Abstract
Sarcomere maintenance, the continual process of replacement of contractile proteins of the myofilament lattice with newly synthesized proteins, in fully differentiated contractile cells is not well understood. Adenoviral-mediated gene transfer of epitope-tagged tropomyosin (Tm) and troponin I (TnI) into adult cardiac myocytes in vitro along with confocal microscopy was used to examine the incorporation of these newly synthesized proteins into myofilaments of a fully differentiated contractile cell. The expression of epitope-tagged TnI resulted in greater replacement of the endogenous TnI than the replacement of the endogenous Tm with the expressed epitope-tagged Tm suggesting that the rates of myofilament replacement are limited by the turnover of the myofilament bound protein. Interestingly, while TnI was first detected in cardiac sarcomeres along the entire length of the thin filament, the epitope-tagged Tm preferentially replaced Tm at the pointed end of the thin filament. These results support a model for sarcomeric maintenance in fully differentiated cardiac myocytes where (a) as myofilament proteins turnover within the cell they are rapidly exchanged with newly synthesized proteins, and (b) the nature of replacement of myofilament proteins (ordered or stochastic) is protein specific, primarily affected by the structural properties of the myofilament proteins, and may have important functional consequences.
Keywords: muscle proteins, tropomyosin, troponin, cardiomyocyte, muscle structure
The muscle sarcomere is a complex three-dimensional array of contractile and regulatory proteins designed to produce both force and motion. It has been well appreciated that during myofibrillogenesis, in embryonic and neonatal muscle cells, the contractile apparatus is very dynamic with coordinate alterations in myofilament gene expression, myofilament protein synthesis/ degradation, and structural incorporation and organization (Rhee et al., 1994; Schiaffino and Reggiani, 1996; Auerbach et al., 1997; Dabiri et al., 1997). However, in fully differentiated, post-mitotic contractile cells, it is important that the contractile apparatus is also dynamic. Under these conditions, the myofilament proteins that make up this contractile apparatus are in a requisite dynamic equilibrium involving mechanisms of incorporating newly synthesized contractile proteins as well as degradation processes that remove old and possibly damaged proteins. In a global sense, myofilament protein turnover in adult muscle cells is well documented. Metabolic labeling studies have indicated that the half-lives of the major contractile proteins in the adult rat heart vary from ∼3 d for troponin I (TnI)1, 5 d for tropomyosin (Tm) and myosin, and up to 10 d for sarcomeric actin (Martin, 1981), which suggests that individual contractile proteins may turn over by different mechanisms. Interestingly, in adult cardiac myocytes, this dynamic process of synthesizing and replacing the myofilament proteins of the contractile apparatus is complicated by the fact that these cells must accomplish this feat while maintaining force production at rates of 70– 200 beats per minute in humans and up to 600–700 beats per minute in small rodents. This mechanism of sarcomere maintenance, collectively defined as the processes of myofilament protein synthesis, incorporation, and degradation, in fully differentiated muscle cells, including adult cardiac myocytes, is not well understood.
There have been several approaches to studying the protein dynamics of the contractile apparatus. These approaches have made important advances in understanding the mechanisms of synthesizing new myofibrils, or myofibrillogenesis. The formation of new myofibrils in embryonic and neonatal cardiac myocytes has been visualized by fluorescence imaging using isoform-specific, myofilament protein antibodies as well as microinjection of fluorescently labeled contractile proteins. These studies have shown the requirement of precise regulation of myofilament protein gene expression and ordered integration of specific myofilament proteins into the contractile apparatus (Dlugosz et al., 1984; Sanger et al., 1986; Wang et al., 1988; Rhee et al., 1994). More recently, transfection techniques have been used to express recombinant epitope-tagged myofilament proteins in neonatal cardiac myocytes in vitro (von Arx et al., 1995; Auerbach et al., 1997; Dabiri et al., 1997). This, along with high resolution imaging techniques, has allowed for visualization of myofibrillogenesis, and mechanisms of myofilament protein isoform sorting in differentiating cardiac myocytes.
Application of these types of studies to adult cardiac myocytes, in order to understand how sarcomere maintenance occurs in the context of a fully differentiated cell, has been limited by several factors. First, adult cardiac myocytes are inherently unstable in primary culture. Adult cardiac myocytes maintained in the presence of fetal bovine serum undergo a complex process of cytoskeletal rearrangements along with myofilament degeneration and regeneration (Jacobson, 1977; Moses and Claycomb, 1982a,b; Eppenberger et al., 1988; Messerli et al., 1993) and reexpression of neonatal myofilament protein isoforms (Eppenberger et al., 1988). Although studies using microinjection of expression plasmids containing cDNAs encoding epitope-tagged myofilament proteins in serum-treated, redifferentiating adult cardiac myocyte cultures have been important in understanding the trafficking of some myofilament proteins to the cardiac sarcomere (Soldati and Perriard, 1991; von Arx et al., 1995), the processes of myofilament protein synthesis and incorporation visualized in these studies more closely resemble myofibrillogenesis in embryonic and neonatal cardiac myocytes (Eppenberger et al., 1988; LoRusso et al., 1997) rather than sarcomere maintenance in adult cardiac myocytes. Second, standard transfection techniques are extremely inefficient and toxic to fully differentiated adult cardiac myocytes (Rust et al., 1998). There have been a few studies that have applied microinjection of mg/ml solutions of fluorescently labeled myofilament proteins into intact, fully differentiated muscle cells or bathing permeabilized muscle fibers in solutions of fluorescently labeled myofilament proteins (Sanger et al., 1984; Dome et al., 1988; LoRusso et al., 1992; Imanaka-Yoshida et al., 1993). These studies have been important in localizing specific myofilament proteins in the adult cardiac sarcomere and suggest that the contractile apparatus is capable of rapidly incorporating exogenous myofilament proteins (seconds to minutes). However, these studies are limited in that they do not measure sarcomere maintenance under normal physiological conditions of myofilament protein transcription and translation while maintaining proper protein stoichiometry and contractile function, processes that occur over several days to weeks.
As a new approach to genetically modify the cardiac sarcomere, recombinant adenoviral vectors have been used to express reporter proteins and myofilament proteins in fully differentiated rat adult cardiac myocytes in vitro while maintaining the normal myofilament protein stoichiometry, adult contractile protein isoform expression, and physiological force production of the myocytes over 6–7 d in primary culture (Westfall et al., 1997, 1998; Rust et al. 1998). In this study, adenoviral-mediated gene transfer was used to express recombinant, epitope-tagged Tm and TnI proteins in adult cardiac myocytes in vitro to visualize the process of thin filament sarcomere maintenance in fully differentiated adult cardiac myocytes. Cardiac TnI and αTm are key thin filament regulatory proteins of the adult cardiac contractile apparatus. TnI, a subunit of the troponin regulatory complex, and Tm, both bind to actin and regulate myosin crossbridge binding to actin in response to changes in intracellular [Ca2+] (Tobacman, 1996). More importantly for the studies presented here, these two proteins could also differ structurally in how they assemble on the contractile apparatus. TnI forms a trimeric complex with the Ca2+-binding subunit, troponin C, and the Tm-binding subunit, troponin T, and this complex is bound to Tm and actin at a spacing of 1 per 7 actin monomers (Tobacman, 1996). In contrast, Tm forms a dimer with itself, binds to 7 actin monomers, and forms a head to tail polymer of 24 Tm proteins along the entire 1-μm length of the thin filament (Trombitas et al., 1990; Tobacman, 1996). Because cTnI and αTm also have different measured half lives in the adult rat heart, we hypothesized that these two proteins might display different mechanisms of myofilament incorporation and therefore may provide two distinct tools for studying sarcomere maintenance.
Using this approach of gene transfer of epitope-tagged myofilament proteins into adult cardiac myocytes in vitro, along with high resolution imaging techniques, it is possible to address several fundamental questions concerning how sarcomere maintenance in adult muscle cells occurs such as: (a) Where do newly synthesized myofilament proteins incorporate into the cardiac sarcomere, i.e., is the process stochastic or ordered? (b) Is myofilament replacement uniform throughout the cell or localized to specific cellular regions? (c) Are all myofilament proteins replaced in the same manner? (d) Does sarcomere maintenance occur by complete breakdown of old thin filaments and formation of new thin filaments? By addressing these fundamental questions, this study shed new light on the mechanisms underlying how sarcomere maintenance can occur in adult cardiac myocytes while maintaining the continual ability of the cell to produce both force and motion.
