Abstract
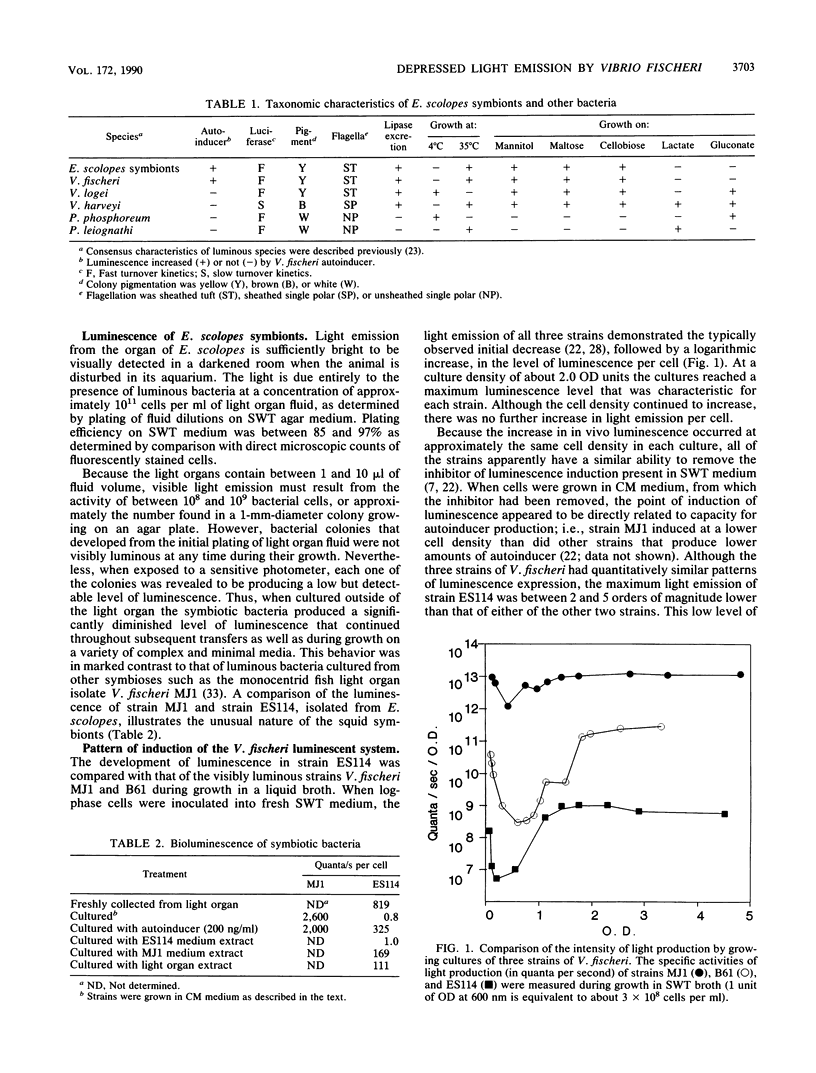
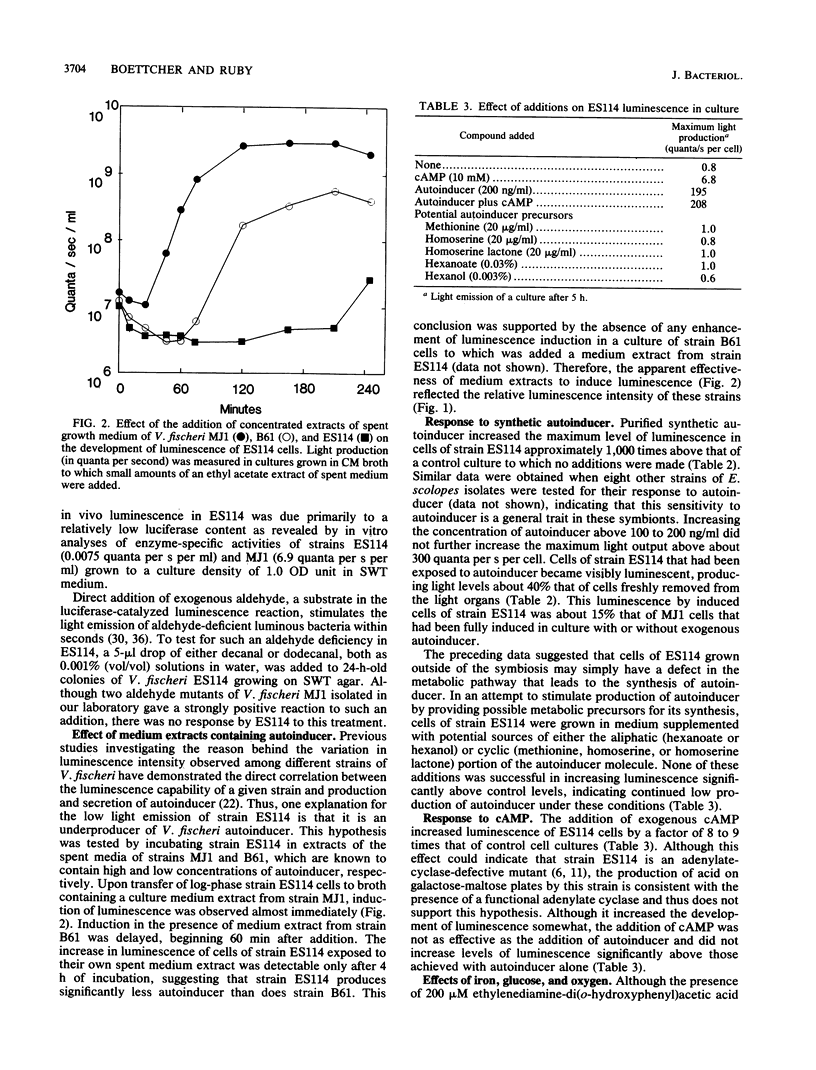
Bioluminescent marine bacteria of the species Vibrio fischeri are the specific light organ symbionts of the sepiolid squid Euprymna scolopes. Although they share morphological and physiological characteristics with other strains of V. fischeri, when cultured away from the light organ association the E. scolopes symbionts depress their maximal luminescence over 1,000-fold. The primary cause of this reduced luminescence is the underproduction by these bacteria of luciferase autoinducer, a molecule involved in the positive transcriptional regulation of the V. fischeri lux operon. Such an absence of visible light production outside of the symbiotic association has not been previously reported among light organ symbionts of this or any other species of luminous bacteria. Levels of luminescence approaching those of the E. scolopes bacteria in the intact association can be restored by the addition of exogenous autoinducer to bacteria in laboratory culture and are affected by the presence of cyclic AMP. We conclude that some condition(s) specific to the internal environment of the light organ is necessary for maximal autoinduction of luminescence in the symbionts of this squid-bacterial association.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Baumann P. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J Bacteriol. 1971 Jul;107(1):295–302. doi: 10.1128/jb.107.1.295-302.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Greenberg E. P. Control of Vibrio fischeri luminescence gene expression in Escherichia coli by cyclic AMP and cyclic AMP receptor protein. J Bacteriol. 1985 Oct;164(1):45–50. doi: 10.1128/jb.164.1.45-50.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V. Osmotic control of luminescence and growth in Photobacterium leiognathi from ponyfish light organs. Arch Microbiol. 