Abstract
AML blasts transmigrate in response to SDF-1α. AMD3100, a novel bicyclam molecule which inhibits SDF-1α/CXCR4 interactions, inhibited the transmigration of AML blasts and inhibited outgrowth of leukemia colony forming units. AMD3100 did not abrogate stroma- mediated protection from cytarabine mediated apoptosis, except in the case of one promyelocytic leukemic sample tested, and it did not influence adhesion of blasts to endothelial monolayers. When AML blasts were pretreated with AMD3100, the positive effects of SDF-1α on NOD-SCID engraftment were diminished. This work confirms that AML is influenced by the SDF-1α/CXCR4 axis and demonstrates that disruption of this axis by the bicyclam AMD3100 can influence AML microenvironmental interactions.
Introduction
Acute myelogenous leukemia blasts and progenitors grow in close contact with a permissive and inductive microenvironment [1]. There is increasing evidence that the stromal-derived factor (SDF)-1α (CXCL12)/CXCR4 axis influences homing and retention of normal stem cells and leukemia cells in the marrow [2]. Furthermore, SDF-1α plays a role in retention and metastasis of brain tumors [3], glioblastoma [4], and breast carcinoma [5] in addition to other tumor types [6]. SDF-CXCR4 interactions are involved in the metastasis of progenitor cells to hypoxic tissue, and SDF-1α gene expression in ischemic tissue is dependent on oxygen tension [7]. This observation has also led to examination of the role that this chemokine receptor axis plays in mediation of cardiac injury after ischemia [8]. Mice which lack CXCR4 and SDF-1 have impaired B-lymphopoiesis, myelopoiesis, and cerebellar neuron migration [9]. Also, tissue-specific muscle, neural and liver stem/progenitor cells reside in the marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury [10]. In this work, we have examined the effect of disruption of the SDF-1α/CXCR4 axis on acute myelogenous leukemia (AML) cells in vitro and upon their engraftment in NOD-SCID mice through exposure to the CXCR4 antagonist, AMD3100.
It has previously been shown that the migratory behavior of leukemic cells is stimulated by SDF-1α [11]. Leukemic cells expressing CD34, CD38, and HLA-DR migrated preferentially, whereas cells with CD14 and CD36 showed diminished migration. Bone marrow derived cells migrated more than blood CD34+ cells. A lower percentage of circulating leukemic blasts was observed in patients with a relatively high level of SDF-1α induced migration, suggesting that SDF-1α is instrumental in retention of AML blasts in the marrow (12).
Furthermore, all AML cells express internal CXCR4 and SDF-1α. Culture of AML cells with SDF-1α promoted their survival, whereas addition of CXCR4 antibodies decreased survival [12]. Pretreatment of primary human AML cells with neutralizing CXCR4 antibodies blocked their homing into the marrow and spleen of transplanted NOD/SCID/β2m mice. Weekly administration dramatically decreased the levels of human AML cells in marrow, blood, and spleen, suggesting that CXCR4 neutralization may be a potential treatment for AML [12].
AMD3100 is a selective antagonist of SDF-1α/CXCL12 which binds to CXCR4, the sole receptor for SDF-1α [13,14]. AMD3100 has been shown to mobilize CD34+ cells and hematopoietic progenitor cells in humans [15], and it enhances G-CSF-induced mobilization of CD34+ cells in humans [16]. It effectively neutralizes CXCL12 function, and in this work its effects on AML blasts and progenitors have been further explored in order to ascertain whether disruption of the CXCL12/CXCR4 axis by this unique bicyclam molecule might result in anti-leukemic effects.
Materials and Methods
Source of AML cell lines and primary cells
All cell lines utilized were purchased form the ATCC (Rockville, MD). Primary AML samples were obtained from patients with AML after informed consent was obtained in accordance with University of Rochester Research Subjects Review Board policies. Samples were subjected to ficoll hypaque density gradient centrifugation. Only samples with high percentages of blast cells were utilized (>70%). Cells were cultured in RPMI plus 10% fetal bovine serum.
Culture of human umbilical vein endothelial cells (HUVECs), marrow endothelial cells (BMECs), and stromal cells
HUVECs were obtained from human term umbilical cords and cultured as previously described [17]. BMECs were isolated from marrow aspirates obtained from normal donors who gave informed consent in accordance with University of Rochester Research Subjects Review Board policies. They were obtained from light density marrow cells using the MiniMACS magnetic bead cell separation device with CD34 and CD105 coated microbeads. Cells were used at early passages (p3–p5).
Stromal cells were grown from the adherent cells persisting after light density marrow populations were plated in long term culture medium (Cell Technologies, Vancouver, BC). Nonadherent cells were demi-populated twice per week. The M210B4 stromal line was obtained from the ATCC (Rockville, MD).
Sources of reagents
AMD3100 was obtained from AnorMED, Inc. through a Materials Transfer Agreement with the University of Rochester. It was provided as freebase with molecular weight 502. It was formulated in 10% HCl and water to pH 6.9. This was stored as a 10 mg/ml solution at −20°C until use. SDF-1α, monocyte chemotactic protein (MCP-1), regulated on activation, normal T expressed and secreted (RANTES), macrophage inflammatory protein-1 alpha (MIP-1α), SDF-1β, interferon inducible protein-10 (IP10), fractalkine, granulocyte colony stimulating factor (G-CSF), interleukin-8 (IL-8), anti-CD45, and anti-CXCR4 were all purchased from R&D Systems, Minneapolis, MN. Staurosporine and cytochalasin B were obtained from Sigma-Aldrich Chemical, St. Louis, MO. Cytarabine (ARA-C) was purchased from Pharmacia Upjohn Co, (Kalamazoo, MI). Fetal bovine serum (FBS) was from Hyclone (Logan, UT) or Atlanta Biologicals (Lawrenceville, GA). All adhesion receptor antibodies were from Becton-Dickinson, and the anti-CD34 antibody was obtained from Caltag (Burlingame, CA).
Transmigration assays
Endothelial or stromal monolayers were grown to confluence on a 3 micron microporous membrane insert and placed in a 24 well tissue culture plate. Normal CD34+ cells or AML cells were placed above the insert at 1 × 105 cells in 300 μl, and various chemoattractants were added to the lower chamber in serum free conditions. After a 24 hour incubation, inserts were removed and cells were counted using a hemacytometer. The percentage of cells migrating was determined. In certain conditions, the plated cells were exposed to AMD3100 at various concentrations 1 hour before plating. The AMD3100 was present during the transmigration assay.
Cell proliferation and cell cycle assays
Cell viability was determined by Trypan blue exclusion, and cell counts were determined by hemacytometer counts or by MTT assay (R&D Systems). For specific determination of apoptosis, staining for Annexin V (R&D Systems) was performed. Simultaneous determination of propidium iodide (PI) staining allowed for determination of apoptosis vs. necrosis. To determine cell cycle status, cells were fixed in 70% EtOH and lysed with 1% Nonidet P-40 and incubated with 10 μg/ml RNase for 30 minutes at 37°C. Nuclear DNA was stained with 100 ug/ml propidium iodide (Sigma). Samples were allowed to equilibrate for at least 1 hour in the dark before analysis. Fluorescence analysis of individual nuclei was performed with a FACSCalibur flow cytometer equipped with an argon-ion laser at 488 and 250 mW light output.
CFU-GM, BFU-E. and CFU-L Assays
Five × 104 normal light density cells for granulocyte-macrophage colony forming units (CFU-GM) or erythroid burst forming units (BFU-E) were plated in 0.9% methylcellulose, 30% FCS, 2 mmol/L L-glutamine, and 10−4 mol/L β-mercaptoethanol, and 1% BSA with 3U/ml human erythropoietin, 10 ng/ml GM-CSF, 10 ng/ml IL-3, and 50 ng/ml stem cell factor (SCF) (c-kit ligand).. For leukemia colony forming units (CFU-Ls), the plating mixture was comparable with the exception that the cytokines utilized were 4U/ml erythropoietin, 10 ng/ml GM-CSF, 10 ng/ml IL-3, 100 ng/ml c-kit ligand, and 100 ng/ml Flt3 ligand. The methylcellulose mixture and associated reagents were purchased from Stem Cell Technologies (Vancouver, BC). Colonies were scored at Day 14 and were defined as >20 grouped cells.
