Abstract
An 11-kilobase-pair element interrupts the nifD gene in vegetative cells of Anabaena sp. strain PCC 7120. The nifD element normally excises only from the chromosomes of cells that differentiate into nitrogen-fixing heterocysts. The xisA gene contained within the element is required for the excision. Shuttle vectors containing the Escherichia coli tac consensus promoter fused to various 5' deletions of the xisA gene were constructed and conjugated into Anabaena sp. strain PCC 7120 cells. Some of the expression plasmids resulted in excision of the nifD element in a high proportion of vegetative cells. Excision of the element required deletion of an xisA 5' regulatory region which presumably blocks expression in Anabaena sp. strain PCC 7120 vegetative cells but not in E. coli. Strains lacking the nifD element grew normally in medium containing a source of combined nitrogen and showed normal growth and heterocyst development in medium lacking combined nitrogen. The xisA gene was shown to be the only Anabaena gene required for the proper rearrangement in E. coli of a plasmid containing the borders of the nifD element.
Full text
PDF
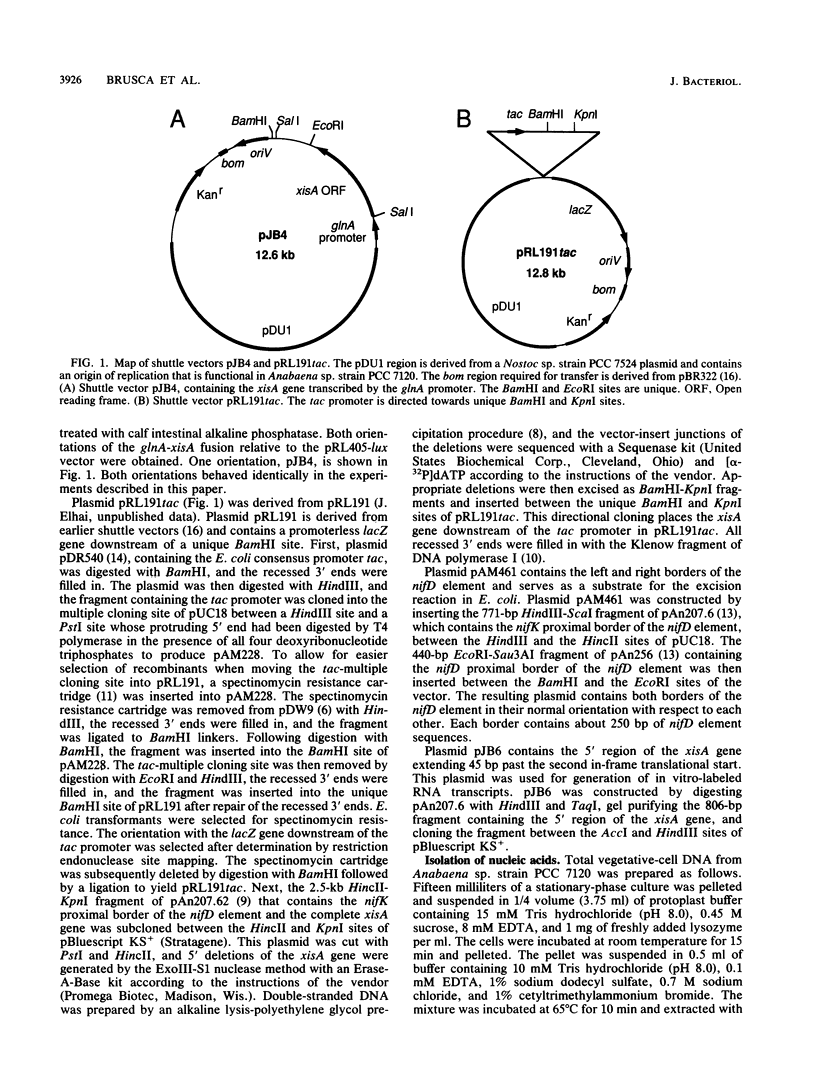


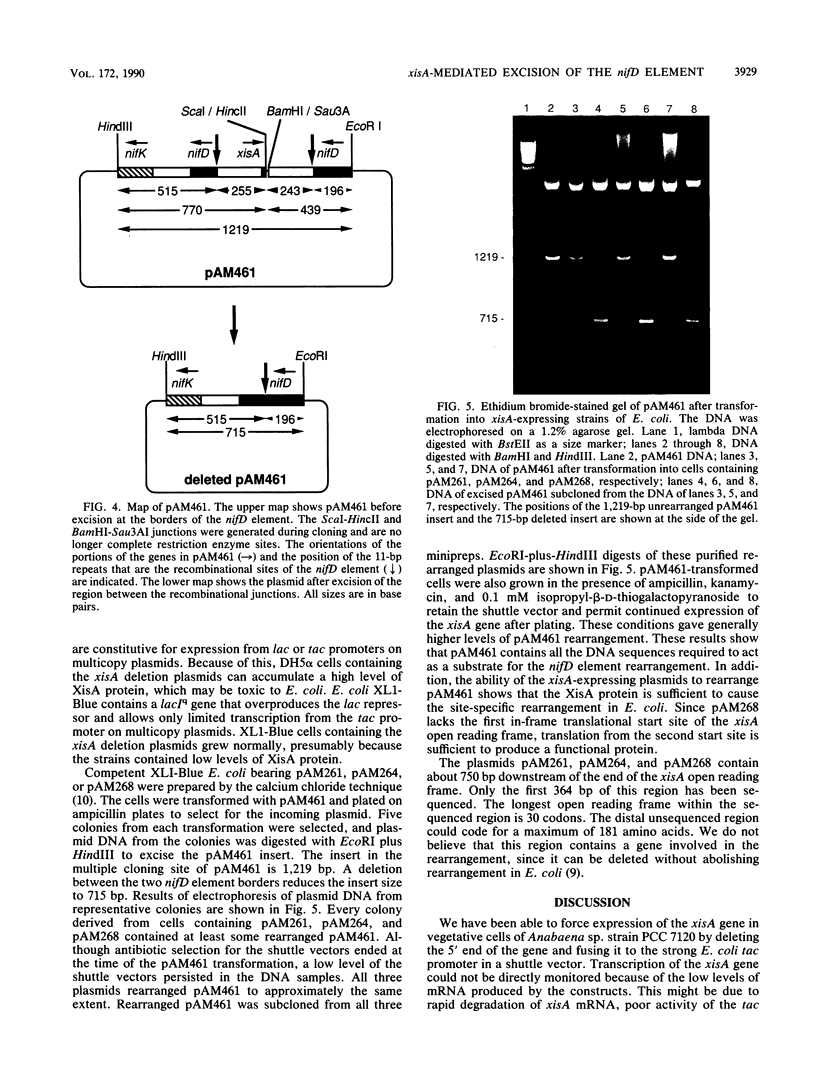


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brusca J. S., Hale M. A., Carrasco C. D., Golden J. W. Excision of an 11-kilobase-pair DNA element from within the nifD gene in anabaena variabilis heterocysts. J Bacteriol. 1989 Aug;171(8):4138–4145. doi: 10.1128/jb.171.8.4138-4145.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Wiest D. R. Genome rearrangement and nitrogen fixation in Anabaena blocked by inactivation of xisA gene. Science. 1988 Dec 9;242(4884):1421–1423. doi: 10.1126/science.3144039. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Lammers P. J., Golden J. W., Haselkorn R. Identification and sequence of a gene required for a developmentally regulated DNA excision in Anabaena. Cell. 1986 Mar 28;44(6):905–911. doi: 10.1016/0092-8674(86)90013-9. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Construction and analysis of in vivo activity of E. coli promoter hybrids and promoter mutants that alter the -35 to -10 spacing. Gene. 1982 Dec;20(2):231–243. doi: 10.1016/0378-1119(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]