Materials and Methods
Generation of Recombinant Adenovirus
The plasmid vector containing the full-length human fast skeletal αTm was a gracious gift from Dr. Clare Gooding (MacLeod and Gooding, 1988). An EcoRI fragment containing the full-length αTm cDNA was subcloned into pCA4 plasmid to add additional restriction enzyme sites for future subcloning (Westfall et al., 1997, 1998). A XbaI, HindIII fragment containing the full-length αTm cDNA was subcloned into pSP72 (Promega) for mutagenesis. The COOH-terminal FLAG epitope (DYKDDDDK) (Sigma) was engineered by PCR mutagenesis using the following primer set to amplify a 204-bp fragment: forward primer 5′ AGAGATCAAGGTCCTTTCCG 3′ and reverse primer 5′ GAAGTGAAGCT*T*AGAAACTTACTTGTCGTCATCGTCTTTGTAGTCTATGGAAGTCA-TATCGTTGAGAG 3′ (underline indicates FLAG epitope sequence). A HindIII site (asterisks) was engineered into the reverse primer to facilitate subcloning. A Ppu M1, HindIII fragment of the PCR product was ligated to the Ppu M1 site in the αTm cDNA and the αTmFLAG cDNA was verified by DNA sequencing. This subcloning step removed 197 bp of 3′ untranslated sequence. The XbaI, HindIII fragment containing the αTmFLAG cDNA was subcloned into pCA4 (pCA4αTmFLAG). The plasmid (pGEM3ZcTnI) containing the full-length cardiac troponin I cDNA was a gracious gift from Dr. Anne Murphy (Murphy et al., 1991). A COOH-terminal FLAG epitope was added by PCR mutagenesis using the following primer sets: forward primer 5′ GCCAAGGAATCCTTGGACCTGAGGG 3′ and reverse primer 5′ CAGTGTGAGAGCCATGGCTCACTTGTCGTCATCGTCTTTGTAGTCGCCCTCG*AACTTTTT- CTTTCGGCC 3′ (underline indicates FLAG epitope sequence). An XmnI site (asterisk) was engineered in the reverse primer to identify the mutagenized clones. An ApaI, NcoI fragment of the PCR product was subcloned into pGEM3ZcTNI and the resulting cTNIFLAG cDNA was verified by DNA sequencing. An EcoRI fragment containing the cTNIFLAG cDNA was subcloned into pCA4 (pCA4cTNIFLAG).
Replication-deficient recombinant adenovirus (Ad5ΔE1) vectors were constructed from the cotransfection shuttle vectors pCA4αTmFLAG or pCA4cTnIFLAG with a vector containing the full-length adenoviral genome, pJM17 followed by homologous recombination in HEK-293 cells as previously described (Westfall et al., 1998). Positive viral lysates were plaque purified and identified by restriction enzyme Southern blot analysis. The viruses were grown to high titer in HEK-293 cells, purified by CsCl centrifugation and the viral stocks (∼1010 pfu/ml) were stored in single use aliquots at −80°C.
Adult Cardiac Myocyte Culture and Gene Transfer
Adult rat ventricular myocytes were isolated and cultured as previously described (Westfall et al., 1998). In brief, female adult rats (∼200 g) were anesthetized with sodium pentobarbitol and the hearts were removed in Krebs-Henseleit buffer containing 1 mM Ca2+ (KHB+Ca2+). The hearts were perfused for 5 min with KHB+Ca2+ on a Langendorff perfusion apparatus followed by a 5-min perfusion with KHB, Ca2+-free (7–10 ml/ min). Collagenase (162 U/ml; Worthington, Type II) and hyaluronidase (0.125 mg/ml; Sigma) were then added to Ca2+-free KHB and perfusion continued for 15 min. CaCl2 was then added to a final concentration of 1 mM and perfusion continued for 10–15 min. The ventricles were then minced and digested in the enzyme solution for 2× 10 min. The tissue was then digested for 2× 15 min with gentle trituration and isolated myocytes were collected by centrifugation. The myocytes were resuspended in KHB+1 mM Ca2+ and 2% BSA and the solution was titrated to 1.75 mM Ca2+ with three additions of CaCl2 over 15 min. The resulting myocytes were collected by centrifugation and resuspended in DME, 5% FBS, 1% penicillin/streptomycin (P/S) (GIBCO BRL), and plated on laminin coated glass coverslips (1 × 105 cells/ml, 200 μl/coverslip) for 2 h. The myocytes were then infected with recombinant adenovirus diluted in 200 μl serum-free DME with P/S at 2.5–5 × 107 pfu/ml or a multiplicity of infection (MOI) of 250–500, for 1 h.
Culture Conditions
After gene transfer, 2 ml serum-free DME with P/S was then added, and for the standard culture conditions, myocytes were maintained (media changed every 48 h) in serum-free DME with P/S unless otherwise indicated. In a subset of experiments (see Fig. 4), in order to generate cultures of redifferentiating adult cardiac myocytes, myocytes were maintained in DME, 20% FBS, P/S from 12 h post infection to the end of time in culture.
Figure 4.
Expression and localization of αTmFLAG in redifferentiating cardiac myocyte cultures (myocytes treated with 20% serum) at day 7 in primary culture. (A) Immunofluorescence localization of αTmFLAG in redifferentiating cardiac myocytes. Note the striated myofibrils around the perinuclear region and the TmFLAG expression extending from Z-line to Z-line. (B) Enlargement of a portion of the perinuclear region boxed in A. (C) Western blot showing expression of Tm isoforms in day 7 redifferentiating cardiac myocytes. Note the near complete replacement of the endogenous Tm with αTmFLAG. Bar, 50 μm.
Protein Gel Electrophoresis and Western Blotting
Intact myocyte samples were prepared by scraping myocytes from coverslips into SDS sample buffer. Permeabilized myocyte samples were prepared by exposing the myocytes on coverslips to relaxing buffer (see below) containing 0.1% Triton X-100 for 3–5 s followed by two washes in 3–4 ml of relaxing buffer. The remaining permeabilized myocytes were scraped into SDS sample buffer. The samples were immediately boiled for 5 min, an equal volume of SDS sample buffer was added, and the samples were stored at −20°C. The protein samples were analyzed on 12% SDS-PAGE followed by transfer to Immobilon-P PVDF membrane (Millipore) for 2,000 V⋅ hr. The membranes were blocked overnight in TBS containing 5% nonfat dry milk. The primary antibodies used for detection of myofilament proteins were as follows: Tm, Tm311, 1:100,000 (Sigma); TnI, MAB1691, 1:1,000 (Chemicon); troponin T, JLT-12, 1:1,000 (Sigma); anti-FLAG M2, 1:2,000 (Sigma); sarcomeric actin, clone 5C5, 1:5,000 (Sigma). Primary antibody binding was detected with a goat anti–mouse IgG– horseradish peroxidase conjugate followed by ECL detection (Amersham). The films were digitized using a transparency scanner and quantitated with Multi-Analyst software (Bio Rad Laboratories). To calculate Tm stoichiometry and to compare the ratios of different myofilament proteins using multiple blots with different exposure times, the ratio of Tm/ actin data was normalized to the mean ratio in the control myocyte samples on each blot ((Tm:actin)sample / (Tm:actin)mean control).
