Abstract
In mouse oocytes, the first meiotic spindle is formed through the action of multiple microtubule organizing centers rather than a pair of centrosomes. Although the chromosomes are thought to play a major role in organizing the meiotic spindle, it remains unclear how a stable bipolar spindle is established. We have studied the formation of the first meiotic spindle in murine oocytes from mice homozygous for a targeted disruption of the DNA mismatch repair gene, Mlh1. In the absence of the MLH1 protein meiotic recombination is dramatically reduced and, as a result, the vast majority of chromosomes are present as unpaired univalents at the first meiotic division. The orientation of these univalent chromosomes at prometaphase suggests that they are unable to establish stable bipolar spindle attachments, presumably due to the inability to differentiate functional kinetochore domains on individual sister chromatids. In the presence of this aberrant chromosome behavior a stable first meiotic spindle is not formed, the spindle poles continue to elongate, and the vast majority of cells never initiate anaphase. These results suggest that, in female meiotic systems in which spindle formation is based on the action of multiple microtubule organizing centers, the chromosomes not only promote microtubule polymerization and organization but their attachment to opposite spindle poles acts to stabilize the forming spindle poles.
Keywords: cell cycle, meiosis, mismatch repair, oocyte, spindle formation
Meiotic cell division requires specialized cell cycle control mechanisms to ensure the segregation of homologous chromosomes at the first meiotic division (MI)1. In organisms that undergo recombination, the sites of meiotic exchange play a key role in chromosome segregation at MI, and mutations that reduce the level of recombination are invariably associated with increased errors in meiotic chromosome segregation. Chiasmata, the sites of recombination, facilitate the orientation of homologues on the spindle at MI and counterbalance the forces directing them to opposite poles, thus allowing the homologous pair to align at the spindle equator at metaphase (for review see Hawley, 1988; Carpenter, 1994). Recently, it has become obvious that, in organisms as evolutionarily diverse as Drosophila and humans, subtle alterations in the placement of recombination events on the chromosome arm can substantially alter the fidelity of meiotic chromosome segregation (Koehler et al., 1996).
Although the role of recombination in chromosome segregation is evolutionarily conserved, it is not the only means of ensuring accuracy in meiotic chromosome segregation. Mechanisms for the segregation of nonexchange homologues have been described in a variety of species. These include mechanisms for the segregation of homologues in the complete absence of recombination, mechanisms for the segregation of a single pair of chromosomes that never recombine, and backup mechanisms for the segregation of homologues in the event of recombination failure (for review see Wolf, 1993).
In addition to recombination and specialized mechanisms for the segregation of nonexchange bivalents, the fidelity of meiotic cell division is ensured by cell cycle control mechanisms that regulate transition points in the cycle. These cell cycle surveillance mechanisms function to detect mistakes and inhibit cell cycle progression under conditions that would result in grave errors in cell division (Murray, 1994; Elledge, 1996; Page and Orr-Weaver, 1997). Of particular interest is the checkpoint mechanism that operates at the metaphase-anaphase transition. This checkpoint delays anaphase onset in cells with defective spindle formation or chromosome alignment, providing an opportunity to correct errors that would predispose to meiotic chromosome missegregation. Recent studies of murine female meiosis suggest that the presence of unaligned chromosomes at first meiotic metaphase does not induce a delay in anaphase onset (Hunt et al., 1995; LeMaire-Adkins et al., 1997; Yin et al., 1998; Hunt, P.A., unpublished observations). It has been hypothesized that the lack of chromosome-mediated metaphase-anaphase checkpoint control in mammalian females may contribute to the high error rate in female meiosis (Hunt et al., 1995; LeMaire-Adkins et al., 1997).
From the standpoint of meiotic chromosome segregation, the DNA mismatch repair proteins provide a particularly interesting class of mutations because in addition to their role in the recognition and repair of DNA mismatches during somatic cell division, at least some of these proteins play a role in meiotic recombination (for review see Chambers et al., 1996; Kolodner, 1996; Modrich and Lahue, 1996). In the mouse, targeted disruptions of several mismatch repair genes have been produced, and mice homozygous for three of these, Pms2, Mlh1, and Msh5, demonstrate a meiotic phenotype (Baker et al., 1995; Baker et al., 1996; Edelmann et al., 1996, 1999; de Vries et al., 1999).
Evidence from studies in yeast suggests that the role of the MutS homologues Msh4 and Msh5 is meiosis-specific. That is, neither is involved in the repair of DNA mismatches in somatic cells but both play a role in meiotic recombination (for review see Modrich and Lahue, 1996). The recent demonstration that Msh5 knockout mice are sterile with germ cell loss at early prophase I due to synaptic failure (de Vries et al., 1999; Edelmann et al., 1999) suggests that these MutS homologues participate in the early events of homologue synapsis. In yeast, the MutL homologues Pms1 (mammalian Pms2) and Mlh1 are involved in DNA mismatch repair during both replication and recombination, but Mlh1 plays a unique role in promoting meiotic recombination and has been proposed to act downstream of Msh4 and Msh5 (Hunter and Borts, 1997). This is consistent with the phenotype of the Mlh1 knockout mouse: in MLH1 null male mice, synapsis of homologous chromosomes proceeds normally, but with the disappearance of the synaptonemal complex at late pachynema, the majority of homologues fail to maintain a physical connection and appear as univalents (Baker et al., 1996). Indeed it has been reported that lack of the MLH1 protein results in a 10-fold reduction in recombination (Baker et al., 1996). Recombination defects in the MLH1 null male mouse result in meiotic arrest, presumably because the presence of univalent chromosomes activates the metaphase-anaphase checkpoint control mechanism, resulting in the arrest of the spermatocyte at metaphase I. Thus, no mature spermatozoa are produced.
MLH1 null females, like null males, are sterile but the meiotic phenotype is apparently different. Although data on homologue synapsis are lacking, previous studies suggest that null females ovulate normal numbers of oocytes in response to exogenous hormones. Ovulated oocytes, however, exhibit a number of abnormalities, including failure to extrude a second polar body, reduced fertilization, poor rate of cleavage to the 2-cell stage, and failure of development beyond the 2-cell stage (Edelmann et al., 1996). Given the recombination failure phenotype of the null male, meiotic studies of oocytes from null females should provide insight to meiotic chromosome segregation and the control of mammalian female meiosis. Specifically, the behavior of univalent chromosomes and their impact on the meiotic process are directly relevant to the hypothesis that female meiosis lacks chromosome-mediated checkpoint control. Additionally, although the importance of genetic exchange in ensuring chromosome segregation at MI during mammalian female meiosis has been clearly demonstrated (Koehler et al., 1996), there are virtually no data on the existence of a backup mechanism of chromosome segregation in mammals.
In this report we summarize the results of meiotic studies in MLH1 null mice to define the recombination defects associated with loss of the MLH1 protein and to determine the impact of reduced recombination on the mammalian female meiotic process. Our meiotic studies suggest that sterility in Mlh1 mutant females results from drastically reduced recombination, leading to gross disruptions in the meiotic process. The orientation of meiotic chromosomes during MI spindle formation and the spindle morphology in oocytes from MLH1 null females provide new insight to the process of meiotic spindle formation and stabilization during mammalian female meiosis. In addition, our results provide further support for the hypothesis that although mammalian female meiosis apparently lacks a checkpoint mechanism to detect the presence of misaligned chromosomes, a functional spindle assembly checkpoint exists.
Materials and Methods
Generation and Identification of MLH1-deficient Mice
MLH1 null animals were produced from matings of male and female animals heterozygous for a targeted disruption of the Mlh1 gene (Baker et al., 1995). The Mlh1 genotype of offspring was determined by PCR from ear punch tissue using conditions and primers as described (Baker et al., 1995).
Isolation and Analysis of Pachytene Stage Oocytes from MLH1 Null Females and Control Siblings
To analyze homologue synapsis, air-dried preparations of oocyte nuclei were made from 16–17-d fetal ovaries using the technique described (Peters et al., 1997). The Mlh1 genotype of individual fetuses was determined by PCR from tail tissue using conditions and primers as described (Baker et al., 1995). Immunostaining was performed as described (Moens et al., 1987) using a goat monoclonal antibody to rat SCP3 (a component of the lateral elements of the synaptonemal complex) generously supplied by T. Ashley (Department of Genetics, Yale University School of Medicine, New Haven, CT), and an FITC-conjugated donkey anti–goat secondary antibody (Jackson ImmunoResearch Laboratories, Inc.). Immunoreacted slides were scored on a Zeiss Axioplan fluorescent microscope.
