Abstract
Cryptophycin-52 (LY355703) is a new synthetic member of the cryptophycin family of antimitotic antitumor agents that is currently undergoing clinical evaluation. At high concentrations (≥10 times the IC50), cryptophycin-52 blocked HeLa cell proliferation at mitosis by depolymerizing spindle microtubules and disrupting chromosome organization. However, low concentrations of cryptophycin-52 inhibited cell proliferation at mitosis (IC50 = 11 pM) without significantly altering spindle microtubule mass or organization. Cryptophycin-52 appears to be the most potent suppressor of microtubule dynamics found thus far. It suppressed the dynamic instability behavior of individual microtubules in vitro (IC50 = 20 nM), reducing the rate and extent of shortening and growing without significantly reducing polymer mass or mean microtubule length. Using [3H]cryptophycin-52, we found that the compound bound to microtubule ends in vitro with high affinity (Kd, 47 nM, maximum of ≈19.5 cryptophycin-52 molecules per microtubule). By analyzing the effects of cryptophycin-52 on dynamics in relation to its binding to microtubules, we determined that ≈5–6 molecules of cryptophycin-52 bound to a microtubule were sufficient to decrease dynamicity by 50%. Cryptophycin-52 became concentrated in cells 730-fold, and the resulting intracellular cryptophycin-52 concentration was similar to that required to stabilize microtubule dynamics in vitro. The data suggest that cryptophycin-52 potently perturbs kinetic events at microtubule ends that are required for microtubule function during mitosis and that it acts by forming a reversible cryptophycin-52-tubulin stabilizing cap at microtubule ends.
A number of drugs that inhibit cell proliferation at mitosis by an action on mitotic spindle microtubules have become highly useful in the treatment of various forms of cancer (1–3). These include paclitaxel and taxotere; the vinca alkaloids, vinblastine, vincristine, and vinorelbine; and estramustine. At relatively high concentrations, these drugs either inhibit or promote spindle microtubule polymerization and thus destroy the ability of the cell to segregate chromosomes to daughter cells at mitosis (2, 3). However, recent evidence indicates that these microtubule-targeted drugs act by a much more sophisticated mechanism than previously thought, an action that occurs at relatively low drug concentrations, which involves stabilization of spindle microtubule dynamics rather than depolymerization or excessive polymerization of microtubules (1, 4–8).
Microtubules exhibit two forms of dynamic behaviors in vitro and in cells. Microtubule ends can switch between episodes of prolonged growing and shortening, a behavior termed “dynamic instability” (9–11). In addition, because of differences in the critical subunit concentration for growth at their opposite ends, microtubules can exhibit treadmilling behavior, in which there is net growth at one microtubule end and shortening at the opposite end (12, 13). Recent studies indicate that the dynamics of microtubules, not just their presence, are critically important for microtubule function in cells (14–16). Microtubule dynamics are especially important for the complex movements of chromosomes during mitosis.
At low concentrations, the antimitotic antitumor drugs vinblastine, paclitaxel, and estramustine all inhibit HeLa cell proliferation at prometaphase/metaphase of mitosis without changing the microtubule polymer mass or appreciably disrupting spindle microtubule organization (1, 4–7). All three of these drugs at their lowest effective concentrations in vitro powerfully suppress microtubule dynamic instability in the absence of appreciable increase or decrease of polymer mass (17–20).
A substantial number of new and structurally diverse compounds have been discovered recently that inhibit cell proliferation at mitosis by an apparent action on microtubules (3). Many of these compounds have shown strong promise as potential chemotherapeutic agents in tumor models (1, 3). Among these are the cryptophycins, cytotoxic depsipeptides originally isolated from cyanobacteria of the genus Nostoc (21–22). The parent compound, cryptophycin-1, inhibits cell proliferation by blocking cell cycle progression at prometaphase/metaphase of mitosis at picomolar concentrations by an apparent action on microtubules (21–25). In vitro, cryptophycin-1 inhibits microtubule polymerization (23, 24, 26) and strongly suppresses the dynamic instability of microtubules (27).
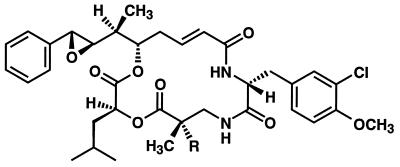
Cryptophycin-52 (LY355703) (Fig. 1) is a new synthetic member of the cryptophycin family that is currently undergoing clinical evaluation (D.W., M. M. Wagner, D. C. Paul, M.A.J., L.W., and C. Shih, unpublished work). It was selected as a clinical candidate from an extensive series of cryptophycin analogs based on its potency, breadth of activity against tumor models, its stability, and its amenability to clinical formulation. It is an extraordinarily potent antiproliferative agent against a broad spectrum of cultured human tumor cells including cells from multidrug-resistant tumors (D.W., M. M. Wagner, D. C. Paul, M.A.J., L.W., and C. Shih, unpublished work). It blocks cell cycle progression at G2/M, causes accumulation of cells at metaphase, and kills cells by apoptosis.