Assessment of AKT Phosphorylation
To assess AKT phosphorylation, leukemic blasts isolated as described above were harvested and lysed after Ficoll-Hypaque density gradient centrifugation, lysed with buffer containing 50 nM Tris, pH 8.0, 120 mM NaCl, 0.5% Nonidet P-40, and protease inhibitor cocktail (Roche). The protein concentration of the lysate was determined by using the Bradford method (Bio-rad, Hercules, CA). Equal amounts of protein were separated on a 4%–20% Tris-Glycine gel (Invitrogen) and transferred to a BioTrace PVDF membrane. Blots were probed with primary antibody to Akt or Phospho-AKT (Cell Signaling). After washing and incubating with secondary antibody, immunoreactive proteins were visualized by using the ECL Plus detection system (Amersham Biosciences).
Endothelial or stromal co-culture assays
Cell lines or AML blasts were suspended in RPMI plus 10% FBS and plated onto tissue culture plastic control surfaces or over confluent HUVEC, BMEC, or stromal layers. Adherent and nonadherent cells were collected using collagenase to maintain an intact endothelial cell layer. Cells were sampled at 24 and 48 hours of co-culture, and cell count, cell viability and apoptosis rates were determined as described above.
NOD-SCID Assays
Animal experiments were approved by the University of Rochester Animal Care and Ethics Committee. Female NOD/SCID (NOD/LtSz-scid/scid) mice aged 6 to 8 weeks were purchased from the Jackson Laboratories or the National Cancer Institute. They were housed in a specific pathogen-free environment for at least 1 week prior to inoculation with human leukemia cells. Mice were supplied with sterile food and water ad libitum. On the day prior to inoculation, mice received 375 cGy total body irradiation from a cesium source (University of Rochester). Immediately prior to inoculation, mice were warmed by an infrared lamp and then inoculated by tail-vein injection with 107 leukemia cells in a maximum volume of 100 μL PBS. Mice were monitored for general well-being on a daily basis.
At 24 hours and 6 weeks following inoculation, mice were euthanized by CO2 intoxication followed by cervical dislocation. Cell suspensions of spleen and liver were prepared by mincing through cell strainers. Bone marrow was collected by flushing femurs with PBS containing heparin, and blood was collected by cardiac puncture. Mononuclear cells were purified by density gradient centrifugation. Cells were washed twice and stained with anti-human CD45-FITC for 45 minutes in the presence of 10% human AB serum. Cells were washed in PBS and fixed in 400 μL of 1% paraformaldehyde at 4°C until analyzed on a Becton Dickinson FACS.
Adhesion assays and flow cytometric quantitation of adhesion receptors
Endothelial or stromal cells were plated in 24 well plates and used at 80–90% confluence. Layers were treated with growth factors or media for 24 hours prior to the adhesion assay which was performed in RPMI with 10% FBS. AML cells were labeled with carboxy-fluorescein diacetate, succinimidyl ester (CFDA SE) (Molecular Probes Inc, Eugene OR) at 2 μM. AML light density cells (5 × 105) were plated over the endothelial or stromal monolayer. In those conditions with AMD3100, cells were exposed at designated concentrations for 1 hour prior to plating over the monolayer for 2 hours. Thereafter, the layers were washed twice with PBS and adherent cells were removed with Trypsin-EDTA and then washed and fixed with 1% paraformaldehyde. Cell counts were determined by hemacytometer, and percentage of adherence was calculated. Blast gating was achieved through analysis of forward vs. side scatter.
To determine whether exposure of endothelial layers to AMD3100 altered expression of adhesion receptors, endothelial cells were trypsinized and stained with monoclonal antibodies to CD31, CD41a, CD42b, CD49d, CD54, CD61, CD62P, CD62L, CD106, CD117, CD154, CD164, and CXCR4. Expression was determined utilizing a FACSCalibur flow cytometer, and isotype specific control staining was performed for each antibody on the whole ungated population.
Statistical considerations
T-tests (two-tailed) or paired t-tests were employed to determine significance of differences between experimental conditions and control conditions. A p-value of <0.05 was required for the determination of statistical significance. In all figures, n refers to the number of experiments performed using distinct samples.
Results
AML blasts transmigrate endothelial monolayers in response to SDF-1α
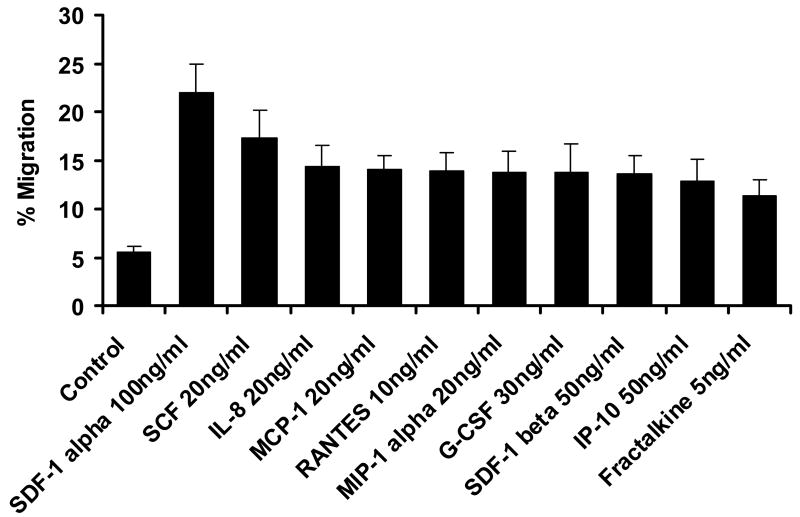
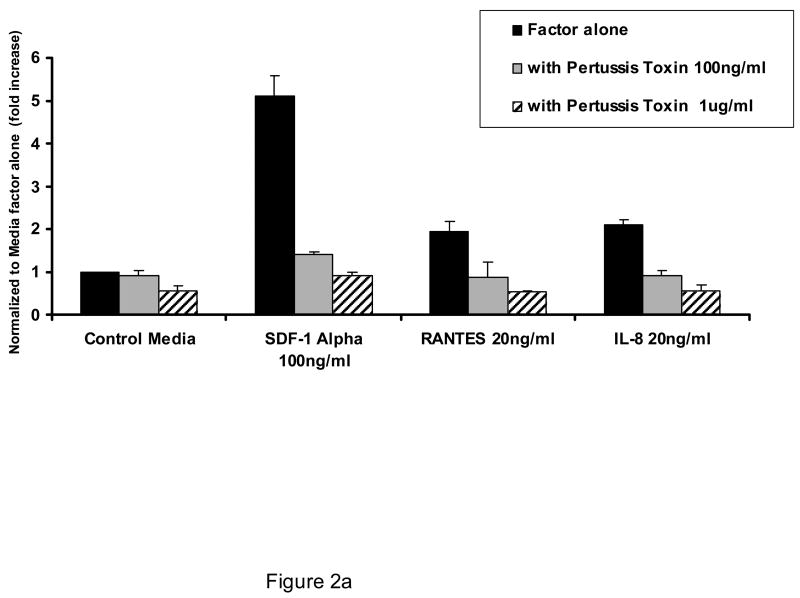
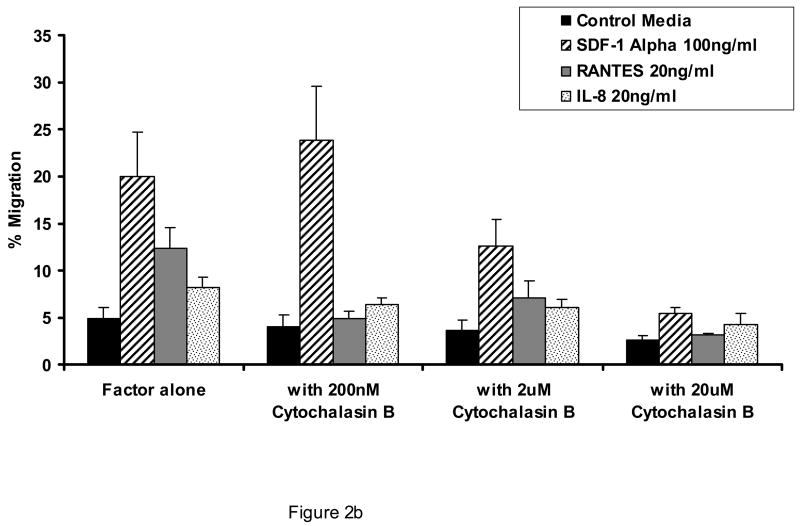
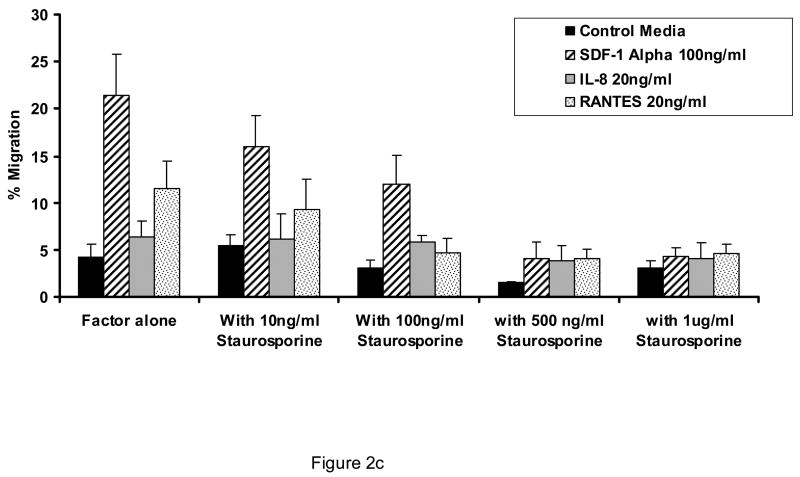
As shown in Figure 1, AML blasts transmigrate endothelial cells in response to SDF-1α to a greater extent than to other chemokines or cytokines, although all are more effective in stimulating transmigration as compared to control conditions (p<0.05 for all cytokines and chemokines shown vs. control conditions). The concentrations of attractants utilized were optimal as determined by dose response experiments. The transmigration with SDF-1 was inhibited by pertussis toxin (Figure 2a), cytochalasin B (Figure 2b), and staurosporine (Figure 2c). (n=3).