Air-dried Preparations of Diakinesis Stage Oocytes and Spermatocytes from MLH1 Null Animals and Sibling Controls
For chiasma counts, air-dried chromosome preparations were made from diakinesis stage oocytes using the technique described (Tarkowski, 1966). Preparations were stained with Giemsa (Harleco) and the number and position of chiasmata was scored by two independent observers. Air-dried testis preparations were made according to the method described (Evans et al., 1964), the preparations were stained with Giemsa (Harleco), and the configuration of the sex chromosomes was scored by two independent observers. For MLH1 null males, the total number of chiasmate bivalents in each cell was also recorded.
Isolation and Culture of Oocytes from MLH1 Null Females and Control Siblings
To obtain ovulated oocytes for first polar body extrusion experiments, females were injected with 2.5 IU of pregnant mare serum gonadotropin (Sigma) followed 42–44 h later by an injection of 5 IU human chorionic gonodotropin (Sigma). Ovulated oocytes were collected from the oviducts 15 h after the human chorionic gonodotropin injection, freed of adherent cumulus cells by a brief exposure to hyaluronidase (200 μg/ml; Sigma), washed, and fixed as described below.
For all studies of MI, prophase arrested oocytes were obtained from 3.5–4-wk-old females. Ovaries were removed and placed in Weymouth's MB752/1 medium (GIBCO BRL) supplemented with 10% fetal calf serum and 0.23 mM sodium pyruvate. Antral follicles were punctured with 26 gauge needles to obtain immature oocytes at the germinal vesicle stage. Germinal vesicle stage oocytes were cultured in microdrops of medium under oil at 37°C in 5% CO2 in air. After 2 h in culture, oocytes were scored for germinal vesicle breakdown, indicating resumption of MI, and any oocytes remaining at the germinal vesicle stage were excluded from the experiment.
For studies of MI spindle pole formation, donor females were injected with 2.5 IU pregnant mare serum gonadotropin 42–44 h before oocyte collection.
Fixation and Staining
For analyses of chromosome behavior and meiotic spindle formation during MI, oocytes were cultured for a total of 2, 4, 6, 8, 10, 12, or 18 h before fixation. At the end of the culture period, oocytes were embedded in a fibrin clot attached to a microscope and fixed as described previously (LeMaire-Adkins et al., 1997). Fixed oocytes were incubated with an antibody to β-tubulin (Sigma) and detected with an FITC-conjugated secondary antibody according to the technique described (Hunt et al., 1995). Meiotic staging, analysis of chromosome behavior, and spindle characteristics and length were scored by two independent observers using a Zeiss Axioplan microscope fitted with a micrometer in one eyepiece.
FISH Analysis of Homologous Chromosome Behavior During MI
Following immunofluorescence staining, a subset of the oocytes fixed after 6 h in culture was hybridized as described previously (Hunt et al., 1995) with the X-specific probe, DXWas 70 (American Tissue Type Culture Collection). The X chromosome probe was labeled with digoxigenin (Boehringer Mannheim Biochemicals) and detected with FITC-conjugated anti-digoxigenin (Boehringer Mannheim Biochemicals). Following hybridization and detection, oocytes were analyzed on a confocal microscope (Bio-Rad Laboratories) to characterize the location of the X chromosome homologues on the MI spindle.
Results
Homologue Synapsis Occurs Normally but Few Functional Chiasmata Are Produced in Oocytes from MLH1 Null Females
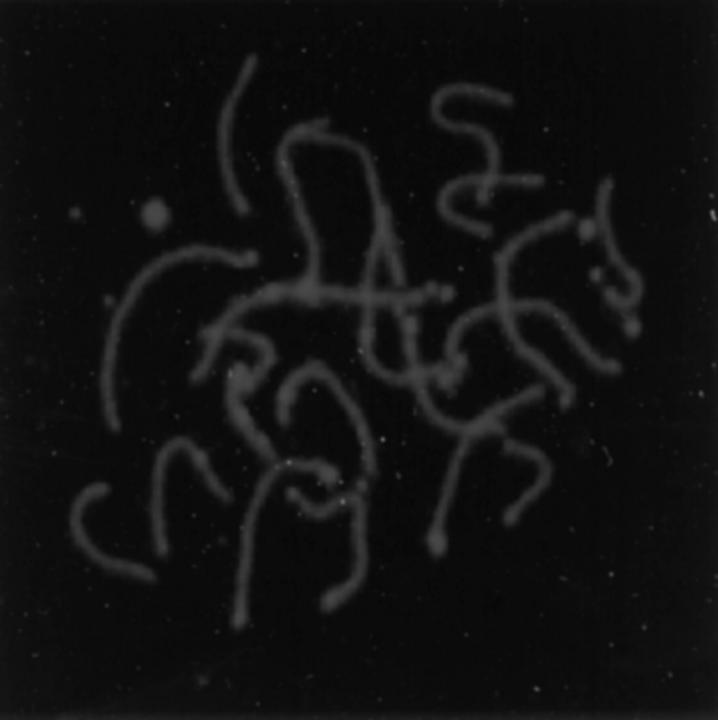
To determine if the process of homologue synapsis occurs normally in oocytes from MLH1 null females, we analyzed air-dried preparations of pachytene stage nuclei from 16– 17-d MLH1 null fetuses and control siblings. Normal pachytene stage oocytes exhibiting fully synapsed homologues were observed (Fig. 1), and no differences in the morphology of the complexes in control and MLH1 null siblings were evident.
Figure 1.
Air-dried preparation of a pachytene stage oocyte from an MLH1 null female. Immunostaining with an antibody against the lateral elements of the synaptonemal complex demonstrates fully synapsed homologues.
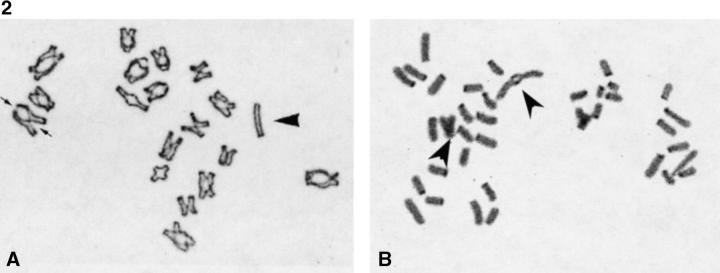
To determine the consequences of MLH1 deficiency on recombination, we analyzed air-dried preparations of oocytes at the diakinesis stage. The number and distribution of chiasmata were virtually identical for wild-type and heterozygous control females (Table I). In contrast, we observed an ∼10-fold reduction in chiasmata in oocytes from MLH1 null females (Table I and Fig. 2). The average number of chiasmata in null females was only 1.9 per oocyte, with a maximum of 6 observed in a single cell and zero exchanges observed in 15% of cells. Moreover, the placement of chiasmata was slightly skewed by comparison with cells from controls, with a preponderance of terminal exchanges and very few interstitial exchanges (Table I).
Table I.
Chiasma Counts from Diakinesis Preparations
| Genotype | Total cells | Chiasma average | Distribution of chiasmata | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Interstitial | Subterm. | Terminal | ||||||||
| +/+ | 43 | 24.1 | 9.2 (38%) | 4.2 (17%) | 10.8 (45%) | |||||
| +/− | 42 | 25 | 9.7 (39%) | 3.3 (13%) | 11.9 (48%) | |||||
| −/− | 54 | 1.9 | 0.33 (18%) | 0.46 (25%) | 1.1 (57%) | |||||
Figure 2.
Chiasma counts of oocytes at the diakinesis stage. (a) Diakinesis preparation from an XO oocyte illustrating the difference in morphology between bivalent and univalent chromosomes. The univalent X (arrowhead) can be clearly distinguished from the paired bivalents. The small arrows marking one bivalent show two chiasmata, one interstitial, and one terminal exchange. (b) Diakinesis cell from an MLH1 null female. Two exchange bivalents are present (arrowheads) and the remaining chromosomes are unpaired univalents.
