Abstract
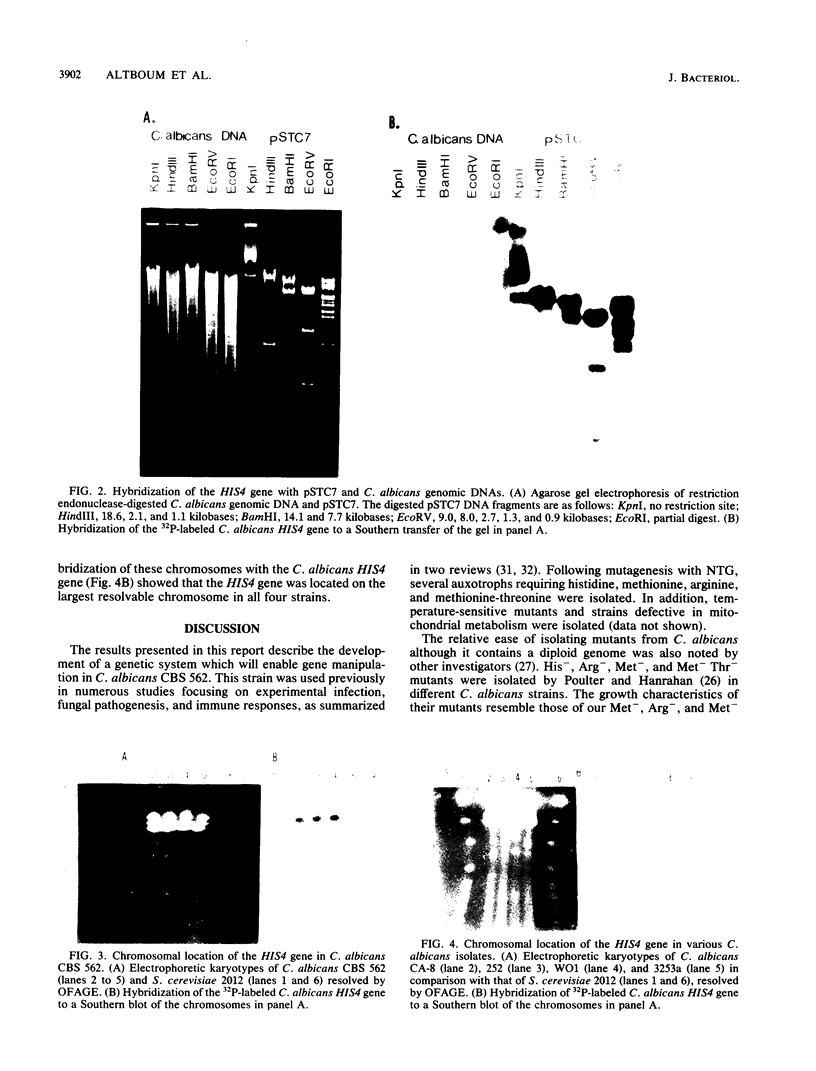
Genetic studies were done with Candida albicans CBS 562. Various auxotrophs were isolated following mutagenesis with N-methyl-N'-nitro-N-nitrosoguanidine. SAG5 (his4C), a stable histidine auxotroph defective in histidinol dehydrogenase activity, was characterized and chosen for further molecular studies. Therefore, the C. albicans HIS4 gene was isolated. The gene was obtained from a genomic library of the wild-type strain, which was constructed in plasmid YEp24. The HIS4 gene was isolated by transformation of a Saccharomyces cerevisiae strain that carried a his4 mutation. The isolated C. albicans HIS4 gene complemented S. cerevisiae his4A, his4B, his4C, and his4ABC mutant strains, which indicates that the clone contains the entire HIS4 gene. The gene was isolated on plasmid pSTC7, whose physical map was constructed with BamHI, SalI, and EcoRV restriction endonucleases, locating the HIS4 gene on a 14-kilobase-pair DNA fragment. Hybridization experiments with HIS4 and C. albicans genomic DNA showed correspondence between the restriction patterns of the gene with that of the chromosomal DNA, indicating that the gene originates from C. albicans and appears in a single copy. Chromosomes of C. albicans CBS562 and four other strains were resolved by orthogonal-field alteration gel electrophoresis. The electrokaryotyping results showed heterogeneity in chromosomal sizes. The electrokaryotyping of CBS 562 showed a resolution of six chromosomal bands, three of which seemed to be doublets. The C. albicans HIS4 gene was located on the largest resolvable chromosome in all of the strains.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., MARTIN R. G., GARRY B. J. The first step of histidine biosynthesis. J Biol Chem. 1961 Jul;236:2019–2026. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Denis C. L., Young E. T. Isolation and characterization of the positive regulatory gene ADR1 from Saccharomyces cerevisiae. Mol Cell Biol. 1983 Mar;3(3):360–370. doi: 10.1128/mcb.3.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. F., Farabaugh P. J., Fink G. R. The nucleotide sequence of the HIS4 region of yeast. Gene. 1982 Apr;18(1):47–59. doi: 10.1016/0378-1119(82)90055-5. [DOI] [PubMed] [Google Scholar]
- FINK G. R. GENE-ENZYME RELATIONS IN HISTIDINE BIOSYNTHESIS IN YEAST. Science. 1964 Oct 23;146(3643):525–527. doi: 10.1126/science.146.3643.525. [DOI] [PubMed] [Google Scholar]
- Fink G. R. A cluster of genes controlling three enzymes in histidine biosynthesis in Saccharomyces cerevisiae. Genetics. 1966 Mar;53(3):445–459. doi: 10.1093/genetics/53.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum A. M., Tsay E. Y., Kirsch D. R. Isolation of the Candida albicans gene for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198(2):179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Hilton C., Markie D., Corner B., Rikkerink E., Poulter R. Heat shock induces chromosome loss in the yeast Candida albicans. Mol Gen Genet. 1985;200(1):162–168. doi: 10.1007/BF00383330. [DOI] [PubMed] [Google Scholar]
- Kakar S. N., Partridge R. M., Magee P. T. A genetic analysis of Candida albicans: isolation of a wide variety of auxotrophs and demonstration of linkage and complementation. Genetics. 1983 Jun;104(2):241–255. doi: 10.1093/genetics/104.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Miller S. M., Kurtz M. B., Kirsch D. R. Directed mutagenesis in Candida albicans: one-step gene disruption to isolate ura3 mutants. Mol Cell Biol. 1987 Jan;7(1):199–208. doi: 10.1128/mcb.7.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Miller S. M., Kurtz M. B. One-step gene disruption by cotransformation to isolate double auxotrophs in Candida albicans. Mol Gen Genet. 1988 Sep;214(1):24–31. doi: 10.1007/BF00340174. [DOI] [PubMed] [Google Scholar]