Indirect Immunofluorescence and Confocal Microscopy
Cardiac myocytes were prepared for confocal imaging as previously described (Westfall et al., 1998) In brief, cardiac myocytes on coverslips were fixed in 3% paraformaldehyde/PBS for 30 min. Myocytes were washed 3× 5 min in PBS and incubated in PBS with 50 mM NH4Cl for 30 min followed by washing 3× 5 min in PBS. Myocytes were blocked with 20% NGS in PBS + 0.5% Triton X-100 for 30 min followed by incubation with primary antibody (Ab) for the FLAG epitope (M2, 1:500), sarcomeric Tm (CH-1, 1:200; Sigma), or TnI (MAB1691, 1:500) diluted in 2% NGS, PBS + Triton X-100) for 1.5 h. Myocytes were washed 3× 5 min in PBS + Triton X-100 and blocked again for 30 min. Myocytes were incubated with secondary Ab (goat anti–mouse IgG, Texas Red, 1:100; Molecular Probes) for 1 h followed by washing 3× 5 min in PBS + Triton X-100. The IgG Ab sites were neutralized overnight with excess whole goat anti– mouse IgG (1:20; Sigma) and followed by neutralization with goat anti– mouse IgG Fab (1:20; Jackson) for 2 h. The second set of Ab incubations were performed as indicated above with anti–α-actinin (EA53, 1:500; Sigma) followed by a goat anti–mouse IgG FITC conjugate (1:200; Sigma). Coverslips were mounted and stored at −80°C. Immunofluorescence was visualized in dual channel mode on a Nikon Diaphot 200 microscope equipped with a Noran confocal laser scanning imaging system and Silicon Graphics Indy workstation and colorized with Adobe Photoshop software. A Leitz Aristoplan fluorescence microscope was used for data presented in Fig. 4.
Cardiac Myocyte Functional Analysis
Cardiac myocyte contractile function was performed on adult cardiac myocytes maintained in serum free media as previously described (Metzger et al., 1993; Westfall et al., 1997).
Solutions.
Relaxing and activating solutions contained in mmol/liter: 7 EGTA, 1 free Mg2+, 4 MgATP, 14.5 creatine phosphate, 20 imidazole, and KCl to yield a total ionic strength of 180 mmol/liter. Solution pH was adjusted to 7.00 with KOH. The pCa (i.e., −log [Ca2+]) of the relaxing solution was set at 9.0 and the pCa of the maximal activating solution was 4.0. Intermediate pCa solutions were generated by mixing the pCa 9.0 and pCa 4.0 solutions as previously described (Metzger et al., 1993).
Cardiac Myocyte Attachment.
Coverslips were removed and washed several times with relaxing solution which results in permeabilization of the myocyte membrane. Single rod-shaped cardiac myocytes were attached to micropipettes coated with an adhesive between a force transducer (model 403A; Cambridge Tech) and moving coil galvanometer (6350; Cambridge Tech) mounted on three-way positioners (Metzger et al., 1993). Sarcomere length was set at 2.2 μm by light microscopy.
Isometric Tension Analysis.
At each pCa, steady state isometric tension was allowed to develop, followed by rapid slackening to obtain the baseline tension. The myocyte was then relaxed. Total tension was measured as the difference in tension just before and after the slack step. Active tension was calculated by subtracting the resting tension (measured at pCa 9.0)
Data Analysis.
Data were acquired on a Nicolet 310 oscilloscope. Tension-pCa curves were fit using the Marquardt-Levenberg nonlinear least squares fitting algorithm and the Hill equation in the form: P = [Ca2+]n/ (Kn + [Ca2+]n) where P is the fraction of maximum tension (Po), K is the [Ca2+] that yields one-half maximum tension, and n is the Hill coefficient (n H). Analysis of variance (ANOVA) with a Student-Neuman Keuls post hoc test were used to examine significant differences with P < 0.05 indicating significance.
Results
Generation of Recombinant Adenovirus AdαTmFLAG and AdcTnIFLAG
Recombinant replication-deficient adenovirus was generated by homologous recombination in HEK-293 cells. Southern blots of restriction enzyme digests of viral DNA using full-length cDNA digoxigenin-labeled (Boehringer Mannheim) probes show the correct insertion of the expression cassettes into the left end of the viral genome (Fig. 1).
Figure 1.
Generation of recombinant adenovirus vectors AdαTmFLAG and AdcTnIFLAG. (A) The schematic of the structure of the recombinant adenoviruses shows the enzyme sites used for restriction enzyme Southern blot analysis: XbaI, XI; HindIII, H3; EcoRV, EV; Eco RI, EI. The sets of enzymes used in lane 2 of each blot cut out the cDNA plus the left end (∼600 bp) of the viral genome indicating the appropriate insertion of the cDNA into the viral genome. The left lane in each blot (U) is uncut viral DNA, and the right lane in each blot is the full-length cDNA. (B) Southern blot of AdαTmFLAG DNA. The cDNA for αTmFLAG is ∼300 bp smaller than the full-length αTm cDNA (see Materials and Methods). (C) Southern blot of AdcTnIFLAG DNA.
Expression of αTmFLAG in Adult Cardiac Myocytes
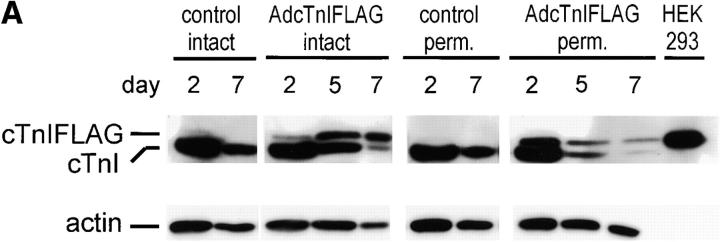
To quantitate the rate of expression and incorporation of newly synthesized αTm in adult cardiac myocytes, protein expression was determined by Western blotting of total cellular protein from AdαTmFLAG infected myocytes at several time points in primary culture (Fig. 2). The addition of the epitope resulted in a slower migration pattern of αTmFLAG than the endogenous αTm on SDS-PAGE allowing direct quantitation of expression using an αTm antibody. Note that the expressed αTmFLAG in cardiac myocytes comigrates with the protein expressed in HEK-293 cells (Fig. 2 C) infected with the same adenovirus indicating the correct processing size of αTmFLAG in two different cell types. The αTmFLAG protein was first detected in adult cardiac myocytes at day 2 in culture and the ratio of αTmFLAG to total Tm (αTmFLAG + endogenous αTm) increased over time in culture. A summary of densitometric analysis of these Western blots is shown in Fig. 2 B. If we assume that by using a strong viral promoter we can outcompete the endogenous Tm gene expression for sites available on the thin filament, then hypothetically the expression of αTmFLAG would be limited by the rate at which Tm is replaced in the thin filament. In that regard, it is interesting that the proportion of αTmFLAG to total Tm correlates well with the half-life of Tm measured in vivo (5.5 d) (Martin, 1981). Permeabilization of adult cardiac myocytes in relaxing buffer containing 0.1% TX-100 before collection for Western blot analysis resulted in no apparent change in the proportion of αTmFLAG to total Tm indicating indirectly that the expressed Tm was bound to the myofilaments (Fig. 2 C). At day 5–7 in primary culture, intact cardiac myocytes contained 39.8 ± 3.3% αTmFLAG (n = 4) and permeabilized cardiac myocytes contained 40.0 ± 2.5% αTmFLAG (n = 7, P > 0.05).
Figure 2.
Expression of αTmFLAG in adult rat cardiac myocytes in primary culture. (A) Time course of αTmFLAG expression. The blot was probed with anti-FLAG Ab shown in the top panel and an Ab recognizing all Tm isoforms shown in the bottom panel. (B) Summary of the time course of αTmFLAG expression in adult cardiac myocytes. The data are expressed as the percentage of total Tm that was αTmFLAG. The solid line is a exponential fit of the data. The dashed line is a hypothetical plot of the expected replacement of Tm with αTmFLAG assuming that all the new Tm binding to the thin filament is αTmFLAG and half-life of Tm bound to the thin filament is 5.5 d (Martin, 1981). (C) Expression of αTmFLAG in day 5 intact myocytes and in myocytes with membranes permeabilized with 0.1% Triton X-100 before sampling. Top panel used an anti-Tm Ab and the bottom panel depicts the same blot reprobed with an anti-sarcomeric actin Ab. (D) Expression of Tm and TnI isoforms in day 7 cardiac myocytes infected with AdαTmFLAG. Samples from soleus muscle show the migration pattern of βTm and ssTnI. Note there is no induction of either fetal cardiac isoform in control myocytes or myocytes treated with AdαTmFLAG over time in culture.