Figure 1.
Transmigration of AML blasts through endothelial monolayers in response to various chemokines. Shown is the percentage ± SE of plated cells which transmigrate a human umbilical endothelial monolayer in 24 hours in response to optimal concentrations of chemokines or cytokines shown. Significant transmigration occurred in response to SDF-1 alpha and all other conditions shown; (p<0.00001 for SDF-1α (n=16) and p <0.05 for all other conditions compared to control condition).
Figure 2.
Effect of pertussis toxin, cytochalasin B, and staurosporine on the ability of AML blasts to transmigrate endothelial cells. Shown is the concentration effect of pertussis toxin (A), cytochalasin B (B), and staurosporine (C) on the 24 hour transmigration of AML blasts through HUVEC monolayers in response to optimal concentrations of SDF-1α, interleukin-8 (IL-8), or RANTES (n=3). Data shown are Means ± SE.
AMD3100 inhibits endothelial transmigration of normal CD34+ cells and AML blasts
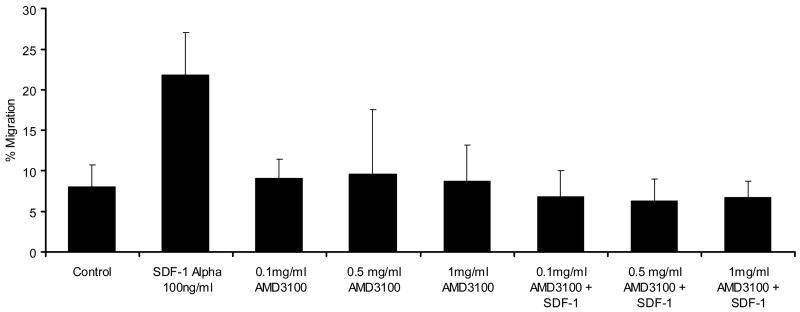
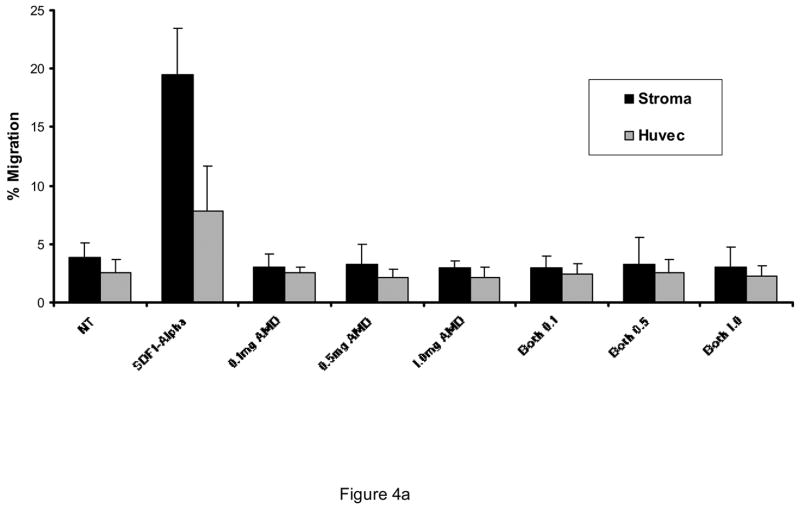
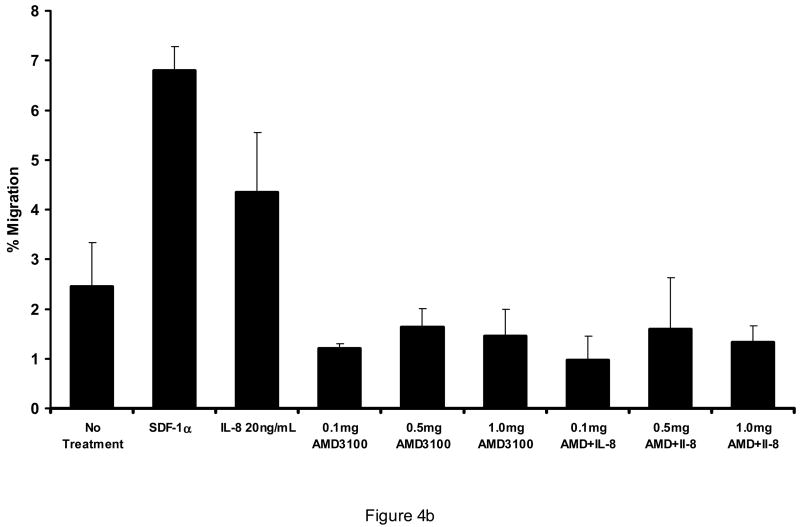
AMD3100 at concentrations from 0.1 mg/ml to 1 mg/ml was capable of inhibiting SDF-1 stimulated transmigration of CD34+ cells through HUVEC monolayers (Figure 3) or through bone marrow endothelial cells (BMECs) (not shown). It did not significantly increase transmigration above that of control conditions at concentrations from 0.01 mg/ml to 1.0 mg/ml. It did not reverse increased migration toward G-CSF at comparable concentrations (not shown). Likewise, with AML blasts, SDF-1α stimulation of transmigration through HUVECs was abrogated by 0.1 mg/ml AMD3100. AMD3100 did not alter baseline transmigration at concentrations up to 1.0 mg/ml (Figure 4A). While the degree of transmigration through marrow stromal layers was not as great as through endothelial monolayers, SDF-1α transmigration through stromal monolayers was also abrogated by AMD3100 (Figure 4A). When AML cells were pretreated with AMD3100 and then placed in the transmigration chamber, no incremental transmigration was noted with SDF-1α stimulation. The transmigration of AML cells in response to the CXC chemokine, IL-8 (20 ng/ml) was also inhibited by AMD3100 (Figure 4b). While this inhibition was nearly complete, the initial stimulation of transmigration was not as great with IL-8 as with SDF-1α.
