Abstract
Oxygen radicals are important components of metazoan apoptosis. We have found that apoptosis can be induced in the yeast Saccharomyces cerevisiae by depletion of glutathione or by low external doses of H2O2. Cycloheximide prevents apoptotic death revealing active participation of the cell. Yeast can also be triggered into apoptosis by a mutation in CDC48 or by expression of mammalian bax. In both cases, we show oxygen radicals to accumulate in the cell, whereas radical depletion or hypoxia prevents apoptosis. These results suggest that the generation of oxygen radicals is a key event in the ancestral apoptotic pathway and offer an explanation for the mechanism of bax-induced apoptosis in the absence of any established apoptotic gene in yeast.
Keywords: apoptosis, glutathione, oxygen stress, reactive oxygen species, Saccharomyces cerevisiae
Apoptosis is an active form of cell death crucial for the development and homeostasis of metazoan organisms. In mammals, apoptosis can be induced by various intra- or extracellular stimuli and via several signaling pathways, depending on, for example, cell type, cellular environment, and differentiation state. Virtually every triggering event or component essential for apoptosis in one system has been shown to be dispensable in some other system, e.g., apoptosis in the presence of caspase inhibitors (Xiang et al., 1996; Monney et al., 1998), or in the absence of bax (Knudson et al., 1995; McCurrach et al., 1997). Apoptosis is characterized by a set of phenotypical alterations such as the exposition of phosphatidylserine on the cell surface (Martin et al., 1995), a characteristic condensation of chromatin to the nuclear envelope (“margination,” Clifford et al., 1996), DNA fragmentation, and the formation of membrane-enclosed cell fragments called apoptotic bodies (Kerr et al., 1972).
Its obviously altruistic function seemed to limit apoptosis to multicellular organisms. Consistently, in a blast database search of the Saccharomyces cerevisiae genome no obvious homologues of any crucial regulator of metazoan apoptosis (members of the bax/bcl-2 family, caspases, Apaf-1/CED-4, p53) were detected. However, it has been noted that yeast cells, both S. cerevisiae and Schizosaccharomyces pombe, can be killed by the expression of a number of proapoptotic mammalian genes, for example, bax (Sato et al., 1994; Hanada et al., 1995; Greenhalf et al., 1996) or p53 (Bischoff et al., 1992; Nigro et al., 1992).
Our recent observations that a certain S. cerevisiae cdc48 mutant as well as cells overexpressing bax coordinately show the phenotypic markers of apoptosis, chromatin condensation and fragmentation, DNA breakage, exposition of phosphatidylserine, and the formation of minicells approximating apoptotic bodies, indicates the presence of a basic apoptotic mechanism in yeast (Madeo et al., 1997; Ligr et al., 1998). This is in accordance with the observation of chromatin condensation and nuclear fragmentation in S. pombe cells dying due to the expression of Bak (Ink et al., 1997) or CED-4 (James et al., 1997). Bax lethality in S. cerevisiae can be suppressed by a defect in mitochondrial F0F1-ATPase or by the expression of the mammalian gene BI-1. Significantly, BI-1 does not interact directly with Bax, suggesting that it acts on elements already present in yeast (Xu and Reed, 1998). Similarly, a mutant form of Bcl-XL, an anti-apoptotic Bcl-2 family member, has been described, which in the absence of physical interaction can prevent Bax-induced death in yeast (Tao et al., 1997). In mammalian cells, both the inhibition of F0F1-ATPase by oligomycin and the expression of BI-1 prevent apoptosis (Matsuyama et al., 1998; Xu and Reed, 1998). Obviously, Bax, Bak, or CED-4 do not simply act as cytotoxic substances in yeast but seem to activate the same or a similar mechanism as in metazoan organisms. Because of their absence in yeast, this mechanism can not depend on Bax relatives, caspases, or caspase-activated deoxyribonuclease.
The observation of cell death accompanied by apoptosis-like features in yeast suggests that apoptosis developed before the evolutionary separation between fungi and metazoans. Elements of the pathway conserved in yeast as well as animals should therefore belong to a basic, evolutionarily old mechanism. Yeast should be useful to trace the roots of apoptosis and solve some of the complications and apparent contradictions inherent to the various models of apoptosis.
Reactive oxygen species (ROS),1 are formed in any organism exposed to molecular oxygen appear to be crucial players in apoptosis (Ghibelli et al., 1995). ROS or H2O2 can act as primary triggers of apoptosis (Hockenbery et al., 1993; Kane et al., 1993; Greenlund et al., 1995; Slater et al., 1995). The anti-apoptotic effect of Bcl-2 appears to be at least partly due to its antioxidant properties (Kane et al., 1993). In addition, oxygen radicals have been shown to act at a late step of the apoptotic pathway in certain neuronal cells, downstream of the effects of bax or caspases (Schulz et al., 1997).
We present proof that ROS accumulate in yeast cells after induction of apoptotic death by various stimuli, and show them to be necessary and sufficient to induce an apoptotic phenotype in yeast. We suggest that the formation of ROS is a key event in the evolutionary original apoptotic mechanism.
Materials and Methods
Strains and Plasmids
S. cerevisiae strain KFY437 (MAT a cdc48::URA3 his4-619 leu2-3,112 ura3-52 YEp52/cdc48S565G containing mutant allele cdc48 S565G on vector YEp52), and the corresponding control strain KFY417 (MAT a cdc48:: URA3 his4-619 leu2-3,112 ura3-52 YEp52/CDC48) with a wild-type CDC48 on YEp52 have been described (Madeo et al., 1997). Strain YPH98gsh1 was constructed from yeast wild-type YPH98 (MAT a ura3-52 lys2-801 ade2-101 leu2-3,112 trp1-Δ1; Sikorski and Hieter, 1989) by deletion of the complete GSH1 reading frame (Brendel et al., 1998). Cell division cycle mutants used as controls were Hartwell (1973) strains LH370 (cdc2 ts) and LH12021 (cdc31 ts), and rE24-15 (cdc48-3ts; Moir et al., 1982). Plasmids pSD10.a-Bax and pSD10.a-Bcl-XL contain murine bax, respectively, bcl-XL under the control of a hybrid GAL1-10/CYC1 promoter (Tao et al., 1997) in a pRS316-based vector with a URA3 marker (Dalton and Treisman, 1992). Plasmid pL009 was constructed by replacing the URA3 marker of plasmid pSD10.a-Bcl-XL by LEU2. Strain WCG4 (MAT α his3-11 ura3-52 leu2-3,112) was transformed with plasmid pSD10.a-Bax (strain WCG4bax), or with both pSD10.a-Bax and pL009 (strain WCG4bax/bcl-XL), or with vector pRS316 as a control.
YEPD consisted of 1% yeast extract (Difco), 2% Bacto peptone (Difco), and 4% glucose. Synthetic complete medium (SC) consisted of 0.67% yeast nitrogen base (Difco) and 2% glucose (SCD) or 2% galactose (SCGal) supplemented with amino acids and nucleotide bases. Strains KFY437 and KFY417 were pregrown on YEP plates with 4% galactose and inoculated in liquid YEPD. WCG4bax, WCG4bax/bcl-XL, and the vector control strain were pregrown in synthetic medium with 2% glucose to a cell density of about 0.5 × 106 cm−3. To induce the expression of Bax or Bcl-XL, cells were washed 3× and resuspended in synthetic medium with 2% galactose.
For growth under anaerobic conditions, strains were incubated in 100 ml culture medium in wash bottles under flow of N2 (Messer Griesheim GmbH) at 100 ml/min. Viability was determined as the portion of cell forming visible colonies on YEPD plates after 3 d at 28°C.
