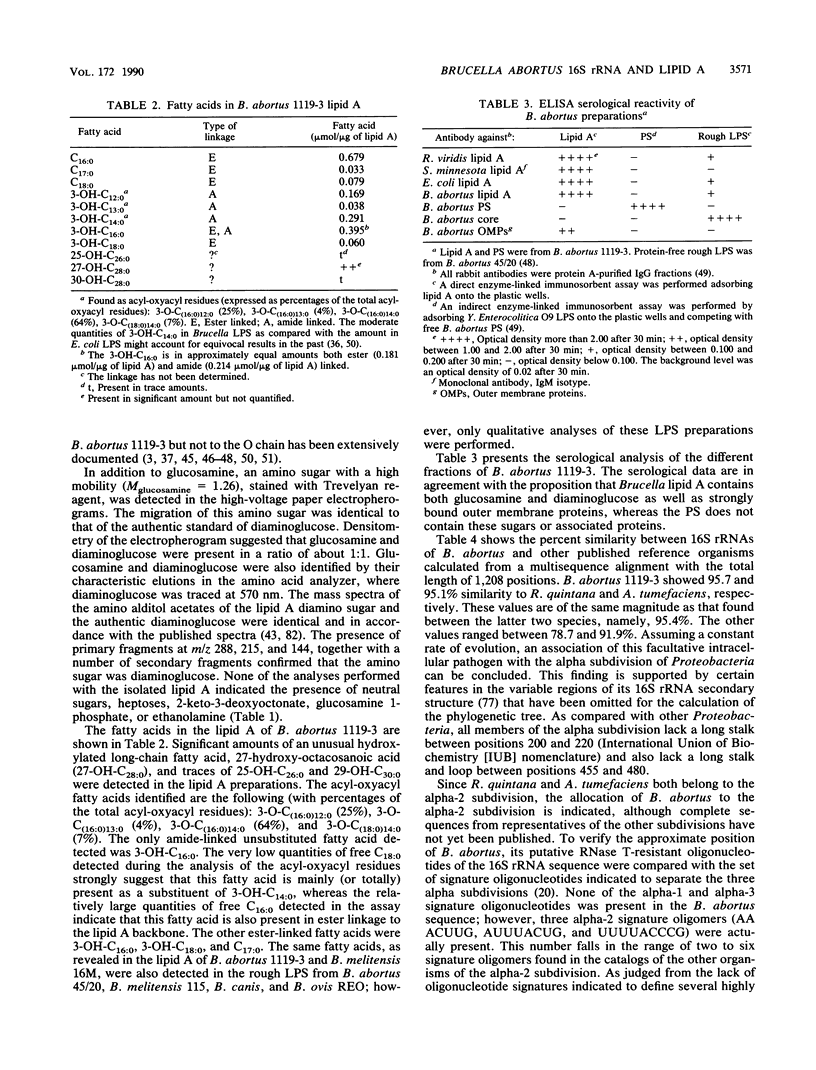
Abstract
On the basis of ribosomal 16S sequence comparison, Brucella abortus has been found to be a member of the alpha-2 subdivision of the class Proteobacteria (formerly named purple photosynthetic bacteria and their nonphototrophic relatives). Within the alpha-2 subgroup, brucellae are specifically related to rickettsiae, agrobacteria, and rhizobiae, organisms that also have the faculty or the obligation of living in close association to eucaryotic cells. The composition of Brucella lipid A suggests a close phylogenetical relationship with members of the alpha-2 group. The chemical analysis of the lipid A fraction revealed that Brucella species contain both glucosamine and diaminoglucose, thus suggesting the presence of a so-called mixed lipid A type. The serological analysis with polyclonal and monoclonal antibodies is in agreement with the existence of mixed lipid A type in B. abortus. The amide-linked fatty acid present as acyl-oxyacyl residues were 3-O-C(16:0)12:0, 3-O-C(16:0)13:0, 3-O-C(16:0)14:0, and 3-O-C(18:0)14:0. The only amide-linked unsubstituted fatty acid detected was 3-OH-C16:0. The ester-linked fatty acids are 3-OH-C16:0, 3-OH-C18:0, C16:0, C17:0, and C18:0. Significant amounts of the large-chain 27-OH-C28:0 were detected together with traces of 25-OH-C26:0 and 29-OH-C30:0. Comparison of the Brucella lipid composition with that of the other Proteobacteria also suggests a close phylogenetical relationship with members of the alpha-2 subdivision. The genealogical grouping of Brucella species with pericellular and intracellular plant and animal pathogens as well as with intracellular plant symbionts suggests a possible evolution of Brucella species from plant-arthropod-associated bacteria.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee D., Basu M., Chatterjee G. C. Physical & chemical studies on lipopolysaccharide of Agrobacterium tumefaciens. Indian J Biochem Biophys. 1983 Jun;20(3):154–159. [PubMed] [Google Scholar]
- Barridge J. K., Shively J. M. Phospholipids of the thiobacilli. J Bacteriol. 1968 Jun;95(6):2182–2185. doi: 10.1128/jb.95.6.2182-2185.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman D. T., Kurtz R. S. Relationship of biological activities to structures of Brucella abortus endotoxin and LPS. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):98–101. doi: 10.1016/0769-2609(87)90084-6. [DOI] [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Perry M. B. Characterization of Brucella polysaccharide B. Infect Immun. 1988 May;56(5):1101–1106. doi: 10.1128/iai.56.5.1101-1106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. R., McNeill J. J., Elkan G. H. Effect of biotin on fatty acids and phospholipids of biotin-sensitive strains of Rhizobium japonicum. J Bacteriol. 1970 Apr;102(1):24–29. doi: 10.1128/jb.102.1.24-29.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Varma M. G. Trans-stadial and transovarial development of disease agents in arthropods. Annu Rev Entomol. 1967;12:347–376. doi: 10.1146/annurev.en.12.010167.002023. [DOI] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B., Cherwonogrodzky J. W., Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984 Nov;46(2):384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester B., Cooper L. H. Achromobacter species (CDC group Vd): morphological and biochemical characterization. J Clin Microbiol. 