Abstract
The formation of axon tracts in nervous system histogenesis is the result of selective axon fasciculation and specific growth cone guidance in embryonic development. One group of proteins implicated in neurite outgrowth, fasciculation, and guidance is the neural members of the Ig superfamily (IgSF). In an attempt to identify and characterize new proteins of this superfamily in the developing nervous system, we used a PCR-based strategy with degenerated primers that represent conserved sequences around the characteristic cysteine residues of Ig-like domains. Using this approach, we identified a novel neural IgSF member, termed neurotractin. This GPI-linked cell surface glycoprotein is composed of three Ig-like domains and belongs to the IgLON subgroup of neural IgSF members. It is expressed in two isoforms with apparent molecular masses of 50 and 37 kD, termed L-form and S-form, respectively. Monoclonal antibodies were used to analyze its biochemical features and histological distribution. Neurotractin is restricted to subsets of developing commissural and longitudinal axon tracts in the chick central nervous system. Recombinant neurotractin promotes neurite outgrowth of telencephalic neurons and interacts with the IgSF members CEPU-1 (K D = 3 × 10−8 M) and LAMP. Our data suggest that neurotractin participates in the regulation of neurite outgrowth in the developing brain.
Keywords: cell adhesion, development, Ig superfamily, neurite outgrowth, nervous system
Wiring of the nervous system in brain embryogenesis is a complex and fascinating process which is dependent on the axonal growth cone's ability to interpret environmental cues along the pathway to its target region. In line with the complexity of the environment through which axons have to navigate, multiple different classes of molecular cues have been implicated in axonal elongation and guidance (Tessier-Lavigne and Goodman, 1996; Drescher et al., 1997).
Currently, the most diversified class of molecules that is involved in contact-dependent regulation of neurite outgrowth and axon guidance are the neural members of the immunoglobulin superfamily (IgSF)1 (Walsh and Doherty, 1997; Sonderegger, 1998; Stoeckli and Landmesser, 1998; Van Vactor, 1998). These proteins show complex and promiscuous extracellular interactions (Brümmendorf and Rathjen, 1996) which appears to be a common feature of contact dependent cell surface molecules implicated in axon guidance (Drescher et al., 1997; Flanagan and Vanderhaeghen, 1998). Recently, functional in vitro analyses of neural IgSF members have been strongly supported by intriguing in vivo observations. For instance, L1-associated hereditary disorders coincide with malformation or even loss of selected axon tracts (reviewed in Kenwrick et al., 1996; Fransen et al., 1997; Kamiguchi et al., 1998; Brümmendorf et al., 1998), and analyses of L1-deficient mice demonstrated that L1 protein plays a role in the formation of the corticospinal tract (Cohen et al., 1998; Dahme et al., 1997). Other recent examples of IgSF members implicated in axon guidance include DCC (Deiner et al., 1997) and neurolin (Ott et al., 1998) in the retina, OCAM in the olfactory system (Yoshihara and Mori, 1997), roundabout-1 at the Drosophila midline (Kidd et al., 1998), NrCAM in the spinal cord (Stoeckli and Landmesser, 1998), and limbic system–associated membrane protein (LAMP) in the hippocampus (Pimenta et al., 1995).
To examine the complex biology of neural IgSF molecules further we are interested in the identification and functional characterization of novel members of this superfamily. Recently, we succeeded to clone cDNAs of novel proteins by a systematic PCR approach, among them CEPU-1, which is strongly expressed by cerebellar Purkinje cells and weakly in other brain regions (Spaltmann and Brümmendorf, 1996). In this study, we describe the cloning and functional characterization of a novel member of this superfamily on subsets of commissural and longitudinal central nervous system axon tracts, termed neurotractin. Recombinant neurotractin promotes neurite outgrowth of telencephalic neurons. It interacts with the CEPU-1 protein (K D = 3 × 10−8 M) and with LAMP which both belong to the same subgroup but not with neural IgSF members of the F11 or L1 subgroup. The spatiotemporal expression pattern and the functional link to axonal elongation suggests that neurotractin plays a role in the development of central nervous system axon tracts.
Materials and Methods
Cloning of Neurotractin cDNA
Sequences encoding fragments of Ig-like molecules were amplified by PCR from embryonic day 11–13 (E11-E13) brain mRNA and subcloned into plasmid pSKII (Stratagene) as outlined previously (Spaltmann and Brümmendorf, 1996). One clone, termed kb14, which contained a 290-bp insert with a novel Ig-like sequence was used to isolate clone pCMV/z14 by hybridization screening of an E16 λZAP library (Plagge and Brümmendorf, 1997) and in vivo excision as a pBK-CMV plasmid (Stratagene). As clone z14 did not contain the complete coding sequence of the novel molecule, a 5′-terminal EcoRI-PstI restriction fragment of 200 bp was used as a hybridization probe to isolate further phages from a chicken spinal cord cDNA library (λUNI-ZAP; Stratagene). Two of them, termed clones sc3 and sc4, which contain the L-form and the S-form, respectively, were subcloned into pSKII (Stratagene). Nucleotide sequences of z14, sc3, and sc4 were determined on both strands by the dideoxy chain termination method using the ALF (automated laser fluorescent) DNA sequencer (Pharmacia). The clones differed in two amino acid positions: V159 (sc4) versus G159 (z14/sc3) and S281 (sc4) versus P281 (z14/sc3).
Eucaryotic Expression of Neurotractin Isoforms
The complete insert of pSKII/sc4 was excised using XbaI and ApaI (partial digest) and ligated into pBK-CMV that had been linearized with NheI and ApaI to obtain pCMV/C11B3. In this step, the E. coli promoter of pBK-CMV which may interfere with eucaryotic expression was deleted. To express the L-form of neurotractin, the COOH-terminal part of pCMV/z14 was excised by digestion with NotI (partial) and MluI and subcloned into NotI-MluI–linearized pCMV/C11B3 to obtain pCMV/C11A1. Plasmids pCMV/C11B3 and pCMV/C11A1, which encode neurotractin-S and -L, respectively, were transfected into COS cells as described previously (Spaltmann and Brümmendorf, 1996). To construct fusion proteins of human IgG Fc domains with neurotractin, the extracellular domains of neurotractin-L and -S were amplified from pCMV/C11A1 and pCMV/ C11B3, respectively, using the primers 5′-AAGAATTCCAGCGCGGAGCGGCGCGGAGAT-3′ and 5′-TTGAATTCTGATCCCATACTGGGCCGTACT-3′. Amplification products were subcloned via EcoRI into plasmid pIG2 (Volkmer et al., 1998), and sequences were confirmed by the dideoxy chain termination method. Expression in COS cells and purification of fusion proteins was done as described (Brümmendorf et al., 1997).
Neurite Outgrowth Assays and Image Analysis
To isolate telencephalic cells, E8 telencephali were incubated for 20 min at 37°C in HBSS with 1 mg/ml trypsin. Tissue was rinsed in HBSS, dissociated in DME and 10% FCS, and then cells were seeded at a density of 50,000 cells/cm2 in tissue culture dishes (Petriperm™; Bachofer). The dishes had been precoated with 5 μl of test protein (12.5–100 μg/ml) for 4 h at 4°C, washed with HBSS, and blocked with DME and 10% FCS for 45 min at 37°C. Cultures were incubated for 40 or 72 h at 37°C, fixed, and stained essentially as described (Treubert and Brümmendorf, 1998) using mAb 5E directed to NCAM (Watanabe et al., 1986). To count attached cells, nuclei were labeled with the DNA-staining reagent H33258 (Boehringer Mannheim). Neurite outgrowth was quantified as follows: images containing nuclei of attached cells were captured separately from images with neurons using appropriate filter settings (images with neurons were edited manually because of a low signal-to-noise ratio). Images were then processed by an automated procedure to count cell nuclei and neurites and to determine neurite lengths essentially as detailed previously (Treubert and Brümmendorf, 1998). In this study low cell densities and numbers of neurites allowed us to measure the exact lengths and numbers of neurites and to normalize with respect to the number of attached cells. Data were pooled from several independent experiments as follows. For quantification of neurite initiation, 80 images (450 × 450 μm) with a total of 2,800 attached cells were evaluated for 25 μg/ml neurotractin-L. For 50 μg/ml L-form, 109 images (5,500 cells) were evaluated, for 100 μg/ml, 125 images (6,700 cells), for the S-form, 31 images (680 cells), and for Fc control substrate, 28 images (890 cells). To quantify neurite elongation, 61 images with a total of 202 neurites were processed for 25 μg/ml neurotractin-L, 102 images (483 neurites) for 50 μg/ml, and 118 images (683 neurites) for 100 μg/ml neurotractin-L. Lower neurotractin-L concentrations in the coating solution (12.5 μg/ml) did not result in significant neurite extension. Statistical significance of differences was evaluated using the Mann-Whitney U Test implemented in the Statview program (Abacus Concepts, Inc.)
Antibodies, Biochemistry, and Immunohistochemistry
Preparation of embryonic and adult chick brain plasma membranes, immunoblots, and release of GPI-linked proteins by phosphatidylinositol-specific phospholipase C (PI-PLC) was performed as outlined previously (Rathjen et al., 1987b, 1991; Wolff et al., 1989). Monoclonal antibodies directed against a 45–55-kD glycoprotein fraction of GPI-linked neural proteins from chicken brain were generated as described previously (Rathjen et al., 1987a,b). To isolate both isoforms of neurotractin by immunoaffinity chromatography from PI-PLC supernatants mAb NTRA-1 was coupled to CNBr-activated Sepharose 4B (Pharmacia Biotech AB). NH2-terminal and internal peptide sequences of neurotractin have been determined essentially as reported previously (Volkmer et al., 1992). Polyclonal antibodies to neurotractin were generated in rabbits by subcutaneous injection of 10 μg neurotractin in 2-wk intervals. N-linked carbohydrates were cleaved from proteins with a mixture of endoglycosidase F and peptide-N-glycosidase F of Flavobacterium meningosepticum (Tarentino and Plummer, 1994) according to instructions of the manufacturer (Oxford GlycoSystems).