Recombination Is Virtually Abolished in MLH1 Null Animals
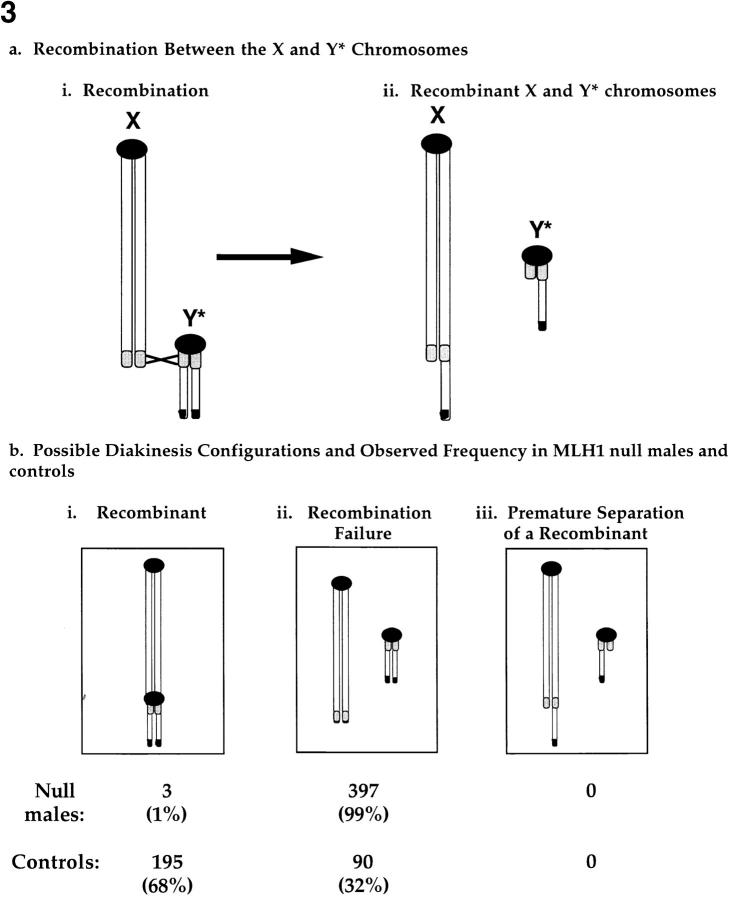
The conclusion that recombination is greatly reduced in MLH1 null males (Baker et al., 1996) and females is based solely on cytological evidence. Thus, the possibility that recombination occurs normally but functional chiasmata are not produced cannot be excluded. To test this hypothesis, we introduced a structurally abnormal Y chromosome that would allow detection of recombination in the absence of chiasmata. As shown in Fig. 3 a, recombination between the X and Y chromosomes in males carrying this structurally rearranged Y chromosome significantly alters the length of the recombinant X and Y chromatids. Thus, premature resolution of an exchange event would be cytologically detectable at diakinesis as univalent X and Y chromosomes with chromatids of different lengths.
Figure 3.
Meiotic chromosome behavior in MLH1 null males carrying the Y* chromosome. (a) Recombination between the X and Y* chromosomes. Centromeres are represented as circles, the pseudoautosomal region is indicated as a shaded segment, and the approximate location of the testis determining gene, Sry, is represented by a blackened region at the distal tip of the Y* chromosome (for further details on the structure, origin, and meiotic behavior of the Y* chromosome see Eicher et al., 1991, and Hale et al., 1991). Recombination in the pseudoautosomal region (i) produces recombinant X and Y* chromosomes (ii) that are segregated to daughter cells at MI. (b) At diakinesis, the recombination history of the XY bivalent is cytologically evident: recombinant bivalents show end-to-end association of the X and Y* chromosomes (i), failure of recombination is evident as X and Y* univalents with chromatids of equal length (ii), and recombination with premature separation would be evident as X and Y* univalents with chromatids of unequal length (iii). The frequency of each diakinesis configuration for MLH1 null males (n = 3) and sibling control males (n = 3) is shown below the schematic of the configuration. Controls include both wild-type (+/+) and heterozygous carriers of the Mlh1 mutation (+/−). Note that failure to detect the Premature Separation of a Recombinant category does not reflect inability to distinguish chromatids of unequal length, since the difference in chromatid length was obvious in MII cells from +/+ and +/− control males (data not shown).
In MLH1 null males (Fig. 3 b), as in null females (Table I), the vast majority of chromosomes were present as unpaired univalents at diakinesis. An average of 2.7 chiasmata per cell was observed, with a maximum of 8 exchanges observed in a single cell and zero exchanges observed in 14% of cells (data not shown). The X chromosome, however, displayed a unique morphology, presumably reflecting its unique chromatin configuration and sequestration in the sex vesicle (for review see Handel and Hunt, 1992), which allowed us to unambiguously distinguish it from univalent autosomal chromosomes. Only 3 cells with recombinant X and Y chromosomes were observed among the 400 diakinesis cells scored (Fig. 3 b). In addition, in all 397 cells in which the X and Y chromosomes were present as univalents, both X chromatids were of equal size (Fig. 3 b), indicating failure of recombination rather than precocious resolution of an exchange event.
The Majority of Oocytes from MLH1 Null Females Fail to Complete MI
Previous studies of oocytes from MLH1 null females suggested a variety of aberrations, including failure to extrude a second polar body and a reduced rate of fertilization and cleavage (Edelmann et al., 1996). Our initial attempts to obtain oocytes arrested at the second meiotic division (MII) from superovulated females suggested that the first polar body extrusion rate is also extremely low. Ovulated oocytes are enclosed in a sticky mass of cumulus cells which are routinely dispersed by brief exposure of the oocyte/cumulus cell mass to a hyaluronidase solution. In initial studies, we noted that hyaluronidase treatment induced polar body extrusion in a small proportion of oocytes from MLH1 null females. Hence, to avoid artificial induction of polar body extrusion, we studied oocytes meiotically matured in vitro.
A large cohort of follicles initiates growth in the neonatal murine ovary. This cohort develops almost synchronously and by 3–4 wk after birth a large population of oocytes can be obtained from the ovary, most of which are meiotically competent and will spontaneously resume and complete MI in vitro (for review see Eppig et al., 1994). When oocytes from control females were collected and cultured in this fashion, 65% had completed MI and extruded a first polar body after 15 h in culture; the remaining oocytes were meiotically incompetent and arrested at metaphase I. In contrast, after 15 h in culture, only 7% of the oocytes collected from MLH1 null females had extruded a polar body and, after extended time in culture (18–20 h), only 18% of the oocytes had completed MI (Table II). Further, immunostaining with an antibody to β-tubulin demonstrated a variety of abnormalities in the vast majority of oocytes that extruded a polar body, including incomplete cytokinesis, lack of chromatin in the polar body, and abnormalities in MII spindle formation (data not shown).
Table II.
Polar Body Extrusion Rate for In Vitro Matured Oocytes
| Donor genotype | 15 h | 18 h | ||||
|---|---|---|---|---|---|---|
| +/− | (n = 3) | 66/101 (65%) | 67/101 (66%) | |||
| −/− | (n = 5) | 15/203 (7%) | 36/203 (18%) |
In the Absence of Functional Exchanges, Chromosomes Do Not Congress and the MI Spindle Is Unstable
To determine why the majority of oocytes failed to extrude a polar body, we analyzed MI in oocytes from MLH1 null females. Oocytes were collected and cultured as described for the polar body experiments and fixed after 2, 4, 6, 8, 10, or 12 h in culture to study successive stages of MI (Table III).
Table III.
Meiotic Progression in Oocytes from MLH1 Null Females and Controls
| Prometaphase | Meta I | Ana/MII | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apolar | Bipolar | Other* | ||||||||||
| +/− | 41 | 4 | — | — | — | |||||||
| 4 h | (91%) | (9%) | ||||||||||
| −/− | 45 | 26 | 1 | — | — | |||||||
| (63%) | (36%) | (1%) | ||||||||||
| +/− | 7 | 8 | — | 9 | — | |||||||
| 6 h | (29%) | (33%) | (38%) | |||||||||
| −/− | 7 | 40 | 7 | — | — | |||||||
| (13%) | (74%) | (13%) | ||||||||||
| +/− | 2 | 6 | — | 56 | 1 | |||||||
| 8 h | (3%) | (9%) | (86%) | (2%) | ||||||||
| −/− | 3 | 38 | 4 | — | — | |||||||
| (7%) | (84%) | (9%) | ||||||||||
| +/− | — | — | — | 39 | 26 | |||||||
| 10 h | (60%) | (40%) | ||||||||||
| −/− | — | 27 | 3 | — | — | |||||||
| (90%) | (10%) | |||||||||||
| +/− | — | — | — | 7‡ | 32 | |||||||
| 12 h | (18%) | (82%) | ||||||||||
| −/− | 3 | 42 | 5 | |||||||||
| (6%) | (84%) | (10%) | ||||||||||
| +/− | — | — | — | — | — | |||||||
| 18 h | ||||||||||||
| −/− | — | 7 | 11 | — | 3 | |||||||
| (33%) | (52%) | (14%) | ||||||||||
Includes cells with collapsed spindles, spindles with grossly unequal poles, and tripolar spindles.
