 10.1099/0022-1317-68-12-3177. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Wary KK, Giancotti FG, Gardner HA. Integrin α1β1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol. 1998;142:587–594. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitano AB, Halpern JR, Hambuch TM, Sawyers CL. The Bcr-Abl leukemia oncogene activates Jun kinase and requires Jun for transformation. Proc Natl Acad Sci USA. 1995;92:11746–11750. doi: 10.1073/pnas.92.25.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S, Rozengurt E. Platelet-derived growth factor modulation of focal adhesion kinase (p125FAK) and paxillin tyrosine phosphorylation in Swiss 3T3 cells. J Biol Chem. 1994;269:704–710. [PubMed] [Google Scholar]
- Renshaw MW, Ren X-D, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO (Eur Mol Biol Organ) J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K, Schiff J, Gilman MZ. Transcription factor AP-1 activity is required for initiation of DNA synthesis and is lost during cellular aging. Proc Natl Acad Sci USA. 1992;89:157–161. doi: 10.1073/pnas.89.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Parsons JT. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK . Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- Rodrigues GA, Park M, Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO (Eur Mol Biol Organ) J. 1997;16:2634–2645. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroek B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as direct target of ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Reed JC. Anchorage-dependence, integrins and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO (Eur Mol Biol Organ) J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase directs SH2-dependent binding of pp60src . Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Integrin signaling and tyrosine phosphorylation: just the facts? . Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase–c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller M, Vuori K, Ruoslahti E. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO (Eur Mol Biol Organ) J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK−cell migration. EMBO (Eur Mol Biol Organ) J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack BE. Tyrosine phosphorylation of paxillin and focal adhesion kinase by activation of muscarinic m3 receptors is dependent on integrin engagement by the extracellular matrix. Proc Natl Acad Sci USA. 1998;95:7281–7286. doi: 10.1073/pnas.95.13.7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal T, Binetruy B, Mercola DA, Birrer M, Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991;354:494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO (Eur Mol Biol Organ) J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton VP, Nichols DW, Laudano AP, Cooper GM. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989;9:639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y-C, Han J, Xu S, Cobb M, Skolnik EY. NIK is a new Ste20-related kinase that binds NCK and MEKK1 and activates the SAPK/JNK cascade via a conserved regulatory domain. EMBO (Eur Mol Biol Organ) J. 1997;16:1279–1290. doi: 10.1093/emboj/16.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Hanafusa H. Guanine-nucleotide exchange protein C3G activates JNK1 by a Ras-independent mechanism: JNK1 activation inhibited by kinase negative forms of MLK3 and DLK mixed lineage kinases. J Biol Chem. 1998;273:1281–1284. doi: 10.1074/jbc.273.3.1281. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Morishita T, Hashimoto Y, Hattori S, Nakamura S, Shibuya M, Matuoka K, Takenawa T, Kurata T, Nagashima K, et al. C3G, a guanine nucleotide-releasing protein expressed ubiquitously, binds to the Src homology 3 domains of CRK and GRB2/ASH proteins. Proc Natl Acad Sci USA. 1994;91:3443–3447. doi: 10.1073/pnas.91.8.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Ouchi T, Hanafusa H. Downstream of Crk adaptor signaling pathway: activation of Jun kinase through the guanine nucleotide exchange protein C3G. Proc Natl Acad Sci USA. 1997;94:2356–2361. doi: 10.1073/pnas.94.6.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Hoeve J, Morris C, Heisterkamp N, Groffen J. Isolation and chromosomal localization of CRKL, a human crk-like gene. Oncogene. 1993;8:2469–2474. [PubMed] [Google Scholar]
- Tokiwa G, Dikic I, Lev S, Schlessinger J. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science. 1996;273:792–794. doi: 10.1126/science.273.5276.792. [DOI] [PubMed] [Google Scholar]
- Vogt, P.K. 1994. Oncogenic transformation by Jun. In The Fos and Jun families of transcription factors. Angel, P.E., and P.A. Herrlich, editors. CRC press, Boca Raton, Florida. pp. 203–219.
- Vuori K, Hirai H, Aizawa S, Ruoslahti E. Induction of p130cassignaling complex formation upon integrin-mediated cell adhesion: a role for Src family kinases. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. 1997;9:76–85. doi: 10.1016/s0955-0674(97)80155-x. [DOI] [PubMed] [Google Scholar]
- Yu H, Li X, Marchetto GS, Dy R, Hunter D, Calvo B, Dawson TL, Wilm M, Anderegg RJ, Graves LM, et al. Activation of a novel calcium-dependent protein-tyrosine kinase. Correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J Biol Chem. 1996;271:29993–29998. doi: 10.1074/jbc.271.47.29993. [DOI] [PubMed] [Google Scholar]
- Zachary I, Sinnett-Smith J, Rozengurt E. Bombesin, vasopressin, and endothelin stimulation of tyrosine phosphorylation in Swiss 3T3 cells. Identification of a novel tyrosine kinase as a major substrate. J Biol Chem. 1992;267:19031–19034. [PubMed] [Google Scholar]
- Zhao J-H, Reiske H, Guan J-L. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]