Generation and characterization of function-blocking polyclonal Fab fragments directed to chicken neural IgSF members Ng-CAM, neurofascin, Nr-CAM, F11, axonin-1, NCAM, or gicerin (Rathjen et al., 1987a,b; Morales et al., 1993; Taira et al., 1994; Volkmer et al., 1998), and of polyclonal antibodies that recognize cell surface–expressed CEPU-1 or LAMP (Spaltmann and Brümmendorf, 1996; Brümmendorf et al., 1997) has been reported in previous studies.
Incubation of chicken eggs, preparation of tissue sections and immunofluorescence analysis were done as outlined in detail previously (Spaltmann and Brümmendorf, 1996).
In Situ Hybridization Analyses
To generate probes specific for the second and the third Ig-like domain of neurotractin, the corresponding sequences were amplified by PCR with the primer pairs 5′-AAGGATCCAACAATGCAGGTGCACCTCAC-3′/5′-TTGAATTCGGACTGAGACGTCGTTTTCTGC-3′ and 5′-AAGGATCCCACAATTCAGGAACTTAAATCC-3′/5′-TTGAATTCGAGGCAGGCTGGCATTGGTCA-3′, respectively, using plasmid pCMV/ C11A1 as a template and the products were subcloned via BamH1/EcoRI into plasmid pKSII (Stratagene). The plasmids were linearized and in vitro transcription was performed on 1 μg of template DNA/reaction using T3- (antisense strand) or T7- (sense strand) RNA polymerase (Fermentas) and a digoxigenin (DIG) nucleotide labeling mixture (Boehringer Mannheim). The resulting RNA was purified on Sephacryl columns (Pharmacia) and the concentration was estimated on an agarose gel.
Brain tissue was dissected from different embryonic stages and either directly frozen and cut on a cryostat (see Fig. 4, A and B) or fixed at first overnight in PBS/4% paraformaldehyde followed by PBS/30% sucrose (see Fig. 4 C). Sections were postfixed in PBS/4% paraformaldehyde for 10 min, washed twice in PBS/0.1% diethylpyrocarbonate for 15 min and equilibrated in 5× SSC for 15 min. Prehybridization was performed for 2 h in hybridization solution (50% formamide, 5× SSC, 40 μg/ml denatured salmon sperm DNA) at 58°C followed by overnight hybridization in the same buffer containing 0.4 μg/ml DIG-labeled RNA at 58°C. Sections were washed in 2× SSC at room temperature (RT) for 30 min, in 2× SSC at 65°C for 1 h, in 0.2× SSC for 1 h, in PBS/0.1% Tween 20 at 65°C for 10 min, and finally in PBS/0.1% Tween 20 at RT for 10 min. Blocking was performed in PBS/0.1% Tween 20/0.5% skimmed milk powder (containing 20% sheep serum) for 2 h at RT followed by anti-DIG alkaline phosphatase-conjugated antibody (Boehringer Mannheim; 1:2,500 diluted in the same buffer) at 4°C overnight. Excess antibody was washed off in PBS/0.1% Tween 20 (three times for 30 min at RT), sections were equilibrated in alkaline phosphatase reaction buffer (100 mM Tris-HCl, 100 mM NaCl, and 50 mM MgCl2, pH 9.5), and then colorized in the same buffer containing 340 μg/ml NBT and 175 μg/ml BCIP overnight at RT. The color reaction was stopped in PBS, sections were dehydrated in ethanol, and then mounted in Eukitt (Kindler).
Figure 4.
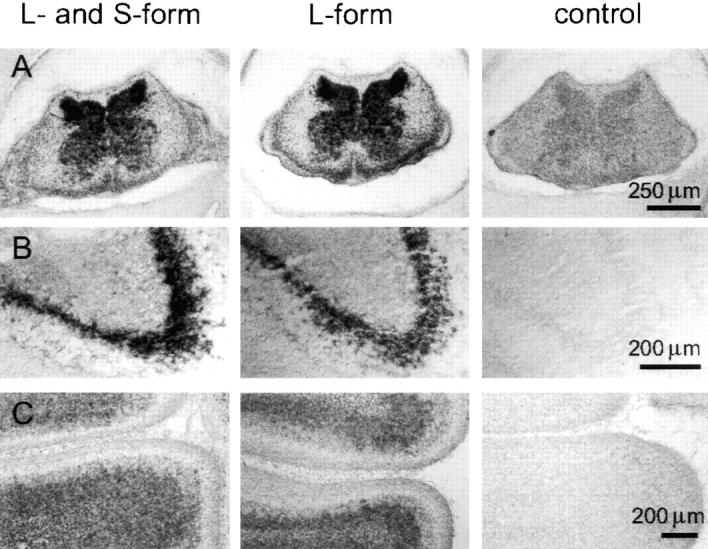
Localization of neurotractin mRNA in the developing nervous system. In situ hybridizations with two different neurotractin antisense probes: one derived from the second Ig-like domain which detects both isoforms (first column) and one derived from the third Ig-like domain which is L-form specific (second row). Both probes gave essentially the same staining patterns in chick E12 cervical spinal cord (A), in a subpopulation of neurons in E12 ventral telencephalon (B), and in the internal granular layer of E16 cerebellum (C). Control hybridizations with sense probes did not give significant signals (third column).
Binding Assays with Recombinant Fusion Proteins
Binding of Fc fusion proteins to transfected CHO and COS cells as well as expression plasmids for chicken CEPU-1 and chicken LAMP have been described previously (Spaltmann and Brümmendorf, 1996; Brümmendorf et al., 1997). Transfection efficiencies and cell surface expression levels were found to be indistinguishable for CEPU-1, LAMP, and NTRA-L, as examined by immunofluorescence analysis using polyclonal antibodies followed by quantification with an image analysis system which has been described previously (Treubert and Brümmendorf, 1998). To estimate binding constants, transfected CHO cells were exposed to increasing concentrations of purified neurotractin-Fc fusion proteins, followed by an excess of Cy3-conjugated secondary antibody directed to human IgG (Dianova). Fluorescence intensity was quantified within the linear response range of the image analysis system and background fluorescence intensity (measured with the same fusion proteins under the same conditions on untransfected cells) was subtracted. To estimate K D values, binding data were fitted to a linearized form of a titration curve as introduced by Heyn and Weischet (1975) and detailed by Bisswanger (1979).
Results
Neurotractin Is a Novel Neural Member of the Ig Superfamily
To identify novel members of the IgSF in the developing brain we defined sequence patterns characteristic for members of this superfamily which has been described in a previous study (Spaltmann and Brümmendorf, 1996). These were used to generate libraries of sequence fragments of neural IgSF members by PCR of reverse-transcribed embryonic chick brain mRNA. Sequencing of one of our initial PCR products indicated that it represents a novel member of the IgSF and it was therefore used to isolate cDNA clones by standard DNA hybridization procedures. Three clones were obtained which encode two isoforms of a novel IgSF protein, termed neurotractin (Fig. 1 A). Both isoforms reveal a hydrophobic COOH-terminal segment which is compatible with anchorage to the plasma membrane via a GPI anchor (Udenfriend and Kodukula, 1995). The large isoform, referred to as neurotractin-L, is composed of three Ig-like domains and the smaller isoform, neurotractin-S, lacks the third, membrane-proximal, Ig-like domain (Fig. 1 B).
Figure 1.