Test for Apoptotic Markers
Electron microscopy, annexin V labeling, and DAPI staining were performed as described previously (Madeo et al., 1997). For the TdT-mediated dUTP nick end labeling (TUNEL) test, cells were prepared as described (Madeo et al., 1997), and the DNA ends were labeled using the In Situ Cell Death Detection Kit, POD (Boehringer Mannheim). Yeast cells were fixed with 3.7% formaldehyde, digested with lyticase, and applied to a polylysine-coated slide as described for immunofluorescence (Adams and Pringle, 1984). The slides were rinsed with PBS and incubated with 0.3% H2O2 in methanol for 30 min at room temperature to block endogenous peroxidases. The slides were rinsed with PBS, incubated in permeabilization solution (0.1% Triton X-100 and 0.1% sodium citrate) for 2 min on ice, rinsed twice with PBS, incubated with 10 μl TUNEL reaction mixture (terminal deoxynucleotidyl transferase 200 U/ml, FITC-labeled dUTP 10 mM, 25 mM Tris-HCl, 200 mM sodium cacodylate, 5 mM cobalt chloride; Boehringer Mannheim) for 60 min at 37°C, and then rinsed 3× with PBS. For the detection of peroxidase, cells were incubated with 10 μl Converter-POD (anti-FITC antibody, Fab fragment from sheep, conjugated with horseradish peroxidase) for 30 min at 37°C, rinsed 3× with PBS, and then stained with DAB-substrate solution (Boehringer Mannheim) for 10 min at room temperature. A coverslip was mounted with a drop of Kaiser's glycerol gelatin (Merck). As staining intensity varies, only samples from the same slide were compared.
Free intracellular radicals were detected with dihydrorhodamine 123, dichlorodihydrofluorescein diacetate (dichlorofluorescin diacetate), or dihydroethidium (hydroethidine; Sigma Chemical Co.). Dihydrorhodamine 123 was added ad-5 μg per ml of cell culture from a 2.5-mg/ml stock solution in ethanol and cells were viewed without further processing through a rhodamine optical filter after a 2-h incubation. Dichlorodihydrofluorescein diacetate was added ad-10 μg per ml of cell culture from a 2.5 mg/ml stock solution in ethanol and cells were viewed through a fluorescein optical filter after a 2-h incubation. Dihydroethidium was added ad-5 μg per ml of cell culture from a 5 mg/ml aqueous stock solution and cells were viewed through a rhodamine optical filter after a 10-min incubation. For flow cytometric analysis, cells were incubated with dihydrorhodamine 123 for 2 h and analyzed using a FACS® Calibur (Becton Dickinson) at low flow rate with excitation and emission settings of 488 and 525–550 nm (filter FL1), respectively.
Free spin trap reagents N-tert-butyl-α−phenylnitrone (PBN; Sigma-Aldrich) and 3,3,5,5,-tetramethyl-pyrroline N-oxide (TMPO; Sigma-Aldrich) were added directly to the cell cultures as 10-mg/ml aqueous stock solutions. Viability was determined as the portion of cell growing to visible colonies within 3 d.
To determine frequencies of morphological phenotypes (TUNEL, Annexin V, DAPI, dihydrorhodamine 123), at least 300 cells of three independent experiments were evaluated.
Inhibition of Protein Synthesis
Cycloheximide was added to exponentially growing yeast cultures at 5, 15, 50, 200 μg/ml, or 0 μg/ml as a control. After 30 min, 10 μCi 4,5-[3H]-l-leucine (Movarek Biochemicals Inc.) was added to a 500-μl aliquot. After further incubation of 200 min at 30°C with shaking, 100-μl aliquots were spotted onto glass fiber filters and incubated in 10% TCA for 15 min in a boiling water bath. Filters were washed twice with 5% TCA, twice with ethanol and once with methanol, dried and counted with 5 ml Ultima Gold scintillation cocktail (Packard BioScience B.V.).
Results
H2O2 Induces an Apoptotic Phenotype in Wild-type S. cerevisiae
Exposure to H2O2 triggers apoptosis in numerous mammalian cells. To examine its effect in yeast, various amounts of H2O2 were added to wild-type cultures (strain YPH98) growing exponentially on YEPD. After a 200-min incubation, cells were examined for markers of apoptosis. DNA cleavage was assayed using the TUNEL test (Gavrieli et al., 1992; Gorczyca et al., 1993), chromatin was visualized by fluorescence microscopy after DAPI staining as well as by electron microscopy.
We found that low concentrations of H2O2 result in a TUNEL-positive phenotype, which vanishes at higher concentrations. Incubation with 3 mM H2O2 produces a strongly TUNEL-positive phenotype (intense black nuclear stain) in 70% of the cells (Fig. 1 I), indicating massive DNA fragmentation. With 0.3 or 1 mM H2O2, only 20– 40% of the cells are stained (Fig. 1 H). TUNEL-positive cells are often remarkably enlarged compared to TUNEL-negative cells from the same batch (Fig. 1, G and H, see also Fig. 3, G and H). Cells incubated in the absence of H2O2 showed unstained or only slightly grayish nuclei (Fig. 1 G). Increasing the H2O2 concentration above 5 mM did not intensify the TUNEL reaction. Instead, with 15 mM H2O2 the TUNEL staining is even much less intense and occurs in fewer cells than with 3 mM H2O2 (Fig. 1 J). Incubation with 180 mM H2O2 results in no detectable TUNEL staining (Fig. 1 K). To demonstrate that the DNA fragmentation is not a result of cell necrosis, membrane integrity was tested by incubating an aliquot of the protoplasts used for TUNEL staining with 23 μg/ml propidium iodide. Only 3–5% of the protoplasts from cultures treated with 0–5 mM H2O2 are stained (not shown). In cultures incubated with 180 mM H2O2, ∼80% of the protoplasts are stained with propidium iodide, indicating the loss of plasma membrane integrity. Cells from stationary cultures tolerate higher concentrations of H2O2. After incubation with 5 mM H2O2 or less, no TUNEL staining is observed (not shown). On the other hand, incubation with 180 mM H2O2 results in a strong TUNEL staining (Fig. 1 L).
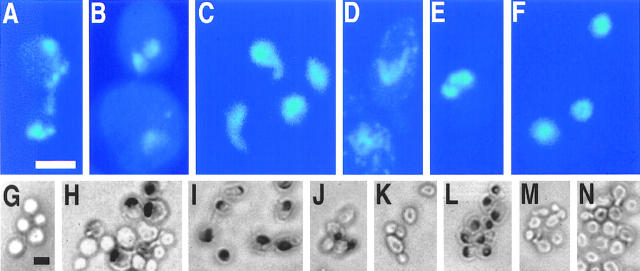
Figure 1.
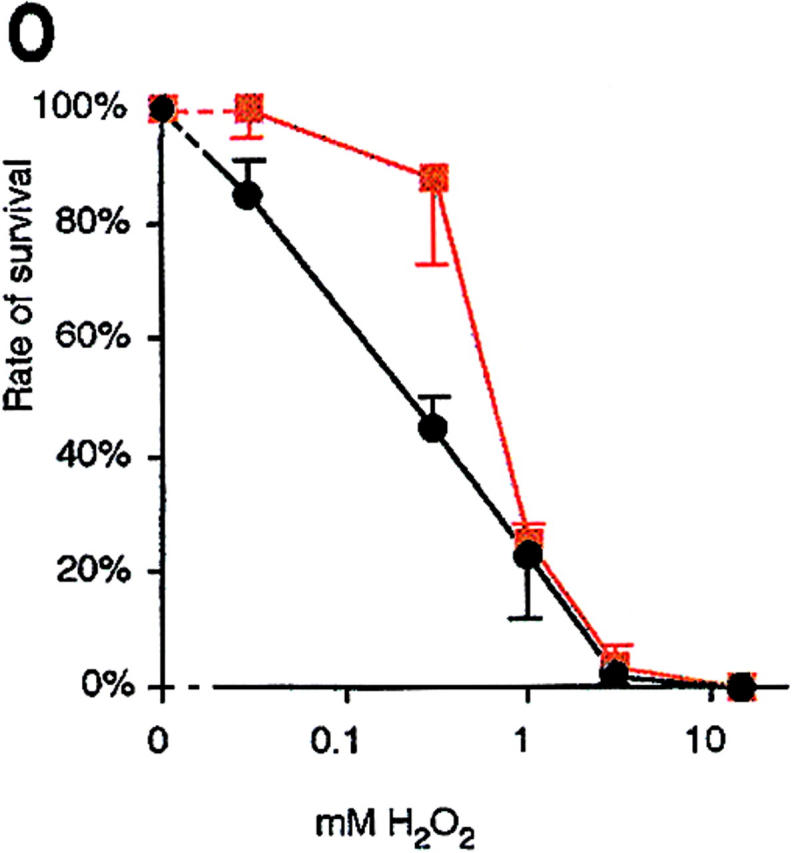
H2O2 induces an apoptotic phenotype in yeast. DAPI-stained wild-type yeast after incubation for 200 min without (F) and with 3 mM H2O2 (A–E) in YEPD. TUNEL reaction after 200 min treatment of exponentially growing cells with 0 mM (G), 1 mM (H), 3 mM (I), 15 mM (J), and 180 mM H2O2 (K), stationary cells treated with 180 mM H2O2 (L), exponentially growing cells treated with 3 mM H2O2 plus 15 μg/ml cycloheximide (M), and exponentially growing cells treated with 15 μg/ml cycloheximide for 230 min (N). Survival (colony formation on YEPD plates, 100% corresponds to the number of plated cells) of wild-type yeast incubated with H2O2 in the absence (black) or presence (red) of 15 μg/ml cycloheximide for 200 min (O). Results were averaged from three experiments. Bars: 5 μm (A–F, G–N).
