Abstract
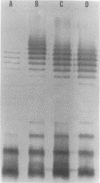
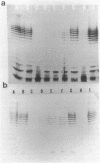
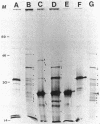
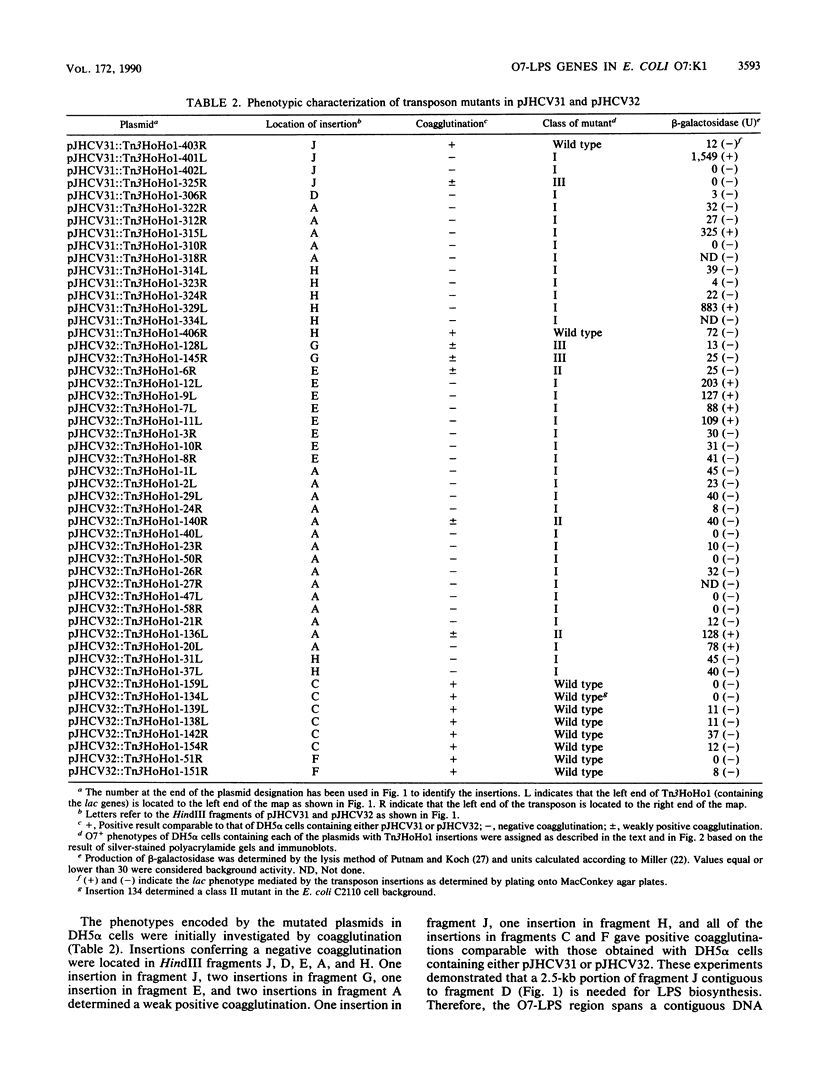
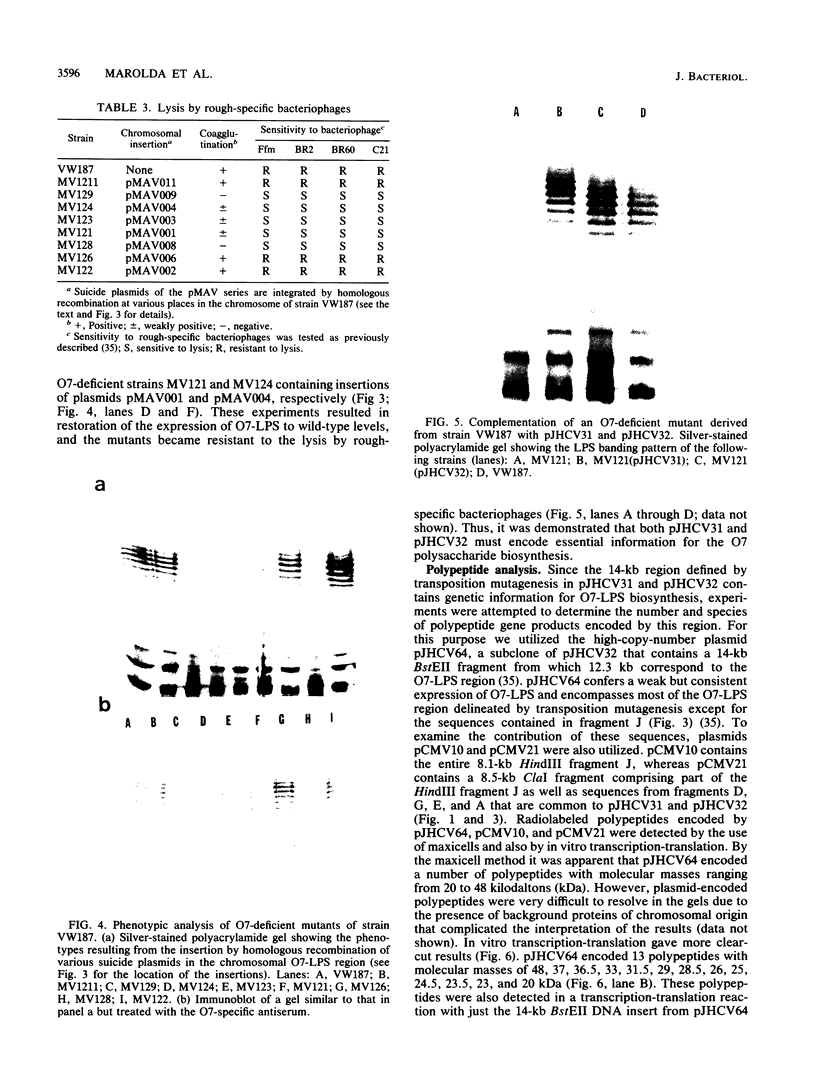
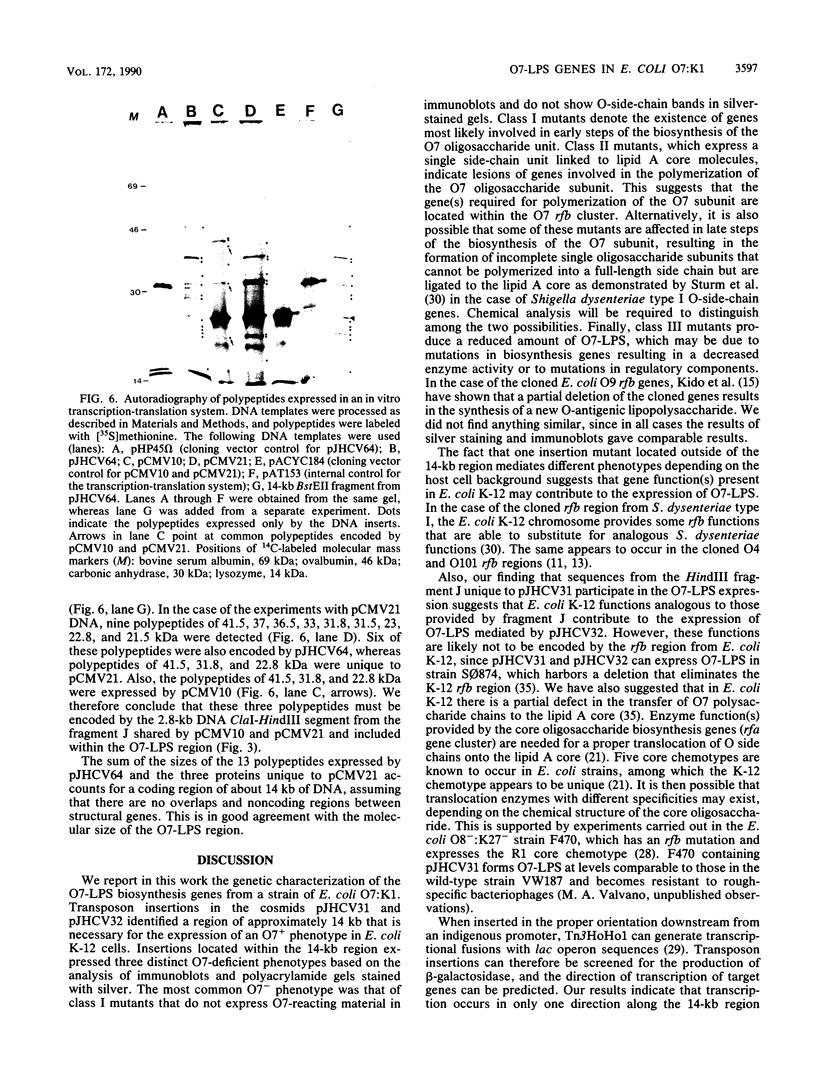
We recently cloned biosynthesis genes for the O7-lipopolysaccharide (O7-LPS) side chain from the Escherichia coli K-1 strain VW187 (M. A. Valvano, and J. H. Crosa, Infect. Immun. 57:937-943, 1989). To characterize the O7-LPS region, the recombinant cosmids pJHCV31 and pJHCV32 were mutagenized by transposon mutagenesis with Tn3HoHo1, which carries a promoterless lac operon and can therefore generate lacZ transcriptional fusions with target DNA sequences. Cells containing mutated plasmids were examined for their ability to react by coagglutination with O7 antiserum. The LPS pattern profiles of the insertion mutants were also investigated by electrophoresis of cell envelope fractions, followed by silver staining and immunoblotting analysis. These experiments identified three phenotypic classes of mutants and defined a region in the cloned DNA of about 14 kilobase pairs that is essential for O7-LPS expression. Analysis of beta-galactosidase production by cells carrying plasmids with transposon insertions indicated that transcription occurs in only one direction along the O7-LPS region. In vitro transcription-translation experiments revealed that the O7-LPS region encodes at least 16 polypeptides with molecular masses ranging from 20 to 48 kilodaltons. Also, the O7-LPS region in VW187 was mutagenized by homologous recombination with subsets of the cloned O7-LPS genes subcloned into a suicide plasmid vector. O7-LPS-deficient mutants of VW187 were complemented with pJHCV31 and pJHCV32, confirming that these cosmids contain genetic information that is essential for the expression of the O7 polysaccharide.