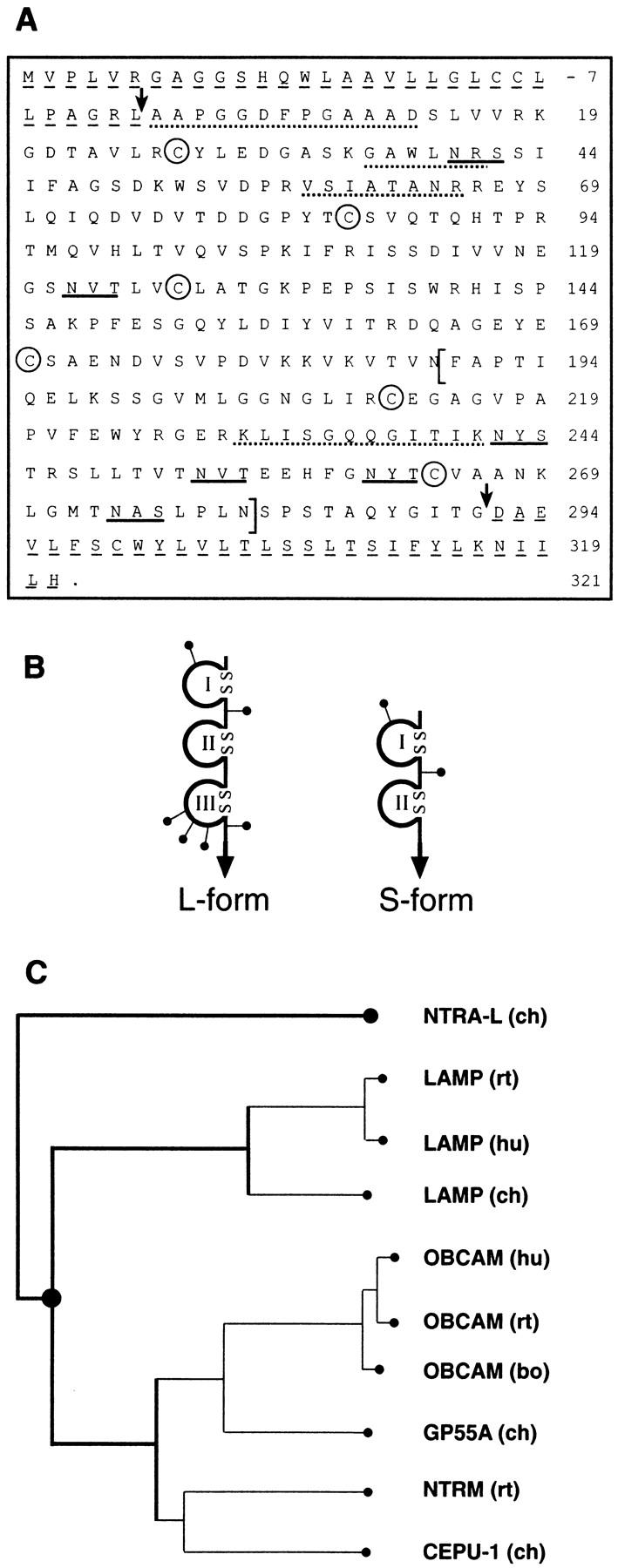
Primary structure, domain models of neurotractin and sequence relationship to other IgLON members. (A) Primary structure of neurotractin. The predicted NH2-terminal signal peptide and the COOH-terminal hydrophobic segment are underlined by dashed lines and arrows indicate the mature NH2 terminus and COOH terminus. Putative N-linked glycosylation sites are underlined and characteristic cysteine residues of Ig-like domains are labeled by circles. To obtain independent evidence that the protein which had been isolated by immunoaffinity chromatography (see Fig. 2 B, lane 1) is identical with that predicted by the cDNA clones, a sample of it as well as peptides derived from a tryptic digest were subjected to Edman degradation. Evaluation of the partial internal as well as the NH2-terminal sequence (indicated by dotted lines) confirms that they match this cDNA sequence. The alternatively spliced and L-form–specific third Ig-like domain is indicated by brackets. The sequences of S-form and L-form are available from GenBank/EMBL/DDBJ under accession numbers AJ132998 and AJ132999, respectively. (B) Domain models of neurotractin-L (large) and -S (small). Ig-like domains are drawn as loops that are closed by disulfide bridges, putative N-linked glycosylation sites are shown as lines ending with dots and the GPI-anchor is represented by an arrow. (C) Neurotractin is a novel protein belonging to the IgLON subgroup. Sequence relationship of neurotractin-L to other members of the IgLON subgroup was examined with the PILEUP program from the GCG package (University of Wisconsin, Madison, WI) and sequences have been taken from Schofield et al., 1989; Lippman et al., 1992; Shark and Lee, 1995; Struyk et al., 1995; Pimenta et al., 1995, 1996a; Spaltmann and Brümmendorf, 1996; Brümmendorf et al., 1997; Hancox et al., 1997. GP55-A is a partial sequence lacking most likely a short stretch at the NH2 terminus (Wilson et al., 1996). ch, chicken; hu, human; bo, bovine; rt, rat.
The overall domain organization and sequence alignments indicate that neurotractin belongs to a subgroup of neural GPI-linked IgSF proteins, termed IgLON subgroup (for LAMP, opioid-binding cell adhesion molecule, neurotrimin; Pimenta et al., 1995). Detailed sequence analyses including all known members of this subgroup reveals that neurotractin represents a novel member of this subgroup and can not be considered as the chicken equivalent of one of the mammalian subgroup members (Fig. 1 C).
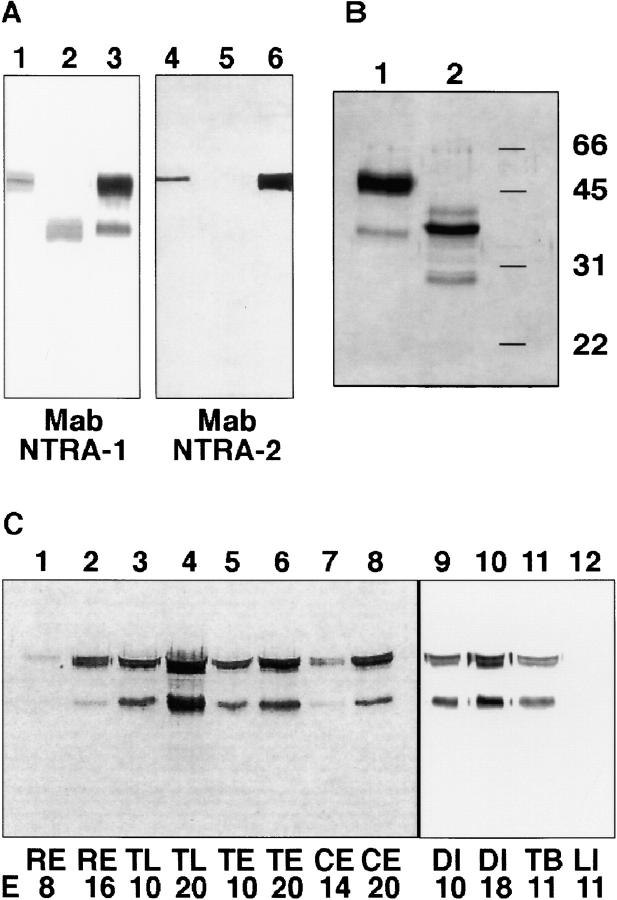
To analyze biochemical features and the histological distribution of neurotractin we used two mAbs, namely NTRA-1 and NTRA-2 (Fig. 2 A). Whereas mAb NTRA-1 reacts with both isoforms, mAb NTRA-2 binds only to the larger form, neurotractin-L, suggesting its epitope is located within the third Ig-like domain. mAb NTRA-1 was used to isolate both isoforms from PI-PLC supernatants of brain plasma membranes by immunoaffinity chromatography. The immunoaffinity isolate was found to comprise two bands, a 50-kD band and a fainter stained 37-kD band (Fig. 2 B, lane 1) that comigrated in SDS-PAGE with neurotractin isoforms expressed in COS cells, suggesting that neurotractin-L is dominating in brain in comparison to the S-form. Members of the IgLON subgroup are found to be highly glycosylated. To determine the degree of N-glycosylation, neurotractin isoforms were subjected to enzymatic deglycosylation which reduced the molecular masses to 38 and 30 kD, respectively (Fig. 2 B, lane 2). Thus, ∼24% of the molecular mass of glycosylated neurotractin-L represents N-linked carbohydrates.
Figure 2.
Neurotractin occurs in two isoforms and is upregulated in development. (A) Lysates of COS cells that had been transfected with the L-form (lanes 1 and 4) or S-form (lanes 2 and 5) of neurotractin were compared with mAb NTRA-1 immunoaffinity isolate (lanes 3 and 6) by SDS-PAGE. In Western blots mAb NTRA-1 stains both isoforms as well as both bands in the immunoaffinity isolate (lanes 1–3) whereas mAb NTRA-2 detects only the L-form and the larger band of the immunoaffinity isolate (lanes 4–6). (B) Neurotractin that was isolated by immunoaffinity chromatography from adult chicken brains using mAb NTRA-1 was subjected to SDS-PAGE and detected by silver staining. Neurotractin resolves in two bands, one of 50 kD and one of 37 kD (lane 1). Deglycosylation by endoglycosidase F/peptide-N-glycosidase F leads to a reduction of the molecular mass to 38 and 30 kD, respectively (lane 2). The 40-kD component (lane 2) represents a deglycosylation intermediate. (C) Samples of different regions of embryonic chick brain from early and late developmental stages were solubilized in SDS-PAGE sample buffer, resolved by SDS-PAGE, and probed with mAb NTRA-1 directed to neurotractin (each lane represents 30 μl of 1% brain homogenate). Comparison of samples from early stages with those from late stages shows that in each analyzed brain region neurotractin expression increases during development (lanes 1–10). Neurotractin can be detected in total brain but not in liver (lanes 11 and 12). E, embryonic day; RE, retina; TL, telencephalon; TE, tectum; CE, cerebellum; DI, diencephalon; TB, total brain; LI, liver.
Taken together, neurotractin is a novel GPI-linked member of the neural IgLON subgroup of the IgSF which occurs in two isoforms, a larger 50-kD form and a less abundant small form of 37 kD.
Expression of Neurotractin Increases during Development and Persists in Adult Brain
To obtain indications of the expression pattern of neurotractin, different brain regions and developmental stages were examined by Western blot analyses. Neurotractin was detectable in embryonic retina, telencephalon, tectum, cerebellum and diencephalon (Fig. 2 C, lanes 1–10). The highest level of neurotractin expression was found in telencephalon whereas the lowest level was determined in retina. In all brain regions examined, expression of the L-form as well as that of the S-form increases during development. In all regions and at all developmental stages examined, neurotractin-L seems to be more abundant than the S-form which is in line with the relationship observed in the immunoaffinity isolate (Fig. 2 B, lane 1).
To examine whether neurotractin is a brain-specific protein, solubilized samples of embryonic liver, muscle and lung tissue were compared with a total brain sample in SDS-PAGE followed by Western blot analyses. Whereas neurotractin can be identified in embryonic brain (Fig. 2 C, lane 11) it is undetectable in embryonic liver (Fig. 2 C, lane 12), muscle or lung (not shown) which suggests that neurotractin is a brain-specific protein.
Neurotractin Is an Axonal Cell Surface Molecule on Subsets of Central Nervous System Axon Tracts
To characterize the histological distribution of neurotractin, distinct chicken brain regions of different developmental stages were examined by immunohistochemical analyses using mAb NTRA-1 which is directed to both neurotractin isoforms (Fig. 2 A). At embryonic day 8, neurotractin is expressed on tectofugal axons in the stratum album centrale of the developing tectum mesencephali (Fig. 3 C). By contrast, neurotractin could not be detected in the plexiform layers or the optic fiber layer of the retina (not shown). It was also undetectable on retinal ganglion cell axons on their pathway to the tectum (Fig. 3, E and G). Therefore, in the retinotectal system at embryonic day 8, neurotractin is restricted to tectal efferents but is lacking on tectal afferents.
Figure 3.