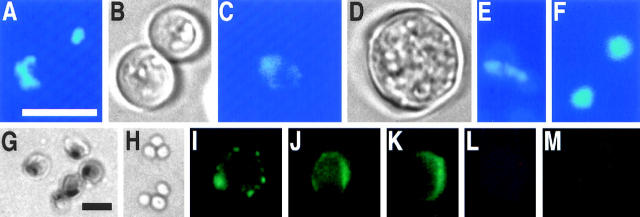
Figure 3.
A yeast lacking glutathione exhibits the typical markers of apoptosis, nuclear breakage, DNA fragmentation, and exposition of phosphatidylserine. gsh1 mutant (A, C, E) and wild-type control (F shows two cells) grown for 3 d on synthetic medium stained with DAPI; B and D are phase contrast pictures corresponding to A and C. TUNEL test of gsh1 mutant (G) and wild-type control (H) grown for 3 d on synthetic medium. Annexin V binding assay of gsh1 mutant (I–K) and wild-type control (M) grown for 3 d on synthetic medium; L shows the propidium iodide staining corresponding to K. Bars: 10 μm (A–F, I–M); 10 μm (G and H).
Analysis of isolated chromosomal DNA from H2O2 cells by agarose electrophoresis showed only a smear at low molecular weights, but not the DNA ladder pattern (not shown) that is found in many apoptotic systems as the result of DNA cleavage between nucleosomes. This confirms our result with the cdc48 mutant showing an apoptotic phenotype (Madeo et al., 1997) and is probably caused by the S. cerevisiae chromatin structure with little or no linker DNA between the nucleosomes (Lowary and Widom, 1989). In addition, apoptosis without the occurrence of a DNA ladder has been described for several metazoan cell types (Oberhammer et al., 1993).
DAPI staining of cells incubated with 3 mM H2O2 shows chromatin fragments arranged in a half-ring (Fig. 1 D) or distributed nuclear fragments (Fig. 1, A, B, and E) in 10– 50% of the cells. In some cells, nuclei seem to degenerate, showing protruding tubes (Fig. 1 C). In untreated cultures, all nuclei appear as single round spots in the cells. In contrast to nuclei or most nuclear fragments, mitochondria are predominantly located near the periphery of the cells and appear as dots of far less intensity and size (Fig. 1 D).
Electron microscopic investigation of cells incubated with 3 mM H2O2 revealed extensive chromatin condensation along the nuclear envelope typical for apoptosis (margination, Fig. 2, D and E), cells containing multiple nuclear fragments (Fig. 2 E), as well as tubular structures protruding from the nucleus (Fig. 2 F) that probably correspond to the tubular structures observed in DAPI-stained cells (Fig. 1 C). Some condensation is already visible after 30 min H2O2 treatment (Fig. 2 A) increasing gradually with time (Fig. 2, B and C). Nuclei of untreated cells are homogeneous in shape and density (Fig. 2 I). In addition, some cells show tiny vesicles on the outer side of the plasma membrane (Fig. 2, D and F). This could be an equivalent of membrane blebbing that is a characteristic marker of apoptosis and has not been observed in yeast cells before. At higher concentrations of H2O2, most intracellular structures are destroyed (Fig. 2 H), indicating necrotic cell death. This corresponds to the diminished TUNEL reaction in these cultures (Fig. 1 K).
Figure 2.
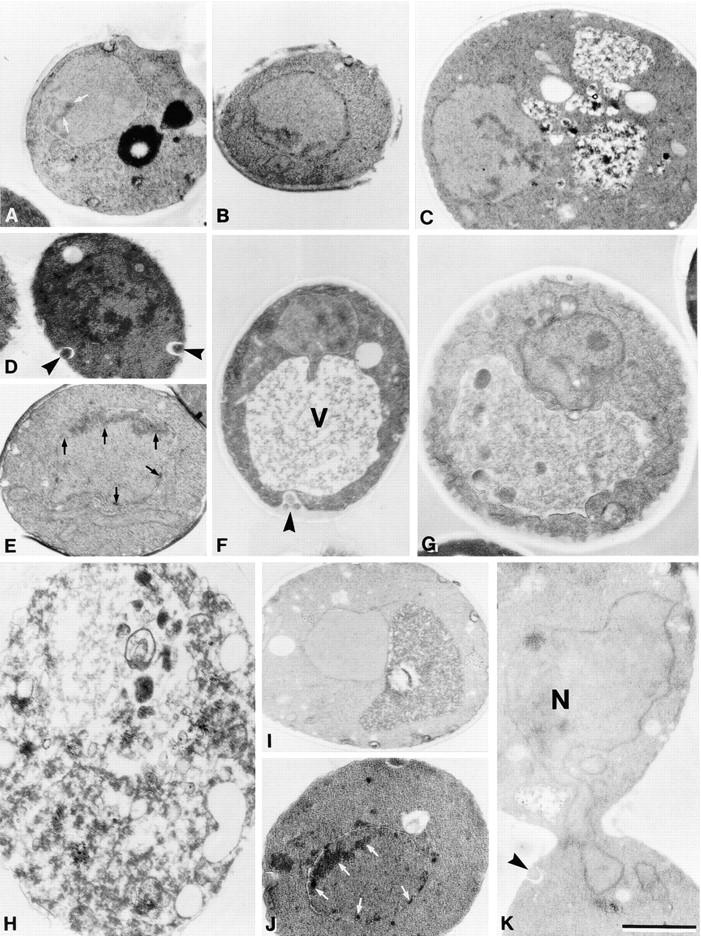
Low concentrations of H2O2 and glutathione depletion induce chromatin condensation and margination in S. cerevisiae. Electron micrographs of exponentially grown wild-type strain YPH98 treated with 3 mM H2O2 for 30 min (A), 60 min (B), 120 min (C), or 200 min (D–F), with 3 mM H2O2 plus 15 μg/ml cycloheximide for 200 min (G, nucleolus visible), with 180 mM H2O2 for 200 min (H), untreated control (I), and of gsh1 deletion strain YPH98gsh1 grown on glutathione-free synthetic medium for 3 d (J and K). N, nucleus; V, vacuole; chromatin condensation is marked by arrows, extracellular vesicles (blebs) are marked by arrowheads. Bar, 1 μm.
Cycloheximide Prevents the Apoptotic Effects of H2O2 on Yeast Cells and Improves the Survival Rate
Apoptosis needs participation of the cell and can be prevented in many cell types by the inhibition of protein synthesis (Hiraoka et al., 1997; Sánchez et al., 1997). To address the question whether the H2O2-induced DNA breakage, chromatin condensation, and cell death are the result of basic radical chemistry or depend on participation of the cellular metabolism, we investigated the effect of cycloheximide on H2O2-treated yeast cells.
To establish which concentration of cycloheximide is sufficient for complete inhibition of protein synthesis, incorporation of labeled leucine into TCA precipitable material was determined. 5 μg/ml cycloheximide reduced leucine incorporation to 23% of the untreated control, 15 μg/ml cycloheximide, or higher concentrations reduced the incorporation to <8% of the untreated control.
Exponentially growing wild-type cultures were preincubated with 15 μg/ml cycloheximide for 30 min. After adding 3 mM H2O2, cultures were incubated for another 200 min. DNA fragmentation was visualized by TUNEL staining (Fig. 1 M). The resulting TUNEL assay stain is only slightly more intense than in untreated controls (Fig. 1 G) and far weaker than in cells treated with 3 mM H2O2 in the absence of cycloheximide (Fig. 1 I). Incubation only with cycloheximide for 230 min (Fig. 1 N) results in approximately the same enhancement of TUNEL staining as the combination of cycloheximide and 3 mM H2O2. This minor increase in DNA fragmentation compared to untreated controls could be due to a reduction of DNA repair as a result of the inhibited protein synthesis.
