Abstract
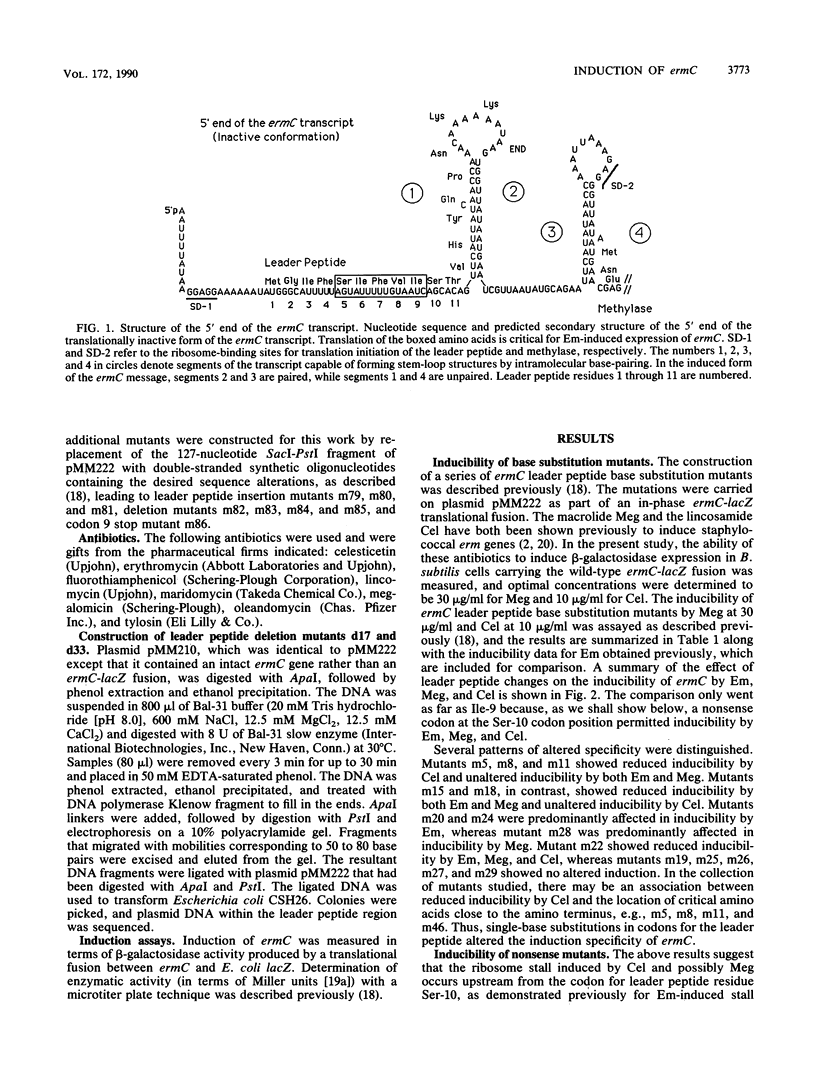
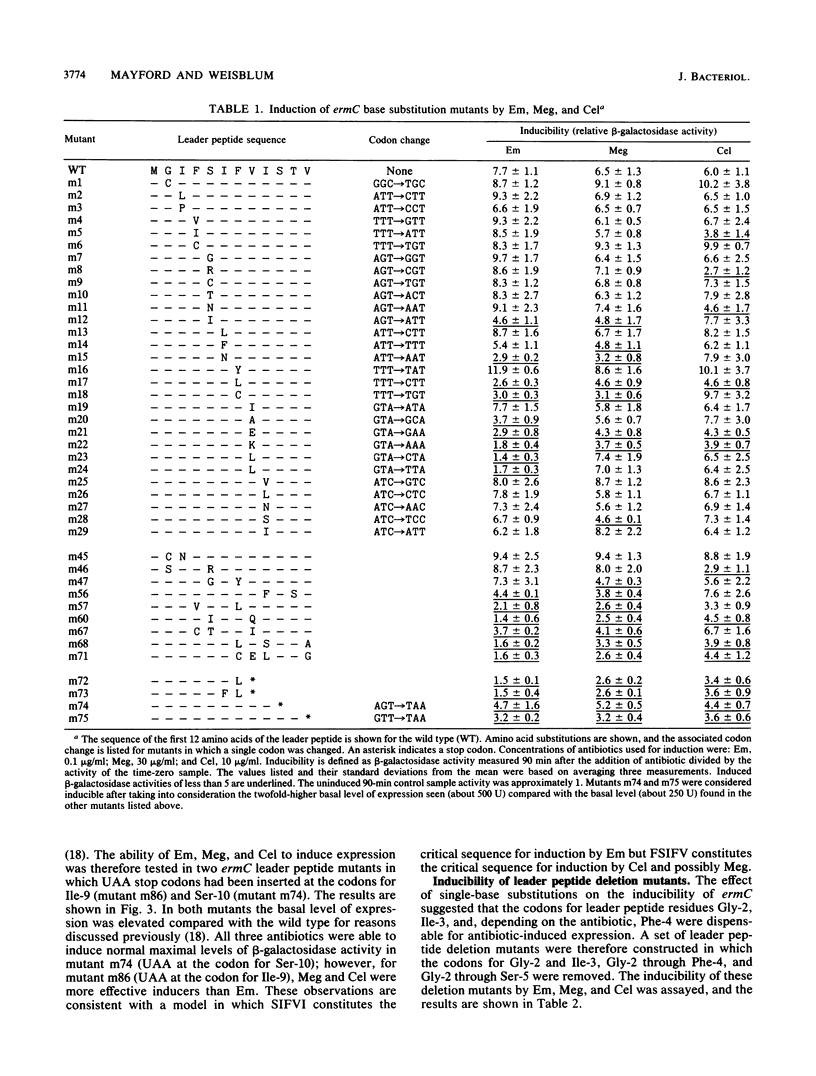
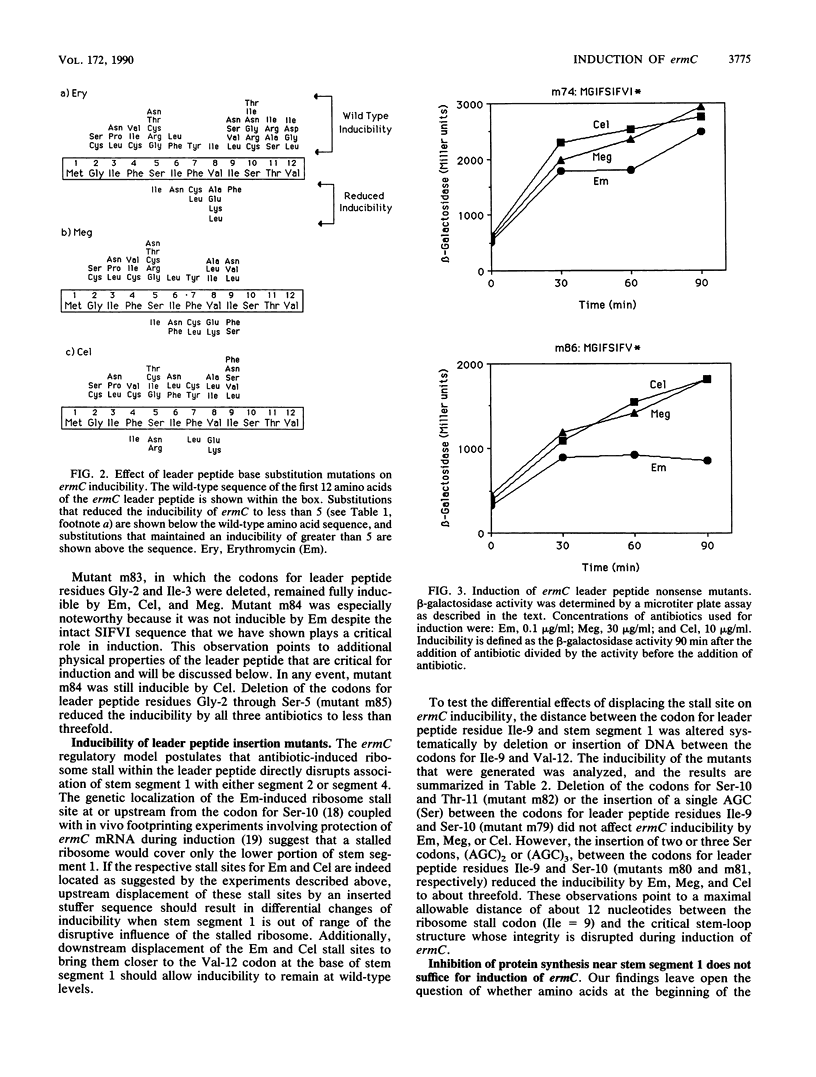
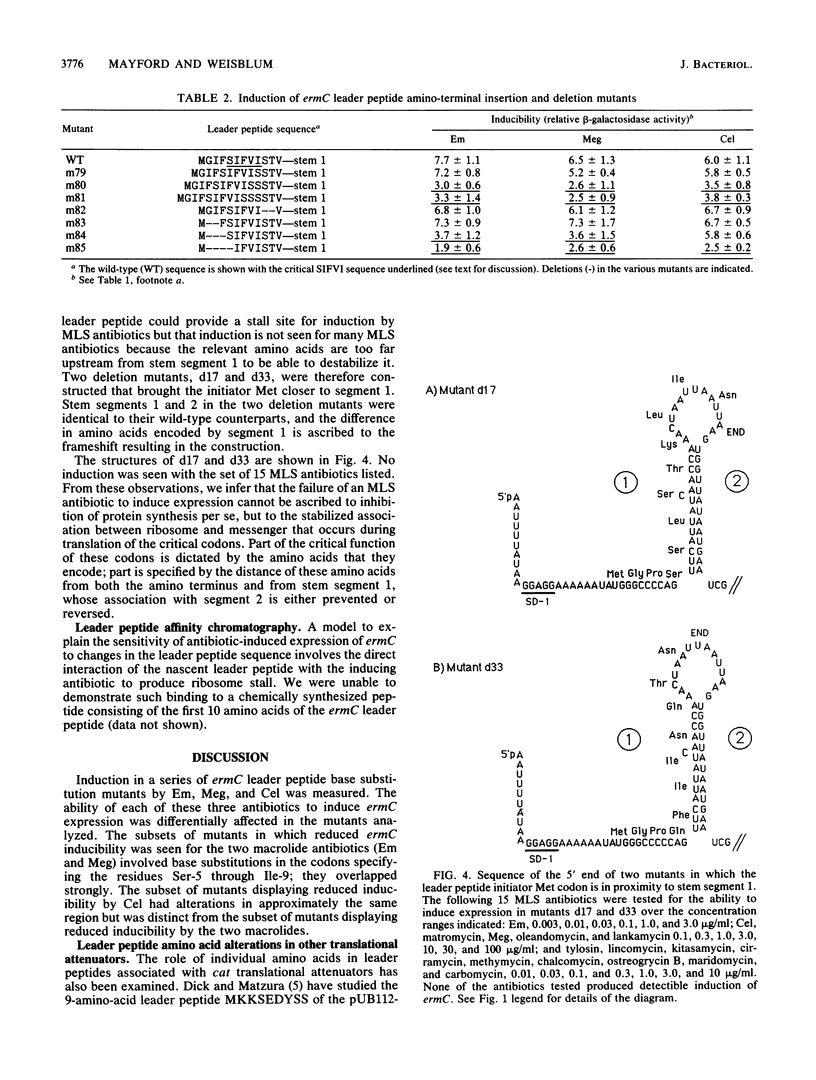
The inducibility of ermC by erythromycin, megalomicin, and celesticetin was tested with both wild-type ermC and several regulatory mutants altered in the 19-amino-acid-residue leader peptide, MGIFSIFVISTVHYQP NKK. In the model test system that was used, the ErmC methylase was translationally fused to beta-galactosidase. Mutational alterations that mapped in the interval encoding Phe-4 through Ile-9 of the leader peptide not only affected induction by individual antibiotics, but did so differentially. The subset of mutations that affected inducibility by the two macrolides erythromycin and megalomicin overlapped and were distinct from the subset of mutations that affected induction by celesticetin. These studies provide a model system for experimentally varying the relative efficiencies with which different antibiotics induce the expression of ermC. The possibility that antibiotics with inducing activity interact directly with the nascent leader peptide was tested by using a chemically synthesized decapeptide, MGIFSIFVIS--, attached at its C-terminus to a solid-phase support. This peptide, however, failed to bind erythromycin in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexieva Z., Duvall E. J., Ambulos N. P., Jr, Kim U. J., Lovett P. S. Chloramphenicol induction of cat-86 requires ribosome stalling at a specific site in the leader. Proc Natl Acad Sci U S A. 1988 May;85(9):3057–3061. doi: 10.1073/pnas.85.9.