Figure 1.
Structure of cryptophycin-52. R = H, cryptophycin-1; R = CH3, cryptophycin-52.
Here we have determined the mechanism of action of cryptophycin-52 in cells and in vitro. At cryptophycin-52 concentrations sufficient to inhibit HeLa cell proliferation at mitosis (3–30 pM), we find that the spindle microtubule mass and microtubule organization remain nearly normal, suggesting that the action of cryptophycin-52 at low concentrations on cell cycle progression at mitosis is a result of suppression of microtubule dynamics rather than inhibition of spindle microtubule polymerization. By analyzing the suppression of individual dynamic instability parameters in relation to the binding of [3H]cryptophycin-52 to microtubules in vitro, we find that ≈5–6 cryptophycin-52 molecules bound to a microtubule can reduce the rate and extent of microtubule shortening by 50%. The data indicate that in cells only a few cryptophycin-52 molecules bound per microtubule may be sufficient to block spindle microtubule function and kill tumor cells.
MATERIALS AND METHODS
Reagents.
Cryptophycin-52 (LY355703) (molecular weight 675) was synthesized by the Lilly Research Laboratories, Indianapolis. [Methoxy-3H]cryptophycin-52 [specific activity, 82 Ci/mmol (3.0 TBq/nmol); radiochemical purity, 99.2%] was synthesized through contract with Amersham. All other reagents were obtained from Sigma.
Cell Culture and Immunofluorescence Microscopy.
HeLa S3 cells (American Type Culture Collection, Manassas, VA) were grown in a monolayer at 37°C without antibiotics in 5% CO2/95% air (4). Cell proliferation was determined by counting cells with a hemocytometer at the time of cryptophycin-52 addition and 20 h later. Mitotic indices and cell morphology were determined by immunofluorescence microscopy both of floating and attached cells (4). Uptake of [3H]cryptophycin-52 into HeLa cells was determined by a scintillation vial monolayer culture procedure as described previously (8). Briefly, HeLa cells grown in scintillation vials were incubated for 20 h with [3H]cryptophycin-52. The quantity of radiolabeled cryptophycin-52 was then determined in the medium after aspirating the medium and in the cells after lysing the cells.
Purification of Tubulin, Assembly of Microtubules, and Determination of Steady-State Microtubule Polymer Mass.
Bovine brain microtubule protein (70% tubulin and 30% microtubule-associated proteins) and phosphocellulose-purified tubulin were isolated and stored as frozen pellets at −70°C (18). Tubulin pellets were thawed and centrifuged at 4°C to remove any aggregated or denatured tubulin. The tubulin (13 μM) was then mixed with Strongylocentrotus purpuratus flagellar axonemal seeds in 87 mM 1,4-piperazinediethanesulfonic acid (Pipes)/36 mM 2-morpholinoethanesulfonic acid (Mes)/1.8 mM MgCl2/1 mM EGTA, pH 6.8 (PMME buffer) containing 2 mM GTP and polymerized to steady state by incubation for 35–45 min at 37°C (17). The microtubules were pelleted by centrifugation at 150,000 × g for 1 h, and pellets were solubilized in PMME buffer at 0°C for protein determination by using BSA as the standard (28).
Stoichiometry of Cryptophycin-52 Binding to Microtubules.
Tubulin (13 μM) was polymerized at the ends of axonemal seeds in the presence of different concentrations of cryptophycin-52 containing a trace amount of [3H]cryptophycin-52. Unbound cryptophycin-52 was separated from the microtubules by centrifugation through 50% sucrose cushions for 75 min at 37°C (190,000 × g). Microtubule pellets were solubilized in PMME buffer at 0°C, the tubulin concentration in the pellets was determined, and the amount of cryptophycin-52 bound to the microtubules was determined by scintillation counting. The molar amount of cryptophycin-52 bound per mole of tubulin dimer in the microtubules was determined by dividing the cryptophycin-52 concentration by the tubulin concentration in the polymer. The mean lengths of the microtubules were determined by video microscopy, and the number of cryptophycin-52 molecules bound per microtubule was calculated by using a value of 1,690 tubulin dimers per μm of microtubule length. Approximately 105 microtubules were measured for each cryptophycin-52 concentration (29).
Analysis of Microtubule Dynamics by Video Microscopy.
Tubulin was polymerized to steady state (35 min, 37°C) in the absence or presence of cryptophycin-52. Dynamics of individual microtubules were recorded by video microscopy (17), and the microtubules were observed for a maximum of 45 min after reaching steady state. Under the experimental conditions used, microtubule growth occurred predominantly at the plus ends of the seeds as determined by the growth rates, the number of microtubules that grew, and the relative lengths of the microtubules at the opposite ends of the seeds (11, 17–20). We considered a microtubule to be in a growing phase if it increased in length by >0.2 μm at a rate >0.15 μm/min, and in a shortening phase if it shortened by >0.2 μm at a rate >0.3 μm/min. Length changes equal to or less than 0.2 μm over the duration of 6 data points were considered as attenuation phases. Twenty-five to 30 microtubules were measured for each experimental condition.