Under the electron microscope, neither chromatin condensation, margination, nor nuclear fragmentation is detected in cells incubated with H2O2 in the presence of cycloheximide. However, numerous cells show vacuoles accumulating autophagic bodies, increased intracellular vacuolization, or misformed nuclei indicating necrotic damage (Fig. 2 G).
To investigate the effect of cycloheximide on yeast cell survival during oxygen stress, we incubated exponentially growing wild-type cultures with 15 μg/ml cycloheximide for 30 min, added various concentrations of H2O2, and incubated the cultures for another 200 min. Survival was determined as the portion of cells forming colonies after 3 d of incubation on YEPD plates. Cycloheximide considerably increased the cell survival rate in the range of 0.03 to 0.3 mM H2O2 (Fig. 1 O).
To investigate whether cycloheximide affects intracellular glutathione concentration by the inhibition of protein synthesis, the level of intracellular glutathione was determined as described (Schmidt et al., 1996). Untreated wild-type cells contained 1.55 μM glutathione per gram of wet twice-washed cells. Incubation with 15 μg/ml cycloheximide for 230 min increased the glutathione level to 140% of the control. The increase is probably caused by accumulation of cysteine. However, even an increase of the glutathione level to 250% of the wild-type level by overexpression of GSH1 has no significant effect on H2O2 tolerance (Grey, M., unpublished result). Also, after 200 min incubation with 3 mM H2O2, cells retained a glutathione level of 1.24–1.36 μmol/g cells (∼80% of the control level) indicating that the intracellular glutathione pool cell is far from exhaustion. Therefore, the observed increase of glutathione by cycloheximide can not be responsible for its protective effect.
A Yeast Strain Lacking Glutathione Exhibits the Characteristic Markers of Apoptosis
Glutathione plays a major role in the protection against ROS. Strain YPH98gsh1 lacks glutathione due to a deletion of the GSH1 gene coding for γ-glutamylcysteine synthetase. It grows on YEPD due to the significant amounts of glutathione contained in the yeast extract, as well as on synthetic media supplemented with glutathione, but dies after 3 d on glutathione-free media (Brendel et al., 1998).
Strain YPH98gsh1 and the isogenic wild-type strain YPH98 were transferred from YEPD plates to glutathione-free synthetic medium plates and incubated for 3 d. Cells were examined for the phenotypic markers of apoptosis: DNA breakage, chromatin condensation and margination, and the exposition of phosphatidylserine.
The deletion strain shows strong staining (black nuclei) with the TUNEL test indicating massive DNA fragmentation in >80% of the cells (Fig. 3 G), while the control strain shows no TUNEL staining (Fig. 3 H). When the deletion strain is grown on YEPD or on synthetic medium supplemented with 20 μg/ml glutathione, nuclear staining is not observed with the TUNEL assay (not shown).
An aliquot of the protoplasts used for TUNEL staining was also tested for membrane integrity by incubation with 23 μg/ml propidium iodide. 10–20% of the protoplasts of both wild-type and glutathione deficient strain are stained (data not shown), confirming that the majority of the cells is intact and that the DNA fragmentation is not a result of cell necrosis.
The deletion strain exhibits an enhanced sensitivity towards H2O2. Incubation with 0.1 mM H2O2 induces a strong TUNEL reaction, as well as condensation and fragmentation of chromatin, whereas little or no TUNEL staining is observed after incubation with 3 mM H2O2 or higher concentrations (not shown).
Analysis of isolated chromosomal DNA from the deletion strain by agarose electrophoresis showed no indication of DNA laddering (not shown).
DAPI staining of the deletion strain shows a strong chromatin condensation with fragments arranged in a ring, a half-ring (Fig. 3, A and C), or as several randomly distributed nuclear fragments (Fig. 3 E). Electron micrographs show intense chromatin margination (Fig. 2 J) and fragmentation of the nucleus (Fig. 2 K). In cells of the deletion strain grown on YEPD medium or on synthetic medium supplemented with 20 μg/ml glutathione, no chromatin condensation or fragmentation occurs (not shown).
In mammalian cells, phosphatidylserine exposure at the outer leaflet of the cytoplasmic membrane is an early marker of apoptosis (Martin et al., 1995). The exposure can be detected with annexin V that specifically binds phosphatidylserine in the presence of Ca2+. Like mammalian cells, S. cerevisiae has an asymmetric distribution of phospholipids within the cytoplasmic membrane with 90% of the phosphatidylserine on the cytoplasmic side of the membrane (Cerbón and Calderón, 1991). We have recently described the exposure of phosphatidylserine in a yeast CDC48 mutant showing characteristics of apoptotic cells (Madeo et al., 1997).
Spheroplasts of the GSH1 deletion strain and the isogenic control strain grown on glutathione-free synthetic medium for 3 d were examined for exposure of phosphatidylserine by incubation with FITC-labeled annexin V and for membrane integrity by incubation with propidium iodide. Approximately 20% of the protoplasts, both wild-type and mutant strain, take up propidium iodide indicating membrane damage. These protoplasts often exhibit annexin V staining of the whole cell. More than 15% of the protoplasts from the deletion strain show a strong fluorescence around the circumference of the cell (Fig. 3, I–K) of which none stain with propidium iodide, demonstrating that phosphatidylserine is indeed exposed at the outer layer of the cytoplasmic membrane (Fig. 3, K and L). All intact protoplasts of the isogenic control show little or no FITC staining in the cell lumen (Fig. 3 M). When the deletion strain was grown on YEPD or on synthetic medium supplemented with 20 μg/ml glutathione, no annexin V fluorescence is visible either (not shown).
The Apoptotic Phenotype of cdc48S565G Mutant Strain KFY437 Is Triggered by an Accumulation of ROS
To investigate whether oxygen stress is involved in other apoptotic scenarios described in S. cerevisiae, we tested apoptotic mutant strain KFY437 for the occurrence of oxygen radicals. Strain KFY437 expresses cdc48 S565G, a mutated allele of CDC48 responsible for the homotypic fusion of ER-derived vesicles, and spontaneously develops an apoptotic phenotype during exponential growth at 28°C while other conditional alleles of CDC48 result in cell death at the restrictive temperature without exhibiting any of these morphological markers (Madeo et al., 1997).
Strain KFY437 was tested for the production of ROS during apoptosis by incubation with dihydrorhodamine 123. This substance accumulates in the cell and is oxidized to the fluorescent chromophore rhodamine 123 by ROS (Schulz et al., 1996).