A proportion of control oocytes arrest at MI; this is a reflection of oocyte immaturity (Eppig et al., 1994).
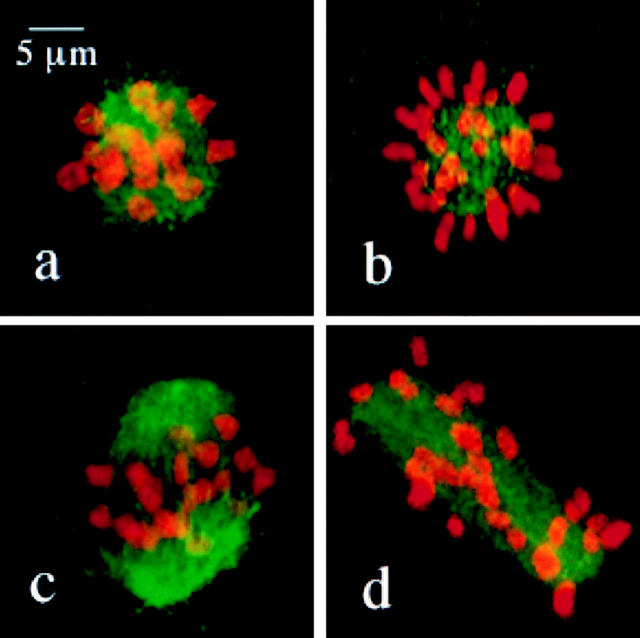
After 4 h in culture, oocytes from control females were at prometaphase of MI and exhibited either a very early prometaphase configuration, with condensed chromosomes surrounded by a mass of microtubules (Fig. 4 a; classified as apolar in Table III), or early spindle formation with chromosomes congressing to the spindle equator (Fig. 4 c; classified as bipolar in Table III). Aberrations in chromosome orientation were evident at both stages of prometaphase in oocytes from MLH1 null females: in early prometaphase cells, the majority of the chromosomes were arranged at the periphery of the microtubule mass, with the chromosome arms radiating outward like the petals of a flower (Fig. 4 b). The few centrally located chromosomes in these cells were almost exclusively bivalents, suggesting that the aberrant chromosome behavior is limited to univalent chromosomes. In cells with evidence of spindle pole formation, there was no evidence of orderly chromosome congression (Fig. 4 d).
Figure 4.
Confocal images of prometaphase configurations in oocytes from control (a and c) and MLH1 null females (b and d). (a) An early prometaphase cell showing a typical rosette configuration with condensed bivalents surrounded by a haze of microtubules. (b) An early prometaphase cell showing the aberrant rosette configuration typical of oocytes from MLH1 null females. The majority of univalent chromosomes are arranged on the periphery of the microtubule mass and the few centrally located chromosomes are predominantly bivalent chromosomes. (c) A late prometaphase cell showing the characteristic loose chromosome arrangement seen on the short, round, early bipolar spindle. (d) A late prometaphase cell from an MLH1 null female showing the characteristic congression failure phenotype.
With increasing time in culture the aberrations in oocytes from MLH1 null females became more pronounced. In control oocytes, organization of the MI spindle and the congression of the chromosomes to the spindle equator occurred within the first few hours of culture and, by 8 h, the majority of oocytes had attained a metaphase configuration (Table III and Fig. 5 a). In contrast, the gross disturbances in chromosome congression evident at the early stages of bipolar spindle formation in oocytes from MLH1 null females persisted over time, and a normal metaphase configuration was never observed (Fig. 5 c). In addition, defects in the organization of the midzone microtubules were a characteristic feature (e.g., compare Fig. 5 a with c and d).
Figure 5.
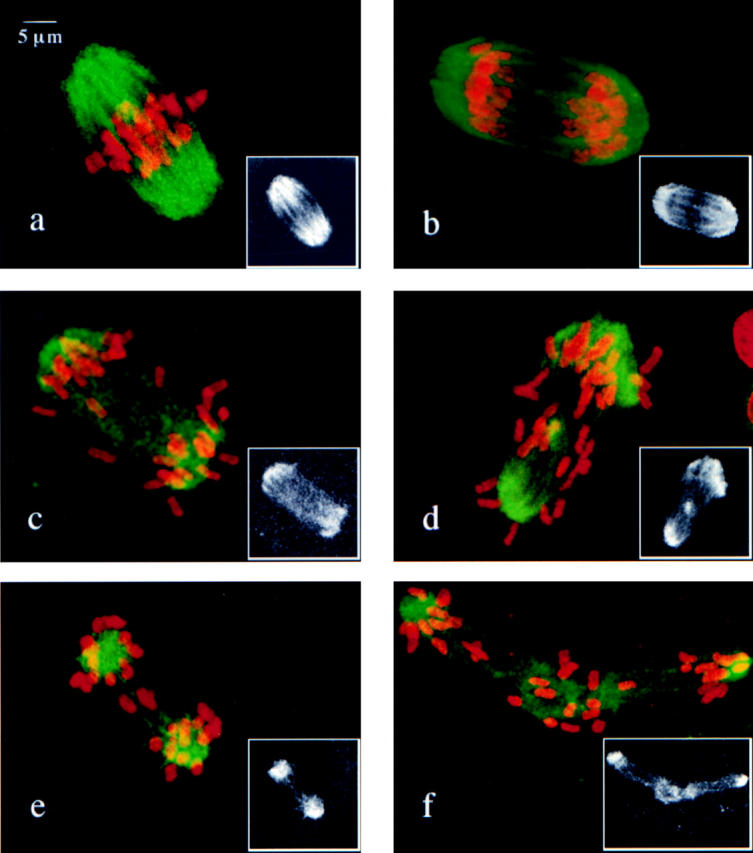
Confocal images of individual oocytes from control (a and b) and MLH1 null females (c–f) collected after >8 h in culture and immunostained with an antibody to tubulin (green) and counterstained with propidium iodide to visualize the chromosomes (red). Black and white inserts show the immunostaining alone to emphasize spindle morphology. (a) Characteristic metaphase configuration from a control oocyte showing chromosomes positioned equidistantly between the poles of a barrel-shaped MI spindle. (b) Characteristic early anaphase configuration from a control oocyte showing two groups of chromosomes moving to opposite spindle poles and early formation of the typical dark spindle midzone. (c–f) Examples of aberrant metaphase figures in oocytes from MLH1 null females. (c) An anaphase-like configuration with two loose groups of chromosomes at opposite spindle poles. Note poor spindle integrity and lack of typical anaphase spindle midzone. (d) Chromosomes scattered along the length of a bipolar spindle. Note different sized spindle poles. (e) Two groups of chromosomes at collapsed spindle poles. Note that the connection between poles is maintained by two chiasmate bivalents. (f) Elongated spindle showing evidence of tripolar formation.
Anaphase figures were first observed in control oocytes after 8 h (Fig. 5 b) and, by 12 h, the majority of oocytes had initiated anaphase or completed the division and arrested at second meiotic metaphase. In oocytes from null females, however, the congression failure phenotype persisted over time, with the only difference being an increase in the frequency of oocytes with gross spindle aberrations, e.g., grossly unequal poles, collapse of the spindle, and extremely elongated spindles, some of which were tripolar (Fig. 5, d, e, and f, respectively).
Oocytes from MLH1 Null Females Exhibit Precocious Pole Formation and Increased Interpolar Distance
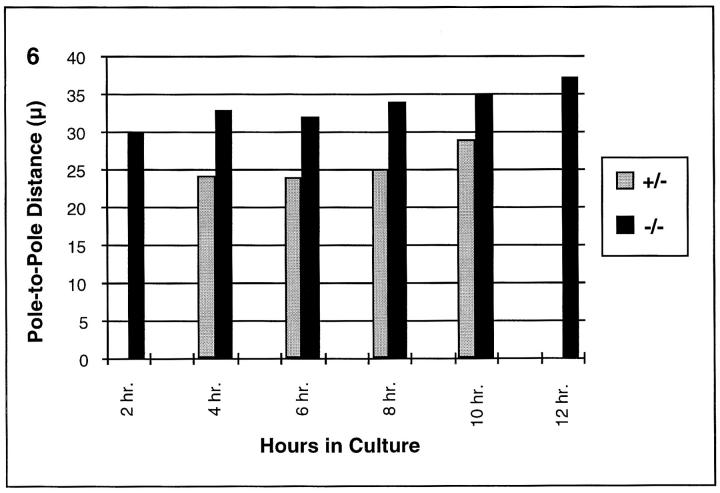
The aberrations evident at early prometaphase and the increase in spindle abnormalities with increasing time in culture suggest that both the organization and maintenance of the MI spindle is aberrant in oocytes from MLH1 null females. To perform a temporal analysis of spindle pole formation and spindle elongation, females were injected with pregnant mare serum gonadotropin 44–48 h before oocyte collection. Priming with exogenous hormones results in a more synchronous population of oocytes and also slightly accelerates progression through MI. Pole-to-pole measurements were obtained for oocytes from MLH1 null females and controls collected after 2–12 h in culture. As shown in Fig. 6, the appearance of organized spindle poles was not delayed, indeed, it was slightly accelerated in oocytes from null females. Although pole formation was not observed in any of the 33 oocytes from control oocytes analyzed after 2 h in culture, 3/43 (7%) of oocytes from null females exhibited organized poles. After 4 h in culture, pole formation was evident in the majority (19/29) of oocytes from null females and, although poles were evident in a significant minority (21/76) of oocytes from control females, the average pole-to-pole distance was significantly greater in oocytes from null females (t = 2.9; P < 0.01). Moreover, the average interpolar distance continued to increase over time in culture and remained significantly greater than the average for controls at all time points (Fig. 6).