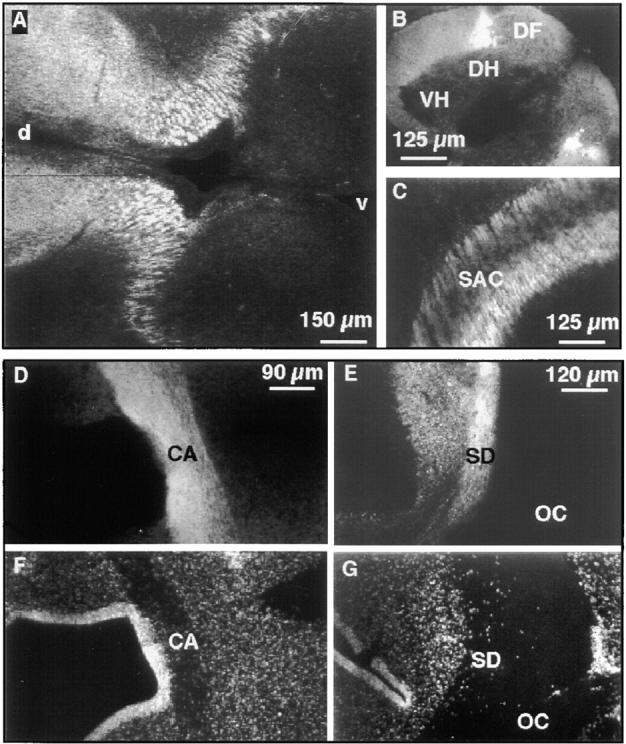
Neurotractin expression on subsets of embryonic axon tracts. Immunohistochemical analysis of E7 diencephalon with mAb NTRA-1 shows crosscut longitudinal neurotractin positive axon tracts (A). In the E9 spinal cord dorsolateral longitudinal axons show strong neurotractin expression (B). In tectum of embryonic day 8 neurotractin is found on axons of the stratum album centrale (C). Staining of a horizontal section of E9 ventral forebrain with mAb NTRA-1 shows strong expression of neurotractin on axons of the anterior commissure (D). This axon tract is also discernible as a nuclei-poor zone as revealed by DNA staining (F). A horizontal section at the level of the E9 optic chiasm reveals neurotractin expression on axons of the supraoptic decussation (E). Bisbenzimide staining of cellular nuclei in the same section outlines the axons of the supraoptic decussation as well as those of retinal ganglion cells in the optic chiasm as an extended nuclei-poor region (G). Note that neurotractin is restricted to the supraoptic decussation and is lacking on the retinal ganglion cell axons. At E9, the supraoptic decussation is not yet subdivided into the dorsal, ventral and subventral regions which can be distinguished later in development (Ehrlich et al., 1988). VH, ventral horn; DH, dorsal horn; DF, dorsal funiculus; SAC, stratum album centrale; d, dorsal; v, ventral; CA, commissura anterior; SD, supraoptic decussation; OC, optic chiasm.
On their pathway from the retina to the tectum, retinal ganglion cell axons cross the midline at the optic chiasm. In this region, they closely approach another axon tract, the supraoptic decussation which represents a major interhemispheric axon tract in the chicken located at the floor of the diencephalon (Ehrlich et al., 1988 and references cited therein). Interestingly, neurotractin is found to be strongly expressed on axons of the supraoptic decussation but it is undetectable on the adjacent retinal ganglion cell axons which demonstrates that neurotractin is restricted to subsets of axon tracts (Fig. 3, E and G). The supraoptic decussation is not the only neurotractin positive axon tract which crosses the midline. Neurotractin was also found on axons of the anterior commissure (Fig. 3, D and F) which is situated in the ventral forebrain and which represents the largest intertelencephalic pathway in chicken (Ehrlich and Mills, 1985).
In addition to axons crossing the midline, neurotractin is also expressed in longitudinal axon tracts, for instance on axon bundles in E7 diencephalon (Fig. 3 A) or on longitudinal axons in the spinal cord (Fig. 3 B). At embryonic day 9, neurotractin expression is pronounced in a small dorsolateral subpopulation of axons in the spinal cord. Later in development, however, this restriction is lost and neurotractin is found on all longitudinal spinal cord axons (data not shown).
As described above, neurotractin occurs in two isoforms which may be differentially expressed in the developing nervous system. Since the L-form–specific mAb NTRA-2 did not recognize the protein in tissue sections we performed in situ hybridizations to investigate a possible differential distribution of the two forms. Two neurotractin probes, one which is L-form–specific and one which detects both forms of neurotractin mRNA were generated. A direct comparison of L-form versus S-form is not possible because the S-form sequence is completely contained within the L-form sequence. Both probes did not reveal detectable differences in the distribution of L-form mRNA versus total neurotractin mRNA in distinct regions of developing chicken brain, for instance in spinal cord (Fig. 4 A), in subpopulations of neurons in ventral telencephalon (Fig. 4 B), in the cerebellum (Fig. 4 C), and in the tectum (data not shown). Thus, the in situ hybridizations suggest that there are no cells which are exclusively expressing the L-form and also support our conclusions drawn from the immunohistochemical analyses that neurotractin expression is restricted to subpopulations of neurons.
Taken together, these results demonstrated that neurotractin is expressed by subpopulations of neurons in distinct regions of the developing chicken brain and that it is an axonal glycoprotein that appears to be restricted to subsets of commissural and longitudinal axon tracts.
Neurotractin-L Promotes Neurite Extension of Telencephalic Neurons
Subpopulations of axons within the anterior commissure, the supraoptic decussation, and longitudinal diencephalic pathways that show prominent neurotractin expression (Fig. 3) originate and terminate in the telencephalon. This might suggest that neurotractin plays a role in fasciculation and/or elongation of telencephalic axons within these pathways. Thus, to get first insights into the function of neurotractin, we tested if it is able to promote neurite outgrowth of telencephalic neurons. Recombinant forms of neurotractin fused to the Fc domains of human IgG1 (Fig. 5, F and G) were immobilized on tissue culture dishes and were used as substrates for embryonic day 8 telencephalic cells. We examined long-term cell attachment, neurite initiation, and neurite elongation of telencephalic neurons by measuring the number of adhering cells, the number of neurites per 100 cells and average neurite length, respectively, using an automated image analysis procedure (see Materials and Methods). These experiments showed that neurotractin-L fusion protein promotes attachment and neurite extension of telencephalic cells (Fig. 5, A, C, and D), whereas on Fc control substrate cell attachment was low and neurite outgrowth was undetectable (Fig. 5, B–D). In contrast, neurotractin-S mediated only weak adhesion which was within the same range as that measured on Fc control substrate and did not induce neurites.
Figure 5.
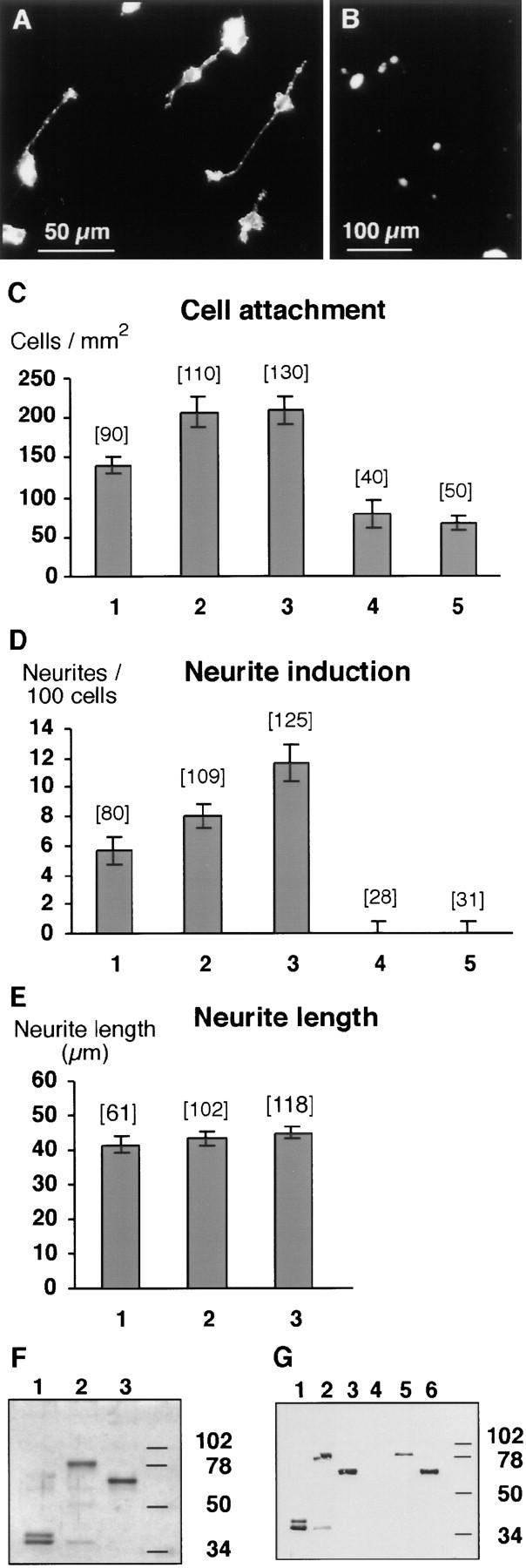
Neurotractin-L promotes neurite outgrowth and cell attachment of telencephalic neurons. (A) Cells isolated from E8 telencephalon attach to a neurotractin-L substratum which promotes neurite outgrowth. (B) Cell attachment to the control substrate consisting of Fc protein without neurotractin domains is shown for comparison. (C) Density of telencephalic neurons adhering to different substrates. Fc fusion proteins were coated as follows: neurotractin L-form with 25 μg/ml (1), 50 μg/ml (2), and 100 μg/ml (3), Fc control protein (4), and neurotractin S-form (5) with 100 μg/ml. Data were pooled from independent experiments, histogram bars show median values, error bars represent SEM, and the number of analyzed images (450 μm × 450 μm) is given in parentheses. The difference between Fc control protein and 25 μg/ml neurotractin-L is statistically significant (P = 0.003, Mann Whitney U test). (D) Neurotractin-induced telencephalic neurite outgrowth after 40 h of incubation on Fc fusion proteins coated as in C. The differences between Fc control protein and 25 μg/ml neurotractin-L (P < 0.001), between 25 μg/ml and 50 μg/ ml (P < 0.05), and between 50 μg/ml and 100 μg/ml (P < 0.05) are statistically significant (Mann Whitney U test). Lower neurotractin-L concentrations in the coating solution (12.5 μg/ml) did not result in significant neurite extension above background values (data not shown). (E) Increasing amounts of neurotractin do not influence the average length of telencephalic neurites. Fc fusion protein of neurotractin L-form was coated as indicated in C. (F) SDS-PAGE (10%) of fusion proteins used in neurite outgrowth experiments and binding studies followed by silver staining. Purified Fc fusion proteins of L-form (lane 2) and S-form (lane 3) neurotractin reveal the expected molecular masses of 80 and 65 kD, respectively. The Fc domains are resolved in a 39-kD component and a 36-kD degradation product (lane 1). (G) SDS-PAGE (10%) and Western blot analyses show that neurotractin-L (lane 2), neurotractin-S (lane 3), and the Fc domains (lane 1) can be detected with polyclonal Fc domain-specific antibodies. The 36-kD component (lanes 2 in F and G) which can also be observed in the Fc control protein (lanes 1 in F and G) is a degradation product. Neurotractin-specific polyclonal antibodies identify both neurotractin isoforms (lanes 5 and 6) but do not react with the Fc domains (lane 4).