1979 Mar;9(3):425–436. doi: 10.1128/jcm.9.3.425-436.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. D., Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981 Jun;45(2):316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dams E., Hendriks L., Van de Peer Y., Neefs J. M., Smits G., Vandenbempt I., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1988;16 (Suppl):r87–173. doi: 10.1093/nar/16.suppl.r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985 Jun 12;822(1):1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Dees S. B., Moss C. W. Identification of Achromobacter species by cellular fatty acids and by production of keto acids. J Clin Microbiol. 1978 Jul;8(1):61–66. doi: 10.1128/jcm.8.1.61-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E. Cytochrome c and the evolution of energy metabolism. Sci Am. 1980 Mar;242(3):137–153. [PubMed] [Google Scholar]
- Dorsch M., Moreno E., Stackebrandt E. Nucleotide sequence of the 16S rRNA from Brucella abortus. Nucleic Acids Res. 1989 Feb 25;17(4):1765–1765. doi: 10.1093/nar/17.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch W. M. A non-sequential method for constructing trees and hierarchical classifications. J Mol Evol. 1981;18(1):30–37. doi: 10.1007/BF01733209. [DOI] [PubMed] [Google Scholar]
- Goldfine H. Comparative aspects of bacterial lipids. Adv Microb Physiol. 1972;8:1–58. doi: 10.1016/s0065-2911(08)60187-3. [DOI] [PubMed] [Google Scholar]
- HILDEBRAND J. G., LAW J. H. FATTY ACID DISTRIBUTION IN BACTERIAL PHOSPHOLIPIDS. THE SPECIFICITY OF THE CYCLOPROPANE SYNTHETASE REACTION. Biochemistry. 1964 Sep;3:1304–1308. doi: 10.1021/bi00897a020. [DOI] [PubMed] [Google Scholar]
- Hase S., Rietschel E. T. Methylation analysis of glucosaminitol and glucosaminyl-glucosaminitol disaccharides. Formation of 2-deoxy-2-(N-acetylacetamido)-glucitol derivatives. Eur J Biochem. 1976 Mar 16;63(1):93–99. doi: 10.1111/j.1432-1033.1976.tb10211.x. [DOI] [PubMed] [Google Scholar]
- Hollingdale M. R., Vinson J. W., Herrmann J. E. Immunochemical and biological properties of the outer membrane-associated lipopolysaccharide and protein of Rochalimaea quintana. J Infect Dis. 1980 May;141(5):672–679. doi: 10.1093/infdis/141.5.672. [DOI] [PubMed] [Google Scholar]
- Holst O., Borowiak D., Weckesser J., Mayer H. Structural studies on the phosphate-free lipid A of Rhodomicrobium vannielii ATCC 17100. Eur J Biochem. 1983 Dec 1;137(1-2):325–332. doi: 10.1111/j.1432-1033.1983.tb07832.x. [DOI] [PubMed] [Google Scholar]
- Imhoff J. F., Kushner D. J., Kushwaha S. C., Kates M. Polar lipids in phototrophic bacteria of the Rhodospirillaceae and Chromatiaceae families. J Bacteriol. 1982 Jun;150(3):1192–1201. doi: 10.1128/jb.150.3.1192-1201.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARLSBAD G., KESSEL R. W., DE PETRIS S., MONACO L. ELECTRON MICROSCOPE OBSERVATIONS OF BRUCELLA ABORTUS GROWN WITHIN MONOCYTES IN VITRO. J Gen Microbiol. 1964 Jun;35:383–390. doi: 10.1099/00221287-35-3-383. [DOI] [PubMed] [Google Scholar]