Figure 6.
Average interpolar spindle distance in oocytes from MLH1 null females and controls. The average pole-to-pole length of bipolar spindles in groups of oocytes from MLH1 null (black bars) and control females (gray bars) analyzed after 2, 4, 6, 8, 10, and 12 h in culture. Note that lack of 2-h control value is due to the fact that none of 33 control oocytes analyzed at this time point showed evidence of pole formation. The lack of control values at 12 h is due to the fact that the vast majority of control oocytes have completed the division by this time.
In mammalian cells, several of the proteins that associate with metaphase chromosomes relocalize to the spindle midzone at anaphase and remain associated with the interzonal microtubules as the chromosomes move to the poles. The interzonal microtubules and associated proteins are thought to be important in positioning of the cleavage furrow (for review see Field et al., 1999). Given the aberrations in both chromosome alignment and midzone microtubule organization in oocytes from MLH1 null females, it remained possible that the extremely elongated spindles observed after extended time in culture represented an “abortive” anaphase. To test this hypothesis, we analyzed extremely elongated spindles for evidence of the chiasma resolution that occurs at the onset of anaphase. From a group of oocytes cultured for 12 h, a subset of 22 oocytes with the longest spindles (i.e., with a pole-to-pole measurement exceeding 80 μm) was selected. In 9 of the 22 oocytes (41%) chiasmate bivalents were obvious, in an additional 9 oocytes a group of chromosomes was present at the spindle equator but it was impossible to distinguish a chiasmate bivalent, and in 4 oocytes (18%) it was possible to conclude that no exchange bivalents were present (e.g., either the chromosomes were randomly dispersed over the length of the spindle and all visible or were present as 2 distinct groups at opposite spindle poles). These observations provide evidence that the elongated spindle phenotype does not represent an attempt to initiate anaphase, a conclusion supported by the results of histone H1 kinase assays (Kubiak et al., 1992) to determine if spindle pole elongation was associated with the characteristic decline in cyclin B levels that accompanies anaphase onset (data not shown). Interestingly, the proportion of cells with no exchange bivalents among the oocytes with extremely elongated spindles (18% of the total) was virtually identical to the frequency of achiasmate cells scored at diakinesis (15% of the total). This suggests that the fate of oocytes with zero chiasmata is no different from that of cells with one or more exchange bivalents.
Do Univalent Chromosomes Maintain a Spatial Relationship on the MI Spindle?
Despite the disturbance in congression, the number and distribution of chromosomes on opposite sides of the bipolar spindle was nearly always equivalent in oocytes from MLH1 null females (e.g., Figs. 4 d and 5 c). The balanced nature of these MI spindles was particularly striking in oocytes fixed during the early stages of MI spindle formation (i.e., oocytes fixed after 4–6 h in culture). To determine if this was due to the fact that homologous chromosomes maintained a spatial orientation during the early stages of spindle formation despite their univalent status, a subset of oocytes fixed after 6 h in culture was hybridized with an X chromosome–specific probe. The signals for the 2 X homologues were in close proximity and located at the spindle equator in 4 of the 44 cells analyzed. This is the expected configuration for an exchange bivalent. In the remaining 40 cells, the 2 X chromosomes were present as univalents with widely separated signals. In these cells the location of the homologues with respect to each other appeared to be random: the two X chromosome signals were located on opposite sides of the spindle equator in 17/40 (43%) cells and on the same side of the spindle equator in 23/40 (58%).
Discussion
The Role of the MLH1 Protein in Mammalian Meiosis
In MLH1 null male mice, meiotic recombination is reportedly reduced 10–100-fold (Baker et al., 1996). Synapsis occurs normally, but homologues separate prematurely due to the absence of functional chiasmata and the cells arrest between pachytene and MI (Baker et al., 1996; Edelmann et al., 1996). Because a reduction in functional chiasmata might reflect the premature separation of exchanged bivalents rather than a primary defect in recombination, we created MLH1 null mice carrying a structurally rearranged Y chromosome that would make it possible to detect recombination even if the resultant chiasma could not be maintained (Fig. 3). The analysis of meiotic cells from these males provides compelling genetic evidence that lack of the MLH1 protein reduces the level of recombination during mammalian meiosis.
Our studies of oocytes from MLH1 null females suggest that lack of the MLH1 protein has a comparable effect on oogenesis. As in the null male, we observed no defects in homologue synapsis in studies of pachytene stage oocytes from MLH1 null females, and analysis at the diakinesis stage demonstrated a 10–100-fold reduction in functional chiasmata. In yeast, the Mlh1 protein plays a unique role in promoting recombination and is involved in both crossover and gene conversion pathways (for review see Hunter and Borts, 1997); Mlh1-deficient yeast show a high level of postmeiotic segregation, indicating failure to correct mismatches in DNA heteroduplexes formed during recombination (Hunter and Borts, 1997). Unlike the situation in yeast where the mechanism of resolution of recombination intermediates can be inferred from the analysis of the products of meiosis, failure to complete MI in the MLH1 null female mouse precludes this type of analysis. Nevertheless, our results are consistent with the interpretation that—as is the case in yeast and in the male mouse—absence of the MLH1 protein disrupts the normal recombination pathway.
Interestingly, our studies demonstrate that oocytes with few or no functional exchanges can initiate but not complete MI. The ensuing discussion of this finding is predicated on the assumption that defects in MI result from the reduction in recombination levels.
Congression Failure Is a Reflection of the Specialized Nature of Sister Kinetochores at MI
In the absence of chiasmata, the majority of chromosomes were present as univalents at prometaphase of MI (Fig. 4, b and d). Although virtually all oocytes from control animals had reached metaphase after 8 h in culture, a normal metaphase configuration was never observed in oocytes from MLH1 null females. Even after an extensive period of time (as long as 18 h after nuclear envelop breakdown) the orientation of the majority of univalent chromosomes on the MI spindle was random, suggesting that most were unable to form stable bipolar attachments necessary for congression to the spindle equator.
Proper segregation at MI requires that the kinetochores on homologous chromosomes—not sister chromatids— attach to opposite spindle poles. Chromosome transfer experiments in grasshopper spermatocytes in the mid-1970s suggested that the MI segregation pattern is a specialized property of MI chromosomes (Nicklas, 1977). This conclusion has been supported by subsequent morphological evaluations of meiotic kinetochores that indicate that sister kinetochores are not present as physically separate domains until anaphase I (Brinkley et al., 1986; Rufas et al., 1989).
Studies in rat kangaroo kidney cells have demonstrated that a chromosome fragment created by laser microsurgery that contains a single kinetochore can form stable attachments to opposite spindle poles and congress to the spindle equator during mitotic cell division (Khodjakov et al., 1997). In contrast, in our meiotic studies, univalent chromosomes with two kinetochores were only rarely oriented at the equator in a manner that suggested attachment to opposite spindle poles. This suggests that the constraint on sister kinetochores at MI is strong. It is not, however, absolute. Equational segregation of univalent chromosomes at MI has been reported in a variety of species, including mammals (Angell, 1991; Angell et al., 1994; Hunt et al., 1995). Further, previous studies of a murine univalent X chromosome have demonstrated that bipolar attachment and resultant equational segregation at MI occurs during female meiosis but that it is not the favored mechanism of segregation (Hunt et al., 1995). The present results extend these observations and suggest that, even after extended time, the majority of murine univalent chromosomes are incapable of differentiating separate functional sister kinetochore domains before anaphase I.