Figure 3.
Effect of AMD3100 on Transmigration of CD34+ cells through HUVEC monolayers. AMD3100 inhibited CD34+ cells isolated from a Miltenyi column from transmigrating HUVEC monolayers in response to 100 ng/ml SDF1-α. AMD3100 by itself did not significantly influence transmigration as compared to control conditions. Data shown is Mean ± SD. P<0.05 for all conditions with AMD3100 plus SDF-1α as compared with SDF-1α alone conditions (n=5).
Figure 4.
AMD3100 inhibited SDF-1α induced transmigration of AML blasts through A) HUVEC monolayers and marrow stromal layers (n=3), and B) was also able to inhibit the transmigration stimulated by IL-8 (n=3). AMD3100 itself did not alter migration of AML blasts significantly from control conditions. A different set of AML blasts was utilized for each of these figures (A and B).
AMD3100 did not affect proliferation or survival or AML blasts in liquid culture but did decrease outgrowth of CFU-Ls
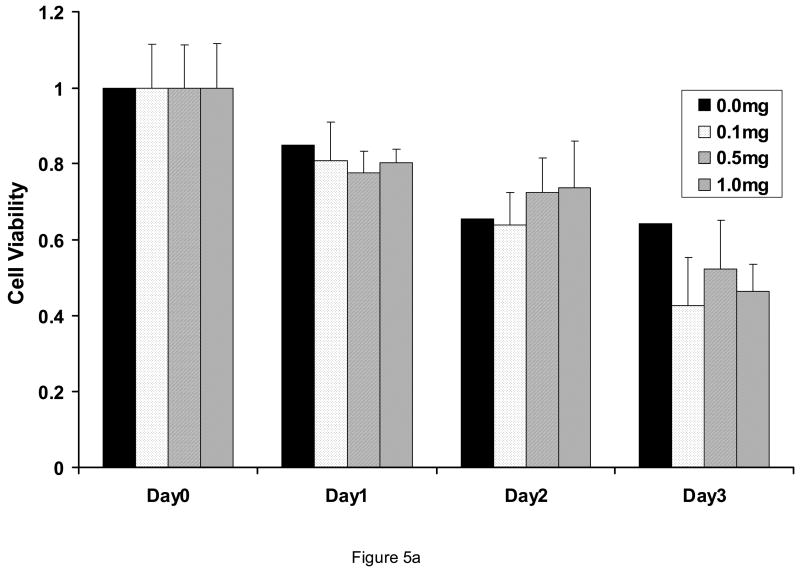
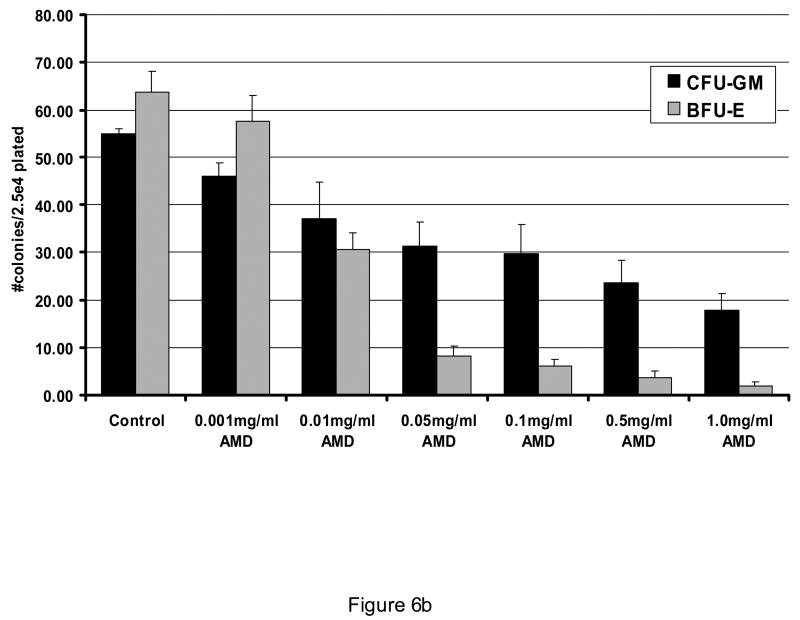
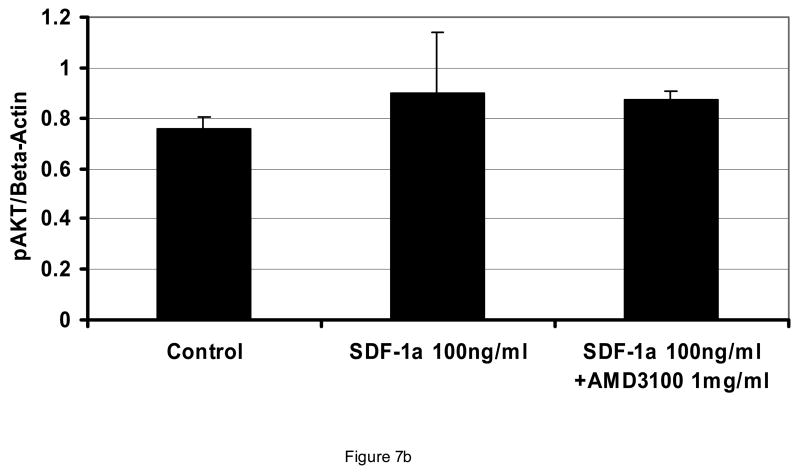
At concentrations from 0.1 to 1.0 mg/ml, AMD3100 did not affect proliferation or viability of AML blasts (Figure 5; n=4). All AML cases utilized in these experiments expressed CXCR4 (>25% blasts positive by flow cytometry). Also, when AML blasts were pre-exposed to SDF-1 alpha at 100 ng/ml, AMD3100 had no influence on AML blast proliferation (Figure 5b). This was also found to be the case in various leukemic cell lines expressing CXCR4 such as the MV411 line. Cell cycle status of AML cells was also not affected by AMD3100 exposure (not shown). AMD3100 inhibited CFU-L outgrowth of AML cells when plated into a semi-solid medium. Inhibition was statistically significantat 0.05 mg/ml and higher (Figure 6A). When light density marrow from normal donors was plated in the presence of AMD3100, mild depression of CFU-GM was noted but there was a significant decrease in BFU-Es at concentrations ≥ 0.01 mg/ml (Figure 6B). SDF is known to signal through the AKT pathway, but AMD3100 of itself did not inhibit AKT activation in AML blasts (Figure 7A). It had only a minimal effect on inhibiting activated AKT expression in the presence of SDF-1 which was not statistically significant (Figure 7B). In all samples utilized here, baseline AKT activation was present.
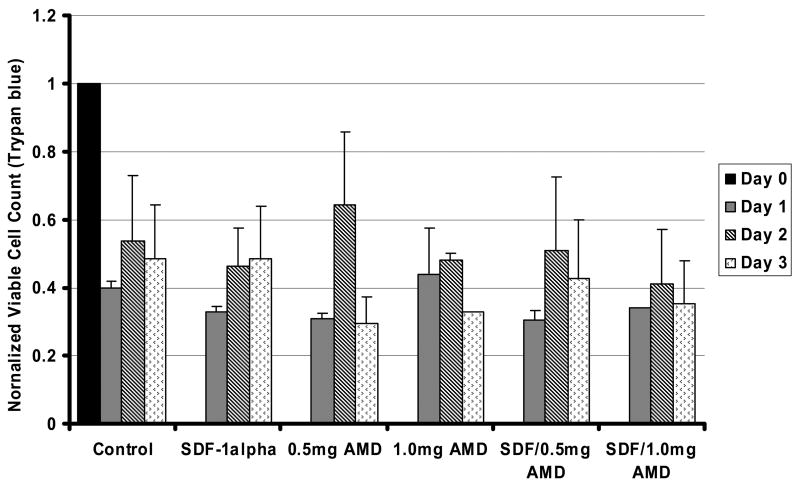
Figure 5.