Expression of αTmFLAG Does Not Alter Myofilament Protein Isoform Expression or Stoichiometry
To determine if the newly synthesized Tm was replacing the endogenous Tm, Tm stoichiometry was analyzed by reprobing Western blots with antibodies recognizing sarcomeric actin and normalizing the total amount of Tm (Tm + αTmFLAG) to the amount of actin in each lane (Fig. 2 C). To compare several different Western blots from different experiments the ratios of Tm/actin were normalized to the average of the Tm/actin ratio in control myocytes on each blot (see Materials and Methods). There was no significant change in the ratio of total Tm to actin in uninfected cells (1.00 ± 0.03, mean ± SEM, n = 4) compared with the total Tm in cells at days 5–7 expressing αTmFLAG (1.21 ± 0.12, n = 8, P > 0.05). In addition, there were no detected changes in isoform expression of troponin I (Fig. 2 D) or troponin T (data not shown) and no induction of βTm in controls and AdαTmFLAG infected cells after 7 d in culture (Fig. 2 D) indicating that the adult cardiac myocytes were maintained in fully differentiated state throughout the experiments. In support of this result, it has been shown previously that similar culture conditions and adenoviral mediated gene transfer of reporter proteins has no effect of the stability and differentiated state of adult rat cardiac myocytes over 7 d in culture (Rust et al., 1998).
αTmFLAG First Incorporates into the Pointed End of the Thin Filament
Indirect immunofluorescence using an anti-FLAG mAb and confocal microscope imaging was used to follow the incorporation of the expressed αTmFLAG into the myofilaments of adult cardiac myocytes over time in culture. No αTmFLAG protein was detected by indirect immunofluorescence in AdαTmFLAG infected myocytes at day 1 after infection (data not shown). In Fig. 3 the immunofluorescence three-dimensional reconstructions of representative myocytes at days 2 and 4 after treatment with AdαTmFLAG are shown. Several interesting points can be noted from these experiments. First, the αTmFLAG incorporation is uniform throughout the entire width, length, and depth of the cardiac myocytes. Second, the αTmFLAG decorates the thin filament between, but not including, the Z-line structures (as noted by the α-actinin staining). Finally, αTmFLAG immunofluorescence always appears first at the center of the sarcomere (Fig. 3 B, inset), with absence of αTmFLAG immunofluorescence between the center of the sarcomere and the Z-line. This is quite different from the immunofluorescence pattern of endogenous Tm in uninfected cells (data not shown) and the immunofluorescence pattern of cardiac TnI which shows labeling of the entire thin filament from Z-line to Z-line (see Fig. 7). It should be noted that the resting sarcomere length in cultured fully differentiated adult cardiac myocytes is 1.8–1.9 μm. Because at this sarcomere length the thin filaments are partially overlapped, one would not expect to see a gap at the center of the sarcomere as seen in immunofluorescence of thin filament proteins in skeletal muscle fibers or neonatal cardiac myocytes (Dome et al., 1988; Helfman et al., 1999) where clearly segregated I-bands are evident. The region that is void of αTmFLAG immunofluorescence, as shown in Fig. 3, C and D, appeared to decrease slightly over time in culture which is associated with increased αTmFLAG protein expression by Western analysis. These results suggest the αTmFLAG incorporates most readily into the pointed end of the thin filament and incorporates in a direction from the pointed end to the barbed end of the thin filament.
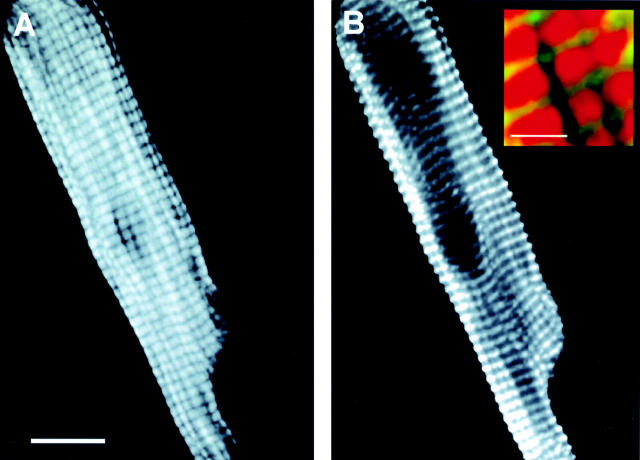
Figure 3.

Confocal three-dimensional reconstructions of representative adult cardiac myocytes expressing αTmFLAG at day 2 (A and B) and day 4 (C and D) after AdαTmFLAG treatment in primary culture. A and C show the immunofluorescence from the anti FLAG-Texas Red set of Abs. B and D show the immunofluorescence from the anti-α actinin-FITC set of Abs. Insets in B and D show an enlarged view of the merged image. Bars: (A–D) 10 μm; (B and D, insets) 2 μm.
Figure 7.
Confocal three-dimensional reconstructions of representative adult cardiac myocytes expressing cTnIFLAG at day 2 (A and B) after infection in primary culture. A shows the immunofluorescence from the anti– FLAG-Texas Red set of Abs. B shows the immunofluorescence from the anti-α actinin-FITC set of Abs. The inset in B shows a magnified view of the merged image. Similar results were seen at day 4 and 6 in culture (data not shown). Bars: (A and B) 10 μm; (B, inset) 2 μm.
The FLAG Epitope on Tm Does Not Limit Tm Incorporation into Myofilaments or Alter Myocyte Contractile Function
To determine if the addition of the eight–amino acid (DYKDDDDK) epitope somehow alters or limits the incorporation of Tm into myofilaments, a second experimental protocol was used. In this protocol, cardiac myocytes infected with AdαTmFLAG were treated with 20% serum added to the media. Treatment of cardiac myocytes with 20% serum increases myofilament protein turnover and results in redifferentiation, or the breakdown of existing myofibrils and myofibrillogenesis with reinduction of embryonic myofilament protein isoform expression (Eppenberger et al., 1988). Western blot analysis shown in Fig. 4 C indicates that treatment of AdαTmFLAG infected myocytes with 20% serum resulted in nearly complete replacement of the endogenous Tm with αTmFLAG after 6 d in culture. Small amounts of expression of βTm, the fetal Tm isoform, was induced by the treatment of cells with 20% serum, but was not different between control serum–treated cells and AdαTmFLAG serum–treated cardiac myocytes. Indirect immunofluorescence on AdαTmFLAG-infected myocytes showed αTmFLAG immunofluorescence patterns that now resemble the pattern of Tm immunofluorescence in uninfected serum–treated myocytes with striated wide bands of staining from Z-line to Z-line around the perinuclear mature myofibrillar region (Fig. 4 B) of the redifferentiating cardiac myocytes and αTmFLAG staining premyofibrils in the periphery (Fig. 4 A). These results together suggest that the FLAG epitope does not limit the structural replacement of the endogenous Tm with the adenoviral delivered αTmFLAG protein.
In addition, if the epitope was altering the structural integrity of Tm, it might be expected that myocytes expressing and incorporating TmFLAG might show altered mechanical function. To determine if the FLAG epitope alters Tm regulation of mechanical function, single cardiac myocyte isometric force measurements were used to determine if expression of αTmFLAG in fully differentiated, serum-free cardiac myocytes, resulted in alterations in contractile functions. As shown in Fig. 5, no significant changes in contractile function (maximum force, pCa50, and Hill coefficient, P > 0.05) were detected in AdαTmFLAG infected myocytes compared with control uninfected cardiac myocytes. This result was likely not due to selection of a large population of uninfected cardiac myocytes, because of the high efficiency of adenoviral-mediated gene transfer to adult cardiac myocytes in vitro using similar preparations of cardiac myocytes and infection protocols (Rust et al., 1998). In support of this point, immunofluorescence staining of time-paired myocytes indicated >85% of the AdαTmFLAG infected rod-shaped myocytes were expressing αTmFLAG (data not shown).
Figure 5.