In yeast mutants with a single-division meiotic phenotype, the ability of sister kinetochores to act independently and direct equational segregation of sister chromatids at MI varies for individual centromeres (for review see Simchen and Hugerat, 1993). This suggests that the degree to which sister kinetochores are physically constrained at MI may be at least partially dependent upon centromere and centromere-associated sequences. In the human, the inappropriate segregation of sister chromatids at MI has been postulated to be a major mechanism of meiotic nondisjunction (Angell, 1991; Angell et al., 1994). Further study of the MLH1 null mouse to identify chromosomes with a propensity to differentiate separate kinetochore domains at MI may provide a means of understanding the structural features that facilitate the coordinated behavior of sister chromatids during MI.
The Chromosomes Organize and Stabilize the MI Spindle
The meiotic spindle in oocytes from a variety of evolutionarily diverse animal species, including mouse (for review see Messinger and Albertini, 1991), Drosophila (Theurkauf and Hawley, 1992), Xenopus (Gard, 1993; Duesbery, N., personal communication), Ascidians (Sawada and Schatten, 1988), and Caenorhabditus elegans (Albertson and Thomson, 1993), forms through the action of multiple microtubule organizing centers rather than from a pair of centrosomes. The fact that chromatin promotes microtubule organization and the observation that the presence of several individual groups of chromosomes can result in the formation of multiple independent spindles (Maro et al., 1986) suggest that, in these meiotic systems, chromosomes play a fundamental role in the early events of spindle organization. In the present study of meiosis in MLH1 null female mice, the aberrant situation created by the presence of multiple univalent chromosomes has made it possible to discern an additional role of the chromosomes in meiotic spindle formation. At very early prometaphase, we observed an abnormal rosette configuration, with univalent chromosomes located at the periphery of the microtubule mass (Fig. 4 b). The unusual positioning of univalent chromosomes in comparison with bivalents during early prometaphase suggests that, in normal oocytes, bivalent chromosomes must be establishing bipolar attachments as the antiparallel arrays of microtubules form. Indeed, consistent with this suggestion, the chromosomes on very early bipolar spindles in oocytes from normal females are frequently nearly aligned at the spindle equator (Fig. 4 c). If bipolar attachment of most bivalents is, in fact, coincident with spindle pole formation, then the attachment and congression of the chromosomes is a very different process during female meiosis than during mitotic cell division.
The simultaneous formation of spindle poles and the establishment of bipolar attachments may be a characteristic feature of meiotic systems in which the MI spindle forms through the action of multiple microtubule organizing centers. In the Xenopus oocyte, each bivalent appears to form a mini spindle and the MI spindle is established from the coalescence of the individual mini spindles (Duesbery, N., personal communication). In the mouse, detailed studies of very early stages of meiotic spindle formation have been possible in an extremely protracted MI division resulting from the microinjection of Mos into oocytes held in meiotic arrest with the drug IBMX (Choi et al., 1996). Interestingly, immunostaining with a centrosomal antibody indicates that multiple foci become regionalized to opposite sides of the condensed chromosome mass, as though one or several bivalents organize individual bipolar spindles that subsequently coalesce into the two discrete poles of a bipolar spindle. These observations support our conclusion that the bipolar attachment of most bivalents occurs concurrently with spindle pole organization.
Our results also suggest that the chromosomes play a role in the organization and stabilization of the meiotic spindle. In both meiotic progression studies (Table III) and subsequent studies of pole formation and spindle elongation (Fig. 6) precocious appearance of the spindle poles was observed in oocytes from MLH1 null females. In view of the compromised spindle integrity apparent at late prometaphase (Fig. 5, c–f), the apparent acceleration in the organization of the MI spindle is puzzling. It is possible that under normal conditions the process of bivalent attachment, detachment, and reorientation necessary to achieve stable bipolar attachments influences the process of pole formation, slowing the process. However, we think it more likely that the early appearance of spindle poles in oocytes from MLH1 null females reflects an inability to tether the newly forming spindle poles. This is partially analogous to the situation in mitotic cells where the bipolar attachment of at least one chromosome is necessary to tether the centrosomes (for review see Waters et al., 1993). In contrast to mitotic cell division, however, our studies suggest that a critical mass is required to tether the forming spindle poles during mammalian female meiosis. That is, both precocious pole formation among cells scored after several hours in culture and spindle defects (i.e., spindles with unequal poles or elongated, collapsed, and tripolar spindles) among cells scored after extended time in culture were a feature of cells with zero, one, or several bivalent chromosomes present at the spindle equator (Fig. 5, d–f). Thus, we propose that the chromosomes not only play a role in organizing the MI spindle, but that the formation of stable bipolar attachments is necessary both to stabilize the interzonal microtubules and to control the movement of the spindle poles. This implies that tension is used differently in female meiotic systems that utilize multiple microtubule organizing centers, and is consistent with recent studies of the cell cycle checkpoint protein MAD2 in maize, where the meiotic spindle also forms by an inside-out mechanism. In contrast to mitotic cells where loss of MAD2 staining was correlated with initial microtubule attachment, loss of MAD2 staining in meiotic cells appeared to be tension-dependent (Yu et al., 1999).
According to our hypothesis, the extremely long spindles that are characteristic of oocytes from MLH1 null females are not the result of congression failure per se, but rather of the failure of the vast majority of univalent chromosomes to form stable bipolar attachments. Several lines of evidence support this conclusion: first, when normal meiotic bivalents or replicated mitotic chromosomes are present, defects in chromosome congression induced by the microinjection of kinetochore antibodies (Simerly et al., 1990) or immunodepletion of the kinetochore-associated motor protein, CENP-E (Wood et al., 1997), apparently are not associated with spindle pole elongation; second, in meiotic studies of human oocytes (Volarcik et al., 1998) and in oocytes from a mouse mutant (Hunt, P.A., manuscript in preparation) we have observed defects in meiotic chromosome congression without corresponding spindle pole elongation. In all of these situations normal MI bivalents are present and the ability of these bivalents to form bipolar attachments is presumably normal. Importantly, in the mouse mutant, although congression to the spindle equator was disrupted, anaphase onset occurred normally (Hunt, P.A., manuscript in preparation).
The suggestion that the tension created by the bipolar attachment of chromosomes plays an essential role in the formation of a stable MI spindle appears to be at odds with several previous observations that suggest that the chromosomes are passive players in the process. First, stable bipolar spindles can be assembled around chromatin-coated beads in Xenopus extracts and, in the absence of kinetochores, the beads can align at the spindle equator in a metaphase-like configuration (Heald et al., 1996). Second, studies of mechanically bisected MI and MII stage mouse oocytes suggest that normal-appearing, stable bipolar spindles can form in chromosome-free oocyte fragments (Brunet et al., 1998). Finally, in grasshopper spermatocytes, the removal of the chromosomes from bipolar spindles does not interfere with the onset of anaphase (Zhang and Nicklas, 1996). These results clearly demonstrate that microtubule self-assembly of a bipolar spindle is possible and that anaphase onset can occur in the absence of cues from the chromosomes. They do not, however, provide any indication of the complexity that may be imposed on the process by the presence of chromosomes. For example, in the grasshopper spermatocyte, although a stable MI spindle persists and can initiate anaphase after the removal of the chromosomes (Zhang and Nicklas, 1996), the presence of a single chromosome that is not removed but merely dragged off the spindle and left in the cytoplasm results in spindle depolymerization (Zhang and Nicklas, 1995). Thus, in the MLH1 null mouse, the presence of multiple univalent chromosomes creates an aberrant situation that provides a means of unraveling the complexity of the normal process of MI spindle formation in a centrosome-free system.
Previous attention has focused on the role of tension in the alignment of the chromosomes at the spindle equator. The formation of a bipolar attachment places opposing kinetochores of a bivalent (or sister kinetochores at MII or mitosis) under tension, stabilizing the microtubule connections to the kinetochores (for review see Moore and Orr-Weaver, 1998). However, to our knowledge an effect of tension on pole formation and spindle integrity has not been described previously. Is the role of the chromosomes in the stabilization of the MI spindle unique to mammalian female meiosis? Tension is used in a very different way in Drosophila oogenesis: metaphase I is a normal cell cycle arrest point and the tension resulting from the bipolar attachment of one or more exchange bivalents is necessary to achieve this arrest (Jang et al., 1995). However, when tension is lacking, not only is the MI arrest voided but the MI spindle becomes significantly elongated (Hawley, R.S., personal communication). Thus, the role of tension in stabilizing the MI spindle may be a generalized feature of oogenesis in many animal species that reflects the manner in which the spindle is organized.