Effects of AMD3100 on AML blast proliferation and viability. When AML blasts were exposed to AMD3100, 0 to 1.0 mg/ml, the viable cell count was not changed significantly for up to 3 days of incubation as compared with control cultures (A) (n=4). Data shown is Mean ± SD. Viability (percentage of cells non-apoptotic) was determined by Annexin V staining. Likewise, AMD3100 had no effects on viable blast counts in AML cells exposed to SDF-1 over a 3 days period (B) (n=3) (p>0.05 for all conditions with SDF-1α).
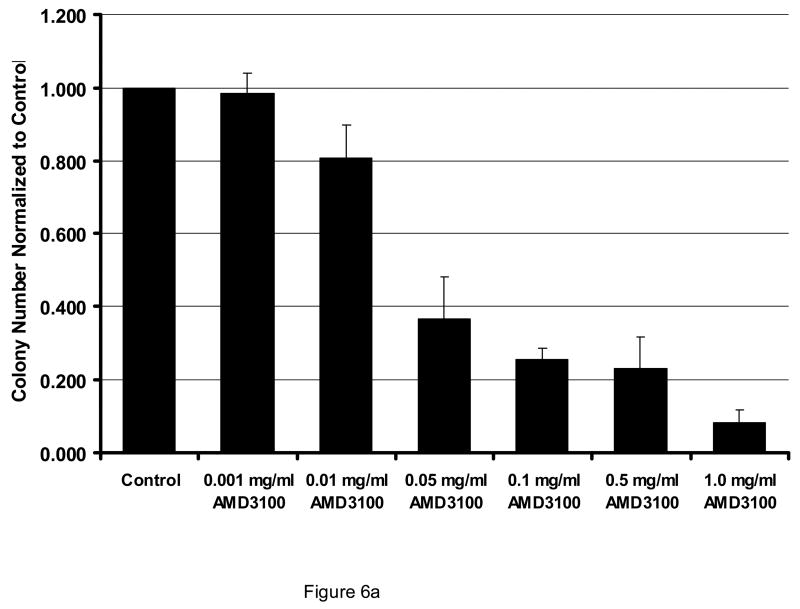
Figure 6.
Effect of AMD3100 on CFU-L outgrowth. When AML cells were exposed to AMD3100 in a CFU-L assay, outgrowth of colonies was significantly inhibited (p<0.01 for all conditions ≥ 0.05 mg/ml). Data shown is normalized to colony numbers observed under control conditions (Figure 6A; n=4). When light density marrow was plated for CFU-GM or BFU-E outgrowth, AMD3100 significantly inhibited BFU-Es (p < 0.02 for all AMD concentrations ≥ 0.01 as compared with control). CFU-GMs were only inhibited significantly at 1 mg/ml (p=0.02) as compared to control conditions (Figure 6B; n=3). All data are shown as Mean ± SEM.
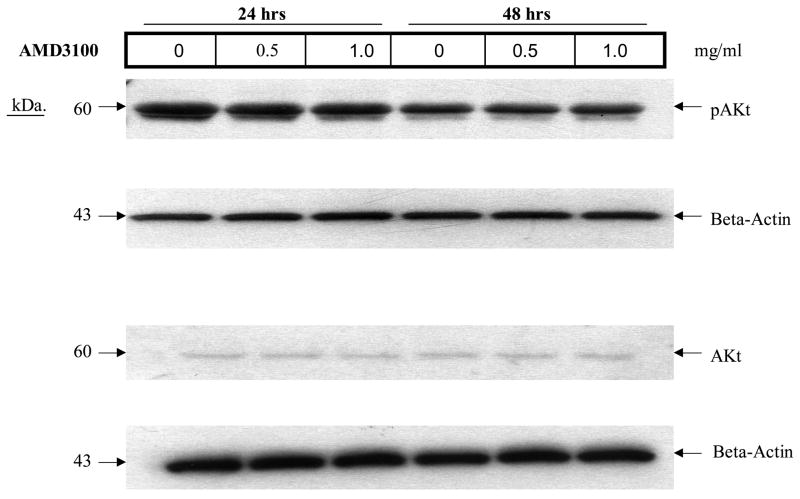
Figure 7.
Effect of AMD3100 on activated AKT expression. Incubation of AML blasts with AMD3100 at the indicated concentrations for 24 or 48 hours did not affect expression of activated AKT (A) (shown is a Western blot for 1 of 3 AML samples tested wherein the leukemia had baseline expression of phosphorylated AKT). When AML blasts were incubated with 100 ng/ml SDF-1α, the minimal increase in AKT activation was not significantly inhibited by AMD3100 (p>0.05 for all conditions compared to control) (B). Only leukemia cases with baseline activation of AKT were studied.
Effects of AMD3100 on Stromal and Leukemic Cell Interactions
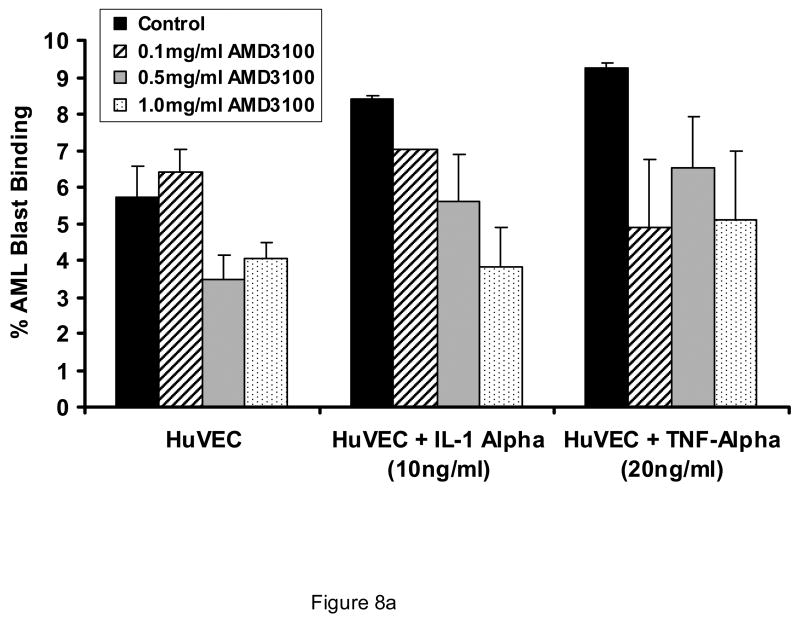
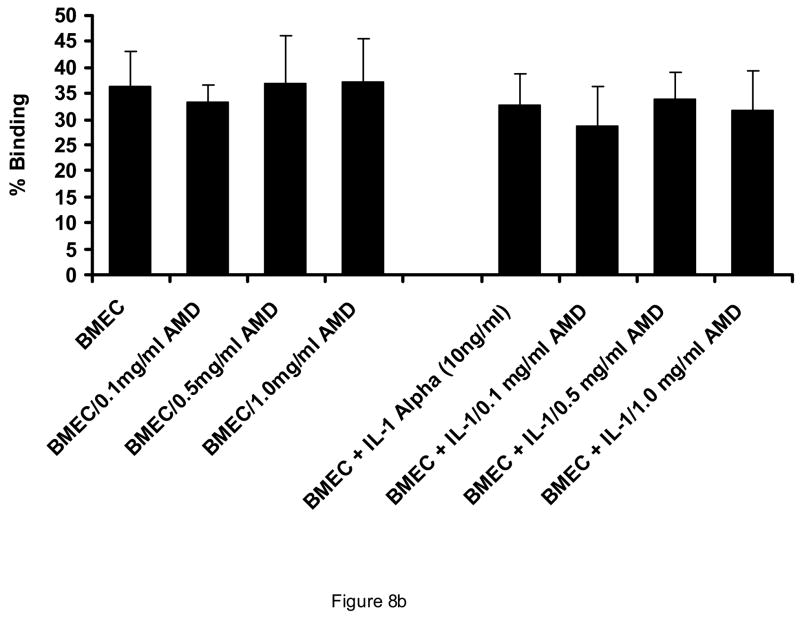
AMD3100 did not affect binding of AML light density cells or AML blasts to endothelial monolayers (Figure 8A). Likewise, AMD3100 at concentrations from 0.1 mg/ml to 1 mg/ml did not influence the adhesion of CD34+ cells to HUVEC or BMEC monolayers. (Figure 8B). Despite the lack of influence on adhesion in a static functional assay, AMD3100 did result in a decrease in CD106 (VCAM), CD164 (Mucin-like receptor), and CD61 (beta 3 integrin receptor) expression on BMEC layers, although only the change in CD164 reached statistical significance (Table 1). Effects on other receptors involved in stem cell/stromal cell adhesion were not significantly affected by AMD3100 exposure.