We calculated the catastrophe frequency [a catastrophe is a transition from the growing or attenuated state to shortening (11)] by dividing the number of catastrophes by the sum of the total time spent in the growing plus attenuated states for all microtubules for a particular condition. The rescue frequency [a rescue is a transition from shortening to growing or attenuation, excluding new growth from a seed (11)] was calculated by dividing the total number of rescue events by the total time spent shortening for all microtubules for a particular condition.
RESULTS
Inhibition of HeLa Cell Proliferation and Induction of Mitotic Block by Cryptophycin-52.
Cryptophycin-52 powerfully inhibited proliferation of HeLa cells in a concentration-dependent manner with half-maximal inhibition occurring at 11 pM (data not shown). Concomitantly, we found that cryptophycin-52 induced a block in mitosis at the transition from metaphase to anaphase. Fifty percent of the cells were blocked in metaphase at 26 pM cryptophycin-52, and the block was maximal (95%) at 100 pM cryptophycin-52 (data not shown). The effective concentrations for induction of mitotic block paralleled those required for inhibition of cell proliferation, with mitotic block requiring somewhat higher cryptophycin-52 concentrations than inhibition of proliferation.
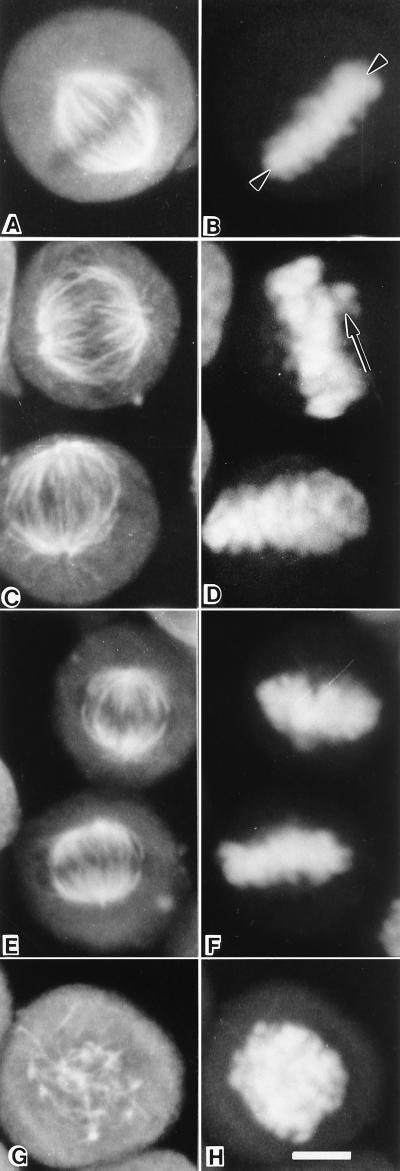
Low picomolar concentrations of cryptophycin-52 inhibited cell proliferation and mitosis without significantly altering the microtubule content or organization of the mitotic spindles, as determined by immunofluorescence microscopy. At 20 pM cryptophycin-52, a concentration that inhibited HeLa cell proliferation by 72% and induced ≈43% mitotic accumulation, the mass of microtubules in the mitotic spindles appeared similar to those in controls (Fig. 2A, B, E, and F). Most of the chromosomes in most cells were present as a compact metaphase plate, whereas a few chromosomes in most cells were found at one or both mitotic poles. At higher cryptophycin-52 concentrations, the mass of mitotic microtubules was decreased substantially, along with increased disorganization of the chromosomes (Fig. 2 G and H). For example, 300 pM cryptophycin-52 depolymerized all microtubules in mitotic cells (Fig. 2G). These results suggest that the antiproliferative mechanism of action of cryptophycin-52 might involve stabilization of spindle microtubule dynamics as has been found for other effective antimitotic cancer drugs including paclitaxel, vinblastine, and estramustine (1, 4–8, 17–20).
Figure 2.
The effects of cryptophycin-52 on Hela cell mitotic spindles. Microtubules (A, C, E, and G) and chromosomes (B, D, F, and H). (A and B) A control cell spindle with a well defined compact metaphase plate of chromosomes (arrowhead). Spindles of cells treated either with 10 or 20 pM cryptophycin-52 were similar. Shown in C and D are cells treated with 10 pM cyptophycin-52 (20 h); one spindle is normal and bipolar whereas the other spindle has chromosomes nearer one spindle pole (arrow in D). Shown in E and F are two cells treated for 20 h at 20 pM cryptophycin-52. Both spindles are relatively normal in organization. (G and H) At 300 pM cryptophycin-52 (20 h), most microtubules are depolymerized and chromosomes are in a ball-shaped mass. (Bar = 2 μm; ×5,500.)