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Pluschke G. Clonal analysis of descent and virulence among selected Escherichia coli. Annu Rev Microbiol. 1986;40:185–210. doi: 10.1146/annurev.mi.40.100186.001153. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmbhatt H. N., Quigley N. B., Reeves P. R. Cloning part of the region encoding biosynthetic enzymes for surface antigen (O-antigen) of Salmonella typhimurium. Mol Gen Genet. 1986 Apr;203(1):172–176. doi: 10.1007/BF00330399. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt H. N., Wyk P., Quigley N. B., Reeves P. R. Complete physical map of the rfb gene cluster encoding biosynthetic enzymes for the O antigen of Salmonella typhimurium LT2. J Bacteriol. 1988 Jan;170(1):98–102. doi: 10.1128/jb.170.1.98-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. G., Jr, Leive L. Two mutations which affect the barrier function of the Escherichia coli K-12 outer membrane. J Bacteriol. 1979 Sep;139(3):899–910. doi: 10.1128/jb.139.3.899-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goto N., Shoji A., Horiuchi S., Nakaya R. Conduction of nonconjugative plasmids by F' lac is not necessarily associated with transposition of the gamma delta sequence. J Bacteriol. 1984 Aug;159(2):590–596. doi: 10.1128/jb.159.2.590-596.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi G. E., Hull R. A., Krallmann-Wenzel U., Hull S. I. Molecular cloning and expression of the O4 polysaccharide gene cluster from Escherichia coli. Microb Pathog. 1989 Feb;6(2):123–132. doi: 10.1016/0882-4010(89)90015-6. [DOI] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Beger D. W., Thomas C. J., Manning P. A. Molecular cloning and expression in Escherichia coli K-12 of the O101 rfb region from E. coli B41 (O101:K99/F41) and the genetic relationship to other O101 rfb loci. Mol Microbiol. 