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen N. E. Macrolide resistance in Staphylococcus aureus: inducers of macrolide resistance. Antimicrob Agents Chemother. 1977 Apr;11(4):669–674. doi: 10.1128/aac.11.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. W., Weiss D. L., Weith H. L., Calvo J. M. Mutations that convert the four leucine codons of the Salmonella typhimurium leu leader to four threonine codons. J Bacteriol. 1985 Jun;162(3):943–949. doi: 10.1128/jb.162.3.943-949.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E., McQuillen K. Bacterial protein synthesis: the effects of antibiotics. J Mol Biol. 1967 Nov 28;30(1):137–146. doi: 10.1016/0022-2836(67)90249-5. [DOI] [PubMed] [Google Scholar]
- Dick T., Matzura H. Positioning ribosomes on leader mRNA for translational activation of the message of an inducible Staphylococcus aureus cat gene. Mol Gen Genet. 1988 Sep;214(1):108–111. doi: 10.1007/BF00340187. [DOI] [PubMed] [Google Scholar]
- Docherty A., Grandi G., Grandi R., Gryczan T. J., Shivakumar A. G., Dubnau D. Naturally occurring macrolide-lincosamide-streptogramin B resistance in Bacillus licheniformis. J Bacteriol. 1981 Jan;145(1):129–137. doi: 10.1128/jb.145.1.129-137.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L. Polysome metabolism in Escherichia coli: effect of antibiotics on polysome stability. Antimicrob Agents Chemother. 1972 Mar;1(3):197–203. doi: 10.1128/aac.1.