Functional analysis of single adult cardiac myocytes at day 5 and 6 in primary culture. The closed circles show the isometric tension developed in control cardiac myocytes and the open circles show the isometric tension developed in cardiac myocytes expressing αTmFLAG. The data shown is the mean ± SEM and the average fit to the Hill equation as described in the methods. There were no significant differences in any of the parameters of Ca2+-activated isometric tension development between control and AdαTmFLAG cardiac myocytes (P > 0.05). The maximal active tension (kN/m2), the Hill coefficient (n H), and the pCa50 were 26.8 ± 13.5, 1.6 ± 0.2, and 5.83 ± 0.03, respectively, for control cardiac myocytes (n = 4) and 26.7 ± 3.7, 2.0 ± 0.2, and 5.89 ± 0.03, respectively, for myocytes expressing αTmFLAG (n = 5).
Cardiac Troponin I Incorporates Randomly along the Entire Length of the Thin Filament
To assess if the mechanism of incorporation of Tm into myofilaments was unique to Tm or if a similar mechanism exists for all thin filament regulatory proteins, myocytes were treated with the AdcTnIFLAG vector and analyzed for protein expression and myofilament protein incorporation. Fig. 6 shows the expression of cTnIFLAG in adult cardiac myocytes over time in primary culture. The ratio of cTnIFLAG to total TnI increases over time in culture indicating the cTnIFLAG protein is being expressed. Note that the ratio of cTnIFLAG to the endogenous TnI over time in culture (Fig. 6 B) is much greater than the expression of αTmFLAG (Fig. 2 B) which is consistent with the greater turnover (shorter half-life) of this protein in cardiac myocytes (Martin, 1981). Permeabilization of the myocytes before sampling does not appear to affect the ratio of cTnIFLAG to the endogenous cTnI indicating that the cTnIFLAG protein was bound to myofilaments (Fig. 6 A). In addition, there was no significant change in the ratio of total TnI to actin in untreated cells (1.00 ± 0.08, mean ± SEM, n = 8) compared with the total TnI in cells at days 5–7 expressing cTnIFLAG (0.95 ± 0.16, n = 10, P > 0.05). Confocal three-dimensional reconstructions of a representative AdcTnIFLAG-treated cardiac myocyte is shown in Fig. 7. Most notably, the first detectable immunofluorescence from cTnIFLAG at day 2 after treatment with viral vector extends the entire length of the thin filament from Z-line to Z-line (Fig. 7, inset). This pattern of immunofluorescence does not change over time in culture and appears to be identical to the immunofluorescence pattern of cTnI labeling in control cells (data not shown). This indicates that TnI turnover and incorporation occurs randomly along the entire length of the adult cardiac thin filament.
Figure 6.
Expression of cTnIFLAG in adult cardiac myocytes in primary culture. (A) Western blot analysis of intact and permeabilized cardiac myocytes infected with AdcTnIFLAG over time in primary culture. The blot was probed with an anti-TnI Ab shown in the top panel and reprobed with an anti-sarcomeric actin Ab shown in the bottom panel. (B) Summary of the densitometric analysis of cTnIFLAG expression. Data is represented as the percentage of the total TnI that was TnIFLAG. The solid line depicts the best fit exponential curve through the data. The dashed line depicts a hypothetical plot of the expected TnIFLAG expression assuming all the new TnI binding to the thin filament is cTnIFLAG and the half-life of the cTnI bound to the thin filament is 3.2 d (Martin, 1981).
To more clearly highlight the protein-specific mechanisms of replacement of endogenous myofilament proteins with newly synthesized myofilament proteins, Fig. 8 compares the immunofluorescence pattern of αTmFLAG and cTnIFLAG at day 2 after gene transfer. It should be noted that day 2 is the first day at which TmFLAG or cTnIFLAG can be detected in adult cardiac myocytes by Western blotting or immunofluorescence analysis. This comparison clearly shows that while the initial replacement of the endogenous TnI with cTnIFLAG (Fig. 8 B) occurs along the entire length of the thin filament from Z-line to Z-line (Z-lines marked by arrows), the initial replacement of αTm with αTmFLAG occurs at distinct regions of the thin filament near the pointed end (Fig. 8 A).
Figure 8.
Comparison of initial localization of newly synthesized αTmFLAG (A) and cTnIFLAG (B) in confocal 3D reconstructions of fully differentiated adult cardiac myocytes at day 2 in primary culture. A and B show the immunofluorescence from the anti–FLAG-Texas Red set of Abs. Arrows indicate position of the Z-line revealed by α-actinin immunofluorescence staining (not shown). Bar, 10 μm.
Discussion
In this study, the mechanism of sarcomere maintenance, collectively defined as the processes of myofilament protein synthesis, incorporation, and degradation, in a fully differentiated muscle cell, the adult cardiac myocyte, was examined. Using adenoviral-mediated gene transfer into adult cardiac myocytes in vitro, the expression and incorporation of newly synthesized epitope-tagged contractile proteins into myofilaments was visualized in order to understand the process of sarcomere maintenance in fully differentiated contractile cells. The results from the expression of epitope-tagged αTm and cTnI in adult cardiac myocytes suggest common mechanisms and protein specific mechanisms of sarcomeric maintenance that shed new light on how sarcomeric maintenance occurs while the cell is still able to maintain force production.
Common Features of Sarcomere Maintenance in Fully Differentiated Cardiac Myocytes
The results presented here suggest several common mechanisms of sarcomeric maintenance. Given the high efficiency of adenoviral mediated myofilament gene transfer into adult cardiac myocytes in vitro (Westfall et al., 1997, 1998; Rust et al., 1998; this study), it is possible to estimate the average protein replacement in single cardiac myocytes by measuring the protein expression in a large sample of cardiac myocytes. From the results shown in Figs. 2 and 6, it is apparent that the expression of both epitope-tagged αTm and cTnI proteins results in expression and incorporation rates that are similar to their measured half-life in vivo (Martin, 1981). Interestingly, in both cases the expression of exogenous cTnI or Tm does not change the total amount of either TnI or Tm indicating that the expression of the endogenous protein is downregulated. A similar result was obtained previously using adenoviral-mediated gene transfer of slow skeletal TnI into adult cardiac myocytes in vitro (Westfall et al., 1997). This maintenance of myofilament protein stoichiometry during changing levels of gene expression has been seen in several mouse models where ablation of one allele of the cardiac expressed αTm1 gene or overexpression the fetal isoform βTm of in the heart results in no change in the total amount of myofilament bound Tm protein (Muthuchamy et al., 1995; Blanchard et al., 1997; Rethinasamy et al., 1998). Interestingly, while ablation of one allele of the αTm gene has no effect on total Tm in the heart and doesn't result in any marked changes in cardiac function (Blanchard et al., 1997; Rethinasamy et al., 1998), ablation of a single copy of αTm gene in yeast disrupts cellular function (Liu and Bretscher, 1989), and in Drosophila a similar disruption of one Tm allele results in impairment of indirect flight muscle function (Molloy et al., 1992; Kreuz et al., 1996). This suggests that the ability of myofilament proteins and the contractile apparatus to adapt to altered levels of myofilament gene expression may not be evolutionarily conserved. Taken together, these results also suggest that the rate of myofilament incorporation of newly synthesized contractile proteins is limited by the turnover rate of the endogenous myofilament protein already residing on sites on the myofilament lattice. If sites on myofilaments are unavailable, the newly synthesized contractile protein is likely not made or is rapidly degraded. In support of these results, a previous study by Dome et al. (1988) showed that binding of fluorescently labeled brain Tm to permeabilized muscle fibers could only occur if the endogenous Tm was extracted by high salt treatment. The mechanism of regulation of myofilament protein stoichiometry in adult muscle cells is not well understood, although mouse models of overexpression and ablation of myofilament genes in the heart suggest that multiple regulatory mechanisms, including transcriptional and translation regulation, may be involved (James and Robbins, 1997; Rethinasamy et al., 1998).
Another common feature of sarcomere maintenance shown in this study is that replacement of endogenous myofilament proteins is uniform throughout the entire cell. This suggests that in mature adult cardiac myocytes where the myofilament lattice is already formed, the myofilaments throughout the muscle cell are being replaced simultaneously. A previous report using 3H-leucine pulse–chase techniques suggested that unidentified newly synthesized protein in cultured, growing skeletal muscle myotubes appears throughout the muscle fiber but also showed that newly synthesized proteins appear somewhat more readily around the periphery of myofibrils (Morkin, 1970). Although we did not find any direct evidence in support of this latter result in fully differentiated adult cardiac myocytes, the resolution needed to address this question is probably beyond the limits of confocal microscopy especially in the axial direction.