1985 Feb;141(1):44–50. doi: 10.1007/BF00446738. [DOI] [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972 Mar;109(3):1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Haygood M. G., Nealson K. H. Mechanisms of iron regulation of luminescence in Vibrio fischeri. J Bacteriol. 1985 Apr;162(1):209–216. doi: 10.1128/jb.162.1.209-216.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron D. S., Pueppke S. G. Mode of infection, nodulation specificity, and indigenous plasmids of 11 fast-growing Rhizobium japonicum strains. J Bacteriol. 1984 Dec;160(3):1061–1066. doi: 10.1128/jb.160.3.1061-1066.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. B., Greenberg E. P. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985 Sep;163(3):1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977 Feb 4;112(1):73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Hastings J. W. Low oxygen is optimal for luciferase synthesis in some bacteria. Ecological implications. Arch Microbiol. 1977 Feb 4;112(1):9–16. doi: 10.1007/BF00446648. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riendeau D., Meighen E. Evidence for a fatty acid reductase catalyzing the synthesis of aldehydes for the bacterial bioluminescent reaction. Resolution from luciferase and dependence on fatty acids. J Biol Chem. 1979 Aug 25;254(16):7488–7490. [PubMed] [Google Scholar]
- Ruby E. G., Greenberg E. P., Hastings J. W. Planktonic marine luminous bacteria: species distribution in the water column. Appl Environ Microbiol. 1980 Feb;39(2):302–306. doi: 10.1128/aem.39.2.302-306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica; a model of symbiosis based on bacterial studies. Biol Bull. 1976 Dec;151(3):574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]