Figure 8.
Effect of AMD3100 on adhesion of CD34+ cells and AML blasts to marrow endothelial monolayers. AMD3100 at concentrations up to 1.0 mg/ml did not significantly affect adhesion of AML cells to HUVECs (A). This was also the case in the presence of Interleukin-1 and Tumor necrosis factor-alpha, both of which upmodulate stromal cell expression of VLA-4 (CD49d) and other integrins. Likewise, adhesion of normal CD34+ cells was not affected by presence of AMD3100 when BMECs were utilized as the substratum (B). N=3. Data shown is Mean ± SD.
TABLE 1.
EFFECTS OF AMD3100 ON ENDOTHELIAL CELL ADHESION RECEPTOR EXPRESSION
| Antigen Name | CD Designation | No AMD | 1.0 mg/ml AMD | p-value |
|---|---|---|---|---|
| ICAM-1 | CD54 | 7.6±3 | 5.8±1.3 | NS |
| VCAM-1 | CD106 | 19.3±6 | 13±2.5 | p=.10 |
| Mucin like | CD164 | 15.1±1.2 | 7±.3 | p<0.02 |
| B3 integrin | CD61 | 22±9.1 | 9.9±3.2 | NS |
Shown is the Mean ± SEM percentage of cells positive for the indicated antigen. n=4. NS=not significant.
In the presence of a stromal monolayer, AMD3100 did not abrogate the positive effects of stromal cells on non-M3 AML survival (51±17% viable vs. 50± 16% viable with AMD3100 0.5 mg/ml at 7 days; n=3). In one case of acute promyelocytic leukemia available for study, the presence of AMD3100 abrogated the protective effect of stomal monolayers on ARA-C induced apoptosis at 2 and 7 days of culture. This abrogation of apoptosis protection against ARA-C toxicity was not seen consistently with other AML cases tested (n=5; not shown).
Effect of AMD3100 exposure on engraftment in NOD/SCID mice
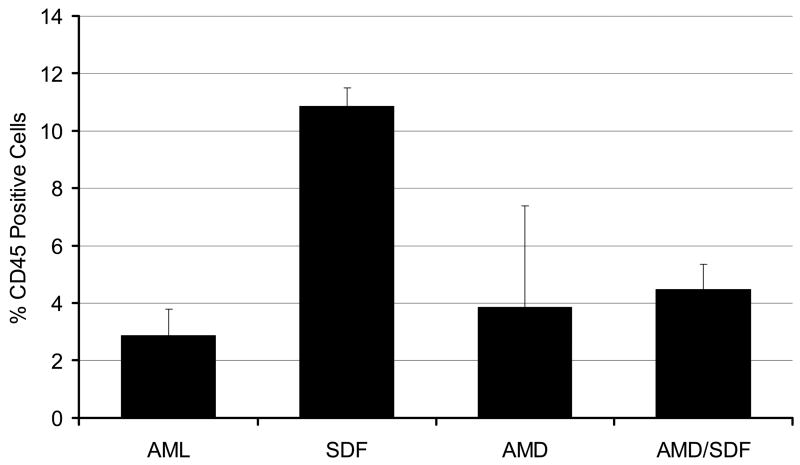
When AML cells (whole mononuclear populations with >80% blasts) were treated with SDF-1α, 100 ng//ml or AMD3100, 1.0 mg/ml for 24 hours and then injected into sublethally irradiated NOD/SCID mice, at 24 hours, less than 0.5% of marrow cells were CD45+ under any conditions. Less than 1% of spleen and liver cells were CD45 positive at 24 hours. At 6 weeks, the percentage of CD45+ cells in marrow did not differ depending upon whether the cells had been exposed to AMD3100 or to a combination of the both SCF-1α and AMD3100 (Figure 9). Cells pre-exposed to SDF-1α engrafted in higher numbers (p< 0.05). Engraftment in the liver was not affected by exposure to SDF-1α or AMD3100, and only minimal cells were present in the spleen.
Figure 9.
Effect of AMD3100 exposure on AML engraftment in NOD/SCID mice. Shown is the percentage engraftment of AML cells expressing CXCR4 in NOD-SCID mice when cells were preincubated in media alone (AML), SDF-1 alpha, AMD3100, or the combination of both (AMD/SDF). Only cells exposed to SDF engrafted at higher levels (p<0.05) as compared to control cells. n=10 mice; two AML samples with high CXCR4 expression were utilized in these studies. Data shown is Mean ± SEM.
Discussion
SDF-1α/CXCL12 and its receptor, CXCR4 are implicated in chemotaxis, homing, and survival/apoptosis of hematopoietic stem cells and progenitor cells [18]. All AML cells express CXCR4 and SDF-1α, sometimes intracellularly. [19]. In normal progenitors, AMD3100, a bicyclam which specifically and reversibly blocks SDF-1/CXCL12 binding to and signaling through CXCR4, results in progenitor and SCID-reconstituting cell mobilization 6 hours after exposure [16]. When humans are given a subcutaneous injection of AMD3100, a rapid generalized leukocytosis with increased CD34+ cells and CFU-Cs occurs [20], and this has led to its utilization in protocols for stem cell mobilization for autologous stem cell reconstitution purposes [16, 21]. These clinical observations correspond well to the observation that mobilization of hematopoietic progenitor cells by G-CSF coincides in vivo with the cleavage of the N-terminus of the chemokine receptor CXCR4 on progenitors resident in the marrow and mobilized into the peripheral blood. This cleavage results in the loss of chemotaxis in response to SDF-1/CXCL12 [22].
In this work, we have attempted to begin exploration of the effects of AMD3100 on AML blasts and progenitors in ex vivo experiments. AMD3100 was able to inhibit the SDF-1α stimulated transmigration of AML cells through endothelial and stromal cell monolayers, and it was able to partially inhibit IL-3 induced transmigration of endothelial cells, possibly by inhibiting SDF-1 enhancement of IL-8 chemotactic effects [23]. It did not have a direct effect on AML blast proliferation or apoptosis in liquid culture systems, but it did decrease the outgrowth of leukemia colony forming units in a short-term semi-solid methylcellulose assay. In normal CD34+ progenitors, overexpression of CXCR4 via a lentiviral gene transfer technique has been reported to increase chemotaxis and actin polymerization, proliferation, migration, and NOD/SCID repopulation [24]. Also, SDF-1 regulates primitive hematopoiesis by suppressing apoptosis and facilitating cell cycle transition in normal CD34+ cells. In AML cells, CXCR4 expression has been associated with poor prognosis in terms of both overall patient survival and relapse probability [25]. Nonetheless, exposure to AMD3100 did not affect survival or alter the cell cycle composition of AML blast cells but did result in decreased output of leukemic progenitors in an assay dependent on a semi-solid medium where multiple cytokines are present. Interestingly, when normal progenitors were plated in these assays, exposure to AMD3100 also affected CFU-GM proliferation and inhibited erythroid colony growth substantially. This is consistent with reports that the CXCR4 gene is upregulated by erythroid sensitive cytokines such as erythropoietin and stem cell factor, suggesting that inhibition of this upregulated expression might have bearing on in vitro erythropoieisis where cell-cell contact is important [26].