Quantification of the neurotractin-L–mediated neurite outgrowth response after 40 h of incubation showed that the number of neurites per 100 cells increased in a dose-dependent manner (Fig. 5 D) suggesting that neurotractin modulates neurite initiation. On average only ∼1 of 10 cells was found to elaborate a neurite suggesting that the responsive neurons might represent a subpopulation of telencephalic cells. To investigate if prolonged incubation might recruit a larger neuronal subpopulation to extend neurites, cultures were evaluated after 72 h. However, no additional increase in the number of neurites per 100 cells could be observed for the highest neurotractin concentration that was tested (data not shown).
A neurite outgrowth–promoting molecule may regulate the initiation of neurites and/or their elongation. Therefore, we investigated if immobilized neurotractin has an impact on the average length of telencephalic neurites, in addition to its effect on neurite initiation. However, no significant effect of increasing neurotractin-L amounts on the average neurite length could be demonstrated after 40 h (Fig. 5 E) or 72 h (data not shown) suggesting that neurotractin primarily influences the initiation of neurites of telencephalic neurons in vitro. Consistently, the average neurite length increased by <10% from 40 to 72 h of incubation (data not shown).
As a first step to characterize the cellular receptor on telencephalic neurons responsible for the neurite outgrowth–promoting activity of neurotractin-L, polyclonal antibodies specific for various cell surface proteins were applied in these in vitro assays. We tested antibodies directed to L1 subgroup members (Ng-CAM, neurofascin, and Nr-CAM), F11 subgroup members (F11 and axonin-1), IgLON subgroup members (LAMP and CEPU-1), NCAM, or gicerin. None of these antibodies which have been previously documented to interfere functionally in distinct experimental paradigms using chicken neurons (Rathjen et al., 1987a,b; Morales et al., 1993; Taira et al., 1994; Volkmer et al., 1998), or to recognize the respective proteins on cell surfaces (Spaltmann and Brümmendorf, 1996; Brümmendorf et al., 1997), blocked neurite outgrowth on neurotractin-L (data not shown). Therefore, the cellular receptor on telencephalic neurons that mediates the neurite outgrowth–promoting activity of neurotractin-L remains unknown at present. To characterize the neurotractin-responsive cells we have analyzed their profile of expression of known adhesion proteins. Consistent with the above-mentioned antibody perturbation experiments, these responding cells are NCAM and F11 positive, but are, however, negative for NgCAM, neurofascin, NrCAM, and axonin-1 (data not shown).
Interaction of Neurotractin with CEPU-1 and with LAMP
Several Ig superfamily members on axons reveal homophilic and/or heterophilic binding to other IgSF members within the same or across plasma membranes to regulate cellular interactions (Brümmendorf and Rathjen, 1996; Drescher et al., 1997; Sonderegger, 1997). To further characterize the molecular function of neurotractin isoforms we examined if recombinant neurotractin interacts with itself or with other neural members of the IgSF. To this end, purified Fc fusion proteins of neurotractin-L and -S were incubated with CHO cell transfectants that express putative interaction partners on their surface and, after washing, bound fusion proteins were detected with fluorochrome-conjugated secondary antibodies specific for their Fc portion. Soluble neurotractin-L fusion protein was found to bind strongly to CEPU-1 transfectants and, in comparison, weakly to LAMP transfectants, whereas no homophilic binding to neurotractin-L transfectants could be detected (Fig. 6 A). The S-form of neurotractin also interacts with CEPU-1, however, binding was clearly weaker than that of the L-form while binding to LAMP could not be detected by this method. As a control, interaction of neurotractin-L with other IgSF members was examined under the same conditions but no binding could be observed to F11 (Fig. 6 D), NgCAM, axonin-1, neurofascin (data not shown), or GPI-linked Fc domains alone (Fig. 6 E). As an additional control, CEPU-1–expressing CHO cells were treated with PI-PLC to release CEPU-1 from the cell surface and were then incubated with neurotractin-L fusion protein. A strongly decreased binding to PI-PLC treated transfectants supports the interpretation that neurotractin-L binds to CEPU-1 on the cell surface and not unspecifically to other cell surface components (Fig. 6, B and C). Furthermore, binding of soluble neurotractin-L to surface-expressed CEPU-1 and LAMP could also be demonstrated with other eucaryotic cells, namely transfected COS cells (Fig. 6, G and F).
Figure 6.
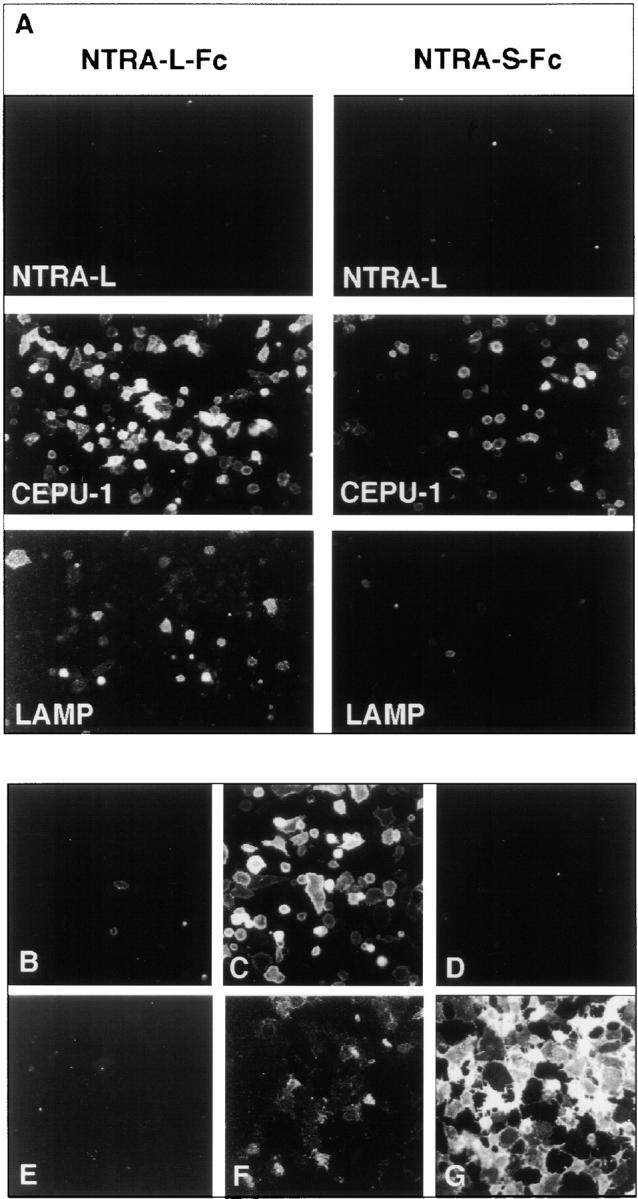
Molecular interaction of neurotractin with CEPU-1 and LAMP. (A) Neurotractin-L (first row), CEPU-1 (middle row), and LAMP (bottom row) were expressed on the surface of CHO cells by transient transfection. Confluent layers of cells were incubated with 10 μg/ml of purified Fc fusion proteins of neurotractin L-form (left column) and S-form (right column). After washing, Fc domains of fusion proteins were detected by Cy3-conjugated secondary antibody directed to human IgG. Control experiments showed that the percentage of transfected cells and their surface expression levels were indistinguishable for NTRA-L, CEPU-1, and LAMP. CHO cells expressing CEPU-1 on their surface that had been preincubated with PI-PLC (B) or control buffer (C) were tested with COS cell supernatants containing neurotractin-L Fc fusion protein. CHO cells expressing F11 (D) or GPI-linked Fc domains (E) do not bind neurotractin-L fusion protein. Furthermore, COS cells which express CEPU-1 (G) or LAMP (F) on their surface were also found to bind soluble recombinant neurotractin-L.