1989 Mar;3(3):295–302. doi: 10.1111/j.1365-2958.1989.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido N., Ohta M., Iida K., Hasegawa T., Ito H., Arakawa Y., Komatsu T., Kato N. Partial deletion of the cloned rfb gene of Escherichia coli O9 results in synthesis of a new O-antigenic lipopolysaccharide. J Bacteriol. 1989 Jul;171(7):3629–3633. doi: 10.1128/jb.171.7.3629-3633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Inuzuka M., Helinski D. R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978 Dec;15(4):1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- L'vov V. L., Shashkov A. S., Dmitriev B. A., Kochetkov N. K., Jann B., Jann K. Structural studies of the O-specific side chain of the lipopolysaccharide from Escherichia coli O:7. Carbohydr Res. 1984 Mar 15;126(2):249–259. doi: 10.1016/0008-6215(84)85382-3. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Borman P. A rapid biochemical method for purifying high molecular weight bacterial chromosomal DNA for restriction enzyme analysis. Nucleic Acids Res. 1987 Apr 24;15(8):3631–3631. doi: 10.1093/nar/15.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Putnam S. L., Koch A. L. Complications in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of beta-galactosidase. Anal Biochem. 1975 Feb;63(2):350–360. doi: 10.1016/0003-2697(75)90357-7. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Different core regions in the lipopolysaccharides of O group 8. Eur J Biochem. 1969 Oct;10(3):501–510. doi: 10.1111/j.1432-1033.1969.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., An G., Flores C., Nester E. W. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985 Apr;4(4):891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm S., Jann B., Jann K., Fortnagel P., Timmis K. N. Genetic and biochemical analysis of Shigella dysenteriae 1 O antigen polysaccharide biosynthesis in Escherichia coli K-12: structure and functions of the rfb gene cluster. Microb Pathog. 1986 Jun;1(3):307–324. doi: 10.1016/0882-4010(86)90056-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Valvano M. A., Crosa J. H. Aerobactin iron transport genes commonly encoded by certain ColV plasmids occur in the chromosome of a human invasive strain of Escherichia coli K1. Infect Immun. 1984 Oct;46(1):159–167. doi: 10.1128/iai.46.1.159-167.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano M. A., Crosa J. H. Molecular cloning and expression in Escherichia coli K-12 of chromosomal genes determining the O7 lipopolysaccharide antigen of a human invasive strain of E. coli O7:K1. Infect Immun. 1989 Mar;57(3):937–943. doi: 10.1128/iai.57.3.937-943.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano M. A., Crosa J. H. Molecular cloning, expression, and regulation in Escherichia coli K-12 of a chromosome-mediated aerobactin iron transport system from a human invasive isolate of E. coli K1. J Bacteriol. 1988 Dec;170(12):5529–5538. doi: 10.1128/jb.170.12.5529-5538.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano M. A., Silver R. P., Crosa J. H. Occurrence of chromosome- or plasmid-mediated aerobactin iron transport systems and hemolysin production among clonal groups of human invasive strains of Escherichia coli K1. Infect Immun. 1986 Apr;52(1):192–199. doi: 10.1128/iai.52.1.192-199.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N. K., Quigley N. B., Reeves P. R. O-antigen variation in Salmonella spp.: rfb gene clusters of three strains. J Bacteriol. 1988 Jan;170(1):103–107. doi: 10.1128/jb.170.1.103-107.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]