Cryptophycin-52 Accumulates in Cells to High Concentrations.
Several antimitotic antitumor agents such as vinblastine and paclitaxel are concentrated intracellularly (200- to 1,000-fold) (4, 6, 8). Thus, we determined the extent to which cryptophycin-52 accumulated intracellularly. Incubation of HeLa cells with 11 pM [3H]cryptophycin-52 resulted in rapid uptake of the compound. An intracellular concentration of 8 nM cryptophycin-52 was attained at 20 h, a 730-fold-higher concentration than that added to the medium (data not shown).
Effects of Cryptophycin-52 on Microtubule Polymerization.
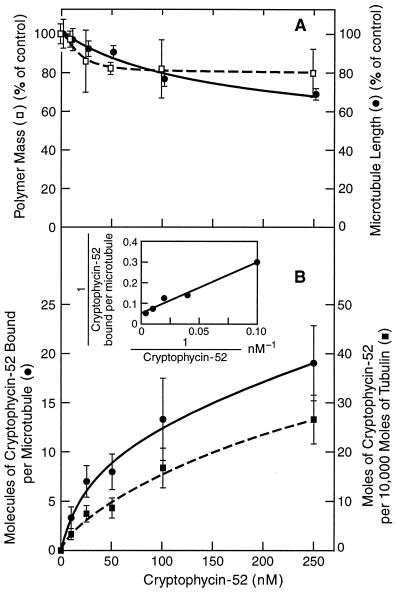
We wanted to understand how cryptophycin-52 affects microtubule polymer mass, microtubule lengths, and dynamics at steady state in relation to the binding of cryptophycin-52 to the microtubules. We assembled microtubules from bovine brain tubulin under conditions required for analysis of cryptophycin-52’s effects on dynamics (Materials and Methods). Polymerization of microtubules in the presence of low concentrations of cryptophycin-52 (≤10 nM) had no effect on the mass of polymer formed as compared with controls (Fig. 3A). At 250 nM cryptophycin-52, the polymer mass was reduced by only 20% (Fig. 3A). Significant inhibition of polymerization occurred at cryptophycin-52 concentrations above 1 μM, and no microtubules formed at concentrations above 2 μM as determined by video microscopy (data not shown). Cryptophycin-52 had no detectable effect on the mean microtubule length at or below a concentration of 50 nM, and 250 nM cryptophycin-52 reduced the mean length of microtubules by 30% (Fig. 3A).
Figure 3.
Effects of cryptophycin-52 on microtubule assembly. Tubulin (13 μM) was mixed with S. purpuratus flagellar seeds in PMME buffer containing 2 mM GTP and incubated at 37°C in the absence or presence of different concentrations of cryptophycin-52 for 35 min to polymerize the microtubules to steady state. (A) The effects of cryptophycin-52 on polymer mass and on mean microtubule length were determined as described in the Materials and Methods. (B) The stoichiometries of cryptophycin-52 binding per microtubule and per mole of tubulin in microtubules were determined as described under Materials and Methods. Mean length of control microtubules was 6.06 ± 2.3 μm. Inset shows a double-reciprocal plot of cryptophycin-52 binding to microtubules. Error bars = SEM.
The Binding of Cryptophycin-52 to Microtubules.
The binding of cryptophycin-52 to microtubules as a function of cryptophycin-52 concentration was determined by polymerizing tubulin in the presence of unlabeled cryptophycin-52 along with a trace quantity of [3H]cryptophycin-52 (Materials and Methods). The stoichiometry of cryptophycin-52 binding to the microtubules was extremely low. At 10 nM cryptophycin-52, only 3.3 ± 1.1 moles of cryptophycin-52 were bound per 10,000 moles of tubulin dimers in the microtubules. At the highest cryptophycin-52 concentration used (250 nM), only 26 ± 5 moles of cryptophycin-52 was bound per 10,000 moles of tubulin in the microtubules (Fig. 3B). Calculating the binding stoichiometry in terms of the number of microtubules in suspension and plotting the data as a double-reciprocal plot yielded a straight line with a maximum binding stoichiometry of 19.5 molecules of cryptophycin-52 per microtubule and a dissociation constant of 47 nM (Fig. 3B Inset). The low maximum stoichiometry of cryptophycin-52 binding to microtubules (Fig. 3B) suggests that cryptophycin-52 binds at the microtubule ends.
We also determined whether with time cryptophycin-52 could become incorporated into microtubules to higher levels by polymerizing microtubule protein into microtubules with 200 nM [3H]cryptophycin-52 and incubating continuously for 2 h, and measuring binding at four different time points. The extent of cryptophycin-52 binding to the microtubules did not increase beyond the initial 30-min time point; specifically, 4.1 ± 1.6 molecules of cryptophycin-52 were bound per microtubule at 30 min and 3.6 ± 1.2 molecules of cryptophycin-52 were bound at 120 min. The data indicate that cryptophycin-52 does not become increasingly incorporated into the microtubule with time, further supporting the idea that the compound binds strictly at the microtubule ends.