In mobilization studies with AMD3100, the percentage of CD34+ cells which express CD62L and the level of CD62L expression were lower in the G-CSF plus AMD3100 mobilized cells as compared with G-CSF mobilized cells [16]. Also, SDF-1α has been found to rapidly increase the adhesion of CD34 (hi) marrow cells to the CS-1 domain of fibronectin, to VCAM-1, and to marrow stromal cells. This increase was only transient, however, and was inhibited by pertussis toxin and cytochalasin D [27]. No differences in the ability of AML cells to adhere to endothelial or stromal monolayers in the presence of AMD3100 were detected in the work reported here. This was also the case when the monolayers were activated with IL-1α or TNF-α, both of which increase stromal layer and HUVEC expression of integrins [17]. When BMECs were exposed to AMD3100, the expression of VCAM-1 (CD106) and the mucin like receptor (CD164) was decreased but no statistically significant alteration in other adhesion receptors was noted. This is consistent with the role that CD106 has been found to play in G-CSF induced mobilization [28]. CD44 and hyaluronic acid have also been reported to cooperate with SDF-1 in CD34+ trafficking [29,30], but whether these play a role in AML mobilization induced by SDF-1 has not been examined [31].
The process of stem cell mobilization and presumably of leukemic blast and leukemic progenitor mobilization is a complex one. It involves cytoskeletal elements, integrins, chemokines such as SDF-1α and IL-8, and proteolytic enzymes such as elastase, cathepsin G, hyaluronic acid and matrix metalloproteases (MMPs) [32–35]. AMD3100 appears to have impact on AML transmigration, and presumably by disruption of the CXCR4-SDF-1α axis, would have impact on the retention of AML blasts and progenitors in the marrow. Since it inhibits transmigration of these cells through stromal and endothelial monolayers, its final effects on AML migration and survival would probably be dependent on existent gradients of SDF-1α which in turn would be dependent on marrow activation by cytokines and other chemokines.
Co-culture of AML blasts with stromal cell layers is known to enhance their survival and to protect blasts from the cytotoxicity of cytarabine through prevention of apoptosis induction [36–39]. When AML blasts were cultured for up to 7 days over stromal monolayers, viability was enhanced in the present of stromal layers, but the presence of AMD-3100 did not consistently affect survival nor did it reverse the effects of cytarabine at 0.1 μM or 1.0 μM. In liquid culture systems, the presence of AMD3100 did not affect the ability of cytarabine to kill AML cells in either a positive or negative direction. In one case of acute promyelocytic leukemia which we were able to study, AMD3100 did appear to restore cytarabine apoptosis induction in the presence of stromal monolayers. This is consistent with other reports of AMD3100 effects in a murine model of human APML [40].
Engraftment of AML in NOD-SCID mice is independent of CXCR4 expression (41) yet predicts poor patient survival. Anti-CXCR4 antibody failed to block the engraftment of AML cells into NOD/SCID mice [41]. In contrast, with normal CD34+ cells, treatment with antibodies to CXCR4 prevented engraftment in NOD/SCID mice, and in vitro CXCR4 dependent migration to SDF-1 of CD34+/CD38− cells correlated with in vivo engraftment [42, 43]. AMD3100 did inhibit the ability of SDF-1 to induce engraftment of AML cells, however we did not detect a difference in AMD3100 exposed AML cells vs. controls in ability to home to marrow and survive to 6 weeks in a leukemic NOD-SCID reconstitution assay in the absence of exogenous SDF exposure. It remains to be determined whether this would also be the case if AMD3100 were present continuously during circulation, homing, engraftment, and retention phases of the leukemic cell homing process in the NOD-SCID model.
Retention of AML progenitors in the marrow is thought to contribute to their survival, so it is possible that interference with blast retention in marrow and other tissues might interfere with survival and proliferation of those progenitors. Furthermore, circulating blasts and progenitor cells in the blood of leukemic patients are also supported by cytokines such as SDF-1α and vascular endothelial growth factor, the levels of which are found to be increased in mobilized stem cell blood products vs. baseline serum [44]. Interfering with this axis which is important to leukemic progenitor cell retention in marrow and extramedullary tissues could play a part in increasing apoptosis or reducing proliferation of those progenitors. It might therefore have a role as an adjunct to chemotherapy to enhance destruction of those progenitors without compromising the re-mergence of normal stem cells which appear to have the ability to return to marrow once mobilized. In fact, in vitro migratory capacity of CD34+ cells is related to hematopoeitic recovery after autologous stem cell transplantation [45]. Effects of AMD3100 on long term progenitor outgrowth after cytoreductive AML chemotherapy would therefore need to be explored.
In the case of ALL, SDF-1α has been found to activate precursor B cell ALL [46], and CXCR4 expression mediates marrow adhesion and engraftment [47]. Furthermore, inhibitors of CXCR4 inhibit ALL cells in vitro [48]. In this work, we have found that AML cells are also influenced by inhibition of the SDF-1α/CXCR4 axis. This would suggest that antagonists such as AMD3100 might have a therapeutic role in AML through modulation of SDF-1α effects on AML progenitors both in marrow and in the circulation and extramedullary tissues. As AML is a heterogeneous disease, it is likely that final effects of AMD3100 on leukemic progenitors in vivo will be a summation of the diverse effects on migratory capacity, retention capacity, and proliferation.
Acknowledgments
This work was supported by in part by R21 CA 112835-02 (JLL) and Strong Memorial Hospital. JLL was the Douglas Kroll Research Foundation Translational Researcher of the Leukemia and Lymphoma Society of America.
Footnotes
Conflicts of interest: None. Gary Bridger is an employee of AnorMED, Inc.
Author contributions: Jane Liesveld—Manuscript preparation and experimental design
Jeremy Bechelli—Performed experiments
Chaohui Lu—Performed Western blots
Karen Rosell—Performed experiments and prepared figures
Gary Bridger—Supplied compound and overall guidance
Gordon Phillips—Manuscript aid
Camille Abboud—Experimental design and manuscript aid
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jane L. Liesveld, James P. Wilmot Cancer Center and the Department of Medicine, University of Rochester Medical Center Rochester, NY
Jeremy Bechelli, James P. Wilmot Cancer Center and the Department of Medicine, University of Rochester Medical Center Rochester, NY.
Karen Rosell, James P. Wilmot Cancer Center and the Department of Medicine, University of Rochester Medical Center Rochester, NY.
Chaohui Lu, James P. Wilmot Cancer Center and the Department of Medicine, University of Rochester Medical Center Rochester, NY.
Gary Bridger, AnorMED, Inc., Langley, British Columbia.
Gordon Phillips, II, James P. Wilmot Cancer Center and the Department of Medicine, University of Rochester Medical Center Rochester, NY.