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa Y., Weisblum B. A family of r-determinants in Streptomyces spp. that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol. 1981 May;146(2):621–631. doi: 10.1128/jb.146.2.621-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Grandi G., Hahn J., Grandi R., Dubnau D. Conformational alteration of mRNA structure and the posttranscriptional regulation of erythromycin-induced drug resistance. Nucleic Acids Res. 1980 Dec 20;8(24):6081–6097. doi: 10.1093/nar/8.24.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Beppu T. Construction and application of a promoter-probe plasmid that allows chromogenic identification in Streptomyces lividans. J Bacteriol. 1985 Apr;162(1):406–412. doi: 10.1128/jb.162.1.406-412.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Byeon W. H., Weisblum B. A complex attenuator regulates inducible resistance to macrolides, lincosamides, and streptogramin type B antibiotics in Streptococcus sanguis. J Bacteriol. 1983 Jun;154(3):1252–1262. doi: 10.1128/jb.154.3.1252-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimiya S., Weisblum B. Translational attenuation control of ermSF, an inducible resistance determinant encoding rRNA N-methyltransferase from Streptomyces fradiae. J Bacteriol. 1988 Apr;170(4):1800–1811. doi: 10.1128/jb.170.4.1800-1811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Attenuation in amino acid biosynthetic operons. Annu Rev Genet. 1982;16:113–134. doi: 10.1146/annurev.ge.16.120182.000553. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Effects of macrolides on peptide-bond formation and translocation. Biochemistry. 1971 May 25;10(11):2054–2061. doi: 10.1021/bi00787a014. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Erythromycin, a peptidyltransferase effector. Biochemistry. 1972 Dec 5;11(25):4864–4872. doi: 10.1021/bi00775a035. [DOI] [PubMed] [Google Scholar]