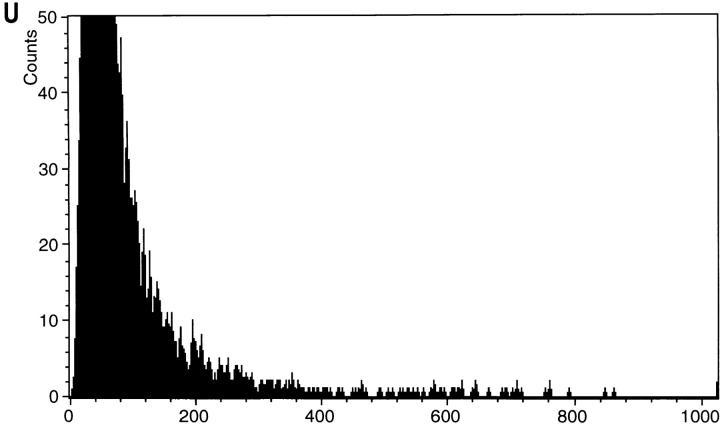
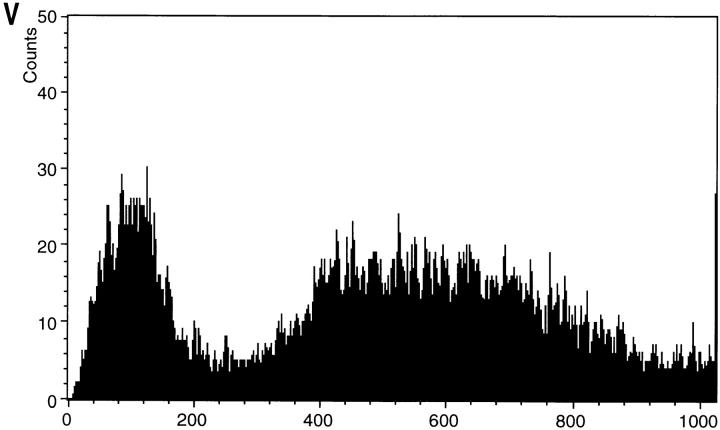
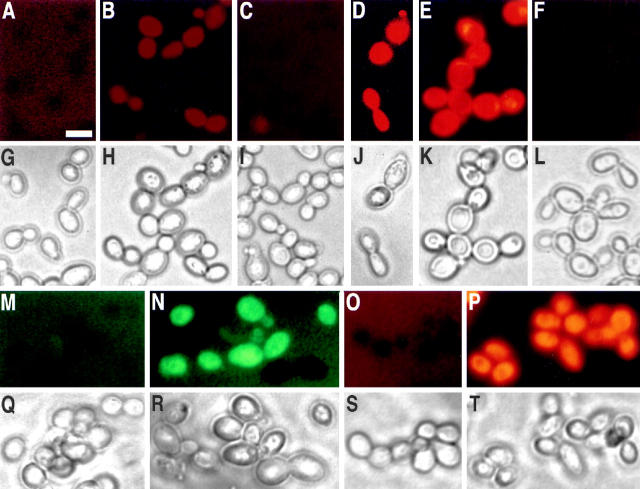
Strain KFY437 growing exponentially on YEPD was incubated with 5 μg/ml dihydrorhodamine 123 for 2 h at 28°C. More than 50% of the cells show a rhodamine 123 fluorescence (Fig. 4 B). Most of the cells of the corresponding wild-type strain show no fluorescence, appearing dark against the faint background fluorescence. Marginal fluorescence occurs in <1% of the cells (Fig. 4 A). Incubation for 2 h with 10 μg/ml dichlorodihydrofluorescein diacetate, which is deacylated to dichlorodihydrofluorescein and oxidized by ROS to fluorescent dichlorofluorescein (Hockenbery et al., 1993), or with 5 μg/ml dihydroethidium for 10 min, which is oxidized specifically by superoxide ions to the fluorescent ethidium (Budd et al., 1997), gave similar results (not shown). Flow cytometric analysis of dihydrorhodamine 123–stained cells confirms that strain KFY437 accumulates oxygen radicals. A majority of the mutants cells show an increased signal (Fig. 4 V), while all wild-type cells display a low fluorescence (Fig. 4 U).
Figure 4.
Yeast mutant KFY437 (allele cdc48 S565G) and yeast-expressing bax accumulate ROS. Rhodamine 123 fluorescence (A–F) and the corresponding phase contrast displays (G–L) after 2 h incubation with dihydrorhodamine 123. Wild-type control (A and G) and mutant KFY437 (B and H) grown at 28°C, wild-type control (C and I) and mutant KFY437 (D and J) grown at 37°C, strain WCG4bax-expressing bax (E and K), strain WCG4bax/bcl-XL expressing bax and bcl-XL (F and L). Fluorescence (M and N) and the corresponding phase contrast displays (Q and R) of wild-type control (M and Q) and mutant KFY437 (N and R) grown at 37°C after 2 h incubation with dichlorodihydrofluorescein diacetate. Fluorescence (O and P) and the corresponding phase contrast displays (S and T) of wild-type control (O and S) and mutant KFY437 (P and T) grown at 37°C after 10 min incubation with dihydroethidium. Flow cytometric analysis of wild-type control (U) and mutant KFY437 (V) after 2 h incubation with dihydrorhodamine 123.
We found that elevated temperature (37°C) induces apoptotic cell death in most cells of strain KFY437. After 4 h at 37°C, >80% of the KFY437 cells show a strong TUNEL reaction as well as condensed and fragmented chromatin. The elevated temperature induces a higher intensity of TUNEL staining as compared to cells grown at 28°C (Fig. 5, B and D). When strain KFY437 is incubated at 37°C for 4 h, more than 80% of the cells show a strong rhodamine 123 fluorescence (Fig. 4 D) of a significantly higher intensity than at 28°C. The majority of cells also show intense fluorescence after incubation with dichlorodihydrofluorescein (Fig. 4 N) or dihydroethidium (Fig. 4 P). The isogenic wild-type strain shows no increased fluorescence at 37°C with either dye (Fig. 4, M and O).
Figure 5.
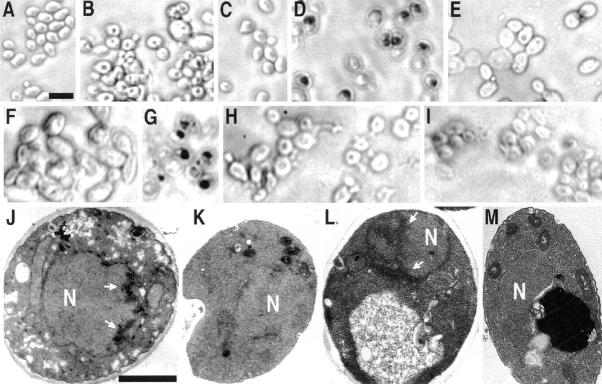
DNA strand breakage and chromatin margination in strain KFY437 and in bax-expressing WCG4bax is prevented by free radical spin traps or anaerobic culture conditions. TUNEL reaction of wild-type control (A) and mutant KFY437 (B) grown at 28°C, of wild-type control (C) and mutant KFY437 (D) incubated at 37°C for 4 h, of mutant KFY437 incubated at 37°C for 4 h in the presence of 5 mM N-tert-butyl-α−phenylnitrone (E), of mutant KFY437 incubated at 37°C in a nitrogen atmosphere (F), of bax-expressing WCG4bax (G), of bax-expressing WCG4bax in the presence of 5 mM N-tert-butyl-α−phenylnitrone (H), of bax-expressing WCG4bax incubated in a nitrogen atmosphere (I). Electron micrographs of mutant KFY437 incubated at 37°C for 4 h without addition (J) or in the presence of 5 mM N-tert-butyl-α−phenylnitrone (K), and of bax-expressing WCG4bax without addition (L) or in the presence of 5 mM N-tert-butyl-α−phenylnitrone (M). Bars: (A and J) 10 μm.
To verify that the accumulation of ROS is not an effect of any cell cycle arrest, temperature sensitive mutants in cell division cycle genes were arrested at 37°C for 4 h, incubated with dihydrorhodamine 123 and tested for fluorescence. Neither a non-apoptotic allele of CDC48 (cdc48-3), nor mutants in cdc2 (arresting as large-budded cells like cdc48 mutants) or cdc31 (arresting with an abnormal spindle-like cdc48 mutants) show an increase of intracellular fluorescence (not shown).
Oxygen Radical Scavengers and Anaerobic Conditions Suppress Temperature Sensitivity and DNA Fragmentation of Apoptotic cdc48 Mutant KFY437
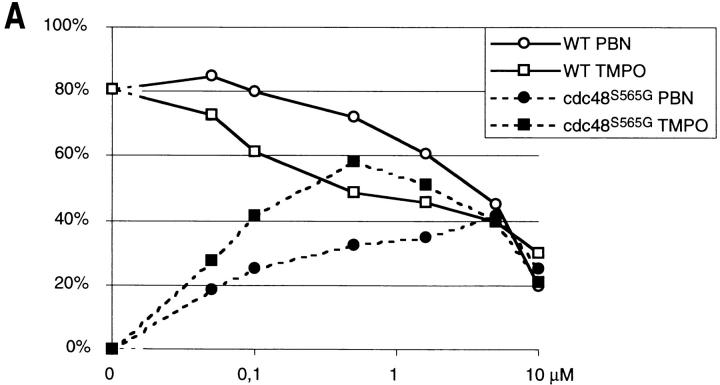
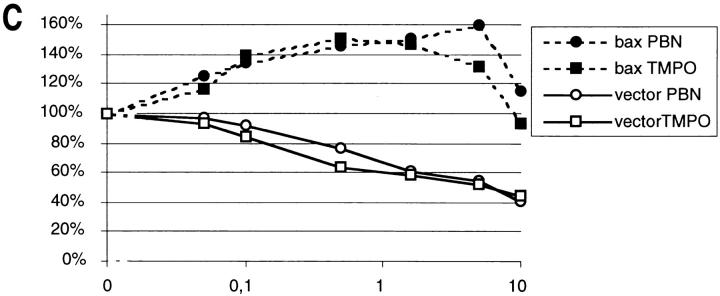
To discriminate whether the accumulation of ROS is just a byproduct of the induction of apoptosis in the cdc48 mutant or whether it is essential for the process, oxygen radicals were scavenged with free radical spin traps (Knecht and Mason, 1993). Strain KFY437 and the corresponding wild-type strain KFY417 were grown on YEPD with shaking at 28°C and then incubated with various concentrations of PBN or TMPO for 4 h at 37°C. To evaluate effects on survival, cell viability was determined as the number of colonies formed after plating a defined number of cells. Aliquots of the same sample were used for TUNEL reaction and electron microscopic investigation. 5 mM PBN (Fig. 5 E) and 0.5 mM TMPO suppress TUNEL staining dramatically, whereas 0.5 mM PBN and 5 mM TMPO have less effect (not shown). Electron microscopy shows that the intense chromatin margination of strain KFY437 at 37°C (Fig. 5 J) is prevented almost completely by 5 mM PBN (Fig. 5 K). These observations correspond to the protective effect of the spin traps on cell viability at 37°C, which is strongest with 0.5 mM TMPO or 5 mM PBN. Viability of the wild-type control is inhibited by the spin trap substances, indicating a certain cytotoxicity (Fig. 6 A). At some concentrations of TMPO, proliferation of the control strain is even lower than that of the apoptotic strain. This might be due to neutralization of radicals and spin traps in the apoptotic cdc48 mutant.