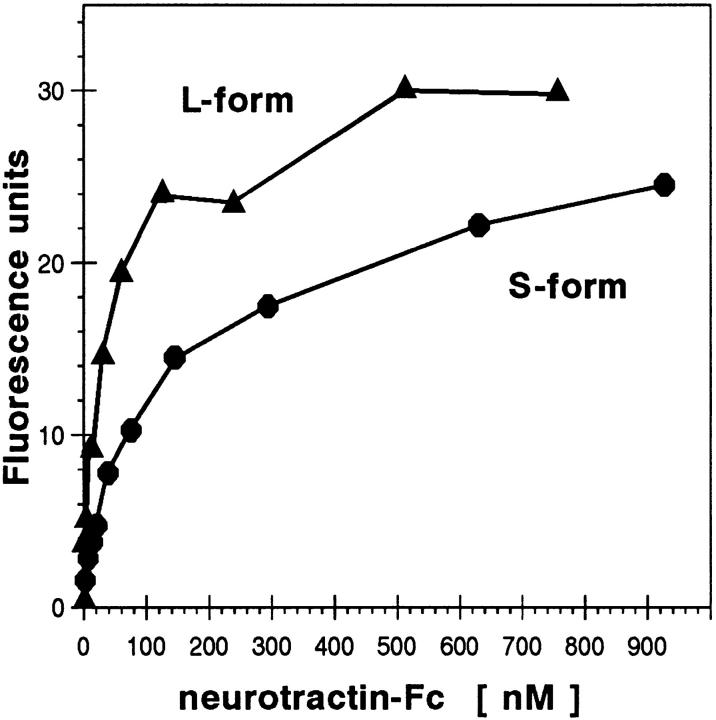
To estimate the apparent dissociation constant for the neurotractin-L–CEPU-1 interaction, we quantified binding of neurotractin-L fusion protein to CEPU-1 which was expressed on the surface of transfected CHO cells. In this assay system, one of the interacting proteins is in a native and membrane-bound form and binding of the interacting partner can be monitored by immunofluorescence analysis and quantified by digital image processing. Binding of neurotractin-L fusion protein to CEPU-1–transfected CHO cells was saturable and gave an apparent dissociation constant of 3 × 10−8 M (Fig. 7). The interactions of neurotractin-L with LAMP and of the S-form with CEPU-1 could not be reliably quantified but were estimated to be at least five times weaker than L-form binding to CEPU-1 (Fig. 6 A). Furthermore, no significant fluorescence signal could be observed if soluble Fc control protein was incubated with CEPU-1–transfected cells (data not shown).
Figure 7.
Equilibrium binding of recombinant neurotractin. CHO cell transfectants expressing CEPU-1 were incubated with increasing concentrations of neurotractin fusion proteins, followed by a constant saturating amount of fluorochrome-conjugated Fc domain–specific antibody. Average fluorescence intensity that was measured in at least three independent experiments is given. Soluble Fc control protein gave a signal of <5 fluorescence units if applied to CEPU-1 transfectants at a concentration of 6.2 μM under the same conditions.
In conclusion, neurotractin-L interacts with the structurally related molecule CEPU-1 and, though more weakly, with LAMP, whereas neither homophilic binding nor binding to other neural IgSF members could be detected. However, these interactions are not required for neurotractin-L–mediated neurite initiation as revealed by antibody perturbation experiments (see above) which suggests that the neurotractin–CEPU-1 or the neurotractin– LAMP binding might be implicated in other cellular activities.
Discussion
A Family of GPI-linked Neural Cell Surface Proteins with Three Ig-like Domains
In this study, we identified neurotractin as a novel GPI-linked neural member of the IgSF with three Ig-like domains. It is associated with specific axon tracts and is implicated in neurite initiation as demonstrated by in vitro assays. Comparison of the neurotractin sequence with sequences in GenBank database revealed that it shows 48– 56% sequence identity to members of a neural subfamily of the IgSF which is termed IgLON subgroup (Pimenta et al., 1995). This group comprises limbic system-associated membrane protein (LAMP) (Pimenta et al., 1995, 1996a), opioid-binding cell adhesion molecule (OBCAM; Schofield et al., 1989; Lippman et al., 1992; Shark and Lee, 1995), and neurotrimin (Struyk et al., 1995) in mammalia as well as CEPU-1 (Spaltmann and Brümmendorf, 1996), LAMP (chLAMP/AvGP50/E19S; Wilson et al., 1996; Brümmendorf et al., 1997; Hancox et al., 1997), GP55-A (Wilson et al., 1996), and neurotractin (this study) which have been identified in chicken (Fig. 1 C). All IgLON subgroup members share with neurotractin the 3-domain organization, the plasma membrane attachment via GPI, and the restricted expression in partially overlapping regions of the nervous system (Struyk et al., 1995; Pimenta et al., 1996b; Wilson et al., 1996; Brümmendorf et al., 1997; Hancox et al., 1997). Furthermore, neurotractin has 26– 29% sequence identity with distinct Drosophila proteins, including lachesin (Karlstrom et al., 1993), amalgam (Seeger et al., 1988), and klingon (Butler et al., 1997).
Pairwise comparisons of single domains of IgLON members shows that the corresponding domains are most highly conserved between different molecules (data not shown). This colinear relationship of individual domains suggests an evolutionary origin of these proteins from a common ancestor. LAMP is currently the most thoroughly characterized representative of this subgroup. It reveals a restricted pattern of expression in functionally related areas of the nervous system and might serve as a guidance cue during the development of the septo-hippocampal pathway (Keller et al., 1989) and the hippocampal mossy fiber projection (Pimenta et al., 1995). Cloning of LAMP from human (Pimenta et al., 1996a), rat (Pimenta et al., 1995), and chicken (Wilson et al., 1996; Brümmendorf et al., 1997; Hancox et al., 1997) revealed that it is highly conserved in evolution, e.g., human LAMP shows 99 and 91% sequence identity with the rat and chicken proteins, respectively. The structural similarity of vertebrate IgLON molecules and their spatiotemporal expression pattern suggests that they might have related functions in the context of cell–cell interactions in nervous system histogenesis and in the adult brain.
Binding of Neurotractin to the GPI-linked Proteins CEPU-1 and LAMP
Whereas we could demonstrate that neurotractin-L is involved in initiation of telencephalic neurite growth, the axonal receptor protein important for this activity could not be identified at present. Our attempts to characterize proteins that interact with neurotractin resulted in the finding that neurotractin-L Fc fusion protein binds to CEPU-1 and, more weakly, to LAMP (Fig. 6). Therefore, it appears to be a characteristic feature of IgLON molecules that some members of this subgroup bind to other members of the same subgroup, however, not to other IgSF proteins. A K D of 3 × 10−8 M was measured for the binding of neurotractin to the GPI-linked molecule CEPU-1 (Fig. 7), an affinity that is lower than that usually reported for soluble polypeptide ligands that bind to cell surface receptors, for instance the neurotrophins (Dechant et al., 1994). However, cell–cell communication via membrane-bound ligands is likely to depend not only on receptor affinity but also on avidity effects and receptor density. Indeed, other receptors which also interact with membrane-bound ligands have binding constants which are comparable to that measured for neurotractin. For example, Fc fusion proteins of the Eph-like receptor tyrosine kinases that bind to cell surface ligands, termed ephrins, show dissociation constants of 4 × 10−10 M to 4 × 10−8 M (Gale et al., 1996; Beckmann et al., 1994). Nevertheless, these K D values should not be overinterpreted. On the one hand, potential bivalency of Fc fusion proteins may decrease their dissociation rate constant which may lead to overestimated affinities. On the other hand, analyses using soluble ligands most likely underestimate the true avidity between receptors and membrane bound ligands in their membrane-associated state. Regardless, the interaction of neurotractin with CEPU-1 is of a strength which is of physiological relevance in other receptor/ligand systems. The biological functions of the neurotractin–CEPU-1 or the neurotractin–LAMP interactions are currently unknown. Our antibody perturbations experiments indicate that they are not required for neurite extension of telencephalic neurons on immobilized neurotractin. However, it is conceivable that these interactions are important for other neurons, in contrast with the telencephalic neurons used here. For example, CEPU-1, which is expressed by Purkinje cells, and neurotractin are also colocalized in the molecular layer of the cerebellum suggesting that the neurotractin–CEPU-1 interaction may play a role in development of the Purkinje cell dendritic tree.
Neurotractin May Participate in the Regulation of Neurite Outgrowth in the Developing Brain
Cell surface molecules that promote neurite outgrowth can be interpreted as membrane-bound neuronal differentiation factors because neurite initiation and elongation are part of the neuronal differentiation program. For instance, an outgrowth-initiating molecule that is restricted to a particular cortical layer may influence neuronal differentiation of precursor cells invading this layer and switch on dendritic growth. On the other hand, a protein that promotes elongation of neurites may provide a permissive environment for advancing growth cones. Our in vitro analyses suggest that neurotractin-L is more likely to be related to neurite initiation rather than elongation for two reasons. First, within 40 h of incubation we observed a dose-dependent influence on the number of neurites per 100 cells but no significant impact on the average length of the neurites (Fig. 5). Second, we did not observe a significant increase of average neurite length between 40 and 72 h of incubation (data not shown).
Our observation that neurotractin has a stronger effect on neurite initiation than on neurite elongation appears to be inconsistent with its expression in axon tracts (Fig. 3) that suggests a role in neurite elongation. However, neurotractin is only one component of a complex network of interacting receptors and ligands that regulate neurite outgrowth in vivo and it is reasonable to assume that other important factors are missing in our in vitro assay system. Thus, a more conclusive interpretation of the role of neurotractin in the context of neurite outgrowth requires additional studies, for instance antibody perturbation experiments in ovo or histological analyses of neurotractin-deficient knockout mice.
Other hints to the putative role of neurotractin in neurohistogenesis come from functional analyses of structurally related proteins. Many members of the IgSF that are expressed in the nervous system are implicated in processes like neurite outgrowth (Brümmendorf and Rathjen, 1995; Kamiguchi and Lemmon, 1997), fasciculation (Van Vactor, 1998), and guidance (Deiner et al., 1997; Stoeckli and Landmesser, 1998; Kidd et al., 1998). In particular, neurotractin is closely related to LAMP (Pimenta et al., 1995), which has been shown to induce neurite outgrowth from specific subpopulations of neurons: transfected CHO cells that express LAMP on their surface have only weak effects on neurons from olfactory bulb or visual cortex but promote neurite outgrowth from perirhinal and hippocampal neurons significantly (Pimenta et al., 1995; Zhukareva et al., 1997). This is reminiscent to neurotractin which also promotes outgrowth only of subsets of neurons, in this case subpopulations of telencephalic neurons. Further experiments are needed to characterize the neurotractin-responsive subpopulations of neurons in the developing brain and to identify the receptor(s) involved in the outgrowth response.