Effects of Cryptophycin-52 on Dynamic Instability at Microtubule Plus Ends.
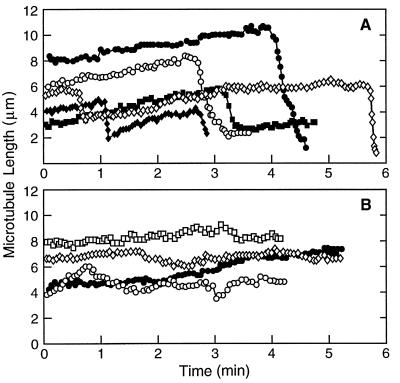
We analyzed the effects of cryptophycin-52 on dynamics at the plus ends of individual microtubules by video microscopy. Several life history traces of control microtubule length changes with time are shown in Fig. 4A. As previously documented, control microtubules alternated between phases of growing and shortening and spent a small fraction of time in an attenuated (or paused) state, neither growing nor shortening detectably. Addition of cryptophycin-52 visually reduced the rate and extent of growth and shortening and increased the fraction of time the microtubules remained in an attenuated state (Fig. 4B).
Figure 4.
Length changes of individual microtubules at their plus ends at steady state in the absence (A) and presence (B) of 50 nM cryptophycin-52. The lengths of individual microtubules were measured from real-time video tape recordings as described under Materials and Methods.
As shown quantitatively in Table 1, cryptophycin-52 powerfully inhibited the rate and extent of microtubule shortening. The effects of the compound on shortening were considerably more potent than its effects on growth (Table 1). For example, 25 nM cryptophycin-52 reduced the shortening rate by 63% from 14.6 ± 2.3 μm/min to 5.4 ± 1.3 μm/min whereas it reduced the growing rate by only 26% from 0.94 ± .11 μm/min to 0.7 ± 0.11 μm/min. At low cryptophycin-52 concentrations (25 nM), suppression of the rate and extent of growing and shortening occurred in the absence of a significant decrease in the microtubule polymer mass or the mean microtubule length. Thus, the rate and extent of microtubule growing and shortening are more sensitive to low nanomolar concentrations of cryptophycin-52 than bulk phase polymerization.
Table 1.
Effects of cryptophycin-52 on the dynamics of individual microtubules
| Concentration, nM
|
||||||
|---|---|---|---|---|---|---|
| 0 | 10 | 25 | 50 | 100 | 250 | |
| Rate, μm/min | ||||||
| Growing | 0.94 ± 0.11 | 0.81 ± 0.12 | 0.70 ± 0.11 | 0.73 ± 0.11 | 0.65 ± 0.10 | 0.44 ± 0.06 |
| Shortening | 14.6 ± 2.3 | 9.6 ± 1.8 | 5.4 ± 1.3 | 4.6 ± 0.9 | 3.6 ± 0.8 | 2.2 ± 2.2 |
| % time in phase | ||||||
| Growing | 75.0 | 64.8 | 70.1 | 57.0 | 58.5 | 49.3 |
| Shortening | 6.9 | 8.2 | 9.6 | 11.0 | 10.0 | 4.6 |
| Attenuation | 18.1 | 27.0 | 20.3 | 32.0 | 31.5 | 46.1 |
| Transition frequencies, events per min | ||||||
| Catastrophe | 0.23 ± 0.05 | 0.31 ± 0.06 | 0.30 ± 0.07 | 0.27 ± 0.06 | 0.25 ± 0.05 | 0.13 ± 0.04 |
| Rescue | 2.4 ± 0.62 | 3.0 ± 0.61 | 2.9 ± 0.7 | 2.2 ± 0.47 | 2.3 ± 0.46 | 2.6 ± 0.80 |
| Transition frequencies, events per μm | ||||||
| Catastrophe | 0.32 ± 0.07 | 0.63 ± 0.13 | 0.56 ± 0.13 | 0.77 ± 0.16 | 0.85 ± 0.17 | 0.71 ± 0.21 |
| Rescue | 0.18 ± 0.04 | 0.38 ± 0.08 | 0.53 ± 0.12 | 0.89 ± 0.19 | 0.90 ± 0.18 | 1.6 ± 0.48 |
± are SEM.
At or near steady state, microtubules in vitro and in cells spend a considerable fraction of time in an attenuated or paused state (7, 17–20, 30). Cryptophycin-52 significantly increased the percentage of time that the microtubules spent in the attenuated state while strongly decreasing the percentage of time the microtubules spent growing (Table 1).