Protein-specific Features of Sarcomere Maintenance in Fully Differentiated Cardiac Myocytes
In this study, the visualization of the incorporation of newly synthesized αTmFLAG and cTnIFLAG with confocal microscopy yielded some interesting and surprising results. As shown in Fig. 3, the incorporation of newly synthesized Tm appears at the free end or pointed end of the thin filament. Two important conclusions can be drawn from this result. First, the newly synthesized αTmFLAG protein is capable of binding to the appropriate location of Tm in the sarcomere, namely the thin filament regions with no binding to the Z-line (Endo et al., 1966; Trombitas, 1990). Second, the replacement of the endogenous Tm with newly synthesized Tm occurs more readily at the pointed end of the thin filament and continues toward the Z-line.
Several explanations could explain this result of preferred pointed end replacement of the endogenous Tm with newly synthesized Tm. The first and most likely explanation is that Tm turnover occurs more readily at the pointed end of the thin filament. We speculate that this is due to the structural properties of Tm, in that it may be more favorable to remove Tm from one end of the head-to-tail polymer than in the center of the polymer, especially if one end of the polymer is anchored in the Z-line by binding to α-actinin (Puskin et al., 1977) or other Z-line proteins. Interestingly, Trombitas et al. (1990) reported that when localizing Tm in frog skeletal muscle with immunoelectron microscopy, there were differences in the ability of certain Tm antibodies to recognize the Tm proteins nearest the Z-line. In particular, the 24th Tm (nearest the Z-line) was only recognized by an antibody that preferentially binds to phosphorylated Tm. These results suggest there may be structural differences in Tm that may affect how rapidly Tm can exchange with newly synthesized Tm along the length of the thin filament.
A second possible explanation is that the result is an artifact due to the addition of the COOH-terminal FLAG epitope. Previous biochemical work has shown that modification of the ends of Tm by acetylation or deletion can alter actin binding affinity (Hitchock-DeGregori, 1994). If the addition of the COOH-terminal FLAG epitope merely disrupted end-to-end interactions of Tm preventing polymerization, we would expect to be able to replace Tm equally well on both ends of the thin filament, a result which was not seen. To confirm further that the epitope does not limit or alter the incorporation of Tm into myofilaments two additional control experiments were performed. First, under serum-treated conditions, the αTmFLAG protein nearly completely replaced the endogenous Tm protein from Z-line to Z-line in the mature myofibrillar regions indicating that the epitope itself is not limiting the incorporation of the αTmFLAG protein into the myofilaments. Second, if the epitope itself was significantly altering the Tm structure, we would expect contractile function would be altered in myocytes expressing the epitope-tagged Tm. This result was not seen as there was no significant difference in Ca2+-activated contractile function (e.g., pCa50, P 0, n H) between control cardiac myocytes and cardiac myocytes expressing αTmFLAG. In support of this result, it was also observed that serum-treated adult cardiac myocytes expressing the TmFLAG protein spontaneously beat in culture similar to control serum-treated cardiac myocytes while in these cells nearly all the Tm in these cardiac myocytes has been replaced with αTmFLAG.
A third possible explanation for the preferred pointed end replacement of the endogenous Tm with newly synthesized Tm is that this process of Tm replacement is not characteristic of the turnover of Tm protein alone but a visualization of what is happening globally to the thin filaments. In other words, is what is visualized with Tm incorporation specific to Tm or a manifestation of thin filaments completely breaking down and reforming from their pointed ends? If the latter were the case, it would be hypothesized that other newly synthesized thin filament proteins would show similar patterns of incorporation into myofilaments. The results presented here show that incorporation of the newly synthesized cTnIFLAG protein does not show preferred pointed end incorporation. Rather, cTnIFLAG is incorporated in all sarcomeres throughout the cell in a stochastic fashion across the length of the thin filament. Interestingly, the half-lives of the subunits of the troponin complex in the rat heart in vivo are varied with TnI and TnT at ∼3 d and TnC at ∼5 d (Martin, 1994). This would suggest, along with our results, that the replacement of Tn subunits may either occur rapidly while the complex remains attached to the thin filament, or that dissociated TnC subunits are capable of reassociating with newly synthesized TnI and TnT subunits followed by rebinding stochastically to the thin filament. The lack of kinetic evidence from isotope studies for precursor pools of TnT and TnC in adult rat heart favors that subunit exchange might take place while the troponin complex remains attached to actin (Martin, 1981).
Comparison of Tm Replacement during Sarcomere Maintenance and Myofibrillogenesis
The pointed end incorporation of newly synthesized αTm in fully differentiated adult cardiac myocytes presented here differs significantly from recent studies in serum-treated neonatal cardiac myocytes. The expression of transfected green fluorescent protein-tagged Tm (Tm-GFP) in neonatal myocytes (Helfman et al., 1999) showed localization of Tm after 48 h along the entire length of the thin filament. There are several possible explanations for these different observations. First, since complete replacement of the endogenous Tm by newly synthesized Tm does not occur in our 7-d culture period in fully differentiated adult cardiac myocytes, we would hypothesize that if culture conditions could be extended further, complete replacement of Tm from Z-line to Z-line would eventually occur (see model below). If the turnover of myofilaments is much faster in neonatal cardiac myocytes (which is likely since neonatal myocytes undergo shape changes and cell division) full replacement of Tm from Z-line to Z-line could be complete in 48 h. However, the average percentage of Tm replaced by Western blotting cannot be estimated in the Tm-GFP experiments due to the low transfection efficiency of neonatal cardiac myocytes (Helfman et al., 1999). Second, Morkin (1970) found in growing muscle myotubes (neonatal skeletal muscle) that new myofibrillar proteins were added preferentially to the periphery of the myofibrils. If myofibrils are adding more filaments in parallel as they grow wider in differentiating neonatal muscle cells, the addition of new filaments from the Z-line could explain the end to end incorporation. Indeed, our results of αTmFLAG incorporation into myofilaments of serum-treated redifferentiating adult cardiac myocytes show very similar incorporation patterns to GFP-Tm incorporation into neonatal adult cardiac myocytes (Helfman et al., 1999). By switching adult cardiac myocytes from a state of sarcomere maintenance (i.e., serum-free conditions) to increased turnover and myofibrillogenesis (i.e., serum conditions) incorporation of αTmFLAG from Z-line to Z-line and complete replacement of the endogenous Tm can and does occur (Fig. 4).
Models of Sarcomere Maintenance in Fully Differentiated Cardiac Myocytes
The differential incorporation of newly synthesized Tm and TnI proteins into sarcomeres of adult cardiac myocytes not only suggests that there are contractile protein specific mechanisms for sarcomere maintenance but also suggests some important basic mechanisms for sarcomere maintenance in fully differentiated contractile cells. Fig. 9 shows several possible models of how replacement of thin filament proteins during sarcomere maintenance could occur.
Figure 9.
Models of thin filament sarcomere maintenance in adult cardiac myocytes. In each model, newly synthesized contractile proteins are shown in black. The contractile proteins are shown schematically and labeled in the model shown in A. (A) In model one, thin filaments remain intact with ordered (Tm) or stochastic (TnI) exchange of newly synthesized contractile proteins with older proteins bound to the thin filament. The exchange of TnI and Tm is shown separately on the left and right, respectively, for simplicity. The mechanism of exchange for TnI and Tm is influenced by structural properties of these proteins. (B) In model two, sarcomere maintenance occurs by formation of new thin filaments by actin nucleation and polymerization from the Z-line with simultaneous addition of newly synthesized regulatory proteins. (C) In model three, thin filaments are broken down from the free or pointed end followed by repolymerization and addition of regulatory proteins.
