Abstract
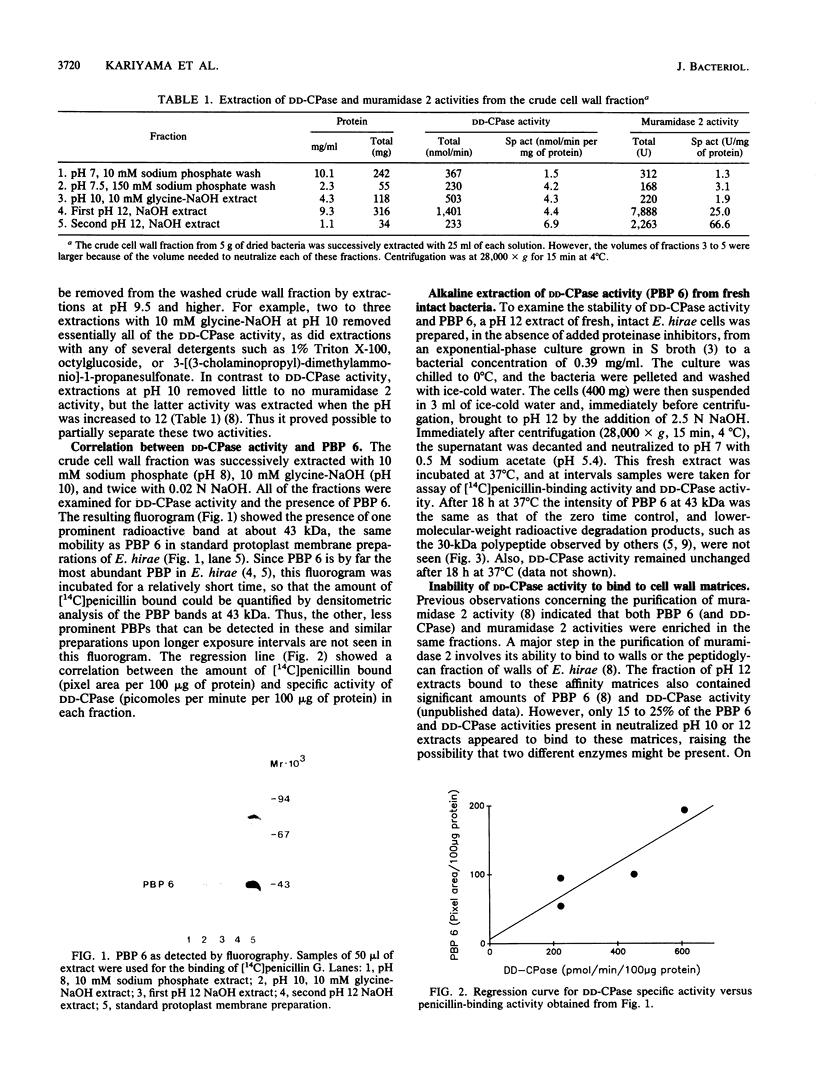
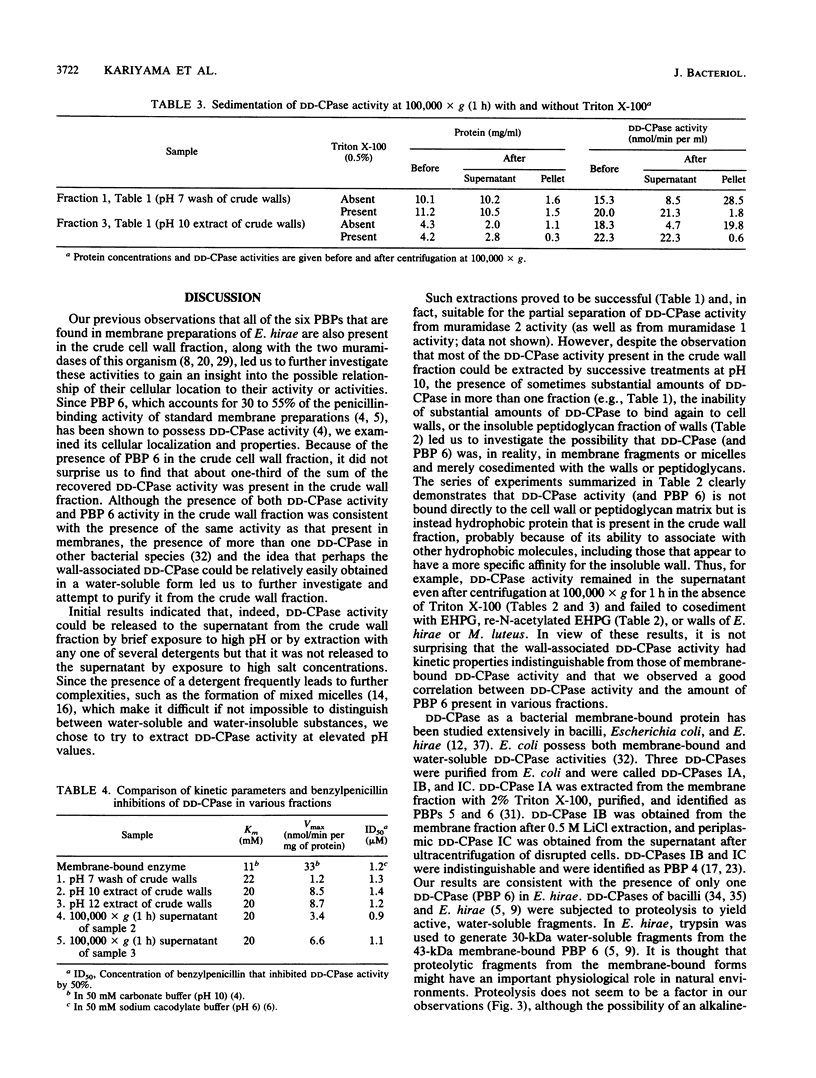
DD-Carboxypeptidase (DD-CPase) activity of Enterococcus hirae (Streptococcus faecium) ATCC 9790 was extracted from intact bacteria and from the insoluble residue (crude cell wall fraction) of mechanically disrupted bacteria by a brief treatment at pH 10.0 (10 mM glycine-NaOH) at 0 degrees C or by extraction with any of several detergents. Extractions with high salt concentrations failed to remove DD-CPase activity from the crude wall fraction. In contrast to N-acetylmuramoylhydrolase (both muramidase 2 and muramidase 1) activities, DD-CPase activity failed to bind to insoluble cell walls or peptidoglycan matrices. Thus, whereas muramidase 1 and muramidase 2 activities can be considered to be cell wall proteins, the bulk of the data are consistent with the interpretation that the DD-CPase of this species is a membrane protein that is sometimes found in the cell wall fraction, presumably because of hydrophobic interactions with other proteins and cell wall polymers. The binding of [14C]penicillin to penicillin-binding protein 6 (43 kilodaltons) was proportional to DD-CPase activity. Kinetic parameters were also consistent with the presence of only one DD-CPase (penicillin-binding protein 6) in E. hirae.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter C. V., Goyer S., Neuhaus F. C. Steric effects on penicillin-sensitive peptidoglycan synthesis in a membrane-wall system Gaffkya homari. Biochemistry. 1976 Jul 13;15(14):3146–3152. doi: 10.1021/bi00659a031. [DOI] [PubMed] [Google Scholar]
- Cornett J. B., Johnson C. A., Shockman G. D. Release of autolytic enzyme from Streptococcus, faecium cell walls by treatment with dilute alkali. J Bacteriol. 1979 Jun;138(3):699–704. doi: 10.1128/jb.138.3.699-704.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. Solubilization and isolation of the membrane-bound DD-carboxypeptidase of Streptococcus faecalis ATCC9790. Properties of the purified enzyme. Eur J Biochem. 1978 Jul 17;88(1):297–305. doi: 10.1111/j.1432-1033.1978.tb12450.x. [DOI] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. The penicillin-binding proteins in Streptococcus faecalis ATCC 9790. Eur J Biochem. 1980 Sep;110(2):445–456. doi: 10.1111/j.1432-1033.1980.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Coyette J., Perkins H. R., Polacheck I., Shockman G. D., Ghuysen J. M. Membrane-bound DD-carboxypeptidase and LD-transpeptidase of Streptococcus faecalis ATCC 9790. Eur J Biochem. 1974 May 15;44(2):459–468. doi: 10.1111/j.1432-1033.1974.tb03504.x. [DOI] [PubMed] [Google Scholar]