Camille N. Abboud, James P. Wilmot Cancer Center and the Department of Medicine, University of Rochester Medical Center Rochester, NY
References
- 1.Mayani H. composition and function of the hemopoietic microenvironment in human myeloid leukemia. Leukemia. 1996;10:1041–1047. [PubMed] [Google Scholar]
- 2.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8483. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 3.Rubin JB, Kung Al, Klein RS, Chan JA, Sun Y, Schmidt K, Kieran MW, Luster AD, Segal RA. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100:13513–8. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarini F, Tham TN, Casanova P, Arenzan-Seisdedos F, Dubois-Dalcq M. Role of the alpha-cheomkine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 5.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 6.Kucia M, Reca R, Mierkus K, Wanzeck J, Wojakawski W, Janowska-Wiecorek A, Ratajczak J, Ratajczak MA. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 7.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capia JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 8.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;11:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 9.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronsons RT, Springer TA. Impaired B-lymphopoiesis, myelopoisis, and derailed cerebellar neuron migration in CXCR4 and SDF-1 deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis. 2004;32:52–7. doi: 10.1016/j.bcmd.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Voermans C, van Heese WP, de Jong I, Gerritsen WR, van der Schoot CE. Migratory behavior of leukemic cells from acute myeloid leukemia patients. Leukemia. 2002;16:650–657. doi: 10.1038/sj.leu.2402431. [DOI] [PubMed] [Google Scholar]
- 12.Tavor S, Petit I, Proozov S, Avigdor A, Dar A, Leider-Trejo L, Shemtov N, Deutsch V, Naparstek E, Nagler A, Lapidot T. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004;64:12817–24. doi: 10.1158/0008-5472.can-03-3693. [DOI] [PubMed] [Google Scholar]
- 13.DeClercq E. The bicyclam AMD3100 story. Nature Reviews. 2003:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 14.Hatse S, Princen K, Bridger G, DeClercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–62. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 15.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper Sc, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, Vesole DH, Badel K, Calandra G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–74. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 17.Liesveld JL, Frediani KE, Harbol AW, DiPersio JF, Abboud CN. Characterization of the adherence of normal and leukemic CD34+ cells to endothelial monolayers. Leukemia. 1994;8:2111–7. [PubMed] [Google Scholar]
- 18.Broxmeyer HE, Cooper S, Kohli L, Hangoc G, Lee Y, Mantel C, Clapp DW, Kim CH. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol. 2003;170:421–9. doi: 10.4049/jimmunol.170.1.421. [DOI] [PubMed] [Google Scholar]
- 19.Kollet O, Petit I, Kahn J, Samira S, Dar A, Peled A, Deutsch V, Gunetti M, Piacibello W, Nagler A, Lapidot T. Human CD34+CXCR4- sorted cells harbor intracellular CXCR4, which can be functionally expressed and provide NOD/SCID repopulation. Blood. 2002;100:2778–2786. doi: 10.1182/blood-2002-02-0564. [DOI] [PubMed] [Google Scholar]
- 20.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, Dale DC. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2627–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 21.Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, Calandra G, DiPersio JF. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s’ lymphoma. J Clin Oncol. 2004;15:1095–102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 22.Levesque J-P, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoieitc stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;110:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gouwy M, Struyf S, Catuese J, Proost P, Van Damme J. Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. J Leukoc Biol. 2004;76:185–94. doi: 10.1189/jlb.1003479. [DOI] [PubMed] [Google Scholar]
- 24.Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutxch V, Gazit Z, Gazit D, Karlsson S, Lapidot T. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–2949. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 25.Rombouts EJC, Pavic B, Lowenberg B, Ploemacher RE. Relation between CXCR-4 expression, FLT3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood. 2004;104:550–557. doi: 10.1182/blood-2004-02-0566. [DOI] [PubMed] [Google Scholar]
- 26.Kolbus A, Blazquez-Domingo M, Carotta S, Bakker W, Luekemann S, van Lindern M, Stenlein P, Beug H. Cooperative signaling between cytokine receptors and the glucocorticoid receptor in the expansion of erythroid progenitors: molecular analysis by expression profiling. Blood. 2003;102:3136–46. doi: 10.1182/blood-2003-03-0923. [DOI] [PubMed] [Google Scholar]
- 27.Hidalgo A, Sanz-Rodriguez F, Rodriguez-Fernandez JL, Albella B, Blaya C, Wright N, Cabanas C, Prosper F, Gutierrez-Ramos JC, Teixido J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoeitic progenitor cells. Exp Hematol. 2001;29:345–355. doi: 10.1016/s0301-472x(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 28.Levesque J-P, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoieitc progenitor cell mobilization by granulocyte-colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 29.Avigdor A, Geichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet G, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 30.Gadhoum Z, Delaunay J, Maquarre E, Durand L, Lancereaux V, Qi J, Robert-Lezenes J, Chomienne C, Smadja-Joffe F. The effect of anti-CD44 monoclonal antibodies on differentiation and proliferation of human acute myeloid leukemia cells. Leuk Lymphoma. 2004;43:1501–10. doi: 10.1080/1042819042000206687. [DOI] [PubMed] [Google Scholar]
- 31.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–81. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 32.Mohle R, Bautz F, Denzlinger G, Kanz L. Transendothelail migration of hematopoieitc progenitor cells, Role of chemotactic factors. Ann NY Acad Sci. 2001;938:26–34. doi: 10.1111/j.1749-6632.2001.tb03571.x. [DOI] [PubMed] [Google Scholar]
- 33.Janowska-Wieczorek A, Matsuzaki AA, Marquez L. The hematopoietic microenvironment: matrix metalloproteinases in the hematopoietic microenvironment. Hematology. 2000;4:515–527. [PubMed] [Google Scholar]
- 34.Fukuda S, Broxmeyer HE, Pelus LM. Flt3 ligand and the Flt3 receptor regulate hematopoietic cell migration aby modulating the SDF-1alpha (CXCL12)/CXCR4 axis. Blood. 2005;105:3117–26. doi: 10.1182/blood-2004-04-1440. [DOI] [PubMed] [Google Scholar]
- 35.Janowska-Wieczorek A, Marquez LA, Nabholtz JM, Cabuhat Ml, Chang H, Rozmus J, Russell JA, Edwards DR, Turner AR. Growth factors and cytokines upregulate gelatinase expression of bone marrow CD34 (+) cells and their transmigration through reconstituted basement membrane. Blood. 1999;93:3379–90. [PubMed] [Google Scholar]
- 36.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanaslev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–24. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 37.Garrido SM, Appelbaum FR, Willman CL, Banker DE. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5) Exp Hematol. 2001;29:448–57. doi: 10.1016/s0301-472x(01)00612-9. [DOI] [PubMed] [Google Scholar]
- 38.Liesveld JL, Rosell KE, Lu C, Bechelli J, Phillips G, Lancet JE, Abboud CN. Acute myelogenous leukemia-microenvironment interactions: role of endothelial cells and proteasome inhibition. Hematology. 2005;10:483–94. doi: 10.1080/10245330500233452. [DOI] [PubMed] [Google Scholar]
- 39.Ryningen A, Wergeland L, Glenjen N, Gjertsen BT, Bruserud O. In vitro crosstalk between fibroblasts and native human acute myelogenous leukemia (AML) blasts via local cytokine networks results in increased proliferation and decreased apoptosis of AML cells as well as increased levels of proangiogenic Interleukin 8. Leuk Res. 2005;29:185–96. doi: 10.1016/j.leukres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Nervi B, Holt M, Rettig MP, Bridger G, Ley TJ, DiPersio JF. AMD3100 mobilizes acute promyelocytic leukemia cells from the bone marrow into the peripheral blood and sensitizes leukemia cells to chemotherapy. Blood. 2005;106:75a. abstract. [Google Scholar]
- 41.Monaco G, Konopleva M, Munsell M, Leysath C, Wang RY, Jackson CE, Korbling M, Estey E, Belmont J, Andreeff M. Engraftment of acute myeloid leukemia in NOD/SCID mice is independent of CXCR4 and predicts poor patient survival. Stem Cells. 2004;22:188–201. doi: 10.1634/stemcells.22-2-188. [DOI] [PubMed] [Google Scholar]
- 42.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 43.Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, Guetta E, Barkai G, Nagler A, Lapidot T. Rapid and efficient homing of human CD34(+)CD38(−/low) CXCR4 (+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–91. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 44.Foss B, Mentzoni L, Bruserud O. Effects of vascular endothelial growth factor on acute myelogenous leukemia blasts. 2001;10:81–93. doi: 10.1089/152581601750098291. [DOI] [PubMed] [Google Scholar]
- 45.Voermans C, Kool ML, Rodenhuis S, et al. In vitro migratory capacity of CD34+ cells is related to hematopoieitc recovery after autologous stem cell transplantation. Blood. 2001;97:799–804. doi: 10.1182/blood.v97.3.799. [DOI] [PubMed] [Google Scholar]
- 46.Spiegel A, Kollet O, Peled A, Abel L, Nagler A, Bielorai B, Rechavi G, Vormoor J, Lapidot T. Unique SDF-1-induced activation of human precursor-B ALL cells as a result of altered CXCR4 expression and signaling. Blood. 2004;103:2900–7. doi: 10.1182/blood-2003-06-1891. [DOI] [PubMed] [Google Scholar]
- 47.Shen W, Bendall LJ, Gottlieb DJ, Bradstock KF. The chemokine receptor CXCR4 enhances integrin mediated in vitro adhesion and facilitates engraftment of leukemic precursor B cells in the bone marrow. Exp Hematol. 2001;29:1439–47. doi: 10.1016/s0301-472x(01)00741-x. [DOI] [PubMed] [Google Scholar]
- 48.Juarez J, Bradstock KF, Gottlieb DF, Bendall LJ. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003;17:1294–300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]