Figure 6.
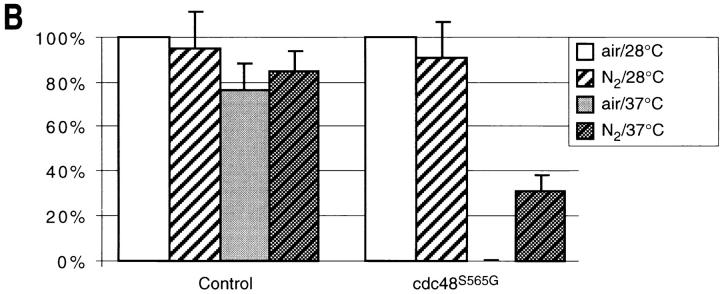
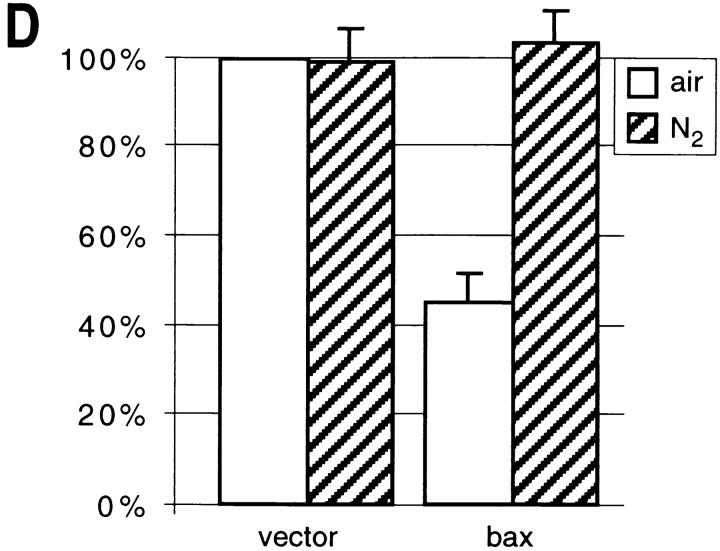
Free radical spin traps or anaerobic culture conditions partially suppress temperature sensitivity in strain KFY437 and bax lethality. (A) Strain KFY437 (allele cdc48 S565G) and the corresponding wild-type were adjusted to a cell titer of 2 × 106/ml and incubated with various concentrations of PBN or TMPO for 4 h at 37°C in YEPD. The portion of colony-forming units is plotted against spin trap concentrations. 100% corresponds to the respective strain grown at 28°C without spin traps. (B) Strain KFY437 (allele cdc48 S565G) and the corresponding wild-type were adjusted to a cell titer of 2 × 106/ml in YEPD, incubated with aeration or in a nitrogen stream at 28°C for 30 min, and further incubated at 37 or 28°C for 4 h. Viability was determined as the portion of colony-forming units. 100% corresponds to the respective strain grown aerobically at 28°C. (C) WCG4bax and the corresponding control strain grown on synthetic medium with glucose were transferred to synthetic medium with galactose, diluted to a cell titer of 2 × 106/ml, and incubated for 15 h either with various concentrations of PBN or TMPO. The portion of colony-forming units is plotted against spin trap concentrations. 100% corresponds to the respective strain grown without spin traps. (D) WCG4bax and the corresponding control strain grown on synthetic medium with glucose were transferred to synthetic medium with galactose, diluted to a cell titer of 2 × 106/ml, and incubated for 15 h with aeration or in a nitrogen stream. Viability was determined as the portion of colony-forming units. 100% corresponds to the aerobically grown control strain. The portion of colony-forming units was determined by incubating YEPD plates with 1,000 cells each for 3 d at 28°C and counting visible colonies. Results were averaged from three experiments each. Standard deviations are below 10% for all data points.
A similar protective effect was observed after incubation in an anaerobic environment. Mutant cultures and controls were incubated in a nitrogen atmosphere at 28°C for 30 min and further incubated in a 37°C waterbath for 4 h under nitrogen. Their viability was tested in a plating assay. While the control was not affected by the anaerobic conditions, the mutant strain showed significant survival after anaerobic conditions (Fig. 6 B). Incubation in a nitrogen atmosphere also prevented accumulation of DNA strand breaks, as shown by TUNEL staining (Fig. 5 F).
Bax Expression Induces Accumulation of Reactive Oxygen Species in S. cerevisiae
Expression of bax in S. cerevisiae results in cell death with an apoptotic phenotype that is suppressed by the coexpression of bcl-XL (Ligr et al., 1998). To determine whether Bax-induced apoptosis in yeast is also accompanied by oxygen stress, strain WCG4bax carrying a bax gene controlled by the GAL1 promoter was used. Bax expression was induced on galactose medium for 4 h or 13 h. 5 μg/ml dihydrorhodamine were then added and the fluorescence determined after 2 more hours of incubation. After a total of 6 h induction, strain WCG4bax shows little fluorescence (not shown). After 15 h, >80% of WCG4bax cells show an intense rhodamine 123 fluorescence (Fig. 4 E), while the control strain (empty vector) shows no detectable fluorescence (not shown). Coexpression of bcl-XL suppresses the bax-induced accumulation of radicals. The strain WCG4bax/bcl-XL shows almost no rhodamine 123 fluorescence after 15 h incubation with galactose (Fig. 4 F). Incubation with dichlorodihydrofluorescein diacetate or dihydroethidium gave similar results (not shown). Flow cytometric analysis of dihydrorhodamine 123–stained cells confirms that a majority of bax-expressing cells show an increased signal, while all control cells display a low fluorescence (not shown).
To determine whether spin traps can prevent Bax-triggered cell death WCG4bax and the corresponding wild-type strain (empty vector) were incubated with the free radical spin traps PBN or TMPO at 28°C on galactose media for 15 h. Viability was determined as the portion of colony-forming units. While spin traps have a toxic effect on the control strain, WCG4bax survival is partially restored. As with the apoptotic cdc48 mutant, 5 mM PBN has the strongest protective effect on WCG4bax (Fig. 6 C). Accordingly, 5 mM PBN prevents DNA strand breakage (Fig. 5 H) and chromatin condensation (Fig. 5 M) occurring in bax-expressing WCG4bax in the absence of spin traps (Fig. 5, G and L).
Anaerobic conditions also strongly reduce cell death and DNA breakage after induction of bax. Strain WCG4bax and the vector control strain were incubated in galactose medium in a nitrogen atmosphere at 28°C for 15 h. Viability of the bax-expressing strain is restored to control levels under these anaerobic conditions (Fig. 6 D). The corresponding TUNEL test shows no indication of strand breaks (Fig. 5 I).
Discussion
In metazoan apoptosis, ROS have been shown to participate in both early and late steps of the regulatory chain. In several apoptotic models, for example in ischemia-induced apoptosis (Maulik et al., 1998), ROS act upstream of bax and caspases. In these models, radical trapping prevents the activation of caspases, and an inhibition of ensuing steps, e.g., with caspase inhibitors, prevents cell death indicating that the radicals act as signal molecules and do not simply cause lethal damage to DNA, lipids or proteins (Hara et al., 1997; Boggs et al., 1998; Tan et al., 1998). However, radicals have also been shown to play a role in the late steps of apoptosis. K+ deprivation induces apoptosis in cerebellar granule neurons via an accumulation of ROS. ROS production is prevented by actinomycin D, cycloheximide, and caspase inhibitors Ac-YVAD-CMK, suggesting that ROS act downstream of gene transcription, mRNA translation, and activation of caspases (Schulz et al., 1997, 1996).