- Lacave C., Asselineau J., Serre A., Roux J. Comparaison de la composition chimique d'une fraction lipopolysaccharidique et d'une fraction polysaccharidique isolées de Brucella melitensis. Eur J Biochem. 1969 Jun;9(2):189–198. doi: 10.1111/j.1432-1033.1969.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Leong D., Diaz R., Milner K., Rudbach J., Wilson J. B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970 Feb;1(2):174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R. A., Finnerty W. R. Isolation and characterization of an ornithine-containing lipid from Desulfovibrio gigas. J Bacteriol. 1975 Aug;123(2):523–529. doi: 10.1128/jb.123.2.523-529.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R. A., Finnerty W. R. Phospholipid composition of Desulfovibrio species. J Bacteriol. 1974 Dec;120(3):1279–1283. doi: 10.1128/jb.120.3.1279-1283.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse R. J., Corpe W. A. Chemical composition of cell envelopes from Agrobacterium tumefaciens. Can J Microbiol. 1967 Dec;13(12):1591–1603. doi: 10.1139/m67-209. [DOI] [PubMed] [Google Scholar]
- Mayer H., Krauss J. H., Urbanik-Sypniewska T., Puvanesarajah V., Stacey G., Auling G. Lipid A with 2,3-diamino-2,3-dideoxy-glucose in lipopolysaccharides from slow-growing members of Rhizobiaceae and from "Pseudomonas carboxydovorans". Arch Microbiol. 1989;151(2):111–116. doi: 10.1007/BF00414423. [DOI] [PubMed] [Google Scholar]
- Merrell B. R., Weiss E., Dasch G. A. Morphological and cell association characteristics of Rochalimaea quintana: comparison of the Vole and Fuller strains. J Bacteriol. 1978 Aug;135(2):633–640. doi: 10.1128/jb.135.2.633-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Berman D. T., Boettcher L. A. Biological activities of Brucella abortus lipopolysaccharides. Infect Immun. 1981 Jan;31(1):362–370. doi: 10.1128/iai.31.1.362-370.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Borowiak D., Mayer H. Brucella lipopolysaccharides and polysaccharides. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):102–105. doi: 10.1016/0769-2609(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Moreno E., Jones L. M., Berman D. T. Immunochemical characterization of rough Brucella lipopolysaccharides. Infect Immun. 1984 Mar;43(3):779–782. doi: 10.1128/iai.43.3.779-782.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Mayer H., Moriyon I. Characterization of a native polysaccharide hapten from Brucella melitensis. Infect Immun. 1987 Nov;55(11):2850–2853. doi: 10.1128/iai.55.11.2850-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Speth S. L., Jones L. M., Berman D. T. Immunochemical characterization of Brucella lipopolysaccharides and polysaccharides. Infect Immun. 1981 Jan;31(1):214–222. doi: 10.1128/iai.31.1.214-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- PHILIP C. B., BURGDORFER W. Arthropod vectors as reservoirs of microbial disease agents. Annu Rev Entomol. 1961;6:391–412. doi: 10.1146/annurev.en.06.010161.002135. [DOI] [PubMed] [Google Scholar]
- Riley P. S., Weaver R. E. Comparison of thirty-seven strains of Vd-3 bacteria with Agrobacterium radiobacter: morphological and physiological observations. J Clin Microbiol. 1977 Feb;5(2):172–177. doi: 10.1128/jcm.5.2.172-177.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossau R., Heyndrickx L., Van Heuverswyn H. Nucleotide sequence of a 16S ribosomal RNA gene from Neisseria gonorrhoeae. Nucleic Acids Res. 1988 Jul 11;16(13):6227–6227. doi: 10.1093/nar/16.13.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russa R., Lorkiewicz Z. Fatty acids present in the lipopolysaccharide of Rhizobium trifolii. J Bacteriol. 