Several lines of evidence suggest that congression of all chromosomes to the spindle equator is not a prerequisite for anaphase onset (Hunt et al., 1995; LeMaire-Adkins et al., 1997; Yin et al., 1998; Hunt, P.A., unpublished observations) in murine oocytes, and mammalian female meiosis has been hypothesized to lack an important cell cycle control mechanism to detect misaligned chromosomes (Hunt et al., 1995; LeMaire-Adkins et al., 1997). Our current studies demonstrate that if the majority of chromosomes fail to form a bipolar attachment, a stable metaphase spindle cannot be assembled and anaphase onset is prevented. These observations are consistent with a number of previous studies which demonstrate that exposure of MI oocytes to spindle disrupting drugs prevents or significantly delays anaphase onset (Eichenlaub-Ritter and Boll, 1989; Pesty et al., 1994; Eichenlaub-Ritter and Betzendahl, 1995; Can and Albertini, 1997). Taken together, these results suggest that the cell cycle checkpoint that monitors both spindle assembly and chromosome alignment in mitotic cells functions only to detect spindle aberrations during female meiosis. We believe that this important difference in cell cycle control reflects differences in spindle assembly: in mitosis, where specialized structures organize the spindle and the attachment and congression of each chromosome occurs independently, stringent mechanisms have evolved to ensure that all chromosomes have congressed to the spindle equator before anaphase onset is initiated. In contrast, in female meiosis where the spindle is formed from multiple microtubule organizing centers and the bipolar attachment of most bivalents appears to be coincident with the formation of the spindle poles, control mechanisms emphasizing spindle stabilization rather than chromosome head counting have evolved.
Is There a Backup Mechanism of Chromosome Segregation in Mammals?
Although mechanisms for the segregation of nonexchange homologues have been described in a variety of species (for review see Wolf, 1993), it remains unclear whether mammalian female meiosis has a backup mechanism to ensure the segregation of homologues that fail to recombine. Interestingly, despite the lack of chromosome congression, in the majority of oocytes from MLH1 null females fixed during the early phases of meiotic spindle formation the chromosomes appeared to be spatially balanced on the spindle (Fig. 5, c and e). Although we made no attempt to quantify the amount of chromosomal balancing, hybridization with an X chromosome–specific probe suggested that the placement of the two X homologues with respect to each other in these cells was random. Thus, we believe that the balanced orientation of the univalent chromosomes in oocytes from MLH1 null females is a property of the meiotic spindle. In Drosophila, the crowded spindle model has been proposed to explain the segregation of chromosomes in the absence of physical attachments (Hawley et al., 1993). According to this model, a given nonexchange chromosome is more likely to make an attachment to the spindle pole that is not already occupied by a univalent. The situation is more complex in the mouse with its 20 pairs of chromosomes than in Drosophila with its 4 pairs. Nevertheless, the behavior of the chromosomes in MLH1 null females suggests that some mechanism of balancing the number of chromosomal bodies on the MI spindle exists, although in MLH1-deficient females it appears to be unrelated to homology.
Acknowledgments
We are grateful to T. Hassold, S. Hawley, and H. Willard for helpful discussions and comments on the manuscript, to T. Ashley for the generous gift of the SCP3 antibody, and to Arlene Ilagan for technical assistance. In addition, we thank N. Duesbery and S. Hawley for allowing us to cite unpublished observations.
These studies were supported by National Institutes of Health grant R01 HD31866 and a March of Dimes research grant to P.A. Hunt.
Abbreviations used in this paper
- MI
first meiotic division
- MII
second meiotic division
References
- Albertson DG, Thomson JN. Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. . Chromosome Res. 1993;1:15–26. doi: 10.1007/BF00710603. [DOI] [PubMed] [Google Scholar]
- Angell RR. Predivision in human oocytes at meiosis I: a mechanism for trisomy formation in man. Hum Genet. 1991;86:383–387. doi: 10.1007/BF00201839. [DOI] [PubMed] [Google Scholar]
- Angell RR, Xian J, Keith J, Ledger W, Baird DT. First meiotic abnormalities in human oocytes: mechanism of trisomy formation. Cytogenet Cell Genet. 1994;65:194–202. doi: 10.1159/000133631. [DOI] [PubMed] [Google Scholar]
- Baker S, Plug A, Prolla T, Bronner C, Harris A, Yao X, Christie D-M, Monell C, Arnheim N, Bradley A, et al. Involvement of mouse MLH1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- Baker SM, Bronner CE, Zhang L, Plug AW, Robatzek M, Warren G, Elliott EA, Yu J, Ashley T, Arnheim N, et al. Male mice defective in the DNA repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell. 1995;82:309–319. doi: 10.1016/0092-8674(95)90318-6. [DOI] [PubMed] [Google Scholar]
- Brinkley BR, Brenner SL, Hall JM, Tousson A, Balczon RD, Valdivia MM. Arrangements of kinetochores in mouse cells during meiosis and spermiogenesis. Chromosoma. 1986;94:309–317. doi: 10.1007/BF00290861. [DOI] [PubMed] [Google Scholar]
- Brunet S, Polanski Z, Verlhac M-H, Kubiak JZ, Maro B. Bipolar meiotic spindle formation without chromatin. Curr Biol. 1998;8:1231–1234. doi: 10.1016/s0960-9822(07)00516-7. [DOI] [PubMed] [Google Scholar]
- Can A, Albertini DF. Stage specific effects of carbendazim (MBC) on meiotic cell cycle progression in mouse oocytes. Mol Reprod Dev. 1997;46:351–362. doi: 10.1002/(SICI)1098-2795(199703)46:3<351::AID-MRD14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Carpenter ATC. Chiasma function. Cell. 1994;77:959–962. doi: 10.1016/0092-8674(94)90434-0. [DOI] [PubMed] [Google Scholar]
- Chambers SR, Hunter N, Louis E, Borts RH. The mismatch repair system reduces meiotic homologous recombination and stimulates recombination-dependent chromosome loss. Mol Cell Biol. 1996;16:6110–6120. doi: 10.1128/mcb.16.11.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T, Rulong S, Resau J, Fukasawa K, Matten W, Kuriyama R, Mansour S, Ahn N, Vande GF, Woude Mos/mitogen-activated protein kinase can induce early meiotic phenotypes in the absence of maturation-promoting factor: a novel system for analyzing spindle formation during meiosis I. Proc Natl Acad Sci USA. 1996;93:4730–4735. doi: 10.1073/pnas.93.10.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries SS, Baart SB, Dekker M, Siezen A, de Rooij DG, de Boer P, te Riele H. Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes Dev. 1999;13:523–531. doi: 10.1101/gad.13.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann W, Cohen PE, Kane M, Lau K, Morrow B, Bennett S, Umar A, Kunkel T, Cattoretti G, Chaganti R, et al. Meiotic pachytene arrest in MLH1-deficient mice. Cell. 1996;85:1125–1134. doi: 10.1016/s0092-8674(00)81312-4. [DOI] [PubMed] [Google Scholar]
- Edelmann W, Cohen PE, Kneitz B, Winand N, Lia M, Heyer J, Kolodner R, Pollard JW, Kucherlapati R. Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat Genet. 1999;21:123–127. doi: 10.1038/5075. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Betzendahl I. Chloral hydrate induced spindle aberrations, metaphase I arrest and aneuploidy in mouse oocytes. Mutagenesis. 1995;10:477–486. doi: 10.1093/mutage/10.6.477. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Boll I. Nocodazole sensitivity, age-related aneuploidy, and alterations in the cell cycle during maturation of mouse oocytes. Cytogenet Cell Genet. 1989;52:170–176. doi: 10.1159/000132871. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Hale DW, Hunt PA, Lee BK, Tucker PK, King TR, Eppig JT, Washburn LL. The mouse Y* chromosome involves a complex rearrangement including interstitial positioning of the Y-pseudoautosomal region. Cytogenet Cell Genet. 1991;57:221–230. doi: 10.1159/000133152. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1671. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Shultz RM, O'Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164:1–9. doi: 10.1006/dbio.1994.1175. [DOI] [PubMed] [Google Scholar]
- Evans EP, Breckon G, Ford CE. An air-drying method for meiotic preparations from mammalian testes. Cytogenet Cell Genet. 1964;3:289–294. doi: 10.1159/000129818. [DOI] [PubMed] [Google Scholar]
- Field C, Li R, Oegema K. Cytokinesis in eukaryotes: a mechanistic comparison. Curr Opin Cell Biol. 1999;11:68–80. doi: 10.1016/s0955-0674(99)80009-x. [DOI] [PubMed] [Google Scholar]
- Gard DL. Ectopic spindle assembly during maturation of Xenopusoocytes: evidence for functional polarization of the oocyte cortex. Dev Biol. 1993;159:298–310. doi: 10.1006/dbio.1993.1242. [DOI] [PubMed] [Google Scholar]
- Hale DW, Hunt PA, Tucker PK, Eicher EM. Synapsis and obligate recombination between the sex chromosome of male laboratory mice carrying the Y* mutation. Cytogenet Cell Genet. 1991;57:231–239. doi: 10.1159/000133153. [DOI] [PubMed] [Google Scholar]
- Handel MA, Hunt PA. Sex chromosome pairing and activity during mammalian meiosis. Bioessays. 1992;14:817–822. doi: 10.1002/bies.950141205. [DOI] [PubMed] [Google Scholar]
- Hawley, R.S. 1988. Exchange and chromosome segregation in eukaryotes. In Genetic Recombination. R. Kucherlapati and G. Smith, editors. American Society for Microbiology, Washington, D.C. 497–525.