The transition frequencies among the growing, shortening, and attenuated states are considered to be important parameters involved in regulation of microtubule function in cells (30–33). Low concentrations of cryptophycin-52 (at or below 100 nM) did not affect the time-based catastrophe frequency, and this parameter was reduced by only ≈1.8-fold at a concentration of 250 nM. Cryptophycin-52 also had no apparent effect on the time-based rescue frequency (Table 1). These data suggest that cryptophycin-52 does not strongly modulate the mechanism responsible for gain and loss of the stabilizing cap at microtubule ends. However, cryptophycin strongly suppressed the growth and shortening rates, and, thus, it did increase the catastrophe frequency per μm of growth (2.24-fold at 250 nM) and it strongly increased the rescue frequency per μm of shortening (8.7-fold at 250 nM). These increases in the catastrophe and rescue frequencies are associated with reductions of the length excursions.
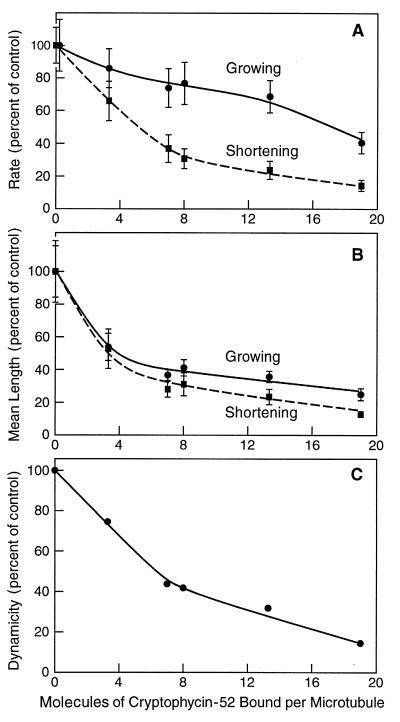
By correlating the stoichiometry of binding of cryptophycin-52 with the effects of cryptophycin-52 on microtubule dynamics, we found that very few molecules of cryptophycin-52 bound to microtubules had a potent suppressive effect on microtubule dynamics (Fig. 5). For example, only ≈5 molecules of cryptophycin-52 bound per microtubule were necessary to reduce the shortening rate by 50% (Fig. 5A). In addition, very few cryptophycin-52 molecules bound per microtubule strongly reduced the average length the microtubules grew per growing event and shortened per shortening event (Fig. 5B). For example, ≈7 moles of cryptophycin-52 bound per microtubule reduced the mean length grown by 63% and the mean length shortened by 71%.
Figure 5.
Effects of bound cryptophycin-52 on rates of growing and shortening (A), mean length grown or shortened (B), and dynamicity (C). The average lengths that microtubules grew during growing events were determined by dividing the summed growing lengths for all microtubules for a particular condition by the total number of growing events measured for that condition. The mean shortening length per shortening excursion was determined similarly. Error bars = SEM.
Dynamicity, the rate of total detectable tubulin dimer exchange at a microtubule end (7, 17, 18), is a reflection of the overall dynamic instability behavior of a microtubule. The binding of a few molecules of cryptophycin-52 to a microtubule powerfully suppressed dynamicity in the absence of significant changes in polymer mass (Fig. 5C). Specifically, ≈6 molecules of cryptophycin-52 bound per microtubule suppressed the dynamicity by 50% while reducing the polymer mass by only ≈15% (Figs. 3A and Fig. 5C).
DISCUSSION
Cryptophycin-52 (LY355703) powerfully inhibited proliferation of HeLa cells at metaphase of mitosis (IC50, 11 pM). At relatively high concentrations (≈100–300 pM), the compound depolymerized the spindle microtubules. However, inhibition of proliferation at low cryptophycin-52 concentrations occurred in the absence of significant spindle microtubule depolymerization or spindle disorganization, suggesting that the drug inhibits mitosis by suppressing spindle microtubule dynamics. Cryptophycin-52 powerfully suppressed microtubule dynamic instability in vitro, and it appears that the cryptophycins are the most potent suppressors of microtubule dynamics discovered thus far. It strongly inhibited the rate and extent of shortening (IC50, 20 nM), the common parameters inhibited most strongly by successful antimitotic antitumor drugs. Only ≈5 molecules of cryptophycin-52 bound per microtubule, apparently at microtubule ends, reduced the shortening rate by 50%. Cryptophycin-52 also induced a large increase in the percentage of time that microtubules spent in an attenuated (paused) state and strongly increased the rescue frequency per μm of length shortened during a shortening event.
The added cryptophycin-52 concentration required to inhibit cell proliferation and block mitotic progression (1–30 pM) was ≈1,000-fold lower than that required to suppress the dynamics of individual microtubules in vitro (10–100 nM). However, the intracellular concentration of cryptophycin-52 after 20 h of continuous incubation with 11 pM compound was ≈8 nM, approximately a 730-fold increase over the concentration in the medium. The intracellular cryptophycin-52 concentration achieved was similar to the concentration required to inhibit microtubule dynamics in vitro (the IC50 for inhibition of dynamicity was 20 nM). Considering that the total tubulin concentration in HeLa cells is ≈2 mg/ml (4) and the microtubule concentration is ≈0.7 mg/ml, the ratio of total cryptophycin-52 to polymeric tubulin in the cells at the IC50 would be ≈1:875 and the ratio of cryptophycin-52 to total tubulin would be ≈1:2,500. Twenty nanomolar cryptophycin-52 suppressed microtubule dynamics in vitro by 50% at a similar cryptophycin-52 to polymeric tubulin ratio of ≈1:1,350. Thus, the data strongly indicate that in cells, as in vitro, the binding of only a few cryptophycin-52 molecules per microtubule are sufficient to powerfully block spindle microtubule function.