- Kurtz M. B., Cortelyou M. W., Miller S. M., Lai M., Kirsch D. R. Development of autonomously replicating plasmids for Candida albicans. Mol Cell Biol. 1987 Jan;7(1):209–217. doi: 10.1128/mcb.7.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz M. B., Kirsch D. R., Kelly R. The molecular genetics of Candida albicans. Microbiol Sci. 1988 Feb;5(2):58–63. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lasker B. A., Carle G. F., Kobayashi G. S., Medoff G. Comparison of the separation of Candida albicans chromosome-sized DNA by pulsed-field gel electrophoresis techniques. Nucleic Acids Res. 1989 May 25;17(10):3783–3793. doi: 10.1093/nar/17.10.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee B. B., Koltin Y., Gorman J. A., Magee P. T. Assignment of cloned genes to the seven electrophoretically separated Candida albicans chromosomes. Mol Cell Biol. 1988 Nov;8(11):4721–4726. doi: 10.1128/mcb.8.11.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson A. C., Creaser E. H. Purification of a trifunctional enzyme, catalysing three steps of the histidine pathway, from Neurospora crassa. Biochem J. 1969 Aug;114(1):49–56. doi: 10.1042/bj1140049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck I., Lebens G. A. Electrophoretic karyotype of the pathogenic yeast Cryptococcus neoformans. J Gen Microbiol. 1989 Jan;135(1):65–71. doi: 10.1099/00221287-135-1-65. [DOI] [PubMed] [Google Scholar]
- Poulter R. T., Rikkerink E. H. Genetic analysis of red, adenine-requiring mutants of Candida albicans. J Bacteriol. 1983 Dec;156(3):1066–1077. doi: 10.1128/jb.156.3.1066-1077.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter R., Hanrahan V. Conservation of genetic linkage in nonisogenic isolates of Candida albicans. J Bacteriol. 1983 Nov;156(2):498–506. doi: 10.1128/jb.156.2.498-506.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter R., Hanrahan V., Jeffery K., Markie D., Shepherd M. G., Sullivan P. A. Recombination analysis of naturally diploid Candida albicans. J Bacteriol. 1982 Dec;152(3):969–975. doi: 10.1128/jb.152.3.969-975.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh A., Mevarech M., Koltin Y., Gorman J. A. Isolation of genes from Candida albicans by complementation in Saccharomyces cerevisiae. Mol Gen Genet. 1985;200(3):500–502. doi: 10.1007/BF00425739. [DOI] [PubMed] [Google Scholar]
- SMITH D. W., AMES B. N. INTERMEDIATES IN THE EARLY STEPS OF HISTIDINE BIOSYNTHESIS. J Biol Chem. 1964 Jun;239:1848–1855. [PubMed] [Google Scholar]
- Segal E., Nussbaum S., Barr-Nea L. Protection against systemic infections with various Candida species elicited by vaccination with Candida albicans ribosomes. Sabouraudia. 1985 Aug;23(4):275–285. [PubMed] [Google Scholar]
- Segal E. Pathogenesis of human mycoses: role of adhesion to host surfaces. Microbiol Sci. 1987 Nov;4(11):344–347. [PubMed] [Google Scholar]
- Segal E. Vaccines against fungal infections. Crit Rev Microbiol. 1987;14(3):229–271. doi: 10.3109/10408418709104440. [DOI] [PubMed] [Google Scholar]
- Shaffer B., Rytka J., Fink G. R. Nonsense mutations affecting the his4 enzyme complex of yeast. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1198–1205. doi: 10.1073/pnas.63.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. A., Allaudeen H. S., Whitman M. H., Koltin Y., Gorman J. A. Isolation and characterization of a beta-tubulin gene from Candida albicans. Gene. 1988;63(1):53–63. doi: 10.1016/0378-1119(88)90545-8. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Galask R., Isley S., Rao T. V., Stone D., Hicks J., Schmid J., Mac K., Hanna C. Switching of Candida albicans during successive episodes of recurrent vaginitis. J Clin Microbiol. 1989 Apr;27(4):681–690. doi: 10.1128/jcm.27.4.681-690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Soll D. R. Mitotic recombination in Candida albicans: recessive lethal alleles linked to a gene required for methionine biosynthesis. Mol Gen Genet. 1982;187(3):477–485. doi: 10.1007/BF00332632. [DOI] [PubMed] [Google Scholar]
- de Jonge P., de Jongh F. C., Meijers R., Steensma H. Y., Scheffers W. A. Orthogonal-field-alternation gel electrophoresis banding patterns of DNA from yeasts. Yeast. 1986 Sep;2(3):193–204. doi: 10.1002/yea.320020307. [DOI] [PubMed] [Google Scholar]