- Coyette J., Shockman G. D. Some properties of the autolytic N-acetylmuramidase of Lactobacillus acidophilus. J Bacteriol. 1973 Apr;114(1):34–41. doi: 10.1128/jb.114.1.34-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinger D. L., Daneo-Moore L., Shockman G. D. The second peptidoglycan hydrolase of Streptococcus faecium ATCC 9790 covalently binds penicillin. J Bacteriol. 1989 Aug;171(8):4355–4361. doi: 10.1128/jb.171.8.4355-4361.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L. C., Schwarz U., Keck W., Charlier P., Dideberg O., Ghuysen J. M. Properties and crystallization of a genetically engineered, water-soluble derivative of penicillin-binding protein 5 of Escherichia coli K12. Eur J Biochem. 1988 Jan 15;171(1-2):11–16. doi: 10.1111/j.1432-1033.1988.tb13751.x. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Frére J. M., Leyh-Bouille M., Ghuysen J. M., Nieto M., Perkins H. R. Exocellular DD-carboxypeptidases-transpeptidases from Streptomyces. Methods Enzymol. 1976;45:610–636. doi: 10.1016/s0076-6879(76)45054-1. [DOI] [PubMed] [Google Scholar]
- Furth A. J., Bolton H., Potter J., Priddle J. D. Separating detergent from proteins. Methods Enzymol. 1984;104:318–328. doi: 10.1016/s0076-6879(84)04098-2. [DOI] [PubMed] [Google Scholar]
- HEYMANN H., MANNIELLO J. M., BARKULIS S. S. STRUCTURE OF STREPTOCOCCAL CELL WALLS. 3. CHARACTERIZATION OF AN ALANINE-CONTAINING GLUCOSAMINYLMURAMIC ACID DERIVATIVE LIBERATED BY LYSOZYME FROM STREPTOCOCCAL GLYCOPEPTIDE. J Biol Chem. 1964 Sep;239:2981–2985. [PubMed] [Google Scholar]
- Hjelmeland L. M., Chrambach A. Solubilization of functional membrane proteins. Methods Enzymol. 1984;104:305–318. doi: 10.1016/s0076-6879(84)04097-0. [DOI] [PubMed] [Google Scholar]
- Iwaya M., Strominger J. L. Simultaneous deletion of D-alanine carboxypeptidase IB-C and penicillin-binding component IV in a mutant of Escherichia coli K12. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2980–2984. doi: 10.1073/pnas.74.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. E., Pratt J. M. Analysis of the membrane-binding domain of penicillin-binding protein 5 of Escherichia coli. Mol Microbiol. 1988 Sep;2(5):563–568. doi: 10.1111/j.1365-2958.1988.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Kalomiris E., Bardin C., Neuhaus F. C. Biosynthesis of peptidoglycan in Gaffkya homari: reactivation of membranes by freeze-thawing in the presence and absence of walls. J Bacteriol. 1982 May;150(2):535–544. doi: 10.1128/jb.150.2.535-544.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T., Shockman G. D. Purification and some properties of the endogenous, autolytic N-acetylmuramoylhydrolase of Streptococcus faecium, a bacterial glycoenzyme. J Biol Chem. 1983 Aug 10;258(15):9514–9521. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Takagaki Y., Maruyama I. N., Tamaki S., Nishimura Y., Suzuki H., Ogino U., Hirota Y. Mutants of Escherichia coli lacking in highly penicillin-sensitive D-alanine carboxypeptidase activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2976–2979. doi: 10.1073/pnas.74.