Neurotractin Occurs in Two Isoforms which Are Upregulated in Development
Neurotractin is expressed in two isoforms, termed L-form and S-form, differing with respect to the presence of the membraneproximal Ig-like domain (Fig. 1 B). Both forms show the same spatiotemporal expression profile, as examined by Western blot analyses (Fig. 2 C) and also at the level of in situ hybridization analyses (Fig. 4). Alternative splicing of complete Ig-like domains has not been observed previously for IgLON molecules and its functional significance remains unclear at present. One possible reason for expression of different isoforms might be that they differ functionally, for instance with respect to binding of receptors and ligands. In this regard it is of interest that only the L-form has been found to mediate adhesion and neurite initiation of telencephalic neurons and that the L-form binds stronger to CEPU-1 or LAMP than the S-form. This may suggest that these cells express a receptor which binds to the membraneproximal domain of neurotractin which is lacking in the S-form. However, this may be argued against since the membraneproximal domains of IgLON members are least conserved in evolution (Pimenta et al., 1996a; Brümmendorf et al., 1997) and ligand or receptor binding sites are frequently located in NH2-terminal regions of cell adhesion receptors (Brümmendorf and Rathjen, 1996). Thus, it is also possible that the receptor binding site of neurotractin is located in the aminoproximal domains but may be sterically inaccessible in the S-form Fc fusion protein.
In addition to the membrane-bound L- and S-forms, soluble variants of neurotractin may also exist. The immunohistochemical analyses show that there are two aspects of neurotractin expression: First, a prominent labeling of axon tracts (Fig. 3) and second, for instance in the cerebellum, a weak and diffuse staining (data not shown) which can be confirmed in Western blot analyses (Fig. 2 C) and in situ hybridizations (Fig. 4 C). One explanation for the diffuse staining may be that neurotractin is released from the cell membrane by an endogenous phospholipase as it has also been described for axonin-1 (Lierheimer et al., 1997), another neurite outgrowth–related IgSF member (Sonderegger, 1997). In the case of axonin-1, the soluble form may act as a competitive inhibitor of neurite fasciculation (Stoeckli et al., 1991). For neurotractin-L, this question will be addressed in future investigations.
Western blot analyses of different brain regions showed that neurotractin is expressed in retina, telencephalon, tectum, cerebellum, and diencephalon. Furthermore, analysis of different developmental stages revealed that expression is increasing in all regions during development (Fig. 2 C). Upregulation in development has also been observed for other IgLON molecules, for instance LAMP (Brümmendorf et al., 1997; Hancox et al., 1997) or CEPU-1 (Spaltmann and Brümmendorf, 1996), and suggests that these molecules may also have a function in the mature brain. Consistently, LAMP has also been found in the adult brain of human (Pimenta et al., 1996a), rat (Reinoso et al., 1996), and chick (Hancox et al., 1992). Furthermore, neurotrimin and OBCAM have been detected in postnatal day 20 rat brain (Struyk et al., 1995). Functions of neurotractin in the adult brain are unknown at present but may include phenomena which are similar to those in the developing brain, for instance functions related to neuronal remodelling and plasticity.
Acknowledgments
We would like to thank Dr. Alfred Gierer for support and discussions, Dr. Stefan Schumacher for help in initial phases of this project, Dr. Ullrich Treubert for helpful discussions, Dieter Jobsky for excellent technical assistance, and Birgit Cloos for secretarial help. We are grateful to Dr. Peter Sonderegger and Dr. Hansjürgen Volkmer for providing plasmids encoding axonin-1, NgCAM, and neurofascin, respectively, and to Dr. Naomasa Miki for providing gicerin-specific antibodies. We would like to thank Hidemasa Kato for help with the in situ hybridization method.
Abbreviations used in this paper
- ALF
automated laser fluorescent
- IgLON
LAMP/OBCAM/neurotrimin subgroup of the IgSF
- IgSF
immunoglobulin superfamily
- LAMP
limbic system-associated membrane protein
- NgCAM
neuron-glia cell adhesion molecule
- NrCAM
NgCAM-related cell adhesion molecule
- OBCAM
opioid binding cell adhesion molecule
- PI-PLC
phosphatidylinositol-specific phospholipase C
- RT
room temperature
Footnotes
This study was in part supported by grant SFB 515 (Deutsche Forschungsgemeinschaft) to F.G. Rathjen and by grant Br1217/3-1 (Deutsche Forschungsgemeinschaft) to T. Brümmendorf.
Pinar Sirim's current address is GeneCenter, University of Munich, Munich, Germany.
Frank Spaltmann's current address is BAYER AG, Pharma Research, Wuppertal, Germany.
Gunther Kauselmann's current address is Artemis Pharmaceuticals, Cologne, Germany.
Friedrich Buck's current address is Institute for Cell Biochemistry and Clinical Neurobiology, University of Hamburg, Hamburg, Germany.
References
- Beckmann MP, Cerretti DP, Baum P, Vanden T, Bos, James L, Farrah T, Kozlosky C, Hollingsworth T, Shilling H, Maraskovsky E, et al. Molecular characterization of a family of ligands for Eph-related tyrosine kinase receptors. EMBO (Eur Mol Biol Organ) J. 1994;13:3757–3762. doi: 10.1002/j.1460-2075.1994.tb06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisswanger, H. 1979. Theorie und Methoden der Enzymkinetik. Verlag Chemie, Weinheim.
- Brümmendorf T, Spaltmann F, Treubert U. Cloning and characterization of a neural cell recognition molecule on axons of the retinotectal system and spinal cord. Eur J Neurosci. 1997;9:1105–1116. doi: 10.1111/j.1460-9568.1997.tb01463.x. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T, Kenwrick S, Rathjen FG. Neural cell recognition molecule L1: from cell biology to human hereditary brain malformations. Curr Opin Neurobiol. 1998;8:87–97. doi: 10.1016/s0959-4388(98)80012-3. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T, Rathjen FG. Cell adhesion molecules 1: immunoglobulin superfamily. Prot Profile. 1995;2:963–1108. [PubMed] [Google Scholar]
- Brümmendorf T, Rathjen FG. Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr Opin Neurobiol. 1996;6:584–593. doi: 10.1016/s0959-4388(96)80089-4. [DOI] [PubMed] [Google Scholar]
- Butler SJ, Ray S, Hiromi Y. Klingon, a novel member of the Drosophilaimmunoglobulin superfamily, is required for the development of the R7 photoreceptor neuron. Development. 1997;124:781–792. doi: 10.1242/dev.124.4.781. [DOI] [PubMed] [Google Scholar]
- Cohen NR, Taylor JS, Scott LB, Guillery RW, Soriano P, Furley AJ. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol. 1998;8:26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17:346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- Dechant G, Rodriguez A, Tebar, Barde YA. Neurotrophin receptors. Prog Neurobiol. 1994;42:347–352. doi: 10.1016/0301-0082(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier M, Lavigne, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Drescher, U., A. Faissner, R. Klein, F.G. Rathjen, and C.A.O. Stuermer, editors. 1997. Molecular bases of axonal growth and pathfinding. Springer Verlag, Berlin. [DOI] [PubMed]
- Ehrlich D, Zappia JV, Saleh CN. Development of the supraoptic decussation in the chick (Gallus gallus) . Anat Embryol Berl. 1988;177:361–370. doi: 10.1007/BF00315845. [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Mills D. Myelogenesis and estimation of the number of axons in the anterior commissure of the chick (Gallus gallus) . Cell Tissue Res. 1985;239:661–666. doi: 10.1007/BF00219246. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Fransen E, Van Camp G, Vits L, Willems PJ. L1-associated diseases: clinical geneticists divide, molecular geneticists unite. Hum Mol Genet. 1997;6:1625–1632. doi: 10.1093/hmg/6.10.1625. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Hancox KA, Sheppard AM, Jeffrey PL. Characterisation of a novel glycoprotein (AvGp50) in the avian nervous system, with a monoclonal antibody. Dev Brain Res. 1992;70:25–37. doi: 10.1016/0165-3806(92)90100-b. [DOI] [PubMed] [Google Scholar]
- Hancox KA, Gooley AA, Jeffrey PL. AvGp50, a predominantly axonally expressed glycoprotein, is a member of the IgLON's subfamily of cell adhesion molecules (CAMs) Brain Res Mol Brain Res. 1997;44:273–285. doi: 10.1016/s0169-328x(96)00228-8. [DOI] [PubMed] [Google Scholar]
- Heyn MP, Weischet WO. Circular dichroism and fluorescence studies on the binding of ligands to the α subunit of tryptophan synthase. Biochemistry. 1975;14:2962–2968. doi: 10.1021/bi00684a026. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Hlavin ML, Yamasaki M, Lemmon V. Adhesion molecules and inherited diseases of the human nervous system. Annu Rev Neurosci. 1998;21:97–125. doi: 10.1146/annurev.neuro.21.1.97. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. Neural cell adhesion molecule L1: signaling pathways and growth cone motility. J Neurosci Res. 1997;49:1–8. doi: 10.1002/(sici)1097-4547(19970701)49:1<1::aid-jnr1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Karlstrom RO, Wilder LP, Bastiani MJ. Lachesin: an immunoglobulin superfamily protein whose expression correlates with neurogenesis in grasshopper embryos. Development. 1993;118:509–522. doi: 10.1242/dev.118.2.509. [DOI] [PubMed] [Google Scholar]