Two scenarios connecting yeast with metazoan apoptosis have been described previously. The expression of some metazoan proapoptotic genes (bak, bax, ced-4) results in cell death (Sato et al., 1994; Hanada et al., 1995; Greenhalf et al., 1996; James et al., 1997) accompanied by an apoptotic phenotype (Ink et al., 1997; Ligr et al., 1998). The mutant allele cdc48 S565G induces the appearance of typical phenotypic markers of apoptosis (Madeo et al., 1997).
We found that in both cases ROS accumulate in the cell. Radical trapping or growth under strictly anaerobic conditions prevent cell death and the accompanying apoptotic effects, demonstrating that the radicals are not a byproduct but a promoter of the apoptotic-like features in these cells. In addition, apoptotic cell death could be induced by exposing yeast cells to oxidative stress, either with low concentrations of exogenous H2O2 or by growing a gsh1 deletion mutant in the absence of glutathione. These results illustrate a central role of ROS in all cases of apoptosis in yeast known to date.
We observed that with low concentrations of H2O2, cycloheximide enhances the survival rate of S. cerevisiae cells. This phenomenon has been observed before, but at the time remained unexplained (Collinson and Dawes, 1992). Our electron microscopic investigation and TUNEL test show that cycloheximide prevents both apoptotic chromatin condensation and DNA fragmentation, and increases the number of necrotic cells. The prevention of cell death by inhibition of protein synthesis is a specific indicator to distinguish apoptosis from necrosis in metazoan systems (el Azzouzi et al., 1994; Hiraoka et al., 1997). Our findings indicate that yeast cell death triggered by low H2O2 concentrations is not caused by cell damage but involves an active cooperation of the cell. Massive corruption of cellular structures and metabolism prevents the cooperation of the cell in its death process. As we have shown, high concentrations of H2O2 result in cell death associated with a disintegration of intracellular structures but without the phenotypic markers of apoptosis. Stationary cells of S. cerevisiae are much less sensitive to oxidative stress than exponentially growing cells (Jamieson et al., 1994; Izawa et al., 1996). We find that they show no detectable effect at concentrations of H2O2 that cause an apoptotic phenotype in exponentially growing cells and give strong TUNEL staining at high concentrations of H2O2.
The observation that numerous cytotoxic substances that cause necrosis at high concentrations induce apoptosis at lower concentrations (Lieberthal and Levine, 1996) give a clue to the origin of apoptosis. ROS are natural inducers of fatal cell damage, aging, and cell death. A likely evolutionary mechanism for the development of apoptosis might be based on that phenomenon. Even if a cell survives the damage caused by radicals, it will probably become a useless mouth to feed, consuming resources but producing no or impaired offspring. For the total clonal cell population, the altruistic death of these cells spares energy sources for the undamaged cells and therefore poses an evolutionary advantage. A potentially altruistic death has been described for stationary cultures of S. cerevisiae that can survive for very long times in pure water, but quickly lose viability in nutrient depleted media, perhaps in order to keep the few resources for the best adapted isogenic relatives. Bcl-2 delays the loss of viability (Longo et al., 1997).
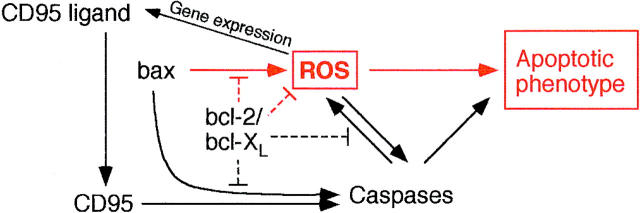
In evolution, more complex regulatory pathways probably developed upstream of the basic mechanism, resulting in a complex signaling network with a seemingly contradictory behavior in different apoptotic models (Fig. 7). The phenomenon that glutathione is actively extruded during apoptosis of human monocytic cells (Ghibelli et al., 1995) may be a strategy to enhance ROS signaling– induced apoptosis. Some apoptotic pathways retain the usage of ROS in early regulatory steps, other pathways still use it in late steps. In addition, alternative pathways lacking the involvement of ROS developed that cannot be blocked by radical trapping. But even in the case of the ROS-independent apoptotic pathway via CD95 (Hug et al., 1994), the expression of the proapoptotic CD95 ligand is induced by ROS (Hug et al., 1997).
Figure 7.
ROS participate in the regulation of apoptosis at various levels. Important established regulators of apoptosis. Components and functions present in yeast are marked in red.
While none of the established apoptotic proteins are encoded in the S. cerevisiae genome, some of them will perform similar functions when expressed in yeast cells. In mammals, one of the effects of Bax expression is a caspase-independent production of ROS (Xiang et al., 1996). Our finding that the expression of Bax protein in yeast also results in an accumulation of ROS that is prevented by the coexpression of Bcl-XL indicates a mechanism for the induction of cell death and apoptotic phenotype by Bax in yeast. Anorganic ROS are therefore the first regulators of apoptosis found to be shared between yeast and higher animals (Fig. 7). Future research should address whether the mechanism downstream of this signal uses conserved elements.
Acknowledgments
We thank J.I. Morgan for the gift of Bax and Bcl-XL plasmids, and Dieter Mecke for discussion and support. We acknowledge the support of Jürgen Voigt with radio labeling and of Matthias Rose with FACS® analysis. We are grateful to Harold Taylor for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations used in this paper
- PBN
N-tert-butyl-α−phenylnitrone
- ROS
reactive oxygen species
- SC
synthetic complete medium
- TMPO
3,3,5,5,-tetramethyl-pyrroline N-oxide
- TUNEL
TdT-mediated dUTP nick end labeling
References
- Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. . J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff JR, Casso D, Beach D. Human p53 inhibits growth in Schizosaccharomyces pombe. . Mol Cell Biol. 1992;12:1405–1411. doi: 10.1128/mcb.12.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs SE, McCormick TS, Lapetina EG. Glutathione levels determine apoptosis in macrophages. Biochem Biophys Res Commun. 1998;247:229–233. doi: 10.1006/bbrc.1998.8765. [DOI] [PubMed] [Google Scholar]
- Brendel M, Grey M, Maris AF, Hietkamp J, Fesus Z, Pich CT, Dafré AL, Schmidt M, Eckardt-Schupp F, Henriques JA. Low glutathione pools in the original pso3 mutant of Saccharomyces cerevisiaeare responsible for its pleiotropic sensitivity phenotype. Curr Genet. 1998;33:4–9. doi: 10.1007/s002940050301. [DOI] [PubMed] [Google Scholar]
- Budd SL, Castilho RF, Nicholls DG. Mitochondrial membrane potential and hydroethidine-monitored superoxide generation in cultured cerebellar granule cells. FEBS Lett. 1997;415:21–24. doi: 10.1016/s0014-5793(97)01088-0. [DOI] [PubMed] [Google Scholar]
- Cerbón J, Calderón V. Changes of the compositional asymmetry of phospholipids associated to the increment in the membrane surface potential. Biochim Biophys Acta. 1991;1067:139–144. doi: 10.1016/0005-2736(91)90035-7. [DOI] [PubMed] [Google Scholar]
- Clifford J, Chiba H, Sobieszczuk D, Metzger D, Chambon P. RXRα-null F9 embryonal carcinoma cells are resistant to the differentiation, anti-proliferative and apoptotic effects of retinoids. EMBO (Eur Mol Biol Organ) J. 1996;15:4142–4155. [PMC free article] [PubMed] [Google Scholar]
- Collinson LP, Dawes IW. Inducibility of the response of yeast cells to peroxide stress. J Gen Microbiol. 1992;138:329–335. doi: 10.1099/00221287-138-2-329. [DOI] [PubMed] [Google Scholar]
- Dalton S, Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992;68:597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- el Azzouzi, B., G.T. Tsangaris, O. Pellegrini, Y. Manuel, J. Benveniste, and Y. Thomas. Cadmium induces apoptosis in a human T cell line. Toxicology. 1994;88:127–139. doi: 10.1016/0300-483x(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibelli L, Coppola S, Rotilio G, Lafavia E, Maresca V, Ciriolo MR. Non-oxidative loss of glutathione in apoptosis via GSH extrusion. Biochem Biophys Res Commun. 1995;216:313–320. doi: 10.1006/bbrc.1995.2626. [DOI] [PubMed] [Google Scholar]
- Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situterminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- Greenhalf W, Stephan C, Chaudhuri B. Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality in Saccharomyces cerevisiae. . FEBS Letts. 1996;380:169–175. doi: 10.1016/0014-5793(96)00044-0. [DOI] [PubMed] [Google Scholar]
- Greenlund LJS, Deckwerth TL, Johnson EM., Jr Superoxide dismutase delays neuronal apoptosis: a role for reactive oxygen species in programmed neuronal death. Neuron. 1995;14:303–315. doi: 10.1016/0896-6273(95)90287-2. [DOI] [PubMed] [Google Scholar]
- Hanada M, Aimé-Sempé C, Sato T, Reed JC. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem. 1995;270:11962–11969. doi: 10.1074/jbc.270.20.11962. [DOI] [PubMed] [Google Scholar]
- Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz MA. Inhibition of interleukin 1β converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Mortimer RK, Culotti J, Culotti M. Genetic control of the cell-division cycle in yeast. V. Genetic analysis of cdcmutants. Genetics. 1973;74:267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka W, Fuma K, Kuwabara M. Concentration-dependent modes of cell death in Chinese hamster V79 cells after treatments with H2O2 . J Radiat Res Tokyo. 1997;38:95–102. doi: 10.1269/jrr.38.95. [DOI] [PubMed] [Google Scholar]
- Hockenbery DM, Oltvai ZN, Yin X-M, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hug H, Enari M, Nagata S. No requirement of reactive oxygen intermediates in Fas-mediated apoptosis. FEBS Lett. 1994;351:311–313. doi: 10.1016/0014-5793(94)00852-3. [DOI] [PubMed] [Google Scholar]
- Hug H, Strand S, Grambihler A, Galle J, Hack V, Stremmel W, Krammer PH, Galle PR. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J Biol Chem. 1997;272:28191–28193. doi: 10.1074/jbc.272.45.28191. [DOI] [PubMed] [Google Scholar]
- Ink B, Zörnig M, Baum B, Hajibagheri N, James C, Chittenden T, Evan G. Human bak induces cell death in Schizosaccharomyces pombewith morphological changes similar to those with apoptosis in mammalian cells. Mol Cell Biol. 1997;17:2468–2474. doi: 10.1128/mcb.17.5.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S, Inoue Y, Kimura A. Importance of catalase in the adaptive response to hydrogen peroxide: analysis of acatalasaemic Saccharomyces cerevisiae. . Biochem J. 1996;320:61–67. doi: 10.1042/bj3200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C, Gschmeissner S, Fraser A, Evan GI. CED-4 induces chromatin condensation in Schizosaccharomyces pombeand is inhibited by direct physical association with CED-9. Curr Biol. 1997;7:246–252. doi: 10.1016/s0960-9822(06)00120-5. [DOI] [PubMed] [Google Scholar]
- Jamieson DJ, Rivers SL, Stephen DWS. Analysis of Saccharomyces cerevisiaeproteins induced by peroxide and superoxide stress. Microbiol. 1994;140:3277–3283. doi: 10.1099/13500872-140-12-3277. [DOI] [PubMed] [Google Scholar]
- Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Örd T, Bredesen DE. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht KT, Mason RP. In vivospin trapping of xenobiotic free radical metabolites. Arch Biochem Biophys. 1993;303:185–194. doi: 10.1006/abbi.1993.1272. [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol. 1996;271:F477–F488. doi: 10.1152/ajprenal.1996.271.3.F477. [DOI] [PubMed] [Google Scholar]
- Ligr M, Madeo F, Fröhlich E, Hilt W, Fröhlich K-U, Wolf DH. Mammalian Bax triggers apoptotic changes in yeast. FEBS Lett. 1998;438:61–65. doi: 10.1016/s0014-5793(98)01227-7. [DOI] [PubMed] [Google Scholar]
- Longo VD, Ellerby LM, Bredesen DE, Valentine JS, Gralla EB. Human bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J Cell Biol. 1997;137:1581–1588. doi: 10.1083/jcb.137.7.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowary PT, Widom J. Higher-order structure of Saccharomyces cerevisiaechromatin. Proc Natl Acad Sci USA. 1989;86:8266–8270. doi: 10.1073/pnas.86.21.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Fröhlich E, Fröhlich K-U. A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CPM, McGahon AJ, Rader JA, van Schie RCAA, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Xu Q, Velours J, Reed JC. The mitochondrial F0F1-ATPase proton pump is required for function of the proapoptotic protein Bax in yeast and mammalian cells. Mol Cell. 1998;1:327–336. doi: 10.1016/s1097-2765(00)80033-7. [DOI] [PubMed] [Google Scholar]
- Maulik N, Yoshida T, Das DK. Oxidative stress developed during the reperfusion of ischemic myocardium induces apoptosis. Free Radic Biol Med. 1998;24:869–875. doi: 10.1016/s0891-5849(97)00388-2. [DOI] [PubMed] [Google Scholar]
- McCurrach ME, Connor TM, Knudson CM, Korsmeyer SJ, Lowe SW. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc Natl Acad Sci USA. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir D, Steward SE, Osmond BC, Botstein D. Cold-sensitive cell-division-cycle mutants of yeast: isolation, properties, and pseudoreversion studies. Genetics. 1982;100:547–563. doi: 10.1093/genetics/100.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monney L, Otter I, Olivier R, Ozer HL, Haas AL, Omura S, Borner C. Defects in the ubiquitin pathway induce caspase-independent apoptosis blocked by Bcl-2. J Biol Chem. 1998;273:6121–6131. doi: 10.1074/jbc.273.11.6121. [DOI] [PubMed] [Google Scholar]
- Nigro JM, Sikorski R, Reed SI, Vogelstein B. Human p53 and CDC2Hs genes combine to inhibit the proliferation of Saccharomyces cerevisiae. . Mol Cell Biol. 1992;12:1357–1365. doi: 10.1128/mcb.12.3.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO (Eur Mol Biol Organ) J. 1993;12:3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez A, Álvarez AM, Benito M, Fabregat I. Cycloheximide prevents apoptosis, reactive oxygen species production, and glutathione depletion induced by transforming growth factor β in fetal rat hepatocytes in primary culture. Hepatology. 1997;26:935–943. doi: 10.1002/hep.510260420. [DOI] [PubMed] [Google Scholar]
- Sato T, Hanada M, Bodrug S, Irie S, Iwama N, Boise LH, Thompson CB, Golemis E, Fong L, Wang H-G, Reed JC. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc Natl Acad Sci USA. 1994;91:9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Grey M, Brendel M. A microbiological assay for the quantitative determination of glutathione. Biotechniques. 1996;21:881–886. doi: 10.2144/96215st07. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Weller M, Klockgether T. Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like protease activity, and reactive oxygen species. J Neurosci. 1996;16:4696–4706. doi: 10.1523/JNEUROSCI.16-15-04696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JB, Bremen D, Reed JC, Lommatzsch J, Takayama S, Wüllner U, Löschmann P-A, Klockgether T, Weller M. Cooperative interception of neuronal apoptosis by BCL-2 and BAG-1 expression: prevention of caspase activation and reduced production of reactive oxygen species. J Neurochem. 1997;69:2075–2086. doi: 10.1046/j.1471-4159.1997.69052075.x. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. . Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater, A.F.G., C. Stefan, I. Nobel, D.J. van den Dobbelsteen, and S. Orrenius. 1995. Signalling mechanisms and oxidative stress in apoptosis. Toxicol. Lett. 82/83:149–153. [DOI] [PubMed]
- Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998;141:1423–1432. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Kurschner C, Morgan JI. Modulation of cell death in yeast by the Bcl-2 family of proteins. J Biol Chem. 1997;272:15547–15552. doi: 10.1074/jbc.272.24.15547. [DOI] [PubMed] [Google Scholar]
- Xiang J, Chao DT, Korsmeyer SJ. BAX-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Reed JC. Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol Cell. 1998;1:337–346. doi: 10.1016/s1097-2765(00)80034-9. [DOI] [PubMed] [Google Scholar]