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Sharon N. Biosynthesis of peptidoglycan by a cell wall preparation of Staphylococcus aureus and its inhibition by penicillin. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1909–1917. doi: 10.1016/0006-291x(72)90069-1. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Shaw D. R., Park J. T. Nature and origins of phosphorus compounds in isolated cell walls of Staphylococcus aureus. J Bacteriol. 1971 Jul;107(1):239–244. doi: 10.1128/jb.107.1.239-244.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus F. C., Tobin C. E., Ahlgren J. A. Membrane-wall interrelationship in Gaffkya homari: sulfhydryl sensitivity and heat lability of nascent peptidoglycan incorporation into walls. J Bacteriol. 1980 Jul;143(1):112–119. doi: 10.1128/jb.143.1.112-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Porres-Juan J. M., Shockman G. D. Dissociation of an autolytic enzyme-cell wall complex by treatment with unusually high concentrations of salt. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1134–1140. doi: 10.1016/0006-291x(70)90357-8. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Barrett J. F. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol. 1983;37:501–527. doi: 10.1146/annurev.mi.37.100183.002441. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Todd J. A., Roberts A. N., Johnstone K., Piggot P. J., Winter G., Ellar D. J. Reduced heat resistance of mutant spores after cloning and mutagenesis of the Bacillus subtilis gene encoding penicillin-binding protein 5. J Bacteriol. 1986 Jul;167(1):257–264. doi: 10.1128/jb.167.1.257-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Cleavage of a COOH-terminal hydrophobic region from D-alanine carboxypeptidase, a penicillin-sensitive bacterial membrane enzyme. Characterization of active, water-soluble fragments. J Biol Chem. 1979 Jun 10;254(11):4863–4875. [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Limited proteolysis of the penicillin-sensitive D-alanine carboxypeptidase purified from Bacillus subtilis membranes. Active water-soluble fragments generated by cleavage of a COOH-terminal membrane anchor. J Biol Chem. 1981 Feb 25;256(4):2059–2066. [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Primary structure of the COOH-terminal membranous segment of a penicillin-sensitive enzyme purified from two Bacilli. J Biol Chem. 1981 Feb 25;256(4):2067–2077. [PubMed] [Google Scholar]
- el Kharroubi A., Piras G., Jacques P., Szabo I., Van Beeumen J., Coyette J., Ghuysen J. M. Active-site and membrane topology of the DD-peptidase/penicillin-binding protein no. 6 of Enterococcus hirae (Streptococcus faecium) A.T.C.C. 9790. Biochem J. 1989 Sep 1;262(2):457–462. doi: 10.1042/bj2620457. [DOI] [PMC free article] [PubMed] [Google Scholar]