The results from this study favor the model shown in Fig. 9 A in which sarcomere maintenance occurs while maintaining a nearly intact thin filament. More specifically, endogenous contractile proteins of the thin filament are capable of rapidly exchanging with newly synthesized contractile proteins, so rapidly that the thin filament structure and function is not dramatically altered. For instance, it has been shown previously that extraction of myofilament proteins such as TnC from skeletal muscle fibers results in a dramatic alterations in the ability to produce force in response to a change in [Ca2+] (Moss, 1992). Consequently, if sites remained vacant for a substantial period of time, this would lead to a dramatic destabilization of sarcomeric structure and alterations in force production of the cell, processes which did not occur as shown in Fig. 5. In addition, this maintenance of intact thin filaments could explain how myofilament protein turnover can occur while maintaining continuous and near maximal force production of the adult cardiac myocyte in vivo.
The mechanisms of exchange of newly synthesized regulatory proteins with endogenous proteins of the thin filament in the first model are protein isoform specific. Cardiac TnI, as a subunit of the ternary troponin complex exchanges stochastically along the length of the thin filament, possibly while the complex remains attached to the thin filament through interactions of TnT and tropomyosin. Tm, which forms head to tail polymers, exchanges with the endogenous Tm in a more ordered fashion. Assuming the Tm polymer is anchored into the Z-line by binding α-actinin, or the Tm nearest the Z-line is structurally different, it is more favorable to remove and replace Tm proteins from the pointed end.
Whereas the results presented here support the model detailed in Fig. 9 A, there are at least two other possible models of sarcomere maintenance that could be considered. In the second model shown in Fig. 9 B, older thin filaments are replaced by formation of entirely new thin filaments. This process could occur by nucleation of new actin polymers from the Z-line, polymerization of new thin filaments with coordinate addition of regulatory proteins followed by removal and breakdown of older thin filaments. This model was proposed by LoRusso et al. (1992) to explain the rapid incorporation (30 s) of microinjected fluorescently labeled actin into myofilaments of freshly isolated adult cardiac myocytes. If sarcomere maintenance occurred by formation of new thin filaments, we would have expected to see the newly synthesized Tm and TnI first binding near either side of the Z-line and extending toward the pointed end over time in culture as these new thin filaments polymerized, a result which was not obtained. It could be argued that actin cables are fully formed from the Z-line followed by addition of newly synthesized Tm from the pointed end. However, this would require a large portion of actin polymers to remain stable in the absence of any Tm binding for several days, and it has been shown previously in yeast and in the hearts of mutant axolotls that in the absence of Tm, actin filaments are not stable and/or do not readily form (Liu and Bretscher, 1989; Lemanski et al., 1976).
In the hypothetical third model of sarcomere maintenance shown in Fig 9 C, the pointed ends of the thin filament are most readily turned over by a process of breaking down thin filaments from the free end, followed by repolymerization of the actin filament and association of regulatory proteins. If the extent or length of myofilament breakdown from the pointed end was stochastic, gradually over time, more and more of the thin filaments on average would be replaced. This model could explain the results seen for the incorporation of newly synthesized Tm into myofilaments. However, if this model was accurate in describing sarcomere maintenance, it would be expected to see similar pointed end incorporation of newly synthesized TnI, a result which was not obtained.
In conclusion, the results presented here support a model for sarcomere maintenance (Fig. 9 A) in which the replacement of thin filament proteins by newly synthesized proteins (a) is a ordered process for Tm and a stochastic process for TnI, and (b) exchange with newly synthesized proteins occurs rapidly enough for thin filament structure to be maintained which allows the adult cardiac myocyte to undergo sarcomere maintenance while maintaining the continual ability to produce force and motion. These results and this model suggest that Tm proteins nearest the Z-line are older than the Tm on the pointed ends of the thin filament. The functional consequences of this property of Tm replacement are unknown. However, it is well known that in cardiac muscle there is a sarcomere length dependence of Ca2+ activation where at longer sarcomere length the myofilaments are more sensitive to activating Ca2+ (Hibberd and Jewell, 1982). Whether or not structural, functional, or age differences in Tm along the length of the thin filament contribute to or reflect properties of this phenomenon remains to be tested. In addition, it is unknown how other contractile proteins of the contractile apparatus are replaced in fully differentiated cardiac myocytes. For example, it will be interesting in future studies to determine how the subunits of the troponin complex are replaced. The components of this complex have been shown to have different turnover rates in vivo (Martin, 1981). This suggests that a single troponin subunit may either turnover while the other subunits remain attached to the thin filament (allowing different rates of total protein turnover), or that troponin subunits with longer half-lives (TnC ∼5 d) can reassociate with newly synthesized troponin subunits and rebind to the thin filament. Finally, another interesting outcome of this work will be to determine if sarcomere maintenance of contractile proteins is altered under pathophysiological conditions such as heart failure and aging.
Acknowledgments
We thank Dr. Clare Gooding and Dr. Anne Murphy for their gifts of the αTm and cTnI cDNAs, respectively, and Dr. Margaret Westfall for her contribution to the designing of the primers for the cTnIFLAG mutagenesis. We thank the Tom Komorowski and the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center for assistance and training with the confocal microscope.
Abbreviations used in this paper
- Ab
antibody
- KHB
Krebs-Henseleit buffer
- MOI
multiplicity of infection
- Tm
tropomyosin
- TnC
troponin C
- TnI
troponin I
- TnT
troponin T
Footnotes
This research was funded by grants from the National Institutes of Health (NIH) and the American Heart Association to J.M. Metzger and NIH Training Grants to D.E. Michele. J.M. Metzger is an Established Investigator for the American Heart Association.
References
- Auerbach D, Rothen-Ruthishauser B, Bantle S, Leu M, Ehler E, Helfman D, Perriard J-C. Molecular mechanisms of myofibril assembly in heart. Cell Struct Funct. 1997;22:139–146. doi: 10.1247/csf.22.139. [DOI] [PubMed] [Google Scholar]
- Blanchard EM, Iizuka K, Christe M, Conner DA, Geisterfer-Lowrance A, Schoen FJ, Maughn DW, Seidman CE, Seidman JG. Targeted ablation of the murine alpha-tropomyosin gene. Circ Res. 1997;81:1005–1010. doi: 10.1161/01.res.81.6.1005. [DOI] [PubMed] [Google Scholar]
- Dabiri GA, Turnacioglu KK, Sanger JM, Sanger JW. Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proc Natl Acad Sci USA. 1997;94:9493–9498. doi: 10.1073/pnas.94.17.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dome JS, Mittal B, Pochapin MB, Sanger JM, Sanger JW. Incorporation of fluorescently labeled actin and tropomyosin into muscle cells. Cell Differ. 1988;23:37–52. doi: 10.1016/0045-6039(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Antin PB, Nachmias VT, Holzer H. The relationship between stress-fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol. 1984;99:2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Nonomura Y, Masaki T, Ohtsuki I, Ebashi S. Localization of native tropomyosin in relation to striation patterns. J Biochem. 1966;60:605–608. [Google Scholar]
- Eppenberger ME, Hauser I, Bächi T, Schaub MC, Brunner UT, Dechesne CA, Eppenberger HM. Immunocytochemical analysis of the regeneration of myofibrils in long-term cultures of adult cardiac myocytes of the rat. Dev Biol. 1988;130:1–15. doi: 10.1016/0012-1606(88)90408-3. [DOI] [PubMed] [Google Scholar]