Cryptophycin-52 “kinetically caps” microtubule ends. From a double-reciprocal plot of cryptophycin-52 binding to tubulin in microtubules (Fig. 3B Inset), we calculated that a maximum of ≈19–20 molecules of cryptophycin could bind per microtubule. These data indicate that the compound binds to microtubule ends, rather than binding stoichiometrically to tubulin along the length of the microtubule. Because the amount of cryptophycin-52 bound per microtubule did not increase with time of incubation, it seems likely that the compound cannot incorporate continuously at the microtubule end but, rather, it remains bound at the extreme end.
Cryptophycin-52 strongly reduced both shortening and growing dynamics, suggesting that it acts by a reversible kinetic capping mechanism. Cryptophycin-52 binds strongly to tubulin with a Kd of 0.5 μM, and the tubulin-cryptophycin-52 complex is very poorly reversible (D.P., V. Ananthnarayan, J. Shih, and L.W., unpublished data). Twenty nanomolar cryptophycin-52 suppressed dynamicity by 50%, and, under the conditions used, the total tubulin concentration (13 μM) far exceeded the concentration of cryptophycin-52 (by approximately 600-fold). Thus, under the conditions used, most of the cryptophycin-52 was complexed with tubulin, and it is reasonable to postulate that the compound binds to the microtubule end as a cryptophycin-52-tubulin complex.
The strong affinity of the cryptophycin-52-tubulin complex for microtubules (Kd = 47 nM) indicates that the cryptophycin-52-tubulin complex must dissociate very slowly from the microtubule end. The presence of a cryptophycin-52-tubulin complex at the microtubule end might reduce growth by causing a conformational change in the microtubule lattice that sterically prevents further tubulin addition. Because the cryptophycin-52-tubulin complex does not become incorporated into the microtubule, dissociation of the tubulin-cryptophycin 52 complex from the microtubule end must occur before further tubulin addition can occur. To reduce the shortening rate and to induce rescue, cryptophycin-52-tubulin complex must stabilize the depolymerizing microtubule at least transiently, perhaps by increasing the interactions between adjacent tubulin dimers at the end.
Interestingly, cryptophycin-52 suppressed the shortening rate more potently than the growing rate and increased the rescue frequency per μm 8.7-fold. One possible explanation for this action is that a cryptophycin-52-tubulin complex may have a higher affinity for depolymerizing microtubule ends, which are composed of GDP-liganded tubulin, than for growing ends, which are composed of GTP-liganded tubulin.
The ratio of total cryptophycin-52 to total tubulin in HeLa cells required to inhibit proliferation by 50% was ≈1:2,500. Based on the strong binding constant, one would predict that in cells, most or all of the cellular cryptophycin-52 would be complexed with tubulin at the IC50 and that it would be difficult to remove the compound from the cells. It was shown previously that after washing with drug-free media, the effects of high cryptophycin-1 concentration (200 nM) on microtubules persisted and proliferation of cryptophycin-1-treated cells was slowed for a prolonged period of time (21). Such poor reversibility of the effects of the cryptophycins may be extremely important in their therapeutic effectiveness. Also, the cryptophycins are effective against multidrug-resistance cell lines, indicating that the cryptophycins are poor substrates for the P-glycoprotein efflux pump. Because the cryptophycins appear to become tightly complexed with tubulin, they may not be readily available for the drug efflux system. In summary, the data indicate that cryptophycin-52-tubulin complexes bind to microtubule ends with high affinity and exert powerful suppressive effects on microtubule dynamics. Cryptophycin-52 appears to be the most potent mitotic inhibitor yet characterized that acts by suppression of spindle microtubule dynamics.
Acknowledgments
Bovine brain tubulin and flagellar axonemes were kindly provided by Mr. Herb Miller. We gratefully acknowledge a grant from Eli Lilly Research Laboratories in support of this work and National Institutes of Health Grants NS13560 and CA57291.