- Hawley RS, McKim KS, Arbel T. Meiotic segregation in Drosophila melanogasterfemales: molecules, mechanisms, and myths. Annu Rev Genet. 1993;27:281–317. doi: 10.1146/annurev.ge.27.120193.001433. [DOI] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopusegg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Hunt PA, LeMaire R, Embury P, Mroz K, Sheean L. Analysis of chromosome behavior in intact mammalian oocytes: monitoring the segregation of a univalent chromosome during mammalian female meiosis. Hum Mol Genet. 1995;4:2007–2012. doi: 10.1093/hmg/4.11.2007. [DOI] [PubMed] [Google Scholar]
- Hunter N, Borts RH. Mlh1 is unique among mismatch repair proteins in its ability to promote crossing-over during meiosis. Genes Dev. 1997;11:1573–1582. doi: 10.1101/gad.11.12.1573. [DOI] [PubMed] [Google Scholar]
- Jang JK, Messina L, Erdman MB, Arbel T, Hawley RS. Induction of metaphase arrest in Drosophilaoocytes by chiasma-based kinetochore tension. Science. 1995;268:1917–1919. doi: 10.1126/science.7604267. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, McEwen BF, Buttle KF, Rieder CL. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J Cell Biol. 1997;136:229–240. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler K, Hawley R, Sherman S, Hassold T. Recombination and non-disjunction in flies and humans. Hum Mol Genet. 1996;5:1495–1504. doi: 10.1093/hmg/5.supplement_1.1495. [DOI] [PubMed] [Google Scholar]
- Kolodner R. Biochemistry and genetics of eukaryotic mismatch repair. Genes Dev. 1996;10:1433–1442. doi: 10.1101/gad.10.12.1433. [DOI] [PubMed] [Google Scholar]
- Kubiak J, Weber M, Geraud G, Maro B. Cell cycle modification during the transitions between meiotic M-phases in mouse oocytes. J Cell Sci. 1992;102:457–467. doi: 10.1242/jcs.102.3.457. [DOI] [PubMed] [Google Scholar]
- LeMaire-Adkins R, Radke K, Hunt PA. Lack of checkpoint control at the metaphase-anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J Cell Biol. 1997;139:1611–1619. doi: 10.1083/jcb.139.7.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro B, Johnson MH, Webb M, Flach G. Mechanism of polar body formation in the mouse oocyte: an interaction between the chromosomes, the cytoskeleton and the plasma membrane. J Embryol Exp Morph. 1986;92:11–32. [PubMed] [Google Scholar]
- Messinger SM, Albertini DF. Centrosome and microtubule dynamics during meiotic progression in the mouse oocyte. J Cell Sci. 1991;100:289–298. doi: 10.1242/jcs.100.2.289. [DOI] [PubMed] [Google Scholar]
- Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu Rev Biochem. 1996;65:101–103. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- Moens PB, Heyting C, Dietrich AJ, van Raamsdonk W, Chen Q. Synaptonemal complex antigen location and conservation. J Cell Biol. 1987;105:93–103. doi: 10.1083/jcb.105.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, D.P., and T.L. Orr-Weaver. 1998. Chromosome segregation during meiosis: building an unambivalent bivalent. In Meiosis and Gametogenesis. Vol. 37. M.A. Handel, editor. Academic Press, San Diego. 263–299. [DOI] [PubMed]
- Murray A. Cell cycle checkpoints. Curr Opin Cell Biol. 1994;6:872–876. doi: 10.1016/0955-0674(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Nicklas RB. Chromosome distribution: experiments on cell hybrids and in vitro. . Phil Trans R Soc Lond B Biol Sci. 1977;277:267–276. doi: 10.1098/rstb.1977.0017. [DOI] [PubMed] [Google Scholar]
- Page AW, Orr-Weaver TL. Stopping and starting the meiotic cell cycle. Curr Biol. 1997;7:23–31. doi: 10.1016/s0959-437x(97)80105-0. [DOI] [PubMed] [Google Scholar]
- Pesty A, Lefevre B, Kubiak J, Geraud G, Tesarik J, Maro B. Mouse oocyte maturation is affected by lithium via the polyphosphoinositide metabolism and the microtubule network. Mol Reprod Dev. 1994;38:187–199. doi: 10.1002/mrd.1080380210. [DOI] [PubMed] [Google Scholar]
- Peters AHFM, Plug AW, van Vugt MJ, de Boer P. A drying down technique for spreading of mammalian meiocytes from the male and female germ line. Chromosome Res. 1997;5:1–3. doi: 10.1023/a:1018445520117. [DOI] [PubMed] [Google Scholar]
- Rufas JS, Mazzella C, Suja JA, de la Vega CG. Kinetochore structures are duplicated prior to the first meiotic metaphase. A model of meiotic behavior of kinetochores in grasshoppers. Eur J Cell Biol. 1989;48:220–226. [Google Scholar]
- Sawada T, Schatten G. Microtubules in ascidian eggs during meiosis, fertilization, and mitosis. Cell Motil Cytoskeleton. 1988;9:219–230. doi: 10.1002/cm.970090304. [DOI] [PubMed] [Google Scholar]
- Simchen G, Hugerat Y. What determines whether chromosomes segregate reductionally or equationally in meiosis? . Bioessays. 1993;15:1–8. doi: 10.1002/bies.950150102. [DOI] [PubMed] [Google Scholar]
- Simerly C, Balczon R, Brinkley B, Shatten G. Microinjected kinetochore antibodies interfere with chromosome movement in meiotic and mitotic mouse oocytes. J Cell Biol. 1990;111:1491–1504. doi: 10.1083/jcb.111.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski AK. An air drying method for chromosome preparations from mouse eggs. Cytogenetics. 1966;5:394–400. [Google Scholar]
- Theurkauf WE, Hawley RS. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nodkinesin-like protein. J Cell Biol. 1992;116:1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul-Karim F, Hunt PA. The meiotic competence of in vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum Reprod. 1998;13:154–160. doi: 10.1093/humrep/13.1.154. [DOI] [PubMed] [Google Scholar]
- Waters JC, Cole RW, Rieder CL. The force-producing mechanism for centrosome separation during spindle formation in vertebrates is intrinsic to each aster. J Cell Biol. 1993;122:361–372. doi: 10.1083/jcb.122.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf KW. How meiotic cells deal with non-exchange chromosomes. Bioessays. 1993;16:107–114. doi: 10.1002/bies.950160207. [DOI] [PubMed] [Google Scholar]
- Wood KW, Sakowicz R, Goldstein LSB, Cleveland DW. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Yin H, Cukurcam S, Betzendahl I, Adler ID, Eichenlaub-Ritter U. Trichlorfon exposure, spindle aberrations and nondisjunction in mammalian oocytes. Chromosoma. 1998;107:514–522. doi: 10.1007/s004120050337. [DOI] [PubMed] [Google Scholar]
- Yu HG, Muszynski MG, Dawe RK. The maize homologue of the cell cycle checkpoint protein MAD2 reveals kinetochore substructure and contrasting mitotic and meiotic localization patterns. J Cell Biol. 1999;145:425–435. doi: 10.1083/jcb.145.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Nicklas RB. The impact of chromosomes and centrosomes on spindle assembly as observed in living cells. J Cell Biol. 1995;129:1287–1300. doi: 10.1083/jcb.129.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Nicklas RB. “Anaphase” and cytokinesis in the absence of chromosomes. Nature. 1996;382:466–468. doi: 10.1038/382466a0. [DOI] [PubMed] [Google Scholar]