- Helfman DM, Berthier C, Grossman J, Leu M, Ehler E, Perriard E, Perriard J-C. Nonmuscle tropomyosin-4 requires co-expression with other low molecular weight isoforms for binding to the thin filaments in cardiac myocytes. J Cell Sci. 1999;112:371–380. doi: 10.1242/jcs.112.3.371. [DOI] [PubMed] [Google Scholar]
- Hibberd MG, Jewell BR. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol Lond. 1982;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock-DeGregori, S.E. 1994. Structural requirements of tropomyosin for binding to filamentous actin. In Actin: Biophysics, Biochemistry and Cell Biology. J.E. Estes, and P.J. Higgins, editors. Plenum Press, NY. 85–96. [DOI] [PubMed]
- Imanaka-Yoshida K, Sanger JM, Sanger JW. Contractile protein dynamics of myofibrils in paired adult rat cardiomyocytes. Cell Motil Cytoskelet. 1993;26:301–312. doi: 10.1002/cm.970260405. [DOI] [PubMed] [Google Scholar]
- Jacobson SL. Culture of spontaneously contracting myocardial cells from adult rats. Cell Struct Funct. 1977;2:1–9. [Google Scholar]
- James J, Robbins J. Molecular remodeling of contractile function. Am J Physiol. 1997;273:H2105–H2118. doi: 10.1152/ajpheart.1997.273.5.H2105. [DOI] [PubMed] [Google Scholar]
- Kreuz AJ, Simcox A, Maughan D. Alterations in flight muscle ultrastructure and function in Drosophilatropomyosin mutants. J Cell Biol. 1996;135:673–687. doi: 10.1083/jcb.135.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemanski LF, Mooseker MS, Peachey LD, Iyengar MR. Studies of muscle proteins in embryonic myocardial cells of cardiac lethal mutant Mexican axolotls by use of heavy meromyosin binding and sodium dodecyl sulfate polyacrylamide gel electrophoresis. J Cell Biol. 1976;68:375–388. doi: 10.1083/jcb.68.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Bretscher A. Disruption of a single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell. 1989;57:233–242. doi: 10.1016/0092-8674(89)90961-6. [DOI] [PubMed] [Google Scholar]
- LoRusso SM, Imanaka-Yoshida K, Shuman H, Sanger JM, Sanger JW. Incorporation of fluorescently labeled contractile proteins into freshly isolated living adult cardiac myocytes. Cell Motil Cytoskelet. 1992;21:111–122. doi: 10.1002/cm.970210204. [DOI] [PubMed] [Google Scholar]
- LoRusso SM, Rhee D, Sanger JM, Sanger JW. Premyofibrils in spreading adult cardiomyocytes in tissue culture: Evidence for reexpression of the embryonic program for myofibrillogenesis in adult cells. Cell Motil Cytoskelet. 1997;37:183–198. doi: 10.1002/(SICI)1097-0169(1997)37:3<183::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Martin AF. Turnover of cardiac troponin subunits. J Biol Chem. 1981;236:964–968. [PubMed] [Google Scholar]
- MacLeod AR, Gooding C. Human hTmα gene: expression in muscle and nonmuscle tissue. Mol Cell Biol. 1988;8:433–440. doi: 10.1128/mcb.8.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli JM, Eppenberger-Eberhardt ME, Rutishauser BM, Schwarb P, von Arx P, Koch-Schneidemann SK, Eppenberger HM, Perriard JC. Remodelling of the cardiomyocyte architecture visualized by three-dimensional confocal microscopy. Histochemistry. 1993;100:193–202. doi: 10.1007/BF00269092. [DOI] [PubMed] [Google Scholar]
- Metzger JM, Parmacek MS, Barr E, Pasyk K, Lin W-I, Cochrane KL, Field LJ, Leiden JM. Skeletal troponin C reduces contractile sensitivity to acidosis in adult cardiac myocytes from transgenic mice. Proc Natl Acad Sci USA. 1993;90:9036–9040. doi: 10.1073/pnas.90.19.9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy, J., A. Kreuz, R. Miller, T. Tansey, and D. Maughan. 1992. Effects of tropomyosin deficiency in flight muscle of Drosophila melanogaster. In Mechanism of Myofilament Sliding in Muscle. H. Sugi, and G. Pollack, editors. Plenum Press, New York. 165–172. [DOI] [PubMed]
- Morkin E. Postnatal muscle fiber assembly: localization of newly synthesized myofibrillar proteins. Science. 1970;167:1499–1501. doi: 10.1126/science.167.3924.1499. [DOI] [PubMed] [Google Scholar]
- Moses RL, Claycomb WC. Culture of terminally and differentiated adult cardiac cells in culture. Am J Anat. 1982a;164:113–131. doi: 10.1002/aja.1001640203. [DOI] [PubMed] [Google Scholar]
- Moses RL, Claycomb WC. Disorganization and reestablishment of cardiac cell ultrastructure in adult rat ventricular muscle cells. J Ultrastruct Res. 1982b;81:358–374. doi: 10.1016/s0022-5320(82)90064-8. [DOI] [PubMed] [Google Scholar]
- Moss RL. Ca2+regulation of mechanical properties of striated muscle: mechanistic studies using extraction and replacement of regulatory proteins. Circ Res. 1992;70:865–884. doi: 10.1161/01.res.70.5.865. [DOI] [PubMed] [Google Scholar]
- Murphy AM, Jones L, II, Sims HF, Strauss AW. Molecular cloning of rat cardiac troponin I and analysis of troponin I isoform expression in developing rat heart. Biochemistry. 1991;30:707–712. doi: 10.1021/bi00217a018. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Grupp IL, Grupp G, O'Toole BA, Kier AB, Boivin GP, Neumann J, Wieczorek DF. Molecular and physiological effects of overexpressing striated muscle β-tropomyosin in the murine adult heart. J Biol Chem. 1995;270:30593–30603. doi: 10.1074/jbc.270.51.30593. [DOI] [PubMed] [Google Scholar]
- Puszkin S, Puszkin E, Maimon J, Roualt C, Schook W, Ores C, Kochwa S, Rosenfield R. α-Actinin and tropomyosin interactions with a hybrid complex of erythrocyte actin and muscle myosin. J Biol Chem. 1977;252:5529–5537. [PubMed] [Google Scholar]
- Rethinasamy P, Muthuchamy M, Hewett T, Boivin G, Wolska BM, Evans C, Solaro RJ, Wieczorek DF. Molecular and physiological effects of α-tropomyosin ablation in the mouse. Circ Res. 1998;82:116–123. doi: 10.1161/01.res.82.1.116. [DOI] [PubMed] [Google Scholar]
- Rhee D, Sanger JM, Sanger JW. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskelet. 1994;28:1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- Rust EM, Westfall MV, Metzger JM. Stability of the contractile assembly and Ca2+activated tension in adenovirus infected adult cardiac myocytes. J Mol Cell Biochem. 1998;181:143–155. doi: 10.1023/a:1006802719136. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Mittal B, Sanger JM. Analysis of myofibrillar structure and assembly using fluorescently labeled contractile proteins. J Cell Biol. 1984;98:825–833. doi: 10.1083/jcb.98.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JM, Mittal B, Pochapin M, Sanger JW. Observations of microfilament bundles in living cells microinjected with fluorescently labelled contractile proteins. J Cell Sci Suppl. 1986;5:17–44. doi: 10.1242/jcs.1986.supplement_5.2. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Soldati T, Perriard J-C. Intracompartmental sorting of essential myosin light chains: molecular dissection and in vivomonitoring by epitope tagging. Cell. 1991;66:277–289. doi: 10.1016/0092-8674(91)90618-9. [DOI] [PubMed] [Google Scholar]
- Tobacman LS. Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol. 1996;58:447–481. doi: 10.1146/annurev.ph.58.030196.002311. [DOI] [PubMed] [Google Scholar]
- Trombitás K, Baatesen PHWW, Lin JJ-C, Lemanski LF, Pollack GH. Immunoelectron microscopic observations of tropomyosin localization in striated muscle. J Musc Res Cell Motil. 1990;11:445–452. doi: 10.1007/BF01739764. [DOI] [PubMed] [Google Scholar]
- von Arx P, Bantle S, Soldati T, Perriard JC. Dominant negative effect of cytoplasmic actin isoproteins on cardiomyocyte cytoarchitecture and function. J Cell Biol. 1995;131:1759–1773. doi: 10.1083/jcb.131.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-M ML, Greaser, Schultz E, Bulinski JC, Lin JJ-C, Lessard JL. Studies on cardiac myofibrillogenesis with antibodies to titin, actin, tropomyosin and myosin. J Cell Biol. 1988;107:1075–1083. doi: 10.1083/jcb.107.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall MV, Rust EM, Metzger JM. Slow skeletal troponin I gene transfer, expression and myofilament incorporation enhances adult cardiac myocyte function. 1997. Proc Natl Acad Sci USA. 1997;94:5444–5449. doi: 10.1073/pnas.94.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall MV, Rust EM, Albayya F, Metzger JM. Adenovirus-mediated myofilament gene transfer into adult cardiac myocytes. Methods Cell Biol. 1998;52:307–322. [PubMed] [Google Scholar]