ABBREVIATION
- PMME
87 mM Pipes/36 mM Mes/1.8 mM MgCl2/1 mM EGTA
References
- 1.Wilson L, Jordan M A. Chem Biol. 1995;2:569–573. doi: 10.1016/1074-5521(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 2.Wilson L, Jordan M A. In: Microtubules. Hyams J S, Lloyd C W, editors. New York: Wiley; 1994. pp. 59–83. [Google Scholar]
- 3.Hamel E. In: Microtubule Proteins. Avila J, editor. Boca Raton, FL: CRC; 1990. pp. 89–191. [Google Scholar]
- 4.Jordan M A, Thrower D, Wilson L. Cancer Res. 1991;51:2212–2222. [PubMed] [Google Scholar]
- 5.Jordan M A, Thrower D, Wilson L. J Cell Sci. 1992;102:401–416. doi: 10.1242/jcs.102.3.401. [DOI] [PubMed] [Google Scholar]
- 6.Jordan M A, Toso R J, Thrower D, Wilson L. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhamodharan R I, Jordan M A, Thrower D, Wilson L, Wadsworth P. Mol Biol Cell. 1995;6:1215–1229. doi: 10.1091/mbc.6.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan M A, Wendell K L, Gardiner S, Derry W B, Copp H, Wilson L. Cancer Res. 1996;56:816–825. [PubMed] [Google Scholar]
- 9.Mitchison T, Kirschner M W. Nature (London) 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 10.Horio T, Hotani H. Nature (London) 1986;321:605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- 11.Walker R A, O’Brien E T, Pryer N K, Soboeiro M F, Voter W A, Erickson H P, Salmon E D. J Cell Biol. 1988;107:1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolis R, Wilson L. Nature (London) 1981;293:705–771. doi: 10.1038/293705a0. [DOI] [PubMed] [Google Scholar]
- 13.Rodionov V I, Borisy G G. Science. 1997;275:215–218. doi: 10.1126/science.275.5297.215. [DOI] [PubMed] [Google Scholar]
- 14.Haydeon J J, Bowser S S, Rieder C. J Cell Biol. 1990;111:1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skibbens R V, Skeen V P, Salmon E D. J Cell Biol. 1993;122:859–875. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wordeman L, Mitchison T J. In: Microtubules. Hyams J, Lloyd C, editors. New York: Wiley; 1994. pp. 287–301. [Google Scholar]
- 17.Panda D, Miller H P, Islam K, Wilson L. Proc Natl Acad Sci USA. 1997;94:10560–10564. doi: 10.1073/pnas.94.20.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toso R J, Jordan M A, Farrell K W, Matsumoto B, Wilson L. Biochemistry. 1993;32:1285–1293. doi: 10.1021/bi00056a013. [DOI] [PubMed] [Google Scholar]
- 19.Panda D, Jordan M A, Chu K C, Wilson L. J Biol Chem. 1996;271:29807–29812. doi: 10.1074/jbc.271.47.29807. [DOI] [PubMed] [Google Scholar]
- 20.Derry W B, Wilson L, Jordan M A. Biochemistry. 1995;34:2203–2211. doi: 10.1021/bi00007a014. [DOI] [PubMed] [Google Scholar]
- 21.Smith C D, Zhang X, Mooberry S L, Patterson G M L, Moore R E. Cancer Res. 1994;54:3779–3784. [PubMed] [Google Scholar]
- 22.Trimurtulu G, Ohtani I, Patterson G M L, Moore R E, Corbett T H, Valeriote F A, Demchik L. J Am Chem Soc. 1994;116:4729–4737. [Google Scholar]
- 23.Smith C D, Zhang X. J Biol Chem. 1996;271:6192–6196. doi: 10.1074/jbc.271.11.6192. [DOI] [PubMed] [Google Scholar]
- 24.Bai R, Schwartz R E, Kepler J A, Pettit G R, Hamel E. Cancer Res. 1996;56:4398–4406. [PubMed] [Google Scholar]
- 25.Trimurtulu G, J, O, Heltzel C E, Husebo T L, Jensen C M, Larsen L K, Patterson G M L, Moore R E, Mooberry S L, Corbett T H, Valeriote F A. J Am Chem Soc. 1995;117:12030–12049. [Google Scholar]
- 26.Kerksiek K, Mejillano M R, Schwartz R E, Georg G I, Himes R H. FEBS Lett. 1995;377:59–61. doi: 10.1016/0014-5793(95)01271-0. [DOI] [PubMed] [Google Scholar]
- 27.Panda D, Himes R H, Moore R E, Wilson L, Jordan M A. Biochemistry. 1997;36:12948–12953. doi: 10.1021/bi971302p. [DOI] [PubMed] [Google Scholar]
- 28.Bradford M M. Anal Biochem. 1976;72:248–354. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Panda D, Daijo J E, Jordan M A, Wilson L. Biochemistry. 1995;34:9921–9929. doi: 10.1021/bi00031a014. [DOI] [PubMed] [Google Scholar]
- 30.Shelden E, Wadsworth P. J Cell Biol. 1993;120:935–945. doi: 10.1083/jcb.120.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belmont L, Hyman A A, Sawin K E, Mitchison T J. Cell. 1990;62:579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- 32.Gliksman N R, Skibbens R V, Salmon E D. Mol Biol Cell. 1993;4:1035–1050. doi: 10.1091/mbc.4.10.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belmont L D, Mitchison T J. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]