- Keller F, Rimvall K, Barbe MF, Levitt P. A membrane glycoprotein associated with the limbic system mediates the formation of the septo-hippocampal pathway in vitro. Neuron. 1989;3:551–561. doi: 10.1016/0896-6273(89)90265-1. [DOI] [PubMed] [Google Scholar]
- Kenwrick S, Jouet M, Donnai D. X linked hydrocephalus and MASA syndrome. J Med Genet. 1996;33:59–65. doi: 10.1136/jmg.33.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Lierheimer R, Kunz B, Vogt L, Savoca R, Brodbeck U, Sonderegger P. The neuronal cell-adhesion molecule axonin-1 is specifically released by an endogenous glycosylphosphatidylinositol-specific phospholipase. Eur J Biochem. 1997;243:502–510. doi: 10.1111/j.1432-1033.1997.0502a.x. [DOI] [PubMed] [Google Scholar]
- Lippman DA, Lee NM, Loh HH. Opioid-binding cell adhesion molecule (OBCAM)-related clones from a rat brain cDNA library. Gene. 1992;117:249–254. doi: 10.1016/0378-1119(92)90734-7. [DOI] [PubMed] [Google Scholar]
- Morales G, Hubert M, Brümmendorf T, Treubert U, Tarnok A, Schwarz U, Rathjen FG. Induction of axonal growth by heterophilic interactions between the cell surface recognition proteins F11 and Nr-CAM/Bravo. Neuron. 1993;11:1113–1122. doi: 10.1016/0896-6273(93)90224-f. [DOI] [PubMed] [Google Scholar]
- Ott H, Bastmeyer M, Stuermer CA. Neurolin, the goldfish homolog of DM-GRASP, is involved in retinal axon pathfinding to the optic disk. J Neurosci. 1998;18:3363–3372. doi: 10.1523/JNEUROSCI.18-09-03363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta AF, Zhukareva V, Barbe MF, Reinoso BS, Grimley C, Henzel W, Fischer I, Levitt P. The limbic system-associated membrane protein is an Ig superfamily member that mediates selective neuronal growth and axon targeting. Neuron. 1995;15:287–297. doi: 10.1016/0896-6273(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Pimenta AF, Fischer I, Levitt P. CDNA cloning and structural analysis of the human limbic-system-associated membrane protein (LAMP) Gene. 1996a;170:189–195. doi: 10.1016/0378-1119(96)84698-1. [DOI] [PubMed] [Google Scholar]
- Pimenta AF, Reinoso BS, Levitt P. Expression of the mRNAs encoding the limbic system-associated membrane protein (LAMP): II. Fetal rat brain. J Comp Neurol. 1996b;375:289–302. doi: 10.1002/(SICI)1096-9861(19961111)375:2<289::AID-CNE8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Plagge A, Brümmendorf T. The gene of the neural cell recognition molecule F11: conserved exon–intron arrangement in genes of neural members of the immunoglobulin superfamily. Gene. 1997;192:215–225. doi: 10.1016/s0378-1119(97)00066-8. [DOI] [PubMed] [Google Scholar]
- Rathjen FG, Wolff JM, Chang S, Bonhoeffer F, Raper JA. Neurofascin: a novel chick cell-surface glycoprotein involved in neurite-neurite interactions. Cell. 1987a;51:841–849. doi: 10.1016/0092-8674(87)90107-3. [DOI] [PubMed] [Google Scholar]
- Rathjen FG, Wolff JM, Frank R, Bonhoeffer F, Rutishauser U. Membrane glycoproteins involved in neurite fasciculation. J Cell Biol. 1987b;104:343–353. doi: 10.1083/jcb.104.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen FG, Wolff JM, Chiquet R, Ehrismann Restrictin: a chick neural extracellular matrix protein involved in cell attachment co-purifies with the cell recognition molecule F11. Development. 1991;113:151–164. doi: 10.1242/dev.113.1.151. [DOI] [PubMed] [Google Scholar]
- Reinoso BS, Pimenta AF, Levitt P. Expression of the mRNAs encoding the limbic system-associated membrane protein (LAMP): I. Adult rat brain. J Comp Neurol. 1996;375:274–288. doi: 10.1002/(SICI)1096-9861(19961111)375:2<274::AID-CNE7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schofield PR, McFarland KC, Hayflick JS, Wilcox JN, Cho TM, Roy S, Lee NM, Loh HH, Seeburg PH. Molecular characterization of a new immunoglobulin superfamily protein with potential roles in opioid binding and cell contact. EMBO (Eur Mol Biol Organ) J. 1989;8:489–495. doi: 10.1002/j.1460-2075.1989.tb03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger MA, Haffley L, Kaufman TC. Characterization of amalgam: a member of the immunoglobulin superfamily from Drosophila. . Cell. 1988;55:589–600. doi: 10.1016/0092-8674(88)90217-6. [DOI] [PubMed] [Google Scholar]
- Shark KB, Lee NM. Cloning, sequencing and localization to chromosome 11 of a cDNA encoding a human opioid-binding cell adhesion molecule (OBCAM) Gene. 1995;155:213–217. doi: 10.1016/0378-1119(94)00830-l. [DOI] [PubMed] [Google Scholar]
- Sonderegger P. Axonin-1 and NgCAM as “recognition” components of the pathway sensor apparatus of growth cones: a synopsis. Cell Tissue Res. 1997;290:429–439. doi: 10.1007/s004410050950. [DOI] [PubMed] [Google Scholar]
- Sonderegger, P., editor. 1998. Ig Superfamily Molecules in the Nervous System. Harwood Academic Publishers, Amsterdam.
- Spaltmann F, Brümmendorf T. CEPU-1, a novel immunoglobulin superfamily molecule, is expressed by developing cerebellar Purkinje cells. J Neurosci. 1996;16:1770–1779. doi: 10.1523/JNEUROSCI.16-05-01770.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli ET, Kuhn TB, Duc CO, Ruegg MA, Sonderegger P. The axonally secreted protein axonin-1 is a potent substratum for neurite growth. J Cell Biol. 1991;112:449–455. doi: 10.1083/jcb.112.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT. Axon guidance at choice points. Curr Opin Neurobiol. 1998;8:73–79. doi: 10.1016/s0959-4388(98)80010-x. [DOI] [PubMed] [Google Scholar]
- Struyk AF, Canoll PD, Wolfgang MJ, Rosen CL, Deustachio P, Salzer JL. Cloning of neurotrimin defines a new subfamily of differentially expressed neural cell adhesion molecules. J Neurosci. 1995;15:2141–2156. doi: 10.1523/JNEUROSCI.15-03-02141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira E, Takaha N, Taniura H, Kim CH, Miki N. Molecular cloning and functional expression of gicerin, a novel cell adhesion molecule that binds to neurite outgrowth factor. Neuron. 1994;12:861–872. doi: 10.1016/0896-6273(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Tarentino AL, Plummer TH. Enzymatic deglycosylation of asparagine-linked glycans: purification, properties and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. . Methods Enzymol. 1994;230:44–57. doi: 10.1016/0076-6879(94)30006-2. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Treubert U, Brümmendorf T. Functional cooperation of β1-integrins and members of the Ig superfamily in neurite outgrowth induction. J Neurosci. 1998;18:1795–1805. doi: 10.1523/JNEUROSCI.18-05-01795.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S, Kodukula K. How glycosyl-phosphatidylinositol-anchored membrane proteins are made. Annu Rev Biochem. 1995;64:563–591. doi: 10.1146/annurev.bi.64.070195.003023. [DOI] [PubMed] [Google Scholar]
- Van Vactor D. Adhesion and signaling in axonal fasciculation. Curr Opin Neurobiol. 1998;8:80–86. doi: 10.1016/s0959-4388(98)80011-1. [DOI] [PubMed] [Google Scholar]
- Volkmer H, Hassel B, Wolff JM, Frank R, Rathjen FG. Structure of the axonal surface recognition molecule neurofascin and its relationship to a neural subgroup of the immunoglobulin superfamily. J Cell Biol. 1992;142:1083–1093. doi: 10.1083/jcb.118.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmer H, Zacharias U, Nörenberg U, Rathjen FG. Dissection of complex molecular interactions of neurofascin with axonin-1, F11, and tenascin-R, which promote attachment and neurite formation of tectal cells. J Cell Biol. 1998;142:1083–1093. doi: 10.1083/jcb.142.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh FS, Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu Rev Cell Dev Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Frelinger AL, Rutishauser U. Topography of N-CAM structural and functional determinants. I. Classification of monoclonal antibody epitopes. J Cell Biol. 1986;103:1721–1727. doi: 10.1083/jcb.103.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DJA, Kim DS, Clarke GA, Marshall S, Clarke, Moss DJ. A family of glycoproteins (GP55), which inhibit neurite outgrowth, are members of the Ig superfamily and are related to OBCAM, neurotrimin, LAMP AND CEPU-1. J Cell Sci. 1996;109:3129–3138. doi: 10.1242/jcs.109.13.3129. [DOI] [PubMed] [Google Scholar]
- Wolff JM, Brümmendorf T, Rathjen FG. Neural cell recognition molecule F11: membrane interaction by covalently attached phosphatidylinositol. Biochem Biophys Res Commun. 1989;161:931–938. doi: 10.1016/0006-291x(89)92688-0. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Mori K. Basic principles and molecular mechanisms of olfactory axon pathfinding. Cell Tissue Res. 1997;290:457–463. doi: 10.1007/s004410050953. [DOI] [PubMed] [Google Scholar]
- Zhukareva V, Chernevskaya N, Pimenta A, Nowycky M, Levitt P. Limbic system-associated membrane protein (LAMP) induces neurite outgrowth and intracellular Ca2+increase in primary fetal neurons. Mol Cell Neurosci. 1997;10:43–55. doi: 10.1006/mcne.1997.0639. [DOI] [PubMed] [Google Scholar]