- Mayford M., Weisblum B. Conformational alterations in the ermC transcript in vivo during induction. EMBO J. 1989 Dec 20;8(13):4307–4314. doi: 10.1002/j.1460-2075.1989.tb08617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M., Weisblum B. ermC leader peptide. Amino acid sequence critical for induction by translational attenuation. J Mol Biol. 1989 Mar 5;206(1):69–79. doi: 10.1016/0022-2836(89)90524-x. [DOI] [PubMed] [Google Scholar]
- Saito T., Mitsuhashi S. Antibacterial activity of megalomicin and its inducer activity for macrolide resistance in Staphylococci. J Antibiot (Tokyo) 1971 Dec;24(12):850–854. doi: 10.7164/antibiotics.24.850. [DOI] [PubMed] [Google Scholar]
- Sandler P., Weisblum B. Erythromycin-induced ribosome stall in the ermA leader: a barricade to 5'-to-3' nucleolytic cleavage of the ermA transcript. J Bacteriol. 1989 Dec;171(12):6680–6688. doi: 10.1128/jb.171.12.6680-6688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar A. G., Hahn J., Dubnau D. Studies on the synthesis of plasmid-coded proteins and their control in Bacillus subtilis minicells. Plasmid. 1979 Apr;2(2):279–289. doi: 10.1016/0147-619x(79)90046-5. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Wallace B. J., Davis B. D. Selective action of erythromycin on initiating ribosomes. Biochemistry. 1974 Oct 22;13(22):4653–4659. doi: 10.1021/bi00719a029. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