1974 Sep;119(3):771–775. doi: 10.1128/jb.119.3.771-775.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shaw N. Lipid composition as a guide to the classification of bacteria. Adv Appl Microbiol. 1974;17(0):63–108. doi: 10.1016/s0065-2164(08)70555-0. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E., Fischer A., Roggentin T., Wehmeyer U., Bomar D., Smida J. A phylogenetic survey of budding, and/or prosthecate, non-phototrophic eubacteria: membership of Hyphomicrobium, Hyphomonas, Pedomicrobium, Filomicrobium, Caulobacter and "dichotomicrobium" to the alpha-subdivision of purple non-sulfur bacteria. Arch Microbiol. 1988;149(6):547–556. doi: 10.1007/BF00446759. [DOI] [PubMed] [Google Scholar]
- Tempst P., van Beeumen J. The complete amino-acid sequence of the low-spin class II cytochrome c-556 from Agrobacterium tumefaciens strain B2a. Eur J Biochem. 1983 Jan 1;129(3):603–614. doi: 10.1111/j.1432-1033.1983.tb07091.x. [DOI] [PubMed] [Google Scholar]
- Thiele O. W., Asselineau J., Lacave C. On the fatty acids of Brucella abortus and Brucella melitensis. Eur J Biochem. 1969 Jan;7(3):393–396. doi: 10.1111/j.1432-1033.1969.tb19621.x. [DOI] [PubMed] [Google Scholar]
- Toschka H. Y., Höpfl P., Ludwig W., Schleifer K. H., Ulbrich N., Erdmann V. A. Complete nucleotide sequence of a 16S ribosomal RNA gene from Pseudomonas aeruginosa. Nucleic Acids Res. 1988 Mar 25;16(5):2348–2348. doi: 10.1093/nar/16.5.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos T., Moss C. W., McDade J. E. Fatty acid composition of rickettsiae. J Clin Microbiol. 1981 Mar;13(3):603–605. doi: 10.1128/jcm.13.3.603-605.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Mayer H. Different lipid A types in lipopolysaccharides of phototrophic and related non-phototrophic bacteria. FEMS Microbiol Rev. 1988 Apr-Jun;4(2):143–153. doi: 10.1111/j.1574-6968.1988.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Woese C. R., Dobson M. E., Weiss E. A common origin of rickettsiae and certain plant pathogens. Science. 1985 Nov 1;230(4725):556–558. doi: 10.1126/science.3931222. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Miller E. T. Phospholipid composition of Rickettsia prowazeki grown in chicken embryo yolk sacs. J Bacteriol. 1978 Oct;136(1):175–178. doi: 10.1128/jb.136.1.175-178.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Weisburg W. G., Paster B. J., Madigan M. T., Fowler V. J., Hahn C. M., Blanz P., Gupta R., Nealson K. H. The phylogeny of purple bacteria: the alpha subdivision. Syst Appl Microbiol. 1984;5:315–326. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Broady K. W., Lüderitz O., Rietschel E. T. The chemical structure of lipid A. Demonstration of amide-linked 3-acyloxyacyl residues in Salmonella minnesota Re lipopolysaccharide. Eur J Biochem. 1982 May;124(1):191–198. doi: 10.1111/j.1432-1033.1982.tb05924.x. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Seydel U., Lindner B., Lüderitz O., Rietschel E. T. Nature and location of amide-bound (R)-3-acyloxyacyl groups in lipid A of lipopolysaccharides from various gram-negative bacteria. Eur J Biochem. 1984 Dec 3;145(2):265–272. doi: 10.1111/j.1432-1033.1984.tb08547.x. [DOI] [PubMed] [Google Scholar]
- Yang D., Oyaizu Y., Oyaizu H., Olsen G. J., Woese C. R. Mitochondrial origins. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]



